- 1Department of Physiology, Faculty of Medical Health and Sciences, University of Auckland, Auckland, New Zealand
- 2Departments of Molecular Medicine and Pathology, Faculty of Medical Health and Sciences, University of Auckland, Auckland, New Zealand
In human beings the immature brain is highly plastic and depending on the stage of gestation is particularly vulnerable to a range of insults that if sufficiently severe, can result in long-term motor, cognitive and behavioral impairment. With improved neonatal care, the incidence of major motor deficits such as cerebral palsy has declined with prematurity. Unfortunately, however, milder forms of injury characterized by diffuse non-cystic white matter lesions within the periventricular region and surrounding white matter, involving loss of oligodendrocyte progenitors and subsequent axonal hypomyelination as the brain matures have not. Existing therapeutic options for treatment of preterm infants have proved inadequate, partly owing to an incomplete understanding of underlying post-injury cellular and molecular changes that lead to poor neurodevelopmental outcomes. This has reinforced the need to improve our understanding of brain plasticity, explore novel solutions for the development of protective strategies, and identify biomarkers. Compelling evidence exists supporting the involvement of microRNAs (miRNAs), a class of small non-coding RNAs, as important post-transcriptional regulators of gene expression with functions including cell fate specification and plasticity of synaptic connections. Importantly, miRNAs are differentially expressed following brain injury, and can be packaged within exosomes/extracellular vesicles, which play a pivotal role in assuring their intercellular communication and passage across the blood–brain barrier. Indeed, an increasing number of investigations have examined the roles of specific miRNAs following injury and regeneration and it is apparent that this field of research could potentially identify protective therapeutic strategies to ameliorate perinatal brain injury. In this review, we discuss the most recent findings of some important miRNAs in relation to the development of the brain, their dysregulation, functions and regulatory roles following brain injury, and discuss how these can be targeted either as biomarkers of injury or neuroprotective agents.
Introduction
MicroRNAs (miRNAs) are a class of endogenous small single-stranded non-protein coding RNA molecules (20–24 nucleotides), often phylogenetically conserved, which play a critical role in the control of gene expression at the post-transcriptional level. Specifically, miRNAs mainly function post-transcriptionally by binding to the 3′ untranslated region (3′UTR) of target messenger RNAs (mRNA) and induce mRNA degradation or translational repression (Bartel, 2009). In addition to their repressive role there is considerable evidence to support post-transcriptional stimulation of gene expression by miRNAs either in specific situations by direct or indirect mechanisms (Vasudevan, 2012).
Given their abundance in the central nervous system (CNS) and their specific patterns of expression within all of the major cell types during development (Sempere et al., 2004; Cao et al., 2006; Cherubini et al., 2006; Narayan et al., 2015), it is unsurprising that a number of miRNAs have emerged as potential regulators of CNS development and homeostatic function and under pathological conditions of hypoxia-ischemia, as mediators of neuroinflammation and neurodegeneration (Bhalala et al., 2013; Moon et al., 2013). Investigation of the possible relationships between miRNAs and their importance to the developing brain, however, remain in its infancy, since the majority of studies have not biologically validated the effects of miRNAs beyond the predicted mRNA targets. Nevertheless, a growing body of studies have demonstrated a critical role of miRNAs in the maturation of oligodendrocytes and myelin formation including the pathophysiology of hypoxia-ischemia-induced brain injury in the developing brain (Barca-Mayo and Lu, 2012; Fitzpatrick et al., 2015; Galloway and Moore, 2016; Su et al., 2016). In relation to the latter, it is presently unknown whether the roles of specific miRNAs or their profiles differ in response to injury with increasing gestational age. However, it is plausible that differences do indeed exist given their importance developmentally and since the neuropathology of brain injury differs as a function of gestational age. Among term infants the spectrum of injuries is dominated by selective necrosis, accompanied by parasagittal cerebral injury involving the paracentral cerebral cortex and associated white matter and represents a watershed injury in a vascular distribution (Ferriero, 2016; Kinney and Volpe, 2018b). In contrast, preterm infants born between 23 and 32 weeks gestation are at greatest risk of injury to the cerebral white matter. Depending on the severity of the insult, the spectrum of white matter injury in the preterm population can differ markedly. In its most severe form, all cell types are affected including oligodendrocytes, glia and axons resulting in focal cystic necrotic lesions (periventricular leukomalacia) forming within regions of the periventricular white matter adjacent to the lateral ventricular wall, which can extend into the centrum semiovale and the subcortical white matter (Back et al., 2002; Kinney, Volpe, 2018a). Milder forms are typically of a diffuse non-cystic variety and are now the most common type of injury observed in the preterm population. Moreover, the predominant pathology underlying diffuse white matter injury in the preterm infant is loss and subsequent arrested differentiation of pre-myelinating oligodendrocyte progenitors, (Volpe et al., 2011; Buser et al., 2012; Back and Miller, 2014; van Tilborg et al., 2016) which results in reduced brain myelination and potentially could be an avenue for miRNA targeted therapy.
In addition to the aforementioned role of miRNAs in the pathophysiology of perinatal brain injury evidence now suggests CNS cells secrete stable miRNAs into the plasma, which are bound to protein, HDL, or packaged within exosomes/microvesicles following stroke (Rao et al., 2013; Chen et al., 2015; Mondello et al., 2018). As their release is intimately related to genomic changes in the brain, they have immense potential as biomarkers of perinatal brain injury and may lead to early diagnosis, thereby allowing early implementation of treatment. This section will review emerging concepts associated with miRNA control of brain development and discuss their connection to perinatal brain injury impacted by inflammation and hypoxia-ischemia and those, which may serve as potential diagnostic biomarkers of injury and therapeutic targets.
miRNAs in CNS Development
Development of the mammalian CNS involves a series of intricately coordinated events that requires precise spatial and temporal control of gene expression at both the transcriptional and translational levels (Taverna et al., 2014; Gotz et al., 2016). As previously mentioned, the brain has an abundance of miRNAs; many are specific to a given cell lineage or cell type with some being shown to vary dynamically within the brain both prior to and after birth, suggesting a need for different miRNAs throughout development (Lagos-Quintana et al., 2003; Miska et al., 2004; Bak et al., 2008; Smith et al., 2010; Podolska et al., 2011; Ziats and Rennert, 2014; Chen and Qin, 2015). The interplay between miRNAs and their target mRNAs have a critical regulatory role during neural development, from early neurogenesis to synaptogenesis as well as maintenance of neural function (Figure 1) (Davis et al., 2015). miRNAs interact mainly through downregulation of expression of both intrinsic and extrinsic factors and activities of cell-specific signaling mechanisms, and therefore regulate the establishment and maintenance of cell fate specification and differentiation of neural stem cells and neurogenic niches (Shi et al., 2010; Brett et al., 2011; Barca-Mayo and Lu, 2012).
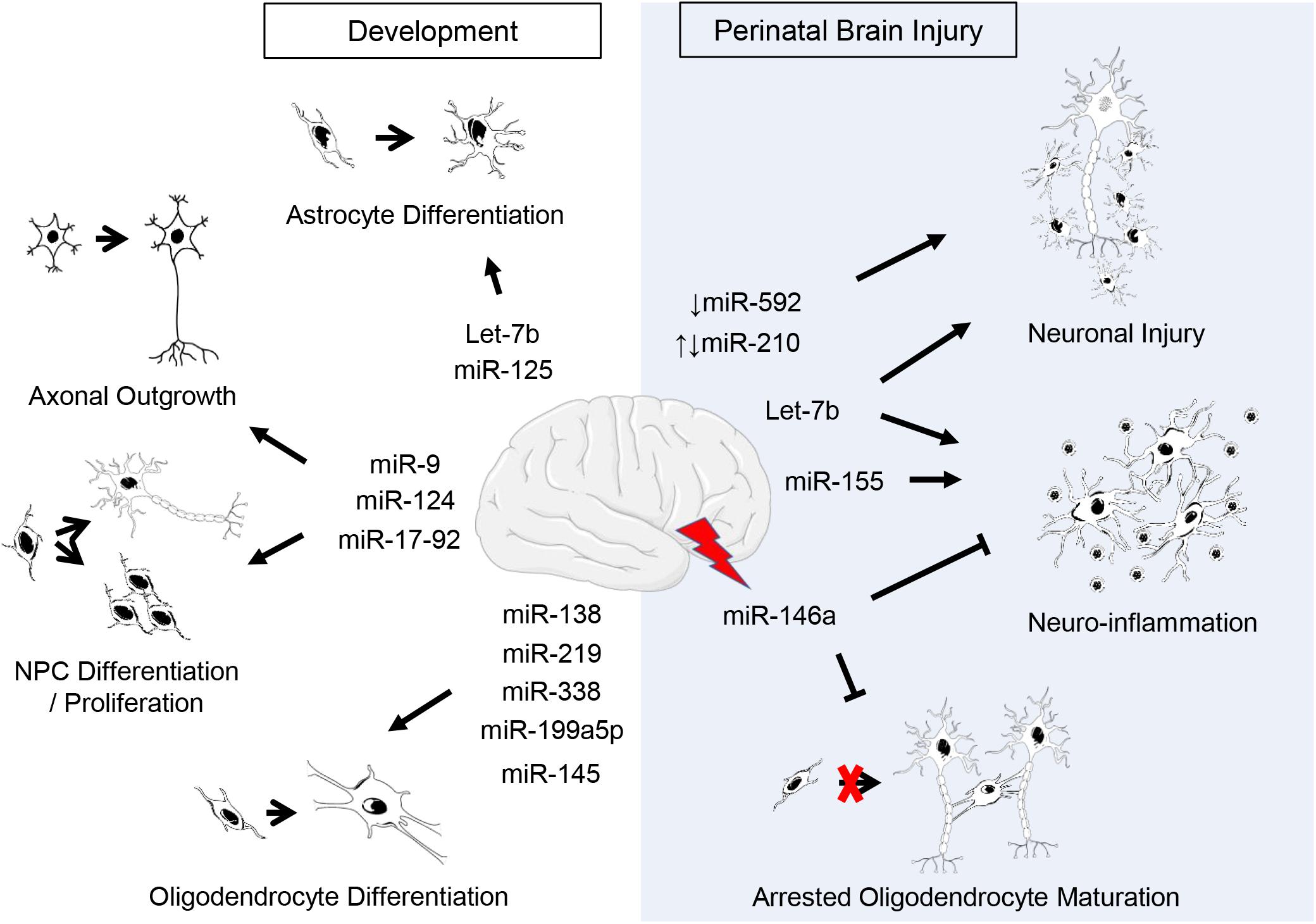
Figure 1. Putative roles of miRNAs in CNS development and perinatal brain injury. Listed are miRNAs covered in this review. Supporting citations are reviewed in text and summarised below. Development: Let-7b and miR-125 are regulators of astrocyte differentiation (Shenoy et al., 2015). miR-17-92 cluster is involved in neural progenitor cell (NPC) proliferation (Bian et al., 2013) and axonal growth (Zhang et al., 2013). miR-124 regulates neurogenesis (Krichevsky et al., 2006; Makeyev et al., 2007) and axonal growth of retinal ganglion cells (He et al., 2018). miR-9 is implicated in the regulation of NPC differentiation and proliferation (Krichevsky et al., 2006; Radhakrishnan and Alwin Prem Anand, 2016) as well as axonal development and neuronal migration (Dajas-Bailador et al., 2012; Otaegi et al., 2011). miR-219 (Dugas et al., 2010; Shin et al., 2009), miR-338 (Zhao et al., 2010), miR-138 (Dugas et al., 2010), miR-199a-5p (Letzen et al., 2010), miR-145 (Letzen et al., 2010) regulate oligodendrocyte (OL) differentiation. Perinatal brain injury: The downregulation of miR-210 is associated with an increase in neuronal apoptosis following hypoxic-ischemic injury (Qiu et al., 2013b) and the upregulation of miR-210 exacerbates cortical injury (Ma et al., 2016; Wang et al., 2017). Post-injury, miR-146a negatively regulates inflammation (Gaudet et al., 2018; Omran et al., 2012) and promotes oligodendrogenesis (Liu et al., 2017). Silencing or inhibition of miR-155 ameliorates inflammation post-injury (Ashhab et al., 2013; Caballero-Garrido et al., 2015; Pena-Philippides et al., 2016; Roitbak, 2018). Let-7b released from neurons and immune cells following injury exacerbates neuronal cell death and induces neuroinflammation (Lehmann et al., 2012; Mueller et al., 2014). The downregulation of miR-592 following hypoxic-ischemic injury induces apoptosis in hippocampal neurons (Sun et al., 2018).
miRNAs in Neuronal Cortical Development
The biological importance of miRNAs in neural development was first demonstrated by conditional knockout experiments of enzymes involved in miRNA biogenesis (Bernstein et al., 2003). The double-stranded RNA (dsRNA) nuclease Dicer is essential to this process (Petri et al., 2014). In mice, targeted ablation of the Dicer1 gene affects brain development including impaired cortical neuron migration, microcephaly, and precursor differentiation in the spinal cord (Davis et al., 2008; De Pietri Tonelli et al., 2008). However, such studies do not readily assign roles for specific miRNAs since a deficiency in Dicer will affect the full complement of miRNAs in the targeted cells. Moreover, knockouts of specific miRNAs are often complicated, since bioinformatics analyses predict hundreds of targets for mammalian miRNAs, and it seems likely that many are indeed true targets (Lewis et al., 2003; Lim et al., 2005).
Despite these drawbacks, valuable information has been deemed regarding numerous miRNAs during brain development through loss-of-function and gain-of-function experiments (Figure 1). Evidence suggests that miRNAs play an important role in cortical development. For example, the miR-17-92 cluster, together with its paralogs miR-106a-363 and miR-106b-25, is required for appropriate development of embryonic fetal cells (Suh et al., 2004; Ventura et al., 2008). It consists of six miRNAs, processed from a common precursor transcript and grouped in four subfamilies, miR-17, miR-18, miR-19, and miR-92 (Ventura et al., 2008; Bian et al., 2013). A role for miR-17-92 in proliferation has been suggested since phosphatase and tensin homolog (PTEN; tumor suppressor gene) is one of its targets (Concepcion et al., 2012). Further, functional role studies have revealed that overexpression of the miR-17-92 cluster in axons of embryonic cortical cells modulates PTEN protein levels and increases axonal growth (Zhang et al., 2013). To confirm additional roles knockout studies of the miR-17-92 cluster and its paralogs have demonstrated an essential role of the miR-17-92 cluster in controlling expansion of neural stem cells and radial glial cells, and transition to intermediate progenitors, which are critical for normal cortical development and function (Bian et al., 2013). Moreover, knockout of miR-17-92 was associated with an upregulation of miR-17-92 target RNAs, PTEN and T-box transcription factor Eomes/Tbr2 (Tbr2; a key regulator of neurogenesis in the SVZ), resulting in an increase in intermediate progenitors and suppression of cortical radial glial cells, respectively (Bian et al., 2013).
miR-124, the most abundant miRNA in the brain, is another well-studied regulator of neurogenesis, whose expression increases with commencement of neural differentiation and peaks in mature neurons (Krichevsky et al., 2006; Makeyev et al., 2007; Visvanathan et al., 2007; Cheng et al., 2009; Maiorano and Mallamaci, 2009; Ponomarev et al., 2011; Sanuki et al., 2011; Åkerblom et al., 2012; Sun et al., 2015). Targets of miR-124 include protein jagged-1 (Jag-1), Sry-type high mobility group box 9 (Sox9; involved in adult neurogenesis) and DLX2 (transcription factor regulating neuronal subtype specification) (Cheng et al., 2009; Liu et al., 2011). Inhibition in vivo of miR-124 blocks neurogenesis resulting in a switch to gliogenesis, specifically inducing formation of ectopic astrocytes in the olfactory bulb derived from the subventricular zone (Åkerblom et al., 2012). Furthermore, overexpression experiments both in vivo and in vitro suggest that miR-124 plays a role in neural fate specification (Smirnova et al., 2005; Krichevsky et al., 2006; Silber et al., 2008; Åkerblom et al., 2012; Xia et al., 2012; Akerblom and Jakobsson, 2014) and most recently promotes axon growth of retinal ganglion cells differentiated from retinal stem cells (He et al., 2018).
Similarly, miR-9, a neuronal specific miRNA, with a prominent role in development, has also been implicated in the regulation of whether neural precursors will adopt a neuronal or glial fate (Krichevsky et al., 2006; Radhakrishnan and Alwin Prem Anand, 2016). miR-9 is highly expressed within the brain, primarily within neural precursors where it controls neural stem cell numbers (Delaloy et al., 2010; Akerblom et al., 2013; Coolen et al., 2013). Overexpression of miR-9 negatively regulates proliferation and accelerates neural differentiation through suppression of the orphan receptor TLX (human homolog of the tailless gene; also known as nuclear receptor subfamily 2, group E member 1 [Nr2e1]) suggesting that TLX and miR-9 participate in a feedback regulatory loop (Zhao et al., 2009). miR-9 is also involved in cortical axonal development via its target, microtubule-associated protein 1b (Map1b) (Dajas-Bailador et al., 2012). Furthermore, neuronal migration and outgrowth is also controlled by miR-9 through its interaction with forkhead transcription factors 1 and 2 (Foxp1 and Foxp2) (Otaegi et al., 2011; Clovis et al., 2012).
miRNAs in Oligodendrocyte Development
Due to the critical roles of miRNAs in neurogenesis, it is unsurprising that miRNAs have also emerged as important regulators of oligodendrocyte development (Figure 1). Microarray analysis of miRNA profiles in normal CNS development and Dicer1 knockout models have identified miR-219 as a crucial regulator of oligodendrocyte differentiation (Shin et al., 2009; Dugas et al., 2010; Zhao et al., 2010). miR-219 is highly expressed in the white matter areas of the brain and expression persists in mature oligodendrocytes (Dugas et al., 2010). Its mechanism of action is via direct repression of expression of its predicted targets, namely platelet-derived growth factor receptor alpha (PDGFRα), SRY-box containing gene 6 (Sox6), forkhead box J3 (FoxJ3), and zinc finger protein 238 (ZFP238), all of which promote oligodendrocyte proliferation and inhibit oligodendrocyte differentiation (Barres et al., 1994; Stolt et al., 2006; Dugas et al., 2010). Transfecting purified oligodendrocytes with miR-219 mimic increases expression levels of early (2′,3′-cyclic nucleotide 3′-phosphodiesterase, CNP; myelin basic protein, MBP) and late (myelin oligodendrocyte glycoprotein, MOG) oligodendrocyte specific differentiation markers (Dugas et al., 2010; Zhao et al., 2010). Furthermore, addition of miR-219 mimic to oligodendrocyte progenitor cells lacking functional Dicer1 expression and which display deficits in myelin gene expression (CNP, MBP, and MOG), markedly enhanced maturation and restored their expression levels to control transfected cells (Dugas et al., 2010; Zhao et al., 2010). Cumulatively, these data indicate that miR-219 is critical for the coordinated transition of oligodendrocyte progenitor cells to oligodendrocytes and subsequent myelin formation and thus may have potential as a therapeutic strategy to promote myelination following injury.
Other important regulators of oligodendrocyte progenitor differentiation are miR-338 and miR-138 (Lau et al., 2008; Dugas et al., 2010). miR-338 is equally as significant as miR-219 in controlling oligodendrogenesis and shares common targets notably Sox6 and Hes Family BHLH Transcription Factor 5 (Hes5); both of which are negative regulators of myelin gene expression (Liu et al., 2006; Stolt et al., 2006; Dugas et al., 2010; Zhao et al., 2010). Furthermore, miR-338 is upregulated in mature oligodendrocytes (Lau et al., 2008) and its overexpression increases oligodendrocyte differentiation (Zhao et al., 2010). However, the role of miR-138 is somewhat incongruous. While miR-138 expression is also elevated in oligodendrocyte precursors its impact on oligodendrocyte development is less significant than miR-219 and miR-338 (Dugas et al., 2010). In contrast to miR-219, oligodendrocyte progenitors induced to differentiate by miR-138 mimic, only express early oligodendrocyte differentiation markers (CNP, MBP) but not late differentiation markers (Dugas et al., 2010). Moreover, miR-138 inhibits Sox4 transcription factor, a repressor of oligodendrocyte maturation (Potzner et al., 2007; Yeh et al., 2013). Together these findings suggest that miR-138 may play a role in extending the period oligodendrocytes are maintained in the early phase of oligodendrocyte differentiation thereby providing a suitable time frame for terminally differentiating oligodendrocytes to myelinate neighboring axons.
Elegant studies by Letzen et al. (2010), using human embryonic stem cells to investigate miRNA expression profiles have revealed unique patterns of expression during the various stages of oligodendrocyte differentiation and maturation. Specifically, four main clusters of miRNA expression were identified encompassing the breadth of the oligodendrocyte lineage scheme (early, mid, and late progenitors and mature oligodendrocytes). Predicted targets of the top differentially expressed genes included myelin-associated genes namely chromosome 11 open reading frame 9 (C11Orf9), myelin gene regulatory factor (MRF), claudin-11 (CLDN11), myelin transcription factor 1-like (MYTL1), myelin-associated oligodendrocyte basic protein (MOBP), myelin protein zero-like 2 (MPZL2), and discoidin domain receptor tyrosine kinase 1 (DDR1). Of interest, the authors showed that within the top 10 differentially expressed miRNAs, spanning early to mid-oligodendrocyte progenitor stages, both miR-199a-5p and miR-145 were strongly biased to C11Orf9, a gene considered to play a critical role in oligodendrocyte maturation and myelin production.
Evidence discussed above, thus highlights the need to define the role of miRNAs in normal neurodevelopment since they may lay the foundations for novel miRNA-based therapies for preterm infants at risk of brain injury.
miRNAs in Astroglial and Microglial Development
Within cells of the neural lineage, information on the function of miRNAs is predominately limited to neuronal and oligodendrocyte differentiation. Only a relatively few studies have been conducted to investigate the role of miRNAs in astrogliogenesis. This is somewhat surprising given astrocytes represent a major glial cell type in the CNS and are powerful homeostatic regulators of brain function (Giaume et al., 2010). Presumably, the difficulty encountered in isolating astrocyte progenitors in vivo has been a major constraint when investigating the functions of astrocyte miRNAs. However, in a recent study of glial progenitors induced to differentiate into astrocytes, deletion of all canonical miRNAs by conditional knockout of Dgcr8 (the RNA binding protein involved in processing of all canonical miRNAs) blocked astrocyte differentiation in vitro (Shenoy et al., 2015). Such results were also in keeping with Dicer-knockout studies of in vivo derived multipotent neural stem cells (Andersson et al., 2010). Furthermore, in the study conducted by Shenoy et al. (2015), let-7 and miR-125, operating through several targets, restored astrocyte differentiation. Additional studies of disruption of both astrogliogenesis and oligodendrogenesis with inhibition of miRNA formation in ventral spinal progenitors from Olig1Cre – mediated Dicer conditional knockout mice provide further support for miRNAs role in gliogenesis (Zheng et al., 2010, 2012). It is also important to note that a recent study has provided unprecedented evidence of miRNA expression profiles of astrocytes isolated by laser capture microdissection from various regions within the human second trimester fetal brain (17–20 weeks gestation) and adult brain (24–76 years) with no discernible pathology (Rao et al., 2016). Regional differences were noted in these studies, as well as lower expression of miRNAs in fetal vs. adult white matter astrocytes and high expression of miRNAs in the fetal germinal matrix, which presumably is of relevance in pathological conditions.
Microglia are another major glial population. Depending on their location within the CNS, microglia can vary in morphology and density and have important functions in immune surveillance, mediating innate immune responses. In recent years there has been an exponential increase in investigations focussing on the function and regulation of microglia by intrinsic and extrinsic factors within the developing and adult brain under both normal and abnormal physiological conditions (Baburamani et al., 2014; Katsumoto et al., 2014; Nayak et al., 2014; Hagberg et al., 2015; Michell-Robinson et al., 2015; Reemst et al., 2016; Li et al., 2017; Tay et al., 2017; Thion et al., 2018). However, to date, Ponomarev et al. (2011, 2013) have performed the only studies thus far on the role of miRNAs in microgliogenesis within the CNS and have demonstrated that miR-124 is highly expressed in normal CNS-resident microglia, but absent in peripheral monocytes and macrophages. As discussed previously (see section “miRNAs in Neuronal Cortical Development”), miR-124 is also highly expressed in other regions of the CNS and is an important regulator of neurogenesis and neuronal differentiation through its regulation of neuronal gene expression.
Ponomarev et al. (2011, 2013) also showed that miR-124 is a key promoter of the quiescent state of microglia. By forced overexpression of miR-124 in macrophages they were able to demonstrate that miR-124 negatively modulates CCAAT/enhancer-binding protein-α (C/EBP-α) transcription factor, and its downstream target PU.1, resulting in their transformation from an activated to a quiescent phenotype (Ponomarev et al., 2011, 2013). Furthermore, knockdown of miR-124 in microglia and macrophages returned both cells into an activated state (Ponomarev et al., 2011). Thus, this supports a role for miR-124 in the maintenance of a resting phenotype through targeting of the CEBPα/PU.1 pathway and possibly is a way to establish an “alternative” activation (M2) phenotype in resident microglia as part of the reparative response to hypoxia-ischemia- or infection-related neuroinflammation (see section “miRNAs and Neuroinflammation”). Finally, it is apparent that there is a need to identify other candidate miRNAs who may participate in developmental regulation of astrocytes and microglia.
Role of miRNAs in Perinatal Brain Injury
An extensive body of literature is now available to suggest dysregulation of miRNA biogenesis and their regulatory role is a common theme associated with the development of neurological injury and disorders from adult experimental models and patients (Dharap et al., 2009; Tan et al., 2009; Liu et al., 2010; Yuan et al., 2010; Bhalala et al., 2013; Eacker et al., 2013; Khanna et al., 2013; Moon et al., 2013; Wang and Yang, 2013; Ouyang et al., 2014). Accordingly, given this and evidence of miRNAs regulatory role during all stages of CNS development, there has been an emerging interest into the implications of miRNAs in perinatal brain injury (Figure 1).
HypoxamiRs
Insults such as impaired oxygen delivery or hypoxia has the potential to elicit expression of a distinct group of miRNAs known as hypoxamiRs, that according to the miRbase database (Griffiths-Jones, 2006; Kozomara and Griffiths-Jones, 2014) are in excess of a 100. Importantly, the specific hypoxamiR signature in response to hypoxia varies according to cell type affected and physiological response (Kulshreshtha et al., 2007; Nallamshetty et al., 2013).
Hypoxic regulation of miR-210, considered to be the master hypoxamiR, was first identified by miRNA microarray over a decade ago (Kulshreshtha et al., 2007) and has been shown to be consistently upregulated under various hypoxic conditions (Huang et al., 2010; Chan et al., 2012). Indeed, multiple studies involving adult models of ischemic stroke have consistently shown that miR-210 induction is a feature of the hypoxic response (Jeyaseelan et al., 2008; Dharap et al., 2009; Qiu et al., 2013b; Liu et al., 2018; Meng et al., 2018). Furthermore, in terms of neurogenesis, studies are contradictory in relation as to whether miR-210 inhibition increases neurogenesis following ischemia (Zeng et al., 2014; Ma et al., 2016; Voloboueva et al., 2017). Such differences presumably relate to timing of miR-210 inhibition, since evidence points to a reduction in proliferation with early post-ischemic inhibition, whereas later it increases neurogenesis (Voloboueva et al., 2017).
Similar controversy exists in relation to the immature brain, as evidence from various neonatal stroke models suggest miR-210 may play either a protective or a detrimental role (Ma et al., 2016). For instance, Qiu et al. (2013a), using a PC12 cell model of oxygen glucose deprivation reported that miR-210 reduced PC12 cell death. The same group demonstrated that in PD (postnatal day; day of birth = postnatal day 0) 7 neonatal rats, miR-210 expression is downregulated in response to hypoxia-ischemia in association with increased brain edema (Qiu et al., 2013b) and that pretreatment with miR-210 mimic significantly reduced edema indicating a possible protective role in response to ischemia.
In contrast, Ma et al. (2016) reported that miR-210 is upregulated following a 2.5 h period of hypoxia-ischemia in PD10 neonatal rats. Furthermore, they demonstrated that miR-210 directly targets the 3′UTR region of the glucocorticoid receptor (GR) in the neonatal rat brain and down regulates GR protein following hypoxia-ischemia resulting in increased susceptibility to injury. In the same study, silencing of miR-210 by intracerebroventricular (ICV) administration of complementary locked nucleic oligonucleotides (miR-210-LNA, miR-210 inhibitor), 4 h after hypoxia-ischemia, significantly ameliorated neuronal injury and infarct size in association with a reduction in brain miR-210 levels. Interestingly, intranasal administration of miR-210-LNA under the same conditions resulted in similar effects. Additional studies by Ma et al. (2017), revealed ICV administration of miR-210 mimic in neonatal rats 48 h prior to hypoxic-ischemic injury compromised blood–brain barrier integrity by suppressing junction proteins, thus resulting in increased susceptibility to brain edema and immunoglobulin G (IgG) parenchyma leakage across the blood–brain barrier.
Finally, in a neonatal rat model of perinatal nicotine-sensitized hypoxic-ischemic brain injury, prior treatment with nicotine was associated with increased miR-210 expression, decreased brain-derived neurotrophic factor/tropomyosin receptor kinase B (BDNF/TRKB) protein expression, and increased susceptibility to hypoxic-ischemic injury (Wang et al., 2017). Moreover, ICV administration of miR-210-LNA 48 h before hypoxia-ischemia significantly decreased brain infarct size in both saline control and nicotine-treated cohorts to levels comparable. To conclude, since a number of verified and putative targets have been identified for miR-210 (Chan and Loscalzo, 2010), it is likely that there are contradictions found with respect to miR-210-specific effects as mentioned above. Such putative roles in relation to perinatal brain injury await corroboration that is more definitive.
miRNAs and Oligodendroglial Response to Hypoxia-Ischemia
Studies highlighting miRNAs as key regulators of oligodendrocyte development may have significant clinical implications with respect to further understanding the pathogenesis of preterm hypoxia-ischemia brain injury, since loss and subsequent arrested differentiation of oligodendrocyte progenitors is a hallmark of injury. Presently, there is a paucity of information with regard to how miRNA expression contributes to critical events of oligodendrogenesis occurring in response to hypoxia-ischemia within the developing brain.
Recently, however, the role of miRNAs in perinatal hypoxia-ischemia has been evaluated in NG2 specific Dicer1 knockout mice (Birch et al., 2014). Loss of Dicer within oligodendrocyte progenitors following hypoxia-ischemia increased both the number of mature oligodendrocytes and MBP expression, which was associated with improved motor co-ordination performance. Furthermore, in the same study, miRNA profiling within lesion sites of wild-type mice, demonstrated delayed but significant increases in miR-138 and miR-338, 7 days following hypoxia-ischemia. These findings are difficult to resolve since Dicer1 knockout would normally result in myelin loss and since miR-138 and miR-338 increases with oligodendrocyte differentiation, which was shown to be impaired with hypoxia-ischemia. The authors, however, proposed that mature miRNAs upregulated in response to hypoxia-ischemia may increase oligodendrocyte progenitor proliferation rate and thus decrease inversely differentiation. Further studies are required to address the roles of these miRNAs in this model of perinatal hypoxia-ischemia.
miRNAs and Neuroinflammation
Inflammatory responses play key roles in the regulation of neurodevelopment, neurodegeneration and injury. Due to their capacity to regulate simultaneously a cascade of different genes, miRNAs are well placed as key regulators of neuroinflammation and their dysfunction is equally recognized as contributing to adverse neuroinflammatory processes (Su et al., 2016). Depending upon the target mRNAs and stimulant involved, miRNAs can exhibit functions that are either pro-inflammatory, anti-inflammatory, and/or mixed immunomodulatory in nature.
The most notable of these miRNAs are miR-155 and miR-146a. While miR-155 has both pro- and anti-inflammatory functions (Duan et al., 2016), it is widely considered to be the most potent pro-inflammatory miRNA (Gaudet et al., 2018), and recognized as a key regulator of microglial-mediated immune responses (Cardoso et al., 2012; Butovsky et al., 2015). In the context of adult cerebral ischemia, there is substantial evidence that silencing or inhibition of miR-155 ameliorates the damaging effects of neuroinflammation (Liu et al., 2010; Caballero-Garrido et al., 2015; Pena-Philippides et al., 2016; Roitbak, 2018). Importantly, miR-146a, a negative regulator of inflammation, is characteristically upregulated in the pathogenesis of various neurological conditions (Gaudet et al., 2018) and considered to play a key role in the regulation of cell survival responses by negative regulation of Toll-like receptor 4 (TLR4) through targeting tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) and interleukin-1 receptor-associated kinase 1 (IRAK1) genes in innate and adaptive immune cells (Taganov et al., 2006; Baltimore et al., 2008; Mann et al., 2017). In addition, miR-146a is a key of regulator of oligodendrogenesis both in the normal (Galloway and Moore, 2016) and ischemic brain (Liu et al., 2017) and a negative-feedback regulator of astrocyte-mediated inflammation (Taganov et al., 2006; Iyer et al., 2012).
While growing evidence has revealed that several miRNAs including miR-155 and miR-146a regulate the extent and timing of TLR responses and innate immune pathways (Taganov et al., 2006; O’Connell et al., 2007; Nahid et al., 2011; O’Neill et al., 2011; Quinn and O’Neill, 2011; Lehmann et al., 2012), little is known about their roles in modulation of neuroinflammation within the immature brain following injury. A study to examine the effect of inflammation on epileptogenesis revealed that miR-146a is upregulated in both a PD11 neonatal rat pilocarpine model of mesial temporal lobe epilepsy (MTLE) and children with MTLE and suggest miR-146a modulates the inflammatory response triggered by interleukin-1β (IL-1β) by inhibiting its expression level thus supporting a neuroprotective role for miR-146a (Omran et al., 2012). Further, studies from the same group and animal model revealed that miR-155 and TNF-alpha (TNF-α) is upregulated in seizure-related acute and chronic stages of MTLE (Ashhab et al., 2013). Similar dysregulation was also observed in children with MTLE, thus supporting a role for miR-155 and TNF-α in the development of seizure susceptibility in the immature brain.
Additional support for miR-146a pro-survival functions is also provided by a recent study conducted in PD1 neonatal rats exposed to hypoxia, in conjunction with BV-2 cells (Zhou et al., 2015). The authors showed that treatment with thymosin β4 (Tβ4), a major actin-sequestering protein, known to reduce inflammation and stimulate remyelination after neurological injury (Morris et al., 2010; Xiong et al., 2012), inhibited microglial activation and was associated with in vitro upregulation of miR-146a expression (Zhou et al., 2015). Interestingly, Tβ4 upregulation of miR-146a has been shown to promote oligodendrocyte differentiation and suppression of TLR pathways, thus adding to its therapeutic implications (Santra et al., 2014). Furthermore, lipopolysaccharide (LPS) in vitro stimulation of newborn cord blood results in upregulation of miR-146a expression in monocytes implicating its involvement in neonatal innate immune responses (Lederhuber et al., 2011). Similarly, studies of miRNA expression profiles from leukocytes isolated from newborn whole cord blood following LPS in vitro stimulation show a total of 85 miRNAs are differentially expressed of which several are proposed to modulate TLR inflammatory pathways (Chen J. et al., 2014). As previously discussed (see section “miRNAs in Astroglial and Microglial Development”), miR-124 is another example of a miRNA that regulates CNS inflammation and is highly expressed in microglia and can reduce CNS inflammation through promotion of microglia quiescence via the C/EBP-α-PU.1 pathway. Consequently, overexpression of miR-124 in microglia can induce a switch to M2 polarization, shown by expression of interleukin-10 (IL-10) and transforming growth factor β (TGF-β) (Ponomarev et al., 2011, 2013). Indeed, miR-124 could potentially become a powerful therapeutic strategy for alleviating brain injury in the perinatal period.
Let-7b, a highly abundant miRNA (Pena et al., 2009) and regulator of gene expression in the CNS, released from injured neurons and immune cells, has been demonstrated to exacerbate CNS injury through activation of TLR7 and induce neurodegeneration through neuronal TLR7 (Lehmann et al., 2012). Furthermore, ICV administration of an antagomir to let-7f, another let-7 family member, has been demonstrated to reduce cortical and striatal infarcts in an adult rat stroke model and be preferentially expressed in microglia within the ischemic boundary zone (Selvamani et al., 2012). Recently Mueller et al. (2014), demonstrated that a synthetic peptide analogous to the mammalian preimplantation factor (PIF) secreted by embryos and which is present in the maternal circulation during pregnancy inhibits let-7 miRNA biogenesis in both murine N2a neuroblastoma cells and RAW 264.7 macrophage cell lines. Using a PD3 neonatal rat hypoxic-ischemic brain injury model these authors then showed that subcutaneous administration of synthetic PIF 3 days after injury, significantly abolished the cortical volume reduction, neuronal loss and microgliosis associated with injury in this model 10 days after injury. Although the neuroprotective mechanism remains unclear these authors provided data to suggest that TLR4 may play an important role in synthetic PIF-induced let-7 repression and that KH-type splicing regulatory protein (KSRP) known to be involved with the biogenesis of the let-7 family of miRNAs and a mediator of mRNA decay (Repetto et al., 2012) may be an interacting cofactor involved with this process. Characterization of the specific signaling pathways activated is required to elucidate the significance of this potential pathway in mediating neuroprotection of the developing brain.
miRNAs as Potential Biomarkers
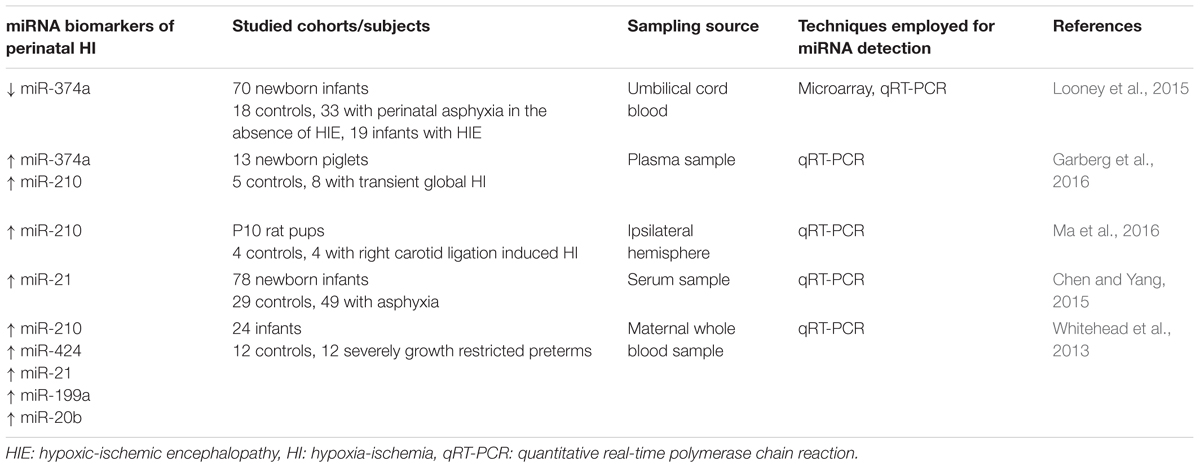
There are numerous other brain-specific miRNAs known to play potentially crucial roles in the pathological processes of adult brain injury, whose roles in relation in perinatal brain injury have yet to be determined. Nevertheless, recent studies have focussed on identification of several miRNAs as potential biomarkers of perinatal brain injury to enable early diagnosis of the severity of injury (Table 1).
Studies, conducted by Looney et al. (2015), involving the analysis of umbilical cord blood miRNA profiles from a cohort of 70 newborn infants [18 controls, 33 with perinatal asphyxia in the absence of hypoxic ischemic encephalopathy (HIE), and 19 infants with HIE analysis], have revealed 70 miRNAs that are differentially expressed with injury. Notably, miR-374a was significantly downregulated in infants with electroencephalographic (EEG) confirmed HIE vs. controls, and further substantiated by quantitative real-time PCR analysis. While no functional mechanism of action and pathways were confirmed, target analysis revealed specific pathways and biological processes associated with neurological injury. Further research from the same group identified several potential downstream targets of this miRNA, namely activin-A receptor type IIb (ACVR2B) (Looney et al., 2017). Despite the lack of confirmation of a significant increase in activin-A levels, as previously demonstrated in biological fluids following perinatal asphyxia and HIE (Florio et al., 2004; Florio et al., 2007; Douglas-Escobar and Weiss, 2012), significantly increased levels of ACVR2B were detected in infants with severe HIE. Of interest, however, is the recent demonstration by Dillenburg et al. (2018) that overexpression of Acvr2b in oligodendroglial lineage cells impairs Acvr2a-regulated oligodendrocyte differentiation and myelin formation, thus supporting the possibility of restoration of Acvr2a-mediated signaling as a strategy to combat perinatal white matter injury.
Aside from the above clinical investigation, a recent study of global hypoxia-ischemia in newborn piglets has also provided evidence to support circulating plasma miR-374a and the hypoxamiR miR-210 as potential biomarkers (Garberg et al., 2016). However, in contrast to Looney et al. (2015), these authors reported a significant upregulation of miR-374a 9.5 h after hypoxia-ischemia and noted that correlations were found between miR-374a and arterial pH, base excess and lactate levels over the study period. Since miR-374a is directly regulated by lactate dehydrogenase A with hypoxia (Wang et al., 2015), the authors concluded that miR-374a might play a role in metabolic adaptive responses to hypoxia-ischemia. Nevertheless, the increase in miR-210 is in congruence with previous studies under a hypoxic environment (Huang et al., 2010; Chan et al., 2012) and those observed by Ma et al. (2016) following hypoxia-ischemia in PD10 neonatal rats.
Other candidate miRNAs have been investigated, namely let7b, miR-29b, miR-124, miR-155, and miR-21 (Ponnusamy et al., 2016). Quantification of these miRNAs in dried blood spots, EDTA-blood, plasma and urine collected from a small cohort of newborns, failed to demonstrate significant differences with injury. However, miR-21, which is expressed in astrocytes, and been shown in adult plasma to be a potential early stage marker of acute cerebral infarction (Zhou and Zhang, 2014), was found to be elevated in serum of 49 neonates with HIE, thus providing support also for an early diagnosis biomarker of neonatal HIE (Chen and Yang, 2015).
Additional studies that warrant mention are preliminary studies conducted by Whitehead et al. (2013). An analysis was made of maternal whole blood expression levels of six miRNAs known to be associated with hypoxia in which fetuses had either experienced acute hypoxia during labor or chronic hypoxia associated with fetal growth retardation. Compared to gestational matched controls there was an upregulation of miR-210, miR-424, miR-21, miR-199a, and miR-20b. Furthermore, correlation with Doppler velocimetry assessments of hypoxia, confirmed the increase was associated with increased severity of hypoxia. The changes observed in miR-210 agree well with that of Ma et al. (2016) and those conducted in the piglet (Garberg et al., 2016) thus supporting the use as a maternally based biomarker of fetal hypoxia.
A caveat: while the field of miRNA research is constantly expanding one must appreciate that the endogenous source of miRNAs within available body fluids including, plasma, serum, urine and saliva can be from a diverse array of peripheral tissue cellular types including those of the brain, thus decreasing in essence the reliability of results. The other point to note is the problem of cell specification of miRNAs; the reality for many miRNAs as biomarkers in body fluids is that they may not be expressed exclusively within one particular cell type. In the last few years, however, since the identification of exosomes [cell-derived vesicles; typically ∼40–100 nm in diameter (Raposo and Stoorvogel, 2013)], as a carrier of protein, lipids, mRNAs and miRNAs, with an important role in cell–cell-communication, there has been intense interest into whether brain-derived exosomes could serve better as biomarkers in the clinical diagnosis and management of brain injury (Valadi et al., 2007; Ludwig and Giebel, 2012; Patz et al., 2013; Taylor and Gercel-Taylor, 2014; Werner and Stevens, 2015). Additionally, unlike free circulating miRNAs, miRNAs are inherently enriched and stable within exosomes (Cheng et al., 2014). In the adult, increased levels of exosomes are released from cells following stroke and traumatic brain injury (Patz et al., 2013; Chiva-Blanch et al., 2016). Based on current information, it is apparent that brain-derived exosomes can traverse across the blood–brain barrier following injury. Their presence in the peripheral circulation places them in an ideal position to provide an informative platform for real-time assessment of newborns who have sustained brain injury or those who are at risk of adverse outcomes and to spearhead therapeutic discovery. However, such enthusiasm must be tempered by the harsh reality that proportionally brain-derived exosomes may represent only a small population of circulating exosomes whereas the contribution from peripheral sources may be relatively high in comparison. Although still in its infancy, platforms that employ microscale structures (e.g., microfluidics or acoustofluidics) (Contreras-Naranjo et al., 2017; Guo et al., 2018; Hisey et al., 2018; Li et al., 2018; Wu et al., 2018) and high-throughput phenotypic and functional analyses (e.g., advanced imaging flow cytometry) (Mastoridis et al., 2018) could circumvent this problem. If advanced to such a degree as to provide a rapid and effective means to selectively sort and detect brain-derived exosomes at a nanoscale level they would be of significant value for clinical evaluation.
In the adult, only a few clinical studies have been performed to assess the potential of exosomal-derived miRNAs as biomarkers of acute brain injury. Studies by Chen et al. (2017) demonstrated there was an association between increased circulating levels of exosomal miR-223 and acute ischemic stroke occurrence, stroke severity, and short-term outcomes. Furthermore, studies conducted by Ji et al. (2016) showed that serum exosomal miR-9 and miR-124 levels were positively associated with adverse scores of acute stroke injury, infarct volumes, and serum concentrations of the pro-inflammatory cytokine, interleukin-6 (IL-6). Currently, however, little is known of the usefulness of exosomal-derived miRNAs in the diagnosis of perinatal brain injury. Nevertheless, in a recent study conducted to investigate whether exosomal protein biomarkers would be valuable in the diagnosis of brain injury and assessment of the effectiveness of hypothermia, it was shown that neutral or decreasing serum neuronally derived exosomal synaptopodin protein levels occurred in neonates with abnormal neuroimaging scores (Goetzl et al., 2018).
There is also a growing interest as to the regenerative utility of exosomal-derived contents with and without loading with therapeutics (Doeppner et al., 2015; Luarte et al., 2016; Xiong et al., 2017; Kim et al., 2018). Several studies have also documented the neuron healing and protective abilities of stem cell derived exosomes (Lee et al., 2013; Zhang et al., 2015b; Long et al., 2017; Willis et al., 2017). Importantly, studies recently reported highlight the regenerative potential of mesenchymal stem cell (MSC)-derived extracellular vesicles in a preterm fetal sheep model of hypoxia-ischemia (Ophelders et al., 2016). In these studies, systemic administration to the fetus of MSC-extracellular vesicles resulted in improved brain activity namely a reduction in duration and number of seizures. Finally, as discussed later in Section “In vivo Evidence,” it warrants mentioning that because exosomes can potentially act as a therapeutic delivery system they hold great promise in revolutionizing the way we can effectively treat perinatal brain injury.
miRNAs as Potential Therapeutic Targets
In alliance with the discovery of miRNAs as functional regulators of cell development, miRNAs have also been shown to orchestrate a variety of critical signaling pathways involved in injury progression and recovery (Shi et al., 2010; Gaudet et al., 2017). Given the recent demonstration that modulation of miRNA expression occurs following a hypoxic-ischemic insult in the developing brain, several therapeutic targets have emerged (Figure 2).
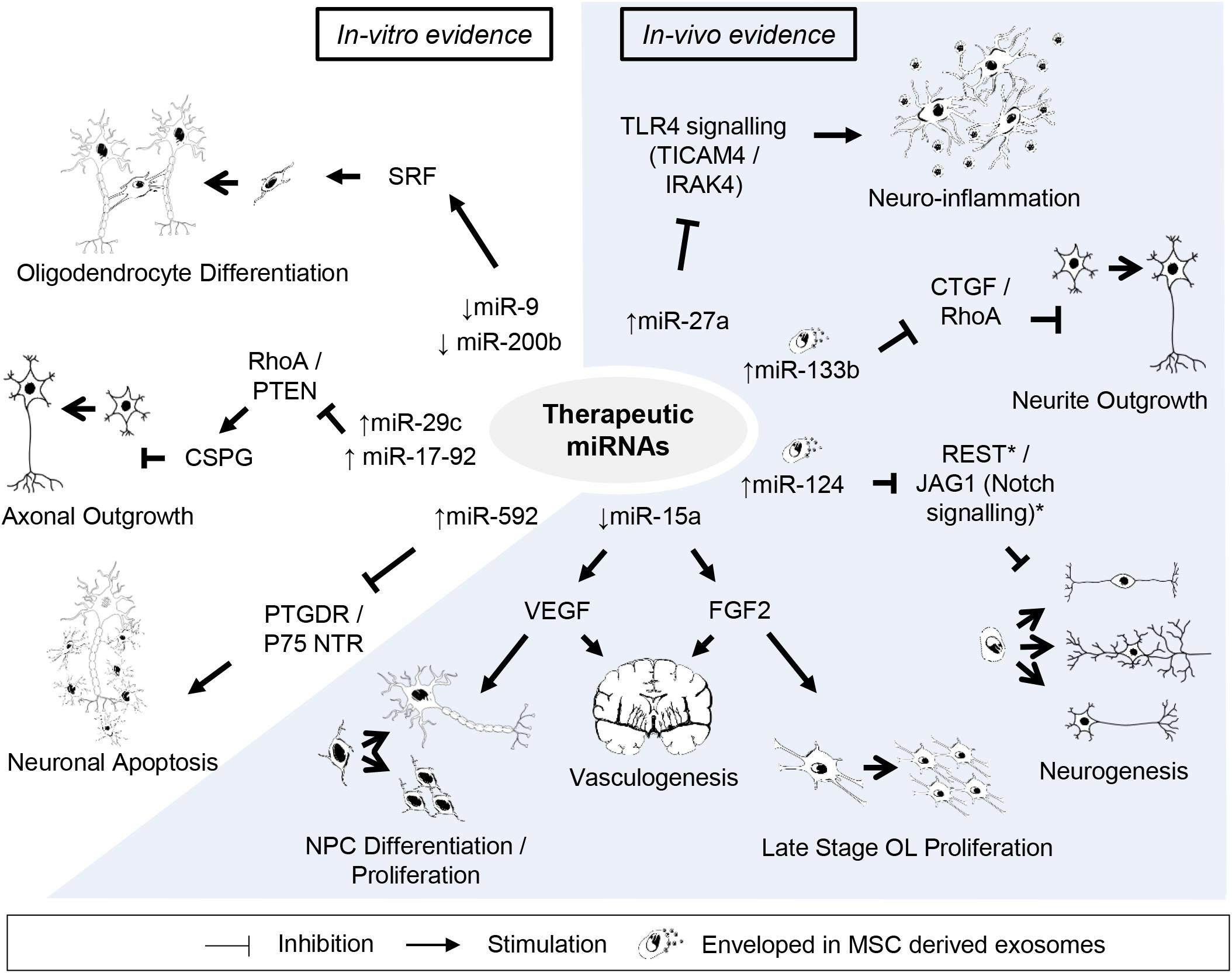
Figure 2. Potential candidates of miRNA-based therapy against perinatal hypoxic-ischemic brain injury. Listed are miRNAs and their downstream targets supporting potentially beneficial outcomes after perinatal hypoxic-ischemic brain injury. Supporting citations are reviewed in text and summarised below. In-vitro evidence: Downregulation of miR-9 and miR-200b mediates serum response factor (SRF) induced oligodendrocyte progenitor cell (OPC) differentiation (Buller et al., 2012). miR-29c (Yi Zhang et al., 2015) and miR-17-92 cluster (Zhang et al., 2013) attenuates the inhibitory effect of chondroitin sulphate proteoglycans (CSPG) on axonal regrowth by stimulating ras homolog family member A (RhoA) and phosphate and tensin homolog (PTEN) protein levels. miR-592 attenuates the activation of pro-apoptotic signalling and neuronal death by targeting the prostaglandin D2 receptor (PTGDR) and neurotrophin receptor (NTR) p75 (Irmady et al., 2014; Sun et al., 2018). In-vivo evidence: miR-27a reduces TLR4-mediated inflammation (Li et al., 2015). Downregulation of miR-15a expression promote vasculogenesis by stimulating neurotrophic factors, fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF) (Yin et al., 2012), which can further support late stage oligodendrocyte (OL) proliferation/migration (Shindo et al., 2016) and neural progenitor cell (NPC) differentiation/proliferation (Teng et al., 2008), respectively. Mesenchymal stem cell (MSC) derived exosomal transfer of miR-133b enhanced neurite outgrowth by inhibiting connective tissue growth factor (CTGF) and RhoA expression (Xin et al., 2012, 2013). Exosome mediated neuronal delivery of miR-124 induces neurogenesis (Yang et al., 2017) speculatively via Usp14-dependent REST degradation (Doeppner et al., 2013) and inhibition of the JAG/Notch signalling pathway (Liu et al., 2011).
In vitro Evidence
In vitro studies have provided insights into the therapeutic potential of miRNAs that regulate reparative processes following a hypoxic-ischemic insult. For example, stroke-induced downregulation of miR-9 and miR-200b expression in the ischemic white matter region mediated serum response factor (SRF) induced differentiation of oligodendrocyte precursor cells (OPCs) into oligodendrocytes (Buller et al., 2012). Accordingly, in vitro overexpression of miR-9 and miR-200b suppressed SRF expression and inhibited OPC differentiation (Buller et al., 2012). Upon validation in vivo, the inhibition of miR-9 and miR-200b following injury may indicate a potential therapeutic strategy in the future given that myelination disturbances in the cerebral white matter represent a hallmark of perinatal brain injury (Pandit et al., 2013; Back and Miller, 2014).
Chondroitin sulfate proteoglycans (CSPGs) are well-characterized inhibitory extracellular matrix molecules expressed by reactive astrocytes, endothelial and oligodendrocyte progenitor cells that inhibit axonal regeneration after injury and are associated with adverse neurological outcome in preterm infants (McKeon et al., 1999; Jones et al., 2002, 2003; Chow et al., 2005). In vitro overexpression of miR29c and miR-17-92 cluster in embryonic cortical neurons has been shown to attenuate the inhibitory effect of CSPG by stimulating intrinsic axonal signals, suppressing Ras homolog gene family, member A (RhoA) and phosphate and tensin homolog (PTEN) protein levels, thereby promoting axonal outgrowth (Park et al., 2008; Zhang et al., 2013, 2015a). Thus, the potential loss or impaired axonal growth observed in focal necrotic white matter injury in the preterm brain could be feasibly targeted (Riddle et al., 2012).
miR-592 was originally suggested as a possible target for promoting cell apoptosis in various cancers (Liu M. et al., 2015; Liu Z. et al., 2015; Fu et al., 2016). Unsurprisingly, recent studies have also supported its regulatory role of cell death following cerebral ischemic injury (Irmady et al., 2014; Sun et al., 2018). In two studies carried out by Irmady et al. (2014) and Sun et al. (2018), both authors observed reduced expression of miR-592 following cerebral ischemic injury in the hippocampus of neonatal and juvenile mice, respectively. Concordantly, overexpression of miR-592 in cultured hippocampal neurons attenuated the activation of pro-apoptotic signaling and cell death (Irmady et al., 2014; Sun et al., 2018). The mechanism underlying this protective mechanism speculates the multi-functional role of miR-592. Sun et al. (2018) demonstrated that miR-592 affords neuroprotection by selectively targeting prostaglandin D2 receptor (PTGDR) and inhibiting prostaglandin D2 (PGD2)-DP signaling, an inflammatory pathway involving the release of glutamate (Weaver-Mikaere et al., 2013). Irmady et al. (2014), on the other hand revealed that vector mediated transfection of miR-592 in embryonic hippocampal neurons attenuated the level of neurotrophin receptor (NTR) p75 induced by ischemic injury and subsequent apoptotic cell death. The NTR p75 is a member of the TNF receptor superfamily closely implicated with neuronal apoptosis following experimental perinatal brain injury (Volosin et al., 2006; Griesmaier et al., 2010). Given the prospective dual anti-apoptotic mechanism of miR-592, results of future in vivo studies are eagerly awaited.
In vivo Evidence
miR-27 is a potential regulator of cortical neuronal apoptosis whose expression in embryonic mouse cerebral cortices is attenuated in response to maternal hypoxia (Chen Q. et al., 2014). Furthermore, neuron-specific over-expression of miR-27b in the mouse cortex increased resistance to hypoxia induced apoptosis by inhibiting apoptotic protease-activating factor 1 (Apaf-1) (Chen Q. et al., 2014). Similar observations have been reported in rat primary embryonic hippocampal neuron cultures (Cai et al., 2016) and further potential targets and mechanisms of the miR-27 family have been alluded. For example, miR-27a, directly modulates components of the TLR4 signaling cascade, including TIR domain-containing adaptor molecule-2 (TICAM2) and interleukin-1 receptor-associated kinase 4 (IRAK4), cytoplasmic proteins that link TLR4 and recruit to adaptor protein MyD88 following TLR4 activation, respectively, and coordinates gene transcription and inflammation (Li et al., 2015; Lv et al., 2017). Prophylaxis treatment with miR-27a mimics in an ischemic reperfusion model results in reduced mRNA and protein expression of TICAM2 accompanied by attenuation of TLR4 activation and pro-inflammatory cytokine production, while pretreatment with miR-27a inhibitory oligonucleotides show opposite effects (Li et al., 2015). Comparable anti-inflammatory effects of miR-27a have also been observed in cultured neonatal microglial cells which were achieved by targeting IRAK4 and TLR4 (Lv et al., 2017). Speculatively, miR-27a can target multiple genes and regulate the TLR4 signaling pathway to prevent an excessive inflammatory response to injury. Indeed, various animal models of perinatal brain injury have shown that TLR4 activation and the ensuing inflammatory response can result in cell death and a pattern of injury similar to that seen in human infants, including hypomyelination, glia activation and disruption of thalamocortical function (Dean et al., 2011; Kannan et al., 2011; Dhillon et al., 2015). Therefore, the anti-apoptotic and anti-inflammatory effects of miR-27a/b may prove to be a potential therapeutic target in the future.
Cerebral angiogenesis is a critical reparative process of the microvasculature following hypoxic-ischemic injury, involving cellular cross-talk through neurotropic factors, improving regional blood supply, and facilitating the migration of neurons toward damaged regions (Ohab et al., 2006; Yin et al., 2015). Modulating this reparative process holds promise since the perinatal brain has the greatest potential for repair and recovery (Dzietko et al., 2013). miR-15a in vascular endothelial cells has emerged as a key regulator of angiogenesis, such that downregulation of miR-15a promotes vasculogenesis by increasing neurotrophic factors, including fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF) (Yin et al., 2012). Critically, VEGF released by angiogenic endothelial cells can promote proliferation and differentiation of neural progenitor cells via vascular endothelial growth factor receptor 2 (VEGFR2) (Teng et al., 2008). FGF2 is also an important growth factor involved in neurogenesis and gliogenesis during embryonic and postnatal development (Vaccarino et al., 1999). Crosstalk between cerebral endothelium and oligodendrocytes can promote proliferation and migration of late-stage OPCs through FGF2 (Shindo et al., 2016). Given that delayed treatment with VEGF and FGF2 have proven to be neuroprotective in perinatal models of brain injury (Monfils et al., 2006; Dzietko et al., 2013), miR-15a may be an attractive therapeutic target in the tertiary phase of injury (Fleiss and Gressens, 2012). In fact, it may pose advantages given its ability to target multiple genes in addition to delivering a synergistic effect.
MSCs have been extensively applied in both experimental and clinical settings of CNS diseases owing to their immunomodulatory, regenerative and reparative properties including stroke (Koh et al., 2008; Steinberg et al., 2016), multiple sclerosis (Zhang et al., 2005; Gerdoni et al., 2007; Uccelli et al., 2011) and perinatal brain injury (van Velthoven et al., 2010; Jellema et al., 2013; Drommelschmidt et al., 2017). Currently, it is proposed that MSCs exert their therapeutic potency at least in part through a paracrine mechanism involving the release of extracellular vesicles, which based on their size and intracellular origin include microvesicles (∼100–1000 nm in diameter) and exosomes (∼40–100 nm in diameter) (Hass and Otte, 2012; Mokarizadeh et al., 2012; Xin et al., 2012; Lee et al., 2013; Koniusz et al., 2016). Indeed, MSCs are prolific producers of extracellular vesicles; a feature that is maintained with immortalization of cells to generate permanent cell lines, making them an ideal option for biological tissue replacement regeneration (Yeo et al., 2013).
MSC-derived extracellular vesicles are enriched with a variety of proteins and different RNA species (mainly mRNA and miRNA) as well as trophic factors whose functions are linked to MSCs biological effects. Importantly, evidence now suggests that specific miRNAs are necessary to mediate MSC-derived extracellular vesicles neuroprotective effect (Xin et al., 2012). While miRNAs encapsulated within MSC derived microvesicles are predominantly in their precursor form (pre-miRNAs) (Chen et al., 2009), studies have demonstrated the presence and biological functional roles of exosomal mature miRNAs (Koh et al., 2010; Katakowski et al., 2013; Ono et al., 2014). The transfer of miR-133b from exosomal MSCs directly enhanced neurite outgrowth and functional recovery in adult stroke models (Xin et al., 2012, 2013). Given the putative occurrence of impaired neurite outgrowth in perinatal brain injury (Robinson et al., 2006; Dean et al., 2013), exosomal miR-133b may be important for brain connectivity and function. In an elegant series of studies conducted by Xin et al. (2012), miR-133b was substantially downregulated in the ischemic rat brain and increased following MSC intravenous administration. Connective tissue growth factor (CTGF) and RhoA are both inhibitors of neurite growth and are selective targets of miR-133b (Xin et al., 2014). Critically, administration of MSC-derived exosomes enriched with miR-133b reduced CTGF and RhoA expression and exhibited enhanced axonal plasticity, neurite remodeling and functional recovery compared to naturally occurring MSC-derived exosomes (Xin et al., 2013). These changes were confirmed in primary cultured neurons and astrocytes (Xin et al., 2013). Transfer of miR-133b enriched MSC derived exosomes in cultured neurons, inhibited RhoA expression and stimulated neurite outgrowth, while the transfer in astrocytes, downregulated CTGF expression, a known inhibitor of axonal growth and contributor to glial scar formation in human cerebral infarction (Schwab et al., 2000; Xin et al., 2013).
In a recent conducted study by Yang et al. (2017), rabies virus glycoprotein modified exosomes were employed to achieve neuron-specific delivery of miR-124 across the blood–brain barrier. Previously, invasive cerebral administration of miR-124, a regulator of neurogenesis (Makeyev et al., 2007; Cheng et al., 2009; Åkerblom et al., 2012), was reported to reduce infarct area and improve neuronal survival against ischemic injury in mice (Liu et al., 2011; Doeppner et al., 2013). In the study conducted by Yang et al. (2017), rabies virus glycoprotein exosomes effectively carried miR-124 to neurons of the ischemic region and supported neuronal identity of cortical neural progenitors and reduced ischemic cortical injury by robust neurogenesis. Thus the above evidence supports the therapeutic potential to ameliorate neuronal injury by exploiting the neurodevelopmental function of miRNAs.
Additionally, in concordance with Xin et al. (2012, 2013), MSC derived exosomes provide a therapeutically viable delivery of gene drugs to the brain and possibly specific cells across the blood–brain barrier. Since MSCs produce an abundant source of extracellular vesicles that contain a selection of miRNAs with the potential to elicit neuroprotective biological processes in response to injury, including the ability to modulate the action of neighboring cells, it seems worthwhile to investigate whether MSC-extracellular vesicles would be a promising therapy to promote neurological functional recovery in the developing brain following injury. Indeed, recent in vivo investigations using animal models of perinatal and neonatal brain injury support their application (Drommelschmidt et al., 2017); however, further investigations are required to characterize what specific miRNA profiles potentially contribute to protection.
Finally, it is pertinent to mention that exosomes/extracellular vesicles, viewed as potent vehicles by which to deliver potentially therapeutic miRNAs to the brain, can equally participate in the pathophysiological processes of blood-CSF-brain-communication. Recent studies undertaken in both an in vivo, in vitro, and ex vivo mouse model of endotoxemia have shown that miRNA-containing extracellular vesicles originating from the choroid plexus epithelium can enter brain parenchymal cells and via astrocytic and microglial processes induce miRNA target repression and inflammatory gene expression (Balusu et al., 2016). The transfer of potentially adverse proinflammatory driven extracellular vesicle-derived miRNAs to the brain via this route of communication would seem of considerable importance for the advancement of our understanding of the pathophysiological mechanisms of intrauterine infection-related preterm brain injury (Dammann and Leviton, 1997; Malaeb and Dammann, 2009), including the role of placental vesicle-derived miRNAs (Ilekis et al., 2016; Wei et al., 2017; Salomon et al., 2018) in this process and warrants further investigation.
Conclusion and Future Directions
In the past decade, numerous articles have been published on the role of miRNAs within the brain. As post-transcriptional regulators of gene expression, miRNAs most definitely play a crucial role in the development of the brain. Nevertheless, research conducted to define their impact on the developing and injured brain is still in its infancy. Given specific miRNAs can exhibit diverse functional roles throughout development and can act synergistically, identification of their precise functional roles is fraught with difficulties. This is particularly relevant when considering adopting specific miRNAs as biomarkers of perinatal brain injury. Thus, careful interpretation of data is required not only in the context of biomarker potential, but also application as a therapeutic strategy since off-target effects can confound the latter. One attractive possibility to ensuring, at least targeted delivery, is the fast developing field of research involving exosome-based miRNA therapies for neurological injuries and disorders. Exosomes are considered a key carrier of circulating miRNAs. Since they mediate the exchange of miRNAs between cells, readily cross the blood–brain barrier and fuse with cell membranes, they hold promise not only as a miRNA biomarker carrier, but also as a means to deliver miRNA-based therapies to the developing injured brain. Clearly, continued developments in this field of research has the potential to enhance future prospects of effectively treating perinatal brain injury especially those vulnerable to premature injury.
Author Contributions
KHTC and MF devised main conceptual ideas and outlines and took the lead in writing the manuscript. All authors contributed to the manuscript and provided feedback and discussed the manuscript.
Funding
This work was supported in part by the Neurological Foundation of NZ 1519-PG (MF), Health Research Council of New Zealand 18/183 (MF), Cure Kids 3581 (MF), Auckland Medical Research Foundation 1117009 (MF), and the Barbara Basham Doctoral Scholarship – Auckland Medical Research Foundation 1216004 (KHTC).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to those authors whose excellent studies we have not dealt with within the scope and limitation of this review.
References
Akerblom, M., and Jakobsson, J. (2014). MicroRNAs as neuronal fate determinants. Neuroscientist 20, 235–242. doi: 10.1177/1073858413497265
Åkerblom, M., Sachdeva, R., Barde, I., Verp, S., Gentner, B., Trono, D., et al. (2012). MicroRNA-124 is a subventricular zone neuronal fate determinant. J. Neurosci. 32, 8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012
Akerblom, M., Sachdeva, R., Quintino, L., Wettergren, E. E., Chapman, K. Z., Manfre, G., et al. (2013). Visualization and genetic modification of resident brain microglia using lentiviral vectors regulated by microRNA-9. Nat. Commun. 4:1770. doi: 10.1038/ncomms2801
Andersson, T., Rahman, S., Sansom, S. N., Alsio, J. M., Kaneda, M., Smith, J., et al. (2010). Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS One 5:e13453. doi: 10.1371/journal.pone.0013453
Ashhab, M. U., Omran, A., Kong, H., Gan, N., He, F., Peng, J., et al. (2013). Expressions of tumor necrosis factor alpha and microRNA-155 in immature rat model of status epilepticus and children with mesial temporal lobe epilepsy. J. Mol. Neurosci. 51, 950–958. doi: 10.1007/s12031-013-0013-9
Baburamani, A. A., Supramaniam, V. G., Hagberg, H., and Mallard, C. (2014). Microglia toxicity in preterm brain injury. Reprod. Toxicol. 48, 106–112. doi: 10.1016/j.reprotox.2014.04.002
Back, S. A., Luo, N. L., Borenstein, N. S., Volpe, J. J., and Kinney, H. C. (2002). Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J. Neuropathol. Exp. Neurol. 61, 197–211. doi: 10.1093/jnen/61.2.197
Back, S. A., and Miller, S. P. (2014). Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann. Neurol. 75, 469–486. doi: 10.1002/ana.24132
Bak, M., Silahtaroglu, A., Moller, M., Christensen, M., Rath, M. F., Skryabin, B., et al. (2008). MicroRNA expression in the adult mouse central nervous system. RNA 14, 432–444. doi: 10.1261/rna.783108
Baltimore, D., Boldin, M. P., O’Connell, R. M., Rao, D. S., and Taganov, K. D. (2008). MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 9, 839–845. doi: 10.1038/ni.f.209
Balusu, S., Van Wonterghem, E., De Rycke, R., Raemdonck, K., Stremersch, S., Gevaert, K., et al. (2016). Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol. Med. 8, 1162–1183. doi: 10.15252/emmm.201606271
Barca-Mayo, O., and Lu, Q. R. (2012). Fine-tuning oligodendrocyte development by microRNAs. Front. Neurosci. 6:13. doi: 10.3389/fnins.2012.00013
Barres, B. A., Lazar, M. A., and Raff, M. C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108.
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Bernstein, E., Kim, S. Y., Carmell, M. A., Murchison, E. P., Alcorn, H., Li, M. Z., et al. (2003). Dicer is essential for mouse development. Nat. Genet. 35, 215–217. doi: 10.1038/ng1253
Bhalala, O. G., Srikanth, M., and Kessler, J. A. (2013). The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 9, 328–339. doi: 10.1038/nrneurol.2013.67
Bian, S., Hong, J., Li, Q., Schebelle, L., Pollock, A., Knauss, J. L., et al. (2013). MicroRNA cluster miR-17-92 regulates neural stem cell expansion and transition to intermediate progenitors in the developing mouse neocortex. Cell Rep. 3, 1398–1406. doi: 10.1016/j.celrep.2013.03.037
Birch, D., Britt, B. C., Dukes, S. C., Kessler, J. A., and Dizon, M. L. (2014). MicroRNAs participate in the murine oligodendroglial response to perinatal hypoxia-ischemia. Pediatr. Res. 76, 334–340. doi: 10.1038/pr.2014.104
Brett, J. O., Renault, V. M., Rafalski, V. A., Webb, A. E., and Brunet, A. (2011). The microRNA cluster miR-106b∼25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging 3, 108–124. doi: 10.18632/aging.100285
Buller, B., Chopp, M., Ueno, Y., Zhang, L., Zhang, R. L., Morris, D., et al. (2012). Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia 60, 1906–1914. doi: 10.1002/glia.22406
Buser, J. R., Maire, J., Riddle, A., Gong, X., Nguyen, T., Nelson, K., et al. (2012). Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109. doi: 10.1002/ana.22627
Butovsky, O., Jedrychowski, M. P., Cialic, R., Krasemann, S., Murugaiyan, G., Fanek, Z., et al. (2015). Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 77, 75–99. doi: 10.1002/ana.24304
Caballero-Garrido, E., Pena-Philippides, J.C., Lordkipanidze, T., Bragin, D., Yang, Y., Erhardt, E.B., et al. (2015). In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J. Neurosci. 35, 12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015
Cai, Q., Wang, T., Yang, W.-J., and Fen, X. (2016). Protective mechanisms of microRNA-27a against oxygen-glucose deprivation-induced injuries in hippocampal neurons. Neural Regen. Res. 11:1285. doi: 10.4103/1673-5374.189194
Cao, X., Yeo, G., Muotri, A. R., Kuwabara, T., and Gage, F. H. (2006). Noncoding RNAs in the mammalian central nervous system. Annu. Rev. Neurosci. 29, 77–103. doi: 10.1146/annurev.neuro.29.051605.112839
Cardoso, A. L., Guedes, J. R., Pereira de Almeida, L., and Pedroso de Lima, M. C. (2012). miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135, 73–88. doi: 10.1111/j.1365-2567.2011.03514.x
Chan, S. Y., and Loscalzo, J. (2010). MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9, 1072–1083. doi: 10.4161/cc.9.6.11006
Chan, Y. C., Banerjee, J., Choi, S. Y., and Sen, C. K. (2012). miR-210: the master hypoxamir. Microcirculation 19, 215–223. doi: 10.1111/j.1549-8719.2011.00154.x
Chen, F., Du, Y., Esposito, E., Liu, Y., Guo, S., Wang, X., et al. (2015). Effects of focal cerebral ischemia on exosomal versus serum miR126. Transl. Stroke Res. 6, 478–484. doi: 10.1007/s12975-015-0429-3
Chen, H., and Yang, T. T. (2015). Expression and significance of serum miRNA-21 control HIF-1a in newborn with asphyxia. Chin. J. Child Health Care 23, 32–34.
Chen, J., Liu, Z., and Yang, Y. (2014). In vitro screening of LPS-induced miRNAs in leukocytes derived from cord blood and their possible roles in regulating TLR signals. Pediatr. Res. 75, 595–602. doi: 10.1038/pr.2014.18
Chen, Q., Xu, J., Li, L., Li, H., Mao, S., Zhang, F., et al. (2014). MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 5:e1132. doi: 10.1038/cddis.2014.92
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B. H., Lee, C. N., and Lim, S. K. (2009). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38, 215–224. doi: 10.1093/nar/gkp857
Chen, W., and Qin, C. (2015). General hallmarks of microRNAs in brain evolution and development. RNA Biol. 12, 701–708. doi: 10.1080/15476286.2015.1048954
Chen, Y., Song, Y., Huang, J., Qu, M., Zhang, Y., Geng, J., et al. (2017). Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front. Neurol. 8:57. doi: 10.3389/fneur.2017.00057
Cheng, L., Sharples, R. A., Scicluna, B. J., and Hill, A. F. (2014). Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 3:10.3402/jev.v3.23743. doi: 10.3402/jev.v3.23743
Cheng, L.-C., Pastrana, E., Tavazoie, M., and Doetsch, F. (2009). miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 12:399. doi: 10.1038/nn.2294
Cherubini, E., Gustincich, S., and Robinson, H. (2006). The mammalian transcriptome and the cellular complexity of the brain. J. Physiol. 575(Pt 2), 319–320. doi: 10.1113/jphysiol.2006.118364
Chiva-Blanch, G., Suades, R., Crespo, J., Pena, E., Padro, T., Jimenez-Xarrie, E., et al. (2016). Microparticle shedding from neural progenitor cells and vascular compartment cells is increased in ischemic stroke. PLoS One 11:e0148176. doi: 10.1371/journal.pone.0148176
Chow, L. C., Soliman, A., Zandian, M., Danielpour, M., and Krueger, R. C. Jr (2005). Accumulation of transforming growth factor-β2 and nitrated chondroitin sulfate proteoglycans in cerebrospinal fluid correlates with poor neurologic outcome in preterm hydrocephalus. Neonatology 88, 1–11. doi: 10.1159/000083945
Clovis, Y. M., Enard, W., Marinaro, F., Huttner, W. B., and De Pietri Tonelli, D. (2012). Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons. Development 139, 3332–3342. doi: 10.1242/dev.078063
Concepcion, C. P., Bonetti, C., and Ventura, A. (2012). The microRNA-17-92 family of microRNA clusters in development and disease. Cancer J. 18, 262–267. doi: 10.1097/PPO.0b013e318258b60a
Contreras-Naranjo, J. C., Wu, H. J., and Ugaz, V. M. (2017). Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab. Chip 17, 3558–3577. doi: 10.1039/c7lc00592j
Coolen, M., Katz, S., and Bally-Cuif, L. (2013). miR-9: a versatile regulator of neurogenesis. Front. Cell Neurosci. 7:220. doi: 10.3389/fncel.2013.00220
Dajas-Bailador, F., Bonev, B., Garcez, P., Stanley, P., Guillemot, F., and Papalopulu, N. (2012). microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci. doi: 10.1038/nn.3082 [Epub ahead of print].
Dammann, O., and Leviton, A. (1997). Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res. 42, 1–8. doi: 10.1203/00006450-199707000-00001
Davis, G. M., Haas, M. A., and Pocock, R. (2015). MicroRNAs: not “fine-tuners” but key regulators of neuronal development and function. Front. Neurol. 6:245. doi: 10.3389/fneur.2015.00245
Davis, T. H., Cuellar, T. L., Koch, S. M., Barker, A. J., Harfe, B. D., McManus, M. T., et al. (2008). Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 28, 4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008
De Pietri Tonelli, D., Pulvers, J. N., Haffner, C., Murchison, E. P., Hannon, G. J., and Huttner, W. B. (2008). miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development 135, 3911–3921. doi: 10.1242/dev.025080
Dean, J. M., McClendon, E., Hansen, K., Azimi-Zonooz, A., Chen, K., Riddle, A., et al. (2013). Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med. 5:168ra167. doi: 10.1126/scitranslmed.3004669
Dean, J. M., Van De Looij, Y., Sizonenko, S. V., Lodygensky, G. A., Lazeyras, F., Bolouri, H., et al. (2011). Delayed cortical impairment following lipopolysaccharide exposure in preterm fetal sheep. Ann. Neurol. 70, 846–856. doi: 10.1002/ana.22480
Delaloy, C., Liu, L., Lee, J. A., Su, H., Shen, F., Yang, G. Y., et al. (2010). MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 6, 323–335. doi: 10.1016/j.stem.2010.02.015
Dharap, A., Bowen, K., Place, R., Li, L. C., and Vemuganti, R. (2009). Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 29, 675–687. doi: 10.1038/jcbfm.2008.157
Dhillon, S. K., Gunn, A. J., Jung, Y., Mathai, S., Bennet, L., and Fraser, M. (2015). Lipopolysaccharide-induced preconditioning attenuates apoptosis and differentially regulates TLR4 and TLR7 gene expression after ischemia in the preterm ovine fetal brain. Dev. Neurosci. 37, 497–514. doi: 10.1159/000433422
Dillenburg, A., Ireland, G., Holloway, R. K., Davies, C. L., Evans, F. L., Swire, M., et al. (2018). Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathol. 135, 887–906. doi: 10.1007/s00401-018-1813-3
Doeppner, T. R., Doehring, M., Bretschneider, E., Zechariah, A., Kaltwasser, B., Müller, B., et al. (2013). MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol. 126, 251–265. doi: 10.1007/s00401-013-1142-5
Doeppner, T. R., Herz, J., Gorgens, A., Schlechter, J., Ludwig, A. K., Radtke, S., et al. (2015). Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 4, 1131–1143. doi: 10.5966/sctm.2015-0078
Douglas-Escobar, M., and Weiss, M. D. (2012). Biomarkers of brain injury in the premature infant. Front. Neurol. 3:185. doi: 10.3389/fneur.2012.00185
Drommelschmidt, K., Serdar, M., Bendix, I., Herz, J., Bertling, F., Prager, S., et al. (2017). Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 60, 220–232. doi: 10.1016/j.bbi.2016.11.011
Duan, Q., Mao, X., Xiao, Y., Liu, Z., Wang, Y., Zhou, H., et al. (2016). Super enhancers at the miR-146a and miR-155 genes contribute to self-regulation of inflammation. Biochim. Biophys. Acta 1859, 564–571. doi: 10.1016/j.bbagrm.2016.02.004
Dugas, J. C., Cuellar, T. L., Scholze, A., Ason, B., Ibrahim, A., Emery, B., et al. (2010). Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65, 597–611. doi: 10.1016/j.neuron.2010.01.027
Dzietko, M., Derugin, N., Wendland, M., Vexler, Z., and Ferriero, D. (2013). Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl. Stroke Res. 4, 189–200. doi: 10.1007/s12975-012-0221-6
Eacker, S. M., Dawson, T. M., and Dawson, V. L. (2013). The interplay of microRNA and neuronal activity in health and disease. Front. Cell Neurosci. 7:136. doi: 10.3389/fncel.2013.00136
Ferriero, D. M. (2016). The vulnerable newborn brain: imaging patterns of acquired perinatal injury. Neonatology 109, 345–351. doi: 10.1159/000444896
Fitzpatrick, J. M., Anderson, R. C., and McDermott, K. W. (2015). MicroRNA: key regulators of oligodendrocyte development and pathobiology. Int. J. Biochem. Cell Biol. 65, 134–138. doi: 10.1016/j.biocel.2015.05.021
Fleiss, B., and Gressens, P. (2012). Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 11, 556–566. doi: 10.1016/S1474-4422(12)70058-3
Florio, P., Luisi, S., Bruschettini, M., Grutzfeld, D., Dobrzanska, A., Bruschettini, P., et al. (2004). Cerebrospinal fluid activin a measurement in asphyxiated full-term newborns predicts hypoxic ischemic encephalopathy. Clin. Chem. 50, 2386–2389. doi: 10.1373/clinchem.2004.035774
Florio, P., Luisi, S., Moataza, B., Torricelli, M., Iman, I., Hala, M., et al. (2007). High urinary concentrations of activin A in asphyxiated full-term newborns with moderate or severe hypoxic ischemic encephalopathy. Clin. Chem. 53, 520–522. doi: 10.1373/clinchem.2005.062604
Fu, Q., Du, Y., Yang, C., Zhang, D., Zhang, N., Liu, X., et al. (2016). An oncogenic role of miR-592 in tumorigenesis of human colorectal cancer by targeting Forkhead Box O3A (FoxO3A). Expert Opin. Ther. Targets 20, 771–782. doi: 10.1080/14728222.2016.1181753
Galloway, D. A., and Moore, C. S. (2016). miRNAs as emerging regulators of oligodendrocyte development and differentiation. Front. Cell Dev. Biol. 4:59. doi: 10.3389/fcell.2016.00059
Garberg, H. T., Huun, M. U., Baumbusch, L. O., Asegg-Atneosen, M., Solberg, R., and Saugstad, O. D. (2016). Temporal profile of circulating microRNAs after global hypoxia-ischemia in newborn piglets. Neonatology 111, 133–139. doi: 10.1159/000449032
Gaudet, A. D., Fonken, L. K., Watkins, L. R., Nelson, R. J., and Popovich, P. G. (2017). MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24, 221–245. doi: 10.1177/1073858417721150
Gaudet, A. D., Fonken, L. K., Watkins, L. R., Nelson, R. J., and Popovich, P. G. (2018). MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24, 221–245. doi: 10.1177/1073858417721150
Gerdoni, E., Gallo, B., Casazza, S., Musio, S., Bonanni, I., Pedemonte, E., et al. (2007). Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 61, 219–227. doi: 10.1002/ana.21076
Giaume, C., Koulakoff, A., Roux, L., Holcman, D., and Rouach, N. (2010). Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 11, 87–99. doi: 10.1038/nrn2757
Goetzl, L., Merabova, N., Darbinian, N., Martirosyan, D., Poletto, E., Fugarolas, K., et al. (2018). Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann. Clin. Transl. Neurol. 5, 4–10. doi: 10.1002/acn3.499
Gotz, M., Nakafuku, M., and Petrik, D. (2016). Neurogenesis in the developing and adult brain-similarities and key differences. Cold Spring Harb. Perspect. Biol. 8:a018853. doi: 10.1101/cshperspect.a018853
Griesmaier, E., Schlager, G., Wegleiter, K., Hermann, M., Urbanek, M., Simbruner, G., et al. (2010). Role of p75NTR in NMDAR-mediated excitotoxic brain injury in neonatal mice. Brain Res. 1355, 31–40. doi: 10.1016/j.brainres.2010.07.095
Griffiths-Jones, S. (2006). miRBase: the microRNA sequence database. Methods Mol. Biol. 342, 129–138. doi: 10.1385/1-59745-123-1:129
Guo, S. C., Tao, S. C., and Dawn, H. (2018). Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J. Extracell. Vesicles 7:1508271. doi: 10.1080/20013078.2018.1508271
Hagberg, H., Mallard, C., Ferriero, D. M., Vannucci, S. J., Levison, S. W., Vexler, Z. S., et al. (2015). The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208. doi: 10.1038/nrneurol.2015.13
Hass, R., and Otte, A. (2012). Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun. Signal. 10:26. doi: 10.1186/1478-811X-10-26
He, Y., Li, H. B., Li, X., Zhou, Y., Xia, X. B., and Song, W. T. (2018). MiR-124 promotes the growth of retinal ganglion cells derived from muller cells. Cell Physiol. Biochem. 45, 973–983. doi: 10.1159/000487292
Hisey, C. L., Dorayappan, K. D. P., Cohn, D. E., Selvendiran, K., and Hansford, D. J. (2018). Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab. Chip 18, 3144–3153. doi: 10.1039/c8lc00834e
Huang, X., Le, Q.T., and Giaccia, A.J. (2010). MiR-210–micromanager of the hypoxia pathway. Trends Mol. Med. 16, 230–237. doi: 10.1016/j.molmed.2010.03.004
Ilekis, J. V., Tsilou, E., Fisher, S., Abrahams, V. M., Soares, M. J., Cross, J. C., et al. (2016). Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 215(Suppl. 1), S1–S46. doi: 10.1016/j.ajog.2016.03.001
Irmady, K., Jackman, K. A., Padow, V. A., Shahani, N., Martin, L. A., Cerchietti, L., et al. (2014). Mir-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury. J. Neurosci. 34, 3419–3428. doi: 10.1523/JNEUROSCI.1982-13.2014
Iyer, A., Zurolo, E., Prabowo, A., Fluiter, K., Spliet, W. G., van Rijen, P. C., et al. (2012). MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One 7:e44789. doi: 10.1371/journal.pone.0044789
Jellema, R. K., Wolfs, T. G., Passos, V. L., Zwanenburg, A., Ophelders, D. R., Kuypers, E., et al. (2013). Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One 8:e73031. doi: 10.1371/journal.pone.0073031
Jeyaseelan, K., Lim, K. Y., and Armugam, A. (2008). MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39, 959–966. doi: 10.1161/STROKEAHA.107.500736
Ji, Q., Ji, Y., Peng, J., Zhou, X., Chen, X., Zhao, H., et al. (2016). Increased Brain-Specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One 11:e0163645. doi: 10.1371/journal.pone.0163645
Jones, L. L., Sajed, D., and Tuszynski, M. H. (2003). Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J. Neurosci. 23, 9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003
Jones, L. L., Yamaguchi, Y., Stallcup, W. B., and Tuszynski, M. H. (2002). NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 22, 2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002
Kannan, S., Saadani-Makki, F., Balakrishnan, B., Dai, H., Chakraborty, P. K., Janisse, J., et al. (2011). Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J. Cereb. Blood Flow Metab. 31, 738–749. doi: 10.1038/jcbfm.2010.156
Katakowski, M., Buller, B., Zheng, X., Lu, Y., Rogers, T., Osobamiro, O., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 335, 201–204. doi: 10.1016/j.canlet.2013.02.019
Katsumoto, A., Lu, H., Miranda, A. S., and Ransohoff, R. M. (2014). Ontogeny and functions of central nervous system macrophages. J. Immunol. 193, 2615–2621. doi: 10.4049/jimmunol.1400716
Khanna, S., Rink, C., Ghoorkhanian, R., Gnyawali, S., Heigel, M., Wijesinghe, D. S., et al. (2013). Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J. Cereb. Blood Flow Metab. 33, 1197–1206. doi: 10.1038/jcbfm.2013.68
Kim, H. Y., Kumar, H., Jo, M. J., Kim, J., Yoon, J. K., Lee, J. R., et al. (2018). Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 18, 4965–4975. doi: 10.1021/acs.nanolett.8b01816
Kinney, H.C., Volpe, J.J. (2018a). “Encephalopathy of prematurity: neuropathology,” in Volpe’s Neurology of the Newborn, 6th Edn, eds J. Joseph, M. D. Volpe, T. Basil, S. Darras Linda, J. Adré, J, Jeffrey et al. (Amsterdam: Elsevier), 389–404.
Kinney, H.C., and Volpe, J.J. (2018b). “Hypoxic-ischemic injury in the term infant: neuropathology,” in Volpe’s Neurology of the Newborn, 6th Edn, eds J.J. Volpe, T.E. Inder, B.T. Darras, L.S. de Vries, A.J. du Plessis, J.J. Neil et al. (Amsterdam: Elsevier), 484–499. doi: 10.1016/B978-0-323-42876-7.00018-1
Koh, S.-H., Kim, K. S., Choi, M. R., Jung, K. H., Park, K. S., Chai, Y. G., et al. (2008). Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 1229, 233–248. doi: 10.1016/j.brainres.2008.06.087
Koh, W., Sheng, C. T., Tan, B., Lee, Q. Y., Kuznetsov, V., Kiang, L. S., et al. (2010). Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics 11:S6. doi: 10.1186/1471-2164-11-S1-S6
Koniusz, S., Andrzejewska, A., Muraca, M., Srivastava, A. K., Janowski, M., and Lukomska, B. (2016). Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front. Cell Neurosci. 10:109. doi: 10.3389/fncel.2016.00109
Kozomara, A., and Griffiths-Jones, S. (2014). miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42(Database issue), D68–D73. doi: 10.1093/nar/gkt1181
Krichevsky, A. M., Sonntag, K. C., Isacson, O., and Kosik, K. S. (2006). Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24, 857–864. doi: 10.1634/stemcells.2005-0441
Kulshreshtha, R., Ferracin, M., Wojcik, S. E., Garzon, R., Alder, H., Agosto-Perez, F. J., et al. (2007). A microRNA signature of hypoxia. Mol. Cell Biol. 27, 1859–1867. doi: 10.1128/MCB.01395-06
Lagos-Quintana, M., Rauhut, R., Meyer, J., Borkhardt, A., and Tuschl, T. (2003). New microRNAs from mouse and human. RNA 9, 175–179. doi: 10.1261/rna.2146903
Lau, P., Verrier, J. D., Nielsen, J. A., Johnson, K. R., Notterpek, L., and Hudson, L. D. (2008). Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci. 28, 11720–11730. doi: 10.1523/JNEUROSCI.1932-08.2008
Lederhuber, H., Baer, K., Altiok, I., Sadeghi, K., Herkner, K. R., and Kasper, D. C. (2011). MicroRNA-146: tiny player in neonatal innate immunity? Neonatology 99, 51–56. doi: 10.1159/000301938
Lee, J.-K., Park, S.-R., Jung, B.-K., Jeon, Y.-K., Lee, Y.-S., Kim, M.-K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8:e84256. doi: 10.1371/journal.pone.0084256
Lehmann, S. M., Kruger, C., Park, B., Derkow, K., Rosenberger, K., Baumgart, J., et al. (2012). An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 15, 827–835. doi: 10.1038/nn.3113
Letzen, B. S., Liu, C., Thakor, N. V., Gearhart, J. D., All, A. H., and Kerr, C. L. (2010). MicroRNA expression profiling of oligodendrocyte differentiation from human embryonic stem cells. PLoS One 5:e10480. doi: 10.1371/journal.pone.0010480
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P., and Burge, C. B. (2003). Prediction of mammalian microRNA targets. Cell 115, 787–798. doi: 10.1016/S0092-8674(03)01018-3
Li, B., Concepcion, K., Meng, X., and Zhang, L. (2017). Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog. Neurobiol. 159, 50–68. doi: 10.1016/j.pneurobio.2017.10.006
Li, K., Rodosthenous, R. S., Kashanchi, F., Gingeras, T., Gould, S. J., Kuo, L. S., et al. (2018). Advances, challenges, and opportunities in extracellular RNA biology: insights from the NIH exRNA Strategic Workshop. JCI Insight 3:98942. doi: 10.1172/jci.insight.98942
Li, X.-Q., Lv, H.-W., Wang, Z.-L., Tan, W.-F., Fang, B., and Ma, H. (2015). MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR 4 signaling pathway. J. Neuroinflam. 12:25. doi: 10.1186/s12974-015-0246-3
Lim, L. P., Lau, N. C., Garrett-Engele, P., Grimson, A., Schelter, J. M., Castle, J., et al. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773. doi: 10.1038/nature03315
Liu, A., Li, J., Marin-Husstege, M., Kageyama, R., Fan, Y., Gelinas, C., et al. (2006). A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 25, 4833–4842. doi: 10.1038/sj.emboj.7601352
Liu, D. Z., Tian, Y., Ander, B. P., Xu, H., Stamova, B. S., Zhan, X., et al. (2010). Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 30, 92–101. doi: 10.1038/jcbfm.2009.186
Liu, J., Zhang, K. S., Hu, B., Li, S. G., Li, Q., Luo, Y. P., et al. (2018). Systematic analysis of RNA regulatory network in rat brain after ischemic stroke. Biomed. Res. Int. 2018:8354350. doi: 10.1155/2018/8354350
Liu, M., Zhi, Q., Wang, W., Zhang, Q., Fang, T., and Ma, Q. (2015). Up-regulation of miR-592 correlates with tumor progression and poor prognosis in patients with colorectal cancer. Biomed. Pharmacother. 69, 214–220. doi: 10.1016/j.biopha.2014.12.001
Liu, X. S., Chopp, M., Pan, W. L., Wang, X. L., Fan, B. Y., Zhang, Y., et al. (2017). MicroRNA-146a promotes oligodendrogenesis in stroke. Mol. Neurobiol. 54, 227–237. doi: 10.1007/s12035-015-9655-7
Liu, X. S., Chopp, M., Zhang, R. L., Tao, T., Wang, X. L., Kassis, H., et al. (2011). MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6:e23461. doi: 10.1371/journal.pone.0023461
Liu, Z., Wu, R., Li, G., Sun, P., Xu, Q., and Liu, Z. (2015). MiR-592 inhibited cell proliferation of human colorectal cancer cells by suppressing of CCND3 expression. Int. J. Clin. Exp. Med. 8:3490.
Long, Q., Upadhya, D., Hattiangady, B., Kim, D. K., An, S. Y., Shuai, B., et al. (2017). Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. U.S.A. 114, E3536–E3545. doi: 10.1073/pnas.1703920114
Looney, A. M., Ahearne, C. E., Hallberg, B., Boylan, G. B., and Murray, D. M. (2017). Downstream mRNA target analysis in neonatal hypoxic-ischaemic encephalopathy identifies novel marker of severe injury: a proof of concept paper. Mol. Neurobiol. 54, 8420–8428. doi: 10.1007/s12035-016-0330-4
Looney, A. M., Walsh, B. H., Moloney, G., Grenham, S., Fagan, A., O’Keeffe, G. W., et al. (2015). Downregulation of umbilical cord blood levels of miR-374a in neonatal hypoxic ischemic encephalopathy. J. Pediatr. 167, 269–273.e2. doi: 10.1016/j.jpeds.2015.04.060
Luarte, A., Batiz, L. F., Wyneken, U., and Lafourcade, C. (2016). Potential therapies by stem cell-derived exosomes in cns diseases: focusing on the neurogenic niche. Stem Cells Int. 2016:5736059. doi: 10.1155/2016/5736059
Ludwig, A. K., and Giebel, B. (2012). Exosomes: small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 44, 11–15. doi: 10.1016/j.biocel.2011.10.005
Lv, Y.-N., Ou-yang, A.-J., and Fu, L.-S. (2017). MicroRNA-27a negatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cell. Mol. Neurobiol. 37, 195–210. doi: 10.1007/s10571-016-0361-4
Ma, Q., Dasgupta, C., Li, Y., Bajwa, N. M., Xiong, F., Harding, B., et al. (2016). Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol. Dis. 89, 202–212. doi: 10.1016/j.nbd.2016.02.011
Ma, Q., Dasgupta, C., Li, Y., Huang, L., and Zhang, L. (2017). MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int. J. Mol. Sci. 18:E1356. doi: 10.3390/ijms18071356
Maiorano, N. A., and Mallamaci, A. (2009). Promotion of embryonic cortico-cerebral neuronogenesis by miR-124. Neural Dev. 4:40. doi: 10.1186/1749-8104-4-40
Makeyev, E. V., Zhang, J., Carrasco, M. A., and Maniatis, T. (2007). The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 27, 435–448. doi: 10.1016/j.molcel.2007.07.015
Malaeb, S., and Dammann, O. (2009). Fetal inflammatory response and brain injury in the preterm newborn. J. Child Neurol. 24, 1119–1126. doi: 10.1177/0883073809338066
Mann, M., Mehta, A., Zhao, J. L., Lee, K., Marinov, G. K., Garcia-Flores, Y., et al. (2017). An NF-kappaB-microRNA regulatory network tunes macrophage inflammatory responses. Nat. Commun. 8:851. doi: 10.1038/s41467-017-00972-z
Mastoridis, S., Bertolino, G. M., Whitehouse, G., Dazzi, F., Sanchez-Fueyo, A., and Martinez-Llordella, M. (2018). Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front. Immunol. 9:1583. doi: 10.3389/fimmu.2018.01583
McKeon, R. J., Jurynec, M. J., and Buck, C. R. (1999). The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 19, 10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999
Meng, Z. Y., Kang, H. L., Duan, W., Zheng, J., Li, Q. N., and Zhou, Z. J. (2018). MicroRNA-210 promotes accumulation of neural precursor cells around ischemic foci after cerebral ischemia by regulating the SOCS1-STAT3-VEGF-C Pathway. J. Am. Heart Assoc. 7:e005052. doi: 10.1161/JAHA.116.005052
Michell-Robinson, M. A., Touil, H., Healy, L. M., Owen, D. R., Durafourt, B. A., Bar-Or, A., et al. (2015). Roles of microglia in brain development, tissue maintenance and repair. Brain 138(Pt 5), 1138–1159. doi: 10.1093/brain/awv066
Miska, E. A., Alvarez-Saavedra, E., Townsend, M., Yoshii, A., Sestan, N., Rakic, P., et al. (2004). Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 5:R68. doi: 10.1186/gb-2004-5-9-r68
Mokarizadeh, A., Delirezh, N., Morshedi, A., Mosayebi, G., Farshid, A.-A., and Mardani, K. (2012). Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol. Lett. 147, 47–54. doi: 10.1016/j.imlet.2012.06.001
Mondello, S., Thelin, E. P., Shaw, G., Salzet, M., Visalli, C., Cizkova, D., et al. (2018). Extracellular vesicles: pathogenetic, diagnostic and therapeutic value in traumatic brain injury. Expert Rev. Proteomics 15, 451–461. doi: 10.1080/14789450.2018.1464914
Monfils, M. H., Driscoll, I., Kamitakahara, H., Wilson, B., Flynn, C., Teskey, G. C., et al. (2006). FGF-2-induced cell proliferation stimulates anatomical, neurophysiological and functional recovery from neonatal motor cortex injury. Eur. J. Neurosci. 24, 739–749. doi: 10.1111/j.1460-9568.2006.04939.x
Moon, J. M., Xu, L., and Giffard, R. G. (2013). Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J. Cereb. Blood Flow Metab. 33, 1976–1982. doi: 10.1038/jcbfm.2013.157
Morris, D. C., Chopp, M., Zhang, L., Lu, M., and Zhang, Z. G. (2010). Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience 169, 674–682. doi: 10.1016/j.neuroscience.2010.05.017
Mueller, M., Zhou, J., Yang, L., Gao, Y., Wu, F., Schoeberlein, A., et al. (2014). PreImplantation factor promotes neuroprotection by targeting microRNA let-7. Proc. Natl. Acad. Sci. U.S.A. 111, 13882–13887. doi: 10.1073/pnas.1411674111
Nahid, M. A., Satoh, M., and Chan, E. K. (2011). MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol. Immunol. 8, 388–403. doi: 10.1038/cmi.2011.26
Nallamshetty, S., Chan, S.Y., and Loscalzo, J. (2013). Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic. Biol. Med. 64, 20–30. doi: 10.1016/j.freeradbiomed.2013.05.022
Narayan, A., Bommakanti, A., and Patel, A. A. (2015). High-throughput RNA profiling via up-front sample parallelization. Nat. Methods 12, 343–346. doi: 10.1038/nmeth.3311
Nayak, D., Roth, T. L., and McGavern, D. B. (2014). Microglia development and function. Annu. Rev. Immunol. 32, 367–402. doi: 10.1146/annurev-immunol-032713-120240
O’Connell, R. M., Taganov, K. D., Boldin, M. P., Cheng, G., and Baltimore, D. (2007). MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 104, 1604–1609. doi: 10.1073/pnas.0610731104
Ohab, J. J., Fleming, S., Blesch, A., and Carmichael, S. T. (2006). A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006
Omran, A., Peng, J., Zhang, C., Xiang, Q. L., Xue, J., Gan, N., et al. (2012). Interleukin-1beta and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53, 1215–1224. doi: 10.1111/j.1528-1167.2012.03540.x
O’Neill, L. A., Sheedy, F. J., and McCoy, C. E. (2011). MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 11, 163–175. doi: 10.1038/nri2957
Ono, M., Kosaka, N., Tominaga, N., Yoshioka, Y., Takeshita, F., Takahashi, R.-U., et al. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 7:ra63 doi: 10.1126/scisignal.2005231
Ophelders, D. R., Wolfs, T. G., Jellema, R. K., Zwanenburg, A., Andriessen, P., Delhaas, T., et al. (2016). Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 5, 754–763. doi: 10.5966/sctm.2015-0197
Otaegi, G., Pollock, A., Hong, J., and Sun, T. (2011). MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J. Neurosci. 31, 809–818. doi: 10.1523/JNEUROSCI.4330-10.2011
Ouyang, Y. B., Xu, L., Yue, S., Liu, S., and Giffard, R. G. (2014). Neuroprotection by astrocytes in brain ischemia: importance of microRNAs. Neurosci. Lett. 565, 53–58. doi: 10.1016/j.neulet.2013.11.015
Pandit, A. S., Ball, G., Edwards, A. D., and Counsell, S. J. (2013). Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology 55, 65–95. doi: 10.1007/s00234-013-1242-x
Park, K. K., Liu, K., Hu, Y., Smith, P. D., Wang, C., Cai, B., et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. doi: 10.1126/science.1161566
Patz, S., Trattnig, C., Grunbacher, G., Ebner, B., Gully, C., Novak, A., et al. (2013). More than cell dust: microparticles isolated from cerebrospinal fluid of brain injured patients are messengers carrying mRNAs, miRNAs, and proteins. J. Neurotrauma 30, 1232–1242. doi: 10.1089/neu.2012.2596
Pena, J. T., Sohn-Lee, C., Rouhanifard, S. H., Ludwig, J., Hafner, M., Mihailovic, A., et al. (2009). miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nat. Methods 6, 139–141. doi: 10.1038/nmeth.1294
Pena-Philippides, J.C., Caballero-Garrido, E., Lordkipanidze, T., and Roitbak, T. (2016). In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J. Neuroinflam. 13:287. doi: 10.1186/s12974-016-0753-x
Petri, R., Malmevik, J., Fasching, L., Akerblom, M., and Jakobsson, J. (2014). miRNAs in brain development. Exp. Cell Res. 321, 84–89. doi: 10.1016/j.yexcr.2013.09.022
Podolska, A., Kaczkowski, B., Kamp Busk, P., Sokilde, R., Litman, T., Fredholm, M., et al. (2011). MicroRNA expression profiling of the porcine developing brain. PLoS One 6:e14494. doi: 10.1371/journal.pone.0014494
Ponnusamy, V., Kapellou, O., Yip, E., Evanson, J., Wong, L. F., Michael-Titus, A., et al. (2016). A study of microRNAs from dried blood spots in newborns after perinatal asphyxia: a simple and feasible biosampling method. Pediatr. Res. 79, 799–805. doi: 10.1038/pr.2015.276
Ponomarev, E. D., Veremeyko, T., Barteneva, N., Krichevsky, A. M., and Weiner, H. L. (2011). MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat. Med. 17, 64–70. doi: 10.1038/nm.2266
Ponomarev, E. D., Veremeyko, T., and Weiner, H. L. (2013). MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61, 91–103. doi: 10.1002/glia.22363
Potzner, M. R., Griffel, C., Lutjen-Drecoll, E., Bosl, M. R., Wegner, M., and Sock, E. (2007). Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol. Cell Biol. 27, 5316–5326. doi: 10.1128/MCB.00339-07
Qiu, J., Zhou, X. Y., Zhou, X. G., Cheng, R., Liu, H. Y., and Li, Y. (2013a). Neuroprotective effects of microRNA-210 against oxygen-glucose deprivation through inhibition of apoptosis in PC12 cells. Mol. Med. Rep. 7, 1955–1959. doi: 10.3892/mmr.2013.1431
Qiu, J., Zhou, X. Y., Zhou, X. G., Cheng, R., Liu, H. Y., and Li, Y. (2013b). Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed. Res. Int. 2013:350419. doi: 10.1155/2013/350419
Quinn, S. R., and O’Neill, L. A. (2011). A trio of microRNAs that control Toll-like receptor signalling. Int. Immunol. 23, 421–425. doi: 10.1093/intimm/dxr034
Radhakrishnan, B., and Alwin Prem Anand, A. (2016). Role of miRNA-9 in Brain Development. J. Exp. Neurosci. 10, 101–120. doi: 10.4137/JEN.S32843
Rao, P., Benito, E., and Fischer, A. (2013). MicroRNAs as biomarkers for CNS disease. Front. Mol. Neurosci. 6:39. doi: 10.3389/fnmol.2013.00039
Rao, V. T., Ludwin, S. K., Fuh, S. C., Sawaya, R., Moore, C. S., Ho, M. K., et al. (2016). MicroRNA expression patterns in human astrocytes in relation to anatomical location and age. J. Neuropathol. Exp. Neurol. 75, 156–166. doi: 10.1093/jnen/nlv016
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. doi: 10.1083/jcb.201211138
Reemst, K., Noctor, S. C., Lucassen, P. J., and Hol, E. M. (2016). The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci. 10:566. doi: 10.3389/fnhum.2016.00566
Repetto, E., Briata, P., Kuziner, N., Harfe, B. D., McManus, M. T., Gherzi, R., et al. (2012). Let-7b/c enhance the stability of a tissue-specific mRNA during mammalian organogenesis as part of a feedback loop involving KSRP. PLoS Genet. 8:e1002823. doi: 10.1371/journal.pgen.1002823
Riddle, A., Maire, J., Gong, X., Chen, K. X., Kroenke, C. D., Hohimer, A. R., et al. (2012). Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke 43, 178–184. doi: 10.1161/STROKEAHA.111.632265
Robinson, S., Li, Q., DeChant, A., and Cohen, M. L. (2006). Neonatal loss of γ–aminobutyric acid pathway expression after human perinatal brain injury. J. Neurosurg. 104, 396–408. doi: 10.3171/ped.2006.104.6.396
Roitbak, T. (2018). Silencing a Multifunctional microrna is beneficial for stroke recovery. Front. Mol. Neurosci. 11:58. doi: 10.3389/fnmol.2018.00058
Salomon, C., Nuzhat, Z., Dixon, C. L., and Menon, R. (2018). Placental exosomes during gestation: liquid biopsies carrying signals for the regulation of human parturition. Curr. Pharm. Des. 24, 974–982. doi: 10.2174/1381612824666180125164429
Santra, M., Zhang, Z. G., Yang, J., Santra, S., Santra, S., Chopp, M., et al. (2014). Thymosin beta4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway. J. Biol. Chem. 289, 19508–19518. doi: 10.1074/jbc.M113.529966
Sanuki, R., Onishi, A., Koike, C., Muramatsu, R., Watanabe, S., Muranishi, Y., et al. (2011). miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 14, 1125–1134. doi: 10.1038/nn.2897
Schwab, J. M., Postler, E., Nguyen, T. D., Mittelbronn, M., Meyermann, R., and Schluesener, H. J. (2000). Connective tissue growth factor is expressed by a subset of reactive astrocytes in human cerebral infarction. Neuropathol. Appl. Neurobiol. 26, 434–440. doi: 10.1046/j.1365-2990.2000.00271.x
Selvamani, A., Sathyan, P., Miranda, R. C., and Sohrabji, F. (2012). An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One 7:e32662. doi: 10.1371/journal.pone.0032662
Sempere, L. F., Freemantle, S., Pitha-Rowe, I., Moss, E., Dmitrovsky, E., and Ambros, V. (2004). Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 5:R13. doi: 10.1186/gb-2004-5-3-r13
Shenoy, A., Danial, M., and Blelloch, R. H. (2015). Let-7 and miR-125 cooperate to prime progenitors for astrogliogenesis. EMBO J. 34, 1180–1194. doi: 10.15252/embj.201489504
Shi, Y., Zhao, X., Hsieh, J., Wichterle, H., Impey, S., Banerjee, S., et al. (2010). MicroRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 30, 14931–14936. doi: 10.1523/JNEUROSCI.4280-10.2010
Shin, D., Shin, J. Y., McManus, M. T., Ptacek, L. J., and Fu, Y. H. (2009). Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann. Neurol. 66, 843–857. doi: 10.1002/ana.21927
Shindo, A., Maki, T., Itoh, K., Miyamoto, N., Egawa, N., Liang, A. C., et al. (2016). “Crosstalk between cerebral endothelium and oligodendrocyte after stroke,” in Non-Neuronal Mechanisms of Brain Damage and Repair After Stroke, eds J. Chen, J. Zhang, and X. Hu (Cham: Springer), 151–170.
Silber, J., Lim, D. A., Petritsch, C., Persson, A. I., Maunakea, A. K., Yu, M., et al. (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 6:14. doi: 10.1186/1741-7015-6-14
Smirnova, L., Grafe, A., Seiler, A., Schumacher, S., Nitsch, R., and Wulczyn, F. G. (2005). Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 21, 1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x
Smith, B., Treadwell, J., Zhang, D., Ly, D., McKinnell, I., Walker, P. R., et al. (2010). Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PLoS One 5:e11109. doi: 10.1371/journal.pone.0011109
Steinberg, G. K., Kondziolka, D., Wechsler, L. R., Lunsford, L. D., Coburn, M. L., Billigen, J. B., et al. (2016). Clinical outcomes of transplanted modified bone marrow–derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 47, 1817–1824. doi: 10.1161/STROKEAHA.116.012995
Stolt, C. C., Schlierf, A., Lommes, P., Hillgartner, S., Werner, T., Kosian, T., et al. (2006). SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev. Cell 11, 697–709. doi: 10.1016/j.devcel.2006.08.011
Su, W., Aloi, M. S., and Garden, G. A. (2016). MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav. Immun. 52, 1–8. doi: 10.1016/j.bbi.2015.07.003
Suh, M. R., Lee, Y., Kim, J. Y., Kim, S. K., Moon, S. H., Lee, J. Y., et al. (2004). Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 270, 488–498. doi: 10.1016/j.ydbio.2004.02.019
Sun, L.-Q., Guo, G.-L., Zhang, S., and Yang, L.-L. (2018). Effects of MicroRNA-592-5p on hippocampal neuron injury following hypoxic-ischemic brain damage in neonatal mice-involvement of PGD2/DP and PTGDR. Cell. Physiol. Biochem. 45, 458–473. doi: 10.1159/000486923
Sun, Y., Luo, Z. M., Guo, X. M., Su, D. F., and Liu, X. (2015). An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell Neurosci. 9:193. doi: 10.3389/fncel.2015.00193
Taganov, K. D., Boldin, M. P., Chang, K. J., and Baltimore, D. (2006). NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486. doi: 10.1073/pnas.0605298103
Tan, K. S., Armugam, A., Sepramaniam, S., Lim, K. Y., Setyowati, K. D., Wang, C. W., et al. (2009). Expression profile of MicroRNAs in young stroke patients. PLoS One 4:e7689. doi: 10.1371/journal.pone.0007689
Taverna, E., Gotz, M., and Huttner, W. B. (2014). The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502. doi: 10.1146/annurev-cellbio-101011-155801
Tay, T. L., Savage, J. C., Hui, C. W., Bisht, K., and Tremblay, M. E. (2017). Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 595, 1929–1945. doi: 10.1113/JP272134
Taylor, D. D., and Gercel-Taylor, C. (2014). Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130503. doi: 10.1098/rstb.2013.0503
Teng, H., Zhang, Z. G., Wang, L., Zhang, R. L., Zhang, L., Morris, D., et al. (2008). Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. 28, 764–771. doi: 10.1038/sj.jcbfm.9600573
Thion, M. S., Low, D., Silvin, A., Chen, J., Grisel, P., Schulte-Schrepping, J., et al. (2018). Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16. doi: 10.1016/j.cell.2017.11.042
Uccelli, A., Laroni, A., and Freedman, M. S. (2011). Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 10, 649–656. doi: 10.1016/S1474-4422(11)70121-1
Vaccarino, F. M., Schwartz, M. L., Raballo, R., Rhee, J., and Lyn-Cook, R. (1999). Fibroblast growth factor signaling regulates growth and morphogenesis at multiple steps during brain development. Curr. Top. Dev. Biol. 46, 179–200. doi: 10.1016/S0070-2153(08)60329-4
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. doi: 10.1038/ncb1596
van Tilborg, E., Heijnen, C. J., Benders, M. J., van Bel, F., Fleiss, B., Gressens, P., et al. (2016). Impaired oligodendrocyte maturation in preterm infants: potential therapeutic targets. Prog. Neurobiol. 136, 28–49. doi: 10.1016/j.pneurobio.2015.11.002
van Velthoven, C. T., Kavelaars, A., van Bel, F., and Heijnen, C. J. (2010). Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 24, 387–393. doi: 10.1016/j.bbi.2009.10.017
Vasudevan, S. (2012). Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 3, 311–330. doi: 10.1002/wrna.121
Ventura, A., Young, A. G., Winslow, M. M., Lintault, L., Meissner, A., Erkeland, S. J., et al. (2008). Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886. doi: 10.1016/j.cell.2008.02.019
Visvanathan, J., Lee, S., Lee, B., Lee, J. W., and Lee, S. K. (2007). The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–749. doi: 10.1101/gad.1519107
Voloboueva, L. A., Sun, X., Xu, L., Ouyang, Y. B., and Giffard, R. G. (2017). Distinct Effects of miR-210 reduction on neurogenesis: increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J. Neurosci. 37, 3072–3084. doi: 10.1523/JNEUROSCI.1777-16.2017
Volosin, M., Song, W., Almeida, R. D., Kaplan, D. R., Hempstead, B. L., and Friedman, W. J. (2006). Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J. Neurosci. 26, 7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006
Volpe, J. J., Kinney, H. C., Jensen, F. E., and Rosenberg, P. A. (2011). The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440. doi: 10.1016/j.ijdevneu.2011.02.012
Wang, J., Wang, H., Liu, A., Fang, C., Hao, J., and Wang, Z. (2015). Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget 6, 19456–19468. doi: 10.18632/oncotarget.3318
Wang, L., Ke, J., Li, Y., Ma, Q., Dasgupta, C., Huang, X., et al. (2017). Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int. J. Biol. Sci. 13, 76–84. doi: 10.7150/ijbs.17278
Wang, Y., and Yang, G. Y. (2013). MicroRNAs in Cerebral Ischemia. Stroke Treat. 2013:276540. doi: 10.1155/2013/276540
Weaver-Mikaere, L., Gunn, A. J., Mitchell, M. D., Bennet, L., and Fraser, M. (2013). LPS and TNF alpha modulate AMPA/NMDA receptor subunit expression and induce PGE2 and glutamate release in preterm fetal ovine mixed glial cultures. J. Neuroinflam. 10:916. doi: 10.1186/1742-2094-10-153
Wei, J., Blenkiron, C., Tsai, P., James, J. L., Chen, Q., Stone, P. R., et al. (2017). Placental trophoblast debris mediated feto-maternal signalling via small RNA delivery: implications for preeclampsia. Sci. Rep. 7:14681. doi: 10.1038/s41598-017-14180-8
Werner, J. K., and Stevens, R. D. (2015). Traumatic brain injury: recent advances in plasticity and regeneration. Curr. Opin. Neurol. 28, 565–573. doi: 10.1097/WCO.0000000000000265
Whitehead, C. L., Teh, W. T., Walker, S. P., Leung, C., Larmour, L., and Tong, S. (2013). Circulating MicroRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One 8:e78487. doi: 10.1371/journal.pone.0078487
Willis, G. R., Kourembanas, S., and Mitsialis, S. A. (2017). Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4:63. doi: 10.3389/fcvm.2017.00063
Wu, M. L., Zhu, C. C., Qi, Y. Y., Shi, Y. X., Xu, H. N., and Yang, J. R. (2018). [Isolation, Identification and Degradation Characteristics of a 17beta-estradiol Degrading Strain Fusarium sp. KY123915]. Huan Jing Ke Xue 39, 4802–4808. doi: 10.13227/j.hjkx.201711077
Xia, H., Cheung, W. K., Ng, S. S., Jiang, X., Jiang, S., Sze, J., et al. (2012). Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J. Biol. Chem. 287, 9962–9971. doi: 10.1074/jbc.M111.332627
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., et al. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556–1564. doi: 10.1002/stem.1129
Xin, H., Li, Y., and Chopp, M. (2014). Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 8:377. doi: 10.3389/fncel.2014.00377
Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., Cui, Y., et al. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31, 2737–2746. doi: 10.1002/stem.1409
Xiong, Y., Mahmood, A., and Chopp, M. (2017). Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen. Res. 12, 19–22. doi: 10.4103/1673-5374.198966
Xiong, Y., Mahmood, A., Meng, Y., Zhang, Y., Zhang, Z. G., Morris, D. C., et al. (2012). Neuroprotective and neurorestorative effects of thymosin beta4 treatment following experimental traumatic brain injury. Ann. N. Y. Acad. Sci. 1270, 51–58. doi: 10.1111/j.1749-6632.2012.06683.x
Yang, J., Zhang, X., Chen, X., Wang, L., and Yang, G. (2017). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther. Nucleic Acids 7, 278–287. doi: 10.1016/j.omtn.2017.04.010
Yeh, Y. M., Chuang, C. M., Chao, K. C., and Wang, L. H. (2013). MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1alpha. Int. J. Cancer 133, 867–878. doi: 10.1002/ijc.28086
Yeo, R. W., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 65, 336–341. doi: 10.1016/j.addr.2012.07.001
Yin, K.-J., Hamblin, M., and Eugene Chen, Y. (2015). Angiogenesis-regulating microRNAs and ischemic stroke. Curr. Vasc. Pharmacol. 13, 352–365. doi: 10.2174/15701611113119990016
Yin, K.-J., Olsen, K., Hamblin, M., Zhang, J., Schwendeman, S. P., and Chen, Y. E. (2012). Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J. Biol. Chem. 287, 27055–27064. doi: 10.1074/jbc.M112.364414
Yuan, Y., Wang, J. Y., Xu, L. Y., Cai, R., Chen, Z., and Luo, B. Y. (2010). MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J. Clin. Neurosci. 17, 774–778. doi: 10.1016/j.jocn.2009.10.009
Zeng, L., He, X., Wang, Y., Tang, Y., Zheng, C., Cai, H., et al. (2014). MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 21, 37–43. doi: 10.1038/gt.2013.55
Zhang, J., Li, Y., Chen, J., Cui, Y., Lu, M., Elias, S. B., et al. (2005). Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 195, 16–26. doi: 10.1016/j.expneurol.2005.03.018
Zhang, Y., Chopp, M., Liu, X. S., Kassis, H., Wang, X., Li, C., et al. (2015a). MicroRNAs in the axon locally mediate the effects of chondroitin sulfate proteoglycans and cGMP on axonal growth. Dev. Neurobiol. 75, 1402–1419. doi: 10.1002/dneu.22292
Zhang, Y., Chopp, M., Meng, Y., Katakowski, M., Xin, H., Mahmood, A., et al. (2015b). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867. doi: 10.3171/2014.11.Jns14770
Zhang, Y., Ueno, Y., Liu, X. S., Buller, B., Wang, X., Chopp, M., et al. (2013). The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci. 33, 6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013
Zhao, C., Sun, G., Li, S., and Shi, Y. (2009). A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 16, 365–371. doi: 10.1038/nsmb.1576
Zhao, X., He, X., Han, X., Yu, Y., Ye, F., Chen, Y., et al. (2010). MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626. doi: 10.1016/j.neuron.2010.02.018
Zheng, K., Li, H., Huang, H., and Qiu, M. (2012). MicroRNAs and glial cell development. Neuroscientist 18, 114–118. doi: 10.1177/1073858411398322
Zheng, K., Li, H., Zhu, Y., Zhu, Q., and Qiu, M. (2010). MicroRNAs are essential for the developmental switch from neurogenesis to gliogenesis in the developing spinal cord. J. Neurosci. 30, 8245–8250. doi: 10.1523/JNEUROSCI.1169-10.2010
Zhou, J., and Zhang, J. (2014). Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Mol. Med. Rep. 10, 971–976. doi: 10.3892/mmr.2014.2245
Zhou, T., Huang, Y. X., Song, J. W., and Ma, Q. M. (2015). Thymosin beta4 inhibits microglia activation through microRNA 146a in neonatal rats following hypoxia injury. Neuroreport 26, 1032–1038. doi: 10.1097/WNR.0000000000000463
Keywords: perinatal, development, brain injury, miRNAs, biomarkers, exosomes, therapies
Citation: Cho KHT, Xu B, Blenkiron C and Fraser M (2019) Emerging Roles of miRNAs in Brain Development and Perinatal Brain Injury. Front. Physiol. 10:227. doi: 10.3389/fphys.2019.00227
Received: 19 August 2018; Accepted: 21 February 2019;
Published: 28 March 2019.
Edited by:
Carina Mallard, University of Gothenburg, SwedenReviewed by:
Angela Leigh Cumberland, RMIT University, AustraliaAmin Mottahedin, University of Cambridge, United Kingdom
Copyright © 2019 Cho, Xu, Blenkiron and Fraser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mhoyra Fraser, bS5mcmFzZXJAYXVja2xhbmQuYWMubno=
 Kenta Hyeon Tae Cho
Kenta Hyeon Tae Cho Bing Xu1
Bing Xu1 Cherie Blenkiron
Cherie Blenkiron Mhoyra Fraser
Mhoyra Fraser