- 1Department of Agricultural, Forest and Food Sciences, University of Torino, Grugliasco, Italy
- 2Department of Pharmacy, University of Pisa, Pisa, Italy
Lavandula angustifolia Mill. has a great economic importance in perfumery, cosmetics, food manufacturing, aromatherapy, and pharmaceutical industry. This species finds its phytosociological optimum in the sub-Mediterranean region. Latitudinal and altitudinal gradients are expected to affect species diversification in peripheral alpine populations. In this study, phenotypic traits including morphometric parameters, volatile organic compounds (VOCs) and essential oils (EOs) were analyzed in lavender peripheral populations selected in order to explore different ecological conditions. Plants were cultivated under uniform conditions to observe variations due to the genetic adaptation to native environments and to exclude the short-term response to environmental factors. Results showed qualitatively and quantitatively intra-specific variations in secondary metabolites, mainly along the latitudinal gradient, while minor effect was attributable to the altitude. This latter affected more the morphometric parameters. As the latitude augmented, VOCs showed lower content of monoterpene hydrocarbon (mh) and higher content of oxygenated monoterpenes (om); whereas EOs showed higher content of mh and non-terpene derivatives (nt) and lower content of sesquiterpene hydrocarbons (sh). Lavender aroma and EO composition varied in every population, for a total of 88 and 104 compounds identified, respectively. Eleven and 13 compounds were responsible for 95% of the dissimilarity, with linalool, linalyl acetate and 1,8-cineole as major contributors. As the latitude augmented, linalool decreased and 1,8-cineole increased while linalyl acetate content was unaffected. These results are discussed with regards to the potential adoption of the lavender peripheral alpine populations for the improvement of quality and productivity of lavender cultivations, especially in mountainous areas.
Introduction
The genus Lavandula (Lamiaceae family) comprises approximately 39 species, with a natural occurrence in the Mediterranean region, to the Arabian Peninsula, South West Asia and India (Lis-Balchin, 2003). This genus is constituted by small evergreen shrubs, with aromatic foliage and flowers, and due to its great economic importance this genus was the subject of several studies. Indeed, 1,000 tons of lavandin essential oil (L. × intermedia Emeric ex Loisel., EO) are produced worldwide every year, while only 200 tons of EO from lavender (L. angustifolia Mill.) together with 200 tons of EO from spike lavender (L. latifolia Medik.) are produced (Lesage-Meessen et al., 2015). Lavender dried flowers and EOs are commonly employed in perfumery and cosmetics, food manufacturing and aromatherapy (Cavanagh and Wilkinson, 2002; Gonçalves and Romano, 2013; Prusinowska and Smigielski, 2014). Biological and antioxidant properties of lavender EO have been extensively assessed (Cavanagh and Wilkinson, 2002; Raut and Karuppayil, 2014; Shahdadi et al., 2017). Numerous therapeutic activities have been reported, such as convulsion relief, anxiety and depression improvement, along with a positive effect to treat several neurological disorders (Cavanagh and Wilkinson, 2002; Caputo et al., 2016; López et al., 2017; Rahmati et al., 2017). The highest quality lavender oil comes from L. angustifolia (syn. L. spica L., L. officinalis Chaix., L. vera DC.) inflorescences, which contain high levels of linalyl acetate and linalool and low amount of camphor, considered positive features by both cosmetic and pharmaceutical industry (Cavanagh and Wilkinson, 2002; Kim and Lee, 2002; Shellie et al., 2002). Although the genus Lavandula has been widely studied in wild and controlled environment (Da Porto and Decorti, 2008; Da Porto et al., 2009; González-Coloma et al., 2011; Gonçalves and Romano, 2013; Pistelli et al., 2013, 2017; Hassiotis et al., 2014; Lesage-Meessen et al., 2015; Caputo et al., 2016; Kirimer et al., 2017; López et al., 2017; Rahmati et al., 2017), lavender aroma profile and its EO composition are continuously subjected to many investigations (Prusinowska and Smigielski, 2014).
Secondary metabolites from plants are involved in several functions such as attraction for pollinators, signaling between plants, defense and protection against biotic and abiotic stresses (Knudsen et al., 2006; Bakkali et al., 2008; Dudareva et al., 2013; Raut and Karuppayil, 2014; Nogués et al., 2015; Caser et al., 2016, 2017). The plant-environment interaction largely contributes to the phytochemicals expression (Kesselmeier and Staudt, 1999; Binns et al., 2002; Dudareva et al., 2013; Holopainen et al., 2013; Selmar and Kleinwächter, 2013; Loreto et al., 2014), and has already been proved to affect the chemical composition of lavender (Prusinowska and Smigielski, 2014). However, the variability in the emission of volatile organic compounds (VOCs) and in the EO biosynthesis is also evidently regulated by genetics, even though the mechanisms involved still need clarifications (Kesselmeier and Staudt, 1999; Sangwan et al., 2001; Brahmkshatriya and Brahmkshatriya, 2013). The intra-specific variation in secondary metabolite production in plants has been reviewed and explained by genetic drift, relaxed selective pressure, introgression of traits through hybridization, gene pleiotropic effects, and phenotypic plasticity (Eckert et al., 2008; Raguso, 2008; Dicke and Loreto, 2010). For instance, qualitatively and quantitatively differences in secondary metabolites have been reported not only between wild and cultivated plants (L. luisieri, González-Coloma et al., 2011), but also among plants of the same wild population, as described in lavandin of northwest Italy (Maffei and Peracino, 1993; Peracino et al., 1994) and in L. latifolia (Muñoz-Bertomeu et al., 2007).
The populations of plants that are geographically situated at the peripheral limit of a species distribution range are usually expected to exhibit lower abundance and intra-genetic diversity, according to the widely accepted “abundant center model.” Moreover, a higher genetic differentiation than populations at the center is likely to occur (Eckert et al., 2008). Altitudinal gradient was also proved to affect species diversification in peripheral populations of L. latifolia (Herrera and Bazaga, 2008). Unfortunately, intra-specific studies on secondary metabolites production performed in plants in different environmental conditions do not inform whether the observed variations represent the genetic adaptation to specific environments, or the short-term response to environmental factors (Spitaler et al., 2006). Thus, trials under uniform cultivation practices are preferable to limit environmental influence on plant secondary metabolite production.
This study aimed to investigate if the environment differences, such as latitude and altitude, affected peripheral population diversification of L. angustifolia. Morphology, VOCs, and EOs were analyzed in lavender plants collected along both latitudinal and altitudinal gradients in West Alps (Italy), and grown in pots under uniform conditions, looking for traits of productive interest.
Materials and Methods
Native Environment
Lavender (L. angustifolia) is widespread throughout Italy (Pignatti, 1982), where it is a common component of low-growing shrub vegetation on calcareous soils. Lavandula angustifolia finds its phytosociological optimum in the sub-Mediterranean region within the order Ononidetalia, that describes meso-xerophile, basophile, supra-oromediterranean vegetation communities (Theurillat et al., 1995). It is the only species naturally occurring in the West Italian Alps (Piedmont region). Nine peripheral populations (Figure 1) were selected according to the natural range of distribution of the species (Pignatti, 1982), in order to explore different ecological conditions (Table 1, Supplementary Figure 1) in terms of latitude (Susa Valley in the northern range, Stura Valley in the core areas, and Tanaro Valley in the southern part of the Piedmont region) and altitude (low, medium and high altitude). Susa Valley represents the highest latitudinal range of distribution of the species in the Alps (Aeschimann et al., 2004). As the latitude decreases (from Susa to Tanaro Valley), the annual rainfalls decrease and yearly temperatures increase, thus there is a clear ecological gradient moving toward the typical Mediterranean conditions (Fick and Hijmans, 2017).
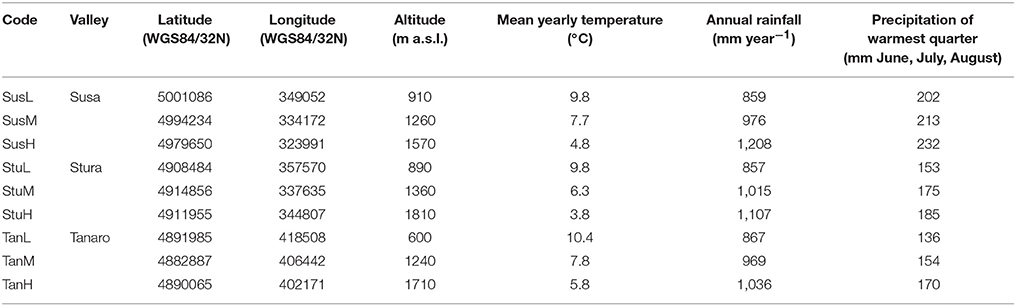
Table 1. Geographical characteristics and climatic conditions of L. angustifolia sampling sites in Piedmont region (West Italian Alps).
Figure 1. Geographical origin of the nine L. angustifolia populations analyzed in this study. For the significance of the population codes, see Table 1.
Plant Material and Pot Cultivation
Portions of lavender branches were collected from wild plants during the flowering season of 2014, from August to September. In each of the nine selected sites, the main area of lavender distribution was identified as a central point and from there, an area with a radius of 10 m was plotted. Inside that area, 10 individuals were randomly chosen and twenty cuttings were immediately prepared from each individual. Specimens are currently available and cultivated in the DISAFA facilities (45°03′58.5″N; 7°35′29.1″E, WGS84 System). Cuttings were allowed to root in peat substrate under plastic tunnels in an organic nursery, specialized in medicinal and aromatic plants (Frat.lli Gramaglia, Collegno, Italy; 45°05′22.4″N, 7°34′26.4″E, 302 m. a.s.l.). In spring 2015, the percentage of successful rooting was calculated and the rooted plants were transplanted in pots, with a mixture of peat and green compost (70-30, % v/v) and transferred outdoors. Irrigation was provided when needed and manual weed control was performed. Plants were fertigated with NPK fertilizer (1:2:2) three times during spring and once during autumn. The cultivation cycle lasted two years and during the first one (Summer 2015), non-destructive VOC analysis was performed in vivo on flowered pot plants to characterize lavender aroma and evaluate their potential differences. As ecological characteristics of the sampling site did have an influence on lavender performance, during the second year (Summer 2016), biometric parameters and EOs were analyzed.
Plant Performance Evaluation
Biometric and morphological characteristics of 123 rooted flowered plants were evaluated once a week from the beginning of blooming, according to and implementing the guidelines of the International Union for the Protection of New Varieties of Plants (UPOV) proposed for lavenders. The selected parameters were: number of spikes per plant (n), spike length (cm), flowering time (% of flowered plants in each population per week), together with plant height (cm) and width (cm), used to calculate the Growth Index (i.e., the plant volume; cm3) according to Demasi et al. (2017). The survival percentage was evaluated at the end of the second cultivation cycle.
VOCs Detection and Analysis
Emitted volatiles were analyzed using a Supelco (Bellofonte, USA) SPME device coated with polydimethylsiloxane (PDMS, 100 μm) in order to sample the headspace of each living/flowering plant. Plants of StuM population did not bloom, thus data on VOCs concerned eight populations, for a total of 33 plants. Sample was introduced individually into a 30 ml glass conical flask and allowed to equilibrate for 30 min. After the equilibration time, the fiber was exposed to the headspace for 15 min at room temperature; once sampling was finished, the fiber was withdrawn into the needle and transferred to the injector of the GC system, where the fiber was desorbed. GC-FID and GC-MS analyses were performed according to Pistelli et al. (2017) using a Varian CP-3800 apparatus (Paolo Alto, California, USA) equipped with a DB-5 capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm) and a Varian Saturn 2000 ion-trap mass detector. The oven temperature was programmed rising from 60°C to 240°C at 3°C/min; injector temperature, 220°C; transfer-line temperature, 240°C; carrier gas, He (1 ml/min).
Essential Oil Extraction and Analysis
Essential oil of lavender plants was obtained by hydro distillation of dried aerial parts of each sample using a Clevenger-type apparatus according to the Italian Pharmacopoeia (Helrich, 1990). Flowered plants of StuM population did not produce enough material to obtain EO, thus data on EOs concerned eight populations, for a total of 122 plants. The obtained EOs were immediately injected in GC-FID and GC-MS according to the method described in Pistelli et al. (2017). Identification of the constituents was based on the comparison of the retention times with those of authentic samples in comparing their linear retention indices (LRI) relative to a series of n-hydrocarbons, and on computer matching against commercial (Adams, 2007; NIST/EPA/NIH Mass Spectral Library, 2014) and home-made mass spectra library built up from pure substances and components of known EOs as well as MS literature data.
Statistical Analyses
Data were tested for the homogeneity of variance, then one-way ANOVA was performed to analyze latitude and altitude effect on biometric parameters and means were separated according to Ryan-Einot-Gabriel-Welsch-F post-hoc test (REGW-F). Statistically significant differences induced by latitude on chemical classes and compounds of VOCs and EO were assessed with the one-way ANOSIM with Euclidean-based similarity. The percentage contribution of each compound to the observed dissimilarity was assessed through the similarity percentage analysis (SIMPER, Euclidean distance). For each compound the difference between valleys were tested with Mann-Whitney pairwise test. Besides, VOC and EO chemical classes were subjected to the regression analysis to investigate their eventual link to altitude or latitude of native environment. The value for statistical significance was P < 0.05. The ANOVA analysis was performed with SPSS software (version 22), while all other analyses were performed with Past software (version 3).
Results and Discussion
Morphology and Performance of Cultivated Lavender
Lavenders of West Alps differently performed under cultivation, and influence of sea distance (latitude) or altitude of the native environment was observed in some biometric parameters (Table 2). Rooting percentage after 3 months in seed cells was generally below 40% and ranged among populations (SusH 23.9%, SusM 17.0%, SusL 38.0%; StuH 15.8%, StuM 9.6%, StuL 35.3%; TanH 37.5%, TanM 23.0%, TanL 39.5%). Rooting ability was therefore generally low, especially considering that selected lavender cultivars often reach 95% of successful rooting (Lis-Balchin, 2003). However, the propagation of wild adult plants by cutting is needed to produce genetically homogeneous individuals and the rooting percentage achieved in this study on L. angustifolia is considered of interest by local nurseries. Significant effects were observed due to both altitude and latitude of native environment, with a more successful rooting in plants evolved closer to the Mediterranean conditions (Tanaro) and from lower altitudes. The survival rate of rooted cuttings after 2 years of cultivation ranged between 54% (SusL) and 83% (StuL), but was independent from the geographical origin of the plants (Table 2). During the first cultivation cycle, 9% of pot lavenders bloomed, while in the second year, all plants bloomed (Figure 2); the first flowered plants were recorded on June 6th 2016 and the last on August 19th 2016. Interestingly, almost all lavenders of high populations flowered simultaneously in 1 week (SusH 100%, StuH 100%, and TanH 96%) regardless the latitude, followed by medium and low altitude populations, with the latter group defining a more gradual trend during summer. Knowledge of flowering times is essential to plan nursery activities (Lis-Balchin, 2003) or harvesting time for the production of EOs or dried flowers. Indeed, the nurseries can exploit lavenders with a flower production concentrated in a short period, since higher number of plants are available per sale. Furthermore, producers can optimize flower harvesting in the field when a simultaneous blooming occurs. Nonetheless, a gradual blooming can ensure flowered plants availability in the nursery and gardens for a longer period.
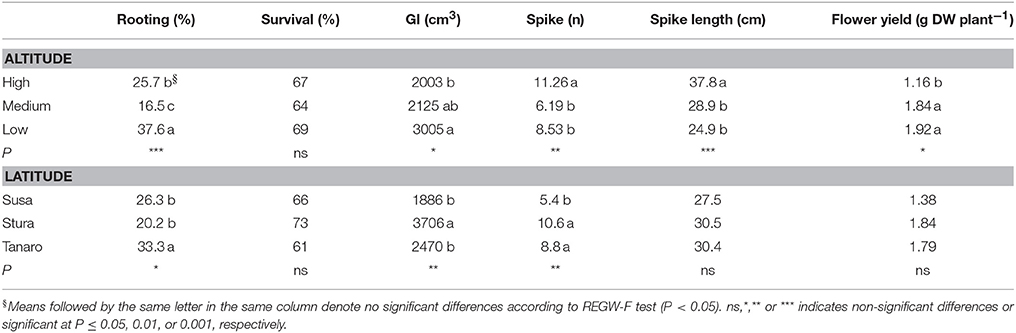
Table 2. Differences in rooting percentage, survival rate, growth index (GI), spike number and spike length recorded in the cultivated lavenders, according to altitude (High, Medium, and Low) and latitude (Susa, Stura, and Tanaro) of the native environment.
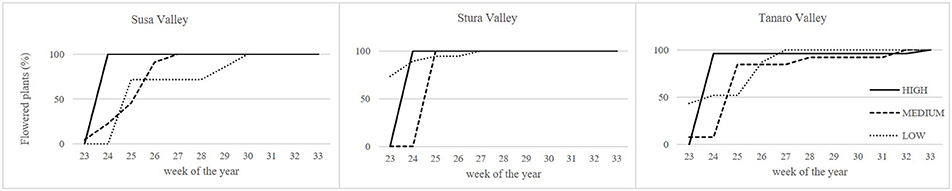
Figure 2. Blooming trend of lavender plants during the second cultivation cycle under uniform conditions. Solid lines, higher altitudes; dashed lines, medium altitudes; dotted lines, lower altitudes.
Altitude affected also morphological parameters and flower yield (Table 2). Contrariwise to low altitude groups, plants from higher elevation developed a more compact shrub (GI = 2,003 cm3), with higher number (11.26) and longer spikes (37.8 cm) during cultivation, but producing lower yield of inflorescences (1.16 gDW/plant), attributable to a less number of flowers per spike. Altitudinal gradient effect has been previously observed in other Lamiaceae species, displaying lower height at high altitudes, to avoid wind damages and improve photosynthetic conditions (Kofidis and Bosabalidis, 2008 and references in it). However, compact plants can facilitate field operations, especially harvesting. Growth and number of flower spikes were influenced also by the population distance from the sea. Plants from Susa Valley showed a compact growth (GI = 1,886 cm3) and a low number of flower spikes (5.4), contrary to what observed in Stura plants (GI = 3,706 cm3 and 10.6). Tanaro plants were compact (GI = 2,470 cm3) and produced a high number of spikes (8.8), showing an interesting habit for ornamental and field cultivation purposes. A latitudinal gradient in spike length and flower yield were not detected in the second cultivation cycle. These data could be helpful in the selection of alpine peripheral L. angustifolia populations with interesting traits for nurseries both for ornamental and production purposes.
Influence of Native Environment on Volatile Emission
The aroma profile represents the volatiles spontaneous emitted from the living plants and is a highly complex component of floral phenotype (Raguso, 2008). High intra and inter-specific variations in secondary metabolite production and aroma composition were reported in Lavandula genus, including L. latifolia and L. luisieri (Sanz et al., 2004; Muñoz-Bertomeu et al., 2007; González-Coloma et al., 2011) and other ornamental plants, such as rose, magnolia, and butterfly bush (Azuma et al., 2001; Raguso, 2008; Gong et al., 2014).
The volatile chemical classes of lavender fragrance were mainly represented by terpenes, with low amount of non-terpene derivatives (NT, 1.1–6.1%) and apocarotenoids (AC, 0.1–0.5%) (Figure 3, Supplementary Table 1). Oxygenated monoterpenes were the most abundant VOCs in all the populations (OM, 61.2–78.1%), followed by monoterpene hydrocarbons (MH, 8.9–22.4%), sesquiterpene hydrocarbons (SH, 4.8–20.5%), and oxygenated sesquiterpenes (OS, 0.1–1.9%). Interestingly, SH were generally present in higher amounts in the aroma of the lowest-altitude populations (SusL 15.6%, StuL 18.3%, TanL 20.5%), and AC were not detected in SusM, SusL, and in TanM populations. One-way ANOSIM performed on chemical classes showed that populations far from the sea (Susa) significantly differed from the closest ones (Tanaro) in their phytochemical composition (p = 0.038), indicating a clear distinction of aroma profiles. Moreover, a linear negative regression fitted between latitude and MH and a positive with OM (Figures 4A,B), whereas a negative regression model fitted between SH and altitude (Figure 4C). Monoterpenes are high volatile terpenoids emitted with warm temperature (>20°C). Their amount in flower fragrance is variable and often exceeds 50% of the total flower emission (Holopainen et al., 2013), as found in our study. Sesquiterpenes are the most diverse class of terpenoids (Holopainen et al., 2013); they are more abundant in plant volatiles than oils and are effectively used in aromatherapy (Cavanagh and Wilkinson, 2002), giving a sweet aromatic note (Prusinowska and Smigielski, 2014). Therefore, altitude influence on sesquiterpenes content found in this study could be an interesting starting point to explore the selection of fragrance characteristics.
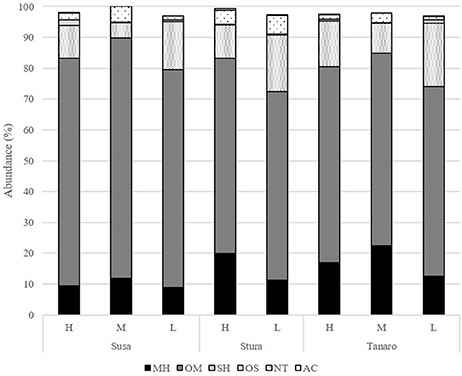
Figure 3. Chemical classes composition (%) of the L. angustifolia aroma after one cultivation cycle in uniform conditions. H, high altitude; M, medium altitude; L, low altitude; MH, monoterpene hydrocarbons; OM, oxygenated monoterpenes; SH, sesquiterpene hydrocarbons; OS, oxygenated sesquiterpenes; NT, non terpene derivatives; AC, apocarotenoids.
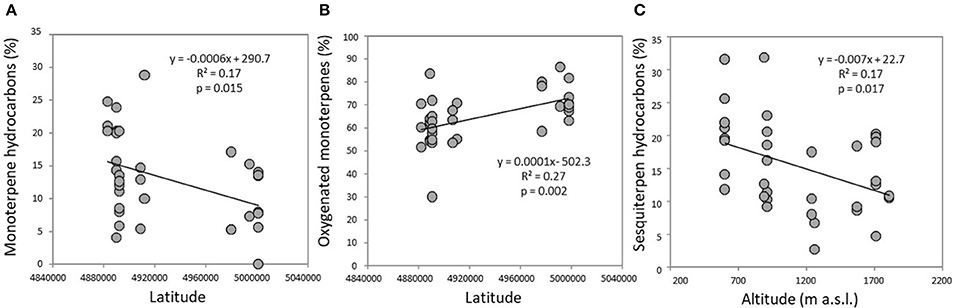
Figure 4. Significant linear regressions detected in the headspace of flowering lavender plants between (A) monoterpene hydrocarbons and latitude; (B) oxygenated monoterpenes and altitude; (C) sesquiterpene hydrocarbons and altitude.
Considering single compounds, 88 VOCs were identified in lavender aroma profile (Supplementary Table 1). The identified compounds accounted for more than 97% of the total composition. The number of compounds in each population varied from 42 (TanM) to 62 (StuL). Interestingly, every population had a peculiar aroma composition and all populations shared only 27 compounds. Among these compounds, the sum of linalyl acetate, linalool and 1,8-cineole were present more than 50% in SusM and SusL populations and more than 40% in the others; 1,8-cineole was less abundant in TanM and TanL; 4-terpineol and (E)-β-ocimene occurred in high percentages only in TanM lavender (7.7% and 7.5%, respectively), which showed also higher amount of lavandulyl acetate (4.5%). Linalool (22.9%), β-caryophyllene (13.9%), as well as borneol (6.7%) evidenced the highest amount in TanL. Da Porto and Decorti (2008) found a different arrangement in VOCs of lavender cultivated in Northeast Italy, in particular with 1,8-cineole, linalool, camphor, linalyl acetate, and β-caryophyllene being the 70% of the total aroma. Moreover, (E)-β-ocimene was present in smaller amounts (0.2–0.7%) compared to our study (1.6–7.5%).
The one-way ANOSIM performed using VOCs composition showed a similar trend to what observed in chemical classes, with a clear differentiation between Susa and Tanaro populations (p = 0.018), respectively the farthest and the closest sites to Mediterranean conditions. According to the SIMPER analysis, 11 compounds were responsible for the 95% of this dissimilarity in lavender fragrance (Table 3), with 1,8-cineole, linalyl acetate and linalool as major contributors (50.65, 16.33, and 13.16% of the contribution, respectively). The effect of sea distance was particularly seen on five out of 11 compounds. In detail, linalool, (E)-β-ocimene and 4-terpineol increased in the populations closer to the Mediterranean Sea, whereas 1,8-cineole and bornyl acetate had an opposite behavior. These latter constituents usually adversely affect lavender fragrance (Prusinowska and Smigielski, 2014), while linalool is responsible for the fresh and floral smell (Chizzola, 2013; Prusinowska and Smigielski, 2014). It is one of the best-examined and most often reported monoterpene in flowers (Buchbauer and Ilic, 2013; Holopainen et al., 2013). Moreover, there are evidences that linalool acts synergistically with linalyl-acetate and both compounds are required for the anxiolytic effect of the inhaled EOs (Buchbauer and Ilic, 2013). Thereof peripheral populations adapted to Mediterranean ecological conditions (Tanaro) appear more interesting for the selection of fragrance genotypes.
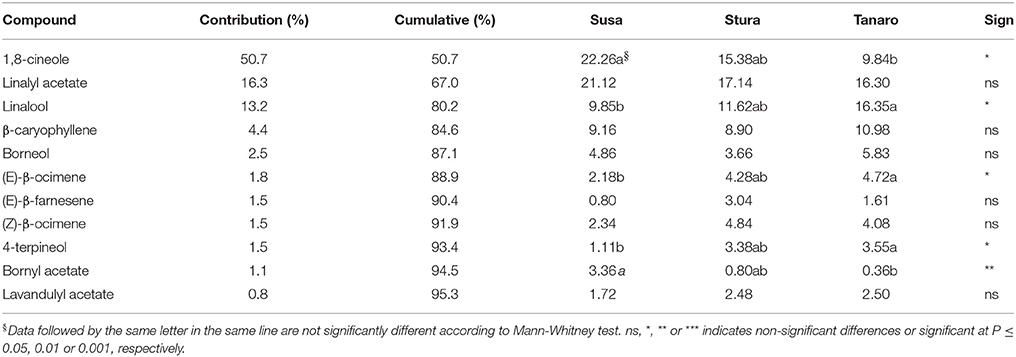
Table 3. List of VOCs responsible for dissimilarity induced by latitude in lavender aroma according to the SIMPER analysis.
Both isomers of β-ocimene, together with linalool, are frequently found in species pollinated by butterflies, bees, and moths (Andersson, 2003; Dötterl and Schäffler, 2007; Gong et al., 2014). TanM population emitted the highest quantity of both isomers [(Z)-β-ocimene, 6.4% and (E)-β-ocimene, 7.5%] and could have therefore differentiated its VOC emission to attract its peculiar type of pollinators (Dudareva et al., 2013).
Qualitative and quantitative variation was found in this study in the fragrance composition of L. angustifolia species according to its distance from the sea, while Stark et al. (2008) found only qualitative differences concerning other secondary metabolites (flavonoids and tannins). Several phenological events and environmental parameters may drive intra-specific floral scent variation, as observed also between and within Phlox cultivars (Majetic et al., 2014), highlighting that species contain a great potential of variation. Nonetheless, the uniform cultivation conditions adopted in our trial pointed out that variation in lavender fragrance composition could be partly attributed to its genetic adaptation to ecological conditions of peripheral alpine sites.
Influence of Native Environment on EO Composition
Generally, EO yields were consistent with previous studies on L. angustifolia (Da Porto et al., 2009; Prusinowska and Smigielski, 2014) which are very low in SusH and TanM to reach 0.69% in SusL (Supplementary Table 2). Plants from the lowest altitudes showed higher yields in Susa and Tanaro valley (0.69 and 0.37%, respectively). Previous studies highlighted the same behavior in other aromatic plants such as Origanum vulgare (Vokou et al., 1993; Giuliani et al., 2013), Coriandrum sativum (Shams et al., 2016), and Rosmarinus officinalis (Tuttolomondo et al., 2015). Giuliani et al. (2013) found a correlation between higher yields at decreasing altitudes and, but not limited to, an increased presence of glandular peltate trichomes, the structures responsible for the accumulation of EOs. Considering distance from Mediterranean conditions, Susa lavenders were the most productive in terms of EO, followed by Tanaro and Stura plants.
The mean of the relative abundance of chemical classes was distributed as follows (Figure 5, Supplementary Table 2): OM (71.2%), OS (13.7%), SH (6.1%), NT (4.9%), MH (3.2%), and AC (0.3%). A different distribution was recorded by Pistelli et al. (2017) in L. angustifolia ‘Maillette’, where the amount of OS and SH were lower than that observed in this study (0.5 and 1.8%, respectively). Commonly, monoterpenes represent the major class in the oil of Lavandula species (Lis-Balchin, 2003) and our data are consistent with values reviewed by Prusinowska and Smigielski (2014). In this study, significant positive linear regression fitted between MH and NT concentration with latitude (Figures 6A,B) and AC content with altitude (Figure 6D); instead, a negative linear regression fitted between SH and latitude (Figure 6C), an opposite behavior than that observed in Teucrium polium EO by Sadeghi et al. (2014).
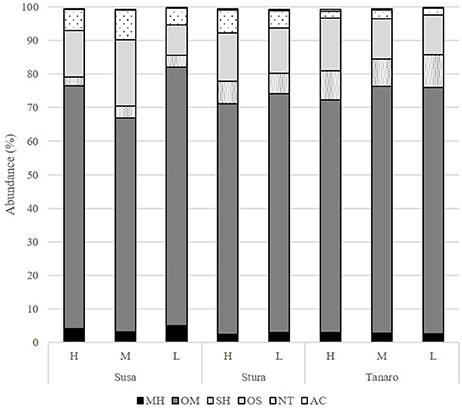
Figure 5. Chemical classes composition (%) of the L. angustifolia essential oils after two cultivation cycle in uniform conditions. H, high altitude; M, medium altitude; L, low altitude; MH, monoterpene hydrocarbons; OM, oxygenated monoterpenes; SH, sesquiterpene hydrocarbons; OS, oxygenated sesquiterpenes; NT, non terpene derivatives; AC, apocarotenoids.
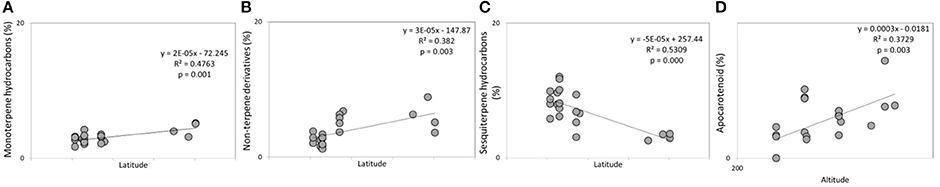
Figure 6. Significant linear regressions detected in lavender EOs between (A) monoterpene hydrocarbons and latitude; (B) non-terpene derivatives and latitude; (C) sesquiterpene hydrocarbons and latitude; (D) apocarotenoids and altitude.
One-hundred-four compounds were identified in lavender EO (Supplementary Table 2), accounting for more than 99% of the total EO composition. The number of compounds in each population varied from 63 (SusH) to 83 (StuL). These numbers were higher than those previously reported in L. angustifolia species and cultivars cultivated in the Tyrrhenian coast (Venskutonis et al., 1997; Pistelli et al., 2017). Interestingly, each EO was a mixture of different compounds, but all populations shared at least 40 constituents. The EO composition of L. angustifolia is reported to be the most variable inside the genus (Lis-Balchin, 2003). However, the characteristic compounds found in Lavandula oil usually are linalool, linalyl acetate, 1,8-cineole, β-ocimene, 4-terpineol, and camphor (Cavanagh and Wilkinson, 2002). Accordingly, in the present study linalool and linalyl acetate were the most abundant in all the EOs analyzed, with the highest amount recorded in TanL (linalool 35.0%) and StuH (linalyl acetate 26.3%). However, linalool content was present in lower percentage than in previous studies on lavender (Venskutonis et al., 1997; Pistelli et al., 2013, 2017). In fact, linalool production in L. angustifolia varies widely (Prusinowska and Smigielski, 2014) depending on temperature, flower development, and rainfall (Hassiotis et al., 2014). Linalyl acetate content registered in this work is in accordance with that reported for lavender ‘Maillette’ (Pistelli et al., 2017), but lower than in lavender species grow in Lithuania (Venskutonis et al., 1997). According to the percentage content of the characteristic compounds of lavender essential oil reported in the European Pharmacopoeia 6.0 (Ph. Eur.) none of the analyzed EOs completely fulfill the expected limits. StuH and TanL were closely related to the established ranges except for linalool (less than 20%) in StuH and linalyl acetate (less than 25%) in TanL. The percentages of 4-terpineol, 3-octanone, limonene, and lavandulyl acetate were in the range fixed by Ph. Eur. for all the tested EOs. Among the compounds reported in ISO 3515:2002 for lavender EO1 only 3-octanone, limonene and lavandulyl acetate respected the fixed limits in all the analyzed EOs. The analysis of flower fragrance has been suggested as a good indicator of the EO composition (An et al., 2001). However, EO composition frequently differed from the aroma profiles (see section Influence of Native Environment on Volatile Emission), as already highlighted in several studies on lavenders (Cavanagh and Wilkinson, 2002). These differences were caused by processes such as hydrolysis, thermal degradation, and molecular rearrangements that are promoted during the hydro distillation method (Da Porto and Decorti, 2008).
The one-way ANOSIM performed on EO compounds revealed significant differences among populations at different latitudes (p = 0.009), likewise to what observed in lavender aroma (see section Influence of Native Environment on Volatile Emission), with a clear separation between populations with more (Tanaro) and less (Susa) Mediterranean ecological conditions. According to the SIMPER analysis, thirteen compounds were responsible for 95% of this difference (Table 4), with linalool being the major contributor (45.98%). The effect of sea distance was seen on nine out of the 13 compounds. In particular, linalool, 4-terpinenol, β-caryophyllene, thujapsan-2-α-ol, and (Z)-γ-bisabolene were higher in populations closer to the Mediterranean Sea (Tanaro), while 1,8-cineole, τ-cadinol, 1-octen-3-yl acetate and α-terpineol where higher in the furthest populations (Susa). Linalool and terpineol are of great interest for medicinal industry due to their positive effects on the central nervous system (Prusinowska and Smigielski, 2014). β-caryophyllene, with its woody and spicy aroma, is commonly used in the fragrance and cosmetic industry. However, it has also antibiotic, anesthetic, anti-inflammatory, antioxidant, and anti-spasmodic activity (Buchbauer and Ilic, 2013). Therefore, similarly to results on fragrance, the populations closer to Mediterranean conditions (Tanaro) could be considered valuable germplasm for selection and breeding purposes, aimed to the production of EO, despite the low yields obtained. Indeed it is needed to consider that the cultivation occurred in pot. In proper open field conditions higher biomass and EO yields are expected. No significant differences were detected in EO compounds according to altitude, likewise to VOCs composition (see Section Influence of Native Environment on Volatile Emission), despite there are evidence that altitude significantly affected chemical composition of EO in other Lamiaceae plants (Giuliani et al., 2013; Sadeghi et al., 2015). Chograni et al. (2010) observed also a phytochemical variation in the leaf EO of wild L. multifida belonging to the same bioclimatic zone, possibly explained by genetic factors. Similarly, in the present study, the ecological conditions of peripheral sites may have induced different adaptation mechanisms in lavender, leading to different phytochemical compositions. Since the EO obtained in this work did not respect the limit established by Ph. Eur., they can be used for other industrial application, or for cosmetic purposes.
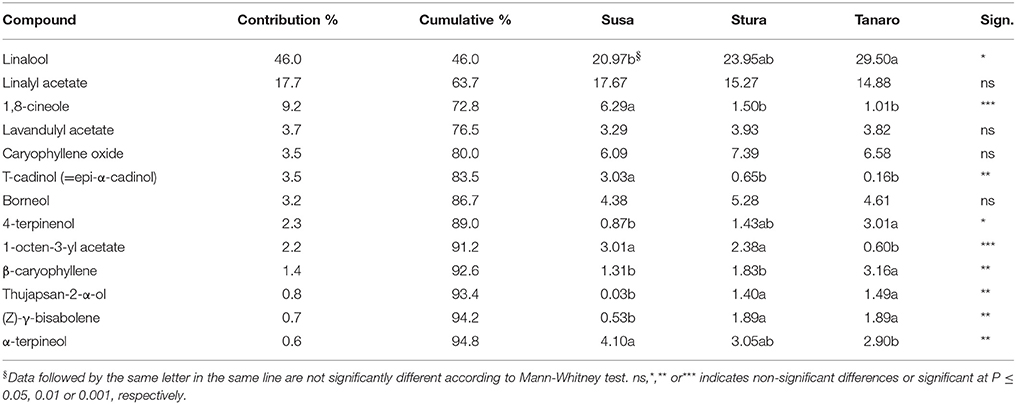
Table 4. List of compounds responsible for dissimilarity induced in lavender EO by latitude according to the SIMPER analysis.
Conclusions
We hereby provide the first phytochemical description of secondary metabolites in L. angustifolia peripheral populations of the West Italian Alps. The influence of sea distance (latitude) and altitude on VOC and EO composition was analyzed in plants grown under uniform cultivation conditions. Interestingly, results showed that the ecological gradient created germplasm heterogeneity. Compared to other studies, altitude seemed to affect mainly biometric parameters, and to a low extent the phytochemical composition of L. angustifolia, while sea distance had an influence on both morphological and phytochemical traits. Lavenders of West Italian Alps disclosed a great potential for the development of a valuable local product, where the cultivation of the thermophilous species L. × intermedia cannot be applied due to the lower average temperature of the studied alpine areas. Among the studied alpine peripheral populations, plants of Tanaro Valley, which have evolved in almost Mediterranean ecological conditions, generally performed better in both morphological and phytochemical characteristics. To obtain uniform productions, the study of the phytochemical variation and the selection and propagation of interesting genotypes must be promoted, together with the improvement of cultivation protocols.
Author Contributions
SD and MC equally contributed to this work in the acquisition of data, analysis and interpretation of data, and drafting of manuscript. BN and PC carried out phytochemical analyses. ML contributed to lavender population selection and data analysis. LP and BN contributed to data interpretation and discussion. VS conceived, designed and coordinated the work. LP and VS critically revised and approved the manuscript.
Funding
This work was partially supported by Fondazione Cassa di Risparmio di Torino (Project Le colture officinali: un approccio di filiera per aumentare la competitività delle aziende piemontesi e valorizzare il territorio, grant number 2014.0976) and by the program Interreg V-A Francia Italia Alcotra Attività innovative per lo sviluppo della filiera transfrontaliera del fiore edule–Antea n. 1139.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Walter Gaino and Paolo Lo Turco for their contribution in the collection and propagation of lavender plants; and Frat.lli Gramaglia nursery for hosting cultivation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00983/full#supplementary-material
Abbreviations
AC, Apocarotenoids; EO, Essential Oils; GC-MS, Gas Cromatography-Mass Spectrometry; GI, Growth Index; MH, Monoterpene Hydrocarbons; NT, Non Terpene Derivatives; OM, Oxygenated Monoterpenes; OS, Oxygenated Sesquiterpenes; SH, Sesquiterpene Hydrocarbons; SPME, Solid Phase Micro Extraction; VOCs, Volatile Organic Compounds.
Footnotes
1. ^ISO 3515:2002 Oil of Lavender (Lavandula angustifolia Mill.).
References
Adams, R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL: Allured Publishign Corp.
Aeschimann, D., Lauber, K., Martin Moser, D., and Theurillat, J. P. (2004). Flora Alpina. Bologna: Zanichelli.
An, M., Haig, T., and Hatfield, P. (2001). On-site field sampling and analysis of fragrance from living Lavender (Lavandula angustifolia L.) flowers by solid-phase microextraction coupled to gas chromatography and ion-trap mass spectrometry. J. Chromatogr. A 917, 245–250. doi: 10.1016/S0021-9673(01)00657-4
Andersson, S. (2003). Antennal responses to floral scents in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepteryx rhamni (Pieridae). Chemoecology 13, 13–20. doi: 10.1007/s000490300001
Azuma, H., Toyota, M., and Asakawa, Y. (2001). Intraspecific variation of floral scent chemistry in Magnolia kobus DC.(Magnoliaceae). J. Plant Res. 114, 411–422. doi: 10.1007/PL00014006
Bakkali, F., Averbeck, S., Averbeck, D., and Idaomar, M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475. doi: 10.1016/j.fct.2007.09.106
Binns, S. E., Arnason, J. T., and Baum, B. R. (2002). Phytochemical variation within populations of Echinacea angustifolia (Asteraceae). Biochem. Syst. Ecol. 30, 837–854. doi: 10.1016/S0305-1978(02)00029-7
Brahmkshatriya, P. P., and Brahmkshatriya, P. S. (2013). “Terpenes: Chemistry, biological role, and therapeutic applications,” in Natural Products eds K. G. Ramawat, J.-M. Mérillon (Berlin: Springer), 2665–2691.
Buchbauer, G., and Ilic, A. (2013). “Biological activities of selected mono-and sesquiterpenes: possible uses in medicine,” in Natural Products eds K. G. Ramawat, J.-M. Mérillon (Berlin: Springer), 4109–4159.
Caputo, L., Souza, L. F., Alloisio, S., Cornara, L., and De Feo, V. (2016). Coriandrum sativum and Lavandula angustifolia essential oils: chemical composition and activity on central nervous system. Int. J. Mol. Sci. 17:1999. doi: 10.3390/ijms17121999
Caser, M., D'Angiolillo, F., Chitarra, W., Lovisolo, C., Ruffoni, B., Pistelli, L., et al. (2016). Water deficit regimes trigger changes in valuable physiological and phytochemical parameters in Helichrysum petiolare Hilliard and BL Burtt. Ind. Crops Prod. 83, 680–692. doi: 10.1016/j.indcrop.2015.12.053
Caser, M., D'Angiolillo, F., Chitarra, W., Lovisolo, C., Ruffoni, B., Pistelli, L., et al. (2017). Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul. 84, 383–394. doi: 10.1007/s10725-017-0349-1
Cavanagh, H. M. A., and Wilkinson, J. M. (2002). Biological activities of lavender essential oil. Phyther. Res. 16, 301–308. doi: 10.1002/ptr.1103
Chizzola, R. (2013). “Regular monoterpenes and sesquiterpenes (Essential oils),” in Natural products eds K. G. Ramawat, J.-M. Mérillon (Berlin: Springer), 2973–3008.
Chograni, H., Zaouali, Y., Rajeb, C., and Boussaid, M. (2010). Essential oil variation among natural populations of Lavandula multifida L.(Lamiaceae). Chem. Biodivers. 7, 933–942. doi: 10.1002/cbdv.200900201
Da Porto, C., and Decorti, D. (2008). Analysis of the volatile compounds of flowers and essential oils from Lavandula angustifolia cultivated in northeastern Italy by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry. Planta Med. 74, 182–187. doi: 10.1055/s-2008-1034295
Da Porto, C., Decorti, D., and Kikic, I. (2009). Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: comparison of three different extraction methods. Food Chem. 112, 1072–1078. doi: 10.1016/j.foodchem.2008.07.015
Demasi, S., Caser, M., Handa, T., Kobayashi, N., De Pascale, S., and Scariot, V. (2017). Adaptation to iron deficiency and high pH in evergreen azaleas (Rhododendron spp.): potential resources for breeding. Euphytica 213, 148–162. doi: 10.1007/s10681-017-1931-3
Dicke, M., and Loreto, F. (2010). Induced plant volatiles: from genes to climate change. Trends Plant Sci. 15, 115–117. doi: 10.1016/j.tplants.2010.01.007
Dötterl, S., and Schäffler, I. (2007). Flower scent of floral oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. J. Chem. Ecol. 33, 441–445. doi: 10.1007/s10886-006-9237-2
Dudareva, N., Klempien, A., Muhlemann, J. K., and Kaplan, I. (2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 198, 16–32. doi: 10.1111/nph.12145
Eckert, C. G., Samis, K. E., and Lougheed, S. C. (2008). Genetic variation across species' geographical ranges: The central-marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x
Fick, S. E., and Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. doi: 10.1002/joc.5086
Giuliani, C., Maggi, F., Papa, F., and Maleci Bini, L. (2013). Congruence of phytochemical and morphological profiles along an altitudinal gradient in Origanum vulgare ssp. vulgare from Venetian region (NE Italy). Chem. Biodivers. 10, 569–583. doi: 10.1002/cbdv.201300019
Gonçalves, S., and Romano, A. (2013). In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol. Adv. 31, 166–174. doi: 10.1016/j.biotechadv.2012.09.006
Gong, W. C., Chen, G., Liu, C.-Q., Dunn, B. L., and Sun, W.-B. (2014). Comparison of floral scent between and within Buddleja fallowiana and Buddleja officinalis (Scrophulariaceae). Biochem. Syst. Ecol. 55, 322–328. doi: 10.1016/j.bse.2014.03.029
González-Coloma, A., Delgado, F., Rodilla, J. M., Silva, L., Sanz, J., and Burillo, J. (2011). Chemical and biological profiles of Lavandula luisieri essential oils from western Iberia Peninsula populations. Biochem. Syst. Ecol. 39, 1–8. doi: 10.1016/j.bse.2010.08.010
Hassiotis, C. N., Ntana, F., Lazari, D. M., Poulios, S., and Vlachonasios, K. E. (2014). Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 62, 359–366. doi: 10.1016/j.indcrop.2014.08.048
Herrera, C. M., and Bazaga, P. (2008). Adding a third dimension to the edge of a species' range: altitude and genetic structuring in mountainous landscapes. Heredity 100, 275–285. doi: 10.1038/sj.hdy.6801072
Holopainen, J. K., Himanen, S. J., Yuan, J. S., Chen, F., and Stewart Jr, C. N. (2013). “Ecological functions of terpenoids in changing climates,” in Natural Products eds K. G. Ramawat, J.-M. Mérillon (Berlin: Springer), 2913–2940.
Kesselmeier, J., and Staudt, M. (1999). Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 33, 23–88. doi: 10.1023/A:1006127516791
Kim, N. S., and Lee, D. S. (2002). Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J. Chromatogr. A 982, 31–47. doi: 10.1016/S0021-9673(02)01445-0
Kirimer, N., Mokhtarzadeh, S., Demirci, B., Goger, F., Khawar, K. M., and Demirci, F. (2017). Phytochemical profiling of volatile components of Lavandula angustifolia Miller propagated under in vitro conditions. Ind. Crops Prod. 96, 120–125. doi: 10.1016/j.indcrop.2016.11.061
Knudsen, J. T., Eriksson, R., Gershenzon, J., and Ståhl, B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2
Kofidis, G., and Bosabalidis, A. M. (2008). Effects of altitude and season on glandular hairs and leaf structural traits of Nepeta nuda L. Bot. Stud. 49, 363–372. Available online at: https://ejournal.sinica.edu.tw/bbas/content/2008/4/Bot494-08.pdf
Lesage-Meessen, L., Bou, M., Sigoillot, J.-C., Faulds, C. B., and Lomascolo, A. (2015). Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 99, 3375–3385. doi: 10.1007/s00253-015-6511-7
López, V., Nielsen, B., Solas, M., Ramírez, M. J., and Jäger, A. K. (2017). Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 8:280. doi: 10.3389/fphar.2017.00280
Loreto, F., Dicke, M., Schnitzler, J., and Turlings, T. C. J. (2014). Plant volatiles and the environment. Plant. Cell Environ. 37, 1905–1908. doi: 10.1111/pce.12369
Maffei, M., and Peracino, V. (1993). Fatty acids from some Lavandula hybrids growing spontaneously in north west italy. Phytochemistry 33, 373–376. doi: 10.1016/0031-9422(93)85521-R
Majetic, C. J., Levin, D. A., and Raguso, R. A. (2014). Divergence in floral scent profiles among and within cultivated species of Phlox. Sci. Hortic. 172, 285–291. doi: 10.1016/j.scienta.2014.04.024
Muñoz-Bertomeu, J., Arrillaga, I., and Segura, J. (2007). Essential oil variation within and among natural populations of Lavandula latifolia and its relation to their ecological areas. Biochem. Syst. Ecol. 35, 479–488. doi: 10.1016/j.bse.2007.03.006
Nogués, I., Muzzini, V., Loreto, F., and Bustamante, M. A. (2015). Drought and soil amendment effects on monoterpene emission in rosemary plants. Sci. Total Environ. 538, 768–778. doi: 10.1016/j.scitotenv.2015.08.080
Peracino, V., Caramiello, R., and Maffei, M. (1994). Essential oils from some lavandula hybrids growing spontaneously in north west italy. Flavour Fragr. J. 9, 11–17. doi: 10.1002/ffj.2730090104
Pistelli, L., Najar, B., Giovanelli, S., Lorenzini, L., Tavarini, S., and Angelini, L. G. (2017). Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 109, 37–44. doi: 10.1016/j.indcrop.2017.07.041
Pistelli, L., Noccioli, C., D'Angiolillo, F., and Pistelli, L. (2013). Composition of volatile in micropropagated and field grown aromatic plants from tuscany islands. Acta Biochim. Pol. 60, 43–50. Available online at: http://www.actabp.pl/pdf/1_2013/43.pdf
Prusinowska, R., and Smigielski, K. B. (2014). Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Pol. 60, 56–66. doi: 10.2478/hepo-2014-0010
Raguso, R. A. (2008). Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 39, 549–569. doi: 10.1146/annurev.ecolsys.38.091206.095601
Rahmati, B., Kiasalari, Z., Roghani, M., Khalili, M., and Ansari, F. (2017). Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 55, 958–965. doi: 10.1080/13880209.2017.1285320
Raut, J. S., and Karuppayil, S. M. (2014). A status review on the medicinal properties of essential oils. Ind. Crops Prod. 62, 250–264. doi: 10.1016/j.indcrop.2014.05.055
Sadeghi, H., Jamalpoor, S., and Shirzadi, M. H. (2014). Variability in essential oil of Teucrium polium L. of different latitudinal populations. Ind. Crops Prod. 54, 130–134. doi: 10.1016/j.indcrop.2014.01.015
Sadeghi, H., Robati, Z., and Saharkhiz, M. J. (2015). Variability in Zataria multiflora Bioss. essential oil of twelve populations from Fars province. Iran. Ind. Crops Prod. 67, 221–226. doi: 10.1016/j.indcrop.2015.01.021
Sangwan, N. S., Farooqi, A. H. A., Shabih, F., and Sangwan, R. S. (2001). Regulation of essential oil production in plants. Plant Growth Regul. 34, 3–21. doi: 10.1023/A:1013386921596
Sanz, J., Soria, A. C., and Garcia-Vallejo, M. C. (2004). Analysis of volatile components of Lavandula luisieri L. by direct thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 1024, 139–146. doi: 10.1016/j.chroma.2003.10.024
Selmar, D., and Kleinwächter, M. (2013). Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 42, 558–566. doi: 10.1016/j.indcrop.2012.06.020
Shahdadi, H., Bahador, R. S., Eteghadi, A., and Boraiinejad, S. (2017). Lavender a plant for medical uses: a literature review. Indian J. Public Heal. Res. Dev. 8, 328–332. doi: 10.5958/0976-5506.2017.00065.1
Shams, M., Esfahan, S. Z., Esfahan, E. Z., Dashtaki, H. N., Dursun, A., and Yildirim, E. (2016). Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). Indian J. Sci. Technol. 9, 1–4. doi: 10.17485/ijst/2016/v9i6/61301
Shellie, R., Mondello, L., Marriott, P., and Dugo, G. (2002). Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 970, 225–234. doi: 10.1016/S0021-9673(02)00653-2
Spitaler, R., Schlorhaufer, P. D., Ellmerer, E. P., Merfort, I., Bortenschlager, S., Stuppner, H., et al. (2006). Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO Phytochemistry 67, 409–417. doi: 10.1016/j.phytochem.2005.11.018
Stark, S., Julkunen-Tiitto, R., Holappa, E., Mikkola, K., and Nikula, A. (2008). Concentrations of foliar quercetin in natural populations of white birch (Betula pubescens) increase with latitude. J. Chem. Ecol. 34, 1382–1391. doi: 10.1007/s10886-008-9554-8
Theurillat, J.-P., Aeschimann, D., Küpfer, P., and Spichiger, R. (1995). “The higher vegetation units of the Alps,” in Colloques Phytosociologiques, 23, 189–239.
Tuttolomondo, T., Dugo, G., Ruberto, G., Leto, C., Napoli, E. M., Cicero, N., et al. (2015). Study of quantitative and qualitative variations in essential oils of Sicilian Rosmarinus officinalis L. Nat. Prod. Res. 29, 1928–1934. doi: 10.1080/14786419.2015.1010084
Venskutonis, P. R., Dapkevicius, A., and Baranauskiene, M. (1997). Composition of the essential oil of Lavender (Lavandula angustifolia Mill.) from Lithuania. J. Essent. Oil Res. 9, 107–110. doi: 10.1080/10412905.1997.9700727
Keywords: lavender, Lamiaceae, volatile organic compounds, essential oils, ecological gradient
Citation: Demasi S, Caser M, Lonati M, Cioni PL, Pistelli L, Najar B and Scariot V (2018) Latitude and Altitude Influence Secondary Metabolite Production in Peripheral Alpine Populations of the Mediterranean Species Lavandula angustifolia Mill. Front. Plant Sci. 9:983. doi: 10.3389/fpls.2018.00983
Received: 20 April 2018; Accepted: 18 June 2018;
Published: 05 July 2018.
Edited by:
Marta Sousa Silva, Universidade de Lisboa, PortugalReviewed by:
Ligia Salgueiro, University of Coimbra, PortugalInger Martinussen, Norwegian Institute of Bioeconomy Research (NIBIO), Norway
Copyright © 2018 Demasi, Caser, Lonati, Cioni, Pistelli, Najar and Scariot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Scariot, dmFsZW50aW5hLnNjYXJpb3RAdW5pdG8uaXQ=
 Sonia Demasi
Sonia Demasi Matteo Caser
Matteo Caser Michele Lonati
Michele Lonati Pier L. Cioni2
Pier L. Cioni2 Luisa Pistelli
Luisa Pistelli Basma Najar
Basma Najar Valentina Scariot
Valentina Scariot