
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 30 June 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00963
Aims: Our object was to find the most appropriate, most effective, and most readily available of four induction regimens for HIV-associated cryptococcal meningitis (CM) (Regimen A: 1 week of AmB plus 5-FC followed by 1 week of fluconazole, Regimen B: 1 week of AmB plus fluconazole followed by 1 week of fluconazole, Regimen C: 2 weeks of AmB plus 5-FC, Regimen D: 2 weeks of AmB plus fluconazole), given the vast differences between resource-limited and resource-abundant settings regarding therapeutic drug accessibility, availability, and affordability for HIV-associated (CM).
Methods: We conducted a network meta-analysis to compare the therapeutic efficacy and safety of four different induction treatment regimens.
Results: The 10-week mortality of Regimen A was significantly lower than that of Regimen B and D, and the 2-week mortality of Regimen A was significantly lower than that of Regimen B. Furthermore, there were no statistically significant differences in 10-week mortality, 2-week mortality, as well as in effective fungicidal activity (EFA) over the first 2 weeks among Regimens B, C, and D. The statistical differences in adverse events between Regimen B and Regimen D, and Regimen C and Regimen D were not calculated to be significant.
Conclusions: Our results indicate that, 1 week of AmB plus 5-FC followed by 1 week of fluconazole is superior to the three other studied regimens, and that when 5-FC is not available, accessible, or affordable, 2 weeks of AmB plus fluconazole or 1 week of AmB plus fluconazole followed by 1 week of fluconazole is an appropriate substitution for 2 weeks of AmB plus 5-FC.
Cryptococcal meningitis (CM) remains a significant contributor to human immunodeficiency virus- (HIV-) associated mortality (Lawrence et al., 2019), accounting for 15% mortality overall globally (Rajasingham et al., 2017). In 2014, an estimated 223,100 HIV-associated CM cases and 181,100 deaths occurred globally (Lawrence et al., 2019), 163,000 of these HIV-associated CM (73%) patients were diagnosed in sub-Saharan Africa and Southeast Asia (Oladele et al., 2017; Rajasingham et al., 2017).
Prompt, rational, and effective antifungal treatment is imperative to HIV-associated CM management (WHO, 2018). HIV-associated CM treatment can be divided into an induction phase, a consolidation phase, and a maintenance phase (Rhein et al., 2019). The object of the induction phase is to drastically reduce cerebrospinal fluid (CSF) fungal burden, and is critical for patients’ survival (Boyer-Chammard et al., 2019). The WHO (2018) guidelines for the preferred induction treatment regimens for HIV-associated CM was updated in March 2018, changing Amphotericin (AmB) plus flucytosine (5-FC) for 2 weeks or AmB plus fluconazole for 2 weeks, to 1 week of AmB plus 5-FC, followed by 1 week of fluconazole. The reason for this change was to reduce the potential for therapeutic drug toxicity, and to reduce the cost of treatment while maintaining efficacy (Tenforde et al., 2018).
The WHO guidelines recommendation change was mainly based on the evidence of a network meta-analysis evaluating the best regimen for patients with HIV-associated CM (Tenforde et al., 2018). This meta-analysis found that 1 week of AmB plus 5-FC, followed by fluconazole for a week was probably superior to other regimens. However, early phase study (Jarvis et al., 2019) of novel liposomal AmB (as opposed to AmB deoxycholate) at many African centers will determine the relative efficacy of this new regimen, but does not offer any new information beyond the network meta-analysis, and some results will be included in our study for analysis. In addition, 5-FC is neither readily accessible nor affordable in resource-limited settings, where a typically heavy burden of HIV-associated CM prevails. Another network meta-analysis (Yao et al., 2014) investigating the efficacy of 5-FC plus AmB and fluconazole plus AmB, demonstrated no difference in mortality between the two regimens at 3 months of treatment. These studies indicate that the most appropriate, effective, and readily available induction regimen for HIV-associated CM in resource-limited settings has, as yet, not been fully addressed, and therefore warrants further investigation.
In the present network meta-analysis, we compared three WHO recommended regimens (Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children.SourceGeneva: World Health Organization, 2011; WHO, 2018), and a commonly chosen 5-FC-free regimen in clinical practice, with the objective of finding the most appropriate antifungal regimen for the induction treatment of HIV-associated CM. The regimens we chose to compare included 1 week of AmB plus 5-FC followed by 1 week of fluconazole (Regimen A), 1 week of AmB plus fluconazole followed by 1 week of fluconazole (Regimen B), 2 weeks of AmB plus 5-FC (Regimen C), and 2 weeks of AmB plus fluconazole (Regimen D).
Randomized controlled trials (RCTs) or cohort studies recruiting HIV-associated CM patients were searched and screened in Pubmed, the Cochrane Library, Web of Science, and EBSCOhost/MEDLINE from inception until Nov 15th, 2019. Keywords connected as “HIV OR AIDS OR human immunodeficiency virus AND cryptococcal meningitis AND (RCT or cohort study)” were used for search in titles and abstracts (Bicanic et al., 2009; Moher et al., 2015).
RCTs or cohort studies were included if they satisfied the following criteria:
1. Study participants were HIV-positive patients with their first episode of CM, and CM was diagnosed by isolation of cryptococcus from CSF cultures, positive CSF India ink staining, and/or positive CSF cryptococcal antigen tests, or isolation of cryptococcus in blood culture with clinical presentations of meningo-encephalitis and typical CSF features (Zheng et al., 2015; Kanters et al., 2016; Spec and Powderly, 2018).
2. Patients receiving 1 week of AmB plus 5-FC followed by 1 week of fluconazole, 1 week of AmB plus fluconazole followed by 1 week of fluconazole, 2 weeks of AmB plus 5-FC, and 2 weeks of AmB plus fluconazole were included.
3. Studies reported 10-week mortality and one of the following outcomes: 2-week mortality, effective fungicidal activity (EFA) over the first 2 weeks, adverse events, and drug cost.
Studies were excluded if:
1. They were not RCTs or cohort studies.
2. Study participants were HIV-negative.
3. The diagnosis of CM was not clearly established by positive CSF cultures, positive India ink staining, or positive blood cultures of cryptococcus in association with clinical presentation and CSF features of CM.
4. The study regimens were for consolidation treatment, maintenance treatment, pre-emptive antifungal treatment, or secondary prophylaxis, in the absence of data for induction treatment.
5. Cases were those of recurrence or retreatment, and not those of first episode of CM.
6. They were not published in English.
We extracted the following data for further analysis: (1) characteristics of studies: first author, date of publication, study type, study duration, and country/area; (2) characteristics of patients: number, age, timing of ART initiation after antifungal therapy; (3) interventions included induction therapeutic regimens and consolidation therapeutic regimens; (4) outcomes included 10-week mortality, 2-week mortality, EFA over the first 2 weeks (Pullen et al., 2020), adverse events, and drug cost. Induction therapeutic regimens were as follows: 1 week of AmB plus 5-FC followed by 1 week of fluconazole (Regimen A), 1 week of AmB plus fluconazole followed by 1 week of fluconazole (Regimen B), 2 weeks of AmB plus 5-FC (Regimen C), and 2 weeks of AmB plus fluconazole (Regimen D).
Two review authors (YL and YQ) independently evaluated the methodological quality of the six RCTs included in our network meta-analysis by means of the Cochrane “risk of bias” tool (Higgins et al., 2011; Huang et al., 2018). The following seven measures of bias were assessed at “low risk,” “unclear risk,” or “high risk”: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and researchers (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
Ten-week mortality and 2-week mortality for Regimens A, B, C, and D was assessed using forest plots. Risk ratios (RRs) with 95% confidence intervals (CIs) in each pair comparison were generated for treatment effect measurements (Bicanic et al., 2015). Adverse events were also assessed using forest plots and risk ratio (RRs) with 95% Cis (Beardsley et al., 2016; Rhein et al., 2016). EFA over the first 2 weeks was assessed by mean differences (MDs) with 95% Cis (Loyse et al., 2012; Tenforde et al., 2018).
The cumulative ranking probabilities were summarized using the surface under the cumulative ranking area (SUCRA) curve (Salanti et al., 2011; Chaimani et al., 2013). The SUCRA value represents the surface under the cumulative ranking curve, and the probability for each regimen to be the best option for HIV-associated CM. The larger the SUCRA value, the higher the ranking probability for the regimen in the network (Tenforde et al., 2018).
Consistency of network meta-analysis was assessed by global and local consistency (Cipriani et al., 2009). Global consistency was assessed by the consistency model and inconsistency model (Tonin et al., 2017). Local consistency was assessed by the node-splitting method (White, 2015).
Statistical heterogeneity was assessed using the inconsistency factor (IF) and p-values in loop-specific heterogeneity (Shim et al., 2017). Factors such as dosing within each regimen in the induction and consolidation therapeutic phase, ART status, and risk of bias (assessed by “risk of bias” tool) were considered likely to cause clinical and methodological heterogeneity (Tenforde et al., 2018).
Reporting bias was assessed by examining asymmetry in funnel plots of pairwise comparisons (Tenforde et al., 2018).
Pairwise meta-analysis was conducted with the traditional frequentist approach (Tu and Wu, 2017). The command “networkplot” was used for pairwise meta-analysis by STATA Version 15.1 (Statacorp, Texas, USA) (StataCorp, 2017; White, 2015).
For dichotomous outcome measures, RRs were calculated with 95% CIs. For continuous outcomes, MDs were calculated with 95% CIs. These two types of outcomes were both analyzed by Review Manager Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed through all phases in our study (Bicanic et al., 2009; WHO, 2018). Registration number CRD42019124942 was obtained after registering the protocol in the PROSPERO international prospective register of systematic reviews (PROSPERO International prospective register of systematic reviews).
A total of 926 articles were retrieved from four electronic databases through our search strategy, and 89 of these articles were eligible for inclusion after screening titles and abstracts. Among the 89 articles, 82 were excluded due to not reporting 10-week mortality, or one of the following criteria: 2-week mortality, EFA, adverse events and drug cost, absence of data for induction treatment, or recurrence and retreatment. Finally, seven articles (Brouwer et al., 2004; Tansuphaswadikul et al., 2006; Loyse et al., 2012; Rajasingham et al., 2012; Day et al., 2013; Molloy et al., 2018; Jarvis et al., 2019) were included into our network meta-analysis after full-text reviewing (Figure 1). In the seven incorporated studies, one arm received 1 week of AmB plus 5-FC followed by 1 week of fluconazole, four arms received 1 week of AmB plus fluconazole followed by 1 week of fluconazole, five arms received 2 weeks of AmB plus 5-FC, and seven arms received 2 weeks of AmB plus fluconazole (Table 1).
The risk bias of 6 RCTs described as “low risk,” “high risk,” or “unclear risk” with supporting evidence is presented in Supplementary Material Table. The results showed that the high risk of performance bias (non-blinding of participants and researchers) of six included RCTs may have contributed to the risk of bias.
Ten-week mortality of seven trials with six pairwise comparisons (1,448 persons) was assessed by STATA 15.1, forming a rhombus-shaped network (Figure 2). An evidence contribution graph denoting the degree of influence of direct comparisons on the whole network is shown in Supplementary Material Figure 1. This suggested that comparison C vs. D contributed most to the entire network (66.0%), followed by comparison B vs. D (35.3%) and comparison A vs. B (30.7%).
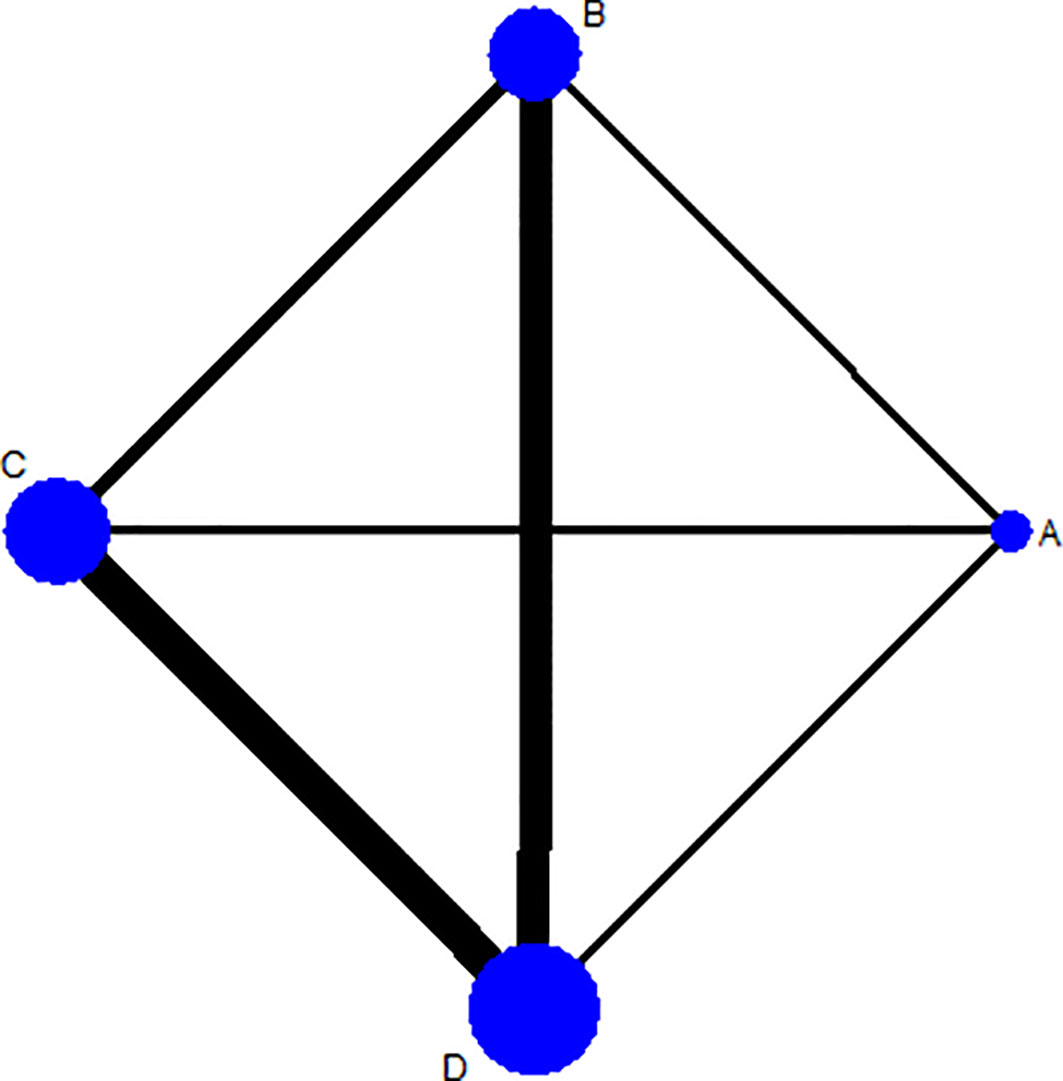
Figure 2 Network plot for 10-week mortality among Regimens A, B, C, and D. Regimen A: 1 week of AmB plus 5-FC followed by 1 week of fluconazole; Regimen B: 1 week of AmB plus fluconazole followed by 1 week of fluconazole; Regimen C: 2 weeks of AmB plus 5-FC; Regimen D: 2 weeks of AmB plus fluconazole. Note: The thicker the line, the more studies of the comparison between 2 interventions.
The 10-week mortality of the six pairwise comparisons was displayed in a forest plot (Figure 3A). The RRs of Regimen A vs. Regimen B, and Regimen A vs. Regimen D with 95% CIs were 2.04 (1.39, 2.98) and 1.60 (1.07, 2.40), all greater than 1, indicating the 10-week mortality of each of the comparisons all favored Regimen A. Meanwhile, the calculated differences in 10-week mortality between regimens A and B, and regimens A and D, were both statistically significant. However, the wide enough CIs of comparisons A vs. C, B vs. C, B vs. D, and C vs. D crossed the line of no effect (Figure 3A), suggesting that the differences in 10-week mortality between these specific pairwise comparisons were all not significant.
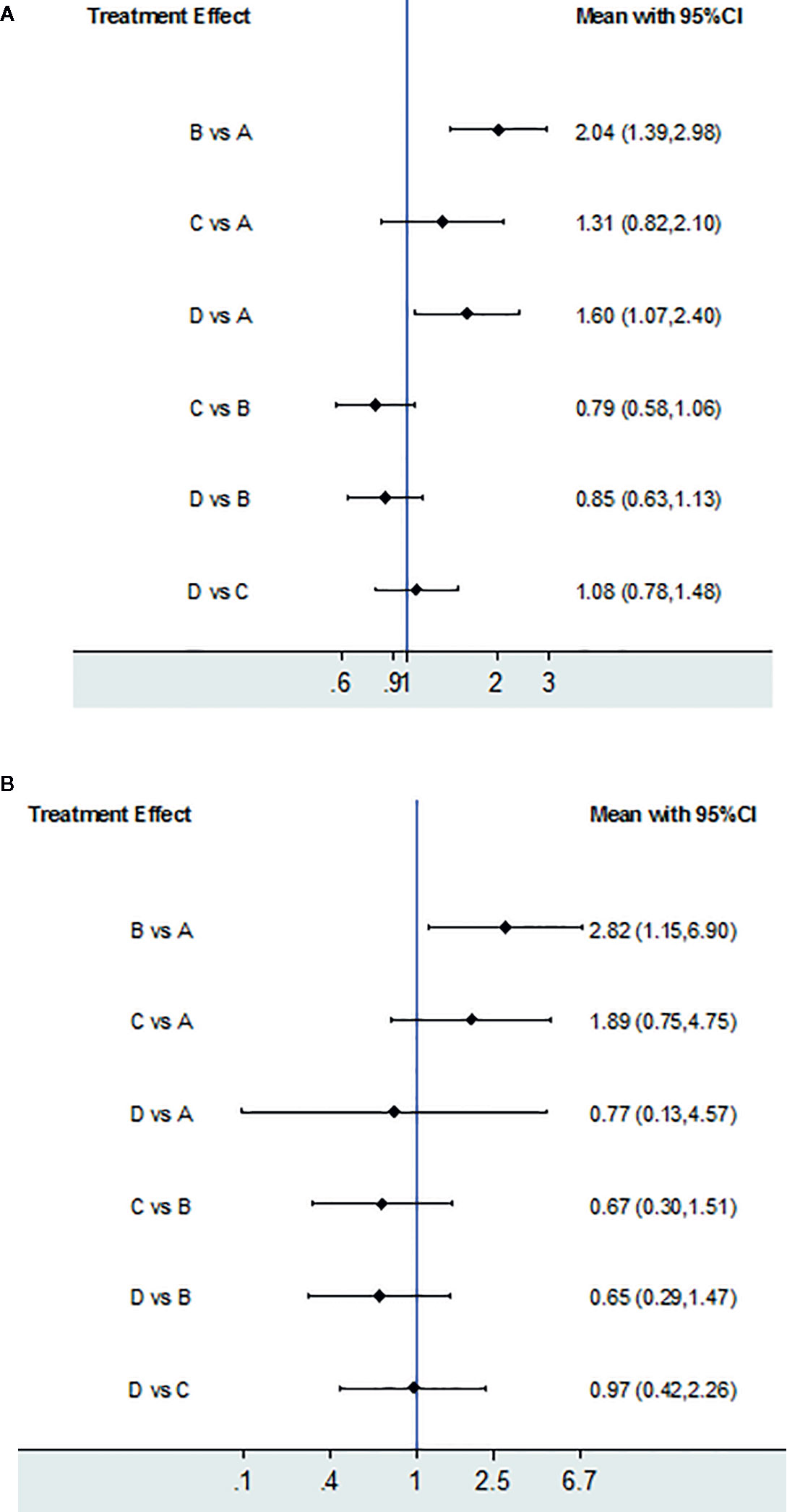
Figure 3 Treatment effect. (A) 10-week mortality for each comparison; (B) 2-week mortality for each comparison. If the risk ratio with 95%CI of a comparison is greater than 1, indicating the results of comparison favored the Regimen.
Two-week mortality of four trials was compared and presented in forest plots (Figure 3B). The 2-week mortality of Regimen A had a noticeable advantage when compared with Regimen B (2.82, 95% CI 1.15 to 6.90). There were no significant statistical differences in 2-week mortality among Regimens B, C, and D.
Cumulative ranking probabilities of 10-week mortality for each treatment regimen are shown in Figures 4A, B. The SUCRA values for each regimen were as follows: Regimen A (93.4%), Regimen B (11.0%), Regimen C (46.3%), and Regimen D (49.3%). Thus, the optimal induction treatment regimen options for HIV-associated CM, ranked in the order of largest to smallest SUCRA probabilities, are A>D>C>B.
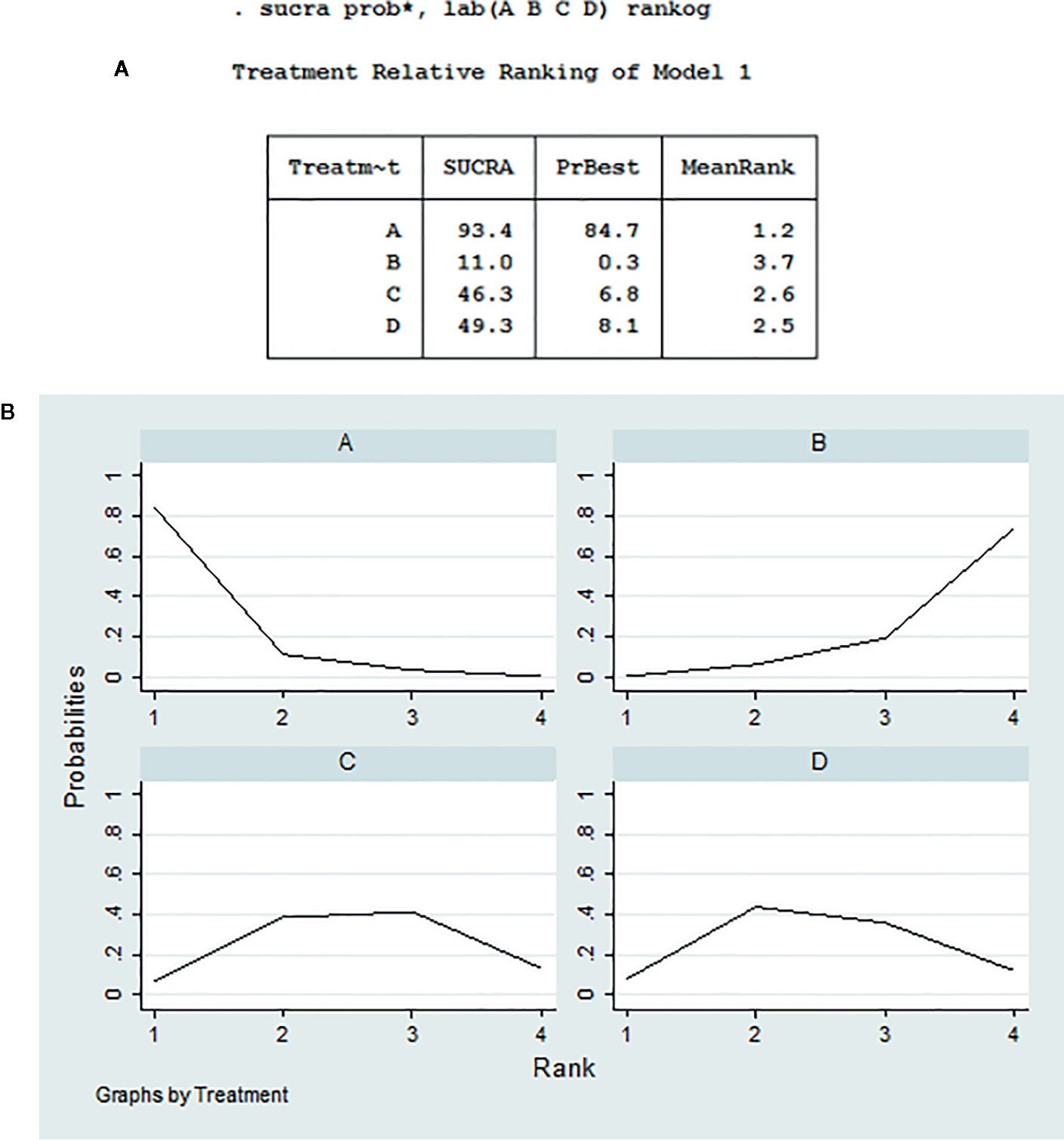
Figure 4 Cumulative ranking probability of 10-week mortality for each regimen. (A) Relative ranking of regimens A, B, C, and D; (B) The surface under the cumulative ranking area of regimens A, B, C, and D.
As displayed in Figure 5, no statistically significant differences were found in EFA among Regimens B, C, and D.
As displayed in Figure 6, there were no observable statistical differences in adverse events between Regimen B and Regimen D, and between Regimen C and Regimen D. None of the included studies reported comparisons of adverse events between regimens B and C.
Consistency was assessed using 10-week mortality data, and was shown in Supplementary Material Figures 2A–C. The result of global consistency, which was conducted via a consistency model, indicated that inconsistency was not significant (p = 2.56, >0.05) (Supplementary Material Figure 2A). Thus, the consistency model could be used for further analysis.
Loop-specific heterogeneity was evaluated by calculating IF via the Lumley method. The closer the IF value is to 0, the better the consistency is between direct and indirect evidence (Supplementary Material Figure 3). In our results, the IF of loop A-C-D was 0.00, and that of loop B-C-D was 0.07, indicating negligible inconsistency between direct and indirect evidence of these two loops. On the other hand, the p- values for loop A-B-C and loop A-B-D were 0.37 and 0.33 (both >0.05) respectively (Supplementary Material Figure 3), indicating that low inconsistency exists between direct and indirect comparisons of these loops (Tenforde et al., 2018).
The reporting bias funnel plot of six comparisons is displayed in Supplementary Material Figure 4. The results showed that most scatter points were located above the funnel plot, and were evenly distributed on both sides of the red indicator line, suggesting that reporting bias was negligible. However, the high risk of detection bias (non-blinding of outcome assessment) and attrition bias (incomplete outcome data) of six included RCTs may have caused some bias to our results (Supplementary Material Table 1).
In this study, we conducted a network meta-analysis to compare the appropriateness of three WHO-recommended induction therapeutic regimens (regimens A, C, and D), and a 5-FC-free induction therapeutic regimen (regimen B) usually chosen in clinical practice in resource-limited settings. All trials included in our study were carried out in resource-limited settings, and therefore, our results would be of clinical significance for the treatment of HIV-associated CM in those specific settings.
The 10-week mortality of Regimen A was significantly lower than that of Regimen B and Regimen D, and the 2-week mortality of Regimen A was significantly lower than that of Regimen B. The above results are consistent with the results of another network meta-analysis conducted by Tenforde et al. (2018), even though the included trials in the Tenforde et al. network meta-analysis, as well as those included in our analysis, were inconsistent. In addition, our relative ranking results showed that the probability for Regimen A to be the most appropriate option amongst the four studied options for the treatment of HIV-associated CM was 93.4%, which reinforces our confidence in the utilization of this latest regimen recommendation by the WHO in resource-limited settings. As for 10-week mortality, consolidation therapy also plays an important role other than induction therapy. In our study, patients in the included six studies were all treated with fluconazole for consolidation therapy. The administered doses ranged from 400 to 800 mg/day, and the consolidation course duration ranged from 8 to 10 weeks.
Our results of drug effectiveness suggest that 5-FC as a second drug is superior to fluconazole when combined with AmB, because Regimen A (1 week of AmB plus 5-FC followed by 1 week of fluconazole) was found to be more efficacious than Regimen B (1 week of AmB plus fluconazole followed by 1 week of fluconazole). Unfortunately, 5-FC remains neither readily accessible/available, nor affordable in resource-limited settings despite of its superior efficacy (Loyse et al., 2013a; Patel et al., 2018). Unfortunately, with widely used fluconazole monotherapy, mortality due to HIV-related CM is approximately 70% in many African low-income and middle-income countries settings (Loyse et al., 2019). In our analysis, there were no statistically significant differences in 10-week mortality and 2-week mortality among Regimen B, Regimen C, and Regimen D. There were also no statistically significant differences between these regimens in EFA over the first 2 weeks, and similarly, the differences in adverse events between Regimen B and Regimen D, and Regimen C and Regimen D were not statistically significant. Our results concur with the results of other researchers, who have observed that the antifungal combination of AmB plus fluconazole resulted in excellent yeast clearance from CSF, with few adverse events in their trials (Brouwer et al., 2004; Loyse et al., 2012), and that there were no statistically significant differences in EFA at 2 weeks of treatment between Regimen C (2 weeks of AmB plus 5-FC) and Regimen D (2 weeks of AmB plus fluconaozle) (Loyse et al., 2012). Our results, together with results of other researchers, indicate that substituting Regimen B or Regimen D for Regimen C may be a sensible choice in terms of efficacy in resource-limited settings, where 5-FC may not be available.
Furthermore, 5-FC is not licensed in 89 (71.2%) of 125 countries, and unavailable in 95 (76.0%) of 125 countries (Loyse et al., 2013b; Kneale et al., 2016). In resource-abundant settings where 5-FC is available, the cost of 5-FC varies from $4.60 to $1,409 per day (Loyse et al., 2013b). By contrast, the total treatment cost of 2 weeks of AmB plus fluconazole is only $402 per patient (Patel et al., 2018). Merry and Boulware (2016) cost-effectiveness analysis suggested 2 weeks of AmB plus fluconazole (Regimen D) was more cost-effective when compared with 2 weeks of AmB plus 5-FC (Regimen C) ($4,4605 vs. $75,121 per quality-adjusted life-year). Rajasingham et al.’s study (Rajasingham et al., 2012), which was based on 18 trials and cohorts in resource-limited settings, showed that 1 week of AmB with fluconazole followed by 1 week of fluconazole (Regimen B) had the best cost-effectiveness ratio, which is $20.24/quality-adjusted life years when compared with 2 weeks of AmB plus 5-FC (Regimen C) or 2 weeks of AmB plus fluconazole (Regimen D) (Rajasingham et al., 2012). The above studies have shown that even from the perspective of cost-effectiveness, substituting Regimens B or D for Regimen C remains a prudent choice in resource-limited settings.
Some limitations of our meta-analysis have to be pointed out. Firstly, one systematic review pooling the 10-week mortality and drug cost of 18 trials and cohorts was incorporated in our meta-analysis, which unfortunately had a paucity of outcome data for direct comparisons between regimens. Secondly, we did not perform an analysis of drug costs due to the limited data for medical expenditure in the included studies. Thirdly, because of the limited number of eligible studies and the limited data extracted from these studies, we did not conduct an analysis of ART use for patients in each study, nor did we evaluate the doses of antifungal medications prescribed to subjects in each study. We also did not assess the utilization of therapeutic lumbar puncture for subjects in each study, which may well have had an impact on heterogeneity in the present study. Finally, the liposomal AmB incorporated in Regimen B, and the high risk of detection bias and attrition bias may have contributed to a degree of bias to our results. Additionally, the SUCRA rankings should be interpreted cautiously, even when accompanied by statistically significant or clinically meaningful effects, due to the imprecision of treatment effects on mortality throughout the network meta-analysis (Tenforde et al., 2018).
Regimen A (1 week of AmB plus 5-FC followed by 1 week of fluconazole) remains the most appropriate induction regimen for the treatment of HIV-associated CM in resource-limited settings, and is superior to Regimen B (1 week of AmB plus fluconazole followed by 1 week of fluconazole) and Regimen D (2 weeks of AmB plus fluconazole) in several aspects. Substituting Regimen B or Regimen D for Regimen C is appropriate in terms of efficacy in resource-limited settings, where 5-FC is likely to be unavailable or unaffordable.
YL, XY, and YC conceived and designed the protocol and study. YL and YQ identified the studies to be screened. XH identified studies for eligibility, extracted data, and assessed the methodological quality of included studies. YL performed the data analysis, with assistance from XY and YC. All authors contributed to the article and approved the submitted version.
This work was supported by the National Science and Technology Major Project of China during the 13th Five-year Plan Period (2018ZX10302104), Key Project of Joint Medical Research Project of Science and Health in Chongqing in 2019 (2019ZDXM012).
All authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potentially causing conflict of interest.
We wish to thank Dr. Vijay Harypursat of the Division of Infectious Diseases, Chongqing Public Health Medical Center for his assistance in proofreading and language improvement for this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00963/full#supplementary-material
Beardsley, J., Wolbers, M., Kibengo, F. M., Ggayi, A. B., Kamali, A., Cuc, N. T., et al. (2016). Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N Engl. J. Med. 374 (6), 542–554. doi: 10.1056/NEJMoa1509024
Bicanic, T., Brouwer, A. E., Meintjes, G., Rebe, K., Limmathurotsakul, D., Chierakul, W., et al. (2009). Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 23 (6), 701–706. doi: 10.1097/QAD.0b013e32832605fe
Bicanic, T., Bottomley, C., Loyse, A., Brouwer, A. E., Muzoora, C., Taseera, K., et al. (2015). Toxicity of Amphotericin B Deoxycholate-Based Induction Therapy in Patients with HIV-Associated Cryptococcal Meningitis. Antimicrob Agents Chemother. 59 (12), 7224–7231. doi: 10.1128/AAC.01698-15
Boyer-Chammard, T., Temfack, E., Alanio, A., Jarvis, J. N., Harrison, T. S., Lortholary, O., et al. (2019). Recent advances in managing HIV-associated cryptococcal meningitis. F1000Res 8, 743. doi: 10.12688/f1000research.17673. F1000 Faculty Rev-743
Brouwer, A. E., Rajanuwong, A., Chierakul, W., Griffin, G. E., Larsen, R. A., White, N. J., et al. (2004). Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363 (9423), 1764–1767. doi: 10.1016/S0140-6736(04)16301-0
Chaimani, A., Higgins, J. P. T., Mayridis, D., Spyridonos, P., Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PloS One 8 (10), e76654. doi: 10.1371/journal.pone.0076654
Cipriani, A., Furukawa, T. A., Salanti, G., Geddes, J. R., Higgins, J. P., Churchill, R., et al. (2009). Comparative efficacy and acceptability of 12 new generation antidepressants: a multiple-treatments meta-analysis. Lancet 373, 746–758. doi: 10.1016/S0140-6736(09)60046-5
Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014). Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. https://community.cochrane.org/help/tools-and-software/revman-5
Day, J. N., Chau, T. T., Wolbers, M., Mai, P. P, Dung, N. T., Mai, N. H., et al. (2013). Combination antifungal therapy for cryptococcal meningitis. N Engl. J. Med. 368 (14), 1291–1302. doi: 10.1056/NEJMc1305981
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi: 10.1136/bmj.d5928
Huang, Y., Huang, X., Luo, Y., Zhou, Y., Tao, X., Chen, H., et al. (2018). Assessing the Efficacy of Lopinavir/Ritonavir-Based Preferred and Alternative Second-Line Regimens in HIV-Infected Patients: A Meta-Analysis of Key Evidence to Support WHO Recommendations. Front. Pharmacol. 9, 890. doi: 10.3389/fphar.2018.00890
Jarvis, J. N., Leeme, T. B., Molefi, M., Chofle, A. A., Bidwell, G., Tsholo, K., et al. (2019). Short-course High-dose Liposomal Amphotericin B for Human Immunodeficiency Virus-associated Cryptococcal Meningitis: A Phase 2 Randomized Controlled Trial. Clin. Infect. Dis. 68 (3), 393–401. doi: 10.1093/cid/ciy51
Kanters, S., Vitoria, M., Doherty, M., Socias, M. E., Ford, N., Forrest, J. I., et al. (2016). Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 3 (11), e510–e520. doi: 10.1016/S2352-3018(16)30091-1
Kneale, M., Bartholomew, J. S., Davies, E., Denning, D. W. (2016). Global access to antifungal therapy and its variable cost. J. Antimicrob Chemother. 71 (12), 3599–3606. doi: 10.1093/jac/dkw325
Lawrence, D. S., Boyer-Chammard, T., Jarvis, J. N. (2019). Emerging concepts in HIV-associated cryptococcal meningitis. Curr. Opin. Infect. Dis. 32 (1), 16–23. doi: 10.1097/QCO.0000000000000514.10.1016/j.ijid.2017.08.004
Loyse, A., Wilson, D., Meintjes, G., Jarvis, J. N., Bicanic, T., Bishop, L., et al. (2012). Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 54 (1), 121–128. doi: 10.1093/cid/cir745
Loyse, A., Dromer, F., Day, J., Lortholary, O., Harrison, T. S. (2013a). Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J. Antimicrob Chemother. 68, 2435–2444. doi: 10.1093/jac/dkt221
Loyse, A., Thangaraj, H., Easterbrook, P., et al. (2013b). Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 13, 629–637. doi: 10.1016/S1473-3099(13)70078-1
Loyse, A., Burry, J., Cohn, J., Ford, N., Chiller, T., Ribeiro, I., et al. (2019). Leave no one behind: response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries. Lancet Infect. Dis. 19 (4), e143–e147. doi: 10.1016/S1473-3099(18)30493-6
Merry, M., Boulware, D. R. (2016). Cryptococcal Meningitis Treatment Strategies Affected by the Explosive Cost of Flucytosine in the United States: A Cost-effectiveness Analysis. Clin. Infect. Dis. 62 (12), 1564–1568. doi: 10.1093/cid/ciw151
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. doi: 10.1186/2046-4053-4-1
Molloy, S. F., Kanyama, C., Heyderman, R. S., Loyse, A., Kouanfack, C., Chanda, D., et al. (2018). Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. N Engl. J. Med. 378 (11), 1004–1017. doi: 10.1056/NEJMoa1710922
Oladele, R. O., Bongomin, F., Gago, S., Denning, D. W. (2017). HIV-Associated Cryptococcal Disease in Resource-Limited Settings: A Case for “Prevention Is Better Than Cure”? J. Fungi (Basel) 3 (4), E67. doi: 10.3390/jof3040067
Patel, R. K. K., Leeme, T., Azzo, C., Tlhako, K., Tsholo, K., Tawanana, E. O., et al. (2018). High Mortality in HIV-Associated Cryptococcal Meningitis Patients Treated With Amphotericin B-Based Therapy Under Routine Care Conditions in Africa. Open Forum Infect. Dis. 5 (11), ofy267. doi: 10.1093/ofid/ofy267
PROSPERO International prospective register of systematic reviews. National Institute for Health Research. https://www.crd.york.ac.uk/prospero/.
Pullen, M. F., Hullsiek, K. H., Rhein, J., Musubire, A. K., Tugume, L., Nuwagira, E., et al. (2020). CSF early fungicidal activity as a surrogate endpoint for cryptococcal meningitis survival in clinical trials. Clin. Infect. Dis., ciaa016. doi: 10.1093/cid/ciaa016
Rajasingham, R., Rolfes, M. A., Birkenkamp, K. E., Meya, D. B., Boulware, D. R. (2012). Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PloS Med. 9 (9), e1001316. doi: 10.1371/journal.pmed.1001316
Rajasingham, R., Smith, R. M., Park, B. J., Jarvis, J. N., Govender, N. P., Chiller, T. M., et al. (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17 (8), 873–881. doi: 10.1016/S1473-3099(17)30243-8
Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children.SourceGeneva: World Health Organization (2011). WHO Guidelines Approved by the Guidelines Review Committee. WHO. pp. 44.
Rhein, J., Huppler Hullsiek, K., Tugume, L., Nuwagira, E., Mpoza, E., Evans, E. E., et al. (2016). Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect. Dis. 16 (7), 809–818. doi: 10.1016/S1473-3099(16)00074-8
Rhein, J., Huppler Hullsiek, K., Tugume, L., Nuwagira, E., Mpoza, E., Evans, E. E., et al. (2019). Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect. Dis. 19 (8), 843–851. doi: 10.1016/S1473-3099(19)30127-6
Salanti, G., Ades, A. E., Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi: 10.1016/j.jclinepi.2010.03.016
Shim, S., Yoon, B. H., Shin, I. S., Bae, J. M. (2017). Network meta-analysis: application and practice using Stata. Epidemiol. Health 39, e2017047. doi: 10.4178/epih.e2017047
Spec, A., Powderly, W. G. (2018). Cryptococcal meningitis in AIDS. Handb. Clin. Neurol. 152, 139–150. doi: 10.1016/B978-0-444-63849-6.00011-6
Tansuphaswadikul, S., Maek-a-Nantawat, W., Phonrat, B., Boonpokbn, L., Mctm, A. G. (2006). Comparison of one week with two week regimens of amphotericin B both followed by fluconazole in the treatment of cryptococcal meningitis among AIDS patients. J. Med. Assoc. Thai 89 (10), 1677–1685.
Tenforde, M. W., Shapiro, A. E., Rouse, B., Jarvis, J. N., Li, T., Eshun-Wilson, I., et al. (2018). Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst. Rev. 7, CD005647. doi: 10.1002/14651858
Tonin, F. S., Rotta, I., Mendes, A. M., Pontarolo, R. (2017). Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm. Pract. (Granada) 15, 943. doi: 10.18549/PharmPract.2017.01.943
Tu, Y. K., Wu, Y. C. (2017). Using structural equation modeling for network meta-analysis. BMC Med. Res. Methodol 17 (1), 104. doi: 10.1186/s12874-017-0390-9
White, I. R. (2015). Network meta-analysis. Stata J. 15 (4), 951–985. doi: 10.1177/1536867X1501500403
WHO (2018). Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. (Geneva: World Health Organisation).
Yao, Z. W., Lu, X., Shen, C., Lin, D. F. (2014). Comparison of flucytosine and fluconazole combined with amphotericin B for the treatment of HIV-associated cryptococcal meningitis: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 33 (8), 1339–1344. doi: 10.1007/s10096-014-2074-2
Keywords: HIV, cryptococcal meningitis, antifungal regimen, induction treatment, network meta-analysis
Citation: Li Y, Huang X, Qin Y, Wu H, Yan X and Chen Y (2020) What Is the Most Appropriate Induction Regimen for the Treatment of HIV-Associated Cryptococcal Meningitis When the Recommended Regimen Is Not Available? Evidence From a Network Meta-Analysis. Front. Pharmacol. 11:963. doi: 10.3389/fphar.2020.00963
Received: 14 March 2020; Accepted: 12 June 2020;
Published: 30 June 2020.
Edited by:
Jean Paul Deslypere, Aesculape CRO, BelgiumReviewed by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyCopyright © 2020 Li, Huang, Qin, Wu, Yan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Yan, MjQyOTkxODM0MkBxcS5jb20=; Yaokai Chen, eWFva2FpY2hlbkBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.