- 1Department of Clinical Sciences and Stomatology, Marche Polytechnic University, Ancona, Italy
- 2Department of Oral and Head-Neck Surgery, Umberto I General Hospital, Marche Polytechnic University, Ancona, Italy
- 3National Institute of Health and Science of Aging, INRCA, Ancona, Italy
Oral squamous cell carcinoma (OSCC) is the most common head and neck malignancy, and despite advances in cancer therapies, the overall 5-year survival rate has remained below 50% over the past decades. OSCC is typically preceded by potentially malignant disorders (PMD), but distinguishing high-risk from low-risk PMD is challenging. In the last years, several diagnostic methods as light-based detection systems (LBDS) have been proposed to facilitate the detection of OSCC and PMD. Furthermore, the recent evolution of nanotechnology may provide new opportunities to detect PMD and OSCC at an early stage. Indeed, several preclinical studies showed the potential of nanotechnology to enhance diagnostic accuracy. For these reasons, it is fundamental to conduct studies to evaluate the efficacy of nanotechnology implementation in LBDS. The aim of this article is to review the current literature on LBDS and to provide a summary of the sensitivity and specificity of each technique, and possible future applications of nanotechnologies. The LBDS showed great potential for screening and monitoring oral lesions, but there are several factors that hinder an extensive use of these devices. These devices seem to be useful in assessing lesion margins that must be biopsied. However, to date, conventional oral examination, and tissue biopsy remain the gold standard for OSCC diagnosis. The use of nanotechnologies could be the next step in the evolution of LBDS, thus providing devices that can help clinicians to detect and better monitor oral lesions.
Introduction
Oral squamous cell carcinoma (OSCC) is the most common head and neck malignancy and the sixth most common tumour worldwide (Warnakulasuriya, 2009). Despite advances in therapies, the overall 5-year survival rate has remained unchanged during the past decades, mainly due to delayed diagnosis (Gomez et al., 2009). OSCC is typically preceded by potentially malignant disorders (PMD), a group of clinically suspicious lesions. Although the majority of PMD do not progress to OSCC, distinguishing high-risk PMD from low-risk PMD is challenging for dental practitioners (Yang et al., 2018). Furthermore, patients treated for OSCC are at risk of developing recurrences and secondary primary tumours, due to field cancerization and/or incomplete surgery (Day and Blot, 1992). Currently, conventional oral examination (COE), consisting in visual and tactile assessment of accessible oral structures, followed by tissue biopsy still constitutes the gold standard for diagnosis of PMD and OSCC. However, there are some limitations of this procedure, such as sampling bias that can lead to underdiagnosis or misdiagnosis, particularly in multifocal lesions (Yang et al., 2018).
The possibility of making an early diagnosis is crucial for reducing high mortality rate and morbidity of OSCC patients. In the last years, several light-based detection systems (LBDS), based on optical properties of biological tissues, have emerged with claims of enhancing oral mucosal examinations and facilitating the detection of PMD and OSCC.
Furthermore, the recent evolution of nanotechnology may provide new opportunities to detect PMD and OSCC at an early stage (El-Sayed et al., 2005). Several preclinical studies showed the potential to enhance diagnostic accuracy of optical diagnostic technologies (e.g., Raman spectroscopy) or imaging techniques (e.g., Magnetic resonance imaging) (Chen et al., 2018). Among the latter techniques, reflectance confocal microscopy seems to improve the evaluation of oral lesions, by detecting backscattered light from illuminated tissue, producing high resolution tissue map. However, several technological limitations need to be resolved to validate diagnostic accuracy (Lucchese et al., 2016). LBDS showed several advantages compared to the aforementioned approaches, such as low cost and ease of use. For these reasons, it is fundamental to conduct studies to evaluate the efficacy of nanotechnology implementation in LBDS.
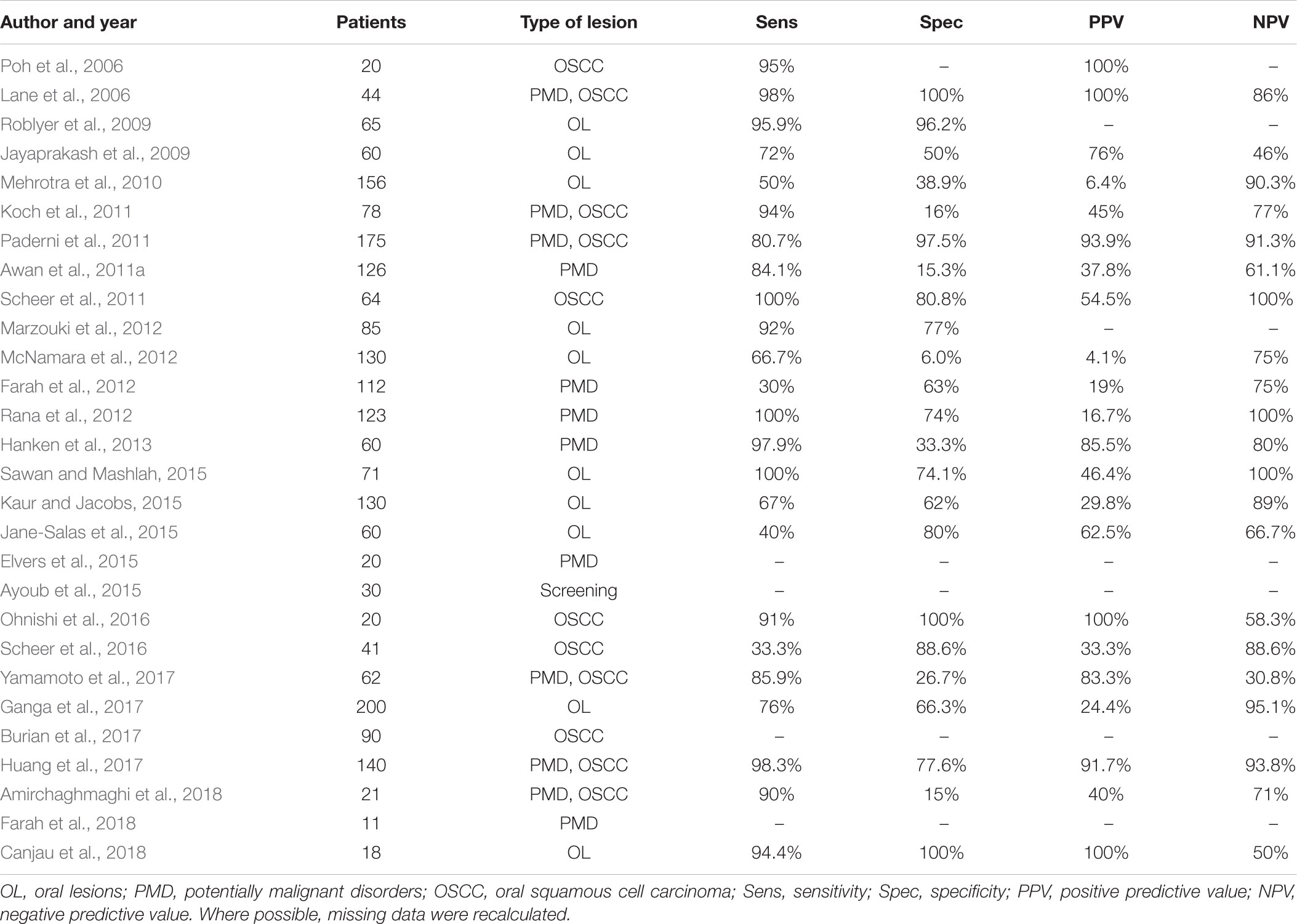
The aim of this article is to review the literature on LBDS currently on the market (Tables 1, 2), providing clinicians with a better understanding of their advantages and limits, and possible future applications of nanotechnologies.
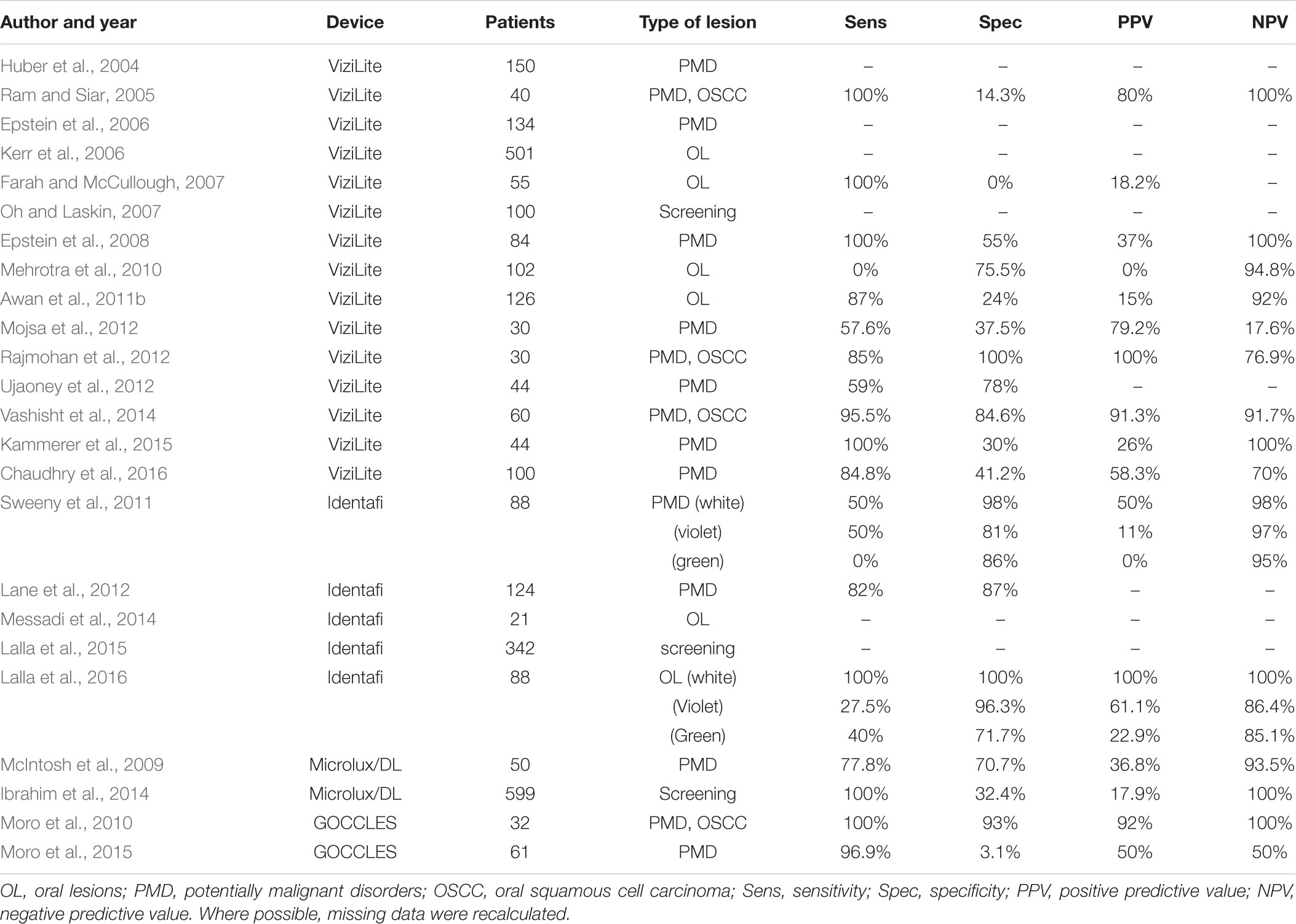
TABLE 2. Published studies on light-based detection systems other than VELscope® for clinical detection of oral lesions.
ViziLite®
ViziLite® (Zila Pharmaceuticals, Phoenix, AZ, United States) is a chemiluminescence-based detection device designed to facilitate the early identification of PMD and OSCC. In 2002 ViziLite® became the first device approved by FDA for this purpose (Oh and Laskin, 2007). This is a disposable capsule formed by an outer shell of flexible plastic containing acetyl salicylic acid and an inner glass vial containing hydrogen peroxide. To activate it, the capsule is bent to break the inner glass vial, triggering the reaction of the chemicals contained in the two compartments. Consequently, a bluish-white light (430–580 nm) is produced, lasting for 10 min (Liu et al., 2016). A modified version (ViziLite® PLUS) consists of a combination of chemiluminescence and toluidine blue (TB) marking system, an acidophilic dye that selectively stains acidic substances such as DNA. Furthermore, an accessory eyewear has been developed, to allow better isolation of chemiluminescent light (Sambandham et al., 2013). Its clinical use requires a 1-min rinse of 1% acetic acid solution, to desiccate oral tissues, followed by oral examination with 430–580 nm wavelength light. The altered epithelial cells, due to higher nuclear/cytoplasmic ratio, reflect the light and cause the appearance of an “aceto-white” lesion, whereas normal cells appear blue (Nagi et al., 2016).
The first studies regarding ViziLite®, published in 2004–2007, were conducted on subjects with different clinical conditions, ranging from normal mucosa to diagnosed OSCC, with the aim to explore the diagnostic utility of chemiluminescence-based strategies (Table 2). In the first reported study, ViziLite® identified a subclinical lesion, suggesting its utility in identifying occult epithelial abnormalities (Huber et al., 2004). In a small cohort of patients with oral lesions, ViziLite® appears to be a better diagnostic tool than TB in detection of OSCC and PMD (Ram and Siar, 2005). Another study highlights the ability of ViziLite® to show brighter and better demarcated lesions than using incandescent light, aiming to enhance the identification of lesions that could be biopsied (Epstein et al., 2006). Unfortunately, these results have not been confirmed, which failed to demonstrate significant improvement in identification and evaluation of oral lesions (Farah and McCullough, 2007; Oh and Laskin, 2007). Interestingly, a cross-sectional study compared ViziLite® and VELscope® to evaluate their clinical utility in diagnosing oral lesions, but the authors failed to demonstrate any superiority to COE (Mehrotra et al., 2010).
For this reason, a new version of this device has been developed (ViziLite® PLUS), aiming to improve the diagnostic power of TB marking system. First results were encouraging, showing that TB reduced the number of false positive cases leaving the false negative rate unchanged (Epstein et al., 2008). On the contrary, ViziLite® PLUS does not seems to be useful to detect malignancies in patients with clearly visible lesions (Mojsa et al., 2012). In fact, some authors described the better diagnostic accuracy of ViziLite® with respect to TB staining alone (Rajmohan et al., 2012; Vashisht et al., 2014), justifying the combined use of these two techniques.
Recently, the results of a clinical study suggested that, although the adjunct of TB to ViziLite® reduced the false positive cases without increasing the number of false negatives, there are little benefits in using this device in general dental practise (Chaudhry et al., 2016).
In conclusion, despite the fact that ViziLite® facilitates the identification of hyperkeratotic areas and may increase the visibility of mucosal lesions, the main limitation is currently the high proportion of false positive and false negative tests, regarding the identification of dysplastic areas rather than hyperkeratosis (Chhabra et al., 2015).
Velscope®
VELscope® (LED Medical Diagnostics, White Rock, BC, Canada) is a hand-held non-magnifying device for direct visualisation of oral mucosa autofluorescence that became commercially available after FDA approval in 2006 (Ayoub et al., 2015). No need of technical measures, such as the use of dimmed light, pre-rinse or lesion-marking solutions, make VELscope® easy to use. It uses a 120 W arc-lamp and a series of philtres and reflectors optimised for producing 400–460 nm wavelength light. The light emitted reaches oral mucosa and excites endogenous autofluorescence substances, called fluorophores (Yamamoto et al., 2017). Preliminary studies, regarding small groups of patients, gave encouraging results (Table 1). In the first reported study, 44 patients with confirmed oral dysplasia or OSCC were evaluated with both COE and VELscope®. The results showed that the device can differentiate PMD and OSCC from normal oral mucosa, with high sensitivity and specificity levels (Lane et al., 2006). These results were confirmed in a small OSCC cohort study, in which the use of autofluorescence-guided examination was able to identify subclinical high-risk fields with cancerous changes (Poh et al., 2006). In a study conducted on 60 patients using a semi-quantitative grading system for autofluorescence, VELscope® demonstrate good sensitivity and a better ability to recognise high-grade lesions than COE (Jayaprakash et al., 2009). Another study evaluated 65 subjects with VELscope®, using a specific algorithm based on the ratio of red-to-green fluorescence. The authors found that 405 nm wavelength light was able to discriminate neoplastic and non-neoplastic tissue with high sensitivity and specificity (Roblyer et al., 2009).
In a cross-sectional study, 175 patients with at least one clinical lesion were evaluated using VELscope®. However, despite the good results, the authors warned that this device could lead to overdiagnosis if used by non-specialists (Paderni et al., 2011). In fact, in the following years several studies on patients with PMD or OSCC reported low specificity values, highlighting this as the primary limitation of VELscope® (Awan et al., 2011a; Koch et al., 2011; Scheer et al., 2011). For these reason, other authors concluded that VELscope® examination alone does not provide significant diagnostic benefit beyond COE in screening for PMD and OSCC, also due to interobserver variability (Farah et al., 2012; McNamara et al., 2012). These results were confirmed by a study on 200 patients, limiting the utility of autofluorescence for OSCC screening (Ganga et al., 2017).
One effort to overcome these shortcomings consists of adding the VELscope® exam to the COE. Indeed, as reported by several authors, the combination of COE and VELscope® examination in patients with oral lesions could provide a significative diagnostic yield (Marzouki et al., 2012; Rana et al., 2012; Hanken et al., 2013). However, these results must be interpreted carefully due to the different inclusion and exclusion criteria used to select the patient cohorts, which can influence both sensitivity and specificity.
Other studies focused on combining VELscope® and other diagnostic tests, aiming to find out better approaches to improve detection of PMD and OSCC. For example, the combination approaches of tissue autofluorescence and salivary protoporphyrin IX levels seems to be effective to distinguish between normal mucosa and high-risk lesions (Kaur and Jacobs, 2015). The use of quantitative analysis of autofluorescence were developed to solve the problem of interobserver variability. Novel methods such as quadratic discriminant analysis or luminance ratio were promising, showing a strong concordance with histopathological diagnosis (Huang et al., 2017; Yamamoto et al., 2017). Recently, a retrospective study based on oral photograph was conducted to find colour distribution patterns related to neoplastic lesions. The fluorescence analysis showed differences in the red-to-green ratios of neoplastic areas, suggesting its clinical utility to detect early OSCC (Burian et al., 2017).
Recently, tissue autofluorescence was used to investigate biological aspects of oral carcinogenesis. In the first in vitro study, VELscope® was used to investigate the autofluorescence in a rat tongue carcinogenesis model. The results showed significant changes in autofluorescence pattern during progression to dysplasia and carcinoma (Ohnishi et al., 2016). In another study, RNA sequencing technique was used to identify molecular differences related to autofluorescence patterns. Results were encouraging, demonstrating that the autofluorescence-based excision was successful in achieving a clear molecular margin when excising PMD (Farah et al., 2018). These results confirmed those previously reported in literature, in which VELscope® demonstrated that the actual sizes of some lesions are significantly larger than they look clinically (Elvers et al., 2015).
In conclusion, several criticisms have been made about VELscope®, mainly focused to the limited capacity to extend the use of this device in general dental practise. Future research directions are aimed at improving the specificity of this device, allowing wider clinical use of VELscope® in routine general practise (Bhatia et al., 2013).
Identafi®
Identafi® (StarDental - DentalEZ, Lancaster, PA, United States) is a probe-like device designed for multispectral screening of PMD, approved by FDA in 2009 as oral screening device (Vigneswaran et al., 2009). Identafi® has three light sources of different wavelengths: white, violet (405 nm), and green-amber (545 nm) lights, that can be sequentially used in oral examination. While white light provides classical visualisation of oral mucosa, violet light excites endogen fluorophores, enabling the assessment of mucosa autofluorescence, like VELscope®. Green-amber light, through the reflectance spectroscopy, excites haemoglobin molecules in the blood, with the aim to visualise the vasculature (Messadi et al., 2014). A mirror is attached to the probe to help visualise relatively obscure areas in oral cavity.
The first clinical trial with Identafi® was conducted on 88 patients who were treated previously for OSCC (Table 2). Screening results with white, violet, and green lights were compared to each other, showing limited benefits of tissue reflectance and autofluorescence in detecting high-risk lesions (Sweeny et al., 2011). In 2012, was reported a case series of PMD patients with the aim to evaluate the efficacy of Identafi®. Although the results are not clearly described, this device seems to be helpful in identifying characteristics not otherwise visible to the COE (Lane et al., 2012).
In a pilot study, Identafi® was used to evaluate tissue vascularity of PMD and to compare with the histological grading of the lesions using a vascular marker (CD34). The results found a correlation between tissue reflectance and histological assessment of vascular structure, in both OSCC and non-cancerous lesions (Messadi et al., 2014).
Two studies on the effectiveness of Identafi® were conducted on Australian population. In the first one, 342 urban Indigenous community members were screened for oral lesions using reflectance spectroscopy and autofluorescence imaging. Identafi® improved the visibility of oral cavity lesions and was capable to find new lesions not seen during COE, although the prevalence of oral pigmentation in this community could hamper the use of autofluorescence screening systems (Lalla et al., 2015). In the second study, 88 patients were evaluated with Identafi®, showing good specificity, negative predictive value, and accuracy (Lalla et al., 2016).
Taken together, the use of Identafi provide the clinician with more data than COE. Unfortunately, the results interpretation requires high level of experience and clinical training in oral pathology, suggesting that its usage should be limited to reference centres for oral pathology (Lalla et al., 2016).
Other Devices
Microlux/DLTM (AdDent Inc., Danbury, CT, United States) is a chemiluminescence-based device which became commercially available after FDA approval in 2005. This device has a diffused blue-white LED light source and a fibre optic light guide (McIntosh et al., 2009). It uses the same principles of ViziLite®: after 1-min rinse with 1% acetic acid, oral examination is performed with 460–555 nm wavelength light. Altered epithelial cells cause the appearance of “aceto-white” lesions, and LED light source makes the lesion more easily recognisable. Furthermore, the use of TB can be used in conjunction with Microlux/DLTM, to enhance the visualisation of dysplastic areas (Ibrahim et al., 2014). In 2009 was conducted a study on 50 patients with oral white lesions to evaluate the efficacy of Microlux/DLTM. The results showed that this device can enhance visualisation of oral mucosa, but no clinical improvement was observed, due to poor ability to distinguish between benign and malignant lesions (McIntosh et al., 2009). Another trial that evaluated the effectiveness of Microlux/DLTM was carried out in 2014. 599 patients were examined with COE and Microlux/DLTM with and without TB, showing high sensitivity but low specificity, indicating that this device is not effective to distinguish between benign and malignant lesions, although seems to be a promising screening test for oral lesions (Ibrahim et al., 2014).
GOCCLES® is a medical device (Pierrel S.p.A, Italy) approved by FDA in 2015. This is a low cost and easy-to-use device consisting in a pair of glasses equipped with special optical philtres that allows autofluorescence detection. Indeed, GOCCLES® was created to provide an easy and low cost mean of identification of autofluorescence abnormalities in oral cavity with the use of any dental curing light (Moro et al., 2015). In 2010 was reported the first study on GOCCLES® in a small cohort of selected patients, showing high sensitivity and specificity (Moro et al., 2010). Five years later, a non-randomised multicentre trial was conducted on patients at risk for OSCC, suggesting the need for further researches to define the diagnostic performance of this device (Moro et al., 2015). Indeed, despite the low cost of GOCCLES® could encourage more careful examinations, its main limitation seems to be the interobserver variability, that could be overcome by proper training.
In recent years, other instruments have been developed and commercialised for facilitate the early identification of oral lesions. Their operating principle is equivalent to the devices described above, using either autofluorescence or chemiluminescence detection. However, their clinical effectiveness is currently hampered by the lack of published studies. For these reasons, they will only be mentioned briefly here. Bio/Screen® (AdDent Inc., Danbury, CT, United States), an instrument with five violet (390–430 nm) high-power LED, designed to enhance the visualisation of mucosal abnormalities through the use of tissue autofluorescence (Kahn et al., 2018).
Orascoptic DKTM system (Orascoptic, Middleton, WI, United States) is another chemiluminescence-based device, designed to improve the visualisation of oral lesions through the use of blue-white LED light and oral rinse of 1% acetic acid solution (Patton et al., 2008).
Sapphire® Plus LD (DenMat Holdings, Lompoc, CA, United States), DentLight DOETM Oral Exam System (DentLight, Richardson, TX, United States), and OralIDTM 2.0 (Forward Science Technologies, Stafford, TX, United States) are other tissue autofluorescence-based devices developed in order to detect oral lesions (Kahn et al., 2018).
Conclusion and Future Perspectives
The diagnostic techniques presented here showed great potential for screening and monitoring oral lesions (Liu et al., 2016). Unfortunately, to date several factors hinder an extensive use of these devices: (1) data do not demonstrate clear superiority of these methods compared to COE; (2) there remains the need for well-designed multicentre prospective studies; (3) these devices exhibit a not-negligible interobserver variability, limiting their use to clinicians with significant experience in oral pathology (Patton et al., 2008; Carreras-Torras and Gay-Escoda, 2015).
However, the current evidence suggests that these devices: (1) seem to be useful in assessing lesion margins that must be biopsied and, therefore, may be useful in surgical management; (2) can be used to investigate biological aspects of oral carcinogenesis, leading to more accurate methods for interpreting data from LBDS; (3) can be enhanced with new approaches used to analyse optical imaging data, with the aim to quantify the results obtained; (4) lowering the costs of these devices could indirectly lead to greater attention for oral lesions among both patients and general dental practitioners, allowing in turn to promote a culture of oral cancer prevention (Carreras-Torras and Gay-Escoda, 2015; Moro et al., 2015); (5) finally, the possibility of implementing LBDS through the use of tissue-marking dyes can in principle allows to develop strategies for the use of nanoparticles. Indeed, nanoparticles can provide molecular targeted imaging, with higher image contrast and resolution. For example, a promising nanotechnology in oral diagnostic research is the quantum dots, consisting in nanometre-sized semiconductor crystals (Walling et al., 2009). The biophysical characteristics of these particles confer several advantages over conventional dyes and fluorescent proteins. The possibility to link the quantum dots to molecules with the ability to target cancer cells make them ideal for diagnostic applications in detecting PMD and OSCC (Chen et al., 2018). Therefore, the use of nanotechnologies could be the next step in the evolution of LBDS, providing devices that can help clinicians to detect and better monitor oral lesions.
Author Contributions
MM, AS, and MP conceived the literature review. MM and VT described the VELscope® system. AS and RM described the ViziLite® system. AB and GO described the Identafi® system. AP, MP, and GO wrote the concluding remarks. All authors discussed and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amirchaghmaghi, M., Mohtasham, N., Delavarian, Z., Shakeri, M. T., Hatami, M., and Mosannen Mozafari, P. (2018). The diagnostic value of the native fluorescence visualization device for early detection of premalignant/malignant lesions of the oral cavity. Photodiagnosis Photodyn. Ther. 21, 19–27. doi: 10.1016/j.pdpdt.2017.10.019
Awan, K. H., Morgan, P. R., and Warnakulasuriya, S. (2011a). Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 47, 274–277. doi: 10.1016/j.oraloncology.2011.02.001
Awan, K. H., Morgan, P. R., and Warnakulasuriya, S. (2011b). Utility of chemiluminescence (ViziLite) in the detection of oral potentially malignant disorders and benign keratoses. J. Oral Pathol. Med. 40, 541–544. doi: 10.1111/j.1600-0714.2011.01048.x
Ayoub, H. M., Newcomb, T. L., Mccombs, G. B., and Bonnie, M. (2015). The use of fluorescence technology versus visual and tactile examination in the detection of oral lesions: a pilot study. J. Dent. Hyg. 89, 63–71.
Bhatia, N., Lalla, Y., Vu, A. N., and Farah, C. S. (2013). Advances in optical adjunctive AIDS for visualisation and detection of oral malignant and potentially malignant lesions. Int. J. Dent. 2013:194029. doi: 10.1155/2013/194029
Burian, E., Schulz, C., Probst, F., Palla, B., Troltzsch, M., Maglitto, F., et al. (2017). Fluorescence based characterization of early oral squamous cell carcinoma using the visually enhanced light scope technique. J. Craniomaxillofac. Surg. 45, 1526–1530. doi: 10.1016/j.jcms.2017.05.021
Canjau, S., Todea, D. C. M., Sinescu, C., Pricop, M. O., and Duma, V. F. (2018). Fluorescence influence on screening decisions for oral malignant lesions. Rom. J. Morphol. Embryol. 59, 203–209.
Carreras-Torras, C., and Gay-Escoda, C. (2015). Techniques for early diagnosis of oral squamous cell carcinoma: systematic review. Med. Oral Patol. Oral Cir. Bucal 20, e305–e315. doi: 10.4317/medoral.20347
Chaudhry, A., Manjunath, M., Ashwatappa, D., Krishna, S., and Krishna, A. G. (2016). Comparison of chemiluminescence and toluidine blue in the diagnosis of dysplasia in leukoplakia: a cross-sectional study. J. Investig. Clin. Dent. 7, 132–140. doi: 10.1111/jicd.12141
Chen, X. J., Zhang, X. Q., Liu, Q., Zhang, J., and Zhou, G. (2018). Nanotechnology: a promising method for oral cancer detection and diagnosis. J. Nanobiotechnology 16:52. doi: 10.1186/s12951-018-0378-6
Chhabra, N., Chhabra, S., and Sapra, N. (2015). Diagnostic modalities for squamous cell carcinoma: an extensive review of literature-considering toluidine blue as a useful adjunct. J. Maxillofac. Oral Surg. 14, 188–200. doi: 10.1007/s12663-014-0660-6
Day, G. L., and Blot, W. J. (1992). Second primary tumors in patients with oral cancer. Cancer 70, 14–19. doi: 10.1002/1097-0142(19920701)70:1<14::AID-CNCR2820700103>3.0.CO;2-S
El-Sayed, I. H., Huang, X., and El-Sayed, M. A. (2005). Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 5, 829–834. doi: 10.1021/nl050074e
Elvers, D., Braunschweig, T., Hilgers, R. D., Ghassemi, A., Mohlhenrich, S. C., Holzle, F., et al. (2015). Margins of oral leukoplakia: autofluorescence and histopathology. Br. J. Oral Maxillofac. Surg. 53, 164–169. doi: 10.1016/j.bjoms.2014.11.004
Epstein, J. B., Gorsky, M., Lonky, S., Silverman, S. Jr., Epstein, J. D., and Bride, M. (2006). The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec. Care Dentist. 26, 171–174. doi: 10.1111/j.1754-4505.2006.tb01720.x
Epstein, J. B., Silverman, S. Jr., Epstein, J. D., Lonky, S. A., and Bride, M. A. (2008). Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 44, 538–544. doi: 10.1016/j.oraloncology.2007.08.011
Farah, C. S., Kordbacheh, F., John, K., Bennett, N., and Fox, S. A. (2018). Molecular classification of autofluorescence excision margins in oral potentially malignant disorders. Oral Dis. 24, 732–740. doi: 10.1111/odi.12818
Farah, C. S., and McCullough, M. J. (2007). A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite) in the visualisation of oral mucosal white lesions. Oral Oncol. 43, 820–824. doi: 10.1016/j.oraloncology.2006.10.005
Farah, C. S., Mcintosh, L., Georgiou, A., and Mccullough, M. J. (2012). Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 34, 856–862. doi: 10.1002/hed.21834
Ganga, R. S., Gundre, D., Bansal, S., Shirsat, P. M., Prasad, P., and Desai, R. S. (2017). Evaluation of the diagnostic efficacy and spectrum of autofluorescence of benign, dysplastic and malignant lesions of the oral cavity using VELscope. Oral Oncol. 75, 67–74. doi: 10.1016/j.oraloncology.2017.10.023
Gomez, I., Seoane, J., Varela-Centelles, P., Diz, P., and Takkouche, B. (2009). Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 117, 541–546. doi: 10.1111/j.1600-0722.2009.00672.x
Hanken, H., Kraatz, J., Smeets, R., Heiland, M., Assaf, A. T., Blessmann, M., et al. (2013). The detection of oral pre- malignant lesions with an autofluorescence based imaging system (VELscope) - a single blinded clinical evaluation. Head Face Med. 9:23. doi: 10.1186/1746-160X-9-23
Huang, T. T., Huang, J. S., Wang, Y. Y., Chen, K. C., Wong, T. Y., Chen, Y. C., et al. (2017). Novel quantitative analysis of autofluorescence images for oral cancer screening. Oral Oncol. 68, 20–26. doi: 10.1016/j.oraloncology.2017.03.003
Huber, M. A., Bsoul, S. A., and Terezhalmy, G. T. (2004). Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 35, 378–384.
Ibrahim, S. S., Al-Attas, S. A., Darwish, Z. E., Amer, H. A., and Hassan, M. H. (2014). Effectiveness of the Microlux/DLTM chemiluminescence device in screening of potentially malignant and malignant oral lesions. Asian Pac. J. Cancer Prev. 15, 6081–6086. doi: 10.7314/APJCP.2014.15.15.6081
Jane-Salas, E., Blanco-Carrion, A., Jover-Armengol, L., and Lopez-Lopez, J. (2015). Autofluorescence and diagnostic accuracy of lesions of oral mucosa: a pilot study. Braz. Dent. J. 26, 580–586. doi: 10.1590/0103-6440201300181
Jayaprakash, V., Sullivan, M., Merzianu, M., Rigual, N. R., Loree, T. R., Popat, S. R., et al. (2009). Autofluorescence-guided surveillance for oral cancer. Cancer Prev. Res. 2, 966–974. doi: 10.1158/1940-6207.CAPR-09-0062
Kahn, M. A., Hall, J. M., and American Dental Association (2018). The ADA Practical Guide to Soft Tissue Oral Disease. Hoboken, NJ: Wiley. doi: 10.1002/9781119437277
Kammerer, P. W., Rahimi-Nedjat, R. K., Ziebart, T., Bemsch, A., Walter, C., Al-Nawas, B., et al. (2015). A chemiluminescent light system in combination with toluidine blue to assess suspicious oral lesions-clinical evaluation and review of the literature. Clin. Oral Investig. 19, 459–466. doi: 10.1007/s00784-014-1252-z
Kaur, J., and Jacobs, R. (2015). Combination of Autofluorescence imaging and salivary protoporphyrin in Oral precancerous and cancerous lesions: Non-invasive tools. J. Clin. Exp. Dent. 7, e187–e191. doi: 10.4317/jced.52100
Kerr, A. R., Sirois, D. A., and Epstein, J. B. (2006). Clinical evaluation of chemiluminescent lighting: an adjunct for oral mucosal examinations. J. Clin. Dent. 17, 59–63.
Koch, F. P., Kaemmerer, P. W., Biesterfeld, S., Kunkel, M., and Wagner, W. (2011). Effectiveness of autofluorescence to identify suspicious oral lesions–a prospective, blinded clinical trial. Clin. Oral Investig. 15, 975–982. doi: 10.1007/s00784-010-0455-1
Lalla, Y., Matias, M., and Farah, C. S. (2015). Oral mucosal disease in an Australian urban Indigenous community using autofluorescence imaging and reflectance spectroscopy. Aust. Dent. J. 60, 216–224. doi: 10.1111/adj.12320
Lalla, Y., Matias, M. A., and Farah, C. S. (2016). Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy. J. Am. Dent. Assoc. 147, 650–660. doi: 10.1016/j.adaj.2016.03.013
Lane, P., Follen, M., and Macaulay, C. (2012). Has fluorescence spectroscopy come of age? A case series of oral precancers and cancers using white light, fluorescent light at 405 nm, and reflected light at 545 nm using the Trimira Identafi 3000. Gend. Med. 9, S25–S35. doi: 10.1016/j.genm.2011.09.031
Lane, P. M., Gilhuly, T., Whitehead, P., Zeng, H., Poh, C. F., Ng, S., et al. (2006). Simple device for the direct visualization of oral-cavity tissue fluorescence. J. Biomed. Opt. 11:024006.
Liu, D., Zhao, X., Zeng, X., Dan, H., and Chen, Q. (2016). Non-Invasive Techniques for Detection and Diagnosis of Oral Potentially Malignant Disorders. Tohoku J. Exp. Med. 238, 165–177. doi: 10.1620/tjem.238.165
Lucchese, A., Gentile, E., Romano, A., Maio, C., Laino, L., and Serpico, R. (2016). The potential role of in vivo reflectance confocal microscopy for evaluating oral cavity lesions: a systematic review. J. Oral Pathol. Med. 45, 723–729. doi: 10.1111/jop.12454
Marzouki, H. Z., Tuong Vi Vu, T., Ywakim, R., Chauvin, P., Hanley, J., and Kost, K. M. (2012). Use of fluorescent light in detecting malignant and premalignant lesions in the oral cavity: a prospective, single-blind study. J. Otolaryngol. Head Neck Surg. 41, 164–168.
McIntosh, L., Mccullough, M. J., and Farah, C. S. (2009). The assessment of diffused light illumination and acetic acid rinse (Microlux/DL) in the visualisation of oral mucosal lesions. Oral Oncol. 45, e227–e231. doi: 10.1016/j.oraloncology.2009.08.001
McNamara, K. K., Martin, B. D., Evans, E. W., and Kalmar, J. R. (2012). The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114, 636–643. doi: 10.1016/j.oooo.2012.07.484
Mehrotra, R., Singh, M., Thomas, S., Nair, P., Pandya, S., Nigam, N. S., et al. (2010). A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. J. Am. Dent. Assoc. 141, 151–156. doi: 10.14219/jada.archive.2010.0132
Messadi, D. V., Younai, F. S., Liu, H. H., Guo, G., and Wang, C. Y. (2014). The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions. Int. J. Oral Sci. 6, 162–167. doi: 10.1038/ijos.2014.39
Mojsa, I., Kaczmarzyk, T., Zaleska, M., Stypulkowska, J., Zapala-Pospiech, A., and Sadecki, D. (2012). Value of the ViziLite Plus System as a diagnostic aid in the early detection of oral cancer/premalignant epithelial lesions. J. Craniofac. Surg. 23, e162–e164. doi: 10.1097/SCS.0b013e31824cdbea
Moro, A., De Waure, C., Di Nardo, F., Spadari, F., Mignogna, M. D., Giuliani, M., et al. (2015). The GOCCLES(R) medical device is effective in detecting oral cancer and dysplasia in dental clinical setting. Results from a multicentre clinical trial. Acta Otorhinolaryngol. Ital. 35, 449–454. doi: 10.14639/0392-100X-922
Moro, A., Di Nardo, F., Boniello, R., Marianetti, T. M., Cervelli, D., Gasparini, G., et al. (2010). Autofluorescence and early detection of mucosal lesions in patients at risk for oral cancer. J. Craniofac. Surg. 21, 1899–1903. doi: 10.1097/SCS.0b013e3181f4afb4
Nagi, R., Reddy-Kantharaj, Y. B., Rakesh, N., Janardhan-Reddy, S., and Sahu, S. (2016). Efficacy of light based detection systems for early detection of oral cancer and oral potentially malignant disorders: systematic review. Med. Oral Patol. Oral Cir. Bucal 21, e447–e455. doi: 10.4317/medoral.21104
Oh, E. S., and Laskin, D. M. (2007). Efficacy of the ViziLite system in the identification of oral lesions. J. Oral Maxillofac. Surg. 65, 424–426. doi: 10.1016/j.joms.2006.10.055
Ohnishi, Y., Fujii, T., Ugaki, Y., Yasui, H., Watanabe, M., Dateoka, S., et al. (2016). Usefulness of a fluorescence visualization system for the detection of oral precancerous and early cancerous lesions. Oncol. Rep. 36, 514–520. doi: 10.3892/or.2016.4776
Paderni, C., Compilato, D., Carinci, F., Nardi, G., Rodolico, V., Lo Muzio, L., et al. (2011). Direct visualization of oral-cavity tissue fluorescence as novel aid for early oral cancer diagnosis and potentially malignant disorders monitoring. Int. J. Immunopathol. Pharmacol. 24, 121–128. doi: 10.1177/03946320110240S221
Patton, L. L., Epstein, J. B., and Kerr, A. R. (2008). Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J. Am. Dent. Assoc. 139, 896–905;quiz993–894. doi: 10.14219/jada.archive.2008.0276
Poh, C. F., Zhang, L., Anderson, D. W., Durham, J. S., Williams, P. M., Priddy, R. W., et al. (2006). Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin. Cancer Res. 12, 6716–6722. doi: 10.1158/1078-0432.CCR-06-1317
Rajmohan, M., Rao, U. K., Joshua, E., Rajasekaran, S. T., and Kannan, R. (2012). Assessment of oral mucosa in normal, precancer and cancer using chemiluminescent illumination, toluidine blue supravital staining and oral exfoliative cytology. J. Oral Maxillofac. Pathol. 16, 325–329. doi: 10.4103/0973-029X.102476
Ram, S., and Siar, C. H. (2005). Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int. J. Oral Maxillofac. Surg. 34, 521–527. doi: 10.1016/j.ijom.2004.10.008
Rana, M., Zapf, A., Kuehle, M., Gellrich, N. C., and Eckardt, A. M. (2012). Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: a prospective randomized diagnostic study. Eur. J. Cancer Prev. 21, 460–466. doi: 10.1097/CEJ.0b013e32834fdb6d
Roblyer, D., Kurachi, C., Stepanek, V., Williams, M. D., El-Naggar, A. K., Lee, J. J., et al. (2009). Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev. Res. 2, 423–431. doi: 10.1158/1940-6207.CAPR-08-0229
Sambandham, T., Masthan, K. M., Kumar, M. S., and Jha, A. (2013). The application of vizilite in oral cancer. J. Clin. Diagn. Res. 7, 185–186. doi: 10.7860/JCDR/2012/5163.2704
Sawan, D., and Mashlah, A. (2015). Evaluation of premalignant and malignant lesions by fluorescent light (VELscope). J. Int. Soc. Prev. Commun. Dent. 5, 248–254. doi: 10.4103/2231-0762.159967
Scheer, M., Fuss, J., Derman, M. A., Kreppel, M., Neugebauer, J., Rothamel, D., et al. (2016). Autofluorescence imaging in recurrent oral squamous cell carcinoma. Oral Maxillofac. Surg. 20, 27–33. doi: 10.1007/s10006-015-0520-7
Scheer, M., Neugebauer, J., Derman, A., Fuss, J., Drebber, U., and Zoeller, J. E. (2011). Autofluorescence imaging of potentially malignant mucosa lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 111, 568–577. doi: 10.1016/j.tripleo.2010.12.010
Sweeny, L., Dean, N. R., Magnuson, J. S., Carroll, W. R., Clemons, L., and Rosenthal, E. L. (2011). Assessment of tissue autofluorescence and reflectance for oral cavity cancer screening. Otolaryngol. Head Neck Surg. 145, 956–960. doi: 10.1177/0194599811416773
Ujaoney, S., Motwani, M. B., Degwekar, S., Wadhwan, V., Zade, P., Chaudhary, M., et al. (2012). Evaluation of chemiluminescence, toluidine blue and histopathology for detection of high risk oral precancerous lesions: a cross-sectional study. BMC Clin. Pathol. 12:6. doi: 10.1186/1472-6890-12-6
Vashisht, N., Ravikiran, A., Samatha, Y., Rao, P. C., Naik, R., and Vashisht, D. (2014). Chemiluminescence and toluidine blue as diagnostic tools for detecting early stages of oral cancer: an invivo study. J. Clin. Diagn. Res. 8, ZC35–ZC38. doi: 10.7860/JCDR/2014/7746.4259
Vigneswaran, N., Koh, S., and Gillenwater, A. (2009). Incidental detection of an occult oral malignancy with autofluorescence imaging: a case report. Head Neck Oncol. 1:37. doi: 10.1186/1758-3284-1-37
Walling, M. A., Novak, J. A., and Shepard, J. R. (2009). Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 10, 441–491. doi: 10.3390/ijms10020441
Warnakulasuriya, S. (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 45, 309–316. doi: 10.1016/j.oraloncology.2008.06.002
Yamamoto, N., Kawaguchi, K., Fujihara, H., Hasebe, M., Kishi, Y., Yasukawa, M., et al. (2017). Detection accuracy for epithelial dysplasia using an objective autofluorescence visualization method based on the luminance ratio. Int. J. Oral Sci. 9:e2. doi: 10.1038/ijos.2017.37
Yang, E. C., Tan, M. T., Schwarz, R. A., Richards-Kortum, R. R., Gillenwater, A. M., and Vigneswaran, N. (2018). Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 670–681. doi: 10.1016/j.oooo.2018.02.020
Keywords: light-based detection system, early diagnosis, OSCC, chemiluminescence, autofluorescence, nanotechnology
Citation: Mascitti M, Orsini G, Tosco V, Monterubbianesi R, Balercia A, Putignano A, Procaccini M and Santarelli A (2018) An Overview on Current Non-invasive Diagnostic Devices in Oral Oncology. Front. Physiol. 9:1510. doi: 10.3389/fphys.2018.01510
Received: 30 July 2018; Accepted: 08 October 2018;
Published: 25 October 2018.
Edited by:
Gianpaolo Papaccio, Università degli Studi della Campania Luigi Vanvitelli, ItalyReviewed by:
Alessandra Pisciotta, Università degli Studi di Modena e Reggio Emilia, ItalyLuigi Mele, Università degli Studi di Napoli Federico II, Italy
Copyright © 2018 Mascitti, Orsini, Tosco, Monterubbianesi, Balercia, Putignano, Procaccini and Santarelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Mascitti, bWFyY29tYXNjaXR0aTg2QGhvdG1haWwuaXQ=
 Marco Mascitti
Marco Mascitti Giovanna Orsini
Giovanna Orsini Vincenzo Tosco
Vincenzo Tosco Riccardo Monterubbianesi1
Riccardo Monterubbianesi1