- 1Research Group Cardiovascular Diseases, Department of Genetics, Pharmacology and Physiopathology of Heart, Blood Vessels and Skeleton, University of Antwerp, Antwerp, Belgium
- 2Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium
- 3Antwerp Surgical Training, Anatomy and Research Centre, University of Antwerp, Antwerp, Belgium
- 4Department of Obstetrics and Gynaecology, Antwerp University Hospital, Antwerp, Belgium
Hypertensive disorders of pregnancy, including gestational hypertension and pre-eclampsia, occur in up to 10% of pregnancies and are associated with increased life-long cardiovascular risk. Physical activity improves cardiovascular health in pregnancy and may lower the risk of developing hypertensive disorders of pregnancy. However, a minority of pregnant women comply with the recommended level of physical activity. Adequate knowledge on the physiological effects of exercise in healthy pregnancy could help to overcome potential barriers as pregnancy is a unique window of opportunity to improve health outcomes for both mother and child. In this mini review, we discuss structural and functional vascular adaptations during healthy and hypertensive pregnancies, we elaborate on the effects of exercise on the vasculature and review the safety and existing evidence of exercise training as preventive therapy for gestational hypertensive disorders.
Introduction
Worldwide guidelines recommend aerobic training during pregnancy from 60 to 150 min/week (Savvaki et al., 2018). Little is known about the number of women practicing this, but numbers as low as 15% have been cited (Kuhrt et al., 2015). Women who exercise as recommended have 30% less risk for developing gestational hypertensive disorders (GHD), including gestational hypertension (GH), characterized by hypertension initiating after the 20th pregnancy week and pre-eclampsia (PE) defined as hypertension and proteinuria after the 20th pregnancy week (Magro-Malosso et al., 2017; Davenport et al., 2018b). Preliminary data suggest that exercise during pregnancy has a lifelong protective effect resulting in a reduced cardiovascular risk profile in the perimenopause (Clapp, 2008). Maternal physical exercise is also beneficial for the fetus, resulting in less macrosomia and consequently improved cardiovascular health of the child at a later age (Alexander et al., 2015).
Pregnancy can be considered a stress test for the cardiovascular system, imposing profound cardiovascular adaptations including increased blood volume, accompanied by a drop in vascular resistance due to increased angiogenesis and vasodilation, generalized reduction in arterial stiffness and improved endothelial function, increased cardiac output associated with increased right and left chamber size and eccentric hypertrophy, resulting in higher stroke volume and heart rate and a fall in systemic blood pressure (Melchiorre et al., 2012; Chung and Leinwand, 2014; Osol and Bernstein, 2014; Tkachenko et al., 2014; Mannaerts et al., 2019). Regular physical exercise can boost these adaptations as has been demonstrated for angiogenesis and endothelial function (Skow et al., 2017). In women with GHD, these functional and structural vascular adaptions fail (Mannaerts et al., 2019), and may persist beyond pregnancy (Kirollos et al., 2019), explaining why these women are at a lifelong increased risk for cardiovascular disease (Lane-Cordova et al., 2019).
In this mini review, we will elucidate the vascular adaptation during normal vs. hypertensive pregnancies and we will focus on the potentially beneficial effects of exercise on the vasculature. Based on this concept, physical exercise prior to and during pregnancy may be a promising therapy to prevent GHD and GHD recurrence, however, current data to underscore this hypothesis are still limited.
Vascular Adaptation During Healthy Pregnancy
An optimal adaptation of the cardiovascular system is crucial for a healthy pregnancy. As early as 5 weeks amenorrhea, a significant fall in systemic vascular tone occurs, altering the set-points of the baroreceptors and the stretch receptors (Tkachenko et al., 2014). As a result, systemic vascular resistance decreases to allow sufficient placental perfusion (Clark et al., 1989). Venous tone decreases as well, resulting in expansion of the venous compartment and increased cardiac preload, ultimately leading to increased cardiac output (Melchiorre et al., 2012; Chung and Leinwand, 2014). To accommodate this blood volume expansion and increased cardiac output, the arterial bed needs to undergo structural and functional changes (Skow et al., 2017).
During pregnancy, structural arterial remodeling is mainly driven by placental growth factor (PlGF)-induced angiogenesis, occurring primarily at the uteroplacental unit (Osol and Bernstein, 2014). Soluble fms-like tyrosine kinase 1 (sFlt-1) is the circulating form of the VEGF receptor-1 and binds VEGF and PlGF thereby reducing their bioavailability.
The ratio of sFlt-1/PlGF is an important indicator of the angiogenic status in pregnancy and is used to predict and diagnose PE. Interestingly, this ratio appears to be indicative of future vascular dysfunction risk (Zeisler et al., 2016). The decrease in total vascular resistance is mediated by VEGF and PlGF as they induce distal angiogenesis (Hasan et al., 2002). Placental growth factor also mediates the cardiac adaptation and insufficient PlGF leads to impaired ventricular remodeling and cardiac dysfunction (Hochholzer et al., 2011).
To accommodate the increased blood volume while maintaining low blood pressure, a generalized reduction in arterial stiffness is of great importance. Central (aortic) pulse wave velocity (PWV), the gold standard for arterial stiffness, is known to be decreased in healthy pregnancy (Mannaerts et al., 2019).
A healthy endothelium controls vasomotor tone, which is essential during pregnancy. The rapidly expanding blood volume and increase in cardiac output pose an increased shear stress on endothelial cells, resulting in increased endothelial nitric oxide (NO) production (Cockell and Poston, 1997; Williams et al., 1997). Together with higher estrogen levels, this leads to a systemic vasodilation (Meah et al., 2016). In healthy pregnancy, endothelial NO synthase (eNOS) activity is significantly increased (Nelson et al., 2000) which is mirrored in improved flow-mediated dilatation (FMD), the gold standard for endothelial function measurement (Iacobaeus et al., 2017; Mannaerts et al., 2019).
Vascular Maladaptation in Gestational Hypertensive Disorders
Women who develop hypertensive disorders during pregnancy such as GH or PE appear to fail the stress test of pregnancy, in part due to insufficient cardiovascular adaptation. Therefore, the risk of developing cardiovascular disease later in life is 9.5 times higher for women with severe early PE [hazard ratio (HR) = 9.5, 95% confidence interval (CI) = 4.5–20.3] (Mongraw-Chaffin et al., 2010). Furthermore, PE has been associated with an increased risk for developing end-stage kidney disease (HR = 4.96, 95% CI = 3.9–6.3) (Khashan et al., 2019). Therefore, long-term cardiovascular monitoring and early preventive therapy are advocated (McDonald et al., 2008; Ahmed et al., 2014).
In PE, insufficient arterial remodeling at the spiral arteries results in placental ischemia-reperfusion damage and the production of high amounts of free radicals causing oxidative stress (Figure 1). Circulating free radicals activate peripheral leucocytes and platelets, resulting in an inflammatory state and disturbing proper endothelial function. The reaction of oxidative products with NO decreases its bioavailability which impairs endothelial function even more (Mannaerts et al., 2018). The abundant placental ischemia and oxidative stress in PE results in an anti-angiogenic state with a three-fold increase in antiangiogenic factors (sFlt-1) and a 90% reduction in angiogenic factors (PlGF and VEGF; Tomimatsu et al., 2017).
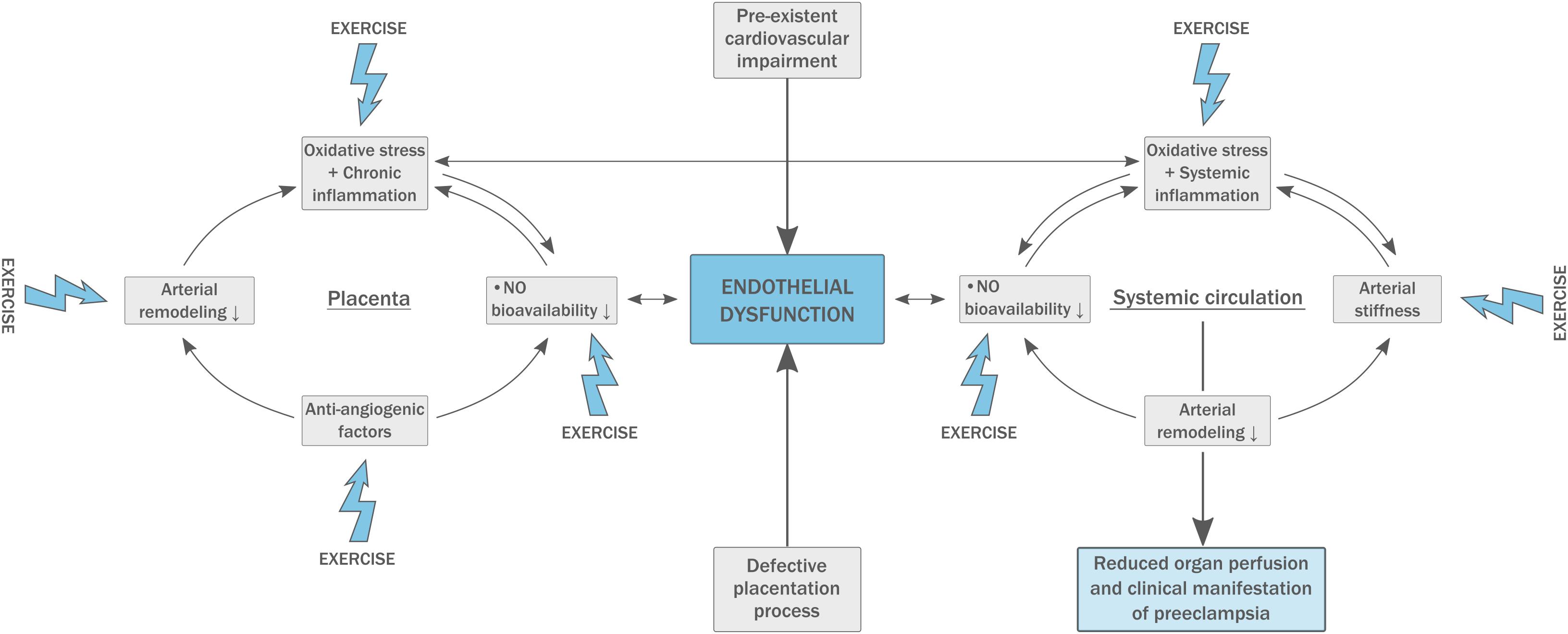
Figure 1. Pathophysiology of pre-eclampsia (PE). A pre-existing fragile endothelial situation leads to defective placentation and high circulating levels of oxidative stress. This inadequate response to pregnancy results in arterial stiffness and exacerbates generalized endothelial dysfunction. Physical exercise has beneficial effects on multiple components of the model.
Women suffering from PE have increased arterial stiffness both during and after pregnancy, and arterial stiffness is directly correlated to the severity of the disease (Figure 1; Hausvater et al., 2012; Mannaerts et al., 2019). Carotid-femoral PWV is abnormal from 11 to 13 weeks in patients who develop PE later in pregnancy, which supports the concept that PE is not caused by dysfunctional placentation alone and underlying vascular disease must be present. Increased arterial stiffness may have an important influence on fetal birth weight and pregnancy outcome (Skow et al., 2017). In addition, central PWV is strongly related to an increased risk for the development of cardiovascular disease later in life, also in PE (Hausvater et al., 2012).
PE is characterized by dysfunction of both resting (L-FMC, low-flow mediated constriction) and recruitable (FMD) endothelial capacity (Mannaerts et al., 2019). Endothelial dysfunction is proven to be present prior to the development of PE, possibly serving as a predictive parameter (Figure 1; Weissgerber, 2014). Further, women with a history of PE appear to have reduced FMD up to 3 years postpartum (Scholten et al., 2014). Endothelial dysfunction impairs vascular smooth muscle relaxation which enhances arterial stiffness and plays an important role in the development of atherosclerosis. This suggests endothelial dysfunction to be the most plausible common link between the pathophysiology of PE and future cardiovascular disease (Mosca et al., 2011; Weissgerber, 2014).
Effects of Exercise on the Vasculature
Repeated exercise bouts effectively benefit vascular function directly by exerting shear forces on the vascular wall (Hambrecht et al., 2003; Adams et al., 2005; Grimm et al., 2018) and indirectly by the release of anti-inflammatory and anabolic mediators in response to increased muscular energy demands (Goldhammer et al., 2005; Kadoglou et al., 2007; Pedersen et al., 2007; Rehm et al., 2015). This results in functional adaptation of the local and systemic vasculature to meet increased perfusion demands and to structural arterial remodeling by engagement of neuro-humoral and metabolic mechanisms (Figure 2; Roveda et al., 2003; Adams et al., 2005; Pedersen et al., 2007; Rehm et al., 2015).
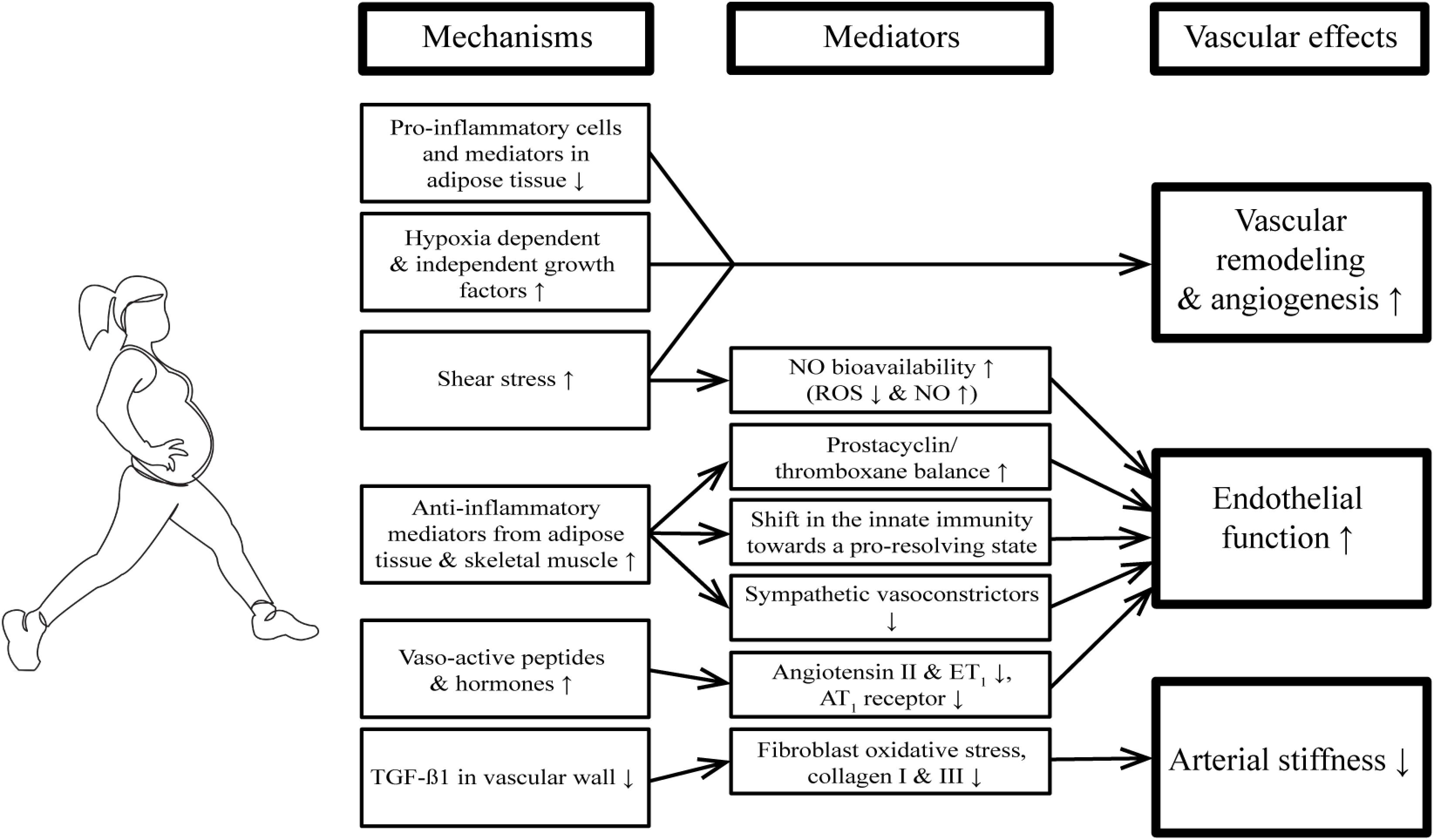
Figure 2. Beneficial effects of repeated exercise bouts on the vasculature. AT1 receptor, Angiotensin II receptor type 1; eNOS, endothelial nitric oxide synthase; ET1, endothelin-1; NO, nitric oxide; ROS, reactive oxygen species; TGF-β, transforming growth factor beta.
There is clear evidence that endothelial function is improved by regular physical activity, both in patients with cardiovascular risk factors (Lavrenčič et al., 2000) and in patients with established cardiovascular disease (Linke et al., 2001; Van Craenenbroeck et al., 2010; Van Craenenbroeck E.M. et al., 2015). This exercise-induced benefit on endothelial function is mediated by different factors.
First, increased shear stress during exercise activates eNOS and reduces NAD(P)H oxidase activity, resulting in decreased reactive oxygen species (ROS) and increased NO bioavailability (Hambrecht et al., 2003; Adams et al., 2005). Furthermore, laminar shear stress prevents inflammation-related alterations in eNOS levels and prostacyclin/thromboxane ratio in an atherogenic environment (Grimm et al., 2018).
Second, endurance training has repeatedly been reported to lower levels of pro-inflammatory cytokines (CRP, IL-18, IL-1β, and IL-8) and increase anti-inflammatory cytokines (IL-10; Goldhammer et al., 2005; Kadoglou et al., 2007). The reduction of body fat and the anti-inflammatory and anabolic mediators released from the active skeletal muscle (referred to as “myokines”; Pedersen et al., 2007) induce systemic shifts in the innate and adaptive immunity toward a more pro-resolving and anti-inflammatory status (Rehm et al., 2015).
Third, exercise training modulates the balance between vasodilating and vasoconstricting factors, overall resulting in more vasodilation. Exercise training reduces levels of endothelin-1 and noradrenalin (Mortensen et al., 2009; Dow et al., 2017), reverses the aging-induced increase in the vasoconstrictor thromboxane (Hellsten et al., 2012) and lowers sympathetic tone (Roveda et al., 2003).
Regular physical activity and exercise interventions have been associated with the prevention of age-related increases in arterial stiffness (Fleenor et al., 2010). In a mouse model, the profibrotic cytokine TGF-β1 increased with aging in the carotid adventitia, where it augmented oxidative stress in fibroblasts. This resulted in increased collagen I and III deposition, and arterial stiffness (Fleenor et al., 2010). The aging-associated elevation in adventitial TGF-β1 is reduced by aerobic exercise both in mice and humans, which in turn reduces large elastic artery stiffening (Fleenor et al., 2010). In addition, increased oxidative stress has been associated with reduced large elastic artery compliance in sedentary vs. habitually exercising postmenopausal women (Moreau et al., 2006).
Exercise has a profound impact on the process of vascular remodeling, which is again driven by increased blood flow and shear stress, by inflammatory cells, as well as by hypoxia-dependent and -independent growth factors (Hoier and Hellsten, 2014; Laughlin, 2016). The pro-angiogenic effect of exercise is not limited to the exercising skeletal muscle, but also induces angiogenesis in adipose tissue (Van Pelt et al., 2017) and increased coronary collateral flow in patients with coronary artery disease (Möbius-Winkler et al., 2016).
Effects of Exercise in Healthy Pregnancy
Regular exercise is known to decrease cardiovascular disease in the non-pregnant population and is implemented in the treatment of heart failure and coronary artery disease patients (Karlsen et al., 2019; Witvrouwen et al., 2019a). Improved vascular health has been suggested as a major contributing factor (Myers, 2003; Van Craenenbroeck E.M. et al. 2010; 2015, Van Craenenbroeck A.H. et al., 2016).
In an uncomplicated pregnancy, current guidelines recommend moderate exercise at a frequency of two to four times a week and with an exercise duration of 30 min, throughout pregnancy (Savvaki et al., 2018). Overall, both aerobic and resistance exercises do not exert any adverse effects during pregnancy. However, evidence on resistance training is scarce and exercise with heavy loads is discommended (Savvaki et al., 2018). Most recreational exercise is safe, but sports that may cause abdominal trauma, falls or excessive joint stress and scuba diving should be avoided (Kuhrt et al., 2015; Bø et al., 2016; Savvaki et al., 2018).
Whereas the guidelines generally recommend 30 min of moderate exercise two to four times per week, 85% of pregnant women are exercising below these levels (Evenson and Wen, 2010). The most frequent barriers are fatigue, lack of time and pregnancy discomforts, but also safety concerns such as low birth weight, preterm labor and inducing fetal bradycardia could withhold pregnant women and health practitioners to prescribe the recommended amount of physical exercise (Kuhrt et al., 2015; Coll et al., 2017; Harrison et al., 2018; Witvrouwen et al., 2019b). Adequate knowledge on the physiological effects of exercise training in healthy pregnancy should help to overcome these barriers as pregnancy is a unique window of opportunity to improve health outcomes for the mother and also the future generations (Kuhrt et al., 2015).
There is no evidence for the induction of preterm delivery by regular physical activity. On the contrary, even a reduction in preterm birth of 20–50% in women performing exercise during pregnancy compared with sedentary pregnant women has been shown (Juhl et al., 2010).
The same is true for the concerns regarding exercise and low birth weight: maternal exercise was not associated with low birth weight or Apgar score at delivery (Davenport et al., 2018a). The normalization of maternal blood glucose, decrease in insulin resistance and increased placental functional capacity and nutrient delivery are suggested mechanisms to explain the beneficial effect of exercise on birth weight (Clapp, 2003; Kuhrt et al., 2015).
During exercise, peripheral vasodilation in the skin and exercising muscles can lead to reduced placental blood flow. In addition to poor autoregulation of the placental circulation, this may cause reduced oxygen and nutrient delivery to the fetus. Other proposed mechanisms for possible fetal distress during maternal exercise include vagal reflex, cord compression or fetal head compression related to malposition (Artal and O’Toole, 2003). Nevertheless, a significant decrease in mean uterine artery blood flow and fetal bradycardia has only been shown in Olympic level athletes exercising at more than 90% of the maximal maternal heart rate (Salvesen et al., 2011). Moreover, it has been shown that regular exercise improves both maternal cardiovascular adaptations and placental function to maintain sufficient fetal oxygenation and growth and does not adversely affect fetal heart rate (Clapp, 2003; Kuhrt et al., 2015).
Effects of Exercise for the Prevention of Hypertensive Disorders of Pregnancy
Even prior to actual pregnancy, physical activity is related to a lower occurrence of PE, with a 22–35% relative risk (RR) reduction for women with the highest vs. lowest physical activity level (Aune et al., 2014). This risk was even further reduced (RR = 0.64, 95% CI = 0.44–0.93) with combined pre- and early pregnancy physical activity. When assessing the dose-response effect of physical activity, 5–6 h of physical activity per week reduced the risk of PE with 40%, but no further reduction with increasing activity levels were reported (Aune et al., 2014). Likewise, sedentary behavior has been related to higher odds for the development of PE and GH (Aune et al., 2014; Fazzi et al., 2017; Davenport et al., 2018b).
Whether physical activity and training during pregnancy can prevent GH and PE, remains to be established. The largest systematic review and meta-analysis to date on GH (22 randomized controlled trials (RCTs), n = 5,316) and PE (15 RCTs, n = 3,322) showed that exercise during pregnancy significantly lowered the risk for GH (OR = 0.61, 95% CI = 0.43–0.85) and PE (OR = 0.59, 95% CI = 0.37–0.94). Moreover, 600 MET-min/week of moderate-intensity exercise (the equivalent of 140 min of brisk walking) was accompanied by a 25% reduction in the odds of developing GH, PE and gestational diabetes mellitus, with a clear dose-dependent effect (Davenport et al., 2018b).
This is in line with findings from three other large meta-analyses, where reductions in PE or GHD were observed (Aune et al., 2014; Di Mascio et al., 2016; Magro-Malosso et al., 2017). However, other systematic reviews and meta-analyses reported conflicting results depending on the type of the study-design (cohort studies vs. case-control studies vs. RCTs) and the exercise exposure that was studied (Kasawara et al., 2012; Wolf et al., 2013; Muktabhant et al., 2015; da Silva et al., 2016; Zheng et al., 2017; Table 1).

Table 1. Summary of meta-analyses and systematic reviews on the effect of exercise before and/or during pregnancy and the occurrence of gestational hypertensive disorders.
This controversy may be caused by methodological issues, such as heterogeneity in study designs or training programs. There is a wide variety in exercise type (strength vs. endurance vs. combined strength and endurance training, or stretching exercises), duration and frequencies of the training programs (with differences in number of sessions per week, the duration of these sessions and the total duration of the training intervention) in the current studies, and also the exercise domain (such as leisure time physical activity, occupational, domestic, or active commuting exercise) often differs. Furthermore, different evaluation of physical activity (objective measures such as accelerometry or subjective self-reported questionnaires), inadequate correction for confounding variables (some studies did not take BMI into account), or low training adherence could contribute to this discrepancy. The slightly stronger association between prepregnancy exercise and PE compared with early pregnancy physical activity, could also be due to higher achievable intensity levels before pregnancy compared with the pregnant state (Aune et al., 2014).
Conceptually, exercise in early pregnancy can reduce the risk of PE by ameliorating placentation since repetitive hypoxia bouts and reduced placental perfusion will stimulate cell proliferation and angiogenesis and lead to an improved sFlt-1/PlGF balance (Skow et al., 2017).
In elite athletes, evidence on a positive effect of vigorous exercise during pregnancy on PE or GH is lacking. A J-shaped relationship between the risk of PE and exercise, with a 40% reduction in risk with up to 5–6 h exercise per week, but no further reductions at higher activity levels has been described (Aune et al., 2014). As stated above, fetal adverse effects have only been shown in athletes exercising at more than 90% of the maximal maternal heart rate (Salvesen et al., 2011). Therefore, pregnant athletes should be referred to gynecologists for individual risk-assessment and recommendations regarding the type and intensity of exercise during pregnancy (Sma Position Statement et al., 2016; Mottola et al., 2018).
To date, only two RCTs evaluated the effect of exercise on the recurrence of PE in a subsequent pregnancy (Yeo et al., 2008; Kasawara et al., 2013). In the study of Kasawara et al., one training session per week in trimester 2 and 3 of pregnancy did not prevent PE recurrence. The low training intensity (heart rate 20% above resting value) and frequency demand for cautious interpretation of these results (Kasawara et al., 2013). Yeo et al. studied the effect of walking vs. stretching (5 × 40 min/week) in 79 women and also did not demonstrate a reduction in the incidence, possibly affected by low adherence (Yeo et al., 2008).
In established PE pregnancies, only one RCT assessed whether exercise (supervised stretching vs. autogenic training) reduced blood pressure. In 40 PE pregnancies, both training modalities equally lowered blood pressure and proteinuria (p < 0.05) over time (Awad et al., 2019).
Current Research GAPS and Future Directions
A large body of evidence demonstrates that exercise improves systemic endothelial function and arterial stiffness in a wide range of subjects, from children to elderly, as well as in several diseases. Surprisingly, effects of exercise on the vasculature in healthy pregnancies is understudied and data in PE pregnancies are virtually non-existent. To our knowledge, only one study examined the effect of exercise training during a healthy pregnancy on endothelial function (Ramírez-Vélez et al., 2011). In that study, FMD improved by 30% by exercise training starting between 16 and 20 weeks, at moderate intensity. Concerning arterial stiffness, a discretely improved PWV in early post-partum period was observed with prenatal exercise, but has not been studied during pregnancy (Kawabata et al., 2012). In women with a history of PE, improved FMD and venous compliance with exercise training have been shown in small patient groups (Krabbendam et al., 2009; Scholten et al., 2014, 2015), and requires confirmation in larger trials.
Whether exercise training can prevent subsequent GHD in high risk patients, is a justified research question that deserves a well-designed clinical trial. Future research should focus on strategies to improve adherence to exercise training during pregnancy (supervised vs. unsupervised training, providing information on training characteristics and safety of exercise, etc.). Also, clear definitions of exercise should be used, using the FITT acronym (frequency, intensity, type, and time). These training characteristics should be compared and their effects on vascular health and the recurrence of GHD should be assessed. The role of gestational weight gain and the socioeconomic state of the women should be explored. Furthermore, confounding variables (age, BMI, parity, and smoking) and pre-pregnancy physical activity levels should be taken into account. Physical activity should be assessed using preferably objective measures. Also, more research on the timing of initiation of exercise (first, second, or third trimester of pregnancy) and more exercise-only interventions in overweight or obese women should be performed. In addition, whether post-partum exercise in women with history of PE can reduce their increased cardiovascular risk, deserves attention.
In the meantime, physical activity in pregnant women should be stimulated, with structured advice from the treating physician. Offering eg. a smartphone-based program while considering the socioeconomic and psychological needs should ultimately lead to fitter pregnant women, with clear benefits for mother and child.
Conclusion
In GHD, structural and functional adaptations of the vascular wall fail by a large amount, leading to measurable effects on blood pressure in the acute phase and increased cardiovascular risk of both mother and child in the long term. Regular physical activity has profound effects on several parts of the vascular wall by improving endothelial function, reducing arterial stiffness and inducing angiogenesis. Nevertheless, whether these beneficial vascular effects of exercise are related to the lower risk on GHD following training remains to be confirmed. However, moderate physical exercise during pregnancy is safe and will benefit both short- and long-term outcome of mother and baby. Therefore, physical activity should be encouraged in every healthy woman considering only a few contra-indications and addressing potential barriers for exercise during pregnancy.
Author Contributions
YJ wrote the introduction. DM wrote the part on vascular adaptation in healthy pregnancy and in hypertensive disorders of pregnancy. EV elaborated on the effects of exercise on the vasculature and edited the manuscript. AV described the effects of exercise in healthy pregnancy. IW discussed the effects of exercise for the prevention of hypertensive disorders of pregnancy and edited the manuscript. DM, IW, and EV wrote the current research gaps and future directions. All authors revised and accepted the final version of the manuscript to be published.
Funding
EV and IW are supported by the fund for scientific research-Flanders (FWO) as Senior clinical investigator (1804320N) and predoctoral fellow (1194918N) respectively.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adams, V., Linke, A., Kränkel, N., Erbs, S., Gielen, S., Möbius-Winkler, S., et al. (2005). Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111, 555–562. doi: 10.1161/01.CIR.0000154560.88933.7E
Ahmed, R., Dunford, J., Mehran, R., Robson, S., and Kunadian, V. (2014). Pre-eclampsia and future cardiovascular risk among women: a review. J. Am. Coll. Cardiol. 63, 1815–1822. doi: 10.1016/j.jacc.2014.02.529
Alexander, B. T., Dasinger, J. H., and Intapad, S. (2015). Fetal programming and cardiovascular pathology. Compr. Physiol. 5, 997–1025. doi: 10.1002/cphy.c140036
Artal, R., and O’Toole, M. (2003). Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br. J. Sport Med. 37, 6–12. doi: 10.1136/bjsm.37.1.6
Aune, D., Saugstad, O. D., Henriksen, T., and Tonstad, S. (2014). Physical activity and the risk of preeclampsia a systematic review and meta-analysis. Epidemiology 25, 331–343. doi: 10.1097/EDE.0000000000000036
Awad, M. A., Hasanin, M. E., Taha, M. M., and Gabr, A. A. (2019). Effect of stretching exercises versus autogenic training on preeclampsia. J. Exerc. Rehabil. 15, 109–113. doi: 10.12965/jer.1836524.262
Bø, K., Artal, R., Barakat, R., Brown, W., Davies, G. A. L., Dooley, M., et al. (2016). Exercise and pregnancy in recreational and elite athletes: 2016 evidence summary from the IOC expert group meeting, Lausanne. Part 1-exercise in women planning pregnancy and those who are pregnant. Br. J. Sports Med. 50, 571–589. doi: 10.1136/bjsports-2016-096218
Chung, E., and Leinwand, L. A. (2014). Pregnancy as a cardiac stress model. Cardiovasc. Res. 101, 561–570. doi: 10.1093/cvr/cvu013
Clapp, J. F. (2003). The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 110, 80–85. doi: 10.1016/S0301-2115(03)00176-173
Clapp, J. F. (2008). Long-term outcome after exercising throughout pregnancy: fitness and cardiovascular risk. Am. J. Obstet. Gynecol. 199, 1–7. doi: 10.1038/jid.2014.371
Clark, S., Cotton, D., and Lee, W. (1989). Central hemodynamic assessment of normal term pregnancy. Am. J. Obstet. Gynecol. 161(6 Pt 1), 1439–1442.
Cockell, A., and Poston, L. (1997). Flow-mediated vasodilation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 30, 247–251.
Coll, C. V. N., Domingues, M. R., Gonçalves, H., and Bertoldi, A. D. (2017). Perceived barriers to leisure-time physical activity during pregnancy: a literature review of quantitative and qualitative evidence. J. Sci. Med. Sport 20, 17–25. doi: 10.1016/j.jsams.2016.06.007
da Silva, S. G., Ricardo, L. I., Evenson, K. R., and Hallal, P. C. (2016). Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sport Med. 47, 295–317. doi: 10.1007/s40279-016-0565-2
Davenport, M. H., Meah, V. L., Ruchat, S. M., Davies, G. A., Skow, R. J., Barrowman, N., et al. (2018a). Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br. J. Sports Med. 52, 1386–1396. doi: 10.1136/bjsports-2018-099836
Davenport, M. H., Ruchat, S., Poitras, V. J., Garcia, A. J., Gray, C. E., Barrowman, N., et al. (2018b). Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy?: a systematic review and meta-analysis. Br. J. Sport Med. 52, 1367–1375. doi: 10.1136/bjsports-2018-099355
Di Mascio, D., Magro-Malosso, E. R., Saccone, G., Marhefka, G. D., and Berghella, V. (2016). Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 215, 561–571. doi: 10.1016/j.ajog.2016.06.014
Dow, C. A., Stauffer, B. L., Brunjes, D. L., Greiner, J. J., and DeSouza, C. A. (2017). Regular aerobic exercise reduces endothelin-1-mediated vasoconstrictor tone in overweight and obese adults. Exp. Physiol. 102, 1133–1142. doi: 10.1113/EP086454
Evenson, K. R., and Wen, F. (2010). Measuring physical activity among pregnant women using a structured one-week recall questionnaire: evidence for validity and reliability. Int. J. Behav. Nutr. Phys. Act. 7, 1–12. doi: 10.1186/1479-5868-7-21
Fazzi, C., Saunders, D. H., Linton, K., Norman, J. E., and Reynolds, R. M. (2017). Sedentary behaviours during pregnancy: a systematic review. Int. J. Behav. Nutr. Phys. Act. 14, 1–13. doi: 10.1186/s12966-017-0485-z
Fleenor, B. S., Marshall, K. D., Durrant, J. R., Lesniewski, L. A., and Seals, D. R. (2010). Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol. 588, 3971–3982. doi: 10.1113/jphysiol.2010.194753
Goldhammer, E., Tanchilevitch, A., Maor, I., Beniamini, Y., Rosenschein, U., and Sagiv, M. (2005). Exercise training modulates cytokines activity in coronary heart disease patients. Int. J. Cardiol. 100, 93–99. doi: 10.1016/j.ijcard.2004.08.073
Grimm, H., Kretzschmar, J., Cook, M. D., and Brown, M. D. (2018). The effects of exercise, aspirin, and celecoxib in an atherogenic environment. Med. Sci. Sports Exerc. 50, 2033–2039. doi: 10.1249/MSS.0000000000001657
Hambrecht, R., Adams, V., Erbs, S., Linke, A., Kränkel, N., Shu, Y., et al. (2003). Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107, 3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C
Harrison, A. L., Taylor, N. F., Shields, N., and Frawley, H. C. (2018). Attitudes, barriers and enablers to physical activity in pregnant women: a systematic review. J. Physiother. 64, 24–32. doi: 10.1016/j.jphys.2017.11.012
Hasan, K. M., Manyonda, I. T., Ng, F. S., Singer, D. R. J., and Antonios, T. F. T. (2002). Skin capillary density changes in normal pregnancy and pre-eclampsia. J. Hypertens. 20, 2439–2443. doi: 10.1097/00004872-200212000-200212024
Hausvater, A., Giannone, T., Sandoval, Y. H. G., Doonan, R. J., Antonopoulos, C. N., Matsoukis, I. L., et al. (2012). The association between preeclampsia and arterial stiffness. J. Hypertens. 30, 17–33. doi: 10.1097/HJH.0b013e32834e4b0f
Hellsten, Y., Jensen, L., Thaning, P., Nyberg, M., and Mortensen, S. (2012). Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J. Hypertens. 30, 2007–2014. doi: 10.1097/HJH.0b013e328356dd57
Hochholzer, W., Reichlin, T., Stelzig, C., Hochholzer, K., Meissner, J., Breidthardt, T., et al. (2011). Impact of soluble fms-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur. Heart J. 32, 326–335. doi: 10.1093/eurheartj/ehq429
Hoier, B., and Hellsten, Y. (2014). Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 21, 301–314. doi: 10.1111/micc.12117
Iacobaeus, C., Andolf, E., Thorsell, M., Bremme, K., Jörneskog, G., Östlund, E., et al. (2017). Longitudinal study of vascular structure and function during normal pregnancy. Ultrasound Obstet. Gynecol. 49, 46–53. doi: 10.1002/uog.17326
Juhl, M., Olsen, J., Andersen, P. K., Nøhr, E. A., and Andersen, A. M. N. (2010). Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. Am. J. Obstet. Gynecol. 202, 63.e1–e63.e8. doi: 10.1016/j.ajog.2009.07.033
Kadoglou, N. P. E., Iliadis, F., Angelopoulou, N., Perrea, D., Ampatzidis, G., Liapis, C. D., et al. (2007). The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Prev. Cardiol. 14, 837–843. doi: 10.1097/HJR.0b013e3282efaf50
Karlsen, T., Videm, V., Halle, M., Ellingsen, Ø, Støylen, A., Dalen, H., et al. (2019). Baseline and exercise predictors of VO2peak in systolic heart failure patients. Med. Sci. Sport Exerc. 52, 810–819. doi: 10.1249/mss.0000000000002193
Kasawara, K. T., Burgos, C. S. G., do Nascimento, S. L., Ferreira, N. O., Surita, F. G., and Pinto e Silva, J. L. (2013). Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: a randomized controlled trial. ISRN Obstet. Gynecol. 2013, 1–8. doi: 10.1155/2013/857047
Kasawara, K. T., Nascimento, S. L., Do Costa, M. L., Surita, F. G., and E Silva, J. L. P. (2012). Exercise and physical activity in the prevention of pre-eclampsia: systematic review. Acta Obstet. Gynecol. Scand. 91, 1147–1157. doi: 10.1111/j.1600-0412.2012.01483.x
Kawabata, I., Nakai, A., Sekiguchi, A., Inoue, Y., and Takeshita, T. (2012). The effect of regular exercise training during pregnancy on postpartum brachial-ankle pulse wave velocity, a measure of arterial stiffness. J. Sport Sci. Med. 11, 489–494.
Khashan, A. S., Evans, M., Kublickas, M., McCarthy, F. P., Kenny, L. C., Stenvinkel, P., et al. (2019). Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med. 16:e1002875. doi: 10.1371/journal.pmed.1002875
Kirollos, S., Skilton, M., Patel, S., and Arnott, C. (2019). A systematic review of vascular structure and function in pre-eclampsia: non-invasive assessment and mechanistic links. Front. Cardiovasc. Med. 6:166. doi: 10.3389/fcvm.2019.00166
Krabbendam, I., Maas, M. L., Thijssen, D. H. J., Oyen, W. J. G., Lotgering, F. K., Hopman, M. T. E., et al. (2009). Exercise-induced changes in venous vascular function in nonpregnant formerly preeclamptic women. Reprod. Sci. 16, 414–420. doi: 10.1177/1933719109332091
Kuhrt, K., Hezelgrave, N. L., and Shennan, A. H. (2015). Exercise in pregnancy. Obstet. Gynaecol. 17, 281–287. doi: 10.1111/tog.12228
Lane-Cordova, A. D., Khan, S. S., Grobman, W. A., Greenland, P., and Shah, S. J. (2019). Long-term cardiovascular risks associated with adverse pregnancy outcomes. J. Am. Coll. Cardiol. 73, 2106–2116. doi: 10.1016/j.jacc.2018.12.092
Laughlin, M. H. (2016). Physical activity-induced remodeling of vasculature in skeletal muscle: role in treatment of type 2 diabetes. J. Appl. Physiol. 120, 1–16. doi: 10.1152/japplphysiol.00789.2015
Lavrenčič, A., Salobir, B. G., and Keber, I. (2000). Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 20, 551–555. doi: 10.1161/01.ATV.20.2.551
Linke, A., Schoene, N., Gielen, S., Hofer, J., Erbs, S., Schuler, G., et al. (2001). Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J. Am. Coll. Cardiol. 37, 392–397. doi: 10.1016/S0735-1097(00)01108-1106
Magro-Malosso, E. R., Saccone, G., Di Tommaso, M., Roman, A., and Berghella, V. (2017). Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 96, 921–931. doi: 10.1111/aogs.13151
Mannaerts, D., Faes, E., Cornette, J., Gyselaers, W., Goovaerts, I., Roelant, E., et al. (2019). Low-flow mediated constriction as a marker of endothelial function in healthy pregnancy and preeclampsia: a pilot study. Pregnancy Hypertens. 17, 75–81. doi: 10.1016/j.preghy.2019.02.001
Mannaerts, D., Faes, E., Cos, P., Briede, J. J., Gyselaers, W., Cornette, J., et al. (2018). Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS One 13:e0202919. doi: 10.1371/journal.pone.0202919
McDonald, S. D., Malinowski, A., Zhou, Q., Yusuf, S., and Devereaux, P. J. (2008). Cardiovascular sequelae of preeclampsia / eclampsia?: a systematic review and meta-analyses. Am. Heart J. 156, 918–930. doi: 10.1016/j.ahj.2008.06.042
Meah, V. L., Cockcroft, J. R., Backx, K., Shave, R., and Stöhr, E. J. (2016). Cardiac output and related haemodynamics during pregnancy: a series of meta-analyses. Heart 102, 518–526. doi: 10.1136/heartjnl-2015-308476
Melchiorre, K., Sharma, R., and Thilaganathan, B. (2012). Cardiac structure and function in normal pregnancy. Curr. Opin. Obstet. Gynecol. 24, 413–421. doi: 10.1097/GCO.0b013e328359826f
Möbius-Winkler, S., Uhlemann, M., Adams, V., Sandri, M., Erbs, S., Lenk, K., et al. (2016). Coronary collateral growth induced by physical exercise: results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) trial. Circulation 133, 1438–1448. doi: 10.1161/CIRCULATIONAHA.115.016442
Mongraw-Chaffin, M. L., Cirillo, P. M., and Cohn, B. A. (2010). Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 56, 166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078.PREECLAMPSIA
Moreau, K. L., Gavin, K. M., Plum, A. E., and Seals, D. R. (2006). Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13, 951–958.
Mortensen, S. P., González-Alonso, J., Nielsen, J. J., Saltin, B., and Hellsten, Y. (2009). Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J. Appl. Physiol. 107, 1757–1762. doi: 10.1152/japplphysiol.00638.2009
Mosca, L., Benjamin, E. J., Berra, K., Bezanson, J. L., Dolor, R. J., Lloyd-Jones, D. M., et al. (2011). Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update. J. Am. Coll. Cardiol. 57, 1404–1423. doi: 10.1016/j.jacc.2011.02.005
Mottola, M. F., Davenport, M. H., Ruchat, S. M., Davies, G. A., Poitras, V., Gray, C., et al. (2018). No. 367-2019 canadian guideline for physical activity throughout pregnancy. J. Obstet. Gynaecol. Can. 40, 1528–1537. doi: 10.1016/j.jogc.2018.07.001
Muktabhant, B., Lawrie, T., Lumbiganon, P., and Laopaiboon, M. (2015). Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 6:CD007145.
Myers, J. (2003). Exercise and cardiovascular health. Circulation 107, e2–e5. doi: 10.1161/01.CIR.0000048890.59383.8D
Nelson, S. H., Steinsland, O. S., Wang, Y., Yallampalli, C., Dong, Y. L., and Sanchez, J. M. (2000). Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ. Res. 87, 406–411. doi: 10.1161/01.RES.87.5.406
Osol, G., and Bernstein, I. (2014). Preeclampsia and maternal cardiovascular disease: consequence or predisposition? J. Vasc. Res. 51, 290–304. doi: 10.1159/000367627
Pedersen, B. K., Åkerström, T. C. A., Nielsen, A. R., and Fischer, C. P. (2007). Role of myokines in exercise and metabolism. J. Appl. Physiol. 103, 1093–1098. doi: 10.1152/japplphysiol.00080.2007
Ramírez-Vélez, R., Aguilar de Plata, A. C., Escudero, M. M., Echeverry, I., Ortega, J. G., Salazar, B., et al. (2011). Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. J. Obstet. Gynaecol. Res. 37, 1601–1608. doi: 10.1111/j.1447-0756.2011.01582.x
Rehm, K., Sunesara, I., and Marshall, G. D. (2015). Increased circulating anti-inflammatory cells in marathon-trained runners. Int. J. Sports Med. 36, 832–836. doi: 10.1055/s-0035-1547218
Roveda, F., Middlekauff, H. R., Rondon, M. U. P. B., Reis, S. F., Souza, M., Nastari, L., et al. (2003). The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J. Am. Coll. Cardiol. 42, 854–860. doi: 10.1016/S0735-1097(03)00831-3
Salvesen, K. A., Hem, E., and Sundgot-Borgen, J. (2011). Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br. J. Sports Med. 46, 279–283. doi: 10.1136/bjsm.2010.080259
Savvaki, D., Taousani, E., Goulis, D. G., Tsirou, E., Voziki, E., Douda, H., et al. (2018). Guidelines for exercise during normal pregnancy and gestational diabetes: a review of international recommendations. Hormones 17, 521–529. doi: 10.1007/s42000-018-0085-6
Scholten, R. R., Hopman, M. T. E., Lotgering, F. K., and Spaanderman, M. E. A. (2015). Aerobic exercise traning in formerly preeclamptic women. effects on venous reserve. Hypertension 66, 1058–1065. doi: 10.1161/HYPERTENSIONAHA.115.05786
Scholten, R. R., Spaanderman, M. E. A., Green, D. J., Hopman, M. T. E., and Thijssen, D. H. J. (2014). Retrograde shear rate in formerly preeclamptic and healthy women before and after exercise training: relationship with endothelial function. Am. J. Physiol. Hear. Circ. Physiol. 307, H418– H425.
Skow, R. J., King, E. C., Steinback, C. D., and Davenport, M. H. (2017). The influence of prenatal exercise and pre-eclampsia on maternal vascular function. Clin. Sci. 131, 2223–2240. doi: 10.1042/CS20171036
Sma Position Statement, Hayman, M., Brown, W., Ferrar, K., Marchese, R., and Tan, J. (2016). Exercise in pregnancy and the postpartum period. Sport Med. Aust. 28, 329–341.
Tkachenko, O., Shchekochikhin, D., and Schrier, R. W. (2014). Hormones and hemodynamics in pregnancy. Int. J. Endocrinol. Metab. 12, 1–8. doi: 10.5812/ijem.14098
Tomimatsu, T., Mimura, K., Endo, M., Kumasawa, K., and Kimura, T. (2017). Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens. Res. 40, 305–310. doi: 10.1038/hr.2016.152
Van Craenenbroeck, A. H., Van Craenenbroeck, E. M., Van Ackeren, K., Hoymans, V. Y., Verpooten, G. A., Vrints, C. J., et al. (2016). Impaired vascular function contributes to exercise intolerance in chronic kidney disease. Nephrol. Dial. Transplant. 31, 2064–2072.
Van Craenenbroeck, E. M., Frederix, G., Pattyn, N., Beckers, P., Van Craenenbroeck, A. H., Gevaert, A., et al. (2015). Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: a SAINTEX-CAD substudy. Am. J. Physiol. Hear. Circ. Physiol. 309, H1876—-H1882.
Van Craenenbroeck, E. M., Hoymans, V. Y., Beckers, P. J., Possemiers, N. M., Wuyts, K., Paelinck, B. P., et al. (2010). Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res. Cardiol. 105, 665–676. doi: 10.1007/s00395-010-0105-4
Van Pelt, D. W., Guth, L. M., and Horowitz, J. F. (2017). Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. 123, 1150–1159. doi: 10.1152/japplphysiol.00614.2017
Weissgerber, T. L. (2014). Flow-mediated dilation: can new approaches provide greater mechanistic insight into vascular dysfunction in preeclampsia and other diseases? Curr. Hypertens. Rep. 16:487. doi: 10.1007/s11906-014-0487-z
Williams, D. J., Vallance, P. J. T., Neild, G. H., Spencer, J. A. D., and Imms, F. J. (1997). Nitric oxide-mediated vasodilation in human pregnancy. Am. J. Physiol. Hear. Circ. Physiol. 272, 748–752. doi: 10.1152/ajpheart.1997.272.2.h748
Witvrouwen, I., Pattyn, N., Gevaert, A. B., Possemiers, N. M., Van Craenenbroeck, A. H., Cornelissen, V. A., et al. (2019a). Predictors of response to exercise training in patients with coronary artery disease: a subanalysis of the SAINTEX-CAD study. Eur. J. Prev. Cardiol. 26, 1158–1163. doi: 10.1177/2047487318786176
Witvrouwen, I., Van Craenenbroeck, E. M., Abreu, A., Moholdt, T., and Kränkel, N. (2019b). Exercise training in women with cardiovascular disease: differential response and barriers – review and perspective. Eur. J. Prev. Cardiol. [Epub ahead of print]. doi: 10.1177/2047487319838221
Wolf, H. T., Owe, K. M., Juhl, M., and Hegaard, H. K. (2013). Leisure time physical activity and the risk of pre-eclampsia: a systematic review. Matern. Child Health J. 18, 899–910. doi: 10.1007/s10995-013-1316-8
Yeo, S. A., Davidge, S., Ronis, D. L., Antonakos, C. L., Hayashi, R., and O’Leary, S. (2008). A comparison of walking versus stretching exercises to reduce the incidence of preeclampsia: a randomized clinical trial. Hypertens. Pregnancy 27, 113–130. doi: 10.1080/10641950701826778
Zeisler, H., Llurba, E., Chantraine, F., Vatish, M., Staff, A. C., Sennström, M., et al. (2016). Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 374, 13–22. doi: 10.1056/NEJMoa1414838
Keywords: exercise, pregnancy, vascular adaptation, pre-eclampsia, gestational hypertension
Citation: Witvrouwen I, Mannaerts D, Van Berendoncks AM, Jacquemyn Y and Van Craenenbroeck EM (2020) The Effect of Exercise Training During Pregnancy to Improve Maternal Vascular Health: Focus on Gestational Hypertensive Disorders. Front. Physiol. 11:450. doi: 10.3389/fphys.2020.00450
Received: 03 February 2020; Accepted: 09 April 2020;
Published: 08 May 2020.
Edited by:
Angelica Lindén Hirschberg, Karolinska Institutet, SwedenReviewed by:
Kirsten Legerlotz, Humboldt University of Berlin, GermanyMasaki Mizuno, University of Texas Southwestern Medical Center, United States
Copyright © 2020 Witvrouwen, Mannaerts, Van Berendoncks, Jacquemyn and Van Craenenbroeck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emeline M. Van Craenenbroeck, RW1lbGluZS5WYW5jcmFlbmVuYnJvZWNrQHVhbnR3ZXJwZW4uYmU=
 Isabel Witvrouwen
Isabel Witvrouwen Dominique Mannaerts
Dominique Mannaerts An M. Van Berendoncks
An M. Van Berendoncks Yves Jacquemyn
Yves Jacquemyn Emeline M. Van Craenenbroeck
Emeline M. Van Craenenbroeck