- School of Health Sciences, Liverpool Hope University, Liverpool, United Kingdom
Objective: To investigate the effects of a 16-week concurrent exercise regimen [resistance exercise (RE) + functional exercise (FE)] in combination with, or without, a leucine-enriched whey protein isolate supplement on muscle strength, physical functioning, aerobic capacity, and cardiometabolic health in older adults (≥60 years). Physical activity levels were also evaluated 6 months post-cessation of the intervention.
Methods: Forty-six, community-dwelling, previously untrained males, and females [age: 68 ± 5 years (mean ± SD); BMI: 27.8 ± 6.2 kg/m2] who completed the trial were initially randomized to one of two independent arms [Exercise n = 24 (E); Exercise+Protein n = 22 (EP)]. Both arms completed 16 weeks of RE (performed to fatigue) (2 times/week) with FE (1 time/week) on non-consecutive days. Additionally, EP were administered a leucine-enriched whey protein supplement (3 times/day) for 16 weeks based on individual body-weight (1.5 g/kg/day).
Results: As a result of dietary supplementation, protein intake increased in EP (∼1.2 ± 0.4 to 1.5 ± 0.7 g/kg/day) during the intervention. Maximal strength (1RM) values for leg press (E: +39 ± 7 kg, p = 0.006; EP: +63 ± 7 kg, p < 0.001), chest press (E: +22 ± 4 kg, p < 0.001; EP: +21 ± 6 kg, p < 0.001), and bicep curl (E: +7 ± 0 kg, p = 0.002; EP: +6 ± 1 kg, p = 0.008) significantly increased in E and EP respectively, with no differences between arms (p > 0.05). Physical functioning in the obstacle course (E: -5.1 ± 6.8 s, p < 0.001; EP: -2.8 ± 0.8 s, p < 0.001) and short-physical performance battery scores (E: +0.5 ± 0.5, p = <0.001; EP: +0.4 ± 0.5, p = 0.038), and aerobic capacity in the 6-min walk test (E: +37 ± 24 m, p = 0.014; EP: +36 ± 3 m, p = 0.005) improved in E and EP respectively, with no differences between arms (p > 0.05). No significant change was observed for markers of cardiometabolic health (glycaemic control or blood pressure) (p > 0.05). At follow-up, 86% of older adults reported to performing physical activity ≥1 per week. Of those, 61% were still participating in strength- and cardiovascular- based exercise.
Conclusion: Concurrent exercise (RE + FE) offers a potent method to combat age-related muscle weakness, and our results suggest a high proportion of older adults may continue to exercise unsupervised. However, leucine-enriched whey protein isolate supplementation did not confer any additional benefit in those already consuming ample amounts of dietary protein at trial enrolment. Future trials should utilize a whole-foods approach and investigate the effects in frail and non-frail older adults habitually consuming the RDA of protein, to assess if a higher intake of protein is needed to delay the onset of muscle weakness.
Trial Registration: Clinicaltrials.gov Identifier: NCT02912130.
Introduction
The aging epidemic has led to increased awareness of frailty phenotypes, notably muscle weakness (Fried et al., 2001), which manifests around 50 years of age, and occurs at a 2–5 times more rapid rate than muscle mass loss (Goodpaster et al., 2006). In the United Kingdom alone, estimated annual costs attributed to muscle weakness are $2.5 billion (Pinedo-Villanueva et al., 2018) which emphasizes the urgent need for prevention strategies.
Two prophylactics suggested to curtail muscle weakness are resistance exercise (RE) and dietary-protein. RE is a potent stimulus to increase muscle strength and physical functioning (Fiatarone et al., 1990; Stec et al., 2017) whilst epidemiological data show higher quantities of dietary-protein (>1 g/kg/day) can curb declines in grip strength (McLean et al., 2016) and mobility (Mustafa et al., 2018). Nonetheless, the body of evidence to support the increased requirement of dietary-protein to augment RE effects on muscle strength is inconclusive. Individual trials have failed to show benefits (Verdijk et al., 2009; Leenders et al., 2013; Holwerda et al., 2018) and only when trials are pooled in a meta-analysis does a positive effect appear (Cermak et al., 2012; Morton et al., 2017) although this has not always been the case (Finger et al., 2015; Gade et al., 2018). Disparate findings may be due to total amount, type and timing of supplemented protein, and in particular, sub-optimal intakes of the essential amino acid leucine, the key regulator of muscle anabolism (Devries et al., 2018). Acute trials utilizing isotope tracers have demonstrated an anabolic resistance in older adults, whereby higher dosages of dietary-protein rich in leucine are suggested to overcome this phenomenon (Moore et al., 2015).
Regarding the optimal intensity of RE, similar increases in strength have been evident when comparing moderate and heavy loads in the range of 40–90% of maximum (Morton et al., 2016; da Silva et al., 2018) once total volume is equated for, and lower loads are carried out to fatigue. Nevertheless, as 45.1% of 14,807 older adults (>75 years) suffer chronic musculoskeletal pain (Cimas et al., 2018) refraining from heavy repetitive loading may be a more practical choice to maintain adherence long term. Similar to RE intensity, comparable improvements in strength are apparent with two compared to three weekly sessions in older adults (Silva et al., 2017; Stec et al., 2017).
A Cochrane review (Sherrington et al., 2019) recently highlighted that combining multiple exercises (muscle strengthening, functional, and balance) offset falls in community-dwelling older adults by 34%. Considering this, in addition to the principle of specificity effect (Hawley, 2008; Reilly et al., 2009), there is strong evidence to include RE and functional exercise (FE) in a regimen to obtain the synergistic benefits on muscle strength and physical functioning. In addition, including FE may act as an added stimulus to confer cardiometabolic health benefits on blood pressure, glycaemic control, and aerobic capacity (Whitehurst et al., 2005; Pollock et al., 2018).
With the aforementioned research in mind, the aim of the present two-arm trial [Exercise (E); Exercise+Protein (EP)] was to investigate the synergistic effects of 16-weeks of RE (to fatigue) with FE, in combination with, or without, a leucine-enriched whey protein isolate supplement on muscle strength, physical functioning, and cardiometabolic health in older adults. It was hypothesized EP would demonstrate superior increases in muscle strength (our primary outcome) compared to E. Secondary aims included the effect of treatments on (a) physical functioning, (b) aerobic capacity, and (c) markers of cardiometabolic health which we anticipated to be superior in EP compared to E. Of tertiary interest was to examine physical activity levels 6 months post-cessation of the trial, which we envisaged to be low.
Materials and Methods
Subjects
Sample size was based on an average pooled effect size of 0.5 (range 0.1–0.9) from a previous meta-analysis (Cermak et al., 2012), which found greater increases in leg strength with combined RE and dietary-protein vs. RE alone in older adults. Using G∗Power (Faul et al., 2007) software and setting power to 80% with alpha at 0.05 (two-tailed) to observe a treatment effect n ≥ 32 participants were required for final analysis. Recruitment was conducted via online advertisement detailing trial information and enrolment was based on initial telephone screening outlining inclusion and exclusion criteria1. To confirm eligibility, participants completed a physical activity readiness questionnaire (PAR-Q) (Thompson et al., 2013) to screen for pre-existing medical conditions. During this time participants were briefed on the nature of the trial, associated risks and benefits before written informed consent was obtained. Participants were excluded with uncontrolled hypertension (160/100 mmHg), hypotension (≤100 mmHg), hyperglycaemia (HbA1c ≥ 10%), on prescribed hormonal and/or anti-inflammatory medication, previous history of scheduled exercise (past 12 months), recent musculoskeletal injury, intolerance to dairy and/or lactose products (for exhaustive list see text footnote 1). For the duration of the trial, participants were instructed to refrain from exercise, and/or nutritional supplements other than administered by the intervention. Ethical approval was sought from the North-West of England NHS Research Ethics Committee United Kingdom (REC No. 16/NW/0480) and the trial was registered at clinicaltrials.gov as NCT02912130.
Trial Design
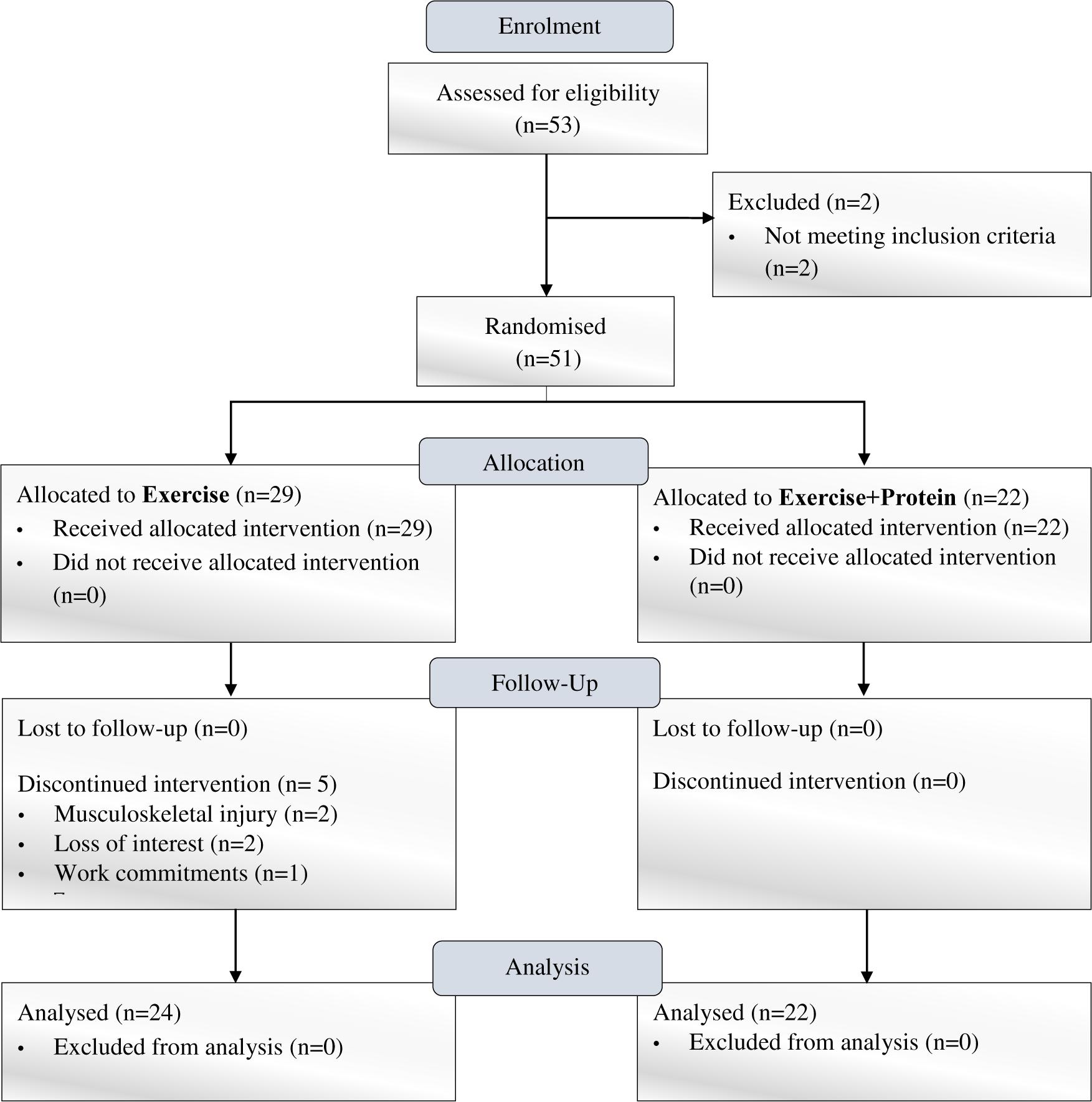
Following enrolment, forty-six, non-frail, community-dwelling, and previously untrained males and females (aged ≥60–86 years) who completed the trial were initially randomized in a single-blind design to one of two independent arms [Exercise n = 24 (E); Exercise+Protein n = 22 (EP); see Figure 1]. All participants attended the clinical laboratories at two separate time points (pre- and post- intervention) where outcome measures were performed. During the intervention, E and EP attended the university sports complex gymnasium thrice weekly for one FE and two RE sessions (supervised by certified exercise trainers) on non-consecutive days for the duration of 16-weeks. EP were administered a leucine-enriched whey protein supplement thrice daily (at breakfast, lunch, and dinner) for 16 weeks based on individual body-weight. Protein supplements were consumed in addition to normal dietary intake. To minimize diurnal variation, the outcome measures were carried out at the same time of day pre- and post- intervention.
Pre- and Post-outcome Measures
Anthropometry
Participants removed shoes, socks, watches, jewelry, and any heavy clothing prior to height (nearest 0.1 m; SECA 213 Stadiometer) and weight (nearest 0.1 kg; TANITA MC-180MA) measurements. Body mass index (BMI) was calculated from the above measurements using the following validated equation: body-weight (kg)/height (m2).
Muscle Strength
Strength was evaluated via 5-repetition maximum (RM) using established guidelines (Baechle and Earle, 2008) on the following exercises in orderly fashion: leg press, chest press, and bicep curl. Testing was performed on resistance machines (leg press and chest press) and with a barbell (bicep curl) using Technogym equipment at the university sports complex gymnasium. A ∼5 min low-intensity cardiovascular warm up was first conducted on either a motorized treadmill, cross-trainer, or bike. Lifting began with a self-selected moderate weight for 15 repetitions followed by 2 min rest before participants completed a further 10 repetitions with an increased weight selected by the exercise trainer. If full range of motion with correct posture was achieved the load was increased by 5 kg and 10 kg for upper- and lower- body, respectively. This process was continued with 2 min breaks until the true 5RM was obtained. 5RM values were then transformed to 1RM values using the previously validated equation (Brzycki, 1993) for strength testing in older adults (Wood et al., 2002). Final 1RM values in kilograms (kg) were used for analysis.
Physical Functioning and Aerobic Capacity
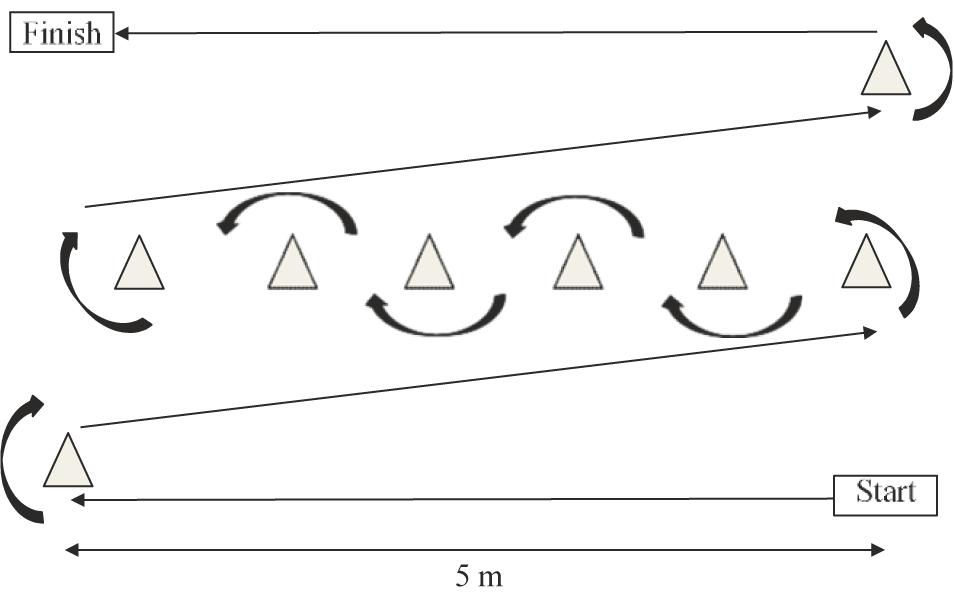
Standardized operating procedures were followed for the short-physical performance battery (SPPB) (Guralnik et al., 1994) which consisted of three timed components: standing balance, 4-m gait speed, and time to complete five chair-stands. Participant scores for each component were totalled between 0 and 12 used for analysis. The obstacle course was re-adapted from Steele et al. (2017) and consisted of a 25 m marked course incorporating 90 and 180 degree turns (Figure 2). Using a stopwatch to record time, participants were instructed to rise from the floor and carry a kettlebell weight (10 kg for males, 5 kg for females) as fast as possible around the course. The stopwatch stopped once the participant was re-seated on the floor at the finish line. Time in seconds (s) was used for analysis. For the 6 min walk test (6MWT) standardized operating procedures were followed (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories et al., 2002). A 30 m track was marked in an environmentally controlled laboratory (17°C) with chairs placed at both ends. Participants were instructed to walk up and down the track covering as much ground as possible within 6 min. Participants were reminded the test was self-paced and if needed a rest was permitted; however, the stopwatch would continue to run. Participants completed two 6MWT with a 10 min break between tests, Average of the two distances in meters (m) was used for analysis.
Blood Pressure and Fasting Blood Samples
Participants were laying rested on a medical bed for ∼5 min prior to blood pressure measurement. An inflatable cuff (SphygmoCor® CPV system; ScanMed Medical) was applied to the upper arm directly over the brachial artery and subsequent systolic and diastolic blood pressure readings (mmHg) were taken and used for analysis. A 35 μm capillary fingerstick blood sample was then collected in sterile conditions for the subsequent determination of plasma glucose (mmol/L; Alere Cholestech LDX Analyzer, Chesire, United Kingdom) and glycated hemoglobin (HbA1c %; Alere, AfinionTM, AS100, Cheshire, United Kingdom).
Physical Activity Follow-Up Survey
Six months post-cessation of the intervention all participants were re-contacted and asked to fill out an online survey (designed via Bristol Online Survey2). Survey was re-adapted from Forkan et al. (2006) and consisted of three multiple-choice questions. (1) How many times have you exercised in the past 4 weeks? (2) If you exercised in the past 4 weeks what type of exercise was it? (3) How long did each session last? Individual responses were totalled and analyzed to illustrate a %.
Exercise Intervention
Participants completed a gym induction and attended a familiarization day where the correct range of motion for each RE exercise was demonstrated to ensure technique and minimize injury risk. Participants also practiced lifting the weight to fatigue (defined as the point where the weight could no longer be lifted with correct posture). Participants were provided with a booklet detailing weekly sessions, specific exercises, and shown how to track weights. For FE, participants were shown the correct movement for each exercise and familiarized with the Borg scale during a practice session. Session attendance was recorded on arrival at the gymnasium reception desk. Average attendance was totalled to give a %.
Resistance Exercise
Each sessions lasted ∼50 min; with 5 min warm up of low-intensity exercise on either a motorized treadmill, cross-trainer or bike, then continued with 45 min of whole-body REs. Participants first completed one upper- and lower- body warm up with a lightweight. Participants then self-selected a moderate weight and completed 2 sets to fatigue separated by 3 min/between sets and 3 min/between exercises on each of the following machines in orderly fashion: leg press, chest press, calf press, shoulder press, seated row, and back extension. Bicep curl was performed last using a free weighted barbell due to no machine-based option. Weight was increased for upper- and lower- body exercises by 2.5 and 5 kg, respectively, once the participant completed ≥12 repetitions in both working sets. Maximal effort and progressive overload was encouraged by the exercise trainer.
Functional Exercise
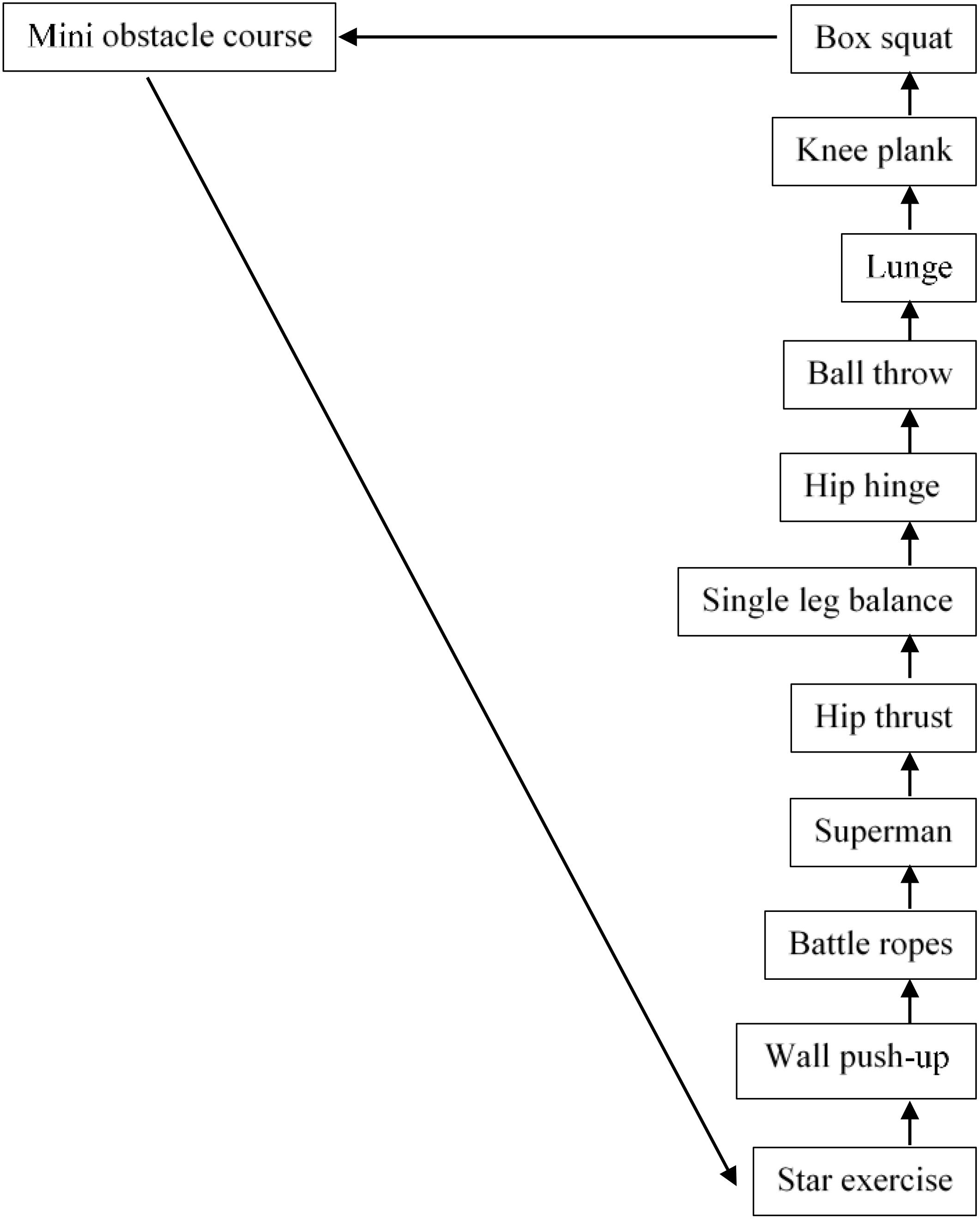
Warm-up began with ∼10 min of low-intensity dancing to participants preferred choice of music. FE session consisted of 12 stations re-adapted from Whitehurst et al. (2005) with 1 min of exercise performed at each individual station before moving in order to the next. Each station was marked with the exercise station name, assigned a station number (between 1 and 12) and marked with a visible Borg CR-10 scale effort sheet. Participants completed the FE circuit 3 times with 3 min breaks between sets (see Figure 3). Participants were instructed to provide high effort throughout the session demonstrating a level of 7–10 on the Borg scale (Borg and Kaijser, 2006).
Protein Supplementation and Dietary Control
All participants recorded their energy intake via 4-day food diaries pre- and post- intervention. Instructions were given how to correctly weigh food, measure liquids, and fill in the diaries. Protein supplements were weighed on scales (Weighstation Electronic Platform Scale, Devon, United Kingdom) and sealed in sachet bags (Tesco Stores, United Kingdom) according to participants’ individual body-mass (g/kg/body-weight). Participants in EP were administered a Vanilla flavored Whey Isolate Protein supplement (MyProtein, Northwich, Cheshire, United Kingdom) (at: 1.5 g/kg/day; 0.5 g/kg/meal) enriched with Leucine (MyProtein, Northwich, United Kingdom) (at: 0.09 g/kg/day; 0.03 g/kg/meal) and mixed with 200 ml of water which was ingested thrice daily (breakfast, lunch, dinner) for 16-weeks. This dosage has previously shown to overcome the anabolic resistance among older adults (Moore et al., 2015). Participants were reminded the protein supplement was to be consumed in addition to normal dietary intake. Adherence was assessed via self-report supplement logs and by counting returned sachets. Compliance with the protein supplement was totalled across the intervention to show a %.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 24 (IBM Corporation, New York, United States). Food diaries were analyzed for energy and protein content through dietary analysis software (Nutritics LTD., Ireland). All data were checked for normality via Shapiro-Wilk test, which were violated for muscle strength and physical function measures. Percentage change and log transformations were unsuccessful at normalizing the data therefore non-parametric methods were utilized. Within-arm time effects (pre- and post- intervention) were analyzed by Wilcoxon-ranked paired tests. Between-arm differences (E vs. EP) were analyzed by Kruskal-Wallis (H) tests. Normality tests showed normal distribution for anthropometry, blood pressure, blood glucose, glycated hemoglobin, and food diary measures therefore parametric testing was utilized. Baseline comparisons were analyzed by students unpaired (t) tests. Independent arms were analyzed using a mixed model ANOVA with two arm levels (E vs. EP) and two time levels (pre- and post- intervention). If between arm effects were present they were followed up using Bonferroni post hoc comparisons. Mauchly’s test of sphericity was used to check homogeneity of variance; where necessary, any violations of the assumption were corrected using the Greenhouse–Geisser adjustment. Data are expressed as mean (±) standard deviation throughout. For descriptive purposes, percentage (±) is calculated from mean values. The alpha level for statistical significance was set at p < 0.05 a priori.
Results
Subjects
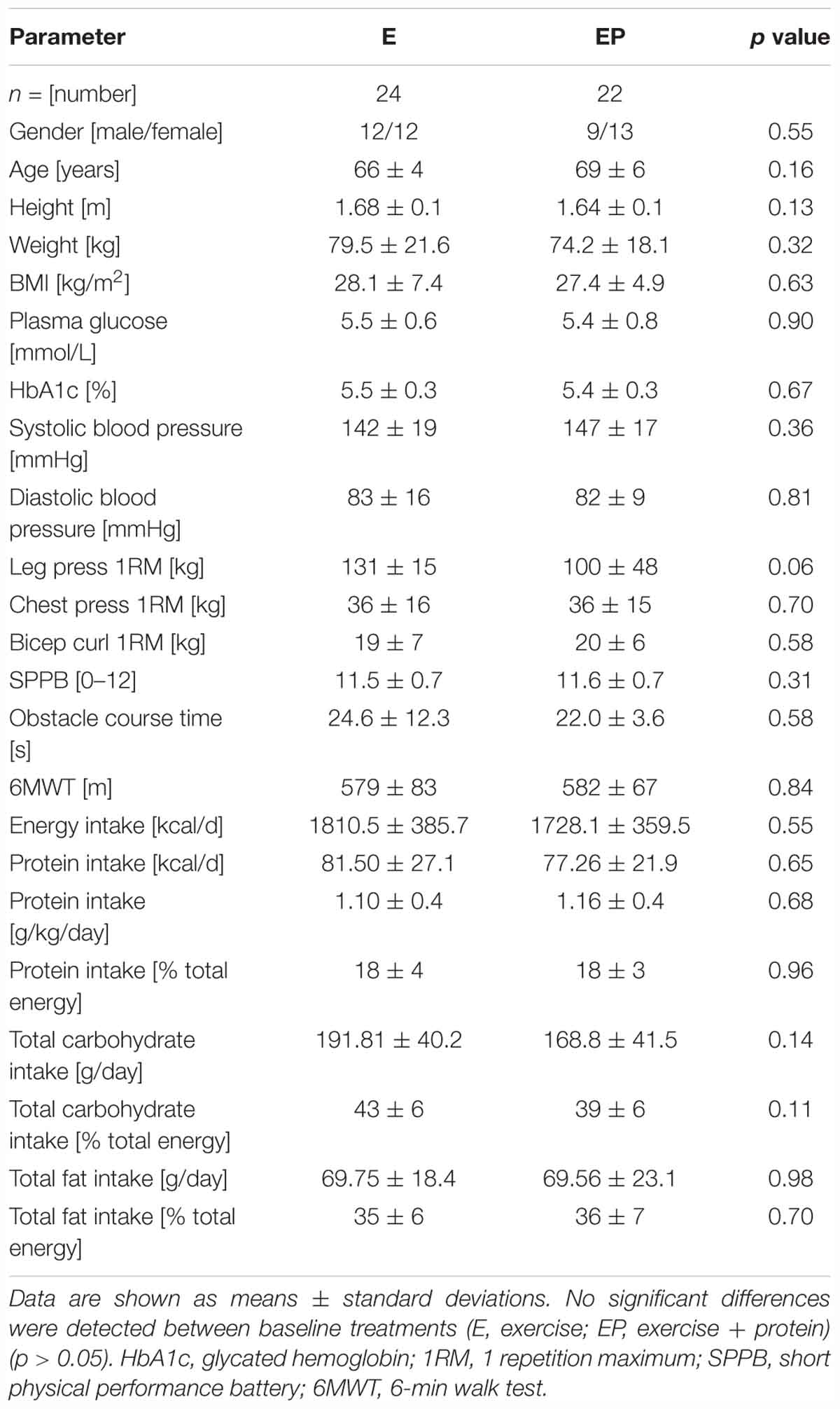
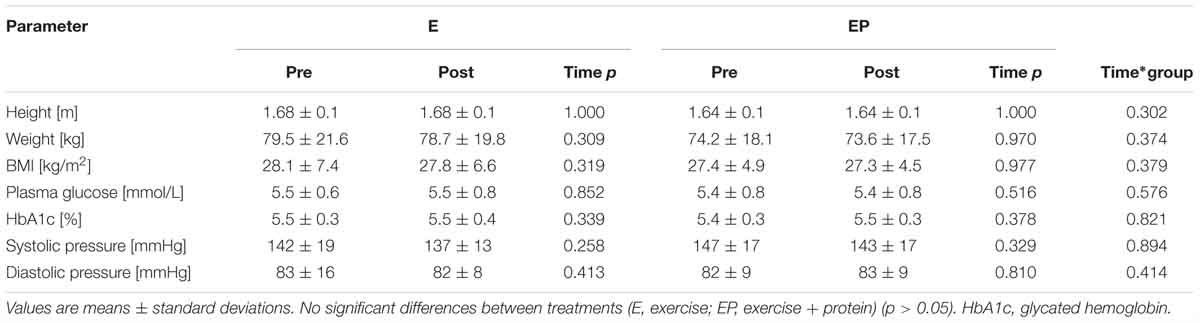
Participants included in the final analysis were distributed similarly in each arm and when split by gender no difference was detected (p = 0.55). Additionally, arms did not differ in any baseline measure (p > 0.05) (see Table 1).
Exercise and Dietary Adherence
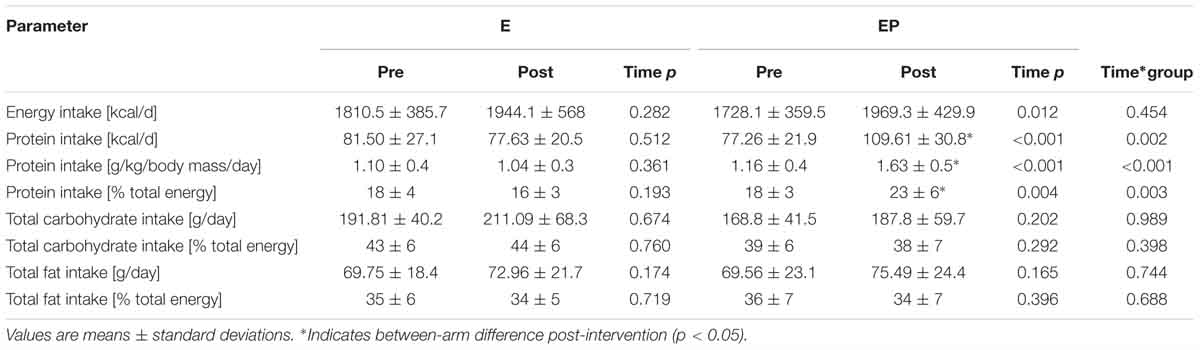
Participants in E and EP attended 77 ± 10% and 78 ± 10% of their prescribed exercise sessions, respectively. A lower degree of compliance was observed with dietary- protein supplementation: EP = 43 ± 14%. As a result of supplementation, protein intake increased from ∼1.2 ± 0.4 at baseline to 1.5 ± 0.7g/kg/day in EP during the intervention period.
Effect of Intervention
Anthropometry, Blood Pressure, and Blood Measures
No within- or between- arm differences were observed for height, weight, BMI, blood pressure, plasma glucose or glycated hemoglobin (p > 0.05) (Table 2). Although minor (non-significant) decreases in systolic blood pressure (E: 142 ± 19 to 137 ± 13, -5 mmHg; EP: 147 ± 17 to 143 ± 17, -4 mmHg) were evident from pre- to post-intervention in E and EP, respectively.
Muscle Strength
Following 16 weeks of progressive resistance and FE 1RM values for leg press (E: 131 ± 58 to 170 ± 51 kg, +30%, p = 0.006; EP: 100 ± 48 to 163 ± 55 kg,+63%, p < 0.001), chest press (E: 36 ± 16 to 58 ± 20 kg, +60%, p < 0.001; EP: 36 ± 15 to 57 ± 21 kg, +58%, p < 0.001) and bicep curl (E: 19 ± 7 to 26 ± 7 kg,+37%, p = 0.002; EP: 20 ± 6 to 26 ± 7 kg, +30%, p = 0.008) significantly increased from pre- to post-intervention in E and EP, respectively. However, no between-arm differences were observed(p > 0.05; Figure 4).
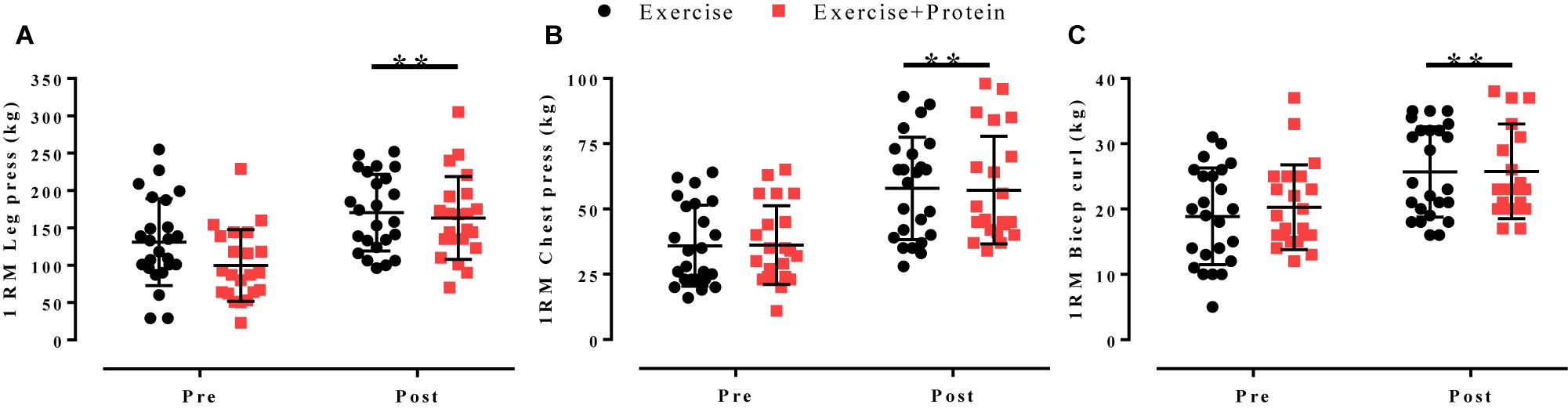
Figure 4. 1RM values for (A) leg press, (B) chest press, and (C) bicep curl in response to independent treatments (E, black; EP, red). Individual data points are shown with horizontal line indicating the mean and error bars representing the standard deviation. Within-arm time effects were evident post-intervention for leg press, chest press, and bicep curl all (∗p < 0.05). No between-arm effects observed (p > 0.05).
Physical Functioning and Aerobic Capacity
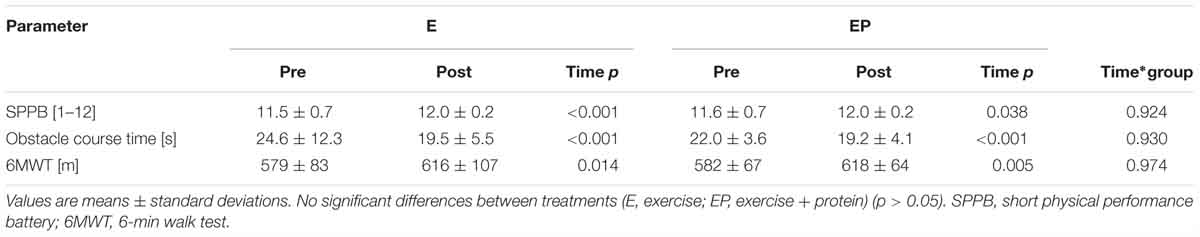
Time to complete the obstacle course (E: 24.6 ± 12.3 to 19.5 ± 5.5 s, +21%, p < 0.001; EP: 22.0 ± 3.6 to 19.2 ± 4.1 s, +13%, p = p < 0.001), performance in the SPPB (E: 11.5 ± 0.7 to 12.0 ± 0.2points, +4%, p = <0.001; EP: 11.6 ± 0.7 to 12.0 ± 0.2 points, +3%, p = 0.038) and aerobic capacity in 6MWT (E: 579 ± 83 to 616 ± 107 m, +6%, p = 0.014; EP: 582 ± 67 to 618 ± 64 m, +6%, p = 0.005) significantly improved from pre- to post-intervention in E and EP, respectively. No between-arm differences were observed (p > 0.05; Table 3).
Physical Activity Levels: Post-trial Follow-Up
Forty-two out of 46 participants completed the 6-months post-trial physical activity survey. No significant differences were observed between arms for any survey question (p > 0.05). Pooled results showed 86% (36/42) were still exercising at least 1/week with 14% (6/42) not exercising. Of those subjects still exercising 25% (9/36) reported to performing aerobic exercise (cardiovascular based, i.e., walking, cycling, jogging, swimming, and yoga), 14% (5/36) reported performing RE (weight-bearing, i.e., lifting weights, body-weight exercises) and 61% (22/36) reported to performing both. The duration of these exercise sessions varied between 45 min (33%) (12/36), 60 min (33%) (12/36), and >60 min (33%) (12/36) (see Figure 5).
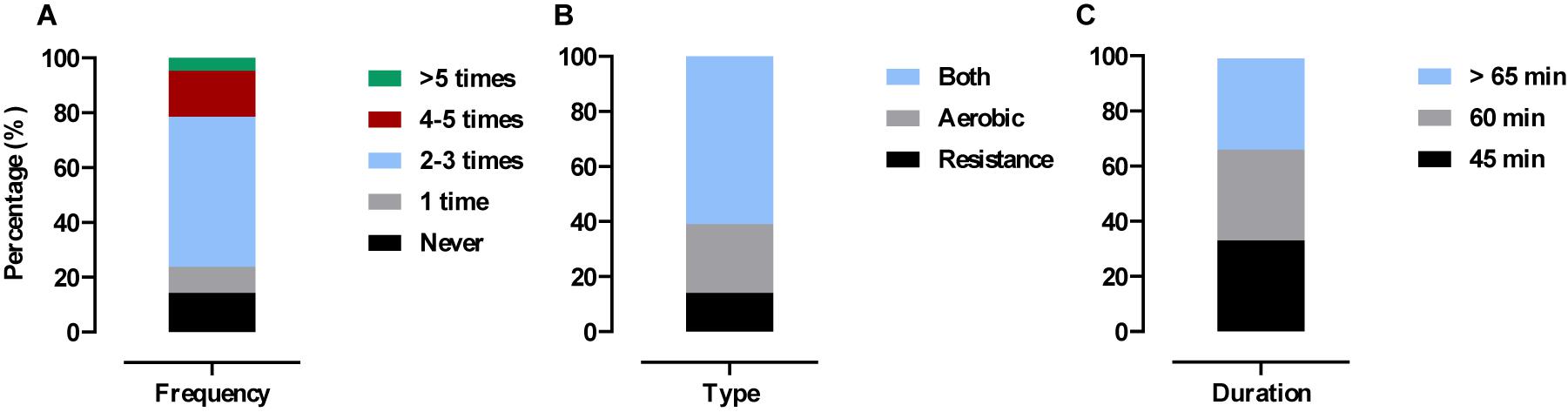
Figure 5. Physical activity levels 6 months post-trial. Values are presented as pooled responses (%) from survey questions. (A) How many times have you exercised in the past 4-weeks? (B) If you exercised in the past 4-weeks what type of exercise was it? (C) If you exercised in the past 4-weeks how long did each session last?
Discussion
We report 16 weeks of progressive resistance and FE (3 times/week) significantly improved muscle strength, physical functioning, and aerobic capacity without influencing blood pressure or glycaemic control in previously untrained older adults. In addition, leucine enriched-whey protein supplementation (3 times/day) did not confer any additional benefit on these outcomes.
We primarily sought to investigate if leucine enriched-whey protein supplementation would augment muscle strength during combined exercise training in older adults. Following recommendations (Paddon-Jones and Rasmussen, 2009; Bauer et al., 2013) we provided ample amounts of dietary-protein (0.5 g/kg/meal) enriched with leucine (0.03 g/kg/meal; >3 g per serving) thrice daily to maximize the muscle protein synthetic response (Moore et al., 2015). Despite substantial increases in muscle strength (Figure 4) and physical/aerobic performance (Table 3) we observed no difference between treatments. This finding is in line with existing data (Kukuljan et al., 2009; Verdijk et al., 2009; Leenders et al., 2013; Stragier et al., 2016; Holwerda et al., 2018) which failed to show a synergistic effect of RE and dietary-protein in strength among community-dwelling older adults. Similar to the above trials, our population of older adults were non-frail i.e., demonstrated high baseline SPPB (11.5 ± 0.7) and 6MWT (583 ± 75) scores. In contrast, benefits have been observed in pre-frail/functionally impaired older adults with lower habitual levels of dietary-protein (Cawood et al., 2012; Tieland et al., 2012). Thus, the relative good health of our population who were habitually consuming adequate amounts of dietary-protein (∼1.2 ± 0.4 g/kg/day) may have masked any effect of supplementation (Table 4). Despite increasing dietary-protein intake from ∼1.2 ± 0.4 to 1.5 ± 0.7 g/kg/day during the present trial, adherence (43 ± 14%) was considerably lower than others (Verdijk et al., 2009; Bell et al., 2017) although similar in those attempting to supplement 3 times/day (Norton et al., 2016). Considering this, coupled with the undesirable verbal feedback relating to supplement taste we recommend future trials use a whole-food approach to increase palatability and adherence as previously described (Haub et al., 2002; Wright et al., 2018).
All strength measures improved from pre- to post- intervention by >30% (Figure 4) adding to the current body of research (Charette et al., 1991; Latham et al., 2004; Nilwik et al., 2013; Bell et al., 2017) demonstrating prolonged resistive exercise modalities (≥12 weeks) are a potent method to combat age-related muscle weakness. Together, these data offer an alternative approach for older adults who may be reluctant to use heavy loads due to health or personal constraints.
The observed increases in strength were accompanied by a favorable shift in physical functioning and aerobic capacity (Table 3). Whilst difficult to distinguish which part of the multifaceted exercise regimen contributed specifically to these improvements, each may have played a complementary role. For instance, RE increases in strength can improve SPPB performance (Tieland et al., 2012, 2015) whereas FE may have predominately enhanced mobility on the obstacle course (Rosendahl et al., 2008) and provided that added stimulus to increase endurance on the 6MWT (Whitehurst et al., 2005). In support, three studies (Arnarson et al., 2013; Kawada et al., 2013; Oesen et al., 2015) found no effect of RE on 6MWT distance, whilst in the present trial and in others (Bell et al., 2017) combining RE with endurance elements of training resulted in improved 6MWT distance. It is difficult to elaborate further as it was not the purpose of the trial to compare these exercise modalities, and associations between neuromuscular attributes and performance indices are not fully understood (Jacob et al., 2018). Nonetheless, the above findings are clinically relevant considering muscle strength declines at an annual rate of ∼2–3% after the fifth decade of life (Goodpaster et al., 2006) and is adversely characterized by reductions in functional capacity (Pavasini et al., 2016), and activities of daily living (Rantanen et al., 2002).
Our multifaceted exercise regimen was designed to optimize muscle strength, physical functioning, aerobic capacity and metabolic health all of which deteriorate with age (Pendergast et al., 1993; Niccoli and Partridge, 2012). Regarding the latter, we failed to observe a change in markers of cardiometabolic health (Table 2) which is in contrast to others (Bell et al., 2017) employing combined strength and high-intensity interval exercise. Thus, we postulate the lack of adaptation in glycaemic control/blood pressure may be due to an insufficient intensity of the exercise regimen employed, or alternatively, due to a lack of reduction in body-weight which may have concealed alterations.
Exercise adherence was high (78 ± 10%) across the 16-week intervention period and was even higher during follow-up (6 months post-intervention) with 86% (36/46) of previously untrained older adults reporting to performing physical activity ≥1 per week (Figure 5). Of those, 61% (22/36) were participating in strength- and cardiovascular- based exercise which aligns with current exercise recommendations for older adults (Nelson et al., 2007). The above figures are promising considering older adults are highlighted as the least active section of society with astonishingly low numbers (<5%) meeting guidelines (Davis et al., 2011; Loustalot et al., 2013; Sun et al., 2013; Van Holle et al., 2014; Dalbo et al., 2015). By continuing to perform concurrent exercise our older adults are inevitably reducing the risk of age-related disease (Vellas et al., 2018) and mortality (García-Hermoso et al., 2018). Even slight increases in RE participation rates (as achieved here) may significantly relieve the economic burden of aging as costs attributed to muscle weakness are estimated at an annual $2,707 per person in the United Kingdom alone (Pinedo-Villanueva et al., 2018).
Limitations
A clear drawback of our trial was the lack of compliance (43 ± 14%) to dietary-protein supplementation. As mentioned, future research should use a whole-food approach as greater adherence rates (>90%) have been evident (Haub et al., 2002; Wright et al., 2018). Another perceived limitation may relate to our population of older adults who were non-frail. By incorporating frail older adults, perhaps greater effects of treatments may have been observed. However, as mounting commentary (Paddon-Jones and Rasmussen, 2009; Bauer et al., 2013) advocate higher dietary-protein intakes (≥1.2 g/kg/day) for older adults it would be unwise to examine the effects in functionally impaired populations alone. For public health mandates to endorse a greater intake of dietary-protein above the current RDA (0.8 g/kg/day); evidence needs to be established across various populations (i.e., in community-dwelling and institutionalized older adults).
Conclusion
To conclude, 16 weeks of progressive resistance and FE (3 times/week) significantly improved muscle strength, physical functioning and aerobic capacity without affecting blood pressure or glycaemic control in previously untrained older adults. In addition, leucine-enriched whey protein supplementation (3 times/day) did not yield further benefits. Nonetheless, 86% (42/46) of older adults were still performing strength- and cardiovascular- based exercise 6-months post-trial demonstrating clinical relevance. Finally, future research should focus on methods to incorporate high dietary-protein intakes (∼1.5 g/kg/day) through naturally occurring food sources in frail and non-frail older adults habitually consuming the RDA of protein. In turn, this may improve adherence rates and enable the efficacy of combined RE with dietary-protein on muscle strength to be evaluated.
Author Contributions
BK, KM, FA, and OK have made substantial contributions to the trial design, data collection and interpretation, and are fully conversant with its content. BK wrote the full manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the participants for their dedication and continuous contribution throughout the trial.
Footnotes
References
Arnarson, A., Gudny Geirsdottir, O., Ramel, A., Briem, K., Jonsson, P. V., and Thorsdottir, I. (2013). Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. Eur. J. Clin. Nutr. 67, 821–826. doi: 10.1038/ejcn.2013.40
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, Crapo, R. O., Casaburi, R., Coates, A. L., Enright, P. L., MacIntyre, N. R., et al. (2002). ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117. doi: 10.1164/rccm.166/1/111
Baechle, T. R., and Earle, R. W. (2008). Essentials of Strength Training and Conditioning. Champaign, IL: Human Kinetics Publishers. doi: 10.1164/ajrccm.166.1.at1102
Bauer, J., Biolo, G., Cederholm, T., Cesari, M., Cruz-Jentoft, A. J., Morley, J. E., et al. (2013). Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J. Am. Med. Dir. Assoc. 14, 542–559. doi: 10.1016/J.JAMDA.2013.05.021
Bell, K. E., Snijders, T., Zulyniak, M., Kumbhare, D., Parise, G., Chabowski, A., et al. (2017). A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One 12:e0181387. doi: 10.1371/journal.pone.0181387
Borg, E., and Kaijser, L. (2006). A comparison between three rating scales for perceived exertion and two different work tests. Scand. J. Med. Sci. Sport 16, 57–69. doi: 10.1111/j.1600-0838.2005.00448.x
Brzycki, M. (1993). Strength testing—predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 64, 88–90. doi: 10.1080/07303084.1993.10606684
Cawood, A. L., Elia, M., and Stratton, R. J. (2012). Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 11, 278–296. doi: 10.1016/J.ARR.2011.12.008
Cermak, N. M., Res, P. T., Groot, L. C., De Saris, W. H. M., and Van Loon, L. J. C. (2012). Protein supplementation augments the adaptive response of skeletal muscle to resistance type exercise training a meta analysis.pdf. Am. J. Clin. Nutr. 96, 1454–1464. doi: 10.3945/ajcn.112.037556.INTRODUCTION
Charette, S. L., McEvoy, L., Pyka, G., Snow-Harter, C., Guido, D., Wiswell, R. A., et al. (1991). Muscle hypertrophy response to resistance training in older women. J. Appl. Physiol. 70, 1912–1916. doi: 10.1152/jappl.1991.70.5.1912
Cimas, M., Ayala, A., Sanz, B., Agulló-Tomás, M. S., Escobar, A., and Forjaz, M. J. (2018). Chronic musculoskeletal pain in European older adults: cross-national and gender differences. Eur. J. Pain 22, 333–345. doi: 10.1002/ejp.1123
da Silva, L. X. N., Teodoro, J. L., Menger, E., Lopez, P., Grazioli, R., Farinha, J., et al. (2018). Repetitions to failure versus not to failure during concurrent training in healthy elderly men: a randomized clinical trial. Exp. Gerontol. 108, 18–27. doi: 10.1016/j.exger.2018.03.017
Dalbo, V. J., Czerepusko, J. B., Tucker, P. S., Kingsley, M. I., Moon, J. R., Young, K., et al. (2015). Not sending the message: a low prevalence of strength-based exercise participation in rural and regional central queensland. Aust. J. Rural Health 23, 295–301. doi: 10.1111/ajr.12207
Davis, A. G., Fox, K. R., Hillsdon, M., Sharp, D. J., Coulson, J. C., and Thompson, J. L. (2011). Objectively measured physical activity in a diverse sample of older urban UK adults. Med. Sci. Sport Exerc 43, 647–654. doi: 10.1249/MSS.0b013e3181f36196
Devries, M. C., McGlory, C., Bolster, D. R., Kamil, A., Rahn, M., Harkness, L., et al. (2018). Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J. Nutr. 148, 1088–1095. doi: 10.1093/jn/nxy091
Faul, F., Erdfelder, E., Lang, A.-G., and Buchner, A. (2007). G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fiatarone, M. A., Marks, E. C., Ryan, N. D., Meredith, C. N., Lipsitz, L. A., and Evans, W. J. (1990). High-intensity strength training in nonagenarians. JAMA 263:3029. doi: 10.1001/jama.1990.03440220053029
Finger, D., Goltz, F. R., Umpierre, D., Meyer, E., Rosa, L. H. T., and Schneider, C. D. (2015). Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sport Med. 45, 245–255. doi: 10.1007/s40279-014-0269-4
Forkan, R., Pumper, B., Smyth, N., Wirkkala, H., Ciol, M. A., and Shumway-Cook, A. (2006). Exercise adherence following physical therapy intervention in older adults with impaired balance. Phys. Ther. 86, 401–410. doi: 10.1093/ptj/86.3.401
Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., et al. (2001). Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 56, M146–M157. doi: 10.1093/gerona/56.3.M146
Gade, J., Pedersen, R. J., and Beck, A. M. (2018). Effect of protein or essential amino acid supplementation during prolonged resistance exercise training in older adults on body composition, muscle strength, and physical performance parameters: a systematic review. Rehabil. Process Outcome 7:117957271876576. doi: 10.1177/1179572718765760
García-Hermoso, A., Cavero-Redondo, I., Ramírez-Vélez, R., Ruiz, J. R., Ortega, F. B., Lee, D.-C., et al. (2018). Muscular strength as a predictor of all-cause mortality in an apparently healthy population: a systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 99, 2100.e5–2113.e5. doi: 10.1016/J.APMR.2018.01.008
Goodpaster, B. H., Park, S. W., Harris, T. B., Kritchevsky, S. B., Nevitt, M., Schwartz, A. V., et al. (2006). The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 61, 1059–1064. doi: 10.1093/gerona/61.10.1059
Guralnik, J. M., Simonsick, E. M., Ferrucci, L., Glynn, R. J., Berkman, L. F., Blazer, D. G., et al. (1994). A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 49, M85–M94. doi: 10.1093/geronj/49.2.M85
Haub, M. D., Wells, A. M., Tarnopolsky, M. A., and Campbell, W. W. (2002). Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 76, 511–517. doi: 10.1093/ajcn/76.3.511
Hawley, J. A. (2008). Specificity of training adaptation: time for a rethink? J. Physiol. 586, 1–2. doi: 10.1113/jphysiol.2007.147397
Holwerda, A. M., Overkamp, M., Paulussen, K. J. M., Smeets, J. S. J., van Kranenburg, J., Backx, E. M. P., et al. (2018). Protein supplementation after exercise and before sleep does not further augment muscle mass and strength gains during resistance exercise training in active older men. J. Nutr. 148, 1723–1732. doi: 10.1093/jn/nxy169
Jacob, M. E., Travison, T. G., Ward, R. E., Latham, N. K., Leveille, S. G., Jette, A. M., et al. (2018). Neuromuscular attributes associated with lower extremity mobility among community-dwelling older adults. J. Gerontol. Ser. A 74, 544–549. doi: 10.1093/gerona/gly102
Kawada, S., Okamoto, Y., Ogasahara, K., Yanagisawa, S., Ohtani, M., and Kobayashi, K. (2013). Resistance exercise combined with essential amino acid supplementation improved walking ability in elderly people. Acta Physiol. Hung. 100, 329–339. doi: 10.1556/APhysiol.100.2013.008
Kukuljan, S., Nowson, C. A., Sanders, K., and Daly, R. M. (2009). Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J. Appl. Physiol. 107, 1864–1873. doi: 10.1152/japplphysiol.00392.2009
Latham, N. K., Bennett, D. A., Stretton, C. M., and Anderson, C. S. (2004). Systematic review of progressive resistance strength training in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 59, M48–M61. doi: 10.1093/gerona/59.1.M48
Leenders, M., Verdijk, L. B., Van Der Hoeven, L., Van Kranenburg, J., Nilwik, R., Wodzig, W. K. W. H., et al. (2013). Protein supplementation during resistance-type exercise training in the elderly. Med. Sci. Sports Exerc. 45, 542–552. doi: 10.1249/MSS.0b013e318272fcdb
Loustalot, F., Carlson, S. A., Kruger, J., Buchner, D. M., and Fulton, J. E. (2013). Muscle-strengthening activities and participation among adults in the United States. Res. Q. Exerc. Sport 84, 30–38. doi: 10.1080/02701367.2013.762289
McLean, R. R., Mangano, K. M., Hannan, M. T., Kiel, D. P., and Sahni, S. (2016). Dietary protein intake is protective against loss of grip strength among older adults in the framingham offspring cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 71, 356–361. doi: 10.1093/gerona/glv184
Moore, D. R., Churchward-Venne, T. A., Witard, O., Breen, L., Burd, N. A., Tipton, K. D., et al. (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 70, 57–62. doi: 10.1093/gerona/glu103
Morton, R. W., Murphy, K. T., McKellar, S. R., Schoenfeld, B. J., Henselmans, M., Helms, E., et al. (2017). A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 52, 376–384. doi: 10.1136/bjsports-2017-097608
Morton, R. W., Oikawa, S. Y., Wavell, C. G., Mazara, N., McGlory, C., Quadrilatero, J., et al. (2016). Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 121, 129–138. doi: 10.1152/japplphysiol.00154.2016
Mustafa, J., Ellison, R. C., Singer, M. R., Bradlee, M. L., Kalesan, B., Holick, M. F., et al. (2018). Dietary protein and preservation of physical functioning among middle-aged and older adults in the framingham offspring study. Am. J. Epidemiol. 187, 1411–1419. doi: 10.1093/aje/kwy014
Nelson, M., Rejeski, W., Blair, S., Duncan, P., Judge, J., King, A., et al. (2007). Physical activity and public health in older adults: recommendation from the american college of sports medicine and the american heart association. Circulation 116, 1094–1105. doi: 10.1161/circulationaha.107.185650
Niccoli, T., and Partridge, L. (2012). Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752. doi: 10.1016/j.cub.2012.07.024
Nilwik, R., Snijders, T., Leenders, M., Groen, B. B. L., van Kranenburg, J., Verdijk, L. B., et al. (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 48, 492–498. doi: 10.1016/J.EXGER.2013.02.012
Norton, C., Toomey, C., McCormack, W. G., Francis, P., Saunders, J., Kerin, E., et al. (2016). Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole-body lean tissue mass in healthy older adults. J. Nutr. 146, 65–69. doi: 10.3945/jn.115.219022
Oesen, S., Halper, B., Hofmann, M., Jandrasits, W., Franzke, B., Strasser, E.-M. M., et al. (2015). Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly - A randomized controlled trial. Exp. Gerontol. 72, 99–108. doi: 10.1016/j.exger.2015.08.013
Paddon-Jones, D., and Rasmussen, B. B. (2009). Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 12, 86–90. doi: 10.1097/MCO.0b013e32831cef8b
Pavasini, R., Guralnik, J., Brown, J. C., di Bari, M., Cesari, M., Landi, F., et al. (2016). Short physical performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 14:215. doi: 10.1186/s12916-016-0763-7
Pendergast, D. R., Fisher, N. M., and Calkins, E. (1993). Cardiovascular, neuromuscular, and metabolic alterations with age leading to frailty. J. Gerontol. 48, 61–67. doi: 10.1093/geronj/48.special_issue.61
Pinedo-Villanueva, R., Westbury, L. D., Syddall, H. E., Sanchez-Santos, M. T., Dennison, E. M., Robinson, S. M., et al. (2018). Health care costs associated with muscle weakness: a UK population-based estimate. Calcif. Tissue Int. 104, 137–144. doi: 10.1007/s00223-018-0478-1
Pollock, R. D., Duggal, N. A., Lazarus, N. R., Lord, J. M., and Harridge, S. D. R. (2018). Cardiorespiratory fitness not sedentary time or physical activity is associated with cardiometabolic risk in active older adults. Scand. J. Med. Sci. Sports 28, 1653–1660. doi: 10.1111/sms.13071
Rantanen, T., Avlund, K., Suominen, H., Schroll, M., Frändin, K., and Pertti, E. (2002). Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin. Exp. Res. 14, 10–15.
Reilly, T., Morris, T., and Whyte, G. (2009). The specificity of training prescription and physiological assessment: a review. J. Sports Sci. 27, 575–589. doi: 10.1080/02640410902729741
Rosendahl, E., Gustafson, Y., Nordin, E., Lundin-Olsson, L., and Nyberg, L. (2008). A randomized controlled trial of fall prevention by a high-intensity functional exercise program for older people living in residential care facilities. Aging Clin. Exp. Res. 20, 67–75. doi: 10.1007/BF03324750
Sherrington, C., Fairhall, N. J., Wallbank, G. K., Tiedemann, A., Michaleff, Z. A., Howard, K., et al. (2019). Exercise for preventing falls in older people living in the community. Coch. Database Syst. Rev. 1:CD012424. doi: 10.1002/14651858.CD012424.pub2
Silva, R. G., da Silva, D. R. P., da Pina, F. L. C., Nascimento, M. A., do Ribeiro, A. S., Cyrino, E. S., et al. (2017). Effect of two different weekly resistance training frequencies on muscle strength and blood pressure in normotensive older women. Rev. Bras. Cineantropometria Desempenho Hum. 19, 118–127. doi: 10.5007/1980-0037.2017v19n1p118
Stec, M. J., Thalacker-Mercer, A., Mayhew, D. L., Kelly, N. A., Tuggle, C. S., Merritt, E. K., et al. (2017). Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp. Gerontol. 99, 98–109. doi: 10.1016/j.exger.2017.09.018
Steele, J., Raubold, K., Kemmler, W., Fisher, J., Gentil, P., and Giessing, J. (2017). The effects of 6 months of progressive high effort resistance training methods upon strength, body composition, function, and wellbeing of elderly adults. Biomed. Res. Int. 2017:2541090. doi: 10.1155/2017/2541090
Stragier, S., Baudry, S., Poortmans, J., Duchateau, J., and Carpentier, A. (2016). Leucine-enriched protein supplementation does not influence neuromuscular adaptations in response to a 6-month strength training programme in older adults. Exp. Gerontol. 82, 58–66. doi: 10.1016/j.exger.2016.06.002
Sun, F., Norman, I. J., and While, A. E. (2013). Physical activity in older people: a systematic review. BMC Publ. Health 13:449. doi: 10.1186/1471-2458-13-449
Thompson, P. D., Arena, R., Riebe, D., and Pescatello, L. S. (2013). ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 12, 215–217. doi: 10.1249/JSR.0b013e31829a68cf
Tieland, M., van der Zwaluw, N., Verdijk, L. B., van de Rest, O., de Groot, L. C. P. G. M., and van Loon, L. J. C. (2012). Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 13, 713–719. doi: 10.1016/j.jamda.2012.05.020
Tieland, M., Verdijk, L. B., de Groot, L. C. P. G. M., and van Loon, L. J. C. (2015). Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. Metab. 25, 27–36. doi: 10.1123/ijsnem.2013-0123
Van Holle, V., Van Cauwenberg, J., Van Dyck, D., Deforche, B., Van de Weghe, N., and De Bourdeaudhuij, I. (2014). Relationship between neighborhood walkability and older adults’ physical activity: results from the Belgian environmental physical activity study in seniors (BEPAS Seniors). Int. J. Behav. Nutr. Phys. Act. 11:110. doi: 10.1186/s12966-014-0110-3
Vellas, B., Fielding, R. A., Bens, C., Bernabei, R., Cawthon, P. M., Cederholm, T., et al. (2018). Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the international conference on frailty and sarcopenia research task force. J. Frail. Aging 7, 2–9. doi: 10.14283/jfa.2017.30
Verdijk, L., Jonkers, R. A., Gleeson, B. G., Beelen, M., Meijer, K., Savelberg, H. H., et al. (2009). Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 89, 608–616. doi: 10.3945/ajcn.2008.26626
Whitehurst, M. A., Johnson, B. L., Parker, C. M., Brown, L. E., and Ford, A. M. (2005). The benefits of a functional exercise circuit for older adults. J. Strength Cond. Res. 19, 647–651.
Wood, T. M., Maddalozzo, G. F., and Harter, R. A. (2002). Accuracy of seven equations for predicting 1-RM performance of apparently healthy, sedentary older adults. Meas. Phys. Educ. Exerc. Sci. 6, 67–94. doi: 10.1207/S15327841MPEE0602_1
Wright, C., Zhou, J., Sayer, R., Kim, J., Campbell, W., Wright, C. S., et al. (2018). Effects of a high-protein diet including whole eggs on muscle composition and indices of cardiometabolic health and systemic inflammation in older adults with overweight or obesity: a randomized controlled trial. Nutrients 10:946. doi: 10.3390/nu10070946
Keywords: aging, muscle weakness, exercise, dietary-protein, leucine
Citation: Kirk B, Mooney K, Amirabdollahian F and Khaiyat O (2019) Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University – Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 10:445. doi: 10.3389/fphys.2019.00445
Received: 04 November 2018; Accepted: 01 April 2019;
Published: 25 April 2019.
Edited by:
Martin Burtscher, University of Innsbruck, AustriaReviewed by:
Susan V. Brooks, University of Michigan, United StatesVandre Casagrande Figueiredo, University of Kentucky, United States
Copyright © 2019 Kirk, Mooney, Amirabdollahian and Khaiyat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Kirk, a2lya2JAaG9wZS5hYy51aw==
 Ben Kirk
Ben Kirk Kate Mooney
Kate Mooney Farzad Amirabdollahian
Farzad Amirabdollahian Omid Khaiyat
Omid Khaiyat