
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 17 June 2020
Sec. Red Blood Cell Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.00577
This article is part of the Research TopicRed Blood Cells at the Mount of Truth: Highlights of the 22nd Meeting of the European Red Cell Research SocietyView all 11 articles
The N-methyl-D-aspartate receptor (NMDAR) provides a pathway for glutamate-mediated inter-cellular communication, best known for its role in the brain but with multiple examples of functionality in non-neuronal cells. Data previously published by others and us provided ex vivo evidence that NMDARs regulate platelet and red blood cell (RBC) production. Here, we summarize what is known about these hematopoietic roles of the NMDAR. Types of NMDAR subunits expressed in megakaryocytes (platelet precursors) and erythroid cells are more commonly found in the developing rather than adult brain, suggesting trophic functions. Nevertheless, similar to their neuronal counterparts, hematopoietic NMDARs function as ion channels, and are permeable to calcium ions (Ca2+). Inhibitors that block open NMDAR (memantine and MK-801) interfere with megakaryocytic maturation and proplatelet formation in primary culture. The effect on proplatelet formation appears to involve Ca2+ influx-dependent regulation of the cytoskeletal remodeling. In contrast to normal megakaryocytes, NMDAR effects in leukemic Meg-01 cells are diverted away from differentiation to increase proliferation. NMDAR hypofunction triggers differentiation of Meg-01 cells with the bias toward erythropoiesis. The underlying mechanism involves changes in the intracellular Ca2+ homeostasis, cell stress pathways, and hematopoietic transcription factors that upon NMDAR inhibition shift from the predominance of megakaryocytic toward erythroid regulators. This ability of NMDAR to balance both megakaryocytic and erythroid cell fates suggests receptor involvement at the level of a bipotential megakaryocyte-erythroid progenitor. In human erythroid precursors and circulating RBCs, NMDAR regulates intracellular Ca2+ homeostasis. NMDAR activity supports survival of early proerythroblasts, and in mature RBCs NMDARs impact cellular hydration state, hemoglobin oxygen affinity, and nitric oxide synthase activity. Overexcitation of NMDAR in mature RBCs leads to Ca2+ overload, K+ loss, RBC dehydration, and oxidative stress, which may contribute to the pathogenesis of sickle cell disease. In summary, there is growing evidence that glutamate-NMDAR signaling regulates megakaryocytic and erythroid cells at different stages of maturation, with some intriguing differences emerging in NMDAR expression and function between normal and diseased cells. NMDAR signaling may provide new therapeutic opportunities in hematological disease, but in vivo applicability needs to be confirmed.
This review summarizes what has been learned about the roles of N-methyl-D-aspartate receptor (NMDAR) in megakaryocytic and erythroid cells. NMDARs are best known for their functions as glutamate-gated cation channels in the central nervous system (Traynelis et al., 2010). It appears that the NMDAR ion channel functionality is maintained in blood progenitors but NMDAR channel properties and its downstream pathways await further characterization in these cells. This paper starts with a brief overview of glutamate signaling in the brain. On this background, we highlight distinctive features of NMDAR in hematopoietic cells. Other glutamate receptors and mature blood cells are not discussed in detail but the appropriate background is provided to place this emerging field of research in a meaningful context. We describe NMDAR effects on hematopoietic differentiation, including some of our recent observations that suggest a novel role for the receptor in balancing megakaryocytic and erythroid cell fates (Hearn et al., 2020).
Glutamate is synthesized from glutamine as a part of normal cellular metabolism in all cells (Yelamanchi et al., 2016). In neurons, vesicular glutamate transporters (VGLUT) pump glutamate into pre-synaptic vesicles (Daikhin and Yudkoff, 2000; Zhou and Danbolt, 2014). Upon membrane depolarization, vesicles fuse with the pre-synaptic plasma membrane and glutamate is released into the synaptic cleft. This process engages soluble N-ethyl-maleimide-sensitive factor attachment protein receptor (SNARE) proteins that are activated by Ca2+ entry through voltage-gated Ca2+ channels. Following release, glutamate concentrations in the synaptic cleft increase markedly, from 2–5 μM to approximately 1.1 mM. While in the synaptic cleft, glutamate activates ionotropic and metabotropic receptors located on the post-synaptic plasma membrane (Reiner and Levitz, 2018). Ionotropic receptors function as ion channels (for Na+, K+, and Ca2+), and metabotropic receptors activate G-proteins that modulate ion channels directly and indirectly. The main purpose of the ionic flux is to generate and propagate action potentials characteristic of excitable tissues. The synaptic glutamate signal is terminated by the excitatory amino acid transporters (EAAT) present on astrocytes that remove glutamate from the synaptic cleft (Featherstone, 2010).
The family of ionotropic glutamate receptors includes NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors, each named after a distinct, synthetic agonist that activates them (Traynelis et al., 2010). AMPA and kainate receptors respond to glutamate first. They mediate intracellular influx of mostly Na+, which leads to membrane depolarization and if large enough, triggers action potential. Membrane depolarization releases a Mg2+ ion blocking the pore of NMDAR, enabling receptor function. This order of events highlights that neuronal NMDAR can activate only when glutamate binding and membrane depolarization coincide (which is named “coincidence detection”). NMDAR-mediated Ca2+ influx contributes little to membrane depolarization but modifies synaptic strength through molecular events related to the Ca2+ role as “second messenger” (Traynelis et al., 2010; Hansen et al., 2018).
Typical NMDARs are built as tetramers that combine two obligate GluN1 subunits with another two GluN2 (A–D) or GluN3 (A or B) subunits, in various combinations. It is believed that GluN1 subunit is an essential component of all NMDARs, and variable GluN2 and GluN3 subunits are modulatory. NMDAR activation requires binding of L-glutamate on each of the GluN2 subunits, as well as glycine (co-agonist) on the GluN1 and GluN3 subunits. The alternative NMDAR ligands include D- and L- aspartate, homocysteine, homocysteic acid, and D-serine. NMDAR subunit composition varies substantially in different areas of the brain, and changes during development (Monyer et al., 1994; Wenzel et al., 1997). NMDAR subunits define the current amplitude and inactivation time, as well as cation selectivity and the regulation patterns, such as agonist affinity, mechano-sensitivity, Mg2+-sensitivity, and responsiveness to polyamines. GluN2A and GluN2B subunits contribute high channel conductance and relatively fast de-activation kinetics compared to GluN2C- and GluN2D- containing NMDAR (Traynelis et al., 2010). In addition, NMDARs containing GluN2C, GluN2D, and GluN3 subunits display low affinity for Mg2+ blocking the pore, making activation of such receptors independent of membrane depolarization (Monyer et al., 1994; Chatterton et al., 2002; Wrighton et al., 2008).
NMDAR sensitivity (EC50) to agonists is high, ranging from 0.4 to 1.7 μM for glutamate (in GluN1–GluN2D and GluN1–GluN2A receptors, respectively), and 0.1 to 2.1 μM for glycine (in GluN1–GluN2D and GluN1–GluN2A receptors, respectively) (Yamakura and Shimoji, 1999). These concentrations lie within the range that is normal in an inactive synaptic cleft. However, all types of NMDAR are extremely sensitive to the inhibition by protons (IC50 around 7.4 μM for most of the subunits) (Yamakura and Shimoji, 1999; Low et al., 2003; Cavara et al., 2009), and Zn2+ [IC50 of 10 nM, 1 μM, and 10 μM for the NMDAR containing GluN2A, GluN2B, and GluN2D, respectively (Gielen et al., 2009)].
NMDAR-mediated Ca2+ entry activates a number of intracellular signaling pathways, including Ca2+/calmodulin-dependent kinase (CaMK), mitogen-activated protein kinase (MAPK) [including extracellular signal-regulated kinase (ERK), Jun kinase, and p38 MAPK], and phosphoinositide 3-kinase (PI3K) (Hardingham, 2006). NMDARs regulate activity-dependent gene expression through cAMP response element binding protein (CREB) transcription factor (Hardingham et al., 2001). Other mediators downstream of NMDAR include Ras, Fyn, striatal-enriched protein tyrosine phosphatase, and nitric oxide synthase. Highly coordinated (albeit incompletely elucidated) NMDAR signaling plays critical roles in embryonic brain development and later, in neuronal plasticity, which allows the brain to respond to new experiences and changing environment (Traynelis et al., 2010).
During the past 10–20 years, NMDARs have been reported in multiple non-neuronal cell types, including hematopoietic (Bozic and Valdivielso, 2015; Hogan-Cann and Anderson, 2016), which raised a principal question of why non-excitable cells need these receptors. We admit this area of research is not very clear, sometimes even controversial, mainly due to the very low abundance of NMDAR in non-neuronal cells. Nevertheless, some progress has been achieved in the characterization of the subunit composition and currents mediated by non-neuronal NMDAR, in particular in red blood cells (RBC) (Makhro et al., 2010), platelets (Kalev-Zylinska et al., 2014), lymphocytes (Fenninger and Jefferies, 2019), and hematopoietic precursors, erythroblasts (Makhro et al., 2013; Hanggi et al., 2014, 2015) and megakaryocytes (Genever et al., 1999; Kamal et al., 2015). Information on the potential physiological role of these receptors is accumulating as well, including in erythroid cells (Makhro et al., 2013, 2016), and megakaryocytes (Hitchcock et al., 2003; Green et al., 2017; Kamal et al., 2018; Hearn et al., 2020). The subsequent sections will focus on the subunit composition, properties and the roles of NMDAR in megakaryocytic and erythroid precursors, and their mature progeny, platelets and RBCs.
Peripheral blood platelets store and respond to a number of regulatory molecules best known for their roles in neurotransmission, including serotonin, epinephrine, dopamine, histamine, γ-aminobutyric acid, and glutamate (Todrick et al., 1960; Ponomarev, 2018; Canobbio, 2019). In psychiatric patients, there is evidence of a crosstalk between abnormal NMDAR function in the brain and platelet responsiveness to glutamate (Berk et al., 1999). Platelets bind glutamate with similar kinetics to neurons (Almazov et al., 1988), store it in dense granules, and express AMPA, kainate, and NMDA receptors (Franconi et al., 1996, 1998; Morrell et al., 2008; Sun et al., 2009; Kalev-Zylinska et al., 2014; Green et al., 2017). Although there are variations between studies, all main types of ionotropic glutamate receptors have now been shown to be functional in platelets. Morrell et al. demonstrated that AMPA and kainate (but not NMDA) receptors amplify platelet activation by contributing Na+ influx to membrane depolarization, but not Ca2+ influx (Morrell et al., 2008; Sun et al., 2009). Franconi et al. provided the first evidence of NMDAR functionality in platelets, demonstrating that NMDARs induce Ca2+ influx into platelets but inhibit platelet function in the presence of adenosine diphosphate (ADP) and arachidonic acid (Franconi et al., 1996, 1998). Our own work demonstrated that NMDAR inhibitors (memantine, MK-801, and anti-GluN1 antibodies) interfere with platelet activation, aggregation and thrombus formation ex vivo (Kalev-Zylinska et al., 2014; Green et al., 2017). It is likely that methodological differences contributed to variable NMDAR effects between studies.
Intriguingly, in schizophrenia and bipolar disorders that are driven by deregulated NMDAR signaling, platelet Ca2+ levels are elevated, including in response to glutamate (Berk et al., 2000; Ruljancic et al., 2013; Harrison et al., 2019). Schizophrenia is characterized by NMDAR hypofunction in the limbic system (Coyle, 2012; Nakazawa et al., 2017), compensated by high glutamate levels and NMDAR hypersensitivity in other areas of the brain (Merritt et al., 2016). The fact that platelets from patients with schizophrenia also show glutamate hypersensitivity further argues that NMDAR functioning in platelets is similar to that in neurons (Berk et al., 2000).
Because platelets have limited protein synthesis, one would expect a similar range of glutamate receptors to be present in megakaryocytes. However, most data thus far indicate regulation of megakaryocytic differentiation by NMDAR, with little or no data on AMPA and kainate receptors (Genever et al., 1999; Hitchcock et al., 2003; Kamal et al., 2018). Nevertheless, electrophysiological recordings from freshly isolated mouse megakaryocytes support expression of functional AMPA receptors in megakaryocytes, most likely GluR2-containing and Ca2+-impermeable (Morrell et al., 2008).
The first evidence that NMDARs operate as ion channels in megakaryocytes was obtained by demonstrating that [3H]MK-801 binds to native mouse megakaryocytes in vivo. Mice were injected with [3H]MK-801 intracardially, followed by bone marrow examination 15 min later (Genever et al., 1999). Because MK-801 is a non-competitive, use-dependent NMDAR inhibitor that can only bind within an open NMDAR pore (Traynelis et al., 2010), its labeling of megakaryocytes was consistent with the NMDAR function as an ion channel in megakaryocytic cells. Later, we showed that glutamate, NMDA, and glycine induce Ca2+ fluxes in Meg-01 cells, and NMDAR antagonists (MK-801, memantine, and AP5 [D-2-amino-5-phosphonopentanoate]) counteract this effect, indicating that NMDARs operate as Ca2+ channels in these cells (Kamal et al., 2015, 2018).
Table 1 provides a summary of the NMDAR subunit expression in megakaryocytic and erythroid cells, reported at either protein or transcript level. Unfortunately, testing for GluN proteins in hematopoietic cells has been difficult due to (a) very low abundance, (b) various protein isoforms and post-translational modifications, and (c) the lack of antibodies optimized for use in non-neuronal cells. Human megakaryocytes were first shown to express GluN1 using immunocytochemistry and Western blotting, the latter indicated that GluN1 was non-glycosylated, which may affect NMDAR distribution in the plasma membrane (Genever et al., 1999). Our group demonstrated expression of GluN1, GluN2A, and GluN2D in Meg-01, K-562, and Set-2 cells using flow cytometry and a modified Western blotting procedure that employed membrane enrichment and high-sensitivity peroxidase substrates (Kamal et al., 2015).
The composition of NMDAR in megakaryocytes differs from that in neurons. In the brain, NMDARs are built mostly from GluN1, GluN2A, and/or GluN2B subunits, but human, native and culture-derived megakaryocytes express predominantly GluN2D, with some GluN2A and GluN1 (Table 1; Genever et al., 1999; Hitchcock et al., 2003; Kamal et al., 2015, 2018). The dominant expression of GluN2D in normal megakaryocytes will affect NMDAR functioning, however no electrophysiological recordings are available from these cells to document the effect. In other systems, GluN2D-containing NMDAR displays the following differences compared with GluN2A and GluN2B containing receptors: approximately 5-fold higher sensitivity to glutamate, 10-fold higher sensitivity to glycine, 100-fold longer deactivation time, lower conductance, lower Ca2+ permeability and weaker Mg2+ block (Paoletti et al., 2013; Wyllie et al., 2013; Hansen et al., 2018). The weak Mg2+ block suggests that NMDAR in megakaryocytes may not require membrane depolarization to become active; meaning the principle of “coincidence detection” may not apply. The unique functionality of the GluN2D subunit is underscored by its dominant expression in the embryonic and postnatal brain; however, the mechanism through which GluN2D subunits provide trophic effects remains incompletely understood (Watanabe et al., 1992; Akazawa et al., 1994).
For those of us working with mouse models, it is relevant to note that there are differences in the NMDAR expression patterns between human and mouse cells. In contrast to human megakaryocytes (that express only GluN1, GluN2A, and GluN2D), mouse megakaryocytes also express GluN2C and GluN3B (Table 1; Kamal et al., 2018). Small numbers of other, yet un-identified mononuclear cells in the mouse bone marrow also express NMDAR, but there is no documented expression in mouse erythroid precursors or mature RBCs (Genever et al., 1999), which differs from human cells (Table 1; Makhro et al., 2013; Hanggi et al., 2014, 2015).
In contrast to normal megakaryocytes, patient-derived leukemic megakaryoblasts and megakaryocyte leukemia cell lines (Meg-01, K-562, and Set-2) carry all possible NMDAR subunits, including GluN2B, GluN3A, and GluN3B (Table 1; Kamal et al., 2015). Meg-01 and K-562 cell lines are derived from patients with chronic myeloid leukemia in megakaryocytic and myeloid blast crisis respectively, and carry oncogenic BCR-ABL1 gene fusion (Lozzio and Lozzio, 1975; Ogura et al., 1985). Both Meg-01 and K-562 cell lines express thrombopoietin (TPO) and erythropoietin (EPO) receptors and can be induced to differentiate into megakaryocytic (Ogura et al., 1988, Herrera et al., 1998) and erythroid cells (Andersson et al., 1979; Morle et al., 1992), thus providing experimental models of bipotential megakaryocyte-erythroid progenitors. Set-2 cell line is derived from a leukemic transformation of essential thrombocythemia and carries JAK2 V617F mutation, an established driver in myeloproliferative neoplasms. Set-2 differentiates spontaneously into megakaryocyte-like cells (Uozumi et al., 2000). Biological characteristics of leukemic cell lines are obviously very different from normal progenitors, which we should keep in mind while interpreting cell line data.
We found that Meg-01 cells are better suited for studies of NMDAR function than K-562 and Set-2 cells, mostly because of their higher levels of NMDAR expression. Upon differentiation with phorbol-12-myristate-13-acetate (PMA), Meg-01 cells up-regulate NMDAR expression further, providing a model in which to examine NMDAR involvement in megakaryocytic differentiation (Genever et al., 1999; Kamal et al., 2018).
The role of GluN3 subunits (highly expressed in leukemic cells; Table 1) is poorly understood, including in the brain, but its functions have already been described as exquisite, peculiar, unconventional, and transformative (Kehoe et al., 2013; Perez-Otano et al., 2016; Grand et al., 2018). This is because GluN3 subunits do not require glutamate for activation (Nilsson et al., 2007). In GluN1-GluN3 receptors, glycine acts both as the sole agonist binding on GluN3, and provides feedback inhibition through GluN1. In GluN1-GluN2-GluN3 receptors, the presence of GluN3 reduces Mg2+ block and Ca2+ entry (Matsuda et al., 2002; Cavara and Hollmann, 2008).
Overall, the presence of nonconventional GluN subunits (in particular GluN2D and GluN3) in megakaryocytic cells, normal and leukemic, suggests that NMDAR generates weaker but more sustained Ca2+ influx, and may allow stronger modulation by glycine than glutamate, in particular in leukemic cells. There is also a possibility of regulation by metabolic factors through GluN1. This is because megakaryocytic cells express h1-1 to h1-4 GluN1 isoforms, all of type “a” (Kamal et al., 2015). GluN1a isoforms lack the N1-cassette from the N-terminal domain (encoded by exon 5) that if present, reduces inhibition by protons and zinc, and potentiation by polyamines (Traynelis et al., 1995; Yi et al., 2018). Because the bone marrow environment is intrinsically hypoxic (Spencer et al., 2014), this form of metabolic regulation warrants testing in blood progenitors.
Mouse and human megakaryocytes express a range of molecules for glutamate re-uptake, storage, and release, including VGLUT1, VGLUT2, SNARE, and the high-affinity glutamate re-uptake system, EAAT1, shown at both transcript and protein levels (Genever et al., 1999; Thompson et al., 2010). Fluorimetric measurements of glutamate concentrations in culture media suggest that human and mouse megakaryocytes, and Meg-01 cells release glutamate in a constitutive manner (Thompson et al., 2010); congruently, similar has been shown for ADP packaged together with glutamate in dense granules (Balduini et al., 2012). We do not know why megakaryocytes need glutamate sensitivity but it is a critical question to seek answers to in the future. A possible auto-regulatory loop (autocrine or paracrine) is suggested by the observation that Meg-01 cells release more glutamate upon differentiation with PMA (Thompson et al., 2010). Glutamate regulation may be independent of TPO, as NMDAR expression is maintained in megakaryocytes from c-Mpl-(TPO receptor) knockout mice (Hitchcock et al., 2003).
Unfortunately, there is no information on glutamate concentrations in the interstitial fluid of the bone marrow. In peripheral blood plasma, physiological glutamate levels are usually maintained between 20 and 100 μM (Kiessling et al., 2000), but vary widely depending on the diet (Stegink et al., 1983) and exercise (Makhro et al., 2016). In the interstitial fluids, concentrations of glutamate have been observed to be as low as 0.5 μM in the masseter muscle of myofascial temporomandibular joint (Castrillon et al., 2010), to as high as blood plasma levels in the vastus lateralis muscle of the lower limb (Gerdle et al., 2016). There is no experimental data on how plasma/interstitial glutamate levels impact endogenous NMDAR in blood/progenitor cells. Paracrine NMDAR activation in neuron-like fashion appears more likely in tissues with low, steady state glutamate concentrations. On the other hand, conditional NMDAR activation via local pH changes would be more likely in tissues with higher, plasma-like glutamate concentrations. Different types of NMDAR subunits will also affect cellular sensitivity to glutamate, and modulate the receptor response (as described in section Evidence for NMDAR Functionality in Megakaryocytic Cells).
NMDAR channel blockers (memantine and MK-801) induce two types of apparently opposing effects in cultured megakaryocytic cells: inhibition of differentiation in normal megakaryocytes, but induction of differentiation in megakaryocytic leukemia cell lines (Figure 1Ai–ii).
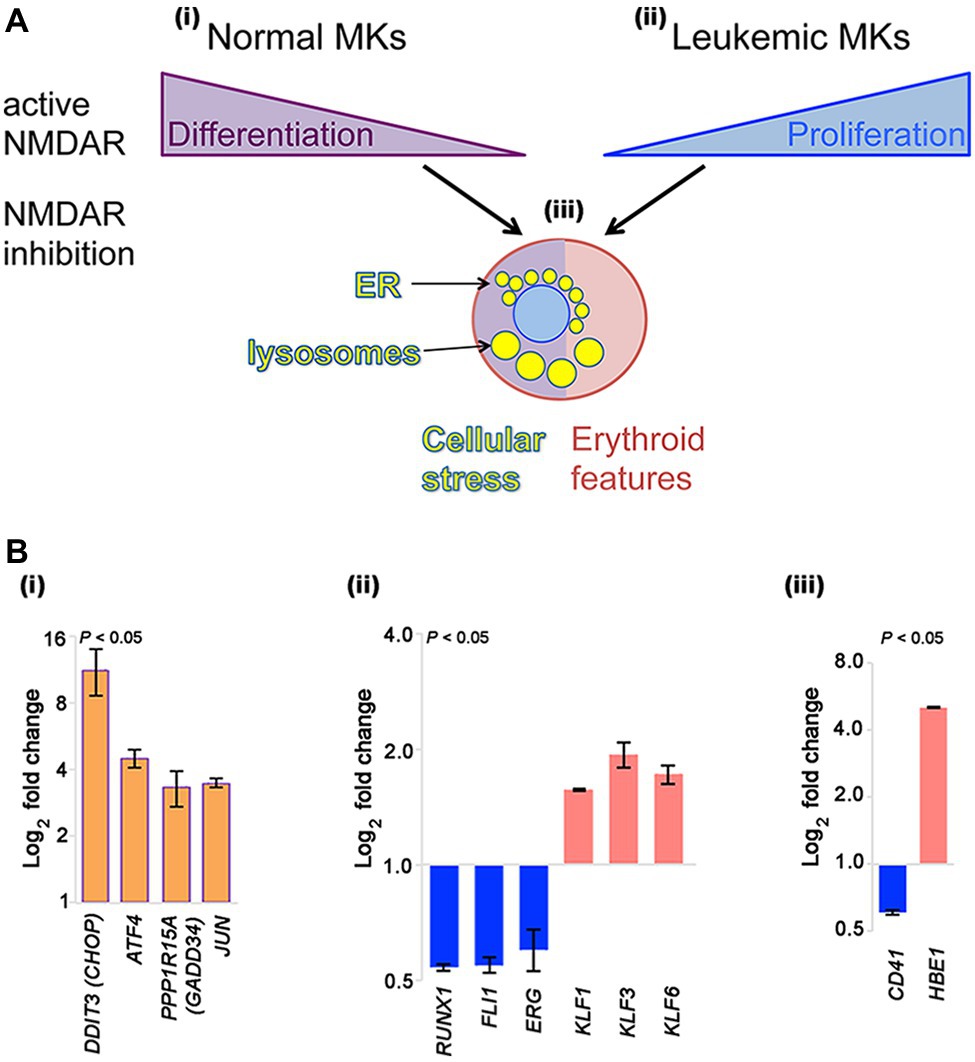
Figure 1. NMDAR effects in normal megakaryocytes and leukemic Meg-01 cells. (A) Schematic indicating that in normal megakaryocytes NMDAR activity supports differentiation, in particular proplatelet formation (i). In contrast, in leukemic cell lines NMDARs increase cell proliferation (ii). In both normal and leukemic cells, NMDAR inhibition induces cellular stress response associated with endoplasmic reticulum (ER) dilatation and accumulation of lysosomes (iii). Red shade in a cell reflects features of erythroid differentiation. (B) Experimental data showing that CRISPR-Cas9-mediated deletion of GRIN1 in Meg-01 cells increased expression of ER stress markers (DDIT3/CHOP, ATF4, PPP1R15A/GADD34, and JUN; Bi; orange bars), associated with decreased expression of megakaryocytic transcription factors (RUNX1, FLI1, ERG; Bii) and megakaryocytic maturation marker, CD41 (Biii) (blue bars). Instead, expression of erythroid transcription factors (KLF1, KLF3, KLF6; Bii) and embryonic hemoglobin (HBE1; Biii) was increased (red bars). Transcript levels were determined by real-time RT-PCR (Bi) and Clariom S microarrays (Bii–iii), as described (Hearn et al., 2020). Statistical significance is shown (p < 0.05 for all markers versus unmodified Meg-01 cells set at 1.0, tested by one-way ANOVA with Dunnett post-hoc. MK, megakaryocyte.
When human megakaryocytes are grown from CD34-positive umbilical cord stem cells, the addition of MK-801 inhibits acquisition of megakaryocytic markers (CD61, CD41a, and CD42a), nuclear ploidy and proplatelet formation; however, progenitor proliferation is unaffected (Hitchcock et al., 2003). Similar effects are seen in cultures of mouse hematopoietic progenitors, and in the native bone marrow milieu of mouse bone marrow explants. MK-801 inhibits actin reorganization in mature mouse megakaryocytes, suggesting that NMDAR-mediated Ca2+ influx is required for the cytoskeletal remodeling that underlies proplatelet formation (Kamal et al., 2018). This process may be similar to dendritic spine formation arising in response to neuronal NMDAR firing (Furuyashiki et al., 2002). NMDAR links with cytoskeletal elements through post-synaptic density (PSD) proteins such as PSD-95 and Yotiao; both of which are expressed in megakaryocytes, suggesting similar interactions may be possible in hematopoietic cells (Hitchcock et al., 2003).
In contrast to normal megakaryocytes that utilize NMDAR function to assist differentiation, leukemic cell lines (Meg-01, K-562, and Set-2) appear to divert NMDAR activity to increase proliferation (Figure 1Ai–ii). In the presence of NMDAR blockers (memantine and MK-801) Meg-01 cells undergo atypical differentiation and accumulate prominent cytoplasmic vacuoles (Figure 1Aiii; Kamal et al., 2015, 2018). The opposing NMDAR effects on cellular phenotype between normal and leukemic cells suggest divergence of NMDAR pathways during leukemogenesis to increase cell proliferation.
To get more insights into the mechanism of this divergence, we recently created a model of NMDAR hypofunction in Meg-01 cells using CRISPR-Cas9 mediated knockout of the GRIN1 gene that encodes the obligate, GluN1 subunit of the NMDAR (Hearn et al., 2020). We found that GRIN1 deletion caused marked changes in the intracellular Ca2+ homeostasis, including higher cytosolic Ca2+ levels at baseline but lower ER Ca2+ release after activation. Deregulated Ca2+ handling led to endoplasmic reticulum (ER) stress and induced autophagy. Prominent cytoplasmic vacuoles accumulated in Meg-01-GRIN1 −/− cells and were found to represent dilated ER and lysosomal organelles (Figure 1Aiii). Microarray analysis revealed that Meg-01-GRIN1 −/− cells had deregulated expression of transcripts involved in Ca2+ metabolism, together with a shift in the pattern of hematopoietic transcription factors toward erythropoiesis (Figure 1Bi–ii). In keeping with the pro-erythroid pattern of transcription factors, Meg-01-GRIN1 −/− cells displayed features of erythroid differentiation (Figure 1Biii). Our data provide the first evidence that NMDARs comprise an integral component of the Ca2+ toolkit in megakaryocytic cells, and argue that intracellular Ca2+ homeostasis may be more important than currently recognized for balancing megakaryocytic with erythroid differentiation at the level of a common progenitor (Hearn et al., 2020).
In support of our findings, Kinney et al. provided computational evidence of NMDAR involvement in erythropoiesis (Kinney et al., 2019). The authors analyzed 164 publicly available erythroid microarray datasets using an enhanced CellNet bioinformatics algorithm to delineate key transitional states of erythroid differentiation at high resolution. This approach identified a role for signaling through epidermal growth factor receptor Erb-B2 receptor tyrosine kinase 4 (ErbB4) in erythroid differentiation, which was further validated experimentally in zebrafish, mouse and human models. The authors linked ErbB4 with NMDAR signaling by finding increased levels of GRIN3B transcripts, coding for GluN3B, in the reticulocyte gene cluster. A similar link between ErbB4 and NMDAR is well-documented in neurons, where ErbB4 and its neuregulin ligands stabilize synaptic NMDAR (Li et al., 2007). In fact, altered neuregulin 1–ErbB4 signaling is a well-established mechanism of NMDAR hypofunction in schizophrenia (Hahn et al., 2006). Encouragingly, we also found that in Meg-01-GRIN1 −/− cells, transcripts for neuregulin 1 and ErbB receptor feedback inhibitor 1 were up-regulated (1.98- and 2.05-fold, respectively), implying the ErbB4-NMDAR link is maintained during megakaryocytic-erythroid differentiation (Hearn et al., 2020).
The interrogation of publicly available transcriptomic data obtained from human megakaryocyte-erythroid progenitors at a single cell level demonstrated the presence of GRINA transcripts (encoding NMDAR-associated protein 1, known to be expressed at relatively high levels) and a scatter of low signals for GRIN1, GRIN2A, GRIN2C, and GRIN2D (Lu et al., 2018). Deep sequencing in that study was performed with approximately 3 million reads per cell, which captured approximately 6,000 of the most highly expressed transcripts in a cell, which may explain why GRIN transcripts were detected at very low levels.
RBCs sense plasma glutamate levels through the NMDAR. Using radiolabeled antagonist ([3H]MK-801) binding assay, basal activity of NMDARs in RBCs suspended in plasma was shown (Makhro et al., 2013). Supplementation of glutamate to plasma caused further activation of the receptors (Hanggi et al., 2014). Other findings suggest that the shear of flowing blood may also activate NMDAR in RBCs of patients with sickle cell disease (Hanggi et al., 2014, 2015).
The abundance of NMDARs is particularly high in erythroid precursors and the UT-7/EPO cell line (Makhro et al., 2013; Hanggi et al., 2015). The receptor density decreases from hundreds of thousands per cell in proerythroblasts and erythroblasts cultured from peripheral blood-derived CD34-positive progenitors, to 35 in young human RBCs, and five in mature and senescent RBCs from healthy people (Makhro et al., 2013; Hanggi et al., 2014). In UT-7/EPO cell line 350,000 NMDARs were detected per cell (Makhro et al., 2010). UT-7/EPO is a subclone of a UT-7 megakaryoblastic leukemia cell line that was maintained in the presence of EPO for more than 6 months to increase erythroid differentiation (Komatsu et al., 1993). In keeping with the NMDAR expression data, the density of currents produced by the NMDAR decreased during differentiation from proerythroblastic to the orthochromatic stage of erythroid progenitors (Hanggi et al., 2015).
Similar to megakaryocytes, the pattern of GluN subunits evolves during erythroid differentiation. Except for GluN2B, all other types of NMDAR subunits have been detected in erythroid cells at either mRNA or protein levels, or both (Table 1). During early stages, (proerythroblasts and basophilic erythroblasts) higher levels of GluN2A and GluN2D were shown along with lower levels of GluN3A, GluN3B, and GluN1 (Makhro et al., 2013; Hanggi et al., 2014, 2015). Orthochromatic erythroblasts switched from GluN2A-containing receptors to those predominantly containing GluN2C (Hanggi et al., 2015). As a result, high amplitude, fast, inactivating currents, mediated by the receptor at early stages of erythroid differentiation were replaced by currents of lesser amplitude but longer duration (Hanggi et al., 2015). This change in receptor subunit composition and its function gave rise to a switch in signal transmitted by the NMDAR, and probably, to the alteration in sensitivity to the physiological stimuli. Whereas GluN2A-containing receptors contributed to the modulation of the transmembrane potential, GluN2C/GluN2D-NMDAR mediated Ca2+ entry through the channels that remained open for a longer time (Hanggi et al., 2015). Mg2+ block is not supposed to control the NMDAR activity in RBCs due to the low transmembrane potential (about −10 mV) and the presence of GluN2C, GluN2D, and GluN3 subunits (Monyer et al., 1994; Wrighton et al., 2008).
Hyperactivation of the NMDAR by repeated stimulation resulted in the channel inactivation and did not affect viability of erythroid precursor cells. However, exposure of erythroid progenitors to the NMDAR channel blockers (MK-801 and memantine) triggered vacuolization and apoptosis, with maximal cell death observed at the early differentiation stages (Hanggi et al., 2014, 2015). This observation is in line with the earlier findings by Miller and Cheung on the importance of Ca2+ signaling for EPO-driven effects in precursor cells (Miller and Cheung, 1994; Tong et al., 2008).
Relatively low numbers of active NMDAR copies are retained by the circulating RBCs. Young RBCs of healthy humans carry 35 NMDARs per cell on average, whereas mature and senescent cells contain about five receptor copies per cell (Makhro et al., 2013; Hanggi et al., 2014). The NMDAR abundance is 3–4-fold higher in RBCs from patients with sickle cell disease (Hanggi et al., 2014). Activation of NMDAR by exposing the cells to the saturating concentrations of agonists (NMDA and glycine, 300 μM each) results in an acute, transient increase in the intracellular free Ca2+ (Makhro et al., 2013). There is striking inter-cellular heterogeneity in responses of the cells to the NMDAR agonists, including changes in transmembrane currents and Ca2+ uptake shown with a fluorescent dye, as well as the level of dehydration and echinocyte formation. These differences cannot be explained solely by the differences in RBC age. Whereas some cells are insensitive to the stimulation, others show a clear response to the NMDAR agonists suggesting inter-cellular heterogeneity in NMDAR numbers/distribution. Along with the changes in RBC volume and density of Ca2+ uptake following the NMDAR stimulation, we observed regulation of nitric oxide production in RBCs by the nitric oxide synthase and modulation of the redox state (Makhro et al., 2010).
Physiological responses to the changes in NMDAR activity in the circulating RBCs include regulation of hemoglobin oxygen affinity, cell rheology, and most likely, longevity. Pathophysiological downstream effects associated with NMDAR hyperactivation were revealed ex vivo for RBCs of patients with sickle cell disease. These included Ca2+ overload, dehydration, and increase in cell density, and oxidative stress (Hanggi et al., 2014). There are no reports of abnormal RBC counts in patients with Alzheimer’s disease taking memantine to protect the brain from glutamatergic excitotoxicity (Kavirajan, 2009). One pilot clinical trial was performed at the University Hospital Zurich, in which patients with sickle cell disease received memantine over a year (Makhro et al., 2020; trial identifier NCT02615847), and the other is currently ongoing (trial identifier NCT03247218). These trials provide an opportunity to explore long-term effects of NMDAR inhibition on RBC and platelet production, and cell properties in humans.
In summary, megakaryocytic and erythroid precursors carry nonconventional NMDAR subunits, therefore NMDAR activity during hematopoiesis may be unique and should be tested. NMDARs regulate megakaryocytic and erythroid differentiation ex vivo, and balance both fates during differentiation of Meg-01 cells, suggesting NMDAR role at the level of a bipotential megakaryocyte-erythroid progenitor. NMDAR effects in hematopoietic cells are mediated by Ca2+ influx, which in early megakaryoblasts affects transcriptional program of differentiation, and in mature megakaryocytes induces cytoskeletal rearrangements required for proplatelet formation. In contrast to normal progenitors, leukemic cell lines re-direct NMDAR signaling to increase proliferation. The shift in the dominant NMDAR effect in leukemic cells may be at least partially related to different GluN subunits these cells express, which may offer therapeutic opportunities.
In keeping with the proliferative NMDAR effects in leukemic cells, other prominent groups found that GluN2B-containing NMDAR promotes growth of pancreatic tumors (Li and Hanahan, 2013; Li et al., 2018), and enable brain metastases by breast cancer (Zeng et al., 2019). The following molecules acting downstream of NMDAR were shown to assist cancer spread in these studies: CaMKII, MAPK, guanylate-kinase-associated protein, heat shock factor 1, and fragile X mental retardation protein (Li and Hanahan, 2013; Li et al., 2018); we should examine similar pathways in leukemic cells as they may provide novel therapeutic targets.
In cultured human proerythroblasts, GluN2A-containing NMDAR provides depolarization, and inward Ca2+ current of high amplitude and fast inactivation kinetics. The presence of active NMDAR supports survival of early erythroid progenitors, which likely contributes to the EPO-driven signaling; however, this link is still to be demonstrated. Expression of GluN2C and GluN2D in the late erythroid precursors coincides with the onset of hemoglobinization. Thus, the NMDAR role in iron uptake warrants investigation. In mature RBCs, NMDAR regulates basal intracellular Ca2+ levels, contributing toward the regulation of cell volume, density, redox balance, and nitric oxide production by RBCs, which most likely contributes to the regulation of RBC longevity and oxygen carrying capacity.
NMDAR signaling can be modulated using small molecules. Memantine is an approved drug for neurological patients and could be repurposed against certain hematological disorders, such as sickle cell disease, megakaryocytic cancers and thrombosis. Preclinical studies have already advanced to stage I clinical trials in sickle cell disease, but a lot more needs to be done to determine if NMDAR modulation could be useful in patients with certain myeloid blood cancers or thrombotic disease. Neurological side effects may limit the use of memantine in hematological patients; therefore, alternative strategies may need to be considered. These include subunit-specific NMDAR inhibitors, compounds that do not cross the blood-brain-barrier, and drugs that target pathways downstream, or glutamate release upstream of NMDAR.
Considering that the NMDAR role in megakaryocytic cells was first reported in Genever et al. (1999), the progress in this field may be viewed as relatively modest. However, we have reached a state of acceptance that NMDARs provide meaningful biological effects in hematopoietic cells. The field is attracting renewed attention. We await results from the first, stage I clinical trial in patients with sickle cell disease, primarily to establish safety of memantine outside of neurological indications. Further progress into NMDAR role in human leukemia and thrombosis will require studies in more advanced ex vivo and in vivo models. In addition, the overall principle and purpose of peripheral glutamate signaling needs to be determined. We, thus, invite collaborative approaches engaging experts from multiple disciplines to join us forming an interest group focusing on peripheral glutamate signaling.
MK-Z, AM and AB wrote the paper. JH contributed research data. All authors approved the final version for submission.
Auckland Medical Research Foundation (grant reference 1115012) provided working expenses for megakaryocytic studies conducted in the laboratory of MK-Z. JH received a scholarship funded by Anne and Victoria Norman.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ADP, adenosine diphosphate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AP5, D-2-amino-5-phosphonopentanoate; CaMK, Ca2+/calmodulin-dependent kinase; CREB, cAMP response element binding protein; EAAT, excitatory amino acid transporters; EC50, the concentration of an agonist that gives half-maximal response; EPO, erythropoietin; ER, endoplasmic reticulum; ErbB4, epidermal growth factor receptor Erb-B2 receptor tyrosine kinase 4; ERK, extracellular signal-regulated kinase; IC50, the concentration of an inhibitor where the response (or binding) is reduced by half; MAPK, mitogen-activated protein kinase; MEP, megakaryocyte-erythroid progenitor; MK, megakaryocyte; NMDAR, N-methyl-D-aspartate receptor; PI3-K, phosphoinositide 3-kinase; PMA, phorbol 12-myristate 13-acetate; PSD, post-synaptic density; RBC, red blood cell; SNARE, soluble N-ethyl maleimide-sensitive factor attachment protein receptor; TPO, thrombopoietin; VGLUT, vesicular glutamate transporter.
Akazawa, C., Shigemoto, R., Bessho, Y., Nakanishi, S., and Mizuno, N. (1994). Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J. Comp. Neurol. 347, 150–160. doi: 10.1002/cne.903470112
Almazov, V. A., Popov Iu, G., Gorodinskii, A. I., Mikhailova, I. A., and Dambinova, S. A. (1988). The sites of high affinity binding of L-[3H]glutamic acid in human platelets. A new type of platelet receptor? Biokhimiia 53, 848–852.
Andersson, L. C., Jokinen, M., and Gahmberg, C. G. (1979). Induction of erythroid differentiation in the human leukaemia cell line K562. Nature 278, 364–365. doi: 10.1038/278364a0
Balduini, A., Di Buduo, C. A., Malara, A., Lecchi, A., Rebuzzini, P., Currao, M., et al. (2012). Constitutively released adenosine diphosphate regulates proplatelet formation by human megakaryocytes. Haematologica 97, 1657–1665. doi: 10.3324/haematol.2011.059212
Berk, M., Plein, H., and Belsham, B. (2000). The specificity of platelet glutamate receptor supersensitivity in psychotic disorders. Life Sci. 66, 2427–2432. doi: 10.1016/S0024-3205(00)00573-7
Berk, M., Plein, H., and Csizmadia, T. (1999). Supersensitive platelet glutamate receptors as a possible peripheral marker in schizophrenia. Int. Clin. Psychopharmacol. 14, 119–122. doi: 10.1097/00004850-199903000-00009
Bozic, M., and Valdivielso, J. M. (2015). The potential of targeting NMDA receptors outside the CNS. Expert Opin. Ther. Targets 19, 399–413. doi: 10.1517/14728222.2014.983900
Canobbio, I. (2019). Blood platelets: circulating mirrors of neurons? Res. Pract. Thromb. Haemost. 3, 564–565. doi: 10.1002/rth2.12254
Castrillon, E. E., Ernberg, M., Cairns, B. E., Wang, K., Sessle, B. J., Arendt-Nielsen, L., et al. (2010). Interstitial glutamate concentration is elevated in the masseter muscle of myofascial temporomandibular disorder patients. J. Orofac. Pain 24, 350–360.
Cavara, N. A., and Hollmann, M. (2008). Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol. Neurobiol. 38, 16–26. doi: 10.1007/s12035-008-8029-9
Cavara, N. A., Orth, A., and Hollmann, M. (2009). Effects of NR1 splicing on NR1/NR3B-type excitatory glycine receptors. BMC Neurosci. 10:32. doi: 10.1186/1471-2202-10-32
Chatterton, J. E., Awobuluyi, M., Premkumar, L. S., Takahashi, H., Talantova, M., Shin, Y., et al. (2002). Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415, 793–798. doi: 10.1038/nature715
Coyle, J. T. (2012). NMDA receptor and schizophrenia: a brief history. Schizophr. Bull. 38, 920–926. doi: 10.1093/schbul/sbs076
Daikhin, Y., and Yudkoff, M. (2000). Compartmentation of brain glutamate metabolism in neurons and glia. J. Nutr. 130, 1026S–1031S. doi: 10.1093/jn/130.4.1026S
Featherstone, D. E. (2010). Intercellular glutamate signaling in the nervous system and beyond. ACS Chem. Neurosci. 1, 4–12. doi: 10.1021/cn900006n
Fenninger, F., and Jefferies, W. A. (2019). What’s bred in the bone: calcium channels in lymphocytes. J. Immunol. 202, 1021–1030. doi: 10.4049/jimmunol.1800837
Franconi, F., Miceli, M., Alberti, L., Seghieri, G., De Montis, M. G., and Tagliamonte, A. (1998). Further insights into the anti-aggregating activity of NMDA in human platelets. Br. J. Pharmacol. 124, 35–40. doi: 10.1038/sj.bjp.0701790
Franconi, F., Miceli, M., De Montis, M. G., Crisafi, E. L., Bennardini, F., and Tagliamonte, A. (1996). NMDA receptors play an anti-aggregating role in human platelets. Thromb. Haemost. 76, 84–87.
Furuyashiki, T., Arakawa, Y., Takemoto-Kimura, S., Bito, H., and Narumiya, S. (2002). Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. U. S. A. 99, 14458–14463. doi: 10.1073/pnas.212148999
Genever, P. G., Wilkinson, D. J., Patton, A. J., Peet, N. M., Hong, Y., Mathur, A., et al. (1999). Expression of a functional N-methyl-D-aspartate-type glutamate receptor by bone marrow megakaryocytes. Blood 93, 2876–2883. doi: 10.1182/blood.V93.9.2876
Gerdle, B., Ernberg, M., Mannerkorpi, K., Larsson, B., Kosek, E., Christidis, N., et al. (2016). Increased interstitial concentrations of glutamate and pyruvate in vastus lateralis of women with fibromyalgia syndrome are normalized after an exercise intervention—a case-control study. PLoS One 11:e0162010. doi: 10.1371/journal.pone.0162010
Gielen, M., Siegler Retchless, B., Mony, L., Johnson, J. W., and Paoletti, P. (2009). Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459, 703–707. doi: 10.1038/nature07993
Grand, T., Abi Gerges, S., David, M., Diana, M. A., and Paoletti, P. (2018). Unmasking GluN1/GluN3A excitatory glycine NMDA receptors. Nat. Commun. 9:4769. doi: 10.1038/s41467-018-07236-4
Green, T. N., Hamilton, J. R., Morel-Kopp, M. C., Zheng, Z., Chen, T. T., Hearn, J. I., et al. (2017). Inhibition of NMDA receptor function with an anti-GluN1-S2 antibody impairs human platelet function and thrombosis. Platelets 28, 799–811. doi: 10.1080/09537104.2017.1280149
Hahn, C. G., Wang, H. Y., Cho, D. S., Talbot, K., Gur, R. E., Berrettini, W. H., et al. (2006). Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat. Med. 12, 824–828. doi: 10.1038/nm1418
Hanggi, P., Makhro, A., Gassmann, M., Schmugge, M., Goede, J. S., Speer, O., et al. (2014). Red blood cells of sickle cell disease patients exhibit abnormally high abundance of N-methyl D-aspartate receptors mediating excessive calcium uptake. Br. J. Haematol. 167, 252–264. doi: 10.1111/bjh.13028
Hanggi, P., Telezhkin, V., Kemp, P. J., Schmugge, M., Gassmann, M., Goede, J. S., et al. (2015). Functional plasticity of the N-methyl-D-aspartate receptor in differentiating human erythroid precursor cells. Am. J. Physiol. Cell Physiol. 308, C993–C1007. doi: 10.1152/ajpcell.00395.2014
Hansen, K. B., Yi, F., Perszyk, R. E., Furukawa, H., Wollmuth, L. P., Gibb, A. J., et al. (2018). Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 150, 1081–1105. doi: 10.1085/jgp.201812032
Hardingham, G. E. (2006). Pro-survival signalling from the NMDA receptor. Biochem. Soc. Trans. 34, 936–938. doi: 10.1042/BST0340936
Hardingham, G. E., Arnold, F. J., and Bading, H. (2001). Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 4, 261–267. doi: 10.1038/85109
Harrison, P. J., Hall, N., Mould, A., Al-Juffali, N., and Tunbridge, E. M. (2019). Cellular calcium in bipolar disorder: systematic review and meta-analysis. Mol. Psychiatry. doi: 10.1038/s41380-019-0622-y
Hearn, J. I., Green, T. N., Chopra, M., Nursalim, Y. N. S., Ladvanszky, L., Knowlton, N., et al. (2020). NMDA receptor hypofunction in Meg-01 cells reveals a role for intracellular calcium homeostasis in balancing megakaryocytic-erythroid differentiation. Thromb. Haemost. 120, 671–686. doi: 10.1055/s-0040-1708483
Herrera, R., Hubbell, S., Decker, S., and Petruzzelli, L. (1998). A role for the MEK/MAPK pathway in PMA-induced cell cycle arrest: modulation of megakaryocytic differentiation of K562 cells. Exp. Cell Res. 238, 407–414. doi: 10.1006/excr.1997.3847
Hitchcock, I. S., Skerry, T. M., Howard, M. R., and Genever, P. G. (2003). NMDA receptor-mediated regulation of human megakaryocytopoiesis. Blood 102, 1254–1259. doi: 10.1182/blood-2002-11-3553
Hogan-Cann, A. D., and Anderson, C. M. (2016). Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol. Sci. 37, 750–767. doi: 10.1016/j.tips.2016.05.012
Kalev-Zylinska, M. L., Green, T. N., Morel-Kopp, M. C., Sun, P. P., Park, Y. E., Lasham, A., et al. (2014). N-methyl-D-aspartate receptors amplify activation and aggregation of human platelets. Thromb. Res. 133, 837–847. doi: 10.1016/j.thromres.2014.02.011
Kamal, T., Green, T. N., Hearn, J. I., Josefsson, E. C., Morel-Kopp, M. C., Ward, C. M., et al. (2018). N-methyl-D-aspartate receptor mediated calcium influx supports in vitro differentiation of normal mouse megakaryocytes but proliferation of leukemic cell lines. Res. Pract. Thromb. Haemost. 2, 125–138. doi: 10.1002/rth2.12068
Kamal, T., Green, T. N., Morel-Kopp, M. C., Ward, C. M., McGregor, A. L., McGlashan, S. R., et al. (2015). Inhibition of glutamate regulated calcium entry into leukemic megakaryoblasts reduces cell proliferation and supports differentiation. Cell. Signal. 27, 1860–1872. doi: 10.1016/j.cellsig.2015.05.004
Kavirajan, H. (2009). Memantine: a comprehensive review of safety and efficacy. Expert Opin. Drug Saf. 8, 89–109. doi: 10.1517/14740330802528420
Kehoe, L. A., Bernardinelli, Y., and Muller, D. (2013). GluN3A: an NMDA receptor subunit with exquisite properties and functions. Neural Plast. 2013:145387. doi: 10.1155/2013/145387
Kiessling, K., Roberts, N., Gibson, J. S., and Ellory, J. C. (2000). A comparison in normal individuals and sickle cell patients of reduced glutathione precursors and their transport between plasma and red cells. Hematol. J. 1, 243–249. doi: 10.1038/sj.thj.6200033
Kinney, M. A., Vo, L. T., Frame, J. M., Barragan, J., Conway, A. J., Li, S., et al. (2019). A systems biology pipeline identifies regulatory networks for stem cell engineering. Nat. Biotechnol. 37, 810–818. doi: 10.1038/s41587-019-0159-2
Komatsu, N., Yamamoto, M., Fujita, H., Miwa, A., Hatake, K., Endo, T., et al. (1993). Establishment and characterization of an erythropoietin-dependent subline, UT-7/Epo, derived from human leukemia cell line, UT-7. Blood 82, 456–464. doi: 10.1182/blood.V82.2.456.456
Li, B., Woo, R. S., Mei, L., and Malinow, R. (2007). The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron 54, 583–597. doi: 10.1016/j.neuron.2007.03.028
Li, L., and Hanahan, D. (2013). Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell 153, 86–100. doi: 10.1016/j.cell.2013.02.051
Li, L., Zeng, Q., Bhutkar, A., Galvan, J. A., Karamitopoulou, E., Noordermeer, D., et al. (2018). GKAP acts as a genetic modulator of NMDAR signaling to govern invasive tumor growth. Cancer Cell 33, 736.e5–751.e5. doi: 10.1016/j.ccell.2018.02.011
Low, C. M., Lyuboslavsky, P., French, A., Le, P., Wyatte, K., Thiel, W. H., et al. (2003). Molecular determinants of proton-sensitive N-methyl-D-aspartate receptor gating. Mol. Pharmacol. 63, 1212–1222. doi: 10.1124/mol.63.6.1212
Lozzio, C. B., and Lozzio, B. B. (1975). Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45, 321–334. doi: 10.1182/blood.V45.3.321.321
Lu, Y. C., Sanada, C., Xavier-Ferrucio, J., Wang, L., Zhang, P. X., Grimes, H. L., et al. (2018). The molecular signature of megakaryocyte-erythroid progenitors reveals a role for the cell cycle in fate specification. Cell Rep. 25, 2083.e4–2093.e4. doi: 10.1016/j.celrep.2018.10.084
Makhro, A., Hanggi, P., Goede, J. S., Wang, J., Bruggemann, A., Gassmann, M., et al. (2013). N-methyl-D-aspartate receptors in human erythroid precursor cells and in circulating red blood cells contribute to the intracellular calcium regulation. Am. J. Physiol. Cell Physiol. 305, C1123–C1138. doi: 10.1152/ajpcell.00031.2013
Makhro, A., Haider, T., Wang, J., Bogdanov, N., Steffen, P., Wagner, C., et al. (2016). Comparing the impact of an acute exercise bout on plasma amino acid composition, intraerythrocytic Ca(2+) handling, and red cell function in athletes and untrained subjects. Cell Calcium 60, 235–244. doi: 10.1016/j.ceca.2016.05.005
Makhro, A., Hegemann, I., Seiler, E., Simionato, G., Claveria, V., Bogdanov, N., et al. (2020). A pilot clinical phase II trialMemSID: Acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment. eJHaem. 2020, 1–12. doi: 10.1002/jha2.11
Makhro, A., Wang, J., Vogel, J., Boldyrev, A. A., Gassmann, M., Kaestner, L., et al. (2010). Functional NMDA receptors in rat erythrocytes. Am. J. Physiol. Cell Physiol. 298, C1315–C1325. doi: 10.1152/ajpcell.00407.2009
Matsuda, K., Kamiya, Y., Matsuda, S., and Yuzaki, M. (2002). Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res. Mol. Brain Res. 100, 43–52. doi: 10.1016/S0169-328X(02)00173-0
Merritt, K., Egerton, A., Kempton, M. J., Taylor, M. J., and McGuire, P. K. (2016). Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiat. 73, 665–674. doi: 10.1001/jamapsychiatry.2016.0442
Miller, B. A., and Cheung, J. Y. (1994). Mechanisms of erythropoietin signal transduction: involvement of calcium channels. Proc. Soc. Exp. Biol. Med. 206, 263–267. doi: 10.3181/00379727-206-43756
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B., and Seeburg, P. H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540. doi: 10.1016/0896-6273(94)90210-0
Morle, F., Laverriere, A. C., and Godet, J. (1992). Globin genes are actively transcribed in the human megakaryoblastic leukemia cell line MEG-01. Blood 79, 3094–3096. doi: 10.1182/blood.V79.11.3094.3094
Morrell, C. N., Sun, H., Ikeda, M., Beique, J. C., Swaim, A. M., Mason, E., et al. (2008). Glutamate mediates platelet activation through the AMPA receptor. J. Exp. Med. 205, 575–584. doi: 10.1084/jem.20071474
Nakazawa, K., Jeevakumar, V., and Nakao, K. (2017). Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ Schizophr. 3:7. doi: 10.1038/s41537-016-0003-3
Nilsson, A., Duan, J., Mo-Boquist, L. L., Benedikz, E., and Sundstrom, E. (2007). Characterisation of the human NMDA receptor subunit NR3A glycine binding site. Neuropharmacology 52, 1151–1159. doi: 10.1016/j.neuropharm.2006.12.002
Ogura, M., Morishima, Y., Ohno, R., Kato, Y., Hirabayashi, N., Nagura, H., et al. (1985). Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood 66, 1384–1392. doi: 10.1182/blood.V66.6.1384.1384
Ogura, M., Morishima, Y., Okumura, M., Hotta, T., Takamoto, S., Ohno, R., et al. (1988). Functional and morphological differentiation induction of a human megakaryoblastic leukemia cell line (MEG-01s) by phorbol diesters. Blood 72, 49–60. doi: 10.1182/blood.V72.1.49.49
Paoletti, P., Bellone, C., and Zhou, Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400. doi: 10.1038/nrn3504
Perez-Otano, I., Larsen, R. S., and Wesseling, J. F. (2016). Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat. Rev. Neurosci. 17, 623–635. doi: 10.1038/nrn.2016.92
Ponomarev, E. D. (2018). Fresh evidence for platelets as neuronal and innate immune cells: their role in the activation, differentiation, and deactivation of Th1, Th17, and Tregs during tissue inflammation. Front. Immunol. 9:406. doi: 10.3389/fimmu.2018.00406
Reiner, A., and Levitz, J. (2018). Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron 98, 1080–1098. doi: 10.1016/j.neuron.2018.05.018
Ruljancic, N., Mihanovic, M., Cepelak, I., and Bakliza, A. (2013). Platelet and serum calcium and magnesium concentration in suicidal and non-suicidal schizophrenic patients. Psychiatry Clin. Neurosci. 67, 154–159. doi: 10.1111/pcn.12038
Spencer, J. A., Ferraro, F., Roussakis, E., Klein, A., Wu, J., Runnels, J. M., et al. (2014). Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273. doi: 10.1038/nature13034
Stegink, L. D., Baker, G. L., and Filer, L. J. Jr. (1983). Modulating effect of Sustagen on plasma glutamate concentration in humans ingesting monosodium L-glutamate. Am. J. Clin. Nutr. 37, 194–200. doi: 10.1093/ajcn/37.2.194
Sun, H., Swaim, A., Herrera, J. E., Becker, D., Becker, L., Srivastava, K., et al. (2009). Platelet kainate receptor signaling promotes thrombosis by stimulating cyclooxygenase activation. Circ. Res. 105, 595–603. doi: 10.1161/CIRCRESAHA.109.198861
Thompson, C. J., Schilling, T., Howard, M. R., and Genever, P. G. (2010). SNARE-dependent glutamate release in megakaryocytes. Exp. Hematol. 38, 504–515. doi: 10.1016/j.exphem.2010.03.011
Todrick, A., Tait, A. C., and Marshall, E. F. (1960). Blood platelet 5-hydroxytryptamine levels in psychiatric patients. J. Ment. Sci. 106, 884–890. doi: 10.1192/bjp.106.444.884
Tong, Q., Hirschler-Laszkiewicz, I., Zhang, W., Conrad, K., Neagley, D. W., Barber, D. L., et al. (2008). TRPC3 is the erythropoietin-regulated calcium channel in human erythroid cells. J. Biol. Chem. 283, 10385–10395. doi: 10.1074/jbc.M710231200
Traynelis, S. F., Hartley, M., and Heinemann, S. F. (1995). Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268, 873–876. doi: 10.1126/science.7754371
Traynelis, S. F., Wollmuth, L. P., McBain, C. J., Menniti, F. S., Vance, K. M., Ogden, K. K., et al. (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496. doi: 10.1124/pr.109.002451
Uozumi, K., Otsuka, M., Ohno, N., Moriyama, T., Suzuki, S., Shimotakahara, S., et al. (2000). Establishment and characterization of a new human megakaryoblastic cell line (SET-2) that spontaneously matures to megakaryocytes and produces platelet-like particles. Leukemia 14, 142–152. doi: 10.1038/sj.leu.2401608
Watanabe, M., Inoue, Y., Sakimura, K., and Mishina, M. (1992). Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3, 1138–1140. doi: 10.1097/00001756-199212000-00027
Wenzel, A., Fritschy, J. M., Mohler, H., and Benke, D. (1997). NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem. 68, 469–478. doi: 10.1046/j.1471-4159.1997.68020469.x
Wrighton, D. C., Baker, E. J., Chen, P. E., and Wyllie, D. J. (2008). Mg2+ and memantine block of rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J. Physiol. 586, 211–225. doi: 10.1113/jphysiol.2007.143164
Wyllie, D. J., Livesey, M. R., and Hardingham, G. E. (2013). Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 74, 4–17. doi: 10.1016/j.neuropharm.2013.01.016
Yamakura, T., and Shimoji, K. (1999). Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 59, 279–298. doi: 10.1016/S0301-0082(99)00007-6
Yelamanchi, S. D., Jayaram, S., Thomas, J. K., Gundimeda, S., Khan, A. A., Singhal, A., et al. (2016). A pathway map of glutamate metabolism. J. Cell Commun. Signal. 10, 69–75. doi: 10.1007/s12079-015-0315-5
Yi, F., Zachariassen, L. G., Dorsett, K. N., and Hansen, K. B. (2018). Properties of triheteromeric N-methyl-D-aspartate receptors containing two distinct GluN1 isoforms. Mol. Pharmacol. 93, 453–467. doi: 10.1124/mol.117.111427
Zeng, Q., Michael, I. P., Zhang, P., Saghafinia, S., Knott, G., Jiao, W., et al. (2019). Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531. doi: 10.1038/s41586-019-1576-6
Keywords: glutamate, intracellular calcium signaling, megakaryocyte, erythropoiesis, red cells, platelets
Citation: Kalev-Zylinska ML, Hearn JI, Makhro A and Bogdanova A (2020) N-Methyl-D-Aspartate Receptors in Hematopoietic Cells: What Have We Learned? Front. Physiol. 11:577. doi: 10.3389/fphys.2020.00577
Received: 29 October 2019; Accepted: 08 May 2020;
Published: 17 June 2020.
Edited by:
Alan N. Schechter, National Institutes of Health (NIH), United StatesReviewed by:
Kate Hsu, Mackay Memorial Hospital, TaiwanCopyright © 2020 Kalev-Zylinska, Hearn, Makhro and Bogdanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maggie L. Kalev-Zylinska, bS5rYWxldkBhdWNrbGFuZC5hYy5ueg==
Dr. Heimo Mairbäurl, Heidelberg University Hospital, also contributed to the review process for this manuscript.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.