
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol. , 19 December 2018
Sec. Aquatic Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01845
 Claudia Gatta1†
Claudia Gatta1† Elena De Felice2*†
Elena De Felice2*† Livia D’Angelo1,3*
Livia D’Angelo1,3* Lucianna Maruccio1
Lucianna Maruccio1 Adele Leggieri1
Adele Leggieri1 Carla Lucini1
Carla Lucini1 Antonio Palladino4
Antonio Palladino4 Marina Paolucci5
Marina Paolucci5 Paola Scocco2
Paola Scocco2 Ettore Varricchio5
Ettore Varricchio5 Paolo de Girolamo1
Paolo de Girolamo1Nesfatin-1 (Nesf-1) is an anorexigenic peptide involved in the regulation of homeostatic feeding. Nesf-1 is expressed in the central nervous system and other organs, including pancreas, where it promotes the release of insulin from β-cells. This raises the possibility that Nesf-1 dysfunction could be involved in metabolic disorders, particularly in type 2 diabetes mellitus (T2D). Recently, it has been discovered that dolphins can be a natural animal model that fully replicates human T2D, due to its prolonged glucose tolerance curve and maintenance of a state of hyperglycemia similar to human T2D during fasting. This correspondence suggests that dolphins may be a suitable model for investigating physiological and pathological metabolic disorders. Here, we have characterized Nesf-1 distribution in the pancreas of the common bottlenose dolphin (Tursiops truncatus) and measured plasmatic levels of Nesf-1 and glucose during fasting and post-prandial states. The Mediterranean Marine Mammal Tissue Bank (MMMTB) of the University of Padova provided us with pancreas samples, derived from four animals, and plasma samples, collected before and after the main meal. Interestingly, our results showed that Nesf-1-immunoreactive cells were distributed in Langerhans islets, co-localized with glucagon in α-cells. Similar to humans, dolphin plasma Nesf-1 concentration doesn’t show a statistically significant difference when comparing fasting and post-prandial states. On the other hand, blood glucose levels were significantly higher before than after the main meal. Our data provide a comparative analysis for further studies on the involvement of Nesf-1 in mammalian metabolic disorders.
Nesfatin–1 (Nesf-1) is an 82 amino acid (aa) length polypeptide derived from calcium and DNA binding protein NUCB2 (NEFA/nucleobindin-2). Within the central nervous system (CNS), Nucb2 mRNA is present in different nuclei of the brain involved in feeding behavior. Until now, the mechanism of food intake inhibition via Nesf-1 has not been clarified. Intracerebroventricular administration of Nesf-1 and NUCB2 reduces food intake and body weight: Nesf-1 can act via melatonin system activation, independently of leptin pathway (Oh-I et al., 2006) or through the stimulation of neuropeptide Y (Stengel and Tache, 2013).
Outside the CNS, Nucb2 mRNA is mainly expressed in pancreas, gastric mucosa, duodenum, white adipose tissue and testis (Gonzalez et al., 2009; Shimizu et al., 2009; Stengel et al., 2009; Ramanjaneya et al., 2010; Garcia-Galiano et al., 2012; Kim et al., 2014). It has been shown that NUCB2/Nesf-1 is localized in β-cells of human and rodent pancreas, promoting the release of insulin (Gonzalez et al., 2011). This raises the possibility that impairment of Nesf-1 production and/or secretion could be involved in metabolic disorders, particularly in type 2 diabetes mellitus (T2D) (Gonzalez et al., 2009).
Although different animals have been used, a unique model species that fully complements human T2D has not been yet identified (Cefalu, 2006). Recently, it has been discovered that dolphins have a prolonged glucose tolerance curve and during fasting maintain a state of hyperglycemia similarly to human diabetes mellitus (Venn-Watson et al., 2010, 2012). The main difference with diabetic patients is that dolphins seem to alternate a physiological diabetes that goes off with fasting during the night. Therefore, common bottlenose dolphins might constitute a valid spontaneous model of T2D (Venn-Watson et al., 2010).
We decided to characterize Nesf-1 distribution in pancreas of the common bottlenose dolphin (Tursiops truncatus) and evaluate its pre- and post-prandial plasmatic levels. These data continue previous studies of our group on the neuropeptides of the gastro-entero-pancreatic system of marine mammals (Russo et al., 2012; Gatta et al., 2014). Our aim is to establish a morphological basis for understanding the mechanisms involved in the pathogenesis of mammalian endocrine diseases.
Pancreas and frozen plasma samples of two adult male and two adult female specimens of common bottlenose dolphin were retrieved from the Mediterranean Marine Mammal Tissue Bank (MMMTB) of the Department of Comparative Biomedicine and Food Science of the University of Padova. For morphological studies, pancreas paraffin embedded samples were used. Blood was originally drawn from veins of the tail flukes of four trained dolphins for routine veterinary medical controls. Plasma samples were collected at 10:00 a.m. (fasting state) and 17:30 p.m. (post-prandial state) in four different days, in February and August (two blood draws for each month).
Archival samples of male Wistar rat testis (Garcia-Galiano et al., 2012), stored in the Department of Veterinary Medicine and Animal Production (MVPA) of University of Naples Federico II, were used as positive controls. Ethic approval was not required neither for dolphins nor for rat samples because they were obtained, respectively, from the MMMTB and from the MVPA.
Paraffin embedded sections were processed as reported also in Gatta et al. (2014). The primary antiserum employed is a polyclonal antibody raised in rabbit against Nesf-1 (1:500, Phoenix Pharmaceuticals, cat. No. H-003-24). Background was prevented by previous incubating sections in normal goat serum (NGS) [1:5, 30 min, room temperature (RT)]. Qualitative identification of antigens was detected with DAKO EnVisionTM+ System, Peroxidase. Staining was completed by incubation with 3,3′-diaminobenzidine (DAB)+ substrate-chromogen (Sigma, St. Louis, MO, United States).
For the double Nesf-1/insulin immunostaining, the employed primary antisera were: anti-Nesf-1 (1:50, Phoenix Pharmaceuticals, cat. No. H-003-24) and anti-Insulin (1:50, Abcam, cat. No. ab7842). Sections were incubated overnight (ON) at 4°C with each primary antibody. After the incubation with the primary antibodies, the sections were rinsed several times and incubated, respectively, with Lissamine Rhodamine (1:1000, Jackson Immuno Research Labs, cat. No. 111-085-003 conjugated goat anti-rabbit) and fluorescein AffiniPure Donkey Anti-Guinea Pig IgG (H+L) (1:1000, Jackson Immuno Research Labs, cat. No. 706-095-148), for 2 h, RT.
For the double Nesf-1/glucagon-like peptide-1 (GLP-1) immunostaining, the employed primary antisera were: anti-Nesf-1 (1:50, Phoenix Pharmaceuticals, cat. No. H-003-24) and anti-GLP-1 (1:50, Santa Cruz Biotechnology Inc., cat. No. sc-7782). Sections were incubated at 4°C, ON with anti-Nesf-1, and 48 h with anti-GLP-1. After the incubation with the primary antibodies, the sections were rinsed several times and incubated, respectively, with Lissamine Rhodamine (1:1000, Jackson Immuno Research Labs, cat. No. 111-085-003 conjugated goat anti-rabbit) and 488-Affinipure donkey anti-goat (1:1000, Jackson Immuno Research Labs, cat. No. 705-545-147), for 2 h, RT.
For double staining, background was prevented by previous incubating sections in normal serum (1:5, 30 min, RT). Finally, sections were washed and mounted.
The specificity of immunohistochemical reactions was checked in repeated trials via pre-absorption of primary antibody Nesf-1 (H-003-24; Phoenix Pharmaceuticals) with homologous antigen Nesf-1 (1-45)/Nesf-1, N-terminal (Human) (003-24; Phoenix Pharmaceuticals) (up to 50 mg/ml antiserum in the final dilution). Positive controls were made by sections of rat pancreas (data not shown). Internal reaction controls were carried out by substituting primary antisera or secondary antisera with phosphate buffered saline or normal serum in the specific step (de Girolamo and Lucini, 2011).
Fluorescent and light images were analyzed by Nikon Eclipse 90i. The digital raw images were optimized for image resolution, contrast, evenness of illumination, and background by using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, United States).
Bottlenose dolphin pancreas and rat testis samples were processed as previously described (Gatta et al., 2014). The same blot membrane was stripped and re-probed against β-actin (A5060, Sigma, Sant Louis, MO, United States), used as internal marker. In the specific step, primary polyclonal antibody raised in rabbit against Nesf-1 (1:2000, Phoenix Pharmaceuticals, cat. No. H-003-24) was incubated ON at 4 °C. This was followed by incubation with the secondary goat anti-rabbit IgG (1:10000, Santa Cruz Biotechnology Inc., cat. No. sc-2004) for 1 h, RT.
Nesfatin-1 plasma concentration was measured with an enzyme immunoassay kit for Nesf-1 (1-82) (Human) (EK-003-26; Phoenix Pharmaceuticals Inc.) according to the manufacturer’s protocols.
The glucose plasma levels were measured photometrically on the Abaxis VetScan VS2 chemistry analyzer.
Data were analyzed as comparison of media and any significant difference was determined at a significance level of 0.05 via the application of Student’s t-test, and Pearson correlation coefficient to indicate the extent to which Nesf-1 and glucose were linearly related.
Amino acid sequence of Nesf-1 of T. truncatus accounts for 95% of conservation to human Nesf-1 (Figure 1A). The employed polyclonal antibody is raised against human Nesf-1 (1–45). Furthermore, western blot analysis showed a Nesf-1 immunoreactive band at 50 kDa, as also observed in rat testis employed as positive control (Figure 1B). The internal marker β-actin was detected as a band of about 42 kDa (data not shown).
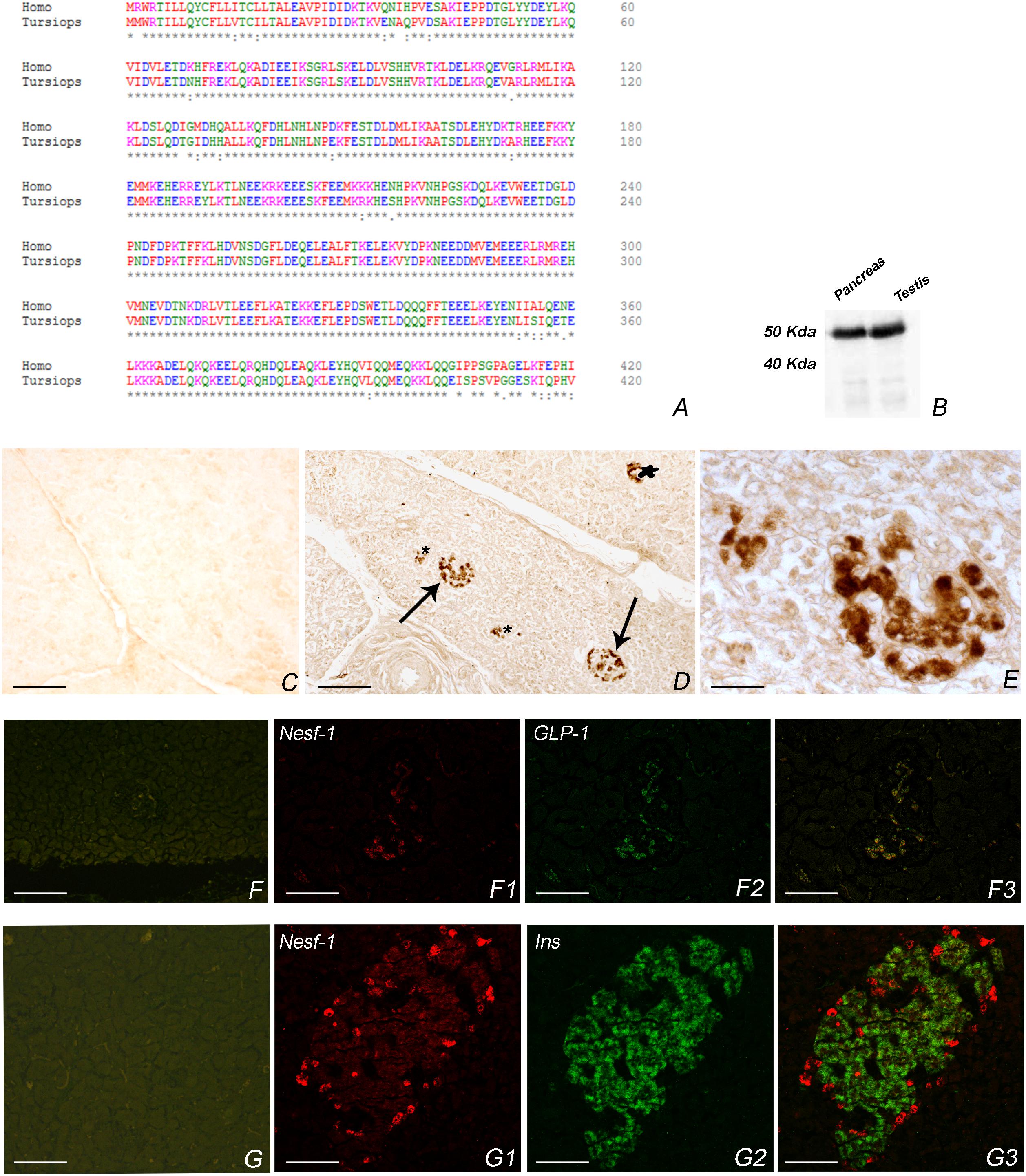
Figure 1. Nesf-1 in Tursiops truncatus. (A) Amino acid sequence alignment T. truncatus and Homo sapiens. Degree of conservation, indicated by asterisks, accounts for 95% (alignment was done with Clustal Omega http://www.ebi.ac.uk/Tools/msa/clustalo/). (B) Western blot in pancreas of bottlenose dolphin and rat testis showing an immunoreactive band of about 50 kDa. (C–G3) Transverse section of pancreas. (C) Negative control of anti-Nesf-1 in the pancreas of T. truncatus. (D) Nesf-1-icPlease cite “Figure 1 F1” inside the text. in pancreatic islet detected by single immunohistochemistry. Arrows indicate large islets; star indicate small islet; asterisks indicate exocrine components. (E) High magnification of (D). (F–F3) Double immunostaining against anti-Nesf-1 and anti-GLP-1. (F) Negative control of anti-Nesf-1 and anti-GLP-1. (F1) Immunofluorescence of anti-Nesf-1 of double anti-Nesf-1/anti-GLP-1. (F2) Immunofluorescence of anti-GLP-1 of double anti-Nesf-1/anti-GLP-1. (F3) Double immunofluorescence of anti-Nesf-1/anti-GLP-1, showing co-localization in α-cells of islet. (G–G3) Double immunostaining against anti-Nesf-1 and anti-Insulin. (G) Negative control of anti-Nesf-1 and anti-Insulin. (G1) Immunofluorescenceof anti-Nesf-1 of double anti-Nesf-1/anti-Insulin. (G2) Immunofluorescence of anti-Insulin of double anti-Nesf-1/anti-Insulin. (G3) Double immunofluorescence of anti-Nesf-1/anti-Insulin showing different distribution pattern. Scale bars C,D,F–F3 = 50 μm, E = 12 μm, G–G3 = 25 μm.
Nesfatin-1-immunoreactive cells (ic) were distributed mainly in the peripheral region of small islets. Additional cords of Nesf-1-ic were detected also in large islets or scattered in the exocrine component (Figures 1D,E,F1,G1).
To characterize the Nesf-1 distribution in pancreatic islets, we performed double immunofluorescence experiments against insulin and GLP-1. In the small islets, insulin-ic were located predominately in the central core while GLP-1 -ic were localized to the periphery; in larger islet cords of insulin-ic are interspersed with cords of glucagon ic-cells (Figures 1F2–G2). The merge results revealed a complete co-localization of Nesf-1 with GLP-1 in alpha cells of islets (Figure 1F3) whereas no co-localization of Nesf-1 and insulin positive beta cells was observed (Figure 1G3).
Negative controls of primary antibodies are in Figures 1C,F,G.
Measurement of Nesf-1 plasma concentration revealed no statistically significant difference (p-value = 0.9328) between pre- and post-prandial states (Figure 2A). Glucose levels were significantly higher (p-value = 0.0039) in the fasting phase than after the meal (Figure 2B). Furthermore, Nesf-1 and glucose plasma levels displayed a slight inverse linear correlation (rpre-prandial = 0.82; rpost-prandial = 0.007) (Figure 2C).
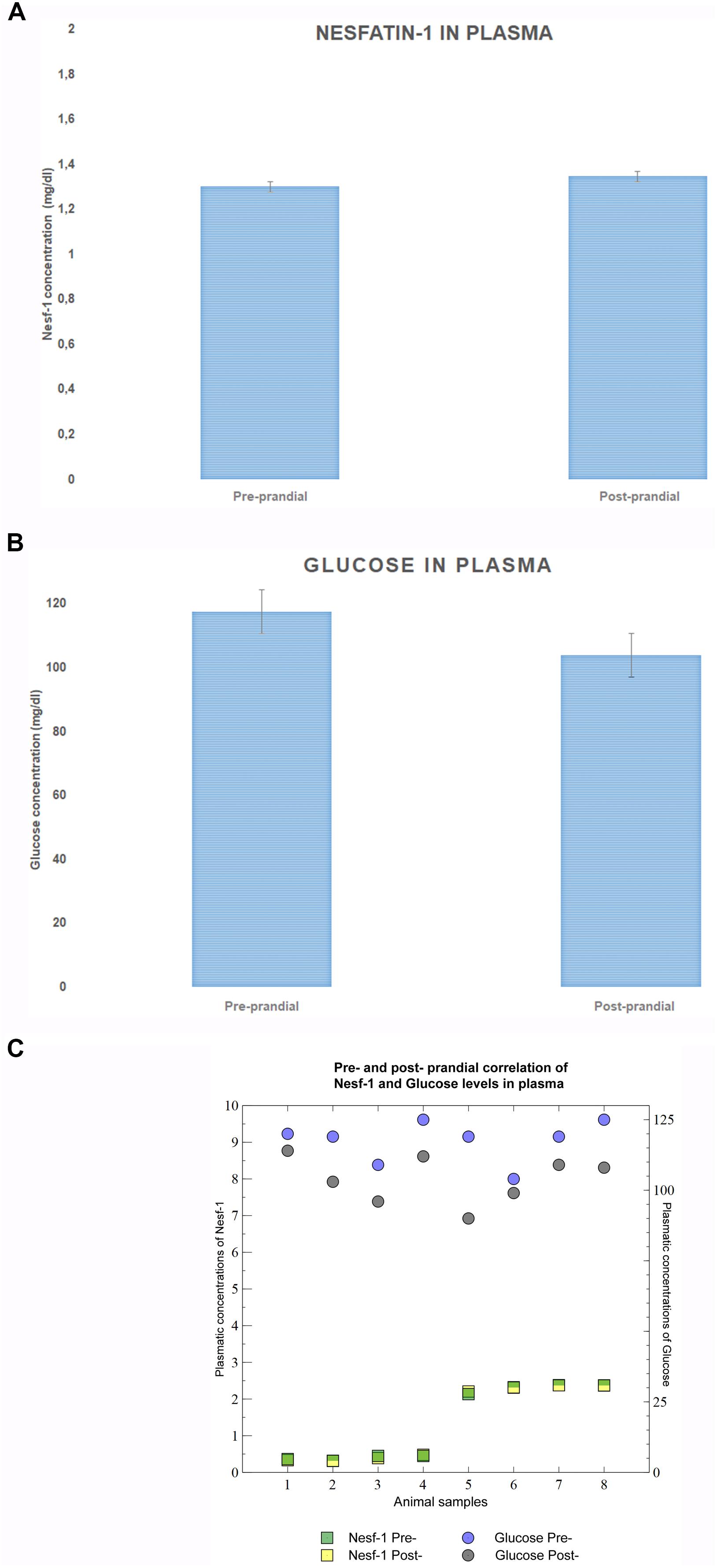
Figure 2. Nesf-1 and glucose plasma levels. (A) Plasma Nesf-1 concentrations didn’t show differences during fasting and post-prandial states. (B) Plasma glucose levels measured during fasting are significantly higher than those post-prandial states (T-test p < 0.01). (C) Pearson correlation coefficient was used to display a slight inverse linear correlation between Nesf-1 and glucose plasma concentration (rpre-prandial = –0.012; rpost-prandial = –0.026).
Here we describe for the first time the presence of Nesf-1 in the pancreas of common bottlenose dolphins. The pancreas of this species, as in most mammals including human, is organized into lobules, with many exocrine acini that produce enzymes necessary for digestion and variable numbers of randomly distributed Langerhans islets, containing glucagon (α-cells) and insulin (β-cells) producing cells (Colegrove and Venn-Watson, 2015; Cozzi et al., 2017).
Our data show that Nesf-1-ic are primarily distributed in the peripheral region of small islets of Langerhans and appear colocalized in glucagon α-cells of pancreas. Previous studies suggested that NUCB2/Nesf-1 is expressed in the endocrine islets of pancreas in rodents (Gonzalez et al., 2009; Stengel et al., 2009) and humans (Foo et al., 2010). Furthermore, it was established that NUCB2/Nesf-1 and insulin are co-localized in β-cells in rodents (Gonzalez et al., 2009) and humans (Foo et al., 2010) suggesting a role in the regulation of glucose homeostasis, particularly promoting the release of insulin (Figlewicz and Benoit, 2009), in concentration-dependent manner (Nakata et al., 2011).
The co-localization of Nesf-1 with GLP-1 we reported in bottlenose dolphin pancreas can be correlated with the peculiar glycemic state of this animal. In addition, our results confirm high serum glucose levels during the fasting state, as already demonstrated (Venn-Watson and Ridgway, 2007), possibly due to the low carbohydrate diet and high demands for cerebral glucose needs. Thus, we hypothesize that Nesf-1 could sustain GLP-1 secretion to maintain high blood sugar levels. In fact, Venn-Watson et al. (2013) showed that dolphins in fasting state had higher levels of glucagon compared to those in post-prandial state (170 and 152 pg/ml, respectively). A recent study (Riva et al., 2011) described a positive correlation between NUCB2 and glucagon gene expression in human, suggesting that Nesf-1 is secreted by β-cell as a response to glucose, and could act via paracrine mode to stimulate glucagon secretion. Riva’s data asserted that Nesf-1 is a stimulator of both insulin and glucagon secretion, hypothesizing different regulatory mechanisms for the two hormones. An analogous regulation can be hypothesized in dolphins. However, future studies are necessary to unravel the mechanism behind biological effects of Nesf-1, including the challenge of identification of the receptor.
Remarkably, when evaluating plasma Nesf-1 levels in common bottlenose dolphins, we do not observe significant differences between fasting or post-prandial states, similarly to data reported in humans (Li et al., 2010). Nesf-1 plasma levels displays a very slight inverse correlation to glucose plasmatic levels, therefore the regulatory mechanisms regulating the blood circulating glucose and Nesf-1 levels requires further investigations.
Overall, the physiological similarities between primates and cetaceans support a shared drive for common glucose metabolism. Only primates and cetaceans have red blood cells ‘extraordinarily’ permeable to glucose (Craik et al., 1998) and share high encephalization quotient (EQ) (Jerison, 1973). These latter relevant aspects can be the key to explain high metabolic demand of the primates and cetaceans’ large brains (Goodwin, 1956).
Our results add new data on the presence and distribution of Nesf-1 in the pancreas of common bottlenose dolphins. These reports highlight the potentiality of this animal species as natural model to unveil the mechanisms involved in human T2D. Further research is needed to fully understand the regulatory mechanisms underlying the glycemic state in dolphins.
All data generated or analyzed during this study are included in this published article.
For the present Please confirm if the Ethics Statement included here is fine.study pancreas and frozen plasma samples of two adult male and two adult female specimens of common bottlenose dolphin (Tursiops truncatus) stored at the Mediterranean Marine Mammal Tissue Bank (MMMTB) of the Department of Comparative Biomedicine and Food Science of the University of Padova (http://www.marinemammals.eu) were used. The MMMTB is a recognized CITES institution (IT020) that stores tissues removed from stranded animals or from marine mammals who died in captivity and were referred for postmortem.
PdG and LDA conceived and supervised the entire study. CG, LM, and AL collected the data. LDA and AP analyzed and interpreted the data. EDF drafted the manuscript. AL and LDA made the figures. CL, PS, MP, and EV critically revised the manuscript. All authors have approved the submitted manuscript.
This work was supported by grants of University of Naples Federico II 409/2017.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We sincerely thank the Mediterranean Marine Mammal Tissue Bank (MMMTB) of the Department of Comparative Biomedicine and Food Science of the University of Padova for providing us tissue and blood samples. We are grateful to professor Bruno Cozzi for his critical revision of the manuscript.
a.m., ante meridiem; CNS, central nervous system; g, relative centrifuge force; GLP-1, glucagon-like peptide-1; h, hour; ic, immunoreactive cells; kDa, kilodalton; min, minute; Nesf-1, Nesfatin-1; NUCB2, NEFA/nucleobindin-2; ON, over-night; p.m., post meridiem; rpm, revolution per minute; RT, room temperature; T2D, type 2 diabetes mellitus; V, Volt; W, Watt; α, alpha; β, beta.
Cefalu, W. T. (2006). Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 47, 186–198. doi: 10.1093/ilar.47.3.186
Colegrove, K. T., and Venn-Watson, S. (2015). Histomorphology of the bottlenose dolphin (Tursiops truncatus) pancreas and association of increasing islet b-cell size with chronic hypercholesterolemia. Gen. Comp. Endocrinol. 214, 17–23. doi: 10.1016/j.ygcen.2015.02.023
Cozzi, B., Huggenberger, S., and Oelschläger, H. H. (2017). Anatomy of Dolphins Insights into Body Structure and Function. Amsterdam: Academic Press.
Craik, J. D., Young, J. D., and Chesseman, C. I. (1998). GLUT-1 mediation of rapid glucose transport in dolphin (Tursiops truncatus) red blood cells. Am. J. Physiol. 274, R112–R119. doi: 10.1152/ajpregu.1998.274.1
de Girolamo, P., and Lucini, C. (2011). Neuropeptide localization in nonmammalian vertebrates. Methods Mol. Biol. 789, 37–56. doi: 10.1007/978-1-61779-310-3_2
Figlewicz, D. P., and Benoit, S. C. (2009). Insulin, leptin, and food reward: update 2008. Am. J. Physiol. Regul. 296, 9–19. doi: 10.1152/ajpregu.90725.2008
Foo, K. S., Brauner, H., Ostenson, C. G., and Broberger, C. (2010). Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J. Endocrinol. 204, 255–263. doi: 10.1677/JOE-09-0254
Garcia-Galiano, D., Pineda, R., Ilhan, T., Castellano, J. M., Ruiz-Pino, F., Sánchez-Garrido, M. A., et al. (2012). Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin 1, in the testis. Endocrinology 153, 1959–1971. doi: 10.1210/en.2011-2032
Gatta, C., Russo, F.,Russolillo, M. G., Varricchio, E., Paolucci, M., Castaldo, L., et al. (2014). The orexin system in the enteric nervous system of the bottlenose dolphin (Tursiops truncatus). PLoS One 9:e105009. doi: 10.1371/journal.pone.0105009
Gonzalez, R., Reingold, B. K., Gao, X., Gaidhu, M. P., Tsushima, R. G., and Unniappan, S. (2011). Nesf-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet beta- and MIN6 cells. J. Endocrinol. 208, R9–R16. doi: 10.1530/JOE-10-0492
Gonzalez, R., Tiwari, A., and Unniappan, S. (2009). Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem. Biophys. Res. Commun. 381, 643–648. doi: 10.1016/j.bbrc.2009.02.104
Goodwin, R. F. (1956). The distribution of sugar between red cells and plasma: variations associated with age and species. J. Physiol. 134, 88–101. doi: 10.1113/jphysiol.1956.sp005625
Jerison, H. J. (1973). Evolution of the Brain and Intelligence. New York, NY: Academic Press, 1–482.
Kim, J., Chung, Y., Kim, H., Im, E., Lee, H., and Yang, H. (2014). The tissue distribution of Nesf-1/NUCB2 in mouse. Dev. Reprod. 18, 301–309. doi: 10.12717/DR.2014.18.4.301
Li, Q. C., Wang, H. Y., Chen, X., Guan, H. Z., and Jiang, Z. Y. (2010). Fasting plasma levels of Nesf-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of Nesf-1 level in normal humans regulat. Peptides 159, 72–77. doi: 10.1016/j.regpep.2009.11.003
Nakata, M., Manaka, K., Yamamoto, S., Mori, M., and Yada, T. (2011). Nesf-1enhances glucose-induced insulin secretion by promoting Ca (2+) influx through L-type channels in mouse islet beta-cells. Endocr. J. 58, 305–313. doi: 10.1507/endocrj.K11E-056
Oh-I, S., Shimizu, H., Satoh, T., Okada, S., Adachi, S., Inoue, K., et al. (2006). Identification of Nesf-1 as a satiety molecule in the hypothalamus. Nature 443, 709–712. doi: 10.1038/nature05162
Ramanjaneya, M., Chen, J., Brown, J. E., Tripathi, G., Hallschmid, M., Patel, S., et al. (2010). Identification of Nesf-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology 151, 3169–3180. doi: 10.1210/en.2009-1358
Riva, M., Nitert, M. D., Voss, U., Sathanoori, R., Lindqvist, A., Ling, C., et al. (2011). Nesf-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res. 346, 393–405. doi: 10.1007/s00441-011-1268-5
Russo, F., Gatta, C., de Girolamo, P., Cozzi, B., Giurisato, M., Lucini, C., et al. (2012). Expression and immunohistochemical detection of leptin-like peptide in the gastrointestinal tract of the South American sea lion (Otaria flavescens) and the bottlenose dolphin (Tursiops truncatus). Anat. Rec. 295, 1482–1493. doi: 10.1002/ar.22532
Shimizu, H., Oh-I, S., Hashimoto, K., Nakata, M., Yamamoto, S., Yoshida, N., et al. (2009). Peripheral administration of Nesf-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology 150, 662–671. doi: 10.1210/en.2008-0598
Stengel, A., Goebel, M., Yakubov, I., Wang, L., Witcher, D., Coskun, T., et al. (2009). Identification and characterization of Nesf-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150, 232–238. doi: 10.1210/en.2008-0747
Stengel, A., and Tache, Y. (2013). Role of brain NUCB2/nesfatin-1 in the regulation of food intake. Curr. Pharm. Des. 19, 6955–6959. doi: 10.2174/138161281939131127125735
Venn-Watson, S., Benham, C., Carlin, K., DeRienzo, D., and StLeger, J. (2012). Hemochromatosis and fatty change: building evidence for insulin resistance in bottlenose dolphins (Tursiops truncatus). J. Zoo Wildl. Med. 43, S35–S47. doi: 10.1638/2011-0146.1
Venn-Watson, S., Carlin, K., and Ridgway, S. (2010). Dolphins as animal models for type 2 diabetes: sustained, post-prandial hyperglycemia and hyperinsulinemia. Gen. Comp. Endocrinol. 170, 193–199. doi: 10.1016/j.ygcen.2010.10.005
Venn-Watson, S., and Ridgway, S. (2007). Big brains and blood glucose: common ground for diabetes mellitus in humans and healthy dolphins. Comp. Med. 57, 390–395.
Keywords: Nesf-1, pancreas, common bottlenose dolphin, glycemic state, type 2 diabetes mellitus
Citation: Gatta C, De Felice E, D’Angelo L, Maruccio L, Leggieri A, Lucini C, Palladino A, Paolucci M, Scocco P, Varricchio E and de Girolamo P (2018) The Case Study of Nesfatin-1 in the Pancreas of Tursiops truncatus. Front. Physiol. 9:1845. doi: 10.3389/fphys.2018.01845
Received: 08 August 2018; Accepted: 07 December 2018;
Published: 19 December 2018.
Edited by:
Jose Pablo Vazquez-Medina, University of California, Berkeley, United StatesReviewed by:
Miwa Suzuki, Nihon University, JapanCopyright © 2018 Gatta, De Felice, D’Angelo, Maruccio, Leggieri, Lucini, Palladino, Paolucci, Scocco, Varricchio and de Girolamo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena De Felice, ZWxlbmEuZGVmZWxpY2VAdW5pY2FtLml0 Livia D’Angelo, bGl2aWEuZGFuZ2Vsb0B1bmluYS5pdA==
†Co-first authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.