- 1Centre of Behavioural Medicine, Department of Practice and Policy, University College London, London, United Kingdom
- 2School of Pharmacy, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
- 3Spoonful of Sugar Ltd, UCL-Business Spin-out Company, London, United Kingdom
Introduction: Practical adherence barriers (e.g., medication frequency) are generally more amenable to intervention than perceptual barriers (e.g., beliefs). Measures which assess adherence barriers exist, however these tend to measure a mix of factors. There is a need to identify what practical barriers are captured by current measures.
Aim: To identify and synthesise the practical adherence barriers which are assessed by currently available self- or observer-report adherence measures.
Methods: A search for systematic reviews of self- or observer-report report adherence measures was conducted. Three electronic databases (Embase, Ovid Medline, and PsycInfo) were searched using terms based on adherence, adherence barriers and measures. Systematic reviews reporting on adherence measures which included at least one self- or observer-report questionnaire or scale were included. Adherence measures were extracted and coded on whether they addressed perceptual or practical barriers, or both. Practical items were then analysed thematically.
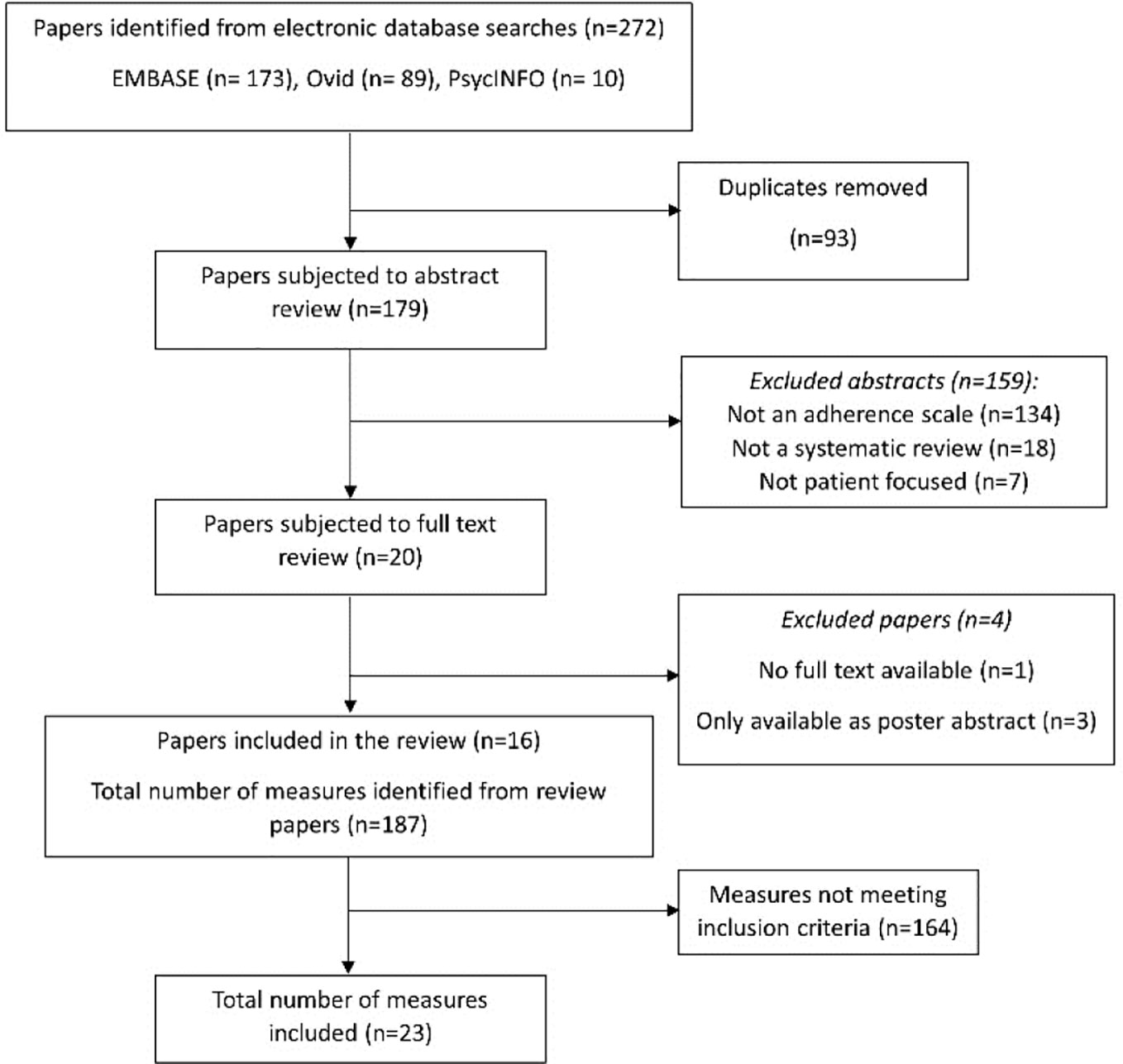
Results: Following screening of 272 initial abstracts, 20 full-text papers were reviewed. Four were excluded after full-text review, leaving 16 systematic reviews for data extraction. From these, 187 different adherence measures were extracted and coded, and 23 unique measures were identified as assessing practical barriers and included in the final analysis. Seven key themes were identified: formulation; instructions for use; issues with remembering; capability—knowledge and skills; financial; medication supply and social environment.
Conclusion: Existing adherence measures capture a variety of practical barriers which can be grouped into seven categories. These findings may be used to inform the development of a measure of practical adherence barriers.
Introduction
Poor adherence to medication in both long- and short-term conditions increases the risk of morbidity, mortality, and cost of care (Roebuck et al., 2011). Despite many years of research into non-adherence and the development of many different interventions to improve poor adherence, medication adherence remains largely an unresolved public health issue. In the European Union (EU) alone, non-adherence is estimated to cost the EU 1.25 billion each year from lost health gains and poor health outcomes (Pharmaceutical Group of the European Union, 2008). Part of the difficulty with addressing non-adherence is its complexity, as adherence can be influenced by multiple factors (Nieuwlaat et al., 2014). These factors can be enablers or barriers to adherence and broadly classified as either perceptual or practical, an approach which has been described in The National Institutes of Clinical Excellence (NICE) adherence guidelines (Horne et al., 2005; Nunes et al., 2009). Perceptual factors refer to factors which arise primarily from internal cognitive processes, such as motivation, emotions, or patient perceptions and beliefs of the illness and treatment, while practical factors refer primarily to external environmental factors relating to the individual, treatment, or society which can affect behaviour (Horne et al., 2019). Examples include the look and feel of the medication, how easy it is to access the medication, and the structure of the healthcare system. These perceptual and practical factors can lead to intentional non-adherence or unintentional non-adherence, respectively (Clifford et al., 2008).
An important first step when addressing any healthcare problem or behavior is to identify and assess the factors which are leading to the behavior, so that interventions can be designed to minimise or remove these factors. While no single type of adherence intervention has been shown to be more effective in improving adherence than others (Nieuwlaat et al., 2014), interventions that are tailored to address the specific adherence barriers faced by an individual are more likely to be effective than a one-size fits all approach (Kassavou and Sutton, 2017). In order to inform the development of a tailored intervention, there is a need for a measure which can (a) identify the types of factors influencing the behavior, and (b) quantify the degree that the various factors are influencing the behavior.
In recent years, there has been an increasing focus on the identification and assessment of perceptual factors influencing adherence, with the application of different theories and models to explain non-adherence (Holmes et al., 2014). The Beliefs about Medicines questionnaire is a valid and reliable measure for quantifying perceptual factors influencing adherence (Horne et al., 1999). A systematic review and meta-analysis showed that non-adherence to medication prescribed for a range of long-term conditions was predicted by doubts about the necessity for treatment (necessity beliefs) and concerns about adverse effects (concerns) (Horne et al., 2013). However, while these perceptual barriers are important factors influencing intentional non-adherence, they may not explain unintentional non-adherence. Even if a person intends to take their medication as prescribed, they may be prevented from doing so by limitations in capacity or resource (i.e., practical adherence barriers) such as deficiencies in memory or access to supply of medicines (Briesacher et al., 2007; Garfield et al., 2011). For example, forgetting has been cited as one of the most common reasons for non-adherence (Taylor et al., 2002; Martin et al., 2005; Shubber et al., 2016; Jamison et al., 2017). There has been a focus on perceptual factors but identifying practical factors are equally important in influencing adherence as perceptual and practical factors influence each other. Changing an individual’s practical barriers (e.g., by making a treatment easier to take) can lead to changes in perceptual barriers (e.g., by increasing motivation to take a treatment and vice versa (e.g., increases in perceived necessity of the treatment can translate into increased efforts in the individual to overcome any previous practical difficulties in taking the medication). Identifying and quantifying practical factors influencing non-adherence is thus an important first step in promoting adherence, as practical factors are often more easily amenable to changes in the physical environment compared to perceptual barriers.
There are currently several self- or observer-reported medication adherence measures available which identify factors which influence adherence (Garfield et al., 2011; Nguyen et al., 2014). These measures range from self- or observer-reported questionnaires to task-based tools which include instruments and surveys. The measures vary in terms of their purpose, what type of factors they measure (perceptual or practical), how they measure these factors (dichotomous or numerical scale), and whether or not the measure has been validated (Nguyen et al., 2014). Some questionnaires also measure adherence itself (i.e., the extent of adherence or medication-taking), as well as the factors influencing adherence (Nguyen et al., 2014). Few measures however distinguish between the different types of non-adherence (i.e., intentional versus unintentional non-adherence), nor measure both perceptual and practical adherence barriers (Garfield et al., 2011), limiting the ability of existing measures to be used to tailor interventions to individual needs.
The aim of this paper is to identify and synthesise the practical factors which are currently measured by self- or observer-reported adherence measures. There have been several studies reporting on adherence measures including systematic reviews of adherence measures; however, the large number of studies, and the variation in breadth and scope of the reviews, make it difficult for those involved in the design of adherence interventions and health policies to identify, extract, and interpret what practical factors are assessed by current adherence measures. As such a review of reviews was deemed necessary to identify what practical factors are currently assessed by adherence measures. Previous reviews of adherence measures or barriers have also not focused on practical barriers to adherence, or included self-report measures only (i.e., observer-rated instruments were excluded) or were limited to measures which have been correlated against a comparison measure of medication-taking behavior (Kardas et al., 2013; Nguyen et al., 2014). Furthermore, prior reviews have focused on synthesising measures which assess adherence per se (i.e., medication-taking), rather than measures which evaluate factors influencing adherence (Garfield et al., 2011).
This review of systematic reviews will identify and synthesise the different practical barriers which influence adherence that are currently included in self- or observer-report adherence measures.
Methods
A search for systematic reviews of self- or observer-report adherence measures was conducted using Embase, Ovid Medline, and PsycInfo electronic databases. Search terms based on adherence, adherence barriers, and questionnaires or measures were used (see Appendix 1 for full search strategy). The search was limited to systematic reviews. This limitation to systematic reviews rather than original scientific papers was considered appropriate due to the large volume of literature in this area, in-line with recommendations by Smith et al. who recommend the use of review of reviews to allow findings from separate reviews to be synthesised when many systematic reviews exist in a single area (Smith et al., 2011). Reviews published from the data of database conception to April 2020 were included. The last date of search was 5th April 2020. To ensure there were no additional relevant measures, the reviews and reference lists of the reviews were also manually checked. The review was registered on PROSPERO under CRD42018085859.
Inclusion and Exclusion Criteria
The paper was included if it was a systematic review, reported on adherence measures which included at least one self- or observer-report questionnaire or scale (though the review could include other types of measures including objective measurements of adherence), and a full text English article was available. Reviews which only reported on factors associated with adherence, without including any adherence measure at all (e.g., discussed only adherence factors, or only included satisfaction measures), or reviewed adherence interventions only (i.e., did not include adherence measures), were excluded. Systematic review protocols without published results were also excluded.
Data Extraction and Analysis
All identified abstracts were screened using the Rayyan tool (Ouzzani et al., 2016) by three researchers (AC, HL, VC) independently, with 100% overlap. Any disagreements were solved by consensus discussion. One researcher (VC) then reviewed and data extracted from the full texts of all identified papers for consistency, with an independent second review and extraction by a second researcher (AC or HL). All papers were therefore reviewed and extracted by at least two researchers independently.
Data extraction was done in two stages. First, data was extracted from the systematic reviews, including: the aim of the review, databases or sources searched in the review, search terms used, questionnaire selection criteria, number of studies included, number of adherence measures used, and names of the adherence measures. Secondly, the original full-text for each measure identified in the reviews was obtained to allow evaluation of what the adherence measure was assessing. Where possible, the full wording of the items in each measure was obtained from the original validation papers or by contacting the author of the measure.
Each measure was then coded according to whether the measure assessed perceptual or practical factors (or both), and whether or not the scale measured adherence (medication-taking) behavior. A measure was considered to measure perceptual factors if it included some reference to necessity beliefs or concerns about treatment, or an individual’s perceptions, attitudes, opinions or emotions towards treatment, or the impact of these perceptions or beliefs on quality of life. A measure was not classified as perceptual if it included only a quantification of the individual’s perceptions or beliefs (e.g., how often or how much the patient was concerned about their medication). Practical measures were any questionnaires which included reference to adherence barriers of a non-perceptual nature (i.e., not related to necessity or concerns). Measures were deemed to include a measure of adherence if it captured past or current medication-taking behavior. Questions relating to future medication-taking behavior were not considered to be measures of adherence (as these were considered to be more relevant to intention to take a medication).
Measures were included for final analysis if they were “generic” (i.e., the measure or items within the measure were not related to, or developed for, a specific named condition or medication e.g., blood pressure medication), and included one or more items relating to practical factors. This formed our final inclusion criteria for the adherence measures. Measures could either be self-administered (i.e., self-reported measures) or administered by another person (e.g., a healthcare professional or carer)—i.e., observer-reported measures. The practical items were summarised then analysed thematically by two independent researchers (AC, HL) (Braun and Clarke, 2006). We elected to use the World Health Organisation’s five interacting dimensions that affect adherence as a guiding framework for categorisation of the items (Sabaté, 2003). In line with Braun and Clarke’s approach to thematic analysis, each researcher independently reviewed and familiarized themselves with the measures, generated initial codes, identified common themes, reviewed the themes, then defined and named the themes (Braun and Clarke, 2006). This process was conducted iteratively, with any discrepancies resolved by in-depth discussion and negotiated consensus.
Results
A total of 272 abstracts were initially identified for inclusion, of which 93 were duplicates, leaving 179 for screening. The PRISMA flow diagram which illustrates the study selection process is shown in Figure 1. After applying the initial inclusion criteria for the systematic reviews, the full texts of 20 systematic reviews were obtained. Four were excluded after full-text review, leaving 16 systematic reviews for data extraction (Vreeman et al., 2008; Elliott and Marriott, 2009; Vandenbroeck et al., 2011; AlGhurair et al., 2012; Paquin et al., 2013; Remor, 2013; Nguyen et al., 2014; Snyder et al., 2014; Akeroyd et al., 2015; Pérez-Escamilla et al., 2015; Huang et al., 2016; Katusiime et al., 2016; Forbes et al., 2018; Pednekar et al., 2019; Pareja-Martinez et al., 2020; Plevinsky et al., 2020). From these 16 systematic reviews, 187 different adherence measures were extracted and coded. Of these, 23 measures met the final inclusion criteria for the selection of adherence measures, and were included in the thematic analysis.
Characteristics of Measures
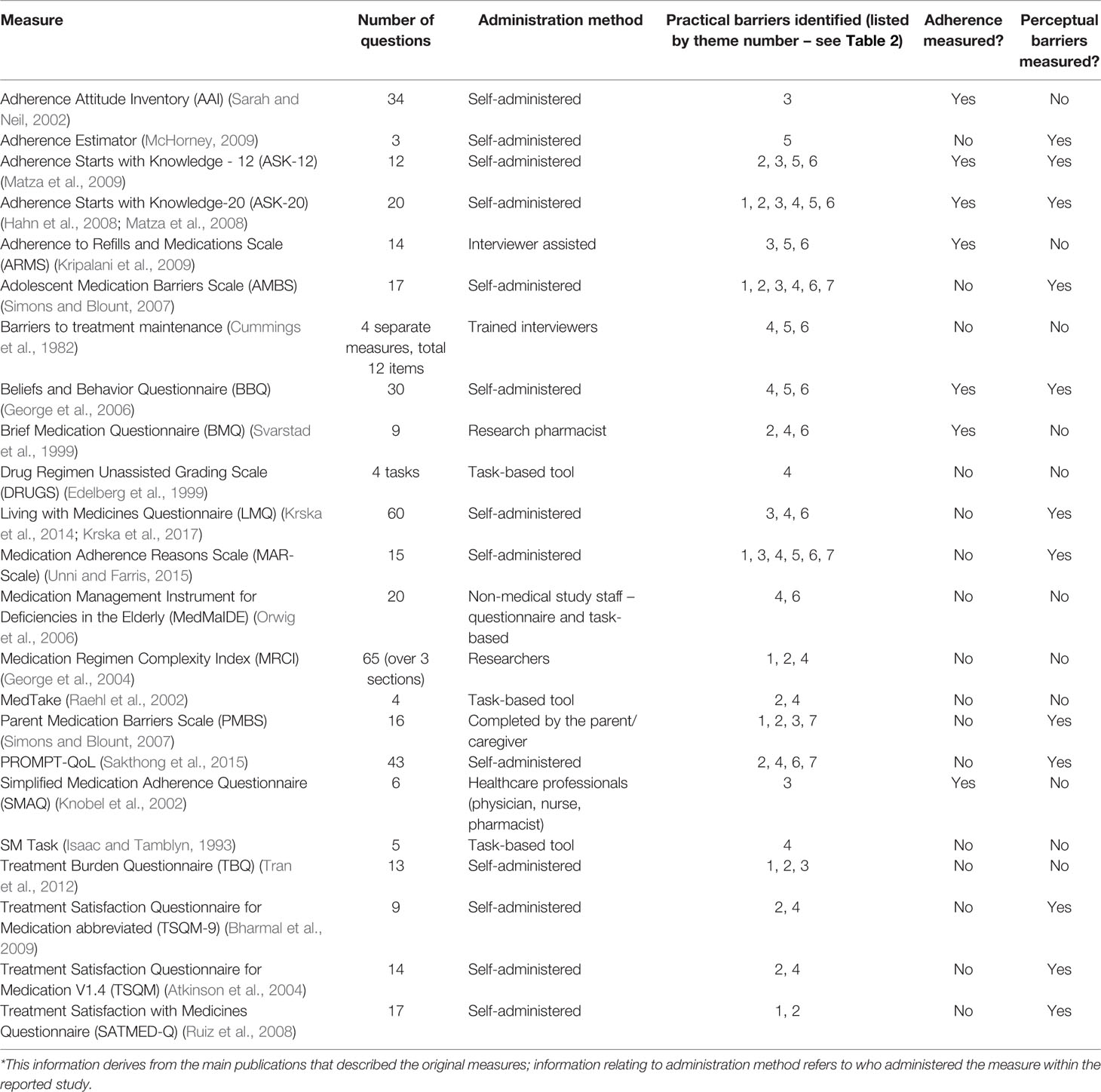
Table 1 summarizes the characteristics of the included measures. Of the 23 included measures, 3 assessed perceptual barriers and adherence behavior as as well as practical adherence barriers. Nine assessed both perceptual and practical adherence barriers (but not adherence), and 4 evaluated adherence behavior and practical barriers (but not perceptual factors). Perceptual barriers addressed included items relating to concern about long-term effects and side effects of the medicine, and beliefs about the importance or necessity of the medication. Although all the included measures included practical barriers to adherence, only 7 measures solely focused on this area without evaluating perceptual barriers or adherence behavior [TBQ (Tran et al., 2012), DRUGS (Edelberg et al., 1999), MedTAKE (Raehl et al., 2002), MedMaIDE (Orwig et al., 2006), SM task (Isaac and Tamblyn, 1993), MRCI (George et al., 2004), Barriers to treatment maintenance (Cummings et al., 1982)]; these were largely task-based tools which involved a second person observing the patient perform a task (e.g., open a container), or recall information (e.g., state dosing frequency). All measures, with the exception of two measures—the AMBS and PBMS (Simons and Blount, 2007)—were developed in adult populations.
The mean ± SD number of items included in the measure was 19 ± 17. Over half of the included measures were self-administered scales (n=13), the remaining 10 were a combination of task-based tools (n=3) and measures administered by study staff or interviewers (n=5), health professionals (n=1), or by the parent/caregiver (n=1). Almost none of the observer-rated scales (with the exception of the parental-completed measure)evaluated perceptual barriers.
Practical Factors—Themes
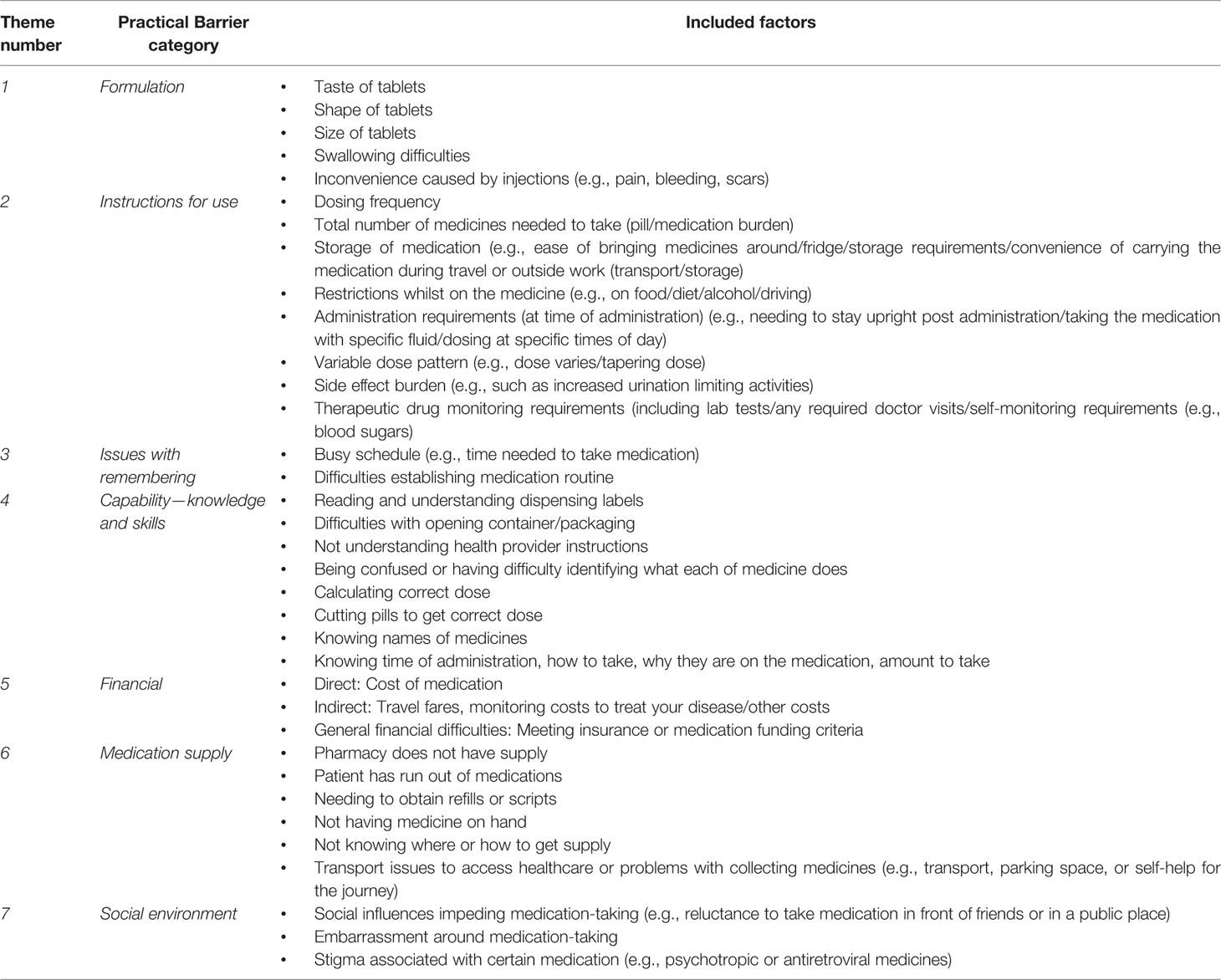
The practical barriers within the included adherence measures fell in seven key themes. These are described below, and listed in Table 2 with examples from each of the measures. These themes were generally spread evenly across all the measures; most measures included items relating to instructions for use (theme 2) or capability (theme 4) with social barriers (theme 7) being captured by only four measures. No measure captured all the practical barriers across the seven themes.
1. Formulation
The formulation theme related to factors around the specific formulation of the medication, such as the size of the oral dosage form (e.g., large tablets) or difficulties associated with injections, which were identified to influence adherence. Six of the included measures had items relating to formulation.
2. Instructions for Use
Many of the measures identified (n=11) included items relating to issues with taking the medication as prescribed, and the burden associated with the medication-taking. This included items ranging from dosing frequency, to medication storage requirements, to specific restrictions with medication administration such as the need to take with food or at certain times of the day. Any items which impacted on daily activities such as an individual’s social life, work, or holidays were included under this category, including indirect medication effects such as the impact of side effects or medication monitoring on daily activities.
3. Issues With Remembering
The third theme relates to issues with remembering or forgetting doses. Ten of the measures included items related to difficulties establishing a medication routine such as forgetting to take medication due to busy schedules or being away on holiday.
4. Capability—Knowledge and Skills
Most measures (n=14) had items which fell into this theme. This relates to an individual’s ability to understand the administration instructions, for example dispensing labels (i.e., relates to health literacy), and to follow the specified instructions (e.g., their ability to open containers, cut pills, or calculate the correct dose). It encompasses an individual’s knowledge and skills which enable them to take the medication as prescribed.
5. Financial
Issues relating to finance or cost were included in a separate theme relating to finance. These included items relating to the direct costs, such as the cost of the medication, or indirect costs relating to medication-taking, such as travelling expenses to obtain the medication supply. Some items related to general finance barriers which are specific to a health system, for example, obtaining health insurance or meeting funding criteria for a medication. Seven of the measures included items on this.
6. Medication Supply
This theme describes adherence barriers which relate to obtaining or accessing medication supplies. It includes issues around the availability of medication and ease of supply of the medication. Eleven of the measures included items in this theme, such as knowing where to obtain medication refills, and being able to get to a pharmacy to obtain ongoing supply.
7. Social Environment
The last theme relates to social influences around medication-taking. It broadly includes any barriers to adherence which arise due to social pressures, such as embarrassment around taking medication in front of other people, and stigma relating to use of medication. Only four of the measures included items relating to this theme, of which two of them related specifically to concerns in adolescents/children about other people noticing them take the medication.
Discussion
This review of reviews is the first to systematically identify and synthesise the types of practical adherence barriers that are currently captured by self- or observer-reported adherence measures. Recent papers examining factors relating to non-adherence identified practical barriers as key factors influencing adherence (Taylor et al., 2002; Weiser et al., 2003; Martin et al., 2005; Shubber et al., 2016; Jamison et al., 2017). Yet, much of the literature focuses either on the measurement of adherence itself, or on perceptual barriers, or both, with few focusing on the practical issues influencing adherence. Whilst it is important to target both perceptual and practical barriers (Linn et al., 2013), and there is often an overlap between these (Horne et al., 2019), practical barriers represent a good starting point for improving adherence. Practical barriers are generally more easily overcome by simple interventions (e.g., changes to the physical environment or medication regimen) (Horne et al., 2019) and may be useful as an initial adherence intervention. Identifying practical barriers may allow health professionals to make simple interventions to improve adherence first before trialling more resource-intensive interventions to shift perceptual barriers. Additionally, changes in practical barriers can lead to changes in perceptual barriers; patients may become more motivated to take their treatment if the medication regimen was made simpler and easier to take. Conversely, many of the practical barriers identified from our review can be overcome if patients are highly motivated—for example, patients are more likely to follow complicated regimens and defy practical barriers if they believe their treatment is necessary. However, shifting an individual’s beliefs about their treatment often requires a more intensive approach that can only be delivered by individuals who have received extra training, e.g., delivery of a health psychology-based intervention, compared to addressing practical barriers, which health professionals may find easier to deliver (Chapman et al., 2015).
This review identified 23 adherence measures which assessed practical barriers to adherence. There were few self- or observer-reported measures identified that addressed only practical barriers, with most measures consisting of only a few items addressing practical barriers. Where measures did focus primarily on practical factors, these were administered in the form of task-based tools, where an observer scored tasks performed by the individual, rather than self-report measures.
Our review builds on previous literature by undertaking a review of systematic reviews of self- and observer-reported adherence measures to ensure that all practical barriers previously identified in adherence measures were identified. It extends the work conducted by Nguyen et al., who conducted a review of primary literature around self-report adherence measures, but did not focus on practical adherence barriers (Nguyen et al., 2014). The findings of this review provide key information for intervention development and the design of future adherence measures, particularly ones that target practical barriers. Previous literature have highlighted the importance of tailoring adherence interventions, with a recent meta-analysis reporting “tailoring” as the most effective behavior change technique to consider when designing an adherence intervention (Kassavou and Sutton, 2017). Valid and reliable measures for tailoring interventions according to individual barriers exist (Horne et al., 1999; Linn et al., 2013), yet from our review, there was no single measure that specifically focused on practical factors nor captured all the barriers represented across the seven themes. From our review, separating perceptual and practical factors is important for intervention development, as perceptual and practical barriers require different types of intervention, with different resource intensity. The themes from this review provide a foundation for the development of a measure that can identify the practical adherence barriers faced by an individual. Used together with measures which evaluate perceptual barriers, such as the Beliefs about Medicines Questionnaire, such a measure would facilitate the development of a tailored adherence intervention that addresses both perceptual and practical barriers—an approach in-line with current guidance from NICE (Horne et al., 2005; Nunes et al., 2009).
The measures identified were broadly consistent with each other, with all statements relating to practical factors falling into one of the seven overarching themes that identified in this review. The themes that were identified aligned well with previous literature including the World Health Organisations’ five dimensions that affect adherence, which we used as a guiding framework for analysis (Sabaté, 2003). Most measures contained items that measured capability, such as opening medication bottles, and whether patients understood the proper administration of their medicines, with items assessing whether patients knew how and when to take the medication. Although self-report is a convenient method of collecting information, a key limitation is that an individual’s perceived ability may not reflect the actual ability of the individual to administer the medication; in this case observer-reported (e.g., task-based) measures would be more accurate. We found that in our current review, over half of the measures were self-reported. There is scope for the development of more observer-reported measures, or for measures to adopt a mix of self- and observer-report within the questionnaire, to increase the accuracy of assessment of practical barriers. Of note, most of these observer-reported measures also did not evaluate perceptual barriers, which makes sense as perceptual barriers are much more likely to be accurately identified by the individual themselves, whereas practical barriers may be more accurately identified by an observer. Observer-reported measures may thus play a more predominant role in identifying practical barriers; whilst self-reported measures may be more suited for perceptual barriers. Further research on observer-reported measures is warranted. Additionally, only two measures focused specifically on adherence barriers in children and adolescents. This represents a gap for further research in identify the barriers that face young people, and how these compare to adults and to the barriers that parents/caregivers perceive their children face. Furthermore, identification of barriers does not necessarily translate into actual adherence; identifying the specific barriers is only the first step towards improving adherence. It is also not known whether certain types of practical barriers (e.g., financial) may have a greater influence on adherence; future work to examine the relationships between the different types of practical barriers reported in our review and how these impact on adherence is needed.
The themes relating to the other items were generally spread evenly across the measures, however, social influences such as embarrassment were identified only in four measures; of these two were social influences considered in the context of child and adolescent adherence. However, as the papers that reported on the development of the adherence measures had little information on where the measure items were derived from, the frequency of occurrence of these items do not necessarily reflect the frequency with which these practical barriers are experienced by individuals. Furthermore, a possible reason for the lack of inclusion of social influences in adherence measures may reflect the difficulty associated with quantifying this construct, compared to more concrete task-based barriers such as cost or obtaining medication supplies.
Strengths and Limitations
We chose to use a review of systematic reviews approach to formulate an understanding of the existing literature and to explore the extent to which practical barriers have been included in current adherence measures. The approach proved valuable for identifying and synthesising the practical factors currently evaluated across multiple adherence measures. However, because the search only captured systematic reviews, any new measures developed which have not been yet been included in prior systematic reviews will not have been picked up in this review of reviews. Our review was also limited to only “generic” scales to maximize the generalizability of our findings across different conditions and disease states. However, with generalisability comes the loss of specificity, and practical barriers which are unique to a particular treatment or condition will not have been included. We also chose not to apply quality screening criteria for the systematic reviews, such as AMSTAR 2 (Shea et al., 2017), as the reviews in this case were used to identify measures used in the literature rather than synthesising outcomes or findings.
Conclusion
This review has identified and categorised practical factors that can lead to unintentional non-adherence. Many of the themes relating to practical adherence barriers reflected situations which are out of the patients’ direct control, yet could be easily addressed if identified. There is currently no self- or observer-reported measure that assesses these barriers independently of other factors, which could lead to many of these practical factors being overlooked. The findings from this review provide a useful foundation on which to develop a measure of practical barriers to adherence, and to inform intervention design.
Author Contributions
AC was involved in the study design and conception, study search, article screening, data extraction, data analysis and interpretation, and manuscript write-up. VC was involved in the study design, study search, article screening, data extraction, data analysis and interpretation, and manuscript write-up. HL was involved in the study search, article screening, data extraction, data analysis and interpretation, and manuscript write-up. RH was involved in the study design, data interpretation, and manuscript write-up and review.
Funding
The authors declare that this study received funding support from Spoonful of Sugar Ltd., a UCL-Business spin-out company. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of Interest
AC reports consultancy fees from Janssen-Cilag, and Spoonful of Sugar Ltd, and speaker fees from Novartis, outside the submitted work. RH reports fees from Medical Innovation Academic Consortium (CASMI), AbbVie, Amgen, Biogen, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Pfizer, Roche, Shire Pharmaceuticals, MSD, Astellas, AstraZeneca, DRSU, Novartis, Universit€atsklinikum Hamburg-Eppendorf, and Teva Pharmaceuticals, and is the Founder and Director of Spoonful of Sugar Ltd., outside the submitted work. VC and HL are employees of Spoonful of Sugar Ltd.
References
Akeroyd, J. M., Chan, W. J., Kamal, A. K., Palaniappan, L., Virani, S. S. (2015). Adherence to cardiovascular medications in the South Asian population: a systematic review of current evidence and future directions. World J. Cardiol. 7 (12), 938. doi: 10.4330/wjc.v7.i12.938
AlGhurair, S. A., Hughes, C. A., Simpson, S. H., Guirguis, L. M. (2012). A systematic review of patient self-reported barriers of adherence to antihypertensive medications using the World Health Organization Multidimensional Adherence Model. J. Clin. Hypertension 14 (12), 877–886. doi: 10.1111/j.1751-7176.2012.00699.x
Atkinson, M. J., Sinha, A., Hass, S. L., Colman, S. S., Kumar, R. N., Brod, M., et al. (2004). Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual. Life Outcomes 2, 12. doi: 10.1186/1477-7525-2-12
Bharmal, M., Payne, K., Atkinson, M. J., Desrosiers, M. P., Morisky, D. E., Gemmen, E. (2009). Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual. Life Outcomes 7, 36. doi: 10.1186/1477-7525-7-36
Braun, V., Clarke, V. (2006). Using thematic analysis in psychology. Qual. Res. Psychol. 3, 77–101. doi: 10.1191/1478088706qp063oa
Briesacher, B. A., Gurwitz, J. H., Soumerai, S. B. (2007). Patients at-risk for cost-related medication nonadherence: a review of the literature. J. Gen. Intern Med. 22 (6), 864–871. doi: 10.1007/s11606-007-0180-x
Chapman, S. C. E., Barnes, N., Barnes, M., Wilkinson, A., Hartley, J., Piddock, C., et al. (2015). Changing adherence-related beliefs about ICS maintenance treatment for asthma: feasibility study of an intervention delivered by asthma nurse specialists. BMJ Open 5 (6), e007354. doi: 10.1136/bmjopen-2014-007354
Clifford, S., Barber, N., Horne, R. (2008). Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. J. Psychosomatic Res. 64 (1), 41–46. doi: 10.1016/j.jpsychores.2007.05.004
Cummings, K. M., Kirscht, J. P., Binder, L. R., Godley, A. J. (1982). Determinants of drug treatment maintenance among hypertensive persons in inner city Detroit. Public Health Rep. (Washington DC: 1974) 97 (2), 99–106.
Edelberg, H. K., Shallenberger, E., Wei, J. Y. (1999). Medication management capacity in highly functioning community-living older adults: detection of early deficits. J. Am. Geriatr. Soc. 47 (5), 592–596. doi: 10.1111/j.1532-5415.1999.tb02574.x
Elliott, R. A., Marriott, J. L. (2009). Standardised assessment of patients’ capacity to manage medications: a systematic review of published instruments. BMC Geriatr. 9 (1), 27. doi: 10.1186/1471-2318-9-27
Forbes, C. A., Deshpande, S., Sorio-Vilela, F., Kutikova, L., Duffy, S., Gouni-Berthold, I., et al. (2018). A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr. Med. Res. Opin. 34 (9), 1613–1625. doi: 10.1080/03007995.2018.1477747
Garfield, S., Clifford, S., Eliasson, L., Barber, N., Willson, A. (2011). Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med. Res. Methodol. 11, 149. doi: 10.1186/1471-2288-11-149
George, J., Phun, Y. T., Bailey, M. J., Kong, D. C., Stewart, K. (2004). Development and validation of the medication regimen complexity index. Ann. Parmacother. 38 (9), 1369–1376. doi: 10.1345/aph.1D479
George, J., Mackinnon, A., Kong, D. C., Stewart, K. (2006). Development and validation of the Beliefs and Behaviour Questionnaire (BBQ). Patient Educ. Couns 64 (1-3), 50–60. doi: 10.1016/j.pec.2005.11.010
Hahn, S. R., Park, J., Skinner, E. P., Yu-Isenberg, K. S., Weaver, M. B., Crawford, B., et al. (2008). Development of the ASK-20 adherence barrier survey. Curr. Med. Res. Opin. 24 (7), 2127–2138. doi: 10.1185/03007990802174769
Holmes, E. A., Hughes, D. A., Morrison, V. L. (2014). Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value Health 17 (8), 863–876. doi: 10.1016/j.jval.2014.08.2671
Horne, R., Weinman, J., Hankins, M. (1999). The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health 14 (1), 1–24. doi: 10.1080/08870449908407311
Horne, R., Weinman, J., Barber, N., Elliott, R., Morgan, M., Cribb, A. (2005). Concordance, adherence and compliance in medicine taking: Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO) (London: NCCSDO).
Horne, R., Chapman, S., Parham, R., Freemantle, N., Forbes, A., Cooper, V. (2013). Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PloS One 8 (12), e80633. doi: 10.1371/journal.pone.0080633
Horne, R., Cooper, V., Wileman, V., Chan, A. (2019). Supporting adherence to medicines for long-term conditions: a Perceptions and Practicalities Approach based on an extended Common Sense Model. Eur. Psychol. 24 (1), 82–96. doi: 10.1027/1016-9040/a000353
Huang, W.-C., Chen, C.-Y., Lin, S.-J., Chang, C.-S. (2016). Medication adherence to oral anticancer drugs: systematic review. Expert Rev. Anticancer Ther. 16 (4), 423–432. doi: 10.1586/14737140.2016.1159515
Isaac, L. M., Tamblyn, R. M. (1993). Compliance and cognitive function: a methodological approach to measuring unintentional errors in medication compliance in the elderly. McGill-Calgary Drug Res. Team Gerontol. 33 (6), 772–781. doi: 10.1093/geront/33.6.772
Jamison, J., Sutton, S., Mant, J., Simoni, A. D. (2017). Barriers and facilitators to adherence to secondary stroke prevention medications after stroke: analysis of survivors and caregivers views from an online stroke forum. BMJ Open 7 (7), e016814. doi: 10.1136/bmjopen-2017-016814
Kardas, P., Lewek, P., Matyjaszczyk, M. (2013). Determinants of patient adherence: a review of systematic reviews. Front. Pharmacol. 4 (91). doi: 10.3389/fphar.2013.00091
Kassavou, A., Sutton, S. (2017). Automated telecommunication interventions to promote adherence to cardio-metabolic medications: meta-analysis of effectiveness and meta-regression of behaviour change techniques. Health Psychol. Rev., 12 (1) 25–42.
Katusiime, B., Corlett, S., Reeve, J., Krska, J. (2016). Measuring medicine-related experiences from the patient perspective: a systematic review. Patient Related Outcome Measures 7, 157. doi: 10.2147/PROM.S102198
Knobel, H., Alonso, J., Casado, J. L., Collazos, J., Gonzalez, J., Ruiz, I., et al. (2002). Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS (London England) 16 (4), 605–613. doi: 10.1097/00002030-200203080-00012
Kripalani, S., Risser, J., Gatti, M. E., Jacobson, T. A. (2009). Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 12 (1), 118–123. doi: 10.1111/j.1524-4733.2008.00400.x
Krska, J., Morecroft, C. W., Rowe, P. H., Poole, H. (2014). Measuring the impact of long-term medicines use from the patient perspective. Int. J. Clin. Pharm. 36 (4), 675–678. doi: 10.1007/s11096-014-9970-5
Krska, J., Katusiime, B., Corlett, S. A. (2017). Validation of an instrument to measure patients’ experiences of medicine use: the Living with Medicines Questionnaire. Patient Prefer. Adherence 11, 671–679. doi: 10.2147/PPA.S126647
Linn, A. J., van Weert, J. C., Smit, E. G., Perry, K., van Dijk, L. (2013). 1+1=3? The systematic development of a theoretical and evidence-based tailored multimedia intervention to improve medication adherence. Patient Educ. Couns. 93 (3), 381–388. doi: 10.1016/j.pec.2013.03.009
Martin, L. R., Williams, S. L., Haskard, K. B., DiMatteo, M. R. (2005). The challenge of patient adherence. Ther. Clin. Risk Manage. 1 (3), 189–199.
Matza, L. S., Yu-Isenberg, K. S., Coyne, K. S., Park, J., Wakefield, J., Skinner, E. P., et al. (2008). Further testing of the reliability and validity of the ASK-20 adherence barrier questionnaire in a medical center outpatient population. Curr. Med. Res. Opin. 24 (11), 3197–3206. doi: 10.1185/03007990802463642
Matza, L. S., Park, J., Coyne, K. S., Skinner, E. P., Malley, K. G., Wolever, R. Q. (2009). Derivation and validation of the ASK-12 adherence barrier survey. Ann. Parmacother. 43 (10), 1621–1630. doi: 10.1345/aph.1M174
McHorney, C. A. (2009). The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr. Med. Res. Opin. 25 (1), 215–238. doi: 10.1185/03007990802619425
Nguyen, T-M-U, Caze, A. L., Cottrell, N. (2014). What are validated self-report adherence scales really measuring?: a systematic review. Br. J. Clin. Pharmacol. 77 (3), 427–445. doi: 10.1111/bcp.12194
Nieuwlaat, R., Wilczynski, N., Navarro, T., Hobson, N., Jeffery, R., Keepanasseril, A., et al. (2014). Interventions for enhancing medication adherence. Cochrane Library. doi: 10.1002/14651858.CD000011.pub4
Nunes, V., Neilson, J., O’Flynn, N., Calvert, N., Kuntze, S., Smithson, H., et al. (2009). Medicines Adherence: involving patients in decisions about prescribed medicines and supporting adherence (London: National Institute for Health and Clinical Excellence).
Orwig, D., Brandt, N., Gruber-Baldini, A. L. (2006). Medication Management Assessment for Older Adults in the Community. Gerontol. 46 (5), 661–668. doi: 10.1093/geront/46.5.661
Ouzzani, M., Hammady, H., Fedorowicz, Z., Elmagarmid, A. (2016). Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5 (1), 210. doi: 10.1186/s13643-016-0384-4
Pérez-Escamilla, B., Franco-Trigo, L., Moullin, J. C., Martínez-Martínez, F., García-Corpas, J. P. (2015). Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer. Adherence 9, 569. doi: 10.2147/PPA.S76139
Paquin, A. M., Zimmerman, K. M., Kostas, T. R., Pelletier, L., Hwang, A., Simone, M., et al. (2013). Complexity perplexity: a systematic review to describe the measurement of medication regimen complexity. Expert Opin. Drug Saf. 12 (6), 829–840. doi: 10.1517/14740338.2013.823944
Pareja-Martinez, E., Esquivel-Prados, E., Martinez-Martinez, F., Garcia-Corpas, J. P. (2020). Questionnaires on adherence to antihypertensive treatment: a systematic review of published questionnaires and their psychometric properties. Int. J. Clin. Pharm. 5, 1–11. doi: 10.1007/s11096-020-00981-x
Pednekar, P. P., Agh, T., Malmenas, M., Raval, A. D., Bennett, B. M., Borah, B. J., et al. (2019). Methods for Measuring Multiple Medication Adherence: A Systematic Review-Report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health 22 (2), 139–156. doi: 10.1016/j.jval.2018.08.006
Pharmaceutical Group of the European Union (2008). Targeting adherence - Improving patient outcomes in Europe through community pharmacists’ intervention (Brussels: Pharmaceutical Group of the European Union).
Plevinsky, J. M., Gutierrez-Colina, A. M., Carmody, J. K., Hommel, K. A., Crosby, L. E., McGrady, M. E., et al. (2020). Patient-Reported Outcomes for Pediatric Adherence and Self-Management: A Systematic Review. J. Pediatr. Psychol. 45 (3), 340–357. doi: 10.1093/jpepsy/jsz096
Raehl, C. L., Bond, C. A., Woods, T., Patry, R. A., Sleeper, R. B. (2002). Individualized drug use assessment in the elderly. Pharmacotherapy 22 (10), 1239–1248. doi: 10.1592/phco.22.15.1239.33473
Remor, E. (2013). Systematic review of the psychometric properties of the questionnaire to evaluate the adherence to HIV therapy (CEAT-VIH). Patient-Patient-Centered Outcomes Res. 6 (2), 61–73. doi: 10.1007/s40271-013-0009-0
Roebuck, M. C., Liberman, J. N., Gemmill-Toyama, M., Brennan, T. A. (2011). Medication Adherence Leads To Lower Health Care Use And Costs Despite Increased Drug Spending. Health Affairs 30 (1), 91–99. doi: 10.1377/hlthaff.2009.1087
Ruiz, M. A., Pardo, A., Rejas, J., Soto, J., Villasante, F., Aranguren, J. L. (2008). Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q). Value Health 11 (5), 913–926. doi: 10.1111/j.1524-4733.2008.00323.x
Sabaté, E. (2003). Adherence to long-term therapies: evidence for action: World Health Organization.
Sakthong, P., Suksanga, P., Sakulbumrungsil, R., Winit-Watjana, W. (2015). Development of Patient-reported Outcomes Measure of Pharmaceutical Therapy for Quality of Life (PROMPT-QoL): A novel instrument for medication management. Res. Soc. Administrative Pharm. : RSAP 11 (3), 315–338. doi: 10.1016/j.sapharm.2014.10.002
Sarah, J. L., Neil, A. (2002). Development and Evaluation of the Adherence Attitude Inventory. Res. Soc. Work Pract. 12 (1), 107–123. doi: 10.1177/104973150201200108
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clin. Res. ed), j4008. doi: 10.1136/bmj.j4008
Shubber, Z., Mills, E. J., Nachega, J. B., Vreeman, R., Freitas, M., Bock, P., et al. (2016). Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. PloS Med. 13 (11), e1002183. doi: 10.1371/journal.pmed.1002183
Simons, L. E., Blount, R. L. (2007). Identifying barriers to medication adherence in adolescent transplant recipients. J. Pediatr. Psychol. 32 (7), 831–844. doi: 10.1093/jpepsy/jsm030
Smith, V., Devane, D., Begley, C. M., Clarke, M. (2011). Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 11 (1), 15. doi: 10.1186/1471-2288-11-15
Snyder, S., Crandell, I., Davis, S. A., Feldman, S. R. (2014). Medical adherence to acne therapy: a systematic review. Am. J. Clin. Dermatol. 15 (2), 87–94. doi: 10.1007/s40257-014-0063-y
Svarstad, B. L., Chewning, B. A., Sleath, B. L., Claesson, C. (1999). The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ. Couns. 37 (2), 113–124. doi: 10.1016/S0738-3991(98)00107-4
Taylor, S. A., Galbraith, S. M., Mills, R. P. (2002). Causes Of Non-Compliance With Drug Regimens In Glaucoma Patients: A Qualitative Study. J. Ocular Pharmacol. Ther. 18 (5), 401–409. doi: 10.1089/10807680260362687
Tran, V. T., Montori, V. M., Eton, D. T., Baruch, D., Falissard, B., Ravaud, P. (2012). Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med. 10, 68. doi: 10.1186/1741-7015-10-68
Unni, E. J., Farris, K. B. (2015). Development of a new scale to measure self-reported medication nonadherence. Res. Soc. Administrative Pharm. : RSAP 11 (3), e133–e143. doi: 10.1016/j.sapharm.2009.06.005
Vandenbroeck, S., De Geest, S., Zeyen, T., Stalmans, I., Dobbels, F. (2011). Patient-reported outcomes (PRO’s) in glaucoma: a systematic review. Eye 25 (5), 555–577. doi: 10.1038/eye.2011.45
Vreeman, R. C., Wiehe, S. E., Pearce, E. C., Nyandiko, W. M. (2008). A systematic review of pediatric adherence to antiretroviral therapy in low-and middle-income countries. Pediatr. Infect. Dis. J. 27 (8), 686–691. doi: 10.1097/INF.0b013e31816dd325
Weiser, S., Wolfe, W., Bangsberg, D., Thior, I., Gilbert, P., Makhema, J., et al. (2003). Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. JAIDS-HAGERSTOWN MD 34 (3), 281–288. doi: 10.1097/00126334-200311010-00004
Appendix 1
Search terms:
1. Measure*.mp. or Patient Reported Outcome Measures/
2. Inventor*.mp.
3. Scale*.mp. or Visual Analog Scale/
4. “Surveys and Questionnaires”/or Questionnaire*.mp.
5. Self Report/
6. instrument.mp. or Psychometrics/
7. 1 or 2 or 3 or 4 or 5 or 6
8. Adherence*.mp. or Medication Adherence/
9. Patient Compliance/or Compliance*.mp.
10. 8 or 9
11. barrier*.mp.
12. factor*.mp.
13. Health Knowledge, Attitudes, Practice/
14. belief*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
15. Percep*.mp.
16. practical*.mp.
17. forget*.mp.
18. enable*.mp.
19. facilitat*.mp.
20. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21. valid*.mp. or Psychometrics/
22. 7 and 10 and 20 and 21
23. (Measure* or Patient Reported Outcome Measures or Inventor* or (Scale* or Visual Analog Scale) or (“Surveys and Questionnaires” or Questionnaire*) or Self Report or (instrument or Psychometrics)).ti. or (Measure* or Patient Reported Outcome Measures or Inventor* or (Scale* or Visual Analog Scale) or (“Surveys and Questionnaires” or Questionnaire*) or Self Report or (instrument or Psychometrics)).ab.
24. (Adherence* or Medication Adherence or (Patient Compliance or Compliance*)).ti. or (Adherence* or Medication Adherence or (Patient Compliance or Compliance*)).ab.
25. (valid* or Psychometrics).ti. or (valid* or Psychometrics).ab.
26. 20 and 23 and 24 and 25
27. systematic review.mp.
28. 26 and 27
Keywords: adherence–compliance–persistence, medication, measurement, self-report measures, practical factors, review (article), patient report, PRO (patient reported outcomes)
Citation: Chan AHY, Cooper V, Lycett H and Horne R (2020) Practical Barriers to Medication Adherence: What Do Current Self- or Observer-Reported Instruments Assess? Front. Pharmacol. 11:572. doi: 10.3389/fphar.2020.00572
Received: 03 February 2020; Accepted: 15 April 2020;
Published: 13 May 2020.
Edited by:
Maria Dimitrova, Medical University-Sofia, BulgariaReviewed by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaNatasa Duborija-Kovacevic, University of Montenegro, Montenegro
Copyright © 2020 Chan, Cooper, Lycett and Horne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rob Horne, ci5ob3JuZUB1Y2wuYWMudWs=
 Amy Hai Yan Chan
Amy Hai Yan Chan Vanessa Cooper1
Vanessa Cooper1