- 1National Demonstration Center for Experimental Crop Science Education, Sichuan Agricultural University, Chengdu, China
- 2Biorational Pesticide Research Laboratory, Sichuan Agricultural University, Chengdu, China
The small brown planthopper [Laodelphax striatellus (Fallén) (Hemiptera, Delphacidae)] is one of the most destructive insect pests of rice and has developed strong resistance to several kinds of chemical insecticides. Triflumezopyrim, a novel mesoionic insecticide developed by Corteva Agriscience (formerly DuPont Crop Protection), has efficient biological activity in controlling sucking insects, such as the planthopper. However, the effects of triflumezopyrim on the growth and reproduction of L. striatellus have not been reported. In this study, an F5 generation was obtained by conducting five rounds of insecticide screening on a sensitive L. striatellus strain (F0 generation). An age-stage life table procedure was used to evaluate the effects of a sublethal concentration (LC50) of triflumezopyrim on the biological parameters of L. striatellus. Compared with those of the F0 generation, the intrinsic rate of increase (r), the finite rate (λ), and the net reproductive rate (R0) of the F5 generation were significantly decreased; nevertheless, the average duration of life (T) was not significantly affected. The results of detoxification enzyme activity assays indicated that the glutathione S-transferase and cytochrome P450 monooxygenase (P450) activities in the F5 generation were significantly higher than those in the F0 generation. The contents of vitellogenin (Vg) and vitellogenin receptor (VgR) were also detected, and the results indicated that the contents of Vg and VgR in the F5 generation were significantly decreased compared to those in the F0 generation. Furthermore, we detected the relative expression of ecdysone receptor (EcR), Vg, and VgR in the F0 and F5 generations and found that the relative expression levels of Vg and VgR in the F5 generation female adults were obviously lower than those in the F0 generation (P < 0.05), whereas the relative expression of EcR was slightly increased, although the difference was not significant (P > 0.05). Based on these results, a sublethal concentration (median lethal concentration, LC50) of triflumezopyrim may inhibit the generational growth and reproduction of L. striatellus. Moreover, our results may provide a reference for further studies of the suitability and resistance mechanisms of L. striatellus subjected to a sublethal dose of triflumezopyrim.
Introduction
The small brown planthopper [Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae)] is one of the most destructive insect pests of rice; its adults and nymphs not only feed directly on rice but also spread viral diseases, such as rice black-streaked dwarf disease (Kisimoto, 1967; Yin et al., 2013). At present, the control of L. striatellus in China depends mainly on chemical insecticides, the use of which has resulted in different levels of resistance to many pesticides, such as deltamethrin, chlorpyrifos, imidacloprid, and buprofezin (Zhang et al., 2016; Abdalla Elzaki et al., 2018; Asaduzzaman et al., 2019). Triflumezopyrim is a newly commercialized molecule from Corteva Agriscience. The biochemical and physiological action of this novel insecticide involves its binding to the orthosteric site of the nicotinic acetylcholine receptor by competitive binding, making the insects lethargic and poisoned, which was highly effective for controlling both imidacloprid-susceptible and imidacloprid-resistant planthopper populations in Malaysia (Cordova et al., 2016). For Chinese rice planthopper populations, triflumezopyrim has extremely high efficacy; additionally, triflumezopyrim is less harmful to natural enemies, such as Anagrus nilaparvatae (Hymenoptera: Mymaridae), Cyrtorhinus lividipennis (Hemiptera: Miridae), and Paederus fuscipes (Coleoptera: Staphylinidae) (Zhu et al., 2018).
The occurrence of resistance is closely related to the sublethal effects of pesticides on insects and the changes in detoxification enzymes in insects (Ma et al., 2019; Meng et al., 2019a, b). The study of sublethal effects can be carried out by establishing an insect’s amphoteric life table. Sublethal effects are manifested mainly in the growth and development of insect individuals and their offspring, feeding behavior, mating and reproduction, oviposition, egg hatching rate, and population growth (Ramirez-Romero et al., 2007). Vitellin (Vn) is a specific protein of the female hemolymph that provides nutritional sources for embryonic development and is closely related to female insect reproduction (Sappington and Raikhel, 1998; Tufail and Takeda, 2008; Veerana et al., 2015; Liao et al., 2019; Xiang et al., 2019; Xu et al., 2019). Vitellogenin (Vg) is the necessary precursor material for synthesizing Vn. When developing oocytes take up Vg, they require membrane-bound Vg receptor (VgR) for transport (Schneider, 1996; Snigirevskaya et al., 1997). Ecdysone receptor (EcR) can inhibit the expression of Vg (Yan et al., 2016; Shen et al., 2018). Hence, the expression of the EcR, Vg, and VgR genes has a significant influence on insect growth and reproduction.
Our objective is to analyze the population dynamics, detoxification enzyme activities, Vg and VgR contents, and relative expression of the EcR, Vg, and VgR genes of L. striatellus under sublethal concentrations of triflumezopyrim. The findings of this study may provide a reference for further research on the suitability and resistance mechanisms of L. striatellus subjected to a sublethal dose of triflumezopyrim and provide theoretical support for the popularization and application of new pesticides.
Materials and Methods
Insects and Insecticide
A susceptible laboratory strain of L. striatellus (F0) was kindly provided by Nanjing Agricultural University in September 2016. These insects had not been exposed to any insecticides for 15 consecutive years and were continuously reared on rice seedlings (TN1) in our laboratory under a temperature of 27 ± 1°C, relative humidity of 70 ± 10%, and photoperiod of 14 L:10 D. Triflumezopyrim SC (10%) was purchased from Corteva Agriscience (Wilmington, DE, United States). All other chemicals and solvents used were analytical reagents.
Bioassays
The toxicity of triflumezopyrim to L. striatellus was assayed using the rice seedling dipping method, with minor modifications (Xiang et al., 2019). A series of concentrations of triflumezopyrim were diluted with water containing 0.1% TritonTM X-100, and 0.1% Triton X-100 solution was used as the control. After soaking the rice seedlings in the prepared liquid for 30 s, they were placed in newspaper to dry. The roots were wrapped with degreased cotton, sufficient water was absorbed, and they were placed in plastic cups. Fifteen healthy and uniform 3rd-instar nymphs were prepared for each treatment, and each concentration was tested in triplicate. After 96 h, the mortality rate of the test insects was counted (Liao et al., 2017), with direct death or incompatible movement as death. The toxicity regression equation Y = a + bX, standard error, LC50 value, 95% confidence interval (95% CI), χ2 value, and Pr were calculated using SAS 9.2 (North Carolina State University, Raleigh, North Carolina, United States).
Sublethal Effects of Triflumezopyrim on L. striatellus
Triflumezopyrim Effects on the Life-History Traits of the F4 Generation
The experimental procedure was performed according to the description of Xiang et al. (2019), with some modifications. The screening operation procedure is shown in Figure 1. According to the bioassay results of the F0 generation, the 3rd-instar nymphs of susceptible L. striatellus were treated with the LC50 of triflumezopyrim. Then, the surviving nymphs were collected, and the F1 generation was obtained after spawning. The toxicity of triflumezopyrim was assessed in the F1 generation, and then, the survived 3rd-instar F1 nymphs were screened with the newer LC50 value. After five rounds of drug screening from the F0 generation, survival of the F4 generation was achieved. Then, 150 surviving nymphs were selected from the F4 generation and transferred into flat-bottomed test tubes (diameter × height: 20.0 mm × 145.0 mm) containing fresh rice seedlings. The first 100 nymphs were numbered and used for formal experiments, and the last 50 nymphs were used as substitutes. After emergence, each pair of male and female adults was transferred one by one to a flat-bottomed test tube containing two fresh rice seedlings. If a pair did not mate, they were provided with substitute partners. Third-instar F0 nymphs were used for the control treatment. The rice seedlings were replaced every day until the adults died, and the fecundity and longevity of the pair were recorded. The life tables of the F4 generation were made at the same time as those of the F0 generation.

Figure 1. The procedure used to produce the F5 generation. According to the bioassay results of the F0 generation, 3rd-instar nymphs of susceptible L. striatellus were treated with the LC50 of triflumezopyrim. Then, the surviving nymphs were collected, and the F1 generation was obtained after spawning. The toxicity of triflumezopyrim was assessed in the F1 generation, and then the remaining 3rd-instar nymphs of F1 were screened with the new LC50 value. After five rounds of drug screening, the F5 generation was obtained. After treatment of the third-instar F4 nymphs, the surviving individuals were selected for observation, as shown in section “Triflumezopyrim Effects on the Life-History Traits of the F4 Generation,” and the results are shown in Table 3. Then, a series of experiments to determine sublethal effects and detoxifying enzyme activity were performed on the F5 generation. The methods are detailed in sections “Triflumezopyrim Effects on the Life-History Traits of the F5 Generation,” “Enzyme Assays,” “Detection of the Contents of Vg and VgR,” and “Reverse Transcriptase-Quantitative Polymerase Chain Reaction of EcR, Vg, and VgR,” and the results are shown in Tables 4–6 and Figures 2–8.
Sublethal Effects of Triflumezopyrim on L. striatellus
Effects of Triflumezopyrim on the Life-History Traits of the F4 Generation
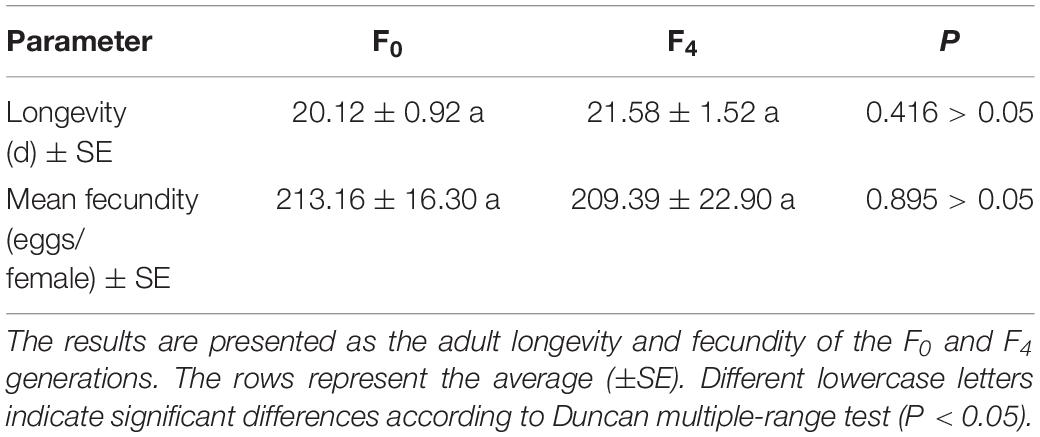
To better understand the effects of triflumezopyrim on the population characteristics of L. striatellus, we used an age-stage life table procedure and found that the mean fecundity of the F4 generation adults was almost unchanged compared with that of the F0 generation adults, and the longevity of the F4 generation adults was extended, although the difference was not significant (P > 0.05), as shown in Table 3.
Effects of Triflumezopyrim on the Life-History Traits of the F5 Generation
All developmental times of the F5 generation are shown in Table 4. The results indicated that the durations of the egg stage and 1st-instar stage (9.21 and 3.09 days, respectively) in the F5 generation were significantly shorter than those in the F0 generation (9.67 and 3.38 days, respectively; P < 0.05); nevertheless, there was no difference between the two strains during the other nymphal stages (P > 0.05).
Triflumezopyrim Effects on the Life-History Traits of the F5 Generation
The sublethal effects of triflumezopyrim on L. striatellus were studied as follows: First, 150 eggs laid on the fifth to sixth day after the emergence and pairing of the sensitive L. striatellus and F4 insects were collected to serve as the F0 (susceptible L. striatellus not exposing triflumezopyrim) and F5 generations, respectively, and kept in separate test tubes, with 100 for formal experiments and an additional 50 retained as potential replacements. Second, when these eggs became adults, pairs were made according to the method described above. Finally, the population characteristics, including developmental time, longevity, fecundity, and hatching ability, were monitored every day until the pair died. The replaced rice seedlings were dissected under a T-type microscope after 12 days to record the number of unhatched eggs. The life tables of the F5 and F0 generations were prepared at the same time. The statistical data of the life table were analyzed by using the age-stage, two-sex life table procedure. The life table parameters were analyzed using the program TWOSEX-MSChart (Chi, 2018) and included female age-specific fecundity (fxj), fx7, population age-specific survival rate (lx), population age-specific fecundity (mx), age-stage survival rate (sxj), age-stage–specific reproductive value (vxj), finite rate (λ), net reproductive rate (R0), intrinsic rate of increase (r), mean generation time (T), developmental time, adult longevity, adult preoviposition period (APOP), and total preoviposition period (TPOP). The values fx7, lx, mx, lxmx, sxj, and vxj were plotted using SigmaPlot 12.3 (Systat Software, Inc, San Jose, California, United States). The mean and standard error of life table parameters were estimated by the bootstrap technique included in TWOSEX-MSChart with 100,000 random resamplings. A paired bootstrap test (TWOSEX-MSChart) program was used to determine the significant differences in population parameters, development duration, and reproductive value of the F5 generation compared to the F0 generation (TWOSEX-MSChart) (P < 0.05).
Enzyme Assays
Third-instar nymphs of the F0 and F5 generations were analyzed for the activity of three detoxifying metabolic enzymes. Carboxylesterase (CarE) activity was determined according to the method described by Asperen (1962). Twenty nymphs (3rd instar) were transferred into a centrifuge tube and stored in liquid nitrogen as quickly as possible, homogenized in 2 mL phosphate buffer (0.04 mol/L phosphate buffer, pH 7.0) ice-bath slurry, and centrifuged at 4°C and 10,000 × g for 15 min. Then, the supernatant was transferred into a clean Eppendorf tube as the crude enzyme solution. A mixture of 0.45 mL of phosphate buffer (0.04 mol/L, pH 7.0), 1.8 mL of 3 × 10–4 mol/L α-NA solution (containing 3 × 10–4 mol/L physostigmine), and 50 μL of diluted enzyme liquid was added to each tube. After thorough mixing, the reaction was performed for 15 min in a constant-temperature bath at 30°C, and then 0.9 mL of staining solution (0.2 g of fast blue-B salt in 20 mL of distilled water plus 50 mL of 5% sodium dodecyl sulfate) was added. The absorbance values were recorded at 600 nm after 5 min in a UV 2000 Spectrophotometer [Unic (Shang Hai) Instruments Incorporated, Shanghai, China].
Glutathione S-transferase (GST) activity was determined by using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate, according to the method of Wang et al. (2018), with minor revisions. Thirty nymphs were placed into a centrifuge tube and stored in liquid nitrogen as quickly as possible. Then, 2 mL of phosphate buffer (0.1 mol/L phosphate buffer containing 1.0 mmol/L EDTA, pH 6.5) was added, and the mixture was centrifuged at 4°C and 10,000 × g for 10 min. Then, the supernatant was used as the enzyme solution. The total volume of the reaction system was 2.7 mL, including 2,470 μL of phosphate buffer (0.1 mol/L, pH 6.5), 90 μL of CDNB (15 mmol/L), 50 μL of the enzyme source, and 90 μL of reduced GSH (30 mmol/L). After rapidly shaking the mixture, the absorbance values were monitored within 2 min with a spectrophotometer at 340 nm.
Cytochrome P450 (P450) activity was assayed using the method of Rose et al. (1995) with some modification. One hundred fifty (3rd-instar) nymphs were homogenized on ice with 2 mL of phosphoric acid buffer (0.1 mol/L, pH 7.6, containing 20% glycerol, 0.1 mmol/L EDTA, 0.1 mmol/L DTT, and 0.4 mmol/L phenylmethylsulfonyl fluoride). The homogenate was centrifuged at 4°C and 10,000 × g for 10 min to obtain the supernatant, which was stored at low temperature as the enzyme solution. The OD value was determined at 405 nm by adding 100 μL of 4-nitroanisole (2 × 10–3 mol/L) and 90 mL of enzyme solution to the enzyme labeling plate, incubating for 3 min at 30°C, and then adding 10 mL of NADPH (9.6 × 10–3 mol/L). The OD values were recorded after every 20 s for 2 min, and a standard curve of p-nitrophenol was prepared in advance. The specific activity of P450s was finally calculated as nanomoles of p-nitrophenol per minute per milligram of protein [nmol/(min mg pro)].
All treatments were performed with three samples (tubes) as biological repetitions, and each enzyme sample was replicated three times as technical repetitions. The total protein content of the enzyme solution was determined by the Bradford method using bovine albumin as a standard (Bradford, 1976). The activities of CarEs, GSTs, and P450s were analyzed using unpaired Student t-tests, and the significance level of the results was set at P < 0.05.
Detection of the Contents of Vg and VgR
Females of the F0 and F5 generations were collected on the second day after emergence, and triplicates were performed for each treatment. Twenty insects of each sample type were frozen with liquid nitrogen and stored at −80°C. The Vg and VgR contents were detected using the double-antibody sandwich method according to the enzyme-linked immunosorbent assay kit of Alpha Enzyme Biotechnology Co., Ltd. (Shanghai, China). The contents of Vg and VgR were analyzed using unpaired Student t-tests, and the significance level of the results was set at P < 0.05.
Reverse Transcriptase-Quantitative Polymerase Chain Reaction of EcR, Vg, and VgR
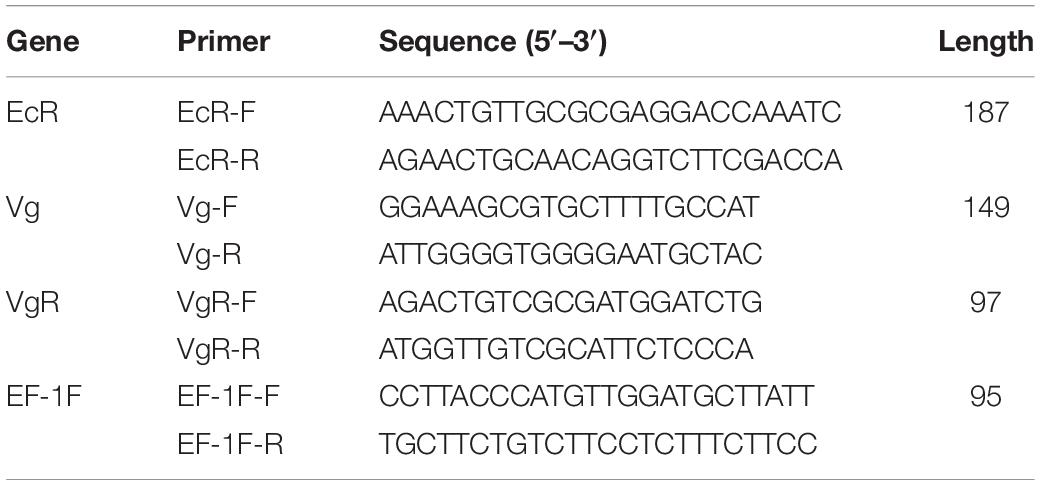
Females of the F0 and F5 generations were collected on the second day after emergence. Total RNA was extracted by using TRIzol reagent (Shanghai Yubo Biological Technology Co. Ltd., Shanghai, China) following the manufacturer’s specifications, and cDNA was synthesized from the total RNA using TransScript cDNA Synthesis SuperMix (Novoprotein Scientific Inc., Shanghai, China) for qPCR. TransStart TopTaq (Novoprotein Scientific Inc.) was used to conduct the reverse transcriptase-quantitative polymerase chain reactions (RT-qPCRs). Primers were designed as shown in Table 1, with the EF-1Fq gene as an internal reference gene (Jia et al., 2015). The RT-qPCRs for each treatment were replicated three times. The relative expressions of EcR (Jia et al., 2015), Vg, and VgR were represented by relative quantification values calculated by the math formula method. The relative expression of the EcR, Vg, and VgR genes was analyzed using unpaired Student t-tests, and the significance level of the results was set at P < 0.05.
Results
Toxicity of Triflumezopyrim on 3rd-Instar Nymphs of L. striatellus
To understand the changes in the susceptibility of L. striatellus under the screening pressure of triflumezopyrim, we used the rice seedling dipping method to evaluate the toxicity of triflumezopyrim and found that the LC50 values of triflumezopyrim on 3rd-instar nymphs of the F0 and F4 generations were 0.443 ± 0.171 μg/mL and 5.682 ± 1.498 μg/mL, respectively, with a resistance ratio of 12.84-fold (Table 2).
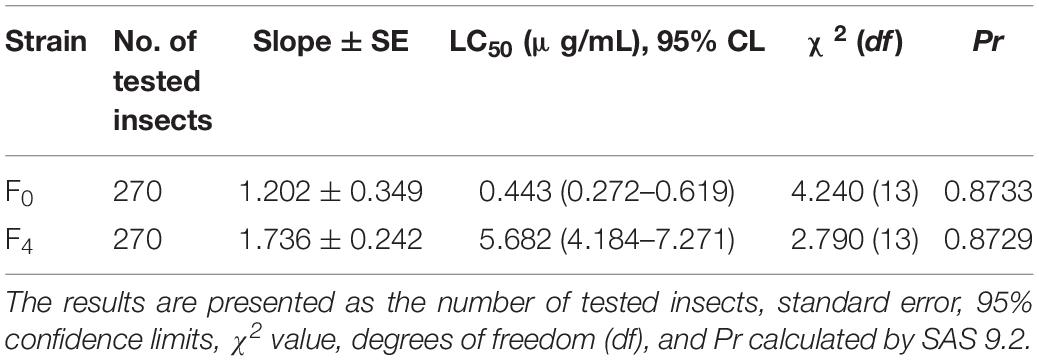
Table 2. The toxicity of triflumezopyrim on the 3rd-instar nymphs of the F0 and F4 generations of L. striatellus.
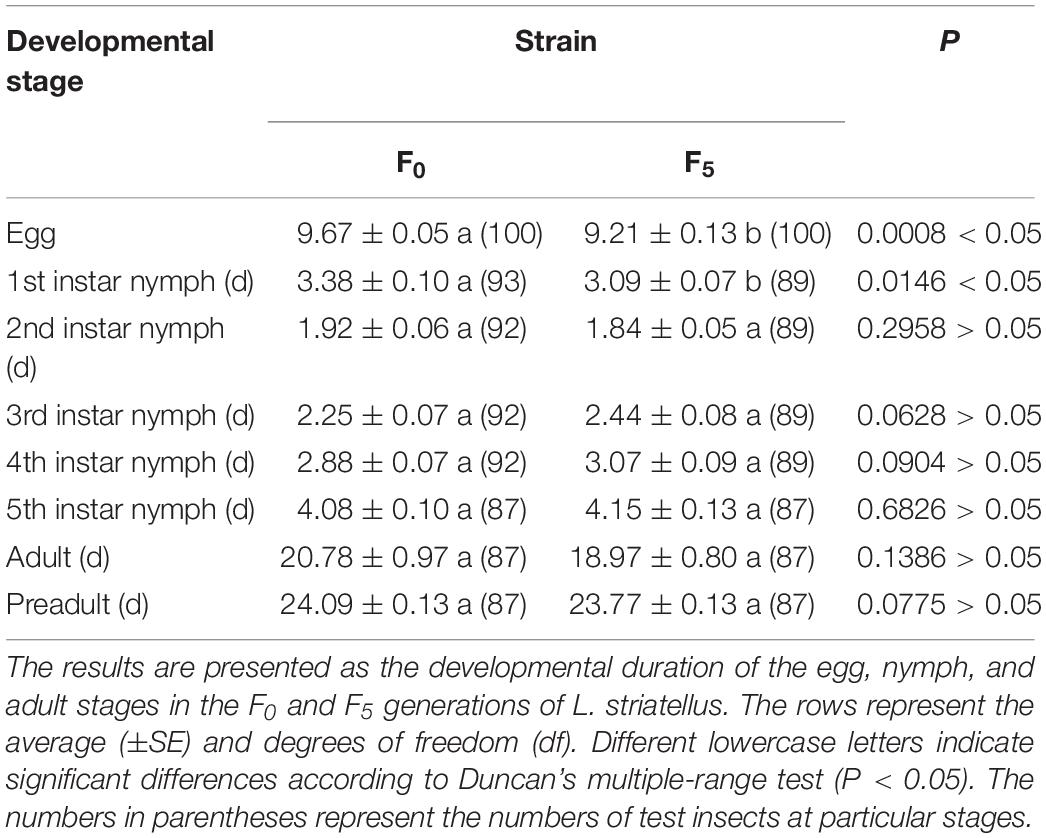
Table 4. The developmental duration of the egg, nymph, and adult stages in the F0 and F5 generations of L. striatellus.
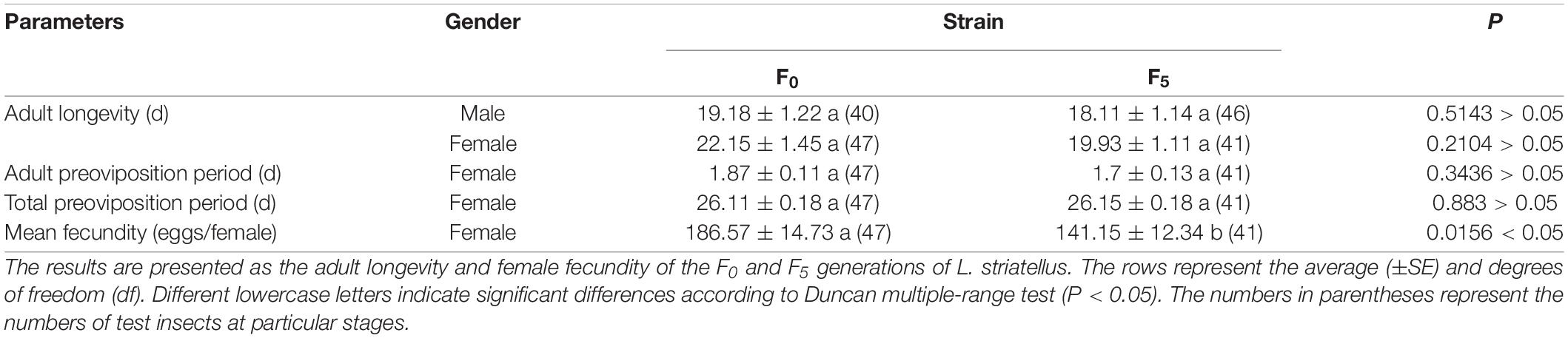
The fecundity, APOP, TPOP, and longevity of the F5 generation are displayed in Table 5. The mean fecundity of the F5 generation (141 eggs) was significantly lower than that of the F0 generation (186 eggs) (P < 0.05), whereas the APOP, TPOP, and adult longevity were not significantly different between the two strains (P > 0.05). The proportions of males to females in the F5 and F0 generations were 1:0.85 and 0.89:1, respectively.
The sublethal effects of triflumezopyrim on population dynamics were calculated with a bootstrapping procedure based on a life table (Table 6). The intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate in the F5 generation (0.12, 1.13, and 57.87, respectively) were all lower than those in the F0 generation (0.14, 1.15, and 87.69, respectively) (P < 0.05). However, there was no difference in the average generation time (T) between these two strains (P > 0.05).
The sxj values in the F0 and F5 generations indicated that their growth curves had substantial overlap and complex growth relationships among individuals. The number of nymphs that could complete development in the two strains was not significantly different (83 and 77%, respectively). In addition, the specific age survival rate of male adults in the F0 generation was higher than that of female adults, whereas a different pattern was observed in the F5 generation, and the sxj values of male adults were similar to those of female adults (Figure 2).
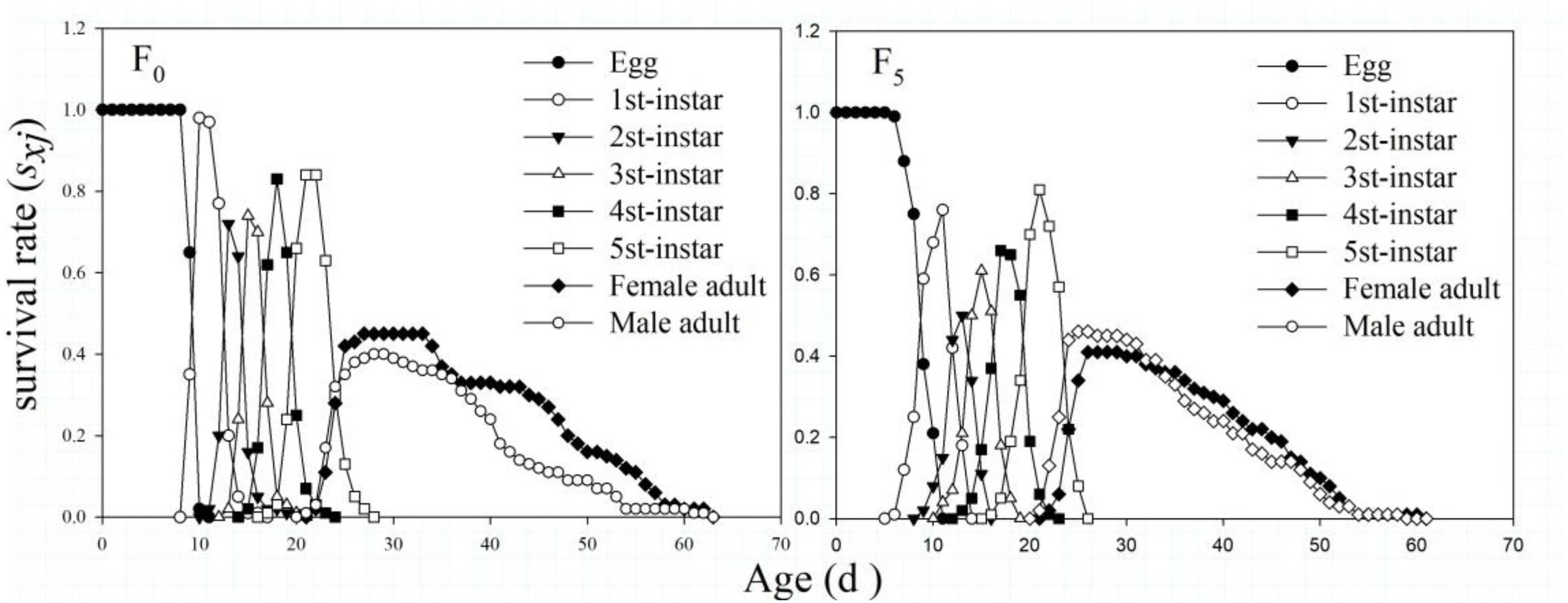
Figure 2. The age-stage-specific survival rate (sxj) of the F0 and F5 generations of L. striatellus.
According to Figure 3, the exj values of the F0 and F5 generations generally decreased with age x and phase j. For the F0 and F5 generations, the highest exj values all appeared at the egg stage, at 41.41 and 38.95 days, respectively. Overall, the exj values of the F5 generation were lower than those of the F0 generation, whereas the exj values of the female adults were higher than those of the male adults. Among all the adults, the exj values of females in the F0 generation were the highest.
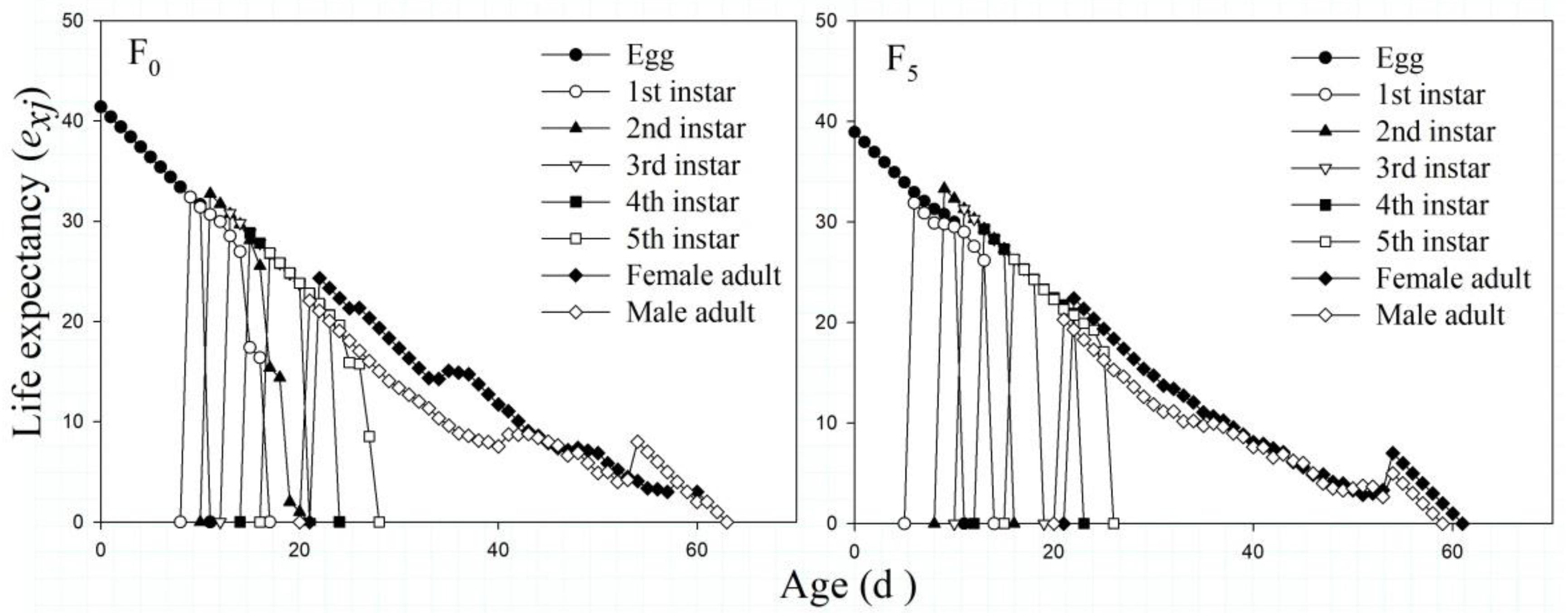
Figure 3. The age-stage-specific life expectancy (exj) of the F0 and F5 generations of L. striatellus.
The lx results indicated that the lx curves of the F0 and F5 generations generally first increased and then decreased. The highest peak fertility in the F0 and F5 generations was on the 28th and 27th days, with the highest average yield of 11.0 and 17.1 hatched eggs, respectively (P < 0.05). The female fx curve of the F5 generation suggested a lower and less variable value than that of the F0 generation. Similarly, the curves of mx and lxmx showed a declining trend in the F5 generation (Figure 4).
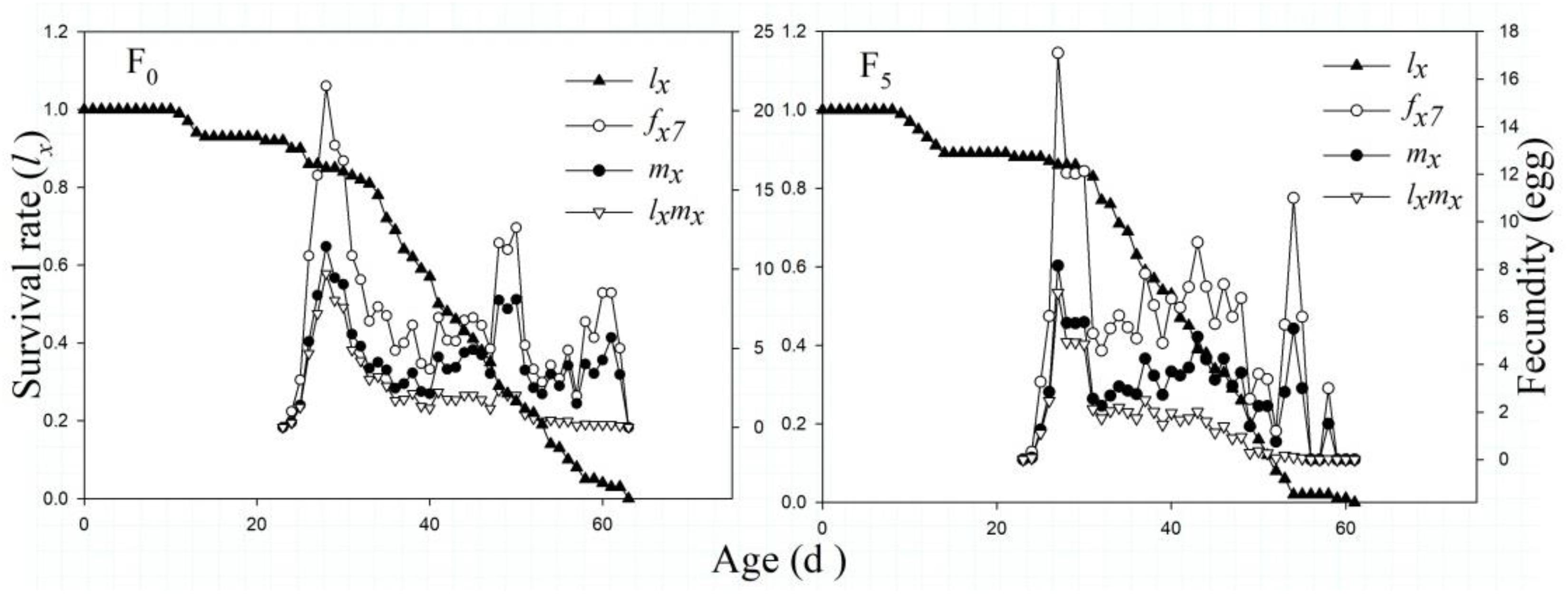
Figure 4. The age-specific survival rate (lx), female age-specific fecundity (fx7), age-specific fecundity of the total population (mx), and age-specific net maternity (lxmx) of the F0 and F5 generations of L. striatellus.
The vxj values in the F5 generation were lower than those in the F0 generation for the female adult stage and total vxj. The vxj values of all strains gradually increased from the egg stage, and that in the F0 generation reached its maximum (124.65 eggs) on the 23rd day, especially for female adult reproduction, which showed a peak value of 86.11 eggs on the 27th day. However, the vxj values in the F5 generation reached their highest peaks (111.45 eggs) on the 25th day, and those of female adults reached their maximum (67.06 eggs) on the 27th day (Figure 5).
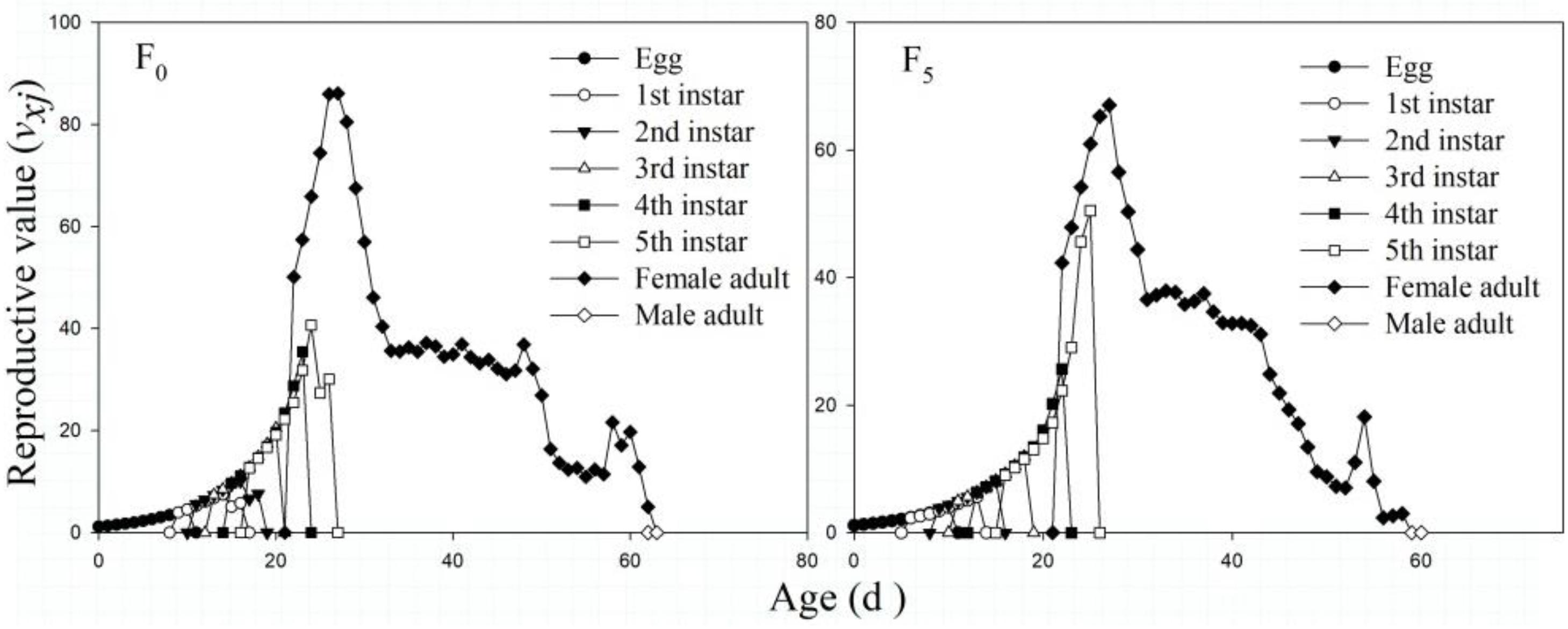
Figure 5. The age-stage–specific reproductive value (vxj) of the F0 and F5 generations of L. striatellus.
Enzymatic Activity in the F0 and F5 Generations
Insect detoxifying metabolic enzymes could be induced by xenobiotics, including insecticides. We assayed the activity of three main detoxifying metabolic enzymes, including CarEs, GSTs, and P450s, in the F0 and F5 generations of L. striatellus. Compared with the F0 generation, the activities of the three detoxifying metabolic enzymes of the F5 generation were increased to varying degrees. The GST [0.78 mmol/(min mg pro)] and P450 activities [58.15 nmol/(min mg pro)] were significantly higher in the F5 generation than in the F0 generation [0.50 mmol/(min mg pro) and 21.84 nmol/(min mg pro), respectively] (P < 0.05), whereas the CarE activity was not significantly different between the F0 and F5 generations (P > 0.05) (Figure 6).
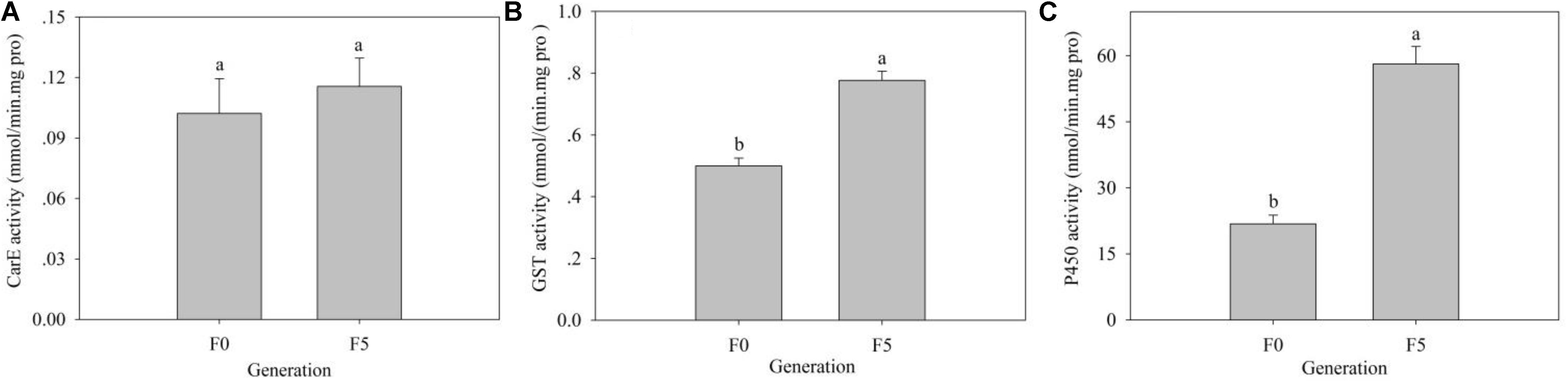
Figure 6. The effects of triflumezopyrim on the activity of the detoxification enzymes CarE (A), GST (B), and P450 (C) in 3rd-instar nymphs of L. striatellus. The activities of CarEs, GSTs, and P450s in 3rd-instar nymphs of L. striatellus are presented as the mean of three replications ± SE. Means followed by the same letters did not differ significantly (P > 0.05) according to the analysis of variance test. The F1,4 values of different treatments of CarEs, GSTs, and P450s in 3rd-instar nymphs of L. striatellus were 0.367, 50.654, and 68.502, and the P values of CarE, GST, and P450 in 3rd-instar nymphs of L. striatellus were 0.577 > 0.05, 0.002 < 0.05, and 0.001 < 0.05, respectively.
Contents of Vg and VgR in the F0 and F5 Generations
To further explore the mechanism of triflumezopyrim on the growth and development of L. striatellus, we detected the contents of Vg and VgR in the F0 and F5 generations. Compared with those in the F0 generation (2,251.80 and 148.33 ng/g), the contents of Vg and VgR in the F5 generation (3,367.35 and 397.35 ng/g) both increased significantly (P < 0.05) (Figure 7).
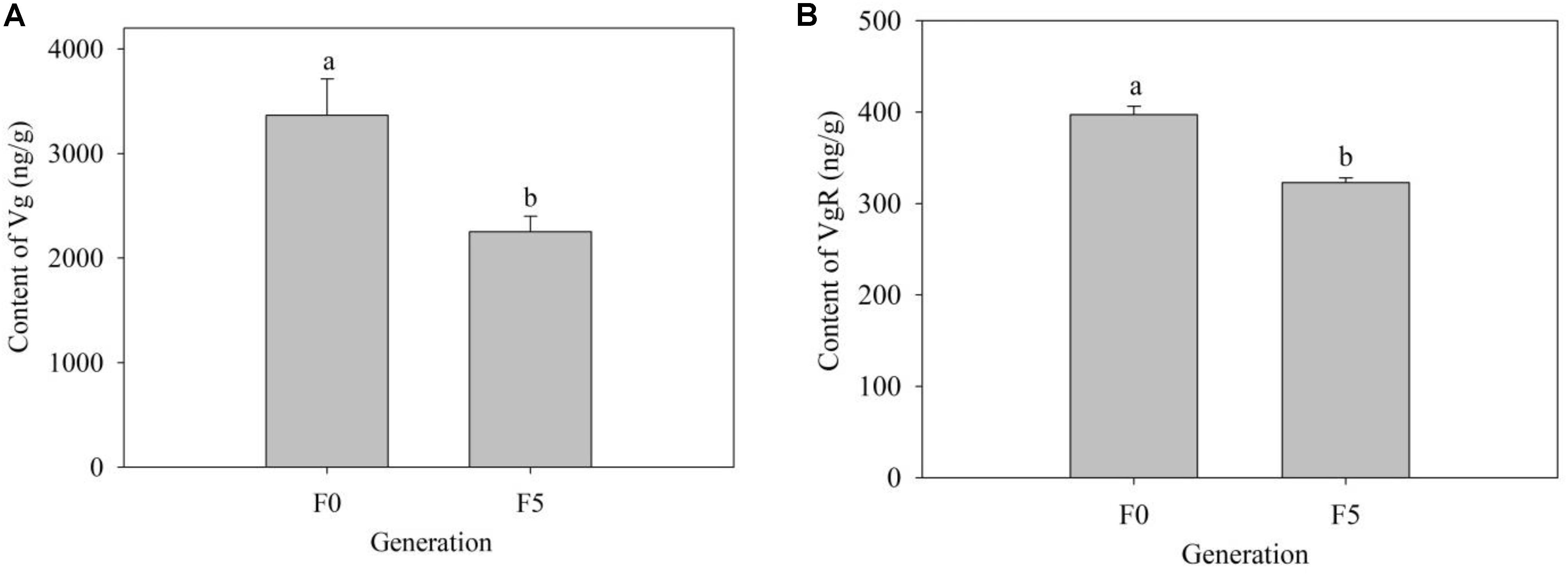
Figure 7. (A,B) Contents of Vg and VgR in female adults of the F0 and F5 generations of L. striatellus. The bars represent the average (±SE). The contents of Vg and VgR are presented as the mean of three replications ± SE. The different lowercase letters above the columns indicate significant differences at the P < 0.05 level according to the analysis of variance test. The F1,4 values of Vg and VgR contents in the female adults of the F0 and F5 generations were 8.786 and 49.943, respectively, and the P values of Vg and VgR contents in the female adults of the F0 and F5 generations were 0.041 < 0.05 and 0.002 < 0.01, respectively.
Relative Expression of EcR, Vg, and VgR
To seek direct evidence of triflumezopyrim having affected the contents of Vg and VgR of L. striatellus, we detected the relative expression of EcR, Vg, and VgR in L. striatellus and found that the relative expression of Vg and VgR (0.27- and 0.23-fold, respectively) in female adults of the F5 generation was significantly decreased compared with that in the F0 generation (P < 0.05); the relative expression of EcR (2.36-fold) was slightly increased, but no significant difference was found between the two treatments (P > 0.05; Figure 8).
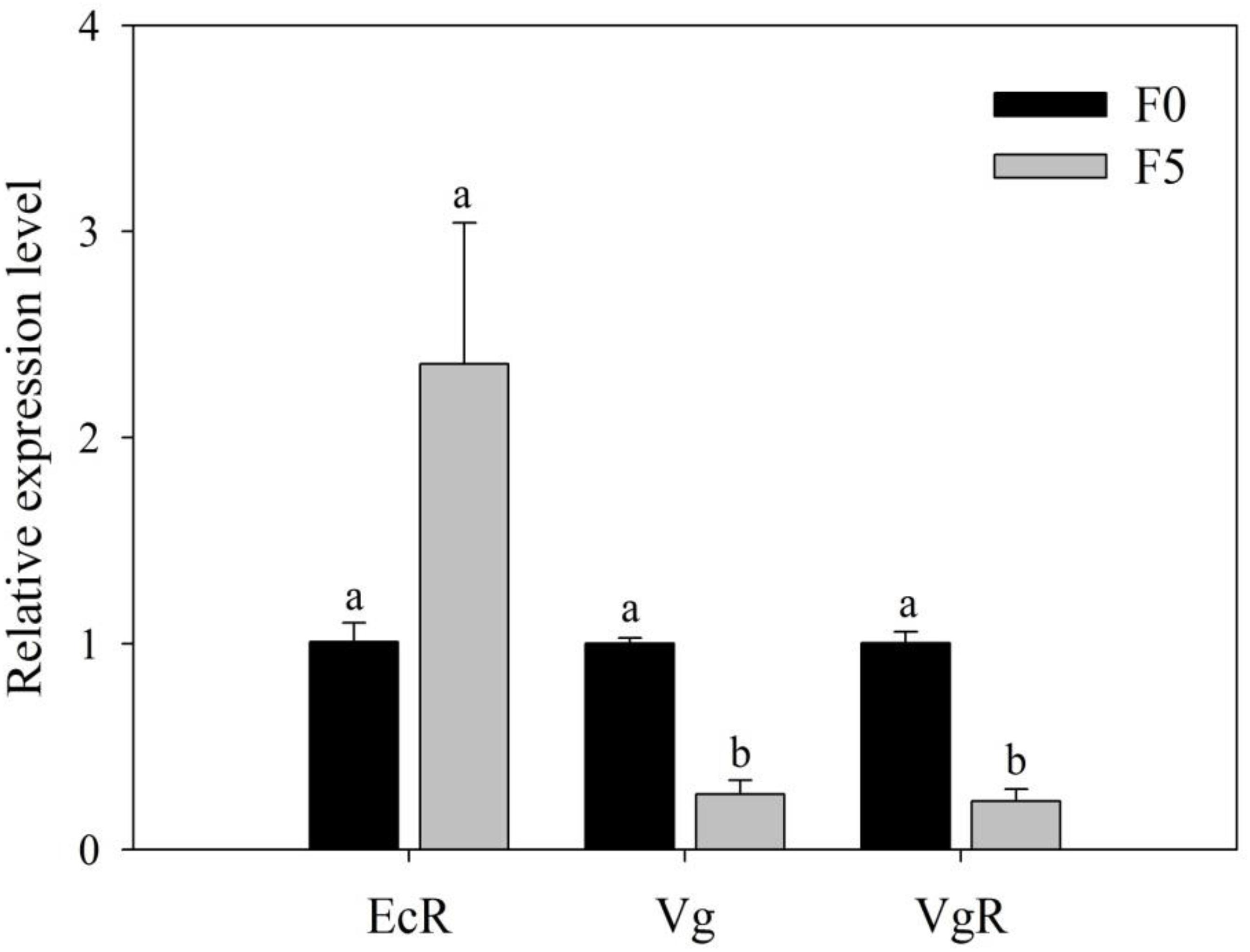
Figure 8. Relative expression level of EcR, Vg, and VgR genes in female adults of the F0 and F5 generations of L. striatellus. The bars represent the SE of three replications. Different lowercase letters indicate significant differences according to Duncan multiple-range test (P < 0.05). The F1,4 values of the relative expression levels of EcR, Vg, and VgR genes in the female adults of the F0 and F5 generations were 3.792, 726.682, and 49.943, respectively, and the P values of the relative expression of the EcR, Vg, and VgR genes in female adults of the F0 and F5 generations were 0.577 > 0.05, 0.041 < 0.05, and 0.002 < 0.01, respectively.
Discussion
Research on insect life tables is one of the imperative aspects of insect population dynamics, and changes in insect populations are influenced by multiple factors, including food, temperature, light, their respective hosts (Tuan et al., 2014; Zhang et al., 2015; Qin et al., 2017), and especially the different kinds of chemical pesticides. Our results showed that the fecundity (including r, λ, and R0) of L. striatellus was significantly decreased following triflumezopyrim treatment and population expansion of L. striatellus was suppressed. This was consistent with an earlier report by Liao et al. (2019), who declared that the sublethal effects of sulfoxaflor reduced the survival and reproductive capability of Nilaparvata lugens in successive generations. Xiang et al. (2019) also found that the number of Sogatella furcifera (Hemiptera: Delphacidae) eggs decreased with the LC25 of sulfoxaflor. In addition, we found that the developmental stage of the F5 generation was not greatly affected, and the values of T, APOP, and TPOP did not change significantly, which was inconsistent with the findings of some previous reports. For instance, low sublethal concentrations of sulfoxaflor could stimulate the fertility of S. furcifera, and the APOP, TPOP, and T values were significantly prolonged, even though the longevity, fecundity, and egg-hatching ability of N. lugens were not significantly affected by the LC30 of triflumezopyrim (Xu et al., 2019). We speculated that different insects respond differently to insecticide stress.
Insects will develop resistance when successively exposed to the same insecticide. Kwon et al. (2019) reported that L. striatellus was reared for nine generations with carbofuran selection, and its resistance was increased 14-fold. The development of resistance is closely related to changes in three major detoxification metabolic enzymes in insects, including CarEs, GSTs, and P450s. According to the results of Asaduzzaman et al. (2019), the overexpression of P450 genes in L. striatellus has resulted in deltamethrin resistance. Abdalla Elzaki et al. (2016) also reported that imidacloprid resistance in L. striatellus was related to increased P450 activity. Meanwhile, there have also been many reports that enhanced GST activity has contributed to the development of resistance in insects, similar to the study of Meng et al. (2019a), who alleged that GSTs played an essential role in the detoxification of malathion in Bactrocera dorsalis. In our research, P450 and GST activities were significantly increased in the F5 generation, and we speculated that the increase in P450 and GST activities could collaboratively enhance the triflumezopyrim resistance of L. striatellus. Whether the susceptibility and detoxification enzyme activities of the F5 generation would return to their previous levels in the F0 generation when the pressure of triflumezopyrim was removed should be further studied, although some reports have declared that resistance levels decreased when insects with high or moderate resistance levels were exposed to no insecticides (Ishtiaq et al., 2014; Abbas et al., 2015).
We found that the reproductive capacity of the F5 generation decreased significantly. It has been reported that the development of drug resistance is often accompanied by a number of adverse factors, such as shortened total life span and reduced fertility (Zhang et al., 2018). To further understand its mechanism, we measured the expression levels of three genes closely related to development and reproduction. Vitellogenin is a vital reproduction-related protein that is traditionally considered to be an adequate parameter for evaluating female fertility in insects (Li et al., 2010; Zhao et al., 2018). For example, the decreased expression of Vg was found to have negative impacts on the fecundity of Chilo suppressalis and Apolygus lucorum (Huang et al., 2016; Zhen et al., 2018). Vitellogenin receptor is a necessary receptor for Vg to function (Schneider, 1996; Snigirevskaya et al., 1997). Notably, a decrease in VgR expression can inhibit the function of Vg. In our experiment, the mRNA expression levels of Vg and VgR in F5 generation female adults were obviously lower than those in the F0 generation. Combined with the fact that the fecundity of the F5 generation was significantly reduced, these data suggested that reduced Vg mRNA expression in the triflumezopyrim-resistant strain might significantly influence the fecundity of L. striatellus. Furthermore, we found that the mRNA expression level of EcR in F5 generation female adults showed a slight increase compared with that in the F0 generation, even though no significant differences were observed. This finding suggested that triflumezopyrim may modulate the molting hormone pathway in L. striatellus, and this result could explain the decreased expression of Vg and the shortened duration of the egg, nymph, and adult stages in the F5 generation to some extent. Moreover, we found that the Vg and VgR contents in the F5 generation were also significantly decreased; these results directly confirm the changing trend of Vg and VgR gene expression.
Conclusion
In this study, an age-stage life table procedure was used to evaluate the effects of a sublethal concentration (LC50) of triflumezopyrim on the biological parameters of L. striatellus for the first time. The detoxification enzyme activities of GSTs and P450s were induced by triflumezopyrim in the F5 generation compared to those in the F0 generation. The contents and relative expression of Vg and VgR in the F5 generation were also significantly decreased compared to those in the F0 generation.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
SZ, FG, CG, YZ, and XW conceived the study. SZ, FG, LC, and LS conducted the experiments. SZ drafted the preliminary manuscript and interpreted the results. XW, SZ, CJ, and AH refined and approved the final manuscript.
Funding
This study received financial support from the National Key R&D Program of China (2018YFD0200300).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the help of Nanjing Agricultural University for providing the susceptible laboratory strain of L. striatellus. We also thank the reviewers for critical reviews of the manuscript.
References
Abbas, N., Khan, H., and Shad, S. A. (2015). Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 114, 247–255. doi: 10.1007/s00436-014-4186-0
Abdalla Elzaki, M. E., Pu, J., Zhu, Y. X., Zhang, W. F., Sun, H., Wu, M., et al. (2018). Cross-resistance among common insecticides and its possible mechanism in Laodelphax striatellus Fallén (Hemiptera: Delphacidae). Orient. Insects 52, 2–15. doi: 10.1080/00305316.2017.1316784
Abdalla Elzaki, M. E., Zhang, W. F., Feng, A., Qiou, X. Y., Zhao, W. X., and Han, Z. J. (2016). Constitutive overexpression of cytochrome P450 associated with imidacloprid resistance in Laodelphax striatellus (Fallén). Pest Manag. Sci. 72, 1051–1058. doi: 10.1002/ps.4155
Asaduzzaman, M. M., Esmail Abdalla, E. M., Asmaul, H., and Han, Z. J. (2019). An overexpressed cytochrome P450 CYP439A1v3 confers deltamethrin resistance in Laodelphax striatellus Fallén (Hemiptera: Delphacidae). Arch. Insect Biochem. 100:e21525. doi: 10.1002/arch.21525
Asperen, K. (1962). A study of housefly esterase by means of sensitive colorimetric method. Insect Physiol. 8, 401–406. doi: 10.1016/0022-1910(62)90074-4
Bradford, M. M. (1976). A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 25, 248–256. doi: 10.1006/abio.1976.9999
Chi, H. (2018). TWOSEX-MSChart: A Computer Program for the Age-Stage, Two Sex Life Table Analysis. Taichung: National Chung Hsing University.
Cordova, D., Benner Eric, A., Schroeder Mark, E., Holyoke Caleb, W., Zhang, W. M., Pahutski Thomas, F., et al. (2016). Mode of action of triflumezopyrim: a novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 74, 32–41. doi: 10.1016/j.ibmb.2016.04.008
Huang, L., Lu, M., Han, G., Du, Y., and Wang, J. (2016). Sublethal effects of chlorantraniliprole on development, reproduction and vitellogenin gene (CsVg) expression in the rice stem borer, Chilo suppressalis. Pest Manag. Sci. 72, 2280–2286. doi: 10.1002/ps.4271
Ishtiaq, M., Razaq, M., Saleem, M. A., Anjum, F., Noor, U. A. M., Raza, A. M., et al. (2014). Stability, cross-resistance and fitness costs of resistance to emamectin benzoate in a re-selected field population of the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae). Crop Prot. 65, 227–231. doi: 10.1016/j.cropro.2014.08.007
Jia, S., Wan, P. J., Zhou, L. T., Mu, L. L., and Li, G. Q. (2015). RNA interference-mediated silencing of a Halloween gene spookier affects nymph performance in the small brown planthopper Laodelphax striatellus. Insect Sci. 22, 191–202. doi: 10.1111/1744-7917.12087
Kisimoto, R. (1967). Genetic variation in the ability of a planthopper vector;, Laodelphax striatellus (Fallén), to acquire the rice stripe virus. Virology 32, 144–152. doi: 10.1016/0042-6822(67)90262-0
Kwon, D. H., Kim, H., Jeong, I. H., and Lee, S. H. (2019). F331H mutation and reduced mRNA in type-1 acetylcholinesterase is associated with carbofuran resistance development in the small brown planthopper (Laodelphax striatellus Fallén). J. Asia Pac. Entomol. 22, 6–14. doi: 10.1016/j.aspen.2018.11.014
Li, J. L., Huang, J. H., Cai, W. Z., Zhao, Z. W., Peng, W. J., and Wu, J. (2010). The vitellogenin of the bumblebee, Bombus hypocrita: studies on structural analysis of the cDNA and expression of the mRNA. J. Comp. Physiol. B 180, 161–170. doi: 10.1007/s00360-009-0434-5
Liao, X., Ali, E., Li, W. H., He, B. Y., Gong, P. P., Xu, P. F., et al. (2019). Sublethal effects of sulfoxaflor on the development and reproduction of the brown planthopper, Nilaparvata lugens (Stål). Crop Prot. 118, 6–14. doi: 10.1016/j.cropro.2018.12.005
Liao, X., Mao, K. K., Ali, E., Zhang, X. L., Wan, H., and Li, J. H. (2017). Temporal variability and resistance correlation of sulfoxaflor susceptibility among Chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Prot. 102, 141–146. doi: 10.1016/j.cropro.2017.08.024
Ma, K. S., Tang, Q. L., Xia, J., Lv, N. N., and Gao, X. W. (2019). Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii glover. Pestic. Biochem. Phys. 158, 40–46. doi: 10.1016/j.pestbp.2019.04.009
Meng, L. W., Yuan, G. R., Lu, X. P., Jing, T. X., Zheng, L. S., Yong, H. X., et al. (2019a). Two delta class glutathione S-transferases involved in the detoxification of malathion in Bactrocera dorsalis (Hendel). Pest Manag. Sci. 75, 1527–1538. doi: 10.1002/ps.5318
Meng, X. K., Zhang, N., Yang, X. Y., Miao, L. J., Jiang, H., Ji, C. H., et al. (2019b). Sublethal effects of chlorantraniliprole on molting hormone levels and mRNA expressions of three Halloween genes in the rice stem borer. Chilo Suppressalis 238:124676. doi: 10.1016/j.chemosphere.2019.124676
Qin, J. Y., Zhang, L., Liu, Y. Q., Sappington, T. W., Cheng, Y. X., Luo, L. Z., et al. (2017). Population projection and development of the Mythimna loreyi (Lepidoptera: Noctuidae) as affected by temperature: application of an Age-stage, two-sex life table. J. Econ. Entomol. 110, 1583–1591. doi: 10.1093/jee/tox138
Ramirez-Romero, R., Desneux, N., Decourtye, A., Chaffiol, A., and Pham-Delègue, M. H. (2007). Does Cry1Ab protein affect learning performances of the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotox. Environ. Safe. 70, 327–333. doi: 10.1016/j.ecoenv.2007.12.002
Rose, R. L., Barbhaiya, L., Roe, R. M., Rock, G. C., and Hodgson, E. (1995). Cytochrome P450-associated insecticide resistance and the development of biochemical diagnostic assays in Heliothis virescens. Pestic. Biochem. Phys. 51, 178–191. doi: 10.1006/pest.1995.1018
Sappington, T. W., and Raikhel, A. S. (1998). Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. 28, 277–300. doi: 10.1016/S0965-1748(97)00110-0
Schneider, W. J. (1996). Vitellogenin receptors: oocyte-specific members of the low-density lipoprotein receptor supergene family. Int. Rev. Cytol. 166, 103–137. doi: 10.1016/S0074-7696(08)62507-3
Shen, G., Wu, J., Han, C., Liu, H., Xu, Y., Zhang, H., et al. (2018). Oestrogen-related receptor reduces vitellogenin expression by crosstalk with the ecdysone receptor pathway in female silkworm, Bombyx mori. Insect Mol. Biol. 27, 454–463. doi: 10.1111/imb.12385
Snigirevskaya, E. S., Sappington, T. W., and Raikhel, A. S. (1997). Internalization and recycling of vitellogenin receptor in the mosquito oocyte. Cell Tissue Res. 290, 175–183. doi: 10.1007/s004410050919
Tuan, S. J., Lee, C. C., and Chi, H. (2014). Erratum: population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 70:1936. doi: 10.1002/ps.3920
Tufail, M., and Takeda, M. (2008). Molecular characteristics of insect vitellogenins. Insect Physiol. 54, 1447–1458. doi: 10.1016/j.jinsphys.2008.08.007
Veerana, M., Kubera, A., and Ngernsiri, L. (2015). Analysis of the Vitellogenin gene of rice moth, Corcyra cephalonica stainton. Arch. Insect Biochem. 87, 126–147. doi: 10.1002/arch.21185
Wang, X. G., Xiang, X., Yu, H. L., Liu, S. H., Yin, Y., Cui, P., et al. (2018). Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Phys. 146, 71–79. doi: 10.1016/j.pestbp.2018.02.008
Xiang, X., Liu, S. H., Wang, X. G., Zhang, Y. M., Gong, C. W., Chen, L., et al. (2019). Sublethal effects of sulfoxaflor on population projection and development of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Crop Prot. 120, 97–102. doi: 10.1016/j.cropro.2019.02.016
Xu, P. F., Shu, R. H., Gong, P. P., Li, W. H., Wan, H., and Li, J. H. (2019). Sublethal and transgenerational effects of triflumezopyrim on the biological traits of the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Crop Prot. 117, 63–68. doi: 10.1016/j.cropro.2018.11.010
Yan, T., Chen, H., Sun, Y., Yu, X., and Xia, L. (2016). RNA Interference of the ecdysone receptor genes EcR and USP in grain aphid (Sitobion avenae F.) affects its survival and fecundity upon feeding on wheat plants. Int. J. Mol. Sci. 17, 2098–2112. doi: 10.3390/ijms17122098
Yin, X., Zheng, F. Q., Tang, W., Zhu, Q. Q., Li, X. D., Zhang, G. M., et al. (2013). Genetic structure of rice black streaked dwarf virus populations in China. Arch. Virol. 158, 2505–2515. doi: 10.1007/s00705-013-1766-8
Zhang, P., Liu, F., Mu, W., Wang, Q. H., and Li, H. (2015). Comparison of Bradysia odoriphaga Yang and Zhang reared on artificial diet and different host plants based on an age-stage, two-sex life table. Phytoparasitica 43, 107–120. doi: 10.1007/s12600-014-0420-7
Zhang, X. L., Mao, K. K., Liao, X., He, B. Y., Jin, R. H., Tang, T., et al. (2018). Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J. Pest Sci. 91, 1145–1151. doi: 10.1007/s10340-018-0972-2
Zhang, Y. L., Wang, Y., Wang, L. H., Yao, J., Guo, H. F., and Fang, J. C. (2016). Knockdown of NADPH- cytochrome P450 reductase results in reduced resistance to buprofezin in the small brown planthopper, Laodelphax striatellus (fallén). Pestic. Biochem. Phys. 127, 21–27. doi: 10.1016/j.pestbp.2015.08.006
Zhao, J., Sun, Y., Xiao, L., Tan, Y., Jiang, Y., and Bai, L. (2018). Vitellogenin and vitellogenin receptor gene expression profiles in Spodoptera exigua are related to host plant suitability. Pest Manag. Sci. 74, 950–958. doi: 10.1002/ps.4794
Zhen, C. G., Miao, L., and Gao, X. W. (2018). Sublethal effects of sulfoxaflor on biological characteristics and vitellogenin gene (AlVg) expression in the mirid bug, Apolygus lucorum (Meyer-Dür). Pestic. Biochem. Phys. 144, 57–63. doi: 10.1016/j.pestbp.2017.11.008
Keywords: Laodelphax striatellus, triflumezopyrim, sublethal effect, detoxification enzyme activity, vitellogenin
Citation: Zhang S, Wang X, Gu F, Gong C, Chen L, Zhang Y, Hasnain A, Shen L and Jiang C (2020) Sublethal Effects of Triflumezopyrim on Biological Traits and Detoxification Enzyme Activities in the Small Brown Planthopper Laodelphax striatellus (Hemiptera: Delphacidae). Front. Physiol. 11:261. doi: 10.3389/fphys.2020.00261
Received: 27 October 2019; Accepted: 06 March 2020;
Published: 07 April 2020.
Edited by:
Fernando Ariel Genta, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Wenwu Zhou, Zhejiang University, ChinaDaniel Cordova, FMC Agricultural Solutions, United States
Copyright © 2020 Zhang, Wang, Gu, Gong, Chen, Zhang, Hasnain, Shen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuegui Wang, d2FuZ3h1ZWd1aUBzaWNhdS5lZHUuY24=
†These authors have contributed equally to this work
 Shuirong Zhang
Shuirong Zhang Xuegui Wang
Xuegui Wang Fuchuan Gu1,2
Fuchuan Gu1,2 Changwei Gong
Changwei Gong Ali Hasnain
Ali Hasnain Litao Shen
Litao Shen