- 1Department of Biochemistry and Biophysics, Institute for Diabetes, Obesity and Metabolism, School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Department of Biophysics and Bioinformatics, Ivan Franko National University of Lviv, Lviv, Ukraine
- 3School of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, PA, United States
Cardiovascular complications are the major cause of mortality and morbidity in diabetic patients. The changes in myocardial structure and function associated with diabetes are collectively called diabetic cardiomyopathy. Numerous molecular mechanisms have been proposed that could contribute to the development of diabetic cardiomyopathy and have been studied in various animal models of type 1 or type 2 diabetes. The current review focuses on the role of sodium (Na+) in diabetic cardiomyopathy and provides unique data on the linkage between Na+ flux and energy metabolism, studied with non-invasive 23Na, and 31P-NMR spectroscopy, polarography, and mass spectroscopy. 23Na NMR studies allow determination of the intracellular and extracellular Na+ pools by splitting the total Na+ peak into two resonances after the addition of a shift reagent to the perfusate. Using this technology, we found that intracellular Na+ is approximately two times higher in diabetic cardiomyocytes than in control possibly due to combined changes in the activity of Na+–K+ pump, Na+/H+ exchanger 1 (NHE1) and Na+-glucose cotransporter. We hypothesized that the increase in Na+ activates the mitochondrial membrane Na+/Ca2+ exchanger, which leads to a loss of intramitochondrial Ca2+, with a subsequent alteration in mitochondrial bioenergetics and function. Using isolated mitochondria, we showed that the addition of Na+ (1–10 mM) led to a dose-dependent decrease in oxidative phosphorylation and that this effect was reversed by providing extramitochondrial Ca2+ or by inhibiting the mitochondrial Na+/Ca2+ exchanger with diltiazem. Similar experiments with 31P-NMR in isolated superfused mitochondria embedded in agarose beads showed that Na+ (3–30 mM) led to significantly decreased ATP levels and that this effect was stronger in diabetic rats. These data suggest that in diabetic cardiomyocytes, increased Na+ leads to abnormalities in oxidative phosphorylation and a subsequent decrease in ATP levels. In support of these data, using 31P-NMR, we showed that the baseline β-ATP and phosphocreatine (PCr) were lower in diabetic cardiomyocytes than in control, suggesting that diabetic cardiomyocytes have depressed bioenergetic function. Thus, both altered intracellular Na+ levels and bioenergetics and their interactions may significantly contribute to the pathology of diabetic cardiomyopathy.
Diabetic Cardiomyopathy
Diabetic cardiomyopathy is a multi-faceted disease. Diabetes is associated with an increased incidence of atherosclerotic heart disease, which results in ischemic cardiomyopathy. In addition to ischemic – heart – disease associated cardiomyopathy, there are other metabolic changes in the heart that are not necessarily related to myocardial ischemia. There is altered substrate utilization and mitochondrial dysfunction; insulin resistance; decreased flexibility in substrate use; changes in oxidative phosphorylation and the citric acid cycle; abnormalities in ketogenesis and glucose free fatty acid (FFA) cycling; and altered Ca2+ handling (Boudina and Abel, 2010; Veeranki et al., 2016; Jia et al., 2018).
Changes in mitochondrial morphology are associated with remodeling of the mitochondrial proteome and decreased respiratory capacity. There are changes in mitochondrial bioenergetics with decreased phosphocreatine (PCr)/ATP (shown with 31P NMR); decreased oxygen consumption and increased H2O2 production; defects in the ATP sensitive K+ channel (KATP); mitochondrial uncoupling resulting in increased state 4 respiration and decreased ATP synthesis and increased oxygen consumption without increased ATP production; and mitochondrial generation of reactive oxygen species (ROS) and lipid peroxides that may activate uncoupling proteins (Boudina and Abel, 2010; Veeranki et al., 2016; Jia et al., 2018).
The combination of these various insults results in left ventricular hypertrophy, interstitial fibrosis, left ventricular diastolic and systolic dysfunction, right ventricular dysfunction, and impaired contractile reserve. The purpose of this paper is to show the importance of maintaining intracellular sodium ([Na+]) homeostasis in the heart and to review some of our early work of the effects of diabetes on metabolism (and vice versa) that are related to ion fluxes (Na+, H+, Ca2+).
Sodium Transport Systems in Cardiomyocytes
Sodium transport processes and [Na+] concentration play important roles in cellular function. [Na+]i concentration regulates Ca2+ cycling, contractility, metabolism, and electrical stability of the heart (Lambert et al., 2015). In the normal cell, there is a large steady-state electrochemical gradient favoring Na+ influx. This potential energy is used by numerous transport mechanisms, including Na+ channels and transporters which couple Na+ influx to either co- or counter-transport of other ions and solutes (Bers et al., 2003). Myocardial [Na+]i is determined by the balance between Na+ influx down a trans-sarcolemmal electrochemical gradient, via Na+/Ca2+ exchanger, Na+/H+ exchanger 1 (NHE1), Na+/Mg2+ exchange, Na+/HCO3- cotransport, Na+/K+/2Cl- cotransport and Na+ channels, and Na+ efflux against an electrochemical gradient, mediated by Na+/K+ pump (Ottolia et al., 2013; Shattock et al., 2015). Under normal conditions, Na+/Ca2+ exchange and Na+ channels are the dominant Na+ influx pathway; however, other transporters may become important during pathological conditions. The Na+/Ca2+ exchanger transports three Na+ ions into the cytoplasm in exchange for one Ca2+ ion using the energy generated from the Na+ gradient as a driving force, and it is one of the main mechanisms for Na+ influx in cardiomyocytes (Shattock et al., 2015). The eukaryotic Na+/Ca2+ exchanger protein, as exemplified by the mammalian cardiac isoform NCX1.1, is organized into 10 transmembrane segments (TMSs; Liao et al., 2012; Ren and Philipson, 2013) and contains a large cytoplasmic loop between TMS 5 and 6 which play a regulatory role (Philipson et al., 2002). Regulation of the mammalian Na+/Ca2+ exchanger has been clearly shown both at the functional and structural levels. Allosteric regulation of the Na+/Ca2+ exchanger, by cytoplasmic Na+ and Ca2+ ions, occurs from within the large cytoplasmic loop that separates TMS 5 from TMS 6 (Philipson et al., 2002). The structures of the two regulatory domains within this region of the eukaryotic exchanger have been described (Hilge et al., 2006; Nicoll et al., 2006; Besserer et al., 2007; Wu et al., 2010). These two contiguous stretches of residues bind cytoplasmic Ca2+, leading to an increase in exchanger activity (Hilgemann et al., 1992; Matsuoka et al., 1995; Chaptal et al., 2009; Ottolia et al., 2009), and are identified as Ca2+ binding domains 1 and 2. Na+ ion regulation of the Na+/Ca2+ exchanger is less well studied; however, it is known that high cytoplasmic Na+ inactivates the exchanger (Hilgemann et al., 1992). Whether Na+/Ca2+ exchanger modulation by cytoplasmic Na+ is relevant to cardiac physiology remains to be established since relatively high intracellular Na+ concentrations (≥20 mM) are required to significantly inactivate the exchanger experimentally (Hilgemann et al., 1992; Matsuoka et al., 1995). Recently, Liu and O’Rourke (2013) revealed a novel mechanism of Na+/Ca2+ exchanger regulation by cytosolic NADH/NAD+ redox potential through a ROS-generating NADH-driven flavoprotein oxidase. The authors proposed that this mechanism may play key roles in Ca2+ homeostasis and the response to the alteration of protein kinase C (PKC) in the cytosolic pyrine nucleotide redox state during cardiovascular diseases, including ischemia–reperfusion (Liu and O’Rourke, 2013). Acting in the opposite direction, the Na+/K+ pump moves Na+ ions from the cytoplasm to the extracellular space against their gradient by utilizing the energy released from ATP hydrolysis. One of the strongest drivers for the activation of the Na+/K+ pump is the elevation of [Na+]i (Shattock et al., 2015). A fine balance between the Na+/Ca2+ exchanger and the Na+/K+ pump controls the net amount of [Na+]i, and aberrations in either of these two systems can have a large impact on cardiac function (Shattock et al., 2015). While the relevance of Ca2+ homeostasis in cardiac function has been extensively investigated (Ottolia et al., 2013), the role of Na+ regulation in heart function and metabolism is often overlooked. Small changes in the cytoplasmic Na+ content have multiple effects on the heart by influencing intracellular Ca2+ and pH levels thereby modulating heart contractility and function. Therefore, it is essential for heart cells to maintain Na+ homeostasis. Despite the large amount of work done in the evaluation of Na+ transport, there is little data that defines the metabolic support (oxidative phosphorylation, glycolysis, and ATPase activity) of Na+ transport under normal and pathophysiological conditions.
[Na+]i and Na+ transport are altered in several diseases, including diabetes mellitus (DM) (Kjeldsen et al., 1987; Makino et al., 1987; Warley, 1991; Regan et al., 1992; Schaffer et al., 1997; Devereux et al., 2000; Hattori et al., 2000; Taegtmeyer et al., 2002; Villa-Abrille et al., 2008; Young et al., 2009; Boudina and Abel, 2010). It has been shown in heart failure myocytes, that resting [Na+]i was increased from 5.2 ± 1.4 to 16.8 ± 3.1 mmol/L (Liu and O’Rourke, 2008). Decreased activity of the Na+/K+ pump (Greene, 1986; Kjeldsen et al., 1987; Hansen et al., 2007) and Na+/Ca2+ exchanger (Chattou et al., 1999; Hattori et al., 2000) were reported in hearts from animals with type 1 diabetes (T1DM). Many studies have also shown that the function and/ or expression of the Na+/K+ pump is reduced in cardiac hypertrophy (Pogwizd et al., 2003; Boguslavskyi et al., 2014). Previously shown, the Na+/Ca2+ exchanger protein and mRNA expression levels were significantly depressed in diabetic animal models (Makino et al., 1987; Hattori et al., 2000) and Na+/Ca2+ exchanger activity, but not mRNA, was decreased in streptozotocin-treated neonatal rats (Schaffer et al., 1997). Because the Na+/Ca2+ exchanger is the main mechanism for systolic Ca2+ removal, the significant reduction in exchanger activity could increase intracellular Ca2+ and may contribute to diabetic cardiomyopathy as a result of altered diastolic Ca2+ removal (Dhalla et al., 1985; Villa-Abrille et al., 2008). It has been shown that the Na+/Ca2+ exchanger activity can be restored by insulin (Villa-Abrille et al., 2008). The myocardial NHE1 was found to be enhanced in the hypertrophied Goto-Kakizaki diabetic rat heart (Darmellah et al., 2007) and led to higher [Na+]i gain during ischemia–reperfusion (Kuo et al., 1983; Pieper et al., 1984; Pierce and Dhalla, 1985; Tanaka et al., 1992; Williams and Howard, 1994; Doliba et al., 1997; Avkiran, 1999; Xiao and Allen, 1999; Babsky et al., 2002; Anzawa et al., 2006, 2012; Williams et al., 2007). It has been suggested that elevated glucose concentrations in DM significantly influence vascular NHE1 activity via glucose induced PKC-dependent mechanisms, thereby providing a biochemical basis for increased NHE1 activity in the vascular tissues of patients with hypertension and DM (Williams and Howard, 1994). In work done by David Allen’s group, it was demonstrated that the major pathway for Na+ entry during ischemia appears to be the so-called persistent Na+ channel and the major pathway for Na+ entry on reperfusion is NHE1 (Xiao and Allen, 1999; Williams et al., 2007). These changes in [Na+]i affect the Na+/Ca2+ exchanger and contribute to Ca2+ influx and to ROS generation, which are the major causes of ischemia/reperfusion damage (Avkiran, 1999). It has been also shown that Na+–glucose cotransport is enhanced in type 2 diabetes (T2DM), which increases Na+ influx and causes [Na+]i overload (Lambert et al., 2015).
One of the causes of altered Na+ transport and increased [Na+]i concentration can be related to the downregulation of bioenergetics. For example, in diabetes, alterations in oxidative phosphorylation may compromise ion transport (Kuo et al., 1983; Pieper et al., 1984; Pierce and Dhalla, 1985; Tanaka et al., 1992; Doliba et al., 1997). Sarcolemmal Na+, K+-ATPase function may also be depressed or down-regulated due to increased serum and intracellular fatty acids (Pieper et al., 1984). Resultant changes in intracellular cation concentrations, specifically Na+ and Ca2+, may in turn cause changes in cellular metabolism (Makino et al., 1987; Allo et al., 1991). In addition, changes in local (autocrine and paracrine) and circulating neurohormones, such as ouabain (OUA)-like (Blaustein, 1993) and natriuretic factors (Kramer et al., 1991), can exacerbate the initial changes in ion transport and result in functional abnormalities found in diabetes.
This review discusses the interdependence of Na+ transport and bioenergetics in the cardiac myocyte. While an energy deficit effects Na+ transport, on other hand, [Na+]i has a strong effect on bioenergetics as evidenced by decreased free concentration of ATP and PCr and reduced mitochondrial respiration and oxidative phosphorylation related to changes in [Na+]i.
Cardiomyocyte Studies in Diabetic Hearts
Dr. Osbakken’s laboratory employed unique non-invasive nuclear magnetic resonance spectroscopy (NMRS) methods for the simultaneous assessment of [Na+]i by 23Na NMRS and adenine nucleotides by 31phosphorus (31P) NMRS in cardiomyocytes embedded in agarose beads (Ivanics et al., 1994; Doliba et al., 1998, 2000). 23Na NMRS allows for the determination of total Na+ signal, and [Na+]i and extracellular Na+ ([Na+]e) pools by splitting into two resonances after the addition of a shift reagent to the perfusate (Doliba et al., 1998; Doliba et al., 2000; Holloway et al., 2011). This method allowed evaluation of changes in [Na+]i in a rat model of streptozotocin-induced DM. Streptozotocin was injected intraperitoneally (60 mg/kg body wt, dissolved in citrate buffer). Myocytes were harvested four weeks after streptozotocin injection. It was found that the baseline [Na+]i in DM cardiomyocytes increased to 0.076 ± 0.01 mmoles/mg protein (or 16.37 mmol/L) from control (Con) levels of 0.04 ± 0.01 mmoles/mg protein (or 9.3 mmol/L); P < 0.05 (Doliba et al., 2000). This observation is similarly reported for heart failure myocytes (Liu and O’Rourke, 2008). Of note, in DM, baseline ATP and PCr were lower compared to Con (peak area/methylene diphosphonate standard area; Doliba et al., 2000): ATP-Con: 0.67 ± 0.08, ATP-DM: 0.31 ± 0.06, P < 0.003; PCr-Con: 0.92 ± 0.08; PCr-DM: 0.46 ± 0.12, P < 0.009. This suggests that DM cardiomyocytes have depressed bioenergetics function, which may contribute to abnormal Na+, K+-ATPase function and thus result in increased [Na+]i.
To further explore these findings, we measured 23Na and 31P spectra from superfused cardiomyocytes subjected to three metabolic inhibitors: 2-deoxyglucose (2DG), 2, 4-dinitrophenol (DNP), and OUA (Figures 1A,B; Doliba et al., 2000).
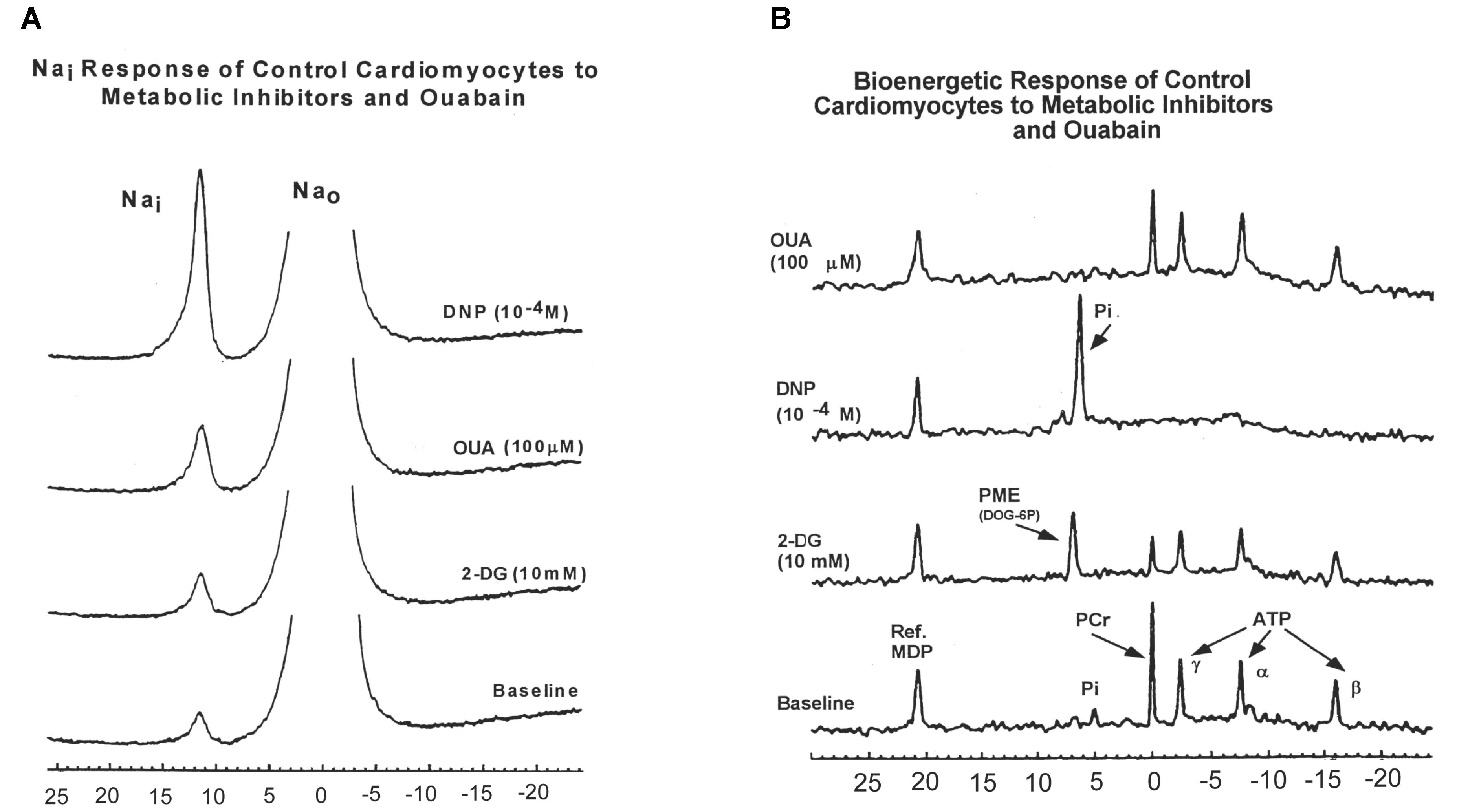
FIGURE 1. (A) A typical 23Na spectra obtained from control rat cardiomyocytes showing intra- and extra-cellular sodium during baseline conditions and during administration of 2-deoxyglucose (2-DG, 10 mM); 2,4-dinitrophenol (DNP, 10-4 M); and ouabain (OUA, 100 μM). (B) Effects of 2-DG, DNP, and OUA on 31P spectra obtained from control rat cardiomyocytes (typical spectra presented). MDP, methylene diphosphonate standard; PME, phosphomonoester; Pi, inorganic phosphate; PCr, phosphocreatine; ATP, adenosine triphosphate (α, γ, β); Nai, intracellular sodium; Na0, extracellular sodium. Data reprinted with permission from Doliba et al. (2000) Translated from Biokhimiya. 2000:65(4) 590-97. Copyright 2000 by MAIK “Nauka/Interperiodica”; DOI 0006-2979/00/6504-0502$25.00; Copyright permission granted by Pleiades Publishing, LLC.
Inhibition of glycolysis with 2-DG was associated with minimal or no change in [Na+]i in DM cardiomyocytes compared to an increase in [Na+]i in Con cardiomyocytes (DM 2DG: -4.6 ± 6%, Con 2-DG: 32.9 ± 8.1% p < 0.05). The Na+, K+-ATPase inhibitor, OUA, produced a smaller change from baseline in [Na+]i in DM cardiomyocytes compared to Con (DM OUA 21.2 ± 9.2%; vs Con OUA: 50.5 ± 8.8% p < 0.05; Doliba et al., 2000). However, despite this apparent lower effect of OUA on DM cardiomyocytes, the absolute [Na+]i after treatment with OUA was still 41% higher in DM cardiomyocytes compared to control due to the higher baseline [Na+]i.
In both animal models, uncoupling of oxidative phosphorylation with DNP was associated with similar large increases in [Na+]i; Con, 119.0 ± 26.9%; DM, 138.2 ± 12.6 (Figure 1A).
Figure 1B presents examples of 31P-NMR spectra for control cardiomyocytes obtained during baseline and 2-DG, OUA, and DNP interventions. In control cardiomyocytes, 2-DG caused a 26.4 ± 4.8% decrease of β-ATP and 35.4 ± 4.9% decrease of PCr compared to baseline. In diabetic cardiomyocytes, 2-DG caused slightly smaller decreases in β-ATP (16.2 ± 5.9%) and PCr (27.96 ± 1.7%) when compared to control. Uncoupling of oxidative phosphorylation with DNP caused apparent complete depletion (i.e., to total NMR invisibility) of both β-ATP and PCr (–100%) in both control and diabetic cardiomyocytes. The large [Na+]i increase due to DNP intervention suggests that both groups of cardiomyocytes require oxidative ATP synthesis to support the cell membrane ion gradient.
Unexpectedly, inhibition of Na, K-ATPase with OUA produced minimal change in bioenergetic parameters in cardiomyocytes from both animal models.
In diabetic cardiomyocytes, the decreased response of [Na+]i to OUA and 2-DG can be related to prior inhibition of Na+/K+ pump (Greene, 1986; Kjeldsen et al., 1987; Hansen et al., 2007) and glycolysis (Boden et al., 1996; Boden, 1997).
Isolated Mitochondrial Studies in Diabetic Hearts
The [Na+]i is tightly coupled to Ca2+ homeostasis and is increasingly recognized as a modulating force of cellular excitability, frequency adaptation, and cardiac contractility (Faber and Rudy, 2000; Grandi et al., 2010; Despa and Bers, 2013; Clancy et al., 2015). Mitochondrial ATP production is continually adjusted to energy demand through increases in oxidative phosphorylation and NADH production mediated by mitochondrial Ca2+ (Liu and O’Rourke, 2008). Mitochondria in cardiac myocytes have been recognized as a Ca2+ storage site, as well as functioning as energy providers that synthesize a large proportion of ATP required for maintaining heart function. In cardiac mitochondria, Ca2+ uptake and removal are mainly mediated via the mitochondrial Ca2+ uniporter and the mitochondrial Na+/Ca2+ exchanger (mNa+/Ca2+) (Gunter and Pfeiffer, 1990; Bernardi, 1999; Brookes et al., 2004; Liu and O’Rourke, 2008; Palty et al., 2010), respectively. The Ca2+ concentration for half-Vmax of the Ca2+ uniporter was estimated as ∼10–20 mM in studies of isolated mitochondria, which far exceeds cytosolic Ca2+ (1–3 mM; Liu and O’Rourke, 2009). By catalyzing Na+-dependent Ca2+ efflux, the putative electrogenic mNa+/Ca2+ exchanger plays a fundamental role in regulating mitochondrial Ca2+ homeostasis (Gunter and Gunter, 2001; Liu and O’Rourke, 2008), oxidative phosphorylation (Cox and Matlib, 1993a,b; Cox et al., 1993; Liu and O’Rourke, 2008), and Ca2+ crosstalk among mitochondria, cytoplasm, and the endoplasmic reticulum (ER; Szabadkai et al., 2006). The dependence of the mNa+/Ca2+ exchanger on [Na+]i is sigmoidal with half-maximal velocity (K0.5) at ∼5–10 mM, which covers the range of physiological [Na+]i in the heart (Bers et al., 2003; Saotome et al., 2005). Mitochondrial Ca2+ activates matrix dehydrogenases (pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and the NAD+-linked isocitrate dehydrogenase) (Hansford and Castro, 1985; McCormack et al., 1990; Balaban, 2002; Gunter et al., 2004) and may also activate F0/F1-ATPase (Yamada and Huzel, 1988; Territo et al., 2000, 2001), and the adenine nucleotide translocase (ANT; Moreno-Sanchez, 1985). The K0.5 for Ca2+ activation of these three dehydrogenases is in the range of 0.7–1 mM (McCormack et al., 1990; Hansford, 1991). The overall effect of elevated mitochondrial Ca2+ may be the upregulation of oxidative phosphorylation and the acceleration of ATP synthesis (McCormack et al., 1990; Balaban, 2002; Matsuoka et al., 2004; Jo et al., 2006). Activation of Ca2+-dependent dehydrogenases by Ca2+ increases NADH production, which is the primary electron donor of the electron transport chain. NADH/NAD+ potential is the driving force of oxidative phosphorylation and an increase in NADH/NAD+ potential leads to a linear increase of maximal respiration rate in isolated heart mitochondria (Moreno-Sanchez, 1985; Mootha et al., 1997). On the other hand, the excessive rise in mitochondrial Ca2+ triggers the mitochondrial permeability transition pore (PTP), resulting in pathological cell injury and death (Hajnoczky et al., 2006). Insufficient mitochondrial Ca2+ accumulation, secondary to cytoplasmic Na+ overload, decreases NAD(P)H/NAD(P)+ redox potential, resulting in compromised NADH supply for oxidative phosphorylation (Liu and O’Rourke, 2008). Since NADPH is required to maintain matrix antioxidant pathway flux, its oxidation causes a cellular overload of ROS (Kohlhaas and Maack, 2010; Kohlhaas et al., 2010; Liu et al., 2010; Clancy et al., 2015). ROS accumulation then contributes to oxidative modification of Ca2+ handling and ion channel targets to promote arrhythmias. This cascade of failures, stemming from [Na+]i overload, is thus hypothesized to provoke triggered arrhythmias (Liu et al., 2010), which, in the context of the altered electrophysiological substrate in HF, may induce sudden cardiac death (SCD). Interestingly, chronic inhibition of the mNa+/Ca2+ exchanger during the induction of HF prevents these mitochondrial defects and abrogates cardiac decompensation and sudden death in a guinea pig model of HF/SCD (Liu et al., 2014). Therefore, the mitochondrial Ca2+ concentration must be kept within the proper range to maintain physiological mitochondrial function.
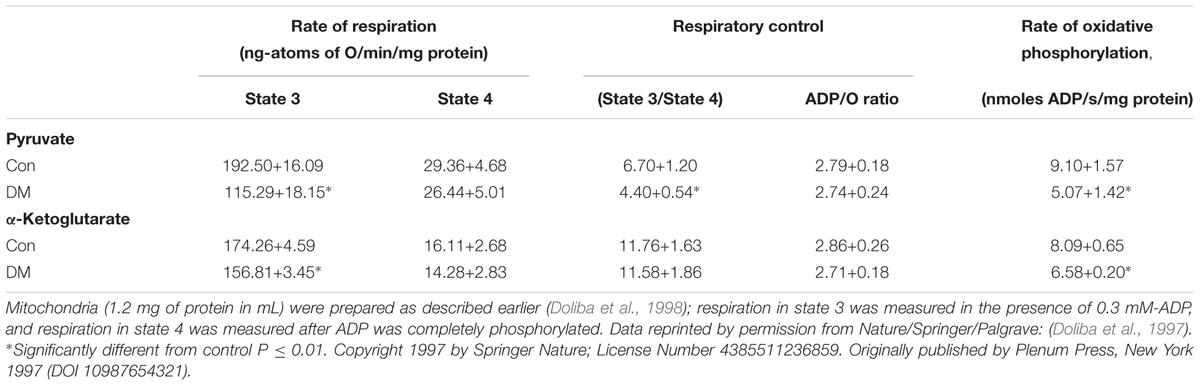
To further evaluate the pathophysiology of DM, our group studied mitochondrial respiratory function [state 3 and state 4 respiration, respiratory control index (RCI), ADP/O ratio, and rate of oxidative phosphorylation (ROP), using different substrates, and ion transport (calcium uptake)] in DM hearts compared to Con hearts. State 3 and RCI and ROP of DM rat heart were decreased when using pyruvate plus malate as substrates (Table 1; Doliba et al., 1997; Babsky et al., 2001). State 3 and ROP were also decreased when α-ketoglutarate was used as substrate (Table 1). The phosphorylation capacity, expressed as ADP/O ratio, appeared to be normal with both sets of substrates. The greatest decrease in substrate oxidation was observed with pyruvate, suggesting that pyruvate dehydrogenase activity is depressed in DM. It should be pointed out that in DM mitochondria, the decrease in state 3 was dependent on the concentration of pyruvate; and that the Km for pyruvate was higher in DM (0.058 ± 0.01 mM) compared to Con (0.0185 ± 0.0014), with no significant difference in Vmax (Doliba et al., 1997). RCI was decreased approximately 35% at all pyruvate concentrations.
To determine whether changes in Ca2+ transport might be the cause of change in oxidative function presented above, state 3 respiration was initially stimulated by ADP, and then by CaCl2 in Con and DM mitochondria during pyruvate plus malate oxidation; Ca2+ uptake was recorded using the change in H+ flux (i.e., Ca2+/2H+ exchange; Figure 2; Doliba et al., 1997). Stimulation of oxygen consumption by ADP or Ca2+ was approximately 50% lower in DM mitochondria compared to Con. In order to measure Ca2+ capacity, 100 mM CaCl2 was added to the incubation medium and Ca2+ uptake was followed by changes in pH. In contrast to Con mitochondria, mitochondria from DM animals did not completely consume even the first addition of CaCl2. These data suggest that the Ca2+ capacity in heart in DM rats is greatly decreased compared to Con.
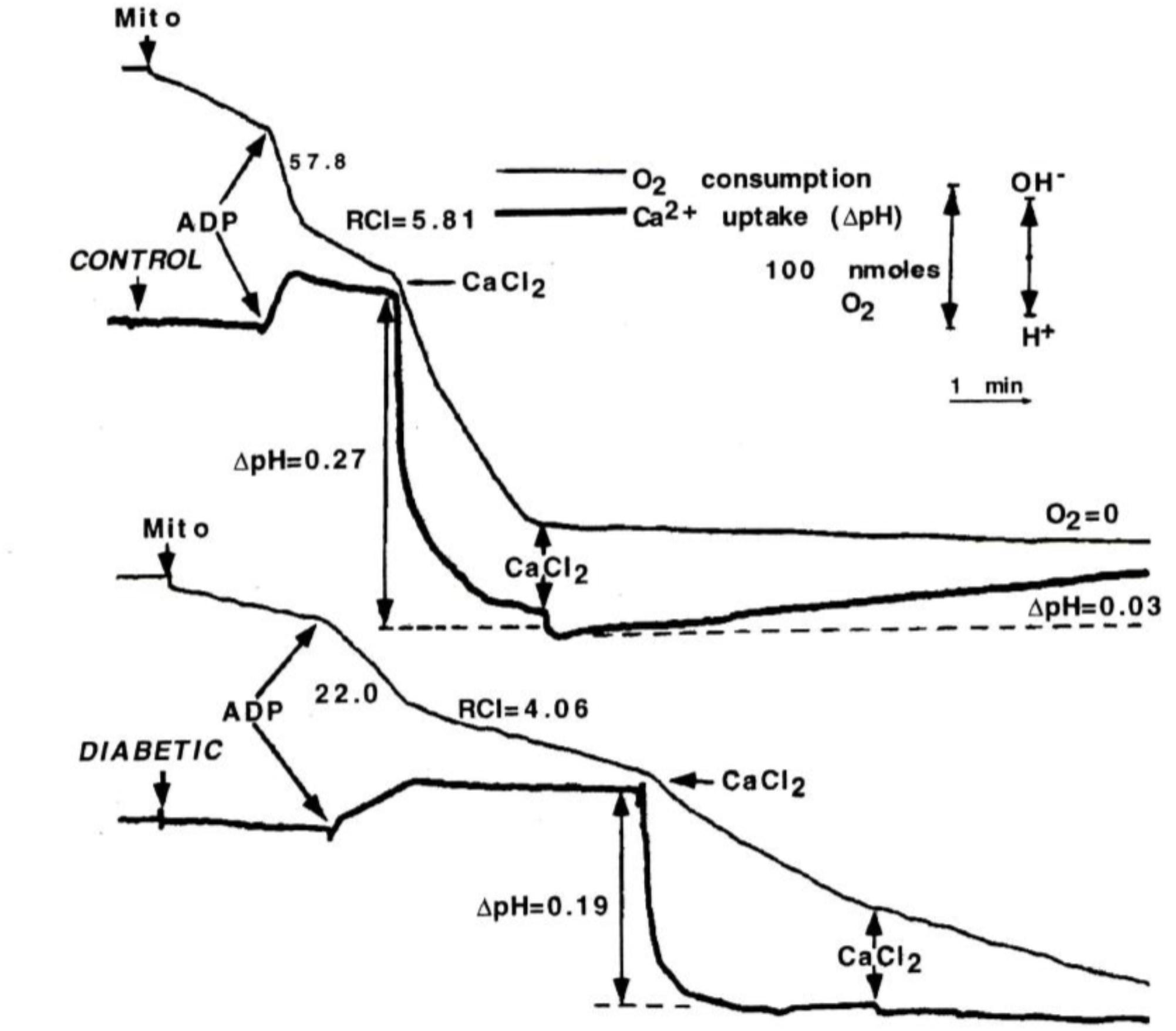
FIGURE 2. ADP and Ca2+-stimulated respiration of mitochondria from control and diabetic rats. Mitochondria (2 mg) were added to assay medium supplemented with 3 mM pyruvate plus 2.5 mM malate. ADP (0.3 mM) or CaCl2 (50 μM) was used to initiate state 3 respiration and Ca-uptake. Ca2+ uptake by mitochondria was monitored by using the change in H+ flux. Stimulation of oxygen consumption by ADP or Ca2+ was approximately 50% lower in DM mitochondria compared to Con. Data reprinted by permission from Nature/Springer/Palgrave: Doliba et al. (1997). Copyright 1997 by Springer Nature; License Number 4385511236859. Originally published by Plenum Press, New York 1997 (DOI 10987654321).
Respiratory Function and Substrate Use Studied by Mass Spectroscopy
Previous studies in our laboratory and laboratories of other investigators have shown abnormalities in pyruvate oxidation in animal models of DM, possibly related to effects of abnormal Ca2+ content on enzymes such as pyruvate dehydrogenase. To evaluate the possible role of abnormal pyruvate dehydrogenase function on respiratory function of heart mitochondria from diabetic rats, mass spectroscopy determination of O2 consumption and 13C16O2 production from [1-13]pyruvate were measured in heart mitochondria from Con (n = 8) and DM (4 weeks after streptozotocin injection; n = 8) rats (Doliba et al., 1997). Figure 3 presents the time course of 13C16O2 production (curve 1) and oxygen consumption (MVO2) (curve 2) during oxidation of [1-13C]pyruvate by heart mitochondria from Con and DM rats (Doliba et al., 1997). Both the 13C16O2 production and MVO2 stimulated by ADP (Figure 3A) or carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP), an uncoupler of respiration and oxidative phosphorylation (Figure 3B), were much less in DM mitochondria compared to Con (with ADP, 35–50% less; FCCP, 20–30% less). Addition of Ca2+ caused minimal changes in 13C16O2 production in DM; whereas Ca2+ increased 13C16O2 production by 33–40% in Con (Figure 4; Doliba et al., 1997). This lack of stimulation of a key enzyme by Ca2+ may be a factor in the development and progression of pathophysiological sequelae in DM and may be related to abnormal Ca2+ transport function. The data presented in the next two paragraphs suggest that abnormal mitochondrial Ca2+ transport and bioenergetics in DM cardiac mitochondria can be related to abnormalities in Na+ flux.
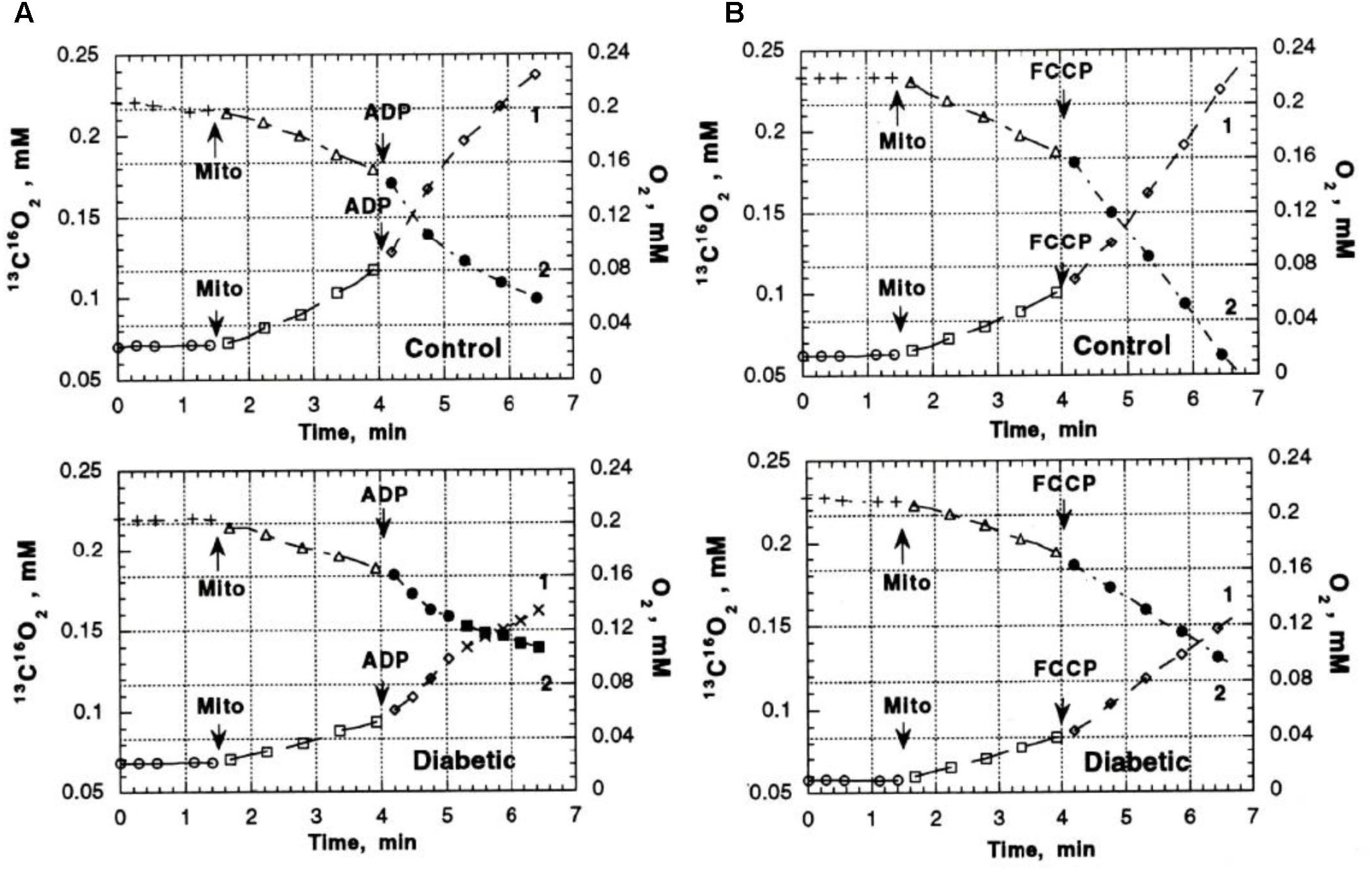
FIGURE 3. 13C16O2 production (Boudina and Abel, 2010) and O2 consumption (MVO2) (Veeranki et al., 2016) during oxidation of [1-13C] pyruvate by heart mitochondria from control and diabetic rats. (A) 13C16O2 production and MVO2 after addition of ADP. (B) 13C16O2 production and MVO2 after addition of FCCP to uncouple oxidative phosphorylation. Both the 13C16O2 production and MVO2 stimulated by ADP FCCP were much less in DM mitochondria compared to Con. Data reprinted by permission from Nature/Springer/Palgrave: Doliba et al. (1997). Copyright 1997 by Springer Nature; License Number 4385511236859. Originally published by Plenum Press, New York 1997 (DOI 10987654321).
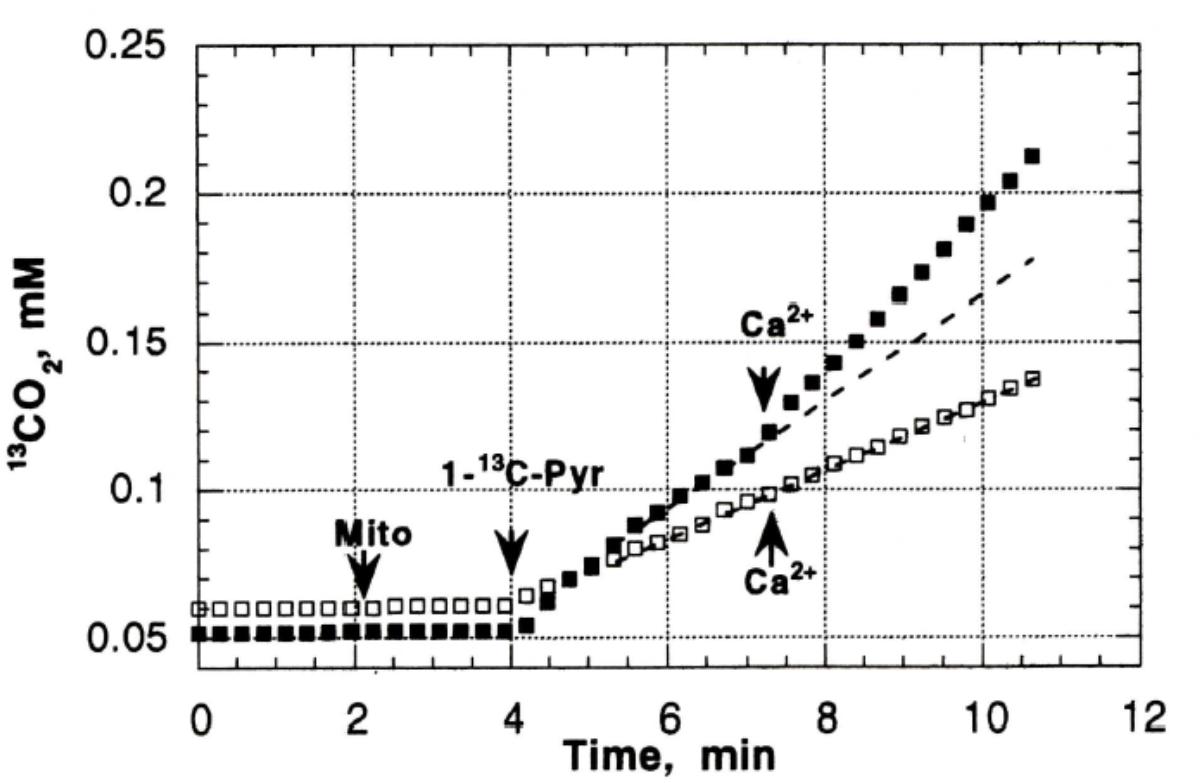
FIGURE 4. The effect of Ca2+ on CO2 production in Con (black squares) and DM mitochondria (open squares). Addition of Ca2+ caused minimal changes in 13C16O2 production in DM; whereas Ca2+ increased 13C16O2 production by 33–40% in Con. Data reprinted by permission from Nature/Springer/ Palgrave: Doliba et al. (1997). Copyright 1997 by Springer Nature; License Number 4385511236859. Originally published by Plenum Press, New York 1997 (DOI 10987654321).
Na+ Regulation of Mitochondrial Energetics: DM Modeling Effort
Previous data reported above, and by others suggest that the etiology of DM end organ damage may be related to abnormalities in Na+ transport. We and others (Cox and Matlib, 1993b; Cox et al., 1993; Maack et al., 2006; Liu and O’Rourke, 2008) proposed that increased [Na+]i is involved in the regulation of mitochondrial oxidative phosphorylation through the Ca2+ metabolism. Mitochondrial Ca2+ ([Ca2+]m) plays a key role in linking ATP production to ATP demand (i.e., mechanical activity) and as Ca2+ rises in the cell, so does [Ca2+]m; this activates mitochondrial enzymes to step-up ATP production (Liu and O’Rourke, 2008; Kohlhaas and Maack, 2010). This relationship, which crucially matches ATP supply to demand, is blocked when [Na+]i is elevated (Liu et al., 2010). The rise in [Na+]i activates Na+/Ca2+ exchange in the inner mitochondrial membrane and keeps [Ca2+]m low preventing ATP supply from meeting demand, leaving the heart metabolically compromised. Not only might this contribute to the known metabolic insufficiency in failing hearts but Kohlhaas et al. (2010) have shown that this mechanism increases mitochondrial free radical formation in failing hearts, further exacerbating injury.
To test this hypothesis, different concentrations of NaCl (in mM: 0.05; 0.1; 0.5; 1; 3; 10) were added to Con and DM mitochondria while respiratory function was monitored (Babsky et al., 2001); 1 mM a-ketoglutarate was used as substrate and mitochondrial respiration was stimulated by 200 mM ADP. Ruthenium red (1 mM), a blocker of Ca2+ uptake, was added to the polarographic cell before Na+ was added. Na+ in concentrations higher than 0.5–1 mM significantly decreased ADP-stimulated mitochondria oxygen consumption (Figure 5; Babsky et al., 2001). Mitochondria from DM rats were more sensitive to increasing extramitochondrial Na+ as demonstrated by more rapid and larger decrease in state 3 respiration (Babsky et al., 2001). The decrease in state 3 in both Con and DM mitochondria was abolished by addition of 10 mM CaCl2 to the polarographic cell before adding NaCl (Babsky et al., 2001). Our data agree with the studies of O’Rourke and colleagues who have shown that the elevation of [Na+]i can impair mitochondrial energetics (Liu and O’Rourke, 2008, 2013; Kohlhaas et al., 2010; Liu et al., 2010).
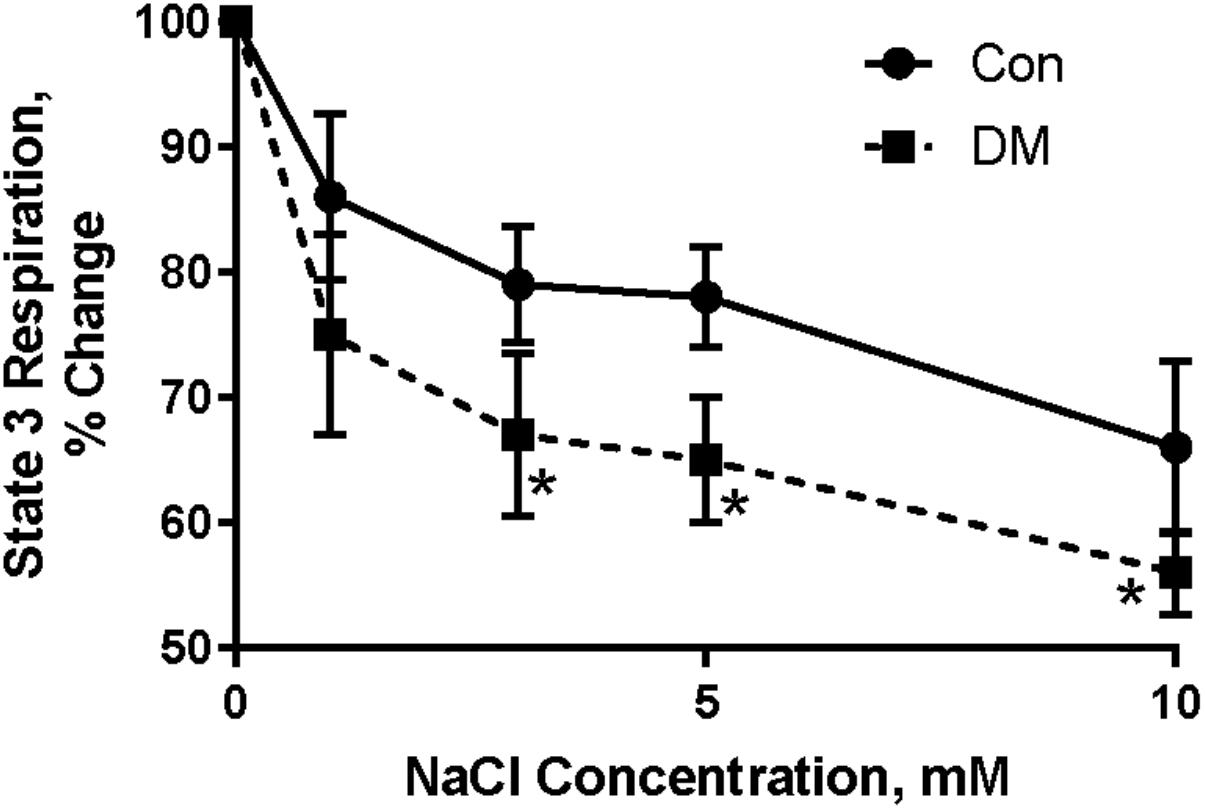
FIGURE 5. Effect of different concentrations of Na+ on ADP stimulated mitochondrial oxygen consumption (state 3) in control (CON) and diabetic (DM) heart mitochondria (means ± SE, n = 5). ∗ < 0.05 CON vs DM. Baseline of state 3 (without Na+) is assumed to be 100%. Data reprinted with permission from Babsky et al. (2001). Copyright 2001 by the Society for Experimental Biology and Medicine; DOI: 0037-9727/01/2266-0543$15.00.
Effect of Na+ on Adenine Nucleotides and Pi in Con and DM Mitochondria
In support of polarographic data, we used 31P NMRS to study the influence of different concentrations of NaCl on ATP synthesis in mitochondria isolated from Con and DM (Babsky et al., 2001). Exposure of DM mitochondria superfused at a rate of 2.7 cc/min with buffer containing Na+ (5–30 mM) led to greater decreases of β-ATP/Pi ratio than that found in Con (Figure 6A; Babsky et al., 2001). Diltiazem (DLTZ), an inhibitor of mitochondrial Na+/Ca2+ exchange, abolished the Na+ (5–30 mM) initiated decrease of β-ATP in DM mitochondria and reduced the increase of Pi with resultant values of β-ATP/Pi similar in both Con and DM mitochondria (Figure 6B; Babsky et al., 2001).
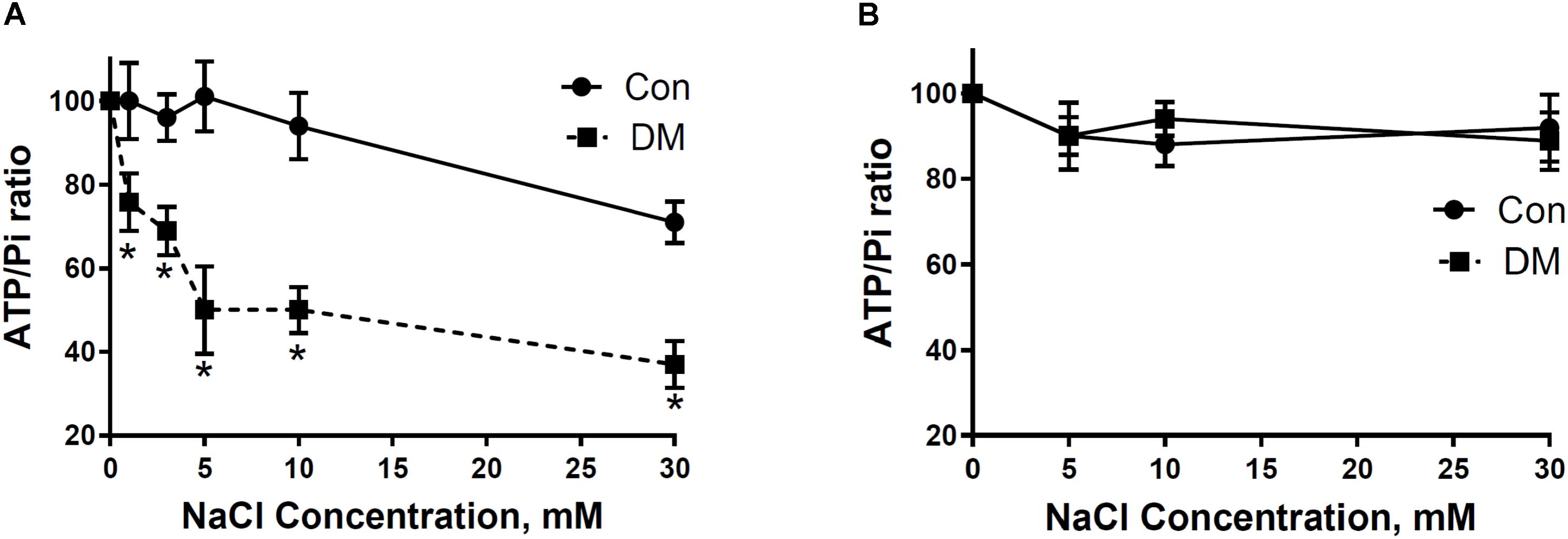
FIGURE 6. (A) The effect of extramitochondrial Na+ on ATP and Pi ratios in CON and DM heart mitochondria. (B) 250 μM DLTZ, an inhibitor of mitochondrial Na+–Ca2+ exchange, was added to perfusate. Baseline (without Na+) is assumed to be 100% (means ± SE. n = 4). Significance: DM vs CON: ∗P < 0.05. Data reprinted with permission from Babsky et al. (2001). Copyright 2001 by the Society for Experimental Biology and Medicine; DOI: 0037-9727/01/2266-0543$15.00.
Ischemia, Preconditioning (IPC), and the Diabetic Heart
One of most important factors of diabetic cardiomyopathy is post-ischemic myocardial injury that is associated with oxygen free radical generation, intracellular acidosis, bioenergetic depletion, as well as with abnormalities in Na+, H+, and Ca2+-transport in cardiomyocytes. Ca2+ overload and ischemic acidosis are also important intracellular alterations that could cause damage to ischemic cardiomyocytes (Bouchard et al., 2000). Sodium ions are involved in regulating both H+ and Ca2+ levels in cardiomyocytes through NHE1, Na+/Ca2+, Na+-K+-2Cl-, and Cl-/HCO3- ion transporters. Furthermore, Na+ is an important regulator of bioenergetic processes in healthy and diseased cardiomyocytes (Babsky et al., 2001).
Ischemic preconditioning (IPC) is a powerful protective mechanism by which exposure to prior episodes of ischemia protects the myocardium against longer and more severe ischemic insults (Murry et al., 1986). The relationship between DM and myocardial IPC is not yet clear (Miki et al., 2012). Some studies have demonstrated that diabetes may impair IPC by producing changes in both sarcolemmal and mitochondrial K-ATP channels, which then alters mitochondrial function (Hassouna et al., 2006). These changes may lead to an elevated superoxide production which produces cellular injuries.
Ishihara et al. (2001) show in 611 patients (including 121 patients with non-insulin treated diabetes) that DM prevents the IPC effect in patients with an acute myocardial infarction. However, a study of Rezende et al. (2015) showed that T2DM was not associated with impairment in IPC in coronary artery disease patients. In fact, there is some evidence that prior short episodes of ischemia that can often occur in the diabetic heart are the substrate for IPC, whereby the heart is protected during longer episode of ischemia.
Tsang et al. (2005) hypothesized that in diabetic hearts, IPC depends on intact signaling through the phosphatidylinositol 3-kinase (PI3K)-Akt pro-survival pathway. The authors concluded that diabetic hearts are less sensitive to the IPC protective effects related to defective components in the PI3k-Akt pathway. For example, in animal models of diabetes, exposure to more prior episodes of IPC were needed to activate PI3K-Akt to a critical level and thus provide cardioprotection during exposure to longer episodes of ischemia–reperfusion than in Con.
Our group studied the effect of IPC on [Na+]i levels in isolated perfused rat hearts (Figure 7; Babsky et al., 2002). We have shown that 20 min ischemia increased the [Na+]i in Con hearts by ∼50% compared to baseline. During 10–20 min of post-ischemic reperfusion the [Na+]i significantly decreased, but was still ∼20% higher compared to baseline levels. Even though IPC significantly improved the post-ischemic recovery of cardiac function (LVDP and heart rate), unexpectedly the [Na+]i levels were higher than Con at end IPC, and during ischemia, and were similar to Con during reperfusion. These results are in agreement with the data reported by Ramasamy et al. (1995). While our studies did not include a DM model, Ramasamy’s studies did; and showed that the % change in [Na+]i from baseline was lower during ischemia in DM than in Con, and that the effect of the NHE1 inhibitor EIPA (similar to preconditioning ischemia) was less in DM than in Con. This suggests that the NHE1 activity was impaired in DM. The topic of NHE1 and ischemia is discussed further below.
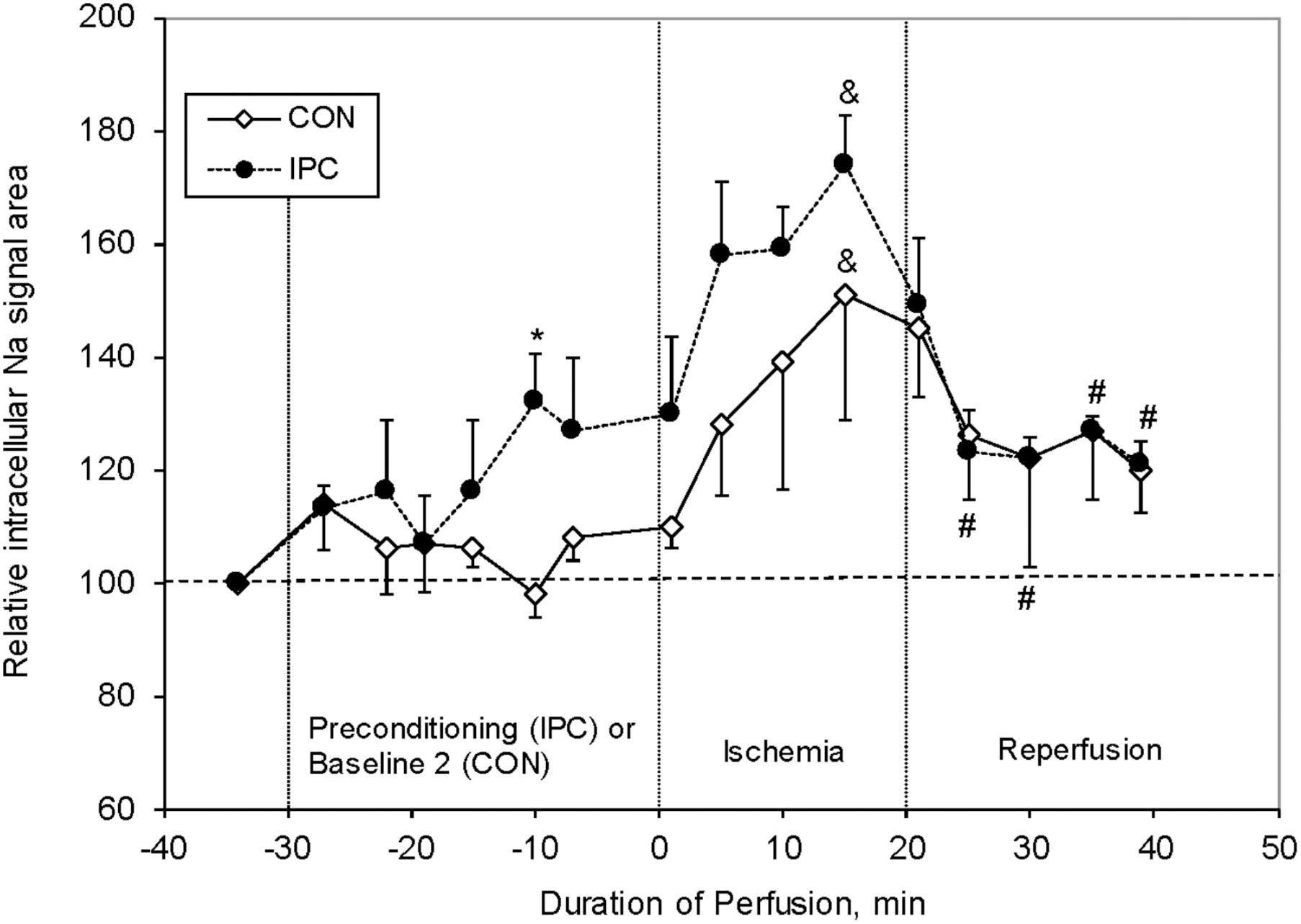
FIGURE 7. Relative changes in intracellular sodium (Nai) resonance areas as a function of time in control (CON, n = 6) and preconditioned (IPC, n = 4) rat hearts. Nai baseline is normalized to 100. Significance: ∗P < 0.01 (IPC vs CON), #P < 0.05 (IPC group vs end of ischemia), and &P < 0.01 (vs pre-ischemic level for each group). Data reprinted with permission from Babsky et al. (2002). Copyright 2002 by the Society for Experimental Biology and Medicine; DOI: 1535-3702/02/2277-0520$15.00.
Although diabetes mostly poses higher cardiovascular risk, the pathophysiology underlying this condition is uncertain. Moreover, though diabetes is believed to alter intracellular pathways related to myocardial protective mechanisms, it is still controversial whether diabetes may interfere with IPC, and whether this might influence clinical outcomes. We believe that ischemia developed in diabetic heart does not produce the same conditions that are developed in animal models when two–three 5-min ischemic episodes are each followed by 5–10 min of reperfusion. This difference may be a reason for the many controversies concerning relationship of IPC and the diabetic heart.
To conclude this discussion, it is likely that the changes in [Na+]i may contribute to ischemic and reperfusion damage, possibly through their effects on Ca2+ overload (Allen and Xiao, 2003; Xiao and Allen, 2003; Williams et al., 2007).
Ischemia and NHE1
Ischemic conditions may activate the NHE1. There are data that show that hyperactivity of NHE1 results of the increase in [Na+]i that leads to Ca2+ overload through the Na+/Ca2+ exchanger, myocardial dysfunction, hypertrophy, apoptosis, and heart failure (Cingolani and Ennis, 2007). David Allen’s group showed that two inhibitors of NHE1, amiloride and zoniporide, cause cardioprotection which was judged by the recovery of LVDP and by the magnitude of the reperfusion contracture (Williams et al., 2007). The authors also showed that there were two different mechanisms for Na+ entry during ischemia and reperfusion: a major pathway for Na+ entry during ischemia is the persistent Na+ channels (INa,P) and the major pathway for Na+ entry on reperfusion is NHE1 (Williams et al., 2007). The optimal therapy may require blocking both pathways. Pisarenko et al. (2005) show that inhibition of NHE1, similar to IPC, protects rat heart. In rabbit hearts, inhibition of NHE1 has been shown to be associated with significant protection during ischemia/reperfusion injury in immature myocardium, mostly by reducing myocardial calcium overload (Cun et al., 2007; Zhou et al., 2008). Furthermore, NHE1 inhibition leads to a decrease of infarct size after coronary artery thrombosis and thrombolysis and provides a comparable to preconditioning degree of cardioprotection against 60 min of regional ischemia (Hennan et al., 2006). NHE1 inhibition attenuates the cardiac hypertrophic response and heart failure in various experimental models. For example, early and transient administration of a NHE1 inhibitor inhibits cardiomyocyte hypertrophy in cultured cells, as well as in vivo cardiac hypertrophy and heart failure, suggesting a critical early NHE1-dependent initiation of hypertrophy (Kilic et al., 2014). However, in a dog model, one NHE1 inhibitor such as EMD 87580 did not protect against ischemia–reperfusion injury, and no additive protection beyond preconditioning was obtained (Kingma, 2018). It appears that NHE1 activity has a biphasic effect on myocardial function. Total blockage of activity provides a beneficial effect, but overexpression also provides cardioprotection. It is important to point out that the mitochondrial KATP channel also plays an important role during ischemia and reperfusion damage (Garlid et al., 1997; Sato and Marban, 2000). The mitochondrial damage, which is in part a consequence of closure of KATP channels, can be partially reversed by mitochondrial KATP channel openers (Xiao and Allen, 2003). Combined treatment of NHE1 by Cariporide and KATP channels by diazoxide provide the most beneficial effect (Xiao and Allen, 2003).
It is interesting to note that the cardioprotective effects of the NHE1 inhibitor, Cariporide, were tested in several clinical trials to protect the heart from ischemia during coronary artery bypass surgery (CABG; Boyce et al., 2003; Mentzer et al., 2008). While Cariporide (at its highest dose of 120 mg) provided protection against all-cause mortality and myocardial infarction at day 36 and 6 months after CABG compared to placebo, there was an increased mortality in the form of cerebrovascular events. Thus, Cariporide was not further developed for clinical use as a cardioprotection agent.
Sodium Transport Inhibitors in Treatment of Diabetic Cardiomyopathy
The NHE1 are integral membrane proteins that may have multiple activities in the heart. Nine different NHEs have been identified. NHE1 is the major isoform found in the heart, and plays an integral role in regulation if intracellular pH, Na+ and Ca2+. Aberrant regulation and over-activation of NHE1 can contribute to heart disease and appears to be involved in acute ischemia–reperfusion damage and cardiac hypertrophy. Changes in intracellular pH related to changes in NHE1 function can stimulate the Na+/Ca2+ exchanger to eliminate intracellular Na+ and increase intracellular Ca2+ (Levitsky et al., 1998; Odunewu-Aderibigbe and Fliegel, 2014).
Pharmacological overload caused by angiotensin II, endothelin-1, and a1-adrenergic agonists can enhance the activity of the NHE1, which leads to an extrusion of H+ and an increase in intracellular Na+. Inhibition of NHE1 can reverse these effects and lead to regression of myocardial hypertrophy that can produce a beneficial effect in heart failure, and can protect against ischemic injury in genetic diabetic rat and non-diabetic rat hearts. However, at present, there are no NHE1 inhibitors that have been found to be therapeutically useful in the treatment of heart disease (Ramasamy and Schaefer, 1999; Cingolani and Ennis, 2007).
More recently, during studies of newer anti-diabetic drugs on cardiac function, it was found that Na+-glucose exchangers used in the treatment of diabetes provided significant cardiac protection. Further investigation into the potential etiology of this protection suggests that at least one of these drugs, Empagliflozin (EMPA) may produce this affect via inhibition of NHE1. This protective effect is apparently unrelated to EMPA effect on HbA1C. In two animal models (rabbit and rat), the effect appears to be related to decreases in cytoplasmic Na+ and Ca2+ and an increase in mitochondrial Ca2+. It is unclear what the effects are due to in humans, but some evidence suggests that they may be similar (Baartscheer et al., 2017; Lytvyn et al., 2017; Packer, 2017; Packer et al., 2017; Bertero et al., 2018; Inzucchi et al., 2018).
Summary
The data presented in this review paper suggest that while changes in bioenergetic function may be a cause of ion transport abnormalities, it is as likely that abnormalities of ion content and transport may contribute to metabolic (bioenergetics and respiratory function) abnormalities. The results also suggest that increased [Na+]i concentration in DM cardiomyocytes may be a factor, leading to chronically decreased myocardial bioenergetics. Further studies in this area may provide insight into some possible cellular and mitochondrial mechanisms which contribute to progressive pathophysiological processes as disease progresses and may set the stage for better therapies in future.
Author Contributions
ND contributed to five sections related to sodium transport and cellular and mitochondrial bioenergetics, AB to “Effect of Sodium on Adenine Nucleotides and Pi”, AB and MO to “Ischemia and NHE1” and “Ischemia, Preconditioning and the Diabetic Heart”, MO to “Diabetes Cardiomyopathy” and “Sodium Transport Inhibitors in Treatment of Diabetic Cardiomyopathy.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allen, D. G., and Xiao, X. H. (2003). Role of the cardiac Na + /H + exchanger during ischemia and reperfusion. Cardiovasc. Res. 57, 934–941. doi: 10.1016/S0008-6363(02)00836-2
Allo, S. N., Lincoln, T. M., Wilson, G. L., Green, F. J., Watanabe, A. M., and Schaffer, S. W. (1991). Non-insulin-dependent diabetes-induced defects in cardiac cellular calcium regulation. Am. J. Physiol. 260(6 Pt 1), C1165–C1171. doi: 10.1152/ajpcell.1991.260.6.C1165
Anzawa, R., Bernard, M., Tamareille, S., Baetz, D., Confort-Gouny, S., Gascard, J. P., et al. (2006). Intracellular sodium increase and susceptibility to ischaemia in hearts from type 2 diabetic db/db mice. Diabetologia 49, 598–606. doi: 10.1007/s00125-005-0091-5
Anzawa, R., Seki, S., Nagoshi, T., Taniguchi, I., Feuvray, D., and Yoshimura, M. (2012). The role of Na + /H + exchanger in Ca2 + overload and ischemic myocardial damage in hearts from type 2 diabetic db/db mice. Cardiovasc. Diabetol. 11:33. doi: 10.1186/1475-2840-11-33
Avkiran, M. (1999). Rational basis for use of sodium-hydrogen exchange inhibitors in myocardial ischemia. Am. J. Cardiol. 83, 10G–17G; discussion 17G–18G.
Baartscheer, A., Schumacher, C. A., Wust, R. C., Fiolet, J. W., Stienen, G. J., Coronel, R., et al. (2017). Empagliflozin decreases myocardial cytoplasmic Na( + ) through inhibition of the cardiac Na( + )/H( + ) exchanger in rats and rabbits. Diabetologia 60, 568–573. doi: 10.1007/s00125-016-4134-x
Babsky, A., Doliba, N., Doliba, N., Savchenko, A., Wehrli, S., and Osbakken, M. (2001). Na + effects on mitochondrial respiration and oxidative phosphorylation in diabetic hearts. Exp. Biol. Med. 226, 543–551. doi: 10.1177/153537020122600606
Babsky, A., Hekmatyar, S., Wehrli, S., Doliba, N., Osbakken, M., and Bansal, N. (2002). Influence of ischemic preconditioning on intracellular sodium, pH, and cellular energy status in isolated perfused heart. Exp. Biol. Med. 227, 520–528. doi: 10.1177/153537020222700717
Balaban, R. S. (2002). Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 34, 1259–1271. doi: 10.1006/jmcc.2002.2082
Bernardi, P. (1999). Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155. doi: 10.1152/physrev.1999.79.4.1127
Bers, D. M., Barry, W. H., and Despa, S. (2003). Intracellular Na + regulation in cardiac myocytes. Cardiovasc. Res. 57, 897–912. doi: 10.1016/S0008-6363(02)00656-9
Bertero, E., Prates Roma, L., Ameri, P., and Maack, C. (2018). Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc. Res. 114, 12–18. doi: 10.1093/cvr/cvx149
Besserer, G. M., Ottolia, M., Nicoll, D. A., Chaptal, V., Cascio, D., Philipson, K. D., et al. (2007). The second Ca2 + -binding domain of the Na + Ca2 + exchanger is essential for regulation: crystal structures and mutational analysis. Proc. Natl. Acad. Sci. U.S.A. 104, 18467–18472. doi: 10.1073/pnas.0707417104
Blaustein, M. P. (1993). Physiological effects of endogenous ouabain: control of intracellular Ca2 + stores and cell responsiveness. Am. J. Physiol. 264(6 Pt 1), C1367–C1387. doi: 10.1152/ajpcell.1993.264.6.C1367
Boden, G. (1997). Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46, 3–10. doi: 10.2337/diab.46.1.3
Boden, G., Chen, X., Mozzoli, M., and Ryan, I. (1996). Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 81, 3419–3423.
Boguslavskyi, A., Pavlovic, D., Aughton, K., Clark, J. E., Howie, J., Fuller, W., et al. (2014). Cardiac hypertrophy in mice expressing unphosphorylatable phospholemman. Cardiovasc. Res. 104, 72–82. doi: 10.1093/cvr/cvu182
Bouchard, J. F., Chouinard, J., and Lamontagne, D. (2000). Participation of prostaglandin E2 in the endothelial protective effect of ischaemic preconditioning in isolated rat heart. Cardiovasc. Res. 45, 418–427. doi: 10.1016/S0008-6363(99)00343-0
Boudina, S., and Abel, E. D. (2010). Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 11, 31–39. doi: 10.1007/s11154-010-9131-7
Boyce, S. W., Bartels, C., Bolli, R., Chaitman, B., Chen, J. C., Chi, E., et al. (2003). Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J. Thorac. Cardiovasc. Surg. 126, 420–427. doi: 10.1016/S0022-5223(03)00209-5
Brookes, P. S., Yoon, Y., Robotham, J. L., Anders, M. W., and Sheu, S. S. (2004). Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287, C817–C833. doi: 10.1152/ajpcell.00139.2004
Chaptal, V., Ottolia, M., Mercado-Besserer, G., Nicoll, D. A., Philipson, K. D., and Abramson, J. (2009). Structure and functional analysis of a Ca2 + sensor mutant of the Na + /Ca2 + exchanger. J. Biol. Chem. 284, 14688–14692. doi: 10.1074/jbc.C900037200
Chattou, S., Diacono, J., and Feuvray, D. (1999). Decrease in sodium-calcium exchange and calcium currents in diabetic rat ventricular myocytes. Acta Physiol. Scand. 166, 137–144. doi: 10.1046/j.1365-201x.1999.00547.x
Cingolani, H. E., and Ennis, I. L. (2007). Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation 115, 1090–1100. doi: 10.1161/CIRCULATIONAHA.106.626929
Clancy, C. E., Chen-Izu, Y., Bers, D. M., Belardinelli, L., Boyden, P. A., Csernoch, L., et al. (2015). Deranged sodium to sudden death. J. Physiol. 593, 1331–1345. doi: 10.1113/jphysiol.2014.281204
Cox, D. A., Conforti, L., Sperelakis, N., and Matlib, M. A. (1993). Selectivity of inhibition of Na( + )-Ca2 + exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 21, 595–599. doi: 10.1097/00005344-199304000-00013
Cox, D. A., and Matlib, M. A. (1993a). A role for the mitochondrial Na( + )-Ca2 + exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J. Biol. Chem. 268, 938–947.
Cox, D. A., and Matlib, M. A. (1993b). Modulation of intramitochondrial free Ca2 + concentration by antagonists of Na( + )-Ca2 + exchange. Trends Pharmacol. Sci. 14, 408–413.
Cun, L., Ronghua, Z., Bin, L., Jin, L., and Shuyi, L. (2007). Preconditioning with Na + /H + exchange inhibitor HOE642 reduces calcium overload and exhibits marked protection on immature rabbit hearts. ASAIO J. 53, 762–765. doi: 10.1097/MAT.0b013e31815766e3
Darmellah, A., Baetz, D., Prunier, F., Tamareille, S., Rucker-Martin, C., and Feuvray, D. (2007). Enhanced activity of the myocardial Na + /H + exchanger contributes to left ventricular hypertrophy in the Goto-Kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia 50, 1335–1344. doi: 10.1007/s00125-007-0628-x
Despa, S., and Bers, D. M. (2013). Na( + ) transport in the normal and failing heart - remember the balance. J. Mol. Cell. Cardiol. 61, 2–10. doi: 10.1016/j.yjmcc.2013.04.011
Devereux, R. B., Roman, M. J., Paranicas, M., O’Grady, M. J., Lee, E. T., Welty, T. K., et al. (2000). Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101, 2271–2276. doi: 10.1161/01.CIR.101.19.2271
Dhalla, N. S., Pierce, G. N., Innes, I. R., and Beamish, R. E. (1985). Pathogenesis of cardiac dysfunction in diabetes mellitus. Can. J. Cardiol. 1, 263–281.
Doliba, N. M., Babsky, A. M., Wehrli, S. L., Ivanics, T. M., Friedman, M. F., and Osbakken, M. D. (2000). Metabolic control of sodium transport in streptozotocin-induced diabetic rat hearts. Biochemistry 65, 502–508.
Doliba, N. M., Sweet, I. R., Babsky, A., Doliba, N., Forster, R. E., and Osbakken, M. (1997). Simultaneous measurement of oxygen consumption and 13C16O2 production from 13C-pyruvate in diabetic rat heart mitochondria. Adv. Exp. Med. Biol. 428, 269–275. doi: 10.1007/978-1-4615-5399-1_37
Doliba, N. M., Wehrli, S. L., Babsky, A. M., Doliba, N. M., and Osbakken, M. D. (1998). Encapsulation and perfusion of mitochondria in agarose beads for functional studies with 31P-NMR spectroscopy. Magn. Reson. Med. 39, 679–684. doi: 10.1002/mrm.1910390502
Faber, G. M., and Rudy, Y. (2000). Action potential and contractility changes in [Na( + )](i) overloaded cardiac myocytes: a simulation study. Biophys. J. 78, 2392–2404. doi: 10.1016/S0006-3495(00)76783-X
Garlid, K. D., Paucek, P., Yarov-Yarovoy, V., Murray, H. N., Darbenzio, R. B., D’Alonzo, A. J., et al. (1997). Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K + channels. Possible mechanism of cardioprotection. Circ. Res. 81, 1072–1082. doi: 10.1161/01.RES.81.6.1072
Grandi, E., Pasqualini, F. S., and Bers, D. M. (2010). A novel computational model of the human ventricular action potential and Ca transient. J. Mol. Cell Cardiol. 48, 112–121. doi: 10.1016/j.yjmcc.2009.09.019
Greene, D. A. (1986). A sodium-pump defect in diabetic peripheral nerve corrected by sorbinil administration: relationship to myo-inositol metabolism and nerve conduction slowing. Metabolism 35(4 Suppl. 1), 60–65.
Gunter, T. E., and Gunter, K. K. (2001). Uptake of calcium by mitochondria: transport and possible function. IUBMB Life 52, 197–204. doi: 10.1080/15216540152846000
Gunter, T. E., and Pfeiffer, D. R. (1990). Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258(5 Pt 1), C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755
Gunter, T. E., Yule, D. I., Gunter, K. K., Eliseev, R. A., and Salter, J. D. (2004). Calcium and mitochondria. FEBS Lett. 567, 96–102. doi: 10.1016/j.febslet.2004.03.071
Hajnoczky, G., Csordas, G., Das, S., Garcia-Perez, C., Saotome, M., Sinha Roy, S., et al. (2006). Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2 + uptake in apoptosis. Cell Calcium 40, 553–560. doi: 10.1016/j.ceca.2006.08.016
Hansen, P. S., Clarke, R. J., Buhagiar, K. A., Hamilton, E., Garcia, A., White, C., et al. (2007). Alloxan-induced diabetes reduces sarcolemmal Na + -K + pump function in rabbit ventricular myocytes. Am. J. Physiol. Cell Physiol. 292, C1070–C1077. doi: 10.1152/ajpcell.00288.2006
Hansford, R. G. (1991). Dehydrogenase activation by Ca2 + in cells and tissues. J Bioenerg. Biomembr. 23, 823–854. doi: 10.1007/BF00786004
Hansford, R. G., and Castro, F. (1985). Role of Ca2 + in pyruvate dehydrogenase interconversion in brain mitochondria and synaptosomes. Biochem. J. 227, 129–136. doi: 10.1042/bj2270129
Hassouna, A., Loubani, M., Matata, B. M., Fowler, A., Standen, N. B., and Galinanes, M. (2006). Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc. Res. 69, 450–458. doi: 10.1016/j.cardiores.2005.11.004
Hattori, Y., Matsuda, N., Kimura, J., Ishitani, T., Tamada, A., Gando, S., et al. (2000). Diminished function and expression of the cardiac Na + -Ca2 + exchanger in diabetic rats: implication in Ca2 + overload. J. Physiol. 527(Pt 1), 85–94.
Hennan, J. K., Driscoll, E. M., Barrett, T. D., Fischbach, P. S., and Lucchesi, B. R. (2006). Effect of sodium/hydrogen exchange inhibition on myocardial infarct size after coronary artery thrombosis and thrombolysis. Pharmacology 78, 27–37. doi: 10.1159/000094874
Hilge, M., Aelen, J., and Vuister, G. W. (2006). Ca2 + regulation in the Na + /Ca2 + exchanger involves two markedly different Ca2 + sensors. Mol. Cell 22, 15–25. doi: 10.1016/j.molcel.2006.03.008
Hilgemann, D. W., Collins, A., and Matsuoka, S. (1992). Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J. Gen. Physiol. 100, 933–961. doi: 10.1085/jgp.100.6.933
Holloway, C. J., Suttie, J., Dass, S., and Neubauer, S. (2011). Clinical cardiac magnetic resonance spectroscopy. Prog. Cardiovasc. Dis. 54, 320–327. doi: 10.1016/j.pcad.2011.08.002
Inzucchi, S. E., Zinman, B., Fitchett, D., Wanner, C., Ferrannini, E., Schumacher, M., et al. (2018). How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41, 356–363. doi: 10.2337/dc17-1096
Ishihara, M., Inoue, I., Kawagoe, T., Shimatani, Y., Kurisu, S., Nishioka, K., et al. (2001). Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J. Am. Coll. Cardiol. 38, 1007–1011. doi: 10.1016/S0735-1097(01)01477-2
Ivanics, T., Blum, H., Wroblewski, K., Wang, D. J., and Osbakken, M. (1994). Intracellular sodium in cardiomyocytes using 23Na nuclear magnetic resonance. Biochim. Biophys. Acta 1221, 133–144. doi: 10.1016/0167-4889(94)90005-1
Jia, G., Hill, M. A., and Sowers, J. R. (2018). Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 122, 624–638. doi: 10.1161/CIRCRESAHA.117.311586
Jo, H., Noma, A., and Matsuoka, S. (2006). Calcium-mediated coupling between mitochondrial substrate dehydrogenation and cardiac workload in single guinea-pig ventricular myocytes. J. Mol. Cell Cardiol. 40, 394–404. doi: 10.1016/j.yjmcc.2005.12.012
Kilic, A., Huang, C. X., Rajapurohitam, V., Madwed, J. B., and Karmazyn, M. (2014). Early and transient sodium-hydrogen exchanger isoform 1 inhibition attenuates subsequent cardiac hypertrophy and heart failure following coronary artery ligation. J. Pharmacol. Exp. Ther. 351, 492–499. doi: 10.1124/jpet.114.217091
Kingma, J. G. (2018). Inhibition of Na( + )/H( + ) exchanger with EMD 87580 does not confer greater cardioprotection beyond preconditioning on ischemia-reperfusion injury in normal dogs. J. Cardiovasc. Pharmacol. Ther. 23, 254–269. doi: 10.1177/1074248418755120
Kjeldsen, K., Braendgaard, H., Sidenius, P., Larsen, J. S., and Norgaard, A. (1987). Diabetes decreases Na + -K + pump concentration in skeletal muscles, heart ventricular muscle, and peripheral nerves of rat. Diabetes 36, 842–848. doi: 10.2337/diab.36.7.842
Kohlhaas, M., Liu, T., Knopp, A., Zeller, T., Ong, M. F., Bohm, M., et al. (2010). Elevated cytosolic Na + increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 121, 1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911
Kohlhaas, M., and Maack, C. (2010). Adverse bioenergetic consequences of Na + -Ca2 + exchanger-mediated Ca2 + influx in cardiac myocytes. Circulation 122, 2273–2280. doi: 10.1161/CIRCULATIONAHA.110.968057
Kramer, H. J., Meyer-Lehnert, H., Michel, H., and Predel, H. G. (1991). Endogenous natriuretic and ouabain-like factors. Their roles in body fluid volume and blood pressure regulation. Am. J. Hypertens. 4(1 Pt 1), 81–89.
Kuo, T. H., Moore, K. H., Giacomelli, F., and Wiener, J. (1983). Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes 32, 781–787. doi: 10.2337/diab.32.9.781
Lambert, R., Srodulski, S., Peng, X., Margulies, K. B., Despa, F., and Despa, S. (2015). Intracellular Na + concentration ([Na + ]i) is elevated in diabetic hearts due to enhanced Na + -Glucose cotransport. J. Am. Heart Assoc. 4:e002183. doi: 10.1161/JAHA.115.002183
Levitsky, J., Gurell, D., and Frishman, W. H. (1998). Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury. J. Clin. Pharmacol. 38, 887–897. doi: 10.1002/j.1552-4604.1998.tb04383.x
Liao, J., Li, H., Zeng, W., Sauer, D. B., Belmares, R., and Jiang, Y. (2012). Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–690. doi: 10.1126/science.1215759
Liu, T., Brown, D. A., and O’Rourke, B. (2010). Role of mitochondrial dysfunction in cardiac glycoside toxicity. J. Mol. Cell Cardiol. 49, 728–736. doi: 10.1016/j.yjmcc.2010.06.012
Liu, T., and O’Rourke, B. (2008). Enhancing mitochondrial Ca2 + uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 103, 279–288. doi: 10.1161/CIRCRESAHA.108.175919
Liu, T., and O’Rourke, B. (2009). Regulation of mitochondrial Ca2 + and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 41, 127–132. doi: 10.1007/s10863-009-9216-8
Liu, T., and O’Rourke, B. (2013). Regulation of the Na + /Ca2 + exchanger by pyridine nucleotide redox potential in ventricular myocytes. J. Biol. Chem. 288, 31984–31992. doi: 10.1074/jbc.M113.496588
Liu, T., Takimoto, E., Dimaano, V. L., DeMazumder, D., Kettlewell, S., Smith, G., et al. (2014). Inhibiting mitochondrial Na + /Ca2 + exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res. 115, 44–54. doi: 10.1161/CIRCRESAHA.115.303062
Lytvyn, Y., Bjornstad, P., Udell, J. A., Lovshin, J. A., and Cherney, D. Z. I. (2017). Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136, 1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012
Maack, C., Cortassa, S., Aon, M. A., Ganesan, A. N., Liu, T., and O’Rourke, B. (2006). Elevated cytosolic Na + decreases mitochondrial Ca2 + uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 99, 172–182. doi: 10.1161/01.RES.0000232546.92777.05
Makino, N., Dhalla, K. S., Elimban, V., and Dhalla, N. S. (1987). Sarcolemmal Ca2 + transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am. J. Physiol. 253(2 Pt 1), E202–E207.
Matsuoka, S., Jo, H., Sarai, N., and Noma, A. (2004). An in silico study of energy metabolism in cardiac excitation-contraction coupling. Jpn. J. Physiol. 54, 517–522. doi: 10.2170/jjphysiol.54.517
Matsuoka, S., Nicoll, D. A., Hryshko, L. V., Levitsky, D. O., Weiss, J. N., and Philipson, K. D. (1995). Regulation of the cardiac Na( + )-Ca2 + exchanger by Ca2 + . Mutational analysis of the Ca(2 + )-binding domain. J. Gen. Physiol. 105, 403–420. doi: 10.1085/jgp.105.3.403
McCormack, J. G., Halestrap, A. P., and Denton, R. M. (1990). Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425. doi: 10.1152/physrev.1990.70.2.391
Mentzer, R. M. Jr., Bartels, C., Bolli, R., Boyce, S., Buckberg, G. D., Chaitman, B., et al. (2008). Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann. Thorac. Surg. 85, 1261–1270. doi: 10.1016/j.athoracsur.2007.10.054
Miki, T., Itoh, T., Sunaga, D., and Miura, T. (2012). Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc. Diabetol. 11:67. doi: 10.1186/1475-2840-11-67
Mootha, V. K., Arai, A. E., and Balaban, R. S. (1997). Maximum oxidative phosphorylation capacity of the mammalian heart. Am. J. Physiol. 272(2 Pt 2), H769–H775.
Moreno-Sanchez, R. (1985). Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. J. Biol. Chem. 260, 12554–12560.
Murry, C. E., Jennings, R. B., and Reimer, K. A. (1986). Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136. doi: 10.1161/01.CIR.74.5.1124
Nicoll, D. A., Sawaya, M. R., Kwon, S., Cascio, D., Philipson, K. D., and Abramson, J. (2006). The crystal structure of the primary Ca2 + sensor of the Na + /Ca2 + exchanger reveals a novel Ca2 + binding motif. J. Biol. Chem. 281, 21577–21581. doi: 10.1074/jbc.C600117200
Odunewu-Aderibigbe, A., and Fliegel, L. (2014). The Na( + ) /H( + ) exchanger and pH regulation in the heart. IUBMB Life 66, 679–685. doi: 10.1002/iub.1323
Ottolia, M., Nicoll, D. A., and Philipson, K. D. (2009). Roles of two Ca2 + -binding domains in regulation of the cardiac Na + -Ca2 + exchanger. J. Biol. Chem. 284, 32735–32741. doi: 10.1074/jbc.M109.055434
Ottolia, M., Torres, N., Bridge, J. H., Philipson, K. D., and Goldhaber, J. I. (2013). Na/Ca exchange and contraction of the heart. J. Mol. Cell Cardiol. 61, 28–33. doi: 10.1016/j.yjmcc.2013.06.001
Packer, M. (2017). Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 136, 1548–1559. doi: 10.1161/CIRCULATIONAHA.117.030418
Packer, M., Anker, S. D., Butler, J., Filippatos, G., and Zannad, F. (2017). Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2, 1025–1029. doi: 10.1001/jamacardio.2017.2275
Palty, R., Silverman, W. F., Hershfinkel, M., Caporale, T., Sensi, S. L., Parnis, J., et al. (2010). NCLX is an essential component of mitochondrial Na + /Ca2 + exchange. Proc. Natl. Acad. Sci. U.S.A. 107, 436–441. doi: 10.1073/pnas.0908099107
Philipson, K. D., Nicoll, D. A., Ottolia, M., Quednau, B. D., Reuter, H., John, S., et al. (2002). The Na + /Ca2 + exchange molecule: an overview. Ann. N. Y. Acad. Sci. 976, 1–10. doi: 10.1111/j.1749-6632.2002.tb04708.x
Pieper, G. M., Salhany, J. M., Murray, W. J., Wu, S. T., and Eliot, R. S. (1984). Lipid-mediated impairment of normal energy metabolism in the isolated perfused diabetic rat heart studied by phosphorus-31 NMR and chemical extraction. Biochim. Biophys. Acta 803, 229–240. doi: 10.1016/0167-4889(84)90112-5
Pierce, G. N., and Dhalla, N. S. (1985). Heart mitochondrial function in chronic experimental diabetes in rats. Can. J. Cardiol. 1, 48–54.
Pisarenko, O. I., Studneva, I. M., Serebriakova, L. I., Tskitishvili, O. V., and Timoshin, A. A. (2005). [Protection of rat heart myocardium with a selective Na( + )/H( + ) exchange inhibitor and ischemic preconditioning]. Kardiologiia 45, 37–44.
Pogwizd, S. M., Sipido, K. R., Verdonck, F., and Bers, D. M. (2003). Intracellular Na in animal models of hypertrophy and heart failure: contractile function and arrhythmogenesis. Cardiovasc Res 57, 887–896. doi: 10.1016/S0008-6363(02)00735-6
Ramasamy, R., Liu, H., Anderson, S., Lundmark, J., and Schaefer, S. (1995). Ischemic preconditioning stimulates sodium and proton transport in isolated rat hearts. J. Clin. Invest. 96, 1464–1472. doi: 10.1172/JCI118183
Ramasamy, R., and Schaefer, S. (1999). Inhibition of Na + -H + exchanger protects diabetic and non-diabetic hearts from ischemic injury: insight into altered susceptibility of diabetic hearts to ischemic injury. J. Mol. Cell. Cardiol. 31, 785–797. doi: 10.1006/jmcc.1998.0908
Regan, T. J., Beyer-Mears, A., Torres, R., and Fusilli, L. D. (1992). Myocardial inositol and sodium in diabetes. Int. J. Cardiol. 37, 309–316. doi: 10.1016/0167-5273(92)90260-A
Ren, X., and Philipson, K. D. (2013). The topology of the cardiac Na( + )/Ca(2)( + ) exchanger, NCX1. J. Mol. Cell Cardiol. 57, 68–71. doi: 10.1016/j.yjmcc.2013.01.010
Rezende, P. C., Rahmi, R. M., Uchida, A. H., da Costa, L. M., Scudeler, T. L., Garzillo, C. L., et al. (2015). Type 2 diabetes mellitus and myocardial ischemic preconditioning in symptomatic coronary artery disease patients. Cardiovasc. Diabetol. 14:66. doi: 10.1186/s12933-015-0228-x
Saotome, M., Katoh, H., Satoh, H., Nagasaka, S., Yoshihara, S., Terada, H., et al. (2005). Mitochondrial membrane potential modulates regulation of mitochondrial Ca2 + in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 288, H1820–H1828. doi: 10.1152/ajpheart.00589.2004
Sato, T., and Marban, E. (2000). The role of mitochondrial K(ATP) channels in cardioprotection. Basic. Res. Cardiol. 95, 285–289. doi: 10.1007/s003950070047
Schaffer, S. W., Ballard-Croft, C., Boerth, S., and Allo, S. N. (1997). Mechanisms underlying depressed Na + /Ca2 + exchanger activity in the diabetic heart. Cardiovasc. Res. 34, 129–136. doi: 10.1016/S0008-6363(97)00020-5
Shattock, M. J., Ottolia, M., Bers, D. M., Blaustein, M. P., Boguslavskyi, A., Bossuyt, J., et al. (2015). Na + /Ca2 + exchange and Na + /K + -ATPase in the heart. J. Physiol. 593, 1361–1382. doi: 10.1113/jphysiol.2014.282319
Szabadkai, G., Simoni, A. M., Bianchi, K., De Stefani, D., Leo, S., Wieckowski, M. R., et al. (2006). Mitochondrial dynamics and Ca2 + signaling. Biochim. Biophys. Acta 1763, 442–449. doi: 10.1016/j.bbamcr.2006.04.002
Taegtmeyer, H., McNulty, P., and Young, M. E. (2002). Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation 105, 1727–1733. doi: 10.1161/01.CIR.0000012466.50373.E8
Tanaka, Y., Konno, N., and Kako, K. J. (1992). Mitochondrial dysfunction observed in situ in cardiomyocytes of rats in experimental diabetes. Cardiovasc. Res. 26, 409–414. doi: 10.1093/cvr/26.4.409
Territo, P. R., French, S. A., Dunleavy, M. C., Evans, F. J., and Balaban, R. S. (2001). Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J. Biol. Chem. 276, 2586–2599. doi: 10.1074/jbc.M002923200
Territo, P. R., Mootha, V. K., French, S. A., and Balaban, R. S. (2000). Ca(2 + ) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 278, C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423
Tsang, A., Hausenloy, D. J., Mocanu, M. M., Carr, R. D., and Yellon, D. M. (2005). Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 54, 2360–2364. doi: 10.2337/diabetes.54.8.2360
Veeranki, S., Givvimani, S., Kundu, S., Metreveli, N., Pushpakumar, S., and Tyagi, S. C. (2016). Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell Cardiol. 92, 163–173. doi: 10.1016/j.yjmcc.2016.01.023
Villa-Abrille, M. C., Sidor, A., and O’Rourke, B. (2008). Insulin effects on cardiac Na + /Ca2 + exchanger activity: role of the cytoplasmic regulatory loop. J. Biol. Chem. 283, 16505–16513. doi: 10.1074/jbc.M801424200
Warley, A. (1991). Changes in sodium concentration in cardiac myocytes from diabetic rats. Scanning Microsc. 5, 239–244; discussion244–245.
Williams, B., and Howard, R. L. (1994). Glucose-induced changes in Na + /H + antiport activity and gene expression in cultured vascular smooth muscle cells. Role of protein kinase C. J Clin Invest 93, 2623–2631. doi: 10.1172/JCI117275
Williams, I. A., Xiao, X. H., Ju, Y. K., and Allen, D. G. (2007). The rise of [Na( + )] (i) during ischemia and reperfusion in the rat heart-underlying mechanisms. Pflugers Arch. 454, 903–912. doi: 10.1007/s00424-007-0241-3
Wu, M., Le, H. D., Wang, M., Yurkov, V., Omelchenko, A., Hnatowich, M., et al. (2010). Crystal structures of progressive Ca2 + binding states of the Ca2 + sensor Ca2 + binding domain 1 (CBD1) from the CALX Na + /Ca2 + exchanger reveal incremental conformational transitions. J. Biol. Chem. 285, 2554–2561. doi: 10.1074/jbc.M109.059162
Xiao, X. H., and Allen, D. G. (1999). Role of Na( + )/H( + ) exchanger during ischemia and preconditioning in the isolated rat heart. Circ. Res. 85, 723–730. doi: 10.1161/01.RES.85.8.723
Xiao, X. H., and Allen, D. G. (2003). The cardioprotective effects of Na + /H + exchange inhibition and mitochondrial KATP channel activation are additive in the isolated rat heart. Pflugers Arch. 447, 272–279. doi: 10.1007/s00424-003-1183-z
Yamada, E. W., and Huzel, N. J. (1988). The calcium-binding ATPase inhibitor protein from bovine heart mitochondria. Purification and properties. J. Biol. Chem. 263, 11498–11503.
Young, L. H., Wackers, F. J., Chyun, D. A., Davey, J. A., Barrett, E. J., Taillefer, R., et al. (2009). Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 301, 1547–1555. doi: 10.1001/jama.2009.476
Keywords: sodium, calcium–sodium exchanger, NMRS, oxygen consumption, mitochondrial bioenergetics
Citation: Doliba NM, Babsky AM and Osbakken MD (2018) The Role of Sodium in Diabetic Cardiomyopathy. Front. Physiol. 9:1473. doi: 10.3389/fphys.2018.01473
Received: 19 June 2018; Accepted: 28 September 2018;
Published: 24 October 2018.
Edited by:
Coert J. Zuurbier, Academic Medical Center (AMC), NetherlandsCopyright © 2018 Doliba, Babsky and Osbakken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary D. Osbakken, bW9zYmFra2VuQHZlcml6b24ubmV0
 Nicolai M. Doliba
Nicolai M. Doliba Andriy M. Babsky2
Andriy M. Babsky2 Mary D. Osbakken
Mary D. Osbakken