- 1Department of Anesthesiology, University Hospital Duesseldorf, Duesseldorf, Germany
- 2Division of Nephrology, University Hospital Zurich, Zurich, Switzerland
During hospital stay, about 20% of adult patients experience an episode of acute kidney injury (AKI), which is characterized by a rapid decrease in kidney function. Diagnostic tools regarding early diagnosis of kidney dysfunction prior to AKI and markers of renal recovery are not available. Additionally, there is no therapeutic option for the treatment of AKI. Thus, better and more specific diagnostic and therapeutic options are urgently needed in daily clinical practice. NoncodingRNAs (ncRNAs) have come into focus of research in the context of AKI in the last decade. The best characterized group of ncRNAs are microRNAs (miRNAs). An increasing body of literature has shown that miRNAs are involved in the pathogenesis of AKI and that they are promising future tools in the diagnosis and therapy of AKI. However, there are obstacles to be overcome before miRNAs can be transferred to patient care. This review will give an overview of our current knowledge of miRNA involvement in the context of AKI while critically evaluating their diagnostic and therapeutic potential.
Introduction
According to Kidney Disease: Improving Global Outcomes (KDIGO), acute kidney injury (AKI) is diagnosis involves elevated levels in creatinine and/or a decrease in urine output (KDIGO, 2012). AKI occurs in up to 50% of critically ill patients (Hoste et al., 2015; Chawla et al., 2017). Its etiology is diverse and involves patient susceptibility (e.g., age, known CKD, diabetes mellitus) as well as exposure to potentially harmful factors contributing to the development of AKI (e.g., sepsis, burns, nephrotoxic drugs, cardiac surgery). AKI remains a major health issue in critically ill patients (Hoste et al., 2015; Chawla et al., 2017). It is an important factor of short-term patient outcome as it can cause electrolyte disorders, fluid accumulation, and acid–base derangement. Indeed, mortality in critically ill patients that experience an episode of AKI raises up to 50% in those that require renal replacement therapy (RRT) (Ympa et al., 2005). AKI furthermore goes along with an increase of cardiovascular events (Chawla et al., 2014a) and the development of CKD (Venkatachalam et al., 2010; Chawla et al., 2014b). There are no specific treatment options in daily clinical practice besides renal replacement therapy (RRT). Morbidity and mortality of AKI patients remains high.
The high incidence of AKI as well as the impairment in outcome indicates that there is an urgent need for new markers and therapeutic options in the prevention and treatment of AKI.
To date, diagnostic markers of AKI are insufficient and late. Therapeutic options are limited to renal replacement therapy. Therefore, noncoding RNAs (nc RNAs) have come into focus of research in the last decade as potential markers and therapeutic targets in AKI. The best investigated class of ncRNAs in this context is microRNAs (miRNAs).
MiRNAs are small ncRNAs that regulate gene expression post transcriptionally (Ambros, 2001). They repress protein translation or induce degradation of messengerRNA (mRNA) (Bartel, 2009) through base-pairing with complementary sequences of the 3´untranslated region (UTR) of mRNAs. A high number of the more than 2000 identified miRNAs is highly conserved between species (www.mirbase.org). In the canonical pathway, miRNAs are transcribed as primary miRNAs (primiRNA) and then cleaved by the enzymes Drosha/Dgcr8 resulting in the so-called premiRNA (Friedman et al., 2009). The enzyme Dicer cleaves the premiRNA which finally results in the mature miRNA with a length of 20-24 nucleotides. The two strands of the mature miRNA are then separated and incorporated into the miRNA induced silencing complex (miRISC) (Bartel, 2009). Within the miRISC, miRNAs bind to the 3'-untranslated region of mRNAs thereby inhibiting translation of messenger RNAs or inducing degradation of mRNAs. In both cases, miRNAs action results in a reduction of protein translation (Baek et al., 2008). In the non-canonical pathway, miRNAs do not originate from their own genes. Instead, they are excised from other larger RNAs, such as tRNAs, introns or small nucleolar RNAs.
It has been shown that miRNAs are essential players in physiological processes from development to death of cells, tissues, and organisms. Moreover, miRNAs are involved in pathophysiological processes including diseases of the kidney. MiRNAs are stable in body fluids (such as plasma and urine) (Gilad et al., 2008) and can be detected easily. These characteristics make them an interesting class of molecules as novel diagnostic markers of myocardial infarction (Goretti et al., 2014), lung cancer (Vencken et al., 2015) or breast cancer (Chan et al., 2013). MiRNA function can be manipulated by the application miRNA-agonists (so-called mimics) or antagonists. They might, therefore, also be future therapeutic targets in the treatment of AKI.
A high number of miRNAs are expressed in mammalian kidneys. A study from 2004 showed five miRNAs that are enriched in human kidneys including miR-192, -194, -215, -216, and miR-204 (Sun et al., 2004). A newer study from 2016 showed miR-449c-5p, -449b-5p, and -449a to be expressed especially in kidneys (Ludwig et al., 2016). It is known that the expression of these miRNAs and others is altered in renal pathologies such as chronic kidney disease (CKD). Several studies have shown that the expression of miRNAs in CKD decreases in higher stages of the disease (Neal et al., 2011). These miRNAs might then be involved in pathological remodeling processes such as abnormal vascularization (Chen et al., 2013).
Besides the well documented involvement of miRNAs in CKD, it has been shown that miRNAs play an essential role in the pathology of AKI as well. Indeed, various miRNAs have been shown to be dysregulated in AKI in animal models as well as in human studies. A number of preclinical and clinical studies have focused on the role of miRNAs in the context of AKI. This review will give an overview of the current knowledge of miRNAs in the context of AKI as well as a perspective of miRNAs as diagnostic and/or therapeutic tools in AKI diagnosis and treatment.
MiRNAs in Experimental AKI Studies
Experimental AKI Studies: Diagnostic Potential of miRNAs
To date, standard clinical markers of renal function are creatinine and urea. Additionally, a reduction in diuresis (oliguria, anuria) is one of the AKI signs. Especially creatinine, the most common marker of renal function, is an unspecific and late marker of AKI (Bagshaw et al., 2008). Oliguria, on the other hand, is influenced by multiple factors such as volume deficiency, heart failure or diuretics.
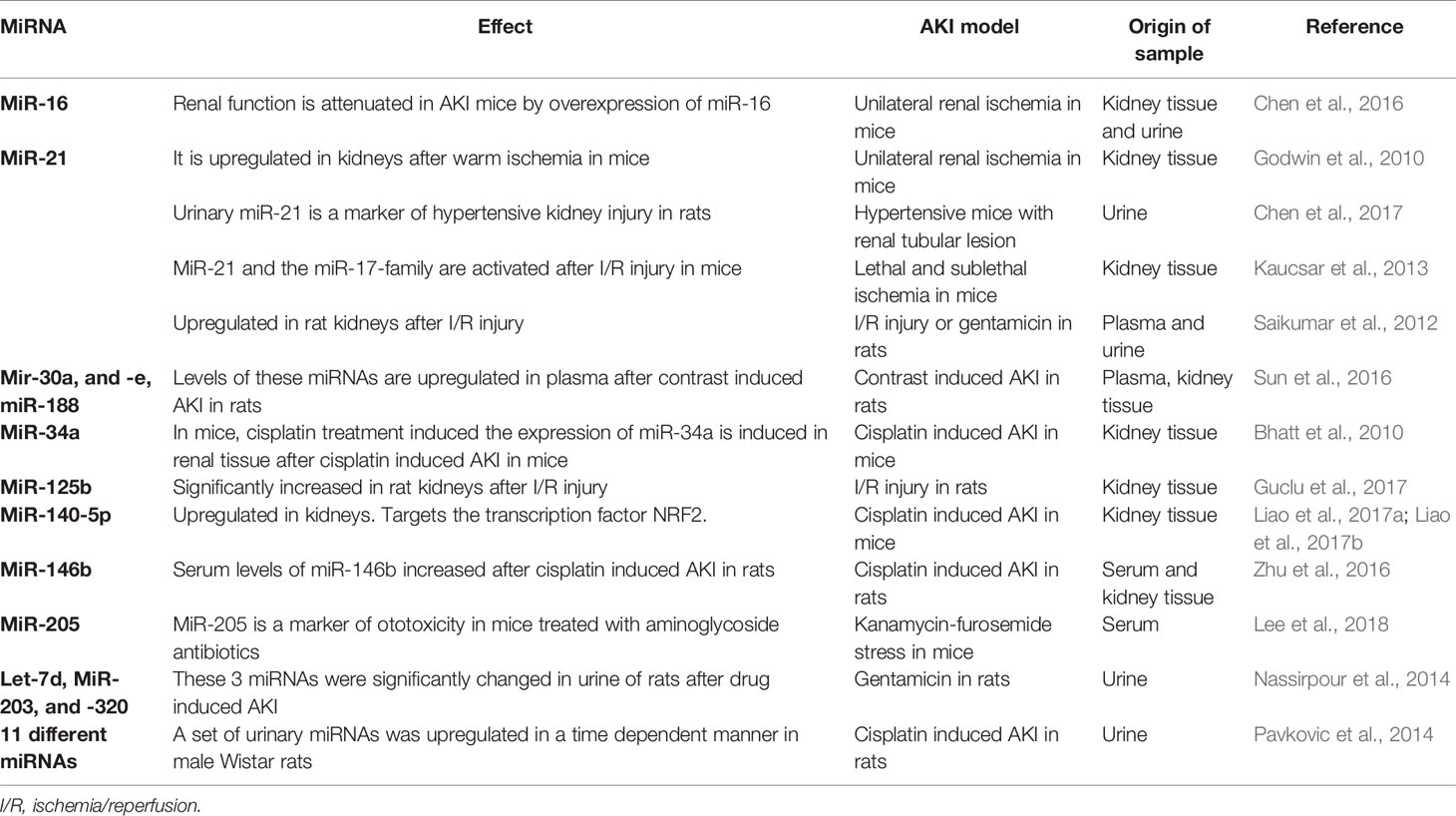
A large number of potential new AKI biomarkers have been extensively evaluated in the last years (reviewed in (Teo and Endre, 2017)). The most relevant markers of these are neutrophil gelatinase-associated lipocalin (NGAL), the cysteine protease inhibitor Cystatin C, kidney injury molecule 1 (KIM-1) and the product of insulin like growth factor binding protein 7 (IGFBP-7) and tissue inhibitor of metalloproteinase 2 (TIMP-2). With areas under the curve (AUC) of 0.67 for NGAL (Han et al., 2009), 0.71 for cystatin C (Koyner et al., 2008), 0.65 for KIM-1 (Han et al., 2009), and 0.80 for the product of IGFBP7 and TIMP-2 (Kashani et al., 2013), these markers perform better than the standard markers creatinine and urea. To date, most of these markers have not been established in clinical practice. A large number of miRNA-studies have, therefore, analyzed the potential of miRNAs as markers and/or potential therapeutics in AKI over the last decade including animal as well as human studies. The most relevant miRNAs as potential markers of AKI are summarized in Table 1.
MiR-21
MiR-21 is expressed in the kidney of mammals and among others in heart and lung tissue (Lagos-Quintana et al., 2002). It has been analyzed in a large number of preclinical and clinical AKI studies. MiR-21 attenuates I/R injury of the kidney by inhibiting apoptosis (Hu et al., 2014). Suppressing miR-21 expression leads to a reduced activity of tumor necrosis factor α and monocyte chemoattractant protein-1 and thereby renal inflammation (Zhong et al., 2013). A number of studies have shown that miR-21 is differentially expressed in plasma and/or urine in AKI (Table 1). However, as miR-21 is expressed ubiquitously, it is likely to be a rather unspecific marker of AKI.
MiR-30a and -c
The miR-30 family consists of five highly conserved members termed miR-30a to –e. This family has been shown to be involved in numerous cancers, such as lung cancer, breast cancer, and colorectal cancer (reviewed in (Yang et al., 2017)). In a rat model of AKI, as well as in patients after cardiac surgery, miR-30c-5p was an early diagnostic marker of AKI in urine (Zou et al., 2017). It has, furthermore, been shown that podocyte injury is facilitated by downregulation of the miR-30 family and that this injury can be prevented by the administration of glucocorticoids (Wu et al., 2014).
MiR-107
MiR-107 is member of the miR-15/107 family. Members of this family share the AGCAGC near the 5′ end and are involved in several pathways which are crucial in AKI, including stress response and angiogenesis (Finnerty et al., 2010). In patients suffering from septic induced AKI, miR-107 was shown to be elevated in Circulating endothelial cells (Wang et al., 2017).
MiR-125b
The miR-125 family is highly conserved between mammals. Of this family, miR-125b has been shown to be involved in ischemia and reperfusion events of several organs, especially the heart (Wang et al., 2014). Additionally, it has been shown that miR-125b is a protective factor in cardiac ischemia and reperfusion injury (Varga et al., 2018). In AKI, miR-125b has been shown to be a potential marker in the diagnosis of ischemic kidney injury (Guclu et al., 2017).
Experimental AKI Studies: Therapeutic Potential of miRNAs
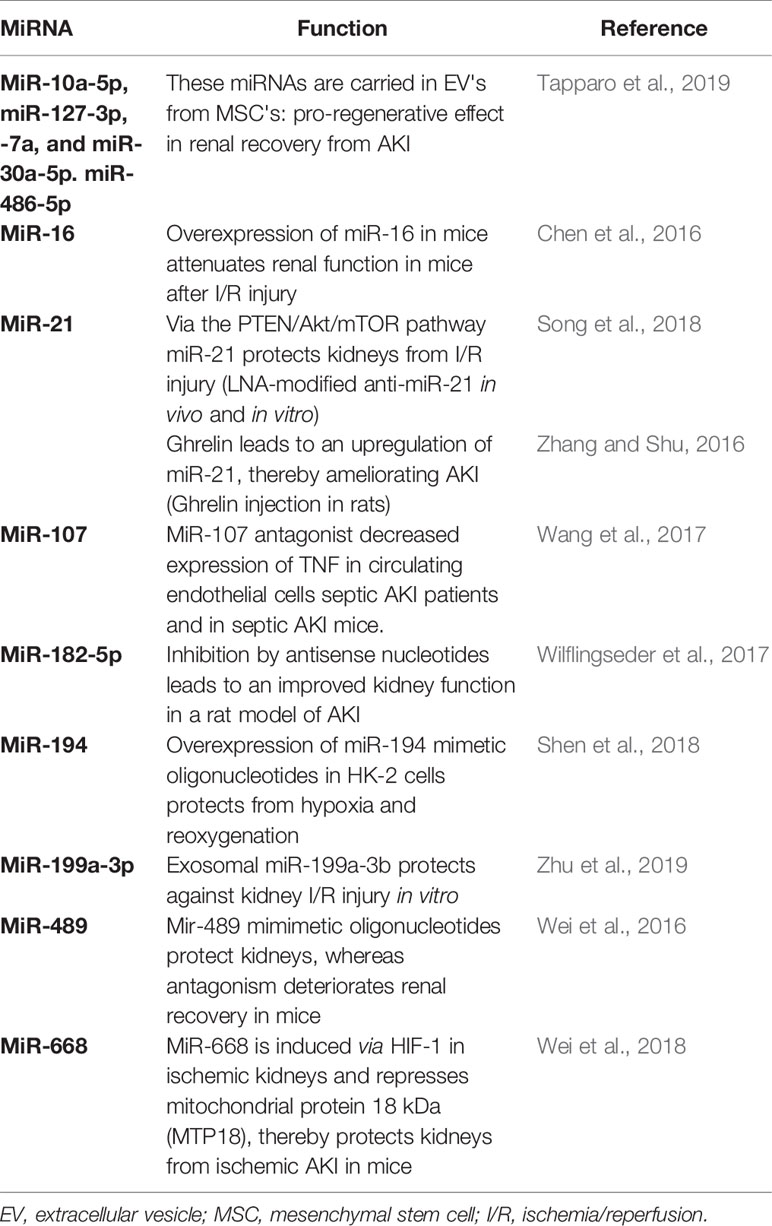
In theory, miRNAs bear a fascinating potential not only as diagnostic markers of AKI but also as therapeutics – summarized in the term theranostics. Dysregulated miRNA-expression can be treated in vivo and in vitro by the application of specific antagonists or agonists vice versa. Obstacles regarding this in vivo application of miRNAs are described in the next passage describing the clinical application of miRNAs.
There are a growing number of animal studies showing an effective modulation of miRNA function in the context of AKI. A selection of these studies is summarized in Table 2. These data show that AKI severity and recovery from AKI can be influenced by the modulation of miRNAs. However the next step from these observation into humans and finally to clinical practice is by far the biggest one. Obstacles and chances of this step will be further discussed in the next paragraph.
Clinical Studies Using miRNAs as Biomarkers and Therapeutics
Extensive research activities regarding preclinical as was patient studies have been forwarded regarding the investigation of the role of miRNAs in kidney injury. Taking into consideration the mechanism of action and downstream effects of miRNAs, their therapeutic silencing or overexpression has become a topic of interest in order to target disease activity. Owing to a high degree of intra-species conservation, miRNAs represent perfect target molecules for investigation, since results of animal studies may be easily translated to the human setting to ameliorate or reverse the progression of disease. The identification of the disease and/or tissue/cell-type-specific regulation of miRNAs using transgenic rodent models or pre-clinical therapeutic silencing/overexpression of relevant miRNAs is the prerequisite to understanding pathological events in the kidney and will ultimately results in novel therapeutic strategies to target diseases. Alterations of specific miRNAs in distinct diseases can be perceived to mirror dysregulation of intertwined pathological signalling, because oftentimes miRNAs have equal mRNA target molecules, thus impacting on similar signalling pathways.
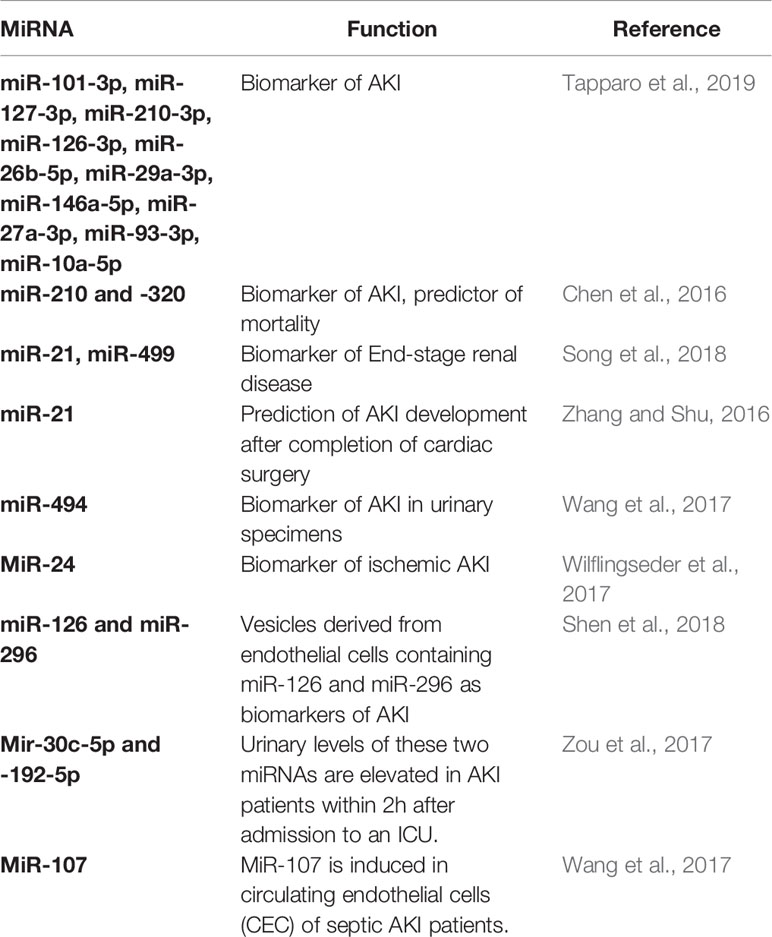
MiRNAs have been analyzed in detail as biomarkers of kidney disease (Table 3). For instance, several miRNAs (including miR-101-3p, miR-127-3p, miR-210-3p, miR-126-3p, miR-26b-5p, miR-29a-3p, miR-146a-5p, miR-27a-3p, miR-93-3p, and miR-10a-5p) have been shown to be altered in serum samples of patients with AKI (Aguado-Fraile et al., 2015). These novel biomarkers showed a near perfect area under the curve of almost 1. Moreover, miR-210 and -320 were demonstrated to be enriched in blood samples of AKI patients (Lorenzen et al., 2011). Here, miR-210 predicted mortality of AKI patients on the intensive care unit. On the contrary, in patients with terminal kidney failure on renal replacement therapy miR-21 (downregulated) and miR-499 (upregulated) seem to be altered (Neal et al., 2011; Emilian et al., 2012). In patients undergoing cardiac surgery, baseline miR-21 before surgery predicted AKI development after completion of cardiac surgery (Gaede et al., 2016). Another study suggested miR-494 to be highly upregulated in urinary specimens of patients with AKI (Lan et al., 2012). MiR-24 has been demonstrated to drive progression of ischemic AKI (Lorenzen et al., 2014).
Contrary to the use of miRNAs as biomarkers, their application as therapeutics is less advanced. A few pre-clinical studies in rodents/cells have investigated the therapeutic potential of miRNAs in AKI (miR-126/miR-296 (Cantaluppi et al., 2012), miR-92a (Henique et al., 2017), miR-709 (Guo et al., 2017)). These preclinical studies suggest that miRNAs may also be applied as powerful therapeutics in the setting of patients with AKI.
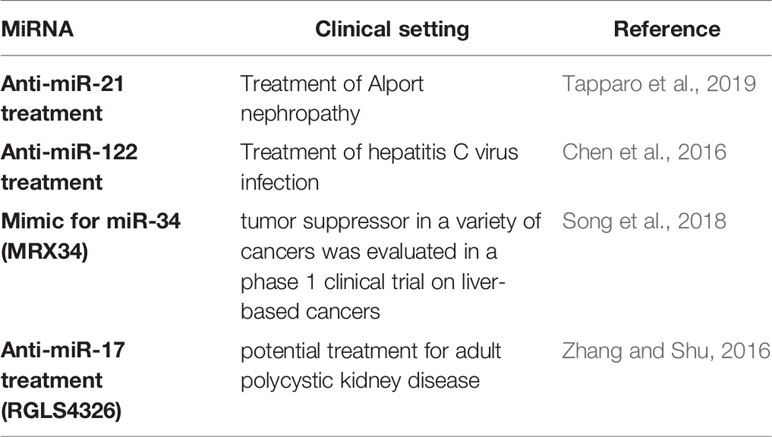
However, translation of pre-clinical findings is complicated by several challenges. A major obstacle to transferring animal data to humans is related to the fact that miRNAs are not regulated in a cell-type or organ specific manner. Therapeutic alteration of a specific miRNA may therefore lead to “off-target” changes in unrelated/untargeted organs, which were initially not aimed for. This problem finds it correlate in the fact that merely few investigations involving patients treated with drugs targeting miRNAs have been forwarded to date. The formulation of cell or tissue-specific miRNA drugs by coupling them with tissue-specific antibodies may be a solution to this predicament and could be a focus of future investigations. A major study in patients with kidney disease, which has just entered Phase II is related to a treatment strategy silencing miR-21 in Alport nephropathy patients (clinicaltrials.gov, NCT03373786). In addition to this important study, miR-122 has been silenced effectively in patients with an infection of the hepatitis C virus (Janssen et al., 2013). The use of so-called miRNA-mimics to overexpress miRNAs in patients with malignant pleural mesothelioma and non-small cell lung cancer (clinicaltrials.gov, NCT02369198) has been forwarded. A mimic for miR-34 (MRX34), which acts as a tumor suppressor in a number of cancers was evaluated in a phase 1 clinical trial on liver-based cancers (Beg et al., 2017). This study was terminated early due to the occurrence of adverse events related to immune-mediated mechanisms. An inhibitor against microRNA-17 (miR-17, named RGLS4326), which targets important disease-relevant genes including PKD1 and PKD2, will soon be evaluated as a potential treatment for adult polycystic kidney disease in a phase 1 clinical trial (Lee et al., 2019). (Table 4) These clinical studies raise hope that miRNAs may be used as therapeutics in future clinical routine. However, the widespread use of miRNA therapeutics still has a long way to go. Nonetheless, the ongoing and detailed analysis of potential therapeutic use of miRNA modulators in kidney disease represents a fascinating avenue of future investigations and might ultimately improve patient care.
Future Outlook Regarding Clinical Studies Using miRNA Modulators in Patients With AKI.
Research into the mechanisms of miRNA-related kidney disease, including AKI, has gained widespread interest over the last decade. The identification and therapeutic applicability of cell- and tissue-specific miRNAs would pave the way for an improvement of the future care of patients. If a disease-specific miRNA is identified, its downstream mechanisms resolved and therapeutic potential proven, it would offer physicians a real alternative for a targeted treatment approach. Aforementioned predicament regarding a lack of tissue-specificity of a number of miRNAs (and still retain its desired function) could be circumvented by a combination of these miRNAs with cell-type specific peptides/antibodies so that the complex may be directed to a tissue of interest.
Several chemistries of miRNA modulators have been described in the past. One of the first studies in the cardiovascular research employed an intravenous treatment approach using Cy3-coupled AntimiRs on a cholesterol based backbone in rodents. This resulted in efficient uptake of the miRNA inhibitor and miRNA suppression (Thum et al., 2008). An additional modification that has been experimentally used includes 2′O-methoxyethylphosphorothioate and 2′ fluoro substitutions. These modifications have been shown to lead to specific and strong silencing of the miRNA of interest (Kim, 2005). Furthermore, miRNAs may be silenced by the use of an approach termed as “erasers”. Using this approach a tandem repeat of a sequence that shows perfect complementarity to the miRNA of interest inhibits its function (Sayed et al., 2008).
As an alternative, miRNA silencing can be achieved by use of a locked nucleic-acid (LNA)-based approach. LNAs have been initially tested in a primate model of hepatitis C infection. Here, they have been shown to be highly effective and non-toxic, while also exhibiting a long half-life (Lanford et al., 2010). This resulted in the design and completion of aforementioned clinical study in patients with hepatitis C (Janssen et al., 2013). MiR-122 can be viewed as a special miRNA, since its expression is highly enriched in the liver, thereby making it an ideal candidate for therapeutic interventions, since off-target effects in distant organs are not expected. A locked nucleic acid approach is now widely used and represents the chemistry of choice regarding future clinical use due to its effectiveness, stability, and low profile regarding potential side effects. Almost all miRNA inhibitors are associated with high specificity regarding its target miRNA. A loss of miRNA downregulation may ensue, if a point mutation is detected within the structure of an AntimiR (Krutzfeldt et al., 2007).
A major concern, as stated above, is the fact that the use of miRNA therapeutics may exert off-target effects in nontarget cells and/or organs. In order to circumvent this predicament miRNA modulators may therefore be locally injected in the future. In addition, miRNA modulators may be coupled to cell and/or tissue-specific antibodies/peptides.
In addition to miRNA antagonists a miRNA of interest may also be therapeutically overexpressed by the use of miRNA-mimics (synthetic double stranded precursor miRNA molecules). Efficient recognition and loading of the guide strand into the RNA-induced silencing complex (RISC) is a prerequisite for its use (Martinez et al., 2002).
In conclusion, miRNA modulation in the setting of AKI represents a field of potential major importance and promise in the future. Critically ill patients with AKI on the intensive care unit might greatly benefit, based on pre-clinical studies involving miRNA therapeutics, from miRNA modulation to halt or even reverse the disease process and limit AKI. If proven successful miRNA modulation might save patients from the installation of renal replacement therapy and its associated complications.
Author Contributions
Both authors wrote the review article.
Funding
This work was supported by a National Center for Excellence in Research NCCR Kidney.ch Junior grant as well as a grant by the Swiss National Science Foundation (SNSF) to JL.
Conflict of Interest
TB has received research grants from Bayer AG and Grünenthal GmbH, Germany, and lecture fees from Fresenius Medical Care Germany.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aguado-Fraile, E., Ramos, E., Conde, E., Rodriguez, M., Martin-Gomez, L., Lietor, A., et al. (2015). A Pilot Study Identifying a Set of microRNAs As Precise Diagnostic Biomarkers of Acute Kidney Injury. PloS One 10, e0127175. doi: 10.1371/journal.pone.0127175
Ambros, V. (2001). microRNAs: tiny regulators with great potential. Cell 107, 823–826. doi: 10.1016/S0092-8674(01)00616-X
Baek, D., Villen, J., Shin, C., Camargo, F. D., Gygi, S. P., Bartel, D. P. (2008). The impact of microRNAs on protein output. Nature 455, 64–71. doi: 10.1038/nature07242
Bagshaw, S. M., George, C., Bellomo, R., Committe, A. D. M. (2008). A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol. Dial Transplant. 23, 1569–1574. doi: 10.1093/ndt/gfn009
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Beg, M. S., Brenner, A. J., Sachdev, J., Borad, M., Kang, Y. K., Stoudemire, J., et al. (2017). Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs 35, 180–188. doi: 10.1007/s10637-016-0407-y
Bhatt, K., Zhou, L., Mi, Q. S., Huang, S., She, J. X., Dong, Z. (2010). MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 16, 409–416. doi: 10.2119/molmed.2010.00002
Cantaluppi, V., Gatti, S., Medica, D., Figliolini, F., Bruno, S., Deregibus, M. C., et al. (2012). Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427. doi: 10.1038/ki.2012.105
Chan, M., Liaw, C. S., Ji, S. M., Tan, H. H., Wong, C. Y., Thike, A. A., et al. (2013). Identification of circulating microRNA signatures for breast cancer detection. Clin. Cancer Res. 19, 4477–4487. doi: 10.1158/1078-0432.CCR-12-3401
Chawla, L. S., Amdur, R. L., Shaw, A. D., Faselis, C., Palant, C. E., Kimmel, P. L. (2014a). Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin. J. Am. Soc. Nephrol. 9, 448–456. doi: 10.2215/CJN.02440213
Chawla, L. S., Eggers, P. W., Star, R. A., Kimmel, P. L. (2014b). Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl. J. Med. 371, 58–66. doi: 10.1056/NEJMra1214243
Chawla, L. S., Bellomo, R., Bihorac, A., Goldstein, S. L., Siew, E. D., Bagshaw, S. M., et al. (2017). Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 13, 241–257. doi: 10.1038/nrneph.2017.2
Chen, N. X., Kiattisunthorn, K., O'neill, K. D., Chen, X., Moorthi, R. N., Gattone, V. H., 2nd, et al. (2013). Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PloS One 8, e64558. doi: 10.1371/journal.pone.0064558
Chen, H. H., Lan, Y. F., Li, H. F., Cheng, C. F., Lai, P. F., Li, W. H., et al. (2016). Urinary miR-16 transactivated by C/EBPbeta reduces kidney function after ischemia/reperfusion-induced injury. Sci. Rep. 6, 27945. doi: 10.1038/srep27945
Chen, C., Lu, C., Qian, Y., Li, H., Tan, Y., Cai, L., et al. (2017). Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci. Rep. 7, 17737. doi: 10.1038/s41598-017-18175-3
Emilian, C., Goretti, E., Prospert, F., Pouthier, D., Duhoux, P., Gilson, G., et al. (2012). MicroRNAs in patients on chronic hemodialysis (MINOS study). Clin. J. Am. Soc. Nephrol. 7, 619–623. doi: 10.2215/CJN.10471011
Finnerty, J. R., Wang, W. X., Hebert, S. S., Wilfred, B. R., Mao, G., Nelson, P. T. (2010). The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J. Mol. Biol. 402, 491–509. doi: 10.1016/j.jmb.2010.07.051
Friedman, R. C., Farh, K. K., Burge, C. B., Bartel, D. P. (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105. doi: 10.1101/gr.082701.108
Gaede, L., Liebetrau, C., Blumenstein, J., Troidl, C., Dorr, O., Kim, W. K., et al. (2016). Plasma microRNA-21 for the early prediction of acute kidney injury in patients undergoing major cardiac surgery. Nephrol. Dial Transplant. 31, 760–766. doi: 10.1093/ndt/gfw007
Gilad, S., Meiri, E., Yogev, Y., Benjamin, S., Lebanony, D., Yerushalmi, N., et al. (2008). Serum microRNAs are promising novel biomarkers. PloS One 3, e3148. doi: 10.1371/journal.pone.0003148
Godwin, J. G., Ge, X., Stephan, K., Jurisch, A., Tullius, S. G., Iacomini, J. (2010). Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 107, 14339–14344. doi: 10.1073/pnas.0912701107
Goretti, E., Wagner, D. R., Devaux, Y. (2014). miRNAs as biomarkers of myocardial infarction: a step forward towards personalized medicine? Trends Mol. Med. 20, 716–725. doi: 10.1016/j.molmed.2014.10.006
Guclu, A., Kocak, C., Kocak, F. E., Akcilar, R., Dodurga, Y., Akcilar, A., et al. (2017). MicroRNA-125b as a new potential biomarker on diagnosis of renal ischemia-reperfusion injury. J. Surg. Res. 207, 241–248. doi: 10.1016/j.jss.2016.08.067
Guo, Y., Ni, J., Chen, S., Bai, M., Lin, J., Ding, G., et al. (2017). MicroRNA-709 Mediates Acute Tubular Injury through Effects on Mitochondrial Function. J. Am. Soc. Nephrol. 29 (2), 449–461. doi: 10.1681/ASN.2017040381
Han, W. K., Wagener, G., Zhu, Y., Wang, S., Lee, H. T. (2009). Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin. J. Am. Soc. Nephrol. 4, 873–882. doi: 10.2215/CJN.04810908
Henique, C., Bollee, G., Loyer, X., Grahammer, F., Dhaun, N., Camus, M., et al. (2017). Genetic and pharmacological inhibition of microRNA-92a maintains podocyte cell cycle quiescence and limits crescentic glomerulonephritis. Nat. Commun. 8, 1829. doi: 10.1038/s41467-017-01885-7
Hoste, E. A., Bagshaw, S. M., Bellomo, R., Cely, C. M., Colman, R., Cruz, D. N., et al. (2015). Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423. doi: 10.1007/s00134-015-3934-7
Hu, H., Jiang, W., Xi, X., Zou, C., Ye, Z. (2014). MicroRNA-21 attenuates renal ischemia reperfusion injury via targeting caspase signaling in mice. Am. J. Nephrol. 40, 215–223. doi: 10.1159/000368202
Janssen, H. L., Reesink, H. W., Lawitz, E. J., Zeuzem, S., Rodriguez-Torres, M., Patel, K., et al. (2013). Treatment of HCV infection by targeting microRNA. N Engl. J. Med. 368, 1685–1694. doi: 10.1056/NEJMoa1209026
Kashani, K., Al-Khafaji, A., Ardiles, T., Artigas, A., Bagshaw, S. M., Bell, M., et al. (2013). Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25. doi: 10.1186/cc12503
Kaucsar, T., Revesz, C., Godo, M., Krenacs, T., Albert, M., Szalay, C. I., et al. (2013). Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther. 23, 344–354. doi: 10.1089/nat.2013.0438
KDIGO (2012). Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2, 19–36. doi: 10.1038/kisup.2012.7
Kim, V. N. (2005). MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 6, 376–385. doi: 10.1038/nrm1644
Koyner, J. L., Bennett, M. R., Worcester, E. M., Ma, Q., Raman, J., Jeevanandam, V., et al. (2008). Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 74, 1059–1069. doi: 10.1038/ki.2008.341
Krutzfeldt, J., Kuwajima, S., Braich, R., Rajeev, K. G., Pena, J., Tuschl, T., et al. (2007). Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 35, 2885–2892. doi: 10.1093/nar/gkm024
Lagos-Quintana, M., Rauhut, R., Yalcin, A., Meyer, J., Lendeckel, W., Tuschl, T. (2002). Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739. doi: 10.1016/S0960-9822(02)00809-6
Lan, Y. F., Chen, H. H., Lai, P. F., Cheng, C. F., Huang, Y. T., Lee, Y. C., et al. (2012). MicroRNA-494 reduces ATF3 expression and promotes AKI. J. Am. Soc. Nephrol. 23, 2012–2023. doi: 10.1681/ASN.2012050438
Lanford, R. E., Hildebrandt-Eriksen, E. S., Petri, A., Persson, R., Lindow, M., Munk, M. E., et al. (2010). Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201. doi: 10.1126/science.1178178
Lee, S. H., Ju, H. M., Choi, J. S., Ahn, Y., Lee, S., Seo, Y. J. (2018). Circulating Serum miRNA-205 as a Diagnostic Biomarker for Ototoxicity in Mice Treated with Aminoglycoside Antibiotics. Int. J. Mol. Sci. 19 (9), E2836. doi: 10.3390/ijms19092836
Lee, E. C., Valencia, T., Allerson, C., Schairer, A., Flaten, A., Yheskel, M., et al. (2019). Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 10, 4148. doi: 10.1038/s41467-019-11918-y
Liao, W., Fu, Z., Zou, Y., Wen, D., Ma, H., Zhou, F., et al. (2017a). Corrigendum to “MicroRNA-140-5p attenuated oxidative stress in Cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism” [Exp. Cell Res., (2017) 292-302]. Exp. Cell Res. 361, 199. doi: 10.1016/j.yexcr.2017.10.023
Liao, W., Fu, Z., Zou, Y., Wen, D., Ma, H., Zhou, F., et al. (2017b). MicroRNA-140-5p attenuated oxidative stress in Cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp. Cell Res. 360, 292–302. doi: 10.1016/j.yexcr.2017.09.019
Lorenzen, J. M., Kielstein, J. T., Hafer, C., Gupta, S. K., Kumpers, P., Faulhaber-Walter, R., et al. (2011). Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin. J. Am. Soc. Nephrol. 6, 1540–1546. doi: 10.2215/CJN.00430111
Lorenzen, J. M., Kaucsar, T., Schauerte, C., Schmitt, R., Rong, S., Hubner, A., et al. (2014). MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 25, 2717–2729. doi: 10.1681/ASN.2013121329
Ludwig, N., Leidinger, P., Becker, K., Backes, C., Fehlmann, T., Pallasch, C., et al. (2016). Distribution of miRNA expression across human tissues. Nucleic Acids Res. 44, 3865–3877. doi: 10.1093/nar/gkw116
Martinez, J., Patkaniowska, A., Urlaub, H., Luhrmann, R., Tuschl, T. (2002). Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110, 563–574. doi: 10.1016/S0092-8674(02)00908-X
Nassirpour, R., Mathur, S., Gosink, M. M., Li, Y., Shoieb, A. M., Wood, J., et al. (2014). Identification of tubular injury microRNA biomarkers in urine: comparison of next-generation sequencing and qPCR-based profiling platforms. BMC Genomics 15, 485. doi: 10.1186/1471-2164-15-485
Neal, C. S., Michael, M. Z., Pimlott, L. K., Yong, T. Y., Li, J. Y., Gleadle, J. M. (2011). Circulating microRNA expression is reduced in chronic kidney disease. Nephrol. Dial Transplant. 26, 3794–3802. doi: 10.1093/ndt/gfr485
Pavkovic, M., Riefke, B., Ellinger-Ziegelbauer, H. (2014). Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology 324, 147–157. doi: 10.1016/j.tox.2014.05.005
Saikumar, J., Hoffmann, D., Kim, T. M., Gonzalez, V. R., Zhang, Q., Goering, P. L., et al. (2012). Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol. Sci. 129, 256–267. doi: 10.1093/toxsci/kfs210
Sayed, D., Rane, S., Lypowy, J., He, M., Chen, I. Y., Vashistha, H., et al. (2008). MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol. Biol. Cell 19, 3272–3282. doi: 10.1091/mbc.e08-02-0159
Shen, Y., Zhao, Y., Wang, L., Zhang, W., Liu, C., Yin, A. (2018). MicroRNA-194 overexpression protects against hypoxia/reperfusion-induced HK-2 cell injury through direct targeting Rheb. J. Cell Biochem. doi: 10.1002/jcb.28114
Song, N., Zhang, T., Xu, X., Lu, Z., Yu, X., Fang, Y., et al. (2018). miR-21 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Front. Physiol. 9, 790. doi: 10.3389/fphys.2018.00790
Sun, Y., Koo, S., White, N., Peralta, E., Esau, C., Dean, N. M., et al. (2004). Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 32, e188. doi: 10.1093/nar/gnh186
Sun, S. Q., Zhang, T., Ding, D., Zhang, W. F., Wang, X. L., Sun, Z., et al. (2016). Circulating MicroRNA-188, -30a, and -30e as Early Biomarkers for Contrast-Induced Acute Kidney Injury. J. Am. Heart Assoc. 5 (8), e004138. doi: 10.1161/JAHA.116.004138
Tapparo, M., Bruno, S., Collino, F., Togliatto, G., Deregibus, M. C., Provero, P., et al. (2019). Renal Regenerative Potential of Extracellular Vesicles Derived from miRNA-Engineered Mesenchymal Stromal Cells. Int. J. Mol. Sci. 20 (10), E2381. doi: 10.3390/ijms20102381
Teo, S. H., Endre, Z. H. (2017). Biomarkers in acute kidney injury (AKI). Best Pract. Res. Clin. Anaesthesiol 31, 331–344. doi: 10.1016/j.bpa.2017.10.003
Thum, T., Gross, C., Fiedler, J., Fischer, T., Kissler, S., Bussen, M., et al. (2008). MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984. doi: 10.1038/nature07511
Varga, Z. V., Agg, B., Ferdinandy, P. (2018). miR-125b is a protectomiR: A rising star for acute cardioprotection. J. Mol. Cell Cardiol. 115, 51–53. doi: 10.1016/j.yjmcc.2017.12.010
Vencken, S. F., Greene, C. M., Mckiernan, P. J. (2015). Non-coding RNA as lung disease biomarkers. Thorax 70, 501–503. doi: 10.1136/thoraxjnl-2014-206193
Venkatachalam, M. A., Griffin, K. A., Lan, R., Geng, H., Saikumar, P., Bidani, A. K. (2010). Acute kidney injury: a springboard for progression in chronic kidney disease. Am. J. Physiol. Renal Physiol. 298, F1078–F1094. doi: 10.1152/ajprenal.00017.2010
Wang, X., Ha, T., Zou, J., Ren, D., Liu, L., Zhang, X., et al. (2014). MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc. Res. 102, 385–395. doi: 10.1093/cvr/cvu044
Wang, S., Zhang, Z., Wang, J., Miao, H. (2017). MiR-107 induces TNF-alpha secretion in endothelial cells causing tubular cell injury in patients with septic acute kidney injury. Biochem. Biophys. Res. Commun. 483, 45–51. doi: 10.1016/j.bbrc.2017.01.013
Wei, Q., Liu, Y., Liu, P., Hao, J., Liang, M., Mi, Q. S., et al. (2016). MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. J. Am. Soc. Nephrol. 27, 2784–2796. doi: 10.1681/ASN.2015080870
Wei, Q., Sun, H., Song, S., Liu, Y., Liu, P., Livingston, M. J., et al. (2018). MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J. Clin. Invest. 128, 5448–5464. doi: 10.1172/JCI121859
Wilflingseder, J., Jelencsics, K., Bergmeister, H., Sunzenauer, J., Regele, H., Eskandary, F., et al. (2017). miR-182-5p Inhibition Ameliorates Ischemic Acute Kidney Injury. Am. J. Pathol. 187, 70–79. doi: 10.1016/j.ajpath.2016.09.011
Wu, J., Zheng, C., Fan, Y., Zeng, C., Chen, Z., Qin, W., et al. (2014). Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J. Am. Soc. Nephrol. 25, 92–104. doi: 10.1681/ASN.2012111101
Yang, X., Chen, Y., Chen, L. (2017). The Versatile Role of microRNA-30a in Human Cancer. Cell Physiol. Biochem. 41, 1616–1632. doi: 10.1159/000471111
Ympa, Y. P., Sakr, Y., Reinhart, K., Vincent, J. L. (2005). Has mortality from acute renal failure decreased? A systematic review of the literature. Am. J. Med. 118, 827–832. doi: 10.1016/j.amjmed.2005.01.069
Zhang, W., Shu, L. (2016). Upregulation of miR-21 by Ghrelin Ameliorates Ischemia/Reperfusion-Induced Acute Kidney Injury by Inhibiting Inflammation and Cell Apoptosis. DNA Cell Biol. 35, 417–425. doi: 10.1089/dna.2016.3231
Zhong, X., Chung, A. C., Chen, H. Y., Dong, Y., Meng, X. M., Li, R., et al. (2013). miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 56, 663–674. doi: 10.1007/s00125-012-2804-x
Zhu, Y., Yu, J., Yin, L., Zhou, Y., Sun, Z., Jia, H., et al. (2016). MicroRNA-146b, a Sensitive Indicator of Mesenchymal Stem Cell Repair of Acute Renal Injury. Stem Cells Transl. Med. 5, 1406–1415. doi: 10.5966/sctm.2015-0355
Zhu, G., Pei, L., Lin, F., Yin, H., Li, X., He, W., et al. (2019). Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell Physiol. 234, 23736–23749. doi: 10.1002/jcp.28941
Keywords: acute kidney injury, microRNA, noncodingRNA, kidney, theranostics
Citation: Brandenburger T and Lorenzen JM (2020) Diagnostic and Therapeutic Potential of microRNAs in Acute Kidney Injury. Front. Pharmacol. 11:657. doi: 10.3389/fphar.2020.00657
Received: 05 December 2019; Accepted: 22 April 2020;
Published: 14 May 2020.
Edited by:
Matthew Griffin, National University of Ireland Galway, IrelandCopyright © 2020 Brandenburger and Lorenzen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timo Brandenburger, dGltby5icmFuZGVuYnVyZ2VyQG1lZC51bmktZHVlc3NlbGRvcmYuZGU=; Johan M. Lorenzen, Sm9oYW4uTG9yZW56ZW5AdXN6LmNo
 Timo Brandenburger
Timo Brandenburger Johan M. Lorenzen
Johan M. Lorenzen