- 1National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan
- 2The Collaboration Unit for Field Epidemiology of State Key Laboratory for Infectious Disease Prevention and Control, Jiangxi Provincial Key Laboratory of Animal-origin and Vector-Borne Diseases, Nanchang Center for Disease Control and Prevention, Nanchang, China
- 3The Ophthalmology Department, The Second Affiliated Hospital of Nanchang University, Nanchang, China
The porin gene is widely disseminated in various organisms and has a pivotal role in the regulation of pathogen infection in blood-sucking arthropods. However, to date, information on the porin gene from the Haemaphysalis longicornis tick, an important vector of human and animal diseases, remains unknown. In this study, we identified the porin gene from H. longicornis and evaluated its expression levels in Babesia microti-infected and -uninfected H. longicornis ticks at developmental stages. We also analyzed porin functions in relation to both tick blood feeding and Babesia infection and the relationship between porin and porin-related apoptosis genes such as B-cell lymphoma (Bcl), cytochrome complex (Cytc), caspase 2 (Cas2), and caspase 8 (Cas8). The coding nucleotide sequence of H. longicornis porin cDNA was found to be 849 bp in length and encoded 282 amino acids. Domain analysis showed the protein to contain six determinants of voltage gating and two polypeptide binding sites. Porin mRNA levels were not significantly different between 1-day-laid and 7-day-laid eggs. In the nymphal stage, higher porin expression levels were found in unfed, 12-h-partially-fed (12 hPF), 1-day-partially-fed (1 dPF), 2 dPF nymphs and nymphs at 0 day post-engorgement (0 dAE) vs. nymphs at 2 dAE. Cytc and Cas2 mRNA levels were higher in 2 dPF nymphs in contrast to nymphs at 2 dAE. Porin expression levels appeared to be higher in the infected vs. uninfected nymphs during blood feeding except at 1 dPF and 0–1 dAE. Especially, the highest B. microti burden negatively affected porin mRNA levels in both nymphs and female adults. Porin knockdown affected body weight and Babesia infection levels and significantly downregulated the expression levels of Cytc and Bcl in H. longicornis female ticks. In addition, this study showed that infection levels of the B. microti Gray strain in nymphal and female H. longicornis peaked at or around engorgement from blood feeding to post engorgement. Taken together, the research conducted in this study suggests that H. longicornis porin might interfere with blood feeding and B. microti infection.
Introduction
The Asian longhorned tick, Haemaphysalis longicornis, is widely distributed in eastern Asia, Australia, and New Zealand and was recently found in the US (Heath, 2016; Rainey et al., 2018; Raghavan et al., 2019; Wormser et al., 2019; Zheng et al., 2019). H. longicornis, known as a harmful ectoparasite for domestic animals, spreads diseases including babesioses to livestock (McFadden et al., 2011). The tick has also been associated with several other tick-borne diseases in humans, including bacterioses and viroses (Chae and Lee, 2010; Fang et al., 2015; Zheng et al., 2018; Zhuang et al., 2018).
Over millions of years, ticks have co-evolved with a variety of microorganisms including Babesia. When Babesia parasites enter the tick body, ticks activate their immune system to inhibit Babesia invasion, and in turn, Babesia parasites hijack various tick molecules to facilitate their own transmission (de la Fuente et al., 2017). Several molecules are essential for tick-Babesia interaction, such as defensins, microplusin/hebraein, Kunitz domain-containing proteins, lipocalins, and proteases (Antunes et al., 2017). It is speculated that porin, also termed a voltage-dependent anion-selective channel (VDAC), plays paramount roles in modulating pathogen infection in vectors, including bacteria and protozoa in ticks, and viruses in mosquitoes (Fongsaran et al., 2014; Alberdi et al., 2015; Rodríguez-Hernández et al., 2015; Jitobaom et al., 2016). To date, porin has been described in at least three tick species, including Ixodes scapularis, Rhipicephalus microplus, and Amblyomma variegatum (Ribeiro et al., 2011; Rodríguez-Hernández et al., 2011; Alberdi et al., 2015). Porin in R. microplus was identified when it was exposed to Babesia bigemina infection (Rodríguez-Hernández et al., 2011).
Various Babesia parasites including Babesia microti have been experimentally transmitted by or detected in the Asian longhorned tick (Ikadai et al., 2007; Sivakumar et al., 2014; Fang et al., 2015; Zhang et al., 2017). B. microti is the most malignant human Babesia parasite with high morbidity and wide distribution around the globe (Vannier and Krause, 2012; Chen et al., 2019; Krause, 2019), and Ixodes ticks have historically been considered as common vectors of B. microti (Mather et al., 1990; Krause, 2019). However, B. microti DNA can be detected in H. longicornis collected from the field (Zhang et al., 2017) and can be acquired by the tick when feeding on mice infected with the B. microti Munich strain (Kusakisako et al., 2015). The transmission of B. microti from H. longicornis to mice has also been achieved (Wu et al., 2017), suggesting that the tick is a potential vector of the protozoan parasite. However, the molecular mechanisms underlying H. longicornis-B. microti interactions remain unclear.
On the basis of the above information, we hypothesized that H. longicornis porin might have roles in modulating B. microti infection in the ticks, and thus we designed experiments to confirm the hypothesis in this study. First, a homolog of porin was identified and characterized in H. longicornis using an Expressed Sequence Tags (ESTs) database, and then the expression levels of porin mRNA in H. longicornis eggs and nymphs were analyzed by real-time PCR. Moreover, we established a H. longicornis-B. microti Gray strain (a human-pathogenic strain) infection model and determined the dynamics of B. microti loads in nymphal and female ticks during the blood feeding stage. Porin mRNA transcripts were then compared between B. microti-infected and -uninfected ticks. Finally, porin functional analyses were carried out by RNA interference (RNAi) to determine its potential roles in blood feeding and B. microti infection.
Materials and Methods
Ticks, Parasites, and Animals
Parthenogenetic H. longicornis ticks (Okayama strain) were kept at the National Research Center for Protozoan Diseases (NRCPD), Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan, and maintained by feeding on the ears of Japanese white rabbits (Japan SLC, Shizuoka, Japan) (Umemiya-Shirafuji et al., 2019a). In the present study, two rabbits were used to maintain the nymphal and female ticks. The B. microti Gray strain was used to produce B. microti-infected ticks. Cryopreserved protozoan parasites were kept in liquid nitrogen in NRCPD and thawed using the methods mentioned in The Global Bioresource Center (ATCC® 30221TM). Seven 8-week-old female hamsters (Japan SLC, Shizuoka, Japan) were inoculated with thawed B. microti and then used for blood feeding to produce B. microti-infected ticks. In parallel, seven uninfected hamsters were used for blood meal and production of uninfected ticks. All animals used in this study were reared in a temperature- and humidity-regulated room under controlled lighting, given water and commercial regular chow, and were cared for in accordance with the guidelines approved by the Animal Care and Use Committee (Animal exp.: 19–74 for rabbits and 19–77 for hamsters) of Obihiro University of Agriculture and Veterinary Medicine.
Identification and Characterization of the cDNA Encoding Porin
ESTs were previously constructed by random partial sequencing of the 5′- terminal of the cDNA clones from cDNA libraries established with salivary glands of 4-day-fed H. longicornis females, and the similarities in the protein databases were examined using the BLASTp program (Liao et al., 2009). The plasmids containing the porin gene-encoding insert were extracted using a Qiagen DNA purification kit (Qiagen, Hilden, Germany) and subsequently subjected to analysis on an ABI PRISM 3100 DNA sequencer (Applied Biosystems, Waltham, MA, United States) using plasmid (pGCAP1 vector)-specific primers and walking primers thereafter.
The full length of the porin coding region was searched with the BLASTx program in the National Center for Biotechnology Information (NCBI)1. The domain structure was determined using the Conserved Protein Domain Family search program in the NCBI2. The deduced amino acid translation of the porin sequence was performed using an online tool Nucleotide Amino acid Derived Visualization3. Alignment of the porin amino acid sequences from different tick species was viewed with the Multiple Align Show4. The identity and similarity between H. longicornis and other tick species were calculated with the Ident and Sim program of the Sequence Manipulation Suite. The similar amino acids were classified into the same group for the similarity calculation: GAVLI, FYW, CM, ST, KRH, DENQ, and P5. The theoretical pI (isoelectric point) and Mw (molecular weight) were determined by the Compute pI/Mw6.
Real-Time PCR Analysis
The expression levels of the porin gene were analyzed in ticks at egg, nymph, or adult stage, in ticks incubated at 15°C or 25°C, and in B. microti-infected or -uninfected ticks. Three duplicates were made for each group of tick samples. After two washes with double distilled water and one wash with 70% ethanol, 10 mg of eggs, whole body of four nymphs, and three unfed and two partially fed or engorged female ticks with host blood removed were homogenized in TRI reagent (Sigma-Aldrich, St. Louis, MO, United States) using pestles. RNA extraction, cDNA synthesis, and real-time PCR were performed as described elsewhere (Umemiya-Shirafuji et al., 2019b). The same amount of cDNA was used in a real-time PCR reaction system to assess the stability of internal control genes in ticks at different developmental stages under unfed, uninfected, or infected conditions. The candidate internal control genes evaluated in this study included glyceraldehyde-3-phosphate dehydrogenase (GAPDH), L23, HlP0, and Hlactin. The most stable one was used for analysis of the relative mRNA level of the porin gene. Porin-related apoptosis genes such as B-cell lymphoma (Bcl), cytochrome complex (Cytc), caspase 2 (Cas2), and caspase 8 (Cas8) were also assessed by real-time PCR. The H. longicornis Bcl sequence was identified using the EST database as described above, and for the other genes, previously published sequences were used (GenBank database under accession number DQ666174 for Cas2, DQ660369 for Cas8, and NC_037493 for Cytc). The primers used in our study are listed in Supplementary Table S1. The mRNA levels were normalized separately against mRNA levels of the internal control gene using the ΔCT {2^[– (CTtarget gene–CTinternal control gene )]} method.
Analyses of B. microti Burdens in Ticks
B. microti burdens were calculated in nymphal and female ticks by standardizing the relative amount of Babesia 18S rRNA against tick ITS-2 in infected ticks with the values obtained in uninfected ones. The amounts of Babesia 18S rRNA and tick ITS-2 in the samples were evaluated using genomic DNA samples for real-time PCR, and the practice was repeated thrice for each group. Tick samples consisted of nymphs, which were allowed to feed on B. microti-infected hamsters with ∼10% parasitemia, and female ticks, which fed on hamsters with ∼5% parasitemia. Genomic DNA was isolated from B. microti-infected ticks using a NucleoSpin® Tissue kit (Macherey-Nagel, Duren, Germany) according to the manufacturer’s manual. In addition, conventional PCR using KOD-Plus-Neo DNA polymerase (Toyobo, Osaka, Japan) and B. microti β-tubulin-specific primers and H. longicornis actin-specific primers (control gene) was performed on nymphal samples to detect B. microti DNA. The PCR products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide (EB). Conventional PCR was performed in triplicate for each group. The primers used in this study are listed in Supplementary Table S1. The genetic amount of B. microti 18S rRNA (Bm18S rRNA) was normalized against that of H. longicornis ITS-2 (HlITS-2) using the ΔCT {2^[–(CT Bm18S rRNA –CT HlITS–2)]} method.
Suppression Subtractive Hybridization (SSH) cDNA Construction and Analysis
The technique of SSH was used to compare the expression levels of the porin gene in B. microti-infected and -uninfected engorged female ticks. Babesia DNA in ticks was detected by conventional PCR with β-tubulin gene primers as described above. Forward and reverse suppression subtraction cDNA libraries were constructed using the Super SMARTTM PCR cDNA synthesis kit according to the manufacturer’s instructions (Clontech, Mountain View, CA, United States). Briefly, in the forward suppression subtraction cDNA library, cDNA prepared from 15 Babesia-infected ticks served as the “Tester,” and cDNA prepared from 15 uninfected ticks served as the “Driver” in the subtraction procedure to enrich for cDNAs preferentially expressed and upregulated in the Babesia-infected ticks. In the reverse suppression subtraction cDNA library, cDNA from 15 infected ticks (driver) was used in excess to hybridize cDNA from 15 uninfected ticks (tester) to enrich for cDNAs preferentially expressed and upregulated in the uninfected ticks. Two PCR amplifications were performed to enrich differentially expressed transcripts in infected ticks from the forward suppression subtraction cDNA library and in uninfected ticks from the reverse suppression subtraction cDNA library. The amounts of porin transcripts in the forward and reverse suppression subtraction cDNA libraries and in unsubtracted cDNA libraries were determined by relative band brightness of its PCR products on an electrophoresed gel stained with EB.
RNAi and the Effect of Porin Knockdown on Tick Blood Feeding and Babesia Infection
RNAi was used to analyze the effect of porin knockdown on blood feeding, Babesia infection, and the porin-related apoptosis signaling pathway. The porin double-strand RNA (dsRNA) was constructed with the primer set including the T7 promoter sequence (underlined with double solid lines) at the 5′-end of both primers (porin RNAiF: 5′-GATA TCTAATACGACTCACTATAGGTGCACACCAACGTGAACG AC-3′; porin RNAiR: 5′-GATATCTAATACGACTCACTATAGG AAAAGATAGGAAGGGTCTGCCG-3′). Female ticks were used for RNAi experiments as described previously (Liao et al., 2009). The dsRNA-injected ticks were allowed to rest 1 day and then put in chambers attached to the hair-shaved back of hamsters. Each hamster was challenged with 15 dsRNA-injected ticks in the control group or experimental group. The practice was repeated three times. To examine porin knockdown efficiency during blood feeding after dsRNA injection, two 0-to-7-day-fed ticks from the infested hamsters were collected from the porin dsRNA-injected group and a firefly luciferase dsRNA-injected group as a control. Determination of the expression of porin was done as described in Section Real-Time PCR Analysis. In contrast, B. microti burdens and the expression levels of porin-related apoptosis genes were assessed by real-time PCR using genomic DNA and cDNA, respectively. The feeding success of the remaining ticks was investigated by measuring the feeding period and body weight at engorgement.
Statistical Analysis
The mean ranks of Babesia burdens in the porin RNAi or control group and mRNA levels of porin or its related apoptosis genes in the uninfected or infected ticks, porin RNAi or control group, 1-day-laid or 7-day-laid eggs, and 2-day-partially-fed (2 dPF) or 2 days after engorgement (2 dAE) nymphs were compared using the Mann-Whitney U test. The difference in the mean ranks of B. microti burdens, and mRNA levels of porin in nymphal and female adult ticks during the blood-feeding process, was analyzed with the Kruskal-Wallis H test followed by the Dunn’s multiple comparisons test. A p-value of <0.05 was considered statistically significant.
Nucleotide Sequence Accession Number
The sequences of the porin gene of H. longicornis and its related apoptosis gene Bcl were submitted to the GenBank database under accession numbers MN584740 and MN584741, respectively.
Results
Porin Characterization
The coding nucleotide sequence of the porin cDNA was found to be 849 bp in length and encoded 282 amino acids with an expected isoelectric point of 8.95 and molecular weight of 30.4 kDa. The protein is glycine-and-leucine rich with 35 glycines and 31 leucines. Domain analysis showed porin to contain six determinants of voltage gating and two polypeptide binding sites (Figure 1). Multiple alignment of the amino acid sequence with the homolog sequences from other tick species, including I. scapularis, R. microplus, and A. variegatum, revealed that the determinants of voltage gating and the polypeptide binding sites are conserved among these four tick species. The H. longicornis porin amino acids showed the highest homology with that of R. microplus, with 90.07% identity and 93.62% similarity, in contrast to 84.75% identity and 92.91% similarity with that of I. scapularis, and 75.18% identity and 79.43% similarity with that of A. variegatum (Figure 2).
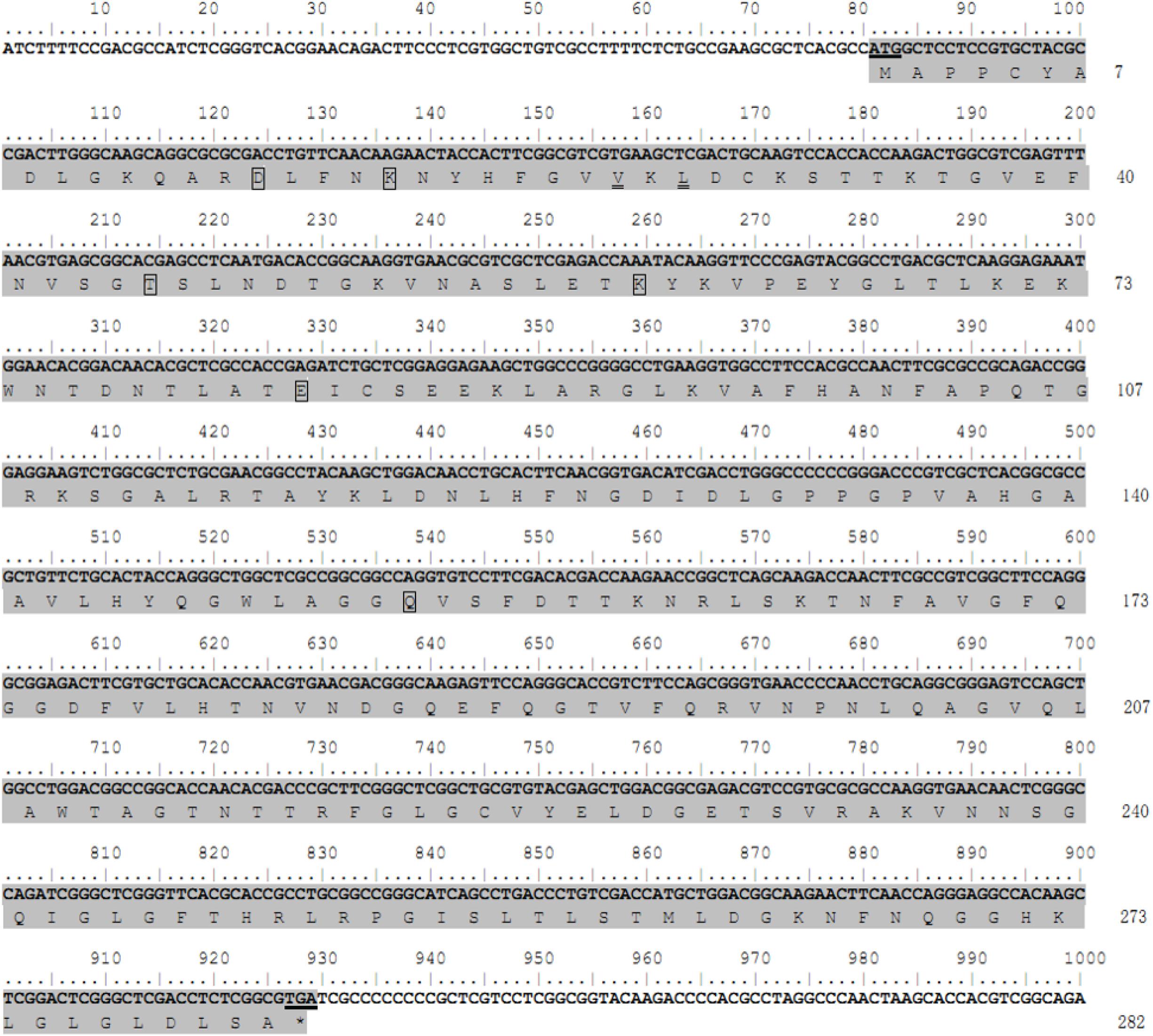
Figure 1. Nucleotide and deduced amino acid sequences of the porin gene from the H. longicornis tick. The protein coding region for the porin gene is indicated by gray shading. The residuals in the boxes show putative determinants of voltage gating, and the residuals with double underlines are putative polypeptide binding sites. The start codon ATG and stop codon TGA are underlined. Nucleotides are numbered above each line, and amino acid numbering is on the right.
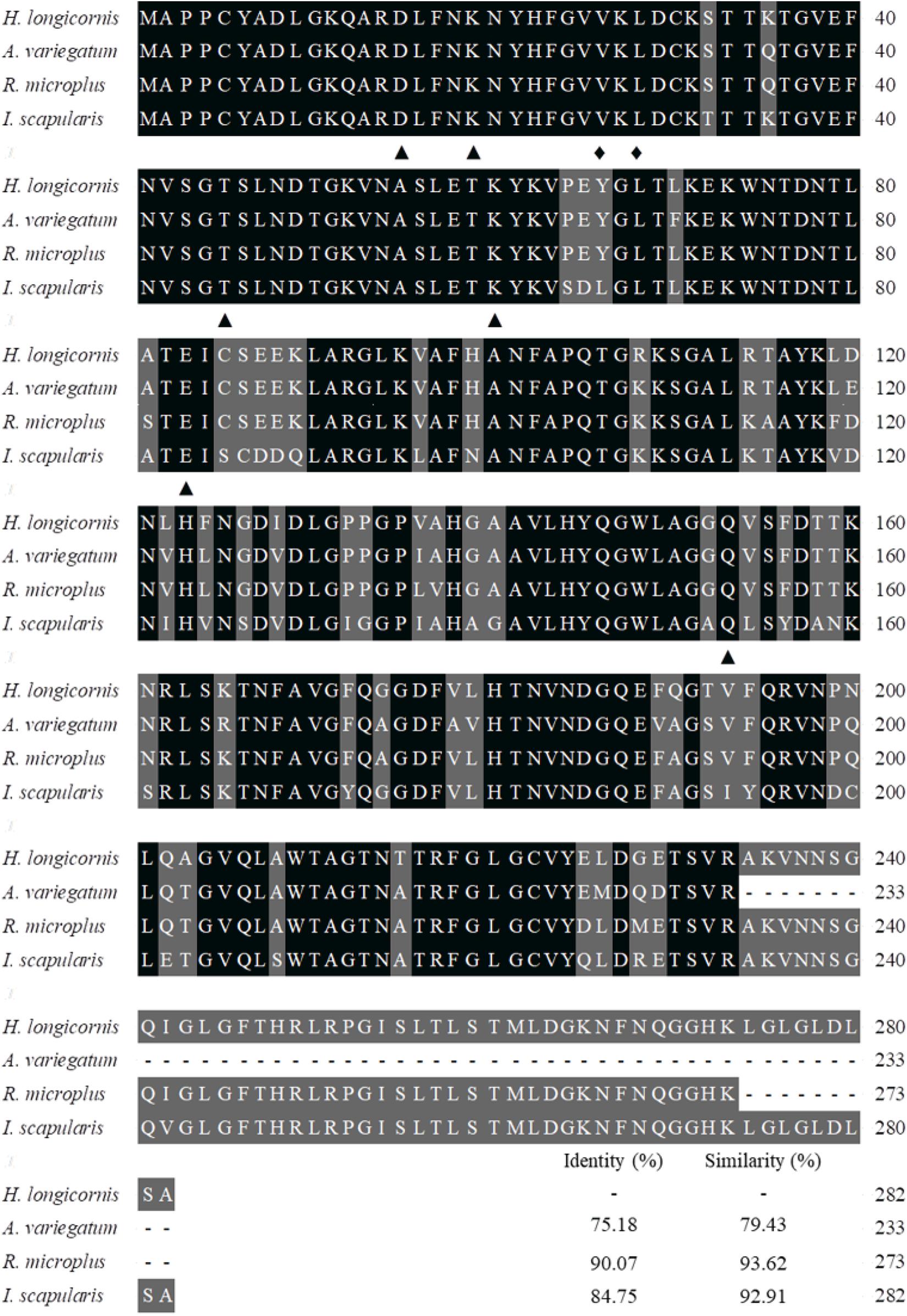
Figure 2. Alignment of the amino acid sequence of the porin gene of H. longicornis was compared with those of the ixodid ticks Ixodes scapularis (XP_002408065), Rhipicephalus microplus (ADT82652), and Amblyomma variegatum (DAA34069). Identical residues are darkly shaded and similarity residues are gray shaded. Amino acid numbering is on the right. The putative determinants of voltage gating and polypeptide binding sites are shown at the bottom of the sub-columns with triangles and diamonds, respectively.
Expression Profiles of Porin Gene and Porin-Related Apoptosis Genes in H. longicornis Ticks
GAPDH was the most stably expressed internal control gene in ticks at developmental stages compared with L23, HlP0, and Hlactin and was used as the internal control gene in this study (Supplementary Figure S1). Real-time PCR revealed that porin mRNA was expressed in the eggs and unfed and fed nymphs (Figures 3A,B). There were no differences in porin mRNA levels between 1-day-laid eggs and 7-day-laid eggs (Figure 3A). Porin showed no significant change in expression levels between unfed nymphs incubated at 15 and 25°C (Supplementary Figure S2). Porin expression levels were higher in the unfed nymphs, 12-h-partially-fed (12 hPF) to 2-d-partially-fed (2 dPF) nymphs, and the nymphs at 0 dAE than the nymphs at 2 dAE (p < 0.05) (Figure 3B). Subsequently, nymphal samples at 2 dPF and 2 dAE were used to examine expression levels of porin-related apoptosis genes. Cytc and Cas2 were significantly less expressed in 2 dAE nymphs than in 2 dPF nymphs (p < 0.05) (Figure 3C). However, mRNA levels of Bcl and Cas8 in nymphs were not significantly different at 2 dAE vs. 2 dPF (Figure 3C).
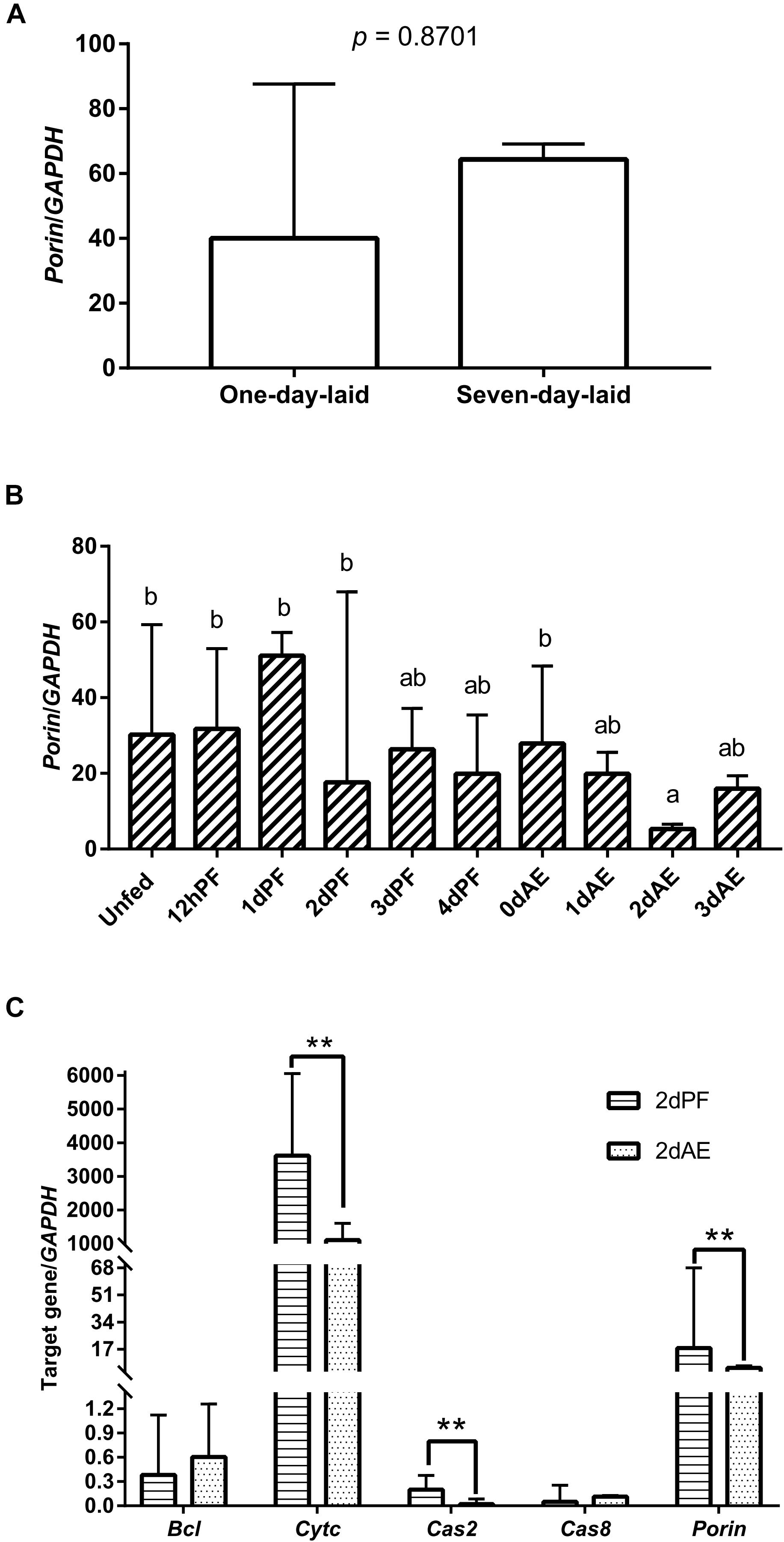
Figure 3. Porin expression levels at developmental stages of H. longicornis ticks. (A) Porin gene expression levels at egg stage (1-day-laid eggs and 7-day-laid eggs). (B) Porin gene expression levels in unfed nymphs, partially fed nymphs (12 hPF to 4 dPF), and nymphs at 0–3 days after engorgement (0 dAE to 3 dAE). (C) Comparison of mRNA expression levels of porin and its caspase-related apoptosis signal pathway genes between 2 dAE nymphs and 2 dPF nymphs. The bar indicates the median with 95% confidence interval (CI) of three biological repeats. Different letters above the bars represent significant differences (p < 0.05). **p < 0.01.
B. microti Gray Strain Burdens in H. longicornis Ticks
Nymphs fed on B. microti-infected hamsters for 12 h and 1–3 days (12 hPF nymphs to 3 dPF nymphs) had lower levels of Babesia burdens compared with those fed for 4 days (4 dPF nymphs) and ticks at the onset of engorgement (0 dAE) (Figure 4). Real-time PCR analysis showed that the largest amount of Babesia DNA was detected at 0 dAE and then decreased at 1–3 dAE, which was further confirmed by conventional PCR analysis (gel electrophoresis image in Figure 4). A similar phenomenon was found in female ticks injected with dsRNA of firefly luciferase (control group) during blood feeding as evidenced by the peak of Babesia burden at 0 dAE and its subsequent reduction (Figure 5).
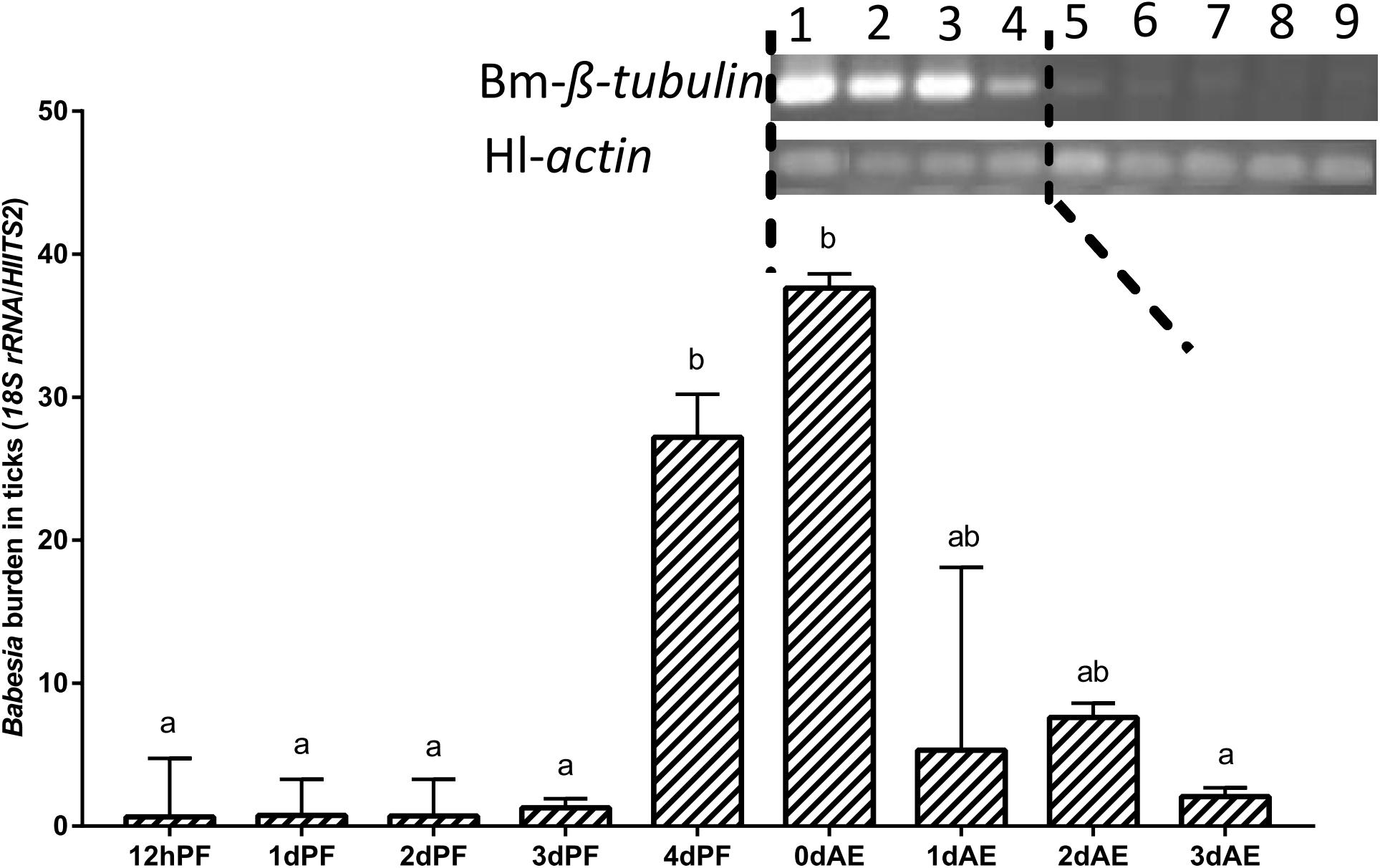
Figure 4. Babesia burdens in nymphal H. longicornis ticks. Gel electrophoresis image shows the result of a conventional PCR analysis of Babesia infection in nymphal ticks at 0–7 days after engorgement (lanes 1–8). Lane 9, negative control. β-tubulin (1,341 bp; Bm-β-tubulin) was amplified to detect Babesia microti, and a 143-bp fragment of the H. longicornis actin was amplified as a control. Data sets plotted in histogram are for 12-h partially fed nymphs to 4-day partially-fed nymphs (12 hPF to 4 dPF) and engorged nymphs at 0–3 days after engorgement (0 dAE to 3 dAE). The bar indicates the median with 95% CI of three biological repeats, and different letters above the bars represent significant differences (p < 0.05).
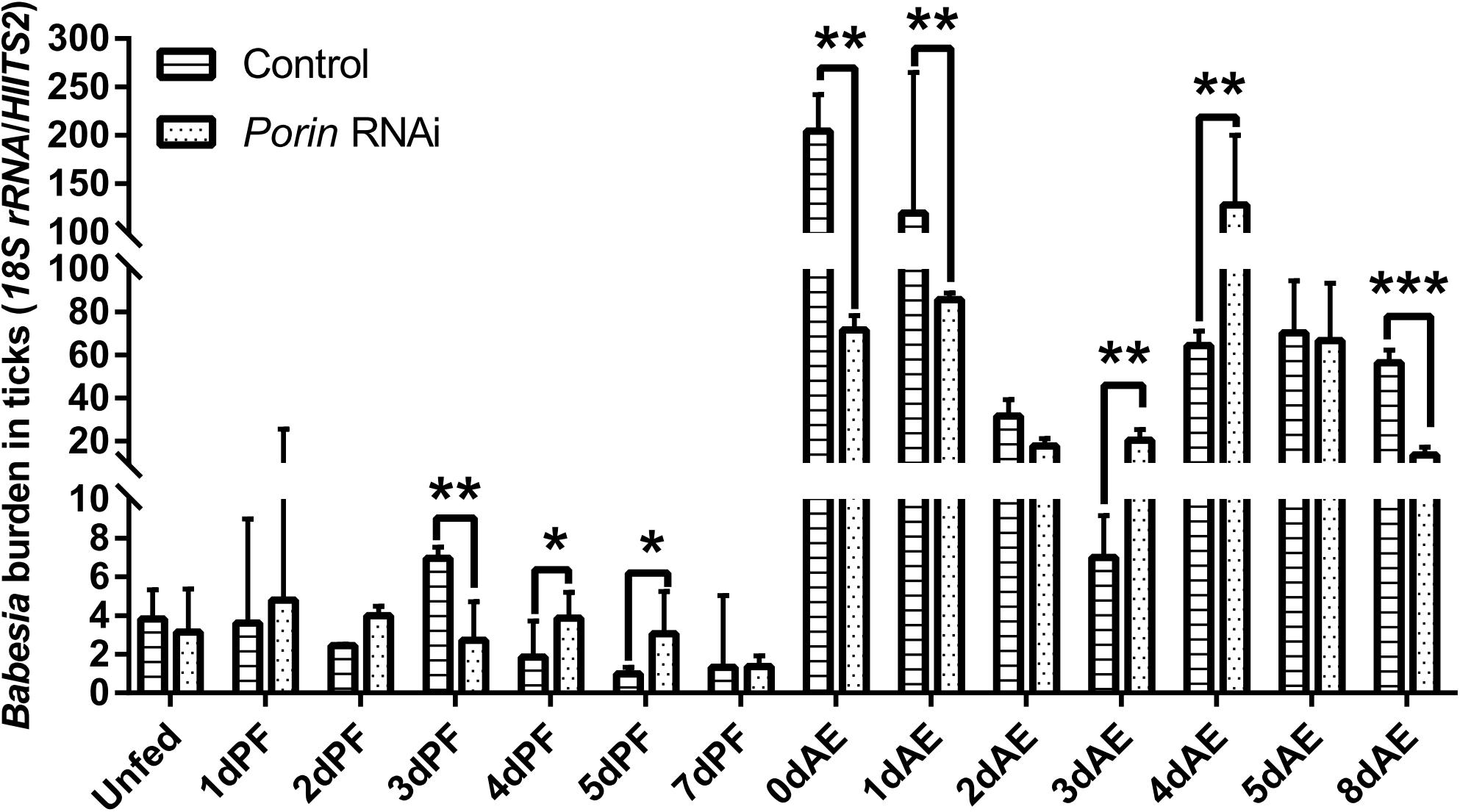
Figure 5. Babesia burdens in porin RNAi and control ticks during blood feeding. The bar indicates the median with 95% CI of three biological repeats. The asterisks above the bars indicate significant differences in Babesia burdens between porin RNAi and control groups. *p < 0.05; **p < 0.01; ***p < 0.001.
Comparison of Expression Levels of Porin Gene Between Uninfected and Infected Nymphal and Female Ticks
Expression of the porin gene was found in B. microti-infected or -uninfected nymphs (Figure 6A). Porin expression levels were higher in the infected vs. uninfected nymphs at 2 and 3 dAE (Figure 6A). The expression levels appeared to be higher in infected nymphs during blood feeding (2, 3, and 4 dPF) compared with uninfected nymphs. When the highest Babesia load was reached at 0 dAE (Figure 4), it appeared that the porin expression level in the infected nymphs was decreased (p = 0.43) (Figure 6A). We then performed an experiment to validate whether the identical phenomenon occurred in female ticks (Figures 6B–D). The band on a gel in porin PCR products amplified from the reverse SSH cDNA library was brighter than those from the forward SSH cDNA library (Figure 6C). The significantly higher mRNA levels of porin in uninfected engorged female ticks were further confirmed by real-time PCR (p = 0.0022) (Figure 6D).
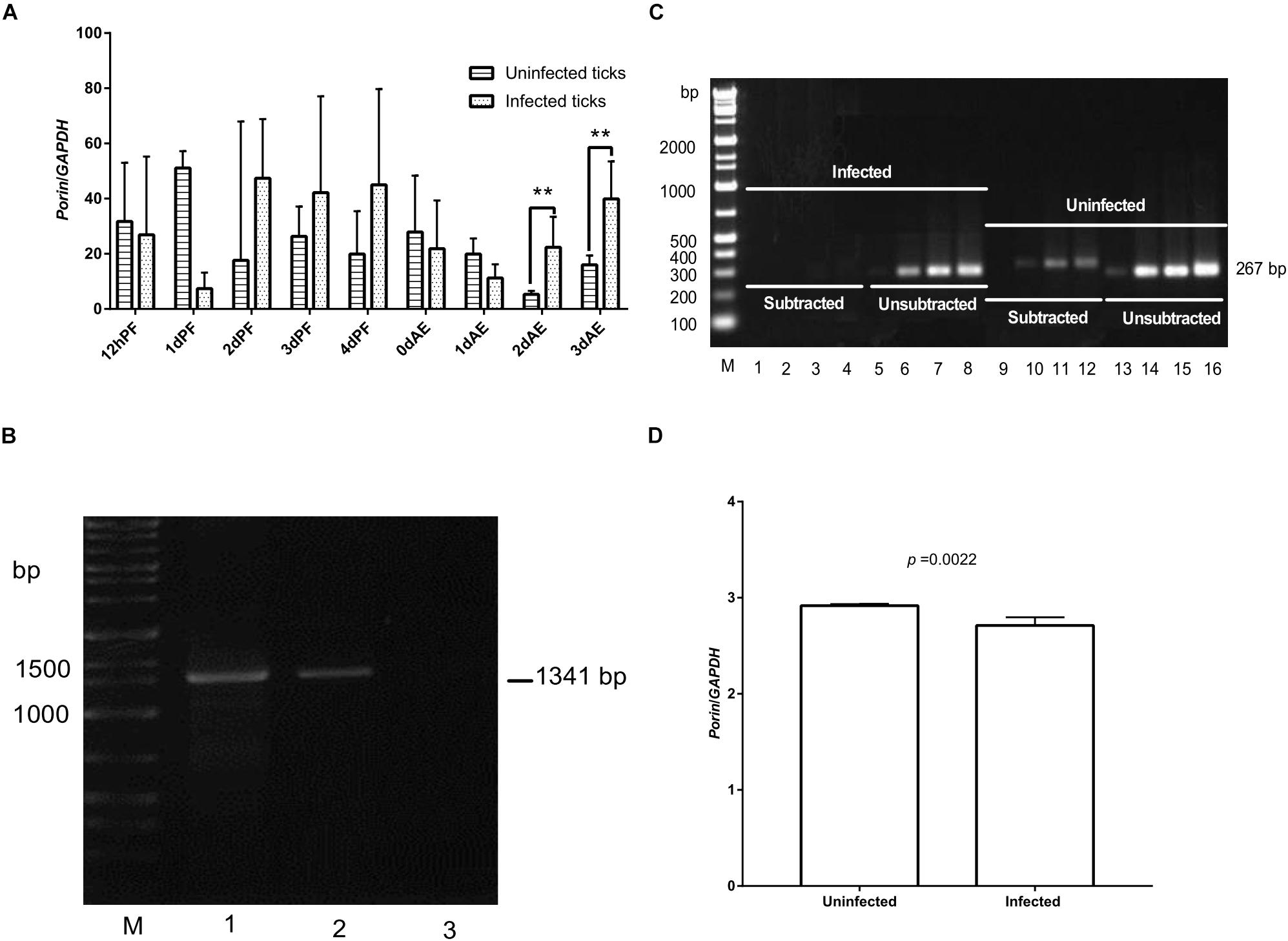
Figure 6. Expression levels of the porin gene between uninfected and infected nymphal and female ticks. (A) Porin gene expression level in B. microti-infected or -uninfected nymphal H. longicornis. (B) Gel electrophoresis analysis of conventional PCR products of Babesia β-tubulin (1,341 bp) in B. microti-infected and -uninfected female ticks at engorgement. Lane 1, infected tick sample; lane 2, positive control; lane 3, uninfected tick sample. (C) SSH analysis of porin expression levels in infected ticks. No or weak bands were visualized on a gel for PCR products of porin amplified from cDNA of infected ticks subtracted with that of uninfected ticks. Bright bands were visualized on a gel for PCR products of porin amplified from cDNA of uninfected ticks subtracted with that of infected ticks. Lanes 1, 5, 9, and 13: PCR products amplified by 18 cycles from porin gene; lanes 2, 6, 10, and 14: PCR products amplified by 23 cycles; lanes 3, 7, 11, and 15: PCR products amplified by 28 cycles; lanes 4, 8, 12, and 16: PCR products amplified by 33 cycles. (D) Real-time PCR analysis of porin mRNA expression in whole body of B. microti-infected and -uninfected engorged female ticks. The bar indicates the median with 95% CI of three biological repeats. The asterisks above the bar indicate a significant difference in porin/GAPDH between uninfected and B. microti-infected ticks (**p < 0.01).
Effect of Porin Knockdown on Blood Feeding, Babesia Infection, and Expression Profiles of Porin-Related Apoptosis Genes in Female Ticks
When each tick was injected with 1 μg of porin dsRNA, a gradual reduction in gene silencing efficiency was seen in the hamster-infested ticks after 2 days from tick attachment (Figure 7A). The gene was knocked down by 90.24% in female ticks fed on hamsters for 2 days (Figure 7A). The body weight of the engorged female ticks in the control group was significantly higher (p < 0.001) than that of the RNAi group (Figure 7B). No differences in feeding period were seen in the control and porin-knockdown groups (data not shown). The effect of porin silencing on Babesia burdens in the female ticks was time-course dependent. At 3dPF, 0 dAE, 1 dAE, and 8 dAE, the RNAi ticks had 2.34, 2.91, 2.16, and 4.26-fold lower Babesia burdens in comparison with the control ticks, respectively (Figure 5). However, at 4 dPF, 5 dPF, 3 dAE and 4 dAE, 2.04, 3.14, 2.82, and 2.41-fold higher amounts of Babesia DNA were detected in the porin-knockdown ticks, respectively (Figure 5). Furthermore, at 0 dAE the expression levels of Cytc and Bcl in the porin-knockdown female ticks significantly decreased in contrast to that in the control ticks (p < 0.01 and p < 0.05), whereas the mRNA levels of Cas2 and Cas8 did not show obvious changes in the porin-RNAi ticks compared with the control ticks (Figure 8).
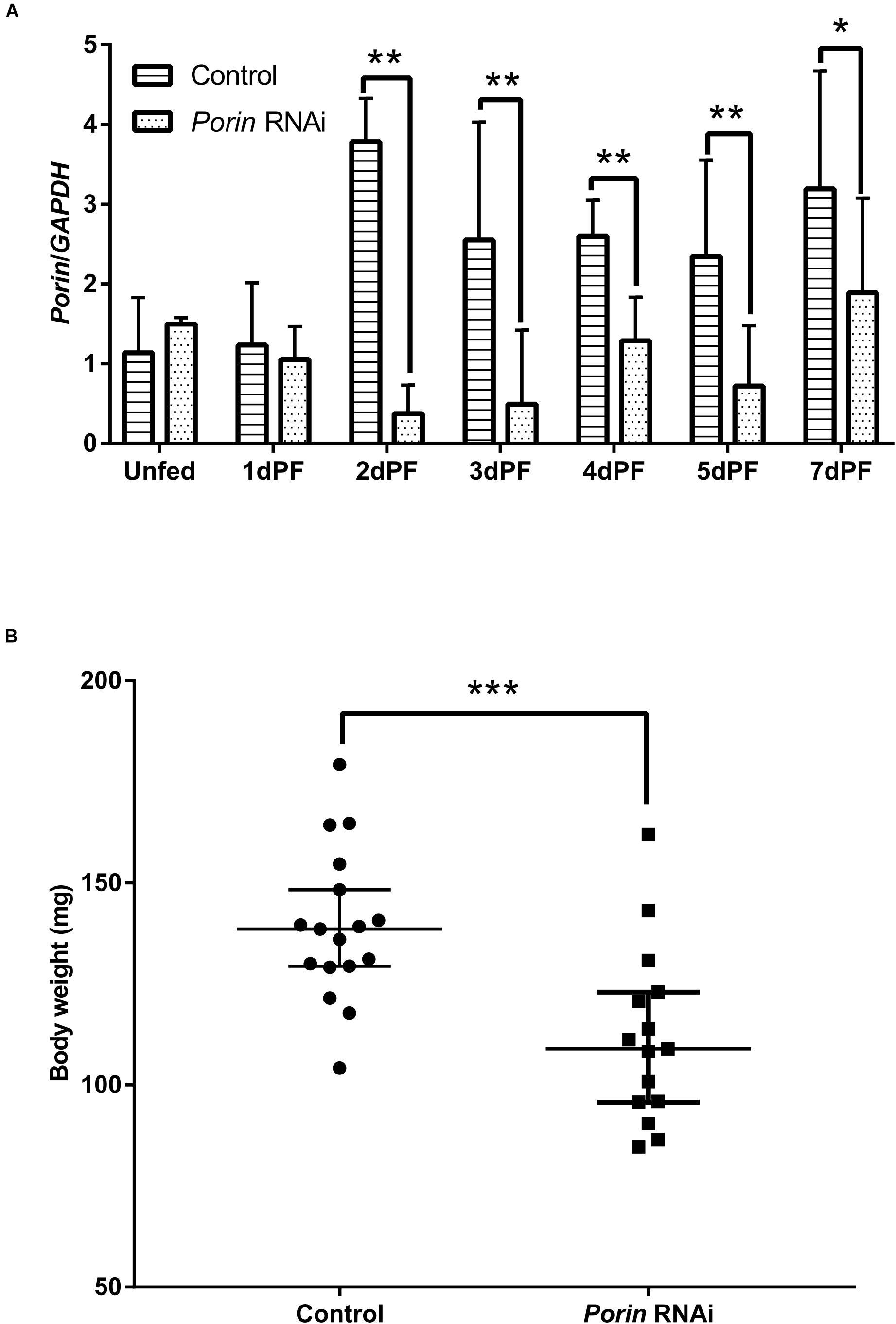
Figure 7. The effect of porin silencing on body weight of female ticks at engorgement. (A) Expression analysis of porin mRNA in whole body of dsRNA-injected female ticks; (B) Body weights of female ticks at engorgement in porin RNAi and control ticks. The bar indicates the median with 95% CI of three biological repeats. The asterisks above the bars indicate significant differences in porin/GAPDH and body weight between porin RNAi and control groups. *p < 0.05; **p < 0.01; ***p < 0.001.
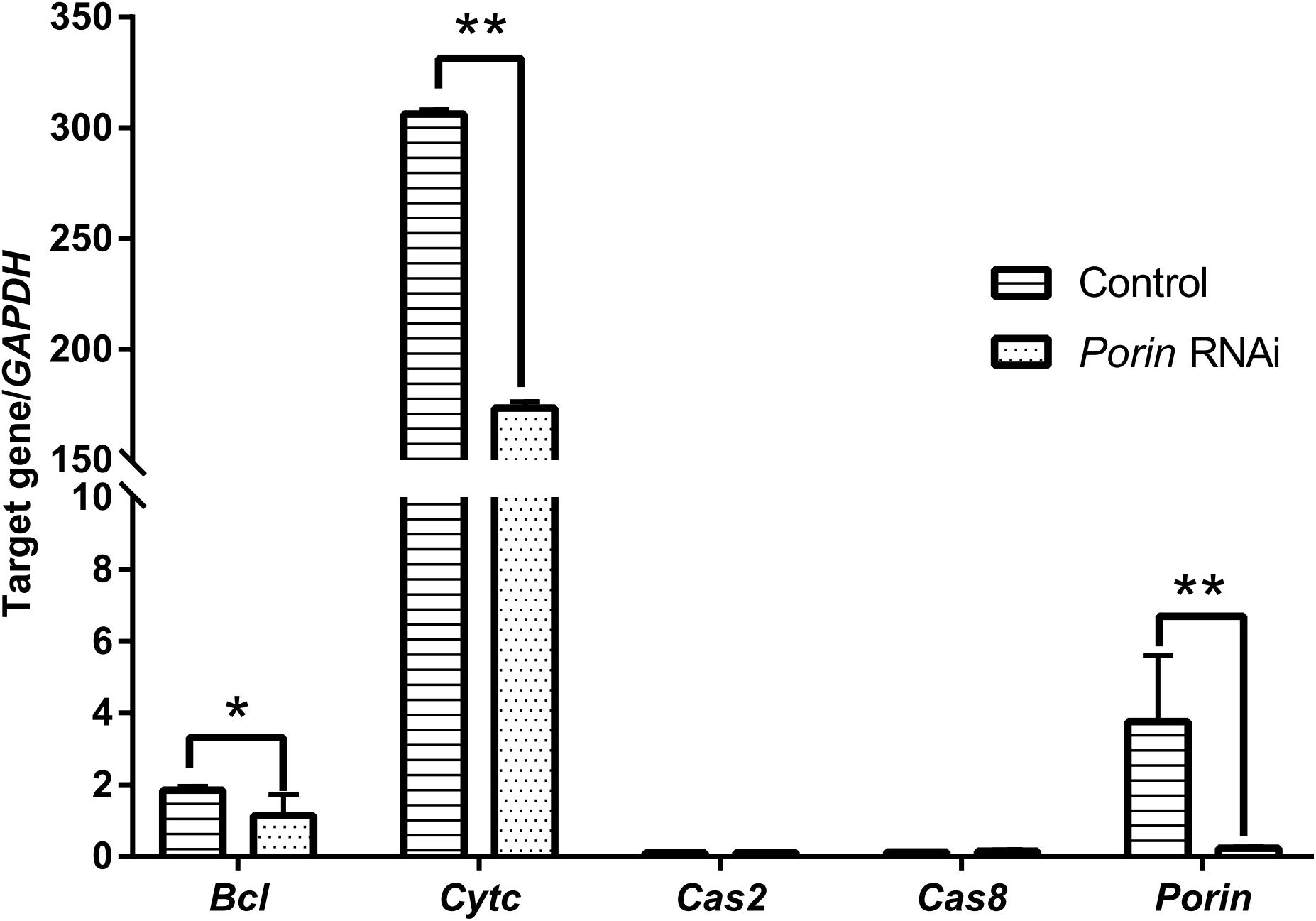
Figure 8. Impact of porin RNAi on porin-related apoptosis gene expression in engorged female ticks. The bar indicates the median with 95% CI of three biological repeats. The asterisks above the bars indicate significant differences in target gene/GAPDH between porin RNAi and control groups. *p < 0.05; **p < 0.01.
Discussion
Porin in H. longicornis is a 30.4 kDa protein with 282 amino acids, as is reported in other organisms (Sardiello et al., 2003; Wang et al., 2010; Rodríguez-Hernández et al., 2011). Additionally, our study showed that determinants of voltage gating and polypeptide binding sites in porin protein are conserved among tick species (Figure 2), suggesting that they play a primary role in the regulation of ion and molecular flow and in metabolism inside and outside the mitochondrial membrane among ticks. It was reported that porin might be involved in tick feeding and/or digestion of blood meals and its development (Ayllón et al., 2013; Rodríguez-Hernández et al., 2015). Porin mRNA levels of I. scapularis increased from egg to adult stages and from the non-feeding to feeding periods of female ticks. Knockdown of the gene resulted in about a 40% reduction in female tick weight after feeding compared to the weight of controls (Ayllón et al., 2013). Porin expression levels in the midgut of adult R. microplus ticks first increased to a maximum and then decreased at 0 to 72 h post repletion (Rodríguez-Hernández et al., 2015). In the present study, a similar expression level of porin mRNA was found in 1-day-laid and 7-day-laid eggs (Figure 3A), and the mRNA levels were appeared to be higher in the unfed nymphs, 12 hPF to 2 dPF nymphs, and the nymphs at 0 dAE than the nymphs at 2 dAE (Figure 3B). However, their expression levels increased in female ticks when taking blood from the hosts (Figure 7A). Porin silencing mediated by RNAi significantly decreased the body weight of engorged adults but did not alter the blood feeding period (Figure 7B).
When confronting stressful situations and adverse conditions, remodeling of the cell skeleton, inhibition of cell apoptosis, and manipulation of the innate or specific immune system can help hosts remove the damaged cells to maintain tissue homeostasis and therefore benefit the remaining cells (de la Fuente et al., 2016). In the regulated process of cell apoptosis, porin plays a pivotal role in releasing an apoptogenic factor, namely Cytc. Pathogen infection activates the Janus kinase/signal transducers and activators of transcription (JAK/STAT) to down-regulate porin expression and therefore inhibit cell apoptosis as an aid to pathogen infection, survival, development, and multiplication inside infected cells (Alberdi et al., 2015; de la Fuente et al., 2016). In the present study, porin mRNA expression levels appeared to be lower in B. microti-infected ticks than in uninfected ticks at engorgement when the highest Babesia burden occurred (Figures 5A,C,D), suggesting that the invasion of a large number of Babesia might inhibit cell apoptosis in ticks via suppression of porin expression. However, some other studies have reported opposite findings, showing the same or higher levels of porin expression in vectors when they have the highest pathogen load (Fongsaran et al., 2014; Rodríguez-Hernández et al., 2015; Jitobaom et al., 2016). These studies showed that porin may function as an activator of pathogen receptors (such as plasminogen) or a part of a pathogen receptor (such as porin plus GRP78 complex). The formation of pathogen receptor facilitates pathogen entry into cells, for example, the dissemination of Borrelia burgdorferi in Ixodes ticks, B. bigemina in Rhipicephalus ticks, and the invasion of Japanese encephalitis virus, dengue virus, and Plasmodium spp. into the midgut cells of mosquitoes. Our data showed that nymphal and adult ticks acquired B. microti via blood sucking. During the blood feeding process, we found that the amount of babesial DNA in ticks increased at 4 dPF and 0 dAE, and then decreased thereafter, suggesting that B. microti infected and proliferated in the tick body at these timings. Moreover, porin mRNA expression levels appeared to be lower at 1 dPF, higher at 2–4 dPF, lower at 0–1 dAE, and higher at 2–3 dAE in the infected nymphs vs. the uninfected nymphs. The porin expression dynamics might be related to Babesia infection in a time-dependent manner. RNAi of porin changed the Babesia infection level in dsRNA-injected ticks in contrast to the control ticks. The peak of Babesia burden in control ticks was observed at 0 dAE, however, the peak in porin dsRNA-injected ticks was found at 4 dAE. Taken together, our results indicate that during the blood feeding Babesia infection might cause the inhibition of cell apoptosis at one time point and/or activation of porin expression for pathogen invasion at another time point (Alberdi et al., 2015; Rodríguez-Hernández et al., 2015; de la Fuente et al., 2016). For better understanding the interactions between B. microti and porin and the related molecules, further analyses will be needed focusing on an important organ for Babesia infection, such as the midgut.
Most caspases play a role in programmed cell death, including apoptosis and pyroptosis, and as initiators, executioners, or inflammatory types (Galluzzi et al., 2016). Hlcaspase-2 (termed Cas2 in our study) and Hlcaspase-8 (termed Cas8) previously identified from H. longicornis (Tanaka et al., 2007) are two members of initiator caspases that might play important roles in inducing cell death by apoptosis. In addition to apoptosis, Cas8 is required for the inhibition of necroptosis (Denecker et al., 2008). During times of cellular stress, mitochondrial Cytc binding to an adaptor protein (APAF-1) recruits initiator caspases, which helps to form a caspase-activating multiprotein complex called the apoptosome. Once activated, initiator caspases will modulate other executioner caspases. This leads to degradation of cellular components for apoptosis (Creagh, 2014). In our study, highly down-regulated Cytc induced by porin silencing did not suppress the expression levels of the initiator caspase, Cas8 (Figure 8). The same phenomenon was observed in nymphs at 2 dAE and 2 dPF (Figure 3C), which might be explained by an extrinsic, and not intrinsic, apoptotic pathway available to Cas8 (Creagh, 2014). Further experiments at protein level will be required to evaluate the role of porin in the activating caspases-interfered apoptotic pathway.
Conclusion
In conclusion, the present experiments identified the porin gene from H. longicornis and evaluated its expression levels in B. microti-infected and -uninfected H. longicornis ticks at developmental stages. Our data suggest that porin might positively regulate expression of the Cytc gene, which is known to be vital for caspases-interfered cell apoptosis. Porin knockdown reduced body weight and changed Babesia infection levels in H. longicornis ticks. In addition, we detected DNA of the B. microti Gray strain in both nymphal and adult stages that fed on infected hamsters by using conventional and real-time PCR analyses. Babesia loads in nymphs and adults remained at low levels before engorgement, peaked at/around onset of engorgement, and then gradually decreased to low levels such as those in initial blood feeding stages, which is consistent with a previous observation of B. microti Munich strain infection in mice (Kusakisako et al., 2015). This H. longicornis-B. microti experimental infection model using hamsters will be used for further investigation of the interaction between ticks and human Babesia. Taken together, our findings will be useful for better understanding the roles of H. longicornis porin in tick development, blood feeding, and B. microti infection.
Data Availability Statement
The datasets generated for this study can be found in the GenBank database under accession numbers MN584740 for porin and MN584741 for Bcl.
Ethics Statement
The animal study was reviewed and approved by Animal Care and Use Committee of Obihiro University of Agriculture and Veterinary Medicine.
Author Contributions
XX, RU-S, HS, QZ, and HC conceived and designed the study. WZ performed most of the experimental work. WZ and RU-S wrote the manuscript. WZ, KO, PAM, and ML collected and analyzed the data.
Funding
This study was financially supported by grants from the Jiangxi Provincial Department of Science and Technology (Grant Nos. 20192BBHL80013, 2016BBG70005), the Health Commission of Jiangxi Province (Grant No. 20162007), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan as a project for Joint Usage/Research Center. WZ was supported by a Sasakawa Medical Fellowship through the Japan-China Medical Association.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate Mr. Hiroyuki Sugawara (Obihiro University of Agriculture and Veterinary Medicine) for his great work in rabbit and hamster rearing and in aiding in attaching chambers to the backs of the hamsters. We thank Ms. Maki Kuniyori (Obihiro University of Agriculture and Veterinary Medicine) for assistance with the methodology.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00502/full#supplementary-material
Footnotes
- ^ https://blast.ncbi.nlm.nih.gov
- ^ https://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi
- ^ http://nadv.herokuapp.com/
- ^ http://www.bioinformatics.org/SMS/multi_align.html
- ^ https://www.bioinformatics.org/sms2/ident_sim.html
- ^ https://web.expasy.org/compute_pi/
References
Alberdi, P., Ayllón, N., Cabezas-Cruz, A., Bell-Sakyi, L., Zweygarth, E., Stuen, S., et al. (2015). Infection of Ixodes spp. tick cells with different Anaplasma phagocytophilum isolates induces the inhibition of apoptotic cell death. Ticks Tick Borne Dis. 6, 758–767. doi: 10.1016/j.ttbdis.2015.07.001
Antunes, S., Rosa, C., Couto, J., Joana, F., and Domingos, A. (2017). Deciphering babesia-vector interactions. Front. Cell. Infect. Microbiol. 7:429. doi: 10.3389/fcimb.2017.00429
Ayllón, N., Villar, M., Busby, A. T., Kocan, K. M., Blouin, E. F., Bonzón-Kulichenko, E., et al. (2013). Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 81, 2415–2425. doi: 10.1128/IAI.00194-13
Chae, J. S., and Lee, M. J. (2010). Molecular detection of Ehrlichia chaffeensis and Anaplasma bovis in the salivary glands from Haemaphysalis longicornis ticks. Vect. Borne Zoon. Dis. 10, 411–443. doi: 10.1089/vbz.2008.0215
Chen, Z., Li, H., Gao, X., Bian, A., Yan, H., Kong, D., et al. (2019). Human babesiosis in China: a systematic review. Parasitol. Res. 118, 1103–1112. doi: 10.1007/s00436-019-06250-9
Creagh, E. M. (2014). Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol. 35, 631–640. doi: 10.1016/j.it.2014.10.004
de la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7:114. doi: 10.3389/fcimb.2017.00114
de la Fuente, J., Estrada-Peña, A., Cabezas-Cruz, A., and Kocan, K. M. (2016). Anaplasma phagocytophilum uses common strategies for infection of ticks and vertebrate hosts. Trends Microbiol. 24, 173–180. doi: 10.1016/j.tim.2015.12.001
Denecker, G., Ovaere, P., Vandenabeele, P., and Declercq, W. (2008). Caspase-14 reveals its secrets. J. Cell. Biol. 180, 451–458. doi: 10.1083/jcb.200709098
Fang, L. Q., Liu, K., Li, X. L., Liang, S., Yang, Y., Yao, H. W., et al. (2015). Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect. Dis. 15, 1467–1479. doi: 10.1016/S1473-3099(15)00177-2
Fongsaran, C., Phaonakrop, N., Roytrakul, S., Thepparit, C., Kuadkitkan, A., and Smith, D. R. (2014). Voltage dependent anion channel is redistributed during Japanese encephalitis virus infection of insect cells. Sci. World J. 2014:976015. doi: 10.1155/2014/976015
Galluzzi, L., López-Soto, A., Kumar, S., and Kroemer, G. (2016). Caspases connect cell-death signaling to organismal homeostasis. Immunity 44, 221–231. doi: 10.1016/j.immuni.2016.01.020
Heath, A. (2016). Biology, ecology and distribution of the tick, Haemaphysalis longicornis neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. 64, 10–20. doi: 10.1080/00480169.2015.1035769
Ikadai, H., Sasaki, M., Ishida, H., Matsuu, A., Igarashi, I., Fujisaki, K., et al. (2007). Molecular evidence of Babesia equi transmission in Haemaphysalis longicornis. Am. J. Trop. Med. Hyg. 76, 694–697.
Jitobaom, K., Tongluan, N., and Smith, D. R. (2016). Involvement of voltage-dependent anion channel (VDAC) in dengue infection. Sci. Rep. 6:35753. doi: 10.1038/srep35753
Krause, P. J. (2019). Human babesiosis. Int. J. Parasitol. 49, 165–174. doi: 10.1016/j.ijpara.2018.11.007
Kusakisako, K., Maeda, H., Galay, R. L., Matsuo, T., Tsujio, M., Umemiya-Shirafuji, R., et al. (2015). The vector potential of Haemaphysalis longicornis ticks for Babesia microti parasites under experimental condition. J. Protozool. Res. 25, 8–17.
Liao, M., Zhou, J., Gong, H., Boldbaatar, D., Shirafuji, R., Battur, B., et al. (2009). Hemalin, a thrombin inhibitor isolated from a midgut cDNA library from the hard tick Haemaphysalis longicornis. J. Insect Physiol. 55, 164–173. doi: 10.1016/j.jinsphys.2008.11.004
Mather, T. N., Telford, S. R., Moore, S. I., and Spielman, A. (1990). Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exp. Parasitol. 70, 55–61. doi: 10.1016/0014-4894(90)90085-q
McFadden, A. M., Rawdon, T. G., Meyer, J., Makin, J., Morley, C. M., Clough, R. R., et al. (2011). An outbreak of Haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N. Z. Vet. J. 59, 79–85. doi: 10.1080/00480169.2011.552857
Raghavan, R. K., Barker, S. C., Cobos, M. E., Barker, D., Teo, E., Foley, D. H., et al. (2019). Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci. Rep. 9:498. doi: 10.1038/s41598-018-37205-2
Rainey, T., Occi, J. L., Robbins, R. G., and Egizi, A. (2018). Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 55, 757–759. doi: 10.1093/jme/tjy006
Ribeiro, J. M., Anderson, J. M., Manoukis, N. C., Meng, Z., and Francischetti, I. M. (2011). A further insight into the sialome of the tropical bont tick, Amblyomma variegatum. BMC Genomics 12:136. doi: 10.1186/1471-2164-12-136
Rodríguez-Hernández, E., Mosqueda, J., Alvarez-Sánchez, M. E., Neri, A. F., Mendoza-Hernández, G., and Camacho-Nuez, M. (2011). The identification of a VDAC-like protein involved in the interaction of Babesia bigemina sexual stages with Rhipicephalus microplus midgut cells. Vet. Parasitol. 187, 538–541. doi: 10.1016/j.vetpar.2012.01.028
Rodríguez-Hernández, E., Mosqueda, J., León-Ávila, G., Castañeda-Ortiz, E. J., Álvarez-Sánchez, M. E., Camacho, A. D., et al. (2015). BmVDAC upregulation in the midgut of Rhipicephalus microplus, during infection with Babesia bigemina. Vet. Parasitol. 212, 368–374. doi: 10.1016/j.vetpar.2015.06.016
Sardiello, M., Tripoli, G., Oliva, M., Santolamazza, F., Moschetti, R., Barsanti, P., et al. (2003). Comparative study of the porin genes encoding VDAC, a voltage-dependent anion channel protein, in Anopheles gambiae and Drosophila melanogaster. Gene 317, 111–112. doi: 10.1016/s0378-1119(03)00658-9
Sivakumar, T., Tattiyapong, M., Okubo, K., Suganuma, K., Hayashida, K., Igarashi, I., et al. (2014). PCR detection of Babesia ovata from questing ticks in Japan. Ticks Tick Borne Dis. 5, 305–310. doi: 10.1016/j.ttbdis.2013.12.006
Tanaka, M., Liao, M., Zhou, J., Nishikawa, Y., Xuan, X., and Fujisaki, K. (2007). Molecular cloning of two caspase-like genes from the hard tick Haemaphysalis longicornis. J. Vet. Med. Sci. 69, 85–90. doi: 10.1292/jvms.69.85
Umemiya-Shirafuji, R., Fujisaki, K., Okado, K., Adjou Moumouni, P. F., Yokoyama, N., Hiroshi, S., et al. (2019a). Hard ticks as research resources for vector biology: from genome to whole-body level. Med. Entomol. Zool. 70, 181–188. doi: 10.7601/mez.70.181
Umemiya-Shirafuji, R., Mihara, R., Fujisaki, K., and Suzuki, H. (2019b). Intracellular localization of vitellogenin receptor mRNA and protein during oogenesis of a parthenogenetic tick, Haemaphysalis longicornis. Parasit. Vect. 12:205. doi: 10.1186/s13071-019-3469-9
Vannier, E., and Krause, P. J. (2012). Human babesiosis. N. Engl. J. Med. 366, 2397–2407. doi: 10.1056/NEJMra1202018
Wang, K. C., Kondo, H., Hirono, I., and Aoki, T. (2010). The Marsupenaeus japonicus voltage-dependent anion channel (MjVDAC) protein is involved in white spot syndrome virus (WSSV) pathogenesis. Fish Shellfish Immunol. 29, 94–103. doi: 10.1016/j.fsi.2010.02.020
Wormser, G. P., McKenna, D., Piedmonte, N., Vinci, V., Egizi, A. M., Backenson, B., et al. (2019). First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis. 70, 314–316. doi: 10.1093/cid/ciz449
Wu, J., Cao, J., Zhou, Y., Zhang, H., Gong, H., and Zhou, J. (2017). Evaluation on infectivity of Babesia microti to domestic animals and ticks outside the Ixodes genus. Front. Microbiol. 8:1915. doi: 10.3389/fmicb.2017.01915
Zhang, H., Sun, Y., Jiang, H., and Huo, X. (2017). Prevalence of severe febrile and thrombocytopenic syndrome virus, Anaplasma spp. and Babesia microti in hard ticks (Acari: Ixodidae) from Jiaodong peninsula, Shandong Province. Vector Borne Zoonotic Dis. 17, 134–140. doi: 10.1089/vbz.2016.1978
Zheng, W., Xuan, X., Fu, R., Tao, H., Xu, R., Liu, Y., et al. (2019). Preliminary investigation of ixodid ticks in jiangxi province of Eastern China. Exp. Appl. Acarol. 77, 93–104. doi: 10.1007/s10493-018-0324-1
Zheng, W. Q., Xuan, X. N., Fu, R. L., Tao, H. Y., Liu, Y. Q., Liu, X. Q., et al. (2018). Tick-borne pathogens in ixodid ticks from Poyang Lake Region, southeastern China. Korean J. Parasitol. 56, 589–596. doi: 10.3347/kjp.2018.56.6.589
Keywords: Haemaphysalis longicornis, tick, Babesia microti, protozoan parasite, porin, expression profiles
Citation: Zheng W, Umemiya-Shirafuji R, Zhang Q, Okado K, Adjou Moumouni PF, Suzuki H, Chen H, Liu M and Xuan X (2020) Porin Expression Profiles in Haemaphysalis longicornis Infected With Babesia microti. Front. Physiol. 11:502. doi: 10.3389/fphys.2020.00502
Received: 14 February 2020; Accepted: 23 April 2020;
Published: 19 May 2020.
Edited by:
Itabajara Silva Vaz Jr., Federal University of Rio Grande do Sul, BrazilReviewed by:
Stephen Lu, National Institute of Allergy and Infectious Diseases (NIH), United StatesJose Octavio Merino, Instituto de Investigacion en Recursos Cinegèticos, Spain
Copyright © 2020 Zheng, Umemiya-Shirafuji, Zhang, Okado, Adjou Moumouni, Suzuki, Chen, Liu and Xuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rika Umemiya-Shirafuji, umemiya@obihiro.ac.jp; Xuenan Xuan, gen@obihiro.ac.jp
 Weiqing Zheng
Weiqing Zheng Rika Umemiya-Shirafuji
Rika Umemiya-Shirafuji Qian Zhang3
Qian Zhang3 Kiyoshi Okado
Kiyoshi Okado