- 1Vagal Afferent Research Group, Adelaide Medical School, The University of Adelaide, Adelaide, SA, Australia
- 2Department of Pharmacodynamics, College of Pharmacy, University of Florida, Gainesville, FL, United States
- 3Center for Integrative Cardiovascular and Metabolic Disease, University of Florida, Gainesville, FL, United States
- 4Nutrition, Diabetes and Gut Health, Lifelong Health Theme, South Australian Health and Medical Research Institute, Adelaide, SA, Australia
Gastrointestinal (GI) vagal afferents convey sensory signals from the GI tract to the brain. Numerous subtypes of GI vagal afferent have been identified but their individual roles in gut function and feeding regulation are unclear. In the past decade, technical approaches to selectively target vagal afferent subtypes and to assess their function has significantly progressed. This review examines the classification of GI vagal afferent subtypes and discusses the current available techniques to study vagal afferents. Investigating the distribution of GI vagal afferent subtypes and understanding how to access and modulate individual populations are essential to dissect their fundamental roles in the gut-brain axis.
Introduction
The vagus nerve provides bidirectional communication between the gut and the brain. The vagus nerve comprises of both sensory and motor neurons with the number of afferent fibres out-numbering the efferent fibres by about 9 to 1 (Agostoni et al., 1957). Vagal sensory pathways facilitate signal transmission from the visceral endings in the gut through the vagal ganglia, where the cell bodies are located, and terminate in the brainstem. Visceral projection of vagal afferents is highly prevalent in the upper gastrointestinal (GI) tract and the density is gradually decreased further down the gut (Berthoud and Neuhuber, 2000). Instead, the lower GI tract is densely innervated by spinal afferents whose cell bodies lie in the dorsal root ganglia (DRG) (Spencer et al., 2016b). The GI vagal afferent cell bodies are located in the nodose ganglia (NG), originating from the epibranchial placode (Baker and Bronner-Fraser, 2001). The predominant function of this traffic is to transmit innocuous signals evoked by food related stimuli in the GI tract. In addition, a small number of jugular vagal afferents, with cell bodies in the jugular ganglia, also project to GI regions (Yu et al., 2005). The jugular neurons are derived from the neural crest, the same cell source as spinal afferent neurons. They share similarities in gene expression of somatosensory markers (Kupari et al., 2019), suggesting a possible similar function of jugular neurons and spinal afferents. Furthermore, vagal afferents also form direct monosynaptic connexions (Rinaman et al., 1989) and indirect interactions (via second order neurons) (Grabauskas and Owyang, 2017) with the efferent fibres in the nucleus tractus solitarius (NTS) to regulate the vago-vagal reflex. Vagal efferents, with the cell bodies located in the dorsal motor nucleus of the vagus (DMV), relay signals from the brain to the gut coordinating motor responses to maintain digestive function (Rogers et al., 1995).
GI vagal afferents play an important role in the regulation of food intake and GI function, orchestrating both physiological and behavioural aspects of food intake in order to ensure energy requirements are maintained. The peripheral afferent endings are specialised to detect mechanical and chemical stimuli evoked within the GI tract in response to food intake. These signals are transmitted to the brainstem and processed before being relayed to different regions of the brain, involved in physiological and behavioural function, or reflexing back to the GI tract to impact on gut motility and enzyme secretion (Travagli and Anselmi, 2016; Browning et al., 2017; Han et al., 2018; Suarez et al., 2018; Waise et al., 2018). It is known that there are numerous subtypes of GI vagal afferent depending on morphology and response to food related stimuli (Berthoud and Powley, 1992; Page et al., 2002). However, their individual role in feeding regulation and GI function is unclear. Identification of the role of specific subtypes of GI vagal afferent will provide critical information for the targeted treatment of disease.
This review will examine the different classifications of GI vagal afferents and discuss the current advances in technology that can improve understanding of the specific role these subclasses of GI vagal afferent play in gut function and appetite regulation.
The Curious Case of Vagal Afferent Subtypes
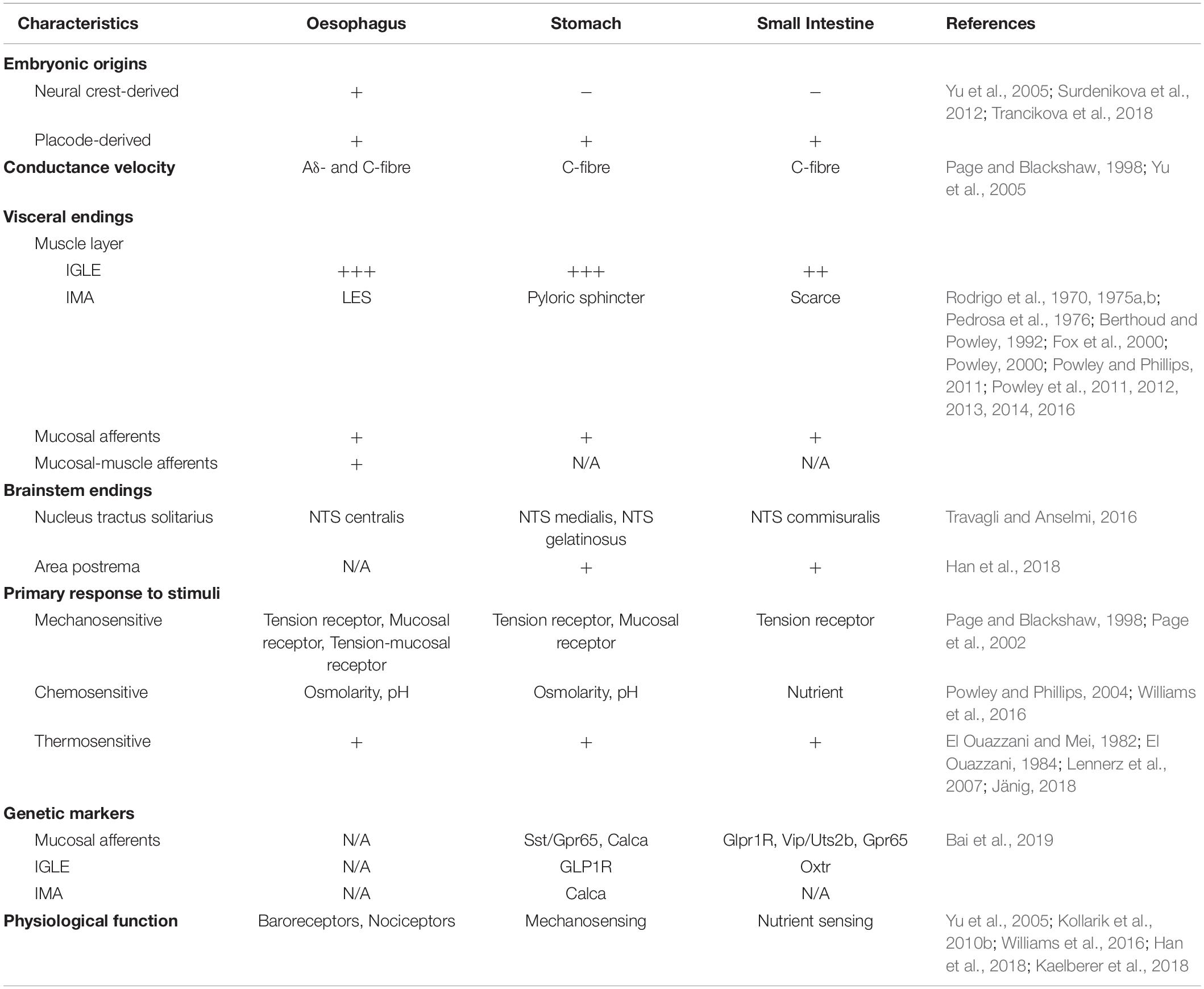
GI vagal afferent subtypes have been previously classified based on their characteristics, such as the embryonic origins, conductance velocity, anatomical and morphological organisation, response to primary stimuli, as well as defined molecular markers (Figure 1 and Table 1). In recent years, gene expression profiles (Egerod et al., 2018; Kupari et al., 2019) and neural circuits (Williams et al., 2016; Han et al., 2018; Kaelberer et al., 2018) have been introduced as new ways to categorise vagal afferent subtypes (Figure 2). Although all of these approaches aim to associate GI vagal afferents to their physiological and behavioural function, each classification has limitations when it comes to in vivo studies.
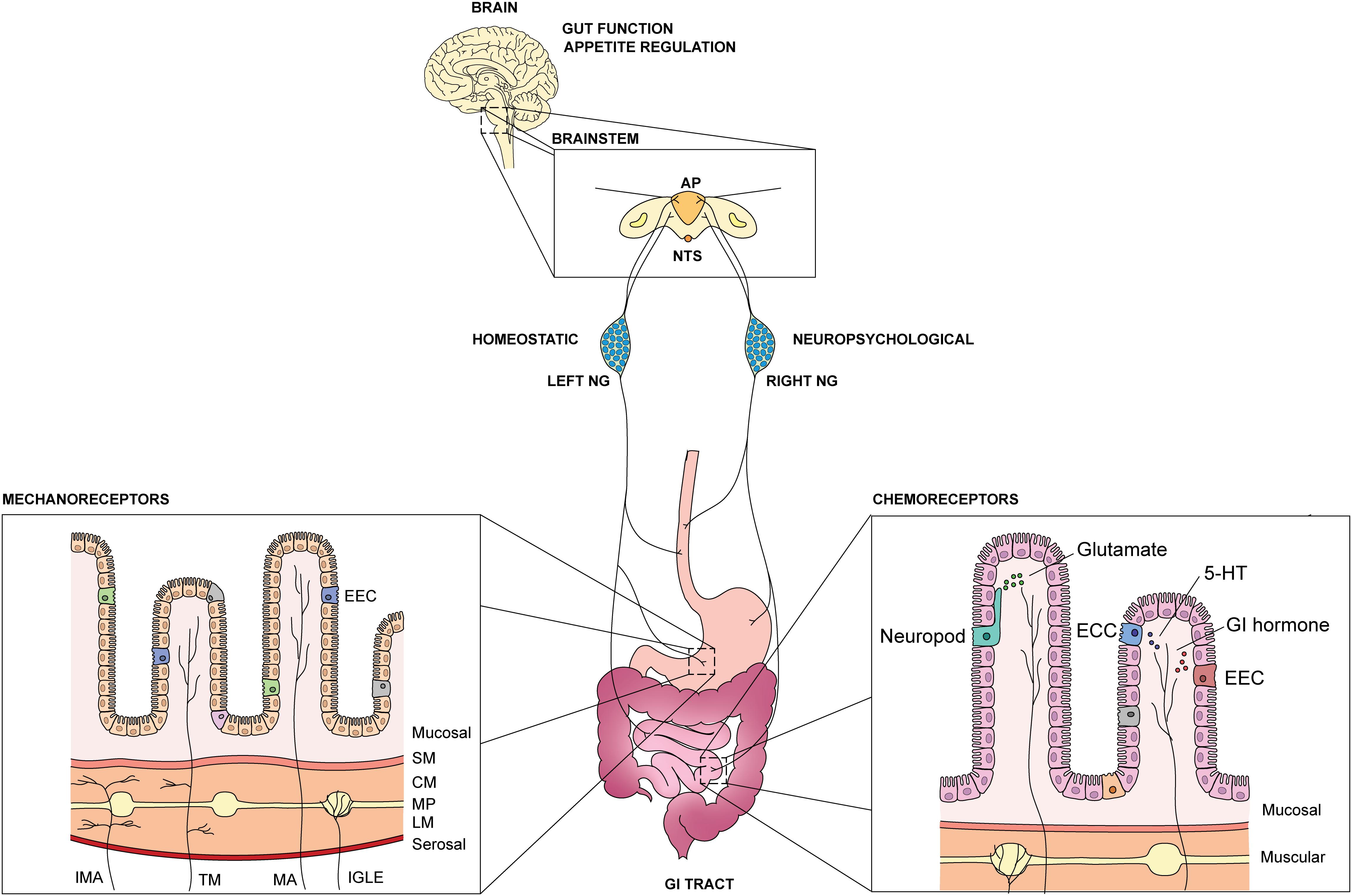
Figure 1. Overview of GI vagal afferents in the gut-brain axis. An illustration of GI vagal afferents neuroanatomy and primary sensory responses in the viscera. NTS: nucleus tractus solitarius, AP: area postrema, NG: nodose ganglia, EEC: enteroendocrine cells, ECC: enterochromaffin cells, 5-HT: 5-hydroxytriptamine, SM: submucosal, CM: circular muscle, MP: myenteric plexus, LM: longitudinal muscle, IMA: intramuscular arrays, TM: tension-mucosal afferents, MA: mucosal afferents, IGLE: intraganglionic laminar endings.
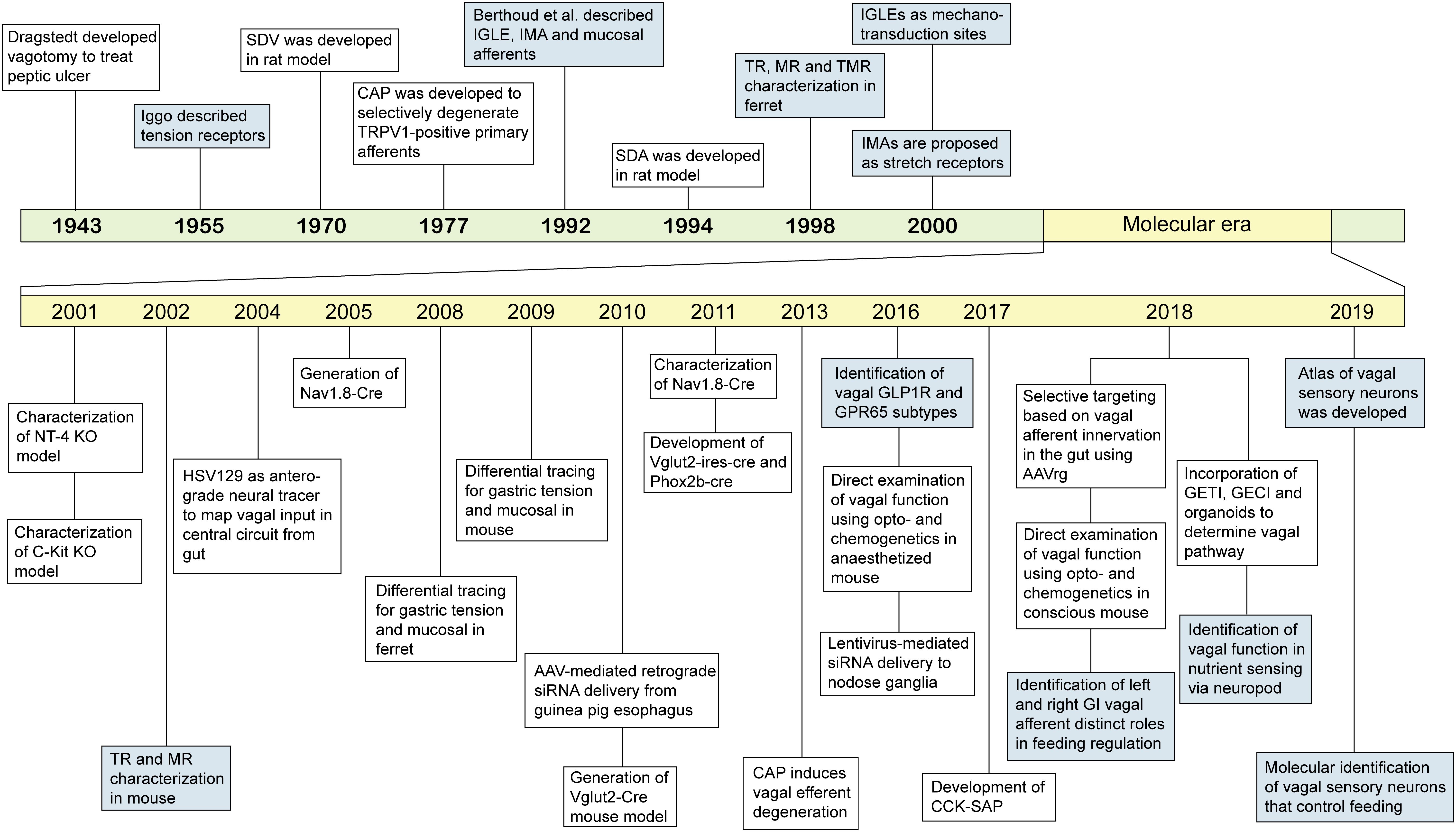
Figure 2. Landmark events of classification and technical advances to study GI vagal afferents. An overview of progress in understanding GI vagal afferents function and technical development to target vagal afferent subtypes. SDV: subdiaphragmatic vagotomy, CAP: capsaicin, TRPV1: transient receptor potential vanilloid 1, IGLE: intraganglionic laminar endings, IMA: intramuscular arrays, SDA: subdiaphragmatic deafferentation, TR: tension receptor, MR: mucosal receptor, TMR: tension-mucosal receptor, NT-4: neurotrophin-4, HSV129: herpes simplex virus strain 129, AAV: adeno-associated virus, GLP1R: glucagon like peptide 1 receptor, GPR65: G-coupled protein receptor 65, siRNA: small interfering RNA, CCK-SAP: cholecystokinin-saporin, AAVrg: adeno-associated virus retrograde, GETI: genetically encoded transmitter indicator, GECI: genetically encoded calcium indicator.
Vagal Afferent Neuroanatomy
Neuroanatomically, vagal afferents are often classified based on their ganglion of origins and conductance velocity. The former differentiates vagal afferents into jugular and nodose neurons, achieved by injecting neural tracers or performing immunostaining. However, this approach appears to be difficult since there are no molecular markers identified to be selectively expressed in the cell bodies of either the nodose or jugular ganglia. Furthermore, anatomical structure in certain animal models, i.e., mouse, limits the accuracy of separation at the level of the vagal ganglia since it is difficult to anatomically distinguish nodose and jugular ganglia (Nassenstein et al., 2010; Surdenikova et al., 2012). In a recent study, Kupari et al. (2019) distinguished nodose and jugular neurons based on the expression of Phox2b and Prdm12, respectively. This study suggests that vagal ganglia consist of 85% of nodose neurons and 15% of jugular neurons, of which eighteen subtypes of nodose neuron and six subtypes of jugular neuron have been identified (Kupari et al., 2019). Although a role for each subtype has been proposed in this study, based on the key molecular markers expressed, the physiological function of these subtypes requires further confirmation in vitro and in vivo.
Defining vagal afferent subtypes based on their signal transmission speed classifies vagal afferent neurons (VAN) into myelinated, fast conducting A-fibres, moderately myelinated, medium conducting B-fibres and unmyelinated, slow conducting C-fibres. This classification follows Erlanger-Gasser rules of afferent and efferent fibres based on their electrophysiological characteristics (Erlanger and Gasser, 1930). In addition, a secondary approach evaluating microscopic cellular structures (e.g., cell shape and surface characteristic) has also been established to identify nodose A- and C-fibre neurons in culture (Lu et al., 2013). However, it seems to be impractical for in vivo usage. Vagal A-fibres convey afferent visceral information and motor input, vagal B-fibres carry parasympathetic input, while vagal C-fibres deliver afferent visceral information (Ruffoli et al., 2011). Vagal afferent A- and C-fibres project to the GI tract, although, the ratio of composition may vary depending on the region of the GI tract innervated. For instance, the oesophagus is innervated by A- and C-fibre to a similar degree while subdiaphragmatic GI organs are predominantly innervated by C-fibres (Yu et al., 2005; Grabauskas and Owyang, 2017). Intriguingly, conductance velocity does not appear to be directly related to vagal afferent function with the location of innervation possibly acting as the main determinant of vagal afferent function (Page et al., 2002; Yu et al., 2005). Furthermore, the threshold of activation of vagal afferent fibres has also been associated with their physiological function. Low threshold activation is related to non-nociceptive function, such as in mechanosensitive tension and mucosal receptors (Page et al., 2002), whilst high threshold activation is linked to nociceptive-like characteristic, such as nodose C-fibres and jugular A-/C-fibres innervating the oesophagus (Yu et al., 2005).
Morphology of Vagal Afferent Visceral Endings
A more specific vagal afferent classification has been made based on morphological specialisation of vagal afferent endings in the gut wall. This approach distinguishes vagal afferent populations into three subtypes, namely the intraganglionic laminar endings (IGLEs), intramuscular arrays (IMAs) and mucosal afferents (Berthoud and Powley, 1992).
IGLEs have been found in the myenteric plexus, forming fine laminar or aggregate puncta surrounding the myenteric ganglia (Fox et al., 2001a). These IGLEs have been shown to be distributed, without any obvious regional specialisation, across the GI tract (Berthoud et al., 1997). In contrast, IMAs are located in discrete locations, such as the longitudinal (longitudinal IMA) and circular (circular IMA) muscle sheets in the sphincter regions and the stomach (Powley et al., 2012, 2013, 2014, 2016). Circular IMAs are predominant in the lesser curvature while longitudinal IMAs are abundant in the greater curvature region of the stomach (Powley et al., 2016). Despite the telodendria-like classical structure, IMA nerve endings also display specialisations, such as modification of arbours, density, and depth of nerve ending penetration, in particular regions of the GI tract. For instance, IMAs that innervate the pyloric sphincter form an annulus ring (Powley et al., 2014), whilst a shorter and denser IMA population has been observed in the sling and clasp of the lower esophageal sphincter (Powley et al., 2013, 2016).
The mucosal layer of the gut wall is also innervated by vagal projections known as mucosal afferents. These endings penetrate into the lamina propria where they may have contact with epithelial cells but not to the luminal content (Wank and Neuhuber, 2001; Powley et al., 2011). Similar to IMAs, mucosal afferents display specialised substructures with regards to its innervated organs. For instance, four classes of mucosal afferent endings have been identified in the upper cervical oesophagus of the rats (Wank and Neuhuber, 2001). In the small intestine, studies in rats have revealed two distinct substructures innervating the crypts and villi of the proximal small intestine, respectively (Berthoud et al., 1995; Powley et al., 2011; Serlin and Fox, 2020). Whilst these previous studies examined distinct regions of the small intestine, accounting for only about 1-2% of the whole length, a recent study by Serlin and Fox described these endings quantitatively and qualitatively for the entire length of the mouse small intestine (Serlin and Fox, 2020). Furthermore, a mucosal afferent ending specialisation was also observed in the antral gland of the stomach (Powley et al., 2011).
Ending specialisation and the existence of distinct substructures in different regions of the GI tract suggest a specific vagal afferent function. However, it is difficult to prove this premise in vivo since separation of each ending is required. To date, muscular and mucosal afferents can be distinguished using a differential retrograde tracing methodology (Young et al., 2008), however, no technical approach has been developed to selectively trace IGLEs or IMAs. A study has established G-protein coupled receptor (GPCR) profiles of GI vagal afferents in the muscular and mucosal layer of the gut wall (Egerod et al., 2018). Although IGLEs and IMAs were not clearly distinguished in this study, two subtypes of vagal afferents, with distinct GPCR profiles, were described in the muscular layer (Egerod et al., 2018). In addition, Bai et al. (2019) has recently profiled and characterised GI vagal afferents based on the correlation between the morphology of the afferent endings and genetic marker expression that revealed distinct populations of IGLE in the stomach (Glp1R+), IGLE in the small intestine (Oxtr+), mucosal afferents in the pyloric antrum (Sst+/Gpr65+), mucosal afferents in the lesser curvature of the corpus (Calca+), IMAs near gastric antrum and large intestine (Calca+), mucosal endings in the small intestine (Gpr65+), and mucosal afferents in the intestinal villi (Vip+/Uts2b+). Therefore, there is potential for this knowledge to be adapted to develop a molecular-based targeting approach to differentiate the physiological roles of distinct vagal afferents population in the GI tract.
Although the focus of this review is vagal afferent innervation, spinal afferents also project to the upper GI tract. Eight distinct ending subtypes have been identified in the stomach after injection of dextran biotin in DRG T8 – T12 (Spencer et al., 2016a). However, their individual functions are still unclear. Spinal afferents are predominantly known for their function in sensing noxious stimuli. Nevertheless, these fibres also detect innocuous mechanical and chemical stimuli that may account to gut physiology (Schwartz and Gebhart, 2014; Spencer et al., 2014). Furthermore, it is possible that spinal afferents contribute to appetite regulation, with a study suggesting the involvement of spinal afferents in hypoglycemic detection in the portal vein (Fujita and Donovan, 2005). Further studies are required to establish whether gastric spinal afferents play a role in food intake regulation.
Physiological Functions of Vagal Afferent
Vagal afferent endings in the GI tract serve as receptive fields towards various type of stimuli, such as mechanical, chemical and thermal. Based on the response, GI vagal afferents are classified into three major classes, namely mechanoreceptors, chemoreceptors and thermoreceptors.
Vagal Afferent Mechanoreceptors
Vagal mechanosensing is an important component in the physiology of digestive function that is prominent in the stomach. This perception is important for the maintenance of energy homeostasis as well as gut motility and secretion, by detecting physical changes during ingestion and digestion of food. Vagal mechanosensors are located in the mucosal and muscular layer of the gut wall, sensing tension and tactile stimuli (Grundy and Scratcherd, 2011). Based on their response to different types of mechanical stimuli, GI vagal afferents are categorised into tension, mucosal, tension-mucosal receptors, and stretch receptors (Phillips and Powley, 2000; Brookes et al., 2013).
Tension receptors
Tension-sensitive vagal afferents were first described by Iggo in 1955, termed as “in-series” tension receptors following their response to passive distension and active contraction of the smooth muscle (Iggo, 1955). Since then, extensive electrophysiological studies have been conducted to characterise these receptors in different GI organs of various species. In general, tension receptors are identified as slowly adapting, low threshold mechanoreceptors which respond to circular tension and high intensity mucosal stroking (Page and Blackshaw, 1998; Page et al., 2002). In 2000, the first evidence correlating tension receptors to a specialised ending structure, i.e., IGLE, was established (Zagorodnyuk and Brookes, 2000). Genetic-based studies have shown that a population of IGLEs in the stomach expresses glucagon-like peptide 1 receptor (GLP1R) (Williams et al., 2016; Bai et al., 2019). This subtype is specifically activated by mechanical distension in vivo (Williams et al., 2016), reinforcing the possible function of IGLEs as a mechanotransduction site. Furthermore, a population of IGLE expressing oxytocin gene (Oxtr) has also been identified in the small intestine (Bai et al., 2019).
The role of tension receptors in sensing distension has been associated with food intake regulation. Studies in humans have shown that mechanical stretch of the stomach limits food intake by inducing satiation and satiety (Marciani et al., 2001, 2015; Feinle-Bisset, 2016). In animal models, a recent study using opto-and chemogenetic approaches in mice has demonstrated that activation of vagal GLP1R subtypes inhibits neurons expressing Agouti-related protein (AgRP neurons) in the hypothalamus and limits food intake (Bai et al., 2019). This inhibition is rapid but transient (Bai et al., 2019). Gastric tension receptors express a variety of GI hormone receptors and ion channels (Christie et al., 2018; Bai et al., 2019), suggesting interactions between neural and humoral pathways in modulating mechanosensation. For instance, Kentish et al. (2015) proposed the role of transient receptor vanilloid 1 (TRPV1) in gastric vagal afferent signalling, given the evidence of dampened tension receptor mechanosensitivity in TRPV1 knockout mouse. In addition, previous studies have shown that in high fat diet-induced obese mice, diurnal rhythms in gastric tension receptor mechanosensitivity are lost and accompanied by a loss of diurnal rhythms in food intake (Kentish et al., 2013). Further, a reduction in food intake was observed in a chronic stress mouse model where gastric tension receptor mechanosensitivity was increased (Li et al., 2019). To date, the mechanism underpinning modulation of gastric tension receptors in a broader physiological context and disease pathophysiology is unclear and remains to be determined. In addition, it has been shown that activation of mechanosensitive vagal Oxtr also limits food intake by inhibiting AgRP neurons (Bai et al., 2019). Interestingly, activation of Oxtr neurons by intestinal distension produced a rapid and sustained inhibition of AgRP neurons and significantly reduced food intake (Bai et al., 2019), suggesting a potential role of intestinal mechanosensation in the central control of food intake besides its canonical function in the intestinal brake mechanism (Alleleyn et al., 2016). Further studies are required to investigate the orchestration of feeding behaviour involving this subtype alongside gastric tension receptors and humoral pathways.
Besides vagal tension receptors, there are populations of mechanosensitive enteric neurons (ENs) that can respond to various types of mechanical stimuli and act as largely tension or tone-sensitive afferents (Furness et al., 2014; Page and Li, 2018), or length-sensitive afferents that are independent of tension or tone (Spencer and Smith, 2004; Spencer and Hu, 2020). In contrast to IGLEs, mechanosensitive ENs are activated by soma deformation and display no specific mechanotransduction sites, suggesting functional specialisation of these neurons in regulating gut motility (Kugler et al., 2015). IGLEs and ENs are located in close proximity within the myenteric plexus, however, no studies have reported the contribution of their interaction in GI mechanosensitivity (Umans and Liberles, 2018). In a broader context, several studies have proposed a role for vagal nerve and EN interactions in disease pathophysiology, e.g., Parkinson’s disease (Ulusoy et al., 2017). In humans, truncal but not selective vagotomy has been suggested to have a protective effect towards Parkinson’s disease (Liu et al., 2017; Breen et al., 2019). Furthermore, accumulation of α-synuclein, a hallmark of Parkinson’s disease, has been detected in ENs (Anselmi et al., 2018) and vagal nerves have been proposed as the key mediator for α-synuclein transport between the ENs and the brain in mice (Santos et al., 2019). α-synuclein was detected in the DMV and substantia nigra (Kim et al., 2019; Van Den Berge et al., 2019) after injection of pathologic α-synuclein into the muscular layer of the pylorus and duodenum, suggesting gut to brain transport of α-synuclein. Evidence suggests the involvement of vagal efferents in the spread of α-synuclein (Phillips et al., 2008). However, a possible role of vagal afferents in this mechanism requires further examination, particularly given that a circuit involving vagal afferent has been identified to connect the gut and the dopaminergic neurons in the substantia nigra (Han et al., 2018).
A population of high threshold vagal mechanoreceptors have also been identified in the GI tract. Whilst low threshold vagal mechanoreceptors have been related to innocuous physiological responses (Paintal, 1953; Iggo, 1955; Blackshaw et al., 1987; Page et al., 2002) the specialised population of high threshold vagal mechanoreceptors, in the oesophagus, has been associated with nociceptive properties similar to spinal afferents (Yu et al., 2005; Kollarik et al., 2010b). However, further studies are required to reveal the mechanisms since distinct receptors and pathways may be involved.
Stretch receptors
To date, the idea that tension and stretch stimuli in the GI tract are detected by independent vagal mechanoreceptors is still in debate. Mechanosensitive vagal afferents in the stomach were initially described as stretch receptors (Paintal, 1954). Tension receptor vagal afferent mechanoreceptors were generally described as a homogenous population of tension-sensitive afferents that detect both muscle stretch and tension (Iggo, 1955; Phillips and Powley, 2000). Stretch and tension are two different types of forces. Stretch reflects the force needed for muscle extension or contraction, while tension is the force given to maintain muscle length (Phillips and Powley, 2000). The discovery of two distinct endings in the muscle layer of the gut wall raises the possibility of the existence of independent stretch receptors (Berthoud and Powley, 1992), with Phillips and Powley proposing IMAs as stretch receptors (Phillips and Powley, 2000).
Studies have shown that IMAs interact with interstitial cells of Cajal (ICC) via synaptic connexions in the muscle layer (ICC-IM) (Powley et al., 2008). In c-Kit and steel mutant mice, lacking ICC-IMs, there was a selective loss of IMAs, whereas stomach and intestinal IGLEs remained unaltered (Fox et al., 2001b, 2002). Studies have used these mouse models to investigate IMAs function in feeding behaviour, where changes in meal patterns, marked by smaller meal size and increased meal frequency, were observed in both mouse models (Fox et al., 2002; Chi and Powley, 2003). However, there were no changes in total daily food intake. Moreover, c-Kit mice displayed increased sensitivity to CCK (Chi and Powley, 2003). This evidence suggests a potential role of IMAs in short-term feeding regulation, presumably through regulation of the gastric accommodation reflex and gastric emptying. Indeed, ICC-IMs are known to play a key role in the initiation and coordination of GI motor activity (Dickens et al., 2001). Therefore, it is plausible that the absence of this structure may lead to the disruption of gut motility which subsequently impacts feeding behaviour. However, further studies are required to establish direct evidence on this relationship in feeding behaviour. In addition, a subset of circular muscle IMAs (collateral IMAs) projecting within the myenteric ganglia and making contact with ENs in the stomach has also been identified (Powley et al., 2016). Altogether evidence suggests conjoint functions of IMAs in local and central regulation of gut motility, IMAs might facilitate communication between ICC-EN and ICC-central nervous system (CNS), or IMAs may act as primary stretch receptors sending cues to the ICCs, ENs and the CNS to regulate gut motility. However, this is highly speculative and further investigation is required.
Mucosal receptors
In contrast to vagal afferent endings in the muscular layer, the physiological roles of mechanosensitive mucosal afferents in the GI tract are relatively overlooked. Mucosal afferents are fast adapting, low threshold mechanoreceptors which are activated by mucosal stroking (Page and Blackshaw, 1998; Page et al., 2002). Only a few studies have focused on mucosal mechanosensation in the last three decades, where functional roles of mucosal mechanoreceptors were mainly determined based on in vitro study through single fibre electrophysiology (Kentish et al., 2015) or measured in vivo in anaesthetized animal models (Becker and Kelly, 1983). In 1983, Becker and Kelly performed gastric emptying measurements in conscious dogs with severed antral mucosal afferents (Becker and Kelly, 1983). The caveat in this approach is that the removal of the mucosal layer of the antrum involved myotomy which disrupt the muscle layer. This could be a confounding factor as other subtypes of vagal afferent mechanoreceptors also innervate the muscle layer of the gastric antrum (Powley, 2000; Bai et al., 2019). Although these studies have suggested a role of mucosal receptors in gastric emptying, by detecting food particle size, and in the regulation of the vomiting vagal reflex (Becker and Kelly, 1983; Andrews and Wood, 1988; Phillips and Powley, 2000; Kentish et al., 2015), none have directly shown the physiological role of mucosal afferents in vivo. Furthermore, mucosal afferents possess a diversity of morphological substructures and the ability to detect different types of tactile mechanical stimuli (Rodrigo et al., 1970, 1975a; Pedrosa et al., 1976; Wank and Neuhuber, 2001), similar to the cutaneous touch receptor characteristics (Abraira and Ginty, 2013). While this suggests that true mucosal mechanoreceptors may act as touch receptors for the viscera, the presence of polymodal (i.e., detect chemical and thermal stimuli) vagal afferent populations may contradict this premise (Iggo, 1955; Clarke and Davison, 1978; Jänig, 1996; Lennerz et al., 2007). Thus, further studies are required to clarify this deliberation.
Tension-mucosal receptors
In addition to vagal tension and mucosal receptors, a novel receptive field termed tension-mucosal receptor has been observed in the oesophagus of ferret (Page and Blackshaw, 1998). A study using a similar approach was conducted in mice, however, tension-mucosal receptors were not identified (Page et al., 2002). This could be due to the thinness of esophageal tissue in mice, where low intensity mucosal stroking (10 mg von Frey hair) also evokes distension and makes differentiation of tension and tension-mucosal receptors impossible (Page et al., 2002). No anatomical studies have reported the structural existence of this vagal subtype in the gut wall, although an analogue, i.e., mucosal-muscular receptor, has been described in the pelvic and sacral spinal pathway of mouse (Brierley et al., 2018). Studies have proposed that mucosal-muscular afferent endings terminate in the mucosal and muscular layer of the gut wall (Page and Blackshaw, 1998; Brierley et al., 2004), however, it has also been suggested that responses to both tension and mucosal stimuli are transduced at the subepithelial plexus (Brookes et al., 2013). Furthermore, recent studies have characterised spinal afferents in the GI tract and discovered that a single DRG neuron can provide complex endings in the mucosa, myenteric ganglia and circular muscle (Spencer et al., 2016a, 2020), suggesting that signal transduction transmitted by a single DRG neuron could be initiated in different layers of the gut wall. To date, the location of the tension-mucosal vagal afferent endings, where the transduction signal is initiated, and their roles in GI function are inconclusive and require further investigation.
Vagal Afferent Chemoreceptors
The role of vagal afferents in gut chemosensation is crucial. Vagal chemoreceptive fields are distributed in the mucosal lamina propria of the gut wall. This sensory nerve detects a wide range of chemical stimuli, such as gut hormones, nutrients, osmolarity and pH change (Powley and Phillips, 2004). Modulation of vagal chemoreceptor activity can occur through nutrient absorption or increasing of mucosal permeability as in leaky gut. Since mucosal afferents do not make direct contact with luminal content, the chemosensing mechanisms are facilitated by the epithelial cells of the gut wall. Early studies have identified subclasses of vagal afferent chemoreceptors based on their activation by specific nutrients, i.e., vagal glucoreceptors (Mei, 1978), amino acid receptors (Jeanningros, 1982) and fatty acids receptors (Lal et al., 2001). Furthermore, it has been shown that vagal activation is potentially mediated by gut hormones released in the presence of specific nutrients (Dockray, 2003, 2013; Raybould, 2010).
Recently, a novel chemosensitive vagal afferent subtype, expressing G protein receptor 65 (GPR65), that detects intestinal nutrients has been discovered (Williams et al., 2016). GPR65, or T cell death-associated gene 8 (TGAD8), is a proton-sensing, psychosine-sensitive, GPCR that detects extracellular pH change (Wang et al., 2004; Ishii et al., 2005). GPR65 is mainly associated with immune cells and inflammatory responses (McGuire et al., 2009). In neurons, GPR65 are expressed in the CNS and dorsal root ganglia neurons with physiological function associated with pH homeostasis (McGuire et al., 2009) and pain (Huang et al., 2007), respectively.
Vagal GPR65 has been shown to innervate the proximal intestine villi, close to the gastroduodenal junction. Activation of vagal GPR65 was exclusively evoked by food entry to the duodenal bulb and resulted in inhibition of gastric motility, limiting food entry to the small intestine (Williams et al., 2016), but has no effect on feeding behaviour (Bai et al., 2019). The entrance of food into the duodenal bulb involves tactile movement of chyme as well as osmolarity and pH change. Serotonin has been proposed as a key mediator of vagal GPR65 activation, given that these neurons are responsive to serotonin but not CCK or glucagon like peptide 1 (GLP1) (Williams et al., 2016). Serotonin has been shown to be released from the enterochromaffin cells (ECC). Mechanical pressure to the intestinal wall has been suggested as a primary trigger of serotonin release (Hansen and Witte, 2008) although chemical stimuli such as pH changes can also trigger serotonin release (Smith et al., 2006). It has been confirmed in a recent study, that mechanical stimuli can trigger the release of serotonin via a Piezo2-dependent mechanism by a population of ECC cells in the small intestine and colon (Alcaino et al., 2018). In addition to vagal GPR65, a new subpopulation of mucosal afferents, expressing Vip/Ust2b, was discovered to be exclusive in the intestinal villi, where activation of this population has no effect on feeding (Bai et al., 2019).
In the distal part of the intestine, vagal afferents have been identified to make a synaptic connexion with enteroendocrine cells (EECs) via a neuropod. Neuropods are axon-like, long cytoplasmic processes that project from the basolateral side of EECs establishing direct contact with vagal afferents, enteric glia, and efferent fibres in the mucosal lamina propria (Bohórquez and Liddle, 2011; Bohórquez et al., 2014, 2015; Kaelberer and Bohorquez, 2018). With the exception of somatostatin secreting EECs, neuropods appear to be a general characteristic of EECs, with basal process length varying depending on the region of the GI tract (Larsson et al., 1979; Gustafsson et al., 2006). Neuropods were first identified in peptide YY-expressing EECs (PYY-EECs), with a high prevalence in the mouse ileum and colon (Bohórquez and Liddle, 2011). A recent study has demonstrated that infusion of sugar (i.e., sucrose) evokes glutamate mediated vagal afferent firing, with EEC glutamate release through the neuropod (Kaelberer et al., 2018). This study provides the first evidence that vagal afferents and EECs establish a direct contact in nutrient sensing transduction. However, the extent of which this occurs and their functional significance for feeding behaviour remain unclear.
It is generally considered that GI hormones mediate the communication between gut epithelial cells and vagal afferents in nutrient sensing. For instance, the presence of glucose in the small intestine induces the release of serotonin and GLP1, which activates vagal afferents in the intestinal mucosa, resulting in the regulation of gastric emptying, pancreatic exocrine and intestinal fluid secretion (Raybould, 2010). Further, CCK release, triggered by the presence of fatty acids and amino acids, has been shown to activate vagal afferents and induce satiety (Dockray, 2003). The finding of a novel pathway of communication via glutamate, occurring in the presence of nutrients, raises a further question of how vagal afferents are involved in satiety regulation via this route. However, functional roles of this pathway in physiology requires further examination. One model posits that it may occur by regulating EEC sensitivity to nutrients. Neuropods have been suggested to make contact with efferent fibres since post-synaptic markers have been identified in these cells (Kaelberer et al., 2018). Although further studies are required to reveal the downstream pathway, this suggests that the nutrient sensing mechanism may occur in a complex manner. The finding of EEC-vagal afferent circuits clarifies one possible transduction mechanism of nutrient sensing in the gut. However, whether GI hormones are involved in this mechanism or act independently remains to be determined.
Vagal Afferent Thermoreceptors
GI vagal afferents have been suggested to play an important role as a visceral thermosensor (Jänig, 2018). A vagal thermoreceptor is described as being an unmyelinated, mechano- and chemo-insensitive neuron, located in the mucosal layer and able to sense either cold (10-36oC) or warm (39-50oC) temperature, or both in some cases (10-35oC and 40-50oC) (El Ouazzani and Mei, 1982; El Ouazzani, 1984). Some populations of vagal mechano- and chemo-receptors also show thermosensitive activity in the noxious heat or cold temperature range (Lennerz et al., 2007). Vagal thermoreceptors are thought to have a role in detecting temperature changes during ingestion that may contribute to the maintenance of body thermal regulation and/or GI protection towards noxious temperature (Jänig, 2018). However, identifying the roles of these subtypes in vivo is hampered by the difficulty in selectively targeting thermosensitive afferents for electrophysiological recording.
Brainstem Projection and Neural Circuitry of GI Vagal Afferents
GI vagal afferents and their visceral endings have been extensively studied in terms of anatomy and their ability to perceive different types of stimuli (Brookes et al., 2013; Browning et al., 2017; Waise et al., 2018). However, their central circuitry is relatively unexplored. Understanding vagal afferent trafficking in the CNS is important since the same type of receptive field, in the GI tract, may have different central endings and generate different feedback responses (Waise et al., 2018). In food intake regulation, left and right vagal ganglia have been shown to terminate in distinct regions of the NTS and regulate different aspects of physiology to control food intake (Han et al., 2018). Recent studies have examined memory control and right NG-mediated reward circuit in food intake (Han et al., 2018; Suarez et al., 2018). However, the role of the left NG neurons and their central circuits, as well as interaction between neural and humoral pathways in regulating food intake require detailed investigation. Therefore, further studies are required to map neural circuits of particular vagal afferent subtypes based on their location in the gut.
Accessing Vagal Afferent Subtypes
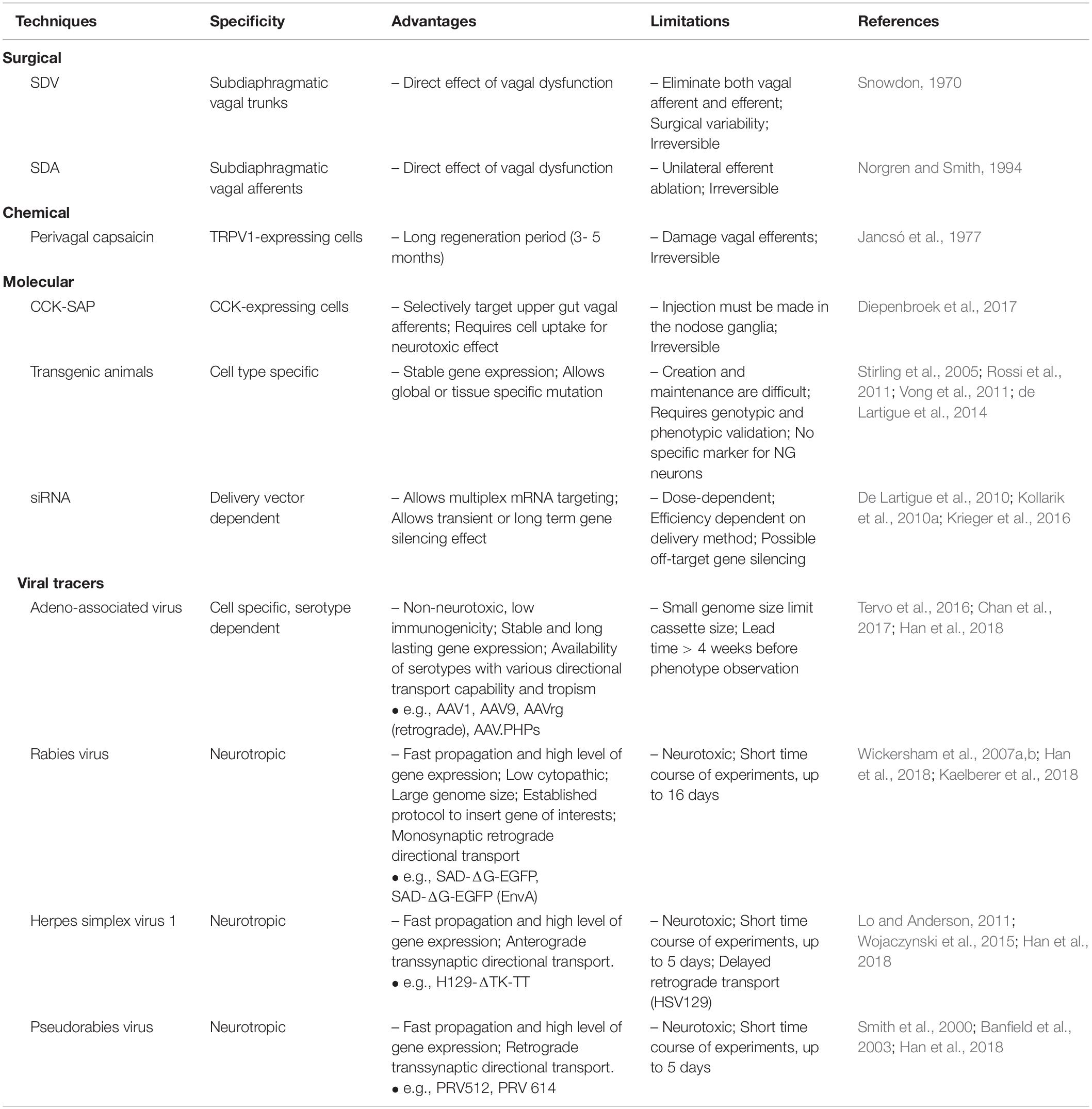
Targeting vagal afferent subtypes has been a longstanding challenge considering the complexity of vagal afferent anatomical organisation, particularly their innervation in the gut (Berthoud and Neuhuber, 2000; Bai et al., 2019). The techniques currently available can be classified into three major classes: surgical, chemical and molecular-based approaches (Figure 3 and Table 2). In this review, we will discuss the basic principles, compare specificity and time course of study, and outline the advantages and limitations for each technique.
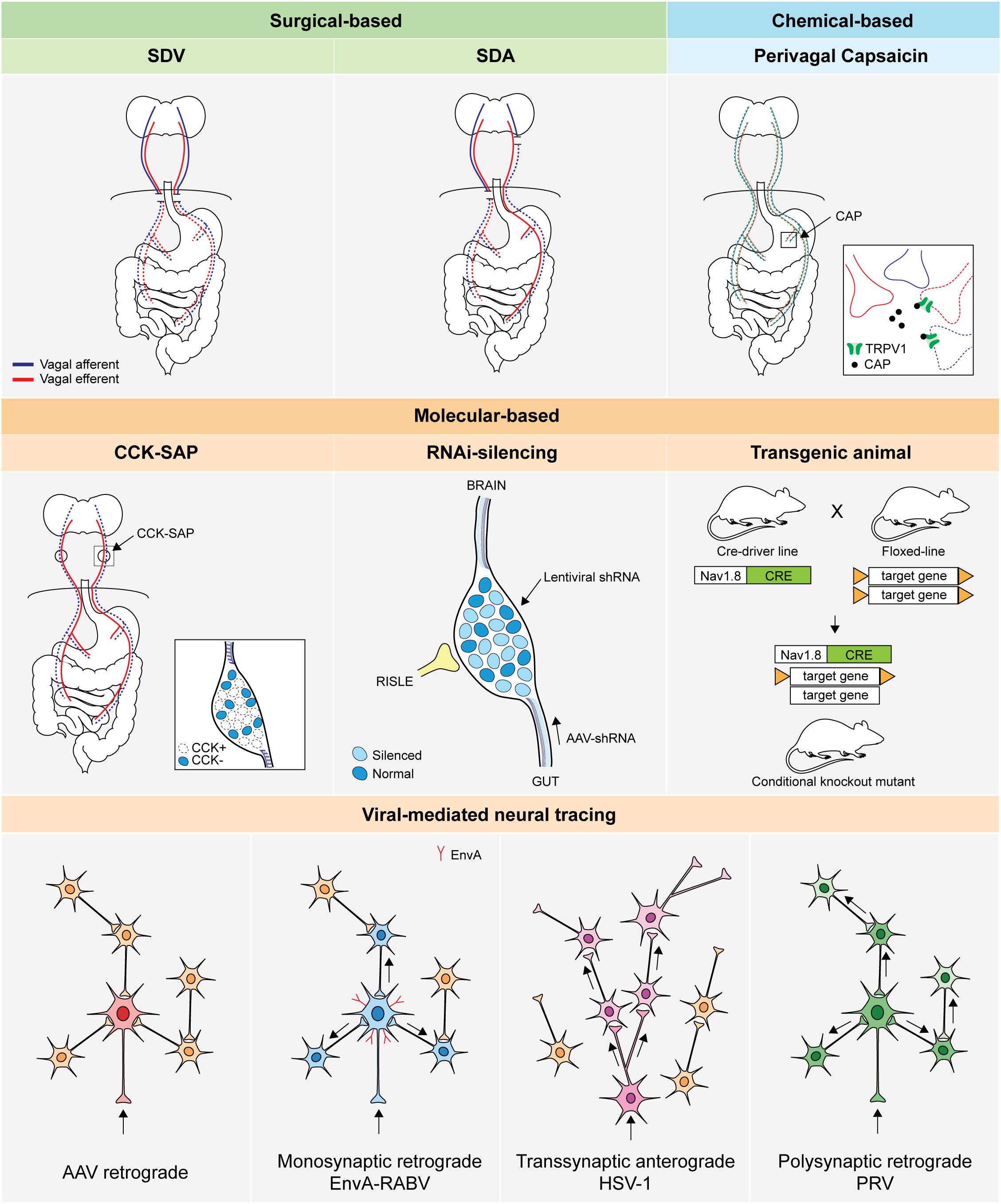
Figure 3. Basic principle of technical approaches to access GI vagal afferents population. An illustration of technical approaches to study vagal afferents. SDV: subdiaphragmatic vagotomy, SDA: subdiaphragmatic deafferentation, CAP: capsaicin, TRPV1: transient receptor potential vanilloid 1,CCK-SAP: cholecystokinin-saporin, RNAi: RNA interference, RISLE: RNAi-induced gene silencing by local electroporation, CRE: Cre recombinase, AAV: adeno-associated virus, EnvA: envelope protein of subgroup A avian sarcoma and leukosis virus, RABV: rabies virus, HSV-1: herpes simplex virus 1, PRV: pseudorabies virus.
Surgical-Based Approaches
Vagotomy is a surgical based approach that removes vagal innervation by cutting the nerve fibres. Dragstedt developed vagotomy as a treatment for peptic ulcer patients in 1943 (Dragstedt and Owens, 1943). The cutting position was initially made on the anterior and posterior vagal trunks using a transthoracic approach (supradiaphragmatic truncal vagotomy) (Dragstedt and Owens, 1943). Since then it has been continuously refined into transabdominal subdiaphragmatic truncal vagotomy, selective vagotomy and highly selective vagotomy (Crile, 1947; Johnston and Goligher, 1976). Peptic ulcer symptoms were improved following supradiaphragmatic truncal vagotomy, however, abnormalities in gastric motility, emptying and secretion were observed (Woodward, 1987). These phenomena have drawn interest on the importance of vagal nerves in GI function and regulation of food intake.
In principle, vagotomy diminishes bidirectional signal traffic between the gut and the brain since both vagal afferent and efferent fibres are excised. Consequently, it becomes difficult to differentiate between the sensory and motor function. In 1970, Snowdon (1970) employed subdiaphragmatic vagotomy (SDV) in a rat model and proposed a role of vagal afferents in peripheral control of food intake. Shortly after, determination of vagal sensory and motor responses was conducted by measuring gastric content and gastric emptying rate respectively, in rats that underwent SDV (Snowdon, 1970). In 1974, Powley and Opsahl demonstrated that SDV neutralised the effect of ventromedial hypothalamus (VMH) lesion to induce obesity in rats but not in genetically obese Zucker rats (Opsahl and Powley, 1974), suggesting a role for the vagus nerve in body weight maintenance. Further examination of vagal afferent and efferent functions was performed by comparing SDV to atropine sulphate treatment. Atropine is an anticholinergic/antimuscarinic agent that abolishes parasympathetic tone via competitive binding to cholinergic or muscarinic receptors (Broadley and Kelly, 2001). This substance is known to inhibit vagal efferent activity (Mittal et al., 1997; Yamakawa et al., 2015). However, studies have shown incomplete motor function blockade due to a non-cholinergic efferent pathway (Powley et al., 1978) and unspecific efferent inhibition in other ganglia (Feldman et al., 1979). Taken together, determination of sensory and motor function using this approach remains problematic.
An improved division of vagal afferent and efferent function using a surgical approach was made in 1994 when subdiaphragmatic vagal deafferentation (SDA) was established in a rat model (Norgren and Smith, 1994). It is known to be the most complete surgical-based vagal deafferentation to date. SDA removes all subdiaphragmatic vagal afferents but leaves 50% of efferent fibres intact by severing at the intracranial vagal afferent or efferent rootlets through a ventral approach (Norgren and Smith, 1994). A similar technique using a dorsal approach, developed earlier, was used to study the role of vagal afferents in the small intestine (Walls et al., 1995).
Completeness of vagotomy can be confirmed using various methods dependent on the type of surgery. Subsets of physiological and anatomical parameters, such as response to insulin, the presence of gastric stasis and impairment of the vago-vagal reflex, were often used to validate the loss of vagal function pre-mortem (Louis-Sylvestre, 1983). After the discovery that the CCK satiety effect is facilitated by gastric vagal branches (Smith et al., 1981), later studies predominantly used a CCK-induced satiety test, administered intraperitoneally at low doses (1 – 6 μg/kg) to validate the success of vagotomy (Moran et al., 1997; Ferrari et al., 2005; Powley et al., 2005; Suarez et al., 2018). In addition, a retrograde tracing protocol using true blue has been developed to validate total and selective vagotomy post-mortem (Powley et al., 1987). This technique provides a complete anatomical evaluation of vagotomized vagal branches and permits validation to the majority of abdominal vagal branches. Furthermore, it has been reported that regeneration may occur following vagotomy. In mice, vagal fibres reinnervate the stomach starting at week 4 post-vagotomy and achieve normal density at week 16 in the corpus, with the optimum time-frame for physiological observation within 8 weeks after vagotomy (Powley et al., 2005). On the other hand, incomplete restoration of vagal afferent innervation in the smooth muscle of rats was observed at 18 weeks (Phillips et al., 2000).
Both SDV and SDA are well-established techniques in rat, however, there are less studies using these techniques in mouse models. Mice undergoing bilateral SDV have been shown to survive for at least two weeks post-surgery (Iwasaki et al., 2015; Yoshii et al., 2017). However, a study has reported the lethality of this procedure in mice due to gastric distension and pyloric stenosis (Dezfuli et al., 2018). Heineke-Mikulicz pyloroplasty has been used to ameliorate pyloric stenosis and increase mouse survival (Dezfuli et al., 2018), however, this may affect the physiological response of the animal. These contradictive outcomes suggest individual variation of the surgical approach.
Chemical-Based Approaches
Capsaicin (CAP) is a pungent component of Capsicum which was isolated in 1876 (Thresh, 1876). CAP has been widely used as an analgesic due to its anti-nociceptive properties, known as the capsaicin desensitisation phenomena (Szolcsányi, 2014). In 1977, Jancsó discovered detrimental effects of CAP on primary sensory neurons of neonatal and adult rats (Jancsó et al., 1977). This study revealed the selective action of CAP in a specific population of primary sensory neurons in the DRG and trigeminal ganglia, referred to as CAP-sensitive sensory neurons. This finding also demonstrated two mechanisms of action of CAP, a short-term excitatory and a long-term neurotoxic effect (Holzer, 1991). However, the molecular mechanism of how CAP generates these effects was unclear at that time.
The excitatory mechanism of CAP was revealed when a capsaicin receptor, termed vanilloid receptor subtype 1 (VR1), was discovered in 1997 (Caterina et al., 1997). This receptor is now known as the transient receptor potential VR1 (TRPV1) channel (Montell et al., 2002), a calcium permeable, non-selective cation channel, that can be activated by numerous agents, e.g., noxious heat, protons, divalent cations (i.e., Mg2+ and Ba2+), exogenous and endogenous TRPV1 agonists, animal toxins and plant secondary metabolites (Caterina et al., 1997; Szolcsányi, 2014; Yang and Zheng, 2017; Christie et al., 2018). CAP binds to the S3-S4 transmembrane regions of the TRPV1 channel and stabilises TRPV1 opening via a “pull and contact” interaction of the S4-S5 linker and vanillyl group (Yang et al., 2015), which increases membrane permeability to cation influx and induces depolarization in a concentration and exposure length dependent manner (Caterina et al., 1997; Chung et al., 2008; Szolcsányi and Sandor, 2012). Furthermore, it has been shown that long-term exposure of capsaicin to HEK293-expressing VR1 cells, DRG and NG neurons induces cell death (Caterina et al., 1997; Czaja et al., 2008). Intracellular acidosis, calcium overload and mitochondrial swelling, due to prolonged opening of TRPV1 channels, have been proposed as underlying mechanisms (Szolcsányi and Pinter, 2013; Chiang et al., 2015).
Systemic injection or application of CAP on vagal afferent visceral endings have been shown to cause neurodegeneration of GI vagal afferents and diminish their sensory signalling (Ritter et al., 1988; Czaja et al., 2008). Commonly, peripheral application of 1% CAP is used to induce vagal afferent lesion in a specific GI organ (Browning et al., 2013). Perivagal CAP has a slower regeneration speed giving a longer experimental time-frame compared to surgical based vagotomy. Studies in rodents have shown a regeneration period of 3 – 5 months post CAP treatment with pronounced loss of vagal neurons within 30 days (Czaja et al., 2008; Gallaher et al., 2011). Since vagal afferents are not the only cells affected by CAP, mineral oil is commonly used to prevent unspecific lesion (Ritter et al., 1988; Patterson et al., 2003). Despite milder intervention and the ease of application compared to surgical based methods, result interpretation should be considered with caution since CAP also destroys vagal efferents in the DMV following perivagal CAP treatment (Browning et al., 2013).
Molecular-Based Approaches
CCK-SAP
In 2017, a novel molecular-based vagal deafferentation technique targeting upper GI tract using CCK conjugated saporin (CCK-SAP) was established. Previously, CCK-SAP has been demonstrated to successfully induce lesion in rostral ventromedial medulla neurons expressing CCK receptor (Zhang et al., 2009). More specific to this review, Diepenbroek et al. (2017) used CCK-SAP to induce neural lesion of VAN in the NG. It has been shown that CCK-SAP ablates ∼80% of muscular and ∼61.7% of mucosal vagal afferents in the upper GI tract of rats and, importantly, leaves the efferent fibres intact.
Cellular selectivity of CCK-SAP action is dependent on CCK, a regulatory peptide hormone in the GI tract, primarily secreted by I-cells and widely known for its function as a satiety hormone (Rehfeld, 2017). CCK consists of 33 amino acids (AA) derived from 115 AA prepro-CCK (Deschenes et al., 1984). Biologically active CCKs emerge in various molecular forms, such as CCK-58, CCK-39, CCK-33, CCK-22, sulphated and unsulphated CCK-8 and CCK-7, CCK-5 and CCK-4 (Noble et al., 1999). CCK-SAP utilises sulphated CCK-8 (CCK8S) as conjugate, which is the predominant structure of the biologically active CCK found in the brain (Schneider et al., 1979).
CCK binds to CCK receptor (CCKR), a member of the GPCR superfamily consisting of a 7 transmembrane domain, to enter the cell. As a GPCR, CCKRs undergo endocytosis following ligand binding, forming a coated vesicle that is transported to the endosome where CCKRs can be recycled back to the plasma membrane or degraded in the lysosome (Koenig and Edwardson, 1997; Weinberg and Puthenveedu, 2019). CCKRs are classified into CCKR-A (CCK1) and CCKR-B (CCK2) based on their affinity to sulphated and amidated CCK (Noble et al., 1999). CCK1 has a high affinity to sulphated/amidated CCK while CCK2 exhibits no preference towards sulphated/non-sulphated CCK. Instead, CCK2 binds to gastrin and is commonly referred to as the gastrin receptor. CCK-SAP demonstrates no significant difference in affinity to CCK1 and CCK2 (Diepenbroek et al., 2017), suggesting neurotoxic effects will occur in cells expressing both receptors. Since CCKRs are widely distributed across the GI tract and nervous system, location of injection becomes a key determinant of CCK-SAP selectivity.
SAP is a type I ribosome inactivating protein (RIP) which causes cell apoptosis, when internalised, due to the impairment of protein synthesis and DNA fragmentation (Stirpe et al., 1983; Bergamaschi et al., 1996; Bagga et al., 2003). Although a small amount of SAP can be internalised via pinocytosis, a conjugate is required for an effective internalisation and cytotoxic effect, since SAP lacks a lectin binding site that facilitates endocytosis (Stirpe et al., 1992). Many neurotoxins have been made by pairing SAP with various conjugates (substance P, isolectin B4 and neuropeptide Y) to selectively ablate neuronal cell populations in the brain (Wiley and Kline, 2000; Wiley, 2001). Besides glycoproteins and neuropeptides, monoclonal antibodies (OX7, 192 IgG, and anti-dopamine beta hydroxylase) have been utilised as conjugates to generate SAP immunotoxins (Wiley and Kline, 2000). These immunotoxins and lectin-toxins exhibit retrograde axonal transport capability, or suicidal transport, via fast axonal transport mediated by microtubules (Wiley and Kline, 2000). However, these properties have not been reported for neurotoxins, including CCK-SAP.
Recent publications have demonstrated the feasibility of CCK-SAP to be used with other techniques to target vagal afferents. For instance, this technique was performed in combination with viral mediated neural tracing in mice to reveal the gut-brain axis in reward mechanisms (Han et al., 2018). Further, CCK-SAP has also been utilised to differentiate vagal sensory and motor signalling in memory control, whilst demonstrating CCK-SAP superiority compared to SDA (Suarez et al., 2018). Besides neuropsychological features, CCK-SAP provides an option to examine vagal function in homeostatic control of appetite. VANs express both CCKRs, with CCK1 more abundant than CCK2 (Moriarty et al., 1997). Studies have shown CCK1 localization with receptors of orexigenic hormones (ghrelin (Date et al., 2005; Burdyga et al., 2006b), orexin-A (Burdyga et al., 2003) and melanin concentrating hormone (Burdyga et al., 2006a), anorexigenic hormones (PYY (Burdyga et al., 2008), GLP-1 (Williams et al., 2016), and leptin (Burdyga et al., 2002; Li et al., 2011), as well as TRPV1 channels (Burdyga et al., 2006b) and cannabinoid CB1 receptors (Burdyga et al., 2004). Thus, lesioning CCK1-positive nodose neurons also impairs satiety signals trafficking between the gut and the brain mediated by these receptors. Furthermore, it is likely that regeneration of vagal afferent fibres may occur after CCK-SAP ablation. In the original study, the blunted effect of CCK-induced satiety was still present 12 weeks after CCK-SAP treatment (Diepenbroek et al., 2017), suggesting vagal function has not recovered within this period. Given that CCK-SAP destroys neuronal cell bodies similar to CAP, it is possible that the regeneration process happens at a slow rate. However, further histological studies are required to examine the regeneration time course.
Cre/LoxP System
The Cre/loxP system plays a vital role in technical advances of vagal afferent targeting. Discovered in 1981, Cre is a 38 kDa, site-specific tyrosine recombinase, isolated from bacteriophage P1 that facilitates double stranded DNA (dsDNA) recombination by targeting loxP, a 34 base-pair (bp) sequence, comprising of two identical 13 bp inverted repeats, separated by a 8 bp spacer region (Sternberg and Hamilton, 1981; Hoess et al., 1982). The mechanism of Cre-mediated recombination will not be discussed since it has been extensively reviewed elsewhere (Lee and Sadowski, 2003; Van Duyne, 2015). Since position, orientation and type of loxP determine the final outcome of Cre-mediated recombination (Hoess et al., 1984; Abremski and Hoess, 1985), several loxP mutants were generated to improve control of gene expression and feasibility to insert gene of interests, such as loxRE and loxLE (Araki et al., 1997), lox511 (Schnutgen et al., 2003), lox2272 and loxFAS (Saunders et al., 2012). Schnutgen et al. developed a flip excision (FLEx) switch system with lox511 which was adapted by Saunders et al. to create Cre-on and Cre-off recombinant adeno-associated virus with lox2272 and loxFAS (Schnutgen et al., 2003; Saunders et al., 2012; Saunders and Sabatini, 2015).
Cre has been shown to effectively facilitate DNA recombination in prokaryotic cells (Sternberg and Hamilton, 1981), yeast (Sauer, 1987), mammalian cells (Sauer and Henderson, 1988) and rodent models. To increase spatiotemporal control of Cre activity, different strategies employing an inducible system were created, e.g., tamoxifen-inducible Cre (Cre-ERT, Cre-ERT1 and Cre-ERT2) (Feil et al., 1996, 1997) and tetracycline-dependent Cre-expression (Tet-On/Tet-Off system) (Gossen and Bujard, 1992; Gossen et al., 1995; Schönig et al., 2002). Tamoxifen is a synthetic agonist of the oestrogen receptor which is converted into its derivatives in the liver by cytochrome P450 that can be administered via oral, subcutaneous or intraperitoneal routes at different doses and forms (Goetz et al., 2008; Jahn et al., 2018). On the other hand, a tetracycline analogue (doxycycline) can be administered via oral (Saam and Gordon, 1999; Lindeberg et al., 2002), intraperitoneal and local injections (Utomo et al., 1999). Further, it is important to note that tamoxifen and doxycycline may introduce confounding factors that should be considered in designing experiment (Moullan et al., 2015; Hammad et al., 2018).
Transgenic Animal Models
The use of transgenic animal models marked the entry of molecular tools to study vagal afferents. In the early 2000s, developmental studies using neutrophin-4 (NT-4) and c-Kit mutant mice demonstrated specific aberration of vagal afferent subtypes in the GI tract (Fox et al., 2001a, b). These mouse models exhibit changes in meal patterns that suggest a role of vagal afferents in short-term feeding regulation. NT-4 is a potent survival factor of CNS and peripheral nervous system (PNS) neuronal development (Huang and Reichardt, 2001). Mice lacking NT-4 exhibit a 55% reduction in size and number of neurons in nodose-petrosal and geniculate sensory ganglia (Conover et al., 1995; Liu et al., 1995). An anterograde labelling study using wheat germ agglutinin-horseradish peroxidase (WGA-HRP) has demonstrated major loss of IGLEs in the duodenum and ileum (90 and 81%, respectively) while the stomach innervation remained unaltered (Fox et al., 2001a). On the other hand, heterozygous c-Kit mutant mice exhibit deficiency of IMA formation in the forestomach (Fox et al., 2001b). This model is also ICC-IM deficient (Burns et al., 1996). c-Kit is a receptor tyrosine kinase, encoded by gene in white spotting (W) locus in chromosome 5 in mice (Bernstein et al., 1990). Spontaneous mutation can occur in W locus, affecting c-Kit expression and altering embryonic development and hematopoiesis (Geissler et al., 1988). c-Kit mutant mice were initially developed as a macrocytic anaemia model (Russell, 1979; Chabot et al., 1988). The absence of specific GI vagal afferent subtypes indicates that these models may be suitable for targeting IGLE or IMA in a particular GI organ. However, apart from the studies above, to date no other studies have reported use of these transgenic animals to investigate vagal afferent function in the gut.
Later studies predominantly utilise Cre/loxP technology to generate a more precise genetic modification in transgenic animals. Development of Cre-driver and Cre-dependent mouse lines are rapidly growing. However, major caveats of using this approach are the difficulties in creating, validating and maintaining the transgenic animal lines. Cre-driver and/or Cre-dependent mouse lines are exposed to the possibility of nonspecific gene expression, variability in breeding efficiency and Cre toxicity (Heffner et al., 2012). Thus, genotypic and phenotypic profiling are required to validate the transgenic animal characteristics. Such information for the majority of established transgenic mouse lines can be obtained from databases, e.g., CrePortal1 (Heffner et al., 2012). Studies by Fox et al. (2013) and Biddinger and Fox (2014) were the first to use the Cre/loxP system to selectively manipulate vagal afferents and investigate their function in feeding behaviour. They targeted nerve growth factor genes that control vagal sensory development in GI smooth muscle and demonstrated changes in meal size without vagal efferent damage. This strategy have been discussed in detail previously (Fox, 2006). Furthermore, transgenic animal models available for anatomical tracing for visualisation of the gut-brain axis has been recently reviewed (Udit and Gautron, 2013). Hence, we will focus on the frequently used Cre-driver line for parental backgrounds to study vagal afferent function, i.e., Nav1.8-Cre, Vglut2-Cre and Phox2b-Cre.
Nav1.8 is a tetrodotoxin resistant voltage-gated sodium channel particularly expressed in peripheral sensory neurons (Akopian et al., 1996). Studies have reported selectivity of Nav1.8 expression in the small-diameter dorsal root ganglia, trigeminal neurons and, importantly, ∼80% of NG neurons (Stirling et al., 2005; Gautron et al., 2011). Stirling et al. (2005) developed a heterozygous Nav1.8-Cre line mouse model which expresses Cre recombinase under Nav1.8 promoter regulation (Stirling et al., 2005). Genetic tracing of Nav1.8-Cre revealed the predominant innervation of Nav1.8 neurons in the mucosa and myenteric plexus of the stomach and small intestine, where IGLE morphology but not IMA was observed in the muscle layer (Gautron et al., 2011). This should be taken into consideration if individuals are aiming to understand a specific population of vagal afferent. Nonetheless, Nav1.8-Cre has become a versatile parental background to generate models for understanding the role of vagal afferents in food intake control. For instance, global knockout of Nav1.8 was generated to investigate vagal role in caloric intake regulation and pain sensing mechanism by crossing Nav1.8-Cre with a mouse line carrying floxed-STOP-DTA (Abrahamsen et al., 2008; Udit et al., 2017). Further, de Lartigue et al. (2014) examine the role of leptin receptor in vagal afferents by selective knockout of leptin receptor in Nav1.8 neurons (Nav1.8/LepRfl/fl). Additionally, other transgenic animals, such as the Nav1.8 null model (Akopian et al., 1999), BAC-Nav1.8-Cre (Agarwal et al., 2004), and heterozygous Nav1.8 Cre-ERT2 (Zhao et al., 2006) have also been developed.
Vesicular glutamate transporter (VGLUT), a membrane-bound protein facilitating glutamate trafficking into presynaptic vesicles, is known as a marker for glutaminergic neurons. There are three transporters that have been characterised so far, namely VGLUT1 (Bellocchio et al., 2000; Takamori et al., 2000), VGLUT2 (Aihara et al., 2000; Bai et al., 2001; Fremeau et al., 2001) and VGLUT3 (Fremeau et al., 2002; Schafer et al., 2002). The functional role of VGLUT2 is associated with autonomic and sensory pathways (Varoqui et al., 2002). In the CNS, expression of VGLUT2 mRNA is distinct to thalamus, brainstem and deep cerebellar nuclei, and transiently expressed in developing hippocampal neurons (Fremeau et al., 2001, 2004). Whereas in the PNS, expression of VGLUT2 has been reported in DRG (Scherrer et al., 2010) and vagal afferents (Tong et al., 2001; Corbett et al., 2005). There are several VGLUT Cre-driver lines, such as BAC-VGLUT2-Cre (Borgius et al., 2010) and Vglut2-ires-Cre (Vong et al., 2011). These models have been used to differentiate vagal sensory and motor neurons functions (Williams et al., 2016; Han et al., 2018).
Paired-like homeobox 2 (Phox2) genes (e.g., Phox2a and Phox2b), encode homeodomain transcription factors that are essential for sympathetic, parasympathetic and ENs development (Pattyn et al., 1999; Brunet and Pattyn, 2002). Whilst Phox2a is responsible for neuron survival (Valarche et al., 1993), Phox2b is vital for cranial ganglia differentiation to acquire visceral neuron characteristics (D’Autreaux et al., 2011). Phox2b expression is observed in the CNS (visceromotor, branchiomotor, NTS, AP, non-adrenergic centres, and serotonergic neurons) and PNS (epibranchial and autonomic ganglia)(D’Autreaux et al., 2011). The absence of Phox2b resulted in the absence of other CNS and PNS neurons, while epibranchial ganglia were still present although atrophic (Morin et al., 1997; Pattyn et al., 1997; D’Autreaux et al., 2011). Phox2b-Cre mouse line has been widely used to target vagal afferents (Rossi et al., 2011; Scott et al., 2011; Liu et al., 2014; Kaelberer et al., 2018). Characterization of Phox2b-Cre illustrates limited Cre expression in the PNS (Rossi et al., 2011) where cre activity was detected in NG and second order visceral sensory neurons in the NTS but absent in other parasympathetic and sympathetic ganglia (Liu et al., 2014). Indeed, expression of Phox2b is known as a marker to differentiate nodose and jugular neurons (Kupari et al., 2019). While this provides high specificity to target nodose neurons in vitro, alteration in other Phox2b-expressing cells in vivo can be a confounding factor.
RNA Interference-Mediated Gene Silencing
RNA interference (RNAi) is a native regulatory mechanism that controls gene expression via post-transcriptional gene silencing in multicellular organisms (Fire et al., 1998; Elbashir et al., 2001). RNAi-mediated gene silencing causes a hypomorphic effect, a reduction but not a complete loss of phenotype. Two small regulatory, double stranded RNAs (dsRNAs), known as small interfering RNAs (siRNAs) and microRNAs (miRNAs), are important for the initiation of RNAi (Carthew and Sontheimer, 2009). A dsRNA-processing enzyme called dicer, converts these small RNAs into shorter fragments (21-23 bp). These short fragments bind to the Argonaute protein, forming siRNA/miRNA-induced silencing complex (si/miRISC) that recognises targeted gene messenger RNAs (mRNAs) and induces degradation (Mello and Conte, 2004; Setten et al., 2019). While miRNAs are mainly produced by the cells, the source of siRNAs can be endogenous (noncoding dsRNAs) or exogenous (synthetic siRNAs). Further, the mechanism of how siRNAs and miRNAs induce RNAi is distinct. Each siRNA has a complementary sequence of a specific mRNA that guides the binding of siRISC precisely and initiates mRNA cleavage (Lam et al., 2015). Conversely, miRNAs are less specific since one miRISC can identify several different mRNAs, and induce gene silencing via translational repression and mRNA destabilisation, followed by mRNA cleavage (Fabian and Sonenberg, 2012; Jonas and Izaurralde, 2015).
Synthetic siRNA has been widely used to specifically knockdown gene expression in vagal afferents. In general, exogenous siRNA can be introduced into the cells as siRNA particles, or as a sequence embedded into a viral genome and endogenously expressed by the cells as siRNA precursors. As a foreign molecule, siRNA often requires a structural modification or a carrier for efficient transport into the cells (Roberts et al., 2016). Different methods, such as lipofectamine for naked siRNA (Heldsinger et al., 2012) and magnetofection for nanoparticle-conjugated siRNA (De Lartigue et al., 2010), have been used to deliver siRNA particles in VAN cell cultures. In addition, a delivery method using local electroporation (RISLE) was developed to facilitate siRNA delivery in vivo in the brain (Akaneya et al., 2005), and this approach has been successfully replicated to deliver siRNA into NG (Zhou et al., 2010). A lead time of 3-6 days is required for gene silencing to occur with knockdown effects lasting for 2 weeks (Akaneya et al., 2005). It is important to note that direct introduction of exogenous siRNA particles results in a transient gene silencing effect.
Currently, viral vectors are predominantly used to deliver siRNA precursors in a form of short-hairpin RNA (shRNA) in vitro and in vivo. The use of viral vectors increases transport efficiency and provides alternatives for a stable silencing effect, given that they utilise a natural mechanism to enter the cells and to express their genome using the host system. Lentivirus is predominantly used as vector to transfer siRNA (Sakuma et al., 2012). For instance, Krieger et al. (2016) delivered GLP1R shRNA to mouse NG, resulting in 80% silencing of GLP1R expression using lentiviral vector measured 20 days after injection. In vitro, lentiviral vector has been shown to efficiently induce gene silencing in nodose neuron cell culture with 3-4 days incubation preceding the observation (Heldsinger et al., 2012). In addition, Kollarik et al. (2010a) used adeno-associated virus (AAV2/8) to deliver TRPV1 shRNA into NG from vagal afferent endings in the guinea pig oesophagus, efficiently silencing TRPV1 expression. Besides its specificity, siRNA-induced gene silencing provides flexibility to target different protein isoforms by introducing a combination of siRNAs (Wang et al., 2017). This allows simultaneous gene silencing that could be beneficial to understand cellular pathways. However, the silencing effect of siRNA-induced RNAi is dose-dependent. Insufficient amounts of siRNA may lead to inadequate silencing, whereas, excessive amounts of siRNA may induce non-specific gene silencing that alters phenotype (Jackson and Linsley, 2010).
Viral-Mediated Neural Tracing
Neural tracing is a classic method to map anatomical distribution of vagal afferents from their cell bodies or axon terminals. An important feature of neural tracing is the ability to selectively target a specific vagal afferent population based on their location in the gut or the brain, by injecting anterograde or retrograde tracer. This allows a specific examination of the functional properties of a particular vagal afferent population. A variety of biochemical-based tracers, such as cholera toxin subunit B, fluorogold, Phaseolus vulgaris-leucoagglutinin, WGA-HRP, lipophilic carbocyanine dyes, and variants of dextran amines, have been utilised to visualise the vagal gut-brain axis (Berthoud and Powley, 1992; Neuhuber et al., 1998; Powley, 2000; Young et al., 2008; Powley et al., 2013). In recent years, development of neurotropic viral vectors has significantly progressed, leading to ground breaking findings on vagal afferent subtypes and their functions in the gut. Here, we will focus on adeno-associated virus, rabies virus, and herpes viruses. We exclude lentivirus-based neural tracers since they mainly target motor neurons (Hirano et al., 2013; Sheikh et al., 2018).
Adeno-associated virus
Adeno-associated virus (AAV) is a 25 nm, non-enveloped, single stranded DNA (ssDNA) virus, isolated from Adenovirus preparation (Atchison et al., 1965; Balakrishnan and Jayandharan, 2014). Recombinant AAV (rAAV) is generated by replacing life cycle genes (i.e., Rep, Cap, and aap) (Sonntag et al., 2010; Balakrishnan and Jayandharan, 2014) between two T-shaped inverted terminal repeat (ITR) sequences with the gene of interests. The total length of rAAV genome should not exceed the wild type AAV genome (∼5 kbp) to avoid reduction in transduction efficiency (Wu et al., 2010) although strategies to deliver large transgene have been developed (McClements and MacLaren, 2017). rAAVs are highly favourable neural tracers, given their nature as a non-pathogenic, low immunogenic, and self-replication defective virus (Weitzman and Linden, 2011) makes rAAVs less neurotoxic compared to other viral vectors. This vector also provides stable gene expression without transgene integration to the host genome.
Serotypes determine AAV tropism and transport directions as neural tracers. Currently, there are 12 AAV (1-12) serotypes with more than one hundred variants (Gao et al., 2005). The majority of native AAV serotypes have tropisms towards neurons at different degrees. However, AAV9 has been shown to profoundly transduce neurons in the CNS, PNS and enteric nervous system (Howard et al., 2008; Schuster et al., 2014). Furthermore, several serotypes (AAV1 and AAV9) also display anterograde transneuronal tracing properties (Zingg et al., 2017).
Characteristics of AAV can be modified using pseudotyping or direct evolution. Pseudotyped AAV is created by combining capsid and ITR sequences from two different AAV serotypes. Kollarik et al. (2010a) utilised this approach to develop AAV2/8 which has an improved retrograde transport capability and transduction efficiency in esophageal vagal afferents compared to AAV2, AAV2/2, AAV2/7 and AAV 2/9. Denotation of pseudotyped AAV, e.g., AAV 2/8, indicates that the virus carries genome from AAV2 and capsid from AAV8. In 2017, a novel method, namely Cre recombinase based AAV targeted evolution (CREATE), was developed (Chan et al., 2017). This technique uses a Cre/loxP system to generate a library of capsid sequences from one AAV serotype which then undergo in vivo selection, termed as direct evolution, to obtain the serotype with desired characteristics. Several robust neurotrophic AAV serotypes, i.e., AAV9-PHPb (Chan et al., 2017), AAV9-PHPs (Chan et al., 2017), and AAV2-retrograde (Tervo et al., 2016), have been developed through this method. AAV9-PHPb and AAV9-PHPs developed tropism towards CNS and PNS neurons, respectively (Chan et al., 2017). Although it has been shown that AAV9-PHPs effectively transduced DRG neurons, there has been no study reporting the capability of this serotype to transduce vagal afferents. In contrast, AAV2-retrograde (AAVrg) has shown tropism to both CNS and PNS neurons (Tervo et al., 2016; Han et al., 2018). Developed by Tervo et al. (2016), AAVrg has an enhanced retrograde transport ability in neurons. A recent study has shown that AAVrg effectively transduce vagal afferents and is transported in a retrograde manner from the gut to the NG (Han et al., 2018).
Despite its robustness, one caveat of using AAV-based neural tracers is that the lead time to observe phenotype or behavioural changes takes at least 2-6 weeks after injection. This is due to a lag time for conversion of AAV ssDNA to dsDNA and genome instability post dsDNA conversion delaying gene expression (Ferrari et al., 1996; Wang et al., 2007). Self-complementary AAV (scAAV), a double stranded DNA variant of AAV, can be used to shorten the lag time (McCarty, 2008).
Rabies virus
Rabies virus (RABV) is an enveloped, retrograde transsynaptic neurotrophic virus from the Rhabdoviridae family, with a 12 kb negative-sense single stranded RNA genome. RABV envelope protein, called rabies glycoprotein (RG) (Conzelmann et al., 1990), with interaction with its receptors compulsory for infection and propagation to occur (Morimoto et al., 2000; Lafon, 2005). In 2007, Wickersham et al. (2007a, b) developed engineered RABVs, a first order retrograde neural tracer SADΔG-EGFP and a monosynaptic retrograde neural tracer SADΔG-EGFP(EnvA). Both engineered viruses lack RG, which hampers their transsynaptic spread ability. SADΔG-EGFP can infect any neurons, however, it cannot spread outside the initially infected neuron (Wickersham et al., 2007a). This virus has been used to map neural circuits in reward pathways that receive vagal input (Han et al., 2018). In contrast, SADΔG-EGFP(EnvA) express envelope protein of subgroup A avian sarcoma and leukosis virus (EnvA) which allows specific infection on cells expressing EnvA receptor, termed as TVA (Barnard et al., 2006). This strategy has been adapted to target nodose neurons in vitro and ECC expressing CCK in vivo to reveal signal transduction between vagal afferents and neuropods in nutrient sensing (Kaelberer et al., 2018).
RABV-based neural tracers are suitable for tracing back vagal projection from its ending in the viscera or region of the CNS. However, they may not be useful for studying neurons receiving vagal inputs from the NG. RABV has a low cytopathic effect, rapid infection in the CNS and high level of gene expression (Wickersham et al., 2007a, b). With a relatively large genome size and an established protocol to generate recombinant ΔG rabies (Osakada and Callaway, 2013), any gene of interest can be inserted to achieve desired aims. However, neural tracing using RABV-based tracers is only ideal for a short term (up to 16 days) experimental time course due to the pathogenic nature of RABV (Wickersham et al., 2007a). Neurotoxic effects, marked by morphological changes (e.g., blebbing) in surviving neurons occur with prolonged incubation (Wickersham et al., 2007a).
Herpes viruses
Two classes of herpes viruses, namely herpes simplex virus-1 (HSV-1) and pseudorabies virus (PRV), are neurotropic viruses with transneuronal spread ability and tropism to sensory and autonomic neurons (Ugolini et al., 1989; Babic et al., 1993). Both viruses are enveloped, have a linear dsDNA genome (HSV-1:150 kbp and PRV:140 kbp), and require interaction between envelope protein and host cell surface to enter the cells and propagate (Ugolini, 2010). The major difference between HSV-1 and PRV is that only HSV-1 can infect primates (Ugolini, 2010).
Herpes viruses-based neural tracers are generally transmitted in a retrograde direction. However, a unique HSV-1 strain, called HSV-1 strain 129 (H129), displays transneuronal spread in an anterograde manner (Zemanick et al., 1991; Rinaman and Schwartz, 2004). Rinaman and Schwartz utilised H129 to map vagal input from the stomach wall to the CNS (Rinaman and Schwartz, 2004) while Krieger et al. (2018) revealed central neurons receiving vagal input from left NG in controlling brown adipose tissue thermogenesis. In 2011, a Cre-dependent H129 recombinant, called H129ΔTK-TT, was generated (Lo and Anderson, 2011). Han et al. (2018) utilised H129ΔTK-TT to map vagal outputs from the right NG and discovered vagal input to dopaminergic neurons in the substantia nigra.
PRV-based neural tracers were developed from the non-virulent PRV strain Bartha. PRV-Bartha are distinct from other retrograde tracer viruses given their retrograde transport occurs exclusively from postsynaptic to presynaptic neurons (Pickard et al., 2002). Two PRV-based tracers, PRV152 and PRV614, were generated by inserting genes encoding GFP and RFP, respectively (Smith et al., 2000; Banfield et al., 2003). These variants were used to confirm the vagal (right NG)-parabrachial-nigrostriatal circuit and its necessity in food intake regulation (Han et al., 2018).
Similar to RABV, the major concern of using HSV-1 and PRV-based neural tracers is their neurotoxicity, resulting in a short experimental time course of approximately 5 days (Brittle et al., 2004; Lo and Anderson, 2011). Since the neurotoxic effect occurs rapidly, prolonged viral incubation may induce cell death in initially infected neurons, making observation of neural circuits difficult. Furthermore, there is a possibility of unspecific infection due to local spread around the injection site (Ugolini et al., 1987). The dose of viral injection is an important factor affecting this local spread and subsequently transneuronal transfer efficiency. In addition, it is important to note that HSV129 can undergo a delayed retrograde transport, approximately 3 days after initial injection, which may confound results (Wojaczynski et al., 2015).
Assessing Vagal Afferent Subtypes Function
In vivo Modulation of Vagal Afferent Activity
The action potential (AP) is a signature of basic forces in neural activity, excitation and inhibition, which is essential for neural communication. The AP relies on ionic balance that regulates membrane potential through depolarization and hyperpolarization. Following the basic principle of an AP, we can modulate neural activity using genetically encoded optogenetic or chemogenetic tools to switch on or off the signal transmission. A recent review has discussed available optogenetic and chemogenetic tools in neurogastroenterology, with a focus on the enteric nervous system (Boesmans et al., 2018). In this review, we provide an update on the current progress in GI vagal afferents.
Optogenetic refers to the use of opsins to modulate neural activity. Opsins are a family of seven-transmembrane, light-sensitive proteins activated by light exposure at a specific wavelength. Current optogenetic tools, such as channelrhodopsins, halorhodopsins and bacteriorhodopsins are derived from microbial opsins (type I opsins). Channelrhodopsin-2 (ChR2) was the first opsin employed as an optogenetic tool. ChR2 is a light-gated proton pump, which is activated by millisecond exposure to blue light driving depolarization and promoting neural excitation (Boyden et al., 2005). The second generation of ChR2, hChR2(H134R), has an improved protein expression and larger steady state current (Nagel et al., 2005). Recent studies have utilised this variant to directly control vagal afferent modulation in vivo. For instance, Williams et al. (2016) activated vagal GLP1R neurons and revealed their function in sensing gastric distension in anaesthetized mice. In 2018, Han et al. (2018) demonstrated distinct roles of right and left NG in food intake in conscious mice by triggering vagal afferent activation, identifying upper gut vagal afferent function in reward circuits and dopamine release via the right NG (Han et al., 2018). In addition, Bai et al. (2019) selectively activated vagal afferent populations expressing Glp1R as well as Oxtr, and discovered GI vagal afferents subtypes that control feeding (Bai et al., 2019). Besides hChR2(H134R), other improved variants of ChR2 with different kinetics, light wavelength and exposure time are currently available for use (Fenno et al., 2011; Yizhar et al., 2011).
In contrast, halorhodopsins (e.g., NpHR) stimulate neuronal hyperpolarization and inhibit neuronal activity upon yellow/green light exposure (Zhang et al., 2007). Halorhodopsins facilitate anion influx (Cl–) into the cells and require constant light exposure to maintain the active state (Zhang et al., 2007). Improved variants of NpHR, such as eNpHR2.0 and eNpHR3.0, exhibit an enhanced membrane localization, increased peak photocurrent, and a high level of gene expression without toxicity compared to the wild type NpHR, in mammalian cells (Gradinaru et al., 2008, 2010). With the opposite function to channelrhodopsins, a combination of ChR2 and NpHR creates a on and off switch to direct neural activity. Kaelberer et al. (2018) demonstrated this scenario using ChR2 to evoke vagal firing, mimicking sucrose-induced vagal activation during signal transduction in the intestine, and eNpHR3.0 to inhibit vagal firing induced by sucrose.
Bacteriorhodopsins (e.g., Arch), in contrast to the other two forms, are proton pumps that enable proton efflux into the extracellular matrix, thus causing hyperpolarization and totally diminishing neural activity (Chow et al., 2010). A major concern with the use of bacteriorhodopsins is the possible extracellular matrix pH alteration due to proton efflux (Chow et al., 2010). Furthermore, a chimeric optogenetic tool, called OptoXR, has been developed by fusing synthetic opsins and GPCRs to facilitate interrogation of biochemical pathways through GPCR Gs and Gq signalling pathways (Airan et al., 2009). To date, no studies have reported the use of these opsins to investigate GI vagal afferent roles.
The use of optogenetics requires a well-designed experimental plan to avoid bias in result interpretation. Variation of protein expression level and activity, side effects of light exposure and tissue heating (Owen et al., 2019), the depth of targeted tissue and instrumental set up can be confounding factors (Fenno et al., 2011). Although optogenetics provides temporal control of neural activity modulation, consideration should be made if long-term stimulation is required. Long term potentiation may cause alteration in neural plasticity and homeostatic adaptation (Allen et al., 2015). Thus, it may alter phenotypes or behavioural responses during observation. The use of slow ChR2 (Schultheis et al., 2011) or chemogenetic can be an alternative if long-term potentiation is required.
Chemogenetics refers to the use of designer receptors activated by designer agonists to modulate neural activity. The majority of chemogenetic tools are based on GPCRs. Designer receptors activated by designer drugs (DREADDs) are the third generation of GPCR-based chemogenetic agents, with downstream effects mediated through classical GPCR (Gq, Gi, or Gs) (Armbruster et al., 2007) or β-arrestin signalling pathways (Allen and Roth, 2011; Nakajima and Wess, 2012). The first DREADD family was created based on human M3 muscarinic receptor (hM1Dq, hM2Di, hM3Dq hM4Di, and hM5Dq) (Armbruster et al., 2007). Gq-DREADDs cause excitatory effects on neuronal activity by mediating calcium (Ca2+) influx, while presynaptic inhibitory effects and silencing are obtained by Gi-DREADD activation mediated by cAMP β/γ-GIRK signalling (Armbruster et al., 2007; Roth, 2016). For instance, Bai et al. (2019) selectively activated vagal afferent subtypes in the stomach and small intestine using hM3D-Gq. On the other hand, Gs-DREADDs influence cAMP-signalling to modulate neural activity (Guettier et al., 2009). Han et al. (2018) used hM3D-Gs to simultaneously activate vagal afferents innervating the upper gut in both the NG and their targets in the brainstem.
Muscarinic receptor-based DREADDs are activated by clozapine-N-oxide (CNO) (Armbruster et al., 2007). CNO is an inert chemical actuator with rapid CNS penetration and distribution in mice (Bender et al., 1994). The use of CNO permits a longer time-course to control neural activity, as well as simultaneous activation of cells expressing DREADD in different locations. The ease of CNO administration (e.g., via oral intake) also makes the DREADD-based approach less laborious compared to optogenetics. If a strict temporal control is required, another class of chemogenetic tool based on ligand-gated ion channels that modulate neural activity via ionic conductance can be used (Magnus et al., 2011; Sternson and Roth, 2014). Furthermore, the level of basal activity, receptor desensitisation or downregulation, and side effects due to clozapine back metabolism should be taken into consideration when designing chemogenetic experiments (Roth, 2016).
In addition, a thermal-driven approach termed as thermogenetic is currently under development. Similar to its predecessors, thermogenetics permits controlled activation or inhibition of neuronal activity using thermal stimulus (Bernstein et al., 2012). Molecules with inhibitory effects, e.g., Drosophila melanogaster dynamin GTPase (Kitamoto, 2001) and excitatory effects from the thermoTRP family (Dhaka et al., 2006), e.g., Drosophila melanogaster TRPA1 (Viswanath et al., 2003; Hamada et al., 2008), rat TRPM8 (Peier et al., 2002; Peabody et al., 2009) and snake TRPA1 (Ermakova et al., 2017), have been discovered. Studies have demonstrated a successful thermal-induced neural activity modulation in drosophila, zebra fish and mouse cultured neurons, but no evidence in in vivo models have been reported (Bath et al., 2014; Ermakova et al., 2017).
Direct Imaging of Vagal Afferent Activity
Communication between neurons occurs via a neurotransmission process. Four classes of genetically encoded indicators have been developed to detect four stages of neurotransmission: pH changes during vesicle fusion (GEPI), neurotransmitter release (GETI), voltage changes (GEVI) and calcium entry (GECI) (Lin and Schnitzer, 2016). These indicators use fluorescence resonance energy transfer (FRET; or single fluorophore-based), of which fluorophore emission will be shifted upon target recognition, allowing visualisation. Recent findings have demonstrated the use of GETI and GECI to decrypt GI vagal afferent signalling. GETI visualises neurotransmitter movement from specific presynaptic to postsynaptic cells and vice versa (Liang et al., 2015). The current available GETIs are glutamate indicators, such as FLIPE, SuperGluSnFR, and iGluSnFR, which exhibit different sensitivity towards glutamate binding (Okumoto et al., 2005; Hires et al., 2008; Marvin et al., 2013). A single fluorophore glutamate sensor, iGluSnFR, has been used to visualise glutamate transmission and identified synaptic connexion between EEC and vagal afferent terminals in vitro (Kaelberer et al., 2018). Furthermore, GECIs detect calcium, a second messenger for neurotransmitters and membrane depolarization in neurons, entry during an AP (Grienberger and Konnerth, 2012). GCaMP, a family of single fluorophore GECI, is often used for in vivo calcium imaging. For instance, GCaMP3 (Tian et al., 2009) was used to identify vagal GLP1R responses to gastric stretch in a non-intact vagal system in mice (Williams et al., 2016).
In vitro Remodelling
The advancement of stem cell technology has made in vitro remodelling at organ level possible. Stem cell-derived three dimensional cell culture or organoids possess some of the key characteristics at the cellular, anatomical or functional level of a real organ (Rossi et al., 2018). The use of organoids to investigate vagal afferent function was recently introduced. Termed as “a gut-brain circuit in a dish”, Kaelberer et al. (2018) simulated the development of synaptic connexions between neuropods and vagal afferents in vitro using epithelial intestinal organoids. To date, generation of epithelium-derived gastrointestinal organoids for the oesophagus (DeWard et al., 2014), stomach fundus (McCracken et al., 2017), corpus (Stange et al., 2013; Bartfeld et al., 2015), and pyloric antrum (McCracken et al., 2014; Noguchi et al., 2015), small intestine (Sato et al., 2009; Spence et al., 2011), and colon (Sato et al., 2011) are possible. Epithelial organoids permit a close investigation of synapse formation and the interactions involving vagal afferent terminals, particularly the mucosal endings with the gut epithelial cells. This system provides a tool to examine mechanisms of signalling transduction as may occur in the viscera, such as the interaction with gut hormone secreting cells, for instance.
Conclusion
Recent advances in technical approaches have made selective targeting and delineating vagal afferent function possible. It is well established that isolating vagal afferent subtypes has been a major challenge that hampers our progress in understanding their function. In recent years, viral-mediated neural tracing, combined with the use of Cre-dependent constructs and Cre-mouse lines, has produced a highly selective approach to target vagal afferent populations. In combination with opto/chemogenetic, calcium imaging and behavioural analysis, we are now able to investigate the role of vagal afferent subtypes in vivo and map the neural circuitry. This combination of tools, in a sequence, creates flexible yet excellent experimental frameworks to thoroughly examine vagal afferent function. Although neural tracing is robust, this approach may not be suitable to address all subtypes of vagal afferent due to feasibility of site-specific injection or the presence of sub-subtypes. In this case, the use of transgenic animal models may be an advantage, given the possibility of generating a new line based on the genetic profile of vagal afferent subtypes. It is important to note that the initial step to identify vagal afferent genetic profiles also involves neural tracing to isolate candidate vagal afferent neurons. This shows that integration of different techniques can boost the development of new tools and aid the progress of understanding vagal afferent function.
Despite the current advancement, there is still much to learn about the role of GI vagal afferents in gut function and feeding behaviour. One interesting question is how GI vagal afferent subtypes interact with each other to maintain gut function or respond to food-related signals. While the current studies mainly focus on manipulating a single subtype, incorporation of other site specific recombinase, such as Flp/FRT, Dre/rox and Vika/vox, may allow us to investigate integrative function of different populations in one mouse in the future. In fact, a study has reported a creation of those four systems in one single reporter mouse line (Karimova et al., 2018). Furthermore, the discovery of an optoribogenetic candidate that allows light-driven gene expression control (Weber et al., 2019) opens the opportunity to manipulate endogenous expression of ion channels and receptors. Although speculative, this could aid investigation of the sensing mechanisms of GI vagal afferents and their plasticity towards meal-related stimuli in vivo. With these tools, currently in the pipeline, our understanding on GI vagal afferent function could be greatly increased in the near future.
Author Contributions
YW wrote the review under the supervision of GL and AP. GL and AP edited the manuscript and provided assistance with the structure of the review. All authors contributed to the manuscript and approved the submitted version.
Funding
YW was supported by the Australia Awards Scholarships from the Department of Foreign Affairs and Trade, The Government of Australia. GL was funded by the NIH grant R01 DK116004.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AAV: adeno-associated virus; AgRP: Agouti-related protein; BAC: bacterial artificial chromosome; CAP: capsaicin; CCK: cholecystokinin; CCKR: cholecystokinin receptor; CCK-SAP: cholecystokinin-saporin; ChR2: channelrhodopsin-2; CNO: clozapine-N-oxide; CNS: central nervous system; DMV: dorsal motor nucleus of the vagus; DREADDs: designer receptors activated by designer drugs; dsDNA: double stranded DNA; ECC: enterochromaffin cells; EEC: enteroendocrine cells; ENs: enteric neurons; GECI: genetically encoded calcium indicator; GETI: genetically encoded neurotransmitter release indicator; GI: gastrointestinal; GLP1R: glucagon-like peptide 1 receptor; GPCR: G-protein coupled receptor; GPR65: G-protein receptor 65; HSV: herpes simplex virus; ICC: interstitial cells of Cajal; IGLE: intraganglionic laminar endings; IMA: intramuscular arrays; mRNA: messenger RNA; NG: nodose ganglia; NpHR: Natromonas pharaonis halorhodopsin; NTS: nucleus tractus solitarius; PNS: peripheral nervous system; PRV: pseudorabies virus; RABV: rabies virus; RNAi: RNA interference; SDA: subdiaphragmatic vagal deafferentation; SDV: subdiaphragmatic vagotomy; siRNA: small interfering RNA; ssRNA: single stranded RNA; TRPA1: transient receptor potential ankyrin 1; TRPM8: transient receptor potential subfamily M member 8; TRPV1: transient receptor potential vanilloid 1; VAN: vagal afferent neurons; VGLUT: vesicular glutamate transporter.
Footnotes
References
Abrahamsen, B., Zhao, J., Asante, C. O., Cendan, C. M., Marsh, S., Martinez-Barbera, J. P., et al. (2008). The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705. doi: 10.1126/science.1156916
Abraira, V. E., and Ginty, D. D. (2013). The sensory neurons of touch. Neuron 79, 618–639. doi: 10.1016/j.neuron.2013.07.051
Abremski, K., and Hoess, R. (1985). Phage P1 Cre-loxP site-specific recombination. Effects of DNA supercoiling on catenation and knotting of recombinant products. J. Mol. Biol. 184, 211–220. doi: 10.1016/0022-2836(85)90374-2
Agarwal, N., Offermanns, S., and Kuner, R. (2004). Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 38, 122–129. doi: 10.1002/gene.20010
Agostoni, E., Chinnock, J. E., De Daly, M. B., and Murray, J. G. (1957). Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J. Physiol. 135, 182–205. doi: 10.1113/jphysiol.1957.sp005703
Aihara, Y., Mashima, H., Onda, H., Hisano, S., Kasuya, H., Hori, T., et al. (2000). Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J. Neurochem. 74, 2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H., and Deisseroth, K. (2009). Temporally precise in vivo control of intracellular signalling. Nature 458, 1025. doi: 10.1038/nature07926
Akaneya, Y., Jiang, B., and Tsumoto, T. (2005). RNAi-induced gene silencing by local electroporation in targeting brain region. J. Neurophysiol. 93, 594–602. doi: 10.1152/jn.00161.2004
Akopian, A. N., Sivilotti, L., and Wood, J. N. (1996). A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379, 257–262. doi: 10.1038/379257a0
Akopian, A. N., Souslova, V., England, S., Okuse, K., Ogata, N., Ure, J., et al. (1999). The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 2, 541–548. doi: 10.1038/9195
Alcaino, C., Knutson, K. R., Treichel, A. J., Yildiz, G., Strege, P. R., Linden, D. R., et al. (2018). A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. U.S.A. 115, E7632–E7641. doi: 10.1073/pnas.1804938115
Alleleyn, A. M. E., Van Avesaat, M., Troost, F. J., and Masclee, A. A. M. (2016). Gastrointestinal nutrient infusion site and eating behavior: evidence for a proximal to distal gradient within the small intestine? Nutrients 8:117. doi: 10.3390/nu8030117
Allen, B. D., Singer, A. C., and Boyden, E. S. (2015). Principles of designing interpretable optogenetic behavior experiments. Learn. Mem. 22, 232–238. doi: 10.1101/lm.038026.114
Allen, J. A., and Roth, B. L. (2011). Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 51, 117–144. doi: 10.1146/annurev-pharmtox-010510-100553
Andrews, P. L., and Wood, K. L. (1988). Vagally mediated gastric motor and emetic reflexes evoked by stimulation of the antral mucosa in anaesthetized ferrets. J. Physiol. 395, 1–16. doi: 10.1113/jphysiol.1988.sp016905
Anselmi, L., Bove, C., Coleman, F. H., Le, K., Subramanian, M. P., Venkiteswaran, K., et al. (2018). Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinson. Dis. 4:30. doi: 10.1038/s41531-018-0066-0
Araki, K., Araki, M., and Yamamura, K. (1997). Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res. 25, 868–872. doi: 10.1093/nar/25.4.868
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S., and Roth, B. L. (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U.S.A. 104, 5163–5168. doi: 10.1073/pnas.0700293104
Atchison, R. W., Casto, B. C., and Hammon, W. M. (1965). Adenovirus-associated defective virus particle. Science 149, 754–756. doi: 10.1126/science.149.3685.754
Babic, N., Mettenleiter, T. C., Flamand, A., and Ugolini, G. (1993). Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J. Virol. 67, 4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993
Bagga, S., Seth, D., and Batra, J. K. (2003). The cytotoxic activity of ribosome-inactivating protein saporin-6 is attributed to its rRNA N-glycosidase and internucleosomal DNA fragmentation activities. J. Biol. Chem. 278, 4813–4820. doi: 10.1074/jbc.M207389200
Bai, L., Mesgarzadeh, S., Ramesh, K. S., Huey, E. L., Liu, Y., Gray, L. A., et al. (2019). Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143. doi: 10.1016/j.cell.2019.10.031
Bai, L., Xu, H., Collins, J. F., and Ghishan, F. K. (2001). Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem. 276, 36764–36769. doi: 10.1074/jbc.M104578200
Baker, C. V., and Bronner-Fraser, M. (2001). Vertebrate cranial placodes I. embryonic induction. Dev. Biol. 232, 1–61. doi: 10.1006/dbio.2001.0156
Balakrishnan, B., and Jayandharan, G. R. (2014). Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr. Gene Ther. 14, 86–100. doi: 10.2174/1566523214666140302193709
Banfield, B. W., Kaufman, J. D., Randall, J. A., and Pickard, G. E. (2003). Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J. Virol. 77, 10106–10112. doi: 10.1128/jvi.77.18.10106-10112.2003
Barnard, R. J., Elleder, D., and Young, J. A. (2006). Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion. Virology 344, 25–29. doi: 10.1016/j.virol.2005.09.021
Bartfeld, S., Bayram, T., Van De Wetering, M., Huch, M., Begthel, H., Kujala, P., et al. (2015). In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136. doi: 10.1053/j.gastro.2014.09.042
Bath, D. E., Stowers, J. R., Hormann, D., Poehlmann, A., Dickson, B. J., and Straw, A. D. (2014). FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat. Methods 11, 756–762. doi: 10.1038/nmeth.2973
Becker, J. M., and Kelly, K. A. (1983). Antral control of canine gastric emptying of solids. Am. J. Physiol. Gastrointest. Liver Physiol. 245, G334–G338. doi: 10.1152/ajpgi.1983.245.3.G334
Bellocchio, E. E., Reimer, R. J., Fremeau, R. T. Jr., and Edwards, R. H. (2000). Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289, 957–960. doi: 10.1126/science.289.5481.957
Bender, D., Holschbach, M., and Stocklin, G. (1994). Synthesis of n.c.a. carbon-11 labelled clozapine and its major metabolite clozapine-N-oxide and comparison of their biodistribution in mice. Nuclear Med. Biol. 21, 921–925. doi: 10.1016/0969-8051(94)90080-9
Bergamaschi, G., Perfetti, V., Tonon, L., Novella, A., Lucotti, C., Danova, M., et al. (1996). Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br. J. Haematol. 93, 789–794. doi: 10.1046/j.1365-2141.1996.d01-1730.x
Bernstein, A., Chabot, B., Dubreuil, P., Reith, A., Nocka, K., Majumder, S., et al. (1990). The mouse W/c-kit locus. Ciba Found Symp. 148, 158–166.
Bernstein, J. G., Garrity, P. A., and Boyden, E. S. (2012). Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 22, 61–71. doi: 10.1016/j.conb.2011.10.023
Berthoud, H. R., Kressel, M., Raybould, H. E., and Neuhuber, W. L. (1995). Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol. 191, 203–212. doi: 10.1007/BF00187819
Berthoud, H. R., and Neuhuber, W. L. (2000). Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17. doi: 10.1016/s1566-0702(00)00215-0
Berthoud, H. R., Patterson, L. M., Neumann, F., and Neuhuber, W. L. (1997). Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol. 195, 183–191. doi: 10.1007/s004290050037
Berthoud, H. R., and Powley, T. L. (1992). Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319, 261–276. doi: 10.1002/cne.903190206
Biddinger, J. E., and Fox, E. A. (2014). Reduced intestinal brain-derived neurotrophic factor increases vagal sensory innervation of the intestine and enhances satiation. J. Neurosci. 34, 10379–10393. doi: 10.1523/jneurosci.1042-14.2014
Blackshaw, L. A., Grundy, D., and Scratcherd, T. (1987). Involvement of gastrointestinal mechano- and intestinal chemoreceptors in vagal reflexes: an electrophysiological study. J. Auton. Nerv. Syst. 18, 225–234. doi: 10.1016/0165-1838(87)90121-4
Boesmans, W., Hao, M. M., and Vanden Berghe, P. (2018). Optogenetic and chemogenetic techniques for neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 15, 21–38. doi: 10.1038/nrgastro.2017.151
Bohórquez, D. V., and Liddle, R. A. (2011). Axon-like basal processes in enteroendocrine cells: characteristics and potential targets. Clin. Trans. Sci. 4, 387–391. doi: 10.1111/j.1752-8062.2011.00299.x
Bohórquez, D. V., Samsa, L. A., Roholt, A., Medicetty, S., Chandra, R., and Liddle, R. A. (2014). An enteroendocrine cell – enteric glia connection revealed by 3D electron microscopy. PLoS One 9:e89881. doi: 10.1371/journal.pone.0089881
Bohórquez, D. V., Shahid, R. A., Erdmann, A., Kreger, A. M., Wang, Y., Calakos, N., et al. (2015). Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest. 125, 782–786. doi: 10.1172/JCI78361
Borgius, L., Restrepo, C. E., Leao, R. N., Saleh, N., and Kiehn, O. (2010). A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol. Cell Neurosci. 45, 245–257. doi: 10.1016/j.mcn.2010.06.016
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., and Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. doi: 10.1038/nn1525
Breen, D. P., Halliday, G. M., and Lang, A. E. (2019). Gut-brain axis and the spread of alpha-synuclein pathology: vagal highway or dead end? Mov. Disord. 34, 307–316. doi: 10.1002/mds.27556
Brierley, S. M., Hibberd, T. J., and Spencer, N. J. (2018). Spinal afferent innervation of the colon and rectum. Front. Cell Neurosci. 12:467. doi: 10.3389/fncel.2018.00467
Brierley, S. M., Jones, R. C. III, Gebhart, G. F., and Blackshaw, L. A. (2004). Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178. doi: 10.1053/j.gastro.2004.04.008
Brittle, E. E., Reynolds, A. E., and Enquist, L. W. (2004). Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 78, 12951–12963. doi: 10.1128/JVI.78.23.12951-12963.2004
Broadley, J. K., and Kelly, R. D. (2001). Muscarinic receptor agonists and antagonists. Molecules 6:142. doi: 10.3390/60300142
Brookes, S. J., Spencer, N. J., Costa, M., and Zagorodnyuk, V. P. (2013). Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 10, 286–296. doi: 10.1038/nrgastro.2013.29
Browning, K. N., Babic, T., Holmes, G. M., Swartz, E., and Travagli, R. A. (2013). A critical re-evaluation of the specificity of action of perivagal capsaicin. J. Physiol. 591, 1563–1580. doi: 10.1113/jphysiol.2012.246827
Browning, K. N., Verheijden, S., and Boeckxstaens, G. E. (2017). The vagus nerve in appetite regulation, mood and intestinal inflammation. Gastroenterology 152, 730–744. doi: 10.1053/j.gastro.2016.10.046
Brunet, J. F., and Pattyn, A. (2002). Phox2 genes - from patterning to connectivity. Curr. Opin. Genet. Dev. 12, 435–440. doi: 10.1016/s0959-437x(02)00322-2
Burdyga, G., De Lartigue, G., Raybould, H. E., Morris, R., Dimaline, R., Varro, A., et al. (2008). Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J. Neurosci. 28, 11583–11592. doi: 10.1523/jneurosci.2493-08.2008
Burdyga, G., Lal, S., Spiller, D., Jiang, W., Thompson, D., Attwood, S., et al. (2003). Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology 124, 129–139. doi: 10.1053/gast.2003.50020
Burdyga, G., Lal, S., Varro, A., Dimaline, R., Thompson, D. G., and Dockray, G. J. (2004). Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J. Neurosci. 24, 2708–2715. doi: 10.1523/jneurosci.5404-03.2004
Burdyga, G., Spiller, D., Morris, R., Lal, S., Thompson, D. G., Saeed, S., et al. (2002). Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience 109, 339–347. doi: 10.1016/S0306-4522(01)00474-2
Burdyga, G., Varro, A., Dimaline, R., Thompson, D. G., and Dockray, G. J. (2006a). Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience 137, 1405–1415. doi: 10.1016/j.neuroscience.2005.10.057
Burdyga, G., Varro, A., Dimaline, R., Thompson, D. G., and Dockray, G. J. (2006b). Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1289–G1297. doi: 10.1152/ajpgi.00543.2005
Burns, A. J., Lomax, A. E., Torihashi, S., Sanders, K. M., and Ward, S. M. (1996). Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. U.S.A. 93, 12008–12013. doi: 10.1073/pnas.93.21.12008
Carthew, R. W., and Sontheimer, E. J. (2009). Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655. doi: 10.1016/j.cell.2009.01.035
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi: 10.1038/39807
Chabot, B., Stephenson, D. A., Chapman, V. M., Besmer, P., and Bernstein, A. (1988). The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 335, 88–89. doi: 10.1038/335088a0
Chan, K. Y., Jang, M. J., Yoo, B. B., Greenbaum, A., Ravi, N., Wu, W. L., et al. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20:1172. doi: 10.1038/nn.4593
Chi, M. M., and Powley, T. L. (2003). c-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R1170–R1183. doi: 10.1152/ajpregu.00015.2003
Chiang, H., Ohno, N., Hsieh, Y. L., Mahad, D. J., Kikuchi, S., Komuro, H., et al. (2015). Mitochondrial fission augments capsaicin-induced axonal degeneration. Acta Neuropathol. 129, 81–96. doi: 10.1007/s00401-014-1354-3
Chow, B. Y., Han, X., Dobry, A. S., Qian, X., Chuong, A. S., Li, M., et al. (2010). High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102. doi: 10.1038/nature08652
Christie, S., Wittert, G. A., Li, H., and Page, A. J. (2018). Involvement of TRPV1 channels in energy homeostasis. Front. Endocrinol. 9:420. doi: 10.3389/fendo.2018.00420
Chung, M. K., Guler, A. D., and Caterina, M. J. (2008). TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 11, 555–564. doi: 10.1038/nn.2102
Clarke, G. D., and Davison, J. S. (1978). Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J. Physiol. 284, 55–67. doi: 10.1113/jphysiol.1978.sp012527
Conover, J. C., Erickson, J. T., Katz, D. M., Bianchi, L. M., Poueymirou, W. T., Mcclain, J., et al. (1995). Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375, 235–238. doi: 10.1038/375235a0
Conzelmann, K. K., Cox, J. H., Schneider, L. G., and Thiel, H. J. (1990). Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175, 485–499. doi: 10.1016/0042-6822(90)90433-r
Corbett, E. K., Sinfield, J. K., Mcwilliam, P. N., Deuchars, J., and Batten, T. F. (2005). Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience 135, 133–145. doi: 10.1016/j.neuroscience.2005.06.010
Crile, G. Jr. (1947). Subdiaphragmatic vagotomy; indications and technic. Cleve Clin. Q. 14, 65–75. doi: 10.3949/ccjm.14.2.65
Czaja, K., Burns, G. A., and Ritter, R. C. (2008). Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience 154, 621–630. doi: 10.1016/j.neuroscience.2008.03.055
Date, Y., Toshinai, K., Koda, S., Miyazato, M., Shimbara, T., Tsuruta, T., et al. (2005). Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 146, 3518–3525. doi: 10.1210/en.2004-1240
D’Autreaux, F., Coppola, E., Hirsch, M. R., Birchmeier, C., and Brunet, J. F. (2011). Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc. Natl. Acad. Sci. U.S.A. 108, 20018–20023. doi: 10.1073/pnas.1110416108
De Lartigue, G., Dimaline, R., Varro, A., Raybould, H., De La Serre, C. B., and Dockray, G. J. (2010). Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138, 1479–1490. doi: 10.1053/j.gastro.2009.10.034
de Lartigue, G., Ronveaux, C. C., and Raybould, H. E. (2014). Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab. 3, 595–607. doi: 10.1016/j.molmet.2014.06.003
Deschenes, R. J., Lorenz, L. J., Haun, R. S., Roos, B. A., Collier, K. J., and Dixon, J. E. (1984). Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc. Natl. Acad. Sci. U.S.A. 81, 726–730. doi: 10.1073/pnas.81.3.726
DeWard, A. D., Cramer, J., and Lagasse, E. (2014). Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9, 701–711. doi: 10.1016/j.celrep.2014.09.027
Dezfuli, G., Gillis, R. A., Tatge, J. E., Duncan, K. R., Dretchen, K. L., Jackson, P. G., et al. (2018). Subdiaphragmatic vagotomy with pyloroplasty ameliorates the obesity caused by genetic deletion of the melanocortin 4 receptor in the mouse. Front. Neurosci. 12:104. doi: 10.3389/fnins.2018.00104
Dhaka, A., Viswanath, V., and Patapoutian, A. (2006). Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161. doi: 10.1146/annurev.neuro.29.051605.112958
Dickens, E. J., Edwards, F. R., and Hirst, G. D. (2001). Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J. Physiol. 531, 827–833. doi: 10.1111/j.1469-7793.2001.0827h.x
Diepenbroek, C., Quinn, D., Stephens, R., Zollinger, B., Anderson, S., Pan, A., et al. (2017). Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G342–G352. doi: 10.1152/ajpgi.00095.2017
Dockray, G. J. (2003). Luminal sensing in the gut: an overview. J. Physiol. Pharmacol. 54(Suppl. 4), 9–17.
Dockray, G. J. (2013). Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 13, 954–958. doi: 10.1016/j.coph.2013.09.007
Dragstedt, L. R., and Owens, F. M. (1943). Supra-diaphragmatic section of the vagus nerves in treatment of duodenal ulcer. Proc. Soc. Exp. Biol. Med. 53, 152–154. doi: 10.3181/00379727-53-14227
Egerod, K. L., Petersen, N., Timshel, P. N., Rekling, J. C., Wang, Y., Liu, Q., et al. (2018). Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol. Metab. 12, 62–75. doi: 10.1016/j.molmet.2018.03.016
El Ouazzani, T. (1984). Thermoreceptors in the digestive tract and their role. J. Auton. Nerv. Syst. 10, 246–254. doi: 10.1016/0165-1838(84)90020-1
El Ouazzani, T., and Mei, N. (1982). Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 83, 995–1001. doi: 10.1016/s0016-5085(82)80065-6
Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498. doi: 10.1038/35078107
Erlanger, J., and Gasser, I. L. S. (1930). The action potential in fibers of slow conduction in spinal roots and somatic nerves. Am. J. Phys. 92, 43–82. doi: 10.3181/00379727-26-4441
Ermakova, Y. G., Lanin, A. A., Fedotov, I. V., Roshchin, M., Kelmanson, I. V., Kulik, D., et al. (2017). Thermogenetic neurostimulation with single-cell resolution. Nat. Commun. 8:15362. doi: 10.1038/ncomms15362
Fabian, M. R., and Sonenberg, N. (2012). The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat. Struct. Mol. Biol. 19, 586–593. doi: 10.1038/nsmb.2296
Feil, R., Brocard, J., Mascrez, B., Lemeur, M., Metzger, D., and Chambon, P. (1996). Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. U.S.A. 93, 10887–10890. doi: 10.1073/pnas.93.20.10887
Feil, R., Wagner, J., Metzger, D., and Chambon, P. (1997). Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757. doi: 10.1006/bbrc.1997.7124
Feinle-Bisset, C. (2016). Upper gastrointestinal sensitivity to meal-related signals in adult humans - relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 162, 69–82. doi: 10.1016/j.physbeh.2016.03.021
Feldman, M., Richardson, C. T., Taylor, I. L., and Walsh, J. H. (1979). Effect of atropine on vagal release of gastrin and pancreatic polypeptide. J. Clin. Invest. 63, 294–298. doi: 10.1172/JCI109302
Fenno, L., Yizhar, O., and Deisseroth, K. (2011). The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412. doi: 10.1146/annurev-neuro-061010-113817
Ferrari, B., Arnold, M., Carr, R. D., Langhans, W., Pacini, G., Bodvarsdóttir, T. B., et al. (2005). Subdiaphragmatic vagal deafferentation affects body weight gain and glucose metabolism in obese male Zucker (fa/fa) rats. Am. J. Physiol. Regulat. Integr. Comp. Physiol. 289, R1027–R1034. doi: 10.1152/ajpregu.00736.2004
Ferrari, F. K., Samulski, T., Shenk, T., and Samulski, R. J. (1996). Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70, 3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996
Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E., and Mello, C. C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811. doi: 10.1038/35888
Fox, E. A. (2006). A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Auton. Neurosci. 12, 9–29. doi: 10.1016/j.autneu.2006.03.005
Fox, E. A., Biddinger, J. E., Baquet, Z. C., Jones, K. R., and Mcadams, J. (2013). Loss of neurotrophin-3 from smooth muscle disrupts vagal gastrointestinal afferent signaling and satiation. Am. J. Physiol. Regul. Integr. Compar. Physiol. 305, R1307–R1322. doi: 10.1152/ajpregu.00337.2013
Fox, E. A., Phillips, R. J., Baronowsky, E. A., Byerly, M. S., Jones, S., and Powley, T. L. (2001a). Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J. Neurosci. 21, 8602–8615. doi: 10.1523/jneurosci.21-21-08602.2001
Fox, E. A., Phillips, R. J., Martinson, F. A., Baronowsky, E. A., and Powley, T. L. (2001b). C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat. Embryol. 204, 11–26. doi: 10.1007/s004290100184
Fox, E. A., Phillips, R. J., Byerly, M. S., Baronowsky, E. A., Chi, M. M., and Powley, T. L. (2002). Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat. Embryol. 205, 325–342. doi: 10.1007/s00429-002-0261-x
Fox, E. A., Phillips, R. J., Martinson, F. A., Baronowsky, E. A., and Powley, T. L. (2000). Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J. Comp. Neurol. 428, 558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m
Fremeau, R. T. Jr., Burman, J., Qureshi, T., Tran, C. H., and Proctor, J. (2002). The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. U.S.A. 99, 14488–14493. doi: 10.1073/pnas.222546799
Fremeau, R. T. Jr., Kam, K., Qureshi, T., Johnson, J., and Copenhagen, D. R. (2004). Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304, 1815–1819. doi: 10.1126/science.1097468
Fremeau, R. T. Jr., Troyer, M. D., Pahner, I., Nygaard, G. O., and Tran, C. H. (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31, 247–260. doi: 10.1016/s0896-6273(01)00344-0
Fujita, S., and Donovan, C. M. (2005). Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes 54, 3258–3264. doi: 10.2337/diabetes.54.11.3258
Furness, J. B., Callaghan, B. P., Rivera, L. R., and Cho, H. J. (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71. doi: 10.1007/978-1-4939-0897-4_3
Gallaher, Z. R., Ryu, V., Larios, R. M., Sprunger, L. K., and Czaja, K. (2011). Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat. Front. Neurosci. 5:12. doi: 10.3389/fnins.2011.00012
Gao, G., Vandenberghe, L. H., and Wilson, J. M. (2005). New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5, 285–297. doi: 10.2174/1566523054065057
Gautron, L., Sakata, I., Udit, S., Zigman, J. M., Wood, J. N., and Elmquist, J. K. (2011). Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J. Comp. Neurol. 519, 3085–3101. doi: 10.1002/cne.22667
Geissler, E. N., Ryan, M. A., and Housman, D. E. (1988). The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 55, 185–192. doi: 10.1016/0092-8674(88)90020-7
Goetz, M. P., Kamal, A., and Ames, M. M. (2008). Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin. Pharmacol. Ther. 83, 160–166. doi: 10.1038/sj.clpt.6100367
Gossen, M., and Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 89, 5547–5551. doi: 10.1073/pnas.89.12.5547
Gossen, M., Freundlieb, S., Bender, G., Muller, G., Hillen, W., and Bujard, H. (1995). Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769. doi: 10.1126/science.7792603
Grabauskas, G., and Owyang, C. (2017). Plasticity of vagal afferent signaling in the gut. Medicina 53, 73–84. doi: 10.1016/j.medici.2017.03.002
Gradinaru, V., Thompson, K. R., and Deisseroth, K. (2008). eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139. doi: 10.1007/s11068-008-9027-6
Gradinaru, V., Zhang, F., Ramakrishnan, C., Mattis, J., Prakash, R., Diester, I., et al. (2010). Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165. doi: 10.1016/j.cell.2010.02.037
Grienberger, C., and Konnerth, A. (2012). Imaging calcium in neurons. Neuron 73, 862–885. doi: 10.1016/j.neuron.2012.02.011
Grundy, D., and Scratcherd, T. (2011). “Sensory afferents from the gastrointestinal tract,” in Comprehensive Physiology, ed. R. Terjung (Hoboken, NJ: Wiley-Blackwell Publishing Ltd), 593–620. doi: 10.1002/cphy.cp060116
Guettier, J. M., Gautam, D., Scarselli, M., Ruiz De Azua, I., Li, J. H., Rosemond, E., et al. (2009). A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 19197–19202. doi: 10.1073/pnas.0906593106
Gustafsson, B. I., Bakke, I., Tommeras, K., and Waldum, H. L. (2006). A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand. J. Gastroenterol. 41, 390–395. doi: 10.1080/00365520500331281
Hamada, F. N., Rosenzweig, M., Kang, K., Pulver, S. R., Ghezzi, A., Jegla, T. J., et al. (2008). An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220. doi: 10.1038/nature07001
Hammad, S., Othman, A., Meyer, C., Telfah, A., Lambert, J., Dewidar, B., et al. (2018). Confounding influence of tamoxifen in mouse models of Cre recombinase-induced gene activity or modulation. Arch. Toxicol. 92, 2549–2561. doi: 10.1007/s00204-018-2254-4
Han, W., Tellez, L. A., Perkins, M. H., Perez, I. O., Qu, T., Ferreira, J., et al. (2018). A neural circuit for gut-induced reward. Cell 175, 665–678.e23. doi: 10.1016/j.cell.2018.08.049
Hansen, M. B., and Witte, A. B. (2008). The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 193, 311–323. doi: 10.1111/j.1748-1716.2008.01870.x
Heffner, C. S., Herbert Pratt, C., Babiuk, R. P., Sharma, Y., Rockwood, S. F., Donahue, L. R., et al. (2012). Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat. Commun. 3:1218. doi: 10.1038/ncomms2186
Heldsinger, A., Lu, Y., Zhou, S. Y., Wu, X., Grabauskas, G., Song, I., et al. (2012). Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1042–G1051. doi: 10.1152/ajpgi.00231.2012
Hirano, M., Kato, S., Kobayashi, K., Okada, T., Yaginuma, H., and Kobayashi, K. (2013). Highly efficient retrograde gene transfer into motor neurons by a lentiviral vector pseudotyped with fusion glycoprotein. PLoS One 8:e75896. doi: 10.1371/journal.pone.0075896
Hires, S. A., Zhu, Y., and Tsien, R. Y. (2008). Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc. Natl. Acad. Sci. U.S.A. 105, 4411–4416. doi: 10.1073/pnas.0712008105
Hoess, R., Abremski, K., and Sternberg, N. (1984). The nature of the interaction of the P1 recombinase Cre with the recombining site loxP. Cold Spring Harb. Symp. Quant. Biol. 49, 761–768. doi: 10.1101/sqb.1984.049.01.086
Hoess, R. H., Ziese, M., and Sternberg, N. (1982). P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. U.S.A. 79, 3398–3402. doi: 10.1073/pnas.79.11.3398
Holzer, P. (1991). Capsaicin as a tool for studying sensory neuron functions. Adv. Exp. Med. Biol. 298, 3–16. doi: 10.1007/978-1-4899-0744-8_1
Howard, D. B., Powers, K., Wang, Y., and Harvey, B. K. (2008). Tropism and toxicity of adeno-associated viral vector serotypes 1,2,5,6,7,8,9 in rat neurons and glia in vitro. Virology 372, 24–34. doi: 10.1016/j.virol.2007.10.007
Huang, C. W., Tzeng, J. N., Chen, Y. J., Tsai, W. F., Chen, C. C., and Sun, W. H. (2007). Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol. Cell Neurosci. 36, 195–210. doi: 10.1016/j.mcn.2007.06.010
Huang, E. J., and Reichardt, L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736. doi: 10.1146/annurev.neuro.24.1.677
Iggo, A. (1955). Tension receptors in the stomach and the urinary bladder. J. Physiol. 128, 593–607. doi: 10.1113/jphysiol.1955.sp005327
Ishii, S., Kihara, Y., and Shimizu, T. (2005). Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 280, 9083–9087. doi: 10.1074/jbc.M407832200
Iwasaki, Y., Maejima, Y., Suyama, S., Yoshida, M., Arai, T., Katsurada, K., et al. (2015). Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R360–R369. doi: 10.1152/ajpregu.00344.2014
Jackson, A. L., and Linsley, P. S. (2010). Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 9, 57–67. doi: 10.1038/nrd3010
Jahn, H. M., Kasakow, C. V., Helfer, A., Michely, J., Verkhratsky, A., Maurer, H. H., et al. (2018). Refined protocols of tamoxifen injection for inducible DNA recombination in mouse astroglia. Sci. Rep. 8:5913. doi: 10.1038/s41598-018-24085-9
Jancsó, G., Kiraly, E., and Jancso-Gabor, A. (1977). Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270, 741–743. doi: 10.1038/270741a0
Jänig, W. (1996). Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol. Psychol. 42, 29–51. doi: 10.1016/0301-0511(95)05145-7
Jänig, W. (2018). “Peripheral thermoreceptors in innocuous temperature detection,” in Handbook of Clinical Neurology, ed. A. A. Romanovsky (Amsterdam: Elsevier Science Inc), 47–56. doi: 10.1016/B978-0-444-63912-7.00002-3
Jeanningros, R. (1982). Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiol. Behav. 28, 9–21. doi: 10.1016/0031-9384(82)90094-4
Johnston, D., and Goligher, J. C. (1976). Selective, highly selective, or truncal vagotomy? In 1976 – a clinical appraisal. Surg. Clin. North Am. 56, 1313–1334. doi: 10.1016/s0039-6109(16)41086-8
Jonas, S., and Izaurralde, E. (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 16, 421–433. doi: 10.1038/nrg3965
Kaelberer, M. M., and Bohorquez, D. V. (2018). The now and then of gut-brain signaling. Brain Res. 1693, 192–196. doi: 10.1016/j.brainres.2018.03.027
Kaelberer, M. M., Buchanan, K. L., Klein, M. E., Barth, B. B., Montoya, M. M., Shen, X., et al. (2018). A gut-brain neural circuit for nutrient sensory transduction. Science 361:eaat5236. doi: 10.1126/science.aat5236
Karimova, M., Baker, O., Camgoz, A., Naumann, R., Buchholz, F., and Anastassiadis, K. (2018). A single reporter mouse line for Vika, Flp, Dre, and Cre-recombination. Sci. Rep. 8:14453. doi: 10.1038/s41598-018-32802-7
Kentish, S. J., Frisby, C. L., Kennaway, D. J., Wittert, G. A., and Page, A. J. (2013). Circadian variation in gastric vagal afferent mechanosensitivity. J. Neurosci. 33, 19238–19242. doi: 10.1523/jneurosci.3846-13.2013
Kentish, S. J., Frisby, C. L., Kritas, S., Li, H., Hatzinikolas, G., O’donnell, T. A., et al. (2015). TRPV1 channels and gastric vagal afferent signalling in lean and high fat diet induced obese mice. PLoS One 10:e0135892. doi: 10.1371/journal.pone.0135892
Kim, S., Kwon, S. H., Kam, T. I., Panicker, N., Karuppagounder, S. S., Lee, S., et al. (2019). Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s Disease. Neuron 103, 627.e–641.e. doi: 10.1016/j.neuron.2019.05.035
Kitamoto, T. (2001). Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92. doi: 10.1002/neu.1018
Koenig, J. A., and Edwardson, J. M. (1997). Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol. Sci. 18, 276–287. doi: 10.1016/s0165-6147(97)01091-2
Kollarik, M., Carr, M. J., Ru, F., Ring, C. J., Hart, V. J., Murdock, P., et al. (2010a). Transgene expression and effective gene silencing in vagal afferent neurons in vivo using recombinant adeno-associated virus vectors. J. Physiol. 588, 4303–4315. doi: 10.1113/jphysiol.2010.192971
Kollarik, M., Ru, F., and Brozmanova, M. (2010b). Vagal afferent nerves with the properties of nociceptors. Auton. Neurosci. 153, 12–20. doi: 10.1016/j.autneu.2009.08.001
Krieger, J. P., Arnold, M., Pettersen, K. G., Lossel, P., Langhans, W., and Lee, S. J. (2016). Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 65, 34–43. doi: 10.2337/db15-0973
Krieger, J. P., Santos Da Conceicao, E. P., Sanchez-Watts, G., Arnold, M., Pettersen, K. G., Mohammed, M., et al. (2018). Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R708–R720. doi: 10.1152/ajpregu.00068.2018
Kugler, E., Michel, K., Zeller, F., Demir, I., Ceyhan, G., Schemann, M., et al. (2015). Mechanical stress activates neurites and somata of myenteric neurons. Front. Cell Neurosci. 9:342. doi: 10.3389/fncel.2015.00342
Kupari, J., Haring, M., Agirre, E., Castelo-Branco, G., and Ernfors, P. (2019). An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27, 2508–2523. doi: 10.1016/j.celrep.2019.04.096
Lal, S., Kirkup, A. J., Brunsden, A. M., Thompson, D. G., and Grundy, D. (2001). Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907
Lam, J. K. W., Chow, M. Y. T., Zhang, Y., and Leung, S. W. S. (2015). siRNA Versus miRNA as therapeutics for gene silencing. Mol. Ther. Nucleic Acids 4:e252. doi: 10.1038/mtna.2015.23
Larsson, L. I., Goltermann, N., De Magistris, L., Rehfeld, J. F., and Schwartz, T. W. (1979). Somatostatin cell processes as pathways for paracrine secretion. Science 205, 1393–1395. doi: 10.1126/science.382360
Lee, L., and Sadowski, P. D. (2003). Sequence of the loxP site determines the order of strand exchange by the cre recombinase. J. Mol. Biol. 326, 397–412. doi: 10.1016/S0022-2836(02)01429-8
Lennerz, J. K., Dentsch, C., Bernardini, N., Hummel, T., Neuhuber, W. L., and Reeh, P. W. (2007). Electrophysiological characterization of vagal afferents relevant to mucosal nociception in the rat upper oesophagus. J. Physiol. 582, 229–242. doi: 10.1113/jphysiol.2007.130823
Li, H., Buisman-Pijlman, F. T. A., Nunez-Salces, M., Christie, S., Frisby, C. L., Inserra, A., et al. (2019). Chronic stress induces hypersensitivity of murine gastric vagal afferents. Neurogastroenterol. Motil. 31:e13669. doi: 10.1111/nmo.13669
Li, Y., Wu, X., Zhou, S., and Owyang, C. (2011). Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G217–G227. doi: 10.1152/ajpgi.00356.2010
Liang, R., Broussard, G. J., and Tian, L. (2015). Imaging chemical neurotransmission with genetically encoded fluorescent sensors. ACS Chem. Neurosci. 6, 84–93. doi: 10.1021/cn500280k
Lin, M. Z., and Schnitzer, M. J. (2016). Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19, 1142–1153. doi: 10.1038/nn.4359
Lindeberg, J., Mattsson, R., and Ebendal, T. (2002). Timing the doxycycline yields different patterns of genomic recombination in brain neurons with a new inducible Cre transgene. J. Neurosci. Res. 68, 248–253. doi: 10.1002/jnr.10213
Liu, B., Fang, F., Pedersen, N. L., Tillander, A., Ludvigsson, J. F., Ekbom, A., et al. (2017). Vagotomy and Parkinson disease: a swedish register-based matched-cohort study. Neurology 88, 1996–2002. doi: 10.1212/wnl.0000000000003961
Liu, C. M., Bookout, A. L., Lee, S., Sun, K., Jia, L., Lee, C., et al. (2014). PPARγ in vagal neurons regulates high-fat diet induced thermogenesis. Cell Metab. 19, 722–730. doi: 10.1016/j.cmet.2014.01.021
Liu, X., Ernfors, P., Wu, H., and Jaenisch, R. (1995). Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature 375, 238–241. doi: 10.1038/375238a0
Lo, L., and Anderson, D. J. (2011). A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950. doi: 10.1016/j.neuron.2011.12.002
Louis-Sylvestre, J. (1983). Validation of tests of completeness of vagotomy in rats. J. Auton. Nerv. Syst. 9, 301–314. doi: 10.1016/0165-1838(83)90149-2
Lu, X.-L., Xu, W.-X., Yan, Z.-Y., Qian, Z., Xu, B., Liu, Y., et al. (2013). Subtype identification in acutely dissociated rat nodose ganglion neurons based on morphologic parameters. Intern. J. Biol. Sci. 9, 716–727. doi: 10.7150/ijbs.7006
Magnus, C. J., Lee, P. H., Atasoy, D., Su, H. H., Looger, L. L., and Sternson, S. M. (2011). Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333, 1292–1296. doi: 10.1126/science.1206606
Marciani, L., Cox, E. F., Pritchard, S. E., Major, G., Hoad, C. L., Mellows, M., et al. (2015). Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur. J. Clin. Nutr. 69, 380–384. doi: 10.1038/ejcn.2014.194
Marciani, L., Gowland, P. A., Spiller, R. C., Manoj, P., Moore, R. J., Young, P., et al. (2001). Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G1227–G1233. doi: 10.1152/ajpgi.2001.280.6.G1227
Marvin, J. S., Borghuis, B. G., Tian, L., Cichon, J., Harnett, M. T., Akerboom, J., et al. (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170. doi: 10.1038/nmeth.2333
McCarty, D. M. (2008). Self-complementary AAV vectors; advances and applications. Mol. Ther. 16, 1648–1656. doi: 10.1038/mt.2008.171
McClements, M. E., and MacLaren, R. E. (2017). Adeno-associated virus (AAV) dual vector strategies for gene therapy encoding large transgenes. Yale J. Biol. Med. 90, 611–623.
McCracken, K. W., Aihara, E., Martin, B., Crawford, C. M., Broda, T., Treguier, J., et al. (2017). Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 541, 182–187. doi: 10.1038/nature21021
McCracken, K. W., Cata, E. M., Crawford, C. M., Sinagoga, K. L., Schumacher, M., Rockich, B. E., et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. doi: 10.1038/nature13863
McGuire, J., Herman, J. P., Ghosal, S., Eaton, K., Sallee, F. R., and Sah, R. (2009). Acid-sensing by the T cell death-associated gene 8 (TDAG8) receptor cloned from rat brain. Biochem. Biophys. Res. Commun. 386, 420–425. doi: 10.1016/j.bbrc.2009.05.133
Mei, N. (1978). Vagal glucoreceptors in the small intestine of the cat. J. Physiol. 282, 485–506. doi: 10.1113/jphysiol.1978.sp012477
Mello, C. C., and Conte, D. Jr. (2004). Revealing the world of RNA interference. Nature 431, 338–342. doi: 10.1038/nature02872
Mittal, R. K., Chiareli, C., Liu, J., Holloway, R. H., and Dixon, W. (1997). Atropine inhibits gastric distension and pharyngeal receptor mediated lower oesophageal sphincter relaxation. Gut 41, 285–290. doi: 10.1136/gut.41.3.285
Montell, C., Birnbaumer, L., Flockerzi, V., Bindels, R. J., Bruford, E. A., Caterina, M. J., et al. (2002). A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 9, 229–231. doi: 10.1016/s1097-2765(02)00448-3
Moran, T. H., Baldessarini, A. R., Salorio, C. F., Lowery, T., and Schwartz, G. J. (1997). Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 272, R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245
Moriarty, P., Dimaline, R., Thompson, D. G., and Dockray, G. J. (1997). Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience 79, 905–913. doi: 10.1016/s0306-4522(96)00675-6
Morimoto, K., Foley, H. D., Mcgettigan, J. P., Schnell, M. J., and Dietzschold, B. (2000). Reinvestigation of the role of the rabies virus glycoprotein in viral pathogenesis using a reverse genetics approach. J. Neurovirol. 6, 373–381. doi: 10.3109/13550280009018301
Morin, X., Cremer, H., Hirsch, M. R., Kapur, R. P., Goridis, C., and Brunet, J. F. (1997). Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron 18, 411–423. doi: 10.1016/s0896-6273(00)81242-8
Moullan, N., Mouchiroud, L., Wang, X., Ryu, D., Williams, E. G., Mottis, A., et al. (2015). Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10, 1681–1691. doi: 10.1016/j.celrep.2015.02.034
Nagel, G., Brauner, M., Liewald, J. F., Adeishvili, N., Bamberg, E., and Gottschalk, A. (2005). Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284. doi: 10.1016/j.cub.2005.11.032
Nakajima, K., and Wess, J. (2012). Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 82, 575–582. doi: 10.1124/mol.112.080358
Nassenstein, C., Taylor-Clark, T. E., Myers, A. C., Ru, F., Nandigama, R., Bettner, W., et al. (2010). Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J. Physiol. 588, 4769–4783. doi: 10.1113/jphysiol.2010.195339
Neuhuber, W. L., Kressel, M., Stark, A., and Berthoud, H. R. (1998). Vagal efferent and afferent innervation of the rat esophagus as demonstrated by anterograde DiI and DiA tracing: focus on myenteric ganglia. J. Auton. Nerv. Syst. 70, 92–102. doi: 10.1016/s0165-1838(98)00034-4
Noble, F., Wank, S. A., Crawley, J. N., Bradwejn, J., Seroogy, K. B., Hamon, M., et al. (1999). International union of pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 51, 745–781.
Noguchi, T. K., Ninomiya, N., Sekine, M., Komazaki, S., Wang, P. C., Asashima, M., et al. (2015). Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 17, 984–993. doi: 10.1038/ncb3200
Norgren, R., and Smith, G. P. (1994). A method for selective section of vagal afferent or efferent axons in the rat. Am. J. Physiol. 267, R1136–R1141. doi: 10.1152/ajpregu.1994.267.4.R1136
Okumoto, S., Looger, L. L., Micheva, K. D., Reimer, R. J., Smith, S. J., and Frommer, W. B. (2005). Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. U.S.A. 102, 8740–8745. doi: 10.1073/pnas.0503274102
Opsahl, C. A., and Powley, T. L. (1974). Failure of vagotomy to reverse obesity in the genetically obese Zucker rat. Am. J. Physiol. 226, 34–38. doi: 10.1152/ajplegacy.1974.226.1.34
Osakada, F., and Callaway, E. M. (2013). Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601. doi: 10.1038/nprot.2013.094
Owen, S. F., Liu, M. H., and Kreitzer, A. C. (2019). Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 22, 1061–1065. doi: 10.1038/s41593-019-0422-3
Page, A. J., and Blackshaw, L. A. (1998). An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol. 512(Pt 3), 907–916. doi: 10.1111/j.1469-7793.1998.907bd.x
Page, A. J., and Li, H. (2018). “Gastrointestinal mechanosensory function in health and disease,” in Mechanobiology in Health and Disease, ed. S. W. Verbruggen (Cambridge, MA: Academic Press), 377–414. doi: 10.1016/B978-0-12-812952-4.00013-1
Page, A. J., Martin, C. M., and Blackshaw, L. A. (2002). Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J. Neurophysiol. 87, 2095–2103. doi: 10.1152/jn.00785.2001
Paintal, A. S. (1953). Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature 172, 1194–1195. doi: 10.1038/1721194a0
Paintal, A. S. (1954). A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J. Physiol. 126, 255–270. doi: 10.1113/jphysiol.1954.sp005207
Patterson, L. M., Zheng, H., Ward, S. M., and Berthoud, H. R. (2003). Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 311, 277–287. doi: 10.1007/s00441-002-0682-0
Pattyn, A., Morin, X., Cremer, H., Goridis, C., and Brunet, J. F. (1997). Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development 124, 4065–4075.
Pattyn, A., Morin, X., Cremer, H., Goridis, C., and Brunet, J. F. (1999). The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370. doi: 10.1038/20700
Peabody, N. C., Pohl, J. B., Diao, F., Vreede, A. P., Sandstrom, D. J., Wang, H., et al. (2009). Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J. Neurosci. 29, 3343–3353. doi: 10.1523/jneurosci.4241-08.2009
Pedrosa, J. A., Hernandez, C. J., Rodrigo, J., and Vidal, M. A. (1976). Vegetative innervation of the esophagus. IV. Endings in the tela submucosa and tunica muscularis. Acta Anat. 95, 452–467. doi: 10.1159/000144633
Peier, A. M., Moqrich, A., Hergarden, A. C., Reeve, A. J., Andersson, D. A., Story, G. M., et al. (2002). A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. doi: 10.1016/s0092-8674(02)00652-9
Phillips, R. J., Baronowsky, E. A., and Powley, T. L. (2000). Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J. Comp. Neurol. 421, 325–346. doi: 10.1002/(sici)1096-9861(20000605)421:3<325::aid-cne3>3.0.co;2-9
Phillips, R. J., and Powley, T. L. (2000). Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Rev. 34, 1–26. doi: 10.1016/S0165-0173(00)00036-9
Phillips, R. J., Walter, G. C., Wilder, S. L., Baronowsky, E. A., and Powley, T. L. (2008). Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience 153, 733–750. doi: 10.1016/j.neuroscience.2008.02.074
Pickard, G. E., Smeraski, C. A., Tomlinson, C. C., Banfield, B. W., Kaufman, J., Wilcox, C. L., et al. (2002). Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J. Neurosci. 22, 2701–2710. doi: 10.1523/jneurosci.22-07-02701.2002
Powley, T. (2000). Vagal input to the enteric nervous system. Gut 47, iv30–iv32. doi: 10.1136/gut.47.suppl_4.iv30
Powley, T. L., Baronowsky, E. A., Gilbert, J. M., Hudson, C. N., Martin, F. N., Mason, J. K., et al. (2013). Vagal afferent innervation of the lower esophageal sphincter. Auton. Neurosci. 177, 129–142. doi: 10.1016/j.autneu.2013.03.008
Powley, T. L., Chi, M. M., Baronowsky, E. A., and Phillips, R. J. (2005). Gastrointestinal tract innervation of the mouse: afferent regeneration and meal patterning after vagotomy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R563–R574. doi: 10.1152/ajpregu.00167.2005
Powley, T. L., Fox, E. A., and Berthoud, H. R. (1987). Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am. J. Physiol. 253, R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361
Powley, T. L., Gilbert, J. M., Baronowsky, E. A., Billingsley, C. N., Martin, F. N., and Phillips, R. J. (2012). Vagal sensory innervation of the gastric sling muscle and antral wall: implications for gastro-esophageal reflux disease? Neurogastroenterol. Motil. 24, e526–e537. doi: 10.1111/nmo.12003
Powley, T. L., Hudson, C. N., Mcadams, J. L., Baronowsky, E. A., Martin, F. N., Mason, J. K., et al. (2014). Organization of vagal afferents in pylorus: mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton. Neurosci. 183, 36–48. doi: 10.1016/j.autneu.2014.02.008
Powley, T. L., Hudson, C. N., Mcadams, J. L., Baronowsky, E. A., and Phillips, R. J. (2016). Vagal intramuscular arrays: the specialized mechanoreceptor arbors that innervate the smooth muscle layers of the stomach examined in the rat. J. Comp. Neurol. 524, 713–737. doi: 10.1002/cne.23892
Powley, T. L., Macfarlane, B. A., Markell, M. S., and Opsahl, C. A. (1978). Different effects of vagotomy and atropine on hypothalamic stimulation-induced feeding. Behav. Biol. 23, 306–325. doi: 10.1016/S0091-6773(78)91337-8
Powley, T. L., and Phillips, R. J. (2004). Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 82, 69–74. doi: 10.1016/j.physbeh.2004.04.037
Powley, T. L., and Phillips, R. J. (2011). Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186, 188–200. doi: 10.1016/j.neuroscience.2011.04.036
Powley, T. L., Spaulding, R. A., and Haglof, S. A. (2011). Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol. 519, 644–660. doi: 10.1002/cne.22541
Powley, T. L., Wang, X. Y., Fox, E. A., Phillips, R. J., Liu, L. W., and Huizinga, J. D. (2008). Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 20, 69–79. doi: 10.1111/j.1365-2982.2007.00990.x
Raybould, H. E. (2010). Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 153, 41–46. doi: 10.1016/j.autneu.2009.07.007
Rehfeld, J. F. (2017). Cholecystokinin-from local gut hormone to ubiquitous messenger. Front. Endocrinol. 8:47. doi: 10.3389/fendo.2017.00047
Rinaman, L., Card, J. P., Schwaber, J. S., and Miselis, R. R. (1989). Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J. Neurosci. 9, 1985–1996. doi: 10.1523/jneurosci.09-06-01985.1989
Rinaman, L., and Schwartz, G. (2004). Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci. 24, 2782–2786. doi: 10.1523/jneurosci.5329-03.2004
Ritter, S., Bugarith, K., Dinh, T. T., and Dinh, T. T. (1988). Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J. Comp. Neurol. 271, 79–90. doi: 10.1002/cne.902710109
Roberts, T. C., Ezzat, K., El Andaloussi, S., and Weinberg, M. S. (2016). Synthetic SiRNA delivery: progress and prospects. Methods Mol. Biol. 1364, 291–310. doi: 10.1007/978-1-4939-3112-5_23
Rodrigo, J., Hernandez, C. J., Vidal, M. A., and Pedrosa, J. A. (1975a). Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat. 92, 79–100. doi: 10.1159/000144431
Rodrigo, J., Hernandez, C. J., Vidal, M. A., and Pedrosa, J. A. (1975b). Vegetative innervation of the esophagus. III. Intraepithelial endings. Acta Anat. 92, 242–258. doi: 10.1159/000144444
Rodrigo, J., Nava, B. E., and Pedrosa, J. (1970). Study of vegetative innervation in the oesophagus. I. Perivascular endings. Trab. Inst. Cajal. Invest. Biol. 62, 39–65.
Rogers, R. C., Mctigue, D. M., and Hermann, G. E. (1995). Vagovagal reflex control of digestion: afferent modulation by neural and “endoneurocrine” factors. Am. J. Physiol. Gastrointest. Liver Physiol. 268, G1–G10. doi: 10.1152/ajpgi.1995.268.1.G1
Rossi, G., Manfrin, A., and Lutolf, M. P. (2018). Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687. doi: 10.1038/s41576-018-0051-9
Rossi, J., Balthasar, N., Olson, D., Scott, M., Berglund, E., Lee, C. E., et al. (2011). Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204. doi: 10.1016/j.cmet.2011.01.010
Roth, B. L. (2016). DREADDs for Neuroscientists. Neuron 89, 683–694. doi: 10.1016/j.neuron.2016.01.040
Ruffoli, R., Giorgi, F. S., Pizzanelli, C., Murri, L., Paparelli, A., and Fornai, F. (2011). The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat. 42, 288–296. doi: 10.1016/j.jchemneu.2010.12.002
Russell, E. S. (1979). Hereditary anemias of the mouse: a review for geneticists. Adv. Genet. 20, 357–359.
Saam, J. R., and Gordon, J. I. (1999). Inducible gene knockouts in the small intestinal and colonic epithelium. J. Biol. Chem. 274, 38071–38082. doi: 10.1074/jbc.274.53.38071
Sakuma, T., Barry, M. A., and Ikeda, Y. (2012). Lentiviral vectors: basic to translational. Biochem. J. 443, 603–618. doi: 10.1042/bj20120146
Santos, S. F., De Oliveira, H. L., Yamada, E. S., and Neves, B. C. (2019). The gut and Parkinson’s disease-A bidirectional pathway. Front. Neurol. 10:574. doi: 10.3389/fneur.2019.00574
Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., Van Es, J. H., Van Den Brink, S., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772. doi: 10.1053/j.gastro.2011.07.050
Sato, T., Vries, R. G., Snippert, H. J., Van De Wetering, M., Barker, N., Stange, D. E., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. doi: 10.1038/nature07935
Sauer, B. (1987). Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol. Cell Biol. 7, 2087–2096. doi: 10.1128/mcb.7.6.2087
Sauer, B., and Henderson, N. (1988). Site-specific DNA recombination in mammalian cells by the cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. U.S.A. 85, 5166–5170. doi: 10.1073/pnas.85.14.5166
Saunders, A., Johnson, C. A., and Sabatini, B. L. (2012). Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circ. 6:47. doi: 10.3389/fncir.2012.00047
Saunders, A., and Sabatini, B. L. (2015). Cre activated and inactivated recombinant adeno-associated viral vectors for neuronal anatomical tracing or activity manipulation. Curr. Protoc. Neurosci. 72, 1.24.21–21.24.15. doi: 10.1002/0471142301.ns0124s72
Schafer, M. K., Varoqui, H., Defamie, N., Weihe, E., and Erickson, J. D. (2002). Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Biol. Chem. 277, 50734–50748. doi: 10.1074/jbc.M206738200
Scherrer, G., Low, S. A., Wang, X., Zhang, J., Yamanaka, H., Urban, R., et al. (2010). VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 107, 22296–22301. doi: 10.1073/pnas.1013413108
Schneider, B. S., Monahan, J. W., and Hirsch, J. (1979). Brain cholecystokinin and nutritional status in rats and mice. J. Clin. Invest. 64, 1348–1356. doi: 10.1172/jci109591
Schnutgen, F., Doerflinger, N., Calleja, C., Wendling, O., Chambon, P., and Ghyselinck, N. B. (2003). A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 21, 562–565. doi: 10.1038/nbt811
Schönig, K., Schwenk, F., Rajewsky, K., and Bujard, H. (2002). Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 30:e134. doi: 10.1093/nar/gnf134
Schultheis, C., Liewald, J. F., Bamberg, E., Nagel, G., and Gottschalk, A. (2011). Optogenetic long-term manipulation of behavior and animal development. PLoS One 6:e18766. doi: 10.1371/journal.pone.0018766
Schuster, D. L. J., Dykstra, J. A., Riedl, M. S., Kitto, K. F., Belur, L. R., Mcivor, R. S., et al. (2014). Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front. Neuroanat. 8:42. doi: 10.3389/fnana.2014.00042
Schwartz, E. S., and Gebhart, G. F. (2014). Visceral pain. Curr. Top. Behav. Neurosci 20, 171–197. doi: 10.1007/7854_2014_315
Scott, M. M., Williams, K. W., Rossi, J., Lee, C. E., and Elmquist, J. K. (2011). Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421. doi: 10.1172/jci43703
Serlin, H. K., and Fox, E. A. (2020). Abdominal vagotomy reveals majority of small intestinal mucosal afferents labeled in nav 1.8cre-rosa26tdTomato mice are vagal in origin. J. Comp. Neurol. 528, 816–839. doi: 10.1002/cne.24791
Setten, R. L., Rossi, J. J., and Han, S. P. (2019). The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18, 421–446. doi: 10.1038/s41573-019-0017-4
Sheikh, I. S., Keefe, K. M., Sterling, N. A., Junker, I. P., Eneanya, C. I., Liu, Y., et al. (2018). Retrogradely transportable lentivirus tracers for mapping spinal cord locomotor circuits. Front. Neural Circ. 12:60. doi: 10.3389/fncir.2018.00060
Smith, A. J., Chappell, A. E., Buret, A. G., Barrett, K. E., and Dong, H. (2006). 5-Hydroxytryptamine contributes significantly to a reflex pathway by which the duodenal mucosa protects itself from gastric acid injury. FASEB 20, 2486–2495. doi: 10.1096/fj.06-6391com
Smith, B. N., Banfield, B. W., Smeraski, C. A., Wilcox, C. L., Dudek, F. E., Enquist, L. W., et al. (2000). Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl. Acad. Sci. U.S.A. 97, 9264–9269. doi: 10.1073/pnas.97.16.9264
Smith, G. P., Jerome, C., Cushin, B. J., Eterno, R., and Simansky, K. J. (1981). Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213, 1036–1037. doi: 10.1126/science.7268408
Snowdon, C. T. (1970). Gastrointestinal sensory and motor control of food intake. J. Comp. Physiol. Psychol. 71, 68–76. doi: 10.1037/h0028964
Sonntag, F., Schmidt, K., and Kleinschmidt, J. A. (2010). A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. U.S.A. 107, 10220–10225. doi: 10.1073/pnas.1001673107
Spence, J. R., Mayhew, C. N., Rankin, S. A., Kuhar, M. F., Vallance, J. E., Tolle, K., et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. doi: 10.1038/nature09691
Spencer, N. J., and Hu, H. (2020). Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 17, 338–351. doi: 10.1038/s41575-020-0271-2
Spencer, N. J., Kyloh, M., Beckett, E. A., Brookes, S., and Hibberd, T. (2016a). Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J. Comp. Neurol. 524, 3064–3083. doi: 10.1002/cne.24006
Spencer, N. J., Zagorodnyuk, V., Brookes, S. J., and Hibberd, T. (2016b). Spinal afferent nerve endings in visceral organs: recent advances. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G1056–G1063. doi: 10.1152/ajpgi.00319.2016
Spencer, N. J., Kyloh, M., and Duffield, M. (2014). Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One 9:e112466. doi: 10.1371/journal.pone.0112466
Spencer, N. J., Kyloh, M. A., Travis, L., and Dodds, K. N. (2020). Identification of spinal afferent nerve endings in the colonic mucosa and submucosa that communicate directly with the spinal cord: the gut-brain axis. J. Comp. Neurol. 528, 1742–1753. doi: 10.1002/cne.24854
Spencer, N. J., and Smith, T. K. (2004). Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol. 558, 577–596. doi: 10.1113/jphysiol.2004.063586
Stange, D. E., Koo, B. K., Huch, M., Sibbel, G., Basak, O., Lyubimova, A., et al. (2013). Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368. doi: 10.1016/j.cell.2013.09.008
Sternberg, N., and Hamilton, D. (1981). Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 150, 467–486. doi: 10.1016/0022-2836(81)90375-2
Sternson, S. M., and Roth, B. L. (2014). Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 37, 387–407. doi: 10.1146/annurev-neuro-071013-014048
Stirling, L. C., Forlani, G., Baker, M. D., Wood, J. N., Matthews, E. A., Dickenson, A. H., et al. (2005). Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain 113, 27–36. doi: 10.1016/j.pain.2004.08.015
Stirpe, F., Barbieri, L., Battelli, M. G., Soria, M., and Lappi, D. A. (1992). Ribosome-inactivating proteins from plants: present status and future prospects. Biotechnology 10, 405–412. doi: 10.1038/nbt0492-405
Stirpe, F., Gasperi-Campani, A., Barbieri, L., Falasca, A., Abbondanza, A., and Stevens, W. A. (1983). Ribosome-inactivating proteins from the seeds of Saponaria officinalis L. (soapwort), of Agrostemma githago L. (corn cockle) and of Asparagus officinalis L. (asparagus), and from the latex of Hura crepitans L. (sandbox tree). Biochem. J. 216, 617–625. doi: 10.1042/bj2160617
Suarez, A. N., Hsu, T. M., Liu, C. M., Noble, E. E., Cortella, A. M., Nakamoto, E. M., et al. (2018). Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 9:2181. doi: 10.1038/s41467-018-04639-1
Surdenikova, L., Ru, F., Nassenstein, C., Tatar, M., and Kollarik, M. (2012). The neural crest- and placodes-derived afferent innervation of the mouse esophagus. Neurogastroenterol. Motil. 24, e517–e525. doi: 10.1111/nmo.12002
Szolcsányi, J. (2014). “Capsaicin and sensory neurones: a historical perspective,” in Capsaicin as a Therapeutic Molecule, ed. O. M. E. Abdel-Salam (Berlin: Springer), 1–37. doi: 10.1007/978-3-0348-0828-6_1
Szolcsányi, J., and Pinter, E. (2013). Transient receptor potential vanilloid 1 as a therapeutic target in analgesia. Expert. Opin. Ther. Targets 17, 641–657. doi: 10.1517/14728222.2013.772580
Szolcsányi, J., and Sandor, Z. (2012). Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol. Sci. 33, 646–655. doi: 10.1016/j.tips.2012.09.002
Takamori, S., Rhee, J. S., Rosenmund, C., and Jahn, R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407, 189–194. doi: 10.1038/35025070
Tervo, D. G., Hwang, B. Y., Viswanathan, S., Gaj, T., Lavzin, M., Ritola, K. D., et al. (2016). A Designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382. doi: 10.1016/j.neuron.2016.09.021
Tian, L., Hires, S. A., Mao, T., Huber, D., Chiappe, M. E., Chalasani, S. H., et al. (2009). Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881. doi: 10.1038/nmeth.1398
Tong, Q., Ma, J., and Kirchgessner, A. L. (2001). Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport 12, 3929–3934. doi: 10.1097/00001756-200112210-00015
Trancikova, A., Kovacova, E., Ru, F., Varga, K., Brozmanova, M., Tatar, M., et al. (2018). Distinct expression of phenotypic markers in placodes- and neural crest-derived afferent neurons innervating the rat stomach. Dig. Dis. Sci. 63, 383–394. doi: 10.1007/s10620-017-4883-5
Travagli, R. A., and Anselmi, L. (2016). Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 13, 389–401. doi: 10.1038/nrgastro.2016.76
Udit, S., Burton, M., Rutkowski, J. M., Lee, S., Bookout, A. L., Scherer, P. E., et al. (2017). Nav1.8 neurons are involved in limiting acute phase responses to dietary fat. Mol. Metab. 6, 1081–1091. doi: 10.1016/j.molmet.2017.07.012
Udit, S., and Gautron, L. (2013). Molecular anatomy of the gut-brain axis revealed with transgenic technologies: implications in metabolic research. Front. Neurosci. 7:134. doi: 10.3389/fnins.2013.00134
Ugolini, G. (2010). Advances in viral transneuronal tracing. J. Neurosci. Methods 194, 2–20. doi: 10.1016/j.jneumeth.2009.12.001
Ugolini, G., Kuypers, H. G., and Simmons, A. (1987). Retrograde transneuronal transfer of herpes simplex virus type 1 (HSV 1) from motoneurones. Brain Res. 422, 242–256. doi: 10.1016/0006-8993(87)90931-0
Ugolini, G., Kuypers, H. G., and Strick, P. L. (1989). Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science 243, 89–91. doi: 10.1126/science.2536188
Ulusoy, A., Phillips, R. J., Helwig, M., Klinkenberg, M., Powley, T. L., and Di Monte, D. A. (2017). Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 133, 381–393. doi: 10.1007/s00401-016-1661-y
Umans, B. D., and Liberles, S. D. (2018). Neural sensing of organ volume. Trends Neurosci. 41, 911–924. doi: 10.1016/j.tins.2018.07.008
Utomo, A. R., Nikitin, A. Y., and Lee, W. H. (1999). Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat. Biotechnol. 17, 1091–1096. doi: 10.1038/15073
Valarche, I., Tissier-Seta, J. P., Hirsch, M. R., Martinez, S., Goridis, C., and Brunet, J. F. (1993). The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development 119, 881–896.
Van Den Berge, N., Ferreira, N., Gram, H., Mikkelsen, T. W., Alstrup, A. K. O., Casadei, N., et al. (2019). Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 138, 535–550. doi: 10.1007/s00401-019-02040-w
Van Duyne, G. D. (2015). Cre recombinase. Microbiol. Spectr. 3:MDNA3-0014-2014. doi: 10.1128/microbiolspec.MDNA3-0014-2014
Varoqui, H., Schafer, M. K., Zhu, H., Weihe, E., and Erickson, J. D. (2002). Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 22, 142–155. doi: 10.1523/jneurosci.22-01-00142.2002
Viswanath, V., Story, G. M., Peier, A. M., Petrus, M. J., Lee, V. M., Hwang, S. W., et al. (2003). Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823. doi: 10.1038/423822a
Vong, L., Ye, C., Yang, Z., Choi, B., and Chua, S. Jr. (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. doi: 10.1016/j.neuron.2011.05.028
Waise, T. M. Z., Dranse, H. J., and Lam, T. K. T. (2018). The metabolic role of vagal afferent innervation. Nat. Rev. Gastroenterol. Hepatol. 15, 625–636. doi: 10.1038/s41575-018-0062-1
Walls, E. K., Wang, F. B., Holst, M. C., Phillips, R. J., Voreis, J. S., Perkins, A. R., et al. (1995). Selective vagal rhizotomies: a new dorsal surgical approach used for intestinal deafferentations. Am. J. Physiol. 269, R1279–R1288. doi: 10.1152/ajpregu.1995.269.5.R1279
Wang, J., Xie, J., Lu, H., Chen, L., Hauck, B., Samulski, R. J., et al. (2007). Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc. Natl. Acad. Sci. U.S.A. 104, 13104–13109. doi: 10.1073/pnas.0702778104
Wang, J. Q., Kon, J., Mogi, C., Tobo, M., Damirin, A., Sato, K., et al. (2004). TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 279, 45626–45633. doi: 10.1074/jbc.M406966200
Wang, R., Lu, Y., Gunasekar, S., Zhang, Y., Benson, C. J., Chapleau, M. W., et al. (2017). The volume-regulated anion channel (LRRC8) in nodose neurons is sensitive to acidic pH. JCI Insight 2:e90632. doi: 10.1172/jci.insight.90632
Wank, M., and Neuhuber, W. L. (2001). Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J. Comp. Neurol. 435, 41–59. doi: 10.1002/cne.1192
Weber, A. M., Kaiser, J., Ziegler, T., Pilsl, S., Renzl, C., Sixt, L., et al. (2019). A blue light receptor that mediates RNA binding and translational regulation. Nat. Chem. Biol. 15, 1085–1092. doi: 10.1038/s41589-019-0346-y
Weinberg, Z. Y., and Puthenveedu, M. A. (2019). Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic 20, 121–129. doi: 10.1111/tra.12628
Weitzman, M. D., and Linden, R. M. (2011). Adeno-associated virus biology. Methods Mol. Biol. 807, 1–23. doi: 10.1007/978-1-61779-370-7_1
Wickersham, I. R., Finke, S., Conzelmann, K. K., and Callaway, E. M. (2007a). Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49. doi: 10.1038/nmeth999
Wickersham, I. R., Lyon, D. C., Barnard, R. J., Mori, T., Finke, S., Conzelmann, K. K., et al. (2007b). Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647. doi: 10.1016/j.neuron.2007.01.033
Wiley, R. G. (2001). Targeting toxins to neural antigens and receptors. Methods Mol. Biol. 166, 267–276. doi: 10.1385/1-59259-114-0:267
Wiley, R. G., and Kline, I. R. (2000). Neuronal lesioning with axonally transported toxins. J. Neurosci. Methods 103, 73–82. doi: 10.1016/s0165-0270(00)00297-1
Williams, E. K., Chang, R. B., Strochlic, D. E., Umans, B. D., Lowell, B. B., and Liberles, S. D. (2016). Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221. doi: 10.1016/j.cell.2016.05.011
Wojaczynski, G. J., Engel, E. A., Steren, K. E., Enquist, L. W., and Patrick Card, J. (2015). The neuroinvasive profiles of H129 (herpes simplex virus type 1) recombinants with putative anterograde-only transneuronal spread properties. Brain Struct. Funct. 220, 1395–1420. doi: 10.1007/s00429-014-0733-9
Woodward, E. R. (1987). The history of vagotomy. Am. J. Surg. 153, 9–17. doi: 10.1016/0002-9610(87)90195-4
Wu, Z., Yang, H., and Colosi, P. (2010). Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86. doi: 10.1038/mt.2009.255
Yamakawa, K., Rajendran, P. S., Takamiya, T., Yagishita, D., So, E. L., Mahajan, A., et al. (2015). Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am. J. Physiol. Heart Circ. Physiol. 309, H1579–H1590. doi: 10.1152/ajpheart.00558.2015
Yang, F., Xiao, X., Cheng, W., Yang, W., Yu, P., Song, Z., et al. (2015). Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 11, 518–524. doi: 10.1038/nchembio.1835
Yang, F., and Zheng, J. (2017). Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell 8, 169–177. doi: 10.1007/s13238-016-0353-7
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M., and Deisseroth, K. (2011). Optogenetics in neural systems. Neuron 71, 9–34. doi: 10.1016/j.neuron.2011.06.004
Yoshii, Y., Inoue, T., Uemura, Y., Iwasaki, Y., Yada, T., Nakabeppu, Y., et al. (2017). Complexity of stomach–brain interaction induced by molecular hydrogen in Parkinson’s Disease model mice. Neurochem. Res. 42, 2658–2665. doi: 10.1007/s11064-017-2281-1
Young, R. L., Cooper, N. J., and Blackshaw, L. A. (2008). Chemical coding and central projections of gastric vagal afferent neurons. Neurogastroenterol. Motil. 20, 708–718. doi: 10.1111/j.1365-2982.2007.01071.x
Yu, S., Undem, B. J., and Kollarik, M. (2005). Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J. Physiol. 563, 831–842. doi: 10.1113/jphysiol.2004.079574
Zagorodnyuk, V. P., and Brookes, S. J. (2000). Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 20, 6249–6255. doi: 10.1523/jneurosci.20-16-06249.2000
Zemanick, M. C., Strick, P. L., and Dix, R. D. (1991). Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc. Natl. Acad. Sci. U.S.A. 88, 8048–8051. doi: 10.1073/pnas.88.18.8048
Zhang, F., Wang, L. P., Brauner, M., Liewald, J. F., Kay, K., Watzke, N., et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639. doi: 10.1038/nature05744
Zhang, W., Gardell, S., Zhang, D., Xie, J. Y., Agnes, R. S., Badghisi, H., et al. (2009). Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain 132, 778–787. doi: 10.1093/brain/awn330
Zhao, J., Nassar, M. A., Gavazzi, I., and Wood, J. N. (2006). Tamoxifen-inducible NaV1.8-CreERT2 recombinase activity in nociceptive neurons of dorsal root ganglia. Genesis 44, 364–371. doi: 10.1002/dvg.20224
Zhou, S. Y., Lu, Y., Song, I., and Owyang, C. (2010). Inhibition of gastric motility by hyperglycemia is mediated by nodose ganglia KATP channels. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G394–G400. doi: 10.1152/ajpgi.00493.2010
Keywords: gastrointestinal tract, vagal afferent subtypes, gut brain axis, molecular tools, feeding behaviour
Citation: Wang YB, de Lartigue G and Page AJ (2020) Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 11:643. doi: 10.3389/fphys.2020.00643
Received: 26 March 2020; Accepted: 20 May 2020;
Published: 11 June 2020.
Edited by:
Catia Sternini, University of California, Los Angeles, United StatesReviewed by:
Nick Spencer, Flinders University, AustraliaEdward A. Fox, Purdue University, United States
Copyright © 2020 Wang, de Lartigue and Page. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda J. Page, YW1hbmRhLnBhZ2VAYWRlbGFpZGUuZWR1LmF1
 Yoko B. Wang
Yoko B. Wang Guillaume de Lartigue
Guillaume de Lartigue Amanda J. Page
Amanda J. Page