- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, South Korea
- 2Department of Biomedical Sciences, Ajou University School of Medicine, Suwon, South Korea
Nonsteroidal antiinflammatory drug (NSAID)-exacerbated respiratory disease (NERD) is characterized by moderate-to-severe asthma and a higher prevalence of chronic rhinosinusitis/nasal polyps, but is a highly heterogeneous disorder with various clinical manifestations. Two major pathogenic mechanisms are: (1) overproduction of cysteinyl leukotrienes with dysregulation of arachidonic acid metabolism and (2) increased type 2 eosinophilic inflammation affected by genetic mechanisms. Aspirin challenge is the gold standard to diagnose NERD, whereas reliable in vitro biomarkers have yet not been identified. Therapeutic approaches have been done on the basis of disease severity with the avoidance of culprit and cross-reacting NSAIDs, and when indicated, aspirin desensitization is an effective treatment option. Biologic approaches targeting Type 2 cytokines are emerging as potential therapeutic options. Here, we summarize the up-to-date evidence of pathophysiologic mechanisms and diagnosis/management approaches to the patients with NERD with its phenotypic classification.
Introduction
Aspirin (acetylsalicylic acid, ASA) and nonsteroidal antiinflammatory drugs (NSAIDs) are the most commonly prescribed drugs in the world (Doña et al., 2012); however, they are considered the most common causes of hypersensitivity reactions to drugs (Blanca-Lopez et al., 2018). Hypersensitivity reactions to NSAIDs have recently been classified by the European Academy of Allergy and Clinical Immunology (EAACI) and European Network of Drug Allergy (ENDA): 1) pharmacologic reactions (mediated by cyclooxygenase [COX]-1 inhibitions) include NSAID-exacerbated respiratory disease (NERD), NSAID-exacerbated cutaneous disease (NECD) and NSAID-induced urticarial/angioedema (NIUA), and present cross-intolerance to various COX-1 inhibitors; 2) selective responses (mediated by immunologic mechanisms) include single NSAIDs-induced urticaria, angioedema and/or anaphylaxis (SNIUAA) and single NSAIDs-induced delayed hypersensitivity reactions (SNIDHR) (Kowalski and Stevenson, 2013). NERD is a major phenotype among cross-intolerant categories of NSAID hypersensitivity and had been called ASA-induced asthma, ASA-intolerant asthma, ASA-sensitive asthma; however, NERD and ASA-exacerbated respiratory disease (AERD) are commonly used (Sánchez-Borges, 2019). The prevalence of NERD is reported to be 5.5% to 12.4% in the general population (Lee et al., 2018a; Chu et al., 2019; Taniguchi et al., 2019), 7.1% among adult asthmatics and 14.9% among severe asthmatics (Rajan et al., 2015), while it rarely occurs in children (Taniguchi et al., 2019). No relationships were found with family history or NSAID administration history (Kowalski et al., 2011; Taniguchi et al., 2019).
NERD is characterized by moderate-to-severe asthma and a higher prevalence of chronic rhinosinusitis (CRS) nasal polyps (NPs) with persistent eosinophilic inflammation in the upper and lower airways (Taniguchi et al., 2019) as well as NSAID hypersensitivity where cysteinyl leukotrienes (CysLTs) over-production and chronic type 2 airway inflammation are key findings (Taniguchi et al., 2019). The diagnosis of NERD is confirmed by ASA challenge (via orally, bronchially or nasally route) and supported by potential biomarkers (Pham et al., 2017; Cingi and Bayar Muluk, 2020). In addition, in vitro cell activation tests and radiological imaging with nasal endoscopy can aid in NERD diagnosis (Taniguchi et al., 2019). This review updates the current knowledge on pathophysiologic mechanisms including molecular genetic mechanisms as well as the diagnosis and treatment of NERD.
Clinical Features
NERD is characterized by chronic type 2 inflammation in the upper and lower airways; therefore, patients suffer from chronic persistent asthmatic symptoms and CRS with/without NPs, which are exacerbated by ASA/NSAID exposure and refractory to conventional medical or surgical treatment. Some patients are accompanied by cutaneous symptoms such as urticaria, angioedema, flushing or gastrointestinal symptoms (Buchheit and Laidlaw, 2016). Previous studies suggested that NERD is more common in females (middle-age onset) and non-atopics (Choi et al., 2015; Trinh et al., 2018). It was reported that rhinitis symptoms appear and then evolve into CRS which worsens asthmatic symptoms, subsequently followed by ASA intolerance (Szczeklik et al., 2000). However, their clinical presentations and courses have been found to be heterogeneous. It has been increasingly required to classify the subphenotypes of NERD according to its clinical features. One study demonstrated 4 subphenotypes by applying a latent class analysis in a Polish cohort: class 1 patients showing moderate asthma with upper airway symptoms and blood eosinophilia; class 2 patients showing mild asthma with low healthcare use; class 3 patients showing severe asthma with severe exacerbation and airway obstruction; and class 4 patients showing poorly controlled asthma with frequent and severe exacerbation (Bochenek et al., 2014). Another study showed 4 subtypes presenting distinct clinical/biochemical findings in a Korean cohort using a 2-step cluster analysis based on 3 clinical phenotypes (urticaria, CRS and atopy status): subtype 1 (NERD with CRS/atopy and no urticaria), subtype 2 (NERD with CRS and no urticaria/atopy), subtype 3 (NERD without CRS/urticaria), and subtype 4 (NERD with acute/chronic urticaria exacerbated by NSAID exposure) (Lee et al., 2017). Each subtype had distinct features in the aspect of female proportion, the degree of eosinophilia, leukotriene (LT) E4 metabolite levels, the frequency of asthma exacerbation, medication requirements (high-dose ICS-LABA or systemic corticosteroids) and asthma severity, suggesting that stratified strategies according to subtype classification may help achieve better clinical outcomes in the management of NERD.
Pathophysiology
The major upper and lower airway symptoms of NERD are mediated by increased levels of CysLTs with dysregulation of arachidonic acid (AA) metabolism and intense type 2/eosinophilic inflammation (Cingi and Bayar Muluk, 2020).
CysLTs Overproduction
In the COX and LOX pathways, AA is metabolized to CysLTs (mostly LTE4, via 5-lipoxygenase [5-LO] and LTC4 synthase [LTC4S]), prostaglandin (PG) pathway (PGE2, PGF2, PGI2 and PGD2) and thromboxanes (TBX) A2 by PG synthase and TBX synthase (Szczeklik, 1990), where enhanced synthesis of CysLTs synthesis with reduced level of PGE2 is a major finding in NERD (Pham et al., 2016; Pham et al., 2017; Lee et al., 2018a; Yin et al., 2020). NERD patients have higher levels of CysLTs (especially LTE4) mainly derived from various inflammatory cells, including neutrophils, monocytes, and basophils, eosinophils and mast cells, which further increases after ASA/NSAID exposure compared to asthmatic patients with ASA/NSAID tolerance (ATA). Moreover, the increased expression of 5-LO and LTC4S was noted in NERD patients with overproduction of CysLTs; increased CysLTs bind to CysLT receptor 1/2, subsequently inducing bronchoconstriction and amplifying inflammatory signal pathways (Jonsson, 1998; Yonetomi et al., 2015; Steinke and Wilson, 2016; Sekioka et al., 2017). Among PGs, PGE2/PGD2 play a major role in the pathogenesis of NERD. Increased PGD2 (released from mast cells and eosinophils) binds to prostanoid receptors to induce bronchoconstriction (Säfholm et al., 2015), and also binds to chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) to induce chemotaxis and activate eosinophils/basophils/Th2 cells/innate lymphoid cells (ILC2) (Hirai et al., 2001; Woessner, 2017), accelerating type 2 airway inflammation (Chang et al., 2014). The down-regulation of PGE2 biosynthesis, especially in peripheral blood leukocytes, nasal epithelial cells and nasal fibroblasts, was noted in patients with NERD (Laidlaw and Boyce, 2013; Cahill et al., 2016; Pham et al., 2017). PGE2 has protective effects against bronchoconstriction, recruitment of eosinophils and degranulation of mast cells after binding to E prostanoid 2 (EP2) receptors (Feng et al., 2006; Sturm et al., 2008); therefore, reduced levels of PGE2 in NERD cannot suppress the signal of 5-LO pathways through IL-10-dependent mechanisms (Harizi et al., 2003). Furthermore, the lower expression of EP2 receptors is closely associated with abnormal regulation of the autocrine loop involved in COX pathways (IL-1R1, COX-2, mPGES) in NERD patients (Cahill et al., 2015; Machado-Carvalho et al., 2016). This can be explained that COX-2 could not sufficiently produce PGH2 (the first unstable precursors of PG products from AA metabolism) without COX-1 (Uematsu et al., 2002). Therefore, reduction in PGE2 and its receptor levels could contribute to CysLTs overproduction in NERD patients. Lipoxin (LX) A4 and its epimer (15-epi-LXA4) are also called as the ASA-triggered lipoxins, and have antiinflammatory effects in airway inflammation (Pham et al., 2017; Sokolowska et al., 2020). Their receptor termed formyl peptide receptor 2 (FPR2) is expressed on human neutrophils, eosinophils, macrophages, T cells, ILCs (ILC2 and NK cells) and epithelial cells of the respiratory tract. After binding their receptors, it leads to the restoration of epithelial barrier function and resolution of allergic inflammation through down-regulation of chemotaxis and cell activation (Barnig et al., 2013; Sokolowska et al., 2020). In the context of NERD, the concentration of LXA4 in the whole blood, sputum and bronchoalveolar lavage fluid, and 15-epi-LXA4 in the urine from NERD patients were lower than those in ATA patients. Additionally, their level has a negative correlation with worsening of airflow obstruction in patients with severe asthma (Christie et al., 1992; Sanak et al., 2000; Kupczyk et al., 2009; Yamaguchi et al., 2011). There was a significant increase in the FPR2 expression of NK cells and ILC2s from patients with severe asthma compared with those with milder asthma (Barnig et al., 2013). All of the studies suggested that LXA4 and its epimer can be considered the potential therapeutics in the treatment of NERD (Figure 1). NSAID-induced inhibition of the COX pathway leads to shunting of AA metabolism down the 5-LO arm (Palikhe et al., 2009; Dominas et al., 2020). This is indirectly evidenced through the decreased level of antiinflammatory PG/LX (LXA4, 15-epi-LXA4, PGE2) and increased levels of the pro-inflammatory CysLTs (Christie et al., 1992; Sanak et al., 2000; Harizi et al., 2003; Kupczyk et al., 2009; Yamaguchi et al., 2011).
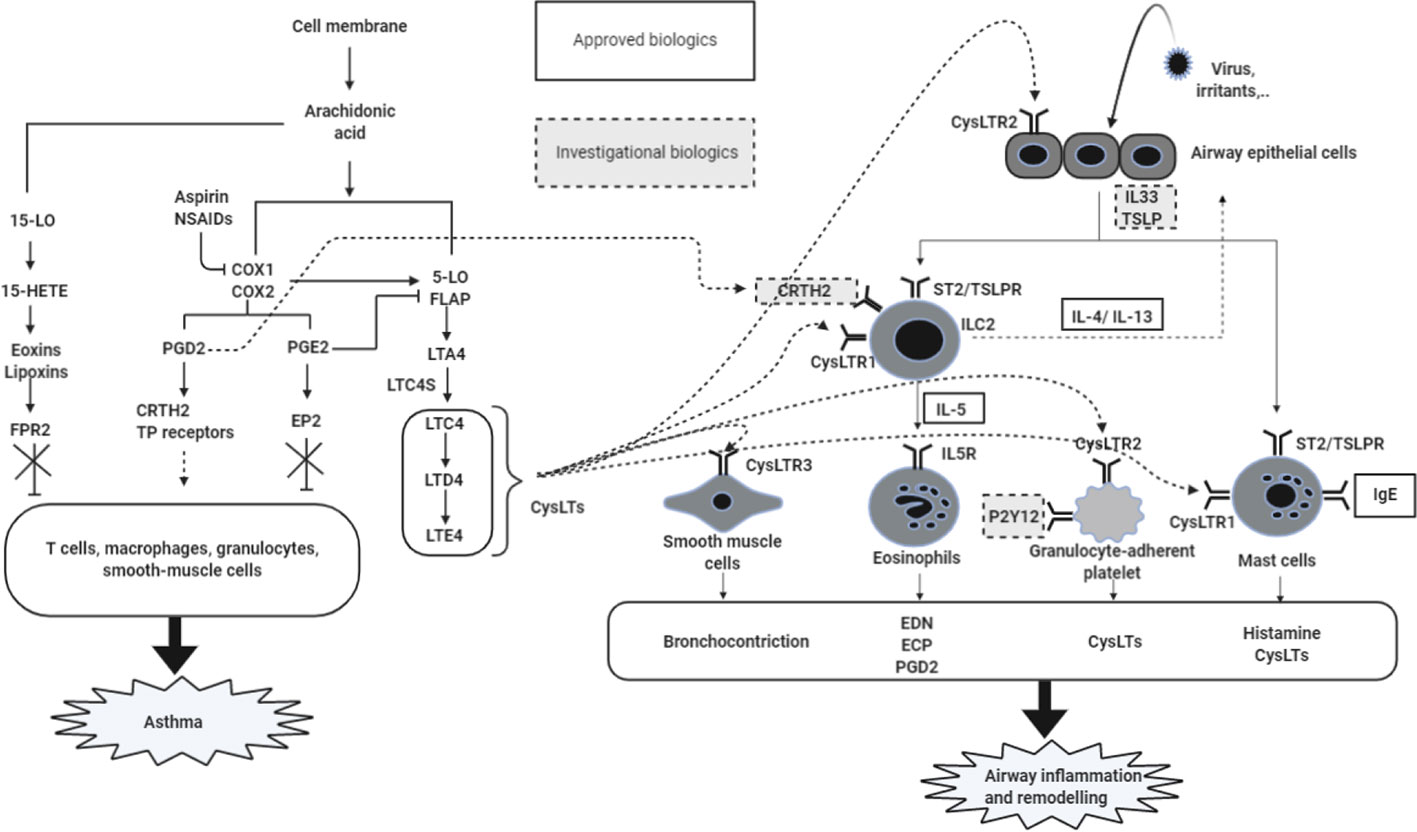
Figure 1 Mechanisms of airway inflammation in NERD. Increased levels of CysLTs and PGD2 as well as a decrease in the PGE2 level caused by the AA metabolism dysregulation are the main mechanism for promoting the severity of NERD. Released CysLTs, PGD2, and PGE2 regulate inflammatory cells via receptors expressed on individual cells (eosinophils, ILC2, mast cells, smooth muscle cells, granulocyte-adherent platelet, and neutrophils). These activated cells release cytokines, histamine, CysLTs, and PGD2, contributing to airway inflammation and remodeling in airway mucosa of NERD patients. 5-LO, 5-lipoxygenase; COX, cyclooxygenase; CysLTs, cysteinyl leukotrienes; PGs, prostaglandins; TBX, thromboxane; LT, leukotrienes; 15-HETE, 15-hydroeicosatetraenoic acid; FPR2, formyl peptide receptor 2; CysLTR, cysteinyl leukotrienes receptors; LTC4S, LTC4 synthase; EP2, E prostanoid 2; CRTH2, chemoattractant receptor-homologous molecule expressed on TH2 cells; TP receptors, T prostanoid receptors; IL, interleukin; TSLP, thymic stromal lymphopoietin; TSLPR, TSLP receptor; ILC2, innate lymphoid type 2 cells; Th2: T helper 2; ECP, eosinophil cationic protein; EDN: eosinophil-derived neurotoxin; IL5R, interleukin 5 receptor.
Enhanced Type 2 Airway Inflammation
NERD is characterized by persistent eosinophil activation (presenting severe asthma, CRS and NPs) and CysLTs overproduction in which increased CysLTs contributes to driving type 2 inflammatory responses (Lee et al., 2018a; Rusznak and Peebles, 2019; Taniguchi et al., 2019). The key inflammatory cells in NERD are eosinophils and mast cells, which are closely interacting with other inflammatory and structural cells including basophils, platelets, neutrophils and epithelial cells. Regarding the activation mechanisms of eosinophils, both Th2 cells and ILC2 could activate eosinophils via release of IL-4, IL-5 and IL-13; moreover, activated eosinophils release the eosinophil extracellular traps (EETs), enhancing type 2 inflammation via interacting with epithelial cells and autocrine functions of eosinophils in the asthmatic airway (Pham et al., 2017; Choi et al., 2019b; Yin et al., 2020). There have been some data demonstrating epithelial dysfunction related to type 2 inflammation in NERD: 1) lower levels of SPD (protective function against eosinophilia) (Choi et al., 2019a), 2) increased epithelial folliculin and periostin levels (Kim M. A. et al., 2014; Trinh et al., 2018; Choi et al., 2019b), 3) increased CysLT-induced signaling (binding to CysLT2R or CysLT3R) in airway epithelial cells to induce the release of pro-inflammatory cytokines including IL-33, TSLP and IL-25 (Corrigan et al., 2005), leading to type 2/eosinophilic inflammation and remodeling in NERD (Ulambayar et al., 2019).
Recent studies suggested that the activation of neutrophils may be related to the severity of airway inflammation in NERD (Kim et al., 2019), although the exact mechanism is still not fully elucidated. Increased LTB4 levels (mostly formed from neutrophils) and reactive oxygen species release after N-formyl-methionyl-leucyl-phenylalanine stimulation were noted in patients with NERD compared to ATA patients (Mita et al., 2004; Kim et al., 2019). In addition, platelets are activated by CysLTR2 on their surfaces to release IL33 and to interact with leukocytes through binding P-selectin (CD62P)–P-selectin glycoprotein ligand 1, GPIIb/IIIa-Mac-1 and CD40 ligand (CD40L)–CD40 (Laidlaw et al., 2012; Mitsui et al., 2016; Liu et al., 2019; Taniguchi et al., 2019). The activation of platelets and adherent leukocytes with platelets leads to the transmigration of leukocytes into inflammatory airway tissue with increased CysLTs, suggesting that platelet-aggregated granulocytes promote severe and persistent airway inflammation in NERD patients (Laidlaw and Boyce, 2013; Laidlaw et al., 2014; Mitsui et al., 2016).
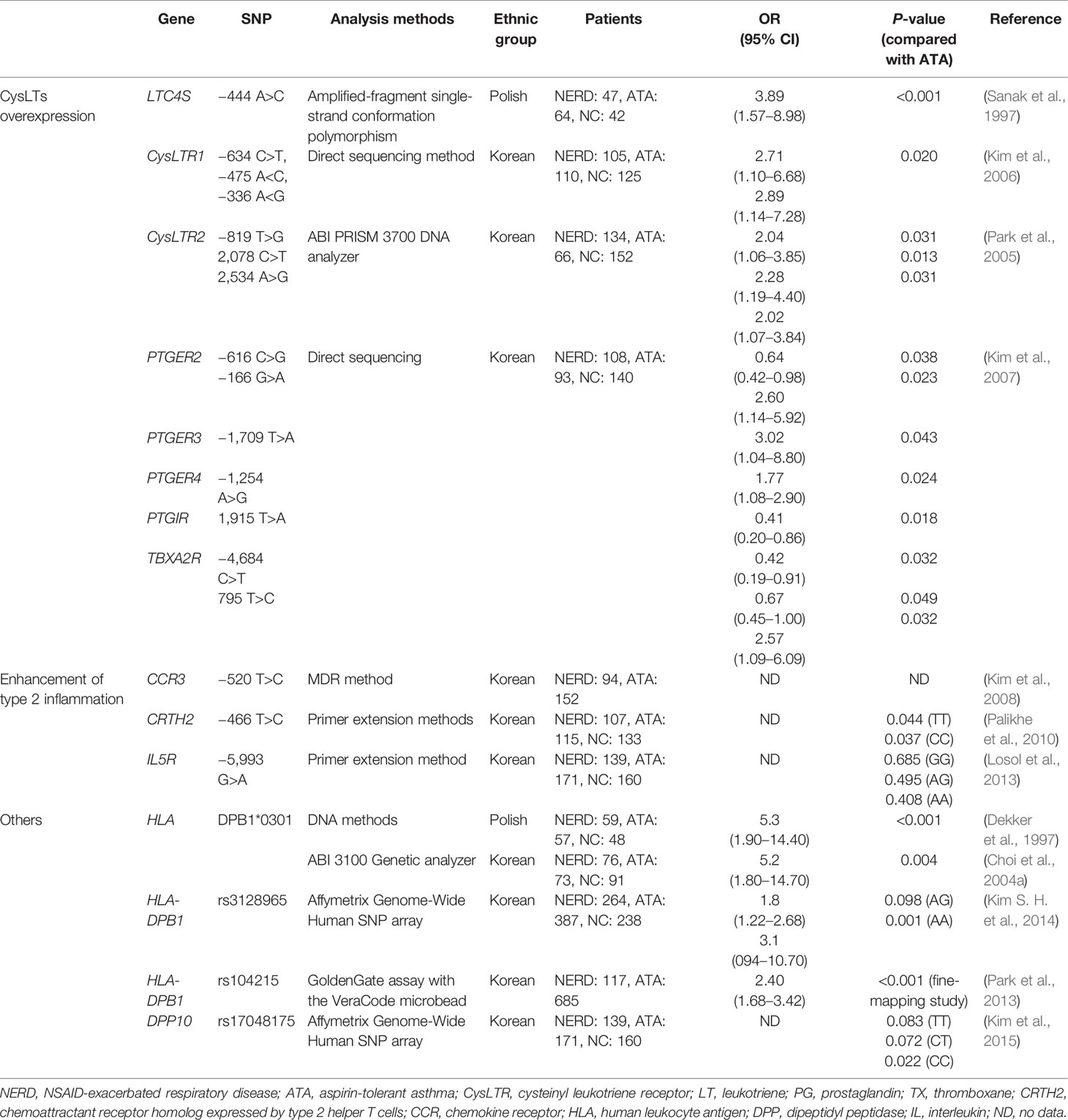
Genetic Mechanisms
Many genetic studies have focused on CysLTs-related and eosinophil activating genes (major pathogenic mechanisms) according to single nucleotide polymorphisms (SNPs) and genome-wide association studies (GWASs) (Pavón-Romero et al., 2017). (Table 1) HLA DPB1*0301 has been regarded as a strong genetic marker and replicated in the 2 ethnic groups Polish and Korean populations (Dekker et al., 1997; Choi et al., 2004a). Patients suffering from this allele manifested the typical clinical characteristics of NERD, and had lower FEV1 levels and a higher prevalence of CRS and/or NPs (Choi et al., 2004a). The GWAS demonstrated several significant SNPs (HLA-DPB1, rs3128965, DPP10 rs17048175 in a Korean population, TSLP rs1837253 in a Japanese population, etc.) which were associated with the phenotypes of NERD (Park et al., 2013; Kim S. H. et al., 2014; Kim et al., 2015). The genetic polymorphism studies identifying the SNPs related to CysLTs synthesis demonstrated several significant SNPs: the promoter polymorphisms at the LTC4S -444 A>C in a Polish population (Sanak et al., 1997), although it was not replicated in the other populations as the US, Japanese and Korean (Van Sambeek et al., 2000; Kawagishi et al., 2002; Choi et al., 2004b). The SNPs of G-coupled receptors (CysLTR1 -634C>T, -475 A>C, -336 A>G, CysLTR2 -819 T>G, 2078 C>T, 2534 A>G) lead to amplify the biological activity of CysLTs, the SNPs of prostanoid receptor genes (PTGER2 -616 C>G, -166 G>A, PTGER3 -1709 T>A, PTGER4 -1254 A>G, PTGIR 1915 T>A, TBXA2R -4684 C>T, 795 T>C) were associated with the development of NERD (Park et al., 2005; Kim et al., 2006; Kim et al., 2007). Regarding the SNPs related to eosinophil activation, including those of the chemokine CC motif receptor (CCR3 −520 T>C), chemoattractant receptor molecular expressed in Th2 cells (CRTH2 −466 T>C) and IL5R (-5993 G>A), were reported (Kim et al., 2008; Palikhe et al., 2010; Losol et al., 2013). Epigenetic factors, including exposure to NSAIDs and other stimuli, be also revealed to contribute to the development of NERD (Pham et al., 2017; Yin et al., 2020); DNA methylation associated with some SNPs (PGE synthesis, PGS, ALOX4AP, LTC4S, etc.) may contribute to presenting more severe phenotypes of NERD (Lee et al., 2019). Further replication studies in diverse ethnic groups are needed to clarify their functional roles in parallel with other omics markers with subphenotype classification.
Diagnosis
A diagnosis of NERD is fundamentally based on the patient’s history. NERD is suspected in patients having a history of upper/lower respiratory reactions after ingestion of ASA/NSAIDs or suffering from asthma along with CRS and NPs, (Choi et al., 2015). Some patients have a definitive history of adverse reactions to ASA/NSAIDs: however, many patients have not experienced hypersensitivity reactions (Palikhe et al., 2009). One study showed that 14% of patients who thought they had NERD based on symptoms were negative for oral aspirin challenge (Dursun et al., 2008). Thus, ASA challenge, as the gold standard for diagnosing NERD, is required to confirm or exclude hypersensitivity in patients with unclear history of adverse reactions.
There are 3 types of the ASA challenge test via the oral, bronchial and nasal routes. The oral challenge test is a more commonly used and convenient approach compared to other challenge tests in that it mimics natural exposure (Adkinson et al., 2013). It may be more suitable for investigating systemic adverse reactions to NSAIDs. Bronchial challenge with lysine-aspirin is safer and quicker, but shows lower sensitivity than the oral test. Nasal challenge is recommended for patients with predominant nasal symptoms, but the sensitivity is lower (Lee et al., 2018a; Kowalski et al., 2019). The EAACI recommended the oral challenge protocol with starting 20-40 mg of aspirin and gradually increasing the dose at 2 hour intervals. When no reactions occur within 3 hours after 325 mg of aspirin, the challenge is considered to be negative (Kowalski et al., 2019). Patients with lower FEV1 (<70% of the predicted value) or unstable asthma status are not recommended, and the test should be performed in a hospital with resuscitative equipment under the supervision of special training physicians (Adkinson et al., 2013). These tests may be influenced by bronchial hypersensitivity, ASA dosage, and the concurrent use of leukotriene modifier drugs and antihistamines (White et al., 2005; White et al., 2006). When patients are false-negative for ASA challenge, subsequent confirmatory challenges are recommended for holding leukotriene modifier drugs, antihistamines and oral corticosteroids for at least 1 week and employing high-dose ASA challenges (White et al., 2013).
There is no in vitro test available for the diagnosis of NERD. LTE4 (especially in urine) is suggested to be the most reliable biomarker for the diagnosis of NERD. Several studies demonstrated that patients with NERD had higher baseline concentrations of urinary LTE4 as well as greater increase after aspirin/NSAID exposure than in patients with ATA, suggesting that urine LTE4 level could be used as a clinical diagnostic test (Hagan et al., 2017; Bochenek et al., 2018). Recent studies demonstrated higher levels of serum periostin, and folliculin as potential biomarkers of NERD, however, further validation studies are needed in other cohorts (Kim M. A. et al., 2014; Trinh et al., 2018). The Polish group proposed the Aspirin-Sensitive Patients Identification Test (ASPI Test), however, it was not replicated in other centers (Kowalski et al., 2005). Despite the basophil activation test (BAT) has been investigated for in vitro diagnosis of NERD, variable values of sensitivity and specificity were reported depending on the protocols used, remaining limitations of the clinical use (Schafer and Maune, 2012). More efforts are needed to establish in vitro diagnostic tests for reducing the risks of challenge tests with identifying reliable biomarkers for the diagnosis of NERD and the classification of its subphenotypes.
Management
The standard management of NERD involves the guidelines established for the management of asthma and CRS with ASA/NSAID avoidance. The complete avoidance of culprit agents and cross-reacting NSAIDs with use of alternative agents (highly selective COX-2 inhibitors such as celecoxib, and partial inhibitors such as acetaminophen, meloxicam or nimesulide) is essential. ASA desensitization can be beneficial for NERD patients when indicated.
Pharmacologic Treatment
Treatment strategies for asthma should follow stepwise management guidelines with maintaining inhaled corticosteroids with or without long-acting beta 2 agonists, leukotriene modifier drugs and/or biologic agents on the basis of disease severity and rescue medications (GINA-guideline, 2020). Because the overproduction of CysLTs is a key feature in the pathogenic mechanisms, targeting the leukotriene pathway with CysLT1 receptor antagonists (montelukast, zafirlukast and pranlukast) and 5-LO inhibitors (zileuton) should be considered to improve upper and lower airway symptoms. Several studies have shown that these leukotriene modifiers lead to improvement in asthma symptoms, pulmonary function, quality of life, nasal function and lower use of bronchodilators (Rodriguez-Jimenez et al., 2018).
Initial treatment for CRS includes intranasal corticosteroids with intranasal saline irrigation. Intranasal corticosteroids have shown to be highly effective in reducing nasal inflammation and in shrinking NPs, which are recommended as a first-line treatment in patients with CRSwNP (Choi et al., 2015; Simon et al., 2015; Rodriguez-Jimenez et al., 2018). Because rinsing the nasal cavities with saline is helpful in removing secretions and washing away allergens and irritants, nasal irrigation prior to administration of topical medications can improve the response to the medications (Simon et al., 2015; Rodriguez-Jimenez et al., 2018). Systemic corticosteroids and broad-spectrum antibiotics can be additionally required according to the severity of nasal symptoms. Adding antihistamines or oral/nasal decongestants may provide symptom relief (Adkinson et al., 2013).
Despite the heterogeneity of NERD, therapeutic approaches have been proposed according to symptom severity. However, these different phenotypes contribute to the variability in response to treatment. A recent study found that clinical severity and courses differ among the 4 subtypes of NERD, which affect antiasthmatic medications required (Lee et al., 2017). Subtype 1/2 patients had severe clinical courses, requiring higher-dose of antiasthmatic medications including higher dose of ICS and systemic corticosteroids, while subtype 3 patients required low doses of these drugs with less frequent asthma exacerbation. These results suggest that a personalized approach according to subtype classification is needed to achieve better outcomes in the management of NERD.
ASA Desensitization
ASA desensitization is an effective treatment option when standard medical treatments are not effective or daily ASA/NSAIDs therapy is required for other medical conditions, such as coronary artery disease or chronic inflammatory disease (Stevenson and Simon, 2006). Multiple studies have demonstrated the effectiveness of ASA desensitization in reducing NP size and the need for sinus surgery as well as in improving nasal and bronchial symptoms with decrease in the doses of topical and oral corticosteroids (Swierczynska-Krepa et al., 2014; Waldram et al., 2018). A recent study showed the long-term safety and efficacy of ASA desensitization in patients who underwent continuous daily ASA therapy for more than 10 years (Walters et al., 2018). ASA desensitization is a provocative procedure by starting at low doses of ASA and gradually increasing to the dose of 650 to 1300 mg over a period of 1 to 3 days, which can induce hypersensitivity reactions (White and Stevenson, 2018). Thus, as safety is an important issue, ASA desensitization should be carried out in a well-equipped hospital under the supervision of special training physicians. The protocol with gradually increasing the dose over 2 days was suggested by the EAACI to secure safety and efficacy of aspirin desensitization (Kowalski et al., 2019).
Biologics
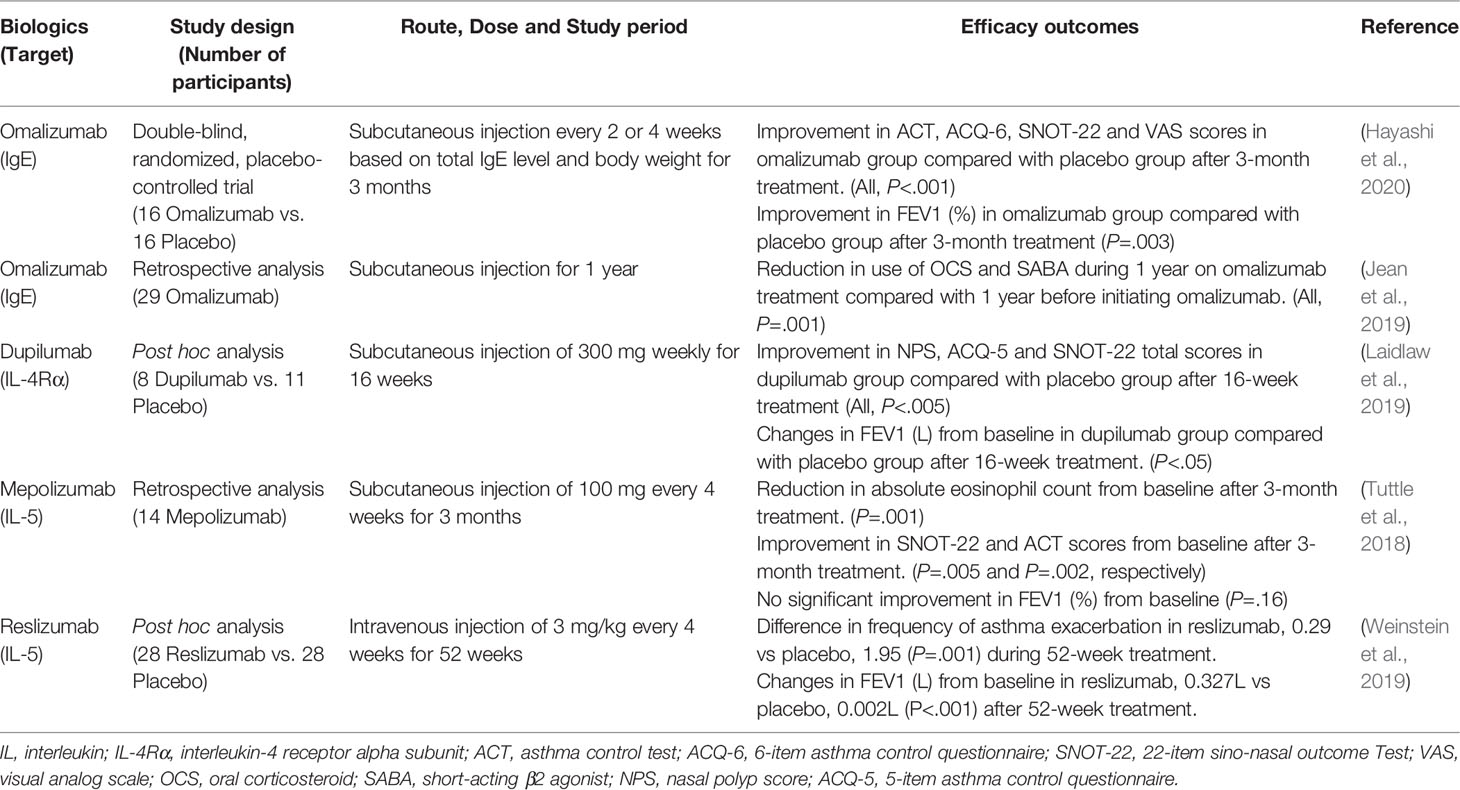
The emergence of biologics in the management of asthma and CRSwNP has represented potential and promising therapy for NERD. New biologics targeting type 2 cytokines, such as IL-4, IL-5 and IL-13 as well as IgE, have been reported in clinical trials, which could reduce asthma exacerbation and oral corticosteroid use, and improve lung function (Kim and Jee, 2018; McGregor et al., 2019). In addition, they have been shown to improve nasal symptom severity and reduce NP size in patients with CRSwNP, leading to a significant increase in quality of life (Bachert et al., 2020). Because NERD is strongly associated with mast cell activation and eosinophilic airway inflammation, the efficacy of biologics may be different from those usually observed in severe asthma (Hayashi et al., 2016). Here, we summarized the available studies for these biologics in patients with NERD (Table 2).
Omalizumab, a humanized recombinant monoclonal anti-IgE antibody, prevents IgE from binding to its high-affinity receptor and reduces Fc receptor expression on mast cells and basophils, subsequently suppressing their activation (Chang et al., 2015). Several studies have suggested the efficacy of omalizumab in the management of NERD, demonstrating a reduction in asthma exacerbation and the need for systemic steroids and short acting beta-2 agonist (SABA) as well as an improvement in upper and lower airway symptoms (Hayashi et al., 2016; (Lee et al., 2018b; Jean et al., 2019). Furthermore, there are some studies suggesting that omalizumab treatment can be beneficial for reducing respiratory symptoms during ASA desensitization and even can restore ASA tolerance without the need for ASA desensitization (Phillips-Angles et al., 2017; Lang et al., 2018; Hayashi et al., 2020). Omalizumab could improve upper and lower airway symptoms with suppression in urinary markers of mast cell activation, LTE4 and PGD2 metabolites, in patients with NERD and lead to the development of ASA tolerance with a reduction in urinary LTE4 concentrations during oral ASA challenge (Hayashi et al., 2016; Hayashi et al., 2020), suggesting that omalizumab has inhibitory effects on mast cell activation in NERD.
Dupilumab is a human monoclonal antibody that targets the IL-4α receptor and inhibits signaling of both IL-4 and IL-13. Although the study was conducted in a small number of patients with NERD, dupilumab could improve nasal and asthma-related symptom scores and lung functions (Laidlaw et al., 2019), although studies with a larger sample size are needed to confirm its effectiveness. Mepolizumab and reslizumab are both monoclonal antibodies that prevent IL-5 from binding to its receptor on eosinophils, and benralizumab is a monoclonal antibody that targets the alpha subunit of the IL-5 receptor. The respiratory tract of NERD patients is characterized by intense eosinophilic inflammation, with higher levels of eosinophils in NPs and bronchial mucosa biopsies than in ATA patients (Tuttle et al., 2018; Eid et al., 2020). These biologics inhibiting IL-5, eosinophilic maturation and differentiation factor could be effective in the management of patients with NERD (Choi et al., 2004b). In addition, based on recent study results on the pathogenic mechanisms, P2Y12 receptor antagonists, CRTH2 antagonists and anti-TSLP/IL-33 antibodies could be potential options in the management of NERD patients (Rodriguez-Jimenez et al., 2018).
Considering the heterogeneity of NERD phenotypes/endotypes, selecting right patients and right targets (biologics) are essential in the management of NERD. In phenotypic clusters of NERD, subtype 4 patients (NERD with urticaria) would need omalizumab as an effective option, which can inhibit activated basophils and mast cells, the key elements of NERD and urticaria (Lee et al., 2017); subtype 2 patients with severe eosinophilia may need anti-IL-5 as a first option. Despite the development of biologic therapies, unmet needs remain in NERD patients to be understood with regard to their comparative efficacy and long-term safety. Further studies are needed to answer questions on the selection of right patients and targets with right safety.
Dietary Interventions
Dietary intervention may be beneficial for controlling symptoms in patients with NERD. Some studies demonstrated that restricting dietary salicylates, including fruits, vegetables, berries, herbs, and spices, improves nasal and asthmatic symptoms, which can be explained by the known contribution of salicylates in the pathogenesis (Ta and White, 2015; Sommer et al., 2016). A previous study showed that alcohol ingestion can more commonly lead to upper and lower respiratory reactions in NERD patients, although the underlying mechanism is not clear (Cardet et al., 2014). Thus, restricting the diet, when experienced respiratory symptoms after the ingestion, can be additionally effective.
Conclusion
Patients with NERD present with a variety of clinical features affected by chronic type 2 eosinophilic inflammation with the overproduction of CysLTs in the upper and lower airways. Although NERD tend to be associated with severe asthma and CRSwNP, an improved understanding of clinical features and underlying pathogenesis of NERD will aid in diagnostic evaluations and new therapeutic strategies for improving clinical outcomes. With the increasing recognition of phenotypic heterogeneity of NERD, efforts are needed to establish precision medicine strategies tailored to individual phenotypes/endotypes with potential biomarkers.
Author Contributions
The clinical features, diagnosis and treatment of NERD were described by S-DW and the pathophysiologic mechanisms including molecular genetic mechanisms were described by QL. This article was written under supervision of H-SP. She, as corresponding author, performed the overall design and review of this article.
Funding
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (H116C0992).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adkinson, N.F., Jr, Bochner, B. S., Burks, A. W., Busse, W. W., Holgate, S. T., Lemanske, R. F., et al. (2013). Middleton"s allergy E-Book: Principles and practice (Elsevier Health Sciences).
Bachert, C., Zhang, N., Cavaliere, C., Weiping, W., Gevaert, E., Krysko, O. (2020). Biologics for chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 145, 725–739. doi: 10.1016/j.jaci.2020.01.020
Barnig, C., Cernadas, M., Dutile, S., Liu, X., Perrella, M. A., Kazani, S., et al. (2013). Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Trans. Med. 5 (174), 174ra26–174ra26. doi: 10.1126/scitranslmed.3004812
Blanca-Lopez, N., Somoza-Alvarez, M. L., Bellon, T., Amo, G., Canto, G., Blanca, M. (2018). NSAIDs hypersensitivity: Questions not resolved. Curr. Opin. Allergy Clin. Immunol. 18, 291–301. doi: 10.1097/ACI.0000000000000454
Bochenek, G., Kuschill-Dziurda, J., Szafraniec, K., Plutecka, H., Szczeklik, A., Nizankowska-Mogilnicka, E. (2014). Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J. Allergy Clin. Immunol. 133, 98–103.e101-106. doi: 10.1016/j.jaci.2013.07.004
Bochenek, G., Stachura, T., Szafraniec, K., Plutecka, H., Sanak, M., Nizankowska-Mogilnicka, E., et al. (2018). Diagnostic Accuracy of Urinary LTE4 Measurement to Predict Aspirin-Exacerbated Respiratory Disease in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 6, 528–535. doi: 10.1016/j.jaip.2017.07.001
Buchheit, K. M., Laidlaw, T. M. (2016). Update on the Management of Aspirin-Exacerbated Respiratory Disease. Allergy Asthma Immunol. Res. 8, 298–304. doi: 10.4168/aair.2016.8.4.298
Cahill, K. N., Bensko, J. C., Boyce, J. A., Laidlaw, T. M. (2015). Prostaglandin D2: A dominant mediator of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 135 (1), 245–252. doi: 10.1016/j.jaci.2014.07.031
Cahill, K. N., Raby, B. A., Zhou, X., Guo, F., Thibault, D., Baccarelli, A., et al. (2016). Impaired E prostanoid2 expression and resistance to prostaglandin E2 in nasal polyp Fibroblasts from subjects with aspirin-exacerbated respiratory disease. Am. J. Respiratory Cell Mol. Biol. 54 (1), 34–40. doi: 10.1165/rcmb.2014-0486OC
Cardet, J. C., White, A. A., Barrett, N. A., Feldweg, A. M., Wickner, P. G., Savage, J., et al. (2014). Alcohol-induced respiratory symptoms are common in patients with aspirin exacerbated respiratory disease. J. Allergy Clin. Immunol. Pract. 2, 208–213. doi: 10.1016/j.jaip.2013.12.003
Chang, J. E., Doherty, T. A., Baum, R., Broide, D. (2014). Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J. Allergy Clin. Immunol. 133 (3), 899–901.e3. doi: 10.1016/j.jaci.2013.09.020
Chang, T. W., Chen, C., Lin, C. J., Metz, M., Church, M. K., Maurer, M. (2015). The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 135, 337–342. doi: 10.1016/j.jaci.2014.04.036
Choi, J. H., Lee, K. W., Oh, H. B., Lee, K. J., Suh, Y. J., Park, C. S., et al. (2004a). HLA association in aspirin-intolerant asthma: DPB1*0301 as a strong marker in a Korean population. J. Allergy Clin. Immunol. 113, 562–564. doi: 10.1016/j.jaci.2003.12.012
Choi, J. H., Park, H. S., Oh, H. B., Lee, J. H., Suh, Y. J., Park, C. S., et al. (2004b). Leukotriene-related gene polymorphisms in ASA-intolerant asthma: An association with a haplotype of 5-lipoxygenase. Hum. Genet. 114 (4), 337–344. doi: 10.1007/s00439-004-1082-1
Choi, J. H., Kim, J. H., Park, H. S. (2015). Upper airways in aspirin-exacerbated respiratory disease. Curr. Opin. Allergy Clin. Immunol. 15, 21–26. doi: 10.1097/aci.0000000000000122
Choi, Y., Lee, D. H., Trinh, H. K. T., Ban, G. Y., Park, H. K., Shin, Y. S., et al. (2019a). Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy: Eur. J. Allergy Clin. Immunol. 74 (1), 78–88. doi: 10.1111/all.13458
Choi, Y., Lee, Y., Park, H. S. (2019b). Which factors associated with activated eosinophils contribute to the pathogenesis of aspirin-exacerbated respiratory disease? Allergy Asthma Immunol. Res. 11, 320–329. doi: 10.4168/aair.2019.11.3.320
Christie, P. E., Spur, B. W., Lee, T. H. (1992). The effects of lipoxin A4 on airway responses in asthmatic subjects. Am. Rev. Respiratory Dis. 145 (6), 1281–1284. doi: 10.1164/ajrccm/145.6.1281
Chu, D. K., Lee, D. J., Lee, K. M., Schünemann, H. J., Szczeklik, W., Lee, J. M. (2019). Benefits and harms of aspirin desensitization for aspirin-exacerbated respiratory disease: a systematic review and meta-analysis. Int. Forum Allergy Rhinol. 9, 1409–1419. doi: 10.1002/alr.22428
Cingi, C., Bayar Muluk, N. (2020). All Around the Nose E-Book Basic Science, Diseases and Surgical Management. (Switzerland: Springer) doi: 10.1007/978-3-030-21217-9
Corrigan, C., Mallett, K., Ying, S., Roberts, D., Parikh, A., Scadding, G., et al. (2005). Expression of the cysteinyl leukotriene receptors cysLT1 and cysLT2 in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J. Allergy Clin. Immunol. 115 (2), 316–322. doi: 10.1016/j.jaci.2004.10.051
Dekker, J. W., Nizankowska, E., Schmitz-Schumann, M., Pile, K., Bochenek, G., Dyczek, A., et al. (1997). Aspirin-induced asthma and HLA-DRB1 and HLA-DPB1 genotypes. Clin. Exp. Allergy 27 (5), 574–577. doi: 10.1111/j.1365-2222.1997.tb00747.x
Dominas, C., Gadkaree, S., Maxfield, A. Z., Gray, S. T., Bergmark, R. W. (2020). Aspirin-exacerbated respiratory disease: A review. Laryngoscope Invest. Otolaryngol. 5, 360–367. doi: 10.1002/lio2.387
Doña, I., Blanca-López, N., Torres, M. J., García-Campos, J., García-Núñez, I., Gómez, F., et al. (2012). Drug hypersensitivity reactions: Response patterns, drug involved, and temporal variations in a large series of patients. J. Investigat. Allergol. Clin. Immunol. 22 (5), 363–371.
Dursun, A. B., Woessner, K. A., Simon, R. A., Karasoy, D., Stevenson, D. D. (2008). Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann. Allergy Asthma Immunol. 100, 420–425. doi: 10.1016/s1081-1206(10)60465-6
Eid, R. C., Wudneh, E., Zahid, S., Cahill, K., Jerschow, E. (2020). Poor control of asthma symptoms with interleukin-5 inhibitors in four patients with aspirin-exacerbated respiratory disease. Ann. Allergy Asthma Immunol. 124, 102–104. doi: 10.1016/j.anai.2019.09.023
Feng, C., Beller, E. M., Bagga, S., Boyce, J. A. (2006). Human mast cells express multiple EP receptors for prostaglandin E 2 that differentially modulate activation responses. Blood 107 (8), 3243–3250. doi: 10.1182/blood-2005-07-2772
GINA-guideline (2020). Global Initiative for Asthma. Global strategy for asthma management and prevention 2020 (Global Initiative for Asthma.; 2020). [cited 2020 Apr]. Available from: http://www.ginasthma.org
Hagan, J. B., Laidlaw, T. M., Divekar, R., O’Brien, E. K., Kita, H., Volcheck, G. W., et al. (2017). Urinary Leukotriene E4 to Determine Aspirin Intolerance in Asthma: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 5, 990–997.e991. doi: 10.1016/j.jaip.2016.11.004
Harizi, H., Juzan, M., Moreau, J.-F., Gualde, N. (2003). Prostaglandins Inhibit 5-Lipoxygenase-Activating Protein Expression and Leukotriene B 4 Production from Dendritic Cells Via an IL-10-Dependent Mechanism. J. Immunol. 170 (1), 139–146. doi: 10.4049/jimmunol.170.1.139
Hayashi, H., Mitsui, C., Nakatani, E., Fukutomi, Y., Kajiwara, K., Watai, K., et al. (2016). Omalizumab reduces cysteinyl leukotriene and 9alpha,11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 137, 1585–1587.e1584. doi: 10.1016/j.jaci.2015.09.034
Hayashi, H., Fukutomi, Y., Mitsui, C., Kajiwara, K., Watai, K., Kamide, Y., et al. (2020). Omalizumab for Aspirin-Hypersensitivity and Leukotriene Overproduction in Aspirin-Exacerbated Respiratory Disease: A Randomized Trial. Am. J. Respiratory Crit. Care Med. 137 (5), 1585–1587. doi: 10.1164/rccm.201906-1215OC
Hirai, H., Tanaka, K., Yoshie, O., Ogawa, K., Kenmotsu, K., Takamori, Y., et al. (2001). Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193 (2), 255–262. doi: 10.1084/jem.193.2.255
Jean, T., Eng, V., Sheikh, J., Kaplan, M. S., Goldberg, B., Jau Yang, S., et al. (2019). Effect of omalizumab on outcomes in patients with aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 40, 316–320. doi: 10.2500/aap.2019.40.4241
Jonsson, E. W. (1998). Functional characterisation of receptors for cysteinyl leukotrienes in smooth muscle. Acta Physiol. Scand. Suppl. 641, 1–55. doi: 10.1006/pupt.1997.0075
Kawagishi, Y., Mita, H., Taniguchi, M., Maruyama, M., Oosaki, R., Higashi, N., et al. (2002). Leukotriene C4 synthase promoter polymorphism in Japanese patients with aspirin-induced asthma. J. Allergy Clin. Immunol. 109 (6), 936–942. doi: 10.1067/mai.2002.124466
Kim, D. H., Jee, Y. K. (2018). Is Omalizumab a Problem-Solving Remedy in Severe Asthma? Allergy Asthma Immunol. Res. 10, 95–96. doi: 10.4168/aair.2018.10.2.95
Kim, S.-H., Oh, J.-M., Kim, Y.-S., Palmer, L. J., Suh, C.-H., Nahm, D.-H., et al. (2006). Cysteinyl leukotriene receptor 1 promoter polymorphism is associated with aspirin-intolerant asthma in males. Clin. Exp. Allergy 36, 433–439. doi: 10.1111/j.1365-2222.2006.02457.x
Kim, S.-H., Kim, Y.-K., Park, H.-W., Jee, Y.-K., Kim, S.-H., Bahn, J.-W., et al. (2007). Association between polymorphisms in prostanoid receptor genes and aspirin-intolerant asthma. Pharmacogenet. Genomics 17, 295–304. doi: 10.1097/01.fpc.0000239977.61841.fe
Kim, S.-H., Jeong, H.-H., Cho, B.-Y., Kim, M., Lee, H.-Y., Lee, J., et al. (2008). Association of Four-locus Gene Interaction with Aspirin-intolerant Asthma in Korean Asthmatics. J. Clin. Immunol. 28, 336–342. doi: 10.1007/s10875-008-9190-7
Kim, M. A., Izuhara, K., Ohta, S., Ono, J., Yoon, M. K., Ban, G. Y., et al. (2014). Association of serum periostin with aspirin-exacerbated respiratory disease. Ann. Allergy Asthma Immunol. 113 (3), 314–320. doi: 10.1016/j.anai.2014.06.014
Kim, S. H., Cho, B. Y., Choi, H., Shin, E. S., Ye, Y. M., Lee, J. E., et al. (2014). The SNP rs3128965 of HLA-DPB1 as a genetic marker of the AERD phenotype. PLoS One. 9 (12), e111220. doi: 10.1371/journal.pone.0111220
Kim, S. H., Choi, H., Yoon, M. G., Ye, Y. M., Park, H. S. (2015). Dipeptidyl-peptidase 10 as a genetic biomarker for the aspirin-exacerbated respiratory disease phenotype. Ann. Allergy Asthma Immunol. 114 (3), 208–213. doi: 10.1016/j.anai.2014.12.003
Kim, S. H., Uuganbayar, U., Trinh, H. K. T., Le Pham, D., Kim, N., Kim, M., et al. (2019). Evaluation of neutrophil activation status according to the phenotypes of adult asthma. Allergy Asthma Immunol. Res. 11 (3), 381–393. doi: 10.4168/aair.2019.11.3.381
Kowalski, M. L., Stevenson, D. D. (2013). Classification of Reactions to Nonsteroidal Antiinflammatory Drugs. Immunol. Allergy Clin. North Am. 33, 135–145. doi: 10.1016/j.iac.2012.10.008
Kowalski, M. L., Ptasinska, A., Jedrzejczak, M., Bienkiewicz, B., Cieslak, M., Grzegorczyk, J., et al. (2005). Aspirin-triggered 15-HETE generation in peripheral blood leukocytes is a specific and sensitive Aspirin-Sensitive Patients Identification Test (ASPITest). Allergy 60, 1139–1145. doi: 10.1111/j.1398-9995.2005.00836.x
Kowalski, M. L., Makowska, J. S., Blanca, M., Bavbek, S., Bochenek, G., Bousquet, J., et al. (2011). Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - Classification, diagnosis and management: Review of the EAACI/ENDA and GA2LEN/HANNA. Allergy 66, 818–829. doi: 10.1111/j.1398-9995.2011.02557.x
Kowalski, M. L., Agache, I., Bavbek, S., Bakirtas, A., Blanca, M., Bochenek, G., et al. (2019). Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy 74, 28–39. doi: 10.1111/all.13599
Kupczyk, M., Antczak, A., Kuprys-Lipinska, I., Kuna, P. (2009). Lipoxin A4 generation is decreased in aspirin-sensitive patients in lysine-aspirin nasal challenge in vivo model. Allergy: Eur. J. Allergy Clin. Immunol. 64 (12), 1746–175. doi: 10.1111/j.1398-9995.2009.02047.x
Laidlaw, T. M., Boyce, J. A. (2013). Pathogenesis of Aspirin-Exacerbated Respiratory Disease and Reactions. Immunol. Allergy Clin. North Am. 33, 195–210. doi: 10.1016/j.iac.2012.11.006
Laidlaw, T. M., Kidder, M. S., Bhattacharyya, N., Xing, W., Shen, S., Milne, G. L., et al. (2012). Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 119 (16), 3790–3798. doi: 10.1182/blood-2011-10-384826
Laidlaw, T. M., Cutler, A. J., Kidder, M. S., Liu, T., Cardet, J. C., Chhay, H., et al. (2014). Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 133 (6), 1692–1701.e3. doi: 10.1016/j.jaci.2013.12.1034
Laidlaw, T. M., Mullol, J., Fan, C., Zhang, D., Amin, N., Khan, A., et al. (2019). Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J. Allergy Clin. Immunol. Pract. 7, 2462–2465.e2461. doi: 10.1016/j.jaip.2019.03.044
Lang, D. M., Aronica, M. A., Maierson, E. S., Wang, X. F., Vasas, D. C., Hazen, S. L. (2018). Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann. Allergy Asthma Immunol. 121, 98–104. doi: 10.1016/j.anai.2018.05.007
Lee, H. Y., Ye, Y. M., Kim, S. H., Ban, G. Y., Kim, S. C., Kim, J. H., et al. (2017). Identification of phenotypic clusters of nonsteroidal anti-inflammatory drugs exacerbated respiratory disease. Allergy 72, 616–626. doi: 10.1111/all.13075
Lee, J. H., Jung, C. G., Park, H. S. (2018a). An update on the management of aspirin-exacerbated respiratory disease. Expert Rev. Respiratory Med. 12, 137–143. doi: 10.1080/17476348.2018.1417843
Lee, J. H., Lee, H. Y., Jung, C. G., Ban, G. Y., Shin, Y. S., Ye, Y. M., et al. (2018b). Therapeutic Effect of Omalizumab in Severe Asthma: A Real-World Study in Korea. Allergy Asthma Immunol. Res. 10, 121–130. doi: 10.4168/aair.2018.10.2.121
Lee, J. U., Park, J. S., Chang, H. S., Park, C. S. (2019). Complementary participation of genetics and epigenetics in development of nsaid-exacerbated respiratory disease. Allergy Asthma Immunol. Res. 11, 779–794. doi: 10.4168/aair.2019.11.6.779
Liu, T., Barrett, N. A., Kanaoka, Y., Laidlaw, T. M., Yoshimoto, E., Garofalo, D., et al. (2019). Cysteinyl Leukotriene Receptor 2 Drives IL-33-Mediated Aspirin Sensitivity Through A Platelet Dependent Mechanism. J. Allergy Clin. Immunol. 143 (2), AB293. doi: 10.1016/j.jaci.2018.12.895
Losol, P., Kim, S.-H., Seob Shin, Y., Min Ye, Y., Park, H.-S. (2013). A genetic effect of IL-5 receptor α polymorphism in patients with aspirin-exacerbated respiratory disease. Exp. Mol. Med. 45, e14–e14. doi: 10.1038/emm.2013.24
Machado-Carvalho, L., Martín, M., Torres, R., Gabasa, M., Alobid, I., Mullol, J., et al. (2016). Low E-prostanoid 2 receptor levels and deficient induction of the IL-1β/IL-1 type i receptor/COX-2 pathway: Vicious circle in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 137 (1), 99–107.e7. doi: 10.1016/j.jaci.2015.09.028
McGregor, M. C., Krings, J. G., Nair, P., Castro, M. (2019). Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 199, 433–445. doi: 10.1164/rccm.201810-1944CI
Mita, H., Higashi, N., Taniguchi, M., Higashi, A., Akiyama, K. (2004). Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin. Exp. Allergy 34 (8), 1262–1269. doi: 10.1111/j.1365-2222.2004.02034.x
Mitsui, C., Kajiwara, K., Hayashi, H., Ito, J., Mita, H., Ono, E., et al. (2016). Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 137 (2), 400–411. doi: 10.1016/j.jaci.2015.05.041
Palikhe, N. S., Kim, J. H., Park, H. S. (2009). Update on recent advances in the management of aspirin exacerbated respiratory disease. Yonsei Med. J. 50, 744–750. doi: 10.3349/ymj.2009.50.6.744
Palikhe, N. S., Kim, S. H., Cho, B. Y., Ye, Y. M., Choi, G. S., Park, H. S. (2010). Genetic variability in CRTH2 polymorphism increases eotaxin-2 levels in patients with aspirin exacerbated respiratory disease. Allergy 65, 338–346. doi: 10.1111/j.1398-9995.2009.02158.x
Park, J. S., Chang, H. S., Park, C.-S., Lee, J.-H., Lee, Y. M., Choi, J. H., et al. (2005). Association analysis of cysteinyl-leukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet. Genomics 15, 483–492. doi: 10.1097/01.fpc.0000166456.84905.a0
Park, B. L., Kim, T. H., Kim, J. H., Bae, J. S., Pasaje, C. F. A., Cheong, H. S., et al. (2013). Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum. Genet. 132 (3), 313–321. doi: 10.1007/s00439-012-1247-2
Pavón-Romero, G. F., Ramírez-Jiménez, F., Roldán-Alvarez, M. A., Terán, L. M., Falfán-Valencia, R. (2017). Physiopathology and genetics in aspirin-exacerbated respiratory disease. Allergol. Int. 69, 138–140. doi: 10.1080/01902148.2017.1358776
Pham, D. L., Kim, J. H., Trinh, T. H. K., Park, H. S. (2016). What we know about nonsteroidal anti-inflammatory drug hypersensitivity. Korean J. Intern Med. 31, 417–432. doi: 10.3904/kjim.2016.085
Pham, D. L., Lee, J. H., Park, H. S. (2017). Aspirin-exacerbated respiratory disease: An update. Curr. Opin. Pulmonary Med. 23, 89–96. doi: 10.1097/MCP.0000000000000328
Phillips-Angles, E., Barranco, P., Lluch-Bernal, M., Dominguez-Ortega, J., Lopez-Carrasco, V., Quirce, S. (2017). Aspirin tolerance in patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease following treatment with omalizumab. J. Allergy Clin. Immunol. Pract. 5, 842–845. doi: 10.1016/j.jaip.2016.12.013
Rajan, J. P., Wineinger, N. E., Stevenson, D. D., White, A. A. (2015). Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J. Allergy Clin. Immunol. 135 (3), 676–681.e1. doi: 10.1016/j.jaci.2014.08.020
Rodriguez-Jimenez, J. C., Moreno-Paz, F. J., Teran, L. M., Guani-Guerra, E. (2018). Aspirin exacerbated respiratory disease: Current topics and trends. Respir. Med. 135, 62–75. doi: 10.1016/j.rmed.2018.01.002
Rusznak, M., Peebles, R. S. (2019). Prostaglandin E2 in NSAID-exacerbated respiratory disease: protection against cysteinyl leukotrienes and group 2 innate lymphoid cells. Curr. Opin. Allergy Clin. Immunol. 19, 38–45. doi: 10.1097/ACI.0000000000000498
Säfholm, J., Manson, M. L., Bood, J., Delin, I., Orre, A. C., Bergman, P., et al. (2015). Prostaglandin E2 inhibits mast cell–dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J. Allergy Clin. Immunol. 136 (5), 1232–1239.e1. doi: 10.1016/j.jaci.2015.04.002
Sanak, M., Simon, H. U., Szczeklik, A. (1997). Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet 350(9091), 1599–1600. doi: 10.1016/S0140-6736(05)64015-9
Sanak, M., Levy, B. D., Clish, C. B., Chiang, N., Gronert, K., Mastalerz, L., et al. (2000). Aspirin-tolerant asthmatics generate more lipoxins than aspirin- intolerant asthmatics. Eur. Respiratory J. 16 (1), 44–49. doi: 10.1034/j.1399-3003.2000.16a08.x
Sánchez-Borges, M. A. (2019). Aspirin or Nonsteroidal Drug-Exacerbated Respiratory Disease (AERD or NERD). Allergy Asthma 1–14. doi: 10.1007/978-3-319-58726-4_15-1
Schafer, D., Maune, S. (2012). Pathogenic Mechanisms and In Vitro Diagnosis of AERD. J. Allergy (Cairo) 2012:789232. doi: 10.1155/2012/789232
Sekioka, T., Kadode, M., Yonetomi, Y., Kamiya, A., Fujita, M., Nabe, T., et al. (2017). CysLT2receptor activation is involved in LTC4-induced lung air-trapping in guinea pigs. Eur. J. Pharmacol 794, 147–153. doi: 10.1016/j.ejphar.2016.11.036
Simon, R. A., Dazy, K. M., Waldram, J. D. (2015). Aspirin-exacerbated respiratory disease: characteristics and management strategies. Expert Rev. Clin. Immunol. 11, 805–817. doi: 10.1586/1744666x.2015.1039940
Sokolowska, M., Rovati, G. E., Diamant, Z., Untersmayr, E., Schwarze, J., Lukasik, Z., et al. (2020). Current perspective on eicosanoids in asthma and allergic diseases - EAACI Task Force consensus report, part I. Allergy. doi: 10.1111/all.14295
Sommer, D. D., Rotenberg, B. W., Sowerby, L. J., Lee, J. M., Janjua, A., Witterick, I. J., et al. (2016). A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. Int. Forum Allergy Rhinol. 6, 385–391. doi: 10.1002/alr.21678
Steinke, J. W., Wilson, J. M. (2016). Aspirin-exacerbated respiratory disease: Pathophysiological insights and clinical advances. J. Asthma Allergy 9, 37–43. doi: 10.2147/JAA.S88739
Stevenson, D. D., Simon, R. A. (2006). Selection of patients for aspirin desensitization treatment. J. Allergy Clin. Immunol. 118, 801–804. doi: 10.1016/j.jaci.2006.06.019
Sturm, E. M., Schratl, P., Schuligoi, R., Konya, V., Sturm, G. J., Lippe, I. T., et al. (2008). Prostaglandin E 2 Inhibits Eosinophil Trafficking through E-Prostanoid 2 Receptors. J. Immunol. 181 (10), 7273–7283. doi: 10.4049/jimmunol.181.10.7273
Swierczynska-Krepa, M., Sanak, M., Bochenek, G., Strek, P., Cmiel, A., Gielicz, A., et al. (2014). Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J. Allergy Clin. Immunol. 134, 883–890. doi: 10.1016/j.jaci.2014.02.041
Szczeklik, A., Nizankowska, E., Duplaga, M. (2000). Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur. Respir. J. 16, 432–436. doi: 10.1034/j.1399-3003.2000.016003432.x
Szczeklik, A. (1990). The cyclooxygenase theory of aspirin-induced asthma. Eur. Respir. J. 3, 588–593.
Ta, V., White, A. A. (2015). Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. Pract. 3, 711–718. doi: 10.1016/j.jaip.2015.03.001
Taniguchi, M., Mitsui, C., Hayashi, H., Ono, E., Kajiwara, K., Mita, H., et al. (2019). Aspirin-exacerbated respiratory disease (AERD): Current understanding of AERD. Allergol. Int. 68, 289–295. doi: 10.1016/j.alit.2019.05.001
Trinh, H. K. T., Pham, D. L., Choi, Y., Kim, H. M., Kim, S. H., Park, H. S. (2018). Epithelial folliculin enhances airway inflammation in aspirin-exacerbated respiratory disease. Clin. Exp. Allergy 48, 1464–1473. doi: 10.1111/cea.13253
Tuttle, K. L., Buchheit, K. M., Laidlaw, T. M., Cahill, K. N. (2018). A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. Pract. 6, 1045–1047. doi: 10.1016/j.jaip.2018.01.038
Uematsu, S., Matsumoto, M., Takeda, K., Akira, S. (2002). Lipopolysaccharide-Dependent Prostaglandin E 2 Production Is Regulated by the Glutathione-Dependent Prostaglandin E 2 Synthase Gene Induced by the Toll-Like Receptor 4/MyD88/NF-IL6 Pathway. J. Immunol. 168 (11), 5811–5816. doi: 10.4049/jimmunol.168.11.5811
Ulambayar, B., Lee, S. H., Yang, E. M., Ye, Y. M., Park, H. S. (2019). Association Between Epithelial Cytokines and Clinical Phenotypes of Elderly Asthma. Allergy Asthma Immunol. Res. 11, 79–89. doi: 10.4168/aair.2019.11.1.79
Van Sambeek, R., Stevenson, D. D., Baldasaro, M., Lam, B. K., Zhao, J., Yoshida, S., et al. (2000). 5’ Flanking region polymorphism of the gene encoding leukotriene C4 synthase does not correlate with the aspirin-intolerant asthma phenotype in the United States. J. Allergy Clin. Immunol. 106 (1), 72–76. doi: 10.1067/mai.2000.107603
Waldram, J., Walters, K., Simon, R., Woessner, K., Waalen, J., White, A. (2018). Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: A single-center study. J. Allergy Clin. Immunol. 141, 250–256. doi: 10.1016/j.jaci.2017.05.006
Walters, K. M., Waldram, J. D., Woessner, K. M., White, A. A. (2018). Long-term Clinical Outcomes of Aspirin Desensitization With Continuous Daily Aspirin Therapy in Aspirin-exacerbated Respiratory Disease. Am. J. Rhinol. Allergy 32, 280–286. doi: 10.1177/1945892418770260
Weinstein, S. F., Katial, R. K., Bardin, P., Korn, S., McDonald, M., Garin, M., et al. (2019). Effects of Reslizumab on Asthma Outcomes in a Subgroup of Eosinophilic Asthma Patients with Self-Reported Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 7, 589–596.e583. doi: 10.1016/j.jaip.2018.08.021
White, A. A., Stevenson, D. D. (2018). Aspirin-Exacerbated Respiratory Disease. N. Engl. J. Med. 379, 1060–1070. doi: 10.1056/NEJMra1712125
White, A. A., Stevenson, D. D., Simon, R. A. (2005). The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann. Allergy Asthma Immunol. 95, 330–335. doi: 10.1016/s1081-1206(10)61150-7
White, A., Ludington, E., Mehra, P., Stevenson, D. D., Simon, R. A. (2006). Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann. Allergy Asthma Immunol. 97, 688–693. doi: 10.1016/s1081-1206(10)61101-5
White, A. A., Bosso, J. V., Stevenson, D. D. (2013). The clinical dilemma of “silent desensitization” in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 34, 378–382. doi: 10.2500/aap.2013.34.3670
Woessner, K. M. (2017). Update on Aspirin-Exacerbated Respiratory Disease. Curr. Allergy Asthma Rep. 17, 1–9. doi: 10.1007/s11882-017-0673-6
Yamaguchi, H., Higashi, N., Mita, H., Ono, E., Komase, Y., Nakagawa, T., et al. (2011). Urinary concentrations of 15-epimer of lipoxin A 4 are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clin. Exp. Allergy 41 (12), 1711–1718. doi: 10.1111/j.1365-2222.2011.03839.x
Yin, W., Yeung, W., Park, H. S. (2020). Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity. Yonsei Med. J. 61, 4–14. doi: 10.3349/ymj.2020.61.1.4
Yonetomi, Y., Sekioka, T., Kadode, M., Kitamine, T., Kamiya, A., Matsumura, N., et al. (2015). Leukotriene C4 induces bronchoconstriction and airway vascular hyperpermeability via the cysteinyl leukotriene receptor 2 in S-hexyl glutathione-treated guinea pigs. Eur. J. Pharmacol. 754, 98–104. doi: 10.1016/j.ejphar.2015.02.014
Keywords: nonsteroidal antiinflammatory drugs, hypersensitivity, asthma, rhinitis, eosinophil, leukotrienes, diagnosis, treatment
Citation: Woo S-D, Luu QQ and Park H-S (2020) NSAID-Exacerbated Respiratory Disease (NERD): From Pathogenesis to Improved Care. Front. Pharmacol. 11:1147. doi: 10.3389/fphar.2020.01147
Received: 24 May 2020; Accepted: 14 July 2020;
Published: 28 July 2020.
Edited by:
Tahia Diana Fernández, University of Málaga, SpainReviewed by:
Craig R. Lee, University of North Carolina at Chapel Hill, United StatesM. Isabel Lucena, University of Malaga, Spain
Copyright © 2020 Woo, Luu and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hae-Sim Park, aHNwYXJrQGFqb3UuYWMua3I=; orcid.org/0000-0003-2614-0303
 Seong-Dae Woo
Seong-Dae Woo Quoc Quang Luu
Quoc Quang Luu Hae-Sim Park
Hae-Sim Park