- 1Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, United States
- 2Escuela de Ingeniería y Ciencias, Tecnológico de Monterrey, Monterrey, Mexico
- 3McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 4Department of Medicine, Weill Cornell Medicine, New York, NY, United States
- 5Department of Cardiology, Houston Methodist DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX, United States
- 6Houston Methodist J.C. Walter Jr. Transplant Center, Houston Methodist Hospital, Houston, TX, United States
- 7Department of Biochemistry and Molecular Biology, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, United States
Pulmonary arterial hypertension (PAH) is a devastating and fatal chronic lung disease. While current pharmacotherapies have improved patient quality of life, PAH drugs suffer from limitations in the form of short-term pharmacokinetics, instability, and poor organ specificity. Traditionally, nanotechnology-based delivery strategies have proven advantageous at increasing both circulation lifetimes of chemotherapeutics and accumulation in tumors due to enhanced permeability through fenestrated vasculature. Importantly, increased nanoparticle (NP) accumulation in diseased tissues has been observed pre-clinically in pathologies characterized by endothelial dysfunction and remodeled vasculature, including myocardial infarction and heart failure. Recently, this phenomenon has also been observed in preclinical models of PAH, leading to the exploration of NP-based drug delivery as a therapeutic modality in PAH. Herein, we discussed the advantages of NPs for efficacious treatment of PAH, including heightened therapeutic delivery to diseased lungs for increased drug bioavailability, as well as highlighted innovative nanotherapeutic approaches for PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and fatal disease arising from restricted blood flow through pulmonary arterial circulation. Defined as having mean pulmonary artery pressures (mPAP) greater than 25 mm Hg (Pauwaa et al., 2011), the increased flow resistance in PAH causes an overload in the right ventricle (RV), leading to hypertrophy, hyperplasia, and fibrosis (Ryan and Archer, 2014). These ultimately lead to right heart failure, the major cause of death in PAH patients (Shah, 2012). PAH pertains to the Group I subset of PH, which encompasses idiopathic and heritable disease affecting pulmonary vasculature (Collum et al., 2017). Pathophysiologically, PAH is characterized by remodeling of the pulmonary vasculature that leads to vessel occlusion, muscularization of previously non-muscular vessels, and formation of complex vascular lesions (Stenmark et al., 2009), with pulmonary arteriole smooth muscle cells (PASMCs) and endothelial cells (PAECs) lying at the crux of these processes (Morrell et al., 2009).
Pulmonary arterial hypertension drug therapies have traditionally relied on regulation of vascular tone (Sahni et al., 2016), principally targeting the prostacyclin (PGI2), endothelin (ET), and nitric oxide signaling pathways (Lang and Gaine, 2015). While pharmacotherapies have resulted in improvements in hemodynamics and quality of life (Lau et al., 2017), they are not without considerable shortcomings, including short drug half-lives and instability (Delcroix and Howard, 2015), as well as adverse side effects (Galie et al., 2009). Moreover, despite combination drug regimens, PAH undoubtedly progresses despite pharmacotherapy. Thus, there are currently no curative treatments available for PAH patients save for lung transplantation (Gottlieb, 2013), highlighting the pressing need to develop innovative treatments that can attenuate or even reverse vascular remodeling.
Nanotechnology-based drug delivery platforms prove effective vectors for packaging of drug and genetic material (Ferrari, 2005). Nanoparticles (NPs) are defined as possessing diameters between 0.1 and 100 nm, which can be composed of either naturally occurring or synthetic, man-made materials (Riehemann et al., 2009). These nanoconstructs can be precisely designed with regards to size and geometry, with versatile chemistry enabling tailorability of properties such as enhanced cellular entry and controlled release (Blanco et al., 2015). NP platforms prolong circulation lifetimes of drugs when administered intravenously (IV), proving pharmacokinetically advantageous when compared to conventional drug formulations (Blanco et al., 2011). Importantly, the myriad of pathophysiological alterations involved in PAH progression, particularly endothelial injury, provides a potential avenue for systemically administered nanotherapies in PAH. NP-based drug delivery has been extensively used in cancer primarily because of the ability of long-circulating NPs to accumulate passively in tumors by extravasating through leaky vasculature (Maeda et al., 2013). This phenomenon is commonly referred to as the enhanced permeability and retention (EPR) effect. Herein, we will discuss conventional pharmacotherapies in PAH. We will also describe the established NP platforms commonly used for drug delivery, and highlight the role that vascular remodeling in PAH can play in enhancing accumulation in lungs. Lastly, we will showcase several nanotherapeutic strategies that prove promising for the treatment of PAH.
Conventional Drug Therapy in PAH
Prostacyclin Agonists
Produced in vascular endothelial cells, the arachidonic acid metabolite PGI2 plays an important role in vasodilation, and inhibits smooth muscle cell (SMC) proliferation and platelet aggregation (Del Pozo et al., 2017). By binding and activating the PGI2 (IP) receptor on SMCs, PGI2 activation increases cyclic adenosine monophosphate (cAMP) levels, which in turn results in vasodilation (Ricciotti and FitzGerald, 2011). In PAH, endogenous PGI2 levels are decreased (Tuder et al., 1999), making PGI2 and prostaglandin analogs attractive therapeutic options for treatment. Prostanoids have been used clinically over the past three decades for PAH therapy, with the synthetic PGI2, epoprostenol sodium (Flolan®), being the first pharmacological agent to gain FDA approval for the treatment of PAH (Safdar, 2011), based on improvements in exercise capacity and hemodynamics in patients (Barst et al., 1996).
Endothelin Receptor Antagonists
Produced by endothelial cells, endothelin-1 (ET-1) promotes SMC vasoconstriction, proliferation, migration, and survival. ET-1 also promotes collagen synthesis by fibroblasts (Rosano et al., 2013). Binding of ET-1 to endothelin receptors (ETA and ETB) on SMCs activates phospholipase C, which in turn increases intracellular calcium, resulting in sustained vasoconstriction (Seo et al., 1994). Patients diagnosed with PAH have increased activation of ET-1 in both plasma and lung tissues (Galié et al., 2004) and elevated plasma levels of ET-1 can be correlated with severity of disease and prognosis (McLaughlin et al., 2009), leading to the exploration of various compounds capable of blocking either ETA or ETA and ETB receptors. Three orally administered ET receptor antagonists (ERAs), ambrisentan (Letairis®, an ETA receptor inhibitor), bosentan (Tracleer®, a dual ETA and ETB receptor inhibitor), and macitentan (Opsumit®, a dual ETA and ETB receptor inhibitor), have been clinically approved by the FDA based on randomized clinical trials where increases in 6-min walk distance (6MWD), improved hemodynamic parameters, and overall quality of life were observed (Raja, 2010).
Nitric Oxide Promoters
Nitric oxide (NO) is a product of endothelial cells and a potent vasodilator. By binding to and subsequent activation of soluble guanylate cyclase (sGC), NO increases levels of cyclic guanosine monophosphate (cGMP) (Russwurm and Koesling, 2004), resulting in reduced intracellular calcium levels and SMC relaxation (Carvajal et al., 2000). NO has also been shown to inhibit SMC proliferation and platelet activation (Tonelli et al., 2013). Levels of NO and NO-products in lungs and bronchoalveolar lavage fluid (BALF) of PAH patients have been shown to be significantly lower compared to control subjects (Kaneko et al., 1998). Therapies targeting the NO pathway in PAH consist of sGC agonists and phosphodiesterase type 5 (PDE5) inhibitors. While NO signaling in PAH patients is aberrant, sGC is expressed in PASMCs of PAH patients (Schermuly et al., 2008), making sGC stimulators attractive agents for increasing cGMP levels in these patients. One such oral sGC agonist, riociguat (Adempas®), was the first drug approved targeting the NO pathway for the treatment of PAH, and activates sGC directly despite the absence of NO (Klinger and Kadowitz, 2017). Findings also demonstrate that PDE5 is overexpressed in PASMCs of PAH patients (Murray et al., 2002). PDE5 inhibitors function by hindering the degradation of cGMP (Giovannoni et al., 2010). Administered orally, PDE5 inhibitors currently approved for the treatment of PAH are sildenafil (Viagra®) and tadalafil (Cialis®). sGC stimulators and PDE5 inhibitors have led to improved 6MWD in patients, as well as lessened time to clinical worsening (Humbert et al., 2014).
Pitfalls of Conventional Pharmacotherapies
Pharmacotherapies in PAH have improved patient hemodynamics and quality of life, but are not without significant shortcomings. Chief among these are drug half-life, stability, and formulation limitations, resulting in deleterious side effects. As an example, epoprostenol has a short half-life of 3–5 min, and instability at low pH values (Mubarak, 2010). As a result, the drug must be continuously infused IV by means of an implanted catheter and infusion pump, and the drug must be constantly maintained under refrigeration and prepared daily. Consequently, patients are at risk of infections, sepsis, and thrombosis (McLaughlin and Palevsky, 2013). Moreover, permanently implanted catheters may malfunction (Ruan et al., 2010). In the case of drugs such as PDE5 inhibitors, a high and continuous dosage is required to achieve beneficial effects, necessitating oral administration of 80 mg up to 3 times a day (Galie et al., 2005).
An additional pitfall is the non-specific distribution of pharmacotherapies, resulting in adverse systemic side effects. Prostanoid therapy is associated with flushing, headaches, and gastrointestinal symptoms, such as nausea and vomiting (Lang and Gaine, 2015). Traditional ET inhibitors result in peripheral edema, anemia, and hepatotoxicity (Aversa et al., 2015). And while the precise mechanism of liver toxicity has not been fully established, abnormal liver function is an indication for treatment discontinuation (McGoon et al., 2006). Lastly, targeting the NO pathway by either PDE5 inhibitors or sGC stimulators causes side effects such as headache, dyspepsia, peripheral edema, nausea, and dizziness (Ishikura et al., 2000; Ghofrani et al., 2013), in addition to retinal vascular disease and myocardial infarction (Duarte et al., 2013).
Novel drug formulations address limitations related to formulation and delivery. As an example, epoprostenol AS (Veletri®), contains arginine and sucrose, and can be stable at room temperature for up to 72 h depending on the concentration of the solution (Sitbon and Vonk Noordegraaf, 2017). More stable prostanoids such as inhaled iloprost (Ventavis®) showed improvements in exercise capacity and beneficial hemodynamic effects (LeVarge, 2015). Recently, a non-prostanoid PGI2 receptor analog, selexipag (Uptravi®) was developed and approved for oral administration in PAH (Duggan et al., 2017). In the case of ERAs, the aforementioned macitentan reduced morbidity and mortality in PAH patients (Sitbon et al., 2014), lowering the incidence of liver toxicity. Despite these improvements, strategies capable of increasing the bioavailability of PAH pharmacotherapies in the lung have the potential to improve patient outcomes and reduce systemic adverse events.
NP Platforms for Drug and Gene Delivery
Liposomes
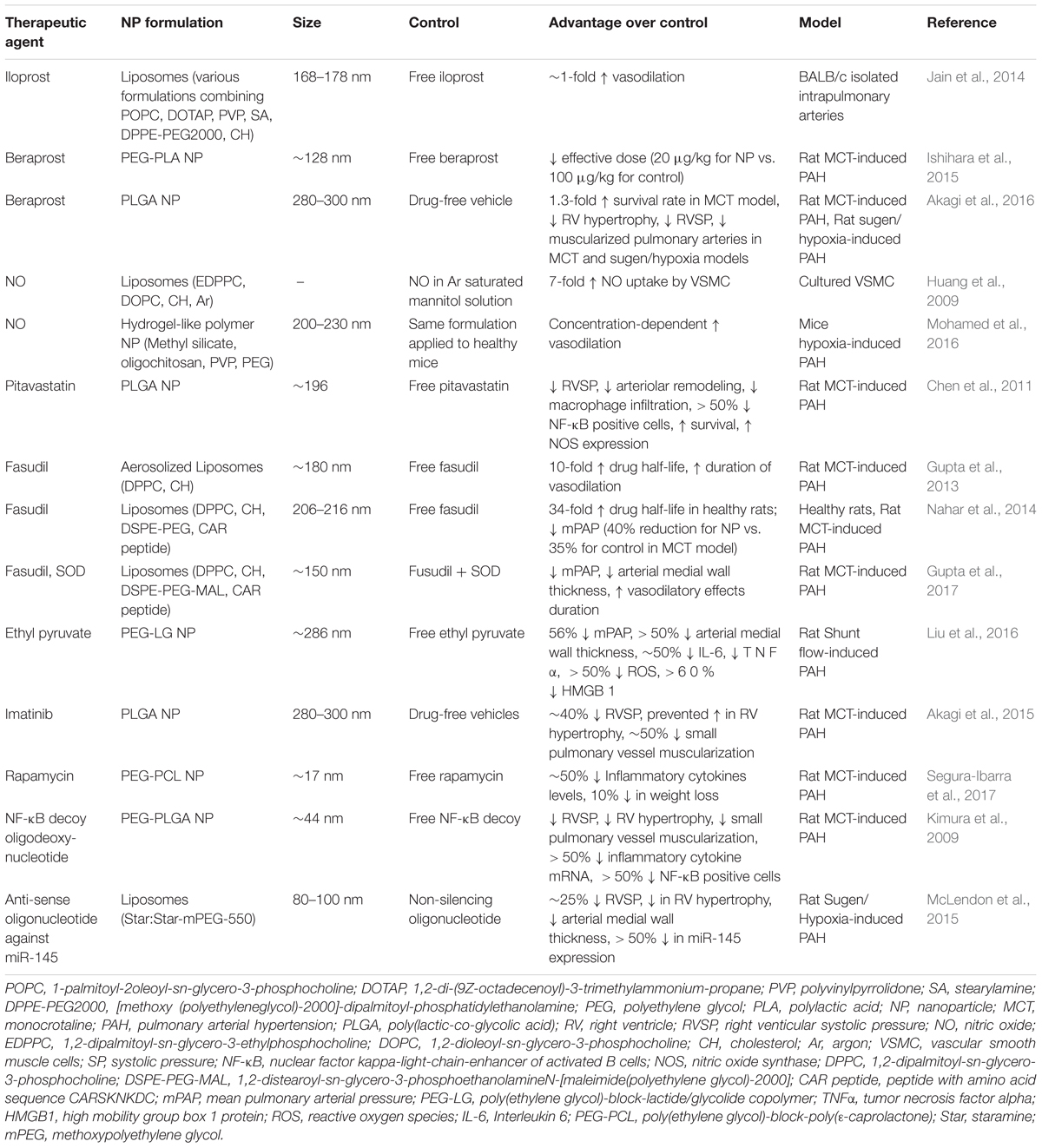
Liposomes are composed of phospholipids with polar heads and hydrophobic tails, forming bilayered constructs with an aqueous core, typically on the order of 100 nm in size (Figure 1A) (Pattni et al., 2015). The aqueous compartment is ideal for accommodation of water-soluble drugs. Hydrophobic drugs can be incorporated within the bi-phospholipid membrane, albeit at the risk of membrane destabilization (Liu et al., 2006). Functionalization of liposomes with polyethylene glycol (PEG) on the surface led to significant enhancement of circulation lifetimes, best demonstrated by DOXIL®, a PEGylated liposomal formulation of doxorubicin (Hamilton et al., 2002). The increase in circulating half-life was a direct result of incorporating PEG onto the surface of liposomes, with the hydrating layer provided by PEG deterring protein adsorption and NP clearance by the mononuclear phagocyte system (MPS) (Harris and Chess, 2003). Importantly, liposomal doxorubicin was shown to reduce doxorubicin-associated cardiotoxicity compared to the conventional, clinically used formulation of doxorubicin (Berry et al., 1998). These advantages led to DOXIL® being the first NP platform approved by the FDA for the treatment of Kaposi’s sarcoma in 1995 (Barenholz, 2012). Liposomes also prove advantageous for efficient delivery of genetic material through incorporation of cationic lipids such as [1,2-bis(oleoyloxy)-3-(trimethylammonio)propane] (DOTAP) (Zhang et al., 2012). Functionalization of liposomes with the thermoresponsive polymer N-isopropylacrylamide (NIPAAm) can be used to induce membrane disruption at high temperatures, resulting in increased local release of drug at specific sites (Ta and Porter, 2013).
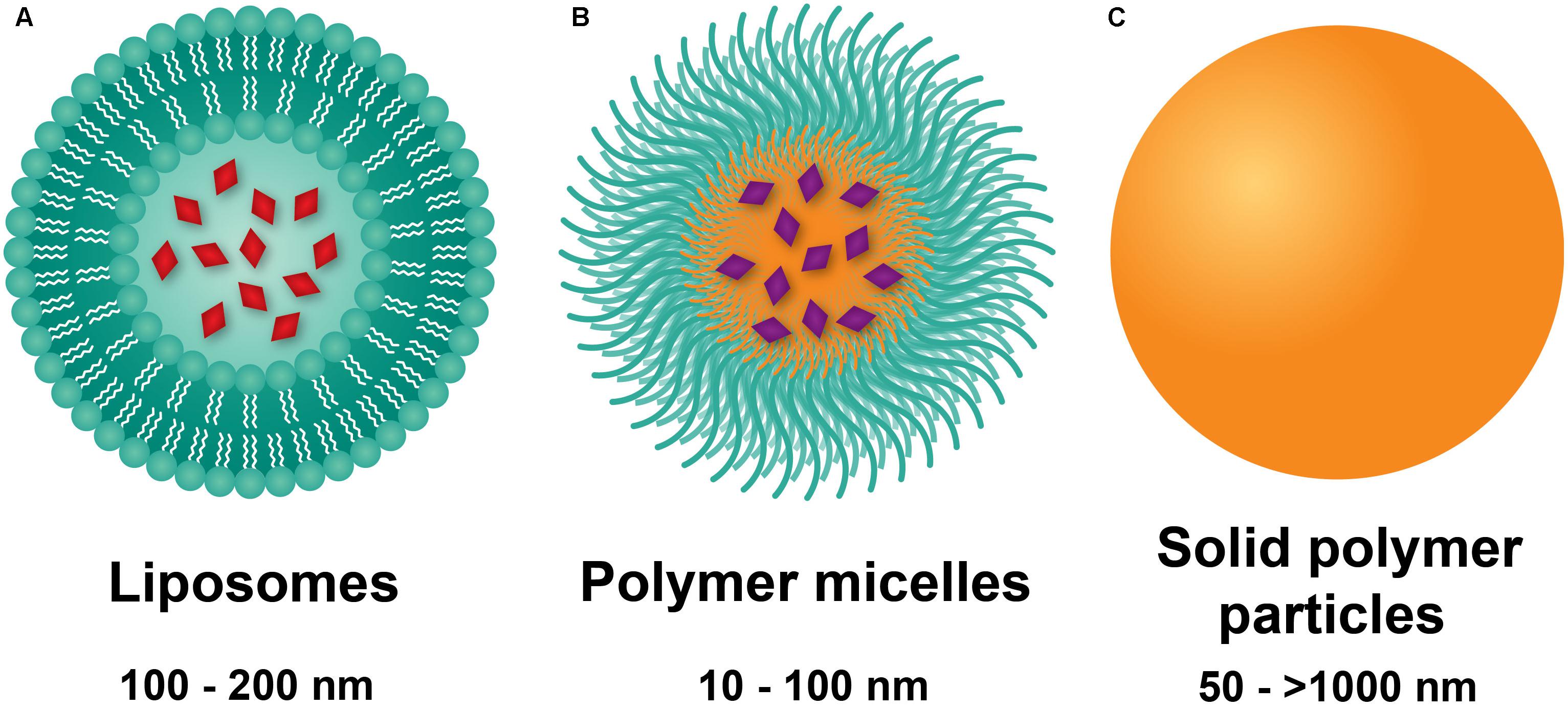
FIGURE 1. Nanoparticle platforms explored in PAH drug and gene delivery. (A) Liposomes are comprised of a lipid bilayer, with an aqueous core ideal for encapsulation of water-soluble drugs (red). (B) Polymer micelles are comprised of amphiphilic block copolymers that self-assemble in water to form a hydrophilic corona and a hydrophobic core for encapsulation of lipophilic drugs (violet). (C) Solid polymer particles have drug dissolved or embedded within a polymer matrix.
Polymer Micelles
Polymer micelles are NPs formed from the self-assembly of amphiphilic-block copolymers in aqueous environments (Blanco et al., 2009). The core-shell morphology of polymer micelles consists of a hydrophobic core and a hydrophilic shell (Figure 1B), wherein the hydrophilic block of the constituent polymer is typically PEG. On the order of 10–100 nm in diameter, polymer micelles have traditionally been used as delivery vehicles for hydrophobic drugs. Of significant note, the tailorability of polymer chemistries makes micelles highly versatile carriers with a myriad of advantages for drug delivery. Cationic polymers such as polyethylenimine (PEI) (Dai et al., 2011b) or poly(L-lysine) (Christie et al., 2012) can be either grafted onto block copolymers or used as the core-forming block for loading of genetic material. Stimuli-responsive, tailored drug release can also be obtained based on the composition of the core forming polymer block. As an example, Bae et al. (2005) used PEG-b-poly(aspartate) (PEG-PAsp) for pH-sensitive release of doxorubicin by conjugating it to PAsp through a hydrazine linkage. Lastly, targeting moieties including antibodies, aptamers, and peptides fashioned onto polymer micelles can be used for active targeting to diseased tissues and cells (Jhaveri and Torchilin, 2014). As an example, the cyclic(Arg-Gly-Asp-DPhe-Lys) (cRGDfK) peptide has been used for polymer micelle targeting to the avb3 integrin found overexpressed on tumor vasculature (Nasongkla et al., 2004; Song et al., 2014). Despite their numerous advantages, polymer micelles are limited by fast release of drug and long-term stability, with strategies such as interlayer-crosslinked cores (Dai et al., 2011a) shown to prevent premature drug release.
Solid Polymer Particles
Solid polymer particles, typically comprised of the polyester polylactide-co-glycolide (PLGA), have long been employed in controlled drug release applications. These particles are spherical in morphology, can range from the nano- to micro-meter dimensions, and can be used for delivery of water soluble and insoluble drugs (Makadia and Siegel, 2011), with agents dissolved or encapsulated within the polymer matrix (Figure 1C; Danhier et al., 2012). PLGA remains the constituent polymer of choice for these NPs due to several advantages. Chief among these is the relative ease of fabrication, as well as the biocompatibility and biodegradability of the PLGA, a material approved by the FDA for a wide range of biomedical applications. In aqueous environments, ester linkages of PLGA undergo hydrolysis, producing the monomers lactic acid and glycolic acid, which are readily metabolized and removed from the body (Acharya and Sahoo, 2011). Moreover, drug release from PLGA NPs occurs through initial diffusion followed by degradation of the polymer matrix, which in turn is affected by crystallinity, composition, molecular weight, and size and shape of the matrix (Makadia and Siegel, 2011). Thus, highly controllable and sustained release profiles can be achieved by employing PLGA copolymers with the more hydrophobic polylactic acid (PLA) than polyglycolic acid (PGA), which give rise to NPs with less water absorption and slower degradation kinetics (Dinarvand et al., 2011). In addition to drugs, PLGA particles can incorporate cationic polymers (e.g., PEI) for delivery of genetic material (Bivas-Benita et al., 2004). PLGA NP drug delivery is limited by rapid initial release of payload due to hydration of the polymer (Kapoor et al., 2015), as well as dose dumping effects at longer timepoints (Khanal et al., 2016). Moreover, peptides and proteins may undergo chemical degradation within polymer matrices (Houchin and Topp, 2008).
Nanoparticle Size Considerations
The relative size of the different NPs influences in vivo fate following intravenous delivery. It is now well known that NPs with diameters < 5 nm are cleared rapidly by the kidneys (Choi et al., 2007). NPs that measure > 100 nm accumulate non-specifically in livers (Braet et al., 2007), those measuring > 200 nm accumulate in the spleen (Chen and Weiss, 1973), and particles >2 μm accumulate in lung capillaries. Resident macrophages of the liver, spleen, and lungs rapidly internalize opsonized NPs in a size-dependent manner. Taken together, smaller sized NPs, measuring 100 nm or less, have been shown to be long circulating following intravenous administration (Blanco et al., 2010).
These size considerations play an important role in the design of nanotherapeutic constructs for purposes of targeting specific tissues. As an example, Xu et al. (2016) used particles with a diameter of 2.5 μm to specifically target breast cancer metastasis in the lung. Long-circulating NPs have a heightened propensity to passively accumulate in tissues with remodeled vasculature by extravasating through submicron sized pores in the endothelium (Hobbs et al., 1998). And while smaller sized NPs are able to extravasate from circulation into these diseased sites, the extent of NP penetration into the tissue depends on the size of the carrier. Cabral et al. (2011) were able to demonstrate that sub-100 nm NPs were able to penetrate into permeable tumors. However, in more fibrotic tumors, only NPs measuring < 50 nm were capable of penetrating into the tissue.
Inhalational delivery of NPs represents an attractive strategy for specifically targeting pulmonary tissues. However, particle size also dictates regional lung deposition after inhalation (Paranjpe and Muller-Goymann, 2014). When administered as a dry powder, large particles in the size range of 1–5 μm deposit in bronchioles and smaller airways, particles in the size range of 0.5–1 μm accumulate in alveolar regions, and smaller NPs (< 0.5 μm) can undergo exhalation.
Enhanced NP Accumulation in Lungs Undergoing PAH
Nanoparticle platforms such as liposomes and polymer micelles have been extensively explored in chemotherapy. While advantageous at increasing the circulation lifetimes of chemotherapeutics, it was the observation by Maeda et al. (2013) regarding the ability of IV-administered macromolecules to accumulate to a large extent in tumors that led to the excitement of NP-based drug delivery strategies in cancer (Matsumura and Maeda, 1986). Passive targeting of macromolecules and NPs to tumors is owed to the high degree of fenestrations (e.g., openings) present in tumor vasculature (McDonald and Choyke, 2003), a direct result of chaotic and ongoing angiogenic processes in tumors (Fang et al., 2011). This enhanced NP accumulation in tumors, combined with NP persistence due to impaired lymphatic drainage (Banerjee et al., 2011) is known as the EPR effect (Maeda et al., 2013).
While passive accumulation of NPs in disease sites is primarily associated with cancer, vascular permeability is prevalent in other diseases characterized by abnormal angiogenesis and vascular remodeling as a consequence of inflammation (Durymanov et al., 2017). As an example, in rheumatoid arthritis, where a combination of angiogenic and inflammatory processes promote vessel leakiness, several groups have reported passive targeting to the synovium (Metselaar et al., 2004; Anderson et al., 2010). Similarly, formation of new blood vessels in atherosclerotic plaques leads to enhanced NP uptake in these lesions (Chono et al., 2005; Stigliano et al., 2017). Vascular injury stemming from local inflammatory processes and hypoxia is present in diseases such as myocardial infarction and heart failure, resulting in enhanced vascular permeability to the heart. Nagaoka et al. (2015) and Nakano et al. (2016) demonstrated increased NP uptake in myocardial infarct areas following IV administration in a model of ischemia-reperfusion (IR) injury in the heart, mirroring previously published findings (Dvir et al., 2011; Paulis et al., 2012). Our laboratory recently demonstrated enhanced accumulation of micron-sized particles in failing hearts compared to healthy hearts (Ruiz-Esparza et al., 2016). It is important to note that the prevalence of immune-related cells in areas of inflammation can also contribute to increased uptake at these sites through macrophage phagocytosis (Ulbrich and Lamprecht, 2010).
Vascular permeability in PAH arises from injurious events such as inflammation and hypoxia, resulting in focal disruptions in endothelial cell basement membranes (McLaughlin and McGoon, 2006; Stenmark et al., 2006; Montani et al., 2014), as well as increased vascular pressure, which leads to fenestrations as a result of greater mechanical and shear stress (Figure 2) (Zhou et al., 2016). Moreover, mutations in bone morphogenetic protein receptor 2 (BMPR2), highly prevalent in heritable PAH, have been shown to contribute to increased vascular permeability through dysregulation of the TGF-β signaling pathway (Morrell, 2006). Our laboratory recently demonstrated that vascular permeability in PAH contributes to enhanced NP accumulation in diseased lungs (Segura-Ibarra et al., 2017), agreeing well with previous findings by Ishihara et al. (2015). In a monocrotaline (MCT)-induced model of PAH, poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-PCL) micelles containing rapamycin (RAP) resulted in increased drug accumulation in diseased lungs compared to healthy lungs 2 h after IV administration (Figure 3A). Moreover, LC/MS analysis comparing RAP-containing micelles and a free drug formulation of RAP showed a significantly higher increase in RAP accumulation in diseased lungs when packaged within NPs (Figure 3A). Upon closer examination of remodeled vasculature using confocal microscopy, heightened accumulation of PEG-PCL NPs was observed within the perivascular region (Figures 3B,C).
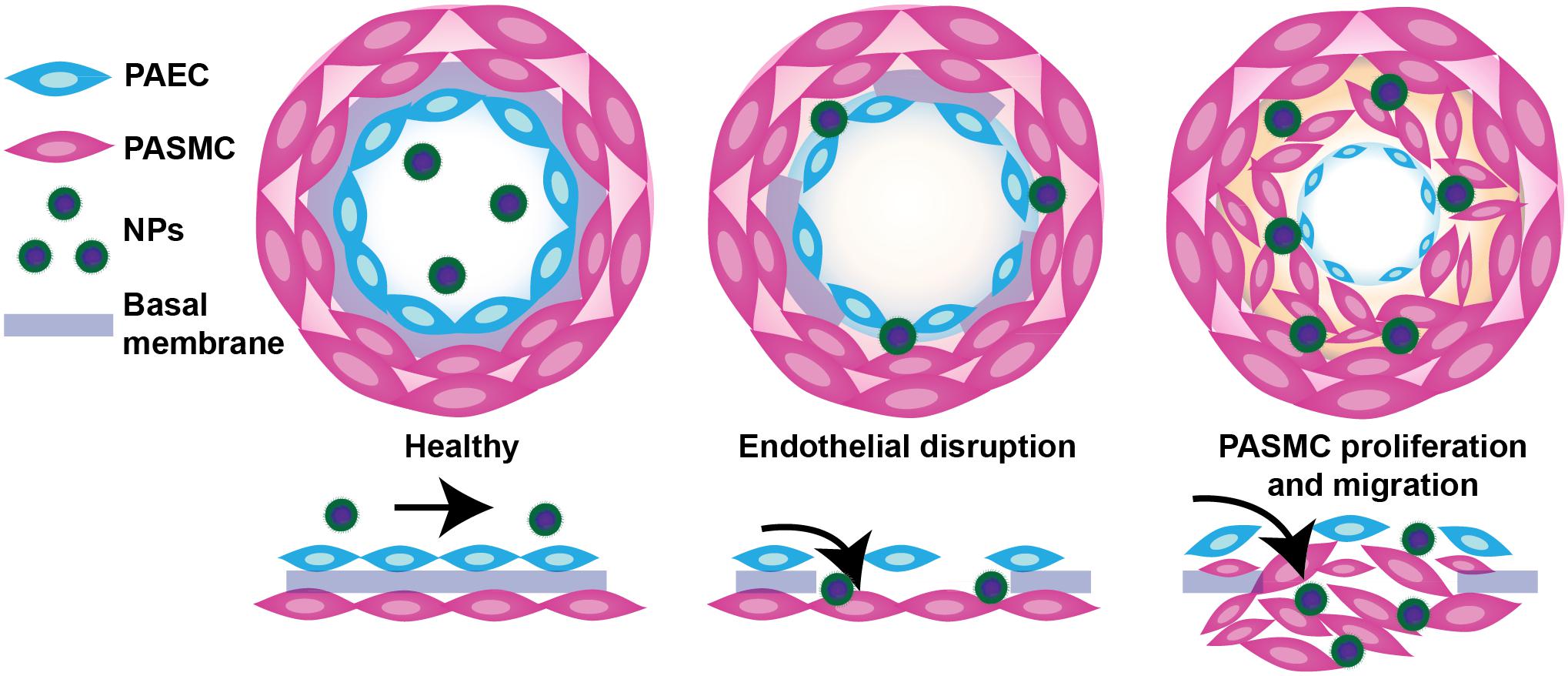
FIGURE 2. Schematic representation of endothelial dysfunction in PAH and a proposed mechanism of NP extravasation into pulmonary vasculature. While healthy pulmonary vasculature is a semipermeable membrane barrier, the vasculature in lungs undergoing PAH exhibits endothelial dysfunction arising from a chronic inflammatory state and hypoxia, leading to fenestrations (openings) in the endothelium and a hyperpermeable state. Such permeability may be exploited by NPs to passively extravasate and accumulate in lungs undergoing PAH. Figure adapted from Segura-Ibarra et al. (2017), reproduced with permission courtesy of Elsevier.
FIGURE 3. NP accumulation in PAH lung vasculature. (A) LC/MS analysis of rapamycin (RAP) concentration in lung tissues 2 and 24 h after a single administration of 15 mg/kg of RAP, either as a free drug formulation (RAP FD) or nanoparticle form (RAP NP) in healthy and MCT-induced model of PH in rats (PH). Results represent mean ± SEM (∗∗∗∗P < 0.0001). (B) Confocal imaging depicting fluorescently loaded NPs in diseased lungs. CD31 positive endothelial cells appear in yellow, NPs are green, while DAPI appears as blue. The scale bar represents 25 μm. (C) Surface intensity plot of the image from panel B representing NP signal. Figure adapted from Segura-Ibarra et al. (2017), reproduced with permission courtesy of Elsevier.
Nanotherapeutics in PAH
Conventional pharmacotherapies for PAH treatment suffer from short half-lives, drug instability, and adverse side effects. NP-based strategies for the treatment of PAH offer advantages of improving short-term pharmacokinetics associated with drugs and increased localization of therapy to diseased tissues, in turn decreasing adverse effects. Herein, we highlight nanotherapeutic approaches aimed at delivering clinically approved PAH drugs, as well as nanoplatforms for delivery of novel agents, including genetic material (Table 1).
Prostanoid-Containing NPs
The clinically approved drug inhaled iloprost has an extremely short half-life, requiring at most 12 inhalations per day (Olschewski et al., 2000), largely impacting patient compliance. In hopes of increasing drug bioavailability, Kleemann et al. (2007) developed a liposomal formulation for sustained release of iloprost for aerosolized PAH therapy. Liposomes consisted of di-palmitoyl-phosphatidyl-choline (DPPC), cholesterol to enhance sustained delivery, and poly(ethylene glycol)-di-palmitoyl-phosphatidyl-ethanolamine (PEG-DPPE) to prevent clearance by alveolar macrophages, which would limit their bioavailability. Resulting liposomes ranged in size from 200 to 400 nm, and contained 11 μg iloprost/ml, which would significantly reduce the number of inhalations required.
Jain et al. (2014) fabricated iloprost-containing liposomes with cationic lipids in hopes of increasing drug loading efficiency and examined their efficacy based on changes in vascular tone of pulmonary arteries isolated from mice by means of a wire myograph. NPs averaged 168–178 nm in diameter and had drug loading efficiencies of ∼50%. Pulmonary arteries were constricted by application of the thromboxane analog, U-46619, and treated either with free or liposomal iloprost. Liposomal iloprost resulted in significant enhancement of vasodilation (29% compared to 16% for free iloprost), with a much lower concentration of liposomal iloprost required to bring about efficacies similar to that of free drug.
The oral PGI2 analog beraprost has proven vasodilatory and anti-platelet activity, but much like other prostanoids, has a very short half-life (∼1 h) (Barst et al., 2003). In attempts to overcome pharmacokinetic limitations of the drug, Ishihara et al. (2015), who previously formulated NPs containing prostaglandin E1 (PGE1) (Takeda et al., 2009), encapsulated beraprost within poly(ethyleneglycol)-block- poly(lactide) (PEG-PLA) micelles and examined their efficacy in an MCT-induced PAH rat model and hypoxia-induced mouse model of PAH. Resulting NPs possessed average diameters of 128 nm and exhibited slow drug release kinetics (∼20% over 1 week). Beraprost NPs showed significantly reduced drug clearance from plasma compared to free beraprost, the former present in circulation at timepoints of 24 h, while the latter was cleared within 6 h. Upon IV administration in an MCT-induced model of PAH in rats, NPs accumulated more in MCT-damaged lungs compared to healthy control lungs, and were found associated with pulmonary peripheral arteries. Importantly, once a week IV administration of beraprost NPs at a dose of 20 μg/kg in an MCT-induced PAH rat model reduced pulmonary arterial remodeling and right ventricular hypertrophy; the efficacy proving similar to that of a daily oral administration of the drug at a much higher dose (100 μg/kg). A similar improvement in pulmonary arterial remodeling was observed in the hypoxia-induced model in mice. This study effectively highlights the advantages afforded by NP-based drug delivery, mainly the need for lower doses and less frequent administrations to achieve similar efficacious responses.
In another study, Akagi et al. (2016) fabricated PLGA NPs containing beraprost and examined the efficacy of the platform in MCT- and Sugen/Hypoxia-induced models of PAH. After a single intratracheal administration of beraprost-containing NPs, RV systolic pressure (RVSP), RV hypertrophy, and the percentage of fully muscularized small pulmonary arteries were significantly reduced compared to disease controls in both PAH models. Moreover, the survival rate increased to 65% following administration of NP-based beraprost, compared to 27.8% in disease controls. Of note, NPs administered intratracheally in the Sugen/Hypoxia-induced model of PAH were found associated with the media of pulmonary arteries and interstitium at timepoints of up to 3 days, whereas no NPs were evident in healthy control lungs.
NP-Based Targeting of the NO Pathway
Nitric oxide plays an important role in healthy pulmonary physiology, driving SMC relaxation (Perez-Zoghbi et al., 2010), with added anti-inflammatory and proliferative properties (Tonelli et al., 2013). Huang et al. (2009) developed a liposomal formulation of NO consisting of 1,2-dipalmitoyl-sn-glycero-3-ethylphosphocholine (EDPPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and cholesterol. These liposomes encapsulated 10 μL of NO per mg of lipids and Argon (Ar) was used as an excipient for NO. Upon examination of release kinetics in vitro, release of NO from liposomes was slower in the presence of Ar, resulting in a sustained release profile. No significant toxicity was observed in vitro in cultured rat vascular smooth muscle cells (VSMCs), and based on a colorimetric NO assay kit, a sevenfold increase in uptake of NO was observed with liposomal NO than NO formulated in Ar saturated mannitol solution. Moreover, liposomes protected NO from microenvironmental scavengers such as hemoglobin. To evaluate in vivo efficacy, a balloon injury was induced in the common carotid arteries of rabbits and liposomes containing NO were administered locally. After 2 weeks, a significant decrease in intimal hyperplasia was observed in rabbits treated with liposomal NO compared to vehicle controls (empty liposomes), demonstrating the feasibility of delivery of bio-active gases NPs.
Recently, Mohamed et al. (2016) developed a novel hydrogel-like polymer composite NP formulation for delivery of NO. NPs released NO in a sustained fashion over time, and showed concentration-dependent vasodilation of U-46619-induced preconstricted pulmonary arteries, with a more pronounced effect observed in arteries from hypoxia-induced PAH mice compared to healthy mice.
Beck-Broichsitter et al. (2012) have explored novel spray-drying techniques to fabricate PLGA microparticles for deposition in the lungs and release of sildenafil. Using a vibrational spray drying procedure, resulting microparticles measured 4–8 μm in size and had a high sildenafil encapsulation efficiency of > 90% (Beck-Broichsitter et al., 2017). Moreover, the formulation resulted in a sustained release of sildenafil over time, making these microparticles potentially beneficial for controlled pulmonary drug delivery in PAH and chronic lung diseases.
Nanotherapeutic Delivery of Novel Agents Targeting PAH
Currently, statins are one of the first-line medications given to patients with elevated cholesterol levels to prevent cardiovascular disease. The mechanism of action involves inhibiting the rate-limiting step of cholesterol biosynthesis by competitive inhibition of HMG-CoA reductase (Istvan, 2003). Statins also improve endothelial function (Beckman and Creager, 2006), displaying anti-tumoral (Crescencio et al., 2009), anti-proliferative (Kamigaki et al., 2011), and anti-inflammatory (Ridker et al., 1999; Lefer, 2002) effects.
Given that inflammation, endothelial injury, and cellular proliferation play a crucial role in PAH progression, Chen et al. (2011) explored the use of statin nanotherapeutics for treatment of PAH. The anti-proliferative effects of different statins (pravastatin, losuvastatin, simvastatin, atorvastatin, fluvastatin, and pitavastatin) were evaluated in human PASMCs, and pitavastatin was selected for PLGA NP encapsulation based on its potent effects. Distribution of PLGA NPs following intratracheal instillation were examined, and FITC-containing NPs were found in lungs of rats undergoing MCT-induced PAH 3 days after administration, specifically in small arteries, bronchi, alveoli, and alveolar macrophages. Of significant note, FITC was detected in lungs at timepoints of up to 14 days after a single administration. A single administration of pitavastatin-containing NPs was performed at the time of PAH induction of rats, and 21 days after administration, right ventricular catheterization revealed a significant decrease in RV systolic pressure compared to rats treated with free pitavastatin or vehicle controls. A significant decrease in systolic pressure in pulmonary arterioles was also observed. Of note, lower levels of macrophages and monocytes were found in rats treated with pitavastatin-containing NPs. Moreover, compared to free pitavastatin, the NP formulation resulted in a > 50% decrease of cells positive for nuclear factor kappa-light-chain-enhancer of activated B (NF-κB), which plays an important role in cell proliferation and survival (Hoesel and Schmid, 2013). The NP formulation increased expression of endothelial NO synthase (eNOS), which can potentially promote endothelial healing. Following NP administration in rats 21 days after MCT induction of PAH, pitavastatin-containing NPs significantly increased survival by 64% compared to control groups (Figure 4A) and significantly decreased RVSP compared to disease controls (Figure 4B). It is important to note that a Phase I clinical trial involving pitavastatin PLGA NP-based delivery for PAH has recently been completed (Nakamura et al., 2017).
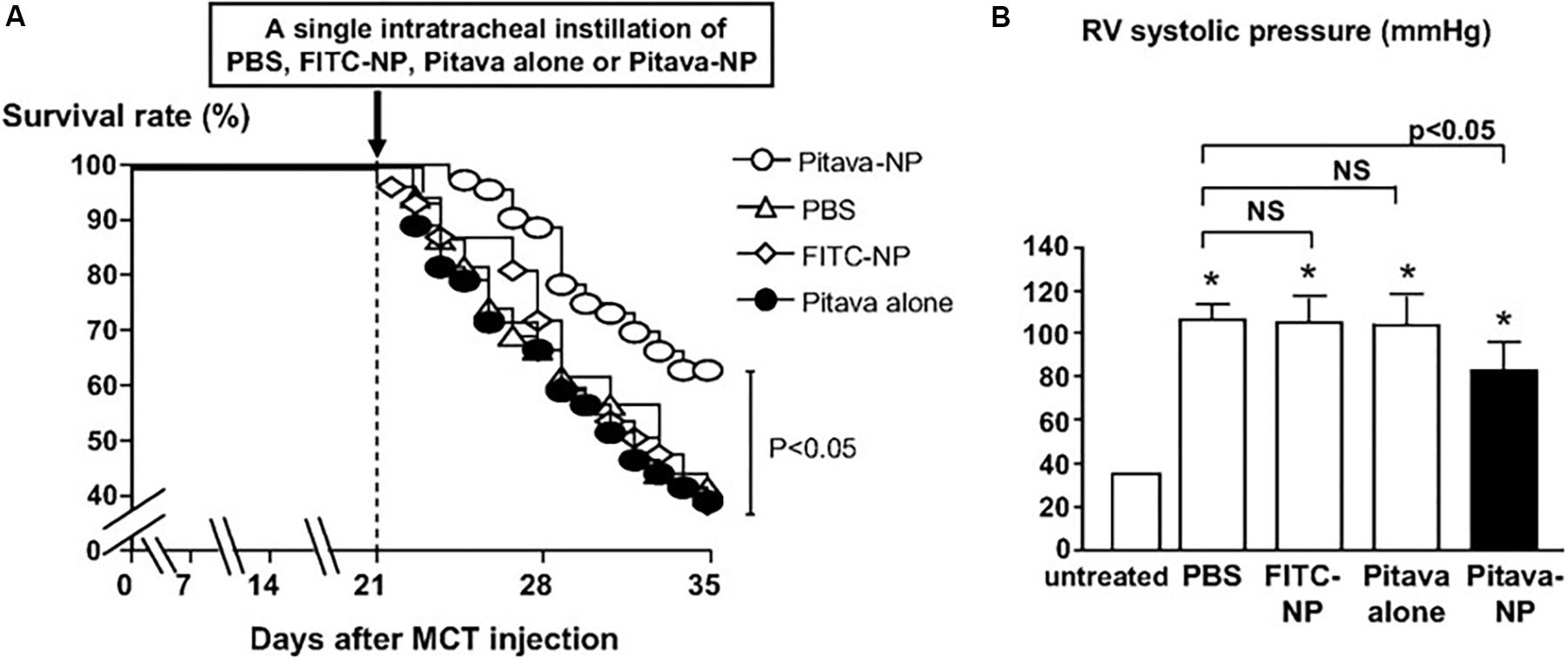
FIGURE 4. Effects on survival and RV systolic pressures following pitavastatin-NP delivery. (A) Kaplan–Meier curve depicting survival of MCT-induced PAH rats following a single intratracheal administration of Pitavastatin-NP compared to controls consisting of intratracheal delivery of PBS, free pitavastatin, and fluorescein isothiocyanate (FITC)-NPs. (B) Effects of pitavastatin-NPs on RV systolic pressure (expressed as mmHg). ∗P < 0.01 vs. untreated control. Figure adapted from Chen et al. (2011), reproduced with permission courtesy of Wolters Kluwer Health, Inc.
Activation of the Ras homolog gene family, member A (RhoA) GTPase and it downstream effector, the Rho-associated kinase (ROCK), have been implicated in several processes driving PAH pathogenesis, including SMC vasoconstriction and proliferation, and endothelial cell contraction (Oka et al., 2008). Thus, inhibitors of RhoA/ROCK signaling such as Fasudil can potentially prove efficacious in the treatment of PAH. However, Fasudil has a short half-life of ∼45 min (Shibuya et al., 2005). In light of these limitations, Gupta et al. (2013) developed a liposomal formulation of fasudil for purposes of aerosolized delivery to lungs undergoing PAH. Resulting liposomes measured ∼180 nm following nebulization, had loading efficiencies > 60%, and released ∼70% of the drug over the course of 35 h. Pulmonary delivery of liposomes via intratracheal administration increased the half-life by more than 10-fold, as well as the bioavailability of the drug, compared to a free drug formulation administered IV. Upon efficacy examination in an MCT-induced PAH model in rats, an intratracheally administered liposomal formulation of fasudil was compared to a free formulation of fasudil administered intratracheally and by IV. Liposomal fasudil resulted in an increase in the duration of vasodilatory effects compared to controls, with a maximal reduction in mPAP of ∼40%.
In an attempt to enhance site-specific accumulation of NPs to the lungs, Nahar et al. (2014) subsequently developed fasudil liposomes with the cyclic peptide CARSKNKDC, which binds to cell surface heparan sulfate found overexpressed in pulmonary vasculature in PAH. Liposomes were in the range of 206–216 nm and had a sustained release of fasudil over the course of 120 h. Peptide-coated liposomes resulted in ∼34-fold increase in half-life of the drug compared to an IV-administered formulation of free drug. As a result, the mPAP in an MCT-induced model and a Sugen/Hypoxia model of PAH in rats was greatly reduced compared to controls. In a recent study, Gupta et al. (2017) incorporated superoxide dismutase (SOD) into their peptide-targeted fasudil liposomes, with the hypothesis that inclusion of a reactive oxygen species (ROS) scavenger would further enhance efficacy, given the role that increased ROS levels play in vascular remodeling in PAH. In an MCT-model of PAH, wherein the liposomal formulation was administered every 72 h for 21 days, the duration of vasodilatory effects was significantly increased in rats receiving targeted liposomes containing both fasudil and SOD compared to free drug controls. In a Sugen/Hypoxia model of PAH, mPAP, RV hypertrophy, fractions of occluded blood vessels, and arterial medial wall thickness were all reduced in rats receiving targeted liposomes containing both fasudil and SOD compared to free drug controls.
Liu et al. (2016) also examined the potential of ROS scavenging nanotherapeutics for the treatment of PAH. In their study, ethyl pyruvate, a derivative of pyruvic acid and an inhibitor of nuclear protein HMGB1, which in turn activates pro-inflammatory cytokines, was incorporated within poly(ethylene glycol)-block-lactide/glycolide (PEG-LG) NPs and examined their efficacy in a hyperkinetic model of PAH induced by shunt flow. At a timepoint of 24 h after intratracheal instillation, NPs were evident in lungs, predominantly in bronchi, alveoli, alveolar macrophages, and small arteries, with evidence of NPs present up to timepoints of 7 days. Following weekly administration of ethyl pyruvate NPs immediately after model induction for a time period of 12 weeks, medial wall thickness index (TI) and medial wall area index (AI) of small pulmonary arteries was significantly reduced by >50% compared to free ethyl pyruvate controls. Moreover, IL-6 and TNFα levels were significantly reduced (∼50%), as were levels of HMGB1 and ROS by more than 50 and 60%, respectively.
PASMC abnormal proliferation is vital to pathogenesis of PAH, with platelet–derived growth factor (PDGF) stimulation resulting in increased growth rate of PASMCs (Ikeda et al., 2010). Akagi et al. (2015) incorporated the PDGF-receptor tyrosine kinase inhibitor imatinib in PLGA NPs and examined their efficacy in an MCT-induced model of PAH. Imatinib is used for the treatment of chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL), and has resulted in 10-year progression-free survivals of 82% in CML (Kalmanti et al., 2015). It is important to note that a limitation of imatinib is patient resistance due to BCR-ABL1 amplification and multidrug-resistant P-glycoprotein (MDR-1) overexpression (Milojkovic and Apperley, 2009). Following a single intratracheal administration immediately after model induction, imatinib-containing NPs significantly reduced RV systolic pressure (∼40% reduction) and RV hypertrophy, as well as muscularization of pulmonary small vessels (∼50% reduction) compared to vehicle controls.
Aberrant activation of the mammalian target of rapamycin (mTOR) plays an important role in diseases such as cancer (Blanco et al., 2014), leading to the therapeutic exploration of mTOR inhibitors such as RAP. mTOR is also a key player in PAH progression due to its effects on PASMC growth and survival (Goncharova, 2013). Rapamycin has been shown to prevent PAH progression pre-clinically (Houssaini et al., 2013) while clinical exploration of everolimus, a rapalog, led to improvements in pulmonary vascular resistance and 6MWD (Seyfarth et al., 2013). Similar to the aforementioned imatinib, resistance to RAP is a limitation of the drug, stemming from mutations in mTOR or mutations in downstream effectors of mTOR (S6K1 or 4E-BP1) (Huang and Houghton, 2001). Our laboratory recently examined the potential of RAP NPs for the treatment of PAH (Segura-Ibarra et al., 2017). RAP was encapsulated within PEG-PCL polymer micelles measuring ∼17 nm in diameter. In an MCT-induced rat model of PAH, RAP NPs led to a significant increase in RAP in diseased lungs compared to healthy lungs. Similarly, RAP NPs led to an increase in RAP in diseased lungs compared to a free drug formulation. Moreover, NPs were localized primarily in pulmonary vasculature. Following twice a week administration of RAP NPs at the time of PAH induction for a duration of 4 weeks, RAP NPs significantly reduced pulmonary arteriole hypertrophy (Figures 5A,B) and RV ventricular remodeling compared to vehicle controls, and prevented increases in right ventricular systolic pressures and phosphorylation of S6, a downstream effector of mTOR. Importantly, compared to a free drug formulation of RAP, a 10% decrease in weight loss associated with RAP was observed in rats receiving RAP NPs, accompanied as well by a decrease (∼50%) in levels of pro-inflammatory cytokines (Figures 5C,D).
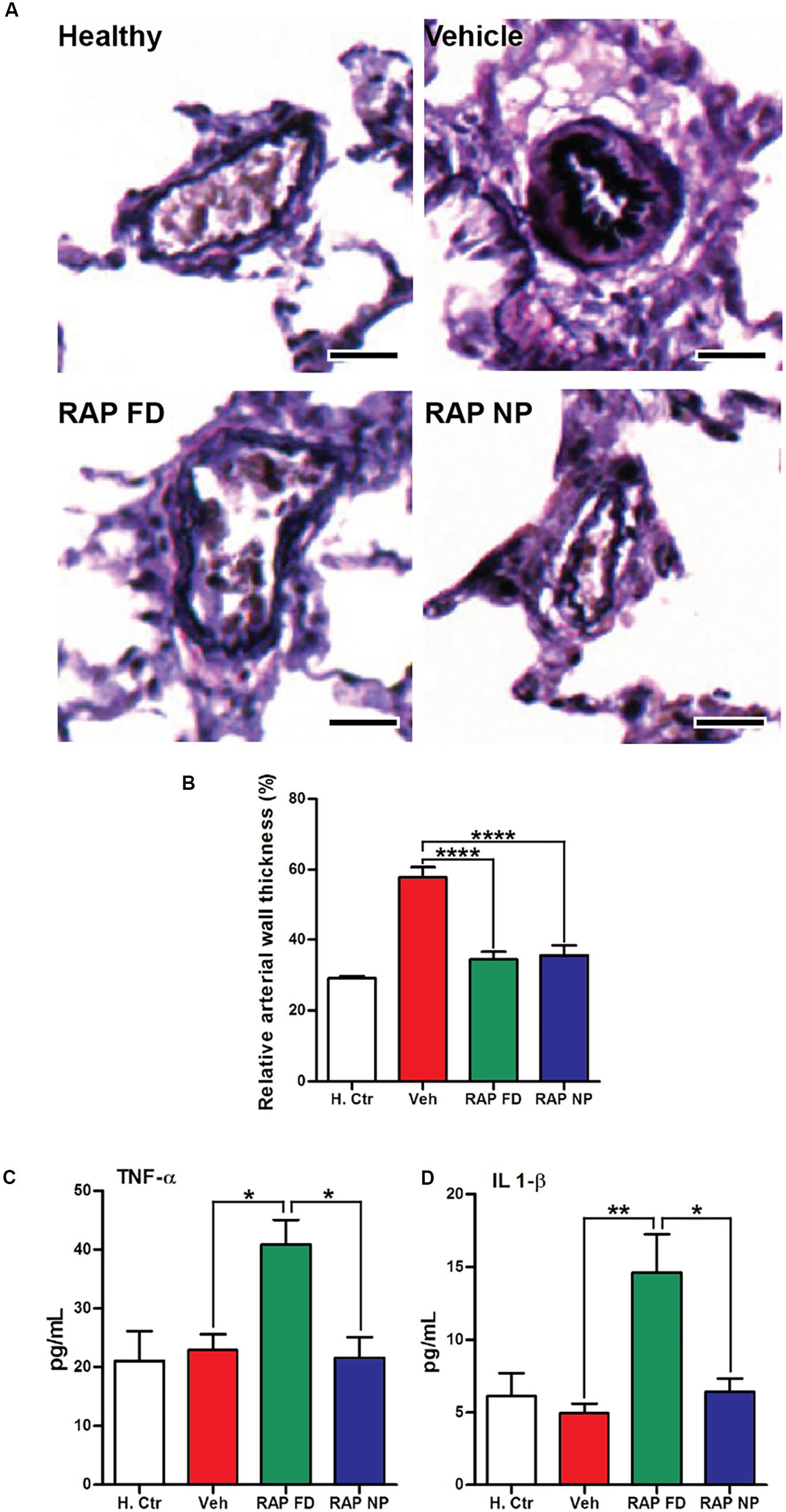
FIGURE 5. Rapamycin NPs prevented pulmonary arteriole hypertrophy in PAH and did not lead to an increase in inflammatory cytokines. (A) Verhoeff–Van Gieson (VVG) stain of pulmonary arterioles from MCT-induced model of PAH in rats treated with free rapamycin (RAP FD), NP vehicle (Vehicle), and RAP NPs. Scale bars represent 50 μm. (B) Quantification of the relative wall thickness among treated groups in (A). Results shown as mean ± SEM (∗∗∗∗P < 0.0001). Serum levels of inflammatory cytokines TNF-α (C) and IL-1β (D) measured after the course of treatment. Results represent mean ± SEM values (∗∗P < 0.01, ∗P < 0.05). Figure adapted from Segura-Ibarra et al. (2017), reproduced with permission courtesy of Elsevier.
NP Delivery of Genetic Material in PAH
Enhanced insights into molecular machinery driving PAH progression, including those involved in inflammation, have resulted in the identification of several viable therapeutic targets, with NP-based delivery platforms enabling gene therapy. As an example, NF-κB is a transcription factor that regulates numerous inflammatory cytokines, including IL-6 and TNF-α, which are involved in PAH (Hoesel and Schmid, 2013). Kimura et al. (2009) examined the role of NF-κB in PAH, as well as the potential for NP-based therapeutics targeting NF-κB as a treatment strategy. An NF-κB decoy oligodeoxynucleotide meant to inhibit binding of NF-κB to the promoter region was encapsulated within poly(ethylene glycol)-block-lactide/glycolide (PEG-PLGA) polymer micelles. Resulting NPs measured 44 nm in diameter, and displayed release of ∼40% of NF-κB decoy over 24 h and sustained release over the course of 28 days. Efficacy of NF-κB NPs were examined in preventive (NP intratracheal administration at the time of model induction) and treatment (NP intratracheal administration 21 days after model induction) protocols in an MCT-induced model of PAH. Using FITC-labeled NF-κB for visualization of NPs, FITC signal was found in small arteries, arterioles, small bronchi, and alveoli of diseased lungs at timepoints of 7 and 14 days after administration. In the preventive study, NF-κB positive cells were significantly reduced (> 50%) compared to free NF-κB decoy controls 7 days after model induction. In the treatment study, NF-κB NPs resulted in a significant decrease in RV systolic pressure, RV hypertrophy, and percentage of muscularized pulmonary arteries compared to PBS controls. Moreover, mRNA levels of inflammatory factors such as monocyte chemoattractant protein (MCP) 1, TNF-α, IL-6, and ICAM-1, were reduced by more than 50% following treatment with NF-κB NPs compared to free NF-κB decoy controls, and animal survival rate was increased compared to vehicle controls.
In a study by McLendon et al. (2015), NPs were used to deliver anti-sense oligonucleotide against microRNA-145 (miR-145) in hopes of exploiting RNA interference (RNAi) as a viable treatment strategy in PAH. Increased expression of miR-145 has been shown in lungs undergoing PAH, playing a vital role in vascular remodeling and pulmonary artery muscularization (Zhou et al., 2015). Moreover, downregulation of miR-145 prevents the onset of PAH in preclinical models (Caruso et al., 2012). Anti-miR-145 oligonucleotides were encapsulated within cationic lipid nanoconstructs in the range of 80–100 nm in size. Efficacy was examined in a Sugen/Hypoxia model of PAH in rats, wherein NPs were administered IV every 2 weeks starting on week 8 after model induction. Liposomes delivered anti-miR-145 to diseased lungs, and decreased the expression of miR-145 by more than 50%. The median wall thickness of pulmonary arteries was reduced following treatment with anti-miR-145 liposomes (Figure 6), with results suggesting that the therapy was capable of repairing vascular remodeling. Moreover, RV systolic pressure decreased by ∼25% and RV hypertrophy was reduced following treatment with anti-miR-145 liposomes compared to non-silencing oligonucleotide controls.
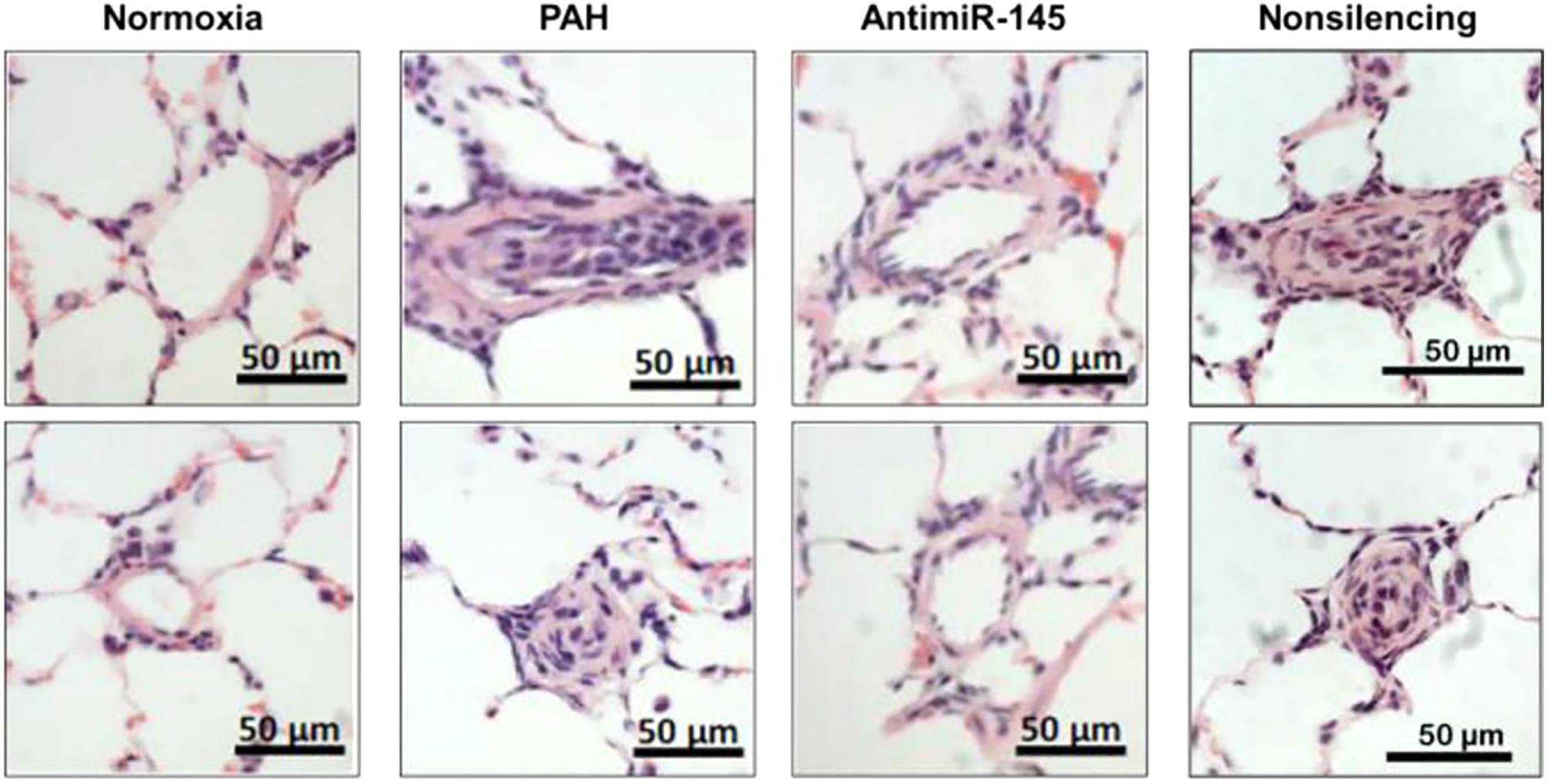
FIGURE 6. Effects of anti-miR-145 loaded liposomes on arteriole hyperplasia in an MCT-induced model of PAH. Hematoxylin and Eosin (H&E) stained histological sections depicting pulmonary arterioles following anti-miR-145 liposome treatment of rats with Sugen/Hypoxia induced PAH. Results highlight anti-miR-145 liposome treatment compared to controls consisting of healthy controls (normoxia), rats undergoing PAH (PAH), and liposomes containing non-silencing control oligonucleotide (non-silencing). Scale bar represents 50 μm. Figure adapted from McLendon et al. (2015), reproduced with permission courtesy of Elsevier.
Conclusion
Pulmonary arterial hypertension results in considerable patient morbidity, proving irreversible and fatal. Present-day pharmacotherapies suffer from considerable limitations. Short-term drug pharmacokinetics, where half-lives are on the order of minutes, contribute to low bioavailability in diseased tissues and adverse side effects. Nanoplatforms have improved the pharmacokinetic profiles of chemotherapeutics, with increased accumulation of NPs in tumors through the EPR effect. Importantly, enhanced accumulation and persistence of NPs has been observed in lungs undergoing PAH following both intravenous and inhalational routes of delivery. Endothelial dysfunction present in diseased lung vasculature results in NP accumulation in pulmonary arterioles, and NPs are found largely associated with vascular cells such as PAECs and PASMCs. Given the vital role these cells play in PAH progression, NPs stand to significantly impact PAH treatment strategies and patient outcomes.
Herein, we have provided an overview of NP-based drug delivery strategies in PAH, with particular emphasis on improvements in vascular remodeling and hemodynamics. Several nanotherapies involved the use of clinically approved drugs for PAH, while others exploited novel signaling pathways and molecular targets. The future of NP-based drug delivery in PAH will surely involve advancements on two fronts. On the one hand, innovations in materials science will lead to sophisticated nanotechnology platforms highly capable of delivering drugs to target cells in diseased lungs. These nanoconstructs will incorporate moieties for successful navigation of barriers to transport to the lungs, facilitate sustained delivery of therapeutics over time, and enable combined delivery of drugs and genetic material for synergistic treatment. Additionally, nanotherapies in PAH will benefit from enhanced understanding of molecular drivers of the disease. Insights into processes of PAH pathogenesis can potentially provide overexpressed surface receptors for active targeting to target cells and provide novel targets for gene therapy. Thus, rational design of NPs that can effectively target diseased lungs combined with molecular-targeted therapeutics will lead to more efficacious treatment outcomes in PAH.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the George and Angelina Kostas Research Center for Cardiovascular Nanomedicine. VS-I is grateful for support from the Instituto Tecnológico y de Estudios Superiores de Monterrey and the Consejo Nacional de Ciencia y Tecnología (CONACyT, 490202/278979). MF is grateful for the Ernest Cockrell Jr. Presidential Distinguished Endowed Chair in the Department of Nanomedicine at the Houston Methodist Research Institute. AG is grateful for funding from the Vaughan Foundation. HK-Q is grateful for funding from the National Institutes of Health (NIH 1R01 HL138510-01) and the UTHealth Pulmonary Center of Excellence Discovery Award Program.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Matthew G. Landry for assistance with schematics.
References
Acharya, S., and Sahoo, S. K. (2011). PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 63, 170–183. doi: 10.1016/j.addr.2010.10.008
Akagi, S., Nakamura, K., Matsubara, H., Kondo, M., Miura, D., Matoba, T., et al. (2016). Intratracheal administration of prostacyclin analogue-incorporated nanoparticles ameliorates the development of monocrotaline and sugen-hypoxia-induced pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 67, 290–298. doi: 10.1097/FJC.0000000000000352
Akagi, S., Nakamura, K., Miura, D., Saito, Y., Matsubara, H., Ogawa, A., et al. (2015). Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension. Int. Heart J. 56, 354–359. doi: 10.1536/ihj.14-338
Anderson, R., Franch, A., Castell, M., Perez-Cano, F. J., Brauer, R., Pohlers, D., et al. (2010). Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res. Ther. 12:R147. doi: 10.1186/ar3089
Aversa, M., Porter, S., and Granton, J. (2015). Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension. Drug Saf. 38, 419–435. doi: 10.1007/s40264-015-0275-y
Bae, Y., Nishiyama, N., Fukushima, S., Koyama, H., Yasuhiro, M., and Kataoka, K. (2005). Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 16, 122–130. doi: 10.1021/bc0498166
Banerjee, D., Harfouche, R., and Sengupta, S. (2011). Nanotechnology-mediated targeting of tumor angiogenesis. Vasc. Cell 3:3. doi: 10.1186/2045-824X-3-3
Barenholz, Y. (2012). Doxil®–the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134. doi: 10.1016/j.jconrel.2012.03.020
Barst, R. J., McGoon, M., McLaughlin, V., Tapson, V., Rich, S., Rubin, L., et al. (2003). Beraprost therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 41, 2119–2125. doi: 10.1016/S0735-1097(03)00463-7
Barst, R. J., Rubin, L. J., Long, W. A., McGoon, M. D., Rich, S., Badesch, D. B., et al. (1996). A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 334, 296–301. doi: 10.1056/NEJM199602013340504
Beck-Broichsitter, M., Bohr, A., Aragao-Santiago, L., Klingl, A., and Kissel, T. (2017). Formulation and process considerations for the design of sildenafil-loaded polymeric microparticles by vibrational spray-drying. Pharm. Dev. Technol. 22, 691–698. doi: 10.3109/10837450.2015.1098661
Beck-Broichsitter, M., Schweiger, C., Schmehl, T., Gessler, T., Seeger, W., and Kissel, T. (2012). Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 158, 329–335. doi: 10.1016/j.jconrel.2011.10.030
Beckman, J. A., and Creager, M. A. (2006). The nonlipid effects of statins on endothelial function. Trends Cardiovasc. Med. 16, 156–162. doi: 10.1016/j.tcm.2006.03.003
Berry, G., Billingham, M., Alderman, E., Richardson, P., Torti, F., Lum, B., et al. (1998). The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann. Oncol. 9, 711–716. doi: 10.1023/A:1008216430806
Bivas-Benita, M., Romeijn, S., Junginger, H. E., and Borchard, G. (2004). PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 58, 1–6. doi: 10.1016/j.ejpb.2004.03.008
Blanco, E., Bey, E. A., Khemtong, C., Yang, S. G., Setti-Guthi, J., Chen, H., et al. (2010). Beta-lapachone micellar nanotherapeutics for non-small cell lung cancer therapy. Cancer Res. 70, 3896–3904. doi: 10.1158/0008-5472.CAN-09-3995
Blanco, E., Hsiao, A., Mann, A. P., Landry, M. G., Meric-Bernstam, F., and Ferrari, M. (2011). Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 102, 1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x
Blanco, E., Kessinger, C. W., Sumer, B. D., and Gao, J. (2009). Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 234, 123–131. doi: 10.3181/0808-MR-250
Blanco, E., Sangai, T., Wu, S., Hsiao, A., Ruiz-Esparza, G. U., Gonzalez-Delgado, C. A., et al. (2014). Colocalized delivery of rapamycin and paclitaxel to tumors enhances synergistic targeting of the PI3K/Akt/mTOR pathway. Mol. Ther. 22, 1310–1319. doi: 10.1038/mt.2014.27
Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951. doi: 10.1038/nbt.3330
Braet, F., Wisse, E., Bomans, P., Frederik, P., Geerts, W., Koster, A., et al. (2007). Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microsc. Res. Tech. 70, 230–242. doi: 10.1002/jemt.20408
Cabral, H., Matsumoto, Y., Mizuno, K., Chen, Q., Murakami, M., Kimura, M., et al. (2011). Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823. doi: 10.1038/nnano.2011.166
Caruso, P., Dempsie, Y., Stevens, H. C., McDonald, R. A., Long, L., Lu, R., et al. (2012). A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ. Res. 111, 290–300. doi: 10.1161/CIRCRESAHA.112.267591
Carvajal, J. A., Germain, A. M., Huidobro-Toro, J. P., and Weiner, C. P. (2000). Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 184, 409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K
Chen, L., Nakano, K., Kimura, S., Matoba, T., Iwata, E., Miyagawa, M., et al. (2011). Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension 57, 343–350. doi: 10.1161/HYPERTENSIONAHA.110.157032
Chen, L. T., and Weiss, L. (1973). The role of the sinus wall in the passage of erythrocytes through the spleen. Blood 41, 529–537.
Choi, H. S., Liu, W., Misra, P., Tanaka, E., Zimmer, J. P., Itty Ipe, B., et al. (2007). Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170. doi: 10.1038/nbt1340
Chono, S., Tauchi, Y., Deguchi, Y., and Morimoto, K. (2005). Efficient drug delivery to atherosclerotic lesions and the antiatherosclerotic effect by dexamethasone incorporated into liposomes in atherogenic mice. J. Drug Target. 13, 267–276. doi: 10.1080/10611860500159030
Christie, R. J., Matsumoto, Y., Miyata, K., Nomoto, T., Fukushima, S., Osada, K., et al. (2012). Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 6, 5174–5189. doi: 10.1021/nn300942b
Collum, S. D., Amione-Guerra, J., Cruz-Solbes, A. S., DiFrancesco, A., Hernandez, A. M., Hanmandlu, A., et al. (2017). Pulmonary hypertension associated with Idiopathic pulmonary fibrosis: current and future perspectives. Can. Respir. J. 2017:1430350. doi: 10.1155/2017/1430350
Crescencio, M. E., Rodríguez, E., Páez, A., Masso, F. A., Montaño, L. F., and López-Marure, R. (2009). Statins inhibit the proliferation and induce cell death of human papilloma virus positive and negative cervical cancer cells. Int. J. Biomed. Sci. 5, 411–420.
Dai, J., Lin, S., Cheng, D., Zou, S., and Shuai, X. (2011a). Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew. Chem. Int. Ed. Engl. 50, 9404–9408. doi: 10.1002/anie.201103806
Dai, J., Zou, S., Pei, Y., Cheng, D., Ai, H., and Shuai, X. (2011b). Polyethylenimine-grafted copolymer of poly(L-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials 32, 1694–1705. doi: 10.1016/j.biomaterials.2010.10.044
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Preat, V. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161, 505–522. doi: 10.1016/j.jconrel.2012.01.043
Del Pozo, R., Hernandez Gonzalez, I., and Escribano-Subias, P. (2017). The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev. Respir. Med. 11, 491–503. doi: 10.1080/17476348.2017.1317599
Delcroix, M., and Howard, L. (2015). Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur. Respir. Rev. 24, 621–629. doi: 10.1183/16000617.0063-2015
Dinarvand, R., Sepehri, N., Manoochehri, S., Rouhani, H., and Atyabi, F. (2011). Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomedicine 6, 877–895. doi: 10.2147/IJN.S18905
Duarte, J. D., Hanson, R. L., and Machado, R. F. (2013). Pharmacologic treatments for pulmonary hypertension: exploring pharmacogenomics. Future Cardiol. 9, 335–349. doi: 10.2217/fca.13.6
Duggan, S. T., Keam, S. J., and Burness, C. B. (2017). Selexipag: a review in pulmonary arterial hypertension. Am. J. Cardiovasc. Drugs 17, 73–80. doi: 10.1007/s40256-016-0209-9
Durymanov, M., Kamaletdinova, T., Lehmann, S. E., and Reineke, J. (2017). Exploiting passive nanomedicine accumulation at sites of enhanced vascular permeability for non-cancerous applications. J. Control. Release 261, 10–22. doi: 10.1016/j.jconrel.2017.06.013
Dvir, T., Bauer, M., Schroeder, A., Tsui, J. H., Anderson, D. G., Langer, R., et al. (2011). Nanoparticles targeting the infarcted heart. Nano Lett. 11, 4411–4414. doi: 10.1021/nl2025882
Fang, J., Nakamura, H., and Maeda, H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151. doi: 10.1016/j.addr.2010.04.009
Ferrari, M. (2005). Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171. doi: 10.1038/nrc1566
Galie, N., Ghofrani, H. A., Torbicki, A., Barst, R. J., Rubin, L. J., Badesch, D., et al. (2005). Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 353, 2148–2157. doi: 10.1056/NEJMoa050010
Galie, N., Hoeper, M. M., Humbert, M., Torbicki, A., Vachiery, J. L., Barbera, J. A., et al. (2009). Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 30, 2493–2537. doi: 10.1093/eurheartj/ehp297
Galié, N., Manes, A., and Branzi, A. (2004). The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 61, 227–237. doi: 10.1016/j.cardiores.2003.11.026
Ghofrani, H. A., Galie, N., Grimminger, F., Grunig, E., Humbert, M., Jing, Z. C., et al. (2013). Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 369, 330–340. doi: 10.1056/NEJMoa1209655
Giovannoni, M. P., Vergelli, C., Graziano, A., and Dal Piaz, V. (2010). PDE5 inhibitors and their applications. Curr. Med. Chem. 17, 2564–2587. doi: 10.2174/092986710791859360
Goncharova, E. A. (2013). mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB J. 27, 1796–1807. doi: 10.1096/fj.12-222224
Gottlieb, J. (2013). Lung transplantation for interstitial lung diseases and pulmonary hypertension. Semin. Respir. Crit. Care Med. 34, 281–287. doi: 10.1055/s-0033-1348462
Gupta, N., Rashid, J., Nozik-Grayck, E., McMurtry, I. F., Stenmark, K. R., and Ahsan, F. (2017). Cocktail of superoxide dismutase and fasudil encapsulated in targeted liposomes slows PAH progression at a reduced dosing frequency. Mol. Pharm. 14, 830–841. doi: 10.1021/acs.molpharmaceut.6b01061
Gupta, V., Gupta, N., Shaik, I. H., Mehvar, R., McMurtry, I. F., Oka, M., et al. (2013). Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control. Release 167, 189–199. doi: 10.1016/j.jconrel.2013.01.011
Hamilton, A., Biganzoli, L., Coleman, R., Mauriac, L., Hennebert, P., Awada, A., et al. (2002). EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with metastatic breast cancer. European organization for research and treatment of cancer. Ann. Oncol. 13, 910–918. doi: 10.1093/annonc/mdf157
Harris, J. M., and Chess, R. B. (2003). Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221. doi: 10.1038/nrd1033
Hobbs, S. K., Monsky, W. L., Yuan, F., Roberts, W. G., Griffith, L., Torchilin, V. P., et al. (1998). Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U.S.A. 95, 4607–4612. doi: 10.1073/pnas.95.8.4607
Hoesel, B., and Schmid, J. A. (2013). The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 12:86. doi: 10.1186/1476-4598-12-86
Houchin, M. L., and Topp, E. M. (2008). Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J. Pharm. Sci. 97, 2395–2404. doi: 10.1002/jps.21176
Houssaini, A., Abid, S., Mouraret, N., Wan, F., Rideau, D., Saker, M., et al. (2013). Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 48, 568–577. doi: 10.1165/rcmb.2012-0429OC
Huang, S., and Houghton, P. J. (2001). Mechanisms of resistance to rapamycins. Drug Resist. Updat. 4, 378–391. doi: 10.1054/drup.2002.0227
Huang, S. L., Kee, P. H., Kim, H., Moody, M. R., Chrzanowski, S. M., Macdonald, R. C., et al. (2009). Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J. Am. Coll. Cardiol. 54, 652–659. doi: 10.1016/j.jacc.2009.04.039
Humbert, M., Lau, E. M., Montani, D., Jais, X., Sitbon, O., and Simonneau, G. (2014). Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 130, 2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974
Ikeda, T., Nakamura, K., Akagi, S., Kusano, K. F., Matsubara, H., Fujio, H., et al. (2010). Inhibitory effects of simvastatin on platelet-derived growth factor signaling in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 55, 39–48. doi: 10.1097/FJC.0b013e3181c0419c
Ishihara, T., Hayashi, E., Yamamoto, S., Kobayashi, C., Tamura, Y., Sawazaki, R., et al. (2015). Encapsulation of beraprost sodium in nanoparticles: analysis of sustained release properties, targeting abilities and pharmacological activities in animal models of pulmonary arterial hypertension. J. Control. Release 197, 97–104. doi: 10.1016/j.jconrel.2014.10.029
Ishikura, F., Beppu, S., Hamada, T., Khandheria, B. K., Seward, J. B., and Nehra, A. (2000). Effects of sildenafil citrate (Viagra) combined with nitrate on the heart. Circulation 102, 2516–2521. doi: 10.1161/01.CIR.102.20.2516
Istvan, E. (2003). Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atheroscler. Suppl. 4, 3–8. doi: 10.1016/S1567-5688(03)00003-5
Jain, P. P., Leber, R., Nagaraj, C., Leitinger, G., Lehofer, B., Olschewski, H., et al. (2014). Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries. Int. J. Nanomedicine 9, 3249–3261. doi: 10.2147/IJN.S63190
Jhaveri, A. M., and Torchilin, V. P. (2014). Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 5:77. doi: 10.3389/fphar.2014.00077
Kalmanti, L., Saussele, S., Lauseker, M., Muller, M. C., Dietz, C. T., Heinrich, L., et al. (2015). Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 29, 1123–1132. doi: 10.1038/leu.2015.36
Kamigaki, M., Sasaki, T., Serikawa, M., Inoue, M., Kobayashi, K., Itsuki, H., et al. (2011). Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int. J. Oncol. 39, 561–568. doi: 10.3892/ijo.2011.1087
Kaneko, F. T., Arroliga, A. C., Dweik, R. A., Comhair, S. A., Laskowski, D., Oppedisano, R., et al. (1998). Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 158, 917–923. doi: 10.1164/ajrccm.158.3.9802066
Kapoor, D. N., Bhatia, A., Kaur, R., Sharma, R., Kaur, G., and Dhawan, S. (2015). PLGA: a unique polymer for drug delivery. Ther. Deliv. 6, 41–58. doi: 10.4155/tde.14.91
Khanal, S., Adhikari, U., Rijal, N. P., Bhattarai, S. R., Sankar, J., and Bhattarai, N. (2016). pH-responsive PLGA nanoparticle for controlled payload delivery of diclofenac sodium. J. Funct. Biomater. 7:E21. doi: 10.3390/jfb7030021
Kimura, S., Egashira, K., Chen, L., Nakano, K., Iwata, E., Miyagawa, M., et al. (2009). Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 53, 877–883. doi: 10.1161/HYPERTENSIONAHA.108.121418
Kleemann, E., Schmehl, T., Gessler, T., Bakowsky, U., Kissel, T., and Seeger, W. (2007). Iloprost-containing liposomes for aerosol application in pulmonary arterial hypertension: formulation aspects and stability. Pharm. Res. 24, 277–287. doi: 10.1007/PL00022055
Klinger, J. R., and Kadowitz, P. J. (2017). The nitric oxide pathway in pulmonary vascular disease. Am. J. Cardiol. 120, S71–S79. doi: 10.1016/j.amjcard.2017.06.012
Lang, I. M., and Gaine, S. P. (2015). Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur. Respir. Rev. 24, 630–641. doi: 10.1183/16000617.0067-2015
Lau, E. M. T., Giannoulatou, E., Celermajer, D. S., and Humbert, M. (2017). Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 14, 603–614. doi: 10.1038/nrcardio.2017.84
Lefer, D. J. (2002). Statins as potent antiinflammatory drugs. Circulation 106, 2041–2042. doi: 10.1161/01.CIR.0000033635.42612.88
LeVarge, B. L. (2015). Prostanoid therapies in the management of pulmonary arterial hypertension. Ther. Clin. Risk Manag. 11, 535–547. doi: 10.2147/TCRM.S75122
Liu, J. B., Lee, H., Huesca, M., Young, A. P., and Allen, C. (2006). Liposome formulation of a novel hydrophobic aryl-imidazole compound for anti-cancer therapy. Cancer Chemother. Pharmacol. 58, 306–318. doi: 10.1007/s00280-005-0161-x
Liu, K., Zhang, X., Cao, G., Liu, Y., Liu, C., Sun, H., et al. (2016). Intratracheal instillation of ethyl pyruvate nanoparticles prevents the development of shunt-flow-induced pulmonary arterial hypertension in a rat model. Int. J. Nanomedicine 11, 2587–2599. doi: 10.2147/IJN.S103183
Maeda, H., Nakamura, H., and Fang, J. (2013). The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 65, 71–79. doi: 10.1016/j.addr.2012.10.002
Makadia, H. K., and Siegel, S. J. (2011). Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397. doi: 10.3390/polym3031377
Matsumura, Y., and Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46(12 Pt 1), 6387–6392.
McDonald, D. M., and Choyke, P. L. (2003). Imaging of angiogenesis: from microscope to clinic. Nat. Med. 9, 713–725. doi: 10.1038/nm0603-713
McGoon, M. D., Frost, A. E., Oudiz, R. J., Badesch, D. B., Galie, N., Olschewski, H., et al. (2006). Ambrisentan rescue therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function abnormalities. Chest 130:254S. doi: 10.1378/chest.130.4_;MeetingAbstracts.254S-a
McLaughlin, V. V., Archer, S. L., Badesch, D. B., Barst, R. J., Farber, H. W., Lindner, J. R., et al. (2009). ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation task force on expert consensus documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119, 2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230
McLaughlin, V. V., and McGoon, M. D. (2006). Pulmonary arterial hypertension. Circulation 114, 1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540
McLaughlin, V. V., and Palevsky, H. I. (2013). Parenteral and inhaled prostanoid therapy in the treatment of pulmonary arterial hypertension. Clin. Chest Med. 34, 825–840. doi: 10.1016/j.ccm.2013.09.003
McLendon, J. M., Joshi, S. R., Sparks, J., Matar, M., Fewell, J. G., Abe, K., et al. (2015). Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control. Release 210, 67–75. doi: 10.1016/j.jconrel.2015.05.261
Metselaar, J. M., van den Berg, W. B., Holthuysen, A. E., Wauben, M. H., Storm, G., and van Lent, P. L. (2004). Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 63, 348–353. doi: 10.1136/ard.2003.009944
Milojkovic, D., and Apperley, J. (2009). Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin. Cancer Res. 15, 7519–7527. doi: 10.1158/1078-0432.CCR-09-1068
Mohamed, N. A., Ahmetaj-Shala, B., Duluc, L., Mackenzie, L. S., Kirkby, N. S., Reed, D. M., et al. (2016). A new NO-releasing nanoformulation for the treatment of pulmonary arterial hypertension. J. Cardiovasc. Transl. Res. 9, 162–164. doi: 10.1007/s12265-016-9684-2
Montani, D., Chaumais, M. C., Guignabert, C., Gunther, S., Girerd, B., Jais, X., et al. (2014). Targeted therapies in pulmonary arterial hypertension. Pharmacol. Ther. 141, 172–191. doi: 10.1016/j.pharmthera.2013.10.002
Morrell, N. W. (2006). Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc. Am. Thorac. Soc. 3, 680–686. doi: 10.1513/pats.200605-118SF
Morrell, N. W., Adnot, S., Archer, S. L., Dupuis, J., Jones, P. L., MacLean, M. R., et al. (2009). Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 54(Suppl. 1), S20–S31. doi: 10.1016/j.jacc.2009.04.018
Mubarak, K. K. (2010). A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir. Med. 104, 9–21. doi: 10.1016/j.rmed.2009.07.015
Murray, F., MacLean, M. R., and Pyne, N. J. (2002). Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br. J. Pharmacol. 137, 1187–1194. doi: 10.1038/sj.bjp.0704984
Nagaoka, K., Matoba, T., Mao, Y., Nakano, Y., Ikeda, G., Egusa, S., et al. (2015). A new therapeutic modality for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin induces cardioprotection from ischemia-reperfusion injury via activation of pi3k/akt pathway and anti-inflammation in a rat model. PLoS One 10:e0132451. doi: 10.1371/journal.pone.0132451
Nahar, K., Absar, S., Gupta, N., Kotamraju, V. R., McMurtry, I. F., Oka, M., et al. (2014). Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol. Pharm. 11, 4374–4384. doi: 10.1021/mp500456k
Nakamura, K., Matsubara, H., Akagi, S., Sarashina, T., Ejiri, K., Kawakita, N., et al. (2017). Nanoparticle-mediated drug delivery system for pulmonary arterial hypertension. J. Clin. Med. 6:E48. doi: 10.3390/jcm6050048
Nakano, Y., Matoba, T., Tokutome, M., Funamoto, D., Katsuki, S., Ikeda, G., et al. (2016). Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci. Rep. 6:29601. doi: 10.1038/srep29601
Nasongkla, N., Shuai, X., Ai, H., Weinberg, B. D., Pink, J., Boothman, D. A., et al. (2004). cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew. Chem. Int. Ed. Engl. 43, 6323–6327. doi: 10.1002/anie.200460800
Oka, M., Fagan, K. A., Jones, P. L., and McMurtry, I. F. (2008). Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br. J. Pharmacol. 155, 444–454. doi: 10.1038/bjp.2008.239
Olschewski, H., Ghofrani, H. A., Schmehl, T., Winkler, J., Wilkens, H., Hoper, M. M., et al. (2000). Inhaled iloprost to treat severe pulmonary hypertension. an uncontrolled trial. German PPH study group. Ann. Intern. Med. 132, 435–443. doi: 10.7326/0003-4819-132-6-200003210-00003
Paranjpe, M., and Muller-Goymann, C. C. (2014). Nanoparticle-mediated pulmonary drug delivery: a review. Int. J. Mol. Sci. 15, 5852–5873. doi: 10.3390/ijms15045852
Pattni, B. S., Chupin, V. V., and Torchilin, V. P. (2015). New developments in liposomal drug delivery. Chem. Rev. 115, 10938–10966. doi: 10.1021/acs.chemrev.5b00046
Paulis, L. E., Geelen, T., Kuhlmann, M. T., Coolen, B. F., Schafers, M., Nicolay, K., et al. (2012). Distribution of lipid-based nanoparticles to infarcted myocardium with potential application for MRI-monitored drug delivery. J. Control. Release 162, 276–285. doi: 10.1016/j.jconrel.2012.06.035
Pauwaa, S., Machado, R. F., and Desai, A. A. (2011). Survival in pulmonary arterial hypertension: a brief review of registry data. Pulm. Circ. 1, 430–431. doi: 10.4103/2045-8932.87314
Perez-Zoghbi, J. F., Bai, Y., and Sanderson, M. J. (2010). Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J. Gen. Physiol. 135, 247–259. doi: 10.1085/jgp.200910365
Raja, S. G. (2010). Endothelin receptor antagonists for pulmonary arterial hypertension: an overview. Cardiovasc. Ther. 28, e65–e71. doi: 10.1111/j.1755-5922.2010.00158.x
Ricciotti, E., and FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000. doi: 10.1161/ATVBAHA.110.207449
Ridker, P. M., Rifai, N., Pfeffer, M. A., Sacks, F., and Braunwald, E. (1999). Long-term effects of pravastatin on plasma concentration of c-reactive protein. The cholesterol and recurrent events (CARE) investigators. Circulation 100, 230–235. doi: 10.1161/01.CIR.100.3.230
Riehemann, K., Schneider, S. W., Luger, T. A., Godin, B., Ferrari, M., and Fuchs, H. (2009). Nanomedicine–challenge and perspectives. Angew. Chem. Int. Ed. Engl. 48, 872–897. doi: 10.1002/anie.200802585
Rosano, L., Spinella, F., and Bagnato, A. (2013). Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 13, 637–651. doi: 10.1038/nrc3546
Ruan, C. H., Dixon, R. A., Willerson, J. T., and Ruan, K. H. (2010). Prostacyclin therapy for pulmonary arterial hypertension. Tex. Heart Inst. J. 37, 391–399.
Ruiz-Esparza, G. U., Segura-Ibarra, V., Cordero-Reyes, A. M., Youker, K. A., Serda, R. E., Cruz-Solbes, A. S., et al. (2016). A specifically designed nanoconstruct associates, internalizes, traffics in cardiovascular cells, and accumulates in failing myocardium: a new strategy for heart failure diagnostics and therapeutics. Eur. J. Heart Fail. 18, 169–178. doi: 10.1002/ejhf.463
Russwurm, M., and Koesling, D. (2004). NO activation of guanylyl cyclase. EMBO J. 23, 4443–4450. doi: 10.1038/sj.emboj.7600422
Ryan, J. J., and Archer, S. L. (2014). The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res. 115, 176–188. doi: 10.1161/CIRCRESAHA.113.301129
Safdar, Z. (2011). Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir. Med. 105, 818–827. doi: 10.1016/j.rmed.2010.12.018
Sahni, S., Ojrzanowski, M., Majewski, S., and Talwar, A. (2016). Pulmonary arterial hypertension: a current review of pharmacological management. Pneumonol. Alergol. Pol. 84, 47–61. doi: 10.5603/PiAP.a2015.0084
Schermuly, R. T., Stasch, J. P., Pullamsetti, S. S., Middendorff, R., Muller, D., Schluter, K. D., et al. (2008). Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur. Respir. J. 32, 881–891. doi: 10.1183/09031936.00114407
Segura-Ibarra, V., Amione-Guerra, J., Cruz-Solbes, A. S., Cara, F. E., Iruegas-Nunez, D. A., Wu, S., et al. (2017). Rapamycin nanoparticles localize in diseased lung vasculature and prevent pulmonary arterial hypertension. Int. J. Pharm. 524, 257–267. doi: 10.1016/j.ijpharm.2017.03.069
Seo, B., Oemar, B. S., Siebenmann, R., von Segesser, L., and Luscher, T. F. (1994). Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 89, 1203–1208. doi: 10.1161/01.CIR.89.3.1203
Seyfarth, H. J., Hammerschmidt, S., Halank, M., Neuhaus, P., and Wirtz, H. R. (2013). Everolimus in patients with severe pulmonary hypertension: a safety and efficacy pilot trial. Pulm. Circ. 3, 632–638. doi: 10.1086/674311
Shibuya, M., Hirai, S., Seto, M., Satoh, S., Ohtomo, E., and Fasudil Ischemic Stroke Study Group (2005). Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J. Neurol. Sci. 238, 31–39. doi: 10.1016/j.jns.2005.06.003
Sitbon, O., Channick, R. N., Delcroix, M., Ghofrani, H.-A., Jansa, P., Le Brun, F.-O., et al. (2014). Macitentan reduces the risk of morbidity and mortality irrespective of the presence or absence of right ventricular (RV) impairment: results from the randomised SERAPHIN trial in pulmonary arterial hypertension (PAH). Eur. Respir. J. 44:3419.
Sitbon, O., and Vonk Noordegraaf, A. (2017). Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur. Respir. Rev. 26:160055. doi: 10.1183/16000617.0055-2016
Song, W., Tang, Z., Zhang, D., Zhang, Y., Yu, H., Li, M., et al. (2014). Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials 35, 3005–3014. doi: 10.1016/j.biomaterials.2013.12.018
Stenmark, K. R., Fagan, K. A., and Frid, M. G. (2006). Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ. Res. 99, 675–691. doi: 10.1161/01.RES.0000243584.45145.3f
Stenmark, K. R., Meyrick, B., Galie, N., Mooi, W. J., and McMurtry, I. F. (2009). Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1013–L1032. doi: 10.1152/ajplung.00217.2009
Stigliano, C., Ramirez, M. R., Singh, J. V., Aryal, S., Key, J., Blanco, E., et al. (2017). Methotraxate-loaded hybrid nanoconstructs target vascular lesions and inhibit atherosclerosis progression in ApoE(-/-) mice. Adv. Healthc. Mater. 6:1601286. doi: 10.1002/adhm.201601286
Ta, T., and Porter, T. M. (2013). Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 169, 112–125. doi: 10.1016/j.jconrel.2013.03.036
Takeda, M., Maeda, T., Ishihara, T., Sakamoto, H., Yuki, K., Takasaki, N., et al. (2009). Synthesis of prostaglandin E-1 phosphate derivatives and their encapsulation in biodegradable nanoparticles. Pharm. Res. 26, 1792–1800. doi: 10.1007/s11095-009-9891-5
Tonelli, A. R., Haserodt, S., Aytekin, M., and Dweik, R. A. (2013). Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm. Circ. 3, 20–30. doi: 10.4103/2045-8932.109911
Tuder, R. M., Cool, C. D., Geraci, M. W., Wang, J., Abman, S. H., Wright, L., et al. (1999). Prostacyclin synthase expression is decreased in lungs front patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 159, 1925–1932. doi: 10.1164/ajrccm.159.6.9804054
Ulbrich, W., and Lamprecht, A. (2010). Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. R. Soc. Interface 7(Suppl. 1), S55–S66. doi: 10.1098/rsif.2009.0285.focus
Xu, R., Zhang, G., Mai, J., Deng, X., Segura-Ibarra, V., Wu, S., et al. (2016). An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 34, 414–418. doi: 10.1038/nbt.3506
Zhang, X. X., McIntosh, T. J., and Grinstaff, M. W. (2012). Functional lipids and lipoplexes for improved gene delivery. Biochimie 94, 42–58. doi: 10.1016/j.biochi.2011.05.005
Zhou, C., Townsley, M. I., Alexeyev, M., Voelkel, N. F., and Stevens, T. (2016). Endothelial hyperpermeability in severe pulmonary arterial hypertension: role of store-operated calcium entry. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L560–L569. doi: 10.1152/ajplung.00057.2016
Keywords: pulmonary arterial hypertension, chronic lung disease, nanomedicine, nanoparticles, drug delivery
Citation: Segura-Ibarra V, Wu S, Hassan N, Moran-Guerrero JA, Ferrari M, Guha A, Karmouty-Quintana H and Blanco E (2018) Nanotherapeutics for Treatment of Pulmonary Arterial Hypertension. Front. Physiol. 9:890. doi: 10.3389/fphys.2018.00890
Received: 08 March 2018; Accepted: 20 June 2018;
Published: 13 July 2018.
Edited by:
Keith Russell Brunt, Dalhousie University, CanadaReviewed by:
Giuseppina Milano, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandMathew J. Platt, University of Guelph, Canada
Natalie Julie Serkova, University of Colorado Denver School of Medicine, United States
Copyright © 2018 Segura-Ibarra, Wu, Hassan, Moran-Guerrero, Ferrari, Guha, Karmouty-Quintana and Blanco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elvin Blanco, ZWJsYW5jb0Bob3VzdG9ubWV0aG9kaXN0Lm9yZw==
 Victor Segura-Ibarra
Victor Segura-Ibarra Suhong Wu1
Suhong Wu1 Harry Karmouty-Quintana
Harry Karmouty-Quintana Elvin Blanco
Elvin Blanco