
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Physiol., 19 October 2020
Sec. Integrative Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.573044
This article is part of the Research TopicThe Tribute of Physiology for the Understanding of COVID-19 DiseaseView all 39 articles
 Elnara Marcia Negri1,2*
Elnara Marcia Negri1,2* Bruna Mamprim Piloto1,3†
Bruna Mamprim Piloto1,3† Luciana Kato Morinaga1,3†
Luciana Kato Morinaga1,3† Carlos Viana Poyares Jardim1,3†
Carlos Viana Poyares Jardim1,3† Shari Anne El-Dash Lamy4†
Shari Anne El-Dash Lamy4† Marcelo Alves Ferreira2†
Marcelo Alves Ferreira2† Elbio Antonio D’Amico5†
Elbio Antonio D’Amico5† Daniel Deheinzelin1†
Daniel Deheinzelin1†Introduction: Elevated D-dimer is a predictor of severity and mortality in COVID-19 patients, and heparin use during in-hospital stay has been associated with decreased mortality. COVID-19 patient autopsies have revealed thrombi in the microvasculature, suggesting that hypercoagulability is a prominent feature of organ failure in these patients. Interestingly, in COVID-19, pulmonary compliance is preserved despite severe hypoxemia corroborating the hypothesis that perfusion mismatch may play a significant role in the development of respiratory failure.
Methods: We describe a series of 27 consecutive COVID-19 patients admitted to Sirio-Libanes Hospital in São Paulo-Brazil and treated with heparin in therapeutic doses tailored to clinical severity.
Results: PaO2/FiO2 ratio increased significantly over the 72 h following the start of anticoagulation, from 254(±90) to 325(±80), p = 0.013, and 92% of the patients were discharged home within a median time of 11 days. There were no bleeding complications or fatal events.
Discussion: Even though this uncontrolled case series does not offer absolute proof that micro thrombosis in the pulmonary circulation is the underlying mechanism of respiratory failure in COVID-19, patient’s positive response to heparinization contributes to the understanding of the pathophysiological mechanism of the disease and provides valuable information for the treatment of these patients while we await the results of further prospective controlled studies.
Since the beginning of the COVID-19 pandemic, disease severity has been linked to markers of coagulation disturbances such as prothrombin time prolongation, elevated fibrin degradation products, reduced platelet count, and specially to elevated D dimer (Chen G. et al., 2020, Chen T. et al., 2020; Han et al., 2020; Tang et al., 2020; Wang D. et al., 2020; Zhang et al., 2020; Zhou et al., 2020). Higher levels of D dimer and the presence of other coagulation disturbances have been independently associated with development of respiratory failure and death in patients with COVID-19 (Wu et al., 2020). The use of heparin, particularly in those patients with more pronounced elevations of D dimer and in those with elevated sepsis induced coagulopathy (SIC) score, has been associated with a better prognosis (Tang et al., 2020; Wu et al., 2020). Diabetic patients, whose levels of D dimer are greater than those of non-diabetic patients, have also been shown to have a worse prognosis regarding COVID-19 (Guo et al., 2020). Moreover, hypercoagulative features can differentiate severe COVID-19 associated pneumonia from that caused by other viruses (Yin et al., 2020).
Over the last months it has been consistently shown that SARS-Cov-2 causes a cytokine storm, endothelial and epithelial dysfunction, which ultimately lead to the activation of the coagulation cascade, causing thrombotic phenomena (Mehta et al., 2020; Tang et al., 2020; Teuwen et al., 2020; Wu et al., 2020). Similarly to what happens in severe sepsis, the widespread deposition of intravascular clots compromises adequate blood supply, contributing to organ failure (Burzynski et al., 2019).
Disseminated intravascular coagulation (DIC) secondary to severe infection is classically associated with gram-positive or gram-negative bacteria, malaria and haemorrhagic fevers, but other viruses, such as dengue (an hemorrhagic virus), SARS-CoV and MERS-CoV, can also be responsible for systemic activation of intravascular coagulation (de Wit et al., 2016; Giannis et al., 2020).
Furthermore, in contrast to the characteristic stiffening of the lung usually seen in acute respiratory distress syndrome (ARDS), in COVID-19 patients the severe hypoxemia observed is accompanied by near normal pulmonary compliance, especially in early stages (Gattinoni et al., 2020). Autopsy findings from COVID-19 patients show microthrombi in the pulmonary microvasculature (Dolhnikoff et al., 2020; Tian et al., 2020; Yao et al., 2020) suggesting that ventilation-perfusion mismatch due to capillary obstruction could be a pivotal feature in the refractory hypoxemia presented by these patients. The anatomical distribution of this peripheral vascular bed mirrors the predominantly distal and patchy distribution of the radiological infiltrates (Ye et al., 2020).
In one of our first COVID-19 patients we noticed a concomitance of peripheral ischaemia (acro-ischemia) with the onset of respiratory distress, an observation that led us to consider the hypothesis that the normal compliance respiratory failure might actually be due to extensive pulmonary capillary obstruction, and that an intense process of intravascular coagulation might be playing a significant role in hypoxemia and outcome of COVID-19 patients.
The treatment of DIC consists in slowing down the coagulation cascade by using low doses of anticoagulation, alongside vigorous specific treatment of the underlying disorder. We therefore considered adding early heparin therapy to our standard care (Chen G. et al., 2020). The present study is a description of the outcome, particularly regarding oxygenation, of the first 27 COVID-19 patients we treated with anticoagulation in the course of the disease.
This study is a case series of 27 consecutive COVID-19 patients seen by our team in Sirio-Libanês Hospital – São Paulo, Brazil, between March 21st and April 12th, 2020. The study was approved by the Sirio-Libanês Hospital Institutional Review Board under the number 3993056 and informed consent was waived.
All patients received initially enoxaparin 0.5 mg/kg SC every 24 h. Patients with a creatinine clearance under 30 mL/min received subcutaneous unfractionated heparin at a dose of 5,000 units every 8 h. If an abrupt decrease in oxygenation or an increase in D Dimer levels was observed, enoxaparin dose was raised to 0.5 mg/kg SC every 12 h and, in the event of thrombotic phenomena or worsening hypoxia, the dose was further increased to 1 mg/kg SC every 12 h. Patients with a BMI (body mass index) of 35 or higher were also considered for the higher dose regimen. Patients in shock or intubated were treated from the beginning with intravenous heparin, targeting an APTT ratio around 1.5 to 2.0 times the normal range. If a patient presented any acute thrombotic event, heparin dosing was increased to obtain an APTT approximately 2.0 to 2.5 times the normal range.
All patients received a 10-day course of azithromycin (500 mg on day 1, then 250 mg daily) (Cramer et al., 2017). Methylprednisolone 40 mg daily was initiated if a worsening in the radiological pattern accompanied by an increase in serum LDH levels was observed. If the patient presented subsequent rise in C-reactive protein, we actively searched for secondary infection and promptly initiated antibiotics.
To evaluate severity of disease during hospital stay, we used the ordinal scale for clinical improvement proposed by the World Health Organization (WHO score): 0. – no clinical or virological evidence of infection; 1. no limitation of activities; 2. limitations of activities; 3. hospitalized, no oxygen therapy; 4. oxygen by mask or nasal prongs; 5. non-invasive ventilation or high-flow oxygen; 6. intubation and mechanical ventilation; 7. ventilation plus additional organ support (pressors, renal replacement therapy, ECMO); 8. death (World Health Organization [WHO], 2020).
We followed a total of 27 hospitalized patients with a diagnosis of COVID-19, all confirmed by PCR. Seventy percent were male, their mean age was 56 ± 17 years, mean BMI was 28.8 ± 6 kg/m2, and comorbidities were present in 67% of them. Individual data from all patients are presented at Table 1. The mean WHO score at admittance was 4.0 ± 1.2 (and the mean maximum score achieved during hospitalization was 4.6 ± 1.6). Entry CT scans showed radiologic infiltrates compromising up to 25% of lung area in 22% of patients, 25–50% of lung area in 48% of patients, and 30% of patients presented infiltrates in over half of lung parenchyma. Symptoms started at an average of 9.6 ± 4.0 days prior to hospitalization, and the anticoagulation protocol was initiated at an average of 3.4 ± 4.0 days after admission. Nineteen patients received methylprednisolone in the course of the disease. Six patients received only the prophylactic dosage of heparin or enoxaparin; three patients started already with enoxaparin 0.5 mg/Kg twice and were kept on this dosage and in 18 patient’s dosages were escalated to either full EV heparin or enoxaparin 1 mg/kg twice a day.
As of August 11th, of the 27 consecutive patients, 25(92%) were discharged from hospital after an median of 11 days. One patient was transferred to another hospital on the 4th day and lost follow-up. Nine patients (33%) were admitted to ICU, 8 (89%) of which have already been discharged to the ward after a median time of 44 days. Eight patients (30%) required intubation, and seven patients have already been successfully weaned after a median time of 11,5 days of mechanical ventilation. One patient is still under mechanical ventilation and required a tracheostomy. This patient had an infected sacral pressure ulcer and required multiple surgical procedures.
Interestingly enough, rotational thromboelastometry (ROTEM) performed in four patients, showed an increase in α-angle, amplitude 10 min after clotting time (A10) and maximum clot firmness (MCF) pointing to a persistent hypercoagulability state, despite their ongoing heparin use.
Figure 1 depicts the gradual improvement in PaO2/FiO2 ratio along the first 72 h in relation to pre-anticoagulation values. Analysis was conducted for the whole series (A) and considering only patients with moderate to severe disease (B) according to the WHO score (p < 0.02 for both groups). For non-mechanically ventilated patients PaO2/FiO2 ratio was calculated according to mask or nasal catheter oxygen flow and oxymetry (Lobete et al., 2013).
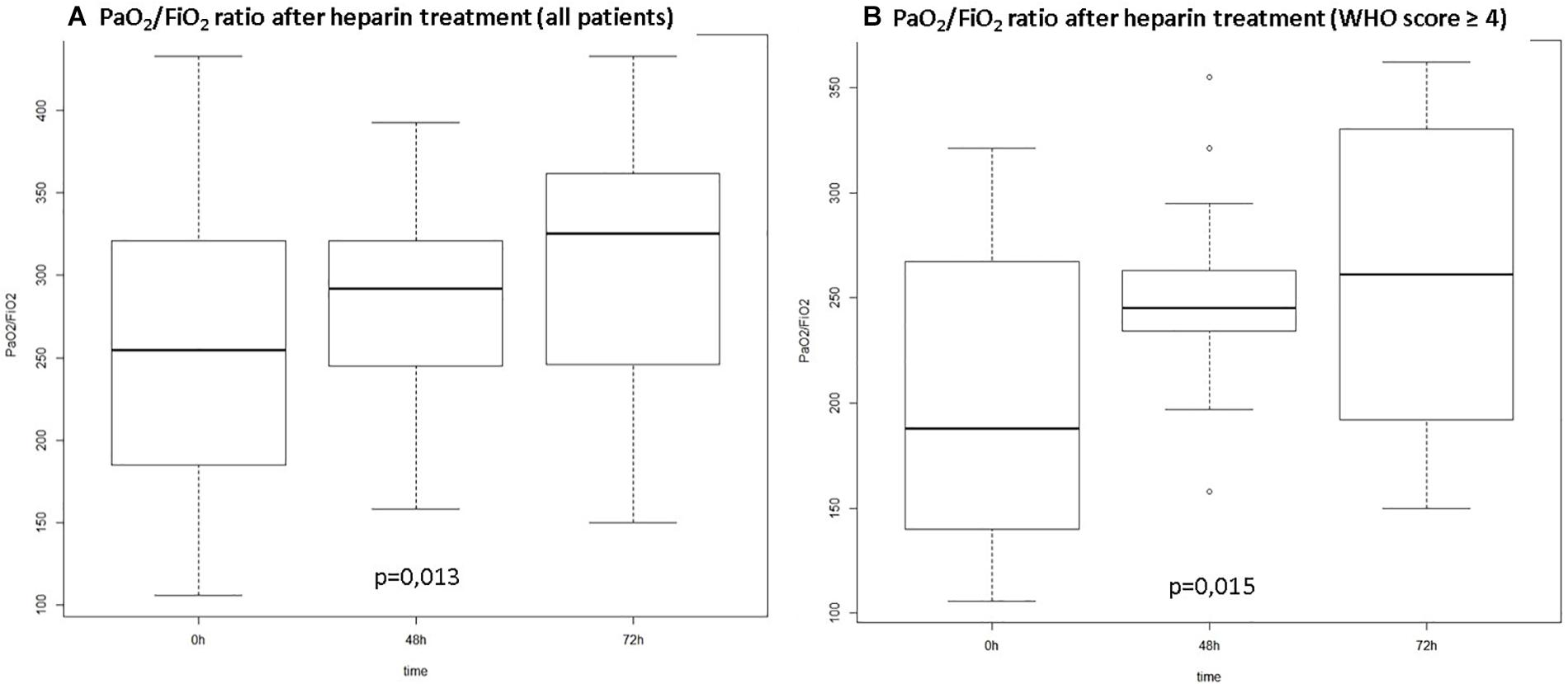
Figure 1. PO2/FiO2 ratio over time from start of anticoagulation. (A) All patients included; (B) Patients with WHO score ≥4 at hospital admission (WHO Score: ordinal scale for clinical improvement proposed by the World Health Organization: 0. – no clinical or virological evidence of infection; 1. no limitation of activities; 2. limitations of activities; 3. hospitalized, no oxygen therapy; 4. oxygen by mask or nasal prongs; 5. non-invasive ventilation or high-flow oxygen; 6. intubation and mechanical ventilation; 7. ventilation plus additional organ support – pressors, renal replacement therapy, ECMO; 8. Death).
We observed no deaths due to any cause or haemorrhagic complications due to anticoagulation during the study period. Moreover, after three months, all but one patient were discharged home without supplementary oxygen.
Our results suggest the important role of hypercoagulative state and microthrombosis as the main mechanisms of organ failure in COVID-19 and the potential response to early anticoagulation therapy.
The significant improvement in oxygen exchange and clinical symptoms observed in these COVID-19 patients, in response to the anticoagulation, points to a potential role for systematic use of heparin in the treatment of such patients. The high incidence of thrombotic events that has been reported in COVID-19 patients (Klok et al., 2020), confirmed more recently, even in the presence of usual heparin prophylaxis (Middeldorp et al., 2020), as well as the fact that similar observations were reported in the other recent coronavirus outbreaks (de Wit et al., 2016; Giannis et al., 2020), further corroborate with this line of reasoning. This is not surprising, as severe cases of COVID-19 meet the laboratory criteria of DIC (Tang et al., 2020; Wu et al., 2020) of thrombotic pattern, in which fibrinogen does not drop and prothrombotic phenomena override the haemorrhagic ones (Wada et al., 2014). Moreover, specifically in patients with respiratory insufficiency caused by COVID-19 under mechanical ventilation, increased d-dimer, abnormal thromboelastography and high levels of fibrinogen point to a hypercoagulative status (Ranucci et al., 2020; Wright et al., 2020). Markers of hypercoagulability has been shown to be independent predictors of increased oxygen requirements in patients with COVID-19 (Rauch et al., 2020).
Thromboelastography showing a pattern of hypercoagulability despite the use of heparin during the course of viral diseases has been previously reported (Wilson et al., 2016). In fact, many viruses known to induce a state of hypercoagulability (Subramaniam and Scharrer, 2018) have a similar pattern of disease, including the timeframe of clinical manifestations (Gai et al., 2012), suggesting a common pattern of response.
Multiple phenomena are involved in the hypercoagulative status in COVID 19: the extensive denudation of epithelial and endothelial spaces causing a massive exposure of tissue factor, production of Von Willeband factor, platelet activation, netosis and pyroptosis, have been described as promoters of extensive microcirculation thrombosis in the severe cases of this disease (Wada et al., 2014; Teuwen et al., 2020). It has been shown that in patients with COVID-19, NETs increased with intubation or death as outcome and were inversely correlated with PaO2/FiO2 (Brinkmann et al., 2004; Middleton et al., 2020). Many autopsy findings confirm this pathophysiological rationale, showing a large amount of microthrombosis as well venous and arterial thrombosis in deceased patients (Dolhnikoff et al., 2020; Yao et al., 2020). Ultrastructural findings also corroborate the endothelial and epithelial destruction in multiple organs (Ackermann et al., 2020). More recently heparin treatment has been pointed to decrease mortality in severe COVID-19 (Ayerbe et al., 2020).
The PaO2/FiO2 ratio improvement observed in our patients after starting heparin is in agreement with the idea of a significant perfusion component explaining the mechanism of respiratory failure with the distinct pattern of marked hypoxia and preserved lung compliance that characterizes severe COVID-19 patients. It has been argued that this could be secondary to the loss of lung perfusion regulation and hypoxic vasoconstriction (Tian et al., 2020), but the clinical response to heparin rather suggests hypoxia due to extensive clogging of pulmonary microcirculation. HRCT studies have shown a consistent reduction in pulmonary blood volume in COVID-19 patients compared to healthy controls, particularly in vessels smaller than 5 mm, again pointing to microthrombi as a cause for hypoxia (Lins et al., 2020). Using electrical impedance tomography, it has been shown that dead space fraction was much more relevant than the shunt fraction as an explanation for the gas exchange derangement observed in the course of disease (Mauri et al., 2020). Moreover when a diagnosis of pulmonary embolism is made in patients with COVID-19, the phenotype is different than embolism in other patients, since it occurs only in areas already affected by the virus, with a lower thrombus load and lower prevalence of proximal embolism of main arteries (van Dam et al., 2020). Interestingly the use of tissue Plasminogen Activator (tPA) has been shown to promote a non-sustained elevation of PaO2/FiO2 ratio (Wang J. et al., 2020). In our opinion, given the marked hypercoagulability seen in these patients – and again in accordance with the autopsy findings – judicious tailoring of heparin doses is needed to prevent capillary reocclusion while avoiding the risks of bleeding complications.
The fact that this is a retrospective study without a control arm does not yet allow us to definitively conclude that heparin in tailored doses should be systematically employed in all COVID-19 patients. Nonetheless, our findings in this early group of patients certainly provide food for thought and perhaps a rationale to justify using a readily available and well-known drug such as heparin, even in larger doses than previously recommended to ameliorate the dim prognosis of such sick patients while we await the more solid data on this subject, as suggested recently (Iba et al., 2020).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Hospital Sirio-Libanes Institutional Review Board – Number of approval: 3.993.056. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors contributed equally on this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the Teaching Research Institute (IEP), Sirio-Libanes Hospital, São Paulo, Brazil. We also would like to acknowledge the hospital staff and their instrumental role in caring for all patients. This manuscript has been released as a pre-print at medRxiv (medRxiv 2020:2020.04.15.20067017) (Negri et al., 2020).
Ackermann, M., Verleden, S. E., Kuehnel, M., Haverich, A., Welte, T., Laenger, F., et al. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 383, 120–128. doi: 10.1056/nejmoa2015432
Ayerbe, L., Risco, C., and Ayis, S. (2020). The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. 50, 298–301. doi: 10.1007/s11239-020-02162-z
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. doi: 10.1126/science.1092385
Burzynski, L. C., Humphry, M., Pyrillou, K., Wiggins, K. A., Chan, J. N. E., Figg, N., et al. (2019). The coagulation and immune systems are directly linked through the activation of interleukin-1alpha by Thrombin. Immunity 50, 1033.e6–1042.e6.
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., Wang, H., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629.
Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., Chen, G., et al. (2020). Clinical characteristics of. (113) deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091. doi: 10.1136/bmj.m1091
Cramer, C. L., Patterson, A., Alchakaki, A., and Soubani, A. O. (2017). Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med. 129, 493–499. doi: 10.1080/00325481.2017.1285677
de Wit, E., van Doremalen, N., Falzarano, D., and Munster, V. J. (2016). SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534. doi: 10.1038/nrmicro.2016.81
Dolhnikoff, M., Duarte-Neto, A. N., de Almeida Monteiro, R. A., Ferraz da Silva, L. F., Pierre de Oliveira, E., Nascimento Saldiva, P. H., et al. (2020). Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 18, 1517–1519. doi: 10.1111/jth.14844
Gai, Z. T., Zhang, Y., Liang, M. F., Jin, C., Zhang, S., Zhu, C. B., et al. (2012). Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J. Infect. Dis. 206, 1095–1102.
Gattinoni, L., Coppola, S., Cressoni, M., Busana, M., Rossi, S., and Chiumello, D. (2020). Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 201, 1299–1300. doi: 10.1164/rccm.202003-0817le
Giannis, D., Ziogas, I. A., and Gianni, P. (2020). Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 127, 1386–1392.
Guo, W., Li, M., Dong, Y., Zhou, H., Zhang, Z., Tian, C., et al. (2020). Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 31e:3319.
Han, H., Yang, L., Liu, R., Liu, F., Wu, K. L., Li, J., et al. (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 58, 1116–1120.
Iba, T., Levy, J. H., Levi, M., and Thachil, J. (2020). Coagulopathy in COVID-19. J. Thromb. Haemost. 18, 2103–2109.
Klok, F., Kruip, M., van der Meer, N., Arbous, M., Gommers, D., Kant, K., et al. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 191, 145–147.
Lins, M., Vandevenne, J., Thillai, M., Lavon, B. R., Lanclus, M., Bonte, S., et al. (2020). Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad. Radiol. 27, 1449–1455.
Lobete, C., Medina, A., Rey, C., Mayordomo-Colunga, J., Concha, A., and Menendez, S. (2013). Correlation of oxygen saturation as measured by pulse oximetry/fraction of inspired oxygen ratio with Pao2/fraction of inspired oxygen ratio in a heterogeneous sample of critically ill children. J. Crit. Care. 28, 538.e1–538.e7.
Mauri, T., Spinelli, E., Scotti, E., Colussi, G., Basile, M. C., Crotti, S., et al. (2020). Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit. Care Med. 48, 1129–1134. doi: 10.1097/ccm.0000000000004386
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. doi: 10.1016/s0140-6736(20)30628-0
Middeldorp, S., Coppens, M., van Haaps, T. F., Foppen, M., Vlaar, A. P., Muller, M. C. A., et al. (2020). Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 18, 1995–2002.
Middleton, E. A., He, X. Y., Denorme, F., Campbell, R. A., Ng, D., Salvatore, S. P., et al. (2020). Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 136, 1169–1179.
Negri, E. M., Piloto, B., Morinaga, L. K., Jardim, C. V. P., Lamy, S. A. E.-D., Ferreira, M. A., et al. (2020). Heparin therapy improving hypoxia in COVID-19 patients - a case series. medRxiv[Preprint]
Ranucci, M., Ballotta, A., Di Dedda, U., Bayshnikova, E., Dei Poli, M., Resta, M., et al. (2020). The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 18, 1747–1751. doi: 10.1111/jth.14854
Rauch, A., Labreuche, J., Lassalle, F., Goutay, J., Caplan, M., Charbonnier, L., et al. (2020). Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J. Thromb. Haemost.[Epub ahead of print].
Subramaniam, S., and Scharrer, I. (2018). Procoagulant activity during viral infections. Front. Biosci. 23:1060–1081. doi: 10.2741/4633
Tang, N., Bai, H., Chen, X., Gong, J., Li, D., and Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost 18, 1094–1099.
Teuwen, L. A., Geldhof, V., Pasut, A., and Carmeliet, P. (2020). COVID-19: the vasculature unleashed. Nat Rev Immunol. 20, 389–391. doi: 10.1038/s41577-020-0343-0
Tian, S., Hu, W., Niu, L., Liu, H., Xu, H., and Xiao, S. Y. (2020). Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 15, 700–704.
van Dam, L. F., Kroft, L. J. M., van der Wal, L. I., Cannegieter, S. C., Eikenboom, J., de Jonge, E., et al. (2020). Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb. Res. 193, 86–89. doi: 10.1016/j.thromres.2020.06.010
Wada, H., Matsumoto, T., Yamashita, Y., and Hatada, T. (2014). Disseminated intravascular coagulation: testing and diagnosis. Clin. Chim. Acta. 436, 130–134. doi: 10.1016/j.cca.2014.04.020
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069.
Wang, J., Hajizadeh, N., Moore, E. E., McIntyre, R. C., Moore, P. K., Veress, L. A., et al. (2020). Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a Case Series. J. Thromb. Haemost 18, 1752–1755.
Wilson, A. J., Martin, D. S., Maddox, V., Rattenbury, S., Bland, D., Bhagani, S., et al. (2016). Thromboelastography in the Management of Coagulopathy Associated With Ebola Virus Disease. Clin Infect Dis. 62, 610–612. doi: 10.1093/cid/civ977
Wright, F. L., Vogler, T. O., Moore, E. E., Moore, H. B., Wohlauer, M. V., Urban, S., et al. (2020). Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J. Am. Coll. Surg. 231, 193.e1–203.e1.
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., et al. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 180, 934–943.
Yao, X. H., Li, T. Y., He, Z. C., Ping, Y. F., Liu, H. W., Yu, S. C., et al. (2020). A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 49:E009.
Ye, Z., Zhang, Y., Wang, Y., Huang, Z., and Song, B. (2020). Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 30, 4381–4389.
Yin, S., Huang, M., Li, D., and Tang, N. (2020). Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thromb. 3, 1–4.
Zhang, J. J., Dong, X., Cao, Y. Y., Yuan, Y. D., Yang, Y. B., Yan, Y. Q., et al. (2020). Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75, 1730–1741.
Keywords: COVID-19, respiratory failure, thrombosis, perfusion mismatch, heparin
Citation: Negri EM, Piloto BM, Morinaga LK, Jardim CVP, Lamy SAE-D, Ferreira MA, D’Amico EA and Deheinzelin D (2020) Heparin Therapy Improving Hypoxia in COVID-19 Patients – A Case Series. Front. Physiol. 11:573044. doi: 10.3389/fphys.2020.573044
Received: 15 June 2020; Accepted: 28 September 2020;
Published: 19 October 2020.
Edited by:
Georges Leftheriotis, Université Côte d’Azur, FranceReviewed by:
Melissa L. Bates, The University of Iowa, United StatesCopyright © 2020 Negri, Piloto, Morinaga, Jardim, Lamy, Ferreira, D’Amico and Deheinzelin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elnara Marcia Negri, ZW1uZWdyaUB5YWhvby5jb20uYnI=; orcid.org/0000-0002-6428-6066
†ORCID: Bruna Mamprim Piloto, orcid.org/0000-0002-8756-0400; Luciana Kato Morinaga, orcid.org/0000-0002-0900-2737; Carlos Viana Poyares Jardim, orcid.org/0000-0003-0425-5548; Shari Anne El-Dash Lamy, orcid.org/0000-0003-2915-4014; Marcelo Alves Ferreira, orcid.org/0000-0003-4181-760X; Elbio Antonio D’Amico, orcid.org/0000-0003-1069-1469; Daniel Deheinzelin, orcid.org/0000-0002-0253-4124
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.