
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 September 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.01081
Children respond differently to atropine treatment, and predicting patient factors associated with better myopia control is important. Therefore, we aimed to evaluate factors related to myopia progression in Chinese children treated with atropine 0.01%. This retrospective study included 133 children who were administered atropine 0.01% eyedrops every night for 1 year. Enrolled children were examined at follow-up visits at 3 and 6 months, and 1 year. The primary outcome was clinically significant myopia progression (over a -0.75 diopter (D) increase in spherical equivalent (SE)). Multivariate logistic analysis was used to identify predictive factors for myopia progression. The mean baseline SE was -3.92 ± 2.76D, and the average increase in SE and axial length at 1 year from baseline were -0.55 ± 0.57D and 0.43 ± 0.52 mm, respectively. The risk of myopia progression significantly increased in children whose mothers had moderate myopia of less than -6D compared to that in children whose mothers had no history of myopia (odd ratio [OR] = 2.76, 95% confidence interval [CI]: 1.06 to 7.19, P = 0.0382). Birth by cesarean section was also a risk factor for myopia progression (odd ratio [OR] = 2.35, 95% CI: 1.30 to 4.27, P = 0.0048). The correlation between SE and treatment efficiency was linear, and the risk of myopia progression significantly decreased with increasing SE. Atropine 0.01% controlled myopia more effectively in children with higher myopia, who were delivered naturally, and whose mothers had no genetic background of myopia.
Myopia is a major public health concern, and refractive error is one of the five major ocular conditions considered to be an immediate priority (McCarty and Taylor, 2000; Pizzarello et al., 2004). The prevalence of high myopia has been increasing with the prevalence of myopia. Due to vision loss and complications potentially leading to blindness associated with high myopia, researchers have assessed intervention methods to slow or halt the progression of myopia (Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia, 2015).
Atropine 0.01% has demonstrated its efficacy in reducing axial length elongation and progression of the spherical equivalent (SE) (Chia et al., 2012; Chia et al., 2014; Chia et al., 2016; Gong et al., 2017; Yam et al., 2019a). Change in the SE of the refractive error per year is now globally accepted as a clinical marker for progressive myopia.
Previous studies have shown that children respond differently to atropine treatment. Therefore, it is difficult to predict participants who have a higher tendency of myopia progression despite atropine treatment. To clarify this, the present study was designed to evaluate factors related to myopia progression in Chinese children treated with atropine 0.01%.
The protocol and investigators of this study were approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University. Written informed consent was obtained from parents or legal guardians of the children before enrolment. The study is registered at the ClinicalTrial.gov website under the identifier ChiCTR1800017154. Our research was conducted in accordance with the tenets of the Declaration of Helsinki.
This was a retrospective clinical study designed to investigate the magnitude of myopia progression and early onset myopia in Chinese children at the age that they are most likely to experience myopia progression. We included children who received atropine 0.01% eyedrops on a treatment-as-usual, once a night basis. Participants were treated with atropine for 1 year. As atropine 0.01% is not commercially available in China, atropine 0.01% eyedrops were produced by adding 1 ml of 0.05% Kg/L atropine sulfate (atropine sulfate injection, Hubei Xinghua Pharmaceutical Co., Ltd., China) to 4 ml of polyethylene glycol eye drops (Systane ULTRA, Alcon Laboratories, Inc., USA) by the Pharmaceutical Department of Eye & ENT Hospital (Chen et al., 2019).
The inclusion criteria were as follows: 1) children aged 3-14 years; 2) baseline SE from 0 to -12.00D, astigmatism less than or equal to -2.50D; 3) eye examinations spanning across at least 12 ± 2 months of treatment; 4) no medical history predisposing severe myopia (e.g., Marfan syndrome, Stickler syndrome, and retinopathy of prematurity), abnormal ocular refractive anatomy (e.g., keratoconus, lenticonus, and spherophakia); 5) no history of other vision-threatening ocular diseases or previous intraocular surgery; and 6) no current or previous use of atropine or pirenzepine, contact lenses or other forms of treatment that may affect myopia progression.
A total of 133 children who were followed-up and reexamined for axial length as well as refractive error at 3 months, 6 months, and 1 year were enrolled; data on demographics (gender, age, parental refractive history, birth month, and method of birth), and medical and ocular treatment history were also collected. Cycloplegic refraction was used to measure the refractive errors of participants before enrollment and at the 6-month and 1-year follow-up visits. Cycloplegia was achieved with four drops of compound tropicamide eyedrops (0.5% tropicamide and 0.5% phenylephrine eyedrops; Mydrin-P, Santen Pharmaceutical, China), administered approximately 5 min apart. Cycloplegic autorefraction was performed using a desktop autorefractor (KR-8800, Topcon Corporation, Tokyo, Japan) 30 min after the last cycloplegic eyedrop was administered. Cycloplegic retinoscopy was then performed by an experienced optometrist. SE was used for statistical computations, and was calculated as the sum of the spherical power of the refraction result and half of the cylinder power.
The efficacy of atropine was assessed by evaluating myopia progression, which is an increase of at least -0.75D from the baseline SE at 1 year after atropine 0.01% treatment. This has been defined as clinically significant worsening of myopia (based on expert opinion from the 2016 FDA Workshop on myopia progression) (FDA Dermatologic and Ophthalmic Drugs Advisory Committee, 2003. https://wayback.archiveit.org/7993/20170405133139/ https://www.fda.gov/ohrms/dockets/ac/03/briefing/3988B1_02_Novartis%20Briefing%20Document.pdf. Accessed 17 Jan 2017).
A stepwise, multivariate logistic regression analysis was performed using the IBM SPSS Statistics for Mac V.24.0. We compared patient demographics and other clinical characteristics between the myopia progressor and non-progressor groups. We compared the two groups using the Mann–Whitney U-test or the χ2 test. Binary logistic regression was also performed to identify the factors that increase the risk of myopia progression. Multivariate logistic regression was also conducted.
A total of 133 eligible participants were qualified for the primary analysis at 1 year. Baseline characteristics are shown in Table 1. The average age of participants at treatment onset was 5.79 ± 2.20 years. Seventy of the participants were male, and 63 were female. As for age, 47.37% of the participants were pre-school children (lower than 6 years old), and 52.63% were school-age children (6 years old or above). 15.79% and 27.07% of the included patients had no mother myopia background or no father myopia background, respectively. 23.31% and 17.29% had mother or father myopia of over -6D, respectively. The mean baseline SE was -3.92 ± 2.76D. Mean changes in SE at 3 months, 6 months, and 1 year from the baseline were -0.036 ± 0.45D, -0.22 ± 0.43D, and -0.55 ± 0.57D, respectively. The mean baseline axial length was 24.79 ± 1.29 mm, and the mean changes in axial length at 6 months and 1 year from baseline were 0.26 ± 0.54 mm and 0.43 ± 0.52 mm, respectively. The mean changes in axial length at 6 months for the non-progressor and progressor groups were 0.21 ± 0.57 mm and 0.38 ± 0.47 mm (P < 0.01), respectively, whereas those at 1 year were 0.34 ± 0.58 mm and 0.62 ± 0.25 mm (P = 0.025), respectively.
As shown in Table 2, there were significant differences in the baseline SE, method of birth, and maternal refractive histories (P = 0.002, 0.004, and 0.045, respectively) between the non-progressor and progressor groups. In the myopia progressor group, baseline SE was significantly lower than the non-progressor group (-3.12 ± 2.03 and -4.26 ± 2.96). However, there were no significant differences in the other variables selected as covariates for myopia progression between the two groups.
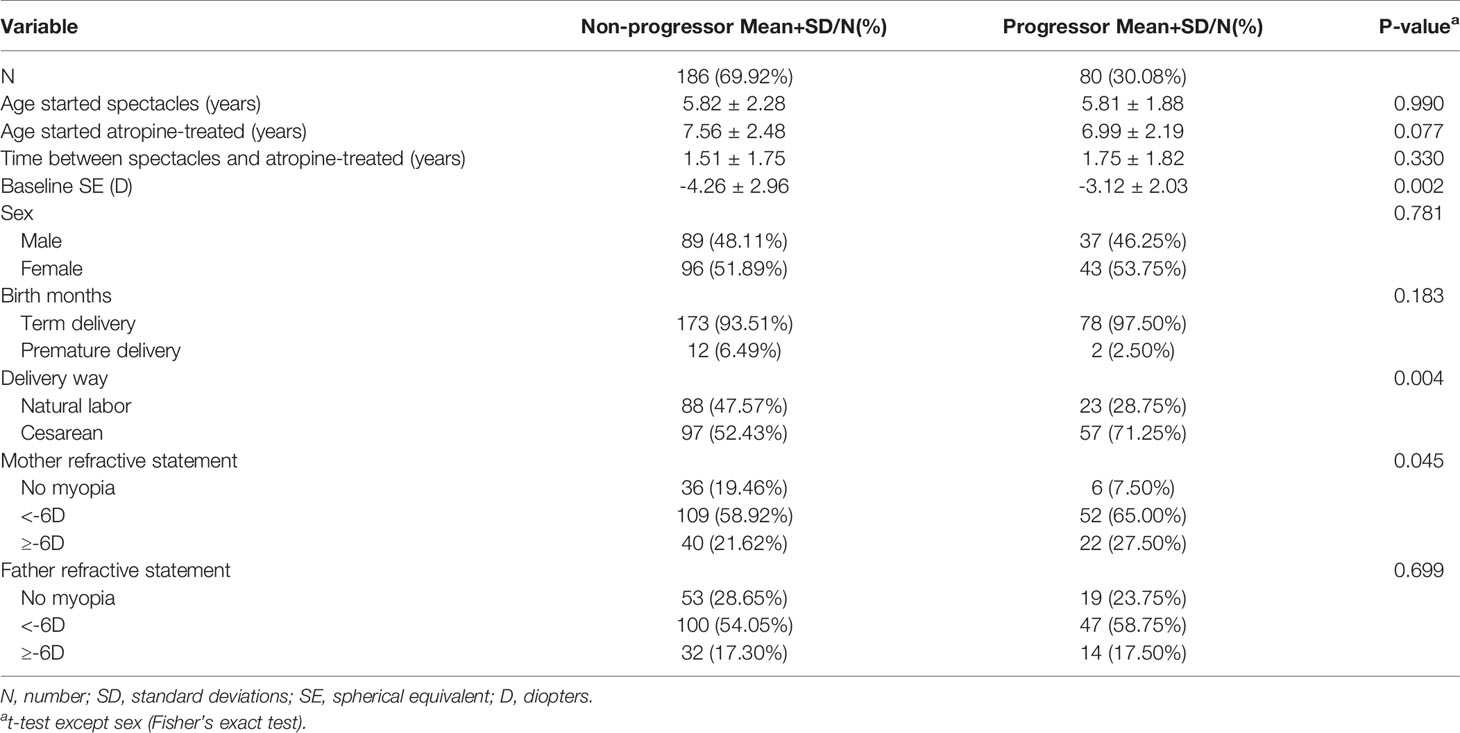
Table 2 Demographics of myopia progression children versus those with no progression at 1-year follow-up after 0.01% atropine applied.
As shown in Table 3, results of the binary logistic regression analysis indicated that birth by cesarean section was independently associated with an elevated risk of myopia progression than birth via vaginal delivery; the odds ratio (OR) was 2.25 (95% confidence interval [CI]: 1.28 to 3.95, P = 0.0048). A maternal genetic background of myopia was independently associated with an increased risk of myopia progression than the absence of a maternal genetic background of myopia; the OR was 2.86 for maternal history of myopia below -6.00D (95% CI: 1.13 to 7.22, P = 0.0259) and 3.30 for maternal history of myopia above or equal to -6.00D (95% CI: 1.20 to 9.05, P = 0.0204). Other variables, including age at the time of first spectacle use, age at the onset of atropine treatment, time between spectacle use and atropine treatment, and paternal refractive history, were not associated with a higher risk of myopia progression.
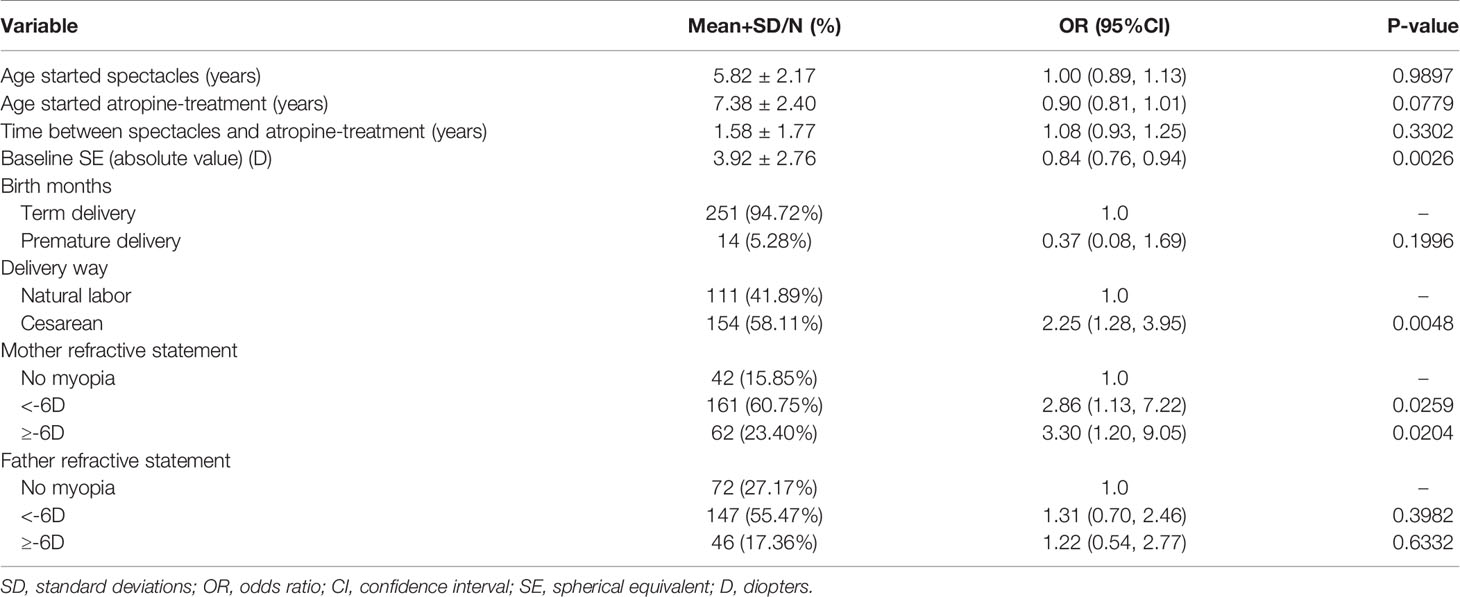
Table 3 Analyses of risk factors for myopia progressor at 1-year follow-up after 0.01% atropine applied, based on univariate analysis.
After adjusting for all variables listed in Table 4, multivariate logistic regression analysis was performed to determine the magnitude of myopia progression despite treatment with atropine 0.01%. Regarding the different birth methods, a significantly higher evaluated risk of myopia progression was associated with birth by cesarean section than with birth via vaginal delivery; the adjusted odds radio (aOR) was 2.35 (95% CI: 1.30 to 4.27, P = 0.0048). Regarding parental genetic background of myopia, a significantly increased risk of myopia progression was related to maternal history of myopia below -6.00D than to the absence of maternal genetic background of myopia; the aOR was 2.76 (95% CI: 1.06 to 7.19, P = 0.0382).
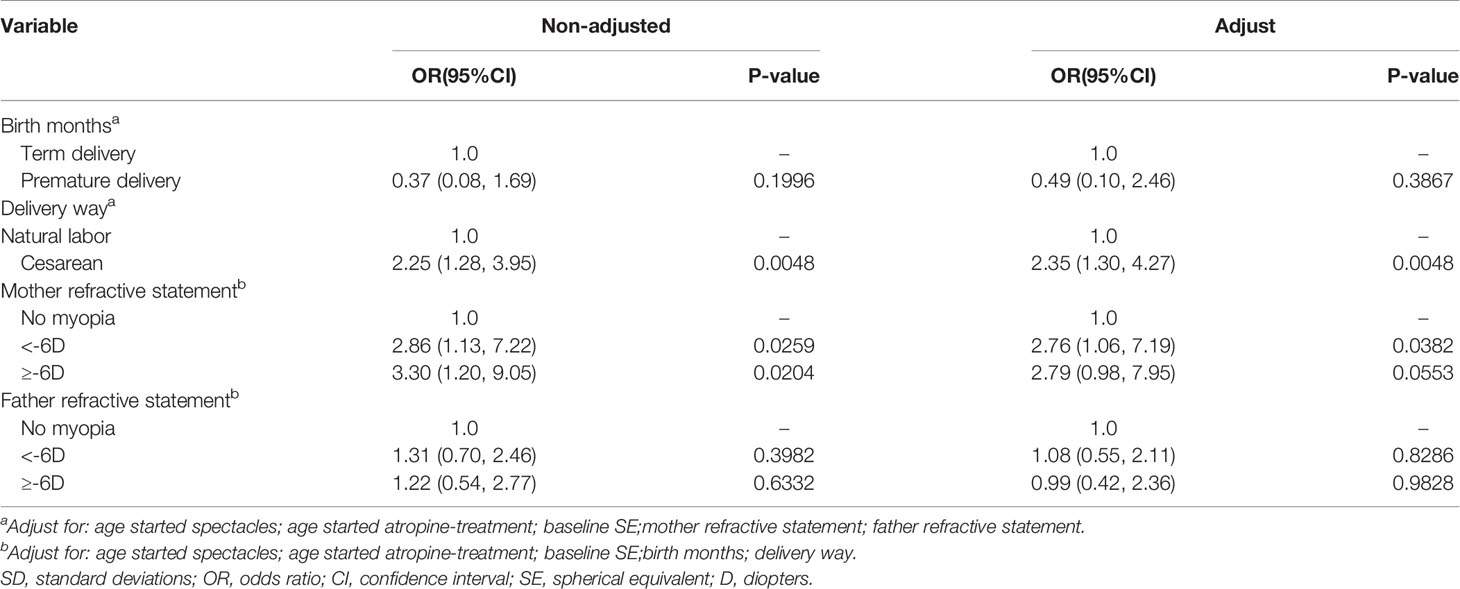
Table 4 Analyses of risk factors for myopia progressor at 1-year follow-up after 0.01% atropine applied with categorized delivery parameters and parental myopia genetic background, based on multivariate analysis.
Smooth curve fitting was performed after adjusting for relevant confounding factors, which were sex, age at the time of first spectacle use, age at the onset of atropine treatment, birth month, method of birth, and parental history of myopia. The resultant curve demonstrated a linear association between the risk of myopia progression and baseline SE. Risk of myopia progression declined by 14% when the baseline SE increased by 1D.
We conducted this retrospective study to evaluate factors related to the progression of childhood myopia in Chinese children treated with atropine 0.01% eyedrops. We analyzed the independent association between predictive factors and the risk of myopia progression using multiple regression analysis after adjusting for potential confounding factors and found that the risk of myopia progression during atropine treatment was significantly increased in children whose mothers had myopia of less than -6D than in those whose mothers had no history of myopia. Birth by cesarean section was also a risk factor for myopia progression during treatment. The correlation between SE and treatment efficiency was linear, and the risk of myopia progression significantly decreased with increasing SE. Myopia control may be more effective in children with higher myopia, who were delivered via natural labor, and who have no maternal genetic background of myopia.
Our study recorded a -0.55D progression of myopia after atropine 0.01% treatment for 1 year, which is similar to the -0.54D progression reported by Sacchi et al. in 2019 (Sacchi et al., 2019), the -0.64D progression reported in the LAMP study (Yam et al., 2019b), and the -0.43D progression reported in the ATOM2 study (Chia et al., 2012). In the present study, the mean changes in axial length at 1 year from baseline was 0.43 ± 0.52 mm. Furthermore, the overall mean change in axial length at 1 year was 0.34 ± 0.58 mm and 0.62 ± 0.25 mm (P = 0.025) the non-progressor and progressor groups, respectively. This increase in axial length was higher than that recorded in the ATOM2 study (0.24 mm) and the LAMP study (0.35 mm) (Chia et al., 2012; Yam et al., 2019b). We noted a relatively high rate of myopia progression (30.08%) in the present study despite atropine treatment. The variation in baseline characteristics of participants may have contributed to this result. Previous studies generally included children aged 5-14 years, whereas in the present study, we included children from the age of 3 years, which lowered the mean age at enrollment to 5.79 ± 2.20 years.
Risk factors for non-responsiveness to atropine treatment have been investigated in previous studies. The atropine 1% therapy (ATOM-1) study focused on variables associated with myopia progression; their results showed that younger children with higher myopia and a parental history of myopia had a greater tendency of myopia progression despite undergoing atropine 1% treatment (Loh et al., 2015). The results of the ATOM-1 study showed that maternal or paternal history of myopia separately had no significant influence on myopia progression in atropine-treated eyes, but the risk of myopia progression was 165% higher when both parents were myopic. This result is somewhat different from the finding of the present study, in that the risk of myopia progression increased by 176% in children whose mothers had myopia of less than -6D. This indicates that maternal history of low to moderate myopia has some predictive power for myopia progression during atropine 0.01% treatment. Univariate analysis showed that maternal history of myopia above or equal to -6D significantly increased the risk of myopia progression, but no significant difference was observed after adjustment for covariates. The authors of previous studies concluded that parental history of myopia increases the risk of myopia in children (Zadnik, 1997; Pacella et al., 1999; Zadnik et al., 2015; Zhang et al., 2015). The effects of maternal and paternal histories of myopia were quantitatively the same, and parental influences were additive (Kurtz et al., 2007). For children without parental genetic backgrounds of myopia, the occurrence of myopia may be related to environment and lifestyle factors, such as less outdoor activity or more near work activity. After the onset of myopia in such children, besides the effect of the use of atropine eyedrops, vision accustomization generally improves further, which weakens the effects of environmental factors that lead to myopia progression and can explain why medication yields better outcomes. Other possibilities are speculative and future studies need to be conducted to explore the association between the parental history of myopia and myopia progression during atropine treatment.
In the present study, children who were delivered via natural labor had better outcomes; a 135% increased risk of myopia progression was observed in children delivered via cesarean section. Birth by cesarean section has never been reported as a risk factor for myopia or myopia control, rather low birth weight and premature birth have been shown to simultaneously affect the development of astigmatism or myopia (Varughese et al., 2005; Wang et al., 2013; Zhu et al., 2017). Regarding the delivery method, previous clinical investigations have mainly focused on the association between cesarean delivery and allergic disorders (Brandão et al., 2016; Chu et al., 2017; Axelsson et al., 2019). It has been hypothesized that children delivered via cesarean section are exposed to microbial colonization which may lead to an altered gut microbiota, and may impair the natural development of the immune system (Kaplan et al., 2011; Dominguez-Bello et al., 2016). The relationship between cesarean birth and myopia progression during atropine treatment could also be due to cesarean indication, as cesarean section, which is performed upon maternal request instead of clinical indications, is very common in China (Zhang et al., 2008). Myopic mothers are more likely to choose a cesarean section; therefore, the method of delivery is also determined by the mother’s refractive status to an extent. Regardless of whether this association between birth method and myopia progression is as a result of clinical indications or the microflora that fetuses come in contact with during the delivery procedure, it still presents a significant concern.
Baseline SE was also found to be associated with myopia progression; with every -1.0D increase in the baseline SE, the risk of myopia progression was 14% lower. A previous risk factor analysis in the ATOM-1 study yielded contrary results; the results showed that children with higher baseline myopia may still experience some myopia progression while undergoing atropine 1% treatment (Loh et al., 2015). The differences between the response profiles of participants in previous studies and those of participants in our study may be due to the lower dose of atropine used in our study. Clinical studies have shown that people with higher myopia are more likely to seek myopia control interventions. Regardless, it cannot be ruled out that participants who had higher myopia had passed the stage of rapid development of myopia. Our results also suggest that atropine indications for clinical use can be broadened.
Our study had a few limitations. Firstly, we did not include any placebo control groups. Secondly, due to the retrospective design of our study, we could not evaluate the safety of atropine, and details on the cessation of medication due to adverse reactions could not be included. Due to the relatively young age of the included children, future prospective clinical studies, with a greater focus on adverse events, need be conducted.
In conclusion, our study focused on further clarifying the variables associated with myopia progression in Chinese children treated with atropine 0.01% for myopia control. Our results show that better myopia control can be achieved in children with higher myopia, who were delivered via natural labor, and who had no maternal genetic background of myopia. Further research to clarify the mechanisms behind these outcomes, and to identify other strategies for myopia control are needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
XYZ and XMQ were responsible for conceptualizing, designing, data collection, extraction, interpretation, manuscript drafting, statistical analysis, and conducting the study. XYZ and YLW were responsible for data collection, extraction, and critical revisions of the manuscript. XYZ and XTZ were responsible for data interpretation, manuscript drafting, supervision, and critical revisions of the manuscript for important intellectual content. This article’s contents are solely the responsibility of the authors. XMQ is the guarantor of this article and takes full responsibility for this study.
This study was supported by the Shanghai Science Popularization Project (Grant No. 17dz2301400), the Key Laboratory of the Ministry of Health in Myopia, Fundamental Research Funds (Grant No. 2018PT32019), and the National Natural Science Foundation of China for Young Scholars (Grant No. 11702063).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Axelsson, P. B., Clausen, T. D., Petersen, A. H., Hageman, I., Pinborg, A., Kessing, L. V., et al. (2019). Relation Between Infant Microbiota and Autism? Results from a National Cohort Sibling Design Study. Epidemiology 30, 52–60. doi: 10.1097/EDE.0000000000000928
Brandão, H. V., Vieira, G. O., de Oliveira Vieira, T., Camargos, P. A., de Souza Teles, C. A., Guimarães, A. C., et al. (2016). Increased risk of allergic rhinitis among children delivered by cesarean section: a cross-sectional study nested in a birth cohort. BMC Pediatr. 16, 57. doi: 10.1186/s12887-016-0594-x
Chen, Z., Huang, S., Zhou, J., Xiaomei, Q., Zhou, X., Xue, F. (2019). Adjunctive effect of orthokeratology and low dose atropine on axial elongation in fast-progressing myopic children—A preliminary retrospective study. Contact Lens Anterior Eye 42, 439–442. doi: 10.1016/j.clae.2018.10.026
Chia, A., Chua, W.-H., Cheung, Y.-B., Wong, W.-L., Lingham, A., Fong, A., et al. (2012). Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology 119, 347–354. doi: 10.1016/j.ophtha.2011.07.031
Chia, A., Chua, W.-H., Wen, L., Fong, A., Goon, Y. Y., Tan, D. (2014). Atropine for the Treatment of Childhood Myopia: Changes after Stopping Atropine 0.01%, 0.1% and 0.5%. Am. J. Ophthalmol. 157, 451–457.e1. doi: 10.1016/j.ajo.2013.09.020
Chia, A., Lu, Q.-S., Tan, D. (2016). Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2. Ophthalmology 123, 391–399. doi: 10.1016/j.ophtha.2015.07.004
Chu, S., Zhang, Y., Jiang, Y., Sun, W., Zhu, Q., Wang, B., et al. (2017). Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by breastfeeding. Sci. Rep. 7, 9762. doi: 10.1038/s41598-017-10206-3
Dominguez-Bello, M. G., De Jesus-Laboy, K. M., Shen, N., Cox, L. M., Amir, A., Gonzalez, A., et al. (2016). Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 22, 250–253. doi: 10.1038/nm.4039
FDA Dermatologic and Ophthalmic Drugs Advisory Committee (2003). Study Designs of Trials in the Treat_ment of Myopia. Available at: https://wayback.archiveit.org/7993/20170405133139/https://www.fda.gov/ohrms/dockets/ac/03/briefing/3988B1_02_Novartis%20Briefing%20Document.pdf (Accessed 17 Jan 2017).
Gong, Q., Janowski, M., Luo, M., Wei, H., Chen, B., Yang, G., et al. (2017). Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 135, 624. doi: 10.1001/jamaophthalmol.2017.1091
Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia. (2015). The impact of myopia and high myopia. World Health Organization. Available at: http://www.who.int/blindness/causes/MyopiaReportforWeb.pdf.
Kaplan, J. L., Shi, H. N., Walker, W. A. (2011). The role of microbes in developmental immunologic programming. Pediatr. Res. 69, 465–472. doi: 10.1203/PDR.0b013e318217638a
Kurtz, D., Hyman, L., Gwiazda, J. E., Manny, R., Dong, L. M., Wang, Y., et al. (2007). Role of Parental Myopia in the Progression of Myopia and Its Interaction with Treatment in COMET Children. Invest. Ophthalmol. Vis. Sci. 48, 562. doi: 10.1167/iovs.06-0408
Loh, K.-L., Lu, Q., Tan, D., Chia, A. (2015). Risk Factors for Progressive Myopia in the Atropine Therapy for Myopia Study. Am. J. Ophthalmol. 159, 945–949. doi: 10.1016/j.ajo.2015.01.029
McCarty, C. A., Taylor, H. R. (2000). Myopia and vision 2020. Am. J. Ophthalmol. 129, 525–527. doi: 10.1016/s0002-9394(99)00444-4
Pacella, R., McLellan, J., Grice, K., Del Bono, E. A., Wiggs, J. L., Gwiazda, J. E. (1999). Role of genetic factors in the etiology of juvenile-onset myopia based on a longitudinal study of refractive error. Optom. Vis. Sci. 76, 381–386. doi: 10.1097/00006324-199906000-00017
Pizzarello, L., Abiose, A., Ffytche, T., Duerksen, R., Thulasiraj, R., Taylor, H., et al. (2004). VISION 2020: The Right to Sight: a global initiative to eliminate avoidable blindness. Arch. Ophthalmol. 122, 615–620. doi: 10.1001/archopht.122.4.615
Sacchi, M., Serafino, M., Villani, E., Tagliabue, E., Luccarelli, S., Bonsignore, F., et al. (2019). Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 97 (8), e1136–e1140. doi: 10.1111/aos.14166
Varughese, S., Varghese, R. M., Gupta, N., Ojha, R., Sreenivas, V., Puliyel, J. M. (2005). Refractive error at birth and its relation to gestational age. Curr. Eye Res. 30, 423–428. doi: 10.1080/02713680590959295
Wang, J., Ren, X., Shen, L., Yanni, S. E., Leffler, J. N., Birch, E. E. (2013). Development of refractive error in individual children with regressed retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 54, 6018–6024. doi: 10.1167/iovs.13-11765
Yam, J. C., Jiang, Y., Tang, S. M., Law, A. K. P., Chan, J. J., Wong, E., et al. (2019a). Low-Concentration Atropine for Myopia Progression (LAMP) Study. Ophthalmology 126, 113–124. doi: 10.1016/j.ophtha.2018.05.029
Yam, J. C., Li, F. F., Zhang, X., Tang, S. M., Yip, B. H. K., Kam, K. W., et al. (2019b). Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology 127 (7), 910–919. doi: 10.1016/j.ophtha.2019.12.011
Zadnik, K., Sinnott, L. T., Cotter, S. A., Jones-Jordan, L. A., Kleinstein, R. N., Manny, R. E., et al. (2015). Prediction of Juvenile-Onset Myopia. JAMA Ophthalmol. 133, 683–689. doi: 10.1001/jamaophthalmol.2015.0471
Zadnik, K. (1997). The Glenn A. Fry Award Lecture, (1995). Myopia development in childhood. Optom. Vis. Sci. 74, 603–608.
Zhang, J., Liu, Y., Meikle, S., Zheng, J., Sun, W., Li, Z. (2008). Cesarean delivery on maternal request in southeast China. Obstet. Gynecol. 111, 1077–1082. doi: 10.1097/AOG.0b013e31816e349e
Zhang, X., Qu, X., Zhou, X. (2015). Association between parental myopia and the risk of myopia in a child. Exp. Ther. Med. 9, 2420–2428. doi: 10.3892/etm.2015.2415
Keywords: myopia, atropine, retrospective study, Chinese children, myopia progression
Citation: Zhang X, Wang Y, Zhou X and Qu X (2020) Analysis of Factors That May Affect the Effect of Atropine 0.01% on Myopia Control. Front. Pharmacol. 11:01081. doi: 10.3389/fphar.2020.01081
Received: 23 March 2020; Accepted: 02 July 2020;
Published: 09 September 2020.
Edited by:
Maria Dimitrova, Medical University-Sofia, BulgariaReviewed by:
Fathi M. Sherif, University of Tripoli, LibyaCopyright © 2020 Zhang, Wang, Zhou and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomei Qu, cXV4aWFvbWVpMjAwMkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.