
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 08 October 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.554847
Background: Oral propranolol has become the first-line treatment for infantile hemangioma (IH). However, combined therapy with topical timolol and oral propranolol has been proposed as a more effective IH treatment strategy. We aimed to compare the safety and efficacy of topical timolol, oral propranolol, and their combination for treating IH in a meta-analysis.
Methods: Relevant randomized controlled trials (RCTs) were obtained after searching the PubMed, Embase, Cochrane’s Library, China National Knowledge Infrastructure, and WanFang databases. A random-effect model was used to pool the results.
Results: Eight RCTs with 759 patients with IH were included in this meta-analysis. Treatment with topical timolol alone showed a similar response rate compared to oral propranolol (risk ratio [RR] = 0.97, p = 0.63), but resulted in fewer adverse events (RR = 0.36, p = 0.002). Combined treatment with topical timolol and oral propranolol showed a favorable response rate compared to treatment with oral propranolol (RR = 1.14, p = 0.03) or topical timolol (RR = 1.36, p = 0.01) alone. Moreover, combined treatment showed similar risks of adverse events compared to oral propranolol (RR = 0.80, p = 0.24) or topical timolol (RR = 1.31, p = 0.25) alone.
Conclusions: Combined treatment with topical timolol and oral propranolol may be more effective than either single treatment strategy in patients with IH. Topical timolol alone conferred similar efficacy for IH compared to oral propranolol, but with less incidence of adverse events.
Infantile hemangioma (IH) is a common congenital vascular malformation that affects 4–5% of full-term infants (Itinteang et al., 2014; Grzesik and Wu, 2017). The incidence of IH is higher in females than in males, with an estimated ratio of 3–5:1 (Harter and Mancini, 2019; Wildgruber et al., 2019). Because of a natural history of spontaneous involution, most IHs do not need treatment (Harter and Mancini, 2019). However, some severe IH cases are associated with complications including bleeding, ulceration, or disfigurement, which require early interventions (Singh et al., 2019). Previous treatments for IHs involve multiple modalities, with glucocorticoids considered the medication of choice (Chinnadurai et al., 2016). Since the first-report of successful treatment of IH with oral propranolol in 2008, this non-selective β-blocker has become the mainstay for managing complex IHs (Leaute-Labreze et al., 2015; Yang et al., 2019). Although oral propranolol is effective and safe in most IH cases, it can cause severe adverse events, such as hypotension, bradycardia, hypoglycemia, sleep disturbance, and possibly central nervous system symptoms (Leaute-Labreze et al., 2016; Raphael et al., 2016; Thai et al., 2019). Timolol, another non-selective β-blocker, which can be applied topically in gel or in solution, has emerged as a novel IH treatment (Zheng and Li, 2018). Topical timolol may confer similar efficacy as propranolol for treating IHs, particularly the superficial type, but causes less adverse events (Cheirif-Wolosky et al., 2019). However, use of topical timolol for IH treatment is mainly based on clinical experience and evidence from observational studies, rather than randomized controlled trials (RCTs) (Wu et al., 2018). Combined therapy with topical timolol and oral propranolol has been proposed to be more effective for treating IHs compared to application of either component alone (Ge et al., 2016; Tong et al., 2016). However, clinical evidence to support this proposal remains limited. Therefore, in this study, we conducted a meta-analysis of head-to-head RCTs to systematically evaluate the efficacy and safety of topical timolol, oral propranolol, and their combination for treating IH.
This meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Moher et al., 2009) and the Cochrane Handbook for Systematic Review and Meta-analysis (Higgins and Green, 2011).
The PubMed, Embase, Cochrane’s Library (Cochrane Center Register of Controlled Trials), China National Knowledge Infrastructure (CNKI, http://www.cnki.net/), and WanFang (http://www.wanfangdata.com.cn/) electronic databases were searched for studies comparing the efficacy and safety among topical timolol, oral propranolol, and their combination for treating IH, using the terms “timolol” AND “propranolol” AND (“hemangioma” OR “haemangioma” OR “vascular malformation” OR “hemangiom*” OR “angiom*” OR “malformation*”). The search was limited to human studies with no restriction of publication languages. We also manually analyzed reference lists of the original and review articles. The final search was performed on June 3, 2020.
Studies were included if they met the following criteria: (1) published as full-length articles with no restriction of languages; (2) reported as RCTs with a parallel design; (3) included patients with IH; (4) directly compared the efficacy and safety of at least two of the following treatment strategies, including topical timolol, oral propranolol, and their combination; (5) reported a treatment duration of at least one month; and (6) reported efficacy outcomes as response rates and safety outcomes of any adverse events that were confirmed to be related to the medications. The response rate was defined as a reduction in tumor size of at least 50% after treatment in accordance with the criteria proposed by Achauer et al., (1997). Reviews, preclinical studies, non-RCTs, and studies that did not report related outcomes were excluded from the current analysis.
Two authors independently performed literature searches, data extraction, and quality assessment according to the inclusion criteria. Discrepancies were resolved by consensus. Extracted data included the country where the study was performed, study design characteristics (blind or open-label), characteristics of the infantile patients (number of participants, sex, and age), lesion characteristics (distribution and clinical classification), and intervention characteristics (medications, routes, dosages, and treatment durations). We used the seven domains of the Cochrane risk of bias tool to evaluate the quality of the included studies (Higgins and Green, 2011), which included criteria concerning sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential threats to validity.
Dichotomous data were analyzed using risk ratios (RRs) with 95% confidence intervals (CIs). The Cochrane’s Q test was applied to evaluate the heterogeneity among the included studies, and significant heterogeneity was considered at p < 0.10 (Higgins et al., 2003). The I2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance, was also calculated to determine study heterogeneity (Higgins and Thompson, 2002). An I2 > 50% indicated significant heterogeneity among the trials. Pooled analyses were calculated using a random-effect model because this model incorporated the potential heterogeneity among the included RCTs and therefore could retrieve a more generalized result (Higgins and Green, 2011). Potential publication bias was assessed by visual inspection of the symmetry of the funnel plots and Egger’s regression asymmetry test (Egger et al., 1997). P values were two-tailed and statistical significance was set at 0.05. We used RevMan software for the meta-analysis and statistical study (Version 5.1; Cochrane, Oxford, UK) and Stata software (Version 12.0; Stata, College Station, TX).
A total of 519 articles were identified through initial database searches, and 431 were obtained after excluding of duplications. Among them, 409 were excluded because they were not relevant studies based on title and abstract screening. Of the 22 potentially relevant articles, 14 articles were excluded because six were not RCTs, six did not compare the interested interventions, one was a repeated report of an already included RCT, and one did not report response rate for the treatment. Finally, eight RCTs were included (Zhao et al., 2013; Li et al., 2014; Chen et al., 2015; Gong et al., 2015; Li et al., 2016; Zhang et al., 2017; Du et al., 2018; Marey et al., 2018) (Figure 1).
Overall, this meta-analysis included 759 patients with IH among eight RCTs (Zhao et al., 2013; Li et al., 2014; Chen et al., 2015; Gong et al., 2015; Li et al., 2016; Zhang et al., 2017; Du et al., 2018; Marey et al., 2018). The characteristics of the included RCTs are summarized in Table 1. Three RCTs were published in English (Gong et al., 2015; Li et al., 2016; Marey et al., 2018), and the other five were published in Chinese (Zhao et al., 2013; Li et al., 2014; Chen et al., 2015; Zhang et al., 2017; Du et al., 2018). All of the included studies were performed in China except one study which was performed in Egypt (Marey et al., 2018). The ages of the included patients varied from 1 to 18 months, with the percentage of males varying from 28–52%. With regards to the distributions of IH lesions, one study included patients with periocular hemangiomas (Marey et al., 2018), three included patients with IH in the facial area (Li et al., 2014; Gong et al., 2015; Li et al., 2016), and the remaining four studies included lesions in the face, scalp, neck, torso (20), scalp, perineum, and extremities (Zhao et al., 2013; Chen et al., 2015; Zhang et al., 2017; Du et al., 2018). The IHs were superficial in five studies (Zhao et al., 2013; Li et al., 2014; Gong et al., 2015; Zhang et al., 2017; Marey et al., 2018) and of mixed clinical classifications in the other threes studies (Chen et al., 2015; Li et al., 2016; Du et al., 2018). Three studies compared the efficacies between topical timolol and oral propranolol (Zhao et al., 2013; Li et al., 2014; Zhang et al., 2017), three compared the efficacies between oral propranolol and a combined therapy with topical timolol and oral propranolol (Chen et al., 2015; Li et al., 2016; Marey et al., 2018), the other two studies compared the efficacies among the three treatments (Gong et al., 2015; Du et al., 2018). The follow-up durations varied from one to eight months.
The details of quality evaluation for the included studies according to the Cochrane assessment tool are listed in Table 2. Briefly, one of the included RCTs was a double-blinded study (Du et al., 2018), one was single-blinded (Marey et al., 2018), and the rest were open-label studies (Zhao et al., 2013; Li et al., 2014; Chen et al., 2015; Gong et al., 2015; Li et al., 2016; Zhang et al., 2017). Details of random sequence generation were reported in five studies (Zhao et al., 2013; Gong et al., 2015; Li et al., 2016; Du et al., 2018; Marey et al., 2018). However, none of the studies reported the efforts of allocation concealment. Details of withdrawals and dropouts were reported in all of the studies.
Five studies including 490 patients compared the efficacy and safety of topical timolol and oral propranolol for treating IH (Zhao et al., 2013; Li et al., 2014; Gong et al., 2015; Zhang et al., 2017; Du et al., 2018). Pooled results with a random-effect model showed that the response rate of IH patients in the two treatment arms was comparable (RR = 0.97, 95% CI: 0.85 to 1.11; p = 0.63; Figure 2A) with no significant heterogeneity (p for Cochrane’s Q test = 0.41, I2 = 0%). However, the incidence of adverse events was less in patients allocated to the topical timolol group (RR = 0.36, p = 0.002; Figure 2B).
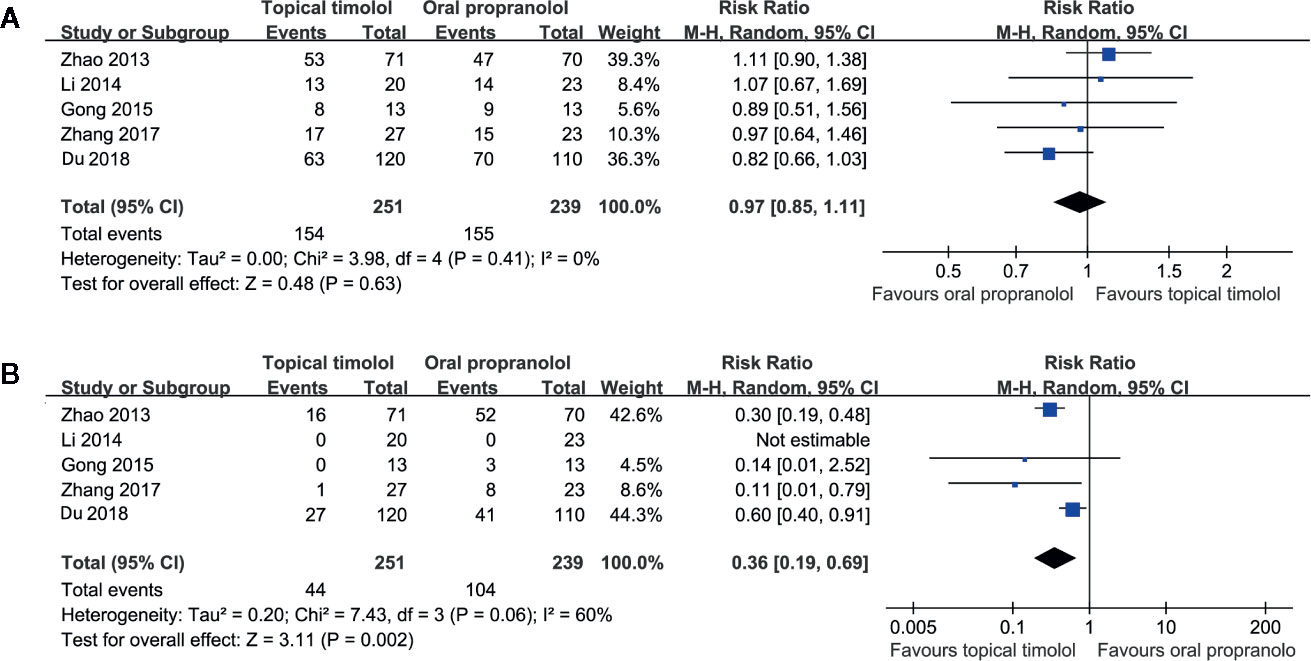
Figure 2 Forest plots for the meta-analyses comparing the efficacy and safety outcomes between topical timolol and oral propranolol for treating IH. (A), response rate after treatment; (B), incidence of adverse events.
Five studies including 392 patients compared the response rate and adverse events in patients allocated to a combined therapy with topical timolol and oral propranolol and those who received oral propranolol only (Chen et al., 2015; Gong et al., 2015; Li et al., 2016; Du et al., 2018; Marey et al., 2018). Pooled results with a random-effect model showed that combined treatment was associated with a higher response rate compared to oral propranolol alone (RR = 1.14, 95% CI: 1.02 to 1.29; p = 0.03; Figure 3A) with no significant heterogeneity (p for Cochrane’s Q test = 0.37, I2 = 6%). Moreover, the incidence of adverse events was comparable between the two groups (RR = 0.80, p = 0.24; Figure 3B).
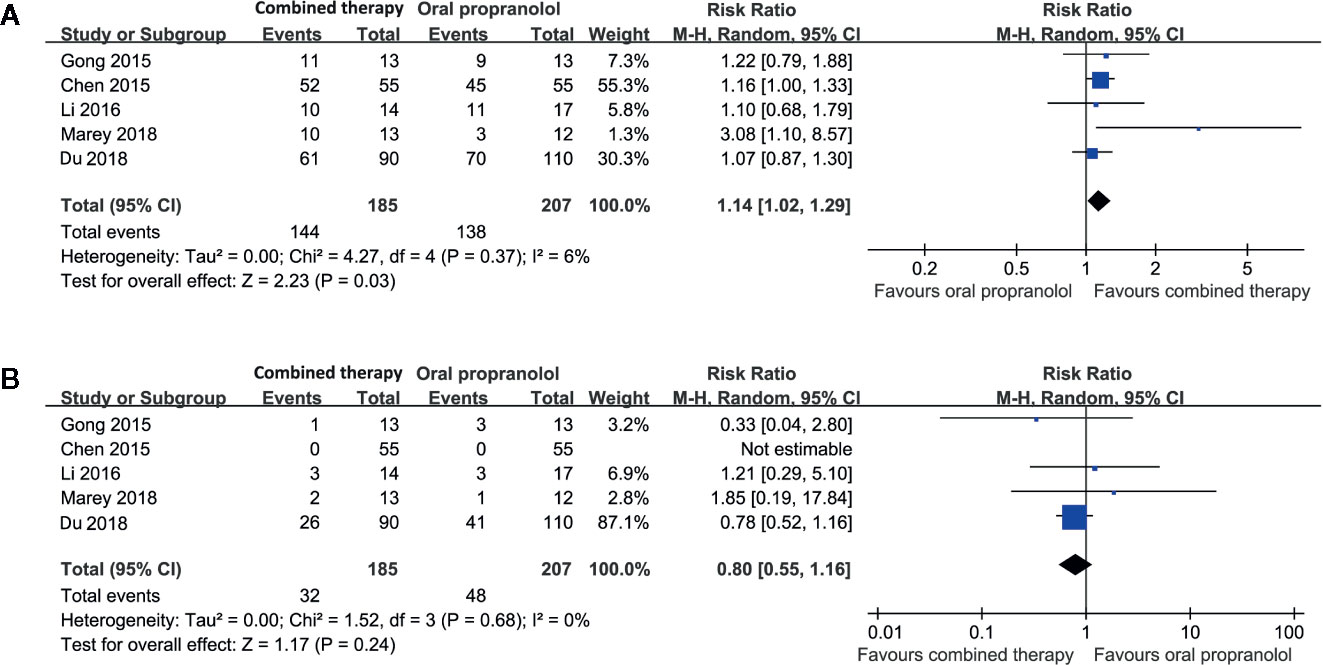
Figure 3 Forest plots for the meta-analyses comparing the efficacy and safety outcomes between the combined therapy and oral propranolol for treating IH. (A), response rate after treatment; (B), incidence of adverse events.
Two studies including 236 patients compared the response rate and adverse events in patients allocated to combined therapy with topical timolol and oral propranolol and those who received topical timolol only (Gong et al., 2015; Du et al., 2018). Pooled results with a random-effect model showed that combined treatment was also associated with a higher response rate compared to topical timolol only (RR = 1.31, 95% CI: 1.07 to 1.60; p = 0.01; Figure 4A) with no significant heterogeneity (p for Cochrane’s Q test = 0.82, I2 = 0%). The incidence of adverse events was comparable between the two groups (RR = 1.31, p = 0.25; Figure 4B).
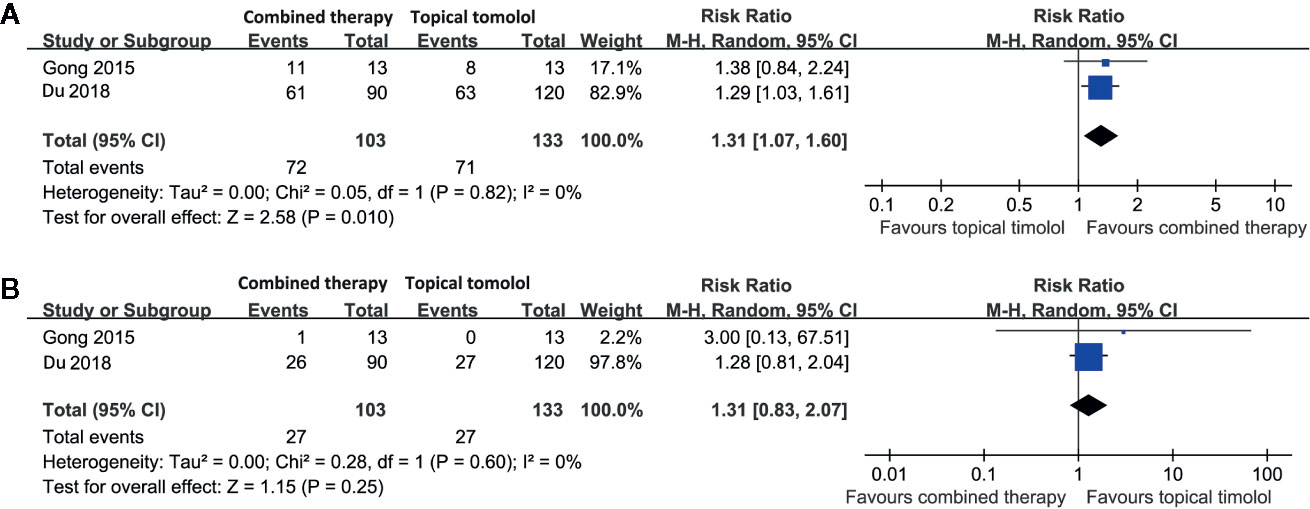
Figure 4 Forest plots for the meta-analyses comparing the efficacy and safety outcomes between the combined therapy and topical timolol for treating IH. (A), response rate after treatment; (B), incidence of adverse events.
The funnel plots for the meta-analyses of safety and efficacy outcomes between topical timolol and oral propranolol, as well as the combined therapy and oral propranolol, were symmetrical on visual inspection (Figures 5A–D), indicating low chance of publication biases. Egger’s regression tests were not performed because of the limited number of studies included. The funnel plots for the meta-analyses comparing the efficacy and safety outcomes between the combined therapy and topical timolol were unavailable because only two studies were included. The publication bias for the meta-analyses of each outcome was difficult to estimate because only a maximum of five studies were included.
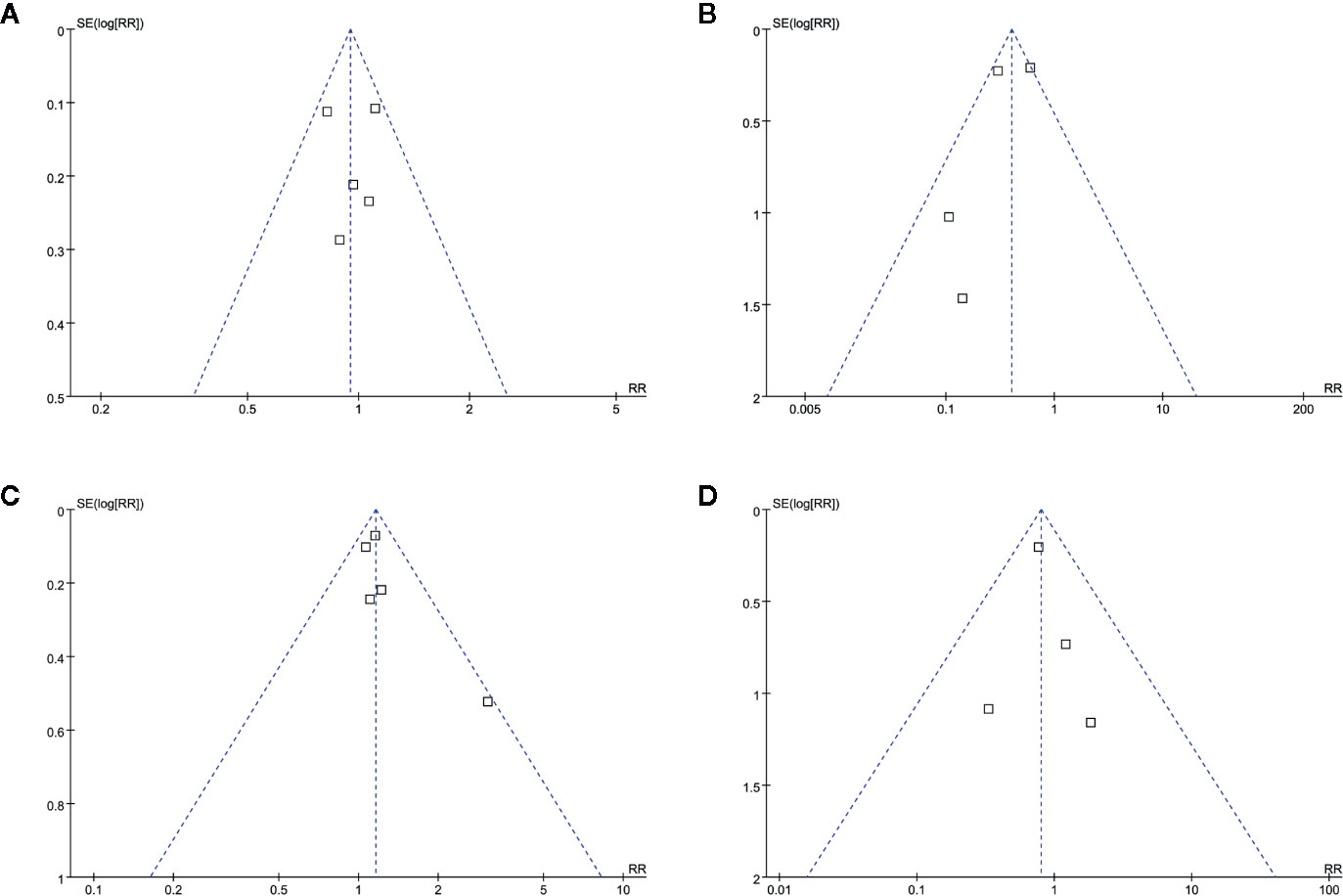
Figure 5 Funnel plots for the meta-analyses. (A), response rate for meta-analysis comparing topical timolol and oral propranolol for treating IH; (B), safety outcome for the meta-analysis comparing topical timolol and oral propranolol for treating IH; (C), response rate for meta-analysis comparing combined therapy and oral propranolol for treating IH; (D), safety outcome for the meta-analysis comparing combined therapy and oral propranolol for treating IH.
In this meta-analysis of head-to-head RCTs, we found that topical timolol and oral propranolol showed similar efficacy for treating IH, as indicated by the response rate. However, topical timolol was associated with fewer incidences of adverse events compared to oral propranolol. Interestingly, we also found that combined therapy with topical timolol and oral propranolol was associated with a favorable response rate for treating IH compared to treatment with either topical timolol or oral propranolol alone. Moreover, the combined treatment was not associated with increased risk of adverse events compared to the treatment of topical timolol or oral propranolol alone. Taken together, these findings suggest that combined treatment with topical timolol and oral propranolol may improve treatment efficacy for patients with large or severe IH. For those with mild or moderate IH, topical timolol may be favorable over oral propranolol, considering the better safety profile of timolol.
Although previous meta-analyses have been conducted to evaluate the potential efficacies and safeties of topical timolol and oral propranolol for treating IH, these analyses generally included a limited number of studies (Liu et al., 2015; Zheng and Li, 2018; Yang et al., 2019). More importantly, these studies included both RCTs and non-randomized studies, which may have led to potential bias and therefore could only provide a limited grade of evidence (Zheng and Li, 2018; Yang et al., 2019). In addition, the control groups in these meta-analyses were heterogeneous, including blank treatment, other active medications, and laser therapy, which makes the interpretation of the results difficult (Novoa et al., 2018). In a recent meta-analysis comparing the efficacy between topical timolol and oral propranolol, the authors included two RCTs and concluded that patients who received these two treatment protocols had similar response rates after treatment (Zheng and Li, 2018). Our study confirmed these results by including five RCTs with 490 patients. Moreover, we found that topical timolol was associated with 64% fewer adverse events compared to oral propranolol, indicating better safety profiles of topical timolol. Notably, four RCTs in our meta-analysis comparing the efficacy and safety between topical timolol and oral propranolol included infants with superficial IHs only (Zhao et al., 2013; Li et al., 2014; Gong et al., 2015; Zhang et al., 2017), which implies that for those with mild or moderate IH, topical timolol may be favorable over oral propranolol considering its better safety profile.
To the best of our knowledge our meta-analysis is first study demonstrating that combined treatment with topical timolol and oral propranolol may be more effective than either single treatment protocol in patients with IH without increasing the risk of adverse events. The potential mechanisms underlying the improved efficacy of combined treatments for IHs are unknown. However, in view of the fact that both medications are non-selective β-blockers, the synergetic treatment efficacy may be primarily caused by the combined routes of medicine administration. It has been proposed that multiple hormonal factors, including β-adrenergic catecholamines, epinephrine, norepinephrine, and vascular endothelial growth factors (VEGF) are involved in the pathogenesis and progression of hemangiomas (Boscolo and Bischoff, 2009). β-blockers may confer therapeutic efficacy for IH via inhibiting various related signaling pathways and cellular components, including thrombospondin-1, nuclear factor-κB, phosphatidylinositol 3-kinase, protein kinase B, mitogen-activated protein kinase, and hypoxia-inducible factor-1 (Munabi et al., 2016; Lin et al., 2018; Xu et al., 2018). Studies are needed to determine the key mechanisms underlying the therapeutic role of β-blockers for IHs and to explore whether timolol and propranolol have synergetic pharmacological mechanisms that protect against the pathogenesis and progression of hemangiomas. Moreover, it has to be mentioned that although propranolol is currently the first-line treatment for IHs, the efficacy of the drug is around 60% as evidenced in a previous RCT (Leaute-Labreze et al., 2015). Therefore, it could be implied that the blockade of beta1 and beta2 adrenoceptor is probably not sufficient, and that other receptors and other pathways are involved in IH pathogenesis, such as basic fibroblast growth factor, vascular endothelial growth factor and its receptor, glucose transporter isoform 1, matrix metalloproteinase-9, proliferating cell nuclear antigen, type IV collagenase, and angiotensin (Chen et al., 2019). The efficacy of combined treatment with propranolol and drugs targeting these pathways for IHs should also be determined in the future.
Our study has limitations that should be considered when interpreting the results. First, there were limited published RCTs available in the databases and we did not have access to individual patient data. Therefore, we were unable to determine the potential influences of study characteristics on the outcomes, such as the distributions and clinical classifications of the lesions, the dosages, administrative routes, and the treatment durations for each protocol. Future RCTs with adequate sample sizes are warranted to evaluate the potential influence of the above factors on therapeutic efficacies of the combined medications. Secondly, the follow-up durations of the included studies were relatively short. Long-term studies are needed to evaluate the influence of these treatment strategies on the risk of relapse of IH. Thirdly, although the statistical heterogeneity was not significant in most of the outcomes of the meta-analyses (I2 = 0 in 5 of the 6 outcomes), clinical heterogeneity existed among the included studies regarding patient characteristics and treatment protocols et al. Therefore, a random-effect model, the more conservative method, was used to pool the results of these studies in order to generate a more common result. The difference of these aforementioned study characteristics may influence the outcome of the meta-analysis. Finally, some of the included RCTs were open-label and of low quality, which may have introduced potential bias. Our results need to be validated in high-quality RCTs in the future.
In conclusion, combined treatment with topical timolol and oral propranolol may be more effective than either single treatment protocol in patients with IH. However, topical timolol alone conferred similar efficacy for treating IH compared to oral propranolol, but with less incidence of adverse events. A combined treatment with topical timolol and oral propranolol may be considered for patients with large or severe IHs to improve the treatment efficacy. For those with mild or moderate IH, topical timolol may be favorable over oral propranolol.
JQ and JjL designed the study. JQ and JjL performed database search, data extraction, and quality evaluation. JQ, DZ, JhL, CC, HY, XL, and BF performed statistical analyses and verified the data. JQ drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Medical Science and Technology Research Project of Henan Province (201601015) and the Research Project of Department of Science and Technology of Henan Province (162102310282).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Achauer, B. M., Chang, C. J., Vander Kam, V. M. (1997). Management of hemangioma of infancy: review of 245 patients. Plast. Reconstr. Surg. 99, 1301–1308. doi: 10.1097/00006534-199704001-00014
Boscolo, E., Bischoff, J. (2009). Vasculogenesis in infantile hemangioma. Angiogenesis 12, 197–207. doi: 10.1007/s10456-009-9148-2
Cheirif-Wolosky, O., Novelo-Soto, A. D., Orozco-Covarrubias, L., Saez-De-Ocariz, M. (2019). Infantile hemangioma: an update in the topical and systemic treatments. Bol. Med. Hosp. Infant Mex. 76, 167–175. doi: 10.24875/BMHIM.19000002
Chen, X. Y., Shu, H., Feng, J. H., Zhang, D. Y. (2015). The fficacy and safety of oral propranolol combination with topical application of timolol maleate eye drops on infant hemangioma. J. Dermatol. Venereol. 37, 314–317. doi: 10.1111/j.1468-3083.2004.00918.x
Chen, Z. Y., Wang, Q. N., Zhu, Y. H., Zhou, L. Y., Xu, T., He, Z. Y., et al. (2019). Progress in the treatment of infantile hemangioma. Ann. Transl. Med. 7, 692. doi: 10.21037/atm.2019.10.47
Chinnadurai, S., Fonnesbeck, C., Snyder, K. M., Sathe, N. A., Morad, A., Likis, F. E., et al. (2016). Pharmacologic Interventions for Infantile Hemangioma: A Meta-analysis. Pediatrics 137, e20153896. doi: 10.1542/peds.2015-3896
Du, J. Y., Wu, S. J., Zhang, Y., Guo, J. (2018). Oral Propranolol and Topical Timolol Treatment in Infantile Hemangioma. Sichuan Med. J. 39, 489–491. doi: 10.16252/j.cnki.issn1004-0501-2018.05.004
Egger, M., Davey Smith, G., Schneider, M., Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Ge, J., Zheng, J., Zhang, L., Yuan, W., Zhao, H. (2016). Oral propranolol combined with topical timolol for compound infantile hemangiomas: a retrospective study. Sci. Rep. 6, 19765. doi: 10.1038/srep19765
Gong, H., Xu, D. P., Li, Y. X., Cheng, C., Li, G., Wang, X. K. (2015). Evaluation of the efficacy and safety of propranolol, timolol maleate, and the combination of the two, in the treatment of superficial infantile haemangiomas. Br. J. Oral. Maxillofac. Surg. 53, 836–840. doi: 10.1016/j.bjoms.2015.09.005
Grzesik, P., Wu, J. K. (2017). Current perspectives on the optimal management of infantile hemangioma. Pediatr. Health Med. Ther. 8, 107–116. doi: 10.2147/PHMT.S115528
Harter, N., Mancini, A. J. (2019). Diagnosis and Management of Infantile Hemangiomas in the Neonate. Pediatr. Clin. North Am. 66, 437–459. doi: 10.1016/j.pcl.2018.12.011
Higgins, J., Green, S. (2011). “Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0,” in The Cochrane Collaboration. Wiley. Available at: www.cochranehandbook.org.
Higgins, J. P., Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Higgins, J. P., Thompson, S. G., Deeks, J. J., Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Itinteang, T., Withers, A. H., Davis, P. F., Tan, S. T. (2014). Biology of infantile hemangioma. Front. Surg. 1, 38. doi: 10.3389/fsurg.2014.00038
Leaute-Labreze, C., Hoeger, P., Mazereeuw-Hautier, J., Guibaud, L., Baselga, E., Posiunas, G., et al. (2015). A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl. J. Med. 372, 735–746. doi: 10.1056/NEJMoa1404710
Leaute-Labreze, C., Boccara, O., Degrugillier-Chopinet, C., Mazereeuw-Hautier, J., Prey, S., Lebbe, G., et al. (2016). Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review. Pediatrics 138 (4), e20160353. doi: 10.1542/peds.2016-0353
Li, Q., Li, J., Yang, T. (2014). The clinical observation and comparion of propranolole and timolol for infant facial hemangioma. Chin. J. Aesthet. Med. 23, 221–223.
Li, G., Xu, D. P., Tong, S., Xue, L., Sun, N. N., Wang, X. K. (2016). Oral Propranolol With Topical Timolol Maleate Therapy for Mixed Infantile Hemangiomas in Oral and Maxillofacial Regions. J. Craniofac. Surg. 27, 56–60. doi: 10.1097/SCS.0000000000002221
Lin, Z., Wang, L., Huang, G., Wang, W., Lin, H. (2018). Propranolol inhibits the activity of PI3K, AKT, and HIF-1alpha in infantile hemangiomas. Pediatr. Surg. Int. 34, 1233–1238. doi: 10.1007/s00383-018-4347-9
Liu, X., Qu, X., Zheng, J., Zhang, L. (2015). Effectiveness and Safety of Oral Propranolol versus Other Treatments for Infantile Hemangiomas: A Meta-Analysis. PloS One 10, e0138100. doi: 10.1145/2818302
Marey, H. M., Elmazar, H. F., Mandour, S. S., Khairy, H. A. (2018). Combined Oral and Topical Beta Blockers for the Treatment of Early Proliferative Superficial Periocular Infantile Capillary Hemangioma. J. Pediatr. Ophthalmol. Strabismus 55, 37–42. doi: 10.3928/01913913-20170703-12
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi: 10.1136/bmj.b2535
Munabi, N. C., England, R. W., Edwards, A. K., Kitajewski, A. A., Tan, Q. K., Weinstein, A., et al. (2016). Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation. Stem Cells Transl. Med. 5, 45–55. doi: 10.5966/sctm.2015-0076
Novoa, M., Baselga, E., Beltran, S., Giraldo, L., Shahbaz, A., Pardo-Hernandez, H., et al. (2018). Interventions for infantile haemangiomas of the skin. Cochrane Database Syst. Rev. 4, CD006545. doi: 10.1002/14651858.CD006545.pub3
Raphael, M. F., Breur, J. M., Vlasveld, F. A., Elbert, N. J., Liem, Y. T., Kon, M., et al. (2016). Treatment of infantile hemangiomas: therapeutic options in regard to side effects and adverse events - a review of the literature. Expert Opin. Drug Saf. 15, 199–214. doi: 10.1517/14740338.2016.1130125
Singh, P., Chamlin, S., Hignett, E., Silverberg, J. I. (2019). Trends in healthcare utilization for infantile hemangioma in the United States. Br. J. Dermatol. 182(2), 509–511. doi: 10.1111/bjd.18513
Thai, T., Wang, C. Y., Chang, C. Y., Brown, J. D. (2019). Central Nervous System Effects of Oral Propranolol for Infantile Hemangioma: A Systematic Review and Meta-Analysis. J. Clin. Med. 8 (2), 268. doi: 10.3390/jcm8020268
Tong, S., Xu, D. P., Liu, Z. M., Du, Y., Wang, X. K. (2016). Evaluation of the efficacy and safety of topical timolol maleate combined with oral propranolol treatment for parotid mixed infantile hemangiomas. Oncol. Lett. 12, 1806–1810. doi: 10.3892/ol.2016.4818
Wildgruber, M., Sadick, M., Muller-Wille, R., Wohlgemuth, W. A. (2019). Vascular tumors in infants and adolescents. Insights Imaging 10, 30. doi: 10.1186/s13244-019-0718-6
Wu, H. W., Wang, X., Zhang, L., Zheng, J. W., Liu, C., Wang, Y. A. (2018). Topical Timolol Vs. Oral Propranolol for the Treatment of Superficial Infantile Hemangiomas. Front. Oncol. 8, 605. doi: 10.3389/fonc.2018.00605
Xu, W., Li, S., Yu, F., Zhang, Y., Yang, X., An, W., et al. (2018). Role of Thrombospondin-1 and Nuclear Factor-kappaB Signaling Pathways in Antiangiogenesis of Infantile Hemangioma. Plast. Reconstr. Surg. 142, 310e–321e. doi: 10.1097/PRS.0000000000004684
Yang, H., Hu, D. L., Shu, Q., Guo, X. D. (2019). Efficacy and adverse effects of oral propranolol in infantile hemangioma: a meta-analysis of comparative studies. World J. Pediatr. 15 (6), 546–558. doi: 10.1007/s12519-019-00285-9
Zhang, K. C., Xu, D. P., Cheng, M. S., Wang, X. K. (2017). Evaluation of the efficacy and safety of 0.5% timolol maleate solution or oral propranolol in the treatment of superficial infantile hemangiomas. Chin. J. Oral. Maxillofacial Surg. 15, 529–533. doi: 10.19438/j.cjoms.2017.06.010
Zhao, Q. F., Liu, X. J., Chen, X. L., Yang, W. C. (2013). Clinical efficacy and safety of topical tomolol for superficial infantile hemangioma: a pilot study. Shandong Yi Yao 53, 86–87. doi: 10.3969/j.issn.1002-266X.2013.37.034
Keywords: timolol, propranolol, infantile hemangioma, effective rate, meta-analysis
Citation: Qiao J, Lin J, Zhang D, Li J, Chen C, Yu H, Li X and Fang B (2020) Efficacy of Combined Topical Timolol and Oral Propranolol for Treating Infantile Hemangioma: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 11:554847. doi: 10.3389/fphar.2020.554847
Received: 23 April 2020; Accepted: 31 August 2020;
Published: 08 October 2020.
Edited by:
Claudio Bucolo, University of Catania, ItalyReviewed by:
Joao Massud, Independent Researcher, São Paulo, BrazilCopyright © 2020 Qiao, Lin, Zhang, Li, Chen, Yu, Li and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junbo Qiao, anVuYm9xaWFvQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.