- 1National Research Council of Italy, Department of Biology, Agriculture and Food Sciences, Trees and Timber Institute, Florence, Italy
- 2Department of Agri-Food Production and Environmental Sciences, University of Florence, Florence, Italy
- 3Department of Agricultural and Environmental Sciences—Production, Landscape, Agroenergy, University of Milan, Milan, Italy
- 4National Research Council of Italy, Department of Biology, Agriculture and Food Sciences, Institute for Sustainable Plant Protection, Florence, Italy
The old observation that plants preferentially synthesize flavonoids with respect to the wide range of phenylpropanoid structures when exposed to high doses of UV-B radiation has supported the view that flavonoids are primarily involved in absorbing the shortest solar wavelengths in photoprotection. However, there is compelling evidence that the biosynthesis of flavonoids is similarly upregulated in response to high photosynthetically active radiation in the presence or in the absence of UV-radiation, as well as in response to excess metal ions and photosynthetic redox unbalance. This supports the hypothesis that flavonoids may play prominent roles as scavengers of reactive oxygen species (ROS) generated by light excess. These ‘antioxidant’ functions of flavonoids appears robust, as maintained between different life kingdoms, e.g., plants and animals. The ability of flavonoids to buffer stress-induced large alterations in ROS homeostasis and, hence, to modulate the ROS-signaling cascade, is at the base of well-known functions of flavonoids as developmental regulators in both plants and animals. There is both long and very recent evidence indeed that, in plants, flavonoids may strongly affect phytohormone signaling, e.g., auxin and abscisic acid signaling. This function is served by flavonoids in a very low (nM) concentration range and involves the ability of flavonoids to inhibit the activity of a wide range of protein kinases, including but not limited to mitogen-activated protein kinases, that operate downstream of ROS in the regulation of cell growth and differentiation. For example, flavonoids inhibit the transport of auxin acting on serine–threonine PINOID (PID) kinases that regulate the localization of auxin efflux facilitators PIN-formed (PIN) proteins. Flavonoids may also determine auxin gradients at cellular and tissue levels, and the consequential developmental processes, by reducing auxin catabolism. Recent observations lead to the hypothesis that regulation/modulation of auxin transport/signaling is likely an ancestral function of flavonoids. The antagonistic functions of flavonoids on ABA-induced stomatal closure also offer novel hypotheses on the functional role of flavonoids in plant–environment interactions, in early as well as in modern terrestrial plants. Here, we surmise that the regulation of phytohormone signaling might have represented a primary function served by flavonols for the conquest of land by plants and it is still of major significance for the successful acclimation of modern terrestrial plants to a severe excess of radiant energy.
Introduction
Flavonoids, a vast class of phenylpropanoids comprising more than 8,000 structures with a wide range of decorations, have the ability to absorb the most energetic solar wavelengths reaching the leaf surface (Agati and Tattini, 2010). Flavonoids have long been considered as primarily synthesized to constitute an effective shield against the penetration of UV-B radiation to sensitive leaf tissues, and greatly involved in protecting plants challenged by the depletion of stratospheric ozone layer (for a review, see Bais et al., 2018). Though this is obvious, as the UV-B (and UV-A) screening properties of flavonoids are long known, recent evidence suggests flavonoids may serve other ‘eco-physiological’ functions in early as well as in modern terrestrial plants (Pollastri and Tattini, 2011; Agati et al., 2012, 2013).
First, it is noted that all phenylpropanoids, not only flavonoids, have the ability to absorb wavelengths over the UV-B region of the solar spectrum. For instance, hydroxycinnamic acid derivatives have greater molar extinction coefficients (𝜀) than flavonoids over the UV-B waveband. Nonetheless, the biosynthesis of flavonoids occur at the expense of hydroxycinnamate biosynthesis in UV-B-treated leaves (Agati and Tattini, 2010; Agati et al., 2012, 2013).
Second, flavonoids have maximum 𝜀s over the 330–355 nm, UV-A region of the solar spectrum, so that the induction spectrum most effective for their biosynthesis (UV-B) does not overlap with their absorption spectrum maxima, thereby suggesting that flavonoids are not synthesized to primarily serve UV-B screening functions in UV-B-treated tissues (Landry et al., 1995; Cockell and Knowland, 1999). This conforms to the observation that the biosynthesis of flavonoids is activated to a similar degree by high solar irradiance, in the presence or in the absence of UV-radiation (Agati et al., 2009, 2011). Moreover, blue light has been recently reported as being more effective than UV-B light in promoting the biosynthesis of flavonoids (Siipola et al., 2015). Finally, flavonoids accumulate to a very similar extent in plants exposed to excess both NaCl and Cu2+ or upon exposure to high UV-B irradiance (Babu et al., 2003; Agati et al., 2011). This is in line with the notion that changes in the redox potential of the cell activate flavonoid (particularly flavonol) biosynthesis (Taylor and Grotewold, 2005; Akhtar et al., 2010), consistent with relatively old findings that R2R3MYB transcription factors that regulate the biosynthesis of flavonoids, are themselves redox controlled (reviewed in Dubos et al., 2010).
Third, the biosynthesis of flavonoids with ortho-dihydroxy B-ring substitution is strongly favored as compared to the biosynthesis of monohydroxy B-ring-substituted flavonoids in response to excess light, irrespective of the relative proportions of solar wavelengths reaching the leaf surface (for a review, see Agati and Tattini, 2010). Monohydroxy and dihydroxy flavonoids do not display different UV-screening abilities, but largely differ in their ability to scavenge reactive oxygen species (ROS), i.e., to behave as antioxidants (Agati et al., 2012, 2013). This is in line with old and recent views that flavonoids may play major functions as antioxidants in plants exposed to excess light and, hence, to photooxidative stress of largely different origin (Landry et al., 1995; Ryan et al., 1998; Lillo et al., 2008; Agati et al., 2012; Tattini et al., 2015). It is a part of the folklore in plant photobiology and plant ecology that UV-B-treated leaves display a steeply higher quercetin to kaempferol (or apigenin) ratio than untreated leaves, and this is for equipping leaves with a more effective antioxidant, not UV-screening potential (Agati and Tattini, 2010; Agati et al., 2012).
The antioxidant properties of flavonoids have long been invoked to explain their health promoting effects in animals (Halliwell et al., 2005; Croft, 2016, for critical reviews), though flavonoid aglycones may also behave as pro-oxidants in high concentrations (Kessler et al., 2010). Since flavonoids are usually glycosylated in plant cells, thereby increasing their solubility and facilitating their transport from the endoplasmic reticulum (ER, the site of their biosynthesis) to different cellular organelles (Agati et al., 2012), their pro-oxidant actions are likely of minor significance in plants. There is an increasing body of evidence indicating that flavonoids may exert complex functions in both animal and plant cell metabolism, going well-beyond the mere chemical quenching of ROS. Certain flavonoids, especially but not limited to quercetin derivatives, have the ability to modulate signaling cascades that regulate cell growth and differentiation. For instance, flavonoids may strongly affect the protein kinase [e.g., mitogen-activated protein kinases (MAPKs)] signaling cascades (Williams et al., 2004; Hou and Kumamoto, 2010), which are at the base of many disorders, including carcinogenesis, in humans. In other words, flavonoids may behave as signaling molecules (Peer and Murphy, 2006), and in a concentration range much lower than that required to both scavenge ROS and effectively absorb UV-radiation (Agati and Tattini, 2010; Pollastri and Tattini, 2011). Though the ability of flavonoids to regulate cell growth and differentiation in plants has been known for decades (Jacobs and Rubery, 1988; Stafford, 1991), the functional significance in the adaptive mechanisms of plants to unfavorable habitats has been explored in less detail (Potters et al., 2009; Di Ferdinando et al., 2014).
Here, we focus our discussion on old and recent evidence of the strong relationship between flavonols (the ancient class of flavonoids already present in mosses, Wolf et al., 2010), auxin (IAA, Peer et al., 2013; Gayomba et al., 2017) and abscisic acid (ABA; Watkins et al., 2014, 2017). We hypothesize that the regulation of phytohormone signaling by flavonoids might have represented a function of primary significance for the conquest of land by plants and it is still of major value for the successful acclimation of modern terrestrial plants to a severe excess of radiant energy.
Regulation of Phytohormone Signaling: a Robust Function of Flavonoids in Terrestrial Plants
One of the molecular innovations that accompanied the water-to-land transition of plants was the replacement of mycosporine like aminoacid (MAA) with the flavonoid, particularly flavonol metabolism (Cockell and Knowland, 1999). The functional reason(s) for such drastic metabolic modification was unlikely used for equipping early land plants with a more effective UV-B shield as compared to algae: MAA are at least as efficient as flavonoids to absorb UV-B radiation (Cockell and Knowland, 1999; Agati et al., 2013). The replacement of ‘nitrogen-rich’ with carbon-based UV-screening pigments might have been functional when early land plants moved to nutrient poor environments (Pollastri and Tattini, 2011). There is consensus that flavonols, particularly derivatives of quercetin, may have enhanced the ability of early land plants to take-up water and nutrients, acting on soil chemistry (Cesco et al., 2012) and, particularly by promoting the symbiosis with nitrogen-fixing bacteria and the plant–mycorrhiza association (Wasson et al., 2006; Hassan and Mathesius, 2012). The plant–mycorrhiza association represented a central event at the origin of land flora (Bonfante and Genre, 2008; Wang et al., 2010; Field et al., 2015). Notably, flavonols play the peculiar role of inhibiting auxin transport during nodulation (Zhang et al., 2009; Ng et al., 2015), thus determining increases in local auxin levels that, in turn, promote nodule organogenesis (Hassan and Mathesius, 2012). This adds further support to the old hypothesis that the origin of land plants resulted from “a union of alga and fungus advanced by flavonoids” Jorgensen (1993).
Flavonols are well-suited to modulate auxin transport and signaling, given their ability to affect the activities of a wide range of proteins (Jacobs and Rubery, 1988; Peer and Murphy, 2006; Santelia et al., 2008; Lewis et al., 2011; Peer et al., 2011), as well as to scavenge ROS (Tattini et al., 2004; Agati et al., 2007; Agati and Tattini, 2010; Peer et al., 2013). Ortho-dihydroxy B-ring-substituted flavonoids, such as quercetin, are particularly effective in affecting the activity serine–threonine PINOID (PID) proteins that control the localization of PINFORMED (PIN) auxin efflux facilitator proteins (PIN) proteins (Figure 1; Peer and Murphy, 2006, 2007; Michniewicz et al., 2007; Adamowski and Friml, 2015). Nonetheless, the significance of flavonoids as direct or indirect inhibitors of auxin transporters in the modulation of auxin gradients at cellular and inter-cellular levels and, hence, of auxin signaling has been questioned in some instances (e.g., Peer et al., 2011). Instead, flavonoids might determine gradients in auxin concentration at both cellular and tissue level by primarily modulating auxin catabolism, i.e., acting as direct and/or indirect scavengers of ROS (Jansen et al., 2001; Peer et al., 2011, 2013; Pollastri and Tattini, 2011; Zhang and Peer, 2017). However, how the ROS-scavenging capacities of flavonols may have an impact on auxin signaling is a matter that deserves further investigation (Gayomba et al., 2017). Antioxidant flavonoids may limit IAA-oxidation, by inhibiting the activity of DIOXYGENASE for AUXIN OXIDATION1 protein (DAO1, Zhang et al., 2016), a member of the 2-oxoglutarate and Fe(II)-dependent [2OG Fe(II)] oxygenase superfamily (Figure 1). Furthermore, flavonols may both reduce IAA radicals generated during IAA-oxidation and chelate Mn(II), which is a cofactor of IAA-oxidase (Mathesius, 2001). As noted above, this may be responsible for determining auxin gradients and the consequential developmental processes. There is evidence, however, that increased IAA levels may promote ROS formation and then stimulate IAA oxidation, thereby transiently repressing auxin signaling (Peer et al., 2013; Zhang and Peer, 2017). Flavonoids might act as general buffers of cellular ROS levels (though how and how much flavonoids may regulate the cytoplasmic ROS levels involved in IAA-oxidation and IAA-signaling is far from being fully elucidated (Figure 1; Gayomba et al., 2017), thus rendering plants in a “receptive state” capable to respond promptly to changes in environmental conditions (Peer et al., 2013). Similarly, stress-induced increase in ROS, e.g., H2O2, levels may activate specific MAPK, such as ANP1 kinase in Arabidopsis and NPK1 in tobacco which, in turn, repress auxin signaling while transducing oxidative stress signaling (Kovtun et al., 2000). In other words, the massive generation of H2O2 triggers ANP1-mediated MAPK cascade, and may help stressed plants to divert energy from auxin-related activities to stress protection (Kovtun et al., 2000).
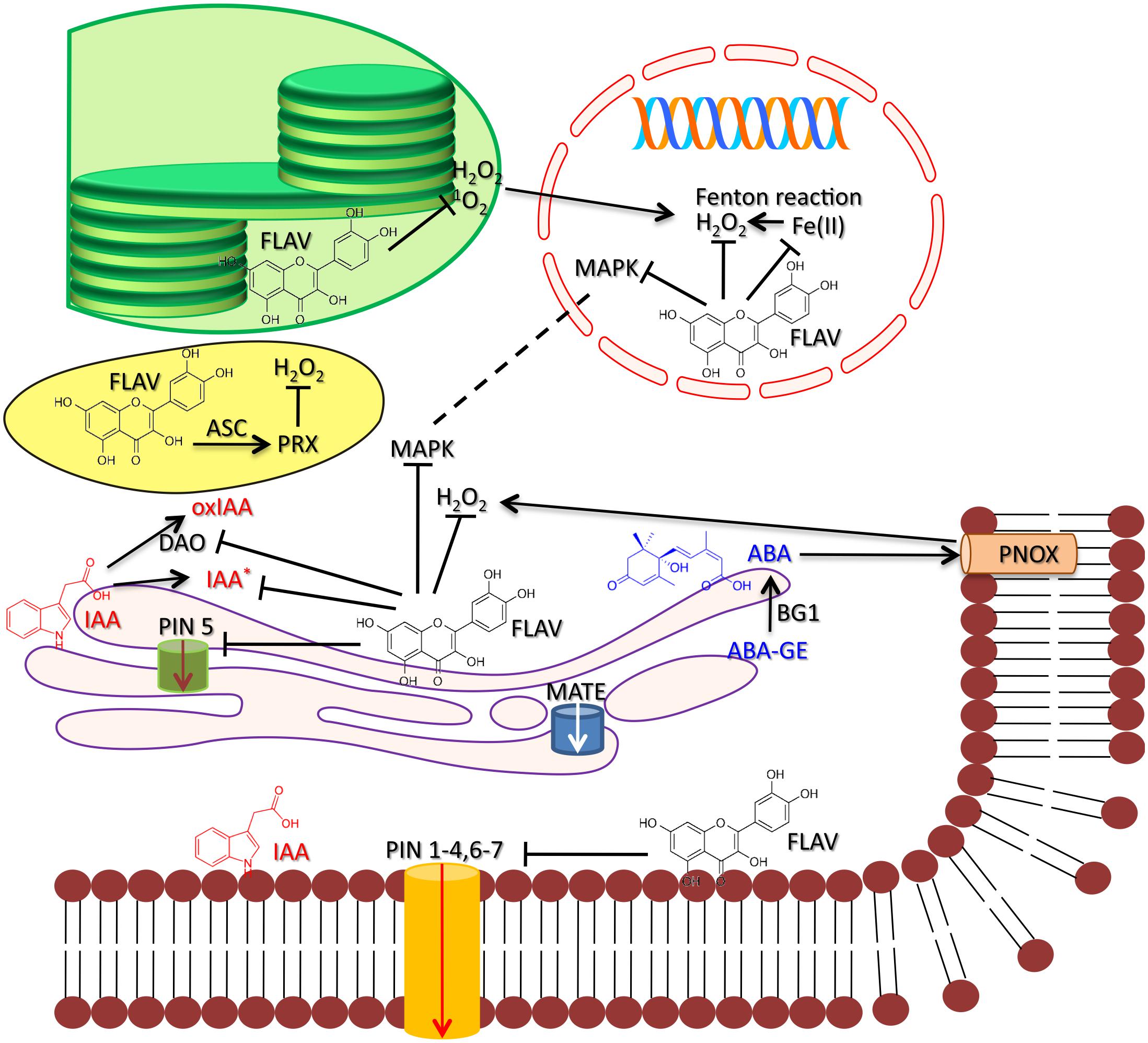
FIGURE 1. A proposed regulatory circuit involving auxin (IAA), abscisic acid (ABA), and flavonols (here represented by quercetin) under high light stress. High light activates the biosynthesis of IAA and ABA and, hence, the biosynthesis of flavonols. IAA is indeed synthesized at the cytoplasmic face of the ER, the very same site of flavonoid biosynthesis, and enhanced ABA biosynthesis under high light conditions mostly originates from de-glucosylation of ABA-GE, through the action of β-glucosidase1 (BG1) located at the ER (Lee et al., 2006; Tattini et al., 2017). The IAA- and ABA-induced flavonol biosynthesis might occur through the involvement of the bZIP transcription factor HY5 that, in turn, activates the expression of MYB12 (Lewis et al., 2011; Tossi et al., 2012). Flavonols distributed in different cellular compartments regulate the IAA and ABA-signaling. ER-located flavonoids may inhibit the activity of PIN5 (and perhaps of PIN6 and PIN8) auxin transport protein that escorts auxin into the ER lumen (Mravec et al., 2009). Flavonols are also transported to the ER lumen, by ABC-type and MATE proteins, and then to the plasma membrane (PM, Kitamura, 2006), where they inhibit the cell-to-cell auxin movement by acting on ‘long’ PINs (but also on PIN6, which has a dual, ER and PM localization, Simon et al., 2016). Flavonols may also alter the auxin catabolism by negatively affecting the activity of DIOXYGENASE for AUXIN OXIDATION (DAO), and hence the production of oxidized auxin (oxIAA, Zhang et al., 2016; Zhang and Peer, 2017), as well as by limiting the generation of IAA radicals (Mathesius, 2001). Chloroplast-located flavonols may complement the action of primary antioxidants (e.g., ascorbate peroxidase) the activity of which decreases under severe light excess (Mullineaux and Karpinski, 2002; Tattini et al., 2015). Flavonols may affect singlet oxygen (1O2) and H2O2-induced retrograde signaling, which, in turn may lead to programmed cell death (Agati et al., 2007, 2013; Fischer et al., 2007). This may occur through not only ROS scavenging, but also by strongly interacting with cytoplasmic- and nuclear-distributed MAPKs. Translocation of MAPKs from the cytoplasm to nucleus assists indeed cell re-programming under stressful conditions (Komis et al., 2018). Nuclear flavonols may indeed chelate transition metal ions (Hernández et al., 2009), such Fe (II), thereby preventing the massive generation of the highly reactive hydroxyl radical (OH∙) through the Fenton reaction [Fe(II) + H2O2 → Fe(III) + OH∙ + OH-]. Finally, vacuolar flavonols may scavenge H2O2 that freely escapes from the chloroplast at considerable rates under severe light excess (Mubarakshina et al., 2010), serving as substrates for vacuolar peroxidase and, then being recycled back to their original (reduced) forms by ascorbate (Sakihama et al., 2000). The tight control of cellular and inter-cellular auxin homeostasis by flavonols determines auxin gradients that regulate cell growth and differentiation, though we cannot exclude a direct involvement of nuclear-located flavonols on cell re-programming in response to severe light excess. Flavonols may scavenge H2O2, generated by the action of NADPH oxidase (Tossi et al., 2009), a key second messenger in the ABA signaling network, as well as by possibly inhibiting the activity of MAPKs, which act downstream of H2O2 and are involved in the ABA-induced regulation of stomata movements (Jammes et al., 2009). This is consistent with flavonols being distributed in the cytoplasm, and mostly in the nucleus (Watkins et al., 2014), but not in the vacuole in stomata guard cells, at least in Arabidopsis.
We speculate that drastic changes in cellular redox homeostasis activate flavonoid (particularly flavonol) biosynthesis (Taylor and Grotewold, 2005; Akhtar et al., 2010; Dubos et al., 2010) and, in turn, antioxidant flavonoids might act as components of a regulatory circuit of auxin signaling pathway. It is worth noting that flavonoids accumulate greatly indeed in regions of high auxin concentration (Peer et al., 2004; Lewis et al., 2011; Grunewald et al., 2012). Further support to our hypothesis is the observation that antioxidant flavonoids have long been reported as having a nuclear location (and perhaps synthesized in the nucleus, for a review see Agati et al., 2012; Watkins et al., 2014) and, hence, optimally suited to strongly affect MAPK activities. This may be of great significance under stressful conditions, when MAPKs re-distribute from the cytoplasm to the nucleus, thus assisting cell re-programming (Komis et al., 2018; Figure 1).
There is relatively recent evidence that a subclade of PIN proteins, characterized by a shorter hydrophilic domain, such as PIN5, PIN6 and PIN8, as compared to ‘long’ plasma membrane (PM) PINs, are located at the ER (Mravec et al., 2009). Notably, the auxin metabolic pathway is also compartmentalized to the ER, as evidenced by the presence of several auxin metabolic enzymes and regulatory proteins in the ER (Bartel and Fink, 1995; Woodward and Bartel, 2005; Friml and Jones, 2010). These ER-located PIN proteins were already present in early land plants, such as Physcomitrella patens and Selaginella moellendorffii, thereby suggesting that mediating auxin homeostasis at the ER is the ancestral function of PIN proteins (Friml and Jones, 2010; Viaene et al., 2014). In particular, PIN5 escorts auxin from the cytoplasmic face of the ER (the site of auxin biosynthesis) to the ER lumen (Figure 1), therefore both increasing auxin compartmentation (Mravec et al., 2009; Kriechbaumer et al., 2012) and establishing auxin gradients at cellular level. Cell-to-cell auxin transport at the PM likely occurred at a later stage during land plant evolution and involved long PINs (e.g., PIN1 and PIN2; Friml and Jones, 2010; Peer et al., 2011; Figure 1). Notably, the cytoplasmic face of the ER is also the site of flavonoid biosynthesis (Burbulis and Winkel-Shirley, 1999), and both ATP binding cassette (ABC)-type and multidrug resistance and toxic ion extrusion (MATE) proteins assist the ‘transport’ of flavonoids to the ER lumen (reviewed in Kitamura, 2006; Figure 1). It is conceivable that the strong relationship between flavonoids (particularly flavonols) and auxin may constitute an ancestral feature of land plants, possibly contributing to enhance their ability to adapt, i.e., evolvability sensu stricto (Wagner, 2011; Lachowiec et al., 2016), to an ever-changing environment (Pollastri and Tattini, 2011).
We hypothesize that the regulation of auxin both movement and signaling, through the regulation of ROS levels and protein activities (Figure 1), i.e., signaling functions sensu lato (Peer and Murphy, 2006) was a function of primary significance served by flavonoids during the colonization of land by plants. These signaling functions are of key significance in the ecology of modern terrestrial plants as well, as capable of greatly affecting the organ as well as the whole-plant morphology. For instance, Arabidopsis transparent testa (tt) mutants, deficient in flavonoid biosynthesis, have elevated auxin transport and display phenotypes with largely impaired apical dominance (Brown et al., 2001). It has been speculated that UVR8-induced preferential activation of quercetin biosynthesis may well-contribute to the regulation of auxin signaling and the compact architecture (bushy phenotypes), of plants long-exposed to UV-B or full solar radiation (Tattini et al., 2000; Hectors et al., 2012; Hayes et al., 2014). Antioxidant flavonoids have the potential to strongly control the plant architecture (Jansen, 2002; Buer and Djordjevic, 2009), and likely play an important role in the stress-induced (particularly high light stress) morphogenic responses (SIMR), the flight strategy of sessile organisms (Tattini et al., 2000; Potters et al., 2007, 2009). It may be not a mere coincidence that environmental stimuli that result into a severe excess of radiant energy, and induce large alterations in the shape of individual organs and the whole-plant, almost exclusively promotes the biosynthesis of quercetin derivatives in all order of taxa, in the presence or in the absence of UV-radiation (Agati et al., 2012). This is also consistent with the observation that both the moss P. patens and the angiosperm A. thaliana respond very similarly to elevated doses of UV-B radiation, mostly enhancing the biosynthesis of quercetin derivatives (Wolf et al., 2010). There is very recent evidence indeed (Soriano et al., 2018) that (1) both the moss P. patens and the liverwort M. polymorpha have functional UVR8 proteins that are regulated similarly to the Arabidopsis UVR8, and (2) in both bryophytes, UVR8 proteins mediate HY5 (ELONGATED HYPOCOTYL5) transcript expression and CHS (CHALCONE SYNTHASE) protein accumulation in response to UV-B. Notably, HY5 regulates the expression of MYB12 and MYB111 genes, known as PRODUCTION OF FLAVONOL GLYCOSIDES (PFG), in response to not only UV-B, but also to high white light (Stracke et al., 2010).
There is scarce evidence of PIN-flavonoid modulation of plant shape in bryophytes. However, recent findings show that the ‘ancestral’ PIN6 protein (Friml and Jones, 2010) in Arabidopsis (Simon et al., 2016) and PINA in P. patens have dual ER and PM localization. The finding that PINs proteins in P. patens are highly responsive to naringenin and strongly controls the shoot architecture (Bennet et al., 2014) is of significance, and opens new perspectives on similar regulation of plant shape by PIN/flavonoids in bryophytes and angiosperms.
There is recent compelling evidence that flavonols, especially derivatives of quercetin may greatly affect the ABA-signaling pathway, by antagonizing the ABA-induced stomatal closure in both Arabidopsis and tomato (Watkins et al., 2014, 2017). Arabidopsis guard cells rich in quercetin have reduced levels of H2O2 and, consequently, greater stomatal aperture than stomata deprived of quercetin (Watkins et al., 2014). Similarly, the anthocyanin reduced (are) tomato mutant, which has low flavonol levels, displays higher ROS content and lower stomatal aperture as compared to the anthocyanin without (aw) mutant, which is rich in quercetin (Watkins et al., 2017). H2O2 is a key second messenger in the ABA-signaling pathway, and it is necessary to promote stomatal closure (reviewed in Wang and Song, 2008). It is conceivable that the antagonistic effect of quercetin on ABA-signaling might result not only from its ability to quench H2O2, but also through the inhibition of MAPKs activities, which act downstream of H2O2 in the regulation of guard cell movements (Jammes et al., 2009; Danquah et al., 2013; Figure 1). Our hypothesis is strongly supported by the observation that flavonols in Arabidopsis guard cells have cytoplasmic and especially nuclear distribution (Watkins et al., 2014).
Notably, ABA has also been reported to promote the biosynthesis of flavonols (Berli et al., 2010, 2011), other than the biosynthesis of anthocyanins (Shen et al., 2014; Kadomura-Ishikawa et al., 2015). This is not surprising, as relatively recent experiments have shown a strong integration between ABA and light signaling (Bechtold et al., 2008; Wang et al., 2017), which may also help to explain the steeply enhanced flavonol biosynthesis in leaves exposed to high light irradiance, even in the absence of UV-radiation. There is intriguing evidence that increases in the levels of foliar ABA upon high light irradiance likely results from enhanced de-glucosylation of the ‘inactive’ ABA-glucoside (ABA-GE), and not from de novo synthesis through the plastidial MEP-pathway (Lee et al., 2006; Tattini et al., 2017). The notion that β-glucosidase1 (BG1), which promotes the release of free-ABA from ABA-GE, is located at the ER (Lee et al., 2006), the site of flavonoid biosynthesis, is of the greatest interest. We speculate that the quick production of free ABA from ABA-GE (Leng et al., 2014) might sustain steep increases in local ABA concentrations that are required for initiating early signaling responses to excess light stress, and these may well-include the biosynthesis of flavonols (Figure 1). The flavonol regulation of the ABA-signaling pathway may offer an additional chemical control to fine tune stomata movement. Nonetheless, how and how much the flavonols–ABA relationship may affect the acclimation/adaptive responses of plants to environmental pressures associated to habitats with contrasting soil water availability and solar irradiance is an interesting matter that needs further investigation. It is, however, plausible that the flavonol-regulation of stomata movements may have allowed early land plants to radiate toward habitats of increasingly excess of solar irradiance and contrasting soil water availability, thereby contributing to their adaptation on land.
Concluding Remarks
Here, we have hypothesized that the mutual regulation of phytohormone signaling and flavonol, particularly quercetin biosynthesis may have been of great value for the adaptation of plants on the land. It may be not a mere coincidence that mosses as well as modern terrestrial plants preferentially synthesize derivatives of quercetin in response to a severe light excess, despite the evolution of flavonoid metabolism has produced 1000s of structures. This is likely for equipping plants with a versatile metabolite capable of serving multiple functions (Pollastri and Tattini, 2011). Here we have shown that quercetin has a superior ability to modulate ROS- and phytohormone signaling, but not a greater capacity to screen-off the most energetic (UV-B) solar wavelengths as compared to other flavonoids. Our reasoning supports the view that modulation of ROS and phytohormone signaling is likely an ancestral, robust function of quercetin (as well as of other ‘antioxidant’ flavonoids), since it has a persisted against environmental, stochastic and genetic perturbations, over an extended time-scale level (Felix and Wagner, 2008; Lachowiec et al., 2016).
The observation that a wide range of abiotic stressors activates the biosynthesis of flavonoids, particularly of the di-hydroxy B-ring-substituted structures when plants suffer from severe excess of radiant energy, suggests that change in cellular ROS homeostasis is one of the main drivers for flavonoid biosynthesis (Taylor and Grotewold, 2005). In turn, flavonoids with effective antioxidant potential (i.e., they are ROS scavengers at very low concentrations, Asensi-Fabado and Munné-Bosch, 2010) may act as buffers of ROS levels, and enhance stress resistance. The ability of flavonoids to modulate ROS as well as phytohormone signaling (Figure 1) may help to explain their involvement in stress-induced morphogenic responses, a general response of plants to ‘fly away’ from adverse environmental conditions. For instance, ‘effective antioxidant’ flavonoids, distributed in the nucleus, the chloroplast, and the vacuole of mesophyll cells, constitutes more than 90% of the total flavonoid pool in plants inhabiting harsh, sunny habitats, and display bushy phenotypes, with small and thick leaves and short internodes. In contrast, monohydroxy-substituted, ‘poor antioxidant’ flavones and flavonols, mostly located in the adaxial epidermal cells, predominate in shade-adapted plants, whose phenotypes are associated to large, thin leaves and long internodes. These are, however, merely correlative observations, and further experimentation is needed to explore the significance of flavonol-phytohormone interactions in the mechanisms regulating the morphology and anatomy of individual organs and the whole plant under natural conditions.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adamowski, M., and Friml, J. (2015). PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27, 20–32. doi: 10.1105/tpc.114.134874
Agati, G., Azzarello, E., Pollastri, S., and Tattini, M. (2012). Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196, 67–76. doi: 10.1016/j.plantsci.2012.07.014
Agati, G., Biricolti, S., Guidi, L., Ferrini, F., Fini, A., and Tattini, M. (2011). The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 168, 204–212. doi: 10.1016/j.jplph.2010.07.016
Agati, G., Brunetti, C., Di Ferdinando, M., Ferrini, F., Pollastri, S., and Tattini, M. (2013). Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol. Biochem. 72, 35–45. doi: 10.1016/j.plaphy.2013.03.014
Agati, G., Matteini, P., Goti, A., and Tattini, M. (2007). Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 174, 77–89. doi: 10.1111/j.1469-8137.2007.01986x
Agati, G., Stefano, G., Biricolti, S., and Tattini, M. (2009). Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 104, 853–861. doi: 10.1093/aob/mcp177
Agati, G., and Tattini, M. (2010). Multiple functional roles of flavonoids in photoprotection. New Phytol. 186, 786–793. doi: 10.1111/j.1469-8137.2010.03269.x
Akhtar, T. A., Lees, H. A., Lampi, M. A., Enstone, D., Brain, R. A., and Greenberg, B. M. (2010). Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemna gibba. Plant Cell Environ. 33, 1205–1219. doi: 10.1111/j.1365-3040.2010.02140.x
Asensi-Fabado, M. A., and Munné-Bosch, S. (2010). Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 15, 582–592. doi: 10.1016/j.tplants.2010.07.003
Babu, S., Akhtar, T. A., Lampi, M. A., Tripuranthakam, S., Dixon, G. R., and Greenberg, B. M. (2003). Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemma gibba: implication of reactive oxygen species as common signals. Plant Cell Physiol. 44, 1320–1329. doi: 10.1093/pcp/pcg160
Bais, A. F., Lucas, R. M., Bornman, J. F., Williamson, C. E., Sulzberger, B., Austin, A. T., et al. (2018). Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP environmental effects assessment panel, update 2017. Photochem. Photobiol. Sci. 17, 127–179. doi: 10.1039/c7pp90043k
Bartel, B., and Fink, G. R. (1995). ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268, 1745–1748. doi: 10.1126/science.7792599
Bechtold, U., Zamboni, R. O., Gapper, C., Geisler, M., Pogson, B., Karpinski, S., et al. (2008). Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J. Exp. Bot. 59, 121–133. doi: 10.1093/jxb/erm289
Bennet, T. A., Liu, M. M., Aoyama, T., Bierfreund, N. M., Braun, M., Coudert, Y., et al. (2014). Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 24, 276–285. doi: 10.1016/j.cub.2014.09.054
Berli, F. J., Fanzone, M., Piccoli, P., and Bottini, R. (2011). Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 59, 4874–4884. doi: 10.1021/jf200040z
Berli, F. J., Moreno, D., Piccoli, P., Hespanhol-Viana, L., Silva, M. F., Bressan-Smith, R., et al. (2010). Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 33, 1–10. doi: 10.1111/j.1365-3040.2009.02044.x
Bonfante, P., and Genre, A. (2008). Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci. 13, 492–498. doi: 10.1016/j.tplants.2008.07.001
Brown, D. E., Rashotte, A. M., Murphy, A. S., Normanly, J., Tague, B. W., Peer, W. A., et al. (2001). Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126, 524–535. doi: 10.1104/pp.126.2.524
Buer, C. S., and Djordjevic, M. A. (2009). Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J. Exp. Bot. 60, 751–763. doi: 10.1093/jxb/ern323
Burbulis, I. E., and Winkel-Shirley, B. (1999). Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 12929–12934. doi: 10.1073/pnas.96.22.12929
Cesco, G., Mimmo, T., Tonon, G., Tomasi, N., Pinton, R., Terzano, R., et al. (2012). Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils 48, 123–149. doi: 10.1007/s00374-011-0653-2
Cockell, C. S., and Knowland, J. (1999). Ultraviolet radiation screening compounds. Biol. Rev. Camb. Philos. Soc. 74, 311–345. doi: 10.1017/S0006323199005356
Croft, K. D. (2016). Dietary polyphenols: antioxidants or not? Arch. Biochem. Biophys. 595, 120–124. doi: 10.1016/j.abb.2015.11.014
Danquah, A., de Zelicourt, A., Colcombet, J., and Hirt, H. (2013). The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 32, 40–52. doi: 10.1016/j.biotechadv.2013.09.006
Di Ferdinando, M., Brunetti, C., Agati, G., and Tattini, M. (2014). Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 103, 107–116. doi: 10.1016/j.envexpbot.2013.09.012
Dubos, C., Stracke, R., Grotewold, E., Weisshaar, B., Martin, C., and Lepiniec, L. (2010). MYB transcription factors in Arabidopsis. Trends Plant Sci. 15, 573–581. doi: 10.1016/j.tplants.2010.06.005
Felix, M. A., and Wagner, A. (2008). Robustness and evolution: concepts, insights and challenges from a developmental model system. Heredity 100, 132–140. doi: 10.1038/sj.hdy.6800915
Field, K. J., Pressel, S., Duckett, J. G., Rimington, W. R., and Bidartondo, M. I. (2015). Symbiotic options for the conquest of land. Trends Ecol. Evol. 30, 477–486. doi: 10.1016/j.tree.2015.05.007
Fischer, B. B., Krieger-Liszkay, A., Hideg, E., Snyrychová, I., Wisendanger, M., and Egger, R. I. L. (2007). Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett. 581, 5555–5560. doi: 10.1016/j.febslet.2007.11.03
Friml, J., and Jones, A. R. (2010). Endoplasmic reticulum: the rising compartment in auxin biology. Plant Physiol. 154, 458–462. doi: 10.1104/pp.110.161380
Gayomba, S. R., Watkins, J. M., and Muday, G. K. (2017). “Flavonols regulate plant growth and development through regulation of auxin transport and cellular redox status,” in Recent Advances in Polyphenol Research, eds K. Yoshida, V. Cheynier, and S. Quideau (Hoboken, NJ: John Wiley & Sons), 143–170.
Grunewald, W., De Smet, I., Lewis, D. R., Löke, C., Jansen, L., Goeminne, G., et al. (2012). Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 109, 1556–1559. doi: 10.1073/pnas.1121134109
Halliwell, B., Rafter, J., and Jenner, A. (2005). Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 81(Suppl. 1), 268S–276S. doi: 10.1093/ajcn/81.1.268S
Hassan, S., and Mathesius, U. (2012). The role of flavonoids in root-rhizosphere signaling: opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 63, 3429–3444. doi: 10.1093/jxb/err430
Hayes, S., Velanis, C. N., Jenking, G. I., and Franklin, K. A. (2014). UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc. Natl. Acad. Sci. U.S.A. 111, 11894–11899. doi: 10.1073/pnas.1403052111
Hectors, K., van Oevelen, S., Guisez, Y., Prinsen, E., and Jansen, M. A. K. (2012). The phytohormone auxin is a component of the regulatory system that controls UV-mediated accumulation of flavonoids and UV-induced morphogenesis. Physiol. Plant. 145, 594–603. doi: 10.1111/j.1399-3054.2012.01590.x
Hernández, I., Alegre, L., van Breusegem, F., and Munnè-Bosch, S. (2009). How relevant are flavonoids as antioxidants in plants. Trends Plant Sci. 14, 125–132. doi: 10.1016/j.tplants.2008.12.003
Hou, D.-X., and Kumamoto, T. (2010). Flavonoids as protein kinase inhibitors for cancer chemoprevention: direct binding and molecular modeling. Antioxid. Redox Signal. 13, 691–719. doi: 10.1089/ars.2009.2816
Jacobs, M., and Rubery, P. H. (1988). Naturally occurring auxin transport regulators. Science 241, 346–349. doi: 10.1126/science.241.4863.346
Jammes, F., Song, C., Shin, D., Munemasa, S., Takeda, K., Gu, K., et al. (2009). MAPK kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 20520–20525. doi: 10.1073/pnas.0907205106
Jansen, M. A. K. (2002). Ultraviolet-B-radiation on plants: induction of morphogenic responses. Physiol. Plant. 116, 423–439. doi: 10.1034/j.1399-3054.2002.1160319.x
Jansen, M. A. K., van der Noort, R. A., Tan, A., Prinsen, E., Lagrimini, L. M., and Thorneley, R. N. F. (2001). Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol. 126, 1012–1023. doi: 10.1104/pp.126.3.1012
Jorgensen, R. (1993). The origin of land plants: a union of alga and fungus advanced by flavonoids? Biosystems 31, 193–207.
Kadomura-Ishikawa, Y., Miyawaki, K., Takahashi, A., Masuda, T., and Noji, S. (2015). Light and abscisic acid independently regulated FaMYB10 in Fragaria × ananassa fruit. Planta 241, 953–965. doi: 10.1007/s00425-014-2228-6
Kessler, M., Ubeaud, G., and Jung, L. (2010). Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 55, 131–142. doi: 10.1211/002235702559
Kitamura, S. (2006). “Transport of flavonoids,” in The Science of Flavonoids, ed. E. Grotewold (New York, NY: Springer), 123–146. doi: 10.1007/978-0-387-28822-2_5
Komis, G., Samajová, O., Oveèka, M., and Samaj, J. (2018). Cell and developmental biology of mitogen-activated protein kinases. Annu. Rev. Plant Biol. 69, 237–265. doi: 10.1146/annurev-arplant-042817-040314
Kovtun, Y., Chiu, W. L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. U.S.A. 97, 2940–2945. doi: 10.1073/pnas.97.6.2940
Kriechbaumer, V., Seo, H., Park, W. J., and Hawes, C. (2012). Endoplasmic reticulum localization and activity of maize auxin biosynthetic enzymes. J. Exp. Bot. 66, 6009–6020. doi: 10.1093/jxb/erv314
Lachowiec, J., Queitsch, C., and Kliebenstein, D. J. (2016). Molecular mechanisms governing differential robustness of development and environmental responses in plants. Ann. Bot. 117, 795–809. doi: 10.1093/aob/mcv151
Landry, L. G., Chapple, C. C. S., and Last, R. L. (1995). Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 109, 1159–1166. doi: 10.1104/pp.109.4.1159
Lee, K. H., Piao, H. L., Kim, H.-Y., Choi, S. M., Jiang, F., Hartung, W., et al. (2006). Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126, 1109–1120. doi: 10.1016/j.cell.2006.07.034
Leng, P., Yuan, B., and Guo, Y. (2014). The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 65, 4577–4588. doi: 10.1093/jxb/eru204
Lewis, D. R., Ramirez, M. V., Miller, N. D., Vallabhaneni, P., Ray, W. K., Helm, R. F., et al. (2011). Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol. 156, 144–164. doi: 10.1104/pp.111.172502
Lillo, C., Lea, U. S., and Ruoff, P. (2008). Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 31, 587–601. doi: 10.1111/j.1365-3040.2007.01748.x
Mathesius, U. (2001). Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 52, 419–426. doi: 10.1093/jexbot/52.suppl_1.419
Michniewicz, M., Zago, M. K., Abas, L., Weijers, D., Schweighofer, A., Meskiene, T., et al. (2007). Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056. doi: 10.1016/j.cell.2007.07.033
Mravec, J., Skupa, P., Bailly, A., Hoyerova, K., Krecewk, P., Bielach, A., et al. (2009). Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136–1140. doi: 10.1038/nature08066
Mubarakshina, M. M., Ivanov, B. N., Naydov, I. A., Hillier, W., Badger, M. R., and Krieger-Liszkay, A. (2010). Production and diffusion of chloroplastic H2O2 and its implication to signalling. J. Exp. Bot. 61, 3577–3587. doi: 10.1093/jxb/erq171
Mullineaux, P., and Karpinski, S. (2002). Signal transduction in response to excess light: getting out of the chloroplast. Curr. Opin. Plant Biol. 5, 43–48. doi: 10.1016/S1369-5266(01)00226-6
Ng, J. L. P., Hassan, S., Truong, T. T., Hocart, C. H., Laffont, C., Frugier, F., et al. (2015). Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 27, 2210–2216. doi: 10.1105/tpc.15.00231
Peer, W. A., Bandyopadhyay, A., Blakeslee, J. J., Makam, S. N., Chen, R. J., Masson, P. H., et al. (2004). Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16, 1898–1911. doi: 10.1105/tpc.021501
Peer, W. A., Blakeslee, J. J., Hanga, H., and Murphy, A. S. (2011). Seven things we think we know about auxin transport. Mol. Plant 4, 487–504. doi: 10.1093/mp/ssr034
Peer, W. A., Cheng, Y., and Murphy, A. S. (2013). Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 64, 2629–2639. doi: 10.1093/jxb/ert152
Peer, W. A., and Murphy, A. S. (2006). “Flavonoids as signaling molecules,” in The Science of Flavonoids, ed. E. Grotewold (New York, NY: Springer), 239–268. doi: 10.1007/978-0-387-28822-2_9
Peer, W. A., and Murphy, A. S. (2007). Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556–563. doi: 10.1016/j.tplants.2007.10.003
Pollastri, S., and Tattini, M. (2011). Flavonols: old compound for old roles. Ann. Bot. 108, 1225–1233. doi: 10.1093/aob/mcr234
Potters, G., Pasternak, T. P., Guisez, Y., and Jansen, M. A. K. (2009). Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ. 32, 158–169. doi: 10.1111/j.1365-3040.2008.01908.x
Potters, G., Pasternak, T. P., Guisez, Y., Palme, K. J., and Jansen, M. A. K. (2007). Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 12, 98–105. doi: 10.1016/j.tplants.2007.01.004
Ryan, K. G., Markham, K. R., Bloor, S. J., Bradley, J. M., Mitchell, K. A., and Jordan, B. R. (1998). UV-B radiation induces increase in quercetin: kaempferol ratio in wild type and transgenic lines of Petunia. Photochem. Photobiol. 68, 323–330.
Sakihama, Y., Mano, J., Sano, S., Asada, K., and Yamasaki, H. (2000). Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem. Biophys. Res. Commun. 279, 949–954. doi: 10.1006/bbrc.2000.4053
Santelia, D., Henrich, S., Vincenzetti, V., Sauer, M., Biglewer, L., Klein, M., et al. (2008). Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J. Biol. Chem. 283, 31218–31226. doi: 10.1074/jbc.M710122200
Shen, X., Zhao, K., Liu, L., Zhang, K., Yuan, H., Liao, X., et al. (2014). A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 55, 862–880. doi: 10.1093/pcp/pcu013
Siipola, S. M., Kotilainen, T., Sipari, N., Morales, L. O., Lindfors, A. V., Robson, T. M., et al. (2015). Epidermal UV-A absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ. 38, 941–952. doi: 10.1111/pce.12403
Simon, S., Skupa, P., Viaene, T., Zwiewka, M., Tejos, R., Klíma, P., et al. (2016). PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 211, 65–74. doi: 10.1111/nph.14019
Soriano, G., Cloix, C., Heilmann, M., Núñez-Olivera, E., Martínez-Abaigar, J., and Jenkins, G. I. (2018). Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. New Phytol. 217, 151–162. doi: 10.1111/nph.14767
Stafford, H. A. (1991). Flavonoid evolution: an enzymic approach. Plant Physiol. 96, 680–685. doi: 10.1104/pp.96.3.680
Stracke, R., Favory, J. J., Gruber, H., Bartelniewoehner, L., Bartels, S., Binkert, M., et al. (2010). The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 33, 88–103. doi: 10.1111/j.1365-3040.2009.02061.x
Tattini, M., Galardi, C., Pinelli, P., Massai, R., Remorini, D., and Agati, G. (2004). Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 163, 547–561. doi: 10.1111/j.1469-8137.2004.01126.x
Tattini, M., Gravano, E., Pinelli, P., Mulinacci, N., and Romani, A. (2000). Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 148, 69–77. doi: 10.1046/j.1469-8137.2000.00743.x
Tattini, M., Loreto, F., Fini, A., Guidi, L., Brunetti, C., Velikova, V., et al. (2015). Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus x acerifolia plants during Mediterranean summers. New Phytol. 37, 1950–1964. doi: 10.1111/nph.13380
Tattini, M., Sebastiani, F., Brunetti, C., Fini, A., Torre, S., Gori, A., et al. (2017). Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: a case study on red and green basil. J. Exp. Bot. 68, 2425–2437. doi: 10.1093/jxb/erx123
Taylor, L. P., and Grotewold, E. (2005). Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8, 317–323. doi: 10.1016/j.pbi.2005.03.005
Tossi, V., Casia, R., Bruzzone, S., Zocchi, E., and Lamattina, L. (2012). ABA says NO to UV-B: a universal response? Trends Plant Sci. 17, 510–517. doi: 10.1016/j.tplants.2012.05.007
Tossi, V., Lamattina, L., and Cassia, R. (2009). An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 181, 871–879. doi: 10.1111/j.1469-8137.2008.02722.x
Viaene, T., Landberg, K., Thelander, M., Medvecka, E., Pederson, E., Feraru, E., et al. (2014). Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 24, 2786–2791. doi: 10.1016/j.cub.2014.09.056
Wagner, A. (2011). The molecular origins of evolutionary innovations. Trends Genet. 27, 397–410. doi: 10.1016/j.tig.2011.06.002
Wang, B., Yeun, L. H., Xue, J.-X., Liu, Y., Ané, J.-M., and Qiu, Y.-L. (2010). Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 181, 514–525. doi: 10.1111/j.1469-8137.2009.03137.x
Wang, F., Wu, N., Zhang, L., Ahammed, G. J., Chen, X., Xiang, X., et al. (2017). Light signaling-dependent regulation of photoinhibition and photoprotection in tomato. Plant Physiol. 176, 1311–1326. doi: 10.1104/pp.17.01143
Wang, P., and Song, C. P. (2008). Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 178, 703–718. doi: 10.1111/j.1469-8137.2008.02431.x
Wasson, A. P., Pellerone, F. I., and Mathesius, U. (2006). Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18, 1617–1626. doi: 10.1105/tpc.105.038232
Watkins, J. M., Chapman, J. M., and Muday, G. K. (2017). Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 175, 1807–1824. doi: 10.1104/pp.17.01010
Watkins, J. M., Hechler, P. J., and Muday, G. K. (2014). Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 164, 1707–1717. doi: 10.1104/pp.113.233528
Williams, R. J., Spencer, J. P. E., and Rice-Evans, C. (2004). Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 36, 838–849. doi: 10.1016/j.freeradbiomed.2004.01.001
Wolf, L., Rizzini, L., Stracke, R., Ulm, R., and Rensing, S. A. (2010). The molecular and physiological responses of Physcomitrella patens to ultraviolet-B radiation. Plant Physiol. 153, 1123–1134. doi: 10.1104/pp.110.154658
Woodward, A. W., and Bartel, B. (2005). Auxin: regulation, action, and interaction. Ann. Bot. 95, 707–735. doi: 10.1093/aob/mci083
Zhang, J., Lin, J. E., Harris, C., Campos Mastrotti Pereira, F., Wu, F., Blakeslee, J. J., et al. (2016). DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 27, 11010–11015. doi: 10.1073/pnas.1604769113
Zhang, J., and Peer, W. A. (2017). Auxin homeostasis. The DAO of catabolism. J. Exp. Bot. 68, 3145–3154. doi: 10.1093/jxb/erx221
Keywords: flavonols, abscisic acid (ABA), auxin, reactive oxygen species (ROS), mitogen-activated protein kinases (MAPKs), early land plants
Citation: Brunetti C, Fini A, Sebastiani F, Gori A and Tattini M (2018) Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant–Environment Interactions. Front. Plant Sci. 9:1042. doi: 10.3389/fpls.2018.01042
Received: 10 April 2018; Accepted: 26 June 2018;
Published: 20 July 2018.
Edited by:
Stefan Martens, Fondazione Edmund Mach, ItalyReviewed by:
Richard Victor Espley, The New Zealand Institute for Plant & Food Research, Ltd., New ZealandChristine Becker, Hochschule Geisenheim University, Germany
Copyright © 2018 Brunetti, Fini, Sebastiani, Gori and Tattini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Tattini, massimiliano.tattini@ipsp.cnr.it
 Cecilia Brunetti
Cecilia Brunetti Alessio Fini
Alessio Fini Federico Sebastiani
Federico Sebastiani Antonella Gori
Antonella Gori Massimiliano Tattini
Massimiliano Tattini