- 1National Key Laboratory of Wheat and Maize Crop Science, Collaborative Innovation Center of Henan Grain Crops, College of Agronomy, Henan Agricultural University, Zhengzhou, China
- 2Department of Biological Sciences, Michigan Technological University, Houghton, MI, United States
- 3Department of Biotechnology, Sharda University, Greater Noida, India
Leaf development is a sequential process that involves initiation, determination, transition, expansion and maturation. Many coding genes and a few non-coding small RNAs (sRNAs) have been identified as being involved in leaf development. sRNAs and their interactions not only determine gene expression and regulation, but also play critical roles in leaf development through their coordination with other genetic networks and physiological pathways. In this review, we first introduce the biogenesis pathways of sRNAs, mainly microRNAs (miRNAs) and trans-acting small interfering RNAs (ta-siRNAs), and then describe the function of miRNA-transcription factors in leaf development, focusing on guidance by interactive sRNA regulatory networks.
Introduction
Leaf is one of the most important organs in flowering plants because of its essential roles in photosynthesis (Efroni et al., 2010). The leaf lamina is typically broad, flat, and thin, and differentiates into the upper (adaxial) surface and the lower (abaxial) surface (Braybrook and Kuhlemeier, 2010). In most plant species, the upper surface facilitates light capture, whereas the lower surface facilitates gas exchange and regulation of transpiration (Husbands et al., 2009; Yamaguchi et al., 2012). Leaf morphology varies greatly among species and within different developmental stages and growth conditions (Rodriguez et al., 2014). Leaf shapes and sizes not only determine plant architecture, but also fundamental agronomic traits for crop productivity.
Small RNAs (sRNAs), including microRNAs (miRNAs) and small interfering RNAs (siRNAs), are 18–30 nt long, endogenous, non-coding RNAs. While substantial differences in the size of sRNAs have been documented, miRNAs and siRNAs have their own typical sizes. sRNAs play important roles in development, metabolism and chromatin modifications (Chen, 2009). In plants, the biogenesis of miRNAs comprises of several sequential steps, including MIRNA transcription, nuclear processing, nuclear export, cytoplasmic processing, and RNA-induced silencing complex (RISC) assembly. The ARGONAUTE 1 (AGO1) protein recruits mature miRNAs in the assembly of RISCs, thereby to cleave target mRNA or block its translation (Rogers and Chen, 2013; Iwakawa and Tomari, 2015).
Trans-acting siRNA (ta-siRNA) is a miRNA-derived siRNA. In Arabidopsis, miR173 initiates the production of TAS1a-c and TAS2 siRNA, miR390 of TAS3, and miR828 of TAS4 siRNA (Allen et al., 2005; Allen and Howell, 2010). The production of ta-siRNA involves two distinct pathways: the ‘one-hit’ and the ‘two-hit’ system (Vazquez et al., 2004; Axtell et al., 2006). The ‘one-hit’ system requires one 22-nt miRNA (miR173 and miR828) and one AGO1 protein, whereas the ‘two-hit’ system requires two 21-nt miRNAs (miR390) and two AGO7 proteins (Chapman and Carrington, 2007).
Compared to animal miRNAs, plant miRNAs have fewer targets. Plant target genes usually encode transcription factors (TFs) and F-box proteins, which places miRNAs at the center of plant gene regulatory networks (Rubio-Somoza and Weigel, 2011). A number of sRNAs and their target TFs have been shown to determine leaf development, such as miR156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) (Xu et al., 2016), miR160-AUXIN RESPONSE FACTOR (ARF) (Ben-Gera et al., 2016), miR165/166-Class III HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIPIII) (Jia et al., 2015), miR319-TEOSINTE BRANCHED/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) (Bresso et al., 2018), miR390-TRANS-ACTING SMALL INTERFERING RNA3 (TAS3) (Husbands et al., 2015) and miR396-GROWTH REGULATING FACTOR (GRF) (Omidbakhshfard et al., 2015). Additionally, multiple crucial components that are required for miRNA and ta-siRNA biogenesis, such as AGO1 (Bohmert et al., 1998), AGO7 (Garcia et al., 2006), SERRATE (SE) (Prigge and Wagner, 2001), and HYPONASTIC LEAVES 1 (HYL1) (Liu et al., 2011), have been suggested to influence leaf development.
Leaf development is a complex process that involves the connections and interactions of many miRNA-pathway components to generate multilayered networks. In this review, we discuss current knowledge of the sRNA-mediated regulatory networks that function during leaf developmental processes, including leaf initiation, polarity determination, phase transition, leaf morphology and leaf senescence (Figure 1). First, we briefly introduce the formation of mature leaves and provide an overview of stages of leaf morphogenesis. Next, we describe the molecular controls of leaf development. In particular, we focus upon miRNA-TF and miRNA-miRNA interactions involved in this multifaceted sequence of leaf development. Finally, we highlight several important aspects that should be addressed to fully understand the functional complexity of such miRNA networks.
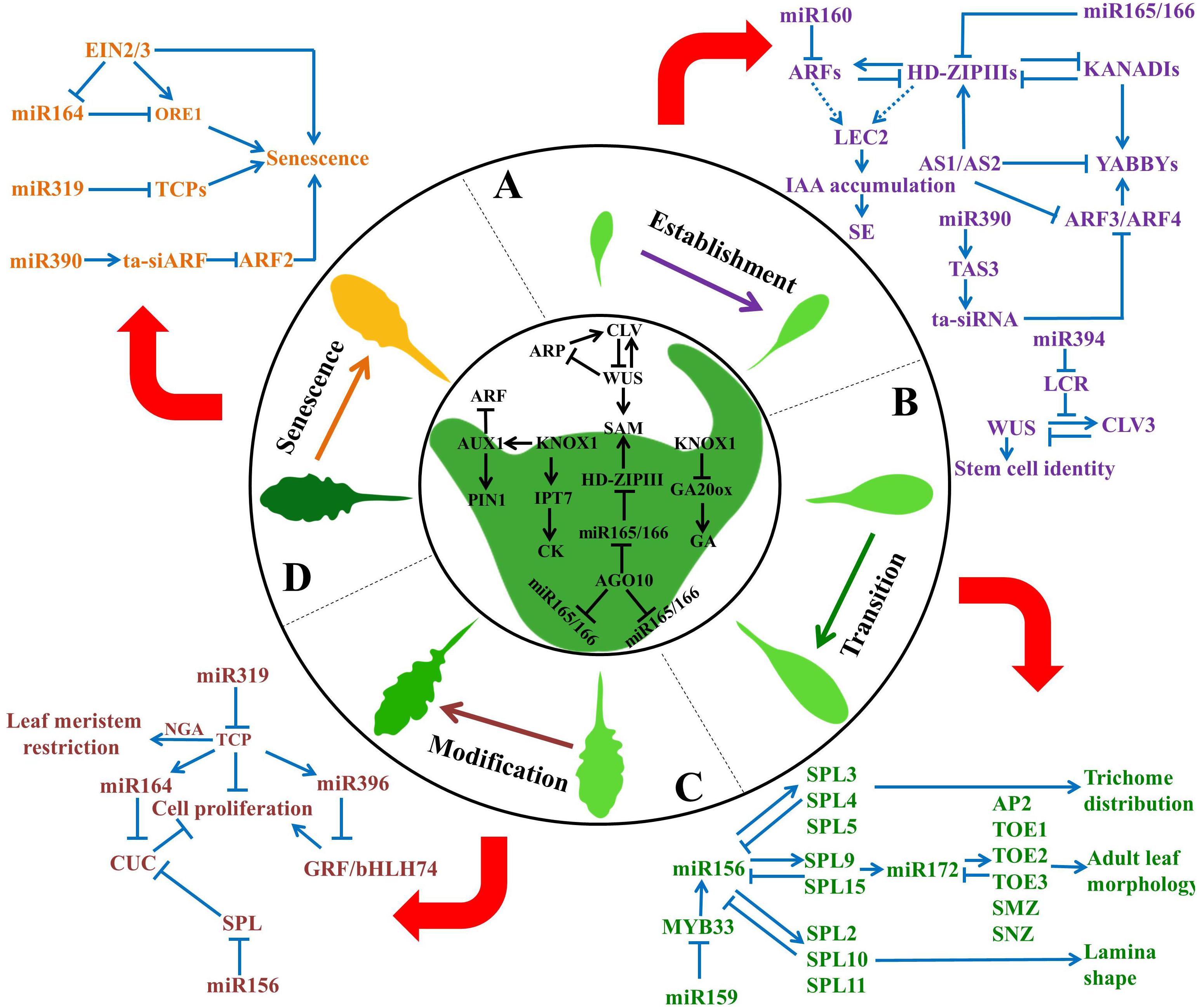
FIGURE 1. Overview of miRNA-mediated regulatory networks in control of leaf development. Leaf development is a complex biological process that is tightly controlled by miRNAs and their target genes. It consists of four stages: (A) estabishment, (B) transition, (C) modification, and (D) senescence. sRNA networks play an important role in each stage of leaf development. For instance, miR160 and miR165/166 regulate leaf initiation; miR165/166 and miR390 control leaf polarity; miR164 and miR319 modulate leaf morphology; miR156 and miR172 determine phase transition; miR164 and miR319 contribute to leaf senescence. Blue arrows indicate positive regulation, while T shaped bars indicate negative regulation. SE, somatic embryogenesis.
The Developmental Processes of Leaf Morphogenesis
Leaves arise from the flanks of the shoot apical meristem (SAM), establish proximo-distal, adaxial-abaxial and medio-lateral axes, followed by subsequent cell division and expansion to reach their final size and shape. Three regulatory pathways which are involved in the establishment of leaf polarity and SAM maintenance are: CLAVATA (CLV)-WUSCHEL (WUS), KNOTTED-LIKE HOMEOBOX (KNOX)-ASYMMETRIC LEAVES1 (AS1) in Arabidopsis, ROUGH SHEATH2 (RS2) in maize and PHANTASTICA (PHAN) in snapdragon, together known as the AS1/RS2/PHAN (ARP) pathway (Waites et al., 1998; Timmermans et al., 1999; Guo et al., 2008; Tabata et al., 2010). Furthermore, HD-ZIP III-KANADI proteins sequentially operate upon three axes to ensure the robust growth and development of leaves. The generation of leaves is largely governed by the repression of Class I KNOX (KNOXI) genes via two pathways. One is the plant hormone dependent pathway, in which auxin polar transport and local accumulation determines the site of leaf primordium initiation, while a low cytokinin (CK)/gibberellin (GA) ratio promotes SAM maintenance (Reinhardt et al., 2000; Fleishon et al., 2011). The other is the ARP genes-dependent pathway. ARP genes encode MYELOBLASTOSIS (MYB)-domain TFs and are expressed in lateral organ founder cells. AS1 recruits a repressor complex with the LATERAL ORGAN BOUNDARIES domain protein, AS2, which binds to the promoter region of KNOX genes (Guo et al., 2008). Thus, leaf development is controlled temporally and spatially by several pivotal factors, such as plant hormones, TFs and sRNAs (Pulido and Laufs, 2010; Byrne, 2012; Dkhar and Pareek, 2014; Kalve et al., 2014).
The Interactions of miRNAs Involved in Leaf Development
The Interplay of miR160, miR165/166, and miR394 Regulates Leaf Initiation in Plants
sRNA transcriptome profiling data revealed that early embryonic development involves the function of several miRNAs, such as miR156, miR160, miR166, miR167, miR390, and miR394 (Figure 1A) (Armenta-Medina et al., 2017). Two miRNA interaction nodes greatly affect meristem maintenance and leaf initiation: the module of miR394 and LEAF CURLING RESPONSIVENESS (LCR) mediated non-cell autonomous pathway, and the interplay of the miR160 and miR165/166-mediated cell autonomous pathway. The protoderm-specific miR394 represses the LCR gene expression to confer stem cell maintenance, which also involves a local feedback loop mediated by WUS and CLV (Knauer et al., 2013).
ARF10, ARF16, and ARF17, which are the targets of miR160, are required for the maintenance of local auxin peaks in the meristem, and phyllotaxis in the rosette (Pulido and Laufs, 2010). Arabidopsis transgenic plants expressing a miR160-resistant version of ARF10 or ARF17 exhibited pleiotropic phenotypes, including abnormal numbers and positions of cotyledons and up-curled, strongly serrated rosettes, indicating that miR160 and ARFs account for leaf initiation (Mallory et al., 2005; Liu et al., 2007). Additionally, miR165/166 is suggested to regulate the organization and maintenance of the SAM. AGO10 sequestrates miR165/166 and thus protects HD-ZIP III transcripts from degradation. AGO10 competes with AGO1 for loading miR165/166 into AGO1-guided RISC complex. This de-represses the expression of HD-ZIP III family genes which helps in SAM maintenance (Zhu et al., 2011). AGO10 gain-of-function mutants displayed varying degrees of leaf hyponasty and serration, whereas loss-of-function of AGO10 resulted in the formation of pin-headed or even empty apexes (Roodbarkelari et al., 2015; Yu et al., 2017). The interaction between miR160 and miR165/166 contributes to somatic embryogenesis via activation of LEAFY COTYLEDON2 (LEC2)-mediated auxin biogenesis pathways. Their targets, ARFs and HD-ZIP IIIs, also interact with each other via a negative feedback loop to further modulate LEC2 expression. HD-ZIP III proteins, PHAVOLUTA (PHV), and ARF10/ARF16 activate LEC2 expression, thereby inducing the up-regulation of several key genes of the auxin synthesis pathway which ultimately triggers indole-3-acetic acid (IAA) accumulation (Wójcik et al., 2017). These findings provide a detailed insight into how miR160-ARF, miR165/166-HD-ZIPIII and miR394-LCR nodes control somatic embryogenesis, meristem maintenance and leaf initiation.
The Interplay of miR165/166, miR390, and miR396 Controls Leaf Polarity in Plants
The establishment of leaf polarity is an important event in leaf development, and several TFs and sRNAs have been proposed to be involved in pattern identity via antagonistic interactions (Figure 1A). Generally, opposite surfaces of the leaf are maintained by two polarity regulators: the adaxial determiners and the abaxial determiners. HD-ZIPIIIs and AS1/AS2 are adaxial determiners where AS1/AS2 promote HD-ZIPIIIs. KANADIs and YABBYs are abaxial determiners, where KANADIs activate YABBYs (Chitwood et al., 2007; Yamaguchi et al., 2012). Additionally, HD-ZIP IIs are involved in the regulation of polarity identity by repressing miR165/166 (Merelo et al., 2016).
Accumulating data have suggested that miR165/166 and miR390-derived ta-siRNAs determine leaf polarity. miR165/166 targets encode five of the HD-ZIP III TFs, including PHB, PHV, REVOLUTA (REV), ATHB-8 and ATHB-15. Of these, PHB, PHV, and REV act redundantly to promote adaxial identity. miR390, which is derived from four loci (MIR390 a-d) of the Arabidopsis genome, also contributes to leaf polarity through the ta-siRNA-dependent pathway. miR390-directed TAS3 cleavage leads to the production of ta-siRNAs which further target ARF3 and ARF4 transcripts to regulate leaf abaxial identity (Pekker et al., 2005). Furthermore, AS1/AS2 repress ARF3/ARF4 to stabilize adaxial-abaxial partitioning in three ways by: directly binding to the promoter region of ARF3, indirectly activating miR390- and RDR6-dependent pathway leading to silencing of ARF3/4, and maintaining DNA methylation in the coding regions of ARF3 (Iwasaki et al., 2013).
Both miR165/166 and miR390 have been shown to move between cells and to interact with each other during the formation of leaf developmental boundaries (Marin-Gonzalez and Suarez-Lopez, 2012). HD-ZIP IIIs and ARF3/4 are required for the fate of the leaf upper and lower surface, respectively. miR165/166 moves from the bottom to top to establish a gradient along the axis, which defines a stable boundary, and limits the localization of HD-ZIP III expression. A similar but opposite pattern was also observed for TAS3-ARF. The movement of miR165/166 and miR390 generates sharp boundaries via a threshold-based readout of their mobility gradients (Robinson and Roeder, 2017; Skopelitis et al., 2017).
In addition to the well-known modules of miR165/166 and miR390 controlling leaf polarity, an interesting study by Das Gupta and Nath (2015) revealed that divergent leaf growth polarity is strongly correlated to the miR396-GRF expression gradient. Positive allometry, or basipetal growth occurs when the growth rate of the base is faster than that at the tip and vice versa for negative allometry, or acropetal growth. The accumulation of miR396 is higher near the tip but lower near the base in basipetal leaves, whereas acropetal leaves exhibit an opposite expression pattern. Consistent with this, the expression level of miR396 is uniform throughout the leaves with diffused growth (Das Gupta and Nath, 2015). Taken together, leaf polarity is regulated in a quantitative manner that involves sRNA production, movement and action.
The Interplay of miR156, miR159, and miR172 Determines Phase Transition in Plants
Plants undergo several developmental changes during their life cycles, including two phase changes, from juvenile to adult and from vegetative to reproductive (Poethig, 2009). Leaf morphology also changes during these transitions, and is characterized by trichome initiation, severe serration, and blade alteration (Usami et al., 2009). Two conserved miRNAs, miR156 and miR172, are involved in phase transition (Figure 1B) (Wu et al., 2009). In Arabidopsis, the targets of miR156/157 encode 10 SPL TFs, SPL2-6, SPL9-11, SPL13, and SPL15. However, the targets of miR172 encode six of the APETALA2 (AP2)-like TFs, including TARGET OF EAT1 (TOE1), TOE2, TOE3, SCHLAFMUTZE (SMZ), and SCHNARCHZAPFEN (SNZ). The targets of miR156 involve several pathways that act redundantly to control leaf phase change. First, SPL3/4/5 determine trichome formation and distribution; second, SPL9/15 modulate adult leaf morphology, of when miR156 interacts with miR172 via SPL9 and SPL10. Finally, SPL2/10/11 regulate the lamina shape and act independently of miR172 (Huijser and Schmid, 2011).
A regulatory model has demonstrated the interaction between miR156 and miR172 (Teotia and Tang, 2015). miR156 is controlled by the positive feedback loop of SPL9, whereas the negative feedback loop is modulated by SPL15. Additionally, miR156 is positively regulated by AP2 and AGAMOUS-LIKE15 (Wu et al., 2009; Serivichyaswat et al., 2015). Furthermore, expression of miR172 is fine-tuned by the positive feedback loop of TOE1/2 and negatively by AP2 via LEUNIG (LUG) and SEUSS (SEU) (Yant et al., 2010; Grigorova et al., 2011). TOE1/2 repress SPL3/4/5, while SPL3 activates TOE3 expression. In addition to the regulatory nodes of miR156 and miR172, miR159 acts as a modifier to control the timing of juvenile to adult transition. MYB33, a target of miR159, directly binds to the promoters of MIR156A, MIR156C, and SPL9 and activates their expression (Guo et al., 2017).
The Interplay of miR164, miR319, and miR396 Modulates Leaf Morphology in Plants
Leaf shape varies greatly among different plant species which is largely attributed to the level and pattern of dissection in the primordium margin. Recent studies have shown miR164 and miR319 as key regulators in leaf shaping (Figure 1C). PIN-FORMED 1 (PIF1) and CUP-SHAPED COTYLEDON (CUC) TFs modify marginal serrations in leaf. The inactivation of CUC2 resulted in reduced serrations and smooth margins, while CUC2 overexpression led to the formation of highly serrated leaves. CUC2 is a target of miR164 and its expression level determines the level of serration in the Arabidopsis leaf (Nikovics et al., 2006). Moreover, CUC1 and CUC3 act redundantly in the formation of incipient serrations in leaves. CUC2 interacts with CUC3 in a feed-forward mechanism. CUC3 gets activated by the CUC2 homodimer, then CUC2 and CUC3 heterodimerize to promote leaf complexity (Rubio-Somoza et al., 2014).
miR319, also known as miRJAW in Arabidopsis, regulates leaf complexity by targeting multiple TCP genes (Palatnik et al., 2003). The overexpression of miR319 promoted the formation of additional serrations whereas MIM319 plants, that repress endogenous miR319 activity, showed opposite phenotypes (Rubio-Somoza et al., 2014). The study of a series of MIR319 and TCP mutants revealed their important roles in cotyledon boundary establishment, leaf serration, and the onset of leaf senescence (Koyama et al., 2017). Furthermore, multiple TCP TFs coupled with NGATHA (NGA) TFs are shown to be involved in sequential developmental processes, including meristem activity localization, marginal persistent differentiation and adaxial-abaxial maintenance (Alvarez et al., 2016).
miR164-CUC and miR319-TCP modules function in a coordinated manner for leaf size regulation. TCP3, a miR319 target, was found to negatively regulate CUC2 by directly activating MIR164A (Koyama et al., 2007). TCPs can also interfere with CUC2 in a miR164-independent manner, resulting in a serration-deficient phenotype. Furthermore, SPLs, targeted by miR156, not only act as timing cues but also regulate TCP-CUC interactions. For example, SPL9 dimerizes with TCP4 and competes with CUC2/3 for TCP4 interaction. This links the temporal development regulator (SPL) with factors responsible for leaf margin modification (CUC) via a common player of leaf cell proliferation and differentiation (TCP) (Chitwood and Sinha, 2014; Rubio-Somoza et al., 2014).
Leaf shape is also modulated by GRFs, which are involved in cell division and expansion. GRF1/2/3 loss-of-function mutants exhibited smaller leaf size, while plants overexpressing them produced bigger leaves (Kim et al., 2003). During leaf development, expression of miR396 increases and that of target GRF decreases, which acts as a signal to determine final leaf size (Rodriguez et al., 2010). GRF-interacting factor 1 (GIF1), interacts with both chromatin-remodeling complexes and GRF proteins, thereby maintaining the cell proliferation activity of vegetative and reproductive organ primordium (Debernardi et al., 2014; Kim and Tsukaya, 2015). An additional target for miR396b, basic helix-loop-helix DNA-binding superfamily protein 74 (bHLH74), also contributes to leaf margin and vein pattern formation (Debernardi et al., 2012). Intriguingly, Mecchia et al. (2013) proposed that the miR396 regulatory pathway cooperates with the ta-siRNA biogenesis pathway in leaf morphogenesis. TCPs simultaneously promote miR164 and miR396, which have opposing functions in leaf cell proliferation (Schommer et al., 2014). Thus, GRFs, negatively regulated by miR396, also lie within this regulatory network, which is guided by miR156 and centered in miR319.
The Interplay of miR164, miR319, and miR390 Contributes to Leaf Senescence in Plants
Leaf senescence is the final stage of leaf development which involves distinctive actions at the cellular, tissue, organ, and organism levels (Lim et al., 2007). miR164 and miR319 are involved in controlling leaf senescence (Figure 1D). A target of miR164, ORESARA1 (ORE1), functions as a positive regulator of aging-induced cell death and leaf senescence in Arabidopsis. ORE1 is induced in an age-dependent manner by ETHYLENE INSENSITIVE2 and 3 (EIN2/3), central components of ethylene signaling (Li et al., 2013). ORE1 expression is gradually down-regulated by miR164 during earlier stages of leaf development, but is up-regulated at later stages by EIN2/3. Additionally, EIN2/3 mediate aging-induced cell death in the absence of both miR164 and ORE1. The trifurcate feed-forward pathway provides a highly robust regulation to ensure coupled leaf senescence and cell death (Kim et al., 2009).
miR319, which targets the TCP genes, is also proposed to be a key player in leaf senescence. TCP4 has been shown to activate the jasmonic acid (JA) biosynthetic gene LIPOXYGENASE2, which increases levels of JA, and accelerates leaf senescence (Schommer et al., 2008). These findings showed that miR319-TCPs control two different processes of leaf development by negatively regulating growth but positively regulating senescence (Schommer et al., 2008). In addition to ethylene and JA, auxin also plays an important role in the control of plant longevity. ARF2, a negative regulator of auxin responses, is believed to regulate leaf senescence (Ellis et al., 2005; Lim et al., 2010). These findings suggest the importance of exploring the complex regulatory networks comprising of miR164/ORE1, miR319/TCP, and miR390/TAS3/ARF2 modules.
Conclusion and Perspectives
In recent years, considerable advances have been made in our understanding of sRNA-mediated regulatory networks in leaf development. In this review, we have provided an overview on miRNA interactions during leaf development. We have emphasized on how sRNAs participate in regulatory networks, involved in various stages of leaf development. We showed that sRNAs can have very specific roles (for example miR160 in leaf initiation) and also very broad roles (for example miR319 in leaf expansion and senescence). Additionally, we explored how different targets of the same miRNA regulate diverse processes (for example miR164 targets, CUC2 in leaf morphology and ORE1 in leaf senescence).
The discovery of more miRNA interacting networks for leaf development, will aid in deciphering important leaf-related traits through manipulation of miRNA/target gene expression. However, our knowledge of sRNA-mediated leaf development networks is far from complete. The systematic study of their roles should integrate several strategies to reconstitute the developmental pathway of single cells from the leaf primordium to their final position in the mature leaf. Such explorations will benefit from the increasing next-generation sequencing techniques, bioinformatics analysis tools, and maturing mathematical modeling methods. Further research could help apply the knowledge gained from the model organisms to crop plants for modulation of leaf shape to promote photosynthesis and increase biomass and yield.
Author Contributions
GT and ZZ conceived the topic and revised the manuscript. TY and YW wrote the first draft of the manuscript and drew the figure. ST further modified the manuscript and the figure with intellectual inputs. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 31571679).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Sarah Williams, Ph.D., from Liwen Bianji, Edanz Group China (https://www.liwenbianji.cn), for editing the English text of a draft of this manuscript. We also thank Ashley Videtich, Ph.D., from Michigan Technological University, for help with language revision.
References
Allen, E., and Howell, M. D. (2010). miRNAs in the biogenesis of trans-acting siRNAs in higher plants. Semin. Cell Dev. Biol. 21, 798–804. doi: 10.1016/j.semcdb.2010.03.008
Allen, E., Xie, Z., Gustafson, A. M., and Carrington, J. C. (2005). microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121, 207–221. doi: 10.1016/j.cell.2005.04.004
Alvarez, J. P., Furumizu, C., Efroni, I., Eshed, Y., and Bowman, J. L. (2016). Active suppression of a leaf meristem orchestrates determinate leaf growth. eLife 5:e15023. doi: 10.7554/eLife.15023
Armenta-Medina, A., Lepe-Soltero, D., Xiang, D. Q., Datla, R., Abreu-Goodger, C., and Gillmor, C. S. (2017). Arabidopsis thaliana miRNAs promote embryo pattern formation beginning in the zygote. Dev. Biol. 431, 145–151. doi: 10.1016/j.ydbio.2017.09.009
Axtell, M. J., Jan, C., Rajagopalan, R., and Bartel, D. P. (2006). A two-hit trigger for siRNA biogenesis in plants. Cell 127, 565–577. doi: 10.1016/j.cell.2006.09.032
Ben-Gera, H., Dafna, A., Alvarez, J. P., Bar, M., Mauerer, M., and Ori, N. (2016). Auxin-mediated lamina growth in tomato leaves is restricted by two parallel mechanisms. Plant J. 86, 443–457. doi: 10.1111/tpj.13188
Bohmert, K., Camus, I., Bellini, C., Bouchez, D., Caboche, M., and Benning, C. (1998). AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 17, 170–180. doi: 10.1093/emboj/17.1.170
Braybrook, S. A., and Kuhlemeier, C. (2010). How a plant builds leaves. Plant Cell 22, 1006–1018. doi: 10.1105/tpc.110.073924
Bresso, E. G., Chorostecki, U., Rodriguez, R. E., Palatnik, J. F., and Schommer, C. (2018). Spatial control of gene expression by miR319-regulated TCP transcription factors in leaf development. Plant Physiol. 176, 1694–1708. doi: 10.1104/pp.17.00823
Byrne, M. E. (2012). Making leaves. Curr. Opin. Plant Biol. 15, 24–30. doi: 10.1016/j.pbi.2011.10.009
Chapman, E. J., and Carrington, J. C. (2007). Specialization and evolution of endogenous small RNA pathways. Nat. Rev. Genet. 8, 884–896. doi: 10.1038/nrg2179
Chen, X. (2009). Small RNAs and their roles in plant development. Annu. Rev. Cell Dev. Biol. 25, 21–44. doi: 10.1146/annurev.cellbio.042308.113417
Chitwood, D. H., Guo, M., Nogueira, F. T., and Timmermans, M. C. P. (2007). Establishing leaf polarity: the role of small RNAs and positional signals in the shoot apex. Development 134, 813–823. doi: 10.1242/dev.000497
Chitwood, D. H., and Sinha, N. R. (2014). Plant development: small RNAs and the metamorphosis of leaves. Curr. Biol. 24, R1087–R1089. doi: 10.1016/j.cub.2014.10.013
Das Gupta, M., and Nath, U. (2015). Divergence in patterns of leaf growth polarity is associated with the expression divergence of miR396. Plant Cell 27, 2785–2799. doi: 10.1105/tpc.15.00196
Debernardi, J. M., Mecchia, M. A., Vercruyssen, L., Smaczniak, C., Kaufmann, K., Inze, D., et al. (2014). Post-transcriptional control of GRF transcription factors by microRNA miR396 and GIF co-activator affects leaf size and longevity. Plant J. 79, 413–426. doi: 10.1111/tpj.12567
Debernardi, J. M., Rodriguez, R. E., Mecchia, M. A., and Palatnik, J. F. (2012). Functional specialization of the plant miR396 regulatory network through distinct microRNA target interactions. PLoS Genet. 8:e1002419. doi: 10.1371/journal.pgen.1002419
Dkhar, J., and Pareek, A. (2014). What determines a leaf’s shape? Evodevo 5:47. doi: 10.1186/2041-9139-5-47
Efroni, I., Eshed, Y., and Lifschitz, E. (2010). Morphogenesis of simple and compound leaves: a critical review. Plant Cell 22, 1019–1032. doi: 10.1105/tpc.109.073601
Ellis, C. M., Nagpal, P., Young, J. C., Hagen, G., Guilfoyle, T. J., and Reed, J. W. (2005). AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132, 4563–4574. doi: 10.1242/dev.02012
Fleishon, S., Shani, E., Ori, N., and Weiss, D. (2011). Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytol. 190, 609–617. doi: 10.1111/j.1469-8137.2010.03616.x
Garcia, D., Collier, S. A., Byrne, M. E., and Martienssen, R. A. (2006). Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr. Biol. 16, 933–938. doi: 10.1016/j.cub.2006.03.064
Grigorova, B., Mara, C., Hollender, C., Sijacic, P., Chen, X., and Liu, Z. (2011). LEUNIG and SEUSS co-repressors regulate miR172 expression in Arabidopsis flowers. Development 138, 2451–2456. doi: 10.1242/dev.058362
Guo, C. K., Xu, Y. M., Shi, M., Lai, Y. M., Wu, X., Wang, H. S., et al. (2017). Repression of miR156 by miR159 regulates the timing of the juvenile-to-adult transition in Arabidopsis. Plant Cell 29, 1293–1304. doi: 10.1105/tpc.16.00975
Guo, M., Thomas, J., Collins, G., and Timmermans, M. C. P. (2008). Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20, 48–58. doi: 10.1105/tpc.107.056127
Huijser, P., and Schmid, M. (2011). The control of developmental phase transitions in plants. Development 138, 4117–4129. doi: 10.1242/dev.063511
Husbands, A. Y., Benkovics, A. H., Nogueira, F. T., Lodha, M., and Timmermans, M. C. P. (2015). The ASYMMETRIC LEAVES complex employs multiple modes of regulation to affect adaxial-abaxial patterning and leaf complexity. Plant Cell 27, 3321–3335. doi: 10.1105/tpc.15.00454
Husbands, A. Y., Chitwood, D. H., Plavskin, Y., and Timmermans, M. C. P. (2009). Signals and prepatterns: new insights into organ polarity in plants. Genes Dev. 23, 1986–1997. doi: 10.1101/gad.1819909
Iwakawa, H. O., and Tomari, Y. (2015). The functions of MicroRNAs: mRNA decay and translational repression. Trends Cell Biol. 25, 651–665. doi: 10.1016/j.tcb.2015.07.011
Iwasaki, M., Takahashi, H., Iwakawa, H., Nakagawa, A., Ishikawa, T., Tanaka, H., et al. (2013). Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 140, 1958–1969. doi: 10.1242/dev.085365
Jia, X., Ding, N., Fan, W., Yan, J., Gu, Y., Tang, X., et al. (2015). Functional plasticity of miR165/166 in plant development revealed by small tandem target mimic. Plant Sci. 233, 11–21. doi: 10.1016/j.plantsci.2014.12.020
Kalve, S., De Vos, D., and Beemster, G. T. (2014). Leaf development: a cellular perspective. Front. Plant Sci. 5:362. doi: 10.3389/fpls.2014.00362
Kim, J. H., Choi, D., and Kende, H. (2003). The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 36, 94–104. doi: 10.1046/j.1365-313X.2003.01862.x
Kim, J. H., and Tsukaya, H. (2015). Regulation of plant growth and development by the GROWTH-REGULATING FACTOR and GRF-INTERACTING FACTOR duo. J. Exp. Bot. 66, 6093–6107. doi: 10.1093/jxb/erv349
Kim, J. H., Woo, H. R., Kim, J., Lim, P. O., Lee, I. C., Choi, S. H., et al. (2009). Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057. doi: 10.1126/science.1166386
Knauer, S., Holt, A. L., Rubio-Somoza, I., Tucker, E. J., Hinze, A., Pisch, M., et al. (2013). A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev. Cell 24, 125–132. doi: 10.1016/j.devcel.2012.12.009
Koyama, T., Furutani, M., Tasaka, M., and Ohme-Takagi, M. (2007). TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19, 473–484. doi: 10.1105/tpc.106.044792
Koyama, T., Sato, F., and Ohme-Takagi, M. (2017). Roles of miR319 and TCP transcription factors in leaf development. Plant Physiol. 175, 874–885. doi: 10.1104/pp.17.00732
Li, Z., Peng, J., Wen, X., and Guo, H. (2013). Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25, 3311–3328. doi: 10.1105/tpc.113
Lim, P. O., Kim, H. J., and Nam, H. G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. doi: 10.1146/annurev.arplant.57.032905.105316
Lim, P. O., Lee, I. C., Kim, J., Kim, H. J., Ryu, J. S., Woo, H. R., et al. (2010). Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 61, 1419–1430. doi: 10.1093/jxb/erq010
Liu, P. P., Montgomery, T. A., Fahlgren, N., Kasschau, K. D., Nonogaki, H., and Carrington, J. C. (2007). Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 52, 133–146. doi: 10.1111/j.1365-313X.2007.03218.x
Liu, Z., Jia, L., Wang, H., and He, Y. (2011). HYL1 regulates the balance between adaxial and abaxial identity for leaf flattening via miRNA-mediated pathways. J. Exp. Bot. 62, 4367–4381. doi: 10.1093/jxb/err167
Mallory, A. C., Bartel, D. P., and Bartel, B. (2005). MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17, 1360–1375. doi: 10.1105/tpc.105.031716
Marin-Gonzalez, E., and Suarez-Lopez, P. (2012). “And yet it moves”: cell-to-cell and long-distance signaling by plant microRNAs. Plant Sci. 196, 18–30. doi: 10.1016/j.plantsci.2012.07.009
Mecchia, M. A., Debernardi, J. M., Rodriguez, R. E., Schommer, C., and Palatnik, J. F. (2013). MicroRNA miR396 and RDR6 synergistically regulate leaf development. Mech. Dev. 130, 2–13. doi: 10.1016/j.mod.2012.07.005
Merelo, P., Ram, H., Caggiano, M. P., Ohno, C., Ott, F., Straub, D., et al. (2016). Regulation of MIR165/166 by class II and class III homeodomain leucine zipper proteins establishes leaf polarity. Proc. Natl. Acad. Sci. U.S.A. 113, 11973–11978. doi: 10.1073/pnas.1516110113
Nikovics, K., Blein, T., Peaucelle, A., Ishida, T., Morin, H., Aida, M., et al. (2006). The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18, 2929–2945. doi: 10.1105/tpc.106.045617
Omidbakhshfard, M. A., Proost, S., Fujikura, U., and Mueller-Roeber, B. (2015). Growth-regulating factors (GRFs): a small transcription factor family with important functions in plant biology. Mol. Plant 8, 998–1010. doi: 10.1016/j.molp.2015.01.013
Palatnik, J. F., Allen, E., Wu, X., Schommer, C., Schwab, R., Carrington, J. C., et al. (2003). Control of leaf morphogenesis by microRNAs. Nature 425, 257–263. doi: 10.1038/nature01958
Pekker, I., Alvarez, J. P., and Eshed, Y. (2005). Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17, 2899–2910. doi: 10.1105/tpc.105.034876
Poethig, R. S. (2009). Small RNAs and developmental timing in plants. Curr. Opin. Genet. Dev. 19, 374–378. doi: 10.1016/j.gde.2009.06.001
Prigge, M. J., and Wagner, D. R. (2001). The Arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13, 1263–1279. doi: 10.1105/TPC.010095
Pulido, A., and Laufs, P. (2010). Co-ordination of developmental processes by small RNAs during leaf development. J. Exp. Bot. 61, 1277–1291. doi: 10.1093/jxb/erp397
Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518. doi: 10.1105/tpc.12.4.507
Robinson, D. O., and Roeder, A. H. K. (2017). Small RNAs turn over a new leaf as morphogens. Dev. Cell 43, 253–254. doi: 10.1016/j.devcel.2017.10.025
Rodriguez, R. E., Debernardi, J. M., and Palatnik, J. F. (2014). Morphogenesis of simple leaves: regulation of leaf size and shape. Wiley Interdiscip. Rev. Dev. Biol. 3, 41–57. doi: 10.1002/wdev.115
Rodriguez, R. E., Mecchia, M. A., Debernardi, J. M., Schommer, C., Weigel, D., and Palatnik, J. F. (2010). Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137, 103–112. doi: 10.1242/dev.043067
Rogers, K., and Chen, X. (2013). Biogenesis, turnover, and mode of action of plant MicroRNAs. Plant Cell 25, 2383–2399. doi: 10.1105/tpc.113.113159
Roodbarkelari, F., Du, F., Truernit, E., and Laux, T. (2015). ZLL/AGO10 maintains shoot meristem stem cells during Arabidopsis embryogenesis by down-regulating ARF2-mediated auxin response. BMC Biol. 13:74. doi: 10.1186/s12915-015-0180-y
Rubio-Somoza, I., and Weigel, D. (2011). MicroRNA networks and developmental plasticity in plants. Trends Plant Sci. 16, 258–264. doi: 10.1016/j.tplants.2011.03.001
Rubio-Somoza, I., Zhou, C. M., Confraria, A., Martinho, C., von Born, P., Baena-Gonzalez, E., et al. (2014). Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr. Biol. 24, 2714–2719. doi: 10.1016/j.cub.2014.09.058
Schommer, C., Debernardi, J. M., Bresso, E. G., Rodriguez, R. E., and Palatnik, J. F. (2014). Repression of cell proliferation by miR319-regulated TCP4. Mol. Plant 7, 1533–1544. doi: 10.1093/mp/ssu084
Schommer, C., Palatnik, J. F., Aggarwal, P., Chetelat, A., Cubas, P., Farmer, E. E., et al. (2008). Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 6:e230. doi: 10.1371/journal.pbio.0060230
Serivichyaswat, P., Ryu, H. S., Kim, W., Kim, S., Chung, K. S., Kim, J. J., et al. (2015). Expression of the floral repressor miRNA156 is positively regulated by the AGAMOUS-like proteins AGL15 and AGL18. Mol. Cells 38, 259–266. doi: 10.14348/molcells.2015.2311
Skopelitis, D. S., Benkovics, A. H., Husbands, A. Y., and Timmermans, M. C. P. (2017). Boundary formation through a direct threshold-based readout of mobile small RNA gradients. Dev. Cell 43, 265–273. doi: 10.1016/j.devcel.2017.10.003
Tabata, R., Ikezaki, M., Fujibe, T., Aida, M., Tian, C. E., Ueno, Y., et al. (2010). Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol. 51, 164–175. doi: 10.1093/pcp/pcp176
Teotia, S., and Tang, G. (2015). To bloom or not to bloom: role of microRNAs in plant flowering. Mol. Plant 8, 359–377. doi: 10.1016/j.molp.2014.12.018
Timmermans, M. C. P., Hudson, A., Becraft, P. W., and Nelson, T. (1999). ROUGH SHEATH2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 284, 151–153. doi: 10.1126/science.284.5411.151
Usami, T., Horiguchi, G., Yano, S., and Tsukaya, H. (2009). The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development 136, 955–964. doi: 10.1242/dev.028613
Vazquez, F., Gasciolli, V., Crete, P., and Vaucheret, H. (2004). The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 14, 346–351. doi: 10.1016/j.cub.2004.01.035
Waites, R., Selvadurai, H. R., Oliver, I. R., and Hudson, A. (1998). The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93, 779–789. doi: 10.1016/S0092-8674(00)81439-7
Wójcik, A. M., Nodine, M. D., and Gaj, M. D. (2017). miR160 and miR166/165 contribute to the LEC2-mediated auxin response involved in the somatic embryogenesis induction in Arabidopsis. Front. Plant Sci. 8:2024. doi: 10.3389/fpls.2017.02024
Wu, G., Park, M. Y., Conway, S. R., Wang, J. W., Weigel, D., and Poethig, R. S. (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759. doi: 10.1016/j.cell.2009.06.031
Xu, M., Hu, T., Zhao, J., Park, M. Y., Earley, K. W., Wu, G., et al. (2016). Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLoS Genet. 12:e1006263. doi: 10.1371/journal.pgen.1006263
Yamaguchi, T., Nukazuka, A., and Tsukaya, H. (2012). Leaf adaxial-abaxial polarity specification and lamina outgrowth: evolution and development. Plant Cell Physiol. 53, 1180–1194. doi: 10.1093/pcp/pcs074
Yant, L., Mathieu, J., Dinh, T. T., Ott, F., Lanz, C., Wollmann, H., et al. (2010). Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22, 2156–2170. doi: 10.1105/tpc.110.075606
Yu, Y., Ji, L., Le, B. H., Zhai, J., Chen, J., Luscher, E., et al. (2017). ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLoS Biol. 15:e2001272. doi: 10.1371/journal.pbio.2001272
Keywords: Arabidopsis thaliana, leaf development, small RNAs, target genes, regulatory network
Citation: Yang T, Wang Y, Teotia S, Zhang Z and Tang G (2018) The Making of Leaves: How Small RNA Networks Modulate Leaf Development. Front. Plant Sci. 9:824. doi: 10.3389/fpls.2018.00824
Received: 09 March 2018; Accepted: 28 May 2018;
Published: 18 June 2018.
Edited by:
Hirokazu Tsukaya, The University of Tokyo, JapanReviewed by:
Jeong Hoe Kim, Kyungpook National University, South KoreaUmesh K. Reddy, West Virginia State University, United States
Gorou Horiguchi, Rikkyo University, Japan
Copyright © 2018 Yang, Wang, Teotia, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanhui Zhang, YW1haXIwNTM0QDE2My5jb20= Guiliang Tang, Z3RhbmcxQG10dS5lZHU=
 Tianxiao Yang
Tianxiao Yang Yongyan Wang
Yongyan Wang Sachin Teotia
Sachin Teotia Zhanhui Zhang
Zhanhui Zhang Guiliang Tang
Guiliang Tang