- 1Dipartimento di Scienze Biomediche, Metaboliche e Neuroscienze, Centro di Neuroscienze e Neurotecnologie, Università di Modena e Reggio Emilia, Modena, Italy
- 2Dipartimento di Scienze del Comportamento e del Sistema Nervoso, Università di Pavia, Pavia, Italy
Multiphoton microscopy is the most widespread method for preclinical brain imaging when sub-micrometer resolution is required. Nonetheless, even in the case of optimal experimental conditions, only a few hundred micrometers under the brain surface can be imaged by multiphoton microscopy. The main limitation preventing the acquisition of images from deep brain structures is the random light scattering which, until recently, was considered an unsurmountable obstacle. When in 2007 a breakthrough work by Vellekoop and Mosk [1] proved it is indeed possible to compensate for random scattering by using high resolution phase modulators, the neuro-photonics community started chasing the dream of a multiphoton microscopy capable of reaching arbitrary depths within the brain. Unfortunately, more than 10 years later, despite a massive improvement of technologies for scattering compensation in terms of speed, performances and reliability, clear images from deep layers of biological tissues are still lacking. In this work, we review recent technological and methodological advances in the field of multiphoton microscopy analyzing the big issue of scattering compensation. We will highlight the limits hampering image acquisition, and we will try to analyze the road scientists must tackle to target one of the most challenging issue in the field of biomedical imaging.
Introduction
The analysis of brain function and dysfunction is inherently bound to the visualization of neuronal morphology in intact tissues and most importantly is tightly related to the investigation of synaptic and cellular activity in extended neuronal networks [2]. The first disruptive advancement in the field of neurophotonics arrived at the beginning of the 90's, when in the Webb laboratory [3] it was proven that the two-photon microscopy (2PM), only theoretically envisaged more than 50 years before [4], was indeed feasible. The advent of femtosecond-pulsed infrared laser sources and the development of advanced scanning methods opened new routes to researchers aiming to perform imaging of thick biological samples [5]. A deeper light penetration, a reduced photodamage together with the possibility to detect non-ballistic fluorescence photons because of the intrinsic confocality of multiphoton excitation, allowed 2PM to become the gold standard technique for in vivo brain imaging. Unfortunately, the strongly turbid media distorting incident light avoids clear and fully resolved images from deeper layer in the brain to be obtained, even in small animals. The maximum imaging depth is in fact related to the ability of incident light to target the focal plane (ballistic photons) in a diffraction limited volume. In particular, the repeated scattering of the incident wavefront is, most of the time, so robust that the spatial coherence is completely lost beyond a small volume with a radius comparable to the wavelength of light [1, 6–8]. While purely morphological imaging of structures deep within a three-dimensional sample can easily be achieved by chemically fixing and optically clearing the tissue [9], functional imaging requires the preservation of physiological condition, and inevitably requires more complex solutions.
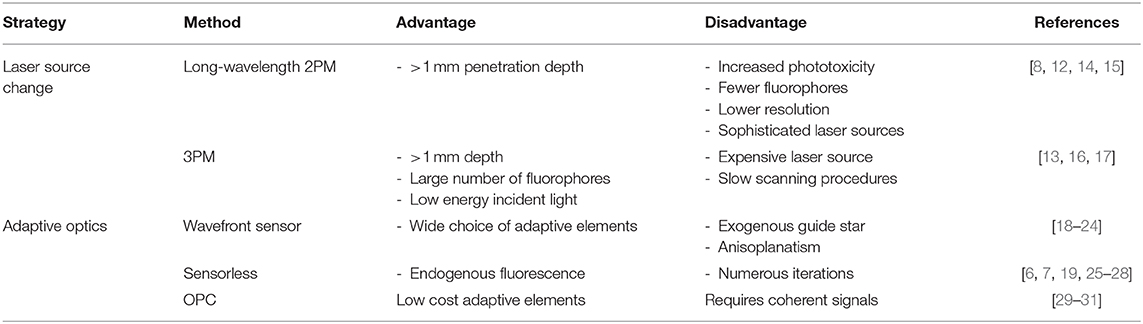
Two non-exclusive strategies are currently adopted to overcome the physical limitations preventing image formation in deep brain layers: (i) changing the laser source to increase the probability of the excitation process to occur; (ii) inserting Adaptive Optics (AO) in the light path to correct the incident wavefront after its determination [10]. In the first case, longer wavelengths, either in the form of two [8] or three photon excitation (3PM) can yield a substantial increase [11] in the penetration depth mainly taking advantage of the lower ratios between scattering and molecule absorption in the mid infrared (see below). In analogy to what was done with astronomy, the second approach proposes the adjustment of the incident wavefront based on the feedback coming from the sample, either through the aid of wavefront sensors or not.
Modulating the Incident Light to Improve Multi-Photon Performances
An extensive analysis of 2PM signal degradation by brain tissues has been conducted by Theer and Denk [12]. In this work, the authors showed that signal rapidly fades during the travel across tissues to disappear when the signal to background ratio (SBR) becomes unitary [12]. This condition typically occurs between 5 and 6 effective attenuation lengths (la) below the surface of the tissue. For instance, in the mouse cortex at 775-nm excitation wavelength the 2PM la is about 130 μm and the resulting penetration depth is 700~μm [13]. This means that, in adult mice, 2PM imaging is limited to cortical layers. The access to subcortical structures is thus restricted to technical approaches encompassing invasive optical probes or the removal of the overlying tissues. A few years after the work by Theer and Denk, in 2013 Horton and colleagues calculated the trade-off between tissue scattering and molecule absorption [13], the two main determinants of signal degradation. This work allowed to determine the optimal spectral window for two-photon excitation. The minimal reduction of 2PE due to brain scattering was in fact registered at wavelengths centered on two narrow regions around 1,300 and 1,700 nm. In addition, the imaging depth scales linearly with the attenuation coefficient, but it scales logarithmically with the average power incident on the tissue surface and on the duty cycle [8]. In 2003 Theer et al. had tried to lower the repetition rate of the laser to increase the pulse power impinging on the sample and therefore limiting the attenuation coefficient [14]. This trick allowed researchers to image down to 1 mm in the mouse brain, with a significant improvement of 2PM performances. Despite the increase in the penetration depth, near infrared excitation wavelengths do not preserve from the strong attenuation due to brain scattering. To further improve the overall image quality, Kobat et al. [8] exploited the 1,300 nm window by performing long wavelength 2PM. The excitation of brain vasculatures filled with dextran molecules allowed researchers to collect signals down to 1.2 mm below the brain surface. The same authors [15], a couple of years later showed images at a depth of 1.6 mm with a relatively low power (120 mW) at the brain surface. Beside the effective increase in the penetration depth, these methods collectively suffer from a few limitations (see Table 1): (I) The phototoxicity scales with the incident average power; (II) long wavelengths coupled with 2PM strongly limit the number of excitable fluorophores, especially excluding the widely employed green fluorescent protein and most of its derivates; (III) the image resolution scales down with the excitation wavelength; (IV) longer wavelength sources are more expensive and sophisticated than a single mode-locked pulsed infrared laser.
Some of these constraints can be overcome by exploiting three-photon absorption at long wavelength (>1,300 nm). In the early 90's two separate demonstrations of the feasibility of 3PE were independently reported [11, 16]. Besides a better penetration depth due to the reduced scattering, 3PM also provides an improvement of image quality through an overall better excitation localization [16]. The emitted fluorescence of 3PE in fact fades off as 1/z4 compared to 1/z2 of 2PM (where z is the distance from the focal plane). This, in turn brings a tremendous increase of the signal to noise ratio. Moreover, the use of long wavelength (>1,500 nm) gives the possibility to employ a large spectrum of fluorescent molecules commonly used with single photon fluorescence imaging. Finally, long wavelength and high-pulse energy excitation at a low repetition rate significantly increases the amount of excited molecules. The main disadvantage of such technique is the light source needed to properly excite fluorophores with three photons [17] which necessarily has to be a high-power laser with an optical parametric amplifier (OPA). Furthermore, the low repetition rate most likely to be used for 3PE may require long scanning procedures to obtain at least one pulse per effective image pixel.
The Impact of Adaptive Optics on 2PM Performances
From a physical standpoint, the effect of a turbid medium on the propagation of light can be described as a spatially dependent phase retardation of the wavefront. While an ideal spherical wavefront, such as that generated by the objective would focus in a diffraction limited spot, any distortion from a spherical form would subtract power from the intended focus and redirect it elsewhere in the sample, reducing excitation efficiency and increasing background noise. The techniques aimed at mitigating such problems are known as adaptive optics (AO). The principle at the basis of adaptive methods is that, by exploiting phase modulating optical components, the incident wavefront impinging onto the surface of a turbid medium, can be shaped until matches the wavefront in the focal plane affected by the medium itself [10]. This is particularly important because the precise focusing of light into the medium at a desired depth is fundamental not only for high-resolution imaging [32] but also for optogenetics [33], functional imaging [34] or photo-dependent therapies [35].
Traditional AO is performed with smooth phase modulators, such as deformable mirrors, capable of compensating relatively low-resolution aberrations defined as a continuous function in the optical system's pupil. The two main roads to get to wavefront determination and correction are with a wavefront sensor of any kind, or “sensorless.” The first group draws inspiration from astronomy, the field which drove the development of AO, where a deformable mirror corrects the wave profile following the feedback given by a wavefront sensor. In vivo wavefront sensing AO must necessarily be guided by a point-like light source in the focal plane inside the sample itself, serving as a guide star for the wavefront sensor. The wavefront from the guide star is then measured by a sensor (e.g., Shack-Hartmann-SHWS) to implement the active correction performed by the adjustable optical components.
In this configuration, the incident wavefront is determined by the acquisition of a de-scanned guidestar that can have the form of exogenous fluorescence molecules [1], second-harmonic radiating nanoparticles [36], photo-acoustic feedback [37], focused ultrasonic waves [29] or kinematics targets to extrapolate intrinsic dynamics [38]. Recently [18], in the Kleinfeld laboratory has been developed a method to perform 2PM imaging 800 μm under the brain surface with the correction of optical aberration through signals coming from brain microvessels labeled with Cy5.5, a strongly red shifted molecule. The robust measurement of aberration is achieved through a descanned Shack Hartmann wavefront sensor (formed by a microlens array and an Electron-Multiplying CCD) producing a spot pattern that feeds the algorithm piloting the shape of a deformable mirror [18]. This method allowed researchers not only to generate high quality images deep inside mouse brain but also to collect functional signals (calcium and glutamate changes) during in vivo sensori-motor tasks. In a different approach, the two-photon emission of previously labeled fluorescent neurons is collected by a SHWS and used to implement an iterative correction of the wavefront through a Liquid Crystal Spatial Light Modulator (LC-SLM) over subsets of large brain volumes [19]. Alternatively, multiple laser lines have been added in the optical setup to excite fluorescent microspheres injected into brain tissue [20] while keeping similar adaptive schemes (SHWS and deformable mirror). Beside the use of SLM and DM, researchers have developed algorithms to control other optical components such as Micromirrors [21, 22], adaptive lenses [23] or ferroelectric SLM [24].
In the case of a sensorless approach, the modulation of the incident beam wavefront is performed through an optimization procedure of the two-photon emission (2PE) intensity as a function of the wavefront correction [25]. The 2PE signal can be also used to measure the quality of images degraded by a priori known trial aberrations [6]. This scheme requires a model-based optimization describing the aberration effect on the chosen metric. Sensorless methods have the main advantage that it is not necessary to introduce neither additional optical components in the detection part of the microscope, nor guide star sources in the sample. On the contrary, these methods typically suffer from longer optimization times compared to wavefront sensing [19], requiring the acquisition of a minimum of N+1 images in order to achieve optimal correction with an adaptive element with N actuators [6, 39, 40], small volumes of proper correction [26], the introduction of fluorescent beads in the scattering medium [27] and may require serial images acquisition [28]. Interestingly, to speed up the optimization process, Galwaduge et al. [7] iteratively determined the correction across the whole pupil instead of working on a subset of pixels. Furthermore, differently from previous approaches, the “whole pupil” scheme takes advantage only on the 2PE intensity to correct the wavefront without image acquisition.
A substantial problem in the application of adaptive optics in microscopy is given by the fact that when scanning, light directed to different areas of the field of view travels through different parts of the sample, and needs therefore a different correction, a phenomenon known as anisoplanatism [41]. Adaptive optics systems generally only correct an average aberration through all the field of view and are effective only on relatively small regions. An approach to mitigate this problem is to conjugate the adaptive element to a plane between the objective and the sample, instead of the pupil of the system [30], or splitting the pupil in multiple subregions for different areas of the field of view [42]. These approaches however require high resolution correctors, which are either very expensive or slow in their correction.
Dynamic Scattering Compensation
Traditionally, only correction of smooth wavefronts was considered possible with adaptive optics, and scattering was considered an unsolvable problem. The work by Vellekoop and Mosk proved [1] that scattering can indeed be compensated through the application of a high resolution, discontinuous phase modulation by means of a high resolution spatial light modulator. While groundbreaking in concept, scattering compensation has a number of technical difficulties which make application in multiphoton microscopy challenging. Due to the discontinuous nature of the correction pattern, Shack Hartmann wavefront sensors cannot be used, while sensorless approaches can require millions of measurements to iteratively adjust the wavefront due to the high number of degrees of freedom of the correction. This is incompatible with microscopy usage, as the brain tissue can rapidly change during measurement on a sub-second timescale [43]. An alternative strategy widely adopted is to measure the scattering response in a parallel manner by adopting the principle of time reversal or “optical phase conjugation” (OPC). The recording of propagating scattered light field both in phase and amplitude should in fact allow the reproduction of a backpropagating phase conjugated field. This field, in turn could retrace its trajectory through the medium and return to the original input light field [31]. The main disadvantage of this approach is that interferometry or alternatively coherent waves mixing is necessary. It is therefore difficult to implement with fluorescence signals which are incoherent unless coupled with ultrasound waves [29].
Discussion
A massive technological effort by the photonics community has produced the development of sophisticated imaging tools. However, the holy grail of neurophotonics has yet to be discovered: it is still almost impossible to perform sub-cellular resolution imaging in deep brain structure without invasive approaches. A proper compensation of scattering in a three-dimensional turbid medium is in fact still an unresolved issue, despite most of the required technologies are available. It is our opinion that the ideal corrector should be a high resolution spatial light modulator, with enough speed to provide sensorless optimization on time scales compatible with experimental needs. While wavefront-sensing correction has long been considered the state of the art in adaptive optics for microscopy, its technical complexity, inability to effectively compensate for anisoplanatism, and its unreliability for high-order aberrations, make it, in our opinion, unsuitable for the future challenges we described in this manuscript. Recent development in pixel overdrive technologies [44] have started reaching the commercial market, providing the scientific community with tools capable of hundreds of Hz modulation. Very fast SLMs would in turn require high performing optimization procedures. In addition, real-time optimization of scattering compensation in dynamically changing scattering media was recently proven possible through an FPGA implementation [45]. Finally, a conjugate adaptive optics configuration, such as the one reported by Park and colleagues [42], would need to be used in order to achieve the widest possible field of view.
Still, the extremely anisoplanatic nature of scattering correction would, in the end, require some form of parallelized correction in multiple, small fields of view. This would require focusing in multiple diffraction limited spots over an extensive field of view (which was already proven possible in the original Vellekoop publication in 2007) and, most importantly, a reliable method to independently collect the fluorescence signal from multiple foci. This last critical step is still an open question while promising initial results have been achieved by exploiting the temporal separation of excitation pulses [46] and, more recently, through speckle demixing [47]. An additional problem is due to the even more pronounced anisoplanatism of the correction, where the size of the corrected field of view can be as small as the resolution itself, if the correction is applied in the pupil.
To our knowledge, the most successful application of scattering compensation reported to date in multiphoton microscopy was performed by conjugating a high speed, high resolution deformable mirror with the highly scattering, but relatively thin intact skull of an adult mice, to image microglial cells underneath [42]. Unfortunately, while extremely impressive, the proposed method cannot be generalized to thicker scattering layers.
Acquiring high quality images with subcellular resolution of deep brain structure is a goal that has not yet been realized. However, the combination of incident scattering compensation with deconvolution algorithms widely adopted in conventional imaging to compensate isotropic emission can substantially improve the performances of 2P systems, giving hope that this dream can be converted into reality into the next few years.
Author Contributions
All authors contributed in the preparation and revision of the manuscript.
Funding
This work was supported by: Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022)—Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia to CP, and by the University of Modena and Reggio Emilia: FAR 2017 to JM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vellekoop M, Mosk AP. Focusing coherent light through opaque strongly scattering media. Opt Lett. (2007) 32:2309. doi: 10.1364/OL.32.002309
2. Gandolfi D, Pozzi P, Tognolina M, Chirico G, Mapelli J, D'Angelo E. The spatiotemporal organization of cerebellar network activity resolved by two-photon imaging of multiple single neurons. Front Cell Neurosci. (2014) 8:92. doi: 10.3389/fncel.2014.00092
3. Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. (1990) 248:73–6. doi: 10.1126/science.2321027
4. Göppert-Mayer M. Über Elementarakte mit zwei Quantensprüngen (Ph.D. dissertation). John Wiley & Sons, Inc., Göttingen, Germany (1931). doi: 10.1002/andp.19314010303
5. Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. (2006). 50:823–39. doi: 10.1016/j.neuron.2006.05.019
6. Débarre D, Botcherby EJ, Watanabe T, Srinivas S, Booth MJ, Wilson T. Image-based adaptive optics for two-photon microscopy. Opt Lett. (2009) 34:2495–7. doi: 10.1364/OL.34.002495
7. Galwaduge PT, Kim SH, Grosberg LE, Hillman EMC. Simple wavefront correction framework for twophoton microscopy of in-vivo brain. Biomed Opt Exp. (2015) 6:2997. doi: 10.1364/BOE.6.002997
8. Kobat D, Durst ME, Nishimura N, Wong AW. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt Exp. (2009) 17:13354. doi: 10.1364/OE.17.013354
9. Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. (2013) 497:332–7. doi: 10.1038/nature12107
10. Kubby JA. Adaptive Optics for Biological Imaging. Boca Raton, FL: CRC Press; Taylor & Francis Group (2013). doi: 10.1201/b14898
11. Hell SW, Bahlmann K, Schrader M, Soini M, Malak H, Gryczynski I, et al. Three-photon excitation in fluorescence microscopy. J Biomed Opt. (1996) 1:71–4. doi: 10.1117/12.229062
12. Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. J Opt Soc Am. (2006) 23:3139–49. doi: 10.1364/JOSAA.23.003139
13. Horton NG, Wang K, Kobat D, Clark CG, Wise FW, Schaffer CB, et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat Photonics. (2013) 7:205–9. doi: 10.1038/nphoton.2012.336
14. Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 μm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt Lett. (2003) 28:2003. doi: 10.1364/OL.28.001022
15. Kobat D, Horton NG, Xu C. In vivo two-photon microscopy to 1.6 μm depth in mouse cortex. J Biomed Opt. (2011) 6:106014. doi: 10.1117/1.3646209
16. Xu C, Zipfel W, Shear JB, Williams RM, Webb WW. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy Proc Natl Acad Sci USA. (1996) 93:10763–8. doi: 10.1073/pnas.93.20.10763
17. Guesmi K, Abdeladim L, Tozer S, Mahou P, Kumamoto T, Jurkus K, et al. Dual-color deep-tissue three-photon microscopy with a multiband infrared laser. Light Sci Appl. (2018) 7:12. doi: 10.1038/s41377-018-0012-2
18. Liu R, Li Z, Marvin JS, Kleinfeld D. Direct wavefront sensing enables functional imaging of infragranular axons and spines. Nat Methods. (2019) 16:615–8. doi: 10.1038/s41592-019-0434-7
19. Wang C, Liu R, Milkie DE, Sun W, Tan Z, Kerlin A, et al. Multiplexed aberration measurement for deep tissue imaging in vivo. Nat Methods. (2014) 11:1037–40. doi: 10.1038/nmeth.3068
20. Tao X, Fernandez BM, Azucena O, Fu M, Garcia D, Zuo Y, et al. Adaptive optics confocal microscopy using direct wavefront sensing. Opt Lett. (2011) 36:2011. doi: 10.1364/OL.36.001062
21. Albert O, Sherman L, Mourou G, Norris TB, Vdovin G. Smart microscope:an adaptive optics learning system for aberration correction in multiphoton confocal microscopy. Opt Lett. (2000) 25:52–4. doi: 10.1364/OL.25.000052
22. Sherman L, Ye JY, Albert O, Norris TB. Adaptive correction of depth-induced aberrations in multiphoton scanning microscopy using a deformable mirror. J Microsc. (2002) 206:65–71. doi: 10.1046/j.1365-2818.2002.01004.x
23. Bueno JM, Skorsetz M, Bonora S, Artali P. Wavefront correction in two-photon microscopy with a multi-actuator adaptive lens. Opt Exp. (2018) 26:14278. doi: 10.1364/OE.26.014278
24. Neil MAA, Kaitis J, Booth MJ, Wilson T, Tanaka T, Kawata S. Adaptive aberration correction in a two-photon microscope. J Microsc. (2000) 200:105–8. doi: 10.1046/j.1365-2818.2000.00770.x
25. Wang K, Sun W, Richie CT, Harvey BK, Betzig E, Ji N. Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nat Commun. (2015) 6:7276. doi: 10.1038/ncomms8276
26. Tang J, Germaina RN, Cuib M. Superpenetration optical microscopy by iterative multiphoton adaptive compensation technique. Proc Natl Acad Sci USA. (2012) 109:22. doi: 10.1073/pnas.1119590109
27. Ji N, Milkie DE, Betzig E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat Methods. (2010) 7:141–7. doi: 10.1038/nmeth.1411
28. Milkie DE, Betzig E, Ji N. Pupil-segmentation-based adaptive optical microscopy with full-pupil illumination. Opt Lett. (2011) 36:4206–8. doi: 10.1364/OL.36.004206
29. Xu X, Liu H, Wang LV. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat Photon. (2011) 5:154–7. doi: 10.1038/nphoton.2010.306
30. Mertz J, Paudel H, Bifano TG. Field of view advantage of conjugate adaptive optics in microscopy applications. Appl Opt. (2015) 101:3498–506. doi: 10.1364/AO.54.003498
31. Yaqoob Z, Psaltis D, Feld MS, Yang C. Optical phase conjugation for turbidity suppression in biological samples. Nat Photon. (2008) 2:110–15. doi: 10.1038/nphoton.2007.297
32. Doi A, Oketani R, Nawa Y, Fujita K. High-resolution imaging in two-photon excitation microscopy using in situ estimations of the point spread function. Biomed Opt Exp. (2018) 9:202. doi: 10.1364/BOE.9.000202
33. Wen-Chen I, Ronzitti E, Lee BR, Daigle TL, Dalkara D, Zeng XH, et al. In vivo submillisecond two-photon optogenetics with temporally focused patterned light. J Neurosci. (2019) 39:3484–97. doi: 10.1523/JNEUROSCI.1785-18.2018
34. Pozzi P, Gandolfi D, Tognolina M, Chirico G, Mapelli J, D'Angelo E. High-throughput spatial light modulation two-photon microscopy for fast functional imaging Neurophotonics. (2015) 2:015005. doi: 10.1117/1.NPh.2.1.015005
35. Sironi L, Freddi S, Caccia M, Pozzi P, Rossetti L, Pallavicini P, et al. Gold branched nanoparticles for cellular treatments. J Phys Chem C. (2012) 116:18407–18. doi: 10.1021/jp305021k
36. Hsieh CL, Pu Y, Grange R, Psaltis D. Digital phase conjugation of second harmonic radiation emitted by nanoparticles in turbid media. Opt Exp. (2014) 18:12283. doi: 10.1364/OE.18.012283
37. Chaigne T, Katz O, Boccara AC, Fink M, Gigan BS. Controlling light in scattering media noninvasively using the photo-acoustic transmission-matrix. Nat Photon. (2014) 8:58–64. doi: 10.1038/nphoton.2013.307
38. Ma C, Xu X, Liu Y, Wang LV. Time-reversed adapted-perturbation (TRAP) optical focusing onto dynamic objects inside scattering media. Nat Photonics. (2014) 8:931–6. doi: 10.1038/nphoton.2014.251
39. Débarre D, Booth MJ, Wilson T. Image-based adaptive optics through optimisation of low spatial frequencies. Opt Exp. (2007) 15:8176–90. doi: 10.1364/OE.15.008176
40. Pozzi. P, Soloviev O, Wilding D, Vdovin G, Verhagen M. Optimal model-based sensorless adaptive optics for epifluorescence microscopy. PLoS ONE. (2018) 13:e0194523. doi: 10.1371/journal.pone.0194523
42. Park JH, Sun W, Cui M. High-resolution in vivo imaging of mouse brain through the intact skull. Proc Natl Acad Sci USA. (2015) 112:9236–41. doi: 10.1073/pnas.1505939112
43. Horstmeyer R, Ruan H, Yang C. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nat Photon. (2015) 9:563–71. doi: 10.1038/nphoton.2015.140
44. Thalhammer G, Bowman RW, Love GD, Padgett MJ, Ritsch-Marte M. Speeding up liquid crystal SLMs using overdrive with phase change reduction. Opt Exp. (2013) 21:1779–97. doi: 10.1364/OE.21.001779
45. Blochet B, Bourdieu L, Gigan S. Focusing light through dynamical samples using fast continuous wavefront optimization. Opt Lett. (2017) 42:4994–7. doi: 10.1364/OL.42.004994
46. David N, Fittinghoff P, Wiseman W, Squier JA. Widefield multiphoton and temporally decorrelated multifocal multiphoton microscopy. Opt Exp. (2000) 7:273–9. doi: 10.1364/OE.7.000273
Keywords: two photon microscopy, scattering compensation, in vivo brain imaging, adaptive optics in biomedical imaging, subcellular and synaptic imaging, neurophotonics
Citation: Pozzi P, Gandolfi D, Porro CA, Bigiani A and Mapelli J (2020) Scattering Compensation for Deep Brain Microscopy: The Long Road to Get Proper Images. Front. Phys. 8:26. doi: 10.3389/fphy.2020.00026
Received: 14 October 2019; Accepted: 27 January 2020;
Published: 13 February 2020.
Edited by:
Bernhard Baumann, Medical University of Vienna, AustriaReviewed by:
Boran Han, Harvard University, United StatesDustin Ryan Osborne, The University of Tennessee, Knoxville, United States
Copyright © 2020 Pozzi, Gandolfi, Porro, Bigiani and Mapelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Mapelli, am9uYXRoYW4ubWFwZWxsaUB1bmltb3JlLml0
†These authors have contributed equally to this work
 Paolo Pozzi
Paolo Pozzi Daniela Gandolfi
Daniela Gandolfi Carlo Adolfo Porro
Carlo Adolfo Porro Albertino Bigiani
Albertino Bigiani Jonathan Mapelli
Jonathan Mapelli