
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 11 September 2020
Sec. Environmental, Aviation and Space Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.541483
Background: Extreme heat events are increasing in frequency, severity, and duration. It is well known that heat stress can have a negative impact on occupational health and productivity, particularly during physical work. However, there are no up-to-date reviews on how vulnerability to heat changes as a function of individual characteristics in relation to the risk of hyperthermia and work capacity loss. The objective of this narrative review is to examine the role of individual characteristics on the human heat stress response, specifically in relation to hyperthermia risk and productivity loss in hot workplaces. Finally, we aim to generate practical guidance for industrial hygienists considering our findings. Factors included in the analysis were body mass, body surface area to mass ratio, body fat, aerobic fitness and training, heat adaptation, aging, sex, and chronic health conditions.
Findings: We found the relevance of any factor to be dynamic, based on the work-type (fixed pace or relative to fitness level), work intensity (low, moderate, or heavy work), climate type (humidity, clothing vapor resistance), and variable of interest (risk of hyperthermia or likelihood of productivity loss). Heat adaptation, high aerobic fitness, and having a large body mass are the most protective factors during heat exposure. Primary detrimental factors include low fitness, low body mass, and lack of heat adaptation. Aging beyond 50 years, being female, and diabetes are less impactful negative factors, since their independent effect is quite small in well matched participants. Skin surface area to mass ratio, body composition, hypertension, and cardiovascular disease are not strong independent predictors of the heat stress response.
Conclusion: Understanding how individual factors impact responses to heat stress is necessary for the prediction of heat wave impacts on occupational health and work capacity. The recommendations provided in this report could be utilized to help curtail hyperthermia risk and productivity losses induced by heat.
Climate change is increasing the frequency, intensity, and duration of extreme heat events. Consequently, the prevalence of occupational heat stress is also increasing, which reduces the ability of workers to live healthy and productive lives (Flouris et al., 2018a). Most affected are those who work with sun exposure, in non-air-conditioned work spaces, those who perform heavy work, or those who require protective clothing. Sustained, daily elevations in body temperature can increase the risk ofkidney injuries, and is also strongly linked to workplace accident rates (Tawatsupa et al., 2012, 2013). Heat stress also decreases physical work productivity (Wyndham, 1969), since workers must reduce their work output to minimize physiological strain and risk of heat stroke (Miller et al., 2011). The health and productivity implications of workplace heat decreases national economic income (Hübler et al., 2008), an effect exacerbated with climate change (Hsiang et al., 2017).
Although the link between heat stress, health, and performance on the macro level is well established, the biophysical and physiological factors that impact the vulnerability of the individual worker is still debated. While past reviews have addressed the impact of some individual characteristics on the heat stress response, an updated synthesis that has practical use is urgently required. Havenith’s report (1985) was extensive for the time, but due to lack of available data the discussion on age and body characteristics were limited, and diabetes was not known to be a relevant factor in thermoregulatory control. Cheung et al. (2000) addressed physiological responses to uncompensable heat stress only, which is relevant in many settings (especially with highly protective clothing) but generally less common than compensable environments (each are defined in the “Clarification of Terms” section). Kenny and Jay (2013) summarized the independent effect of age, sex, and diabetes on the heat stress response as part of a larger review, but their conclusions are drawn mostly from groups matched for all other characteristics apart from that under investigation, rather than the population distribution. They also do not comment on the cardiovascular adjustments to heat stress, which is relevant because workers seem to pace themselves based on their heart rate (HR), a proxy for cardiovascular strain (Miller et al., 2011).
In the present review, we indeed report on individual differences for matched individuals, but also for unmatched groups, which is a better representation of the population distribution. This approach allows for conclusions to be made on a wider scale, facilitating the development of practical advice for policymakers and industrial hygienists. We also recognize the contribution of large, individual lab studies which use heterogeneous groups and multiple regression to document the most relevant factors governing the heat stress response (Havenith et al., 1995b, 1998; Havenith and van Middendorp, 1990; Flouris et al., 2018b; Notley et al., 2019b). These works are addressed throughout this paper, based on their contribution to understanding the influence of each individual factor described below. However, no one single study can answer all the relevant questions needed to determine the importance of any given individual characteristic. The relative importance of each factor changes based on the environment (hot dry or hot humid), work intensity (low, moderate, or high metabolic rate, Table 1), and work type (fixed or self-paced). Equally important to consider is the cardiovascular response to heat (particularly HR), since this can govern perceived work intensity and thus, work output (discussed in the “Physiological and Biophysical Aspects of Heat Transfer” section) (Miller et al., 2011). Any impact a single factor has on work capacity therefore has implications for economic production.
The aim of this review is to synthesize the relative importance of individual factors, based on how they can predict the human heat stress response. We base our conclusions on how each factor may be protective against hyperthermia (rises in Tc) and losses in physical work output during fixed and self-paced work scenarios.
We chose to perform a narrative review due to (i) concerns that the systematic review process will omit many studies based on strict inclusion/exclusion criteria, and (ii) the broad scope of the present review, which is unsuitable if using the systematic process (Misra and Agarwal, 2018). Articles were obtained by searching relevant keywords into Google Scholar and PubMed databases. The reference list of relevant articles was also scanned for their potential inclusion.
This section will aim to improve the translation of findings from laboratory studies to real-world working scenarios. To achieve this aim, we present a clarification of terms used throughout this review.
The term Tc is used to reflect the global internal temperature of the body. The rectal (typically 10−12 cm beyond the anal sphincter) and/or oesophaeal (typically level with the left atrium) temperatures are the most adopted tissues used to estimate Tc. Alternative measurements are intestinal temperature, arterial blood, tympanic, and brain temperature, but each have issues of either cost, invasiveness, logistics during exercise, or accuracy, decreasing their use. A further consideration is that there may be a time lag of 10−30 min for Tc to reflect whole body heat content (Kenny and Jay, 2013).
Various thermoreceptors sense temperature variations throughout the body to generate an appropriate effector response (Romanovsky, 2018), with the global internal temperature best represented whole body heat content (Kenny and Jay, 2013). Whole-body heat content can be measured with a direct calorimeter, a unique tool which generates data on each heat transfer pathway (evaporative, dry, and respiratory), and in combination with indirect calorimetry to measure metabolic rate, whole body heat storage. Using direct calorimetry, differences in whole body heat storage help to identify inter-individual differences in heat exchange pathways (Larose et al., 2013; Stapleton et al., 2013, 2015; Carter et al., 2014; Kenny et al., 2015; Poirier et al., 2015; Flouris et al., 2018b; Notley et al., 2019b). Due to reasons previously described, the device is primarily limited to cycling exercise in hot dry environments, and with high air flow (to minimize sweat drippage) (Cramer and Jay, 2019). Hence, the environment is considered in each study when drawing conclusions about the data from direct calorimetry.
Protocols that require participants to work at a fixed metabolic rate simulate a constant work rate, not allowing for self-pacing of exercise intensity (Havenith et al., 1998). This type of activity may reflect work on an assembly line where the work pace is fixed for all. The approach is often used in regression studies to determine what individual factors best predict the heat stress response (Havenith et al., 1995b, 1998; Havenith and van Middendorp, 1990; Cramer and Jay, 2015).
The term relative exercise intensity means the workload is prescribed based on the individual participant’s maximal work capacity (Havenith et al., 1998; Periard et al., 2012). Here, fitter people will work at a greater metabolic rate than unfit people to achieve equivalent percentage maximum oxygen uptake (V̇O2max). It stands to reason therefore, that results from studies that use a relative intensity can be used to reflect scenarios where physical work is self-paced. This is supported by evidence of self-pacing during actual physical work in the heat (Wyndham et al., 1965; Morrison et al., 1969; Kalkowsky and Kampmann, 2006; Miller et al., 2011; Bröde et al., 2018). In a laboratory setting, the intensity is normally set as a percentage of V̇O2max, normally prescribed relative to body mass (ml O2⋅kg–1⋅min–1).
Environments in the present review are often characterized based on whether they are compensable or uncompensable. A distinction between compensable and uncompensable heat stress is required since it can have implications for the relevance of individual characteristics. These terms describe if metabolic heat production can be matched by heat loss. In compensable heat stress, enough heat can be lost to the environment so that the body is not in a continuous state of heat gain. In hot working scenarios, compensable heat stress is typically associated with work in an environment with low ambient humidity. With uncompensable heat stress, heat production exceeds heat loss potential in that climate, and the body is in a state of continuous heat gain. Thermal compensability can be determined by estimating required evaporative heat loss (Ereq) and the maximum evaporative capacity of the environment (Emax), determined by the humidity, wind speed, and clothing. A work situation is generally considered compensable if Emax > Ereq, indicating the environment can accommodate Ereq for thermal balance.
Physical work capacity defines the ability of an individual to perform maximal physical work. To support SkBF requirements during work, cardiac output (primarily mediated by HR) increases as a function of the heat stress severity (Rowell, 1974). Because the WHO have classified occupational work intensities based on HR (Table 2; Andersen et al., 1978), HR is considered an integral part of the heat stress response. Moreover, there are a number of large scale field observations showing that workers will pace themselves according to the environmental heat, resulting in a relatively stable working HR regardless of the environmental severity (Morrison et al., 1969; Wyndham, 1973; Vogt et al., 1983; Kalkowsky and Kampmann, 2006; Miller et al., 2011). Since self-pacing is primarily driven by HR (Borg, 1982), those with a more stable and lower HR increase during hot work will likely maintain greater physical work capacity (Jay et al., 2019).
Control of human Tc relies on delivery of warm blood from the core to the skin surface. Heat loss from the skin surface to the environment can then occur through dry and/or evaporative pathways. Throughout this review, reference is made to adjustments in SkBF with specific factors, but its contribution to overall heat loss should be nuanced relative to the environment. In resting, normothermic conditions, blood is delivered to the skin at a rate of ∼250 ml/min, warming the skin. Heat from the skin surface is then lost to the environment (dry heat loss) at a rate similar to metabolic heat production, producing heat balance (Charkoudian, 2003). The rate of dry heat loss is therefore modified by SkBF in resting conditions in a cool environment, where it is the primary contributor to overall heat loss. The contribution of dry heat loss (and thus SkBF) to overall heat loss is minimal in hotter conditions due to a narrowing of the skin and air temperature gradient. For instance, at 30°C air temperature, attenuated SkBF causes a faster increase in Tc during activity in the heat, despite similar sweat rates (Balmain et al., 2018a). However, at 35°C air temperature, a reduced SkBF in older participants did not increase Tc, because dry heat loss was similar in the young and older participants (Havenith et al., 1995b).
Thus, if an individual factor is shown to modify SkBF, this in-of-itself is likely to improve heat loss mainly in conditions permitting high rates of dry heat transfer. Such conditions are air temperatures < 30°C, minimal clothing insulation, and high wind speed. All these factors increase rates of dry heat loss from the skin to the environment, rendering an elevation in SkBF beneficial to the heat loss response. A secondary effect of SkBF raising skin temperature is its effect on the saturated vapor pressure on the skin, which increases to a small amount with each degree of increase in skin temperature (Parsons, 2010). This effect increases the vapor pressure gradient from the skin to the environment, increasing sweating efficiency i.e., the proportion off sweat that evaporates, rather than drips from the body (Candas et al., 1979).
Important to note is the role of SkBF in overall cardiovascular strain, which impacts work tolerance time in the heat. In several studies throughout this review, differences in absolute SkBF do not result in different body temperatures, an observation supported and explained by Kenney and Havenith (1993). However, while changes in absolute SkBF may not result in a different Tc (and risk of hyperthermia per se), such differences can have implications for work capacity, depending on the %HRmax required to achieve that SkBF (Rowell et al., 1970; Rowell, 1974). For individual factors that reduce maximum cardiac output (i.e., low fitness, age), a similar, or even lower absolute level of SkBF can still represent a greater relative cardiovascular strain (in terms of %HRmax), which is a major limitation to work capacity in the heat (Drinkwater and Horvath, 1979; Cheung and McLellan, 1998). For example, when comparing young vs. older participants, despite absolute SkBF being lower in older participants and no corresponding change in Tc (Havenith et al., 1995b), older people required a similar %HRmax to achieve their SkBF, placing similar relative stress on the cardiac system to meet the combined oxygen demand of locomotion (active muscle tissues) and thermoregulation (skin tissues). The net result would be a similar “cardiovascular strain” despite reduced SkBF requirements.
Clothing is a pre-requisite of most occupations but varies depending on the level of protection required. Clothing impacts dry and evaporative heat transfer pathways (Havenith et al., 1999; Holmér et al., 1999), such that the potential for heat loss decreases as a function of the total insulation and evaporative resistance of a given ensemble (Potter et al., 2015). Many of the factors discussed in this review impact heat stress vulnerability through adaptation or maladaptation of the sweating response, which impacts the rate of sweat evaporative heat loss. However, with heavy protective clothing (i.e., NBC protective clothing), sweat evaporation is severely diminished, resulting in similar thermoregulatory responses between people of different phenotypes/individual characteristics (Cheung and McLellan, 1998). Clearly, heavy protective clothing that creates an uncompensable environment changes the relevance of individual characteristics, compared with environments where sweat output impacts heat loss. We differentiate between compensable and uncompensable heat stress throughout this review, but the reader is also directed to an earlier review which focuses on individual factors during uncompensable heat stress exclusively (Cheung et al., 2000).
Data is limited regarding the impact of typical clothing (i.e., for non-specialist situations) on the relevance of individual factors. However, in the present review, the impact of added clothing will be like that of increasing ambient humidity since both decrease compensability in a similar way. Studies that create a more uncompensable environment by increasing humidity (i.e., Havenith et al., 1995a) are therefore likely to serve as a proxy for increasing clothing insulation. This relationship is not perfect however, and more studies are required to investigate the importance of individual characteristics in typical, non-specialist work ensembles.
At the end of each section, we use specific terminology to state the overall impact of an individual factor. We also use that terminology to determine the relative importance of an individual factor as shown in Figure 1. If a factor has a “strong” impact on the heat stress response, it is consistently relevant independent of climate type or workload. If a factor has a “moderate” impact on the heat stress response, its relevance is dependent on climate type or workload. If a factor has a “low” impact on the heat stress response, it has minimal independent effect on heat stress vulnerability. With that being said, some factors of “low” impact will be secondary to other factors of strong impact, so may still be important screening tools for individual workers.
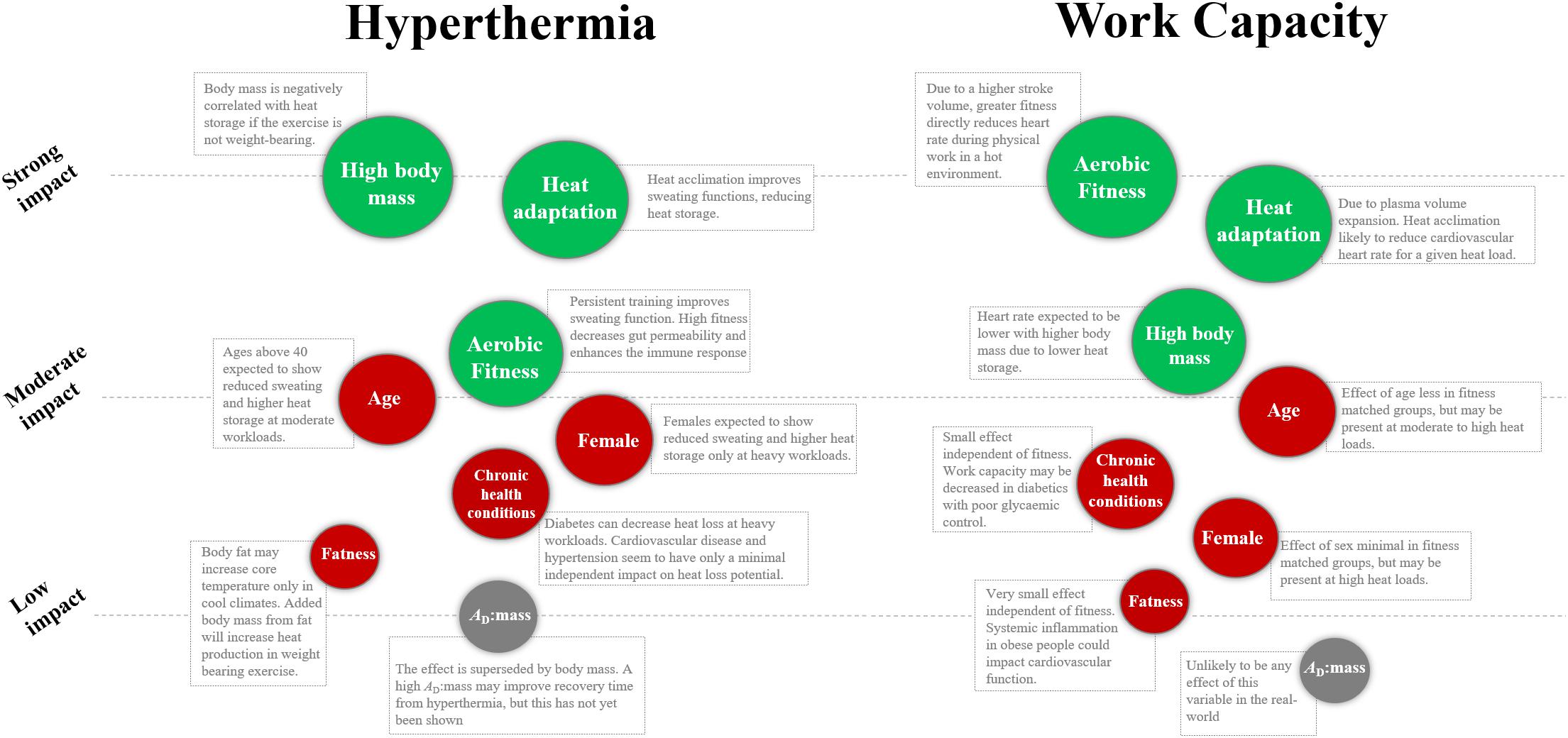
Figure 1. Relative influence of individual characteristics on Tc and work capacity during physical work in the heat. Green variables indicate a positive influence, red variables indicate a negative influence, and the gray variables are deemed not relevant. The orders are based on the authors’ interpretation of the literature, as explained in the clarification of terms section. AD:mass, surface area to mass ratio.
Morphological factors have been described as key modulators of individual heat stress responses (Havenith et al., 1998; Havenith, 2001a). Factors included for discussion are body mass, the body surface area to mass ratio (AD:mass), and body fat.
Bergmann’s rule suggests that typically, species originating from colder climates will have a larger body mass than those originating from warm, tropical climates (Bergmann, 1847). The rule tends to apply to modern human beings, but only when extreme differences in climate are apparent (i.e., 50°C of latitude and/or more than 30°C C difference in air temperature) (Foster and Collard, 2013). Here, we show that Bergmann’s rule does not apply to humans, in that heavier people are not more vulnerable to heat compared with smaller people. Bergmann’s rule may still apply to extreme geographical changes because (i) absolute fluid requirements are lower in smaller people, and (ii) being heavier increases metabolic heat production if the activity has a considerable weight bearing component i.e., climbing, jogging, etc (Dennis and Noakes, 1999; Marino et al., 2000; Smoljanić et al., 2014). In most occupations, there is minimal weight bearing component and water is not typically in short supply. Below, we discuss the effects of body mass with the assumption that an individual is within a healthy range of body fat.
In humans, specific heat capacity defines the amount of energy required to heat the body by 1°C. The specific heat of most tissue in the body is ∼3.65 kJ⋅kg–1, apart from adipose tissue which is ∼2.51 J⋅g–1 (Lipkin and Hardy, 1954). Compared with light people, larger people are at an advantage if they work at the same absolute metabolic rate, since their larger heat sink results in more energy being required to raise Tc (Havenith, 1997). Consequently, total body mass below 50 kg has been highlighted as a major risk factor for hot work in general (Wyndham and Heyns, 1973). Body mass has been shown to explain a large portion of the heat stress responses during fixed and relative exercise intensities (Havenith, 1985; Havenith et al., 1995a, b, 1998; Coso et al., 2011; Cramer and Jay, 2014, 2015). Since a larger body mass allows for greater distribution of internal heat (i.e., “heat sink”), ΔTc is negatively correlated with body mass during hot work (Lind, 1963; Havenith et al., 1995b, 1998; Havenith, 2001a; Gagnon et al., 2009, 2013b; Coso et al., 2011; Cramer and Jay, 2015). During cycling at a fixed work rate, body mass explained ∼40% of ΔTc, where it is negatively correlated i.e., protective (Havenith et al., 1998; Cramer and Jay, 2014). HR was also negatively correlated with body mass during fixed pace work in both dry and humid conditions (Havenith et al., 1995a), indicating a protective impact on physical work capacity. In occupational settings where the workload is externally governed, it can be assumed that heavier people are less vulnerable to heat stress compared with lighter people. However, upon recovery from heat stress, people with a heavy body mass will generally have a slower rate of Tc decrease compared with smaller people (White et al., 1992). This is relevant for occupations that adopt fixed work/rest cycles because heavier people may take longer to recover to their baseline Tc.
The effect of total body mass was determined in hot-dry and warm-humid environments during exercise at a relative workload (Havenith et al., 1998). During exercise in a compensable environment, body mass explained ∼10% of the ΔTc, where there was a negative association. In the more uncompensable environment, body mass explained 30% of the ΔTc, also with a negative association. However, body mass had no independent effect on HR during relative work in dry or humid heat (Havenith et al., 1995a). Therefore, body mass remains protective against hyperthermia at relative workloads, without a strong impact on HR.
In summary, total body mass has a strong impact on the heat stress response in humans, where it is protective against hyperthermia and increased HR during fixed paced work (Havenith et al., 1998). A high body mass remains protective against hyperthermia during self-paced work, without impacting HR (Havenith et al., 1995a). The findings are unlikely to apply to activities with a heavy weight bearing component.
Allen (1907) rule suggests that homeothermic animals adapted to their thermal environment through evolutionary alterations in the skin surface area to body mass ratio (AD:mass). In short, the rule suggests that a high AD:mass decreases heat gain, due to a larger ratio of cooled tissue (from dry and evaporative heat exchange at the skin) to metabolically active tissue (the body mass reflects this component). Geographical adaptations to heat are evident since humans born in hot climates generally show greater limb length compared with those descending from cold climates (Katzmarzyk and Leonard, 1998; Weinstein, 2005).
The literature examining the human heat stress response does not support Allen’s rule. Heavier people mostly have a lower AD:mass than lighter people, because mass and AD do not increase in direct proportion to one another. Hence, the proportion of AD in relation to mass typically decreases as mass increases, unless an individual is exceptionally tall and lean. For example, if an individual was 60 kg and 1.8 m (5.9 ft) tall, their AD:mass would be 294 cm2⋅kg–1. For a heavier person (80 kg) to achieve the same AD:mass, they would need to be 2.2 m (7.2 ft) tall, clearly not a population norm. Ultimately, there is strong collinearity between mass and AD:mass in most population samples, meaning that AD:mass is effectively, another representation of mass itself (White et al., 1992; Havenith, 2001a).
During non-weight bearing activity in the heat, ΔTc is more related to total body mass compared with AD:mass. Havenith (2001a) reports total mass to be the most relevant characteristics for heating rates during hot work at a fixed metabolic rate, where AD:mass was not a stronger predictor. Moreover, AD:mass generally increases as body mass decreases, such that a higher AD:mass is associated with a faster ΔTc. It was shown in the last section that a lower body mass decreases heat-sink, which results in an increased ΔTc for lighter people. Hence, during physical work, participants with a higher AD:mass (smaller people) showed elevated Tc’s compared with the heavier people (Havenith et al., 1998; Havenith, 2001a; Cramer and Jay, 2015). Taken together, these findings contradict Allen’s and Bergmann’s rules. An earlier study analyzed sex differences in responses to heat stress, reporting a lower heat gain in females due to their higher AD:mass (Shapiro et al., 1980). However, the interpretation was shown to be erroneous because the heavier males (i.e., lower AD:mass) were working at higher rates of heat production than the lighter females (Havenith, 2001a). In humans, AD:mass only seems relevant for two individuals of the same mass, where increased limb length alters heat exchange with the environment. In environments where air temperature is below skin temperature, having a larger AD for the same mass increases heat loss by convection and radiation. Conversely, when air temperature exceeds skin temperature, more heat will be gained from these dry heat exchange pathways with increasing AD. An increased AD will also increase Emax, which is beneficial in all heat stress conditions, if sweat can evaporate freely i.e., compensable. It is worth noting that occupational heat stress typically involves short, non-steady state heat stress exposures (Vogt et al., 1983), often not allowing time for steady state sweating to occur. In those scenarios where the skin is not wet, dry heat exchange, primarily determined by the gradient between skin and ambient temperature, will become highly relevant. Overall, body mass is the more relevant characteristic during heat stress.
Similar to the last section, total body mass was a stronger predictor of ΔTc during recovery compared with AD:mass (White et al., 1992). However, that study used cold water immersion during recovery, which is applicable for heat stroke recovery but less commonly adopted in occupational settings. More data on the association between body characteristics during recovery from heat stress in cool and hot air is required as it is more applicable to industry.
Overall, AD:mass is not a strong independent predictor of the heat stress response in humans.
Based on the physical properties of fat tissue (described below), the WHO suggest that being overweight increases vulnerability to hyperthermia during heat stress (Koppe et al., 2004). Body fat can affect the heat stress response in several ways. Firstly, fat tissue has different heat transfer properties compared with muscle (McIntosh and Anderson, 2010). The comparison between these tissues is appropriate in the context of comparing individuals with different body compositions. The properties are shown in Table 3.
The specific heat capacity (c) of a tissue defines the thermal energy required to raise its temperature by 1°C. Fat has a lower value of c, which means it requires less thermal energy to raise its temperature. Fat also has a lower value of k, which means less heat propagates from the tissue into the blood stream. These aspects intuitively lead to the assumption that body fat independently changes an individual’s vulnerability to heat stress. Importantly however, these values are provided for resting conditions only, not taking into account the fact that during activity, the metabolic heat production of active skeletal muscle will far exceed fat, contributing heavily to whole body heat storage rates (Jay et al., 2007; Kenny and Jay, 2013). However, body fat also increases passive mass carried, another form of load carriage, which elevates metabolic heat production for a given task (Pandolf et al., 1977). Finally, obese humans typically have greater levels of systemic inflammation (Fontana et al., 2007), which, theoretically, may predispose this group to heat stroke (Chin et al., 2006). The above factors intuitively lead to the assumption that body fat independently increases vulnerability to heat stress (Havenith, 1997).
A primary supporting article for this assumption showed that overweight army trainees were 70% more likely to develop heat illness in basic training compared with those who have a healthy body fat (Bedno et al., 2010). However, the increased risk of heat illness could have been be due to reduced fitness levels in the overweight recruits (Mondal and Mishra, 2017), which was not accounted for in that study. The extra passive mass carried during the weight bearing activity could have also increased metabolic heat production, contributing to the risk of heat exhaustion. In lab studies which do control for these variables, researchers generally cannot isolate an independent effect of body fat on the heat stress response (Haymes et al., 1974; Havenith et al., 1998; Jay et al., 2011; Adams et al., 2015). Work using a multiple regression approach could not identify body fat as a significant predictor for the heat stress response in dry or humid heat conditions, and at either a fixed or relative exercise intensity (Havenith et al., 1998). Similar findings have been documented in studies using independent matched groups designsin hot (Adams et al., 2015) or warm conditions (Jay et al., 2011). Mechanistically, the potentially insulating effect of fat seems to be outweighed by unimpeded blood flow to the skin surface across the fat layer i.e., blood flow provides a convective short-cut for heat-transport through the fat tissue (Havenith, 2001b). We acknowledge evidence of reduced SkBF in obese vs. lean individuals exercising in the heat (Vroman et al., 1983), but given that sweating is not impaired in obese individuals, any influence of fat on SkBF is unlikely to pose significant increases in risk of heat illness (Dervis et al., 2016). On the population level (i.e., not fitness matched), obese individuals are expected to show an increased HR of 20−30 b⋅min–1 during work in hot conditions compared with those of normal body fat (Bar-Or et al., 1969; Haymes et al., 1975).
During exercise in cool conditions, the insulative effect of body fat can increase heat storage rates. At a relative intensity, body fat independently explained 26% of the Tc response in cold conditions, but not in hot conditions (Havenith et al., 1998). Another study found that large differences in body fat of ∼21% increased heat gain during fixed intensity exercise in warm conditions (Dervis et al., 2016). Therefore, it appears that the difference in heat storage between high and low-fat populations increases as the temperature decreases, because the insulative effect of fat takes precedence in colder conditions. Although less specific, the body mass index (BMI) may be a practical guideline when formulating employment standards for hot work. Work using ROC curve analysis suggest an upper threshold for BMI of 26 kg⋅m2 for protection against heat illness (Flouris et al., 2018b). Since being underweight is also problematic for heat storage capacity, we advise a lower limit of 18.5 kg⋅m2, following WHO guidelines.
Overall, body fat is not a strong independent predictor of the heat stress response, but in cool conditions, will likely cause faster elevations in Tc at heavy workloads. On a population level, obese individuals are likely to experience a higher HR and produce less physical work during heat stress.
In the normal range of body fat, total body mass can be a strong predictor of the heat stress response, where it is protective during hot work. The beneficial effect of a high mass is greater during uncompensable heat stress compared with compensable heat stress. The AD:mass is not a strong predictor of the heat stress response, and is superseded by body mass. On a population level, individuals with high adiposity do not typically show a different ΔTc than leaner males in hot conditions but may have increased HR’s due to (on average) lower fitness levels and more passive mass carried. The independent effect of body fat on thermophysiological responses to exercise are displayed in Table 4.
1. If employment standards for hot work are utilized based on body type, they should be based on body mass, and not AD:mass. Heavier people with a normal body fat are at less risk of hyperthermia if the workload is fixed.
2. Previous research suggests those under 50 kg should not perform physical work in the heat.
3. For general purposes, the BMI should be within 18.5 and 26 kg⋅m2.
Exercise training evokes a plethora of adaptations relevant to thermoregulation, such as increased cardiac function, plasma volume, and microvascular function (Hellsten and Nyberg, 2016). It is logical to assume, therefore, that the physiological adaptations to endurance training directly improve thermoregulatory and cardiovascular performance during heat stress. In exercise physiology, V̇O2max is the most used index of aerobic fitness. It is most commonly measured through analysis of expired air during maximal aerobic exercise, but can be predicted during cycling or treadmill exercise based on the power output, and speed and grade, respectively (American College of Sports Medicine [ACSM], 2013; Ludlow and Weyand, 2017). In line with exercise physiology literature, we use V̇O2max to categorize aerobic fitness levels.
Endurance training increases cardiovascular and thermoregulatory stability during exercise (Ekblom et al., 1968; Ho et al., 1997; Convertino, 1991; Cramer et al., 2012). In older (previously sedentary) participants, physical training decreased Tc and HR during fixed work in the heat, without alterations in body characteristics (Ho et al., 1997). The data indicate that training increased SkBF and plasma volume for the same fixed workload. Endurance training typically increases SkBF for a given Tc and can activate vasodilation for a lower Tc (Roberts et al., 1977; Thomas et al., 1999; Beaudin et al., 2009; Simmons et al., 2011), results not seen with resistance training (Thomas et al., 1999). The increase in SkBF is explained by an expansion of blood volume and increased cardiac output (Simmons et al., 2011), and increases basal production of nitric oxide, an endothelium derived vasodilatory compound (Kingwell et al., 1997; Holowatz and Kenney, 2010).
In addition to the cardiovascular adaptations that are beneficial during hot work, exercise training also enhances sweating function. For instance, endurance training can reduce the Tc threshold for the onset of sweating (Nadel et al., 1974; Henane et al., 1977; Roberts et al., 1977) similar to the effects seen from heat adaptation, but to a lesser extent. Modeling the response based on available literature, Havenith (2001b) suggests that a training-induced increase in V̇O2max by 12−17% will reduce the sweat onset threshold by 0.1°C, although reductions up to 0.4°C have been reported in a low sample size (Henane et al., 1977). With exercise training, there are increases in sweat output for the same Tc increase, in addition to elevations in maximal sweat output (Nadel et al., 1974; Henane et al., 1977; Roberts et al., 1977). Most recently, 8 weeks exercise training (V̇O2max increase from 46 to 52 ml/kg/min) increased local sweat rate, and thus skin wettedness from 72 to 85% surface area (Ravanelli et al., 2018). The increased sweating function in the above studies may not be related to aerobic fitness per se, but more due to frequent and persistent rises in Tc due to the training itself, evoking a mild heat adaptation (Ravanelli et al., 2020).
At a relative intensity, fitter people had a slower increase in Tc in both cool and hot-dry climates, but a faster increase in Tc in very humid heat (Havenith et al., 1998). Thus, when evaporative heat loss is limited by high humidity, the greater heat produced by fitter people makes them more vulnerable to heat. While a similar study documents equivalent thermoregulatory patterns between trained and untrained males in humid heat (Periard et al., 2012), the water vapor pressure was ∼1 kPa higher in the study of Havenith et al. (1998), suggesting an upper critical kPa where a higher metabolic rate can be compensated for by increasing sweat rates. When fully uncompensable conditions are simulated with NBC clothing, fitness has no impact on thermometric responses (Cheung and McLellan, 1998). The above data suggests that impermeable clothing, or an ambient kPa of ∼4 kPa will likely negate any beneficial effect of aerobic fitness on thermoregulatory function. The true upper threshold will depend on the skin temperature and the evaporative resistance of any clothing ensemble.
Some studies used multiple regression analysis to determine what variables explain the thermoregulatory responses to heat (Havenith et al., 1995a, 1998; Cramer and Jay, 2015; Flouris et al., 2018b; Notley et al., 2019b). In a temperate environment, the exercising metabolic rate (in W/kg) explained ∼50% of the heat storage during fixed intensity exercise, with aerobic fitness explaining only a further 1% (Cramer and Jay, 2015). It is worth noting that this work used forward entry stepwise regression without interpretation of standardized regression coefficients, which are useful when comparing the relative contribution of individuals parameters that have different units. Therefore, the impact of V̇O2max on its own could have been higher 1%. During fixed intensity exercise with dry heat stress, aerobic fitness explained 17−25% of ΔTc, where there was a negative association (Notley et al., 2019b). In humid heat and at a fixed intensity, Tc was negatively associated with fitness, suggesting that fitness has a protective impact (Havenith et al., 1998). In that study, fitness was poorly associated with heat storage in dry heat, but this is likely because Tc was only mildly elevated in that condition. During fixed intensity work, HR was negatively correlated with absolute VO2max in dry and humid heat stress, indicating that those with a higher fitness level will be less vulnerable to losses in physical work capacity independant of the climate type (Havenith et al., 1995a).
Using ROC curve analysis, it was shown that V̇O2max thresholds of ≤36.5 and 30 ml⋅kg–1⋅min–1 be used to identify vulnerable males and females, respectively (Flouris et al., 2018b). Although the fitness requirement for females may be lower, this is explained by the fact they were exercising at a lower absolute heat production. In summary, the impact of fitness on the Tc response is determined by work type (fixed or relative workload) and whether the environment is compensable or uncompensable (see Clarification of terms for definition).
An important consideration with respect to fitness level is the effect this has on heat tolerance since this has implications primarily for work capacity. We define heat tolerance as the maximum exposure duration to hot working conditions, which is dictated by global cardiovascular stress and the Tc rise (Cheung and McLellan, 1998). Compared with unfit adults, fitter individuals (V̇O2max 62 vs. 40 ml/kg/min) show a lower HR (∼30 b/min) and perceived exertion while cycling at a fixed heat production (Cramer et al., 2012). In the event workers can self-pace, fitter people have an increased physical work capacity for the same HR (Periard et al., 2012). In support, one study found that the ΔTc was not different between fit and unfit males during uncompensable heat stress, but the fitter individuals had a longer tolerance time, probably explained by their lower HR (Cheung and McLellan, 1998). Another factor relevant to occupational heat stress is that fitter people may have a lower psychological stress for the same rate of heat storage (Tikuisis et al., 2002). A higher fitness level also infers a greater resistance to lipopolysaccharide leakage from the gut (Selkirk et al., 2008), greater cellular tolerance to hyperthermia (Yamada et al., 2008), and a curtailed release of stress hormones (norepinephrine, ACTH) at a given ΔTc (Wright et al., 2010).
Aerobic fitness and endurance training can have a strong impact on heat tolerance and is an important factor for determining work capacity and health during hot work. While research points toward improved sweating function with high fitness, the major benefits stem from an increased cardiovascular function. During fixed intensity work, fitness is associated with a lower rate of heat storage and improved cardiovascular stability. During relative intensity (self-paced) work, fitter people can work harder for an equivalent HR and perceived effort compared with unfit people. Working at a relative intensity (i.e., based on fitness) may elevate the risk of hyperthermia in fitter people if evaporation is impeded by high humidity or protective clothing. The heat stress responses in fit vs. unfit people are shown in Table 5.
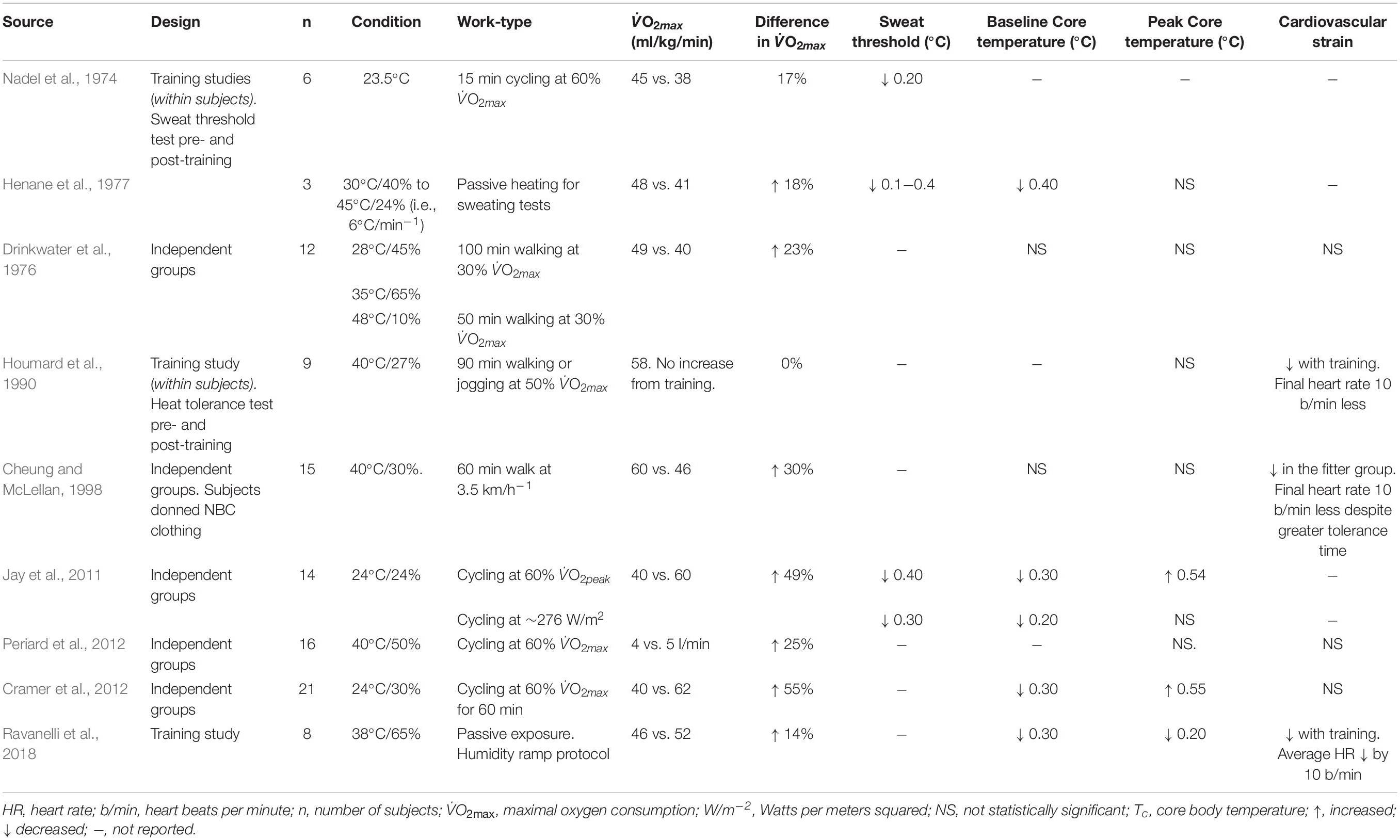
Table 5. Overview of data relating to the effect of aerobic fitness and training on the heat stress response.
• For fixed paced physical work in the heat, fitter people will typically be at reduced risk of hyperthermia and productivity loss.
• The benefits of fitness on Tc will be minimized, and even reversed, during uncompensable heat stress i.e., with heavy protective clothing, or in very humid environments (∼ 4 kPa). Even in those conditions, cardiovascular stability, and overall tolerance is likely to be improved with high fitness.
• For self-paced physical work in the heat, fitter people will typically have higher work output, but this could lead to a higher Tc if workers are unacclimatized or inexperienced. Monitoring of all workers is recommended regardless of fitness level.
• During heavy work, those with a V̇O2max (ml/kg/min) < 36.5 are more at risk of a higher Tc than those above this threshold.
• During moderate work, those with a V̇O2max (ml/kg/min) < 30 are more at risk of a higher Tc than those above this threshold.
When body tissues are repeatedly exposed to a higher temperature than normal, they adapt to that stress so they can better cope with the physiological demand during future exposures. Adaptation to heat is a reversible phenomenon which begins at a genetic level, manifests to a cellular level, and eventually results in whole body physiological adaptations. Knowledge pertaining to the cardiovascular (Taylor, 2014; Périard et al., 2016), epigenetic (Horowitz, 2014, 2016), and performance (Périard et al., 2015; Tyler et al., 2016) adaptations to heat acclimation have been reported in considerable depth. The reader is also directed to an article describing the early use and development of natural (acclimatization) and artificial (acclimation) adaptation in the mining industry (Schneider, 2016), since it has specific occupational relevance. In this section, we provide a summary of the most relevant information which can inform guidance.
Scientific appreciation of man’s adaptability to heat can be traced back to 1768. Observing the adaptability of European’s to hot climates, James Lind remarks on behavioral adaptations such as reduced appetite, and changing exposure time by seeking “repose” during the heat of the day (Lind, 1768). However, it was not until the early 20th century where the study of man’s physiological response to heat adaptation emerged. The following studies form most of the fundamental knowledge in this area (Shaklee, 1917; Dill et al., 1938; Henschel et al., 1943; Robinson et al., 1943, 1965; Eichna et al., 1945; Ladell, 1951; Wyndham, 1951; Hellon et al., 1956a; Wyndham and Jacobs, 1957; Piwonka et al., 1965; Piwonka and Robinson, 1967). Shaklee (1917) published a report on the adaptability of monkeys to heat exposure, and postulated that “If the monkey can become adapted to life in the tropical sun, man could more readily become adapted.” He found that the rectal temperature of monkeys exposed to heat was ≥40°C (and sometimes fatal) for the first 2 weeks but was always <40°C for the next 5 months of heat exposure, indicating that most adaptation occurs in the first 2 weeks. He went on to study his own adaptation to heat over a period of 6 months and concluded anecdotally that “Healthy white men may be more readily acclimatized to the conditions named, that is, to the tropical climate at its worst.” On that note, potential adaptability to heat does not depend on ethnic origin (Taylor, 2014). In the 1930’s, evidence of decreased sweat-induced ion loss throughout the course of heat exposures was one of the first seminal findings (Dill et al., 1933, 1938). Increased ion reabsorption from sweat glands results in more dilute sweat (Chinevere et al., 2008), which reduces the risk of health issues linked to electrolyte depletion. In the 1940’s, evidence of decreased physiological throughout the course of heat adaptation began to emerge, which is linked to increased sweat rates, and decreased HR and Tc (Henschel et al., 1943; Robinson et al., 1943; Horvath and Shelley, 1946). That work used fixed work rates throughout the daily exposures, and generally found that the work was less taxing on the thermoregulatory and cardiovascular system as acquired heat tolerance developed. Henschel et al. (1943) also found the decay of acquired heat adaptation was ∼3 weeks, a notion supported by modern-day literature. In the 1950’s, more precise data on the adaptation of the sweat rate/Tc relation, as well as the cardiovascular adaptations, such as skin and central blood flow, and cardiac output, which ultimately leads to a reduced HR, began to emerge (Ladell, 1951; Wyndham, 1951; Wyndham et al., 1954b; Wyndham and Jacobs, 1957). After the 1960’s, the individual variation in human adaptability to heat was explored. Generally, the scope for heat adaptation does not seem to depend on chronological age (Robinson et al., 1965; Wagner et al., 1972), sex (Hertig et al., 1963; Frye et al., 1982), or physical fitness (Piwonka and Robinson, 1967; Cheung and McLellan, 1998). This concept is highly relevant to occupational heat exposure because most types of people can physiologically adapt to work in the heat.
The extent to which an individual has become heat adapted can be determined through changes in specific physiological, behavioral, and biochemical characteristics. Périard et al. (2015) summarized twenty-five physiological adaptations which occur throughout heat acclimation and the time course for their attainment. Familiar indices include a lower exercising HR, Tc and Tsk at rest and during exercise, an earlier sweating onset and an increased sweat rate for a given Tc (Havenith, 2001b). A plasma volume expansion is a major adaptation which typically peaks after the first week of acclimation (Périard et al., 2016). This adaptation improves cardiovascular stability by increasing vascular filling pressure (Senay et al., 1976) and the specific heat content of blood (Blake et al., 2000). These physiological adaptations allow for improved work performance and comfort during heat stress (Cheung and McLellan, 1998; Lorenzo et al., 2010; Burk et al., 2012; Willmott et al., 2016; James et al., 2017). In support, an early study showed that the risk of syncope during physical work in the heat was due to excessive global cardiovascular strain, but the risk declined throughout the course of acclimation (Eichna et al., 1945). Modeling the sweating adaptation, Havenith (2001b) calculated that acclimation has beneficial effects in terms of (i) reducing sweating onset threshold, and (ii) it can increase the maximum sweat output for the same Tc (see Table 6). Recent work also shows a redistribution of sweat rate toward the limbs, compared to the torso and back with heat acclimation (Smith and Havenith, 2019). Importantly, in an environment which impedes sweat evaporation (i.e., with NBC clothing), heat acclimatized people will typically lose more sweat for the same rate of heat storage, accelerating dehydration (Wyndham et al., 1954a; Cheung and McLellan, 1998).
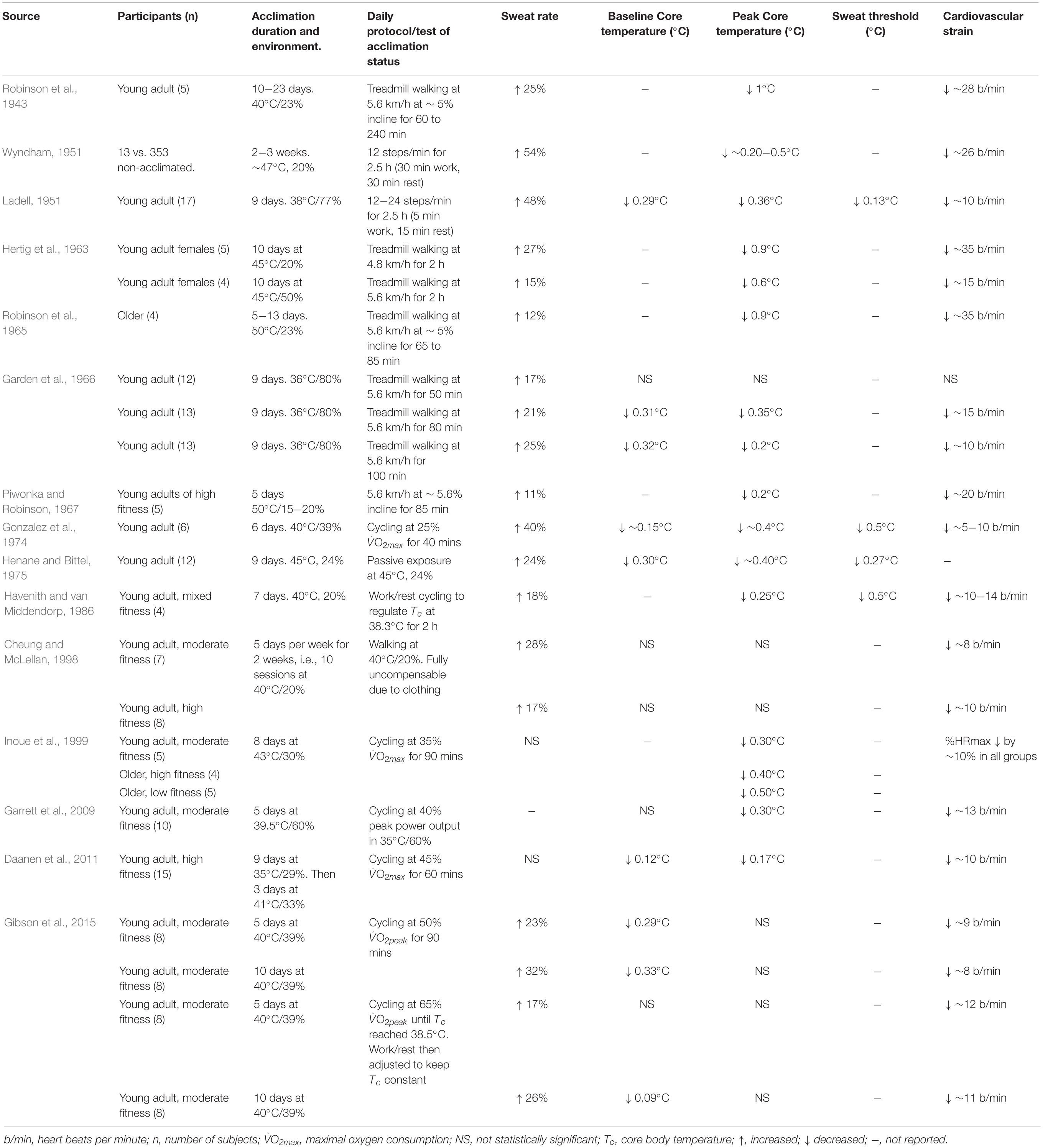
Table 6. Overview of data relating to the effect of acclimation on the heat stress response. Participants are young adult males, unless stated otherwise.
Heat acclimation programs are normally prescribed as short (<7 days), medium (8−12 days) or long (>14 days) durations (Garrett et al., 2011). Generally, the reductions in baseline and exercising Tc, Tsk, and HR occur after only 4−6 days, while a full adaptation of the sweating response requires ∼12−14 days (Périard et al., 2015). Consequently, the ergogenic effects are maximized in line with improvements in the sweating function, owing to greater skin wetness, and an elevated Emax (Fox et al., 1964). The time-course for the decay of heat acclimation has been addressed in several reviews (Armstrong and Maresh, 1991; Pandolf, 1998; Garrett et al., 2011; Daanen et al., 2018). After a heat acclimation, studies suggest that the adaptations to HR and Tc are lost at a rate of ∼2.5% per day of absence from the heat (Daanen et al., 2018). The general conclusion is that humans return to a pre-acclimation phenotype within 3-weeks of absence from the heat, characterized by a return of sweating responses back to baseline levels (Armstrong and Maresh, 1991; Poirier et al., 2015). Following short-term (5-day) heat acclimation, adaptations to exercising HR and Tc were maintained after 1 week but lost after 2 weeks (Garrett et al., 2009). Isothermic protocols ensure the participant’s Tc is consistent throughout the program, which is preferable to a constant daily work rate. Full adaptation may take place a while after the heat acclimation program itself, as shown by lower resting Tc by ∼0.5°C, 6 days after a nine-day program (Daanen et al., 2011). As observed in the German coal mines, new workers should be paired with a more experienced worker during the initial days of exposure to learn optimal pacing and drinking behaviors (Kalkowsky and Kampmann, 2006). Early work demonstrates a memory feature with heat adaptation, since pre-acclimatized workers took only two days to return to an acclimatized phenotype after a 6-day period of working in cool conditions (Wyndham and Jacobs, 1957). Furthermore, Weller et al. (2007) showed that only 2 and 4 days of heat acclimation was required for re-acclimation following 12 and 26 days of non-exposure to heat stress, respectively. A recent systematic review and meta-analysis showed that heat-reacclimation occurs 8−12 times faster than the process of heat acclimation decay (Daanen et al., 2018). In practical terms, this means that heat acclimation can be maintained relatively simply in workers who have previously undergone a recent procedure of heat acclimation.
Adaptation to heat can have a strong impact on the heat stress response, inferring a physiological advantage when sweat evaporation is possible. Strong evidence supports that short-term heat acclimation (<7 days) is beneficial for those required to work in heat stress conditions, reflected by a lower Tc and HR compared with pre-acclimation. Long-term heat acclimation (>14 days) provides further benefit due to adaptation of the sweating mechanism and acquired cellular tolerance to hyperthermia. Evidence of heat acclimation memory suggests that a rapid re-acclimation is likely in individuals previously exposed to long-term heat acclimation. The heat stress responses in non-acclimated and acclimated individuals are shown in Table 6.
Advice for natural adaptation:
• Natural acclimatization to hot work will typically occur over 14−30 days.
• Unacclimatized workers should be considered more at risk and be monitored frequently during acclimatization.
• New workers will typically benefit from working with someone more experienced during acclimatization.
• New workers should try to adopt similar fluid replacement behaviors and pacing strategies to more experienced workers.
Advice for laboratory/artificial adaptation:
• Acclimation should be 5-days minimum, with full adaptation taking place over 14-days.
• Adaptations to heat will be lost after ∼3 weeks no heat exposure, but re-acclimatization will typically only take 3−4 days i.e., workers do not have to go through the full 14-day process twice.
General considerations:
• The positive effect of acclimation/acclimatization will be lower if sweat evaporation is impeded with impermeable protective clothing, or in very humid environments.
• Acclimation/acclimatization increases sweat output, so may result in greater body fluid losses, especially in severe environments, such as those noted above.
Aging is accompanied by several physiological changes which are relevant to human heat stress vulnerability. In general, the changes are maladaptive for thermoregulation, and include a hampered cardiovascular function (Minson et al., 1998; Betik and Hepple, 2008), sweat gland output (Hellon and Lind, 1956; Anderson and Kenney, 1987; Kenney and Fowler, 1988) and reduced thermal perceptual sensitivity (Dufour and Candas, 2007; Inoue et al., 2016; Coull et al., 2017). While there is overwhelming evidence that pre-frail and frail elderly people account for most of the mortality/morbidity statistics during heat waves (Kenney et al., 2014), the relative impact of age on heat stress responses to physical work needs a general clarification, especially as the effective retirement age in most countries has been rising since 2000. Compared with young adults, aging has been shown to reduce heat loss capacity as early as age 40, primarily through a reduction in whole-body sweat losses for certain work-loads (Larose et al., 2013). These findings help explain the documented link between age and heat stroke risk in Bantu miners (Strydom, 1971). Analysis from that work showed those over the age of 40 accounted for over 50% of fatal heat stroke and 25% of non-fatal heat stroke cases, despite accounting for less than 10% of the total working population (Strydom, 1971).
Seminal work in the 1960’s showed that sustained increases in skin temperature can result in a doubling of cardiac output and resting HR (Rowell et al., 1969). Responses like this, which are accompanied by large reductions in peripheral resistance, cause a re-distribution of blood flow from the core to the cutaneous vascular beds for heat dissipation (Rowell et al., 1969). Since that work, numerous studies compared responses of young and older adults to heat stress. During passive, uncompensable heat stress, elderly people (aged ≥ 65 years) showed a 33% reduction in stroke volume and cardiac output, accounting for 53% reduction in total SkBF compared with young adults (Minson et al., 1998). That healthy aging reduces stroke volume is not a consistent finding however (Gagnon et al., 2016), and the explanation for the disparity between studies remains unclear. With advancing age, many studies have shown that vasoreactivity is reduced during heat stress (Holowatz et al., 2003; Stanhewicz et al., 2015, 2017). Nitric oxide is an important vasodilatory molecule but its concentration within the endothelium is reduced in older people, an effect which contributes to decreased SkBF (Holowatz et al., 2006). While folic acid supplementation may potentially rescue some of the age-related declines in vasoreactivity (Stanhewicz et al., 2017), 6-weeks supplementation had no impact on SkBF or Tc during whole body heat stress in older adults (Gagnon et al., 2018). The above data pertains to elderly individuals, and as such cardiovascular perturbations are likely to be present but less severe in healthy workers <60 years of age.
An early study compared heat stress responses in young (19−31) and older (39−45) miners who performed stepping exercise over 4 h (Hellon et al., 1956b). The authors found similar Tc responses the first 3-h, after which the Tc was only 0.3°C higher in the older group. There were greater levels of cardiovascular strain in the older adults, marked by a 10 b⋅min–1 higher HR during work from 40 min to the end of the trial. Consequently, the older adults worked closer to their age-predicted maximum HR by ∼14%.
In the last decade, numerous studies have compared heat stress responses in well-matched participants of different age groups (Anderson and Kenney, 1987; Kenney, 1988; Kenney and Anderson, 1988; Tankersley et al., 1991; Larose et al., 2013, 2014; Stapleton et al., 2015; Kenny et al., 2017). In dry heat, older people store more heat due to reductions in evaporative heat loss, but the difference is proportional to the metabolic heat load. At heat loads > 325 W, sweat evaporation was reduced by ∼14% in those aged 58 compared with well-matched young adults in a hot dry environment (Stapleton et al., 2015). This resulted in greater levels of whole-body heat storage, and an increased Tc. During cycling at 400 W, age-related decrements in sweat loss occurred as early as age 40 compared with young adults (Larose et al., 2013). In summary, there is an independent negative impact of age on sweat evaporation and heat storage during exercise in fully compensable environments.
High humidity decreases the proportion of sweat that evaporates into the environment to provide a cooling effect (Candas et al., 1979). Intuitively, there is less likely to be a different thermoregulatory response to humid heat between age groups, because young people cannot take advantage of their higher sweat rates. Indeed, at low to moderate intensity exercise, the difference in Tc between young and older groups is minimal in high humidity (Havenith et al., 1995b; Kenney, 1988; Kenney and Anderson, 1988; Tankersley et al., 1991; Larose et al., 2014), peaking at around ∼0.4°C after 1.5−2 h work (Kenney, 1988). However, studies do find blunted cardiovascular effector responses in older people during humid heat, such as reduced SkBF and cardiac output (Kenney, 1988; Tankersley et al., 1991; Havenith et al., 1995b). The maintenance of SkBF is particularly relevant for dry heat transfer, especially if ambient temperature < skin temperature.
Compared with study designs that match young and old for all relevant characteristics, using unmatched participants better reflects the differences between age groups on a population level.
Havenith et al. (1995b) documented the relative importance of age compared with V̇O2max, anthropometry, and adiposity on thermoregulatory and cardiovascular responses during cycling in humid heat, at equal absolute work rate. They showed that V̇O2max, body mass and body surface area predicted the Tc and whole body sweat losses during exercise, but age did not. However, age was a strong predictor of the cardiovascular responses to humid heat, particularly SkBF which was lower with age, despite similar levels of cardiovascular strain (%HRmax). While fitness (V̇O2max in L/min) was the primary indicator of HR, aging further reduced working HR in a non-linear fashion (Havenith et al., 1995b), likely due reduced beta-adrenergic responsiveness, calcium handling, and myocyte counts (Olivetti et al., 1991). The aging mediated reduction in HR and SkBF is due to structural and functional changes in the heart and cutaneous vasculature. Notable cardiac changes include reduced beta-adrenergic responsiveness, calcium handling, and myocyte counts (Olivetti et al., 1991). Age-related changes in vasodilatory function were described above. In the study of Havenith et al. (1995b), older people were working at a similar percentage of maximum HR compared with young adults of the same fitness level. Regardless of age, the fitness level was the main determinant of cardiac strain, in terms of both absolute HR and %HR maximum.
Several studies have used an exercise intensity relative to fitness (i.e., %VO2max) to compare thermometric and cardiovascular responses between unmatched young and older adults. Due to reduced fitness level, metabolic rate is normally lower in the older groups, meaning they produce less metabolic heat. Despite this reduced heat production, the negative aging effect on thermoregulatory function can result in similar rates of Tc rise in young and older people, despite the lower heat production in older individuals (Tankersley et al., 1991; Inbar et al., 2004). Consequently, working at a relative intensity yields a similar percentage of maximum HR across age groups, indicating that self-pacing can result in equivalent thermoregulatory and cardiovascular loads between age groups (Tankersley et al., 1991). In terms of protection from hyperthermia, the effectiveness of self-pacing will depend on the heat severity of the climate. Self-pacing may be less effective in uncompensable (high humidity) conditions, since required sweat evaporation for heat balance will typically be high regardless of any reduction of metabolic heat load (Sagawa et al., 1988; Dufour and Candas, 2007; Kenny et al., 2017). In less extreme, compensable conditions, self-pacing seems to be a good measure to prevent hyperthermia in older workers (Kalkowsky and Kampmann, 2006).
Tolerance times to heat are closely related to fitness (Cheung and McLellan, 1998), which is of relevance in this section because (i) young participants are generally more fit than aged participants (Betik and Hepple, 2008), and (ii) exercise training will infer greater tolerance to heat in an older population (Ho et al., 1997). On a population level, older people appear to be less vulnerable to heat if they work at an intensity relative to their fitness. At a fixed exercise intensity, older people are more vulnerable to heat on a population level. Using ROC curve analysis, Flouris et al. (2018b) show that age is a predictor of the heat stress response to fixed pace exercise. For heavy work in males, they suggest those over the age of 52 years are more likely to have a higher Tc than those below this age. For females performing moderate intensity work, they suggest a threshold of 56 years.
Age can have a strong impact on the heat stress response on a population level, but this is primarily linked to reduced cardiovascular fitness. In matched groups, age has a moderate impact on the heat stress response. While exercise training in older people helps maintain cardiovascular responses to heat, reductions in sweat output are apparent at moderate to high heat loads. In a compensable environment, older people are expected to show lower levels of sweat evaporation, and thus a higher level of heat gain at moderate to heavy workloads. In an uncompensable environment, younger people cannot take advantage of a greater delivery of heat to the skin surface for cooling and may store heat at the same rate as older people. In general, absolute V̇O2max peaks at ∼20−29 years and declines as a function of age thereafter. Because tolerance to heat is largely dependent on the aerobic fitness level, one can expect a reduced performance in the older workforce. If older people can self-pace, the risk does not appear to be significant. Comparative heat stress responses between young and older people are shown in Table 7.
• For fixed paced physical work in the heat, older people are more vulnerable to hyperthermia and reduced physical work capacity.
• If self-pacing is allowed, there should be no greater risk in older people compared with young people, if the workload is not heavy (see Table 1), and if the environmental heat is not extreme.
• Those over the age of 50 should be monitored closely upon initial exposure to heat stress. Those under this age are typically at less risk of heat injury.
There are several factors relevant to thermoregulation that may differ between males and females. Studies on large subject numbers show that compared with males, females on average have a lower body mass and lower cardiorespiratory fitness (Kaminsky et al., 2015). In prior sections, these factors were shown to have a strong influence on heat stress vulnerability. Sex differences in thermoregulation have been reviewed previously (Burse, 1979; Havenith, 1985; Kenney, 1985; Kaciuba-Uscilko and Grucza, 2001), but there have been significant advances in this subfield in the last two decades, which are well summarized in a more recent review (Gagnon and Kenny, 2012a). The general conclusions from prior work are that males and females are mostly equal in thermoregulatory control if fitness and body composition are equal, despite small differences in sweat rates. This section will provide an update on the current state of knowledge regarding sex differences in heat stress responses.
Early studies document the comparative responses of unmatched men and women to various types of heat exposures, representing population averages. The earliest comparisons were made in the 1940’s, documenting differential heat stress responses between men and women at rest (Hardy and Du Bois, 1940). Further comparisons demonstrate a delayed sweating onset and a reduction in maximum sweat rates in females (Bittel and Henane, 1975). During physical work, the impact of sex on the heat stress response seems to depend on the environment, fitness, and status of heat adaptation. During physical work in the heat, women initially suffered from greater Tc’s and HR, but the differences subsided following a period of acclimation (Wyndham et al., 1965). In that study, the lighter mass for the women likely contributed to the faster rate of heat gain initially, while their greater sweating with heat adaptation later compensated (Havenith, 2001a). Using multiple regression, Havenith showed that gender was a predictor of the Tc response in dry and humid heat, but lost its predictive power when V̇O2max and body characteristics were added into the model (Havenith and van Middendorp, 1990). Therefore, the effect of sex as an independent variable is minimal in comparison to fitness and body characteristics. In unmatched participants, a heavy work rate caused a faster increase in Tc and HR in females compared with males (Gagnon et al., 2009). In that study, the increased heat vulnerability of the females is explained primarily by their lower body mass, but their lower fitness may have also contributed. On a population level, sex impacts heat vulnerability, owing primarily to the differences in fitness and body characteristics (Havenith and van Middendorp, 1990; Gagnon et al., 2009).
Evidence shows a reduced sweat output in females compared with males. Women have been shown to exert sweat rates as low as 30% to that of males, with the differences increasing as a function of the heat stress severity (Hardy and Du Bois, 1940; Wyndham et al., 1965; Hertig, 1971; Gagnon and Kenny, 2012b; Notley et al., 2017). Importantly, large differences in sweat output can also be due to women working at a lower rate of heat production during relative intensity work, due to their lower fitness level (Smith and Havenith, 2012). The importance of a lower sweat rate depends on the environmental humidity. In a dry environment, women typically show higher rates of Tc rise than men due to their reduced sweat evaporation (Shapiro et al., 1980; Frye and Kamon, 1981). However, greater sweat rates in males can cause a higher Tc and HR in uncompensable environments, due to faster rates of dehydration (Avellini et al., 1980; Shapiro et al., 1980; Havenith, 1985; Kenney, 1985). Importantly, the differences in sweat output between males and females are abolished after heat acclimation (Frye and Kamon, 1981).
Compared with females of equal fitness, males showed a greater sweat rate and a lower Tc rise during treadmill walking in extreme dry heat at the same relative intensity (Frye and Kamon, 1981). The females also showed a greater HR in that study by 10−15 b⋅min–1, implying reduced physical work capacity during self-paced work. It is important to note that the sexes were only matched for fitness, not size, such that the males had a greater body mass and surface area, which are protective against rises in Tc and HR (Havenith and van Middendorp, 1990; Havenith et al., 1995a). However, the differences were abolished once both groups were acclimated. Cycling at a heat production of 500 W in dry heat, females matched for body characteristics and fitness had a lower sweat output for a given change in body temperature (Gagnon and Kenny, 2011). Here, the males activated heat loss responses (evaporation and cutaneous blood flow) at a lower body temperature compared with females, resulting in a lower end-exercise Tc. The mechanism behind these responses are not fully elucidated, but recent evidence suggests that this may be due to differences in maximal sweat gland output (Gagnon et al., 2013a). Females seem to have a lower maximum sweat gland output compared with males, which means they compensate by activating a greater quantity of sweat glands. When the activated number of sweat glands reaches its maximum (i.e., a mean body temperature increase of ∼1°C), the higher maximal sweat gland output in males elevates sweat rate for the same mean body temperature (Gagnon and Kenny, 2012b; Gagnon et al., 2013a). An independent effect of sex on the heat stress response may only appear at heat loads > 250 W⋅m–2 (Gagnon and Kenny, 2012b).
There are detectable differences in Tc throughout the menstrual cycle, specifically between the pre-and post-ovulation phases. Since the thermogenic hormone progesterone is released subsequent to ovulation, there is typically an increase in resting baseline Tc of ∼0.5°C (Kenshalo, 1966). Many studies were conducted in the late 1960’s to determine the impact of the menstruation on the heat stress response. Early work was equivocal, either finding a lower Tc “set-point” for the onset of sweating pre-ovulation (Haslag and Hertzman, 1965; Bittel and Henane, 1975; Stephenson and Kolka, 1985), or finding no meaningful differences in the Tc and sweat relation (Senay, 1973; Wells and Horvath, 1973). Based on a recent analysis, the weight of the evidence suggests that menstrual phase does alter the Tc onset thresholds for sweating and vasodilation, with delays up to 0.5°C in the luteal phase compared with the follicular phase (Charkoudian et al., 2014). Of note, mild rises in Tc have been shown in the luteal phase with combined use of oral contraceptives (Lei et al., 2019). However, measurement of onset thresholds need to be conducted under well-controlled conditions, since their effects are quite small and can be negated by other factors, such as time of day (Stephenson and Kolka, 1985). Also, onset thresholds for vasodilation and sweating are typically conducted in one limb and might well be compensated for in other body areas. Therefore, it is important to consider whole body heat stress responses to physical activity in the heat to determine the true relevance of the menstrual cycle. When the female response to 2-h extreme dry heat was compared at the three menstrual phases, there were no significant differences in Tc, skin temperature, or body heat content (Wells and Horvath, 1973). It was also shown that differences between pre-and post-ovulation are not affected by heat acclimation. During exercise at a fixed rate of heat production, there were no differences in Tc or Tsk when women exercised at the follicular or luteal phase of menstruation. Moreover, there were no differences in biophysical parameters such as Emax, required evaporation, and whole-body heat storage (Notley et al., 2019a). In summary, menstrual phase has a marginal impact on the heat stress response and is unlikely to dictate independently whether an individual is vulnerable to heat stress.
Sex has a moderate impact on the heat stress response which becomes minor if body characteristics and fitness factors are accounted for. The impact of sex on hyperthermia and work capacity are mostly relevant during heavy work in compensable environments. Compared with men of equal fitness and body composition, women may have a higher HR and reduced capacity for sweat evaporation at heavy workloads, but the differences disappear if both groups are heat acclimated. If the heat load is strong enough, this can result in a greater rate of heat storage and thus an elevated Tc in unacclimated females. Menstruation appears to affect resting Tc but does not influence the rate of heat storage or the threshold for sweating onset. The effect of sex on thermoregulatory responses to heat are shown in Table 8.
• On a population scale, males are more suited to hot work compared with females. However, the minimum criteria for hot work should be initially based on fitness and age, not sex.
• Females will typically be more at risk of hyperthermia if the heat load is high (see Table 1).
• Males are likely to dehydrate faster in uncompensable heat stress, due to higher sweat output.
• Once fully acclimatized to the heat, the heat stress response between matched males and females is similar.
Chronic health conditions have an important impact on the human heat stress response. Addressing all health conditions is beyond the scope of this review because our findings are applied to those who perform physical work in the heat. We primarily focus on diabetics due to its current and future global prevalence (Roglic, 2016), and available research investigating its impact on whole body heat stress responses. We discuss hypertension and cardiovascular diseases more briefly because the proportion of the population with clinically relevant hypertension or heart disease performing physical work in the heat is likely to be small, and applies mostly to the elderly population (Kenney et al., 2014). Moreover, research investigating whole body physiological responses to heat in those with these conditions is sparse, and in some cases absent entirely. Nonetheless, understanding the role of cardiovascular disease in vulnerability to heat is relevant for scenarios in which people with these underlying conditions still perform work in the heat.
The World Health Organization estimates that 422 million adults have diabetes, the majority of which have type 2 diabetes. The global prevalence of diabetes has nearly doubled since 1980, rising from 4.7 to 8.5% of the adult population (Roglic, 2016), and prevalence rates may reach an astonishing 33% by 2050 (Boyle et al., 2010). With diabetes being the most prevalent morbidity present in the population, it is pertinent to address whether thermoregulatory function is impaired in these individuals. We should note that more in-depth reviews are available specific to diabetes and thermoregulation, for the interested reader (Yardley et al., 2013; Kenny et al., 2016). Our aim in this section is to give a concise summary of the primary information of relevance for employers and policymakers.
Thermal physiologists have taken interest in diabetics because local SkBF and sweating are negatively correlated with the level of glucose control (Wigington et al., 2004; Petrofsky et al., 2009; Brugler et al., 2011). At a normal Tc of ∼37°C, there is very little difference in SkBF between diabetic and non-diabetic participants, but diabetics have shown up to a 50% reduction in local SkBF stimulated by heat or vasodilatory compounds (Rendell et al., 1989; Brugler et al., 2011; Fujii et al., 2018). The link between diabetes and a reduced SkBF is coined diabetic cutaneous microangiopathy and can affect both type 1 (T1DM) and type 2 (T2DM) diabetes mellitus sufferers. In T1DM, there is no release of C-peptide which is produced in pancreatic β cells, and the peptide has known roles in maintaining microvascular blood flow (Forst et al., 1998; Forst and Kunt, 2004). In T2DM, the reductions in SkBF may be due to a reduced nitric oxide bioavailability (Williams et al., 1996; Beer et al., 2008), which is worsened by the presence of atherosclerotic plaques (Watson et al., 2003; Kawashima and Yokoyama, 2004). There may be additional factors at play, and the interested reader is directed to two reviews for further reading (Ngo et al., 2005; Kenny et al., 2016).
The above evidence is based on local SkBF measurements with laser Doppler, but until recently it was unknown whether they translate into meaningful whole-body responses. The evidence thus far is equivocal and seems to depend on the severity of the condition and physical fitness of the individual. For instance, young-adult recreationally active T1DM sufferers were compared against well-matched healthy controls during 1-h cycling exercise at 400 W metabolic heat production, and the thermoregulatory responses were similar between both groups (Stapleton et al., 2013). When the heat load was increased to 500 W, T1DM sufferers exhibited lower sweat rates in the forearm and chest, which in turn led to an increased Tc by up to 0.5°C (Carter et al., 2014). The findings were repeated in a later study which showed impaired thermoregulation during exercise in T1DM patients (McGinn et al., 2015). Most recently, a study demonstrated reduced evaporative heat loss and higher Tc in young adults with T1DM but only during heavy work (Notley et al., 2019c). For T2DM sufferers working at a high metabolic rate for 1-h in mild heat, an increased heat storage rate was documented due to a lower rate of evaporative heat exchange, compared with health matched controls (Kenny et al., 2013). Overall, it seems that recreationally active diabetes sufferers can show impairments in heat loss if the workload is heavy. Whether or not prolonged work at a lower rate of heat production is dangerous for diabetics has not been investigated.
Cardiovascular disease is a broad term that can encompass several conditions such as chronic heart failure, coronary and valvular heart disease, cardiomyopathy, congenital heart defects, and cerebrovascular and peripheral vascular disease (Kenny et al., 2010). The majority of deaths during heat waves are attributed to cardiovascular issues, and are predominant in the elderly population (Conti et al., 2005; Kenney et al., 2014). During heat stress, the elevated risk of death in those with cardiovascular disease has been described previously (Kenney et al., 2014). Although risk of death is higher in those with cardiovascular disease, its impact on hyperthermia risk and HR during work in the heat is less clear due to the paucity of evidence. Patients with congestive heart failure had similar Tc and HR responses to passive heat stress compared with healthy controls, despite lower SkBF (Cui et al., 2005). In that study, those in the congestive heart failure group did not discontinue any medication, which we consider a strong reflection of real-world responses. In a later study, it was shown that patients with chronic heart failure showed no difference in Tc and HR, with no reduction in sweat rate, again despite a lower SkBF (Cui et al., 2013). That work used a water perfused suit model which limits most excreted sweat from evaporating, making comparisons in Tc problematic. During 3-h exposure to mild heat stress in a climatic chamber, those with ischemic heart disease experienced similar thermoregulatory responses to healthy controls (Andersen et al., 1976). During cycling exercise in mild heat, heart failure resulted in a faster Tc rise compared with control participants, either at relative (Balmain et al., 2016) or fixed intensity work (Balmain et al., 2018a). The faster increase in Tc was primarily due to a reduced SkBF downstream of attenuated cardiac function, and folic acid supplementation (which enhances nitric oxide bioavailability) was shown not to enhance this response in a later study, despite increase vascular function in general (Balmain et al., 2018b). Finally, the thermoregulatory responses to physical activity in the heat was not affected by previous coronary artery bypass surgery, compared with healthy controls (Walsh et al., 2002). Overall, cardiovascular disease is not a strong independent predictor of the heat stress response in the working population. However, individuals with cardiovascular disease are likely to present with very low fitness levels, which is a major risk factor for hot work.
Hypertension is a long-term medical condition characterized by a permanent elevation of peripheral resistance. Hypertension decreases resting SkBF and produces a shallower slope between SkbF and increased Tc (Kenney et al., 1984; Carberry et al., 1992). As noted in the clarification of terms section, such changes in SkBF do not necessarily translate into greater heat vulnerability during physical work. When well matched normotensive and hypertensive participants are compared during physical work in the heat, their Tc and HR responses are similar (Kenney and Kamon, 1984; Fonseca et al., 2015). As noted by Kenny et al. (2010), the use of antihypertensive medications (diuretics, vasodilators, β-blockers) may independently increase susceptibility to heat stress issues. Similarly to cardiovascular disease, hypertension has a marginal independent effect on the heat stress response. However, individuals with hypertension are likely to present with lower fitness levels, which is a major risk factor for hot work.
Diabetes can have a moderate impact on heat stress responses, depending on the heat load and the disease severity. There is strong evidence that SkBF can be reduced in T1DM and T2DM induced by local heating or vasodilator compounds. During whole-body heat stress, impairments in heat loss have been shown in unfit T2DM sufferers and recreationally active T1DM patients if the level of heat production is high (i.e., 500 W). The independent effect of cardiovascular disease and hypertension is marginal during whole body heat stress, but secondary risk factors (i.e., very low fitness levels) are likely to be present in these populations.
• Based on the literature available, people with diabetes may be at risk during hot work.
• People with diabetes who have good glucose control should not be considered more at risk if they meet fitness standards and can acclimatize to the work.
• Cardiovascular disease and hypertension do not increase vulnerability to heat per se, but secondary risk factors such as very low fitness level increase susceptibility to heat stress.
The relative influence of each physical characteristic on the Tc response and physical work capacity during heat stress is displayed in Figure 1. The Tc figure is most relevant from a health and safety perspective, as the International Standard states that average Tc should not exceed 38°C during a typical working day for a group of workers. The key factors for Tc control during heat stress are acclimation status, body mass, and fitness. The work capacity figures focus on how each factor influences work output in the heat, based on the assumption that work output is regulated by the HR, as has indeed been shown in the field (Mairiaux and Malchaire, 1985; Brake and Bates, 2002; Miller and Bates, 2007; Miller et al., 2011).
The present review has determined which individual characteristics are most relevant during physical work in the heat, in the context of health and work capacity. It should be noted that several other factors can independently influence thermophysiological responses to heat, such as dehydration (Cheuvront and Kenefick, 2014), some medications (Martin-Latry et al., 2007), and psychological stress (Boulant, 2010). The present review is written with the assumption that workers are hydrated, not on prescription medication, and are free of severe psychological distress. There is an urgent need to explore the interaction between multiple factors, and the time dependency for certain factors to take effect.
JF drafted versions of the manuscript with input and revisions from GH, SH, and AL. All authors contributed to the article and approved the submitted version.
This literature review forms part of the HEAT-SHIELD project, receiving funding from the European Union’s Horizon 2020 Research and Innovation Program under the grant agreement no. 668786.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AD:mass, skin surface area to mass ratio; BMI, body mass index; Emax, maximum evaporative potential; Ereq, required evaporation for heat balance; HSP72, heat shock protein 72; ISO, International Standardization Organization; kPa, water vapor pressure; NBC clothing, nuclear, biological, and chemical protective clothing; ROC, receiver operator characteristic; SkBF, skin blood flow; T1DM and T2DM, type 1 or 2 diabetes mellitus; Tc, core temperature; V̇O2max, maximal oxygen consumption; W, watts; WBGT, wet-bulb globe temperature; WHO, World Health Organization.
Adams, J. D., Ganio, M. S., Burchfield, J. M., Matthews, A. C., Werner, R. N., Chokbengboun, A. J., et al. (2015). Effects of obesity on body temperature in otherwise-healthy females when controlling hydration and heat production during exercise in the heat. Eur. J. Appl. Physiol. 115, 167–176. doi: 10.1007/s00421-014-3002-y
Allen, J. A. (1907). The influence of physical conditions in the genesis of species. Sci. Am. 63, 26247–26248. doi: 10.1038/scientificamerican05251907-26247supp
American College of Sports Medicine [ACSM] (2013). ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins.
Andersen, I., Jensen, P. L., Junker, P., Thomsen, A., and Wyon, D. P. (1976). The effects of moderate heat stress on patients with ischemic heart disease. Scand. J. Work. Environ. Heal. 2, 256–272. doi: 10.5271/sjweh.2804
Andersen, K. L. Masironi, R., Rutenfranz, J., and Seliger, V. (1978). Habitual Physical Activity and Health. (Copenhagen: WHO), 188.
Anderson, R. K., and Kenney, W. L. (1987). Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. J. Appl. Physiol. 63, 1089–1094. doi: 10.1152/jappl.1987.63.3.1089
Armstrong, L. E., and Maresh, C. M. (1991). The induction and decay of heat acclimatisation in trained athletes. Sports Med. 12, 302–312. doi: 10.2165/00007256-199112050-00003
Avellini, B. A., Kamon, E., and Krajewski, J. T. (1980). Physiological responses of physically fit men and women to acclimation to humid heat. J. Appl. Physiol. 49, 254–261. doi: 10.1152/jappl.1980.49.2.254
Balmain, B. N., Jay, O., Morris, N. R., Shiino, K., Stewart, G. M., Jayasinghe, R., et al. (2018a). Thermoeffector responses at a fixed rate of heat production in heart failure patients. Med. Sci. Sports Exerc. 50, 417–426. doi: 10.1249/MSS.0000000000001455
Balmain, B. N., Jay, O., Morris, N. R., Stewart, G. M., Shiino, K., McFarland, A. J., et al. (2018b). Folic acid supplementation improves vascular endothelial function, yet not skin blood flow during exercise in the heat, in patients with heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R810–R819. doi: 10.1152/ajpregu.00132.2018
Balmain, B. N., Jay, O., Sabapathy, S., Royston, D., Stewart, G. M., Jayasinghe, R., et al. (2016). Altered thermoregulatory responses in heart failure patients exercising in the heat. Physiol. Rep. 4:e13022. doi: 10.14814/phy2.13022
Bar-Or, O., Lundegren, H. M., and Buskirk, E. R. (1969). Heat tolerance of exercising obese and lean women. J. Appl. Physiol. 26, 403–409. doi: 10.1152/jappl.1969.26.4.403
Beaudin, A. E., Clegg, M. E., Walsh, M. L., and White, M. D. (2009). Adaptation of exercise ventilation during an actively-induced hyperthermia following passive heat acclimation. AJP Regul. Integr. Comp. Physiol. 297, R605–R614. doi: 10.1152/ajpregu.90672.2008
Bedno, S. A., Li, Y., Han, W., Cowan, D. N., Scott, C. T., Cavicchia, M. A., et al. (2010). Exertional heat illness among overweight U.S. Army recruits in basic training. Aviat. Space Environ. Med. 81, 107–111. doi: 10.3357/asem.2623.2010
Beer, S., Feihl, F., Ruiz, J., Juhan-Vague, I., Aillaud, M.-F., Wetzel, S. G., et al. (2008). Comparison of skin microvascular reactivity with hemostatic markers of endothelial dysfunction and damage in type 2 diabetes. Vasc. Health Risk Manag. 4, 1449–1458. doi: 10.2147/vhrm.s4175
Bergmann, C. (1847). Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttingen: Vandenhoeck und Ruprecht.
Betik, A. C., and Hepple, R. T. (2008). Determinants of VO2max decline with aging: an integrated perspective. Appl. Physiol. Nutr. Metab. 33, 130–140. doi: 10.1139/H07-174
Bittel, J., and Henane, R. (1975). Comparison of thermal exchanges in men and women under neutral and hot conditions. J. Physiol. 250, 475–489. doi: 10.1113/jphysiol.1975.sp011066
Blake, A. S., Petley, G. W., and Deakin, C. D. (2000). Effects of changes in packed cell volume on the specific heat capacity of blood: implications for studies measuring heat exchange in extracorporeal circuits. Br. J. Anaesth. 84, 28–32. doi: 10.1093/oxfordjournals.bja.a013376
Borg, G. A. (1982). Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381.
Boulant, J. A. (2010). Hypothalamic neurons regulating body temperature. Compr. Physiol 105–126. doi: 10.1002/cphy.cp040106
Boyle, J. P., Thompson, T. J., Gregg, E. W., Barker, L. E., and Williamson, D. F. (2010). Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 8:29.
Brake, D. J., and Bates, G. P. (2002). Deep body core temperatures in industrial workers under thermal stress. J. Occup. Environ. Med. 44, 125–135. doi: 10.1097/00043764-200202000-00007
Bröde, P., Fiala, D., Lemke, B., and Kjellstrom, T. (2018). Estimated work ability in warm outdoor environments depends on the chosen heat stress assessment metric. Int. J. Biometeorol. 62, 331–345. doi: 10.1007/s00484-017-1346-9
Brugler, A., Thompson, S., Turner, S., Ngo, B., and Rendell, M. (2011). Skin blood flow abnormalities in diabetic dermopathy. J. Am. Acad. Dermatol. 65, 559–563. doi: 10.1016/j.jaad.2010.06.010
Burk, A., Timpmann, S., Kreegipuu, K., Tamm, M., Unt, E., and Ööpik, V. (2012). Effects of heat acclimation on endurance capacity and prolactin response to exercise in the heat. Eur. J. Appl. Physiol. 112, 4091–4101. doi: 10.1007/s00421-012-2371-3
Burse, R. L. (1979). Sex Differences in human thermoregulatory response to heat and cold stress. Hum. Factors 21, 687–699. doi: 10.1177/001872087912210606
Candas, V., Libert, J. P., and Vogt, J. J. (1979). Human skin wettedness and evaporative efficiency of sweating. J. Appl. Physiol. 46, 522–528. doi: 10.1152/jappl.1979.46.3.522
Carberry, P. A., Shepherd, A. M. M., and Johnson, J. M. (1992). Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension 20, 349–355. doi: 10.1161/01.HYP.20.3.349
Carter, M. R., Mcginn, R., Barrera-Ramirez, J., Sigal, R. J., and Kenny, G. P. (2014). Impairments in local heat loss in type 1 diabetes during exercise in the heat. Med. Sci. Sports Exerc. 46, 2224–2233. doi: 10.1249/MSS.0000000000000350
Charkoudian, N. (2003). Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin. Proc. 78, 603–612. doi: 10.4065/78.5.603
Charkoudian, N., Stachenfeld, N. S., Charkoudian, N., and Stachenfeld, N. S. (2014). Reproductive hormone influences on thermoregulation in women. Compr. Physiol. 4, 793–804. doi: 10.1002/cphy.c130029
Cheung, S. S., and McLellan, T. M. (1998). Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J. Appl. Physiol. 84, 1731–1739. doi: 10.1152/jappl.1998.84.5.1731
Cheung, S. S., McLellan, T. M., and Tenaglia, S. (2000). The thermophysiology of uncompensable heat stress. Physiological manipulations and individual characteristics. Sports Med. 29, 329–359. doi: 10.2165/00007256-200029050-00004
Cheuvront, S. N., and Kenefick, R. W. (2014). Dehydration: physiology, assessment, and performance effects. Compr. Physiol. 4, 257–285. doi: 10.1002/cphy.c130017
Chin, L. L., Mackinnon, L. T., Lim, C. L., and Mackinnon, L. T. (2006). The roles of exercise-induced immune system disturbances in the pathology of heat stroke: the dual pathway model of heat stroke. Sports Med. 36, 39–64. doi: 10.2165/00007256-200636010-00004
Chinevere, T. D., Kenefick, R. W., Cheuvront, S. N., Lukaski, H. C., and Sawka, M. N. (2008). Effect of heat acclimation on sweat minerals. Med. Sci. Sports Exerc. 40, 886–891. doi: 10.1249/MSS.0b013e3181641c04
Conti, S., Meli, P., Minelli, G., Solimini, R., Toccaceli, V., Vichi, M., et al. (2005). Epidemiologic study of mortality during the Summer 2003 heat wave in Italy. Environ. Res. 98, 390–399. doi: 10.1016/j.envres.2004.10.009
Convertino, V. A. (1991). Blood volume: its adaptation to endurance training. Med. Sci. Sports Exerc. 23, 1338–1348.
Coso, J. D., Hamouti, N., Ortega, J. F., Fernandez-Elias, V., and Mora-Rodriguez, R. (2011). Relevance of individual characteristics for thermoregulation during exercise in a hot-dry environment. Eur. J. Appl. Physiol. 111, 2173–2181. doi: 10.1007/s00421-011-1847-x
Coull, N., West, A., Wheeler, P., Hodder, S. G., and Havenith, G. (2017). “Regional sweat distribution in young and older individuals,” in Proceedings of the 17th International Conference on Environmental Ergonomics (ICEE2017), Kobe, 156.
Cramer, M. N., Bain, A. R., and Jay, O. (2012). Local sweating on the forehead, but not forearm, is influenced by aerobic fitness independently of heat balance requirements during exercise. Exp. Physiol. 97, 572–582. doi: 10.1113/expphysiol.2011.061374
Cramer, M. N., and Jay, O. (2014). Selecting the correct exercise intensity for unbiased comparisons of thermoregulatory responses between groups of different mass and surface area. J. Appl. Physiol. 116, 1123–1132. doi: 10.1152/japplphysiol.01312.2013
Cramer, M. N., and Jay, O. (2015). Explained variance in the thermoregulatory responses to exercise: the independent roles of biophysical and fitness/fatness-related factors. J. Appl. Physiol. 119, 982–989. doi: 10.1152/japplphysiol.00281.2015
Cramer, M. N., and Jay, O. (2019). Partitional calorimetry. J. Appl. Physiol. 126, 267–277. doi: 10.1152/japplphysiol.00191.2018
Cui, J., Arbab-Zadeh, A., Prasad, A., Durand, S., Levine, B. D., and Crandall, C. G. (2005). Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation 112, 2286–2292. doi: 10.1161/CIRCULATIONAHA.105.540773
Cui, J., Boehmer, J. P., Blaha, C., Lucking, R., Kunselman, A. R., and Sinoway, L. I. (2013). Chronic heart failure does not attenuate the total activity of sympathetic outflow to skin during whole-body heating. Circ. Heart Fail. 6, 271–278. doi: 10.1161/CIRCHEARTFAILURE.112.000135
Daanen, H. A. M., Jonkman, A. G., Layden, J. D., Linnane, D. M., and Weller, A. S. (2011). Optimising the acquisition and retention of heat acclimation. Int. J. Sports Med. 32, 822–828. doi: 10.1055/s-0031-1279767
Daanen, H. A. M., Racinais, S., and Périard, J. D. (2018). Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 48, 409–430. doi: 10.1007/s40279-017-0808-x
Dennis, S. C., and Noakes, T. D. (1999). Advantages of a smaller bodymass in humans when distance-running in warm, humid conditions. Eur. J. Appl. Physiol. Occup. Physiol. 79, 280–284. doi: 10.1007/s004210050507
Dervis, S., Coombs, G. B., Chaseling, G. K., Filingeri, D., Smoljanic, J., and Jay, O. (2016). A comparison of thermoregulatory responses to exercise between mass-matched groups with large differences in body fat. J. Appl. Physiol. 120, 615–623. doi: 10.1152/japplphysiol.00906.2015
Dill, D. B., Hall, F. G., and Edwards, H. T. (1938). Changes in composition of sweat during acclimatiztion to heat. Am. J. Physiol. 123, 412–419. doi: 10.1152/ajplegacy.1938.123.2.412
Dill, D. B., Jones, B. F., Edwards, H. T., and Oberg, S. A. (1933). Salt economy in extreme dry heat. J. Biol. Chem. 100, 755–767.
Drinkwater, B., and Horvath, S. (1979). Heat tolerance and aging. Med. Sci. Sports 11, 49–55. doi: 10.1080/03610739408253977
Drinkwater, B. L., Bedi, J. F., and Loucks, A. B. (1982). Sweating sensitivity and capacity of women in relation to age. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 53, 671–676.
Drinkwater, B. L., Denton, J. E., Kupprat, I. C., Talag, T. S., and Horvath, S. M. (1976). Aerobic power as a factor in women’s response to work in hot environments. J. Appl. Physiol. 41, 815–821. doi: 10.1152/jappl.1976.41.6.815
Dufour, A., and Candas, V. (2007). Ageing and thermal responses during passive heat exposure: sweating and sensory aspects. Eur. J. Appl. Physiol. 100, 19–26. doi: 10.1007/s00421-007-0396-9
Eichna, L. W., Bean, W. B., Ashe, W. F., and Nelson, N. (1945). Performance in relation to Environmental Temperature, Reactions of Normal Young Men to Hot, Humid (Simulated Jungle) Environment. Bull. Johns Hopkins Hosp. 76, 25–58.
Ekblom, B., Astrand, P. O., Saltin, B., Stenberg, J., and Wallström, B. (1968). Effect of training on circulatory response to exercise. J. Appl. Physiol. 24, 518–528. doi: 10.1152/jappl.1968.24.4.518
Flouris, A. D., Dinas, P. C., Ioannou, L. G., Nybo, L., Havenith, G., Kenny, G. P., et al. (2018a). Workers’ health and productivity under occupational heat strain: a systematic review and meta-analysis. Lancet Planet. Health 2, e521–e531. doi: 10.1016/S2542-5196(18)30237-7
Flouris, A. D., McGinn, R., Poirier, M. P., Louie, J. C., Ioannou, L. G., Tsoutsoubi, L., et al. (2018b). Screening criteria for increased susceptibility to heat stress during work or leisure in hot environments in healthy individuals aged 31-70 years. Temperature 5, 86–99. doi: 10.1080/23328940.2017.1381800
Fonseca, S. F., Teles, M. C., Ribeiro, V. G. C., Magalhães, F. C., Mendonça, V. A., Peixoto, M. F. D., et al. (2015). Hypertension is associated with greater heat exchange during exercise recovery in a hot environment. Braz. J. Med. Biol. Res. 48, 1122–1129. doi: 10.1590/1414-431X20154532
Fontana, L., Eagon, J. C., Trujillo, M. E., Scherer, P. E., and Klein, S. (2007). Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes Metab. Res. Rev. 56, 1010–1013. doi: 10.2337/db06-1656
Forst, T., and Kunt, T. (2004). Effects of C-peptide on Microvascular Blood Flow and Blood Hemorheology. Exp. Diabesity Res. 5, 51–64. doi: 10.1080/15438600490424532
Forst, T., Kunt, T., Pohlmann, T., Goitom, K., Engelbach, M., Beyer, J., et al. (1998). Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J. Clin. Invest. 101, 2036–2041. doi: 10.1172/JCI2147
Foster, F., and Collard, M. (2013). A reassessment of Bergmann’s rule in modern humans. PLoS One 8:e72269. doi: 10.1371/journal.pone.0072269
Fox, R. H., Goldsmith, R., Hampton, I. F. G., and Lewis, H. E. (1964). The nature of the increase in sweating capacity produced by heat acclimatization. J. Physiol. 171, 368–376. doi: 10.1113/jphysiol.1964.sp007382
Frye, A. J., and Kamon, E. (1981). Responses to dry heat of men and women with similar aerobic capacities. J. Appl. Physiol. 50, 65–70. doi: 10.1152/jappl.1981.50.1.65
Frye, A. J., Kamon, E., and Webb, M. (1982). Responses of menstrual women, amenorrheal women, and men to exercise in a hot, dry environment. Eur. J. Appl. Physiol. Occup. Physiol. 48, 279–288. doi: 10.1007/bf00422988
Fujii, N., Meade, R. D., McNeely, B. D., Nishiyasu, T., Sigal, R. J., and Kenny, G. P. (2018). Type 2 diabetes specifically attenuates purinergic skin vasodilatation without affecting muscarinic and nicotinic skin vasodilatation and sweating. Exp. Physiol. 103, 212–221. doi: 10.1113/EP086694
Gagnon, D., Crandall, C. G., and Kenny, G. P. (2013a). Sex differences in postsynaptic sweating and cutaneous vasodilation. J. Appl. Physiol. 114, 394–401. doi: 10.1152/japplphysiol.00877.2012
Gagnon, D., Dorman, L. E., Jay, O., Hardcastle, S., and Kenny, G. P. (2009). Core temperature differences between males and females during intermittent exercise: physical considerations. Eur. J. Appl. Physiol. 105, 453–461. doi: 10.1007/s00421-008-0923-3
Gagnon, D., Jay, O., and Kenny, G. P. (2013b). The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J. Physiol. 591, 2925–2935. doi: 10.1113/jphysiol.2012.248823
Gagnon, D., and Kenny, G. P. (2011). Sex modulates whole-body sudomotor thermosensitivity during exercise. J. Physiol. 589, 6205–6217. doi: 10.1113/jphysiol.2011.219220
Gagnon, D., and Kenny, G. P. (2012a). Does sex have an independent effect on thermoeffector responses during exercise in the heat? J. Physiol. 590, 5963–5973. doi: 10.1113/jphysiol.2012.240739
Gagnon, D., and Kenny, G. P. (2012b). Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J. Appl. Physiol. 113, 746–757. doi: 10.1152/japplphysiol.00637.2012
Gagnon, D., Romero, S. A., Cramer, M. N., Kouda, K., Poh, P. Y. S., Ngo, H., et al. (2018). Folic acid supplementation does not attenuate thermoregulatory or cardiovascular strain of older adults exposed to extreme heat and humidity. Exp. Physiol. 103, 1123–1131. doi: 10.1113/EP087049
Gagnon, D., Romero, S. A., Ngo, H., Sarma, S., Cornwell, W. K., Poh, P. Y. S., et al. (2016). Healthy aging does not compromise the augmentation of cardiac function during heat stress. J. Appl. Physiol. 121, 885–892. doi: 10.1152/japplphysiol.00643.2016
Garden, J. W., Wilson, I. D., and Rasch, P. J. (1966). Acclimatization of healthy young adult males to a hot-wet environment. J. Appl. Physiol. 21, 665–669. doi: 10.1152/jappl.1966.21.2.665
Garrett, A. T., Goosens, N. G., Rehrer, N. G., Patterson, M. J., Cotter, J. D., and Cotter, J. D. (2009). Induction and decay of short-term heat acclimation. Eur. J. Appl. Physiol. 107, 659–670. doi: 10.1007/s00421-009-1182-7
Garrett, A. T., Rehrer, N. J., and Patterson, M. J. (2011). Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med. 41, 757–771. doi: 10.2165/11587320-000000000-00000
Gibson, O. R., Mee, J. A., Tuttle, J. A., Taylor, L., Watt, P. W., and Maxwell, N. S. (2015). Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J. Therm. Biol. 49–50, 55–65. doi: 10.1016/j.jtherbio.2015.02.005
Gonzalez, R. R., Pandolf, K. B., and Gagge, A. P. (1974). Heat acclimation and decline in sweating during humidity transients. J. Appl. Physiol. 36, 419–425.
Hardy, J. D., and Du Bois, E. F. (1940). Differences between men and women in their response to heat and cold. Proc. Natl. Acad. Sci. U.S.A. 26, 389–398.
Haslag, S., and Hertzman, A. (1965). Temperature regulation in young women. J. Appl. Physiol. 20, 1283–1288. doi: 10.1152/jappl.1965.20.6.1283
Havenith, G. (1985). Individual Differences in Thermoregulatory Control, a Review. Soesterberg: TNO Institute for Perception. doi: 10.13140/RG.2.1.2477.1448
Havenith, G. (2001a). Human surface to mass ratio and body core temperature in exercise heat stress?a concept revisited. J. Therm. Biol. 26, 387–393. doi: 10.1016/S0306-4565(01)00049-3
Havenith, G. (2001b). Individualized model of human thermoregulation for the simulation of heat stress response Individualized model of human thermoregulation for the simulation of heat stress response. J. Appl. Physiol. 90, 1943–1954. doi: 10.1152/jappl.2001.90.5.1943
Havenith, G., and van Middendorp, H. (1986). Determination of the Individual State of Acclimatization. Soesterberg, NL: TNO Institute for Perception, 24.
Havenith, G., Coenen, J. M. L., and Kistemaker, J. (1995a). Climate and Work Load Both Interact with Individual Characteristics in Determining the Human Heat Stress Response. TNO- Report TNO-TM 1995 B-14. Soesterberg: TNO Human Factors Research Institute.
Havenith, G., Coenen, J. M. L., Kistemaker, L., and Kenney, W. L. (1998). Relevance of individual characteristics for human heat stress response is dependent on exercise intensity and climate type. Eur. J. Appl. Physiol. 77, 231–241. doi: 10.1007/s004210050327
Havenith, G., Holmér, I., den Hartog, E. A., and Parsons, K. C. (1999). Clothing evaporative heat resistance—proposal for improved representation in standards and models. Ann. Occup. Hyg. 43, 339–346. doi: 10.1093/annhyg/43.5.339
Havenith, G., Inoue, Y., Luttikholt, V., and Kenney, W. L. (1995b). Age predicts cardiovascular, but not thermoregulatory, responses to humid heat stress. Eur. J. Appl. Physiol. Occup. Physiol. 70, 88–96. doi: 10.1007/bf00601814
Havenith, G., and van Middendorp, H. (1990). The relative influence of physical fitness, acclimatization state, anthropometric measures and gender on individual reactions to heat stress. Eur. J. Appl. Physiol. Occup. Physiol. 61, 419–427. doi: 10.1007/BF00236062
Haymes, E. M., Buskirk, E. R., Hodgson, J. L., Lundegren, H. M., and Nicholas, W. C. (1974). Heat tolerance of exercising lean and heavy prepubertal girls. J. Appl. Physiol. 36, 566–571. doi: 10.1152/jappl.1974.36.5.566
Haymes, E. M., McCormick, R. J., and Buskirk, E. R. (1975). Heat tolerance of exercising lean and obese prepubertal boys. J. Appl. Physiol. 39, 457–461. doi: 10.1152/jappl.1975.39.3.457
Henane, R., and Bittel, J. (1975). Changes of thermal balance induced by passive heating in resting man. J. Appl. Physiol. 38, 294–299.
Hellon, R. F., Jones, R. M., Macpherson, R. K., and Weiner, J. S. (1956a). Natural and artificial acclimatization to hot environments. J. Physiol. 132, 559–576. doi: 10.1113/jphysiol.1956.sp005549
Hellon, R. F., and Lind, A. R. (1956). Observations on the activity of sweat glands with special reference to the influence of ageing. J. Physiol. 133, 132–144. doi: 10.1113/jphysiol.1956.sp005571
Hellon, R. F., Lind, A. R., and Weiner, J. S. (1956b). The physiological reactions of men of two age groups to a hot environment. J. Physiol. 133, 118–131. doi: 10.1113/jphysiol.1956.sp005570
Hellsten, Y., and Nyberg, M. (2016). Cardiovascular adaptations to exercise training. Compr. Physiol. 6, 1–32. doi: 10.1002/cphy.c140080
Henane, R., Flandrois, R., and Charbonnier, J. P. (1977). Increase in sweating sensitivity by endurance conditioning in man. J. Appl. Physiol. 43, 822–828. doi: 10.1152/jappl.1977.43.5.822
Henschel, A., Taylor, H. L., and Keys, A. (1943). The persistence of heat acclimatization in man. Am. J. Physiol. 140, 321–325. doi: 10.1152/ajplegacy.1943.140.3.321
Hertig, B. A. (1971). Human physiological responses to heat stress: males and females compared. J. Physiol. 63, 270–273.
Hertig, B. A., Belding, H. S., Kraning, K. K., Batterton, D. L., Smith, C. R., and Sargent, F. (1963). Artificial acclimatization of women to heat. J. Appl. Physiol. 18, 383–386. doi: 10.1152/jappl.1963.18.2.383
Ho, C. W., Beard, J. L., Farrell, P. A., Minson, C. T., and Kenney, W. L. (1997). Age, fitness, and regional blood flow during exercise in the heat. J. Appl. Physiol. 82, 1126–1135. doi: 10.1152/jappl.1997.82.4.1126
Holmér, I., Nilsson, H., Havenith, G., and Parsons, K. (1999). Clothing convective heat exchange—proposal for improved prediction in standards and models. Ann. Occup. Hyg. 43, 329–337. doi: 10.1093/annhyg/43.5.329
Holowatz, L. A., Houghton, B. L., Wong, B. J., Wilkins, B. W., Harding, A. W., Kenney, W. L., et al. (2003). Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am. J. Physiol. Heart Circ. Physiol. 284, H1662–H1667. doi: 10.1152/ajpheart.00871.2002
Holowatz, L. A., and Kenney, W. L. (2010). Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J. Appl. Physiol. 109, 1538–1544. doi: 10.1152/japplphysiol.00338.2010
Holowatz, L. A., Thompson, C. S., and Kenney, W. L. (2006). l -Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J. Physiol. 574, 573–581. doi: 10.1113/jphysiol.2006.108993
Horowitz, M. (2014). Heat acclimation, epigenetics, and cytoprotection memory. Compr. Physiol 199–230. doi: 10.1002/cphy.c130025
Horowitz, M. (2016). Epigenetics and cytoprotection with heat acclimation. J. Appl. Physiol. 120, 702–710. doi: 10.1152/japplphysiol.00552.2015
Horvath, S. M., and Shelley, W. B. (1946). Acclimatization to extreme heat and its effect on the ability to work in less severe environments. Am. J. Physiol. 146, 336–343. doi: 10.1152/ajplegacy.1946.146.3.336
Houmard, J. A., Costill, D. L., Davis, J. A., Mitchell, J. B., Pascoe, D. D., and Robergs, R. A. (1990). The influence of exercise intensity on heat acclimation in trained subjects. Med. Sci. Sports Exerc. 22, 615–620.
Hsiang, S., Kopp, R., Jina, A., Rising, J., Delgado, M., Mohan, S., et al. (2017). Estimating economic damage from climate change in the United States. Science 356, 1362–1369. doi: 10.1126/science.aal4369
Hübler, M., Klepper, G., and Peterson, S. (2008). Costs of climate change: the effects of rising temperatures on health and productivity in Germany. Ecol. Econ. 68, 381–393. doi: 10.1016/j.ecolecon.2008.04.010
Inbar, O., Morris, N., Epstein, Y., and Gass, G. (2004). Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males. Exp. Physiol. 89, 691–700. doi: 10.1113/expphysiol.2004.027979
Inoue, Y., Gerrett, N., Ichinose-Kuwahara, T., Umino, Y., Kiuchi, S., Amano, T., et al. (2016). Sex differences in age-related changes on peripheral warm and cold innocuous thermal sensitivity. Physiol. Behav. 164, 86–92. doi: 10.1016/j.physbeh.2016.05.045
Inoue, Y., Havenith, G., Kenney, W. L., Loomis, J. L., and Buskirk, E. R. (1999). Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int. J. Biometeorol. 42, 210–216.
International Organisation for Standardisation (ISO) (2004). Ergonomics of the Thermal Environment: Determination of Metabolic Rate (Standard No. 8996:2004). Available online at: https://www.iso.org/standard/34251.html#:~:text=The%20metabolic%20rate%2C%20as%20a,a%20numerical%20index%20of%20activity.&text=ISO%208996%3A2004%20specifies%20different,of%20the%20climatic%20working%20environment
James, C. A., Richardson, A. J., Watt, P. W., Willmott, A. G. B., Gibson, O. R., and Maxwell, N. S. (2017). Short-term heat acclimation improves the determinants of endurance performance and 5-km running performance in the heat. Appl. Physiol. Nutr. Metab. 42, 285–294. doi: 10.1139/apnm-2016-0349
Jay, O., Bain, A. R., Deren, T. M., Sacheli, M., and Cramer, M. N. (2011). Large differences in peak oxygen uptake do not independently alter changes in core temperature and sweating during exercise. AJP Regul. Integr. Comp. Physiol. 301, R832–R841. doi: 10.1152/ajpregu.00257.2011
Jay, O., Gariépy, L. M., Reardon, F. D., Webb, P., Ducharme, M. B., Ramsay, T., et al. (2007). A three-compartment thermometry model for the improved estimation of changes in body heat content. Am. J. Physiol. Integr. Comp. Physiol. 292, R167–R175. doi: 10.1152/ajpregu.00338.2006
Jay, O., Hoelzl, R., Weets, J., Morris, N., English, T., Nybo, L., et al. (2019). Fanning as an alternative to air conditioning – A sustainable solution for reducing indoor occupational heat stress. Energy Build. 193, 92–98. doi: 10.1016/j.enbuild.2019.03.037
Kaciuba-Uscilko, H., and Grucza, R. (2001). Gender differences in thermoregulation. Curr. Opin. Clin. Nutr. Metab. Care 4, 533–536. doi: 10.1097/00075197-200111000-00012
Kalkowsky, B., and Kampmann, B. (2006). Physiological strain of miners at hot working places in German coal mines. Ind. Health 44, 465–473. doi: 10.2486/indhealth.44.465
Kaminsky, L. A., Arena, R., and Myers, J. (2015). Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing. Mayo Clin. Proc. 90, 1515–1523. doi: 10.1016/j.mayocp.2015.07.026
Katzmarzyk, P. T., and Leonard, W. R. (1998). Climatic influences on human body size and proportions: ecological adaptations and secular trends. Am. J. Phys. Anthropol. 106, 483–503. doi: 10.1002/(sici)1096-8644(199808)106:4<483::aid-ajpa4>3.0.co;2-k
Kawashima, S., and Yokoyama, M. (2004). Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 998–1005. doi: 10.1161/01.ATV.0000125114.88079.96
Kenney, W. L. (1985). A review of comparative responses of men and women to heat stress. Environ. Res. 37, 1–11. doi: 10.1016/0013-9351(85)90044-1
Kenney, W. L. (1988). Control of heat-induced cutaneous vasodilatation in relation to age. Eur. J. Appl. Physiol. Occup. Physiol. 57, 120–125. doi: 10.1007/bf00691250
Kenney, W. L., and Anderson, R. K. (1988). Responses of older and younger women to exercise in dry and humid heat without fluid replacement. Med. Sci. Sports Exerc. 20, 155–160. doi: 10.1249/00005768-198820020-00009
Kenney, W. L., Craighead, D. H., and Alexander, L. M. (2014). Heat waves, aging, and human cardiovascular health. Med. Sci. Sports Exerc. 46, 1891–1899. doi: 10.1249/MSS.0000000000000325
Kenney, W. L., and Fowler, S. R. (1988). Methylcholine-activated eccrine sweat gland density and output as a function of age. J. Appl. Physiol. 65, 1082–1086. doi: 10.1152/jappl.1988.65.3.1082
Kenney, W. L., and Havenith, G. (1993). Heat stress and age: skin blood flow and body temperature. J. Therm. Biol. 18, 341–344. doi: 10.1016/0306-4565(93)90056-Y
Kenney, W. L., and Kamon, E. (1984). Comparative physiological responses of normotensive and essentially hypertensive men to exercise in the heat. Eur. J. Appl. Physiol. Occup. Physiol. 52, 196–201. doi: 10.1007/BF00433392
Kenney, W. L., Kamon, E., and Buskirk, E. R. (1984). Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 56, 930–935. doi: 10.1152/jappl.1984.56.4.930
Kenny, G. P., and Jay, O. (2013). Thermometry, calorimetry, and mean body temperature during heat stress. Compr. Physiol. 3, 1689–1719. doi: 10.1002/cphy.c130011
Kenny, G. P., Larose, J., Wright-Beatty, H. E., Boulay, P., Sigal, R. J., and Flouris, A. D. (2015). Older firefighters are susceptible to age-related impairments in heat dissipation. Med. Sci. Sports Exerc. 47, 1281–1290. doi: 10.1249/MSS.0000000000000537
Kenny, G. P., Poirier, M. P., Metsios, G. S., Boulay, P., Dervis, S., Friesen, B. J., et al. (2017). Hyperthermia and cardiovascular strain during an extreme heat exposure in young versus older adults. Temperature 4, 79–88. doi: 10.1080/23328940.2016.1230171
Kenny, G. P., Sigal, R. J., and McGinn, R. (2016). Body temperature regulation in diabetes. Temperature 3, 119–145. doi: 10.1080/23328940.2015.1131506
Kenny, G. P., Stapleton, J. M., Yardley, J. E., Boulay, P., and Sigal, R. J. (2013). Older adults with type 2 diabetes store more heat during exercise. Med. Sci. Sports Exerc. 45, 1906–1914. doi: 10.1249/MSS.0b013e3182940836
Kenny, G. P., Yardley, J., Brown, C., Sigal, R. J., and Jay, O. (2010). Heat stress in older individuals and patients with common chronic diseases. CMAJ 182, 1053–1060. doi: 10.1503/cmaj.081050
Kenshalo, D. R. (1966). Changes in the cool threshold associated with phases of the menstrual cycle. J. Appl. Physiol. 21, 1031–1039.
Kingwell, B. A., Sherrard, B., Jennings, G. L., and Dart, A. M. (1997). Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am. J. Physiol. 272, 1070–1077. doi: 10.1152/ajpheart.1997.272.3.H1070
Koppe, C., Kovats, S., Jendritzky, G., and Menne, B. (2004). Heat-Waves: Risks and Responses. Copenhagen: World Health Organisation.
Ladell, W. S. S. (1951). Assessment of group acclimatization to heat and humidity. J. Physiol. 115, 296–312.
Larose, J., Boulay, P., Sigal, R. J., Wright, H. E., and Kenny, G. P. (2013). Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS One 8:e83148. doi: 10.1371/journal.pone.0083148
Larose, J., Boulay, P., Wright-Beatty, H. E., Sigal, R. J., Hardcastle, S., and Kenny, G. P. (2014). Age-related differences in heat loss capacity occur under both dry and humid heat stress conditions. J. Appl. Physiol. 117, 69–79. doi: 10.1152/japplphysiol.00123.2014
Lei, T. H., Cotter, J. D., Schlader, Z. J., Stannard, S. R., Perry, B. G., Barnes, M. J., et al. (2019). On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J. Physiol. 597, 71–88. doi: 10.1113/JP276233
Limbaugh, J. D., Wimer, G. S., Long, L. H., and Baird, W. H. (2013). Body fatness, body core temperature, and heat loss during moderate-intensity exercise. Aviat. Space. Environ. Med. 84, 1153–1158.
Lind, A. R. (1963). A physiological criterion for setting thermal environmental limits for everyday work. J. Appl. Physiol. 18, 51–56.
Lind, A. R., Humphreys, P. W., Collins, K. J., Foster, K., and Sweetland, K. F. (1970). Influence of age and daily duration of exposure on responses of men to work in heat. J. Appl. Physiol. 28, 50–56. doi: 10.1152/jappl.1970.28.1.50
Lind, J. (1768). An Essay on Diseases Incidental to Europeans in Hot Climates with the Method of Preventing Their Fatal Consequences. Philadelphia, PA: William Duane.
Lipkin, M., and Hardy, J. D. (1954). Measurement of some thermal properties of human tissues. J. Appl. Physiol. 7, 212–217. doi: 10.1152/jappl.1954.7.2.212
Lorenzo, S., Halliwill, J. R., Sawka, M. N., and Minson, C. T. (2010). Heat acclimation improves exercise performance. J. Appl. Physiol. 109, 1140–1147. doi: 10.1152/japplphysiol.00495.2010
Ludlow, L. W., and Weyand, P. G. (2017). Walking economy is predictably determined by speed, grade, and gravitational load. J. Appl. Physiol. 123, 1288–1302. doi: 10.1152/japplphysiol.00504.2017
Mairiaux, P., and Malchaire, J. (1985). Workers self-pacing in hot conditions: a case study. Appl. Ergon. 16, 85–90. doi: 10.1016/0003-6870(85)90209-1
Marino, F. E., Mbambo, Z., Kortekaas, E., Wilson, G., Lambert, M. I., Noakes, T. D., et al. (2000). Advantages of smaller body mass during distance running in warm, humid environments. Pflugers Arch. 441, 359–367. doi: 10.1007/s004240000432
Martin-Latry, K., Goumy, M.-P., Latry, P., Gabinski, C., Bégaud, B., Faure, I., et al. (2007). Psychotropic drugs use and risk of heat-related hospitalisation. Eur. Psychiatry 22, 335–338. doi: 10.1016/J.EURPSY.2007.03.007
McGinn, R., Carter, M. R., Barrera-Ramirez, J., Sigal, R. J., Flouris, A. D., and Kenny, G. P. (2015). Does type 1 diabetes alter post-exercise thermoregulatory and cardiovascular function in young adults? Scand. J. Med. Sci. Sports 25, e504–e514. doi: 10.1111/sms.12344
McIntosh, R. L., and Anderson, V. (2010). A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys. Rev. Lett. 5, 129–151. doi: 10.1142/S1793048010001184
Miller, V., Bates, G., Schneider, J. D., and Thomsen, J. (2011). Self-pacing as a protective mechanism against the effects of heat stress. Ann. Occup. Hyg. 55, 548–555. doi: 10.1093/annhyg/mer012
Miller, V. S., and Bates, G. P. (2007). The thermal work limit is a simple reliable heat index for the protection of workers in thermally stressful environments. Ann. Occup. Hyg. 51, 553–561. doi: 10.1093/annhyg/mem035
Minson, C. T., Wladkowski, S. L., Cardell, A. F., Pawelczyk, J. A., and Kenney, W. L. (1998). Age alters the cardiovascular response to direct passive heating. J. Appl. Physiol. 84, 1323–1332.
Misra, D. P., and Agarwal, V. (2018). Systematic reviews: challenges for their justification, related comprehensive searches, and implications. J. Korean Med. Sci. 33:92. doi: 10.3346/jkms.2018.33.e92
Mondal, H., and Mishra, S. P. (2017). Effect of BMI, body fat percentage and fat free mass on maximal oxygen consumption in healthy young adults. J. Clin. Diagn. Res. 11, 17–20. doi: 10.7860/JCDR/2017/25465.10039
Morrison, J., Wyndham, C., and Viljoen, J. (1969). The effect on the production of Bantu, engaged on tram-shovelling, of different temperature conditions. J. S. Afr. Inst. Min. Metall. 69, 408–416.
Nadel, E. R., Pandolf, K. B., Roberts, M. F., and Stolwijk, J. A. (1974). Mechanisms of thermal acclimation to exercise and heat. J. Appl. Physiol. 37, 515–520.
Ngo, B. T., Hayes, K. D., DiMiao, D. J., Srinivasan, S. K., Huerter, C. J., and Rendell, M. S. (2005). Manifestations of cutaneous diabetic microangiopathy. Am. J. Clin. Dermatol. 6, 225–237.
Notley, S. R., Dervis, S., Poirier, M. P., and Kenny, G. P. (2019a). Menstrual cycle phase does not modulate whole body heat loss during exercise in hot, dry conditions. J. Appl. Physiol. 126, 286–293. doi: 10.1152/japplphysiol.00735.2018
Notley, S. R., Lamarche, D. T., Meade, R. D., Flouris, A. D., and Kenny, G. P. (2019b). Revisiting the influence of individual factors on heat exchange during exercise in dry heat using direct calorimetry. Exp. Physiol. 104, 1038–1050. doi: 10.1113/EP087666
Notley, S. R., Park, J., Tagami, K., Ohnishi, N., and Taylor, N. A. S. (2017). Variations in body morphology explain sex differences in thermoeffector function during compensable heat stress. Exp. Physiol. 102, 545–562. doi: 10.1113/EP086112
Notley, S. R., Poirier, M. P., Yardley, J. E., Sigal, R. J., and Kenny, G. P. (2019c). Impaired whole-body heat loss in type 1 diabetes during exercise in the heat: a cause for concern? Diabetologia 62, 1087–1089. doi: 10.1007/s00125-019-4858-5
Olivetti, G., Melissari, M., Capasso, J. M., and Anversa, P. (1991). Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 68, 1560–1568.
Pandolf, K. B. (1998). Time course of heat acclimation and its decay. Int. J. Sport. Med. Suppl. 19, S157–S160. doi: 10.1055/s-2007-971985
Pandolf, K. B., Givoni, B., and Goldman, R. F. (1977). Predicting energy expenditure with loads while standing or walking very slowly. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 43, 577–581. doi: 10.1152/jappl.1977.43.4.577
Parsons, K. C. (2010). Human Thermal Environments, 2nd Edn. London: Taylor & Francis. doi: 10.4324/9780203302620_chapter_1
Periard, J. D., Caillaud, C., and Thompson, M. W. (2012). The role of aerobic fitness and exercise intensity on endurance performance in uncompensable heat stress conditions. Eur. J. Appl. Physiol. 112, 1989–1999. doi: 10.1007/s00421-011-2165-z
Périard, J. D., Racinais, S., and Sawka, M. N. (2015). Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 25, 20–38. doi: 10.1111/sms.12408
Périard, J. D., Travers, G. J. S., Racinais, S., and Sawka, M. N. (2016). Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. 196, 52–62. doi: 10.1016/j.autneu.2016.02.002
Petrofsky, J. S., Bains, G. S., Prowse, M., Mc Lellan, K., Ethiraju, G., Lee, S., et al. (2009). The influence of age and diabetes on the skin blood flow response to local pressure. Med. Sci. Monit. 15, CR325–CR331.
Piwonka, R. W., and Robinson, S. (1967). Acclimatization of highly trained men to work in severe heat. J. Appl. Physiol. 22, 9–12. doi: 10.1152/jappl.1967.22.1.9
Piwonka, R. W., Robinson, S., Gay, V. L., and Manalis, R. S. (1965). Preacclimatization of men to heat by training. J. Appl. Physiol. 20, 379–383. doi: 10.1152/jappl.1965.20.3.379
Poirier, M. P., Gagnon, D., Frieson, B. J., Hardcastle, S. G., Kenny, G. P., Friesen, B. J., et al. (2015). Whole-body heat exchange during heat acclimation and its decay. Med. Sci. Sports Exerc. 47, 390–400. doi: 10.1249/MSS.0000000000000401
Potter, A. W., Gonzalez, J. A., and Xu, X. (2015). Ebola response: modeling the risk of heat stress from personal protective clothing. PLoS One 10:e0143461. doi: 10.1371/journal.pone.0143461
Ravanelli, N., Coombs, G. B., Imbeault, P., and Jay, O. (2018). Maximum skin wettedness after aerobic training with and without heat acclimation. Med. Sci. Sports Exerc. 50, 299–307. doi: 10.1249/MSS.0000000000001439
Ravanelli, N., Gagnon, D., Imbeault, P., and Jay, O. (2020). A retrospective analysis to determine if exercise training-induced thermoregulatory adaptations are mediated by increased fitness or heat acclimation. Exp. Physiol. doi: 10.1113/EP088385 [Epub ahead of print].
Rendell, M., Bergman, T., O’Donnell, G., Drobny, E., Borgos, J., and Bonner, R. F. (1989). Microvascular blood flow, volume, and velocity measured by laser Doppler techniques in IDDM. Diabetes 38, 819–824.
Roberts, M. F., Wenger, C. B., Stolwijk, J. A., and Nadel, E. R. (1977). Skin blood flow and sweating changes following exercise training and heat acclimation. J. Appl. Physiol. 43, 133–137.
Robinson, S., Belding, H. S., Consolazio, F. C., Horvath, S. M., and Turrell, E. S. (1965). Acclimatization of older men to work in heat. J. Appl. Physiol. 20, 583–586. doi: 10.1152/jappl.1965.20.4.583
Robinson, S., Turrell, E. S., Belding, H. S., and Horvath, S. M. (1943). Rapid acclimatization to work in hot environments. Am. J. Physiol. 140, 168–176. doi: 10.1152/ajplegacy.1943.140.2.168
Roglic, G. (2016). WHO Global report on diabetes: a summary. Int. J. Noncommun. Dis. 1, 3–8. doi: 10.4103/2468-8827.184853
Romanovsky, A. A. (2018). The thermoregulation system and how it works. Handb. Clin. Neurol. 156, 3–43.
Rowell, L. B. (1974). Human cardiovascular adjustments to exercise and thermal-stress. Physiol. Rev. 54, 75–159.
Rowell, L. B., Brengelmann, G. L., Blackmon, J. R., and Murray, J. A. (1970). Redistribution of blood flow during sustained high skin temperature in resting man. J. Appl. Physiol. 28, 415–420. doi: 10.1152/jappl.1970.28.4.415
Rowell, L. B., Brengelmann, G. L., and Murray, J. A. (1969). Cardiovascular responses to sustained high skin temperature in resting man. J. Appl. Physiol. 27, 673–680.
Sagawa, S., Shiraki, K., Yousef, M. K., and Miki, K. (1988). Sweating and cardiovascular responses of aged men to heat exposure. J. Gerontol. 43, M1–M8. doi: 10.1093/geronj/43.1.M1
Schneider, S. M. (2016). Heat acclimation: gold mines and genes. Temperature 3, 527–538. doi: 10.1080/23328940.2016.1240749
Selkirk, G. A., McLellan, T. M., Wright, H. E., and Rhind, S. G. (2008). Mild endotoxemia, NF- B translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R611–R623. doi: 10.1152/ajpregu.00917.2007
Senay, L. (1973). Body fluid and temperature responses of heat exposed women before and after ovulation with and without rehydration. J. Physiol. 232, 209–219.
Senay, L. C., Mitchell, D., and Wyndham, C. H. (1976). Acclimatization in a hot, humid environment: body fluid adjustments. J. Appl. Physiol. 40, 786–796. doi: 10.1152/jappl.1976.40.5.786
Shapiro, Y., Pandolf, K. B., Avellini, B. A., Pimental, N. A., and Goldman, R. F. (1980). Physiological responses of men and women to humid and dry heat. J. Appl. Physiol. 49, 1–8.
Simmons, G. H., Wong, B. J., Holowatz, L. A., and Kenney, W. L. (2011). Changes in the control of skin blood flow with exercise training: Where do cutaneous vascular adaptations fit in? Exp. Physiol. 96, 822–828. doi: 10.1113/expphysiol.2010.056176
Smith, C. J., and Havenith, G. (2012). Body mapping of sweating patterns in athletes: a sex comparison. Med. Sci. Sports Exerc. 44, 2350–2361. doi: 10.1249/MSS.0b013e318267b0c4
Smith, C. J., and Havenith, G. (2019). Upper body sweat mapping provides evidence of relative sweat redistribution towards the periphery following hot-dry heat acclimation. Temperature 6, 50–65. doi: 10.1080/23328940.2019.1570777
Smoljanić, J., Morris, N. B., Dervis, S., and Jay, O. (2014). Running economy, not aerobic fitness, independently alters thermoregulatory responses during treadmill running. J. Appl. Physiol. 117, 1451–1459. doi: 10.1152/japplphysiol.00665.2014
Stanhewicz, A. E., Alexander, L. M., and Kenney, W. L. (2015). Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin. Sci. 129, 159–167. doi: 10.1042/CS20140821
Stanhewicz, A. E., Greaney, J. L., Alexander, L. M., and Kenney, W. L. (2017). Folic acid supplementation increases cutaneous vasodilator sensitivity to sympathetic nerve activity in older adults. Am. J. Physiol. 312, R681–R688. doi: 10.1152/ajpregu.00493.2016
Stapleton, J. M., Poirier, M. P., Flouris, A. D., Boulay, P., Sigal, R. J., Malcolm, J., et al. (2015). At what level of heat load are age-related impairments in the ability to dissipate heat evident in females? PLoS One 10:e0119079. doi: 10.1371/journal.pone.0119079
Stapleton, J. M., Yardley, J. E., Boulay, P., Sigal, R. J., and Kenny, G. P. (2013). Whole-body heat loss during exercise in the heat is not impaired in type 1 diabetes. Med. Sci. Sports Exerc. 45, 1656–1664. doi: 10.1249/MSS.0b013e31829002f3
Stephenson, L. A., and Kolka, M. A. (1985). Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am. J. Physiol. 249, R186–R191. doi: 10.1152/ajpregu.1985.249.2.R186
Strydom, N. B. (1971). Age as a causal factor in heat stroke. J. South Afr. Inst. Min. Metall. 72, 112–114.
Tankersley, C. G., Smolander, J., Kenney, W. L., and Fortney, S. M. (1991). Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake. J. Appl. Physiol. 71, 236–242.
Tawatsupa, B., Lim, L. L. Y., Kjellstrom, T., Seubsman, S. A., Sleigh, A., Chokhanapitak, J., et al. (2012). Association between occupational heat stress and kidney disease among 37,816 workers in the Thai Cohort Study (TCS). J. Epidemiol. 22, 251–260. doi: 10.2188/jea.JE20110082
Tawatsupa, B., Yiengprugsawan, V., Kjellstrom, T., Berecki-gisolf, J., Seubsman, S., and Sleigh, A. (2013). Association between heat stress and occupational injury among Thai workers: findings of the Thai cohort study. Ind. Health 51, 34–46.
Taylor, N. A. S. (2014). Human heat adaptation. Compr. Physiol. 4, 325–365. doi: 10.1002/cphy.c130022
Thomas, C. M., Pierzga, J. M., and Kenney, W. L. (1999). Aerobic training and cutaneous vasodilation in young and older men. J. Appl. Physiol. 86, 1676–1686. doi: 10.1152/jappl.1999.86.5.1676
Tikuisis, P., McLellan, T. M., and Selkirk, G. (2002). Perceptual versus physiological heat strain during exercise-heat stress. Med. Sci. Sports Exerc. 34, 1454–1461. doi: 10.1249/01.mss.0000027764.43430.fe
Tyler, C. J., Reeve, T., Hodges, G. J., and Cheung, S. S. (2016). The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 46, 1699–1724. doi: 10.1007/s40279-016-0538-5
Vogt, J. J., Libert, J. P., Candas, V., Daull, F., and Mairjaux, P. (1983). Heart rate and spontaneous work-rest cycles during exposure to heat. Ergonomics 26, 1173–1185. doi: 10.1080/00140138308963453
Vroman, N. B., Buskirk, E. R., and Hodgson, J. L. (1983). Cardiac output and skin blood flow in lean and obese individuals during exercise in the heat. J. Appl. Physiol. 55, 69–74.
Wagner, J. A., Robinson, S., Tzankoff, S. P., and Marino, R. P. (1972). Heat tolerance and acclimatization to work in the heat in relation to age. J. Appl. Physiol. 33, 616–622. doi: 10.1152/jappl.1972.33.5.616
Walsh, J., Prpic, R., Goodman, C., Dawson, B., and Hung, J. (2002). Thermoregulatory responses in post-coronary artery bypass surgery and healthy males during moderate exercise in warm and cool environments. J. Cardiopulm. Rehabil. 22, 31–37. doi: 10.1097/00008483-200201000-00004
Watson, K. E., Peters Harmel, A. L., and Matson, G. (2003). Atherosclerosis in type 2 diabetes mellitus: the role of insulin resistance. J. Cardiovasc. Pharmacol. Ther. 8, 253–260. doi: 10.1177/107424840300800402
Weinstein, K. J. (2005). Body proportions in ancient Andeans from high and low altitudes. Am. J. Phys. Anthropol. 128, 569–585. doi: 10.1002/ajpa.20137
Weller, A. S., Linnane, D. M., Jonkman, A. G., and Daanen, H. A. M. (2007). Quantification of the decay and re-induction of heat acclimation in dry-heat following 12 and 26 days without exposure to heat stress. Eur. J. Appl. Physiol. 102, 57–66. doi: 10.1007/s00421-007-0563-z
Wells, L., and Horvath, M. (1973). Heat stress reponses the menstrual cycle related. J. Appl. Physiol. 35, 1–5.
White, M. D., Ross, W. D., and Mekjavić, I. B. (1992). Relationship between physique and rectal temperature cooling rate. Undersea Biomed. Res. 19, 121–130.
Wigington, G., Ngo, B., and Rendell, M. (2004). Skin blood flow in diabetic dermopathy. Arch. Dermatol. 140, 1248–1250. doi: 10.1001/archderm.140.10.1248
Williams, S. B., Cusco, J. A., Roddy, M. A., Johnstone, M. T., and Creager, M. A. (1996). Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 27, 567–574.
Willmott, A. G. B., Gibson, O. R., Hayes, M., and Maxwell, N. S. (2016). The effects of single versus twice daily short term heat acclimation on heat strain and 3000m running performance in hot, humid conditions. J. Therm. Biol. 56, 59–67. doi: 10.1016/j.jtherbio.2016.01.001
Wright, H. E., Selkirk, G. A., and McLellan, T. M. (2010). HPA and SAS responses to increasing core temperature during uncompensable exertional heat stress in trained and untrained males. Eur. J. Appl. Physiol. 108, 987–997. doi: 10.1007/s00421-009-1294-0
Wyndham, C. H. (1951). Effect of acclimatization on circulatory responses to high environmental temperatures. J. Appl. Physiol. 4, 383–395. doi: 10.1152/jappl.1951.4.5.383
Wyndham, C. H. (1969). Adaptation to heat and cold. Environ. Res. 2, 442–469. doi: 10.1016/0013-9351(69)90015-2
Wyndham, C. H. (1973). The effects of heat stress upon human productivity. Arch. Sci. Physiol. 27, 491–497.
Wyndham, C. H., and Heyns, A. J. (1973). The probability of heat stroke developing at different levels of heat stress. Arch. Sci. Physiol. 27, 545–562.
Wyndham, C. H., and Jacobs, G. E. (1957). Loss of acclimatization after six days of work in cool conditions on the surface of a mine. J. Appl. Physiol. 11, 197–198.
Wyndham, C. H., Morrison, J. F., and Williams, C. G. (1965). Heat reactions of male and female Caucasians. J. Appl. Physiol. 20, 357–364.
Wyndham, C. H., Strydom, N. B., Morrison, J. F., du Toit, F. D., and Kraan, J. G. (1954a). Responses of unacclimatized men under stress of heat and work. J. Appl. Physiol. 6, 681–686. doi: 10.1152/jappl.1954.6.11.681
Wyndham, C. H., Strydom, N. B., Morrison, J. F., Dutoit, F. D., and Kraan, J. G. (1954b). A new method of acclimatization to heat. Arbeitsphysiologie 15, 373–382. doi: 10.1007/BF00935938
Yamada, P., Amorim, F., Moseley, P., and Schneider, S. (2008). Heat shock protein 72 response to exercise in humans. Sports Med. 38, 715–733. doi: 10.2165/00007256-200838090-00002
Keywords: heat, fitness, sex, age, diabetes, hyperthermia, performance, acclimation
Citation: Foster J, Hodder SG, Lloyd AB and Havenith G (2020) Individual Responses to Heat Stress: Implications for Hyperthermia and Physical Work Capacity. Front. Physiol. 11:541483. doi: 10.3389/fphys.2020.541483
Received: 09 March 2020; Accepted: 18 August 2020;
Published: 11 September 2020.
Edited by:
David Andrew Low, Liverpool John Moores University, United KingdomReviewed by:
Daniel Gagnon, Université de Montréal, CanadaCopyright © 2020 Foster, Hodder, Lloyd and Havenith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josh Foster, ai5mb3N0ZXIyQGxib3JvLmFjLnVr; am9zaGZvc3RlcnBoZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.