- 1Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Ethnic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4School of Pharmacy, Southwest Minzu University, Chengdu, China
- 5Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Cancer is a leading cause of death around the world. Apoptosis, one of the pathways of programmed cell death, is a promising target for cancer therapy. Traditional Tibetan medicine (TTM) has been used by Tibetan people for thousands of years, and many TTMs have been proven to be effective in the treatment of cancer. This paper summarized the medicinal plants with anticancer activity in the Tibetan traditional system of medicine by searching for Tibetan medicine monographs and drug standards and reviewing modern research literatures. Forty species were found to be effective in treating cancer. More importantly, some TTMs (e.g., Ophiocordyceps sinensis, Phyllanthus emblica L. and Rhodiola kirilowii (Regel) Maxim.) and their active ingredients (e.g., cordycepin, salidroside, and gallic acid) have been reported to possess anticancer activity by targeting some apoptosis pathways in cancer, such as Bcl-2/Bax, caspases, PI3K/Akt, JAK2/STAT3, MAPK, and AMPK. These herbs and natural compounds would be potential drug candidates for the treatment of cancer.
Introduction
Apoptosis, which is also known as programmed cell death, is beneficial to normal cell development, organ growth, and the dynamic balance of tissues (Rogers and Almenri, 2019). Apoptosis is a normal physiological process that plays an important role in the development and dynamic balance of organisms (Xu et al., 2015). Defects in apoptosis occur in most types of cancer, such as lung, female breast, prostate, liver, thyroid, and bladder cancers. A large number of studies have shown that regulating and inducing apoptosis are feasible ways for treating cancer (Hoshyar and Mollaei, 2017; Yoon et al., 2018). In vitro and in vivo experiments have demonstrated that the mechanism of apoptosis encompasses extremely complex processes and involve many biological factors, and failure to induce apoptosis is one of the major obstacles to cancer treatment (Li-Weber, 2013). From a mechanistic perspective, apoptosis can be activated by the intrinsic mitochondrial or extrinsic death receptor apoptotic pathway. The intrinsic mitochondrial apoptotic pathway is activated when cells sense directly or indirectly intracellular or extracellular stimuli, such as DNA damage, reactive oxygen species, hypoxia, and Ca2+ (Tompkins and Thorburn, 2019). These stimuli ultimately disrupt mitochondrial function by inducing the expression and activation of proapoptotic Bcl-2 family members, such as Bcl-2, Bcl-xL, Bax, and Bak (Hoshyar and Mollaei, 2017). By contrast, stimulated extrinsic death receptors can induce the sequential activation of caspase-3, which cleaves target proteins and leads to apoptosis (Tompkins and Thorburn, 2019). Therefore, the development of anticancer agents with apoptosis pathway-related targets has become an important strategy for cancer treatment.
Natural medicines, including plants, animals, and minerals, are the gifts of nature to humans and play an important role in fighting various diseases. Many anticancer drugs that are commonly used in modern medicine, such as paclitaxel, camptothecin, matrine and vinblastine, are derived directly or indirectly from natural sources. Therefore, new anticancer drugs can be discovered from natural plants. In the course of more than 2,000 years of history, a complete theoretical system has been established for traditional Tibetan medicine (TTM). TTM has played an important role in the prevention and treatment of various diseases, such as “Zhui-nai” (འབྲས་ནད།), which is similar to cancer in modern medicine (Bauer-Wu et al., 2014). TTM believes that “Zhui-nai” is caused by external factors invading the body, resulting in the dysfunction of the three “stomach fire”. These abnormalities can cause indigestion and increase bad blood, which ultimately lead to the dysfunction of the mei-nian loong (རླུང་མེ་མཉམ།), neng-xiao tripa (མཁྲིས་པ་འཇུ་བྱེད།), and baekan ni-mu-xie (བད་ཀན་མྱག་བྱེད།) (Yutuo, 1983). In TTM, unclean substances in the body and physical weakness are important factors in the development of cancer. Therefore, TTM with tonic, heat-clearing and detoxification functions can be used to treat cancer. In recent years, TTM has received extensive attention worldwide owing to its unique advantages in terms of preventing and treating cancer. TTM can directly inhibit the growth of cancer cells, induce apoptosis, and suppress tumor growth through multi-target pathways (Yadav et al., 2017; Bhardwaj et al., 2018; Tao et al., 2019). In addition, TTM combined with radiotherapy or chemotherapy can significantly reduce adverse reactions and enhance the patient’s immunity and quality of life (Liu et al., 2016a; Liu et al., 2016b; Colapietro et al., 2018). Numerous TTM monographs and research papers have documented some natural medicines and prescriptions for cancer treatment. However, no consensus has been reached in most records, resulting in a lack of systematic summarization, induction, and arrangement.
In this study, information on natural Tibetan medicines used in treating cancer was sampled by performing a bibliographic investigation of TTM monographs and drug standards. The names, species, families, and medicinal parts of TTMs with anticancer effect were introduced in detail. These data can provide a good reference for the development and utilization of TTMs. Moreover, recent research progress on some anticancer TTMs and their active ingredients that can induce apoptosis in cancer cells was introduced in detail. These herbs and natural compounds would be potential drug candidates for the treatment of cancer.
Methods
Some Tibetan medicine monographs and medicinal materials standards, such as “Jing Zhu Materia Medica”, “Drug Standards of Tibetan Medicine” and “Chinese Tibetan Medicine”, were searched for information on natural Tibetan medicine for cancer treatment. Data collected from these documents included names, species, families, and medicinal parts. The botanical names of plants are mainly derived from references, and verified through the “Flora of China (http://frps.eflora.cn/)” and Medicinal Plant Names Services: Royal Botanic Gardens, Kew databases based on their Chinese names. In addition, in order to obtain the active ingredients and biological/pharmacological effects of the selected species, online Chinese databases (e.g., Wanfang and CNKI) and international databases (e.g., NCBI, Web of Science, and Science Direct) were searched with cancer, apoptosis and/or Latin names as search keywords.
Results and Discussion
Understanding of Cancer in Traditional Tibetan Medicine
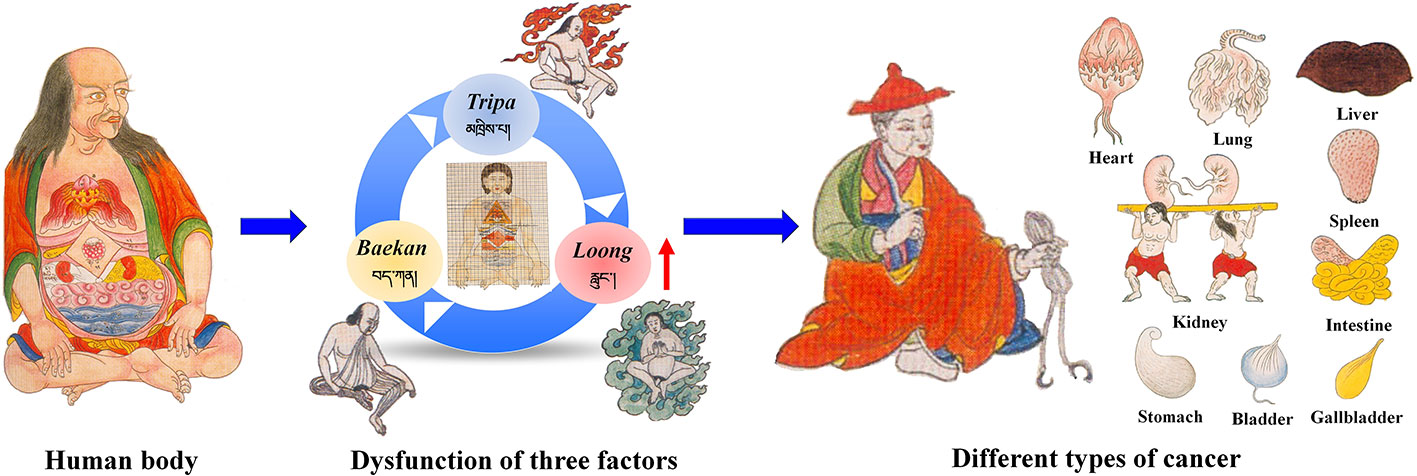
TTM is an important part of traditional medicine worldwide. In the history of more than 2,000 years, TTM has established a complete theoretical system and a unique diagnostic style. It has played an important role in the prevention and treatment of various diseases, including cancer. TTM has a unique understanding of the occurrence and development of cancer. According to the ancient literature of TTM, the hard lump with the size of Qinggang nucleus in the body is called “Zhui-nai” (འབྲས་ནད།) (Yutuo, 1983). “Zhui-nai” is similar to cancer in modern medicine. TTM believes that the occurrence of cancer is closely related to “loong” and “bad blood”. In general, when the loong, tripa and baekan maintain a relative balance in the body, normal physiological and psychological functions can be achieved. When they are in an unbalanced state, especially the “loong” disorder will lead to an increase in “bad blood”, and then the pathological state of “Zhui-nai” is manifested.
The classification of cancer by TTM is generally consistent. According to the “Four Books of Pharmacopeia”, two classification methods, namely, etiology and lesion location classification, are applied (Yutuo, 1983). Eighteen broad types of cancer are classified by etiology classification. By contrast, cancer are classified in to inside and outside according to lesion location classification. Outside cancers can be divided into flesh, bone and pulse cancer, and the inside cancer includes lung, heart, liver, spleen, kidney, stomach, intestine, rectum, and bladder cancers (Figure 1). Outside cancers are equivalent to the superficial and soft tissue tumors of modern medicine. Inside cancers mainly refer to abdominal and organ tumors.
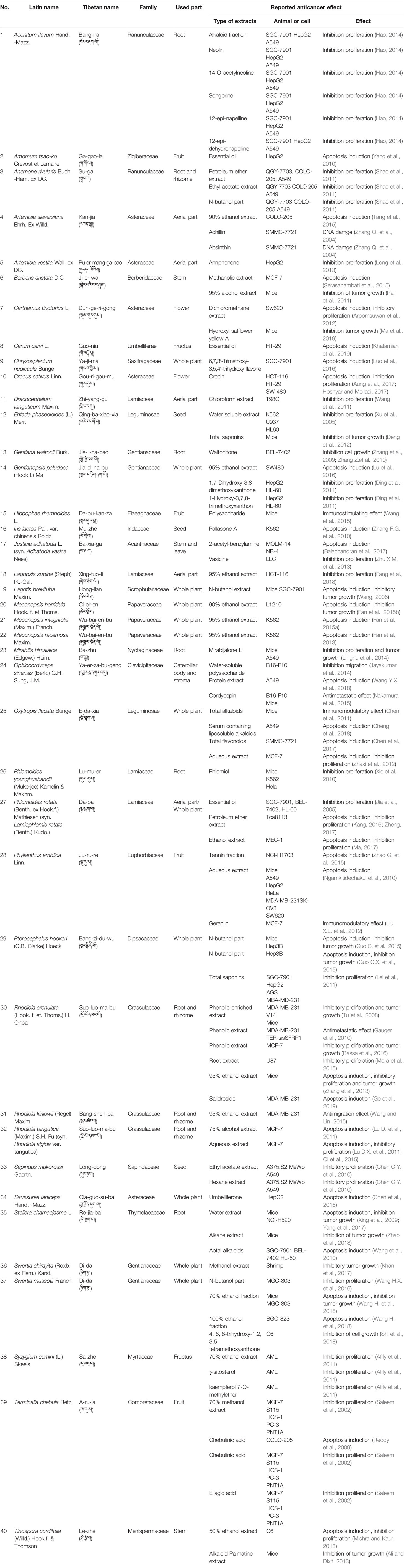
The treatment of cancer by TTM can be summarized as follows: The first step is the inhibition, breaking down, and/or dissolution of tumor growth. The second step is the regulation and maintenance of the balance among the loong, tripa, and baekan, cleaning of diseased tissues, and control of inflammation. Finally, target tissues and organs are healed and repaired, and the systemic immune system is restored to normal. In TTM, unclean substances in the body are considered important factors in the development of cancer. Therefore, TTMs with tonic, and heat-clearing, detoxifying functions are usually used to treat cancer (Table 1 and Figure 2). TTM prescriptions for cancer treatment are mainly based on the six tastes (i.e., sweet, sour, salty, bitter, astringent, and pungent). These tastes transform sequentially into three gastropyretic phases, which become into three post-digestive taste profiles (sweet, sour, and bitter) (Bauer-Wu et al., 2014; Dhondrup et al., 2019). Prescription medicines include medicinal plants, animals, and/or minerals, which are processed by powdering, boiling, concentration, and mixing (Dimaer, 2012). TTM also uses some mineral medicines in the treatment of cancer, such as Margarita, Margaritifera concha, Magnetitum, and Cinnabaris, but their modern research is scarce.

Figure 2 Tibetan medicinal plants with anticancer activity. (A) Ophiocordyceps sinensis (Berk.) G.H. Sung, J.M. (B) Crocus sativus, (C) Rhodiola crenulata, (D) Rhodiola kirilowii, (E) Meconopsis integrifolia, (F) Meconopsis racemosa, (G) Meconopsis horridula, (H) Phyllanthus emblica, (I) Phlomis younghusbandii, (J) Pterocephalus hookeri, (K) Gentianopsis paludosa, (L) Justicia adhatoda L. (syn. Adhatoda vasica Nees), (M) Phlomoides rotata (Benth. ex Hook.f.) Mathiesen (syn. Lamiophlomis rotata (Benth.) Kudo.), (N) Stellera chamaejasme.
Apoptotic Pathway as a Target of TTM in the Treatment of Cancer
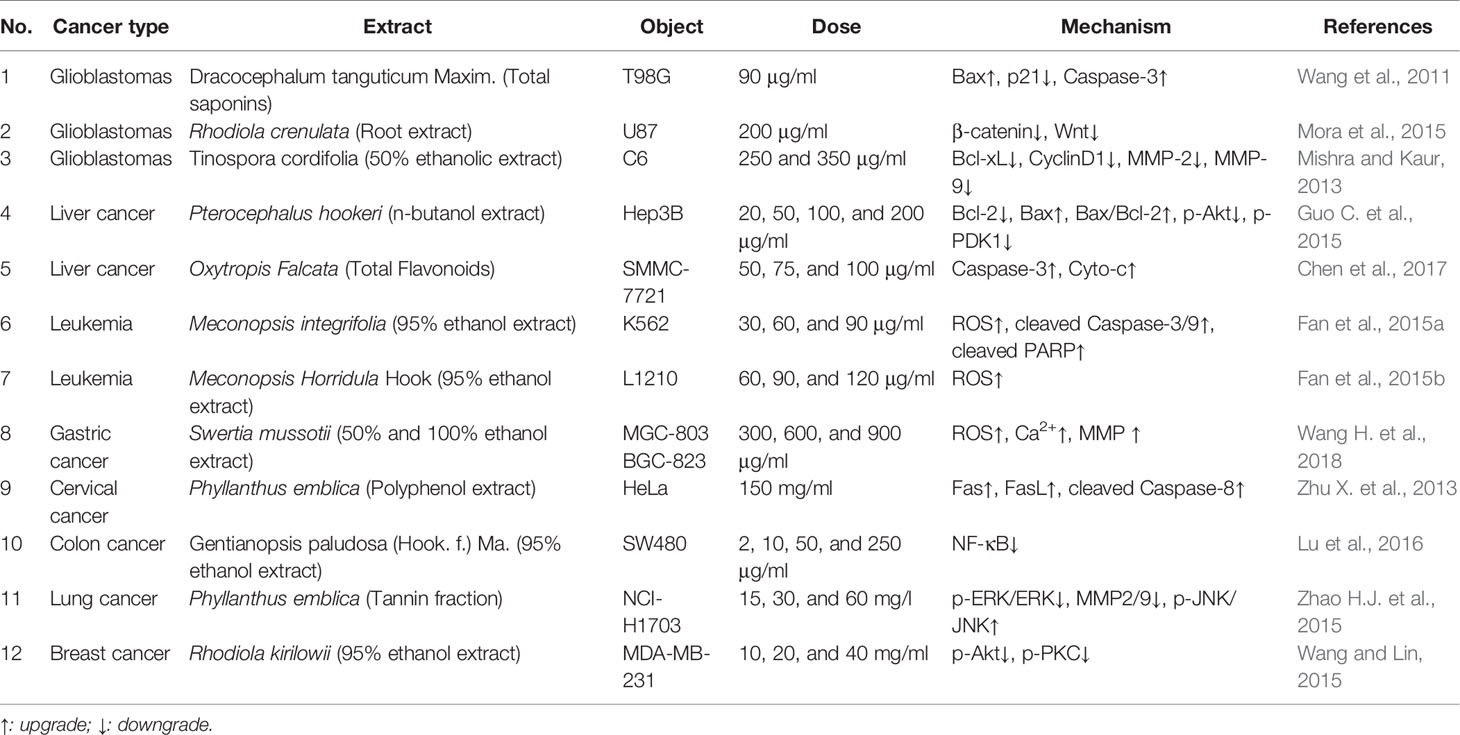
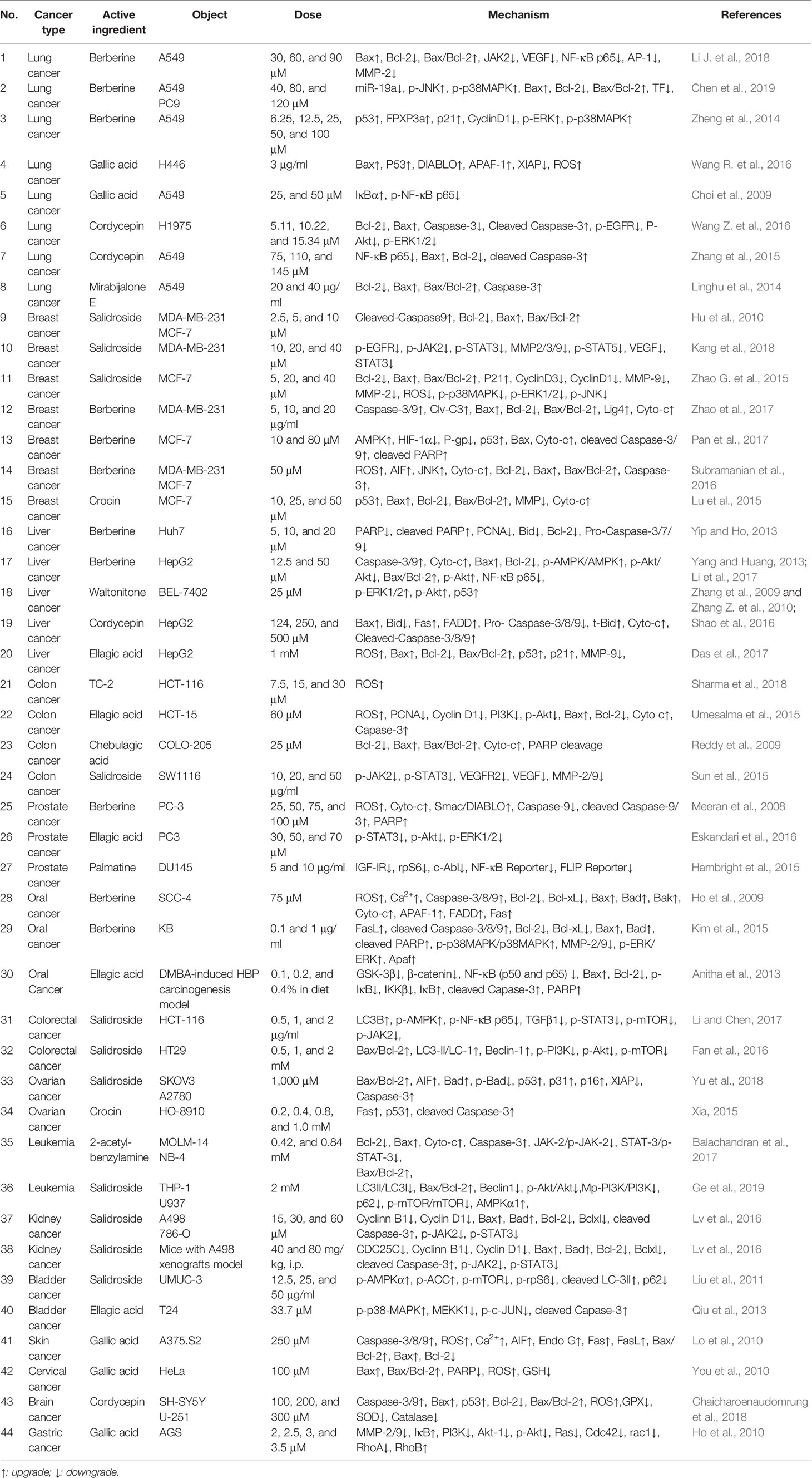
Apoptosis, which is known as programmed cell death, is a widely important mechanism of cell growth inhibition in cancer cells. Therefore, the apoptotic pathway is an important target for cancer treatment (Tompkins and Thorburn, 2019). By collating the literature of Tibetan medicines with anticancer activity, up to now, these anticancer TTMs were found among forty species, such as Ophiocordyceps sinensis (Berk.) G.H. Sung, J.M., Crocus sativus L., Phyllanthus emblica L., Rhodiola species, Mirabilis himalaica (Edgew.) Heimerl, Terminalia chebula Retz. Some TTMs can kill cancer cells by inducing apoptosis. In the following sections, we will focus on introducing the TTMs and their compounds whose therapeutic mechanisms are related to apoptosis (Tables 2 and 3).
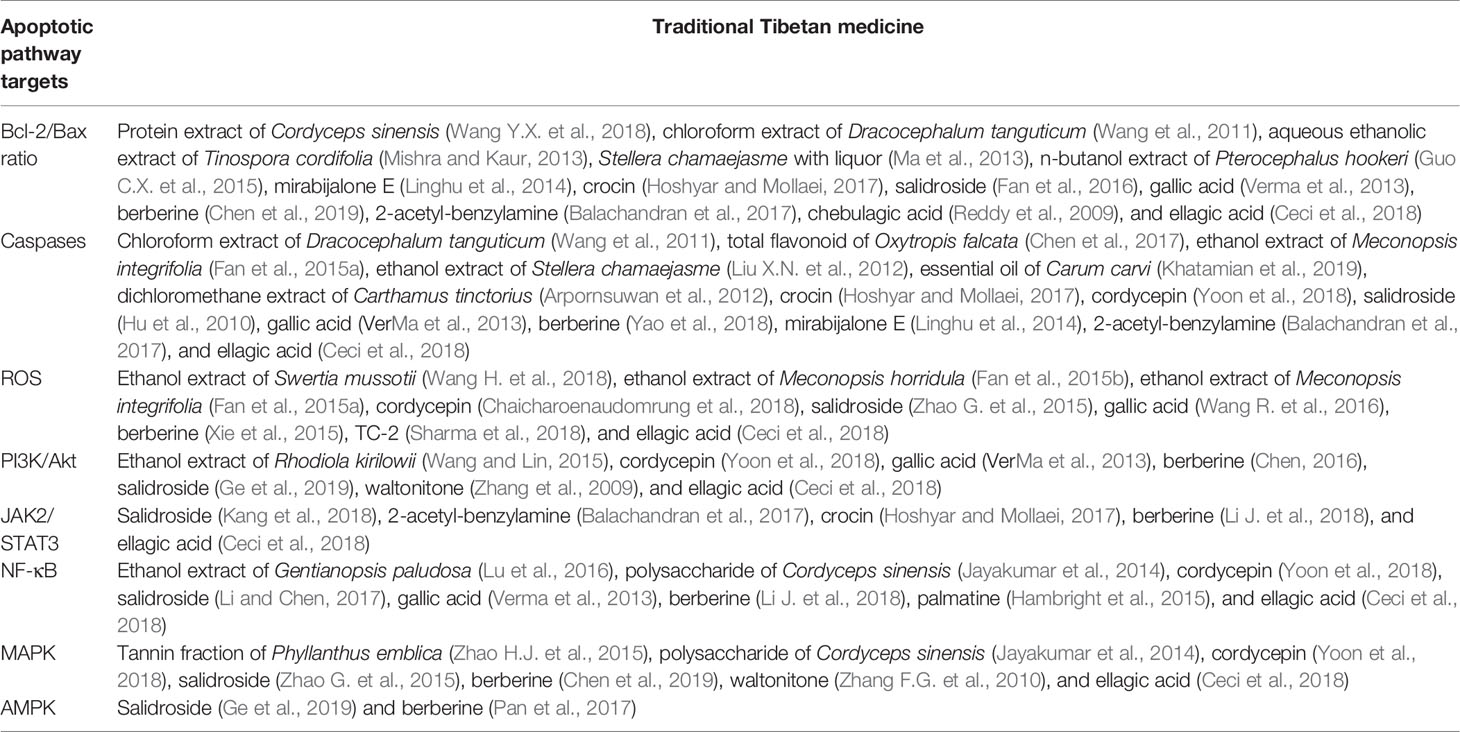
TTMs That Alter the Bcl-2/Bax Ratio
The Bcl-2 family has both proapoptotic and surviving members, which play important roles in regulating apoptosis (Rogers and Almenri, 2019). As an antiapoptotic protein, Bcl-2 is mainly distributed in the outer membrane of mitochondria, the inner surface of cell membrane, endoplasmic reticulum and nuclear membrane. On the contrary, Bax is a proapoptotic member of Bcl-2 family. Therefore, the proportional relationship between Bax/Bcl-2 plays a key role in mitochondrial mediated apoptosis (Li J. et al., 2018). In addition, many TTMs have been found to induce apoptosis by regulating the balance of Bcl-2 family members.
Cancer is recognized to be the result of three factors dysfunction (loong, tripa and baekan), especially under the inverse of the loong. According to the ancient Tibetan medicine classics, TTMs with tonic, clearing heat, and detoxifying functions are used to treat cancer. O. sinensis (Figure 2), which is known as Ya-er-zha-geng-bu (Tibetan: དབྱར་རྩྭ་དགུན་འབུ།), Dong-chong-xia-cao (Chinese name) or cordyceps (English name), is considered as one of the most valued Tibetan medicines (Qinghai Institute for Drug Control, 1996). In the theory of Traditional Chinese medicine and Tibetan medicine, O. sinensis is pungent flavor and warm-natured, and used for hundreds of years in traditional medicine as a tonic for the bronchitis, phthisis, pneumonia, lung heat, and impotence nocturnal emission (Dimaer, 2012). It’s worth noting that O. sinensis has a wide range of pharmacological properties, such as anti-inflammatory, cell cycle disruption, immune enhancement, induction apoptosis, etc. So it is widely used and concerned as an anticancer agent. Previous studies have found that water extract of O. sinensis combined with methotrexate could significantly prolong the survival time of mice inoculated with cancer cell sarcoma and inhibit the metastasis of tumor cells by inducing apoptosis (Nakamura, et al., 2003). An exopolysaccharide fraction from O. sinensis could significantly inhibit the metastasis of B16 melanoma cells and decreased the levels of Bcl-2 in the lungs and livers at a dose concentration of 120 mg/kg (Zhang et al., 2005). Treatment of A549 lung cancer cells with protein extract of O. sinensis could increase in Bax/Bcl-2 ratio, significantly upregulate mRNA levels of Bax, tumor necrosis factor-α (TNF-α), interleukin-1, and interleukin-12 (Wang Y.X. et al., 2018). Tinospora cordifolia (Willd.) Hook.f. & Thomson (Tibetan: Le-zhe) is one of the most widely used in TTM, has immunomodulatory, antitumor, anti-angiogenesis, and antimetastatic activity in various in vivo models. Aqueous ethanolic extract of T. cordifolia could block C6 glioma cells in G0/G1 phase and G2/M phase, inhibit the expression of G1/S phase specific protein cyclin D1 and antiapoptotic protein Bcl-xl, and thus produce its antiproliferation and apoptotic inducing effect in concentration range of 250–350 μg/ml (Ali and Dixit, 2013).
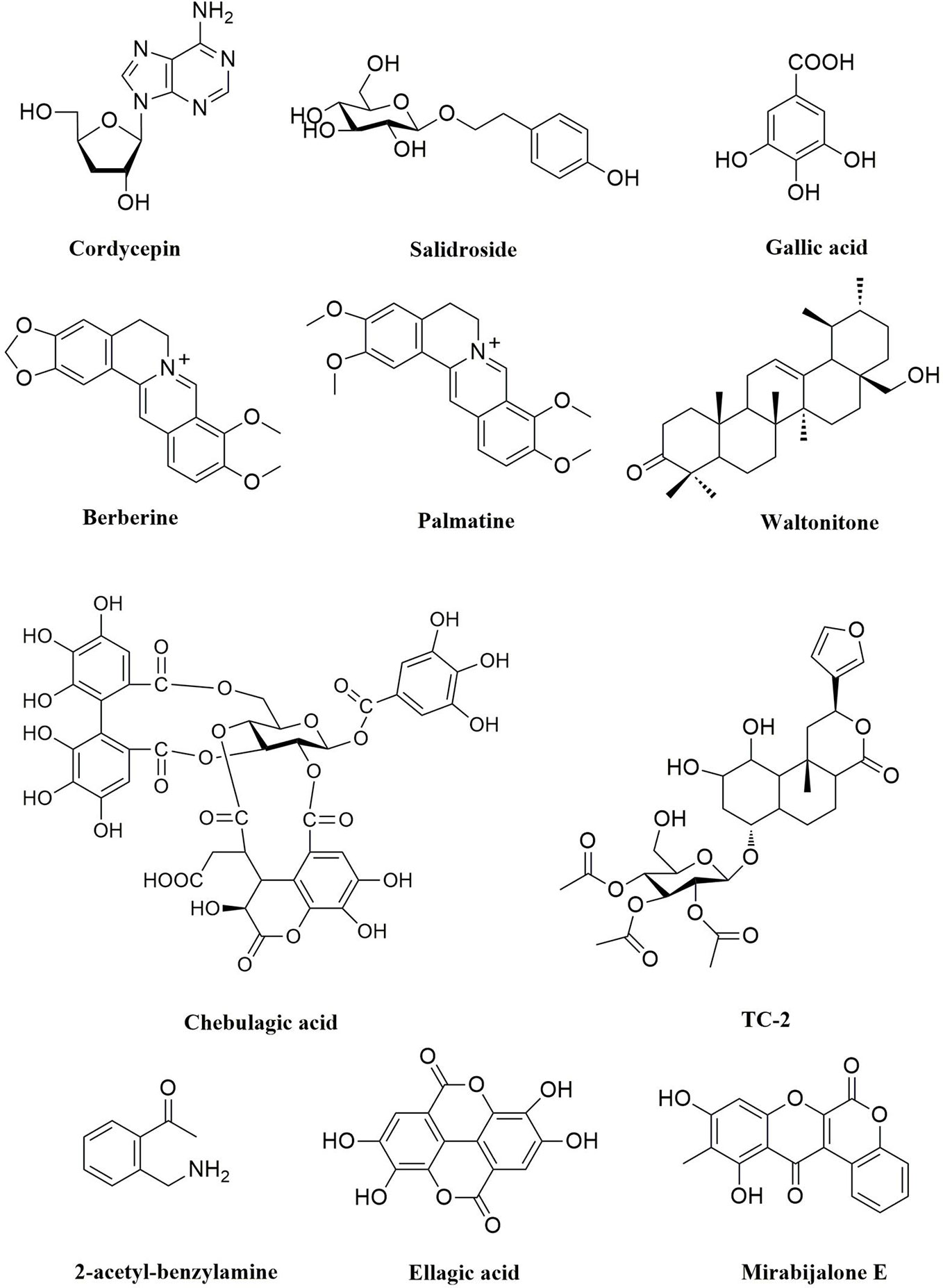
Some active ingredients of TTMs that alter the Bcl-2/Bax ratio have been found and identified. Cordycepin, a 3-deoxyadenosine (Figure 3), is the predominant functional component of the fungus Ophiocordyceps species, has antitumor effects or apoptosis in brain cancer, human oral squamous cancer, thyroid carcinoma cancer, gallbladder cancer, liver cancer, breast cancer, and lung cancer (Wu et al., 2007; Chen Y. et al., 2010; Aramwit et al., 2015; Chaicharoenaudomrung et al., 2018). Cordycepin (5.11–15.34 μM) could inhibit cell proliferation and induce apoptosis in a dose-dependent manner. It was demonstrated that cordycepin could decrease the expression levels of Bcl-2 and caspase-3, increase the expression levels of proapoptotic protein Bax, and cleaved caspase-3 (Wang Z. et al., 2016). Notably, the study showed that cordycepin (125–500 μM) could induce the mitochondria mediated apoptosis signal pathway of human liver cancer HepG2 cells through upregulation of the ratio of Bax/Bcl-2, and initiating the FADD mediated signal pathway (Shao et al., 2016). Mirabijalone E (Figure 3), which was isolated from M. himalaica, has been reported to increase of Bax expression level and decrease of Bcl-2 level and activation of caspase-3 (Linghu et al., 2014). In addition, chebulagic acid (Figure 3) which was isolated from the fruits of T. chebula, could induce apoptosis by DNA fragmentation assay, PARP cleavage, cytochrome c release from the mitochondria and alteration of Bcl-2/Bax ratios in COLO-205 cell line (with an IC50 of 25μM) (Reddy et al., 2009).
TTMs That Activate Caspases
Caspases are a family of cysteine proteases and play an important role in apoptotic and inflammatory signaling pathways. During the process of tumorigenesis, significant loss or inactivation of major members of the caspase family leading to impaired apoptosis induction, causing the serious imbalance of growth dynamics, and eventually to abnormal growth of human tumors (Rogers and Almenri, 2019). Caspases are divided into promoter groups (caspase-8/9/10) and executive groups (caspase-3/6/7). Re-activation of caspase to restore the apoptosis-induced pathway is a key molecular approach to the development of anticancer agents. Restoring apoptosis induction by caspase reactivation is a key molecular mechanism for the development of anticancer agents. Most studies have found that TTMs could induce apoptosis via caspase activation.
Saffron (Figure 2) is an edible spice and colorant found in the dried stigmas of Crocus sativus L. and has been used in TTM as an herb to treat various diseases, such as cancer. Over the past two decades, studies have been conducted on the therapeutic applications of saffron, which have been found to have anticancer, antitumor (in vivo and in vitro) and antimutagenic potential. Modern pharmacological studies have been proved that saffron can treat a variety of cancers, such as lung, breast, skin, and prostate cancers. In human lung cancer (A549 and H446), saffron extract (0.25–8.0 mg/ml) could suppress proliferation and induce apoptosis in a dose- and time-dependent manner and has significant anticancer effects via caspase-3/8/9 mediated cell apoptosis (Liu et al., 2014). The crocin family includes various glycosyl esters of which six types have been detected in saffron and is the main active substance of saffron. Previous studies have shown that crocin (0.2–1.0 mmol/L) could induce ovarian cancer HO-8910 cells’ apoptosis by increasing p53 and Fas/APO-1 expression and activating the apoptotic pathway regulated by Caspase-3 (Xia, 2015). In addition, crocin could induce apoptosis on human breast cancer cells (MCF-7) through a caspase-8-dependent mitochondrial pathway, involving p53 expression, Bax conformation, and mitochondrial membrane potential loss (Lu et al., 2015).
Dracocephalum tanguticum Maxim. (Labiatae) is a commonly used TTM for treating arthritis, hepatitis, and ulcer. In recent years, D. tanguticum has been used to treat glioblastomas. Wang et al. (2011) found that the chloroform extract of D. tanguticum stimulated caspase-3 cleavage and inhibited the expression of p21 protein with induction of glioblastomas cells (T98) apoptosis. The ethanol extract of Meconopsis integrifolia (Maxim.) Franch. and total flavonoid of Oxytropis falcata Bunge could block cell cycle processes and induce mitochondrial dependent apoptosis in human leukemia K562 cells and hepatoma SMMC-7721 cells by the release of cytochrome C, activation of Caspase-3/9 (Fan et al., 2015a; Chen et al., 2017). The ethanol extract of Stellera chamaejasme L. induced apoptosis significantly improved the activity of caspase-3/8/9, increased Fas and TNF-α expression (Liu X.N et al., 2012). Carum carvi L. essential oil has an efficient novel apoptosis inducer for human colon cancer cells (HT-29 and Huvec) by up-regulation Caspase-3 gene expression (Khatamian et al., 2019).
Ellagic acid (Figure 3), an important small molecular compound, was isolated from some TTMs, such as P. emblica, T. chebula, and T. billerica. Similarly, ellagic acid is a polyphenolic compound found in fruits and berries such as pomegranate, strawberry, raspberry, and blackberry. A large number of studies have reported the anticancer effects of ellagic acid on most types of cancer, such as colorectal, breast, prostate, lung, and liver cancers (Ceci et al., 2018). Hagiwara et al. (2010) found that ellagic acid activated apoptosis pathway associated with caspase-3 activation in human leukemia HL-60 cells. Notably, ellagic acid could enhance the chemotherapeutic sensitivity of 5-Fluorouracil and induce apoptosis by regulating the Bcl-2/Bax ratios and activating caspase-3 in colorectal carcinoma cells (HT-29) (Kao et al., 2012). In addition to the mechanisms mentioned above, ellagic acid induced apoptosis by regulating ROS, PI3K/Akt, JAK2/STAT3, MAPK, and NF-κB pathway in cancer cells (Bisen et al., 2012; Ceci et al., 2018).
TTMs That Activate Reactive Oxygen Species
Reactive oxygen species (ROS) are substances produced by all aerobic cells to regulate cell development, growth, survival and death. ROS are generally present in all aerobic cells in relative balance with biochemical antioxidants. When this balance is disrupted by mitochondria excess production of ROS and/or depletion of antioxidants, oxidative stress may occur, which eventually leads to mitochondrial swelling, depolarization of mitochondrial membrane potential, and release of apoptosis-inducing proteins (Wang R. et al., 2016; Chaicharoenaudomrung et al., 2018). Oxidative stress is a major apoptotic stimulus for cancer cells, which require particularly high energy metabolism in the process of rapid growth and proliferation. Therefore, the production of ROS may enhance the proapoptotic mechanism of cancer cells and provide important targets for the treatment of cancer. TTMs have been reported to induce apoptosis of cancer cells by production of ROS.
P. emblica, a euphorbiaceous plant, is widely distributed in subtropical and tropical regions of China, India, Indonesia, and Malay Peninsula (Liu X.L. et al., 2012). The dried fruits of P. emblica is one of the famous plants used in traditional medicinal systems such as Ayurvedic medicine, Tibetan traditional medicine, Chinese herbal medicine, and Thai traditional medicine (Zhang Y.J. et al., 2004; Ngamkitidechakul et al., 2010). In traditional medicine Tibetan system, P. emblica is called “Ju-ru-re” (Tibetan: སྐྱུ་རུ་ར།). It is the most frequently used formulations in TTM (Li et al., 2018a). The extensive use of P. emblica in traditional medicines and food products has led to a large number of pharmacological activity studies. Up to now, a large number of biological activities have been reported, such as anti-inflammatory, antioxidant, antitumor, and immunomodulatory effects. It is noted that the aqueous extract of P. emblica (25–100 μg/ml) could induce apoptosis on human hepatoma cells (HepG2) by reducing production of ROS and increasing the levels of glutathione (Shivananjappa and Josi, 2012).
Swertia mussotii Franch., which is known as “Di-da” (Tibetan: ཏིག་ཏ།), was reported in the classic book of Tibetan medicine “Jing Zhu Materia Medica” that S. mussotii has the clearing heat and detoxifying functions. Recent studies have shown that S. mussotii has significant anticancer activity. Wang H. et al. (2018) reported that ethanol extract of S. mussotii was able to induce apoptosis in gastric cancer cells (MGC-803 and BGC-823) through depolymerization of cytoskeletal filaments, S phase arrest, disrupted mitochondrial transmembrane potential and increased cytoplasmic levels of ROS. Similarly, Meconopsis horridula Hook. f. & Thomson ethanol extract induced murine leukemia L1210 cell apoptosis and inhibited proliferation through G2/M phase arrest, and ROS were involved in the process (Fan et al., 2015b). Gallic acid, 3,4,5-trihydroxybenzoic acid (Figure 3), which can be found in various natural products, such as green tea, grapes, Punica granatum L., P. emblica, Galla chinensis Mill., and many other fruits plants. Gallic acid known to affect several pharmacological and biochemical pathways have strong antioxidant, antimutagenic, anti-inflammatory, and anticancer properties (Karimi-Khouzani et al., 2017; Limpisophon and Schleining, 2017; Silva et al., 2017; Ahmed et al., 2018). Therefore, gallic acid has been recognized as an inducer of apoptosis in cancer cell lines. It has been reported that gallic acid could induce apoptosis by ROS-dependent mitochondrial pathway in most cancer cells, such as colon cancer HCT-15 cells, small cell lung cancer H446 cells, prostate cancer DU145 cells, cervical cancer HeLa cells, melanoma A375.S2 cells (Lo et al., 2010; You et al., 2010; Subramanian et al., 2016; Wang R. et al., 2016). TC-2 (Figure 3) is a new clerodane diterpenoid from Tinospora cordifolia (Willd.) Hook.f. & Thomson. It has been confirmed that TC-2 induced apoptosis of colon cancer cells (HCT) cells by triggering ROS production (Sharma et al., 2018). In addition, cordycepin (Figure 3) inhibited cell growth and induced apoptosis on human brain cancer cells (SH-SY5Y and U251), related to ROS-mediated apoptosis pathway, accompanied by upregulation the expression of P53, Bax, Caspase-3/9, and downregulation the levels of Bcl-2, GPX and SOD (Chaicharoenaudomrung et al., 2018).
TTMs Targeting PI3K/Akt and JAK2/STAT3
The phosphatidylinositol-3 kinase (PI3K) signaling pathway is involved in many cancer processes. Meanwhile, the serine/threonine specific protein kinase Akt, the main downstream effector of PI3K, is frequently activated (Chen, 2016). In addition, Akt is a key regulator of the survival, proliferation, differentiation, apoptosis, and metabolism of cancer cells. Therefore, in recent years, PIK/Akt has received considerable attention in cancer research. Signal transducer and activator of the activator of transcription 3 (STAT3) can regulate the cancer cell proliferation, apoptosis and survival by activating Janus kinase 2 (JAK-2) (Lv et al., 2016; Hoshyar and Mollaei, 2017).
Rhodiola species are genera of perennial plants of the family Crassulaceae, which grow in high-altitude and cold areas in China, such as Tibet, Sichuan, Yunnan, and Qinghai (Xia et al., 2005). Among these species, Rhodiola crenulata and R. kirilowii are the most commonly used species of Hong-jing-tian as folk medicine in China. Modern studies have shown that Rhodiola species possesses a wide range of pharmacological activities, such as anti-altitude sickness, immunomodulatory, anti-inflammatory, antifatigue, and anticancer activities (Kumar et al., 2010; Tao et al., 2019). It is noteworthy that R. kirilowii was reported to show potential anticancer activity. It has been found that ethanol extract of R. kirilowii in the concentration range of 10–40 mg/ml inhibited human breast cancer cells (MDA-MB-231 and HUVEC) migration and invasion, and significantly decreased phosphorylation of Akt and PKC on PI3K/Akt signaling pathway (Wang and Lin 2015). Salidroside (Figure 3), a p-hydroxyphenethyl-β-D-glucoside, was isolated from Rhodiola species, has been reported to exhibit extensive anticancer effects. It has been verified that salidroside induced apoptosis and autophagy in human colorectal cancer cells (HT-29) through inhibition of PI3K/Akt/mTOR pathway at 0.5, 1 and 2 mM (Fan et al., 2016). In addition, salidroside also induced apoptosis in renal cell carcinoma (A498 and 786-0), and reduced the levels of p-STAT3 and p-JAK2 at a concentration of 60 μM (Lv et al., 2016).
Pterocephali herba is the whole herb of the perennial plant Pterocephalus hookeri (C.B. Clarke) Höeck, a member of the Dipsacaceae family. P. hookeri has clearing heat and detoxifying functions in TTM. It is mainly used to treat rheumatoid arthritis and influenza. Recent research found that n-butanol extracts of P. hookeri with 50–200 μg/ml inhibited proliferation and induced apoptosis on Hep3B cancer cells, blocked PI3K/Akt pathway, and regulated the levels of Bcl-2 family proteins (Guo C. et al., 2015). Waltonitone (Figure 3), a pentacyclic triterpenoid of ursane type compound, was isolated from Gentiana waltonii, inhibited the cell growth, and induced apoptosis on hepatocellular carcinoma a BEL-7420 cells by modulating Akt and ERK1/2 pathway (Zhang et al., 2009). In addition, 2-acetyl-benzylamine (Figure 3) isolated from Justicia adhatoda L. (syn. Adhatoda vasica Nees) (0.42, 0.84, and 1.68 mM) could induce apoptosis, inhibit the expression of JAK2/STAT3, and regulate Bcl-2/Bax ratios in MOLM-14 and NB-4 cells (Balachandran, et al., 2017).
TTMs That Downregulate the NF-κB Pathway
The nuclear factor-kappa B (NF-κB) pathway is one of the most important cellular signal transduction pathways involved in immunity, inflammation, proliferation, and apoptosis. Most of studies showed that NF-κB played a key role in cancer progression. Activation of NF-κB leads to either upregulation of antiapoptotic genes (FLIP, cIAP, survivin, Bcl-2, and Bcl-XL) or downregulation of apoptotic genes (Li et al., 2017). Therefore, the combination of chemotherapy drugs with NF-κB inhibitors is considered to be an effective therapeutic strategy for the treatment of cancer.
Berberis aristata, known as Ji-er-wa (Tibetan: སྐྱེར་པའི་བར་ཤུན།) in TTM, has been widely used to treat inflammation and diabetes (Belwal et al., 2020) due to its anti-inflammatory and immune-potentiating properties. Serasanambati et al. (2015) found that different concentrations (125, 250, and 500 μg/ml) of the methanolic extracts of B. aristata could significantly inhibit cell migration and induce apoptosis in human breast cancer cells (MCF-7). Berberine and palmatine are isoquinoline alkaloids (Figure 3), which can be extracted from some medicinal plants, such as Berberis aristata, B. kansuensis, B. diaphana, B. vernae, and Coptis chinensis (Serasanambati et al., 2015; Li et al., 2018b; Neag et al., 2018; Sheng et al., 2018). Berberine exhibits multiple biologic effects with low toxicity, and the antitumor activities in various human cancer cells have been reported (Yip and Ho, 2013; Zhao et al., 2017; Yao et al., 2018). Berberine (80–160 µmol/l) induced apoptosis by suppressing NF-κB nuclear translocation via Set9-mediated lysine methylation, decreasing the levels of miR21 and Bcl-2 (Hu et al., 2012). Meanwhile, berberine (10, 50, and 100 μM) could inhibit the growth of HepG2 cells by promoting apoptosis through the NF-κB p65 pathway (Li et al., 2017). It is worth noting that palmatine-induced apoptosis was associated with decreased activation of NF-κB and downstream target gene FLIP (Hambright et al., 2015).
Gentianopsis paludosa (Hook.f.) Ma is an annual Gentianaceae plant. As a traditional Tibetan medicinal material, it has been widely used as an herb in China because of its clearing heat and detoxifying functions. Lu et al. (2016) found that ethanol extract of G. paludosa could induce apoptosis of colon cancer cells (SW480), and the mechanism might be partly related to the NF-κB signaling pathway. In addition, some compounds of TTMs can also downregulate the NF-κB pathway. Cordycepin (75, 110, and 145 μmol/L) could inhibit the proliferation and induct the apoptosis of A549 cells dose-dependently, increase the expression of Bax and cleaved caspase-3, decrease the expression of Bcl-2, and the mechanism of action was achieved by inhibiting the NF-κB pathway (Zhang et al., 2015). Choi et al. (2009) showed that gallic acid (5, 10, 25, and 50 μM) inhibited inflammatory responses caused in A549 lung cancer cells by other stimuli, including lipopolysaccharide, IFN-γ, and interleukin-1β, and further downregulated the expression of NF-κB-regulated antiapoptotic genes.
TTMs That Mediate the MAPK Pathway
Mitogen-activated protein kinases (MAPKs) belong to an evolutionarily conserved and ubiquitous signal transduction superfamily of Ser/Thr protein kinases. The MAPK pathway is involved in the growth, development, proliferation, and differentiation of various cells. The MAPK pathway, involving its major subgroups ERK1/2, JNK, and p38 MAPK, is involved in physiological processes such as the growth, development, proliferation, and differentiation of various cells (Zhao H. J. et al., 2015). More and more studies have shown that the MAPK pathway plays important roles in the process of apoptosis transduction and is significantly related to the occurrence and development of breast, ovarian, esophageal, colon, stomach, and liver cancers (Yoon et al., 2018).
It is worth noting that Phyllanthus emblica L. can induce cancer cell apoptosis through the MAPK pathway. Zhao H.J. et al. (2015) found that the tannin fraction of P. emblica (15, 30, and 60 mg/L) dose-dependently induced apoptosis of human lung squamous carcinoma cells (NCI-H1703) by suppressing the expression of p-ERK1/2, MMP-2/9, upregulating the expression of p-JNK. Therefore, the tannin fraction of P. emblica induced apoptosis via the MAPK/MMP pathways. Furthermore, berberine, a famous small molecule compound from TTM, also has the function of regulating MAPK pathway. Zheng et al. (2014) and Kim et al. (2015) reported similar results that berberine-induced apoptosis was mediated by activation of the p38 MAPK signaling pathway via the death receptor ligand FOXO3a, p53, and FasL. In another study, berberine also promoted the rate of apoptosis of NSCLC cells by the suppression of the MMP-2, Bcl-2/Bax, and modulating the miR-19a/TF/MAPK signaling pathway (Chen et al., 2019).
TTMs That Activate AMPK Pathway
The AMP-activated protein kinase (AMPK), which is a conserved heterotrimeric protein kinase, is an important “energy sensor” regulating intracellular metabolism and energy balance and is very sensitive to changes in AMP/ATP ratio (Pan et al., 2017). AMPK is rapidly activated when cellular energy metabolism is abnormal, such as starvation, hypoxia, and ischemia (Ortiz et al., 2014). A series of studies have found that AMPK has strong proapoptotic potential under activated conditions. In summary, AMPK can be an important target for the treatment of cancer.
In addition to the mechanisms described above, berberine can also be used to treat cancer by activating AMPK. It was found that after berberine (12.5 and 50 μM) pretreatment of hepatocellular carcinoma cells (HepG2), the levels of p-AMPK and p-Akt were significantly increased. In addition, activation of AMPK was associated with caspase-dependent mitochondrial pathway apoptosis, coupled with mitochondrial cytochrome c release and activation of Caspase-3/9, with a dose-dependent increase in the Bax/Bcl-2 ratio. Therefore, berberine could selectively inhibit HepG2 cells’ growth by inducing AMPK-mediated caspase-dependent mitochondrial pathway apoptosis (Yang and Huang, 2013). Moreover, berberine (10 and 80 μM) enhanced Doxorubicin sensitivity of drug-resistant in MCF-7/MDR breast cancer cells via AMPK/HIF-1α/P-gp pathway and directly induced apoptosis through the AMPK/p53 pathway (Pan et al., 2017).
Salidroside has a wide range of pharmacological activities, especially antiplateau hypoxia and immune-enhancing effects. It has been reported that salidroside can reduce superoxide dismutase (SOD) level in the mitochondria and improve endurance exercise performance. Therefore, it can be considered that salidroside reduces the production of SOD due to its effect on oxygen consumption, resulting in the change of ATP and finally the activation of AMPK. This was discovered in bladder cancer cells (UMUC3) by Liu et al. (2011). It is worth noting that salidroside could induce the autophagy-related apoptosis on human acute monocytic leukemia cells (THP-1 and U937) through AMPK activation via downregulating p62, p-PI3K, p-AKT, and p-mTOR expressions and upregulating Beclin1, LC3II and AMPK expressions (Ge et al., 2019).
Conclusion Remarks
Traditional medicines are the gifts of nature to humans. Many drugs that are commonly used in modern medicine, such as artemisinin, paclitaxel, camptothecin, and ephedrine, are derived directly or indirectly from these natural medicines. TTM is an ancient health system and part of the world’s traditional medical system. This system uses various treatments and personalized approaches to prevent and treat a wide range of diseases, especially chronic diseases, such as cancer.
In this review, we attempt to summarize the traditional Tibetan medical theory on the knowledge and treatment of cancer. The results showed that, in TTM, the direct cause of cancer is the shrinking and aggregating of “bad blood” owing to the reverse effect of loong (Figure 1). In addition, we review the natural Tibetan medicines traditionally used in the Tibetan system of medicine for cancer treatment. More importantly, some TTMs and their effects on apoptotic pathways are summarized in Table 4. Most TTMs exert anticancer effects through multiple components and multiple pathways. As previously mentioned, apoptosis is one of the main mechanisms by which TTM induces cancer cell death. Therefore, the molecular mechanisms of Tibetan medicine targeting apoptosis pathways are worthy of further study. However, in addition to apoptosis pathway targets, other cell death pathways may be triggered by TTMs. For example, some TTMs show anticancer activity by enhancing immunity. In order to fully evaluate the anticancer potential of these TTMs and their active ingredients, multidisciplinary approaches should be integrated to conduct pharmacological studies and reveal their mechanisms of action.
In addition, current research on TTMs is insufficient and limited. First, according to statistics (Jia and Zhang, 2016), 3,105 natural medicines have been used in the Tibetan medicine theory system. However, only 40 species have been demonstrated to possess cancer-related biological activity, and most species still lack sufficient experimental evidence. For example, brag-zhun is a natural exudate from rock stratum, which sometimes contains animal feces. Brag-zhun and its preparations are commonly used in Tibetan medicines for cancer therapy. However, to date, reports on the biological activity of the medicine associated with cancer are unavailable. Similarly, Swertia chirayita (Roxb. ex Fleming) H. Karst. and Halenia elliptica D.Don also lack cancer-related research. Given the high frequency of natural medicines being used in the treatment of cancer, supplementing these gaps in research is necessary. Secondly, although some compounds that are isolated from TTMs exhibit cancer-related biological activities, their cellular and molecular mechanisms, and possible synergies among these compounds have not been clearly elucidated. Third, TTM mainly uses prescriptions to treat cancer in clinic, but relevant research to support their application is limited. Only studies involving Yukyung Karne have been reported. Addressing these limitations in future research is necessary. Moreover, although some Tibetan herbal medicines can induce the death of cancer cells through the apoptotic pathways in vitro, these herbs have a weak anticancer effect on animal models. Therefore, in vivo experiments are necessary to verify the anticancer effects and molecular mechanisms of these TTMs.
In conclusion, this review provides the first compilation of data on TTM for cancer treatment. We found that some TTMs (e.g., O. sinensis, P. emblica, and Rhodiola kirilowii) and their active ingredients (e.g., cordycepin, salidroside, and gallic acid) have good anticancer activity. The molecular mechanisms are mainly through targeting some apoptotic pathways in cancer, for example, Bcl-2/Bax, caspases, PI3K/Akt, JAK2/STAT3, MAPK, and AMPK. These herbs and natural compounds would be potential drug candidates for cancer treatment and deserve further research and development.
Author Contributions
CT and C-CZ: collected and organized the data and wrote the paper. HY and X-YW: collected the data. Z-JG: wrote the Tibetan names of natural medicines. YZ: amended the paper. YL and GF: conceived and designed the study and amended the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 81874370 and 81903922), the Major Cultivation Project of Scientific and Technological Achievements Transformation of Sichuan Provincial Department of Education (No. 18CZ0011), the National Key Research and Development Program of China (No. 2017YFC1703900), and “Xinglin Scholars” Research Promotion Program of Chengdu University of Traditional Chinese Medicine (BSH2019002).
References
Afify, A. E. M. M. R., Fayed, S. A., Shalaby, E. A., EI-Shemy, H. A. (2011). Syzygium cumini (pomposia) active principles exhibit potent anticancer and antioxidant activities. Afr. J. Pharm. Pharmacol. 5, 948–956. doi: 10.5897/AJPP10.420
Ahmed, H. H., Galal, A. F., Shalby, A. B., Abd-Rabou, A. A., Mehaya, F. M. (2018). Improving anti-cancer potentiality and bioavailability of gallic acid by designing polymeric nanocomposite formulation. Asian Pac. J. Cancer Prev. 19, 3137–3146. doi: 10.31557/APJCP.201-8.19.11.3137
Ali, H., Dixit, S. (2013). Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci. World J. 2013, 376216. doi: 10.1155/2013/376216
Anitha, P., Priyadarsini, R. V., Kavitha, K., Thiyagarajan, P., Nagini, S. (2013). Ellagic acid coordinately attenuates Wnt/β-catenin and NF-κB signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur. J. Nutr. 52, 5–84. doi: 10.1007/s00394-011-0288-y
Aramwit, P., Porasuphatana, S., Srichana, T., Nakpheng, T. (2015). Toxicity evaluation of cordycepin and its delivery system for sustained in vitro anti-lung cancer activity. Nanoscale Res. Lett. 10, 152. doi: 10.1186/s11671-015-0851-1
Arpornsuwan, T., Petvises, S., Thim-uam, A., Boondech, A., Roytrakul, S. (2012). Effects of Carthamus tinctorius L. solvent extracts on anti-proliferation of human colon cancer (SW 620 cell line) via apoptosis and the growth promotion of lymphocytes. Songklanakarin J. Sci. Technol. 34, 45–51.
Aung, H. H., Wang, C. Z., Ni, M., Fishbein, A., Mehendale, S. R., Xie, J. T., et al. (2017). Crocin From Crocus Sativus Possesses Significant Anti-Proliferation Effects on Human Colorectal Cancer Cells. Exp. Oncol. 29, 175–180. doi: mdl-18004240
Balachandran, C., Arun, Y., Sangeetha, B., Duraipandiyan, V., Awale, S., Emi, N., et al. (2017). In vitro and in vivo anticancer activity of 2-acetyl-benzylamine isolated from Adhatoda vasica L. leaves. Biomed. Pharmacother. 93, 796–806. doi: 10.1016/j.biopha.2017.06.096
Bassa, L. M., Jacobsm, C., Gregory, K., Henchey, E., Ser-Dolansky, J., Schneider, S. S. (2016). Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine 23, 87–94. doi: 10.1016/j.phymed.2015.11.014
Bauer-Wu, S., Lhundup, T., Tidwell, T., Lhadon, T., Ozawa-de Silva, C., Dolma, J., et al. (2014). Tibetan medicine for cancer: an overview and review of case studies. Integr. Cancer Ther. 13, 502–512. doi: 10.1177/1534735414549624
Belwal, T., Bisht, A., Devkota, H. P., Ullah, H., Khan, H., Bhatt, I. D., et al. (2020). Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharmacol. 11, 41.
Bhardwaj, P., Bhardwaj, G., Raghuvanshi, R., Thakur, M. S., Kumar, R., Chaurasia, O. P. (2018). Rhodiola: An overview of phytochemistry and pharmacological applications. New Age Herbals., 71–113. doi: 10.1007/978-981-10-8291-7-5
Bisen, P. S., Bundela, S. S., Sharma, A. (2012). Ellagic acid–chemopreventive role in oral cancer. J. Cancer Sci. Ther. 4, 23–30. doi: 10.4-172/1948-5956.1000106
Ceci, C., Lacal, P. M., Tentori, L., De Martino, M. G., Miano, R., Graziani, G. (2018). Experimental evidence of the antitumor, antimetast-atic and antiangiogenic activity of ellagic acid. Nutrients 10, E1756. doi: 10.3390/nu10111756
Chaicharoenaudomrung, N., Jaroonwitchawan, T., Noisa, P. (2018). Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol. In Vitro 46, 113–121. doi: 10.1016/j.tiv.2017.10.002
Chen, C. Y., Kuo, P. L., Chen, Y. H., Huang, J. C., Ho, M. L., Lin, R. J., et al. (2010). Tyrosinase inhibition, free radical scavenging, antimicroor-ganism and anticancer proliferation activities of Sapindus mukorossi extracts. J. Taiwan Inst. Chem. E. 41, 129–135. doi: 10.1016/j.jtice.200-9.08.005
Chen, Y., Chen, Y. C., Lin, Y. T., Huang, S. H., Wang, S. M. (2010). Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J. Agric. Food Chem. 58, 11645–11652. doi: 10.1021/jf1028976
Chen, X., Yang, G. M., He, Y. Y., Cai, B. C. (2011). Antitumor effect of alkaloids from Oxytropis Falcata on S180 bearing mice. Chin. J. Tradit. Chin. Med. Pharm. 26, 2540–2542.
Chen, Q. L., Chen, X. Y., Zhu, L., Chen, H. B., Ho, H. M., Yeung, W. P., et al. (2016). Review on Saussurea laniceps, a potent medicinal plant known as “snow lotus”: botany, phytochemistry and bioactivities. Phytochem. Rev. 15, 537–565. doi: 10.1007/s11101-015-9452-y
Chen, X. H., Cheng, H. Q., Deng, Y., Yang, G. M., Pan, Y. (2017). Effect of Tibetan medicine Oxytropis Falcata on mitochondrial transmembrane potential and expression of apoptosis related proteins in SMMC-7721. J. Nanjing Univ. Tradit. Chin. Med. 33, 54–58. doi: 10.14148/j.issn.1672-0482.2017.0054
Chen, Q. Q., Shi, J. M., Ding, Z., Xia, Q., Zheng, T. S., Ren, Y. B., et al. (2019). Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manage. Res. 11, 9005–9015. doi: 10.2147/CMAR.S207677
Chen, Z. Z. (2016). Berberine induced apoptosis of human osteosarcoma cells by inhibiting phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) signal pathway activation. Iran. J. Public Health 45, 578–585.
Cheng, H. Q., Niu, L. R., Li, G. C., Yang, G. M., Pan, Y. (2018). Effect of serum containing liposoluble alkaloids from Oxytropis Falcata on viability, apoptosis and life cycle of human lung adenocarcinoma A549 cells in vitro. Tradit. Chin. Drug Res. Pharmacol. 29, 535–539. doi: 10.19378/j.issn.1003-9783.2018.05.001
Choi, K. C., Lee, Y. H., Jung, M. G., Kwon, S. H., Kim, M. J., Jun, W. J., et al. (2009). Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol. Cancer Res. 7, 2011–2021. doi: 10.1158/1541-7786.MCR-09-0239
Colapietro, A., Mancini, A., D’Alessandro, A. M., Festuccia, C. (2018). Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anticancer Agents Med. Chem. 19, 38–47. doi: 10.2174/187152061966618123111-2453
Das, U., Biswas, S., Chattopadhyay, S., Chakraborty, A., Dey Sharma, R., Banerji, A., et al. (2017). Radiosensitizing effect of ellagic acid on growth of hepatocellular carcinoma cells: an in vitro study. Sci. Rep. 7, 14043. doi: 10.1038/s41598-017-14211-4
Deng, W. H., Xiao, E., Xiong, H., Mo, S. S., Mei, Z. N. (2012). Experimental study on anti-tumor effect of the total saponins from the crude and processed products of Entada phaseoloides. Chin. J. Exp. Tradit. Med. Form. 18, 148–150. doi: 10.13422/j.cnki.syfjx.2012.06.004
Dhondrup, W., Tidwell, T., Wang, X., Tso, D., Dhondrup, G., Luo, Q., et al. (2019). Tibetan medical informatics: An emerging feld in Sowa Rigpa pharmacological & clinical research. J. Ethnopharmacol. 250, 112481. doi: 10.1016/j.jep.2019.112481
Dimaer, D. Z. P. C. (2012). Jing Zhu Materia Medica (Shanghai: Shanghai Science and Technology Press).
Ding, L., Liu, B., Zhang, S. D., Hou, Q., Qi, L. L., Zhou, Q. Y. (2011). Cytotoxicity, apoptosis-inducing effects and structure-activity relationships of four natural xanthones from Gentianopsis paludosa Ma in HepG2 and HL-60 cells. Nat. Prod. Res. 25, 669–683. doi: 10.1080/1476410802497398
Eskandari, E., Heidarian, E., Amini, S. A., Saffari-Chaleshtori, J. (2016). Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J. Cancer Res. Ther. 12, 1266–1271. doi: 10.4103/0973-1482.165873
Fan, J., Wang, P., Wang, X., Tang, W., Liu, C., Wang, Y., et al. (2015a). Induction of mitochondrial dependent apoptosis in human leukemia K562 cells by Meconopsis integrifolia: A species from traditional Tibetan medicine. Molecules 20, 11981–11993. doi: 10.3390/molecules-200711981
Fan, J., Wang, Y., Wang, X., Wang, P., Tang, W., Yuan, W., et al. (2015b). The antitumor activity of Meconopsis horridula Hook, a traditional Tibetan medical plant, in murine leukemia L1210 cells. Cell. Physiol. Biochem. 37, 1055–1065. doi: 10.1159/000430231
Fan, Y., Fan, J. P., Guo, D. Y., Li, T., Ma, X. J., Du, J. H. (2013). The molecular mechanisms of Meconopsis racemosa Maxim. extract on proliferation of K562 cells. Chin. J. Chin. Mater Med. 36, 1143–1146. doi: 10.13863/j.issn1001-4454.2013.07.037
Fan, X. J., Wang, Y., Wang, L., Zhu, M. (2016). Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol. Rep. 36, 3559–3567. doi: 10.3892/or.2016.5138
Fang, H. J., Li, Y., Wu, W., Zhu, Y. J., Zheng, Z. (2018). Effect of Lagopsis Supina ethanol extract on the proliferation and apoptosis of HCT-116 cells. Chin. Med. Mod. Dis. Edu. Chin. 16, 84–86. doi: 10.3969/j.issn.1672-2779.2018.22.037
Gauger, K. J., Rodríguez-Cortés, A., Hartwich, M., Schneider, S. S. (2010). Rhodiola crenulata inhibits the tumorigenic properties of invasive mammary epithelial cells with stem cell characteristics. J. Med. Plant Res. 4, 446–454. doi: 10.5897/JMPR09.395
Ge, C., Zhang, J., Feng, F. (2019). Salidroside enhances the anti-cancerous effect of imatinib on human acute monocytic leukemia via the induction of autophagy-related apoptosis through AMPK activation. RSC Adv. 9, 25022. doi: 10.1039/c9ra01683j
Guo, C., Wu, Y., Zhu, Y., Wang, Y., Tian, L., Lu, Y., et al. (2015). In vitro and In vivo antitumor effects of n-butanol extracts of Pterocephalus hookeri on Hep3B cancer cell. Evid. Based Complement. Alternat. Med. 2015, 159132. doi: 10.1155/2015/159132
Guo, C. X., Wu, Y. C., Zhu, Y. Z., Tian, L. L., Lu, Y., Han, C., et al. (2015). Inhibits human liver hep3B cell proliferation and invasion and metastasis of n-butanol part of Pterocephalus hookeri in vitro. Chin. J. Exp. Tradit. Med. Form. 21, 100–105. doi: 10.13422/j.cnki.syfi-x.2-015030100
Hagiwara, Y., Kasukabe, T., Kaneko, Y., Niitsu, N., Okabe-Kado, J. (2010). Ellagic acid, a natural polyphenolic compound, induces apoptosis and potentiates retinoic acid-induced differentiation of human leukemia HL-60 cells. Int. J. Hematol. 92, 136–143. doi: 10.1007/s12185-010-0627-4
Hambright, H. G., Batth, I. S., Xie, J., Ghosh, R., Kumar, A. P. (2015). Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFκB/FLIP. Mol. Carcinog. 54, 1227–1234. doi: 10.1002/mc.22192
Hao, W. Q. (2014). Study on chemical constituents of the alkaloids from the root of Aconitum flavum Hand.-Mazz and its anti-tumor activities (Yinchuan: Ningxia Medical University).
Ho, H. H., Chang, C. S., Ho, W. C., Liao, S. Y., Wu, C. H., Wang, C. J. (2010). Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 48, 2508–2516. doi: 10.1016/j.fct.2010.06.024
Ho, Y. T., Lu, C. C., Yang, J. S., Chiang, J. H., Li, T. C., Ip, S. W., et al (2009). Berberine Induced Apoptosis via Promoting the Expression of caspase-8, -9 and -3, Apoptosis-Inducing Factor and Endonuclease G in SCC-4 Human Tongue Squamous Carcinoma Cancer Cells. Anticancer Res. 29, 4063–4070.
Hoshyar, R., Mollaei, H. (2017). A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 69, 1419–1427. doi: 10.1111/jphp.12776
Hu, H., Li, K., Wang, X., Liu, Y., Lu, Z., Dong, R., et al. (2012). Set9, NF-κB and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 34, 157–166. doi: 10.1038/aps.2012.161
Hu, X., Zhang, X., Qiu, S., Yu, D., Lin, S. (2010). Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 398, 62–67. doi: 10.1016/j.bbrc.2010.06.033
Jayakumar, T., Chiu, C. C., Wang, S. H., Chou, D. S., Huang, Y. K., Sheu, J. R. (2014). Anti-cancer effects of CME-1, a novel polysaccharide, purified from the mycelia of Cordyceps sinensis against B16-F10 melanoma cells. J. Cancer Res. Ther. 10, 43–49. doi: 10.4103/0973-148-2.131365
Jia, M. R., Zhang, Y. (2016). Dictionary of Chinese ethnic medicine (Beijing: China Medical Science and Technology Press).
Jia, Z. P., Li, M. X., Zhang, R. X., Wang, J. H., Wang, M., Guo, X. N., et al. (2005). Vitro screening of the effective antitumor or components on Herba Lamiophlam is rotate. Med. J. NDFNC. 26, 173–175. doi: 10.3969/j.issn.1007-8622.2005.03.005
Kang, D. Y., Sp, N., Kim, D. H., Joung, Y. H., Lee, H. G., Park, Y. M., et al. (2018). Salidroside inhibits migration, invasion and angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int. J. Oncol. 53, 877–885. doi: 10.3892/ijo.2018.4430
Kang, Y. J. (2016). Effects and mechanisms of petroleum ether extract of Lamiophlomis rotate on proliferation of human tongue squamous cell carcinoma Tca8113 cell line. (Lanzhou: Lanzhou University).
Kao, T. Y., Chung, Y. C., Hou, Y. C., Tsai, Y. W., Chen, C. H., Chang, H. P., et al. (2012). Effects of ellagic acid on chemosensitivity to 5-fluorouracil in colorectal carcinoma cells. Anticancer Res. 32, 4413–4418.
Karimi-Khouzani, O., Heidarian, E., Amini, S. A. (2017). Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol. Rep. 69, 830–835. doi: 10.1016/j.pharep.2017.03.011
Khan, L. U., Khan, R. A., Khan, S., Bano, S. A., Fasim, F. F., Uzair, B. (2017). Phytochemical Screening and Assessment of Pharmacological Properties of Swertia chirayita (Roxb. ex Fleming) Root Methanolic Extract. Int. J. Pharmacol. 13, 1000–1009. doi: 10.3923/ijp.2017.1000.1009
Khatamian, N., Homayouni Tabrizi, M., Ardalan, P., Yadamani, S., Darchini Maragheh, A. (2019). Synthesis of Carum Carvi essential oil nanoemulsion, the cytotoxic effect, and expression of caspase 3 gene. J. Food Biochem. 43, e12956. doi: 10.1111/jfbc.12956
Kim, J. S., Oh, D., Yim, M. J., Park, J. J., Kang, K. R., Cho, I. A., et al. (2015). Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol. Rep. 33, 1775–1782. doi: 10.3892/or.2015.3768
Kumar, R., Tayade, A., Chaurasia, O. P., Sunil, H., Singh, S. B. (2010). Evaluation of anti-oxidant activities and total phenol and flavonoid content of the hydro-alcoholic extracts of Rhodiola sp. Phcog. J. 2, 431–435. doi: 10.1016/s0975-3575(10)80027-6
Lei, X. D., Zhu, G. F., Cui, W. X., Fan, S. J., Zhong, W. C., Jiang, X. Y. (2011). Effects of tumor cell proliferation of total saponins of Pterocephalus hookeri on in vitro. Lishizhen Med. Mater. Med. Res. 22, 1518–1519. doi: 10.3969/j.issn.1008-0805.2011.06.113
Li, H., Chen, C. (2017). Inhibition of autophagy enhances synergistic effects of Salidroside and anti-tumor agents against colorectal cancer. BMC Complement Altern. Med. 17, 538. doi: 10.1186/s12906-017-2046-z
Li, M., Zhang, M., Zhang, Z. L., Liu, N., Han, X. Y., Liu, Q. C., et al. (2017). Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncol. Res. 25, 233–239. doi: 10.3727/096504016X1-4742891049073
Li, J., Liu, F., Jiang, S., Liu, J., Chen, X., Zhang, S., et al. (2018). Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 15, 7409–7414. doi: 10.3892/-ol.2018.8249
Li, Q., Li, H. J., Xu, T., Du, H., Fan, G., Zhang, Y. (2018a). Analysis on medication regularities of traditional Tibetan medicine treating hepatitis based on data mining. Chin. J. Ethnom. Ethnop. 27, 4–7.
Li, Q., Du, H., Wen, H. S., Xu, T., Wang, Y. J., Lai, X. R., et al. (2018b). Determination of six compounds in Berberidis Cortex and comparative study of its different species. Zhongguo Zhong Yao Za Zhi. 44, 968–974. doi: 10.19540/j.cnki.cjcmm.2018-1226.014
Limpisophon, K., Schleining, G. (2017). Use of gallic acid to enhance the antioxidant and mechanical properties of active fish gelatin film. J. Food Sci. 82, 80–89. doi: 10.1111/1750-3841.13578
Linghu, L., Fan, H., Hu, Y., Zou, Y., Yang, P., Lan, X., et al. (2014). Mirabijalone E: a novel rotenoid from Mirabilis himalaica inhibited A549 cell growth in vitro and in vivo. J. Ethnopharmacol. 155, 326–333. doi: 10.1016/j.jep.2014.05.034.
Liu, Z., Li, X., Simoneau, A. R., Jafari, M., Zi, X. (2011). Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol. Carcinog. 51, 257–267. doi: 10.1002/mc.20780
Liu, X. N., Li, Y. J., Yang, Q., Chen, Y., Weng, X. G., Wang, Y. W., et al. (2012). Comparative study on tumor cell apoptosis in vitro induced by extracts of Stellera chamaejasme. Chin. J. Chin. Mater Med. 37, 1440–1444. doi: 10.4268/cjcmm20121020
Liu, X. L., Zhao, M. M., Wu, K. G., Chai, X. H., Yu, H. P., Tao, Z. H., et al. (2012). Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.). Food Chem. 131, 685–690. doi: 10.1016/j.foodchem.2011.09.063
Liu, D. D., Ye, Y. L., Zhang, J., Xu, J. N., Qian, X. D., Zhang, Q. (2014). Distinct pro-apoptotic properties of Zhejiang saffron against human lung cancer via a caspase-8-9-3 cascade. Asian. Pac. J. Cancer Prev. 215, 6075–6080. doi: 10.7314/apjcp.2014.15.15.6075
Liu, Y., Bi, T., Dai, W., Wang, G., Qian, L., Gao, Q., et al. (2016a). Effects of oxymatrine on the proliferation and apoptosis of human hepatoma carcinoma cells. Technol. Cancer Res. Treat. 15, 487–497. doi: 10.1177/1533034615587616
Liu, Y., Bi, T., Dai, W., Wang, G., Qian, L., Gao, Q., et al. (2016b). Oxymatrine synergistically enhances the inhibitory effect of 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Tumour. Biol. 37, 7589–7597. doi: 10.1007/s13277015-4642-1
Li-Weber, M. (2013). Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 332, 304–312. doi: 10.1016/j.canlet.2010.07.0-15
Lo, C., Lai, T. Y., Yang, J. H., Yang, J. S., Ma, Y. S., Weng, S. W., et al. (2010). Gallic acid induces apoptosis in A375.S2 human melanoma cells through caspase-dependent and -independent pathways. Int. J. Oncol. 37, 377–385. doi: 10.3892/ijo00000686
Long, A., Fu, J., Hu, Y., Luo, Y. (2013). Annphenone from Artemisia vestita inhibits HepG2 cell proliferation. Asian J. Chem. 17, 9497–9502. doi: 10.14233/ajchem.2013.15043
Lu, D., Lu, D., Zhang, S., Wang, X., Zhen, J. (2011). The study of cytostatic effect on MCF-7 cells of the alcohol extract of Rhodiola Algida Var. Tangutica. Proc. Environ. Sci. 8, 615–619. doi: 10.1016/j.proenv.2011.10.095
Lu, D., Zhang, S., Ge, R., Lu, D. (2011). The preliminary study of antiproliferative effect on MCF-7 cells of Rhodiola algida Var. tangutica. The 2011 IEEE/ICME International Conference on Complex Medical Engineering. 23–25. doi: 10.1109/iccme.2011.5876698
Lu, D. X., Zhang, S. N., Wang, W. P., Zhen, J. (2011). “The study of cytostatic effect on MCF-7 cells of the alcohol extract of Rhodiola Algida Var. Tangutica,” in 2011 3rd International Conference on Bioinformatics and Biomedical Technology (ICBBT 2011), 228–230.
Lu, P., Lin, H., Gu, Y., Li, L., Guo, H., Wang, F., et al. (2015). Antitumor effects of crocin on human breast cancer cells. Int. J. Clin. Exp. Med. 8, 20316–20322.
Lu, N. H., Zhao, H. Q., Chen, Z. J., Jing, M., Zhang, Y. X. (2016). Study on mechanism of Tibetan Gentianopsis paludosa (Hook. f.) Ma. extraction (GPE) inducing colon cancer cells apoptosis by NF-κB signaling pathway. J. Pharm. Res. 35, 129–132. doi: 10.13506/j.cnki.j-pr.2016.03.002
Luo, Y., Yu, H., Yang, Y., Tian, W., Dong, K., Shan, J., et al. (2016). A flavonoid compound from Chrysosplenium nudicaule inhibits growth and induces apoptosis of the human stomach cancer cell line SGC-7901. Pharm. Biol. 54, 1133–1139. doi: 10.3109/13880209.2015.1055634
Lv, C., Huang, Y., Liu, Z. X., Yu, D., Bai, Z. M. (2016). Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 17, 41–47. doi: 10.3233/CBM-160615
Ma, X. L., Liu, J. Z., Cao, S. Y., Wang, M., Zhang, X. Y. (2013). Anti tumor effect of processing Stellera chamaejasme with liquor on tumor-bearing mice. Chin. Tradit. Pat. Med. 35, 1143–1147. doi: 10.3969/j/issn.1001-1528.2013.06.009
Ma, Y., Feng, C., Wang, J., Chen, Z., Wei, P., Fan, A., et al. (2019). Hydroxyl safflower yellow A regulates the tumor immune microenvironment to produce an anticancer effect in a mouse model of hepatocellular carcinoma. Oncol. Lett. 17, 3503–3510. doi: 10.3892/ol.2019.9946
Ma, C. H. (2017). Effect and mechanism research of ethanolic extracts of Lamiophlomis rotate on proliferation of MEC-1 cells (Lanzhou: Lanzhou University).
Meeran, S. M., Katiyar, S., Katiyar, S. K. (2008). Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 229, 33–43. doi: 10.1016/j.taap.2007.12.027
Mishra, R., Kaur, G. (2013). Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PloS One 8, e78764. doi: 10.1371/journal.pone.0078764
Mora, M. C., Bassa, L. M., Wong, K. E., Tirabassi, M. V., Arenas, R. B., Schneider, S. S. (2015). Rhodiola crenulata inhibits Wnt/β-catenin signaling in glioblastoma. J. Surg. Res. 197, 247–255. doi: 10.1016/j.jss.2015.02.074
Nakamura, K., Konoha, K., Yamaguchi, Y., Kagota, S., Shinozuka, K., Kunitomo, M. (2003). Combined effects of Cordyceps sinensis and methotrexate on hematogenic lung metastasis in mice. Receptors Channels 9, 329–334. doi: 10.3109/713745176
Nakamura, K., Shinozuka, K., Yoshikawa, N. (2015). Anticancer and Antimetastatic Effects of Cordycepin, an Active Component of Cordyceps Sinensis. J. Pharmacol. Sci. 127, 53–056. doi: 10.1016/j.jphs.2014.09.001
Neag, M. A., Mocan, A., Echeverría, J., Pop, R. M., Bocsan, C. I., Crişan, G., et al. (2018). Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 9, 557.
Ngamkitidechakul, C., Jaijoy, K., Hansakul, P., Soonthornchareonnon, N., Sireeratawong, S. (2010). Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother. Res. 24, 1405–1413. doi: 10.1002/ptr.3127
Ortiz, L. M., Lombardi, P., Tillhon, M., Scovassi, A., II (2014). Berberine, an epiphany against cancer. Molecules 19, 12349–12367. doi: 10.3390/molecules19081-2349
Pai, K. S. R., Srilatha, P., Suryakant, K., Setty, M. M., Nayak, P. G., Rao, C. M., et al. (2011). Anticancer activity of Berberis aristatain Ehrlich ascites carcinoma-bearing mice: A preliminary study. Pharm. Biol. 50, 270–277. doi: 10.3109/13880209.2011.599035
Pan, Y., Zhang, F., Zhao, Y., Shao, D., Zheng, X., Chen, Y., et al. (2017). Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J. Cancer. 8, 1679–1689. doi: 10.7150/jca.19106
Qi, Y. J., Cui, S., Lu, D. X., Yang, Y. Z., Luo, Y., Ma, L., et al. (2015). Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1α, HIF-2α expression in MCF-7 cells under hypoxic condition in vitro. Cancer Cell Int. 1515:, 81. doi: 10.1186/s12935-015-0225-x
Qinghai Institute for Drug Control (1996). Chinese Tibetan Medicine Vol. Vol. 1-3 (Shanghai: Shanghai Science and Technology Press).
Qiu, Z., Zhou, B., Jin, L., Yu, H., Liu, L., Liu, Y., et al. (2013). In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem. Toxicol. 59, 428–437. doi: 10.1016/j.fct.2013.06.025
Reddy, D. B., Reddy, T. C., Jyotsna, G., Sharan, S., Priya, N., Lakshmipathi, V., et al. (2009). Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacol. 124, 506–512. doi: 10.1016/j.jep.2009.05.022
Rogers, C., Alnemri, E. S. (2019). Gasdermins in apoptosis: New players in an old game. Yale J. Biol. Med. 92, 603–617.
Saleem, A., Husheem, M., Härkönen, P., Pihlaja, K. (2002). Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J. Ethnopharmacol. 81, 327–336. doi: 10.1016/s0378-8741(02)00099-5
Serasanambati, M., Chilakapati, S. R., Manikonda, P. K., Kanala, J. R. (2015). Anticancer activity of methanolic extract of Berberis aristata in MCF-7 human breast cancer cell lines. Int. J. Life Sci. Biotech. Pharm. Res. 4, 31–35.
Shao, J. H., Zhao, C. C., Liu, M. X. (2011). Separation and structural identification on the anti-tumor activity of Anemone rivularis. J. Yangzhou Univ. 14, 48–50. doi: 10.19411/j.1007-824x.2011.02.014
Shao, L. W., Huang, L. H., Yan, S., Jin, J. D., Ren, S. Y. (2016). Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol. Lett. 12, 995–1000. doi: 10.3892/ol.2016.4706
Sharma, N., Kumar, A., Sharma, P. R., Qayum, A., Singh, S. K., Dutt, P., et al. (2018). A new clerodane furano diterpene glycoside from Tinospora cordifolia triggers autophagy and apoptosis in HCT-116 colon cancer cells. J. Ethnopharmacol. 211, 295–310. doi: 10.1016/j.jep.2017.09.034
Sheng, J., Zou, X., Cheng, Z., Xiang, Y., Yang, W., Lin, Y., et al. (2018). Recent advances in herbal medicines for sigestive system malignancies. Front. Pharmacol. 9, 1249. doi: 10.3389/fphar.2018.01249
Shi, Z., Dai, Y. X., Liang, C., Hu, S., Tan, R. (2018). Study on chemical constitutions and activity of the ethyl acetate extract from Swertia mussotii. Nat. Prod. Res. Dev. 30, 1918–1922. doi: 10.16333/j.1001-6880.2018.11.011
Shivananjappa, M. M., Joshi, M. K. (2012). Influence of Emblica officinalis aqueous extract on growth and antioxidant defense system of human hepatoma cell line (HepG2). Pharm. Biol. 50, 497–505. doi: 10.3109/13880209
Silva, I. C., Polaquini, C. R., Regasini, L. O., Ferreira, H., Pavan, F. R. (2017). Evaluation of cytotoxic, apoptotic, mutagenic, and chemopreventive activities of semi-synthetic esters of gallic acid. Food Chem. Toxicol. 105, 300–307. doi: 10.1016/j.fct.2017.04.033
Subramanian, A. P., Jaganathan, S. K., Mandal, M., Supriyanto, E., Muhamad, I., II (2016). Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J. Gastroenterol. 22, 3952–3961. doi: 10.3748/wjg.v22.i15.3952
Sun, K. X., Xia, H. W., Xia, R. L. (2015). Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway. Int. J. Clin. Exp. Pathol. 8, 615–621.
Tang, J., Zhao, J. J., Li, Z. H. (2015). Ethanol extract of Artemisia sieversiana exhibits anticancer effects and induces apoptosis through a mitochondrial pathway involving DNA damage in COLO-205 colon carcinoma cells. Bangladesh J. Pharmacol. 10, 518–523. doi: 10.3329/bjp.v10i3.23196
Tao, H., Wu, X., Cao, J., Peng, Y., Wang, A., Pei, J., et al. (2019). Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 39, 1–72. doi: 10.1002/med.21564
Tompkins, K. D., Thorburn, A. (2019). Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J. Biol. Med. 92, 707–718.
Tu, Y., Roberts, L., Shetty, K., Schneider, S. S. (2008). Rhodiola crenulata induces death and inhibits growth of breast cancer cell lines. J. Med. Food. 11, 413–423. doi: 10.1089/jmf.2007.0736
Umesalma, S., Nagendraprabhu, P., Sudhandiran, G. (2015). Ellagic acid inhibits proliferation and induced apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells. Mol. Cell Biochem. 399, 303–313. doi: 10.1007/s11010-014-2257-2
Verma, S., Singh, A., Mishra, A. (2013). Gallic acid: molecular rival of cancer. Environ. Toxicol. Pharmacol. 35, 473–485. doi: 10.1016/j.e-tap.2013.02.011
Wang, Y. Y., Lin, H. (2015). Mechanism of anti-tumor metastasis for Rhodiola extraction in vitro. Tianjin Pharm. 27, 1–4. doi: 10.3969/j.issn.1006-5687.2015.06.001
Wang, M., Wang, X. X., Jia, Z. P., Wang, B. (2010). Study on the antitumor activity and mechanism of total alkaloids from Stellera chamaejasme. Chin. Med. Mat. 33, 1919–1922. doi: 10.13863/j.issn1001-4454.2010.12.032
Wang, X., Xu, J., Yang, M., Zhou, H. (2011). Chloroform extract of Tibetan herbal medicine Dracocephalum tanguticum Maxim. inhibits proliferation of T98G glioblastomas cells by modulating Caspase-3 cleavage and expression of Bax and p21. J. Med. Plant Res. 5, 6024–6031. doi: 10.5897/JMPR11.877
Wang, H., Gao, T., Du, Y., Yang, H., Wei, L., Bi, H., et al. (2015). Anticancer and immunostimulating activities of a novel homogalacturonan from Hippophae rhamnoides L. berry. Carbohydr. Polym. 131, 288–296. doi: 10.1016/j.carbpol.2015.06.021
Wang, Z., Wu, X., Liang, Y. N., Wang, L., Song, Z. X., Liu, J. L., et al. (2016). Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR. Molecules 21, E1267. doi: 10.3390/molecu-les21101267
Wang, R., Ma, L., Weng, D., Yao, J., Liu, X., Jin, F. (2016). Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 35, 3075–3083. doi: 10.3892/or.2016.4690
Wang, H. X., Huang, H. M., Yuan, X., Xu, K., Cao, C. N., Zhao, H. P. (2016). Inhibition on proliferation and cell cycle of MGC-803 cancer cells treated with extracts of Tibetan medicine Swertia mussotii. Drug Eval. Res. 39, 735–740. doi: 10.7501/j.issn.1674-6376.2016.05.006
Wang, Y. X., Wang, Y. X., Chen, R., Wan, D. G., Peng, C., Shen, C. H., et al. (2018). Effect of protein extracts of Cordyceps on growth of A549 cells and immune activity of mouse peritoneal macrophages in vitro. Chin. J. Exp. Tradit. Med. Form. 24, 79–84. doi: 10.13422/j.cnki.syfjx.2018010079
Wang, H., Yuan, X., Huang, H. M., Zou, S. H., Li, B., Feng, X. Q., et al. (2018). Swertia mussotii extracts induce mitochondria-dependent apoptosis in gastric cancer cells. Biomed. Pharmacother. 104, 603–612. doi: 10.1016/j.biopha.2018.05.023
Wang, Y. (2006). Research on anti-tumor and immunoregulation activities of the n-butanol extract from Lagotis Brevituba Maxim. (Lanzhou: Lanzhou University).
Wu, W. C., Hsiao, J. R., Lian, Y. Y., Lin, C. Y., Huang, B. M. (2007). The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemother. Pharmacol. 60, 103–111. doi: 10.1007/s00280-006-0354-y
Xia, T., Chen, S., Chen, S., Ge, X. (2005). Genetic variation within and among populations of Rhodiola alsia (Crassulaceae) native to the Tibetan Plateau as detected by ISSR markers. Biochem. Genet. 43, 87–101. doi: 10.1007/s10528-005-1502-5
Xia, D. (2015). Ovarian cancer HO-8910 cell apoptosis induced by crocin in vitro. Nat. Prod. Commun. 10, 249–252. doi: 10.1177/1934578X1501000208
Xie, W. L., Zhu, J., Zhao, Y. W., Li, L. (2010). Antitumor or effect of phlomiol in vivo and in vitro. Chin. J. Chin. Mater. Med. 35, 1189–1192.
Xie, J., Xu, Y., Huang, X., Chen, Y., Fu, J., Xi, M., et al. (2015). Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour. Biol. 36, 1279–1288. doi: 10.1007/s13277-0-14-2754-7
Xing, L. Q., Zhao, J. Q., Li, W., Sun, L. (2009). Effects of water extract of Stellera chamaejasme on survivin expression in human lung squamous cell carcinoma. Lishizhen Med. Mater. Med. Res. 20, 2185–2186.
Xu, T., Xue, C. K., He, X. B., Shen, K., Zhu, J., Jiang, P., et al. (2005). Antitumor effects of water soluble extracts from Entada phaseoloides in vitro. West China J. Pharm. Sci. 20, 487–489. doi: 10.13375/j.cnki.wcjps.2005.06.006
Xu, H., Zhao, X., Liu, X., Xu, P., Zhang, K., Lin, X. (2015). Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des. Devel. Ther. 9, 2735–2544. doi: 10.2147/DDDT.S80902
Yadav, S. S., Singh, M. K., Singh, P. K., Kumar, V. (2017). Traditional knowledge to clinical trials: A review on therapeutic actions of Emblica officinalis. Biomed. Pharmacother. 93, 1292–1302. doi: 10.1016/j.biopha.2017.07.065
Yang, X., Huang, N. (2013). Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol. Med. Rep. 8, 505–510. doi: 10.3892/mmr.2013.1506.
Yang, Y., Yue, Y., Runwei, Y., Guolin, Z. (2010). Cytotoxic, apoptotic and antioxidant activity of the essential oil of Amomum tsao-ko. Bioresour. Technol. 101, 4205–4211. doi: 10.1016/j.biortech.2009.12.131
Yang, X. H., Wang, J. T., Chen, L. X., Yang, Y. M., Yan, L., Ren, L. S. (2017). Life-extending effect of Stellera chamaejasme extract on mice with orthotopic liver cancer transplantation. Chin. J. Med. Clin. 17, 501–503.
Yao, Z., Wan, Y., Li, B., Zhai, C., Yao, F., Kang, Y., et al. (2018). Berberine induces mitochondrial-mediated apoptosis and protective autophagy in human malignant pleural mesothelioma NCI-H2452 cells. Oncol. Rep. 40, 3603–3610. doi: 10.3892/or.2018.6757
Yip, N. K., Ho, W. S. (2013). Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 30, 1107–1112. doi: 10.3892/or.2013.2543
Yoon, S. Y., Park, S. J., Park, Y. J. (2018). The anticancer properties of cordycepin and their underlying mechanisms. Int. J. Mol. Sci. 19, E3027. doi: 10.3390/ijms19103027
You, B. R., Moon, H. J., Han, Y. H., Park, W. H. (2010). Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 48, 1334–1340. doi: 10.1016/j.fct.2010.02.034
Yu, G., Li, N., Zhao, Y., Wang, W., Feng, X. L. (2018). Salidroside induces apoptosis in human ovarian cancer SKOV3 and A2780 cells through the p53 signaling pathway. Oncol. Lett. 15, 6513–6518. doi: 10.3892/ol.2018.8090
Zhang, Y. J., Nagao, T., Tanaka, T., Yang, C. R., Okabe, H., Kouno, I. (2004). Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol. Pharm. Bull. 27, 251–255. doi: 10.1248/bpb.27.251
Zhang, Q., Guo, G. N., Miao, R. D., Chen, N. Y., Wang, Q. (2004). Studies on the chemical constituents of Artemisia sieversiana and their anticancer activities. J. Lanzhou Univ. 40, 68–71. doi: 10.13885/j.issn.0455-2059.2004.04.019
Zhang, W., Yang, J., Chen, J., Hou, Y., Han, X. (2005). Immunomodulatory and anti-tumor effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumour-bearing mice. Biotechnol. Appl. Biochem. 42, 9–15. doi: 10.1042/BA2-0040183
Zhang, Z., Duan, C. H., Ding, K., Wang, Z. T. (2009). WT inhibit human hepatocellular carcinam a BEL-7402 cells growth by modulating Akt and ERK1/2 phosphorylation. Chin. J. Chin. Mater. Med. 34, 3277–3280.
Zhang, Z., Duan, C. H., Ding, K., Wang, Z. T. (2010). Bioactivity and mechanism study of waltonitone on tumor cells growth in vitro. Chin. Pharmacol. J. 45, 259–263.
Zhang, F. G., Li, D. H., Qi, J., Liu, C. X. (2010). In vitro anticancer effects of pallasone A and its induced apoptosis on leukemic K562 cells. Chin. Pharm. 45, 1716–1719.
Zhang, M., Zhao, Y. L., Sun, F. Y. (2013). Inhibitory effect of sachalin rhodiola rhizome extract on CD4+CD25+ regulatory T cells in xenograft tumors of Lewis lung cancer bearing mice. Chin. J. Cancer Biother. 20, 444–448. doi: 10.3872/j.issn.1007-385X.2013.04.011
Zhang, C., Zhong, Q., Zhang, X. F., Hu, D. X., He, X. M., Li, Q. L., et al. (2015). Effects of cordycepin on proliferation, apoptosis and NF-κB signaling pathway in A549 cells. Chin. Med. Mat. 38, 786–789. doi: 10.13863/j.issn1001-4454.2015.04.034
Zhao, G., Shi, A., Fan, Z., Du, Y. (2015). Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol. Rep. 33, 2553–2560. doi: 10.3892/or.2015.3857
Zhao, H. J., Liu, T., Mao, X., Han, S. X., Liang, R. X., Hui, L. Q., et al. (2015). Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways. Acta Pharmacol. Sin. 36, 758–768. doi: 10.1038/aps.2014.130
Zhao, Y., Jing, Z., Lv, J., Zhang, Z., Lin, J., Cao, X., et al. (2017). Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 95, 18–24. doi: 10.1016/j.biopha.2017.08.045
Zhao, L., Yang, X. H., Yang, Y. M. (2018). Chemical components and antitumor activity of root extract of Stellera chamaejasme L. Nat. Prod. Res. Dev. 30, 621–628.
Zhaxi, D. Z., Lu, D. X., He, M., Shi, S. J., Suonan, Z. M., Yu, K. X. (2012). The proliferative inhibition effects of the aqueous extracts of Oxytropis falcate on MCF-7 breast cancer cells. West China J. Pharm. Sci. 27, 131–133. doi: 10.13375/j.cnki.wcjps.2012.02.006
Zheng, F., Tang, Q., Wu, J., Zhao, S., Liang, Z., Li, L., et al. (2014). p38α MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J. Exp. Clin. Cancer Res. 33, 36. doi: 10.1186/1756-9966-33-36
Zheng, X. L. (2017). Preliminary study of petroleum ether extract of Lamiophlomis Rotate on migration, adhesion and invasion of Tca8113 cell line. (Lanzhou: Lanzhou University).
Zhu, X., Wang, J., Ou, Y., Han, W., Li, H. (2013). Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. Eur. J. Med. Res. 18, 46. doi: 10.1186/2047-783X-18-46
Keywords: cancer, traditional Tibetan medicine, anticancer activity, apoptosis, Ophiocordyceps sinensis, salidroside, gallic acid
Citation: Tang C, Zhao C-C, Yi H, Geng Z-J, Wu X-Y, Zhang Y, Liu Y and Fan G (2020) Traditional Tibetan Medicine in Cancer Therapy by Targeting Apoptosis Pathways. Front. Pharmacol. 11:976. doi: 10.3389/fphar.2020.00976
Received: 23 January 2020; Accepted: 15 June 2020;
Published: 07 July 2020.
Edited by:
Hong Zhang, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Hongmei Jia, Institute of Medicinal Plant Development (CAMS), ChinaFeng Xin, China Tibetology Research Center, China
Copyright © 2020 Tang, Zhao, Yi, Geng, Wu, Zhang, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya Liu, bGl1eWF5YTkxOEAxNjMuY29t; Gang Fan, ZmFuZ2FuZzExMTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ce Tang1,2†
Ce Tang1,2† Gang Fan
Gang Fan