- 1School of Human Sciences, University of Western Australia, Perth, WA, Australia
- 2Telethon Kids Institute, University of Western Australia, Perth, WA, Australia
- 3School of Public Health, Curtin University, Perth, WA, Australia
- 4Department of Mathematics, University of Auckland, Auckland, New Zealand
Developmental abnormalities of airways may impact susceptibility to asthma in later life. We used a maternal hypoxia-induced mouse model of intrauterine growth restriction (IUGR) to examine changes in mechanical properties of the airway wall. Pregnant BALB/c mice were housed under hypoxic conditions (10.5% O2) from gestational day (GD) 11 to GD 17.5 (IUGR; term, GD 21). Following hypoxic exposure, mice were returned to a normoxic environment (21% O2). A control group of pregnant mice were housed under normoxic conditions throughout pregnancy. At 8 weeks postnatal age, offspring were euthanized and a tracheasectomy performed. Tracheal segments were studied in organ baths to measure active airway smooth muscle (ASM) stress to carbachol and assess passive mechanical properties (stiffness) from stress-strain curves. In a separate group of anesthetized offspring, the forced oscillation technique was used to examine airway mechanics from relative changes in airway conductance during slow inflation and deflation between 0 and 20 cmH2O transrespiratory pressure. From predicted radius-pressure loops, storage and loss moduli and hysteresivity were calculated. IUGR offspring were lighter at birth (p < 0.05) and remained lighter at 8 weeks of age (p < 0.05) compared with Controls. Maximal stress was reduced in male IUGR offspring compared with Controls (p < 0.05), but not in females. Sensitivity to contractile agonist was not affected by IUGR or sex. Compared with the Control group, airways from IUGR animals were stiffer in vitro (p < 0.05). In vivo, airway hysteresivity (p < 0.05) was increased in the IUGR group, but there was no difference in storage or loss moduli between groups. In summary, the effects of IUGR persist to the mature airway wall, where there are clear abnormalities to ASM contractile properties and passive wall mechanics. We propose that mechanical abnormalities of the airway wall acquired through disrupted fetal growth impact susceptibility to disease.
Introduction
The early life presentation of lung function impairment in asthma implicates a developmental disorder as the driver for disease. Reduced airway function (Turner et al., 2004) and exaggerated airway narrowing to bronchial challenge (Palmer et al., 2001) is reported in infants who go on to develop asthma in childhood, a relationship which has now been shown to extend to adulthood (Owens et al., 2017). These findings are also consistent with early deficits in FEV1 in children with asthma (Bisgaard et al., 2012) with any decline in lung function in later life differing only marginally from the healthy lung (Phelan et al., 2002; Sears et al., 2003; James et al., 2005). Impaired respiratory function may therefore be a risk factor for asthma rather than a consequence of ongoing pathological processes.
At a population level, asthma development is linked with a number of prenatal events that include intrauterine growth restriction (IUGR) (Källén et al., 2013) and low birth weight (Barker et al., 1991). Working on the premise that such associations were mediated by persisting functional changes to the airway, we established a mouse model of maternal hypoxia-induced IUGR to show that IUGR female offspring were hyperresponsive to methacholine and male IUGR offspring hyporesponsive at 8 weeks of age (Wang et al., 2018). Functional abnormalities of the adult airway were therefore the result of an in utero insult, specifically corresponding to the pseudoglandular-canalicular period when the airway develops. Further, sex-dependent effects of IUGR aligned well with differences in the prevalence of asthma between males and females; hyperresponsiveness in adult females and hyporesponsiveness in adult males are broadly consistent with greater prevalence in adult females (Schatz and Camargo, 2003).
Determining the mechanism through which an in utero insult can affect function of the adult airway is important as this represents a physiological determinant of future disease. However, the cause of above changes in airway function after IUGR and low birth weight (Wang et al., 2018) could not be established. Given that airway responsiveness is strongly impacted by airway wall structure (Noble et al., 2013) and lung volume (Ding et al., 1987), these properties were initially assessed; no differences were found in either wall thickness [including airway smooth muscle (ASM)] or plethysmographically determined lung volume (Wang et al., 2018). Other than wall thickness, changes in “contractility” of the ASM (force for a given cross section area) theoretically impacts airway narrowing capacity, as has been documented in subjects with fixed airflow obstruction (Opazo Saez et al., 2000). Airway stiffness may also affect airway responsiveness by blunting protective bronchodilatory effects of breathing stresses (Noble et al., 2007).
This study examined previously unassessed mechanical properties of the airway wall from IUGR-affected adult mice including ASM contractility and airway stiffness. Male and female offspring were studied in order to determine whether sex differences in airway responsiveness in vivo are explained by intrinsic changes to the airway wall. Findings suggest that changes in ASM contractility and passive mechanical properties contribute to functional abnormalities observed in adulthood after IUGR.
Materials and Methods
Ethical Approval
Our experimental approach was to subject pregnant mice to a hypoxic or normoxic environment and, in both male and female offspring, to measure mechanical properties of the ASM and airway wall. This study was carried out in strict accordance with the recommendations of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (7th Edition). The protocol was approved by the Telethon Kids Institute Animal Ethics Committee (Project Number 264).
Maternal Hypoxia-Induced Intrauterine Growth Restriction Mouse Model
Pregnant female BALB/c mice at gestational day (GD) 7 were obtained from Animal Resources Centre (Murdoch, WA, Australia) and housed at the Telethon Kids Institute in specific pathogen-free environments. Mice were maintained on a 15:9-h light:dark cycle and supplied with an allergen-free diet (Specialty Feeds, Glen Forrest, WA, Australia) and water ad libitum. One group of pregnant mice were housed under hypoxic conditions (10.5% O2) from GD 11 to GD 17.5 (pseudoglandular-canalicular stage of mouse lung development) and then returned to a normoxic environment (21% O2) until birth (GD 21) (Wang et al., 2018). A control group of pregnant mice was housed under normoxic conditions throughout pregnancy (Wang et al., 2018). Weights of offspring were recorded at birth and 8 weeks of age, prior to in vitro organ bath experimentation. Mice were euthanized by overdose i.p. ketamine and xylazine. Tracheasectomy was performed and tracheal segments placed immediately into chilled Krebs solution (Ansell et al., 2014).
Organ Bath Studies
A DMT myography system (620 M) was used for the study of tracheal segments. Organ bath chambers contained heated Krebs solution gassed with carbogen (95% O2:5% CO2) (Ansell et al., 2014; Cairncross et al., 2018). The dissected tracheal segment (~2.5 mm in length) was slid onto two stainless steel prongs, one connected to a transducer and the other to a micrometer (Cairncross et al., 2018). Posterior ASM was aligned in series with the transducer. The micrometer was adjusted to distend the tracheal lumen diameter (and therefore ASM). Lumen “diameter” was approximated by the external distance between the prongs, determined under a dissecting microscope and calibrated to a 1-mm graticule. An initial force of 0.2 g was maintained over a 1-min period which was sufficient to ensure that the tissue was held fixed in position. Initial lumen diameter was denoted Di and was not different between groups (data not shown).
Tracheal segments were equilibrated to organ bath conditions for 1 h and regularly flushed with Krebs solution. After this period, viability was confirmed by contraction to 10−4 M acetylcholine; the bath was subsequently washed every 10 min with Krebs solution for a further 30 min. A diameter-force curve was then constructed to identify a reference diameter for contraction (to standardize mechanical history). Tracheal segments were contracted to KCl (80 mM) every 5 min at increasing lumen diameters. Passive and active force (total force − passive force) were determined at each diameter, until the contractile response reached a peak (Figure 1). Once the lumen diameter producing peak response to KCl was identified, the airway was returned to this diameter and adapted a further two times to KCl.
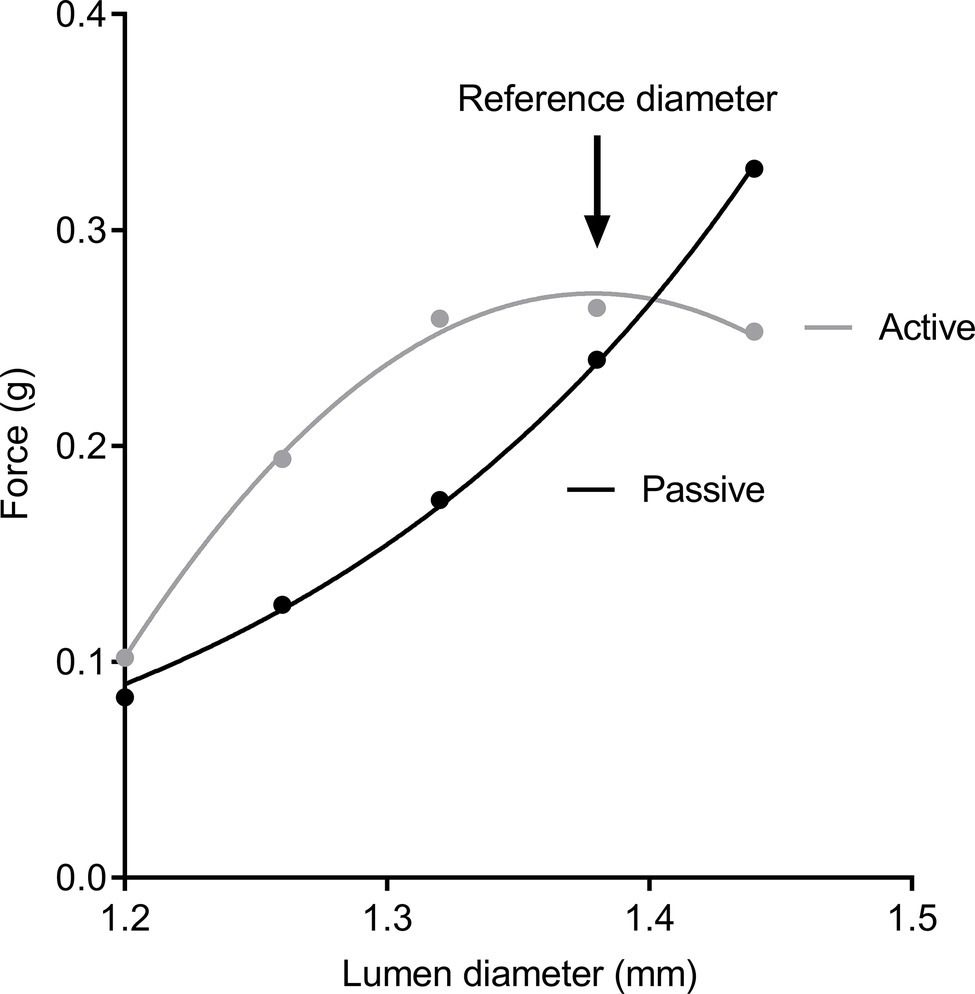
Figure 1. Diameter-force curves. Passive force (black circles) and active force (gray circles) after stimulation to KCl solution. Peak contraction was used to establish a reference diameter for dose-response curves.
A cumulative dose-response curve for carbachol (CCh) was constructed (10−9 to 3 × 10−5 M). At the end of the dose-response curve, the organ bath was flushed with Krebs solution containing theophylline to completely remove muscle tone. A passive diameter-force curve was then constructed, from Di to 1.2 Di, again in 5% increments. Force was recorded >30 s after each incremental diameter change, at which point stress-relaxation had subsided. At the end of the experiment, the length of the tracheal segment was measured under a dissecting microscope and airways were fixed in the organ bath (4% formaldehyde dissolved in Krebs solution).
Airway Morphometry
Five-micrometer-thick transverse sections of the trachea were cut and stained with Masson’s Trichrome. The area and length of the ASM layer, total wall area and internal perimeter of the basement membrane (Pbm) were measured using the newCast stereology software (Visiopharm, Hoersholm, Denmark).
In vivo Assessment
In a separate group of mice (8-week-old offspring), the mechanical response of the airway wall to inflation/deflation was assessed in vivo. Information on the body weights of this group of mice including sex-specific changes in airway responsiveness have previously been described (Wang et al., 2018). Mice were anesthetized (i.p. 0.4 mg/g body weight of ketamine and 0.02 mg/g body weight of xylazine), tracheostomized, placed inside a whole-body plethysmograph, and mechanically ventilated at 400 breaths/min with a tidal volume of 10 ml/kg and 2 cmH2O positive end-expiratory pressure.
Respiratory system impedance (Zrs) was measured using a wave-tube system (Hantos et al., 1992) adapted for use in small animals (Peták et al., 1997; Sly et al., 2003) and a modification of the forced oscillation technique (Sly et al., 2003; Larcombe et al., 2013). During brief apneic periods, oscillatory signals between 4 and 38 Hz were delivered via a 1-m wave-tube to tracheostomized mice. Load impedance and lateral pressure at either end of the wave-tube were used to calculate Zrs which was partitioned into the airway compartment (airway resistance, Raw and inertance, Iaw) and tissue compartment (tissue damping and tissue elastance) (Hantos et al., 1992).
Increases in transrespiratory pressure (Prs) were achieved by evacuating the air in the plethysmograph via a regulated vacuum source, while deflation occurred passively after removal of the pressure gradient. Mice were inflated and deflated between 0 and 20 cmH2O Prs (~40 s) while the FOT signal was applied. The constant phase model was fit to Zrs at 0.5-s intervals to calculate Raw during the inflation-deflation maneuver (Larcombe et al., 2011). Three such maneuvers were performed, the first two to establish a volume history and the third for analysis.
Analysis and Statistics
Active stress (g/mm2) was determined from force divided by ASM cross sectional area: thickness (ASM area/ASM length, mm) × length of tracheal segment (mm). Sigmoidal dose-response curves were fit to the data to estimate sensitivity to CCh, defined as the negative logarithm of the dose producing half maximal response (pD2). Passive stress (g/mm2) was determined from force divided by total wall cross sectional area: wall thickness (wall area/Pbm, mm) × length of tracheal segment (mm). Forces used in the calculations were modified when calculating passive stress: since both sides of the tracheal segment act on the force transducer, the recording is likely double the physiological level and for this reason was halved. The analysis does however assume a flattened lumen which may not be completely true for a tracheal segment that contains stiff C-shaped cartilaginous rings at its anterior surface. Nonetheless, this correction does not affect comparisons between groups, only the absolute values reported. No correction was necessary for active stress since the ASM is only present on the posterior surface of the segment. Stiffness was calculated from the change in stress divided by the change in diameter strain (i.e., ∆stress/0.2).
For in vivo measurements, conductance was calculated from the inverse of Raw (Wong et al., 2012), and subsequently converted into a global airway radius (radius = conductance1/4). Normalized radius-Prs curves (radius/radius at 0 cmH2O Prs) were then constructed and used to calculate the storage modulus, the loss modulus, and the hysteresivity (loss/storage modulus) using well-established methods for assessment of cyclic forcing in nonlinear physiological systems (Oliver et al., 2010).
Graphical and statistical analyses were performed by SigmaPlot (13.0) and Prism version (7.02). Birth weights were compared by t-test. Two-way ANOVA was used to determine the effect of treatment and dose on active ASM stress, and the effect of treatment and sex on all other parameters. Data were transformed where necessary to ensure the assumptions of normality and homoscedasticity of variances for the parametric tests were satisfied. When data could not be normalized, equivalent non-parametric statistical analyses were used. All data are presented as mean ± SEM with *p < 0.05 considered significant. n refers to number of offspring.
Results
Growth Outcomes
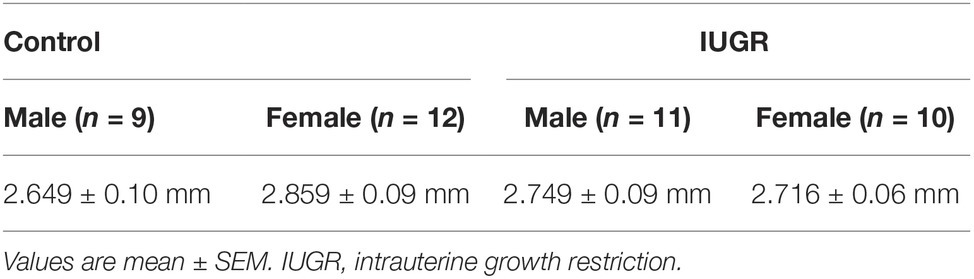
The IUGR offspring (n = 42) were lighter at birth compared with Control (n = 49) offspring (p = 0.03; unsexed) and remained lighter at 8 weeks of age (p < 0.001; Figure 2). Male offspring were also heavier compared with female offspring (p < 0.001). Maternal hypoxia had no effect on litter size (Control, 3.47 ± 0.34 pups; IUGR, 3.23 ± 0.28 pups; p = 0.599) or gestational period (Control, 19.88 ± 0.15 days; IUGR, 19.94 ± 0.006 days; p = 0.507). Tracheal Pbm (an index of airway size) was not affected by IUGR (p = 0.329; Table 1) or sex (p = 0.812).
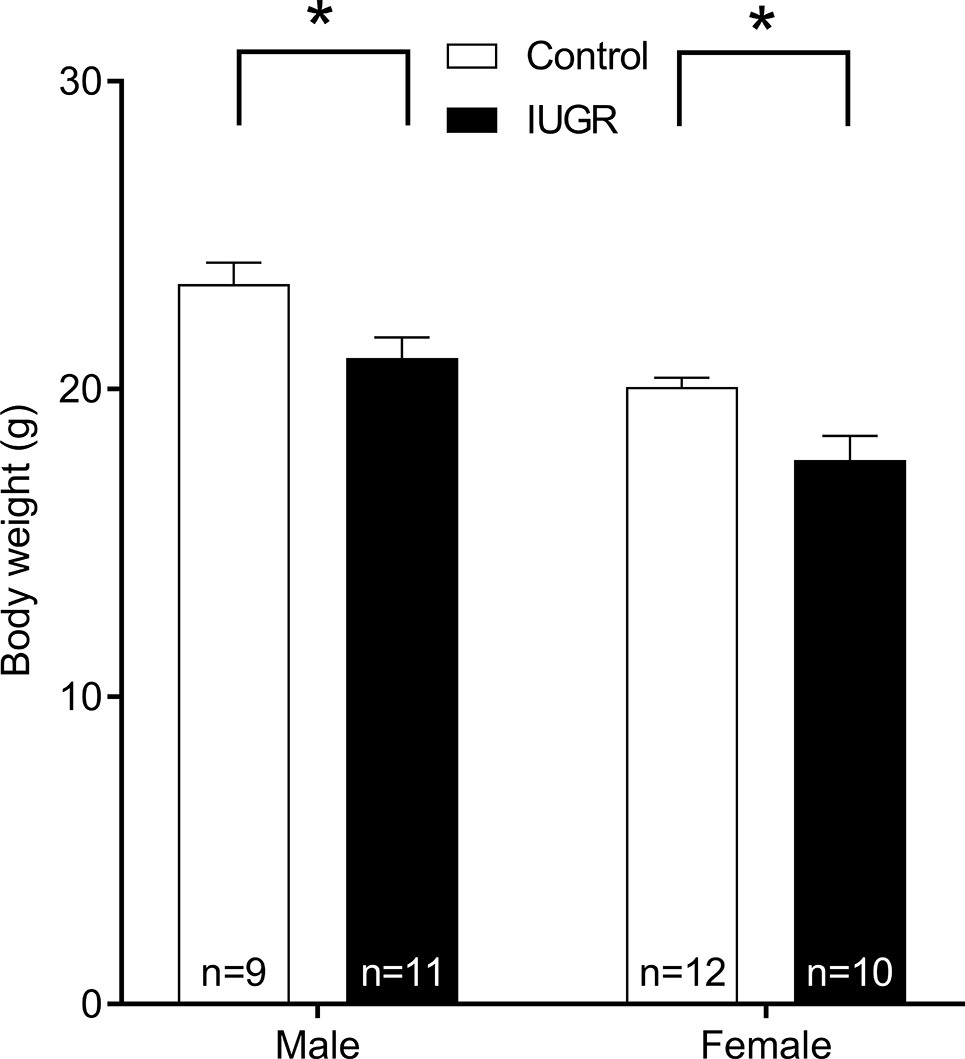
Figure 2. Body weight of Control and IUGR offspring at 8 weeks of age. Values are mean ± SEM. *Significantly different from Control (p < 0.001). Control (white bar); IUGR, intrauterine growth restriction (black bar).
Active Airway Smooth Muscle Stress
CCh-stress dose-response curves are shown in Figure 3. Compared with male Control offspring, active stress was reduced in male IUGR offspring at CCh doses ranging from 3 × 10−7 M to a maximal concentration of 3 × 10−5 M (p < 0.05; Figure 3A). There was no difference in active ASM stress between female groups (p = 0.738; Figure 3B). Sensitivity to CCh, as reflected by pD2, was not different between groups (Control male, 6.52 ± 0.07; Control female, 6.47 ± 0.07; IUGR male, 6.34 ± 0.07; IUGR female, 6.45 ± 0.11; p = 0.22) and was not affected by sex (p = 0.737).
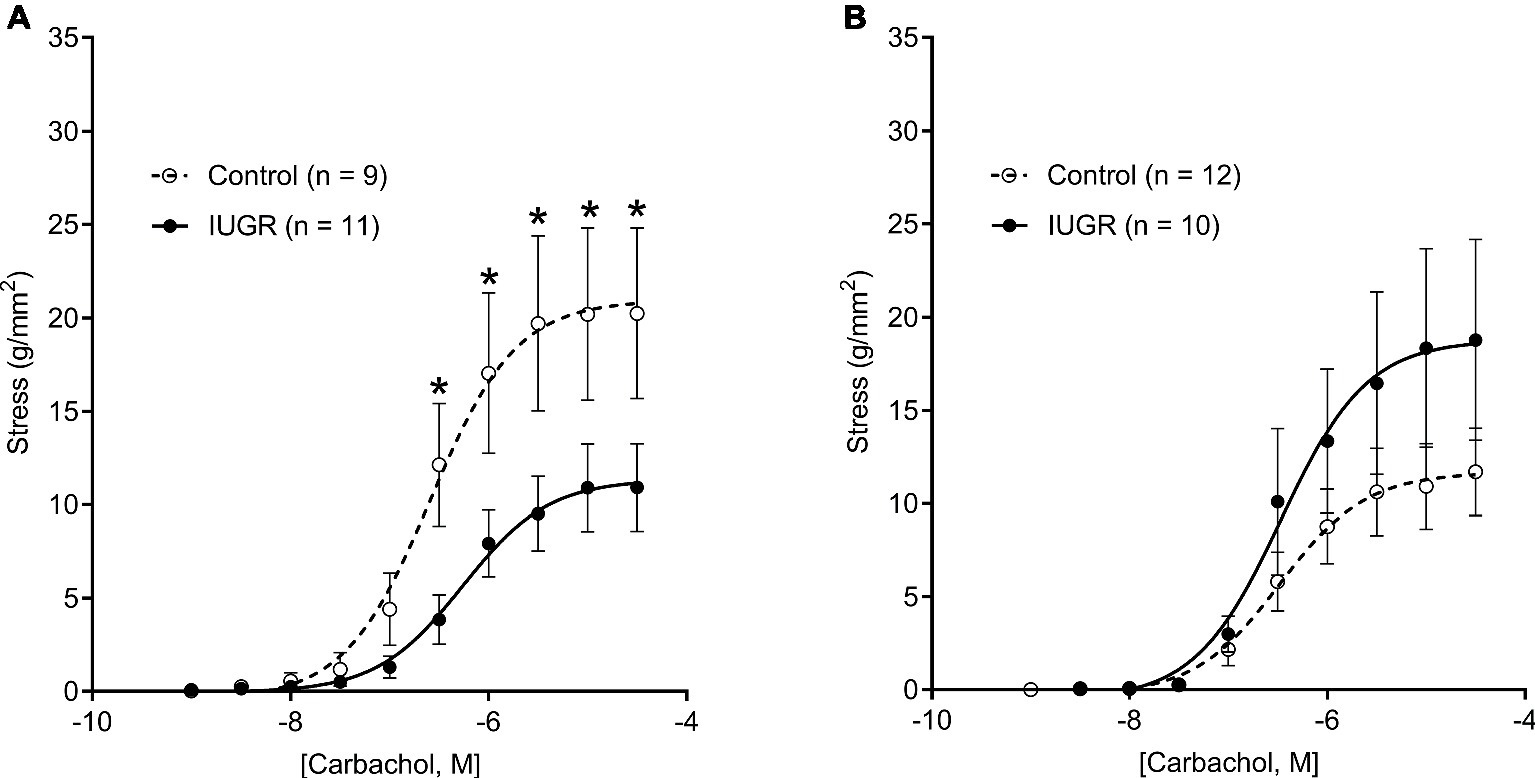
Figure 3. CCh-stress dose-response curves in males (A) and females (B) for Control and IUGR offspring. Values are mean ± SEM. *Significantly different from Control (p < 0.05). CCh, carbachol; Control (white circles); IUGR, intrauterine growth restriction (black circles).
Passive Airway Stiffness in vitro
Passive airway stiffness was calculated from changes in wall stress over the relative change in diameter strain (Figure 4A). Passive airway stiffness was greater in IUGR offspring compared with Control offspring (p = 0.003; Figure 4B). Passive airway stiffness was not affected by sex (p = 0.538).
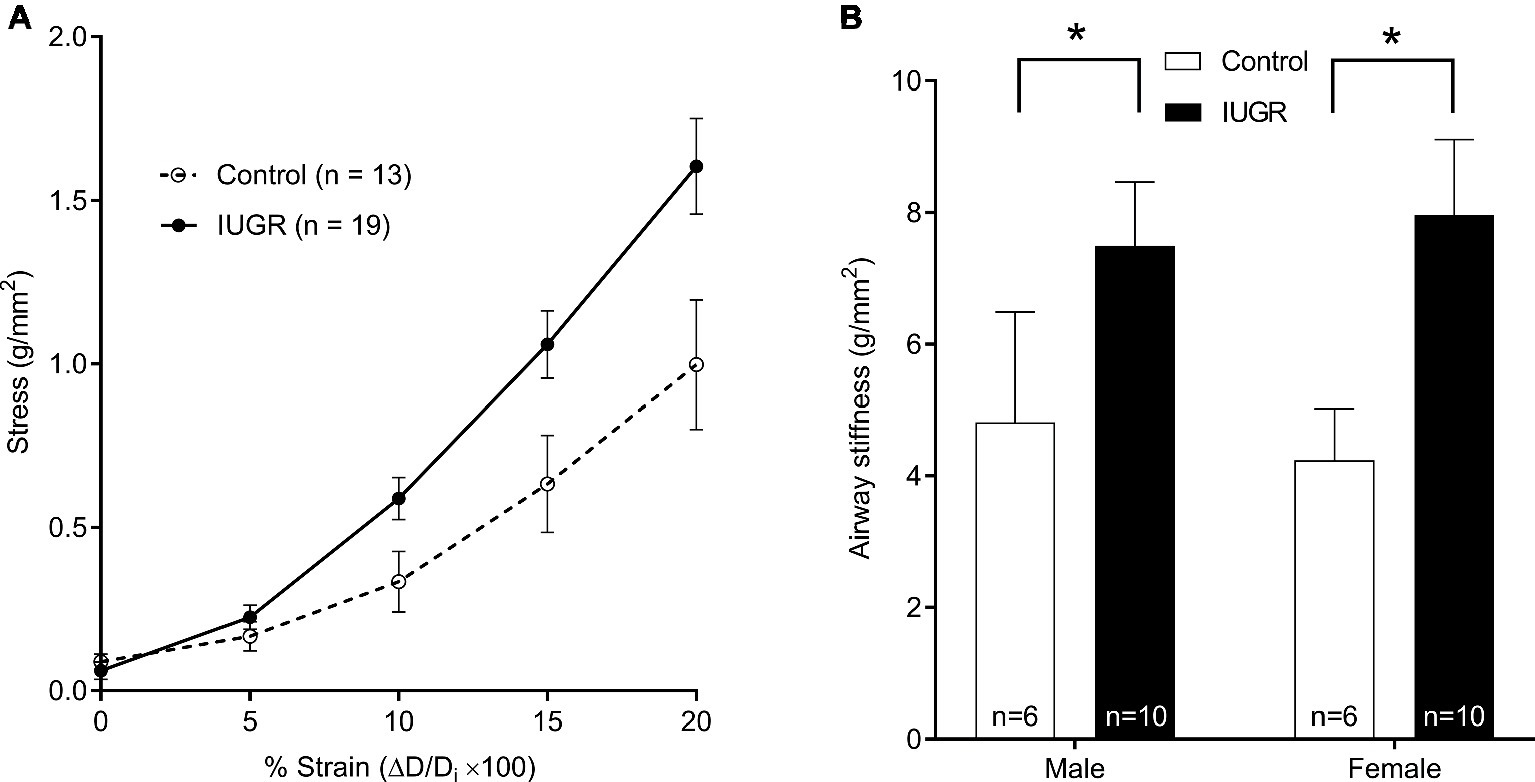
Figure 4. Relationship between wall stress and lumen diameter strain (sexes combined), where Di was the initial diameter after mounting in the organ bath (A). Passive airway stiffness in males and females for Control and IUGR offspring (B). Values are mean ± SEM. *Significantly different from Control (p < 0.05). Control (white circles/bars); IUGR, intrauterine growth restriction (black circles/bars).
Airway Moduli and Hysteresivity in vivo
From the global (normalized) airway radius-Prs curves shown in Figures 5A,B, the compliant region of the curve is between 0 and 5 cmH2O, above which the airway is seen to stiffen. There was no difference in storage (p = 0.187) or loss moduli (p = 0.168) between groups. Hysteresivity was however increased in the IUGR group compared with Control (p = 0.016; Figure 5C) (note that findings were similar when analyzed as Raw or conductance rather than global airway radius). Sex was also an independent predictor; loss modulus (p = 0.038) and hysteresivity (p = 0.018) were increased in males compared with females.
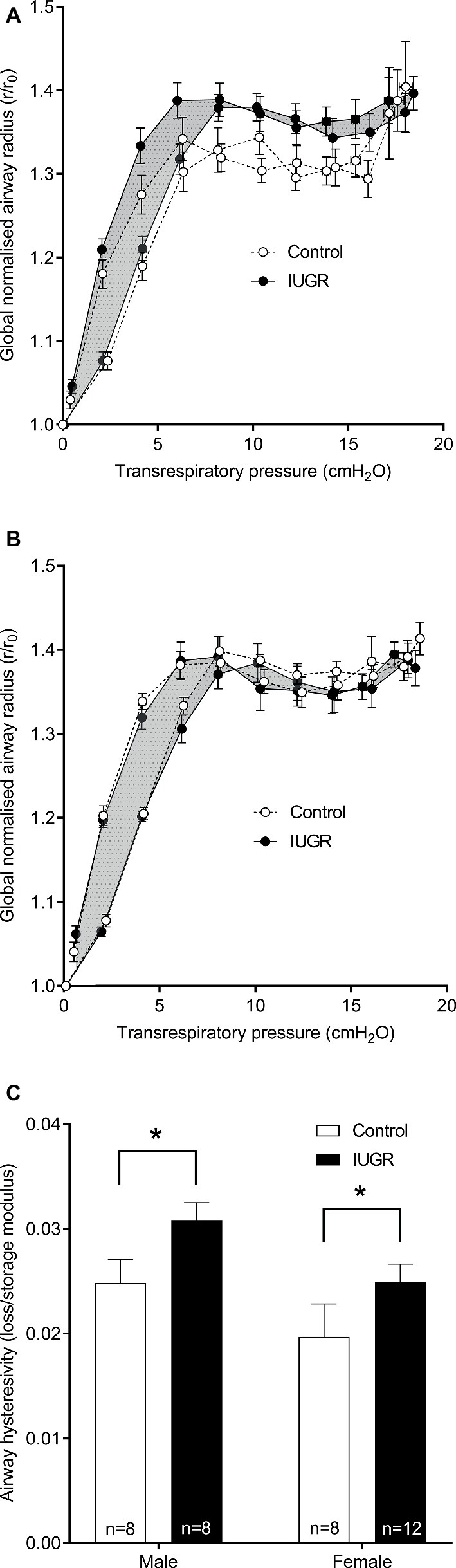
Figure 5. Global airway radius against transrespiratory pressure. Conductance was calculated from the inverse of resistance and subsequently converted into a global airway radius (radius = conductance1/4) normalized to initial radius at 0 cmH2O (r/r0). Inflationary and deflationary curves were plotted for males (A) and females (B). In the IUGR group, the area between inflation and deflation curves is shaded for clarity. Airway hysteresivity for Control and IUGR offspring (C; loss/storage modulus). Values are mean ± SEM. *Significantly different from Control (p < 0.05). Control (white circles/bars); IUGR, intrauterine growth restriction (black circles/bars).
Discussion
There is emerging data demonstrating that IUGR and low birth weight impact airway development and subsequent function, and in turn risk of developing obstructive airway disease in adulthood (Roseboom et al., 2003; Turner et al., 2010). Using our established mouse model of maternal hypoxia-induced IUGR (Wang et al., 2018), we generated offspring with low birth weight and demonstrated changes in the physiology of the airway that may account for changes in airway responsiveness observed in our previous investigation (Wang et al., 2018). Our results suggest that mechanical abnormalities of the airway wall develop after an in utero insult and could alter susceptibility for asthma development.
Our model of IUGR develops after a period of induced maternal hypoxia. Ultimately birth weight is a marker of adverse fetal development and many of the risk factors for IUGR (placental restriction and under nutrition) exert their effects through a hypoxic insult (Wickström, 2007; Belkacemi et al., 2010). Evidence from prenatal sheep studies indicate that partial pressure of oxygen in the fetal carotid artery is positively correlated with birth weight (Botting et al., 2012). The magnitude of birth weight restriction observed in the present study was small (~5%) and approximately half that of our earlier study (Wang et al., 2018). When comparing mean birth weight of IUGR animals to the Control group, growth-restricted animals are placed below the <32nd percentile group of the Control group, whereas the clinical definition of IUGR is birth weight <10th percentile (Peleg et al., 1998). The intervention was nonetheless sufficient to produce physiological abnormalities that persisted to the adult airway.
Other than ASM thickness, the force produced for a given cross sectional area of ASM, the definition of stress or generically “contractility,” is expected to impact airway narrowing capacity to an applied agonist. Force or stress is only feasibly assessed in vitro, specifically in excised segments of airway or strips of muscle preserved in organ bath chambers. One such in vitro study examined ASM responses from guinea pigs exhibiting hyperresponsiveness in vivo, and demonstrated an increase in pressure generation to contractile agonist, which was not accounted for by changes in ASM thickness, consistent with increased ASM contractility (Ishida et al., 1990). Hyperresponsiveness in vivo is however not always accompanied by increased ASM contraction in vitro (Ishida et al., 1990), with some studies even reporting a reduced contractility of ASM from hyperresponsive animals (Turner et al., 2002). Inconsistencies between in vivo and in vitro findings on airway reactivity reflect complexity in the physiology of airway narrowing, which is determined by a balance of forces produced by the ASM layer, mural loads, and airway-lung interdependence in the dynamic breathing environment (Harvey and Lutchen, 2013). Sexual dimorphism in airway responsiveness is also a consideration, as we demonstrated in IUGR offspring where males were hyporesponsive and females hyperresponsive compared with Control offspring (Wang et al., 2018).
The present data on ASM contraction in vitro provides an explanation for hyporresponsiveness in male IUGR offspring in vivo. Maximal contractility of the ASM was reduced relative to male Control offspring and should intuitively decrease ASM shortening and luminal narrowing to bronchial challenge in vivo. In comparison, there were no clear changes in ASM contractility in female IUGR offspring, indicating that active ASM properties are not affected by IUGR. The effect of IUGR on smooth muscle contraction has been previously assessed in the vascular system. Coronary arterial segments isolated from sheep subject to IUGR late in gestation were more responsive to vasoconstrictors than Control group (Bubb et al., 2007). In contrast, carotid arteries of neonatal rats showed blunted contractions following maternal hypoxia, which the authors proposed was not due to changes in muscle thickness (Williams et al., 2005). None of these studies identified the sex of the offspring, and as such we were unable to conclude if sex impacted arteries similar to our findings on the murine airway.
Beyond establishing a mechanism for hyporesponsiveness in male IUGR offspring in vivo, the data generated from the present study do not provide information on why the contractility of the ASM layer was herein modified. Given that the experimental intervention was applied during the fetal period, known maturational changes in ASM contraction are relevant. Sparrow and Mitchell compared contractility of tracheal ASM from fetal, young and mature pigs (Sparrow and Mitchell, 1990). While contractility varied between time points, differences were minimized after accounting for myosin content that increased with maturity. Ontogenetic changes in myosin light chain kinase content are observed in guinea-pig airways (Chitano et al., 2004), which offers an explanation for increased airway reactivity in early life (Montgomery and Tepper, 1990). An effect of IUGR on contractile filament expression, myosin light chain kinase, or other key components of the contractile cycle would impact force production. Changes extrinsic to the ASM cell are also possible; we report increased proportion of extracellular matrix within the ASM layer of subjects with fixed airflow limitation (Jones et al., 2016). If the proportion of matrix to muscle cells within the ASM layer were altered by IUGR, the amount of contractile tissue contained within a given cross sectional area of ASM layer will change and be reflected in our measures of contractility. Matrix changes further impact the micro-mechanical environment of ASM cells which through mechanosensation pathways determine contractility at a cellular level (An et al., 2009). Future investigations should focus on phenotyping the composition of the ASM layer and contractility at a tissue and cellular level.
Due to advancements in our understanding on the physiological behavior of ASM, particularly contraction under conditions of dynamic forces accompanying breathing, it is now prudent to examine properties beyond simple changes in ASM thickness and contractility when attempting to reveal mechanisms for abnormalities in airway responsiveness. Increased airway stiffness has been documented in subjects with asthma (Brackel et al., 2000), possibly due to gross changes in wall structure (Ward et al., 2001) and/or increased passive tension of the ASM layer (Chin et al., 2012). The implications of a stiffer airway wall in asthma is that protective effects of breathing maneuvers including deep inspiration may be blunted, which through distension of the airway wall and ASM layer, normally, reduces ASM force production (Noble et al., 2007) and in turn expands lumen caliber (West et al., 2012). Similar to what is proposed in asthmatic subjects, the IUGR group exhibited increased airway stiffness in vitro. Findings are unlikely due to changes in tissue bulk, since we previously observed no changes in airway wall structure when sampling systematically through the IUGR-affected murine lung (Wang et al., 2018). Intrinsic changes to the ASM layer are possible, which is modifiable without apparent changes in structure (Seow, 2013). Changes in cartilage stiffness are also worth considering, particularly in our system where cartilage and ASM layers are arranged in parallel, such that cartilage may contribute in no small way to the reported stiffness values.
At an airway level, the net effect of changes in ASM contractility and airway stiffness will determine airway responsiveness in IUGR mice in vivo. In male IUGR offspring exhibiting hyporesponsiveness in vivo (Wang et al., 2018), reduced contractility may outweigh the detrimental effects of increased stiffness and/or there may be protective effects in preventing collapse (Noble et al., 2002). In comparison, female IUGR offspring do not show the same attenuation in ASM contractility and, in this scenario, increased stiffness is accompanied by hyperresponsiveness in vivo (Wang et al., 2018). Our observations are consistent with human population studies that report a greater susceptibility of adult females to asthma development (Schatz and Camargo, 2003), an association that may begin in utero.
A limitation of the present study is the use of the trachea to model in vivo lung function which is governed by the behavior of intra-parenchymal airways. Tracheal ASM is a commonly studied tissue due to its relative thickness and ease of dissection. Contractile properties of the tracheal ASM in asthma are consistent with studies performed on large bronchi (Ijpma et al., 2015). There is however some evidence from horses with heaves (an innate model of asthma) to suggest that in the context of disease, peripheral airways behave differently to more proximally located airways (Matusovsky et al., 2016). Moreover, the conclusions drawn from our earlier biological and mathematical modeling study attributed increased heterogeneity of lumen caliber to an anatomical variation in airway compliance (Wang et al., 2017). Assessment at a single location may therefore not be representative of integrated function in vivo.
The above reductionist in vitro approach provides information at an airway level on how tissue changes contribute to previously observed changes in respiratory function after IUGR. In separate experiments, we examined how apparent increases in airway stiffness within the IUGR group in vitro would manifest more globally in vivo. Mechanical properties of the bronchial tree were assessed from the inverse of airway resistance (conductance) to the one-quarter power, approximating changes in global lumen radius. That is, the bronchial tree was represented as a single lumen radius, an approach which itself carries numerous assumptions, albeit different assumptions to studies conducted in vitro. After normalization to baseline caliber, IUGR animals exhibited an increase in hysteresivity, but no change in storage or loss modulus. In other murine models, changes in tissue (lung) hysteresivity have been observed following fibrotic disease (Pillow et al., 2001). The lack of an effect of IUGR on airway storage modulus (equivalent to in vitro measurements of stiffness) may be due the aforementioned heterogeneity in compliance predicted in our earlier study (Wang et al., 2017). In vivo, these data suggest that the dominant effect is an increase in airway viscosity after IUGR, which may also contribute to impaired distension of the airway wall to dynamically applied loads. There is of course the possibility that changes in airway stiffness identified in vitro included some component of viscous load, although this is unlikely as measurements were performed under quasistatic conditions after stress-relaxation forces had subsided. Finally, an interesting observation was the clear sex differences in airway loss modulus which was increased in males compared with females. Such differences may further contribute to sex differences in respiratory pathophysiology.
In conclusion, the present study demonstrates mechanical abnormalities of the ASM layer and airway wall in adult mice after IUGR. Such abnormalities are expected to alter susceptibility for asthma development, in a sex-dependent manner, which has implications for our understanding of the pathogenesis of asthma and early life preventative measures.
Data Availability
All datasets generated for this study are included in the manuscript and/or supplementary files.
Ethics Statement
The animal study was reviewed and approved by the Telethon Kids Institute Animal Ethics Committee.
Author Contributions
PN, AL, and KW designed the study. DK and KW were involved in data collection. PN and KW drafted the manuscript. All authors were involved in data analysis and interpretation and revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Funding
This project was funded by the National Health and Medical Research Council (NHMRC) of Australia (1120128). KW was supported by a NHMRC Early Career Research Fellowship (1090888) and a Western Australia Department of Health – New Independent Researcher Infrastructure Support. PN was supported by a Western Australia Department of Health – Merit Award and a Medical and Health Research Infrastructure Fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Luke J. Berry, Donna L. Savigni, and Carolyn J. Wang for technical and analytical assistance.
References
An, S. S., Kim, J., Ahn, K., Trepat, X., Drake, K. J., Kumar, S., et al. (2009). Cell stiffness, contractile stress and the role of extracellular matrix. Biochem. Biophys. Res. Commun. 382, 697–703. doi: 10.1016/j.bbrc.2009.03.118
Ansell, T. K., Noble, P. B., Mitchell, H. W., and McFawn, P. K. (2014). Pharmacological bronchodilation is partially mediated by reduced airway wall stiffness. Br. J. Pharmacol. 171, 4376–4384. doi: 10.1111/bph.12781
Barker, D. J., Godfrey, K. M., Fall, C., Osmond, C., Winter, P. D., and Shaheen, S. O. (1991). Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 303, 671–675. doi: 10.1136/bmj.303.6804.671
Belkacemi, L., Nelson, D. M., Desai, M., and Ross, M. G. (2010). Maternal undernutrition influences placental-fetal development. Biol. Reprod. 83, 325–331. doi: 10.1095/biolreprod.110.084517
Bisgaard, H., Jensen, S. M., and Bønnelykke, K. (2012). Interaction between asthma and lung function growth in early life. Am. J. Respir. Crit. Care Med. 185, 1183–1189. doi: 10.1164/rccm.201110-1922OC
Botting, K. J., Wang, K. C., Padhee, M., McMillen, I. C., Summers-Pearce, B., Rattanatray, L., et al. (2012). Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin. Exp. Pharmacol. Physiol. 39, 814–823. doi: 10.1111/j.1440-1681.2011.05649.x
Brackel, H. J., Pedersen, O. F., Mulder, P. G., Overbeek, S. E., Kerrebijn, K. F., and Bogaard, J. M. (2000). Central airways behave more stiffly during forced expiration in patients with asthma. Am. J. Respir. Crit. Care Med. 162, 896–904. doi: 10.1164/ajrccm.162.3.9905034
Bubb, K. J., Cock, M. L., Black, M. J., Dodic, M., Boon, W. M., Parkington, H. C., et al. (2007). Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J. Physiol. 578, 871–881. doi: 10.1113/jphysiol.2006.121160
Cairncross, A., Noble, P. B., and McFawn, P. K. (2018). Hyperinflation of bronchi in vitro impairs bronchodilation to simulated breathing and increases sensitivity to contractile activation. Respirology 23, 750–755. doi: 10.1111/resp.13271
Chin, L. Y., Bossé, Y., Pascoe, C., Hackett, T. L., Seow, C. Y., and Paré, P. D. (2012). Mechanical properties of asthmatic airway smooth muscle. Eur. Respir. J. 40, 45–54. doi: 10.1183/09031936.00065411
Chitano, P., Voynow, J. A., Pozzato, V., Cantillana, V., Burch, L. H., Wang, L., et al. (2004). Ontogenesis of myosin light chain kinase mRNA and protein content in guinea pig tracheal smooth muscle. Pediatr. Pulmonol. 38, 456–464. doi: 10.1002/ppul.20118
Ding, D. J., Martin, J. G., and Macklem, P. T. (1987). Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J. Appl. Physiol. 62, 1324–1330.
Hantos, Z., Daróczy, B., Suki, B., Nagy, S., and Fredberg, J. J. (1992). Input impedance and peripheral inhomogeneity of dog lungs. J. Appl. Physiol. 72, 168–178.
Harvey, B. C., and Lutchen, K. R. (2013). Factors determining airway caliber in asthma. Crit. Rev. Biomed. Eng. 41, 515–532. doi: 10.1615/CritRevBiomedEng.2014010687
Ijpma, G., Kachmar, L., Matusovsky, O. S., Bates, J. H., Benedetti, A., Martin, J. G., et al. (2015). Human trachealis and main bronchi smooth muscle are normoresponsive in asthma. Am. J. Respir. Crit. Care Med. 191, 884–893. doi: 10.1164/rccm.201407-1296OC
Ishida, K., Paré, P. D., Thomson, R. J., and Schellenberg, R. R. (1990). Increased in vitro responses of tracheal smooth muscle from hyperresponsive guinea pigs. J. Appl. Physiol. 68, 1316–1320. doi: 10.1152/jappl.1990.68.4.1316
James, A. L., Palmer, L. J., Kicic, E., Maxwell, P. S., Lagan, S. E., Ryan, G. F., et al. (2005). Decline in lung function in the Busselton health study: the effects of asthma and cigarette smoking. Am. J. Respir. Crit. Care Med. 171, 109–114. doi: 10.1164/rccm.200402-230OC
Jones, R. L., Noble, P. B., Elliot, J. G., Mitchell, H. W., McFawn, P. K., Hogg, J. C., et al. (2016). Airflow obstruction is associated with increased smooth muscle extracellular matrix. Eur. Respir. J. 47, 1855–1857. doi: 10.1183/13993003.01709-2015
Källén, B., Finnström, O., Nygren, K. G., and Otterblad Olausson, P. (2013). Association between preterm birth and intrauterine growth retardation and child asthma. Eur. Respir. J. 41, 671–676. doi: 10.1183/09031936.00041912
Larcombe, A. N., Foong, R. E., Boylen, C. E., and Zosky, G. R. (2013). Acute diesel exhaust particle exposure increases viral titre and inflammation associated with existing influenza infection, but does not exacerbate deficits in lung function. Influenza Other Respir. Viruses 7, 701–709. doi: 10.1111/irv.12012
Larcombe, A. N., Foong, R. E., Bozanich, E. M., Berry, L. J., Garratt, L. W., Gualano, R. C., et al. (2011). Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir. Viruses 5, 334–342. doi: 10.1111/j.1750-2659.2011.00236.x
Matusovsky, O. S., Kachmar, L., Ijpma, G., Bates, G., Zitouni, N., Benedetti, A., et al. (2016). Peripheral airway smooth muscle, but not the trachealis, is hypercontractile in an equine model of asthma. Am. J. Respir. Cell Mol. Biol. 54, 718–727. doi: 10.1165/rcmb.2015-0180OC
Montgomery, G. L., and Tepper, R. S. (1990). Changes in airway reactivity with age in normal infants and young children. Am. Rev. Respir. Dis. 142, 1372–1376. doi: 10.1164/ajrccm/142.6_Pt_1.1372
Noble, P. B., Jones, R. L., Cairncross, A., Elliot, J. G., Mitchell, H. W., James, A. L., et al. (2013). Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J. Appl. Physiol. 114, 1460–1471. doi: 10.1152/japplphysiol.01489.2012
Noble, P. B., McFawn, P. K., and Mitchell, H. W. (2007). Responsiveness of the isolated airway during simulated deep inspirations: effect of airway smooth muscle stiffness and strain. J. Appl. Physiol. 103, 787–795. doi: 10.1152/japplphysiol.00314.2007
Noble, P. B., Turner, D. J., and Mitchell, H. W. (2002). Relationship of airway narrowing, compliance, and cartilage in isolated bronchial segments. J. Appl. Physiol. 92, 1119–1124. doi: 10.1152/japplphysiol.00662.2001
Oliver, M., Kováts, T., Mijailovich, S. M., Butler, J. P., Fredberg, J. J., and Lenormand, G. (2010). Remodeling of integrated contractile tissues and its dependence on strain-rate amplitude. Phys. Rev. Lett. 105:158102. doi: 10.1103/PhysRevLett.105.158102
Opazo Saez, A. M., Seow, C. Y., and Paré, P. D. (2000). Peripheral airway smooth muscle mechanics in obstructive airways disease. Am. J. Respir. Crit. Care Med. 161, 910–917. doi: 10.1164/ajrccm.161.3.9903138
Owens, L., Laing, I. A., Zhang, G., and Le Souëf, P. N. (2017). Infant lung function predicts asthma persistence and remission in young adults. Respirology 22, 289–294. doi: 10.1111/resp.12901
Palmer, L. J., Rye, P. J., Gibson, N. A., Burton, P. R., Landau, L. I., and Lesouëf, P. N. (2001). Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am. J. Respir. Crit. Care Med. 163, 37–42. doi: 10.1164/ajrccm.163.1.2005013
Peleg, D., Kennedy, C. M., and Hunter, S. K. (1998). Intrauterine growth restriction: identification and management. Am. Fam. Physician 58, 453–460, 466–457.
Peták, F., Hantos, Z., Adamicza, A., Asztalos, T., and Sly, P. D. (1997). Methacholine-induced bronchoconstriction in rats: effects of intravenous vs. aerosol delivery. J. Appl. Physiol. 82, 1479–1487. doi: 10.1152/jappl.1997.82.5.1479
Phelan, P. D., Robertson, C. F., and Olinsky, A. (2002). The Melbourne asthma study: 1964–1999. J. Allergy Clin. Immunol. 109, 189–194. doi: 10.1067/mai.2002.120951
Pillow, J. J., Korfhagen, T. R., Ikegami, M., and Sly, P. D. (2001). Overexpression of TGF-alpha increases lung tissue hysteresivity in transgenic mice. J. Appl. Physiol. 91, 2730–2734. doi: 10.1152/jappl.2001.91.6.2730
Roseboom, T. J., Van Der Meulen, J. H., Ravelli, A. C., Osmond, C., Barker, D. J., and Bleker, O. P. (2003). Perceived health of adults after prenatal exposure to the Dutch famine. Paediatr. Perinat. Epidemiol. 17, 391–397. doi: 10.1046/j.1365-3016.2003.00516.x
Schatz, M., and Camargo, C. A. Jr. (2003). The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann. Allergy Asthma Immunol. 91, 553–558. doi: 10.1016/S1081-1206(10)61533-5
Sears, M. R., Greene, J. M., Willan, A. R., Wiecek, E. M., Taylor, D. R., Flannery, E. M., et al. (2003). A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 349, 1414–1422. doi: 10.1056/NEJMoa022363
Seow, C. Y. (2013). Passive stiffness of airway smooth muscle: the next target for improving airway distensibility and treatment for asthma? Pulm. Pharmacol. Ther. 26, 37–41. doi: 10.1016/j.pupt.2012.06.012
Sly, P. D., Collins, R. A., Thamrin, C., Turner, D. J., and Hantos, Z. (2003). Volume dependence of airway and tissue impedances in mice. J. Appl. Physiol. 94, 1460–1466. doi: 10.1152/japplphysiol.00596.2002
Sparrow, M. P., and Mitchell, H. W. (1990). Contraction of smooth muscle of pig airway tissues from before birth to maturity. J. Appl. Physiol. 68, 468–477. doi: 10.1152/jappl.1990.68.2.468
Turner, S. W., Campbell, D., Smith, N., Craig, L. C., McNeill, G., Forbes, S. H., et al. (2010). Associations between fetal size, maternal {alpha}-tocopherol and childhood asthma. Thorax 65, 391–397. doi: 10.1136/thx.2008.111385
Turner, D. J., Noble, P. B., Lucas, M. P., and Mitchell, H. W. (2002). Decreased airway narrowing and smooth muscle contraction in hyperresponsive pigs. J. Appl. Physiol. 93, 1296–1300. doi: 10.1152/japplphysiol.00150.2002
Turner, S. W., Palmer, L. J., Rye, P. J., Gibson, N. A., Judge, P. K., Cox, M., et al. (2004). The relationship between infant airway function, childhood airway responsiveness, and asthma. Am. J. Respir. Crit. Care Med. 169, 921–927. doi: 10.1164/rccm.200307-891OC
Wang, K. C. W., Larcombe, A. N., Berry, L. J., Morton, J. S., Davidge, S. T., James, A. L., et al. (2018). Foetal growth restriction in mice modifies postnatal airway responsiveness in an age and sex-dependent manner. Clin. Sci. 132, 273–284. doi: 10.1042/CS20171554
Wang, K. C. W., Morton, J. S., Davidge, S. T., Larcombe, A. N., James, A. L., Donovan, G. M., et al. (2017). Increased heterogeneity of airway calibre in adult rats after hypoxia-induced intrauterine growth restriction. Respirology 22, 1329–1335. doi: 10.1111/resp.13071
Ward, C., Johns, D. P., Bish, R., Pais, M., Reid, D. W., Ingram, C., et al. (2001). Reduced airway distensibility, fixed airflow limitation, and airway wall remodeling in asthma. Am. J. Respir. Crit. Care Med. 164, 1718–1721. doi: 10.1164/ajrccm.164.9.2102039
West, A. R., Needi, E. T., Mitchell, H. W., McFawn, P. K., and Noble, P. B. (2012). Airways dilate to simulated inspiratory but not expiratory manoeuvres. Eur. Respir. J. 40, 455–461. doi: 10.1183/09031936.00187411
Wickström, R. (2007). Effects of nicotine during pregnancy: human and experimental evidence. Curr. Neuropharmacol. 5, 213–222. doi: 10.2174/157015907781695955
Williams, S. J., Campbell, M. E., McMillen, I. C., and Davidge, S. T. (2005). Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R360–R367. doi: 10.1152/ajpregu.00178.2004
Keywords: intrauterine growth restriction, low birth weight, animal models, asthma, respiratory structure and function
Citation: Noble PB, Kowlessur D, Larcombe AN, Donovan GM and Wang KCW (2019) Mechanical Abnormalities of the Airway Wall in Adult Mice After Intrauterine Growth Restriction. Front. Physiol. 10:1073. doi: 10.3389/fphys.2019.01073
Edited by:
Walter Araujo Zin, Federal University of Rio de Janeiro, BrazilReviewed by:
Chun Y. Seow, University of British Columbia, CanadaYnuk Bossé, Laval University, Canada
Copyright © 2019 Noble, Kowlessur, Larcombe, Donovan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kimberley C. W. Wang, a2ltYmVybGV5LndhbmdAdXdhLmVkdS5hdQ==
 Peter B. Noble
Peter B. Noble Darshinee Kowlessur
Darshinee Kowlessur Alexander N. Larcombe
Alexander N. Larcombe Graham M. Donovan4
Graham M. Donovan4 Kimberley C. W. Wang
Kimberley C. W. Wang