
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 June 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00784
 Qi Wang1
Qi Wang1 Zhenyan Zhang1
Zhenyan Zhang1 Weisheng Yu1
Weisheng Yu1 Chenguang Lu1
Chenguang Lu1 Guoming Li1
Guoming Li1 Ziyi Pan1
Ziyi Pan1 Hongying Zhang1
Hongying Zhang1 Wanting Wu1
Wanting Wu1 Tinah Atcha Oubou2
Tinah Atcha Oubou2 Yueming Yuan1,3
Yueming Yuan1,3 Jiawen Guo1,3
Jiawen Guo1,3 Yuan Liang1
Yuan Liang1 Xinan Huang1
Xinan Huang1 Wenfeng Guo1
Wenfeng Guo1 Changqing Li1
Changqing Li1 `Nadia Julie1
`Nadia Julie1 Qin Xu1
Qin Xu1 Logte Sanwogou4
Logte Sanwogou4 Jianping Song1
Jianping Song1 Changsheng Deng1*
Changsheng Deng1*Background: Malaria is a major public health concern in Togo. The Est-Mono district of Togo has a population of 150,000. Accordingly, the Guangzhou University of Chinese Medicine, China and the Ministry of Health and Social Security, Togo launched a nationwide Mass Drug Administration Project with artemisinin–piperaquine (AP) in Est-Mono. Before launching this project, the sensitivity test of AP was conducted in a general clinic in Elawagnon, Togo. With this background, we evaluated the efficacy and safety of AP for the treatment of uncomplicated falciparum malaria in children under the age of 5 years.
Methods: Children aged 6–59 months with uncomplicated falciparum malaria were enrolled in this study. The selected patients were treated with a combination regime of artemisinin–piperaquine. The patients were followed up for 28 days, during which signs of the following were observed for: the duration for fever clearance, parasitemia density, gametophyte generation, cure rate, hemoglobin level, and merozoite surface protein-2 (msp-2) polymorphism. The primary end point was a 28-day cure rate and polymerase chain reaction (PCR)-corrected reinfection and recrudescence. This research followed the standardized World Health Organization (WHO) protocol for the assessment of the efficacy of antimalarial drugs.
Results: A total of 91 children with uncomplicated falciparum malaria were enrolled in this study. Adequate clinical and parasitological responses (ACPRs) before and after PCR-correction were 66 (73%) and 90 (99%), respectively. The average hemoglobin level in the patient increased by 0.05 g/dl per day (p < 0.0001) after the treatment. The gametophyte generation did not decline at the beginning of the treatment; however, after 14 days, it declined (day 21: p < 0.05; day 28: p < 0.01). In the msp-2 polymorphism study of 24 children treated for parasite infection, one case of msp-2 with 3D7 haplotype and FC27 haplotype was noted, indicating its recrudescence, with a frequency of 4%. The remaining 23 cases could have been of reinfection, with a frequency of 96%. No serious adverse reactions occurred, and AP was well-tolerated by all patients.
Conclusion: Artemisinin–piperaquine was found to be an effective combination for treating uncomplicated falciparum malaria in children aged <5 years in Togo, and the drugs were well-tolerated. In Togo, Plasmodium falciparum remains sensitive to artemisinin–piperaquine, necessitating its trial in this region.
Clinical Trial Registration: Trial registration: ECGPHCM No. B2017-054-01; MHSST AVIS N° 0001/2016/CBRS du 07 janvier 2016. Registered 17 March 2014, http://www.chinadrugtrials.org.cn/eap/main.
Malaria remains a major public health concern in Togo (Dorkenoo et al., 2012; Klement et al., 2014). In 2018, 7,889,095 people were at risk for malaria. The same year, 2,108,823 (median) malaria cases were reported, of which 1,090,110 were of falciparum malaria and 5,132 (median) of malarial deaths (World Health Organization (WHO, 2019)). According to the Togo 2014 National Data for the Plateaux region, malaria accounted for 51% of all outpatient cases and 29% of inpatient cases in medical institutions, with children under the age of 5 years accounting for 37% of all malarial incidences and 29% of all mortality cases (Djadou et al., 2006; Djadou et al., 2007). In Togo, > 10% of the children experiencing fever took the aid of informal private care centers, while most remained untreated, which led to a mortality rate of 8.2% (Assimadi et al., 1998; Dorkenoo et al., 2013; WHO, 2019).
The Ministry of Health and Social Security of Togo (MHSST) decided to implement chemoprophylactic measures for handling seasonal malaria in children under the age of 5 years in the Grassland, Kara, and Central regions (Guidelines for the Treatment of Malaria, 3rd edn (WHO, 2015)). In 2015, the Guangzhou University of Chinese Medicine (GUCM), China, signed an agreement with the MHSST to launch a mass drug administration (MDA) program with artemisinin–piperaquine (AP) for approximately 1.5 million people in the Plateaux region and selected approximately 150,000 people in the Est-Mono province as the pilot study area. AP was introduced as an option to treat uncomplicated falciparum malaria by the National Malaria Control Programme (NMCP) and administrated to Est-Mono inhabitants as the above mentioned MDA program. The latest clinical studies have confirmed >97% efficacy and better compliance in treating uncomplicated falciparum malaria post the program (Srivicha et al., 2007; Song et al., 2008; Nguyen et al., 2012). Meanwhile, the MDA program with AP has been implemented with positive outcomes in Cambodia (Song et al., 2011) and the Comoros (Bo et al., 2015; Bo et al., 2016; Chang et al., 2018), where malarial morbidity and mortality has dropped by >90% after the implementation of the AP-MDA program.
The efficacy and safety of AP have not yet been tested in children under the age of 5 years in Togo. Therefore, sensitivity test for AP was conducted at a general clinic in the Elawagnon county before launching the MDA program universally. With this background, we evaluated the efficacy and safety of AP for the treatment of uncomplicated falciparum malaria in children under the age of 5 years in Elawagnon, Prefecture on Est-Mono province, Togo.
The Est-Mono district covers an area of 2,474 km2 in Togo and has a population of 89,060, with a population growth rate of 1.03% in 2010 (Essoya et al., 2012). Est-Mono is one of the nine health districts in the Plateaux region—which is one of the Togo's six such regions. This district is situated in central Togo and has 17 health facilities staffed by nurses, auxiliary nurses, or nonqualified nurses. Each health unit has community health workers (CHWs) trained in health promotion and the management of uncomplicated malaria cases within their communities.
The Est-Mono district (Essoya et al., 2012) and the other central and southern parts of Togo have two rainy seasons in a year, one during April–July and the other during September–November. This district receives an average of 949 mm (± 37.4) annual or 79 mm (± 3.1) monthly precipitation. The climate in Togo is generally tropical, with the average temperatures ranging from 27.5°C (81.5°F) on the coastal areas to approximately 30°C (86°F) in the north. Malarial transmission here is seasonal, with peaks occurring during the rainy seasons. Two villages, EPT and RCPA, in the Elawagnon Prefecture on Est-Mono province were selected as the study area. The time span of the MDA was from March to May 2017.
The study for evaluating suitability of AP in the treatment of uncomplicated falciparum malaria followed drug sensitivity observation, as suggested by the WHO, for 28 days (Methods for surveillance of antimalarial drug efficacy (WHO, 2015), Methods and techniques for assessing exposure to antimalarial drugs in clinical field studies (WHO, 2011)). Based on past researches, the proportion of failed treatments for AP was estimated to be 5%. To ensure that 95% of the cases were treated successfully and that approximately 5% of the patients could not participate in this study, at least 73 people were selected as a sample size at the study site. The calculation was considered under the hypothesis that if 10% of the patients were lost to follow-up and hence excluded, the final sample size would be 80 (Methods for surveillance of antimalarial drug efficacy (WHO, 2015), Methods and techniques for assessing exposure to antimalarial drugs in clinical field studies (WHO, 2008; WHO, 2011)). Therefore, at least 80 participants were considered to be included as the target population for the study site to minimize loss of patient participation.
Children aged 6–59 months with body temperature ≥37.5°C, no signs or symptoms of severe malaria, and without obvious symptoms of fever or other symptoms of severe malnutrition (e.g., upper arm length <11 cm, height and weight ratio <70%) were included in the study. Moreover, the participants should be with a single infection caused by Plasmodium falciparum parasite at densities of 2,000–250,000/μl and with the ability of intaking oral drugs. Informed consent of the patient's parent or legal guardian was obtained after confirming their convenience to attend clinic examinations and confirming no history/allergic reaction/contraindications to AP in the patients.
AP tablets (containing 62.5 mg of artemisinin and 375 mg of piperaquine per tablet, Artequick®, Artepharm, Co., Ltd., batch number: 20160601) were administered in 1/2 tablet dosage at every 24 h over 2 days for children aged 6–24 months and 3/4 tablet dosage at every 24 h over 2 days for children aged 25–59 months. The medicines were administered under adult supervision. All study participants were provided with acetaminophen treatment every 6 h until the fever symptoms resolved completely.
Blood samples were collected from the finger tips on days 0, 2, 3, 7, 14, 21, and 28 to prepare thin and thick blood specimens. The number of asexual bodies/cubic millimeter of the blood sample was calculated before treatment. No asexual protozoa or parasite were detected in 500 fields of thick blood membrane (Methods for surveillance of antimalarial drug efficacy (WHO, 2015)). The hemoglobin level was measured on days 0, 7, 14, 21, and 28 on the Hemoglobin Analyzer URIT-12 (Labon Medical Equipment, China). Blood filter paper was prepared on days 0, 7, 14, 21, and 28, and thin and thick blood glass as well as blood filter paper specimens were made for laboratory testing for patients positive for the parasite. Meanwhile, approximately 3 ml of the venous blood samples was collected on days 0 and 28 from positive patients using heparin vacuum tubes, and the samples were stored at −20°C in the laboratory for white blood cells (WBC) and PCR analysis of polymorphism of merozoite surface protein-2 (msp-2) of P. falciparum in order to distinguish between incidence of recrudescence and reinfection (Adithya et al., 2003; Aubouy et al., 2003; Basco et al., 2004; Dolmazon et al., 2008; Awaga et al., 2012).
Clinical outcomes were evaluated with reference to the classification system suggested by the WHO (Methods for surveillance of antimalarial drug efficacy (WHO, 2015), as follows:
Danger signs or severe malaria on days 1, 2, or 3 in the presence of parasitemia; parasitemia on day 2 being higher than that on day 0, irrespective of the axillary temperature; parasitemia on day 3 with axillary temperature of ≥37.5°C; and parasitemia on day 3 of ≥25% of the count on day 0.
Danger signs or severe malaria in the presence of parasitemia on any day between days 4 and 28 in patients who did not previously meet any of the criteria of ETF; and presence of parasitemia on any day between days 4 and 28 with axillary temperature of ≥37.5°C in patients who did not previously meet any of the criteria of ETF.
Presence of parasitemia on any day between days 7 and 28 with axillary temperature of <37.5°C in patients who did not previously meet any of the criteria of ETF or LCF.
Absence of parasitemia on day 28, irrespective of the axillary temperature, in patients who did not previously meet any of the criteria of ETF, LCF, or LPF.
After every administration, the following question was asked: whether there were any adverse reactions, such as mental disorder, insomnia, headache, tinnitus, deafness, nausea, vomiting, loss of appetite, abdominal pain, diarrhea, itching, rash, or other symptoms, and detailed records were made according to the observation of adverse reactions.
All data was entered twice into Microsoft Excel spreadsheet. The per-protocol (PP) analysis was performed as the primary end-point. The PP population included all participants whose primary endpoint data was at day 28, who received a full course of AP, and who adhered to the follow-up visit schedule.
The GraphPad PRISM 7.0 (GraphPad Software, USA) was employed for all statistical analyses. The Shapiro–Wilk normality test was used to test the normality of the data. For normally distributed data, t-test was used for comparison between two groups in combination with Welch's t-test; and one-way analysis of variance (ANOVA) and Tukey's test were used for multiple comparisons among multiple groups. For nonparametric data, Mann–Whitney test was employed to compare between two groups, while Kruskal–Wallis and Dunn test were used to compare among multiple groups. Bidirectional ANOVA was employed for two-dimensional grouping data. Additionally, parasite clearance time and fever clearance time were represented by the mean ± standard error of the mean (SEM).
Among 208 children aged 6–59 months who were admitted to the general hospital and presented with fever, 73 (35%) tested negative for malaria by microscopy, 7 (3%) tested positive for mixed infections by P. ovale and P. malariae, 33 (16%) tested positive for parasitemia with density <2,000 parasites/μl, two (1%) could not fulfill the 28-day follow-up, one (0.5%) received other antimalarial treatment on the first day of the study duration, and one (0.5%) was lost to follow-up. Finally, 91 (44%) patients who met the inclusion criteria were included in this study (Figure 1).
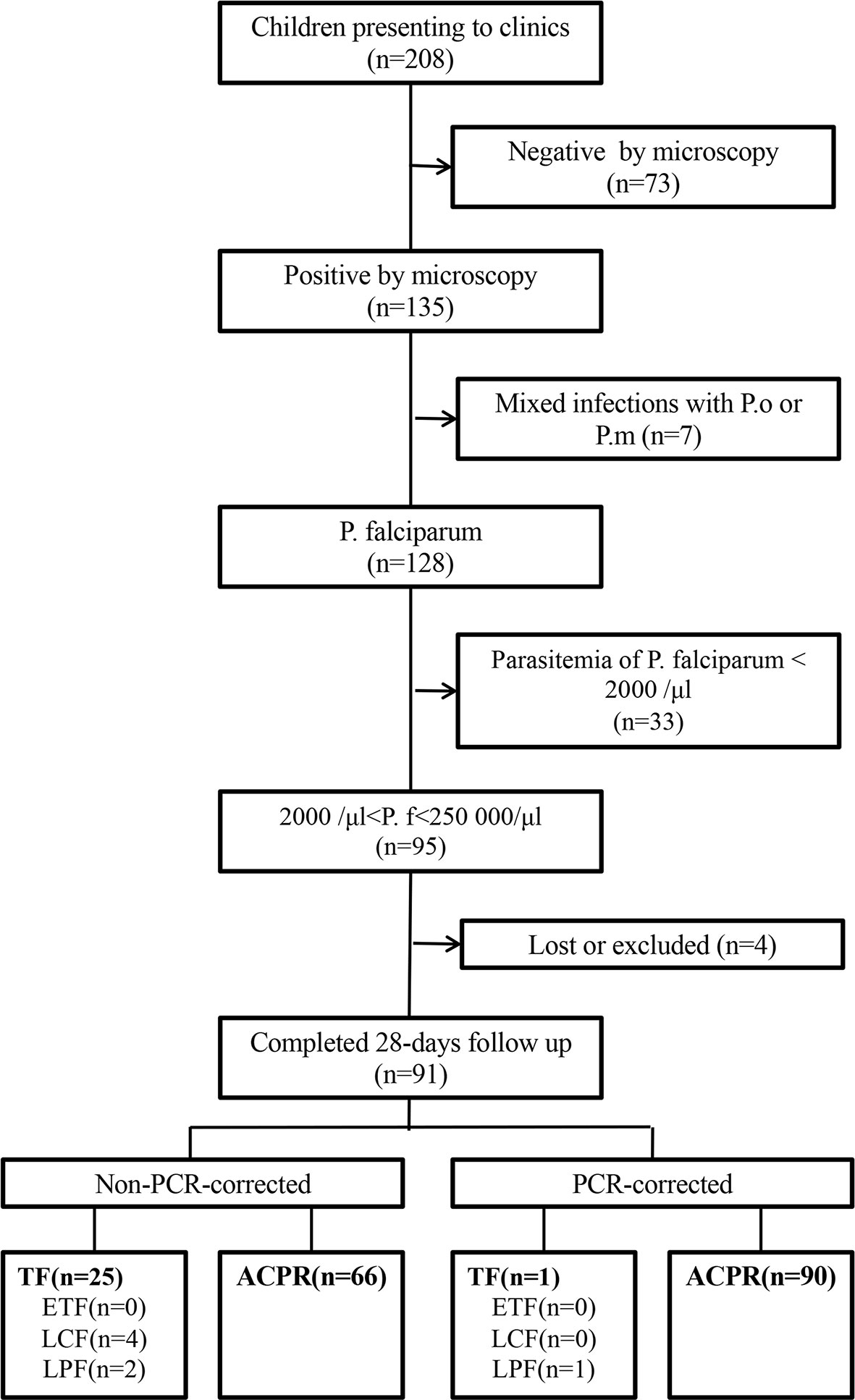
Figure 1 Patient flow chart in the clinical trial. ACPR, adequate clinical and parasitological response; ETF, early treatment failure; LCF, late clinical failure; LPF, late parasitological failure; TF, treatment failure; P.f, Plasmodium falciparum; P.m, Plasmodium malariae; P.o, Plasmodium ovale.
The baseline clinical and laboratory characteristics of the study subjects are shown in Table 1. The patients (n = 91) had an average age of 31.41 months (age range: 6–59 months) and included 44 (48%) girls. Their average weight was 11.73 kg (weight range: 8.73–14.73 kg), and the average body temperature was 38.5°C (body temperature range: 37.6–39.4°C). Their mean hemoglobin level before treatment was 8.2 g/dl (hemoglobin range: 6.6–9.8 g/dl), and the geometric mean of parasitemia was 36,184 (95% CI: 28,249–44,118).

Table 1 Baseline characteristics of the study participants with uncomplicated Plasmodium falciparum malaria treated with artemisinin–piperaquine in Togo.
After AP treatment, most patients experienced rapid remission of clinical symptoms. For instance, their average body temperature of 38.5°C on day 0 fell to 36.5°C on day 2 (p < 0.0001), with 79 (87%) patients reporting a temperature of <37°C (Figure 2A). On day 2 of treatment, 66 (73%) patients showed clearance of parasites in their blood smears, with complete clearance observed on day 3 (Figure 2B).
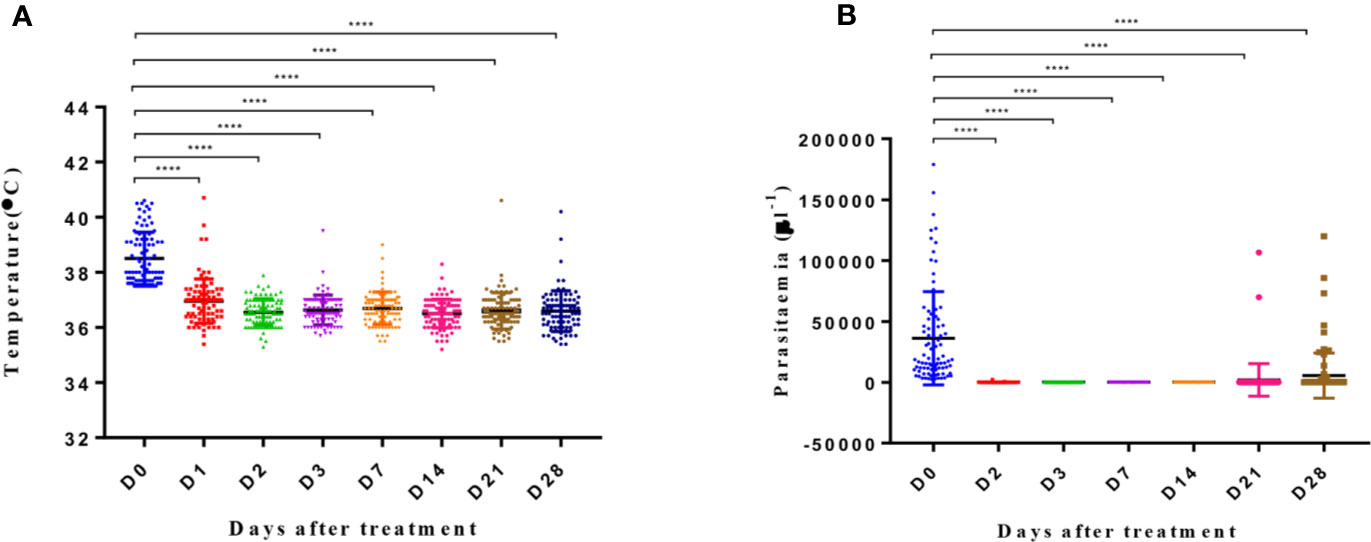
Figure 2 Body temperature and parasitemia across days after treatment. Y axis represents the temperature (A) and parasitemia (B) in patients. The significance levels are shown as significant (****: p < 0.0001) and nonsignificant (NS). All data were analyzed with one-way ANOVA and multiple comparisons. Error bars show the standard error of the mean (SEM).
The gametophyte observation (Figures 3A, B) revealed that the average gametocytemia increased until day 7, and the number of patients with gametophyte did not decrease significantly. Both these parameters decreased synchronously after day 7 of treatment, with significant reduction noted on day 21 (p < 0.05) and day 28 (p < 0.01). Gametophytes were continually observed on blood smears until day 28 in six patients, with a mean value of 8/µl.
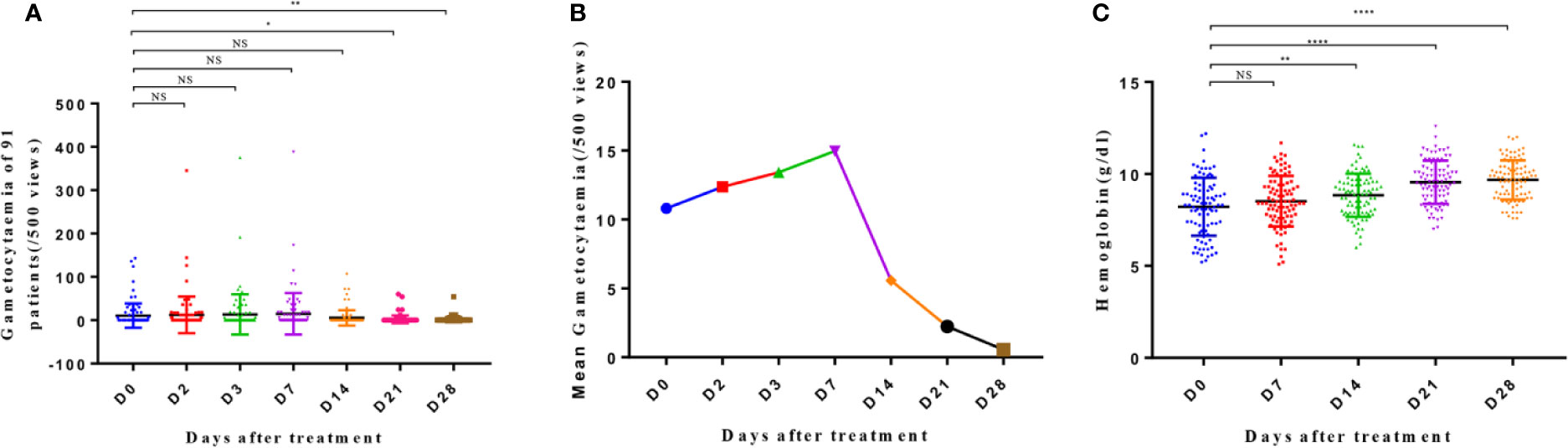
Figure 3 Gametocytemia (A), mean gametocytemia (B), and hemoglobin level (C) of patients from day 0 to day 28. The significance levels are shown as significant (****: p < 0.0001; **: p < 0. 01; *: p < 0. 05) and nonsignificant (NS). The gametocytemia and hemoglobin level of different groups were analyzed with one-way ANOVA and multiple comparisons. Error bars show the standard error of the mean (SEM).
The hemoglobin level of the patients improved progressively from day 0 to day 14 and on days 21 and 28 (Figure 3C). Significant differences were noted in the hemoglobin levels on days 14, 21, and 28 when compared to that on day 0 (p < 0.01; p < 0.0001; p < 0.0001, respectively), with an average increase in the level of 0.05 g/dl per day.
In the PP analyses, PCR-corrected ACPR was found to be 90 (99%) for AP (Table 2), and treatment failure was only one for recrudescence. On the other hand, non-PCR-corrected ACPR was 66 (73%), including four (4%) for LCF and 21 (23%) for LPF.
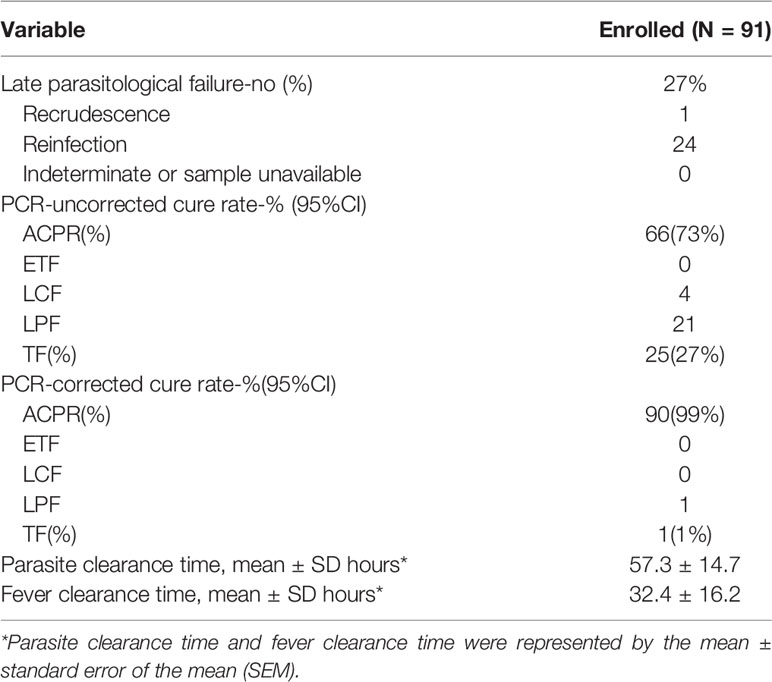
Table 2 Efficacy outcomes and adequate clinical and parasitological response rates of artemisinin–piperaquine treatment by day 28 among the participants.
Two allele genes (3D7 and FC27) were detected in 24 patients who remained positive for malaria despite AP treatment by nested PCR. In these patients, msp-2 of all isolates was analyzed before and after treatment. The condition of only one patient, whose 3D7 and FC27 haplotypes of msp-2 were the same before and after taking the drugs, suggested recrudescence, with a frequency of 4% (1/24). The remaining 23 patients may have been reinfected, with a frequency of 96% (23/24).
Meanwhile, the WBC levels of the 24 positive patients increased on day 28 after AP treatment (p < 0.001) (Figure 4A). The parasitemia density in these patients also decreased (p < 0.05) (Figure 4B). Moreover, the generation of gametophytes decreased from day 21 (p < 0.01) to day 28 (p < 0.001) (Figure 4C).
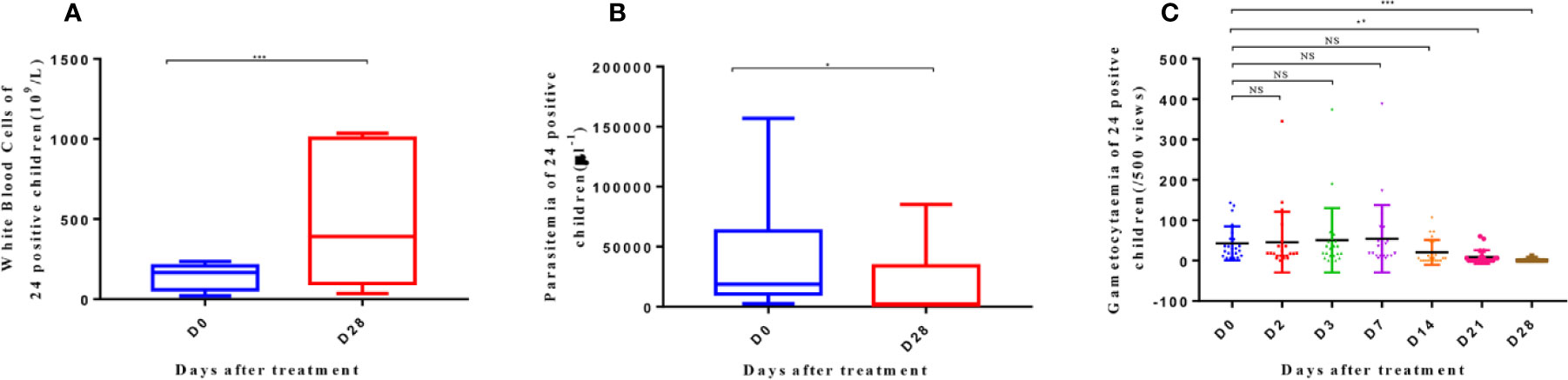
Figure 4 White blood cells (A), parasitemia (B), and gametocytemia (C) of 24 patients with positive parasite detection after treatment on D0 and D28. The significance levels are shown as: significant (***: p < 0. 001; **: p < 0. 01; *: p < 0. 05) and nonsignificant (NS). White blood cells and parasitemia of 24 patients were analyzed with one-way ANOVA, and gametocytemia was analyzed with one-way ANOVA and multiple comparisons. Error bars show the standard error of the mean (SEM).
In this study, the most common adverse reaction recorded was cough, followed by diarrhea and rash in all 91 patients who undertook AP treatment. Other notable adverse reactions included loss of appetite, vomiting, and abdominal pain. No serious adverse reactions occurred, and AP seemed to be well tolerated. The list of adverse reactions in the patients after the treatment is given in Table 3.

Table 3 Summary of adverse reactions of patients with uncomplicated falciparum malaria treated with artemisinin–piperaquine combination regime in Togo.
Malaria is the leading public health concern in Togo. This disease is prevalent throughout the country and strikes in all 12 months of the year. Moreover, the outbreak often occurs in the rainy season. According to the National Health Authority data of Togo in 2014, approximately 11 million malaria cases occur every year in Togo, with children aged <5 years being the most affected, accounting for 60.5% of all cases (Awag et al., 2008). In a secondary data analyses from the regional health information system on confirmed and suspected malaria cases, an annual record of 114,654 malaria cases (19,109 ± 6,622) was reported between January 2005 and December 2010, with the number of cases increasing from 10,299 in 2005 to 26,678 in 2010 (p < 0.001). The prevalence of confirmed malaria cases increased from 23.1‰ in 2005 to 257.5‰ in 2010 (p < 0.001). Despite the implementation of the artemisinin combination therapies (ACTs) and CHWs strategies in Togo, the study demonstrated an increase in the prevalence of malaria.
A few studies have reported the efficacy of malarial treatment and resistance to ACTs in Togo (Djadou et al., 2007; Dorkenoo et al., 2012; Améyo et al., 2016.). Only one article reported about the effect of ACTs (Dorkenoo et al., 2012). The therapeutic effects of artemether–benflumetol (AL) and artesunate–amodiaquine (ASAQ) were monitored in the National Malaria Control Program (NMCP) of Togo in 2013. In this program, a PCR-corrected ACPR of 97–100% was suggested for AL and of 96.3–100% for ASAQ. However, the recorded adverse events were significant: 2.68% for AL and 1.53% for ASAQ. In Benin, a country bordering Togo, a 42-day AL-efficacy study was conducted in 2014 (Aurore et al., 2016). On day 1 of the treatment in their study, the author reported apparent clearance of fever, with a clearance rate of parasites of approximately 90%; on day 2, almost all parasites were cleared, with the hemoglobin level rising slightly in sync with increasing clearance rate of the parasite. In their study, the non-PCR-corrected ACPR was 75.6% after 42-day follow-up, while PCR-corrected ACPR was 100%. These observations indicated that all treatment failures occurred due to refection, which supports the findings of the two previously mentioned papers and the present one (Dorkenoo et al., 2012; Aurore et al., 2016). The WHO recommends changing of the treatment policies only when the PCR-corrected treatment failure is >10%. As this was not the case in Togo, ACTs remain sensitive here.
During the sensitivity test of AP in this paper, fever was observed to reduce in most participating patients on day 1, with only one patient recording a temperature >37.5°C on day 2, and complete parasite clearance was observed on day 3. In addition, the average gametophyte generation increased, and the number of gametophyte cases did not reduce significantly in the first 7 days of the treatment. However, until day 28, the average gametophyte generation rapidly declined, probably because AP only affected parasites in the blood stage and could not directly kill the gametophytes already present. As a result, no obvious change was recorded in the early performance for patients carrying the gametophytes, although mature gametes were produced in some patients. Later, in the blood stage, a large number of parasites were killed, which led to a sharp decline in the number of late mature gametophytes; to a certain extent, this phenomenon inhibited the transmission of parasites by mosquitoes (Sophie et al., 2011). Overall, with respect to parasite clearance reduction, the reduction in gametophytic carrier rate was consistent with the improvements in the hemoglobin levels in all 91 participating patients. In our study, after PCR proofreading genotyping, the efficacy threshold of AP reached 98.90%, which is greater than the 95% efficacy threshold recommended by the WHO for AL (Guidelines for the treatment of malaria (WHO, 2010)). In addition, AP was well-tolerated by the local children in Togo, and no serious or adverse events, including diarrhea, weakness, anorexia, hemoglobinuria, itching, or eyelid edema, were noted during the 28-day follow-up in this study.
Our results conform to those of ACT studies conducted over the past 5 years in the Sub-Saharan Africa. The ACT combination therapy has a high cure rate for falciparum malaria without any complications. A past randomized study (Bernhards et al., 2014) conducted in Kenya in 2014 reported PCR-corrected ACPR of 97.8% for AL and 99.1% for dihydroartemisinin–piperaquine (DP). In addition, a single-arm prospective study (Busiku et al., 2014) conducted in Zambia in 2012 showed that 98% of the participating patients achieved complete clearance of their parasitic diseases on day 3, with PCR-corrected ACPR of 100% for AL. In other words, all participating patients achieved ACPR. In another study (Mateusz et al., 2017) conducted in three provinces of Angola in 2015, the 28-day cure rate of AL after PCR-correction was 88.1–96.3%. No evidence of artemisinin resistance was recorded in this study. However, the pfmdr1 haplotype was detected in all cases of AL treatment failures, indicating its association with decreased sensitivity of phenylfluorene. Another study (Magdalena et al., 2014) conducted in Malawi in 2014 reported a non-PCR-corrected cure rate for AL of 69–82.5%, but the PCR-corrected APCR was 98–100%, which was associated with high reinfection. These authors attributed this phenomenon to the short half-life of phenylfluorene (i.e., 3–6 days). Some researchers (Stephen et al., 2014) evaluated the efficacy of ACT in six geographic regions of Nigeria and found that AL and ASAQ were highly effective in Nigeria, with the PCR-corrected cure rates of 96.9 and 98.3%, respectively.
AP was determined to be effective in treating uncomplicated falciparum malaria and was well-tolerated in affected children under the age of 5 years in Elawagnon, Prefecture on East-Mono province, Togo. Hence, it was confirmed that P. falciparum remains sensitive to AP in Togo, which could be further tested via extensive drug trial in this region.
All datasets generated for this study are included in the article/supplementary material.
The studies involving human participants were reviewed and approved by Trial registration: ECGPHCM No. B2017-054-01; MHSST AVIS N° 0001/2016/CBRS du 07 janvier 2016. Registered 17 March 2014, http://www.chinadrugtrials.org.cn/eap/main. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
QW performed the statistical analysis and drafted the manuscript. CLu, WY, ZZ, WW, GL, YL, and GL collected and interpreted the data. HZ and ZP conducted the research and performed the PCR analysis. XH, WG, CLi, MC, JG, TO, LS, and QX collected the data and conducted the research. JS conceived and designed the study, conducted the research. CD conducted the research. All authors read and approved the final manuscript.
This work was supported by Guangzhou Science and Technology Program [Grant Number 201807010007] to JS and QW, China post-doctoral science foundation[Grant Number A2-2902-19-414-007] to QW, Natural Science Foundation of China [Grant Number 81873218] to JS and QW, National Major Science and Technology Projects of China (CN) [Grant Number 2018ZX09303008] to CD and QW, Project of Traditional Chinese Medicine Bureau of Guangdong [Grant Number 2019(43)] to JS and QW, and YangFan Innovative And Entrepreneurial Research Team Project[Grant Number 2014YT02S008] to CD and QW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to the parents and guardians of the children who participated, as well as the staff of the sentinel sites, especially Guangchao Wu, Longhua Pan, Yanshan Hu, and Xue Wang for their collaboration. The authors also thank the staff of the National Malaria Control Programme of Togo, the members of the Malaria Technical Committee, and Pr. Xinzhuan Su of Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health of U.S.A.
ETF, Early treatment failure; ACPR, Adequate clinical and parasitological response; ACT, Artemisinin-based combination therapy; LCF, Late clinical failure; LPF, Late parasitological failure; NMCP, National Malaria Control Programme; WHO, World Health Organization; DP, Dihydroartemisinin-piperaquine; AL, Artemether-benflumetol; ASAQ, Artesunate-amodiaquine; AP, Artemisinin piperaquine.
Adithya, C., Daniel, K., Alan, H., Philip, J. R., Grant, D. (2003). Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am. J. Trop. Med. Hyg. 68 (2), 33–139. doi: 10.1016/S0001-706X(02)00236-X
Améyo, M. D., Degninou, Y., Yao, M. A., Yao, L., Foli, A., Poukpessi, A., et al. (2016). Therapeutic efficacy trial of artemisinin-based combination therapy for the treatment of uncomplicated malaria and investigation of mutations in k13 propeller domain in Togo 2012-2013. Malaria J. 15 (331). doi: 10.1186/s12936-016-1381-8
Assimadi, J. K., Gbadoe, A. D., Atakouma, D. Y., Agbenowossi, K., Evi, L. K., Gayibor, A., et al. (1998). Severe malaria in Togolese children. Arch. Pediatr. 5 (12), 1310–1315. doi: 10.1016/S0929-693X(99)80048-7
Aubouy, A., Migot-Nabias, F., Deloron, P. (2003). Polymorphism in two merozoite surface proteins of Plasmodium falciparum isolates from Gabon. Malaria J. 9, 2–12. doi: 10.1186/1475-2875-9-S2-O2
Aurore, O. H., Christian, A., Souliatou, L., Georgia, D., Yolande, S., Franck, R., et al. (2016). Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Benin. Malaria J. 15 (37). doi: 10.1186/s12936-016-1091-2
Awaga, K. L., Missihoun, T. D., Karou, S. D., Djadou, K. E., Chabi, N. W., Akati, A., et al. (2008). Genetic diversity and genotype multiplicity of Plasmodium falciparum infections in symptomatic individuals in the maritime region of Togo. Trop. Med. Int. Health 17 (2), 153–160. doi: 10.1016/j.actatropica.2008.04.012
Awaga, K. L., Missihoun, T. D., Karou, S. D., Djadou, K. E., Chabi, N. W., Akati, A, et al. (2012). Genetic diversity and genotype multiplicity of Plasmodium falciparum infections in symptomatic individuals in the maritime region of Togo. Trop. Med. Int. Health 17 (2), 153–160. doi: 10.1111/j.1365-3156.2011.02913.x
Basco, L. K., Tahar, R., Escalante, A. (2004). Molecular epidemiology of malaria in Cameroon. XVIII. Polymorphisms of the Plasmodium falciparum merozoite surface antigen-2 gene in isolates from symptomatic patients. Am. J. Trop. Med. Hygiene 70, 238–244. doi: 10.4269/ajtmh.2004.70.238
Bernhards, R. O., Kevin, O. O., Nelly, K., Edgar, K. O., John, M. O., Godfrey, A. O., et al. (2014). Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malaria J. 13 (33). doi: 10.1186/1475-2875-13-33
Bo, H., Chang, S. D., Tao, Y., Lin, L. X., Qi, W., Shi, G. H., et al. (2015). Polymorphisms of the artemisinin resistant marker (K13) in Plasmodium falciparum parasite populations of Grande Comore Island 10 years after artemisinin combination therapy. Parasites Vectors 8, 634. doi: 10.1186/s13071-015-1253-z
Bo, H., Qi, W., Chang, S. D., Jian, H. W., Tao, Y., Shi, G. H., et al. (2016). Prevalence of crt and mdr-1 mutations in Plasmodium falciparum isolates from Grande Comore island after withdrawal of chloroquine. Malar. J. 15, 414. doi: 10.1186/s12936-016-1474-4
Busiku, H., Freddie, M., Hawela, M., Mulenga, M., Pascalina, C. K., Victor, C., et al. (2014). Therapeutic efficacy of artemether-lumefantrine on treatment of uncomplicated Plasmodium falciparum mono-infection in an area of high malaria transmission in Zambia. Malaria J. 13 (430). doi: 10.1186/1475-2875-13-430
Chang, S. D., Bo, H., Qi, W., Wan, T. W., Shao, Q. Z., Hong, Y. Z., et al. (2018). Large-scale Artemisinin–Piperaquine Mass Drug Administration With or Without Primaquine Dramatically Reduces Malaria in a Highly Endemic Region of Africa. Clin. Infect. Dis. 67 (11), 1670–1676. doi: 10.1093/cid/ciy364
Djadou, K. E., Komlangan, A., Balaka, B., Dokounor, D., Gbadoe, A. D., Atakouma, D., et al. (2006). Management of children severe malaria in Tsevie's (Togo) hospital regional center. Arch. Pediatr. 13 (12), 1555–1556. doi: 10.1016/j.arcped.2006.09.010
Djadou, K. E., Agbodjan, D. A., Azoumah, K. D., Djadou, D., Lawson, E. K., Balaka, B., et al. (2007). Artemether-lumefantrine, treatment of child more than 5 years old uncomplicated malaria in Tsevie's hospital (Togo). Arch. Pediatr. 14 (12), 1463–1464. doi: 10.1016/j.arcped.2007.10.003
Dolmazon, V., Matsika-Claquin, M. D., Manirakiza, A., Yapou, F., Nambot, M., Menard, D., et al. (2008). Genetic diversity and genotype multiplicity of Plasmodium fal- ciparum infections in symptomatic individuals living in Bangui (CAR). Acta Tropica 107, 37–42. doi: 10.1016/j.actatropica.2008.04.012
Dorkenoo, A. M., Barrette, A., Agbo, Y. M., Bogreau, H., Kutoati, S., Sodahlon, Y. K., et al. (2012). Surveillance of the efficacy of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum among children under five in Togo 2005–2009. Malar. J. 11 (338). doi: 10.1186/1475-2875-11-338
Dorkenoo, A. M., Layibo, Y., Agbo, Y. M., Morgah, K., Agbere, D. (2013). White blood cells counts and parasite density in malaria in children aged 6 to 60 months in urban areas of Togo. Med. Et. Sante Tropicales 23 (4), 412–416. doi: 10.1684/mst.2013.0246
Essoya, D. L., Potougnima, T., Bayaki, S., Khin, S. T., Sheba, N. G., Peter, W., et al. (2012). Morbidity and mortality due to malaria in Est Mono district, Togo, from 2005 to 2010: a times series analysis. Malaria J. 11 (389). doi: 10.1186/1475-2875-11-389
Klement, E., Pitche, P., Kendjo, E., Singo, A., D'Almeida, S., Akouete, F., et al. (2014). Effectiveness of Co-trimoxazole to Prevent Plasmodium falciparum Malaria in HIV-Positive Pregnant Women in Sub-Saharan Africa: An Open-Label, Randomized Controlled Trial. Clin. Infect. Dis. 58 (5), 651–665. doi: 10.1093/cid/cit806
Magdalena, P., Dyson, M., Daniel, M., Madalitso, L., Georgina, M., John, S., et al. (2014). In vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine for uncomplicated Plasmodium falciparum malaria in Malawi. Malaria J. 15 (236). doi: 10.1186/s12936-016-1281-y
Mateusz, M. P., Pedro, R. D., Aleixo, P. M., Carolina, M. F., Claudete, S., Joltim, Q., et al. (2017). Efficacy of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola 2017. Malaria J. 16 (62). doi: 10.1186/s12936-018-2290-9
Nguyen, X. T., Trieu, N. T., Nguyen, C. P., Huynh, H. Q., Bui, D., G Dennis, S., et al. (2012). The efficacy and tolerability of artemisinin-piperaquineversus artesunate-amodiaquine (Coarsucam™) for the treatment of uncomplicated Plasmodium falciparum malaria in south-central Vietnam. Malaria J. 11 (217). doi: 10.1186/1475-2875-11-217
Song, J. P., Tan, B., Socheat, D., Suou, S., Xu, Y., Ou, F. Z., et al. (2008). Dosage exploring of artemisinin-piperaquine in treatment of uncomplicated falciparum malaria. Chin. J. New Drugs Clin. Rem. 12, 908–911. doi: 10.1016/S1872-2075(08)60042-4
Song, J. P., Socheat, D., Tan, B., Seila, S., Xu, Y., Ou, F., et al. (2011). .Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia-Thailand border area. Malar. J. 10, 231. doi: 10.1186/1475-2875-10-231
Sophie, H. A., Geoffrey, L. J., Tao, L., Richard, T. E., Eric, H. E., Abraham, G. E., et al. (2011). Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. PNAS 108, 1214–1223. doi: 10.1073/pnas.1112037108
Srivicha, K., Noppadon, T., Vipa, T., Polrat, W., Siripan, S., Nantaporn, P., et al. (2007). Dose ranging studies of new artemisinin-piperaquine fixed combinations compared to standard regimens of artemisisnin combination therapies for acute uncomplicated falciparum malaria. Am. J. Trop. Med. Public Health 38, 971–978. doi: 10.1109/ICSM.2011.6080785
Stephen, O., Henrietta, U. O., Ismaila, W., Martin, M., Philip, A., William, O., et al. (2014). Efficacy of Artemisinin-Based Combination Treatments of Uncomplicated Falciparum Malaria in Under-Five-Year-Old Nigerian Children. Am. J. Trop. Med. Hyg. 91 (5), 925–935. doi: 10.4269/ajtmh.13-0248
WHO (2008). Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations (Geneva: World Health Organization;).
WHO (2011). Methods and techniques for assessing exposure to antimalarial drugs in clinical field studies (Geneva: World Health Organization;).
WHO (2015). Guidelines for the treatment of malaria. 3rd edtion. (Geneva: World Health Organization).
WHO (2015). Methods for surveillance of antimalarial drug efficacy (Geneva: World Health Organization;).
Keywords: artemisinin–piperaquine, Plasmodium falciparum, merozoite surface protein-2, Togo, gametophyte
Citation: Wang Q, Zhang Z, Yu W, Lu C, Li G, Pan Z, Zhang H, Wu W, Oubou TA, Yuan Y, Guo J, Liang Y, Huang X, Guo W, Li C, Julie `N, Xu Q, Sanwogou L, Song J and Deng C (2020) Surveillance of the Efficacy of Artemisinin–Piperaquine in the Treatment of Uncomplicated Plasmodium falciparum Malaria Among Children Under 5 Years of Age in Est-Mono District, Togo, in 2017. Front. Pharmacol. 11:784. doi: 10.3389/fphar.2020.00784
Received: 14 February 2020; Accepted: 12 May 2020;
Published: 05 June 2020.
Edited by:
Joseph O. Fadare, Ekiti State University, NigeriaReviewed by:
Billy Ngasala, Muhimbili University of Health and Allied Sciences, TanzaniaCopyright © 2020 Wang, Zhang, Yu, Lu, Li, Pan, Zhang, Wu, Oubou, Yuan, Guo, Liang, Huang, Guo, Li, Julie, Xu, Sanwogou, Song and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changsheng Deng, ZGNzMTk4MTEyMDJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.