- 1Department of Cardiology, The Second Medical Centre, Chinese PLA General Hospital, National Clinical Research Center for Geriatric Diseases, Beijing, China
- 2The Second Medical Centre, Chinese PLA General Hospital, National Clinical Research Center for Geriatric Diseases, Beijing, China
Aims: This study investigated the association of circulating ceramides in patients with comorbid acute coronary syndrome and type 2 diabetes mellitus (ACS-DM).
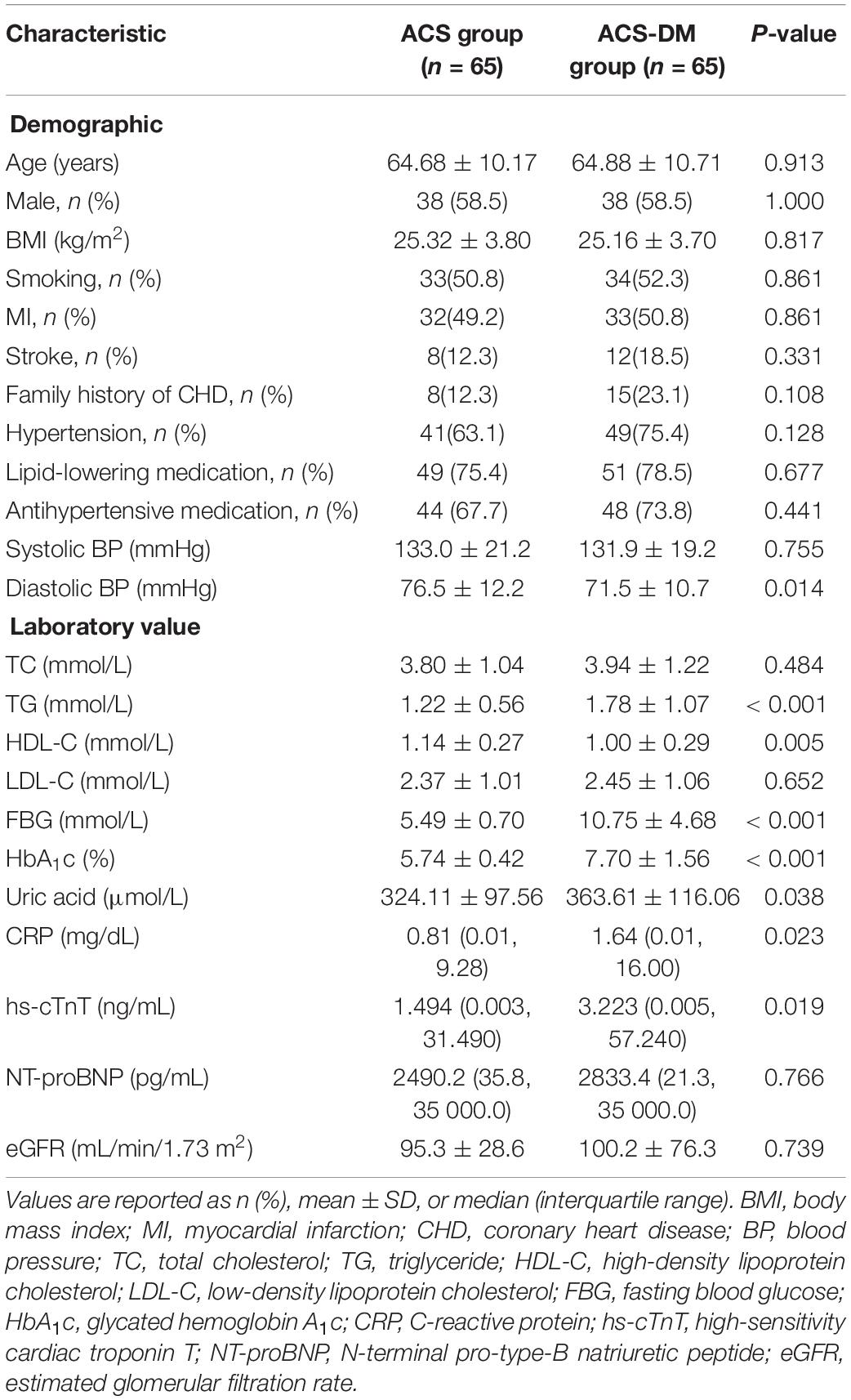
Methods: A total of 761 patients with coronary heart disease who were admitted to the Department of Cardiology at the Chinese PLA General Hospital from March to August 2018 were enrolled in this study. Of these 761 patients, 282 were diagnosed with acute coronary syndrome (ACS). We selected 65 patients with ACS-DM (ACS-DM group; mean age 64.88 years; 38 men) and 65 patients with ACS but without any comorbidities (ACS group; mean age 64.68 years; 38 men); the two groups were matched by age and sex. We determined four circulating ceramides in 130 plasma samples: Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), and Cer(d18:1/24:0). The ceramides in plasma samples from patients with ACS and those from patients with ACS-DM were compared. Pearson correlation coefficients between individual ceramides and traditional cardiovascular risk factors for the whole study population were calculated. Multiple logistic regression models were used to evaluate the relativity between the ceramide and ACS-DM.
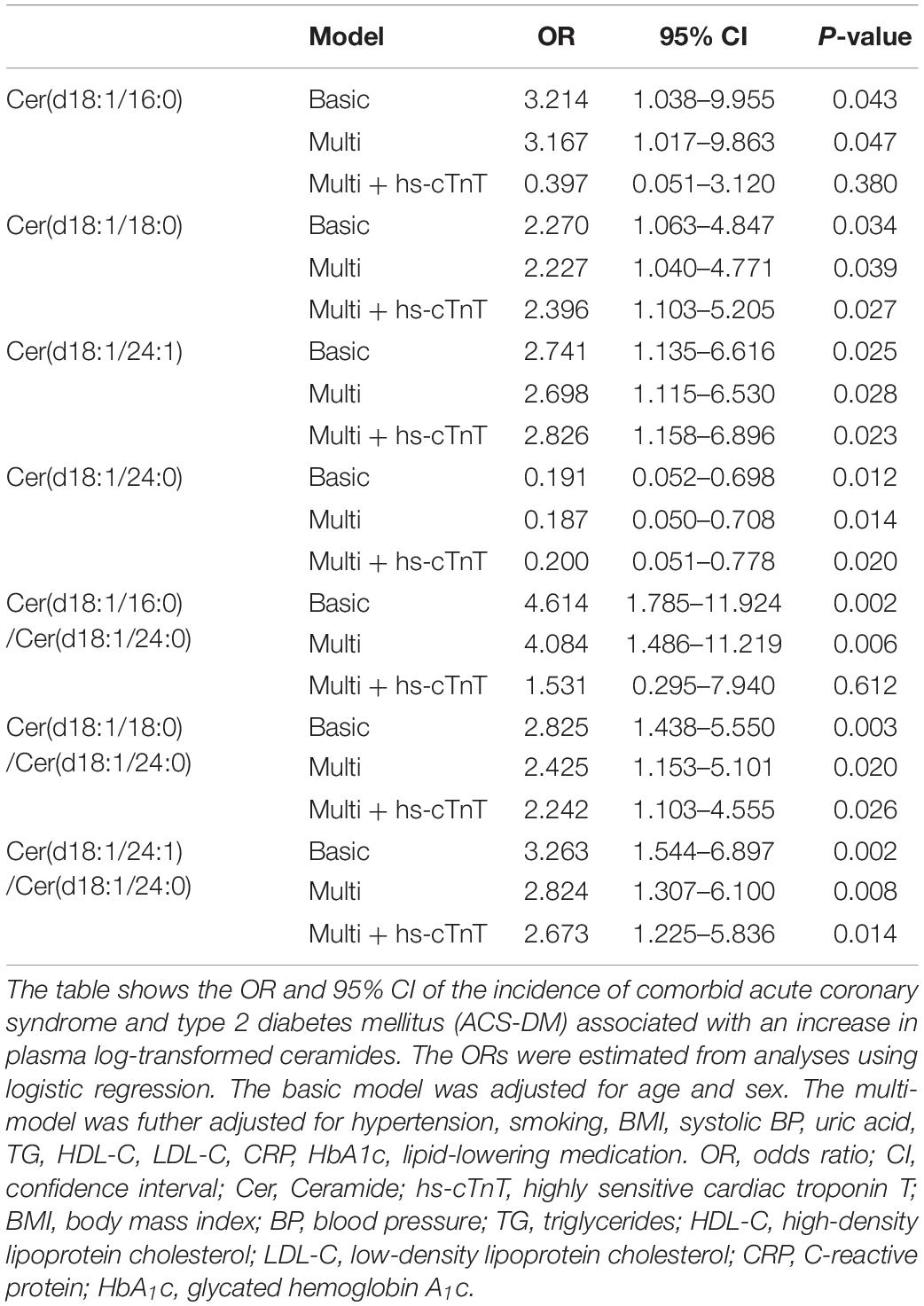
Results: Compared with the ACS group, the levels of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) and their ratios to Cer(d18:1/24:0) were higher in the ACS-DM group and Cer(d18:1/24:0) was lower in the ACS-DM group (P < 0.05). Correlation analysis demonstrated mild-to-moderate correlations of ceramide and traditional cardiovascular risk factors. There were relatively strong correlations of Cer(d18:1/18:0) and Cer(d18:1/24:1) with C-reactive protein, blood lipids, fasting blood glucose, and glycated hemoglobin A1c. In multiple logistic regression models, Cer(d18:1/18:0) [odds ratio (OR) 2.396; 95% confidence interval (CI) 1.103–5.205; P = 0.027], Cer(d18:1/24:1) (OR 2.826; 95% CI 1.158–6.896; P = 0.023), Cer(d18:1/18:0)/Cer(d18:1/24:0) (OR 2.242; 95% CI 1.103–4.555; P = 0.026), and Cer(d18:1/24:1)/Cer(d18:1/24:0) (OR 2.673; 95% CI 1.225–5.836; P = 0.014) were positively correlated with ACS-DM, and Cer(d18:1/24:0) (OR 0.200; 95% CI 0.051–0.778; P = 0.020) was negatively correlated with ACS-DM.
Conclusion: Circulating ceramides are positively correlated with the risk of ACS-DM comorbidity. These results give a new insight into the pathogenesis of ACS-DM comorbidity and could provide new options for risk estimation.
Introduction
The serum concentration of lipids is used to assess the risk of atherosclerotic heart disease. The level of low-density lipoprotein cholesterol (LDL-C) is closely related to atherosclerosis and acute coronary events. Previous studies have indicated that distinct ceramide species are closely related to cardiovascular death in patients with coronary heart disease (CHD). Ceramide molecules Cer(d18:1/16:0), which is abbreviated to Cer16:0; Cer(d18:1/18:0), which is abbreviated to Cer18:0; Cer(d18:1/24:1), which is abbreviated to Cer24:1; and their ratios to Cer(d18:1/24:0), which is abbreviated to Cer24:0, have been investigated as new risk stratification factors in patients with CHD (Meeusen et al., 2017; Peterson et al., 2018). In patients with CHD, a high level of Cer16:0, low level of Cer24:0, and a high Cer16:0/Cer24:0 ratio in plasma are related directly to cardiovascular mortality (Tarasov et al., 2014; Laaksonen et al., 2016). Accumulating evidence has shown that ceramides are closely related to the pathological process of type 2 diabetes mellitus (DM) and its complications (Galadari et al., 2013; Muilwijk et al., 2020).
Coronary heart disease is the main cause of mortality worldwide, resulting in more than 7 million deaths every year. Acute coronary syndrome (ACS) is a severe type of CHD associated with substantial mortality. DM is the main risk factor contributing to CHD, being regarded as having an equivalent risk to CHD (Juutilainen et al., 2005). Over 425 million people worldwide have DM. The number of adults in China with DM has reached 114 million, and China remains the country with the largest number of patients with DM (Cho et al., 2018). Cardiovascular events in patients with CHD and DM are five to six times higher than in patients with CHD alone (Høfsten et al., 2009).
Coronary heart disease and DM are both metabolic diseases and have mutual pathological mechanisms, but the pathological mechanism of the comorbidity is still unclear. Abnormal glucose and lipid metabolism is the pathological basis of many metabolic diseases. The pathophysiological alterations related to DM promote the development of CHD, the important driving mechanisms of which include oxidative stress, alterations in calcium metabolism, the inflammatory response, endothelial dysfunction, and autophagy (Yahagi et al., 2016; Zhang et al., 2018). Although our understanding of the pathogenesis of this comorbidity is increasing, the incidence of cardiovascular death in patients with acute coronary syndrome and type 2 diabetes mellitus (ACS-DM) remains high, and there is a lack of more effective risk stratification and prediction biomarkers for patients with ACS-DM.
It is not known whether the levels of ceramides are higher in patients with comorbid ACS-DM. Therefore, we measured the plasma concentrations of ceramides in patients with ACS-DM to ascertain if they could be novel cardiometabolic biomarkers for ACS-DM.
Materials and Methods
Ethics Approval of the Research Protocol
This study was approved by the ethics committee of the PLA General Hospital. All patients gave written informed consent.
Participants
A total of 761 patients with CHD were admitted to the Cardiology Department of PLA General Hospital from March to August 2018. The inclusion criteria were: (i) patients aged 18 years or older and diagnosed as having ACS according to the ACS diagnostic criteria and (ii) patients had a complete clinical data record. According to the inclusion criteria, 401 adult patients were enrolled in the study. The exclusion criteria were: chronic heart failure, acute cerebrovascular disease, moderate-to-severe renal dysfunction (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2), prolonged bedridden status, mental illness, and malignant tumor. Considering the effect of disease on ceramide levels, 45 patients with chronic heart failure and 12 patients with acute cerebrovascular disease were excluded. After excluding another 62 patients with moderate-to-severe renal dysfunction, 282 participants with ACS were enrolled in the study. Among these, there were 65 patients with ACS-DM, who were selected as the experimental group (ACS-DM group), and 65 patients with only ACS (ACS group), who were selected as the control group after matching for age and sex with patients with ACS-DM. According to a previous study (Yao et al., 2019), ceramide levels were associated with ACS with an odds ratio (OR) of 3.0. According to the sample size calculation formula for a paired case–control study, the sample size required was calculated to be 56 patients by using PASS software (proportioning, two related proportions; tests for two correlated proportions, McNemar test and OR). Consequently, 65 patients in each group met the sample size requirements; therefore, 130 patients were finally selected as the study cohort. Among them, 65 patients (mean age 64.88 years, range 34–84 years; 38 men) were in the ACS-DM group, and 65 patients (mean age 64.68 years, range 34–86 years; 38 men) were in the ACS group (Figure 1).
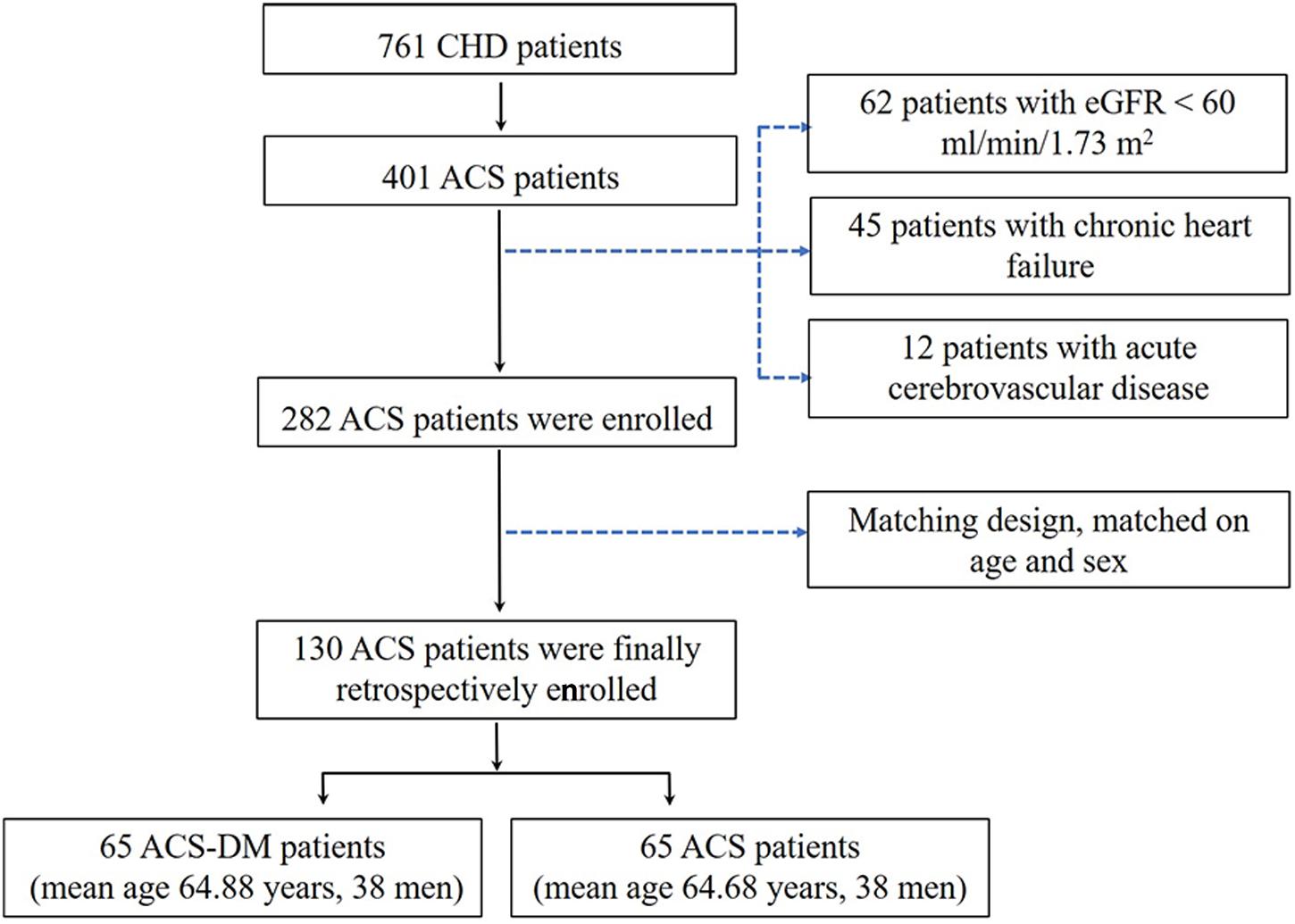
Figure 1. Study frame diagram. ACS, acute coronary syndrome; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate.
Data Collection
Patients’ demographic characteristics, lifestyle information, and medication use were obtained by review of medical records. The definition of “smoking” is having smoked more than 1 cigarette per day for over 1 year. Patients’ body mass index (BMI) was also calculated, and blood pressure was measured using the right arm of seated participants by mercury sphygmomanometer.
Variable Biomarkers Determination
Blood samples were obtained with EDTA anticoagulation between 6:00 and 7:00 after patients fasted overnight. Samples were stored at 4°C for less than 1 h, and then the plasma samples were frozen at –80°C. Concentrations of fasting blood glucose (FBG), uric acid, blood lipid, and C-reactive protein (CRP) were measured by enzymatic assays (Roche Diagnostics, Mannheim, Germany). The glycated hemoglobin A1c (HbA1c) level was tested using high-performance liquid chromatography. The concentration of creatinine was determined by an enzymatic assay (Roche Diagnostics) on an autoanalyzer (7600; Hitachi, Tokyo, Japan). N-terminal pro-type-B natriuretic peptide (NT-proBNP) was measured by an electro-chemiluminescence immunoassay (Roche Diagnostics). The concentration of high-sensitivity cardiac troponin T (hs-cTnT) was measured on an E170 autoanalyzer (Modular Analytics; Roche Diagnostics) (Xiao et al., 2017).
Quantification of Ceramides
The levels of ceramides were determined from the available plasma samples (n = 130). We used liquid chromatography–tandem mass spectrometry (LC-MS/MS) to quantitatively and simultaneously measure the four circulating ceramides in human plasma (Kauhanen et al., 2016). In short, the deuterated internal standards were added to 10 μL plasma: D7-Cer16:0, D7-Cer18:0, D7-Cer24:0, and D7-Cer24:1. The plasma levels of Cer16:0, Cer18:0, Cer24:0, and Cer24:1 were quantified on a Triple QuadTM 3200 (Sciex, Framingham, MA, United States). A 5 μL sample was added, then the ceramide was separated on an Acquity UPLC I-Class column (Waters, Milford, MA, United States) using a 5 min gradient. Quantification was evaluated by calibration line samples containing known amounts of synthetic Cer16:0, Cer18:0, Cer24:0, and Cer24:1 and the deuterated standards. We calculated and plotted the peak area ratios of ceramides to the corresponding deuterated form according to the added ceramide concentration. According to the measured peak area ratios, the plasma concentration of ceramides was calculated by the individual fitting linear regression equation. The final concentration of ceramide is expressed in μmol/L.
Definition of Variables
ACS refers to unstable angina (UA), non-ST elevated myocardial infarction (NSTEMI), and ST elevated myocardial infarction (STEMI). The diagnostic criteria for ACS are according to a comprehensive examination of clinical characteristics, including biomarkers of myocardial injury and an electrocardiogram (ECG). Unstable angina is defined as ischemic symptoms without increased biomarkers but with transient changes on the ECG. Myocardial infarction (MI) refers to myocardial necrosis during acute myocardial ischemia. The difference between STEMI and NSTEMI is whether the ST segment is elevated or not on the ECG (Anderson et al., 2007; Levine et al., 2016).
Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking antihypertensive medication. The diagnostic criteria of DM were FBG ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, non-fasting glucose ≥ 11.1 mmol/L, or taking antihyperglycemic medication (Cosentino et al., 2020). The eGFR was calculated using eGFR (mL/min/1.73 m2) = 175 × plasma creatinine–1.234 × age–0.179 × 0.79 (if female) (Ma et al., 2006).
Statistical Analyses
Continuous variables were expressed as the mean ± standard deviation or median, and categorical variables were expressed as percentages. Differences in risk factors at baseline and clinical characteristics between patients with ACS and those with ACS-DM were compared by Chi-squared test or Student’s t-test. Continuous variables in the skewness distribution were compared with the Wilcoxon rank sum test. Pearson correlation coefficients between individual ceramides and traditional cardiovascular risk factors for the whole study population were calculated. Multiple logistic regression analysis was used to assess the relationship between the concentration of ceramide and the risk of ACS-DM. The ceramide concentration was logarithm transformed. The basic model was adjusted for age and sex. The multi-model was further adjusted for smoking, BMI, hypertension, SBP, uric acid, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, CRP, HbA1c, and lipid-lowering medication. The multi-model + hs-cTnT was further adjusted for hs-cTnT based on the multi-model. The receiver operating characteristic (ROC) curve was used to assess the accuracy of the ceramide concentration for the prediction of ACS-DM; the discrimination was assessed with the area under curve (AUC).
Analyses were implemented with SPSS 23.0 (IBM, NY, United States). P < 0.05 was regarded as significant.
Results
Clinical Characteristics at Baseline
A total of 130 patients were enrolled in this study. The characteristics of the patients according to disease status (ACS group and ACS-DM group) are summarized in Table 1. Higher plasma levels of glucose, uric acid, TG, and CRP and lower levels of DBP and HDL-C were associated with ACS-DM. The proportion of patients with MI between the ACS group and the ACS-DM group did not differ, but the levels of hs-cTnT were higher in the ACS-DM group. Compared with the ACS group, the levels of Cer16:0, Cer18:0, and Cer24:1 and their ratios to Cer24:0 in the ACS-DM group were higher, whereas the level of Cer24:0 was lower (Figure 2).
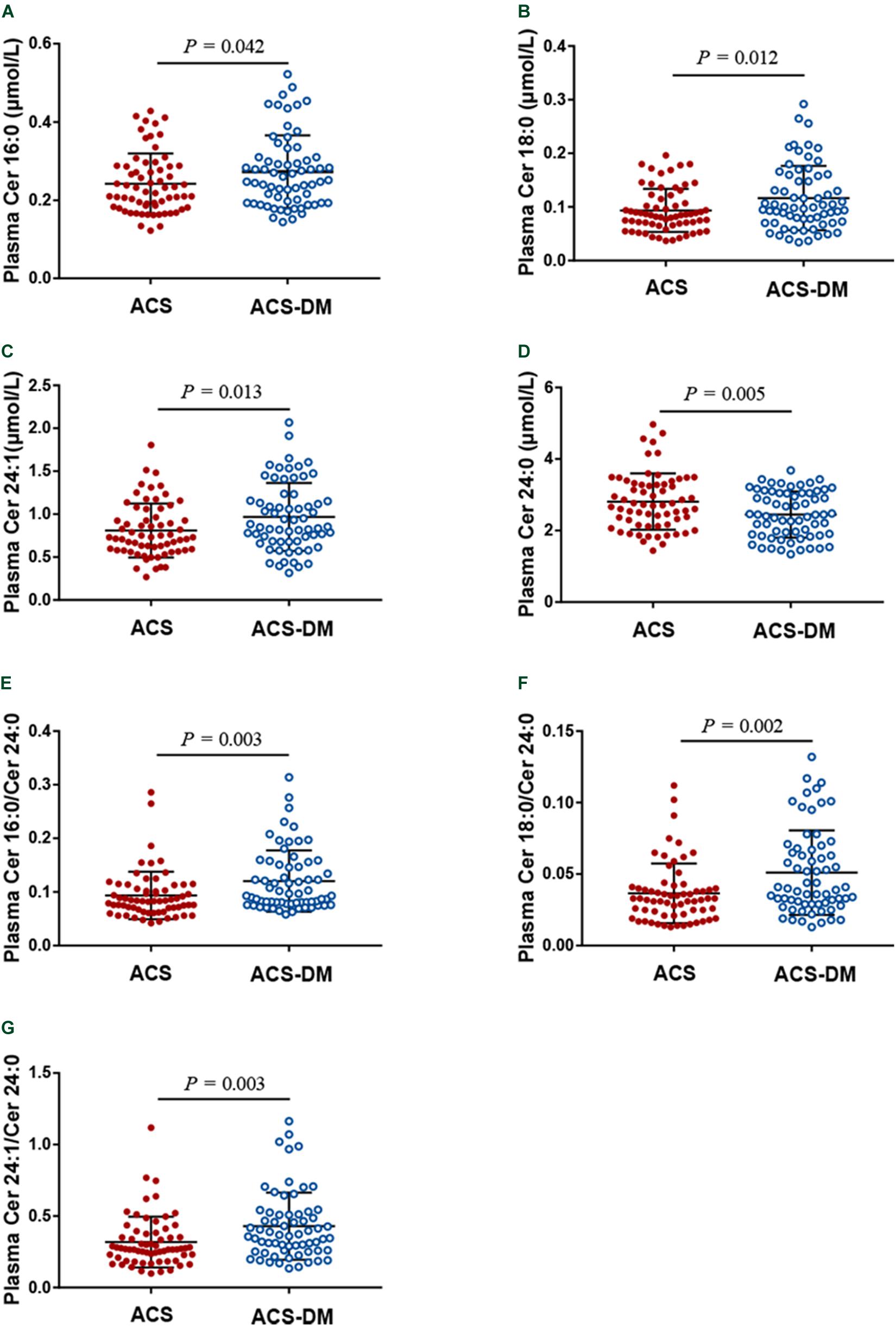
Figure 2. (A–G) A comparison of the levels of ceramides in patients with acute coronary syndrome (ACS) and those with comorbid acute coronary syndrome and type 2 diabetes mellitus (ACS-DM).
Subgroup Analyses
According to whether or not they had MI, patients were divided into the UA and MI subgroups and circulating ceramides in patients with ACS and those with ACS-DM were compared. In the UA subgroup, the levels of Cer18:0, Cer24:1, and Cer16:0/Cer24:0 in patients with ACS-DM were higher (n = 32) than those in patients with ACS (n = 33) (P < 0.05). In the MI subgroup, compared with patients with ACS (n = 32), the levels of Cer24:1, Cer16:0/Cer24:0, Cer18:0/Cer24:0, and Cer24:1/Cer24:0 were higher in patients with ACS-DM (n = 33), and the levels of Cer24:0 were lower in patients with ACS-DM (P < 0.05) (Figure 3).
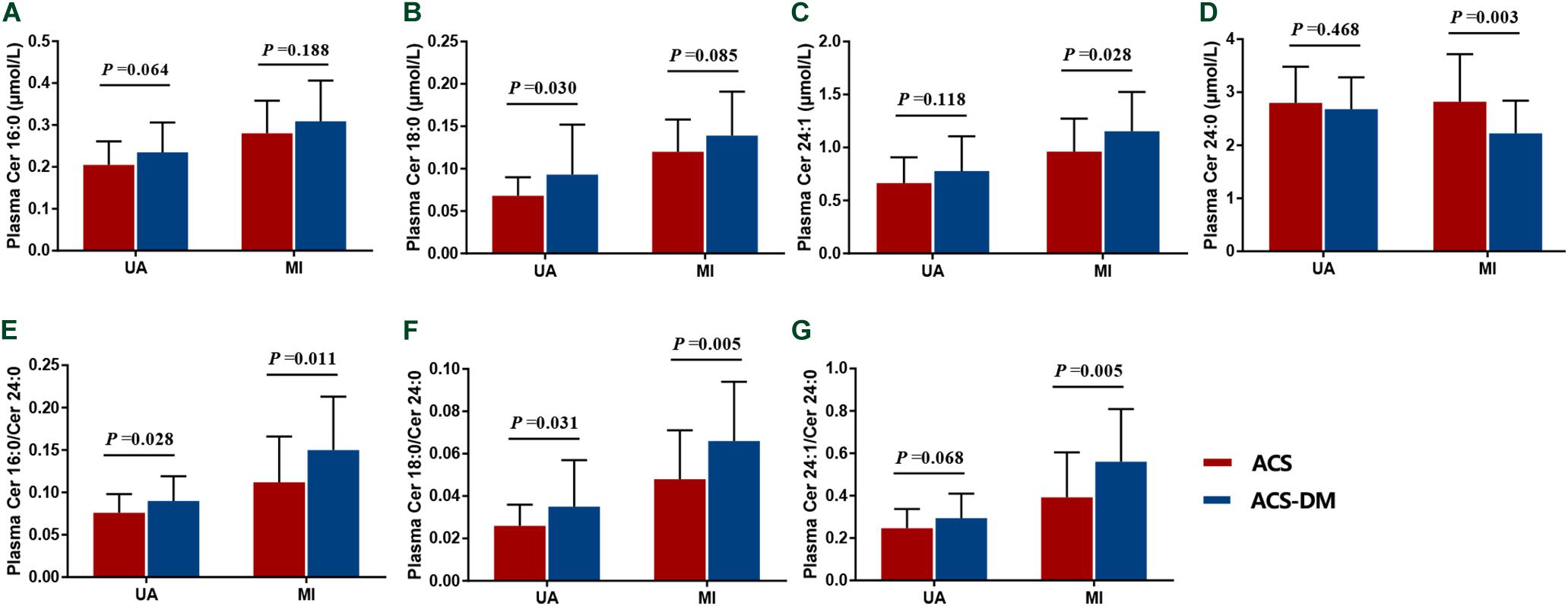
Figure 3. (A–G) Subgroup analysis of the levels of ceramides in patients with acute coronary syndrome (ACS) compared with those with comorbid acute coronary syndrome and type 2 diabetes mellitus (ACS-DM). MI, myocardial infarction; UA, unstable angina.
Correlation of Ceramides and Traditional Cardiovascular Risk Factors
The Pearson correlation coefficients for the levels of individual ceramides and those for traditional cardiovascular risk factors for the whole study population were calculated. Correlation analysis demonstrated that the levels of ceramides and their ratios had a mild-to-moderate correlation with those of traditional cardiovascular risk factors. Of note was the relatively strong correlation of Cer18:0 and Cer24:1 with the levels of CRP, blood lipids, FBG, HbA1c, and hs-cTnT (Table 2).
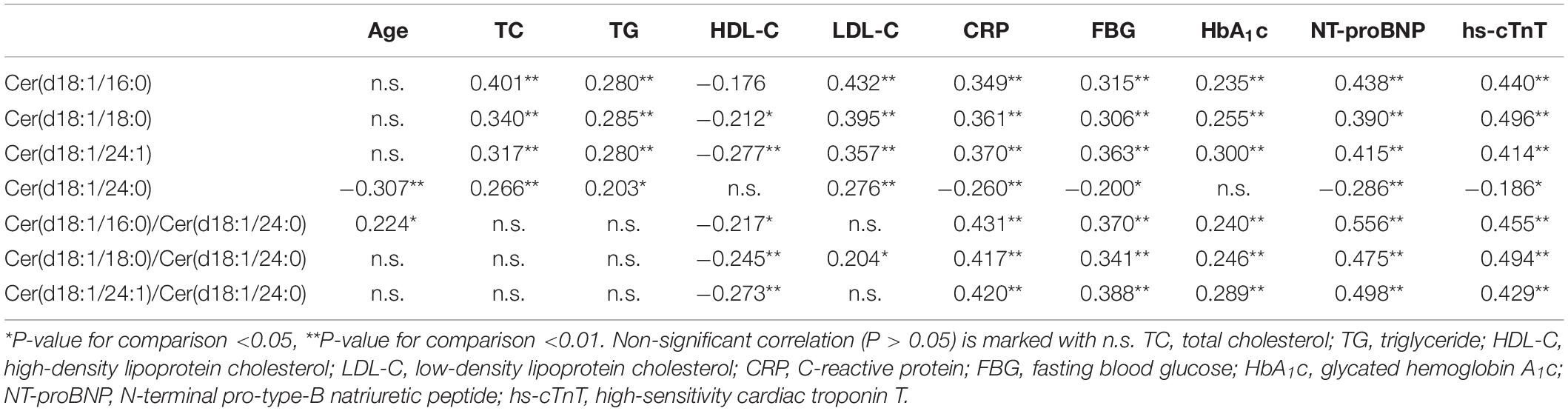
Table 2. Spearman rank correlation coefficients between ceramides and traditional cardiovascular risk factors for the whole study population (n = 130).
Relationship Between Ceramides and the ACS-DM Group
Stepwise multiple logistic regression analysis revealed that the ceramide levels are significantly related to ACS-DM. In the basic model and the multi-model, the relationship between the ceramide level and the ACS-DM group was significant (P < 0.05). In the multi-model + hs-cTnT, Cer(d18:1/18:0) [OR 2.396; 95% confidence interval (CI) 1.103–5.205; P = 0.027], Cer(d18:1/24:1) (OR 2.826; 95% CI 1.158–6.896; P = 0.023), Cer(d18:1/18:0)/Cer(d18:1/24:0) (OR 2.242; 95% CI 1.103–4.555; P = 0.026), and Cer(d18:1/24:1)/Cer(d18:1/24:0) (OR 2.673; 95% CI 1.225–5.836; P = 0.014) were positively correlated with ACS-DM and Cer(d18:1/24:0) (OR 0.200; 95% CI 0.051–0.778; P = 0.020) was negatively correlated with ACS-DM, whereas the association of Cer16:0 and Cer16:0/Cer24:0 with ACS-DM disappeared. The significance of the ceramide levels in the adjusted models (basic model, multi-model, and multi-model + hs-cTnT) is presented in Table 3.
Analyses of ROC Curves on Ceramide Levels in Patients With ACS-DM
Analyses of ROC curves indicated that the level of ceramides (except for Cer16:0 and Cer16:0/Cer24:0) had reasonable accuracy for the prediction of ACS-DM (Figures 4A,B).
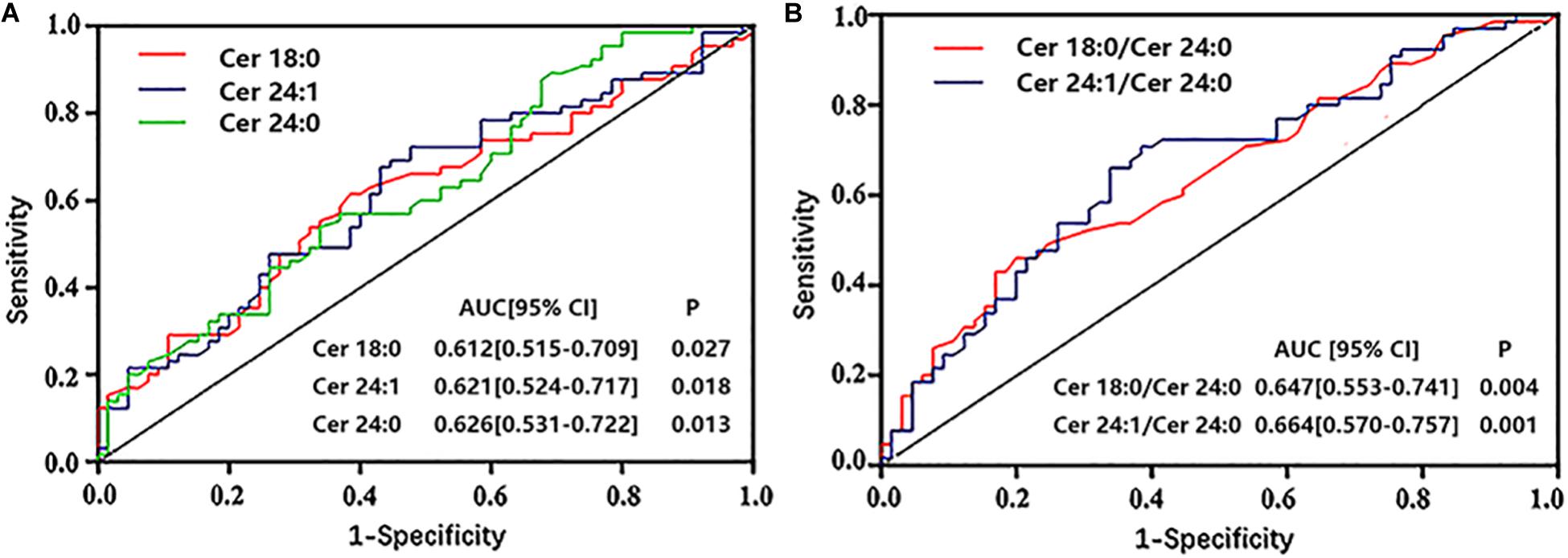
Figure 4. (A,B) Receiver operator characteristic (ROC) curves of ceramides to predict comorbid acute coronary syndrome and type 2 diabetes mellitus. ROC analysis was performed to determine the sensitivity and specificity of the value. AUC, area under the curve; CI, confidence interval.
Discussion
We demonstrated in patients with ACS-DM and patients with ACS matched by age and sex that ceramides were associated with patients with ACS-DM independent of sex, age, or other risk factors of vascular disease. The levels of Cer18:0 and Cer24:1 and the ratios to Cer24:0 had reasonable accuracy for ACS-DM prediction. Cer18:0 and Cer24:1 and their ratios to Cer24:0 were positively correlated with ACS-DM, and Cer24:0 was negatively correlated with ACS-DM. These results suggest that ceramides could be new biomarkers of ACS-DM.
Recent studies have demonstrated that ceramides are associated with an atherosclerotic process (Bismuth et al., 2008). Elevated levels of ceramides are also related to multiple cardiovascular risk factors, such as hypertension (Mielke et al., 2019), heart failure (Lemaitre et al., 2019), and DM (Neeland et al., 2018). Increases in levels of ceramides are predictive of cardiovascular events and are also independently correlated with adverse cardiovascular events in patients with and without CHD. The predictive value of ceramides is better than that of LDL-C levels (Meeusen et al., 2018). Gierens et al. explained the potential cause of this phenomenon. They confirmed that interleukin-6 (IL-6) enhances LDL receptor activity in the liver during the inflammatory response, subsequently increasing the elimination of circulating LDL-C. Thus, the acute inflammatory response in patients with ACS may increase the clearance of LDL-C, leading to a decrease in the circulating LDL-C concentration and affecting the accuracy of risk prediction (Lubrano et al., 2015). Determination of ceramide levels using a high-throughput methodology such as LC-MS/MS is efficient and convenient. Accurate quantification of ceramides can be achieved by isotope-labeled standards, enabling ceramides to be new potential predictors of cardiovascular events. Long-chain ceramides have the effect of promoting apoptosis, and very-long-chain ceramides (Cer24;0) have an anti-apoptotic effect (Grösch et al., 2012). Consistent with the present study, long-chain species (Cer16:0 and Cer18:0) increased cardiovascular risk, and very-long-chain (Cer24:0) species were cardioprotective. Also, in patients with established CHD, Cer24:0 appears to be cardioprotective, and the increase in Cer24:0/Cer24:1 can lower the risk of cardiovascular events in patients with or without DM (Menuz et al., 2009). Our results are consistent with these studies.
Our study demonstrated that the levels of Cer16:0, Cer18:0, and Cer24:1 and their ratios to Cer24:0 in patients with ACS-DM were higher than those in patients with ACS. The levels of Cer24:0, Cer18:0/Cer24:0, and Cer24:1/Cer24:0 had a relatively strong correlation with ACS-DM. The levels of Cer18:0 and Cer24:1 were associated with the levels of CRP, blood lipids, FBG, and HbA1c; these results are consistent with a previous study (Havulinna et al., 2016). Cer18:0 and Cer24:1 may be the main risk factors and pathogenic factors for ACS-DM. The Cer24:0 level was negatively correlated with ACS-DM and appeared to have a protective effect in patients with ACS-DM.
Ceramides are complex sphingolipids widely expressed in cells and lipoproteins. The increase in ceramide content in LDL promotes the atherosclerotic process of the vascular wall (Li et al., 2014). Ceramides are involved in biological processes that may influence atherosclerosis and cardiovascular events, including oxidative stress apoptosis, endothelial dysfunction, inflammation, lipotoxicity, and insulin resistance (Alessenko et al., 2018). The inflammatory cytokines interferon-γ and IL-6 promote ceramide synthesis. Ceramides participate in endothelial damage and platelet activation (Marathe et al., 1998). Ceramides are associated with vulnerable atherosclerotic plaques and participate in the process of atherosclerosis, including lipoprotein aggregation and uptake, the inflammatory response, endothelial damage, and apoptosis. They also inhibit the production of apolipoprotein E in macrophages and lead to cholesterol accumulation in macrophage-derived foam cells (Lucic et al., 2007). The levels of ceramides increased with accumulated lipid loading and inflammation in patients with DM, and ceramides can directly regulate β-cell apoptosis and induce insulin resistance (Kayser et al., 2019). Circulating leptin levels were significantly increased in insulin resistance, which is associated with cardiac dysfunction (Ren and Relling, 2006). The potential toxicity of ceramides and their ability to inhibit contraction in cardiomyocytes are related to exposure time (Relling et al., 2003). Several studies have demonstrated that different ceramide species have different effects. For instance, cell experiments have shown that Cer16:0 can promote apoptosis, while Cer24:0 appears to protect against apoptosis. Evidence is also accumulating on the functions of ceramides with different chain lengths. Increases in Cer16:0 but not Cer24:0 are involved in insulin resistance. C2 ceramide reduced the damage to cardiac function caused by a high glucose concentration (Colligan et al., 2002). In brief, ceramides have a mutual pathologic mechanism in patients with ACS and those with DM that increases the risk of cardiovascular events. Ceramides may be potential targets for the treatment of CHD in patients with DM.
Limitations
There were four main limitations to this study. First, our study was an observational study, unable to establish a causal relationship between ceramides and ACS-DM. Because of this, it is unclear whether cardiovascular risk treatment based on ceramides would improve clinical benefits. Second, although a paired design was used to reduce confounding factors, the sample size was relatively small. The possibility of residual confounding from imprecisely measured or unmeasured variables cannot be ruled out. Third, our study cohort comprised primarily a Chinese Han population. Therefore, it is necessary to conduct further research on an ethnically more diverse population. Fourth, the AUC showed that the individual discriminative power of ceramides was low for ACS-DM detection (AUC < 0.70). Therefore, our observations need to be further confirmed in longitudinal studies with a larger sample.
Conclusion
In conclusion, circulating ceramides are positively correlated with the risk of ACS-DM comorbidity. However, longitudinal follow-up studies are still needed to evaluate the causal relationship between ceramides and comorbid ACS-DM. The results of this study demonstrated new insights into the pathogenesis of comorbid ACS-DM. Circulating ceramides may provide new options for risk evaluation as novel biomarkers of cardiometabolic diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of Chinese PLA General Hospital (Beijing, China). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FC designed the experiments. RC, ZF, SL, MX, JZ, DH, WH, and LY performed the experiments. RC, ZF, and LY analyzed the data. RC prepared the manuscript. RC, YW, and LF supervised data collection. All authors have contributed to the study and approved the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2016YFC1300300 and 2016YFA0100903), the Key Project of Chinese Military Health Care Projects (18BJZ32), the National Natural Science Foundation of China (81530058), and the Science Foundation of the PLA General Hospital (2018XXFC-9).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Department of Laboratory Medicine of the PLA General Hospital for their help in biomarker detection.
References
Alessenko, A. V., Lebedev, ÀÒ, and Kurochkin, I. N. (2018). The role of sphingolipids in cardiovascular pathologies. Biomed. Khim. 64, 487–495. doi: 10.18097/PBMC20186406487
Anderson, J. L., Adams, C. D., Antman, E. M., Bridges, C. R., Califf, R. M., and Casey, D. E. Jr., et al. (2007). ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the american college of cardiology/american heart association task force on practice guidelines (Writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the american college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. J. Am. Coll. Cardiol. 50, e1–e157. doi: 10.1016/j.jacc.2007.02.013
Bismuth, J., Lin, P., Yao, Q., and Chen, C. (2008). Ceramide: a common pathway for atherosclerosis? Atherosclerosis 196, 497–504. doi: 10.1016/j.atherosclerosis.2007.09.018
Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., et al. (2018). IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. doi: 10.1016/j.diabres.2018.02.023
Colligan, P. B., Relling, D. P., and Ren, J. (2002). Ceramide attenuates high glucose-induced cardiac contractile abnormalities in cultured adult rat ventricular myocytes. Cell. Mol. Biol. 48, OL251-7.
Cosentino, F., Grant, P. J., Aboyans, V., Bailey, C. J., Ceriello, A., Delgado, V., et al. (2020). 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323. doi: 10.1093/eurheartj/ehz486
Galadari, S., Rahman, A., Pallichankandy, S., Galadari, A., and Thayyullathil, F. (2013). Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 12:98. doi: 10.1186/1476-511x-12-98
Grösch, S., Schiffmann, S., and Geisslinger, G. (2012). Chain length-specific properties of ceramides. Prog. Lipid Res. 51, 50–62. doi: 10.1016/j.plipres.2011.11.001
Havulinna, A. S., Sysi-Aho, M., Hilvo, M., Kauhanen, D., Hurme, R., Ekroos, K., et al. (2016). Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430. doi: 10.1161/ATVBAHA.116.307497
Høfsten, D. E., Løgstrup, B. B., Møller, J. E., Pellikka, P. A., and Egstrup, K. (2009). Abnormal glucose metabolism in acute myocardial infarction: influence on left ventricular function and prognosis. JACC Cardiovasc. Imaging 2, 592–599. doi: 10.1016/j.jcmg.2009.03.007
Juutilainen, A., Lehto, S., Ronnemaa, T., Pyorala, K., and Laakso, M. (2005). Type 2 diabetes as a “Coronary Heart Disease Equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 28, 2901–2907. doi: 10.2337/diacare.28.12.2901
Kauhanen, D., Sysi-Aho, M., Koistinen, K. M., Laaksonen, R., Sinisalo, J., and Ekroos, K. (2016). Development and validation of a high-throughput LC-MS/MS assay for routine measurement of molecular ceramides. Anal. Bioanal. Chem. 408, 3475–3483. doi: 10.1007/s00216-016-9425-z
Kayser, B. D., Prifti, E., Lhomme, M., Belda, E., Dao, M. C., Aron-Wisnewsky, J., et al. (2019). Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics 15:140. doi: 10.1007/s11306-019-1596-0
Laaksonen, R., Ekroos, K., Sysi-Aho, M., Hilvo, M., Vihervaara, T., Kauhanen, D., et al. (2016). Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart. J. 37, 1967–1976. doi: 10.1093/eurheartj/ehw148
Lemaitre, R. N., Jensen, P. N., Hoofnagle, A., McKnight, B., Fretts, A. M., King, I. B., et al. (2019). Circ. Heart Fail. 12, e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 134, e123–e155. doi: 10.1161/CIR.0000000000000404
Li, W., Yang, X., Xing, S., Bian, F., Yao, W., Bai, X., et al. (2014). Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med. Cell. Longev. 2014:823071. doi: 10.1155/2014/823071
Lubrano, V., Gabriele, M., Puntoni, M. R., Longo, V., and Pucci, L. (2015). Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell. Mol. Biol. Lett. 20, 310–322. doi: 10.1515/cmble-2015-0020
Lucic, D., Huang, Z. H., Gu, D., Subbaiah, P. V., and Mazzone, T. (2007). Cellular sphingolipids regulate macrophage apolipoprotein E secretion. Biochemistry 46, 11196–11204. doi: 10.1021/bi701106v
Ma, Y. C., Zuo, L., Chen, J. H., Luo, Q., Yu, X. Q., Li, Y., et al. (2006). Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 17, 2937–2944. doi: 10.1681/ASN.2006040368
Marathe, S., Schissel, S. L., Yellin, M. J., Beatini, N., Mintzer, R., Williams, K. J., et al. (1998). Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J. Biol. Chem. 273, 4081–4088. doi: 10.1074/jbc.273.7.4081
Meeusen, J. W., Donato, L. J., Bryant, S. C., Baudhuin, L. M., Berger, P. B., and Jaffe, A. S. (2018). Plasma Ceramides. Arterioscler. Thromb. Vasc. Biol. 38, 1933–1939. doi: 10.1161/ATVBAHA.118.311199
Meeusen, J. W., Donato, L. J., and Jaffe, A. S. (2017). Lipid biomarkers for risk assessment in acute coronary syndromes. Curr. Cardiol. Rep. 19:48. doi: 10.1007/s11886-017-0863-9
Menuz, V., Howell, K. S., Gentina, S., Epstein, S., Riezman, I., Fornallaz-Mulhauser, M., et al. (2009). Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 324, 381–384. doi: 10.1126/science.1168532
Mielke, M. M., Syrjanen, J. A., Bui, H. H., Petersen, R. C., Knopman, D. S., and Jack, C. R. Jr., et al, (2019). Elevated plasma ceramides are associated with higher white matter hyperintensity volume-brief Report. Arterioscler. Thromb. Vasc. Biol. 39, 2431–2436. doi: 10.1161/ATVBAHA.119.313099
Muilwijk, M., Goorden, S. M. I., Celis-Morales, C., Hof, M. H., Ghauharali-van der Vlugt, K., Beers-Stet, F. S., et al. (2020). Contributions of amino acid, acylcarnitine and sphingolipid profiles to type 2 diabetes risk among South-Asian Surinamese and Dutch adults. BMJ Open Diabetes Res. Care 8:e001003. doi: 10.1136/bmjdrc-2019-001003
Neeland, I. J., Singh, S., McGuire, D. K., Vega, G. L., Roddy, T., Reilly, D. F., et al. (2018). Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the dallas heart study. Diabetologia 61, 2570–2579. doi: 10.1007/s00125-018-4720-1
Peterson, L. R., Xanthakis, V., Duncan, M. S., Gross, S., Friedrich, N., Völzke, H., et al. (2018). Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 7:e007931. doi: 10.1161/jaha.117.007931
Relling, D. P., Hintz, K. K., and Ren, J. (2003). Acute exposure of ceramide enhances cardiac contractile function in isolated ventricular myocytes. Br. J. Pharmacol. 140, 1163–1168. doi: 10.1038/sj.bjp.0705510
Ren, J., and Relling, D. P. (2006). Leptin-induced suppression of cardiomyocyte contraction is amplified by ceramide. Peptides 27, 1415–1419. doi: 10.1016/j.peptides.2005.11.022
Tarasov, K., Ekroos, K., Suoniemi, M., Kauhanen, D., Sylvänne, T., Hurme, R., et al. (2014). Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99, E45–E52. doi: 10.1210/jc.2013-2559
Xiao, W., Cao, R., Liu, Y., Wang, F., Bai, Y., Wu, H., et al. (2017). Association of high-sensitivity cardiac troponin T with mortality and cardiovascular events in a community-based prospective study in Beijing. BMJ Open 7:e013431. doi: 10.1136/bmjopen-2016-013431
Yahagi, K., Kolodgie, F. D., Lutter, C., Mori, H., Romero, M. E., Finn, A. V., et al. (2016). Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 37, 191–204. doi: 10.1161/atvbaha.116.306256
Yao, K., Wang, Y., Xu, D., Liu, X., Shen, C., Hu, W., et al. (2019). Effect of combined testing of ceramides with high-sensitive troponin T on the detection of acute coronary syndrome in patients with chest pain in China: a prospective observational study. BMJ Open 9:e028211. doi: 10.1136/bmjopen-2018-028211
Keywords: ceramide, acute coronary syndrome with type 2 diabetes mellitus, comorbidity, cardiometabolic diseases, risk factors
Citation: Cao R, Fang Z, Li S, Xu M, Zhang J, Han D, Hu W, Yan L, Wang Y, Fan L and Cao F (2020) Circulating Ceramide: A New Cardiometabolic Biomarker in Patients With Comorbid Acute Coronary Syndrome and Type 2 Diabetes Mellitus. Front. Physiol. 11:1104. doi: 10.3389/fphys.2020.01104
Received: 31 May 2020; Accepted: 10 August 2020;
Published: 15 September 2020.
Edited by:
Xin Wang, University of Manchester, United KingdomReviewed by:
Jun Ren, University of Washington, United StatesXu Yan, Victoria University, Australia
Copyright © 2020 Cao, Fang, Li, Xu, Zhang, Han, Hu, Yan, Wang, Fan and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Cao, ZmVuZ2Nhbzg4MjhAMTYzLmNvbQ==
 Ruihua Cao1
Ruihua Cao1 Zhiyi Fang
Zhiyi Fang Dong Han
Dong Han Yabin Wang
Yabin Wang Feng Cao
Feng Cao