- 1Lilongwe University of Agriculture and Natural Resources, Lilongwe, Malawi
- 2Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
- 3Jodrell Laboratory, Royal Botanic Gardens, Richmond, United Kingdom
- 4Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent, United Kingdom
In the fight against arthropod crop pests using plant secondary metabolites, most research has focussed on the identification of bioactive molecules. Several hundred candidate plant species and compounds are now known to have pesticidal properties against a range of arthropod pest species. Despite this growing body of research, few natural products are commercialized for pest management whilst on-farm use of existing botanically-based pesticides remains a small, but growing, component of crop protection practice. Uptake of natural pesticides is at least partly constrained by limited data on the trade-offs of their use on farm. The research presented here assessed the potential trade-offs of using pesticidal plant extracts on legume crop yields and the regulating ecosystem services of natural pests enemies. The application of six established pesticidal plants (Bidens pilosa, Lantana camara, Lippia javanica, Tephrosia vogelii, Tithonia diversifolia, and Vernonia amygdalina) were compared to positive and negative controls for their impact on yields of bean (Phaseolus vulgaris), cowpea (Vigna unguiculata), and pigeon pea (Cajanus cajan) crops and the abundance of key indicator pest and predatory arthropod species. Analysis of field trials showed that pesticidal plant treatments often resulted in crop yields that were comparable to the use of a synthetic pesticide (lambda-cyhalothrin). The best-performing plant species were T. vogelii, T. diversifolia, and L. javanica. The abundance of pests was very low when using the synthetic pesticide, whilst the plant extracts generally had a higher number of pests than the synthetic but lower numbers than observed on the negative controls. Beneficial arthropod numbers were low with synthetic treated crops, whereas the pesticidal plant treatments appeared to have little effect on beneficials when compared to the negative controls. The outcomes of this research suggest that using extracts of pesticidal plants to control pests can be as effective as synthetic insecticides in terms of crop yields while tritrophic effects were reduced, conserving the non-target arthropods that provide important ecosystem services such as pollination and pest regulation. Thus managing crop pests using plant secondary metabolites can be more easily integrated in to agro-ecologically sustainable crop production systems.
Introduction
The search for novel pest control products from plants continues to grow, but not always with clear outcomes and benefits (Isman and Grieneisen, 2013). However, there are many candidate plant species with known pesticidal properties where much is already known about their chemistry and efficacy under laboratory conditions that could be rapidly developed in to new products (Stevenson et al., 2017). Isman (2017) has argued that increasing farmer use of natural pesticides needs research directed at the practical application of such products under complex agro-ecological conditions, particularly understanding how different pesticidal plant species perform when applied to different crops under different growing conditions. Furthermore, their effects against target and non-target species, safe use and overall socio-economic and agro-ecological benefits need work. Only through their evaluation under field conditions can the evidence for more widespread adoption of natural pest control products be found, particularly as natural compounds are often not as effective as current synthetic pesticides (Casida, 1980). Using unrefined plant extracts for pest control has several advantages in terms of preventing the development of insecticide resistance due to the usual presence of several bio-active compounds, their low persistence in the environment and their generally low cost of use, particularly for smallholder farmers with limited income (Angioni et al., 2005; Caboni et al., 2006; Isman, 2008). However, disadvantages include variable efficacy, and low toxicity and persistence against target pests, which is partly due to the rapid breakdown of bio-active compounds, for example through photodegradation, and due to such extracts easily washing off when it rains. Consumers and policy makers are demanding reduced synthetic inputs in food production, and practices that support agro-ecological intensification and pesticidal plant products may be well suited to this vision (Grzywacz et al., 2014; Sola et al., 2014; Pavela, 2016).
An important trade-off to consider in the fight against arthropod crop pests using plant secondary metabolites, is the impact of crop protection strategies on ecosystem services. Pollination and natural pest regulation by arthropods are affected negatively by the use of synthetic pesticides (Rundlöf et al., 2015; Potts et al., 2016). The value of natural suppression of aphids on soya bean was valued at US$239 million in four US states (Landis et al., 2008), so the benefits of natural pest regulation can be measured in terms of environmental and economic value. Natural pest control is an ecosystem service that can be augmented and sustained by natural or manipulated agro-ecosystems (Gurr et al., 2016, 2017) as well as local land management practices which can impact on availability of pollinators on legume crops such as pigeon pea (Lautenbach et al., 2012). Many factors appear to be causing pollinator decline; however, the increasing use of synthetic pesticides is one of the primary causes (Potts et al., 2016), and policies that facilitate more environmentally benign approaches are required for sustainable agriculture (Dicks et al., 2016). Although some research has been conducted on the impact of pesticidal plant use on non-target arthropods (Mkenda et al., 2015; Mkindi et al., 2017), this remains a neglected area of research that needs further investigation to understand the trade-offs of using more plant-based pest control products.
The research presented here established whether crude extracts of six cosmopolitan pesticidal plant species have potential as the basis of biopesticides on different legume crops and to understand the impacts of using pesticidal plants on non-target arthropods.
Materials and Methods
Study Site
The study was conducted at field sites in Tanzania and Malawi over three years where common bean (Phaseolus vulgaris) was grown during 2015, cowpea (Vigna unguiculata) was grown during 2016 and pigeon pea (Cajanus cajan) was grown during 2017 cropping seasons. Field trials were carried out at Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania (Latitude 3°24′S Longitude 36°47′E and at Lilongwe University of Agriculture and Natural Resources, Bunda, Malawi (Latitude 14°11′S Longitude 33°46′E). In Tanzania, the location was at an elevation of 1,168 masl with a mean annual rainfall of 1,200 mm, mean maximum temperature of 21.7°C and mean minimum temperature of 13.6°C. For Malawi, the location was at an elevation of 1,100 masl with a mean annual rainfall of 700 mm, mean maximum temperature of 29°C and mean minimum temperature of 17°C.
Experimental Design
The farm land where field trials took place was disc harrowed and ridged prior to planting. The common bean seeds used for planting were of the variety Lyamungo 90 in Tanzania and Kalima in Malawi. In Tanzania, the seeds were planted at a spacing of 50 cm between rows and 20 cm within rows in 5 × 5 m plots which were 1 m apart. In Malawi, beans were planted 75 cm part with 2 rows of beans on each ridge, with rows spaced 10 cm apart and ridges 30 cm apart and plants spaced 10 cm within rows in 5 × 5 m plots which were 1 m apart. The cowpea seeds used for planting were of the variety Raha1 in Tanzania and Mkanakaufiti in Malawi. In Tanzania, the seeds were planted at a spacing of 50 cm between rows and 20 cm within rows in 5 × 5 m plots which were 1 m apart. In Malawi, cowpeas were planted on ridges of 75 cm apart with 1 row on each ridge, spaced at 20 cm within rows in 5 × 5 m plots which were 1 m apart. The pigeon pea seeds used for planting were of the variety Mali in Tanzania and Mthawajuni in Malawi. In Tanzania, the pigeon pea seeds were planted at a spacing of 75 cm between rows and 30 cm within rows in 5 × 5 m plots which were 2 m apart. In Malawi, pigeon pea were planted at a spacing of 75 cm between rows and 60 cm within rows in 5 × 5 m plots which were 2 m apart. Diammonium phosphate fertilizer was applied according to manufacturer's instructions during planting of the seeds. Trials were inspected each week with ad hoc hand weeding carried out as necessary. The experimental layout was a randomized complete block design, and the treatments were replicated on four blocks (Anderson and McLean, 1974).
Plant Species Collection and Processing
Fresh leaves of Tephrosia vogelii (Hook f.) (Fabales: Fabaceae), Vernonia amygdalina (Delile) (Asterales: Asteraceae), Lippia javanica (Burm.f.) Spreng. (Lamiales: Verbenaceae), Tithonia diversifolia (Hemsl.) A. Gray (Asterales: Asteraceae), Bidens pilosa L. (Asterales: Asteraceae), and Lantana camara L. (Lamiales: Verbenaceae) were collected from different locations around Hai District, Tanzania and Mitundu District, Malawi (voucher specimens and GPS coordinates lodged at Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania and Lilongwe University of Agriculture and Natural Resources, Bunda, Malawi). Common bean field trials were carried out with four plant species (L. javanica, T. diversifolia, T. vogelii, and V. amygdalina in Tanzania and L. camara, T. diversifolia, T. vogelii, and V. amygdalina in Malawi) whilst cowpea and pigeon pea trials were carried out with all six plant species. These plant species were chosen due to their wide abundance around farms, roadsides, and bushland, their familiarity to farmers and considerable existing knowledge on their efficacy, bioactive constituents and safety (Ganjian et al., 1983; Pereira et al., 1997; Gu et al., 2002; Adedire and Akinneye, 2004; Kawuki et al., 2005; Viljoen et al., 2005; Ambrósio et al., 2008; Asawalam et al., 2008; Mujovo et al., 2008; Oyewole et al., 2008; Bagnarello et al., 2009; Gadzirayi et al., 2009; Adeniyi et al., 2010; Madzimure et al., 2011; Belmain et al., 2012; Stevenson et al., 2012). To ensure uniformity, the leaves from each seasonal collection were mixed together for each species before drying. Leaves were dried under shade for a week and then crushed using a mill and sieved into a fine powder. Powders were stored in black plastic bags in dark, dry conditions until required. New plant material was harvested each year.
Field Treatments
Data on the effect of plant extract concentration has been reported with expected dose response trends on crop yield when applying plant treatments at 0.1, 1.0, and 10% w/v (Mkindi et al., 2017). All data presented here are based on the application of 10% w/v as this was determined to be the most effective concentration for reducing insect damage and maintaining high crop yield (Mkindi et al., 2017). In making 10% extracts, 1 kg of plant powder was weighed and added to 10 L water to extract at ambient temperature (20 ± 5°C) for 24 h. In all cases 0.1% soap was added to the water during extraction as detergent increases the extraction efficiency of non-polar compounds from plant material (Belmain et al., 2012). Extracts were kept in 10 L buckets with lids in the shade and, shortly before application, filtered twice through a coarse and then fine cloth to remove all plant material that may inadvertently clog the sprayer. Negative controls consisted of water +0.1% soap and water only. The positive control in all trials was synthetic pesticide Karate 5 EC (lambda-cyhalothrin pyrethroid, Syngenta) which was applied as per the manufacturers' instructions (20 g/ha). All treatments were replicated across four blocks and were sprayed throughout the growing season at an interval of 7 days starting 1 week after crop plant emergence. A 15 L knapsack sprayer was used to apply the various treatments, and the sprayer was thoroughly cleaned with soap and water prior to being re-filled with another formulation for application.
Sampling for Presence of Arthropod Pest and Beneficial Species
All assessments were carried out the day before treatments were to be sprayed. Three inner rows from each plot were selected for sampling. Five plants in the selected three middle rows were visually examined to record the number of each arthropod type. Preliminary work indicated that a number of pest and beneficial species were documented on legume crops; however, many were only present infrequently or in low numbers. Furthermore, to assist with data collection, enumerators focussed on more obvious life stages and relatively larger insects. For example, parasitoids and larval forms of hoverflies and lacewings were not monitored due to their small size and difficulty to assess quickly. Thus indicator pest and beneficial species were chosen for monitoring that were easily identified and observed to be abundant throughout the cropping season. Thus the main target pest species evaluated were aphids (Aphis fabae Scopoli) (Hemiptera: Aphididae), bean foliage beetle (Ootheca mutabilis (Schönherr) and O. bennigseni Weise) (Chrysomelidae: Galerucinae) and flower beetle (Epicauta albovittata Gestro and E. limbatipennis Pic) (Coleoptera: Meloidae). The target predatory species were lady beetles (adults and larvae) (Coccinellidae), spiders (Araneae), and hoverflies (adults only) (Syrphidae). Due to often very high numbers, a categorical index was used to assess aphid abundance, where 0 = None; 1 = A few scattered individuals; 2 = A few isolated colonies; 3 = Several isolated colonies; 4 = Large isolated colonies; and 5 = Large continuous colonies (Mkindi et al., 2017). For analytical purposes, this index was used as a proxy to report aphid numbers. For all other arthropod species, the actual number of individuals was counted.
Data Analysis
Differences among treatments in insect abundance and bean yield were assessed by analysis of variance (ANOVA) and Tukey's post-hoc Honestly Significant Difference (HSD) test to separate the means at the 95% confidence interval. Analyses were performed in XLSTAT version 2015.1.01 (Addinsoft, Paris, France). Datasets are available on request.
Results
Arthropod Abundance
Field trials carried out across the two countries with three different legume crops over three seasons showed similar trends with respect to pest and beneficial arthropod abundance (Figure 1). The general trend shared across trials was that the positive control synthetic pesticide treatment had very low numbers of both pest and beneficial species, with the negative controls (water only and water with soap) usually having the highest numbers. The pesticidal plant treatments did reduce numbers of both pest and beneficial species but these data more generally followed the abundance observed in the negative controls as opposed to the positive control. An analysis of variance confirms these trends for each crop and location (Table 1). Lippia javanica, T. vogelii, and T. diversifolia were the most able to reduce pest insect numbers, showing similar effects to the synthetic pesticide in cowpea and bean crops but not for pigeon pea. Bidens pilosa, L. camara, and V. amygdalina showed similar pest abundance as to that observed in the negative controls. Pest abundance was higher on pigeon pea than on cowpea or bean crops, and this is likely related to the larger size of each plant where pigeon pea grows to ~1.5 m high whereas cowpea and bean crops are <0.5 m high. However, this trend in relative abundance among crops was not strongly observed for beneficial species. Abundance of beneficial species was highest for bean crops, but this was not found to differ significantly from abundance observed in cowpea or pigeon pea crops (Table 1). Beneficial insect numbers in cowpea crops treated with pesticidal plants were higher than that observed in the negative controls, while lower numbers of beneficials were found in bean and pigeon pea when compared to the negative controls. In all crops, the synthetic positive control significantly reduced beneficial numbers compared to all other treatments.
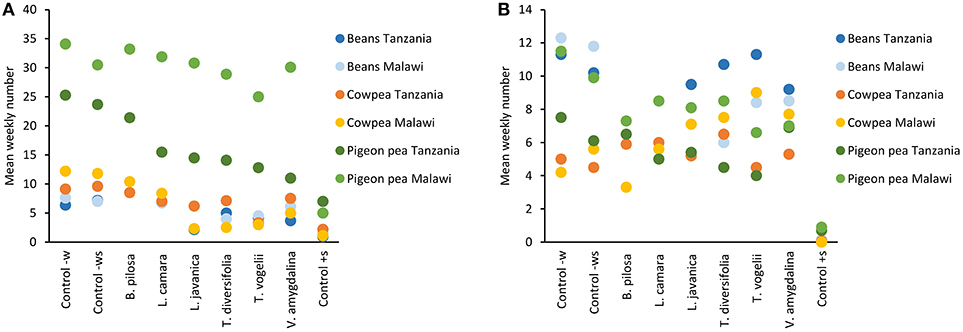
Figure 1. Effect of different pesticidal plant treatments on mean weekly number of (A) key indicator pest species (aphids, flower beetles and foliage beetles) and (B) key indicator beneficial species (lady beetles, spiders and hoverflies). All plant treatments were applied at 10% w/v with 0.1% liquid soap added to the water during the 24 h extraction period. Control–w is the application of water only, Control–ws is the application of water containing 0.1% liquid soap only, and Control+s is the application of the synthetic pesticide Karate 5 EC (lambda-cyhalothrin pyrethroid, Syngenta) which was applied as per the manufacturers' instructions (20 g/ha).
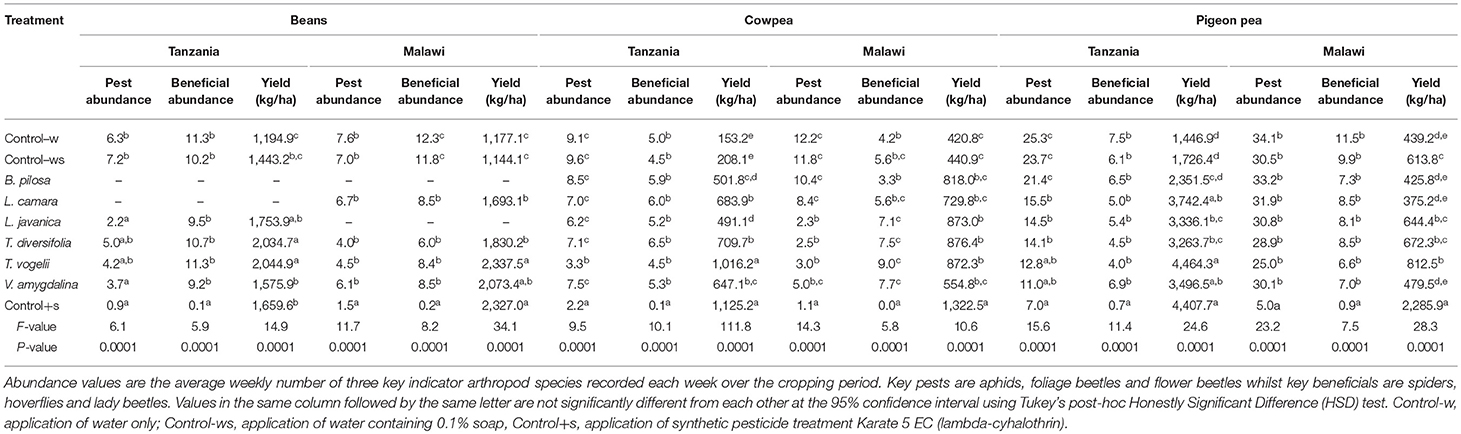
Table 1. Treatment effect on abundance of key pest and beneficial arthropod species and legume crop yield during field cropping trials in the countries of Tanzania and Malawi.
Crop Yield
Despite insect pest abundance on crops treated with pesticidal plants being significantly higher than observed with the synthetic control, crop yields obtained from pesticidal plant treatments were often comparable to the synthetic pesticide treatment (Figure 2). This is most notable with the use of T. vogelii in Tanzania where the yields were statistically comparable for cowpea (1,016–1,125 kg/ha) and pigeon pea (4,407–4,464 kg/ha) and where the bean yield was statistically higher for T. vogelii compared to the positive control (2,044 vs. 1,659 kg/ha). Tephrosia vogelii treated plants also performed as well as the synthetic pesticide treated on beans in Malawi, but its application on cowpea and pigeon pea produced lower yields than the synthetic in Malawi. Other plant treatments that generally performed well across locations and crops were L. javanica and T. diversifolia. With the exception of pigeon pea crop yields in Malawi, the negative control treatments (water only, water + soap) had the lowest yields of all the treatments. In some cases, yields of certain pesticidal plant treatments were no better than the untreated controls, notably B. pilosa on pigeon pea in Tanzania and V. amygdalina on cowpea in Malawi. However, these species did work effectively in other contexts, e.g., B. pilosa was effective on cowpea and V. amygdalina was effective on bean crops. None of the pesticidal plants appeared to be effective on pigeon pea in Malawi. We suspect this was probably caused by frequent high rainfall in Central Malawi during the 2017 cropping season that inadvertently washed off the pesticidal plant treatments before they were able to affect pests. The synthetic pesticide was still effective on pigeon pea in Malawi, arguably because it was more resistant to frequent rainfall events. An analysis of variance performed on crop yields confirms these observations on yield differences among treatments (Table 1). Comparing the crop yields obtained with the water only application with the different treatments showed that the plant treatments were able to significantly increase the percentage crop yield (Table 2). The simple addition of 0.1% soap to the water generally showed a 20–30% increase in crop yields, most likely due to the well-known effects of soaps on arthropod cuticles and water regulation (Butler et al., 1993). The synthetic control showed the largest percentage increase in yield. However, T. vogelii showed comparable yield increases for cowpea and pigeon pea in Tanzania and even exceeded the synthetic on beans in Tanzania. Crop yield will also be affected by the different varieties planted and the slightly different crop spacing employed in each country. Yields are well-known to differ by crop variety (Wallace and Munger, 1966; Talbot, 1984) and other location specific issues such as soil type (Chmelíková et al., 2015), thus preventing statistical analyses of our field trials across locations due to uncontrolled environmental parameters.
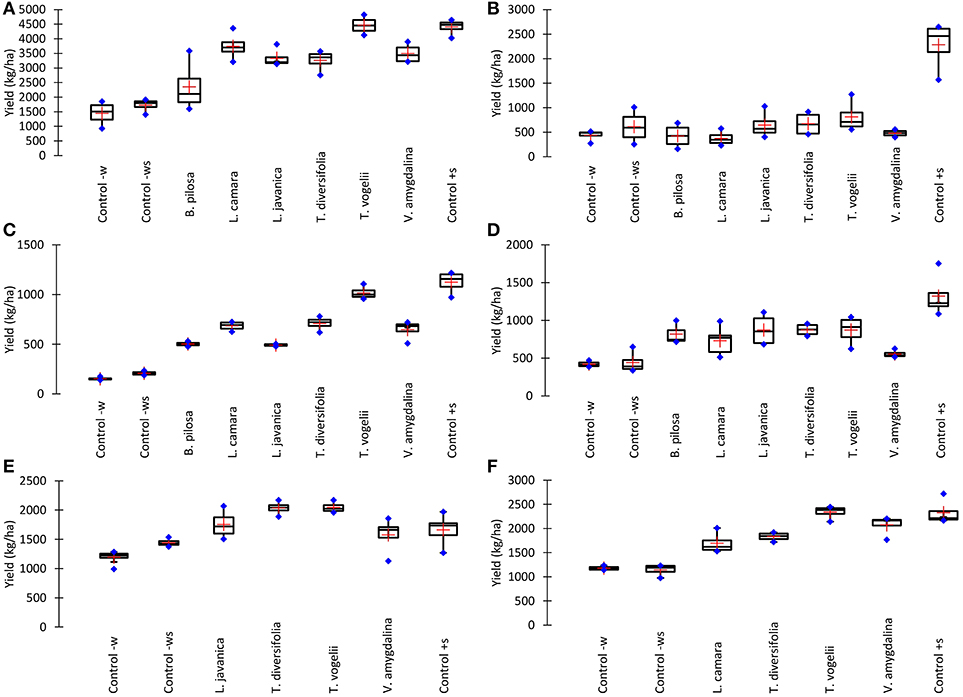
Figure 2. Effect of different pesticidal plant treatments on crop yield of (A) Pigeon pea grown in Tanzania, (B) Pigeon pea grown in Malawi, (C) Cowpea grown in Tanzania, (D) Cowpea grown in Malawi, (E) Common beans grown in Tanzania and (F) Common beans grown in Malawi. All plant treatments were applied at 10% w/v with 0.1% liquid soap added to the water during the 24 h extraction period. Control–w is the application of water only, Control–ws is the application of water containing 0.1% liquid soap only, and Control+s is the application of the synthetic pesticide Karate 5 EC (lambda-cyhalothrin pyrethroid, Syngenta) which was applied as per the manufacturers' instructions (20 g/ha). Boxes represent mean and 95% confidence intervals, blue markers are max. and min. values, orange markers are median values.
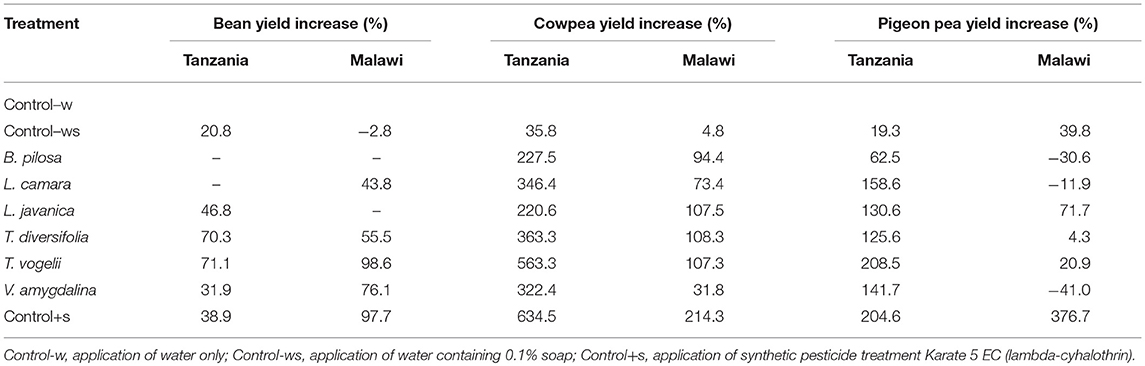
Table 2. Percentage yield increase when comparing the yield obtained from the untreated control applying water only to the yields obtained from applying the other treatments.
Discussion
Previous research on the pesticidal plant species used for pest control in this study all have reported bioactivities against insects, parasites, bacteria and fungi (Ganjian et al., 1983; Jisaka et al., 1992; Lina et al., 1992; Pereira et al., 1997; Gu et al., 2002; Rabe et al., 2002; Ogendo et al., 2003; Adedire and Akinneye, 2004; Boeke et al., 2004; Omolo et al., 2004; Kawuki et al., 2005; Koona and Dorn, 2005; Viljoen et al., 2005; Koona et al., 2007; Ambrósio et al., 2008; Asawalam et al., 2008; Mujovo et al., 2008; Oyewole et al., 2008; Bagnarello et al., 2009; Chukwujekwu et al., 2009; Deng, 2009; Gadzirayi et al., 2009; Koul and Walia, 2009; Madzimure et al., 2011; Tesch et al., 2011; Chagas-Paula et al., 2012; Bartolome et al., 2013; Ellse and Wall, 2013; Nhamo et al., 2013; Utono et al., 2014; Green et al., 2017; Kamanula et al., 2017); however, none of these works have investigated the effects of their application on field crop performance or tritrophic impact. Much is also known about the phytochemistry of the six species evaluated. Previous analysis of L. javanica has shown the major bioactive component to be camphor, along with minor components including camphene, α-pinene, eucalyptol, Z and E α-terpineol, linalool, cymene, thymol, 2-carene, caryophyllene, and α-cubebene (Mkenda et al., 2015). Camphor has well-documented insecticidal properties (Singh et al., 2014). Chemical analysis of T. vogelii has indicated the presence of the rotenoids deguelin, tephrosin and rotenone (with deguelin being the most abundant) (Belmain et al., 2012; Stevenson et al., 2012). Rotenoids are well-known for their anti-insect properties (Ott, 2006). The major compounds in T. diversifolia confirmed to have anti-insect properties were identified as the sesquiterpene lactones tagitinin A and tagitinin C (Green et al., 2017). Both compounds were reported recently to be the major compounds in this species (Miranda et al., 2015) while other research indicated tagitinins to have insecticidal activity (Ambrósio et al., 2008). The main anti-insect components of V. amygdalina were identified as vernodalin and 11,13-dihydrovernodalin as well as several vernoniosides (Green et al., 2017). Like tagitinin A and C, the first two compounds are sesquiterpene lactones which have exhibited antimalarial, antibacterial and cytotoxic activities (Rabe et al., 2002; Chukwujekwu et al., 2009). The major bioactive components of L. camara are germacrene D, β-caryophyllene, a-phellandrene, limonene, and 1,8-cineole (Tesch et al., 2011). Bioactive constituents from B. pilosa include β-caryophyllene and τ-cadinene (Deba et al., 2008). B. pilosa bioactivity is mainly reported as pharmacological, allopathic, anti-fungal, and anti-bacterial, with no clear anti-insect properties previously noted (Bartolome et al., 2013).
Synthetic pesticides are often misused leading to negative effects on ecosystems and human health, particularly in developing countries (Ecobichon, 2001). Using biocontrol options such as extracts of pesticidal plants has long been argued to be more sustainable and appropriate for smallholder farmers in developing countries (Isman, 2006, 2008; Sola et al., 2014), and our data support this and show that use of pesticidal plants can effectively control pests and be integrated into sustainable agricultural practice. Our data also showed that plant pesticide pest control on legume crops could support yields similar to those on which synthetic pesticides were used. This required relatively frequent weekly application of 10% w/v plant extracts highlighting a trade-off of using pesticidal plants since the active components break down quickly and have low persistence (Casida, 1980). This means that labor inputs must increase when using crude preparations of pesticidal plants, although the commercialization of botanical products that incorporate photostabilizers and sticking agents could prolong their efficacy on crops. This sort of trade-off is generally accepted by many smallholder farmers because using synthetic pesticides requires financial outlay, whereas using pesticidal plant materials simply requires labor costs for harvesting and processing. Economic analyses of pesticidal plant use in Africa generally show that they are more cost-beneficial for smallholder farmers than using synthetic pesticides as pesticidal plant use reduces input costs, even when costing in extra labor, with generally little yield loss trade-off (Amoabeng et al., 2014; Mkenda et al., 2015). On the other hand lower persistence of pesticidal plants means that the health of consumers is at less risk owing to reduced exposure to bioactive compounds from the plants which decompose into harmless natural products unlike the synthetic compounds that persist in and on plants for weeks or in soil for months or years. This means that crops can be harvested without the risk of residues remaining due to the rapid breakdown of naturally occurring compounds when exposed to UV light, and micro-organisms in soil/water (Isman, 2000; Angioni et al., 2005; Caboni et al., 2006). Another benefit for smallholder farmers is that it enables production for higher value organic markets and for export.
The research presented highlights another important trade-off when comparing synthetic vs. pesticidal plant crop protection effects on non-target species. The persistence and generic toxicity of synthetic pesticides inevitably means their impact on pollinators, predators, and parasitoids is usually very high (Potts et al., 2010; Stanley and Preetha, 2016). Indeed, our research showed that a synthetic pesticide commonly used on legume crops and other horticultural produce resulted in the absence of nearly all beneficial indicator species monitored over the cropping season. This typically leads to the phenomenon of pest resurgence once the synthetic pesticide wears off enabling pest species populations to expand in the absence of predatory species (Roubos et al., 2014; Welch and Harwood, 2014). However, the pesticidal plant treatments had much less of an impact on indicator beneficial species. Some of the plant materials did reduce the numbers of beneficials in comparison to the untreated (water and water+soap) controls, but in all cases these reductions were not as severe as that observed in the synthetic treatment. Overall, there was very little difference among the pesticidal plant treatments and the untreated controls in terms of beneficial numbers. We expect this is partly due to lower persistence of plant treatments but also due to different modes of action where the plant treatments may be acting against pests as repellents, anti-feedants or through toxicity post-ingestion. Lower toxicity and persistence of the pesticidal plant treatments is supported through their reduced effects on indicator pest species. In only a few cases were the pesticidal plant treatments just as successful as the synthetic in reducing pest abundance. Pest and beneficial species were less affected by the pesticidal plant treatments compared to the synthetic. We would argue that this would help natural pest regulation manage pest numbers more effectively because natural pest regulation is most effective when the ratio between pest and predator numbers is low (Arditi and Ginzburg, 1989; Rusch et al., 2010). The continual knock-down provided by the pesticidal plant treatments allows the beneficial species to contribute to pest regulation at a meaningful scale (Crowder et al., 2010). The protection and facilitation of ecosystem services provided by pollinators, predators and parasitoids through using pesticidal plants is a strong argument for the adoption of pesticidal plant extracts in crop protection.
Despite higher numbers of pests on the pesticidal plant treatments compared to the synthetic treatment, yields were often comparable. This could be due to further pest reduction through natural enemies. However, this may also be because plant species can tolerate a certain amount of damage and are able to physiologically compensate to maintain overall yield (Rubia et al., 1996; Brown, 2005). Compensation usually requires that plants are generally in good health, with access to sufficient nutrients and water, with little other sources of stress (Tardieu and Tuberosa, 2010). Although we did not measure such responses, legume crop compensation may have been facilitated through the frequent application of pesticidal plant extracts. This could involve other forms of plant protection by direct control of bacterial or fungal pathogens (Soylu et al., 2010; Marei et al., 2012; Rasoul et al., 2012), or indirect physiological assistance by acting as a topical green fertilizer (Jama et al., 2000), bio-stimulant (Pretali et al., 2016), or foliar feed (Shaaban, 2001). We are undertaking further field trials to assess the multiple benefits of using pesticidal plants for smallholder crop production, which should provide more evidence for their integration in to agro-ecologically sustainable crop production systems.
Author Contributions
SB, PS, YT, and PN conceived the study. AM, PM, RM, and NM were involved in the study design. SB carried out the statistical analysis and wrote the first draft of the manuscript. YT, AM, PM, RM, and NM carried out field trials and data collection. All authors were involved in writing the manuscript, and gave final approval for publication.
Funding
This research was funded by McKnight Foundation grants (#13-335 and #17-070, http://ccrp.org/) to SB and a Darwin Initiative grant (22-012, http://www.darwininitiative.org.uk/ project/22012) to PS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the technical field assistance provided by Philipo Mashamba, Damas Mnyang'ali, Canisius Kayombo, and Harrison Monyo at NMAIST and technical field assistance by Maxwell Katulu, Haighten Francis, Aubrey Kaphukusi, Luci Ng'ambani, and Late Sempulo Kanthiti at LUANAR. We are grateful to Dr. Ernest Mbega and Prof Vernon Kabambe for providing cowpea seeds for Tanzania and Malawi field trials, respectively.
References
Adedire, C. O., and Akinneye, J. O. (2004). Biological activity of tree marigold, Tithonia diversifolia, on cowpea seed bruchid, Callosobruchus maculatus (Coleoptera: Bruchidae). Ann. Appl. Biol. 144, 185–189. doi: 10.1111/j.1744-7348.2004.tb00332.x
Adeniyi, S. A., Orjiekwe, C. L., Ehiagbonare, J. E., and Arimah, B. D. (2010). Preliminary phytochemical analysis and insecticidal activity of ethanolic extracts of four tropical plants (Vernonia amygdalina, Sida acuta, Ocimum gratissimum and Telfaria occidentalis) against beans weevil (Acanthscelides obtectus). Int. J. Phys. Sci. 5, 753–762.
Ambrósio, S. R., Oki, Y., Heleno, V. C. G., Chaves, J. S., Nascimento, P. G. B. D., Lichston, J. E, et al. (2008). Constituents of glandular trichomes of Tithonia diversifolia: relationships to herbivory and antifeedant activity. Phytochemistry 69, 2052–2060. doi: 10.1016/j.phytochem.2008.03.019
Amoabeng, B. W., Gurr, G. M., Gitau, C. W., and Stevenson, P. C. (2014). Cost:benefit analysis of botanical insecticide use in cabbage: implications for smallholder farmers in developing countries. Crop Prot. 57, 71–76. doi: 10.1016/j.cropro.2013.11.019
Anderson, V. L., and McLean, R. A. (1974). Design of Experiments: A Realistic Approach. New York, NY: M. Dekker.
Angioni, A., Dedola, F., Minelli, E. V., Barra, A., Cabras, P., and Caboni, P. (2005). Residues and half-life times of pyrethrins on peaches after field treatments. J. Agric. Food Chem. 53, 4059–4063. doi: 10.1021/jf0477999
Arditi, R., and Ginzburg, L. R. (1989). Coupling in predator-prey dynamics: ratio-Dependence. J. Theor. Biol. 139, 311–326. doi: 10.1016/S0022-5193(89)80211-5
Asawalam, E. F., Emosairue, S. O., and Hassanali, A. (2008). Contribution of different constituents to the toxicity of the essential oil constituents of Vernonia amygdalina (Compositae) and Xylopia aetiopica (Annonaceae) on maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Afr. J. Biotechnol. 7, 2957–2962. doi: 10.4314/ajb.v7i16.59209
Bagnarello, G., Hilje, L., Bagnarello, V., Cartín, V., and Calvo, M. (2009). Phagodeterrent activity of the plants Tithonia diversifolia and Montanoa hibiscifolia (Asteraceae) on adults of the pest insect Bemisia tabaci (Homoptera: Aleyrodidae). Rev. Biol. Trop. 57, 1201–1215. doi: 10.15517/rbt.v57i4.5457
Bartolome, A. P., Villase-or, I. M., Yang, W. C., and Yang, W. C. (2013). Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evid. Based. Complement. Alternat. Med. 2013:340215. doi: 10.1155/2013/340215
Belmain, S. R., Amoah, B. A., Nyirenda, S. P., Kamanula, J. F., and Stevenson, P. C. (2012). Highly variable insect control efficacy of Tephrosia vogelii chemotypes. J. Agric. Food Chem. 60, 10055–10063. doi: 10.1021/jf3032217
Boeke, S. J., Kossou, D. K., Huis, A. V., Loon, V. J. J., and Dicke, M. (2004). Field trials with plant products to protect stored cowpea against insect damage. Int. J. Pest Manag. 50, 1–9. doi: 10.1080/09670870310001619282
Brown, P. R. (2005). The effect of simulated house mouse damage to wheat in Australia. Crop Prot. 24, 101–109. doi: 10.1016/j.cropro.2004.06.012
Butler, G. D., Henneberry, T. J., Stansly, P. A., Schuster, D. J., and Schuster, D. J. (1993). Insecticidal effects of selected soaps, oils and detergents on the sweetpotato whitefly: (Homoptera: Aleyrodidae). Florida Entomol. 76:161. doi: 10.2307/3496023
Caboni, P., Sarais, G., Angioni, A., Garcia, A. J., Lai, F., Dedola, F., et al. (2006). Residues and persistence of neem formulations on strawberry after field treatment. J. Agric. Food Chem. 54, 10026–10032. doi: 10.1021/jf062461v
Casida, J. E. (1980). Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. 34, 189–202.
Chagas-Paula, D. A., Oliveira, R. B., Rocha, B. A., and Da Costa, F. B. (2012). Ethnobotany, chemistry, and biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 9, 210–235. doi: 10.1002/cbdv.201100019
Chmelíková, L., Wolfrum, S., Schmid, H., Hejcman, M., and Hülsbergen, K. J. (2015). Seasonal development of biomass yield in grass–legume mixtures on different soils and development of above- and belowground organs of Medicago sativa. Arch. Agron. Soil Sci. 61, 329–346. doi: 10.1080/03650340.2014.936854
Chukwujekwu, J. C., Lategan, C. A., Smith, P. J., Van Heerden, F. R., and Van Staden, J. (2009). Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. South Afr. J. Bot. 75, 176–179. doi: 10.1016/j.sajb.2008.10.001
Crowder, D. W., Northfield, T. D., Strand, M. R., and Snyder, W. E. (2010). Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112. doi: 10.1038/nature09183
Deba, F., Xuan, T. D., Yasuda, M., and Tawata, S. (2008). Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. radiata. Food Control 19, 346–352. doi: 10.1016/j.foodcont.2007.04.011
Deng, A. L. (2009). Factors determining the use of botanical insect pest control methods by small-holder farmers in the Lake Victoria basin, Kenya. Afr. J. Environ. Sci. Technol. 3, 108–115. doi: 10.5897/AJEST09.034
Dicks, L. V., Viana, B., Bommarco, R., Brosi, B., Arizmendi, M. D. C., Cunningham, S. A., et al. (2016). Ten policies for pollinators. Science 354, 975–976. doi: 10.1126/science.aai9226
Ecobichon, D. J. (2001). Pesticide use in developing countries. Toxicology 160, 27–33. doi: 10.1016/S0300-483X(00)00452-2
Ellse, L., and Wall, R. (2013). The use of essential oils in veterinary ectoparasite control: a review. Med. Vet. Entomol. 28, 233–243. doi: 10.1111/mve.12033
Gadzirayi, C. T., Mutandwa, E., Mwale, M., and Chindundu, T. (2009). Utilization of Tephrosia vogelii in controlling ticks in dairy cows by small-scale commercial farmers in Zimbabwe. Afr. J. Biotechnol. 8, 4134–4136. doi: 10.5897/AJB2009.000-9396
Ganjian, I., Kubo, I., and Fludzinski, P. (1983). Insect antifeedant elemanolide lactones from Vernonia amygdalina. Phytochemistry 22, 2525–2526. doi: 10.1016/0031-9422(83)80154-X
Green, P. W. C., Belmain, S. R., Ndakidemi, P. A., Farrell, I. W., and Stevenson, P. C. (2017). Insecticidal activity of Tithonia diversifolia and Vernonia amygdalina. Ind. Crops Prod. 110, 15–21. doi: 10.1016/j.indcrop.2017.08.021
Grzywacz, D., Stevenson, P. C., Mushobozi, W. L., Belmain, S. R., and Wilson, K. (2014). The use of indigenous ecological resources for pest control in Africa. Food Secur. 6, 71–86. doi: 10.1007/s12571-013-0313-5
Gu, J. Q., Gills, J. J., Park, E. J., Mata-Greenwood, E., Hawthorne, M. E., Axelrod, F., et al. (2002). Sesquiterpenoids from Tithonia diversifolia with potential cancer chemopreventive activity. J. Nat. Prod. 65, 532–536. doi: 10.1021/np010545m
Gurr, G. M., Lu, Z., Zheng, X., Xu, H., Zhu, P., Chen, G., et al. (2016). Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2:16014. doi: 10.1038/nplants.2016.14
Gurr, G. M., Wratten, S. D., Landis, D. A., and You, M. (2017). Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 62, 91–109. doi: 10.1146/annurev-ento-031616-035050
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Prot. 19, 603–608. doi: 10.1016/S0261-2194(00)00079-X
Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi: 10.1146/annurev.ento.51.110104.151146
Isman, M. B. (2008). Botanical insecticides: for richer, for poorer. Pest Manag. Sci. 64, 8–11. doi: 10.1002/ps.1470
Isman, M. B. (2017). Bridging the gap: moving botanical insecticides from the laboratory to the farm. Ind. Crops Prod. 110, 10–14. doi: 10.1016/j.indcrop.2017.07.012
Isman, M. B., and Grieneisen, M. L. (2013). Botanical insecticide research: many publications, limited useful data. Trends Plant Sci. 19, 140–145. doi: 10.1016/j.tplants.2013.11.005
Jama, B., Palm, C. A., Buresh, R. J., Niang, A., Gachengo, C., Nziguheba, G., et al. (2000). Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: a review. Agrofor. Syst. 49, 201–221. doi: 10.1023/A:1006339025728
Jisaka, M., Ohigashi, H., Takagaki, T., Nozaki, H., Tada, T., Hirota, M., et al. (1992). Bitter steroid glucosides, vernoniosides A1, A2, and A3, and related B1 from a possible medicinal plant, Vernonia amygdalina, used by wild chimpanzees. Tetrahedron 48, 625–632. doi: 10.1016/S0040-4020(01)88123-0
Kamanula, J. F., Belmain, S. R., Hall, D. R., Farman, D. I., Goyder, D. J., Mvumi, B. M., et al. (2017). Chemical variation and insecticidal activity of Lippia javanica (Burm. f.) Spreng essential oil against Sitophilus zeamais Motschulsky. Ind. Crops Prod. 110, 75–82. doi: 10.1016/j.indcrop.2017.06.036
Kawuki, R. S., Agona, A., Nampala, P., and Adipala, E. (2005). A comparison of effectiveness of plant-based and synthetic insecticides in the field management of pod and storage pests of cowpea. Crop Prot. 24, 473–478. doi: 10.1016/j.cropro.2004.09.017
Koona, P., and Dorn, S. (2005). Extracts from Tephrosia vogelii for the protection of stored legume seeds against damage by three bruchid species. Ann. Appl. Biol. 147, 43–48. doi: 10.1111/j.1744-7348.2005.00006.x
Koona, P., Tatchago, V., and Malaa, D. (2007). Impregnated bags for safer storage of legume grains in West and Central Africa. J. Stored Prod. Res. 43, 248–251. doi: 10.1016/j.jspr.2006.06.005
Koul, O., and Walia, S. (2009). Comparing impacts of plant extracts and pure allelochemicals and implications for pest control. CAB Rev. 4:30. doi: 10.1079/PAVSNNR20094049
Landis, D. A., Gardiner, M. M., van der Werf, W., and Swinton, S. M. (2008). Increasing corn for biofuel production reduces biocontrol services in agricultural landscapes. Proc. Natl. Acad. Sci. U. S. A 105, 20552–20557. doi: 10.1073/pnas.0804951106
Lautenbach, S., Seppelt, R., Liebscher, J., and Dormann, C. F. (2012). Spatial and temporal trends of global pollination benefit. PLoS ONE 7:e35954. doi: 10.1371/journal.pone.0035954
Lina, E. C., Dadang, D., Manuwoto, S., Syahbirin, G., and Prijono, D. (1992). Synergistic action of mixed extracts of Brucea javanica (Simaroubaceae), Piper aduncum (Piperaceae), and Tephrosia vogelii (Leguminosae) against cabbage head caterpillar, Crocidolomia pavonana. J. Biopestic. 6, 77–83.
Madzimure, J., Nyahangare, E. T., Hamudikuwanda, H., Hove, T., Stevenson, P. C., Belmain, S. R., et al. (2011). Acaricidal efficacy against cattle ticks and acute oral toxicity of Lippia javanica (Burm F.) Spreng. Trop. Anim. Health Prod. 43, 481–489. doi: 10.1007/s11250-010-9720-1
Marei, G. I. K., Abdel Rasoul, M. A., and Abdelgaleil, S. A. M. (2012). Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 103, 56–61. doi: 10.1016/j.pestbp.2012.03.004
Miranda, M. A., Varela, R. M., Torres, A., Molinillo, J. M. G., Gualtieri, S. C. J., and Macías, F. A. (2015). Phytotoxins from Tithonia diversifolia. J. Nat. Prod. 78, 1083–1092. doi: 10.1021/acs.jnatprod.5b00040
Mkenda, P., Mwanauta, R., Stevenson, P. C., Ndakidemi, P., Mtei, K., and Belmain, S. R. (2015). Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 10:e0143530. doi: 10.1371/journal.pone.0143530
Mkindi, A., Mpumi, N., Tembo, Y., Stevenson, P. C., Ndakidemi, P. A., Mtei, K., et al. (2017). Invasive weeds with pesticidal properties as potential new crops. Ind. Crops Prod. 110, 113–122. doi: 10.1016/j.indcrop.2017.06.002
Mujovo, S. F., Hussein, A. A., Meyer, J. J. M., Fourie, B., Muthivhi, T., and Lall, N. (2008). Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat. Prod. Res. 22, 1047–1054. doi: 10.1080/14786410802250037
Nhamo, N., Rodenburg, J., Zenna, N., Makombe, G., and Luzi-Kilhupi, A. (2013). Narrowing the rice yield gap in East and Southern Africa: using and adapting existing technologies. Agric. Syst. 131, 45–55. doi: 10.1016/j.agsy.2014.08.003
Ogendo, J. O., Belmain, S. R., Deng, A. L., and Walker, D. J. (2003). Comparison of toxic and repellent effects of Lantana camara L. with Tephrosia vogelii hook and a synthetic pesticide against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) in stored maize grain. Insect Sci. Appl. 23, 127–135. doi: 10.1017/S1742758400020348
Omolo, M. O., Okinyo, D., Ndiege, I. O., and Hassanali, A. (2004). Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry 65, 2797–2802. doi: 10.1016/j.phytochem.2004.08.035
Ott, K. C. (2006). Rotenone. A Brief Review of Its Chemistry, Environmental Fate, and the Toxicity of Rotenone Formulations. Available online at: http://www.newmexicotu.org/Rotenonesummary.pdf
Oyewole, I. O., Ibidapo, C. A., Moronkola, D. O., Oduola, A. O., Adeoye, G. O., Anyasor, G. N., et al. (2008). Anti-malarial and repellent activities of Tithonia diversifolia (Hemsl.) leaf extracts. J. Med. Plants Res. 2, 171–175.
Pavela, R. (2016). History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects-a review. Plant Prot. Sci. 52, 229–241. doi: 10.17221/31/2016-PPS
Pereira, P. S., Dias, D. A., Vichnewski, W., Tucci Nasi, A. M. T., and Herz, W. (1997). Sesquiterpene lactones from Brazilian Tithonia diversifolia. Phytochemistry 45, 1445–1448. doi: 10.1016/S0031-9422(97)00142-8
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Potts, S. G., Imperatriz-Fonseca, V., Ngo, H. T., Aizen, M. A., Biesmeijer, J. C., Breeze, T. D., et al. (2016). Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. doi: 10.1038/nature20588
Pretali, L., Bernardo, L., Butterfield, T. S., Trevisan, M., and Lucini, L. (2016). Botanical and biological pesticides elicit a similar induced systemic response in tomato (Solanum lycopersicum) secondary metabolism. Phytochemistry 130, 56–63. doi: 10.1016/j.phytochem.2016.04.002
Rabe, T., Mullholland, D., and van Staden, J. (2002). Isolation and identification of antibacterial compounds from Vernonia colorata leaves. J. Ethnopharmacol. 80, 91–94. doi: 10.1016/S0378-8741(02)00010-7
Rasoul, M. A. A., Marei, G. I. K., and Abdelgaleil, S. A. M. (2012). Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 6, 3667–3672. doi: 10.5897/AJMR12.118
Roubos, C. R., Rodriguez-Saona, C., and Isaacs, R. (2014). Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol. Control 75, 28–38. doi: 10.1016/j.biocontrol.2014.01.006
Rubia, E. G., Heong, K. L., Zalucki, M., Gonzales, B., and Norton, G. A. (1996). Mechanisms of compensation of rice plants to yellow stem borer Scirpophaga incertulas (Walker) injury. Crop Prot. 15, 335–340. doi: 10.1016/0261-2194(95)00102-6
Rundlöf, M., Andersson, G. K. S., Bommarco, R., Fries, I., Hederström, V., Herbertsson, L., et al. (2015). Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. doi: 10.1038/nature14420
Rusch, A., Valantin-Morison, M., Sarthou, J. P., and Roger-Estrade, J. (2010). Biological control of insect pests in agroecosystems: effects of crop management, farming systems, and seminatural habitats at the landscape scale: a review. Adv. Agron. 109, 219–259. doi: 10.1016/B978-0-12-385040-9.00006-2
Shaaban, M. M. (2001). Green microalgae water extract as foliar feeding to wheat plants. Pak. J. Biol. Sci. 4, 628–632. doi: 10.3923/pjbs.2001.628.632
Singh, P., Jayaramaiah, R. H., Sarate, P., Thulasiram, H. V., Kulkarni, M. J., and Giri, A. P. (2014). Insecticidal potential of defense metabolites from Ocimum kilimandscharicum against Helicoverpa armigera. PLoS ONE 9:e104377. doi: 10.1371/journal.pone.0104377
Sola, P., Mvumi, B. M., Ogendo, J. O., Mponda, O., Kamanula, J. F., Nyirenda, S. P., et al. (2014). Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: making a case for plant-based pesticidal products. Food Secur. 6, 369–384. doi: 10.1007/s12571-014-0343-7
Soylu, E. M., Kurt, S., and Soylu, S. (2010). In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 143, 183–189. doi: 10.1016/j.ijfoodmicro.2010.08.015
Stanley, J., and Preetha, G. (2016). Pesticide Toxicity to Non-target Organisms. Dordrecht: Springer Netherlands
Stevenson, P. C., Isman, M. B., and Belmain, S. R. (2017). Pesticidal plants in Africa: a global vision of new biological control products from local uses. Ind. Crops Prod. 110, 2–9. doi: 10.1016/j.indcrop.2017.08.034
Stevenson, P. C., Kite, G. C., Lewis, G. P., Forest, F., Nyirenda, S. P., Belmain, S. R., et al. (2012). Distinct chemotypes of Tephrosia vogelii and implications for their use in pest control and soil enrichment. Phytochemistry 78, 135–146. doi: 10.1016/j.phytochem.2012.02.025
Talbot, M. (1984). Yield variability of crop varieties in the U.K. J. Agric. Sci. 102:315. doi: 10.1017/S0021859600042635
Tardieu, F., and Tuberosa, R. (2010). Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 13, 206–212. doi: 10.1016/j.pbi.2009.12.012
Tesch, N. R., Mora, F., Rojas, L., Díaz, T., Velasco, J., Yánez, C., et al. (2011). Chemical composition and antibacterial activity of the essential oil of Lantana camara var. moritziana. Nat. Prod. Commun. 6, 1031–1034.
Utono, I. M., Coote, C., and Gibson, G. (2014). Field study of the repellent activity of ‘Lem-ocimum’-treated double bags against the insect pests of stored sorghum, Tribolium castaneum and Rhyzopertha dominica, in northern Nigeria. J. Stored Prod. Res. 59, 222–230. doi: 10.1016/j.jspr.2014.03.005
Viljoen, A. M., Subramoney, S., van Vuuren, S. F., Başer, K. H. C., and Demirci, B. (2005). The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J. Ethnopharmacol. 96, 271–277. doi: 10.1016/j.jep.2004.09.017
Wallace, D. H., and Munger, H. M. (1966). Studies of the physiological basis for yield differences. ii. variations in dry matter distribution among aerial organs for several dry bean varieties1. Crop Sci. 6:503.
Keywords: pest control, pesticidal plants, botanical products, ecosystem services, agro-ecological intensification, sustainable agriculture
Citation: Tembo Y, Mkindi AG, Mkenda PA, Mpumi N, Mwanauta R, Stevenson PC, Ndakidemi PA and Belmain SR (2018) Pesticidal Plant Extracts Improve Yield and Reduce Insect Pests on Legume Crops Without Harming Beneficial Arthropods. Front. Plant Sci. 9:1425. doi: 10.3389/fpls.2018.01425
Received: 28 June 2018; Accepted: 07 September 2018;
Published: 28 September 2018.
Edited by:
Giovanni Benelli, Scuola Sant'Anna di Studi Avanzati, ItalyReviewed by:
Marcel Amichot, UMR7254 Institut Sophia Agrobiotech (ISA), FranceCarlos L. Cespedes, University of the Bío Bío, Chile
Roman Pavela, Crop Research Institute (CRI), Czechia
Copyright © 2018 Tembo, Mkindi, Mkenda, Mpumi, Mwanauta, Stevenson, Ndakidemi and Belmain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven R. Belmain, cy5yLmJlbG1haW5AZ3JlLmFjLnVr
 Yolice Tembo
Yolice Tembo Angela G. Mkindi
Angela G. Mkindi Prisila A. Mkenda
Prisila A. Mkenda Nelson Mpumi2
Nelson Mpumi2 Regina Mwanauta
Regina Mwanauta Philip C. Stevenson
Philip C. Stevenson Patrick A. Ndakidemi
Patrick A. Ndakidemi Steven R. Belmain
Steven R. Belmain