- 1National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
- 2State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
- 3College of Life Sciences, Henan Agricultural University, Zhengzhou, China
Wheat leaf rust is one of the most common wheat diseases worldwide and can cause up to 40% wheat yield loss. To combat the growth and spread of leaf rust disease, continual exploration and identification of new and effective resistance genes are needed. Here, we report for the first time a locus conferring leaf rust resistance located on the long arm of Agropyron cristatum chromosome 2P in Triticum aestivum–A. cristatum 2P translocation lines. This study used 50 leaf rust races, including two Chinese major dominant leaf rust races, named by THT and PHT, and other 48 different leaf rust races collected from 11 provinces, 1autonomous region and 1 municipality of China to test the resistance to T. aestivum–A. cristatum 2P chromosome translocation lines and their backcross populations, the results indicated that the novel leaf rust resistance locus was immune or nearly immune to all tested leaf rust races. Four long arm translocation lines with different breakpoints of A. cristatum chromosome 2PL and their backcross populations were tested with leaf rust race THT at the seedling and adult stages and genotyped with 2P-specific STS markers. The results showed that the novel leaf rust resistance locus of the T. aestivum–A. cristatum 2P translocation lines was located in the chromosomal bin FL 0.66–0.86 of 2PL. Therefore, T. aestivum–A. cristatum 2P chromosome translocation lines conferring leaf rust resistance locus could provide a novel disease-resistance resource for future wheat breeding programs.
Introduction
Common wheat (Triticum aestivum L., 2n = 6x = 42, genomes AABBDD) is one of the most important widely planted food crops worldwide (Su et al., 2013). Wheat production plays an important role in the development of the national economy (Costanzo and Barberi, 2014). Wheat leaf rust caused by Puccinia triticina Erikss. is one of the most common diseases of wheat throughout the world (Bolton et al., 2008; Kolmer, 2013), and serious cases can cause up to 40% wheat yield loss (Knott, 2012). Recent changes in climate conditions caused by global warming are more suitable for the spread of wheat leaf rust, increasing concern.
Wild relatives of common wheat contain a large number of desirable genes that can be exploited for wheat improvement (Li et al., 2017). The discovery and utilization of the desirable genes from wild relatives is significant for the genetic improvement of wheat resistance. At present, 76 wheat leaf rust resistance genes have been named and identified in common wheat and its relatives (McIntosh et al., 2016; Bansal et al., 2017). Approximately half of the leaf rust resistance genes are derived from wild relatives of common wheat, including Aegilops L., Secale cereale L., and Elytrigia Desv.. Aegilops L. is the donor of 15 leaf rust resistance genes described in the Catalog of Wheat Gene Symbols, including Lr9, Lr21, Lr22a, Lr28, Lr29, Lr32, Lr35, Lr36, Lr37, Lr39, Lr42, Lr47, Lr51, Lr66, and Lr76 (Marais et al., 2010; Martynov et al., 2015; Bansal et al., 2017). Lr25 (Singh et al., 2012), Lr26 (Singh et al., 2006), and Lr45 (Friebe et al., 1996; Naik et al., 2015) were transferred from S. cereal L., and Lr19 (Sarma and Knott, 1966), Lr24 (Hart et al., 1976) and Lr38 were transferred from Elytrigia Desv. (Friebe et al., 1992, 1993; Mebrate et al., 2007). These genes have played very important roles in wheat resistance breeding, but many of them have been defeated by new virulent races. Therefore, new and effective resistance genes constantly need to be explored and identified by breeders and researchers to combat the growth and spread of leaf rust disease.
Agropyron Gaertn., one of the important wild relatives of common wheat, is a useful genetic resource for wheat genetic improvement (Dewey, 1984; Dong et al., 1992; Lu et al., 2015). The exploitation of desirable genes from the P genome of Agropyron cristatum has mainly been reported by our laboratory. For example, A. cristatum chromosome 2P shows high resistance to powdery mildew caused by Blumeria graminis f. sp. tritici (Bgt) (Li Q. et al., 2016, Li et al., 2017). A. cristatum chromosome 7P confers higher tolerance to drought compared with Fukuho (Lu et al., 2016). A. cristatum chromosome 6P contains genes conferring high numbers of kernels per spike genes (Wu et al., 2006; Han et al., 2014). Besides, it was also found that A. cristatum chromosome 6P carries leaf rust resistance gene(s). The leaf rust resistance gene(s) was mapped to the bin 6PS-0.81-1.00 using the BC1F2 population of 6PS/6PL telosomics at the adult plant stage (Song et al., 2016). Moreover, T. aestivum–A. cristatum 2P addition line was induced by 60Co-γ ray and Aegilops cylindrical gametocidal chromosome 2C, leading to the creation of T. aestivum–A. cristatum 2P translocation lines and deletion lines with different chromosomal fragments and breakpoint positions (Li H. et al., 2016, Li et al., 2017). Then a physical map of A. cristatum chromosome 2P was constructed with specific STS (sequence-tagged site) markers (Li et al., 2017). In addition, we previously showed that the T. aestivum–A. cristatum 2P addition lines are more resistant to leaf rust than the T. aestivum–A. cristatum 6P addition lines. We determined that T. aestivum–A. cristatum 2P addition line II-9-3 is not only highly resistant to powdery mildew but also shows good resistance to leaf rust.
In this study, T. aestivum–A. cristatum 2P disomic addition lines II-9-3 and translocation lines were inoculated with different races at the seedling stage to identify the resistance spectrum and availability of II-9-3 against leaf rust. Then, the BC2F2 or BC3F2 populations of T. aestivum–A. cristatum 2P translocation lines with different chromosomal segments were used to evaluate the resistance and genotype with molecular markers to map the novel locus to a specific region of chromosome 2P. These data provide a scientific basis for effectively using this novel leaf rust resistance locus in wheat improvement and basic research.
Materials and Methods
Plant Materials
The plant materials in this study included T. aestivum cv. Fukuhokomugi (Abbreviation: Fukuho, 2n = 6x = 42, genomes AABBDD); T. aestivum–A. cristatum 2P disomic addition line II-9-3 (2n = 44); 5 T. aestivum–A. cristatum 2P translocation lines 2PT-3 (4DS.2PL), 2PT-5 [3BS.L-2PL(0.6-1)], 2PT-6 [6AS.L-2PL(0.37-0.66)], 2PT-8 [4AS.L-2PL(0.86-1)], and 2PT-10 (2PS.1AL); and 5 backcross populations derived from the crosses between the 5 translocation lines (2PT-3, 2PT-5, 2PT-6, 2PT-8, and 2PT-10) and Fukuho. The sizes of the 2PT-3 and 2PT-10 BC1F2 backcross populations were 131 and 211, respectively. At the seedling stage, each of the 2PT-3, 2PT-5, and 2PT-6 BC3F2 or BC2F2 backcross populations had a size of 200, and the size of the 2PT-8 BC2F2 backcross populations was 196. At the adult plant stage, the size of the 2PT-5, 2PT-6 and 2PT-8 BC2F2 backcross populations were 153, 126, and 106 respectively. The size of the 2PT-3 BC2F2 backcross populations was 159 at the adult plant stage test. The numbers of positive and negative plants of each population were showed in Tables 1, 2. These materials were provided by the Wheat Resources Laboratory, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences. The susceptible control T. aestivum cv. ‘Zhengzhou5389’ was kindly provided by the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences.
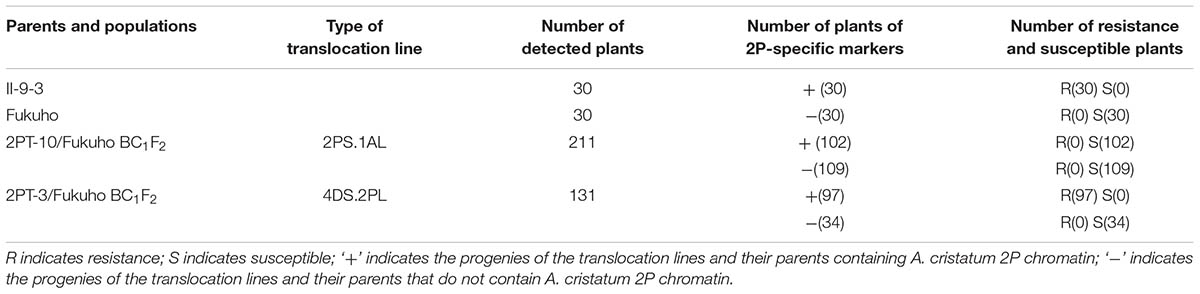
TABLE 1. Response to leaf rust race THT and molecular marker analysis of the 2PL translocation line populations in the field.
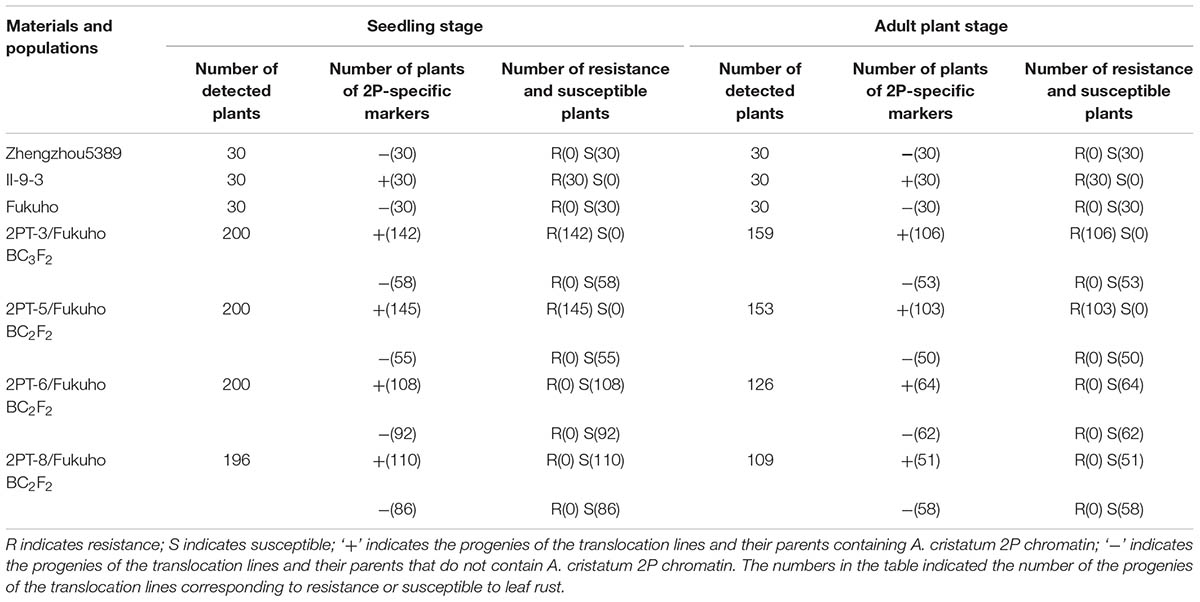
TABLE 2. Response to leaf rust race THT and molecular marker analysis of the 2PL translocation line populations at the seedling and adult plant stages.
Collection of the Leaf Rust Isolates
This study used 50 leaf rust races, including two Chinese major dominant leaf rust races, named by THT and PHT, and other 48 different leaf rust races collected from different locations in Hebei, Henan, Shandong, Inner Mongolia, Jiangsu, Anhui, Hubei, Shaanxi, Xinjiang, Yunnan, Guizhou, Sichuan, and Chongqing of China in 2016 (Figure 1). The 50 races tested were identified with a total of 102 samples collected from the mentioned 11 provinces, 1 autonomous region and 1 municipality, and the detailed information was listed in Table 3. The 50 races were denominated using the Prt code system (Long and Kolmer, 1989) by Liu and Chen (2012). The leaf rust races used in this study were kindly provided and tested by the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, China.
FIGURE 1. Map of China. The provinces, autonomous regions or municipalities from which the collections of Puccinia triticina were obtained are marked out.
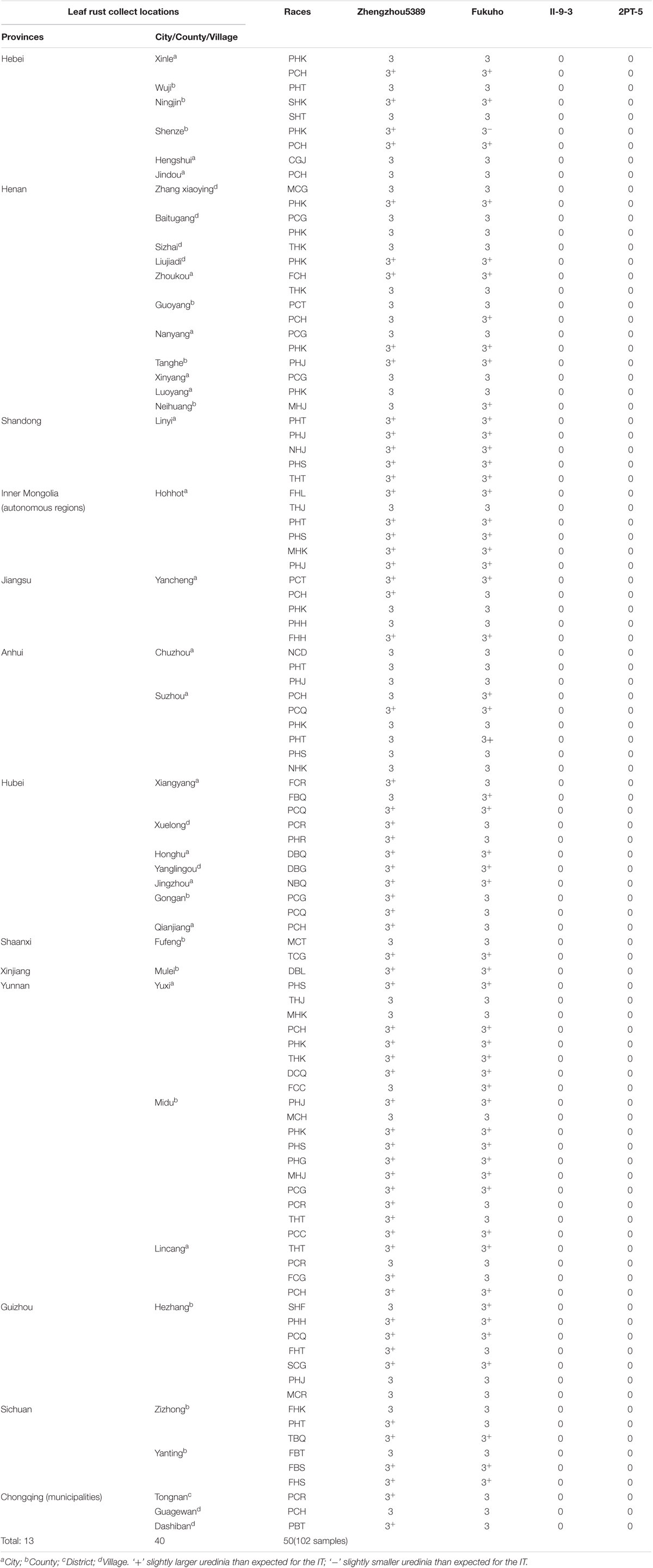
TABLE 3. Infection types of the T. aestivum–A. cristatum 2P addition line and the 2PL translocation line for different leaf rust races at the seedling stage.
Identification of Leaf Rust in Nurseries
Triticum aestivum–A. cristatum 2P addition line II-9-3, the two BC1F2 backcross populations derived from T. aestivum–A. cristatum 2PL translocation lines 2PT-3(4DS.2PL), T. aestivum–A. cristatum 2PS translocation line 2PT-10(2PS.1AL) and Fukuho were planted in the nurseries in Xinxiang, Henan Province of China. The disease was assessed using the 0–4 infection type scoring system described by Roelfs (1992).
Identification of Leaf Rust Resistance at the Seedling Stage
Two Chinese major dominant races THT and PHT (Liu and Chen, 2012), and other 48 races were used for inoculation. The T. aestivum–A. cristatum 2P addition lines, the translocation lines and the backcross populations were infected as described by Liu and Chen (2012). The races were inoculated onto 7-day-old seedling leaves of wheat plants planted in pots with low-nutrient soils. After inoculation, the plants were placed in a dew chamber at 18°C in the dark for 24 h. The inoculated wheat seedlings were then moved to a greenhouse at 24°C. The infection types (ITs) on the first leaves were recorded when Zhengzhou5389 was fully rusted, 15 days after inoculation. A 0 to 4 scale was used for ITs, as described by Roelfs (1992). Compared to the normal IT, larger and smaller uredinia were indicated with plus and minus signs. Plants with ITs 0-2 were considered resistant to leaf rust, whereas plants with ITs 3–4 were considered susceptible to leaf rust. Leaf rust infections were carried out at the greenhouse of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences.
Identification of Leaf Rust Resistance at the Adult Stage
The procedures for leaf rust resistance at the adult stage in the field were carried out as described by Qi et al. (2016). In this study, T. aestivum cv. ‘Zhengzhou5389’ was used as the susceptible control. One row of Zhengzhou5389 was planted every tenth row of tested materials to aid the spread of the spores within the trial (Qi et al., 2016). Zhengzhou5389 was also planted in the spreader rows perpendicular and adjacent to the test rows. The race THT was used for inoculation. Leaf rust infections were established by the spraying method at the wheat jointing stage. The plants were sprayed with an aqueous suspension of uredinia spores of P. triticina containing 0.05% Tween 20. Then, the inoculated plants were covered with plastic film, and the film was removed after 16 h (Song et al., 2016). ITs were recorded on the first leaf of each plant using a 0–4 scale when susceptible control Zhengzhou5389 were fully rusted. ITs were recorded in the same manner as the seedling stage.
Molecular Marker Analysis
Genomic DNA was extracted using a modified CTAB method (Kidwell and Osborn, 1992; Li et al., 2017). A total of 82 A. cristatum chromosome 2P-specific STS markers which were developed based on the A. cristatum transcriptome sequences (Zhang et al., 2015, 2017) were mapped to 17 2P-specific bins, and the information of STS markers was described as Li et al. (2017). Of these, eight markers located on different 2P-specific bins were selected to detect the absence or presence of 2P chromosomal segments in the translocation lines and their backcross populations (Table 4). A. cristatum Z559 and II-9-3 were used as positive controls, whereas Fukuho and Zhengzhou5389 were used as negative controls.

TABLE 4. Primer sequences of the Agropyron cristatum 2P-specific STS markers for chromosome localization of the leaf rust resistance locus.
The PCR amplification was performed as described by Li et al. (2017). The PCR amplification reactions were conducted in a total volume of 10 μl mixture containing 1.0 μl of template DNA (50 ng/μl), 1.0 μl of forward primer (2 μmol/l), 1.0 μl of reverse primer (2 μmol/l), 0.15 μl of 1 × Taq DNA polymerase (5 U/μl), 0.8 μl of MgCl2 (25 mmol/l), 0.8 μl of dNTP (2.5 mmol/l), 1.0 μl of 10 × buffer, and 4.25 μl of ddH2O. The cycling conditions were as follows: 94°C for 5 min followed by 38 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s, followed by a final 10-min extension at 72°C. The amplified products were separated by electrophoresis on a polyacrylamide gel with an acrylamide concentration of 8% s and visualized by silver staining.
Results
Leaf Rust Responses of the T. aestivum–A. cristatum 2P Addition Line and Translocation Lines in the Field
Triticum aestivum–A. cristatum 2P addition line II-9-3, the two backcross populations derived from the cross between 2PL translocation lines 2PT-3, 2PS translocation line 2PT-10 and Fukuho were planted in the nurseries in Xinxiang during 2014 and 2015. Fukuho was highly susceptible to leaf rust, whereas II-9-3 was highly resistant (Figure 2). According to the leaf rust resistance results and molecular marker analysis (Figure 3 and Table 1), in the 2PT-10 BC1F2 populations, all progenies were susceptible to leaf rust, regardless of the presence or absence of 2P-specific STS markers, suggesting that the leaf rust resistance locus is not located on the 2PS arm. In the 2PT-3 BC1F2 populations, all the progenies with 2P-specific STS markers were highly resistant to leaf rust, while other progenies of 2PT-3 without 2P-specific STS markers were as highly susceptible to leaf rust as Fukuho. The 2PT-3 translocation lines contained the whole 2PL arm, suggesting that the leaf rust resistance locus is located on the 2PL arm.
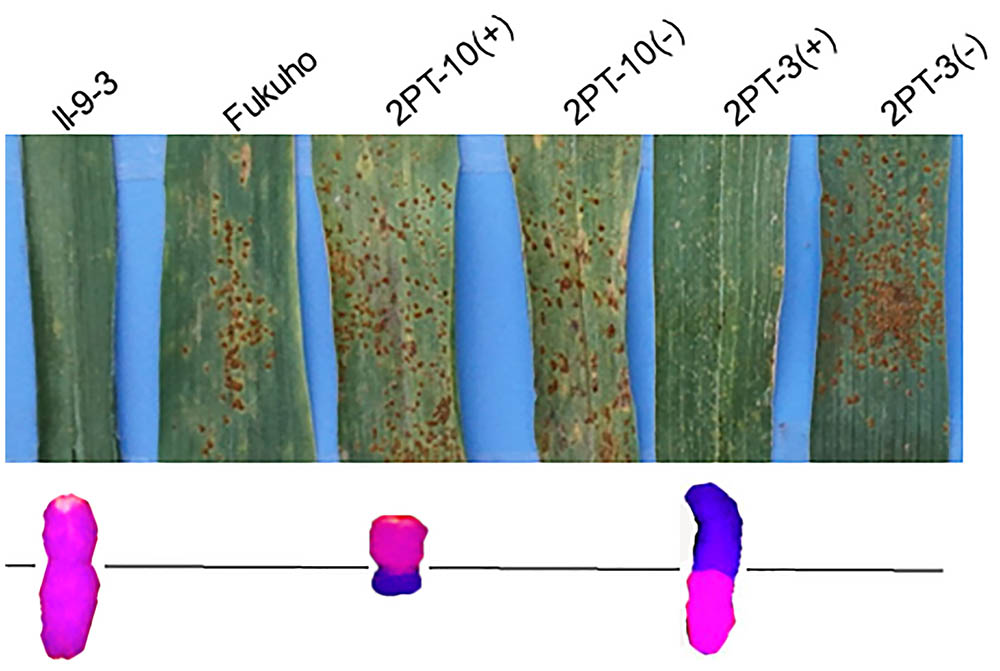
FIGURE 2. Disease responses to P. triticina of the Triticum aestivum–Agropyron cristatum 2P addition line and translocation line populations of wheat in the field. ‘+’ indicated the progenies of the translocation lines and their parents containing A. cristatum 2P chromatin; ‘–’ indicated the progenies of the translocation lines and their parents that do not contain A. cristatum 2P chromatin.
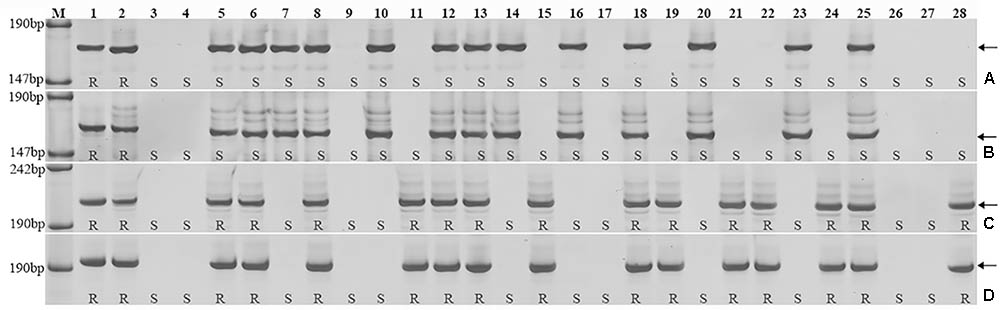
FIGURE 3. Amplification patterns of the A. cristatum 2P translocation lines using 4 STS markers specific for the A. cristatum 2P chromosome. Agc1846 (A), Agc52107 (B), Agc3725 (C), Agc4115 (D). M, pUC19 DNA/MspI (HpaII); 1 A. cristatum accession Z559; 2 II-9-3; 3 Fukuho; 4 Zhengzhou5389; 5–28 the isolates of different populations. (A,B) Shows positive and negative plants of the isolates of 2PT-10, (C,D) shows positive and negative plants of the isolates of 2PT-3. The arrows indicate the diagnostic bands. R indicates resistance; S indicates susceptible.
Evaluation of the Resistance Spectrum and Availability of the 2PL Arm Against Leaf Rust
During 2015 and 2016, the responses of the translocation lines 2PT-5(3BS.L-2PL(0.6-1)), 2PT-3(4DS.2PL), and T. aestivum–A. cristatum 2P addition line II-9-3 were 0;, and all plants were characterized as nearly immune to leaf rust. To evaluate the resistance spectrum of T. aestivum–A. cristatum 2P II-9-3 and 2PT-5, 102 leaf rust samples (Table 3), which collected from 11 provinces, 1 autonomous region and 1 municipality of China in 2016 were used to inoculate II-9-3, 2PT-5, Fukuho and Zhengzhou5389 at the seedling stage in the greenhouse. Fifty races were identified from 102 samples by the State Key Laboratory for Biology of Plant Diseases and Insect Pests. Among these races, PHK, PHT, PCH, PHJ, PHS, THT, and PCG were found in different provinces. II-9-3 and 2PT-5 were nearly immune (IT = 0) to all 50 races, whereas Fukuho and Zhengzhou5389 were highly susceptible (IT = 3+ ∼ 4) to all 50 races at the seedling stage (Figure 4 and Table 3). The disease responses to duplicate races from different locations showed the same ITs. These results suggested that II-9-3 and 2PT-5 have broad-spectrum leaf rust resistance and may be useful for wheat disease resistance improvement.
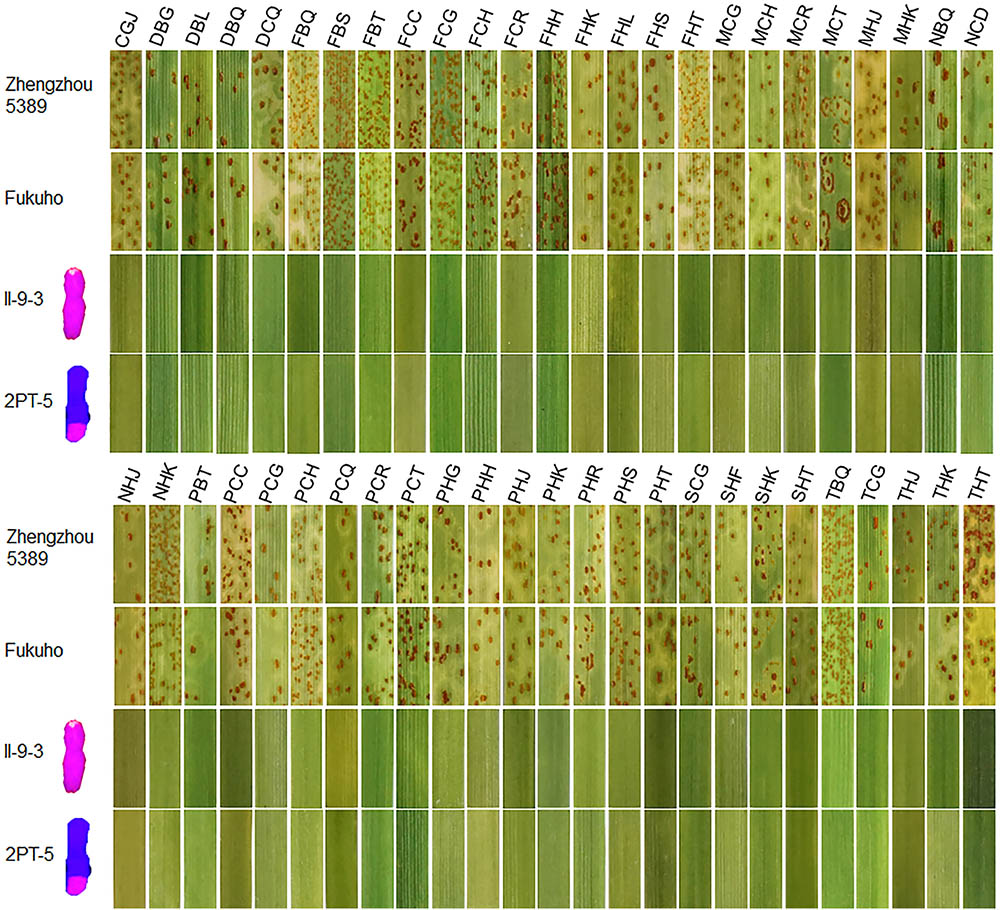
FIGURE 4. Disease responses to P. triticina of T. aestivum–A. cristatum 2P addition line II-9-3 and T. aestivum–A. cristatum 2P translocation line 2PT-5.
Chromosomal Localization of Leaf Rust Resistance Locus on A. cristatum Chromosome 2P
To further pinpoint the chromosomal segment containing the leaf rust resistance locus, three BC2F2 populations (2PT-5, 2PT-6, 2PT-8) and one BC3F2 population (2PT-3) derived from four translocation lines with different chromosomal segments of 2PL were constructed (Table 2). The major dominant races THT (Liu and Chen, 2012) was used to evaluate the leaf rust resistance of T. aestivum–A. cristatum 2P addition line II-9-3, Fukuho and the populations of 2PT-3, 2PT-5, 2PT-6, and 2PT-8 in the greenhouse and field, with Zhengzhou5389 used as the control. At the same time, the plants in each population were identified with the A. cristatum 2P-specific markers. According to the results of the molecular marker analysis (Figure 5) and the leaf rust responses of the four populations at the seedling stage (Figure 6 and Table 2), the responses of T. aestivum–A. cristatum 2P addition line II-9-3 was 0, and all plants were nearly immune to leaf rust. The responses of Fukuho was 4, and all plants were as highly susceptible as the susceptible control Zhengzhou5389. In the 2PT-3 BC3F2 population, 142 positive plants were nearly immune (IT = 0) and 58 negative plants were highly susceptible (IT = 3+). In the 2PT-5 BC2F2 population, 145 positive plants were nearly immune (IT = 0) and 55 negative plants were highly susceptible (IT = 3+). In the 2PT-6 BC2F2 population, all of the 108 positive plants and 92 negative plants were highly susceptible (IT = 3+). In the 2PT-8 BC2F2 population, 110 positive plants and 86 negative plants were also highly susceptible (IT = 3+). These results suggest that the novel leaf rust resistance locus of the T. aestivum–A. cristatum 2P translocation lines is located in the chromosomal bin FL 0.66–0.86 of 2PL. The four populations of translocation lines were assessed for leaf rust resistance at the adult plant stage in the field. The results for the disease response to the P. triticina at the adult stage (Figure 7 and Table 2), indicated that II-9-3 was nearly immune, whereas Fukuho was as highly susceptible as Zhengzhou5389 to leaf rust. In the 2PT-3 BC3F2 population, 106 positive plants of the isolates of 2PT-3 were nearly immune (IT = 0) and 53 negative plants were highly susceptible (IT = 3+). In the 2PT-5 BC2F2 population, 103 positive plants were nearly immune (IT = 0) and 50 negative plants were highly susceptible (IT = 3+). In the 2PT-6 BC2F2 population, all of the 64 positive plants and 62 negative plants were highly susceptible (IT = 3+). In the 2PT-8 BC2F2 population, 51 positive plants and 58 negative plants were also highly susceptible (IT = 3+). According to the evaluation of the leaf rust resistance of the BC2F2 and BC3F2 populations of the four T. aestivum–A. cristatum 2P translocation lines and their parents, II-9-3 and 2PT-5 were nearly immune to leaf rust at both the seedling and adult plant stages. Based on these results, the novel leaf rust resistance locus of T. aestivum–A. cristatum chromosome 2P is located in the chromosomal bin FL 0.66–0.86 of 2PL (Figure 8).
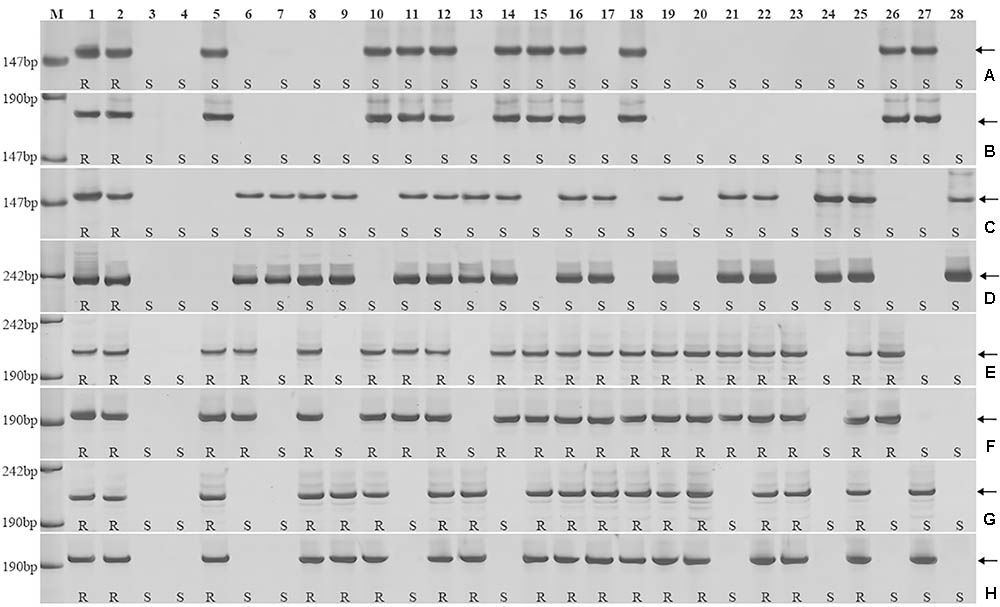
FIGURE 5. Amplification patterns of the A. cristatum 2PL translocation lines using 6 STS markers specific for the A. cristatum 2P chromosome. Agc17451 (A), Agc32544 (B), Agc31475 (C), Agc51085 (D), Agc3725 (E,G), Agc4115 (F,H). M, pUC19 DNA/MspI (HpaII); 1 A. cristatum accession Z559; 2 II-9-3; 3 Fukuho; 4 Zhengzhou5389; 5–28 the isolates of different populations. (A,B) Shows positive and negative plants of the isolates of 2PT-6, (C,D) shows positive and negative plants of the isolates of 2PT-8, (E,F) shows positive and negative plants of the isolates of 2PT-5, (G,H) shows positive and negative plants of the isolates of 2PT-3. The arrows indicate the diagnostic bands. R indicates resistance; S indicates susceptible.
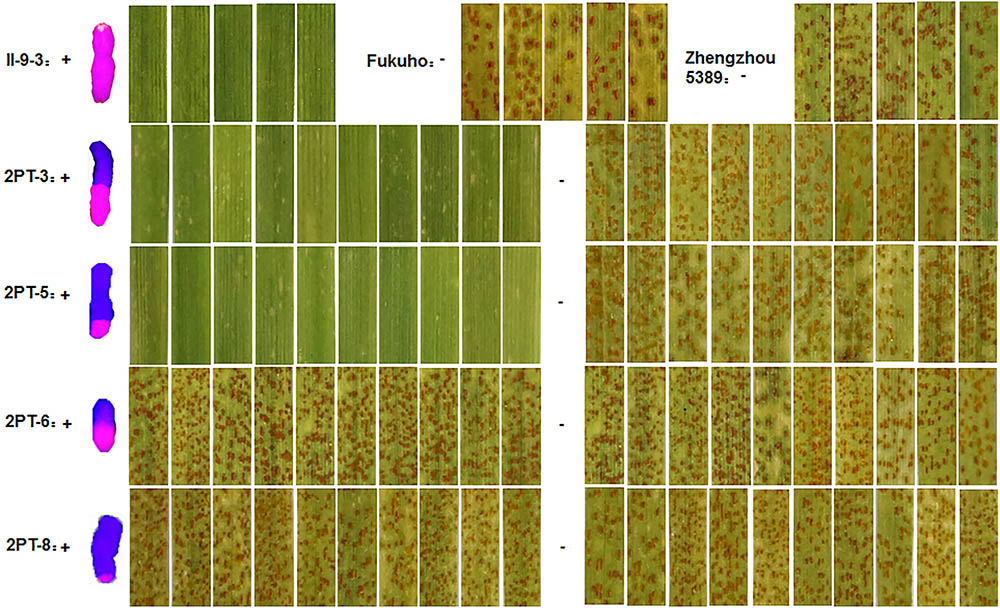
FIGURE 6. Disease responses to the P. triticina of the BC2F2 and BC3F2 populations of 4 T. aestivum–A. cristatum 2PL translocation lines and their parents at the seedling stage. ‘+’ indicates the progenies of the translocation lines and their parents containing A. cristatum 2P chromatin; ‘–’ indicates the progenies of the translocation lines and their parents that do not contain A. cristatum 2P chromatin.
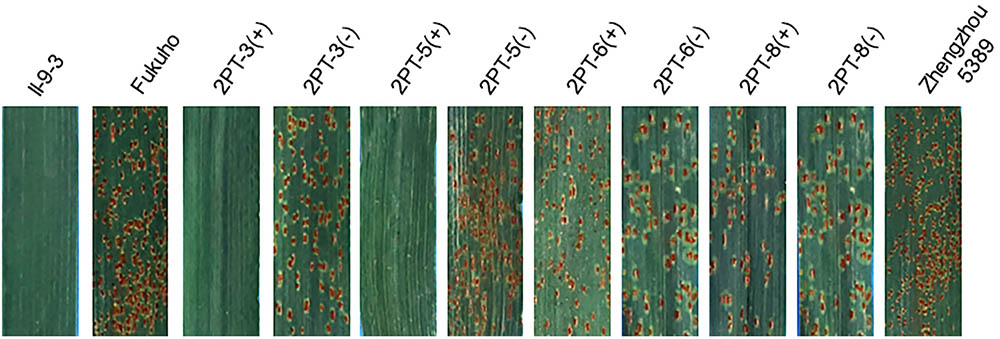
FIGURE 7. Disease responses to the P. triticina of the BC2F2 and BC3F2 populations of 4 T. aestivum–A. cristatum 2PL translocation lines and their parents at the adult plant stage. ‘+’ indicates the progenies of the translocation lines and their parents containing A. cristatum 2P chromatin; ‘–’ indicates the progenies of the translocation lines and their parents that do not contain A. cristatum 2P chromatin.
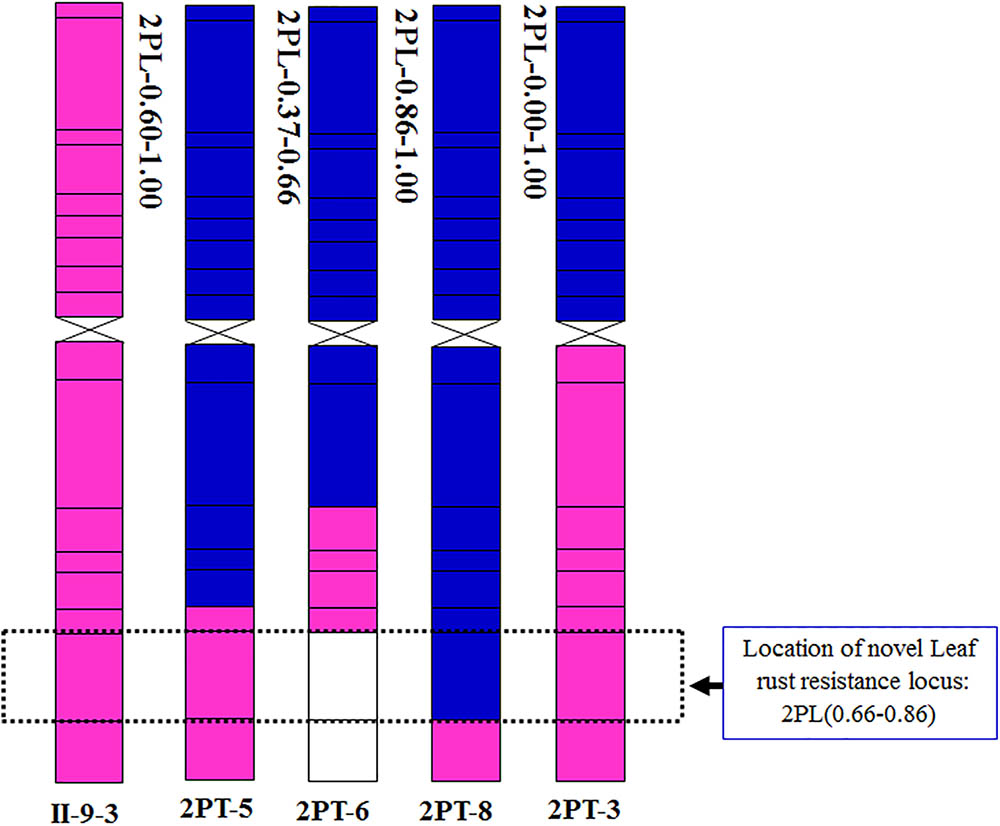
FIGURE 8. Chromosomal localization of the novel leaf rust resistance locus from A. cristatum chromosome 2P.
The leaf rust resistance locus is located in the same region as the powdery mildew resistance locus reported by Li et al. (2017). It is speculated that chromosomal bin FL 0.66–0.86 of 2PL carries two types of resistance loci: the leaf rust resistance locus and powdery mildew resistance locus.
Evaluation of the Resistance Locus in the Chromosomal Bin FL 0.66–0.86 of 2PL
Recently, 2PT-3 was induced by 60Co-γ ray, leading to the creation of thirty-seven 2PL translocation lines with different chromosomal fragments and breakpoint positions. According to the results of molecular marker analysis, leaf rust and powdery mildew responses, eleven 2PL translocation lines displays nearly immune to leaf rust and high susceptible to powdery mildew; ten 2PL translocation lines displays high susceptible to leaf rust and high resistance to powdery mildew. Based on these result, different locus are responsible for resistance to powdery mildew and leaf rust.
Discussion
Wheat leaf rust generally occurs in the world’s wheat-producing areas and is a major diseases in wheat affecting wheat production. Serious disease can significantly reduce yields. Recent increases in temperature and precipitation have gradually increased the prevalence of wheat leaf rust in the main wheat-production areas in China, especially in southwestern and northwestern China, the middle and lower regions of the Yangtze River, and the southern parts of the Huang-Huai-Hai River regions (Huerta-Espino et al., 2011). Yield losses were recorded in the Henan, Sichuan, Anhui, Gansu, and Shaanxi provinces in China in the year 2012 (Zhou et al., 2013). Liu and Chen (2012) studied the race and virulence frequency of P. triticina in China, and found that THT, PHT, THT, PHJ, THJ, and PCG were the main races in the most provinces and wheat areas. They also found that only Lr9, Lr19, Lr24, Lr25, Lr28, and Lr29 were effective leaf rust resistance genes among the identified genes in China (Liu and Chen, 2012). Kolmer (2015) studied the leaf rust resistance genes and virulence of P. triticina from Sichuan, Hebei, Henan, Shandong, Anhui, Hubei, and Shanxi provinces of China. A total of 48 virulence phenotypes were detected and included FCBQQ, PCGLN, and PCGLL. Lr1, Lr2c, Lr3, Lr26, LrB, Lr10, Lr11, Lr3bg, Lr20, and Lr14b showed low disease resistance, indicating that the utilization value of these resistance genes was not significant. Most of the identified leaf rust resistance genes are race-specific resistance genes which easily to lose resistance in breeding (Kolmer, 2015). Because the disease continuously evolves and forms novel virulent races (Kolmer, 2005; Bolton et al., 2008), new varieties with different desirable genes are constantly needed to replace the varieties that have lost effectiveness (Herrera-Foessel et al., 2012). Therefore, mining novel resistance genes, breeding resistant cultivars by the utilization of leaf rust resistance genes, and using wheat cultivars with different resistance genes can extend the effective life of these genes and are of great significance to wheat breeding and production.
In this study, 50 races were collected from 11 provinces, 1 autonomous region and 1 municipality covering the main wheat-producing areas of the Huang-Huai-Hai River regions, the middle and lower regions of the Yangtze River, the northern and southwest parts of China. These races were used to inoculate plants to identify resistance locus. The novel leaf rust resistance locus were first confirmed in the T. aestivum–A. cristatum 2P addition line II-9-3 and 2PL translocation line 2PT-5 with broad-spectrum and strong availability. Lines of Thatcher wheat differing in the single leaf rust resistance genes Lr1, Lr2c, Lr3, Lr16, Lr26, Lr3ka, Lr11, Lr17, and Lr30 were resistant to19, 7, 12, 28, 5, 26, 25, 10, and 25 of the 50 tested races, respectively. Therefore, the novel leaf rust resistance locus exhibited a broader spectrum than the nine genes above. T. aestivum–A. cristatum chromosome 6P addition line 4844-12 was highly resistance to leaf rust at the adult plant stage and the resistance locus was finally located on chromosome arm 6PS (Song et al., 2016). However, in the 6PS telosomic populations, the plants carrying A. cristatum chromosome 6P were not all resistance to leaf rust, in contrast to the resistance locus exploited in chromosome 2P in this study and this characteristic would affects the application of the gene from 6P in breeding. In conclusion, the novel leaf rust resistance locus have high utilization value and not only confer broad-spectrum resistance but also stronger and more stable than the resistance carried by A. cristatum chromosome 6P.
Many leaf rust resistance genes were found to have genetic linkages to genes conferring resistance to powdery mildew, stripe rust caused by P. striiformis f. sp. tritici (Pst) or stem rust caused by P. graminis Pers. f.sp. tritici. (Pgt) in wheat, such as Lr19/Sr25, Lr24/Sr24/Yr71 (McIntosh et al., 1977), Lr27/Sr2 (Mago et al., 2011), Lr34/Yr18/Pm38/Sr57 (Krattinger et al., 2009), Lr37/Yr17/Sr38 (Seah et al., 2001), Lr46/Yr29/Sr58/Pm39 (Rosewarne et al., 2006; Lillemo et al., 2008), Lr57/Yr40 (Kuraparthy et al., 2007), Lr62/Yr42 (Marais et al., 2009), and Lr67/Yr46/Sr55/Pm46 (Herrera-Foessel et al., 2014). The investigation and identification of leaf rust-resistance genes will allow these previously identified resistance genes to be used more effectively. Lr34/Yr18/Pm38/Sr57 is a single wheat gene that confers durable and partial adult plant resistance against leaf rust, stripe rust, powdery mildew, and stem rust (Krattinger et al., 2009). This gene has been widely used in wheat breeding for more than one hundred years, and no pathogen adaptation has been observed so far, indicating that wheat with durable resistance genes in wheat can effectively prolong the effectiveness of resistance genes for different diseases (Krattinger et al., 2009; Keller et al., 2015). In this study, leaf rust resistance locus was located in the chromosomal bin FL 0.66–0.86 of 2PL using translocation line backcross populations that also carried powdery mildew resistance locus (Li et al., 2017). The leaf rust resistance locus, with broad-spectrum resistance, was high resistance to powdery mildew and nearly immune to leaf rust at both the seedling and adult stages. Chromosomal bin FL 0.66–0.86 of 2PL is probably an excellent gene segment with various disease resistance locus that are closely linked. T. aestivum–A. cristatum chromosome 2P translocation line 2PT-5, which is resistant to leaf rust and powdery mildew, is of great value for wheat resistance breeding.
Triticum aestivum–A. cristatum chromosome 2P translocation line 2PT-5 was not only showed nearly immune to wheat leaf rust and highly resistant to powdery mildew but also had many other desirable traits, such as a small and erect flag leaf, compact plant type, closely arranged spikelets, and long uppermost internode. T. aestivum–A. cristatum chromosome 2P translocation line 2PT-5 can be used in wheat breeding (submitted). Therefore, translocation line 2PT-5 is potentially useful for broadening the genetic basis of leaf rust resistance and provides valuable genetic material for wheat breeding. The combination of irradiation, backcrossing and other methods with the ph1b mutant will further delimit the chromosome 2P 0.66–0.86 segment smaller for the next step and even enable deeper study of leaf rust resistance gene(s) and powdery mildew gene(s).
Author Contributions
WL conceived the research. BJ and TL performed the research. BJ wrote the paper. WL and HL created the translocation lines. LL created the T. aestivum–A. cristatum 2P addition line. HH, LL, JZ, XY, SZ, and XL participated in the preparation of the reagents and materials used in this study. All authors read and approved the manuscript.
Funding
This research was supported by Grants from the National Natural Science Foundation of China (Grant No. 31471493) and the National Key Research and Development Program of China (2016YFD0100102).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Ainong Gao (Chinese Academy of Agricultural Sciences) for his constructive suggestions on this manuscript.
References
Bansal, M., Kaur, S., Dhaliwal, H. S., Bains, N. S., Bariana, H. S., Chhuneja, P., et al. (2017). Mapping of Aegilops umbellulata-derived leaf rust and stripe rust resistance loci in wheat. Plant Pathol. 66, 38–44. doi: 10.1111/ppa.12549
Bolton, M. D., Kolmer, J. A., and Garvin, D. F. (2008). Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 9, 563–575. doi: 10.1111/j.1364-3703.2008.00487.x
Costanzo, A., and Barberi, P. (2014). Functional agrobiodiversity and agroecosystem services in sustainable wheat production. A review. Agron. Sustain. Dev. 34, 327–348. doi: 10.1007/s13593-013-0178-1
Dewey, D. R. (1984). “The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae,” in Gene Manipulation in Plant Improvement, ed. P. Gustafson (Berlin: Springer), 209–279.
Dong, Y. S., Zhou, R. H., Xu, S. J., Li, L. H., Cauderon, Y., and Wang, R. R.-C. (1992). Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116, 175–178. doi: 10.1111/j.1601-5223.1992.tb00819.x
Friebe, B., Jiang, J., Gill, B. S., and Dyck, P. L. (1993). Radiation-induced nonhomoeologous wheat-Agropyron intermedium chromosomal translocations conferring resistance to leaf rust. Theor. Appl. Genet. 86, 141–149. doi: 10.1007/BF00222072
Friebe, B., Jiang, J., Raupp, W. J., Mcintosh, R. A., and Gill, B. S. (1996). Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91, 59–87. doi: 10.1007/Bf00035277
Friebe, B., Zeller, F. J., Mukai, Y., Forster, B. P., Bartos, P., and McIntosh, R. A. (1992). Characterization of rust-resistant wheat-Agropyron intermedium derivatives by C-banding, in situ hybridization and isozyme analysis. Theor. Appl. Genet. 83, 775–782. doi: 10.1007/BF00226697
Han, H., Bai, L., Su, J., Zhang, J., Song, L., Gao, A., et al. (2014). Genetic rearrangements of six wheat–Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS One 9:e91066. doi: 10.1371/journal.pone.0091066
Hart, G. E., Mcmillin, D. E., and Sears, E. R. (1976). Determination of the chromosomal location of a glutamate oxaloacetate transaminase structural gene using Triticum-Agropyron translocations. Genetics 83, 49–61.
Herrera-Foessel, S. A., Singh, R. P., Huerta-Espino, J., Rosewarne, G. M., Periyannan, S. K., Viccars, L., et al. (2012). Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 124, 1475–1486. doi: 10.1007/s00122-012-1802-1
Herrera-Foessel, S. A., Singh, R. P., Lillemo, M., Huerta-Espino, J., Bhavani, S., Singh, S., et al. (2014). Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 127, 781–789. doi: 10.1007/s00122-013-2256-9
Huerta-Espino, J., Singh, R. P., German, S., Mccallum, B. D., Park, R. F., Chen, S. C., et al. (2011). Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 179, 143–160. doi: 10.1007/s10681-011-0361-x
Keller, B., Krattinger, S., Selter, L., Harsh, C., Singla, J., et al. (2015). “Genomic approaches towards durable fungal disease resistance in wheat,” in Advances in Wheat Genetics: From Genome to Field, eds Y. Ogihara, S. Takumi, and H. Handa (Berlin: Springer), 369–375. doi: 10.1007/978-4-431-55675-6_42
Kidwell, K. K., and Osborn, T. C. (1992). “Simple plant DNA isolation procedures,” in Plant Genomes: Methods for Genetic and Physical Mapping (Dordrecht: Springer), 1–13.
Knott, D. R. (2012). The Wheat Rusts—Breeding for Resistance. Berlin: Springer Science & Business Media.
Kolmer, J. (2013). Leaf rust of wheat: pathogen biology, variation and host resistance. Forests 4, 70–84. doi: 10.3390/f4010070
Kolmer, J. A. (2005). Tracking wheat rust on a continental scale. Curr. Opin. Plant Biol. 8, 441–449. doi: 10.1016/j.pbi.2005.05.001
Kolmer, J. A. (2015). Collections of Puccinia triticina in different provinces of china are highly related for virulence and molecular genotype. Phytopathology 105, 700–706. doi: 10.1094/PHYTO-11-14-0293-R
Krattinger, S. G., Lagudah, E. S., Spielmeyer, W., Singh, R. P., Huerta-Espino, J., McFadden, H., et al. (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363. doi: 10.1126/science.1166453
Kuraparthy, V., Chhuneja, P., Dhaliwal, H. S., Kaur, S., Bowden, R. L., and Gill, B. S. (2007). Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor. Appl. Genet. 114, 1379–1389. doi: 10.1007/s00122-007-0524-2
Li, H., Jiang, B., Wang, J., Lu, Y., Zhang, J., Pan, C., et al. (2017). Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor. Appl. Genet. 130, 109–121. doi: 10.1007/s00122-016-2797-9
Li, H., Lv, M., Song, L., Zhang, J., Gao, A., Li, L., et al. (2016). Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS One 11:e0145928. doi: 10.1371/journal.pone.0145928
Li, Q., Lu, Y., Pan, C., Zhang, J., Liu, W., Yang, X., et al. (2016). Characterization of a novel wheat–Agropyron cristatum 2P disomic addition line with powdery mildew resistance. Crop Sci. 56, 2390–2400. doi: 10.2135/cropsci2015.10.0638
Lillemo, M., Asalf, B., Singh, R. P., Huerta-Espino, J., Chen, X. M., He, Z. H., et al. (2008). The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 116, 1155–1166. doi: 10.1007/s00122-008-0743-1
Liu, T. G., and Chen, W. Q. (2012). Race and virulence dynamics of Puccinia triticina in China during 2000–2006. Plant Dis. 96, 1601–1607. doi: 10.1094/PDIS-06-10-0460-RE
Long, D. L., and Kolmer, J. A. (1989). A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 79, 525–529. doi: 10.1094/Phyto-79-525
Lu, M., Lu, Y., Li, H., Pan, C., Guo, Y., Zhang, J., et al. (2016). Transferring desirable genes from Agropyron cristatum 7P chromosome into common wheat. PLoS One 11:e0159577. doi: 10.1371/journal.pone.0159577
Lu, Y. Q., Wu, X. Y., Yao, M. M., Zhang, J. P., Liu, W. H., Yang, X. M., et al. (2015). Genetic mapping of a putative Agropyron cristatum-derived powdery mildew resistance gene by a combination of bulked segregant analysis and single nucleotide polymorphism array. Mol. Breed. 35:96. doi: 10.1007/s11032-015-0292-7
Mago, R., Tabe, L., McIntosh, R. A., Pretorius, Z., Kota, R., Paux, E., et al. (2011). A multiple resistance locus on chromosome arm 3BS in wheat confers resistance to stem rust (Sr2), leaf rust (Lr27) and powdery mildew. Theor. Appl. Genet. 123, 615–623. doi: 10.1007/s00122-011-1611-y
Marais, F., Marais, A., Mccallum, B., and Pretorius, Z. (2009). Transfer of leaf rust and stripe rust resistance genes Lr62 and Yr42 from Aegilops neglecta Req. ex Bertol. to common wheat. Crop Sci. 49, 871–879. doi: 10.2135/cropsci2008.06.0317
Marais, G. F., Bekker, T. A., Eksteen, A., Mccallum, B., Fetch, T., and Marais, A. S. (2010). Attempts to remove gametocidal genes co-transferred to common wheat with rust resistance from Aegilops speltoides. Euphytica 171, 71–85. doi: 10.1007/s10681-009-9996-2
Martynov, S. P., Dobrotvorskaya, T. V., and Mitrofanova, O. P. (2015). Genealogical analysis of the use of aegilops (Aegilops L.) genetic material in wheat (Triticum aestivum L.). Russ. J. Genet. 51, 855–862. doi: 10.1134/S1022795415090070
McIntosh, R., Dyck, P., and Green, G. (1977). Inheritance of leaf rust and stem rust resistances in wheat cultivars Agent and Agatha. Aust. J. Agric. Res. 28, 37–45. doi: 10.1071/AR9770037
McIntosh, R. A., Dubcovsky, J., Rogers, W. J., Morris, C., Appels, R., et al. (2016). Catalogue of Gene Symbols for Wheat: 2015-2016 Supplement. Available at: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2015.pdf
Mebrate, S. A., Oerke, E. C., Dehne, H. W., and Pillen, K. (2007). Mapping of the leaf rust resistance gene Lr38 on wheat chromosome arm 6DL using SSR markers. Euphytica 162, 457–466. doi: 10.1007/s10681-007-9615-z
Naik, B. K., Vinod, Sharma, J. B., Sivasamy, M., Prabhu, K. V., Tomar, R. S., et al. (2015). Molecular mapping and validation of the microsatellite markers linked to the Secale cereale-derived leaf rust resistance gene Lr45 in wheat. Mol. Breed. 35:61. doi: 10.1007/s11032-015-0234-4
Qi, A. Y., Zhang, P. P., Zhou, Y., Yao, Z. J., Li, Z. F., and Liu, D. Q. (2016). Mapping of QTL conferring leaf rust resistance in Chinese wheat lines W014204 and Fuyu 3 at adult plant stage. J. Integr. Agric. 15, 18–28. doi: 10.1016/S2095-3119(14)60974-6
Roelfs, A. P. (1992). Rust Diseases of Wheat: Concepts and Methods of Disease Management. Texcoco: CIMMYT.
Rosewarne, G. M., Singh, R. P., Huerta-Espino, J., William, H. M., Bouchet, S., Cloutier, S., et al. (2006). Leaf tip necrosis, molecular markers and beta1-proteasome subunits associated with the slow rusting resistance genes Lr46/Yr29. Theor. Appl. Genet. 112, 500–508. doi: 10.1007/s00122-005-0153-6
Sarma, D., and Knott, D. (1966). The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can. J. Genet. Cytol. 8, 137–143. doi: 10.1139/g66-018
Seah, S., Bariana, H., Jahier, J., Sivasithamparam, K., and Lagudah, E. S. (2001). The introgressed segment carrying rust resistance genes Yr17, Lr37 and Sr38 in wheat can be assayed by a cloned disease resistance gene-like sequence. Theor. Appl. Genet. 102, 600–605. doi: 10.1007/s001220051686
Singh, A., Pallavi, J. K., Gupta, P., and Prabhu, K. V. (2012). Identification of microsatellite markers linked to leaf rust resistance gene Lr25 in wheat. J. Appl. Genet. 53, 19–25. doi: 10.1007/s13353-011-0070-0
Singh, R. P., Hodson, D. P., Jin, Y., Huerta-Espino, J., Kinyua, M. G., Wanyera, R., et al. (2006). Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 1, 1–13. doi: 10.1079/PAVSNNR20061054
Song, L., Lu, Y., Zhang, J., Pan, C., Yang, X., Li, X., et al. (2016). Physical mapping of Agropyron cristatum chromosome 6P using deletion lines in common wheat background. Theor. Appl. Genet. 129, 1023–1034. doi: 10.1007/s00122-016-2680-8
Su, W. Y., Cong, W. W., Shu, Y. J., Wang, D., Xu, G. H., and Guo, C. H. (2013). Gametocidal chromosomes enhancing chromosome aberration in common wheat induced by 5-azacytidine. Genet. Mol. Res. 12, 2227–2233. doi: 10.4238/2013.July.8.4
Wu, J., Yang, X., Wang, H., Li, H., Li, L., Li, X., et al. (2006). The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 114, 13–20. doi: 10.1007/s00122-006-0405-0
Zhang, J., Liu, W., Han, H., Song, L., Bai, L., Gao, Z., et al. (2015). De novo transcriptome sequencing of Agropyron cristatum to identify available gene resources for the enhancement of wheat. Genomics 106, 129–136. doi: 10.1016/j.ygeno.2015.04.003
Zhang, J., Liu, W., Lu, Y., Liu, Q., Yang, X., Li, X., et al. (2017). A resource of large-scale molecular markers for monitoring Agropyron cristatum chromatin introgression in wheat background based on transcriptome sequences. Sci. Rep. 7:11942. doi: 10.1038/s41598-017-12219-4
Keywords: T. aestivum–A. cristatum 2P chromosome translocation lines, leaf rust resistance, physical mapping, molecular marker, novel disease-resistant germplasm
Citation: Jiang B, Liu T, Li H, Han H, Li L, Zhang J, Yang X, Zhou S, Li X and Liu W (2018) Physical Mapping of a Novel Locus Conferring Leaf Rust Resistance on the Long Arm of Agropyron cristatum Chromosome 2P. Front. Plant Sci. 9:817. doi: 10.3389/fpls.2018.00817
Received: 07 December 2017; Accepted: 28 May 2018;
Published: 18 June 2018.
Edited by:
Soren K. Rasmussen, University of Copenhagen, DenmarkCopyright © 2018 Jiang, Liu, Li, Han, Li, Zhang, Yang, Zhou, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihui Li, bGlsaWh1aUBjYWFzLmNu Weihua Liu, bGl1d2VpaHVhQGNhYXMuY24=
 Bo Jiang
Bo Jiang Taiguo Liu
Taiguo Liu Huanhuan Li3
Huanhuan Li3 Shenghui Zhou
Shenghui Zhou