- 1Department of Basic Science, Biomedical Science Research Lab, Faculty of Medicine, Universidad Católica de la Santísima Concepción, Concepción, Chile
- 2Laboratorio de Neuroinmunología, Fundación Ciencia & Vida, Santiago, Chile
- 3Universidad San Sebastián, Santiago, Chile
Dopamine is one of the neurotransmitters whose transmission is altered in a number of neural pathways in the brain of schizophrenic patients. Current evidence indicates that these alterations involve hyperactive dopaminergic transmission in mesolimbic areas, striatum, and hippocampus, whereas hypoactive dopaminergic transmission has been reported in the prefrontal cortex of schizophrenic patients. Consequently, schizophrenia is associated with several cognitive and behavioral alterations. Of note, the immune system has been found to collaborate with the central nervous system in a number of cognitive and behavioral functions, which are dysregulated in schizophrenia. Moreover, emerging evidence has associated schizophrenia and inflammation. Importantly, different lines of evidence have shown dopamine as a major regulator of inflammation. In this regard, dopamine might exert strong regulation in the activity, migration, differentiation, and proliferation of immune cells that have been shown to contribute to cognitive functions, including T-cells, microglial cells, and peripheral monocytes. Thereby, alterations in dopamine levels associated to schizophrenia might affect inflammatory response of immune cells and consequently some behavioral functions, including reference memory, learning, social behavior, and stress resilience. Altogether these findings support the involvement of an active cross-talk between the dopaminergic and immune systems in the physiopathology of schizophrenia. In this review we summarize, integrate, and discuss the current evidence indicating the involvement of an altered dopaminergic regulation of immunity in schizophrenia.
Dysregulation of the Dopaminergic Neural Pathways in the Schizophrenia
Schizophrenia is a mental illness that often appears during late adolescence or early adulthood. It is characterized by thought disorders, perception, cognition and volition. The prevalence of this disorder reaches almost 1% of the world population, with an annual incidence ranging between 3.89 and 4.03 per 1,000 subjects (Moreno-Kustner et al., 2018). Its etiology is still unclear, and includes genetic and environmental components promoting alterations of dopaminergic signaling. The initial dopamine hypothesis stated that hyperactive dopaminergic transmission leads to development of schizophrenia symptoms (hallucinations, delusions, thought disorder, among others). However, several lines of evidence have shown that hypoactivity of frontal dopaminergic neurons in rodents (Pycock et al., 1980), non-human primates (Roberts et al., 1994), and humans (Ragland et al., 2007; Simpson et al., 2010) are also associated with schizophrenia. For instance, a pharmacological lesion of subcortical dopaminergic pathways in rats suggested a correlation between hyperactivation of subcortical dopaminergic neurons with hypoactivity of frontal dopaminergic neurons (Pycock et al., 1980). In addition, evidence obtained from humans has suggested that the polymorphism in the gene encoding catechol-O-methyltransferase, an enzyme involved in the degradation of dopamine, is associated with hypoactivity of prefrontal dopaminergic neurons in schizophrenia (Slifstein et al., 2008). Moreover, patients with frontal lobe damage as well as schizophrenia patients display similar alterations in the executive function (Ragland et al., 2007). Therefore, the current dopaminergic hypothesis involves hyperactive dopaminergic transmission in mesolimbic areas, striatum and hippocampus (Lodge and Grace, 2007; Patel et al., 2010; Weinstein et al., 2017), as well as hypoactive dopaminergic transmission in the prefrontal cortex of schizophrenic patients (Da Silva Alves et al., 2008). In addition, glutamatergic hypofunction has been suggested as one of the mechanisms involved in this dopaminergic dysfunction in schizophrenia (Swerdlow et al., 2009). In this regard, it has been hypothesized that DRD2-antagonism might prevent DRD1-mediated potentiation of N-Methyl-d-aspartate (NMDA) responses in the prefrontal cortex (Paz et al., 2008). Another line of evidence points to the changes in subcortical dopaminergic activity as one of the responsible circuits promoting alterations in glutamatergic neurotransmission in the substantia nigra (Mueller et al., 2004).
This, imbalance in the dopaminergic signaling has been differentially associated with the development of positive (presence of undesired cognitive/emotional functions, such as hallucinations, delusions, thought disorders, trouble concentrating, movement disorders) and negative (deficiency of desired cognitive/emotional effects, such as flattened affect, lack of pleasure, trouble with speech, apathy, concentration problems, and lack of motivation) symptoms. Positive symptoms have been related with stimulation of D2-like receptors, including DRD2, DRD3, and DRD4) (Li et al., 2016). Both primate and rodent brains express a higher density of D1-like (including DRD1 and DRD5) than D2-like receptors in healthy conditions (Weinstein et al., 2017). Meta-analysis of studies using positron emission tomography (PET) and single photon emission computed tomography (SPECT) have shown that presynaptic dopamine release is decreased in most brain regions of schizophrenic patients (Slifstein et al., 2015), except in the striatum, where the synthesis and the levels of dopamine released are increased (Mccutcheon et al., 2018; Avram et al., 2019). Furthermore, PET studies have demonstrated that prefrontal DRD1 expression is decreased in patients with schizophrenia (Kosaka et al., 2010), which has been associated with working memory deficits in the prefrontal cortex (Takahashi et al., 2008). In contrast, DRD1 expression is increased in the temporal and parietal cortex of schizophrenic patients, which might be associated with auditory hallucinations (Domyo et al., 2001). It has been described a moderate increase (10–20%) in the expression of DRD2 and DRD3 in the striatum of a subgroup of schizophrenic patients (Kestler et al., 2001). Moreover, DRD3 expression has been found to be enhanced in the basal ganglia, ventral forebrain (Gurevich et al., 1997), and blood lymphocytes of schizophrenic patients (Ilani et al., 2001). On the other hand, it has been shown that in comparison to healthy subjects, dopamine occupies a higher proportion of striatal D2-like receptors (Kegeles et al., 2010), and a bigger fraction of the dopamine transporters (DAT) in sensorimotor striatum (Weinstein et al., 2017) in schizophrenia.
Interestingly, it has been described a sub-regional heterogeneity in the dopaminergic dysregulation within the striatum. The greatest alterations in dopaminergic transmission have been observed in the associative striatum region. These alterations have been negatively correlated with verbal fluency performance in schizophrenic patients (Howes et al., 2009b). Since this brain region regulates information flow to and from the prefrontal cortex, the authors have suggested a potential link between striatal dopaminergic dysfunction and prefrontal alterations in schizophrenic patients (Howes et al., 2009b). A recent study showed that impaired connectivity between the cortico-striato-thalamo-cortical circuits is associated with cognitive difficulties in schizophrenic patients, including deficits in attention, memory, and executive function (Avram et al., 2018). Moreover, reduced striatal dopamine synthesis correlates with cognitive difficulties in patients during remission of positive symptoms, without an association with negative symptoms (Avram et al., 2019). Of note, the cohort of patients was taking antipsychotic drugs that did not seem to have a short-term clear effect on the results of the study (Avram et al., 2019). In addition, studies performed in schizophrenic patients have analyzed the expression levels of tyrosine hydroxylase (TH), the enzyme that catalyzes the first (and limiting) step in the biosynthesis of dopamine, and have found heterogeneous results, supporting either dopaminergic hyperactivity or hypoactivity (Akil et al., 2000; Mueller et al., 2004). One study has reported regional and laminar specific decrease of TH-immunoreactive axons in the entorhinal cortex of schizophrenic patients (Akil et al., 2000), whereas another study has shown increased TH mRNA levels in the dopaminergic neurons of the substantia nigra pars compacta of schizophrenic patients (Mueller et al., 2004).
Thus, current evidence indicates the involvement of complex alterations in the activity of neural dopaminergic pathways in the brain of schizophrenic patients, which are not completely consolidated. Therefore, further research is still needed to better understand the alterations of dopaminergic circuitry associated to the pathophysiological scenario of schizophrenia.
Targeting the Dopaminergic System in Schizophrenia
The World Health Organization estimates that costs of schizophrenia in Western countries represent 1.6–2.6% of total health care budget, whereas in the US more than $60 billion USD per year are spent in this disorder (Howes et al., 2009a; Chong et al., 2016). The primary targets of many antipsychotic drugs for schizophrenia are striatal DRD2 and DRD3 (Howes et al., 2009a). However, the antagonism of these receptors is not always specific, and current drugs also act over other neurotransmitter receptors in the brain, including receptors for serotonin, histamine, norepinephrine, gamma-aminobutyric acid (GABA), and acetylcholine (Li et al., 2016). A DRD2 occupancy between 50% to 65%, is required in order to achieve clinical response to antipsychotic drugs and to minimize development of side-effects (e.g. extrapyramidal motor side effects) (Kapur et al., 2000). Targeting DRD2 using the antagonists chlorpromazine and haloperidol has been shown to effectively reduce positive symptoms, but ineffective at attenuating negative symptoms, cognitive deficits, and development of extrapyramidal motor side effects (Li et al., 2016). Of note, antipsychotic drugs might also increase the density of D2-like receptors in the striatum (Simpson et al., 2010). Antagonism of serotonin receptor 5-HT2A in combination with DRD2-antagonism (e.g. clozapine and risperidone) have been shown to be more effective attenuating positive and negative symptoms, nevertheless, promoting the development of extrapyramidal motor side effects (Kinon and Lieberman, 1996) and others, such as gain of body weight, increase incidence of diabetes, loss of bladder control, and blurred vision (Snyder et al., 2015).
Moreover, patients who do not respond well to antipsychotic treatment have relatively normal levels of striatal dopamine compared with patients whose symptoms respond to antipsychotics (Demjaha et al., 2012). Treatment with the dopaminergic agonist apomorphine, ameliorates cognitive deficits and improves dopaminergic neurotransmission, which has been associated to the enhancement of prefrontal activity (Dolan et al., 1995), exaggerated stimulation of dopaminergic release, and potentially promoting more occupancy of D2-like receptors by dopamine in schizophrenic patients (Laruelle et al., 1996). On the other hand, the treatment with dopamine receptor blockers is more effective at ameliorating symptoms such as hallucinations or delusions. New antipsychotic drugs antagonizing preferentially DRD3 over DRD2 have shown cognitive performance improvement (Nakajima et al., 2013; Wang et al., 2017). However, further research is needed in this regard to fully understand how dopaminergic transmission, triggered through the stimulation of every single dopamine receptor subtypes, regulates the spectrum of positive, negative, and cognitive symptoms involved in schizophrenia. Of note, schizophrenia is a heterogeneous disorder and no single brain region or neurotransmitter is likely to explain all symptoms observed in all schizophrenic patients (Mccutcheon et al., 2019). Therefore, new drugs aiming to target beyond the dopaminergic system or involving modulation of multiple targets are more likely to effectively tackle positive and negative symptoms of schizophrenia. An example is the newer antipsychotic drug ITI-007, which is able to interact with the serotoninergic, dopaminergic, and glutamatergic pathways (Snyder et al., 2015). This drug has shown promising results either in safety and improving negative symptoms in a phase II randomized double-blind multicenter clinical trial (Lieberman et al., 2016).
The interaction between the dopaminergic and the immune system should also be considered for the development of new therapeutic targets in schizophrenia. In this regard, it is important to consider the role of tetrahydrobiopterin (BH4), which is an essential enzyme cofactor required for the production of tyrosine and dopamine (Felger et al., 2012). Some cytokines involved in inflammation might regulate the expression of GTP-cyclohydrolase I (GCH-1), the enzyme necessary for BH4 synthesis, thus increasing or decreasing dopamine biosynthesis rate. Nevertheless, inflammation may also increase reactive oxygen species and inducible nitric oxide synthase (NOS) activity, which lead to decreased BH4 availability and thereby reducing dopamine synthesis. For instance, the administration of Interferon alpha (IFN-α) in rats has been shown to promote a significant decrease in the levels of dopamine and BH4 in the amygdala and raphe area, an effect that was abolished upon administration of a NOS inhibitor (Kitagami et al., 2003). Similarly, IFN-α, Interleukin-6 (IL-6), and cardiotrophin-1, have also been shown to reduce the levels of BH4 in sympathetic neurons (Li et al., 2003). Conversely, IL-1β, IFN-γ, and TNF-α have been shown to increase BH4 synthesis, by inducing the expression and activity of GCH-1 in endothelial cells (Shi et al., 2004).
In addition to the effect in the biosynthesis of dopamine, some cytokines have shown to regulate dopamine storage in dopaminergic cells. In this regard, the pro-inflammatory cytokines IL-1β and TNF-α have been shown to decrease the expression of the vesicular monoamine transporter 2 (VMAT2), which is responsible for transporting cytosolic dopamine into secretory vesicles, and thereby limiting the availability of presynaptic dopamine. Conversely, TGF-α increases VMAT2 expression, favoring the storage of presynaptic dopamine (Kazumori et al., 2004). Taken together these results indicate that inflammatory cytokines exert a complex regulation in the levels of dopamine available in dopaminergic cells by modifying the biosynthesis rate and the storage of this neurotransmitter.
Adding another level of complexity in the interaction between the dopaminergic and immune system, some studies have shown that some drugs targeting dopaminergic system might regulate inflammation. According to the critical role of dopamine in the regulation of sepsis (Torres-Rosas et al., 2014), it has recently been shown that the antipsychotic drug trifluoperazine (TFP), which suppress dopamine secretion, exerted a strong regulation of pro-inflammatory cytokines and increased the survival rate in animal models of sepsis (Park et al., 2019). Another example illustrating the role of antipsychotic drugs is the study of the effect of paliperidone in neuroinflammation. The authors show that the pre-treatment of rats with paliperidone inhibited the stimulation of toll-like receptor 4 (TLR4) in a model of neuroinflammation induced by stress (Macdowell et al., 2014). In the same direction, another study has shown that haloperidol attenuates the activation of NF-κB, and consequently abrogated the production of pro-inflammatory cytokines in macrophages in response to lipopolysaccharide (LPS) (Yamamoto et al., 2016). Thus, these findings together illustrate how some antipsychotic drugs used for the treatment of schizophrenia might also induce anti-inflammatory effects by targeting the dopaminergic system in immune cells.
Involvement of the Immune System in Cognitive Functions
Cognitive deficits in schizophrenia affect language, working and episodic memory, processing speed, stress resilience, social behavior, attention inhibition, and sensory processing (Kennedy and Adolphs, 2012; Brisch et al., 2014). Proper function of our cognitive and social abilities has partially been associated with the interaction between the central nervous system (CNS) and the immune system. The crosstalk between CNS and the peripheral cells is mediated by the glymphatic and meningeal lymphatic systems (Pape et al., 2019). In addition, the communication through the blood-brain barrier (BBB) and the endocrine system might also contribute to immunological alterations, affecting cognitive function. Here we analyze how some of these cognitive functions need the participation of the immune system.
Memory and Learning
Immune cell infiltration has been considered a pathological hallmark of many CNS conditions. However, cells from either the innate and adaptive immune systems might exert beneficial effects in the CNS, as long as their recruitment and activation are well controlled. Cells from the immune system have been shown to play a role in spatial memory, learning, and neurogenesis (Kipnis et al., 2004b; Ziv et al., 2006).
The hippocampus is partially responsible for spatial learning/memory. Neurogenesis occurring in the hippocampal dentate gyrus has been shown to be dependent on the infiltration of mature CD4+ T-cells in the meninges (Ziv et al., 2006; Wolf et al., 2009), on the activation, phagocytic activity, and recruitment of microglia (Ziv et al., 2006), and on the increased production of brain-derived neurotrophic factor (BDNF) by glial cells (Wolf et al., 2009). Accordingly, the administration of minocycline, a non-specific anti-inflammatory drug, led to decreased neurogenesis in the dentate gyrus and abrogated the activation of meningeal of T-cells (Ziv et al., 2006; Derecki et al., 2010). Interestingly, a later study demonstrated that meningeal CD4+ T-cells participating in the acquisition of spatial memory and neurogenesis in the dentate gyrus are memory T-cells with specificity for self-antigens derived from the CNS (Baruch et al., 2013). Thus, these studies provide evidence that CNS-specific T-cells are required for neurogenesis, and their activation is dependent on microglial cells (Ziv et al., 2006; Derecki et al., 2010; Baruch et al., 2013).
Surgical removal of the deep cervical lymph nodes can result in dysregulated T-cell immunity that correlates with cognitive impairment (Radjavi et al., 2014b). Moreover, a decreased relative number of circulating dendritic cells, HLA-DR+ regulatory T-cells (Tregs), and CD4+ memory T-cells has been associated with more severe negative and cognitive symptoms in schizophrenic patients (Fernandez-Egea et al., 2016). According to the key role described for CNS-specific CD4+ T-cells in spatial memory, mice lacking peripheral mature T-cells manifest impaired spatial learning, memory capabilities (Kipnis et al., 2004b), and neurogenesis (Ziv et al., 2006) compared with the wild-type control group. This cognitive decline is reversed by transfer of T-cells (Kipnis et al., 2004b), but not when other immune cells (i.e. bone marrow-derived immune cells from T-cell depleted donors) are injected (Brynskikh et al., 2008). Similarly, it has been shown that the cognitive impairment developed by the deficiency of adaptive immune system is reversed just by the transfer of CD4+ T-cells, even in the absence of B-lymphocytes and CD8+ T-cells (Wolf et al., 2009; Radjavi et al., 2014b). Accordingly, a particular CD4+ T-lymphocyte subset, which originates from deep cervical lymph nodes (Radjavi et al., 2014a) and resides in the choroid plexus, ventricular margins, and subarachnoid spaces (Derecki et al., 2010; Baruch et al., 2013; Radjavi et al., 2014b) has been implicated in cognitive functions (Wolf et al., 2009; Radjavi et al., 2014b). These cells have been shown to decrease drug-induced psychosis and reduce cognitive impairment (Kipnis et al., 2004b) in mice. The training in spatial memory led to the accumulation of IL-4 producing T-cells in the meninges of experimental mice. Moreover, experiments in which the entrance of T-cells into the meningeal space was attenuated by using FTY720 or an anti-VLA4 antibody showed that reduced recruitment of a subset of memory CD4+ T-cells into the meninges resulted in impaired spatial memory (Derecki et al., 2010). However, these results do not rule out the indirect interaction of CD4+ T-cells with local or systemic antigen presenting cells (i.e. microglia, myeloid cells) and their secreted cytokines. For example, myeloid cells acquire an inflammatory phenotype in response to cognitive tasks in absence of meningeal T-cells. This phenotype can be reversed after injection of T-cells-expressing IL-4, which act on myeloid cells, favoring the acquisition of an anti-inflammatory phenotype (Derecki et al., 2010). These results suggest that T-cell derived IL-4 is the main cytokine regulating the phenotype of myeloid cells. Furthermore, peripheral macrophages alternatively activated in vitro in the presence of IL-4 acquire an anti-inflammatory phenotype that might improve learning and memory in the absence of CD4+ T-cells (Derecki et al., 2011).
Thus, these findings suggest that the key role of meningeal CD4+ T-cells in learning and memory is associated to their participation as a source of IL-4 in the brain. Interestingly, age-related cognitive impairment has been related with a shift on the regulatory cytokines produced in the choroid plexus, which constitute the entrance gate for CD4+ T-cells into the meninges. In this regard, high IL-4-to-IFN-γ ratio promotes CCL11 production, whereas low IL-4-to-IFN-γ ratio favors production of BDNF (Baruch et al., 2013). Remarkably, BDNF has been involved in neurogenesis and promoting learning and spatial memory (Derecki et al., 2010). Conversely, CCL11 is a chemokine associated with age-related cognitive impairments, whose high plasma levels correlate with reduced neurogenesis in mice and aging in humans (Villeda et al., 2011). These results highlight the importance of a cross-talk between meningeal lymphoid and myeloid cells for cognitive function. To add another piece to the puzzle, a recent study has raised new questions regarding the potential role of CD8+ T-cells derived IFN-γ from neurogenic brain niches in the generation of neurogenesis and cognition (Dulken et al., 2019). It has been shown in both rodents and humans that aging involves an increased CNS-infiltration of T-cells expressing IFN-γ, which decreases proliferation of neural stem cells (Dulken et al., 2019).
Importantly, another group of studies has provided evidence of a fundamental role of microglia in shaping neuronal circuitry by four different ways. First, by engulfing presynaptic termini in the healthy brain (Schafer et al., 2012; Meyer, 2013). Secondly, by limiting neurogenesis through the release of soluble factors, such as secretome after phagocytosis (Diaz-Aparicio et al., 2020), BDNF, insulin growth factor-1, TNF-α, pre-micro RNAs, among others (Rodriguez-Iglesias et al., 2019). Third, by inhibition of Sirt1/p65 signaling pathway in the dentate gyrus (Sellner et al., 2016). Fourth, by phagocytosis of apoptotic newborn cells in the dentate gyrus (Sierra et al., 2010). In this regard, the stimulation of the fractalkine receptor (CX3CR1) and the complement receptor 3 (CR3) signaling pathways have been shown to participate in these processes through the pruning of synaptic spines, engulfment of neurons during periods of active synaptic pruning (Schafer et al., 2012; Meyer, 2013). Indeed, the defective interaction between neurons and microglia given in cx3cr1-deficient animals leads to impaired synaptic maturation and reduced efficiency of synaptic transmission (Reshef et al., 2014; Basilico et al., 2019). Consequently, the deficiency in these signaling pathways results in neurological impairment. For instance, cx3cr1-deficient mice display impaired associative and spatial memory (Rogers et al., 2011), reduced neurogenesis in the dentate gyrus (Meyer, 2013), as well as increased levels of IL-1β in the hippocampus (Rogers et al., 2011). Cx3cr1-deficiency also leads to higher spine density, enhanced number of excitatory synapses (Meyer, 2013), and altered microglial morphology (Corona et al., 2010; Basilico et al., 2019) in comparison with wild-type controls. It has been suggested that one of the mechanisms involved in the learning deficits observed in cx3cr1-deficient mice is the inability to achieve long-term potentiation (LTP) in the hippocampus of these animals, which seems to be a consequence of the increased levels of IL-1β (Rogers et al., 2011; Liu et al., 2019). In addition, other studies have shown that CX3CL1 transiently potentiates NMDA-function, but inhibits hippocampal LTP, a process regulated through the stimulation of adenosine receptors (Maggi et al., 2009; Scianni et al., 2013). In the same direction, the pharmacological depletion of microglial cells mediated by the bilateral injection of clodronate into the dorsal hippocampus or by oral administration of PLX3397 showed alterations in spatial learning (Torres et al., 2016). Moreover, depletion of BDNF from microglial cells has been shown to reduce motor learning, recapitulating some of the behavioral alterations reported in microglia-depleted mice (Parkhurst et al., 2013). Regarding CR3, it has been shown that C3 deficiency leads to an enhanced learning and memory in mice when compared with their wild-type littermates (Shi et al., 2015; Shi et al., 2017). However, little is known regarding C3R deficiency in microglial cells. These findings together illustrate how the innate and adaptive immune response in the CNS play a relevant role in the proper development of neuronal circuitry and in cognitive tasks in healthy physiological conditions.
Finally, some studies have shown that BBB might play a relevant role modulating the immune response, thus affecting cognitive function in schizophrenia. For instance, increased expression of genes involved in immune function and inflammation have been detected in the choroid plexus of schizophrenic patients, which correlates with BBB permeability (Kim et al., 2016). On the other hand, decreased expression of the tight-junction protein claudin-5 at the BBB, correlates with impaired learning and memory, depression, anxiety, impaired social behavior, and altered locomotor activity in mice (Greene et al., 2018). Therefore, the BBB should also be considered as an important actor involved in the regulation of the cross-talk between immune system and the cognitive and behavioral impairment associated to schizophrenia.
Stress Resilience
Another behavioral response that might be significantly regulated by immune cells, including T-cells, microglia, and peripheral monocytes, is the adaptation to psychological stress. In this regard, it has been shown that T-cell deficient mice, including severe combined immunodeficient (SCID) and nude mice, develop a worst adaptation than their immunocompetent counterpart in models of post-traumatic stress disorder (Cohen et al., 2006; Scheinert et al., 2016). Adoptive transfer of T-cells into either T-cell deficient (Cohen et al., 2006) or immunocompetent mice (Lewitus et al., 2008) improves the adaptation to psychological stress (Scheinert et al., 2016). Moreover, when immunodeficient mice where reconstituted with T-cells devoid of Tregs, psychological adaptation to stress was better than in mice replenished with the total T-cell compartment, containing both Tregs and effector T-cells (Teff) (Cohen et al., 2006). Further analysis of post-traumatic stress showed an association between lymphocyte recruitment into the choroid plexus and stress resilience, as well as increased hippocampal BDNF levels (Lewitus et al., 2008). Single-cell RNAseq analysis of T-cells recruited into the CNS upon psychological stress suggests a non-encephalitogenic origin, expressing Foxp3, Gata3, and Th2 genes (Kertser et al., 2019). It has been hypothesized that increased lymphocyte recruitment into the CNS might lead to the development of memory T-cells, that are necessary in order to promote homeostasis and enhance resilience to subsequent psychological stressful experiences (Lewitus and Schwartz, 2009). Accordingly, lymphocytes from chronically stressed mice adoptively transferred into immunodeficient mice (Rag2−/−) are able to confer anti-depressant like behavioral effects compared to the adoptive transfer of lymphocytes isolated from unstressed mice (Brachman et al., 2015; Scheinert et al., 2016). Thus, these findings support the hypothesis that psychological stress triggers the generation of memory T-cells, probably with specificity to CNS-derived self-antigens, which are recruited to the choroid plexus and confer resilience to future adverse psychological events.
Recent studies have shown that monocytes might also be involved in stress resilience. In a mouse model of severe psychological stress, leukocyte trafficking through choroid plexus was suppressed. The inhibition of glucocorticoid receptor signaling restored leukocyte trafficking through choroid plexus, which was associated to the recruitment of Th2 and Tregs cells into the CNS, leading to attenuation of post-traumatic behavioral deficit (Kertser et al., 2019). Accordingly, corticosterone mediated an increase of inflammatory circulating monocytes in mice behaviorally susceptible to stress (Niraula et al., 2018; Gururajan et al., 2019). It has been shown that peripheral monocytes might be recruited into the brain by microglial cells and their chemokines secreted (Weber et al., 2019), where they can exacerbate neuroinflammation (Niraula et al., 2018) and anxiety-like behavior (Wohleb et al., 2014). In addition, cx3cr1-deficiency in mice confers resilience to chronic unpredictable stress stimuli, thus suggesting a detrimental role of microglial cells in response to psychological stress (Hellwig et al., 2016; Rimmerman et al., 2017). In this regard, it has been shown that CX3CR1-signaling induces hyper-ramification of microglial cells in response to chronic stress, which was associated with the development of depressive-like behavior (Hellwig et al., 2016). Thus, the emerging evidence indicates that the innate and adaptive immune system play a fundamental role in the behavioral response to psychological stress.
Social Behavior
Social behavior constitutes another response regulated by the immune system. In this regard, mice deficient in adaptive immunity display social deficits, as evidenced by anti-social behavior in validated behavioral tests of preference between another mouse or an inert object. This behavioral impairment has been attributed to the lack of IFN-γ production by meningeal T-cells. Accordingly, this deficit in social behavior might be reversed by administration of IFN-γ in the cerebrospinal fluid (CSF) or by the adoptive transfer of T-lymphocytes isolated from wild-type mice. The authors showed evidence indicating that IFN-γ stimulates GABAergic inhibitory neurons, triggering inhibitory neural circuits and thereby preventing hyper-excitability in the prefrontal cortex (Filiano et al., 2016). On the other hand, microglial cells have been reported to be involved in social behavior (Torres et al., 2016; Kopec et al., 2018), and to drive changes in affective behavior under exposure to psychosocial stress (Lehmann et al., 2019). In this regard, microglia abrogated the development of chronic social defeat-induced anxiety-like and antisocial behavior. Accordingly, microglia replenishment in the brain, just after psychosocial stress, leads to anxiety-like and antisocial behavior. A potential mechanism to explain the role of microglia in social behavior suggested by the authors involves the elevated reactive oxygen species produced by microglial cells during and after stress exposure (Lehmann et al., 2019). Another plausible explanation comes from a recent study involving the CSF-1/CSF-1R axis, which is required for development of most tissue macrophages including osteoclasts, brain microglial cells, and others. In this regard, it has been shown that the interference of the CSF-1/CSF-1R signaling pathway in cerebellar microglia leads to defective motor learning and impaired social interactions (Kana et al., 2019). This latter study is supported by another work that indicates a relevant role of the cerebellum as a regulator of major cognitive functions, such as expectations and reward (Wagner et al., 2017). Furthermore, it has recently been shown that the disruption of connectivity between the cerebellum and the right dorsolateral prefrontal cortex is associated with the severity of negative symptoms in schizophrenia (Brady et al., 2019). Another line of evidence shows microglial cells as key components organizing neuronal circuits involved in sex-associated social behavior during adolescence. In this regard, microglia and their complement-dependent phagocytosis promoted the elimination of D1-like receptors in the nucleus accumbens of male rats, a process that was required for natural developmental changes in male social play behavior (Kopec et al., 2018). Interestingly, a recent study provided evidence that this organized phagocytic process mediated by microglia and complement in the development of sex-associated social behavior during adolescence is promoted by the action of testosterone-induced endocannabinoids (Vanryzin et al., 2019).
Interestingly, maternal immune inflammation has also been linked to an increasing risk of developing schizophrenia in the progeny, where hippocampal microglial cells display an altered gene expression profile, including upregulation of genes involved in embryonic development, long-term neuronal plasticity, angiogenesis, and extracellular matrix organization, whereas genes involved in phagocytosis, cell migration, and inflammatory response were downregulated (Mattei et al., 2017). Thus, emerging evidence indicates that meningeal T-cells and microglial cells play relevant roles regulating social behavior.
Dopaminergic Regulation of the Immune System
A number of catecholamine family members, including dopamine, have been extensively involved in the regulation of the immune response (Tracey, 2009), affecting both the innate and adaptive immune system (Pacheco et al., 2014; Vidal and Pacheco, 2019). In this regard, dopamine receptors are expressed on T and B lymphocytes, dendritic and NK cells, macrophages, microglia, intermediate monocytes, neutrophils, and eosinophils (Levite, 2016; Arce-Sillas et al., 2019). In this section we focused in the analysis of the dopaminergic regulation of T cells, microglia, and monocytes, since these immune cells have been implicated in cognitive functions.
Dopaminergic Regulation of T Cells
The final outcome of dopamine effects on T-lymphocytes depends on dopamine concentrations, type of T-cells and activation status, as well as the dopamine receptors being expressed (Levite, 2016). In addition to the expression of dopamine receptors, the dopaminergic system in T-cells also involves some subsets of T-cells as sources of dopamine. For instance, human Tregs might synthesize and release dopamine, which exerts a negative feedback on their suppressive activity (Cosentino et al., 2007). More recently, another study revealed that follicular helper T (TFH) cells synthesize and store dopamine, which is released upon antigen-recognition to stimulate DRD1-signaling on B-cells as a costimulatory signal to induce antibody production (Papa et al., 2017).
A group of studies has addressed the effect of dopamine in Teffs and Tregs using pharmacologic approaches in experiments in vitro. These studies have shown that the stimulation of D1-like dopamine receptors favors the differentiation of naive CD4+ T-cells toward a Th2 phenotype (Nakano et al., 2009), and attenuates the regulatory activity of Tregs (Kipnis et al., 2004a; Cosentino et al., 2007). Pharmacologic evidence has also shown that DRD4-signaling induces T-cell quiescence (Sarkar et al., 2006). A recent study using genetic approaches revealed that DRD4-signaling in T-cells favors Th2 differentiation, promoting allergic asthma in newborn lungs (Wang et al., 2019). Addressing the role of DRD3-signaling in T-cells, pharmacologic and genetic evidence has recently shown that stimulation of this receptor potentiates T-cell activation, favoring Th1 differentiation and reciprocally dampening the acquisition of the Th2 phenotype, both in vitro and in vivo (Gonzalez et al., 2013; Franz et al., 2015; Contreras et al., 2016; Elgueta et al., 2019). In the same direction, pharmacologic evidence obtained with human T-cells has shown that DRD3-stimulation increases IFN-γ production and concomitantly decreases IL-4 and IL-10 release, along with and exacerbated expression of the activation marker CD25 (Ilani et al., 2004).
Mechanistic analyses carried out through pharmacologic and genetic approaches have shown that this DRD3-mediated potentiation of Th1-differentiation and concomitant repression of Th2-differentiation involves the upregulation of the regulatory protein SOCS5 (Contreras et al., 2016), as well as reduction of intracellular cyclic adenosine monophosphate (cAMP) levels and ERK phosphorylation (Franz et al., 2015). It is important to consider that, under chronic inflammatory conditions, DRD3-signaling in CD4+ T-cells also favors the expansion of the Th17-lienage (Contreras et al., 2016). Regarding DRD5-signaling in T-cells, pharmacologic and genetic evidence has shown DRD5-stimulation on CD4+ T-cells potentiates TCR-signaling (Franz et al., 2015) favoring a stronger T-cell activation in vitro and in vivo (Osorio-Barrios et al., 2018). Further analyses have shown that DRD5-signaling in Teff favors the acquisition of the Th17-lineage, whereas in Tregs increase the potency of their suppressive activity (Osorio-Barrios et al., 2018).
Despite that most studies addressing the dopaminergic regulation of T-cells have been focused in CD4+ T-cells, a few studies have also analyzed CD8+ T-cells. In this regard, a couple of studies using pharmacological approaches have shown that DRD3-stimulation on CD8+ T-cells potentiates IFN-γ transcription (Ilani et al., 2004), increases their integrin-mediated adhesion to fibronectin and intercellular adhesion molecule 1 (ICAM-1), and synergizes lymphocytes migration toward inflammatory chemokines (Watanabe et al., 2006). In addition, a recent study has shown that pharmacologic stimulation of D1-like dopamine receptors attenuates both the generation and the suppressive activity of regulatory CD8+ T-cells (Nasi et al., 2019). Taken together, these findings indicate that both CD8+ and CD4+ T-cells might undergo a complex dopaminergic regulation, which affects their activation, adhesion, migration, differentiation, and effector or suppressive function. Thus, alterations in physiological dopamine levels or the expression of dopamine receptors in T-lymphocytes, such as the case of schizophrenia, may exert strong changes in the behavior of T-cells.
Dopaminergic Regulation of Microglial Cells
Microglial cells constitute a key cell of the innate immune response in the CNS, which play a central role in neuroinflammation (Gonzalez et al., 2015). Since microglial cells reside in the CNS, they are exposed to dopamine released by dopaminergic neural circuits of the CNS. In this regard, dopaminergic signaling has been reported to modulate different microglial functions, including their inflammatory activity (Yan et al., 2015; Dominguez-Meijide et al., 2017; Elgueta et al., 2017), migration (Farber et al., 2005), and cell adhesion (Fan et al., 2018). Accordingly, microglial cells express both dopamine D1-like and D2-like receptors (Farber et al., 2005; Huck et al., 2015). However, under inflammatory conditions the expression of dopamine receptors might strongly change (Huck et al., 2015). This is the case of the DRD2, whose expression is induced upon inflammatory stimulation of microglial cells following stroke (Huck et al., 2015). High dopamine levels attenuate the inflammatory activation of microglia by reducing the release of nitric oxide (Farber et al., 2005) and decreasing the extent of phagocytosis (Fan et al., 2018). It has been suggested that this process may be mediated by the stimulation of low-affinity dopamine receptors in these cells, including DRD1 and DRD2 (Dominguez-Meijide et al., 2017), leading to a reduction in the phosphorylation of ERK1/2 (Fan et al., 2018), and to the inhibition of the angiotensin type-1/NADPH-oxidase/superoxide axis (Dominguez-Meijide et al., 2017). In the same direction, it has been described that DRD1-signaling in microglial cells induces the cAMP-mediated degradation of the NLRP3 inflammasome, thus exerting a potent anti-inflammatory effect in vivo (Yan et al., 2015). Moreover, microglial complement-dependent signaling pathway mediates elimination of D1-like receptors in the nucleus accumbens of adolescent male, leading to the development of social behavior changes in male rats (Kopec et al., 2018). In addition, the stimulation of the DRD2 in homeostatic microglial cells has been shown to attenuate the inflammatory response (Dominguez-Meijide et al., 2017) by increasing p38MAPK and reducing the number of cellular processes (Fan et al., 2018). Furthermore, indirect mechanisms involving DRD2-signaling in astrocytes and triggering anti-inflammatory effects on microglial cells have been described. Accordingly, it has been shown that high-dopamine levels might exert down-regulation of angiotensin II release by astrocytes (Dominguez-Meijide et al., 2017), and also induce the upregulation of the anti-inflammatory molecule αβ-crystallin in astrocytes (Shao et al., 2013), thus attenuating inflammatory behavior in microglial cells.
On the other hand, emerging evidence has suggested that stimulation of high-affinity dopamine receptors in microglia promotes neuroinflammation. Accordingly, the treatment of primary cultures of activated microglia with a DRD2/DRD3 agonist, has been shown to increase the release of nitrite and IFN-γ (Huck et al., 2015). Moreover, inflammatory stimuli such as LPS, IFN-γ, or TNF-α, induced enhanced levels of intracellular BH4 in microglial cells, which promotes a higher rate of dopamine biosynthesis in mouse. A similar situation was observed in peripheral macrophages when stimulated by IFN-γ or LPS, which promoted the activity of the transcription factor NRF2 (Mcneill et al., 2015) and the consequent upregulation of GCH-1 in a rats (Sakai et al., 1995). Thereby, these findings suggest that pro-inflammatory stimuli in microglial cells promote a stronger capacity for dopamine biosynthesis in animal models. However, it is important to keep in mind that human cells are less efficient generating BH4 in comparison to other species (Schmidt et al., 2014).
Interestingly, dopaminergic regulation of microglial activity has also been studied under pathological conditions, such as those associated to amyotrophic lateral sclerosis (ALS) and Parkinson’s disease. For instance, the pharmacologic stimulation of DRD4 has been shown to suppress microglia recruitment to the site of inflammation, and thereby delaying the progression of ALS in a mouse model (Tanaka et al., 2008). Moreover, the systemic DRD3-antagonism has been shown to modulate the inflammatory response of astrocytes, attenuating microglial activation in the striatum in a mouse model of Parkinson’s disease (Elgueta et al., 2017; Elgueta et al., 2019). Taken together, the current evidence indicates that high-dopamine levels promote the stimulation of low-affinity dopamine receptors (including DRD1, DRD2, and DRD4), inducing an anti-inflammatory effect in microglia, while low-dopamine levels selectively stimulates high-affinity dopamine receptors (including DRD3 and DRD5), triggering inflammation, as proposed before (Pacheco, 2017).
Dopaminergic Regulation of Monocytes
Under pathological conditions, such as those associated to neurodegenerative disorders (Gonzalez and Pacheco, 2014), or CNS injury (Wattananit et al., 2016; Olingy et al., 2017; Norden et al., 2019; Vidal et al., 2019) peripheral monocytes play an important role contributing to neuroinflammation. For instance, using a rat model of peripheral inflammation it has been shown that monocytes depletion, mediated by the peripheral administration of clodronate, mitigates the production of inflammatory mediators and microglial activation without affecting dopaminergic neuronal survival (Xie et al., 2017).
Similar to microglial cells, monocytes also express both dopamine D1-like and D2-like receptors (Mckenna et al., 2002; Coley et al., 2015). A few studies have addressed the role of dopaminergic signaling in monocytes function and have found that it is associated with migration, regulation of inflammatory mediators (Gaskill et al., 2012; Coley et al., 2015), and proliferation (Bergquist et al., 2000). In this regard, in vitro experiments using human monocytes have shown that high dopamine levels potentiate the production of IL-10 as well as CXCL8, while low dopamine levels favor the secretion of IL-6 and CCL-2, and decrease TNF-α production in response to LPS (Gaskill et al., 2012). In addition, it has been shown that dopaminergic signaling through D1-like dopamine receptors increases monocytes migration and adhesion (Coley et al., 2015). Interestingly, a recent study has shown that DAT hypofunction in mice, a condition associated with increased dopamine levels, correlates with reduced microglial activation and a lower extent of infiltration of monocyte-derived macrophages into the brain (Castellani et al., 2019), suggesting that high-dopamine levels attenuate neuroinflammation. Furthermore, it has been shown that high dopamine concentrations (10–100 µM) decrease proliferation of peripheral blood monocytes (Bergquist et al., 2000). In summary, the current evidence suggests that high dopamine levels, probably by stimulating DRD1 or DRD2 in monocytes (Pacheco et al., 2014), reduce the production of inflammatory mediators, proliferation and CNS recruitment, thus attenuating neuroinflammation. Conversely, the stimulation of high-affinity dopamine receptors in peripheral monocytes seems to favor the production of some inflammatory cytokines in these cells.
Changes in Dopaminergic Regulation of the Immune System Associated to Schizophrenia
As described in section 3, the immune system plays an important role collaborating with the CNS to carry out some cognitive and behavioral function. Thereby, dysregulation of the immune system function might be involved in the development of neurologic diseases. In this regard, the association between psychiatric disorders, like schizophrenia, with altered immune responses has progressively gained interest over the past decade (Khandaker et al., 2015; Sellgren et al., 2019).
Several studies have reported immunological alterations associated to schizophrenia. In this regard, two meta-analysis have reported dynamic alterations in the profile of cytokine expression of schizophrenic patients depending on the stage of the disease (first episode versus relapsed patients) (Miller et al., 2011; Frydecka et al., 2018). These changes involve both inflammatory and anti-inflammatory cytokines depending on the disease duration, pharmacologic treatment, smoking status, among others (Miller et al., 2011; Muller et al., 2013). Another group of studies addressing gene expression in schizophrenic patients has suggested that dysregulation of the immune response associated to schizophrenia is a consequence of the disease progression or due to the long-term treatment with antipsychotic medication (Kumarasinghe et al., 2013; Frydecka et al., 2018).
A group of studies has analyzed samples of peripheral blood from patients and has found that dopamine receptors are differentially expressed in peripheral blood mononuclear cells (PBMCs) in schizophrenia. It has been reported that DRD3 mRNA levels are increased in peripheral blood lymphocytes (Ilani et al., 2001). Consistently with the dopaminergic nature of drugs used in schizophrenia, it has been shown that pharmacological medication might induce changes in the expression of dopamine receptors in lymphocytes. In this regard, the transcriptional levels of DRD3 and DRD5 are increased in drug-free patients compared to medicated patients (Kwak et al., 2001). Moreover, the percentage of CD4+ and CD8+ T-cells expressing the DRD4, and CD4+ T-cells expressing DRD2 was increased in medicated schizophrenic patients compared to controls (Brito-Melo et al., 2012). Interestingly, two studies have reported changes in the relative composition of PBMCs associated to schizophrenia. The first one showed a higher proportion of circulating Tregs without changes in CD3+ or CD4+ T-cells in medicated-schizophrenic patients in comparison with healthy controls (Kelly et al., 2018). The second one, showed increased frequencies of NK cells, classical monocytes, naive B-cells, and CXCR5+ memory T-cells, and reduced percentages of dendritic cells, CD4+ memory T-cells, and HLA-DR+ regulatory T-cells in the blood of patients resistant to clozapine-treatment (Fernandez-Egea et al., 2016). Of note, the expression of DRD3 was shown significantly increased on peripheral CD4+ T-cells in clozapine-treated schizophrenic patients, which was correlated with reduced frequency of Tregs (Fernandez-Egea et al., 2016). According to this negative correlation, DRD3-signaling in CD4+ T-cells has been involved in promoting inflammatory responses, including Th1 and Th17 mediated immunity (Franz et al., 2015; Contreras et al., 2016). Following the same line, another study has shown an inverse correlation between the proportion of Tregs and the development of negative symptoms in schizophrenic patients (Kelly et al., 2018). Thus, the current evidence suggests an increased expression of high-affinity dopamine receptors, including DRD3 and DRD5, in T-lymphocytes of untreated schizophrenic patients, and enhanced levels of expression of the low-affinity dopamine receptor DRD2 in drug-treated schizophrenic patients. It is noteworthy that evidence obtained from in vivo approaches using animal models, or from in vitro approaches using human samples, has indicated that high affinity dopamine receptors DRD3 and DRD5 exert inflammatory effects while the low affinity dopamine receptor DRD2 promotes anti-inflammatory effects (Pacheco, 2017). Changes in dopaminergic regulation of the immune system associated to schizophrenia are integrated in Figure 1.
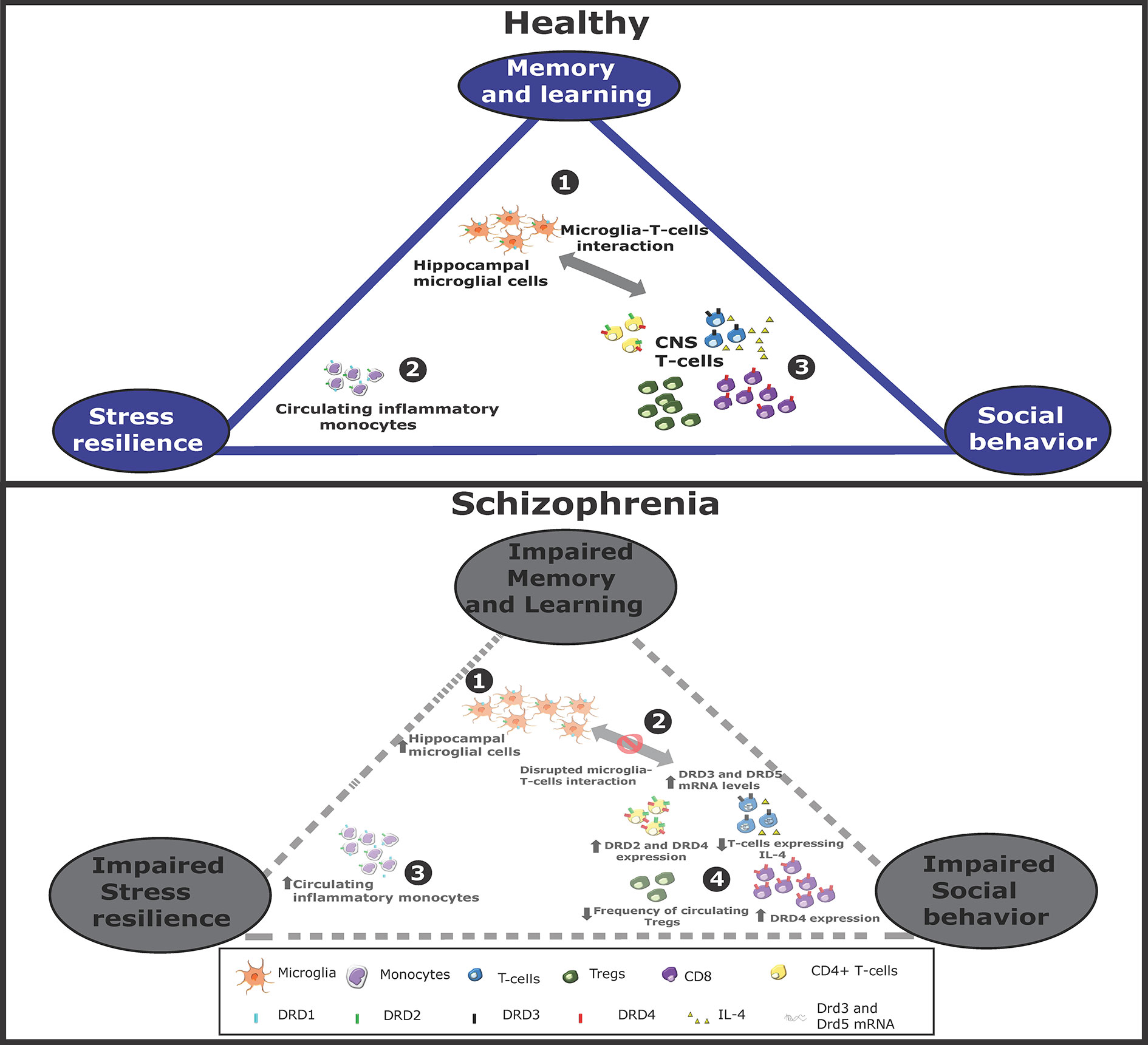
Figure 1 Altered dopaminergic signaling in monocytes, T-cells, and microglia associated to schizophrenia. Schematic representation of immune cells associated to cognitive/behavioral function in healthy conditions (top panel) or in schizophrenia (bottom panel). Top panel: In healthy conditions, 1. microglial cells stimulate to T-cells infiltrated into the CNS. 2. Peripheral monocytes might also infiltrate the brain and favor T-cell activation. 3. Activated T-cells contribute to stress resilience. Some T-cells acquire Th2 phenotype and produce IL-4, which favors neurogenesis and thereby memory and learning. Some T-cells acquire Th1 features, produce IFN-γ and promote social behavior. Bottom panel: In schizophrenia, 1. the interaction between microglia and T-cells is impaired, 2. thereby attenuating T-cell activation. Moreover, the expression of different dopamine receptors is altered, changing the extent of T-cell activation and differentiation. 3. Furthermore, the extent of peripheral monocytes and hippocampal microglia are increased, 4. whereas Treg frequency is reduced. All these changes hypothetically contribute to a reduced social behavior, decreased stress resilience and in impaired memory and learning.
Interestingly, it has been shown that the treatment of schizophrenic patients with anti-inflammatory drugs in combination with anti-psychotic leads to better cognitive outcomes that the treatment with anti-psychotic drugs alone (Muller, 2010; Xiang et al., 2017). This is based on the inflammatory hypothesis of schizophrenia, which states that increased neuroinflammation contributes to the symptoms of schizophrenia. Therefore, combinatorial treatments using anti-inflammatory with anti-psychotic drugs might have synergistic effects at attenuating symptomatology, dampening the production of inflammatory cytokines, reactive oxygen species, prostaglandins and microglia function, among others (Sommer et al., 2014).
For instance, the treatment with the anti-inflammatory drug minocycline in combination with an atypical antipsychotic (risperidone, olanzapine, quetiapine, clozapine, or chlorpromazine), alleviates negative and positive symptoms in patients with schizophrenia (Levkovitz et al., 2010; Chaves et al., 2015). Of note, it has been suggested that minocycline acts not only by attenuating inflammation, but also decreasing synapse engulfment (Sellgren et al., 2019), and might potentially modify the composition of the gut-microbiota due to its antibiotic properties (Pape et al., 2019). The use of other anti-inflammatory therapies for schizophrenia are also being currently investigated in clinical trials, such as the treatment with monoclonal antibodies, such as Natalizumab, Tocilizumab, and Siltuximab (clinicaltrials.gov). It is noteworthy that a dopaminergic drug with anti-inflammatory activity is currently under study in humans as a treatment for schizophrenia. This is the case of a randomized, double-blind clinical trial ongoing in the US using a novel dopaminergic antagonist, I-tetrahydropalmatine, as an adjuvant treatment. This drug displays an anti-inflammatory activity and presents a higher affinity for D1-like receptors (clinicaltrials.gov).
Immunological Alterations During Embryonic or Early Postnatal Development Leading to Imbalance in Dopaminergic Transmission and Neuropsychiatric Disorders in Adulthood
Diverse environmental risk factors associated with the development of schizophrenia during embryonic and early postnatal life have been reported, including perinatal hypoxia, cannabis consumption, stress, maternal infection, among others (Winter et al., 2009; Brown, 2011; Inta et al., 2011; Delpech et al., 2016). In the late 80’s a novel hypothesis for the development of schizophrenia was raised, which suggested that primary cerebral insults occur during early brain development, triggering disease manifestation years later (Meyer, 2013). The revised hypothesis incorporated to the first version the brain changes occurring during the early phases of the disease at the postnatal stage (Meyer, 2013). A study carried out in Denmark showed that both infections requiring hospitalization and autoimmune disease are risk factors for developing schizophrenia in the future (Benros et al., 2011). Moreover, maternal infection or immune activation during critical periods of pregnancy enhances the risk of the offspring to develop neuropsychiatric disorders later in life (Winter et al., 2009). Little is known regarding the molecular mechanisms explaining how a broad range of infectious agents might trigger schizophrenia. Indeed, the evidence suggests that a significant immune response in the mother during pregnancy is sufficient to trigger schizophrenia in the adult offspring, irrespective of the pathogen identity (Zuckerman and Weiner, 2005). To add another level of complexity, it has been shown that exposure to Epstein-Barr virus or throat infection in early childhood have also been associated with increasing risk of experiencing psychotic symptoms and/or subsequent neuropsychiatric disorders during the adolescence (Khandaker et al., 2014; Orlovska et al., 2017). Thus, the period of time in which infections can increase the risk of developing neuropsychiatric disorders is not restricted to the prenatal stage (Khandaker et al., 2012).
Some recent studies have demonstrated how maternal immune activation (MIA) during embryonic life might lead to changes in dopaminergic transmission during adulthood. In this regard, a preliminary study in a non-human primate model of MIA reported that male offspring born to MIA-treated dams had increased striatal dopamine in late adolescence compared with the control group (Bauman et al., 2019), changes similar to those associated with schizophrenia (see section 1). Similarly, prenatal exposure to the inflammatory agents Poly I:C or LPS in mice led to long-lasting changes in neurotransmitter levels and dopamine receptors in the brain of adult offspring. Specifically, levels of TH (Meyer et al., 2008), dopamine, and dopamine-derived metabolites were increased, whereas DRD1 and DRD2 (Meyer et al., 2008), serotonin receptors, and its metabolites were reduced in some specific brain areas (Winter et al., 2009). Moreover, organotypic cultures of ventral mesencephalon and striatum of rat fetuses exposed to LPS at embryonic day (E) E10, E14, or E18 showed a reduction in dopaminergic neurons when the cultures were kept for a long period compared with organotypic cultures of non-exposed fetuses (Snyder-Keller and Stark, 2008). In addition, prenatal exposure to inflammation also induces changes in pre- and post- synaptic GABAergic, glutamatergic, and serotoninergic neuronal circuits. Changes associated to embryonic exposure to inflammatory stimuli are not limited to neurons, but also to glial cells affecting the number, structure, positioning, and survival of these cells, as well as morphological changes in the brain (Boksa, 2010; Chua et al., 2012). With regard to these latter analyses, controversial results have been reported about the effect of embryonic exposure to inflammation in microglial density, proliferation, phagocytic activity, and number of cells in animal models (Boksa, 2010). Some studies have reported an increase in these parameters (Juckel et al., 2011; Hadar et al., 2017) while others have reported no change (Garay et al., 2013; Mattei et al., 2014). This discrepancy can mainly be attributed to the rodent strain used and the stage of gestation where pregnant mothers receive the injection of Poly I:C (Juckel et al., 2011; Garay et al., 2013; Mattei et al., 2014). Of note, although alterations in the number and morphology of microglia cells may be a transient process, they might lead to long-lasting changes at the gene expression level increasing, for example, their phagocytic activity (Delpech et al., 2016; Mattei et al., 2017), and acquiring a “primed” pro-inflammatory phenotype prone to neuroinflammation in the adulthood (Norden et al., 2015). Microglia isolated from mice that underwent maternal immune activation in utero have shown altered expression of genes involved in embryonic development and phagocytosis (Mattei et al., 2017), which resemble those alterations observed upon interruption of the CSF-1/CSF-1R axis in cerebellar microglia associated with motor and social deficits (Kana et al., 2019). Intriguingly, it has been shown that cesarean section births also induce long-term changes in brain dopamine receptors, specially D1-like receptors (El-Khodor and Boksa, 2001). Thus, current evidence suggests that inflammatory events occurring during embryonic or early postnatal age lead to alterations in microglial cells which later in life result in imbalance of dopaminergic transmission and the consequent development of neuropsychiatric disorders.
Anti-inflammatory treatments have shown to partially attenuate or even fully prevent the development of schizophrenia in animal models. For instance, minocycline treatment in the offspring reduced the levels of TNF-α and IL-1β in the hippocampus of rats exposed to MIA (Mattei et al., 2014). Furthermore, the authors showed that the treatment with minocycline promoted neurogenesis, improved working memory, and social behavior as well as increased the phagocytic activity of microglia in the hippocampus (Mattei et al., 2017). In addition, it was shown that deep brain electrical stimulation treatment during the adolescence of MIA rats prevented the behavioral impairment and attenuated the increase of microglial density (Hadar et al., 2017). An important consequence of LPS-induced MIA is the induction of metallothionein, a zinc-binding protein, in the mother’s liver, which promotes fetal zinc deficiency. Accordingly, maternal dietary zinc supplementation has been assessed as a treatment for offspring exposed to MIA. In this regard, it has been shown that zinc supplementation prevented the development of long-term cognitive abnormalities, reduced the number of TNF-α+ cells, and decreased the extent of apoptotic cells in the offspring of LPS-treated mice (Coyle et al., 2009; Chua et al., 2012). Thus, prophylactic anti-inflammatory treatments intended to target maternal inflammation in experimental animal models of schizophrenia have sparked interest in the combinatorial use of anti-inflammatory drugs with atypical antipsychotic drugs during the early phases of schizophrenia. Preliminary studies in human patients with schizophrenia have shown promising beneficial effects by improving negative and cognitive symptoms compared with patients receiving antipsychotic drugs alone (Levkovitz et al., 2010; Muller et al., 2010).
Conclusions
Schizophrenia is associated with dysregulated activity of dopaminergic neural circuits, which consequently promotes several cognitive and behavioral alterations. Immune system cells, specially T-cells, microglial cells and peripheral monocytes, have been described to collaborate with the CNS to carry out some of these cognitive and behavioral functions that are altered in schizophrenia. Furthermore, dopamine might strongly affect the activity of T-cells, microglia, and peripheral monocytes, since all these immune cells express dopamine receptors. The current evidence indicates that high-dopamine levels promote the stimulation of low-affinity dopamine receptors (including DRD1, DRD2, and DRD4), inducing an anti-inflammatory effect on immune cells, while low-dopamine levels selectively stimulates high-affinity dopamine receptors (including DRD3 and DRD5), triggering inflammation. Thus, alterations in dopamine levels associated to schizophrenia might affect inflammatory response of immune cells and consequently some behavioral functions, including reference memory, learning, social behavior, and stress resilience (see a summary in Table 1). Interestingly, studies performed with patients have shown that drug-free schizophrenic patients display exacerbated expression of high-affinity dopamine receptors, and thereby acquiring pro-inflammatory features in response to dopamine. Conversely, medicated patients seem to switch their expression of dopamine receptors favoring the expression of low-affinity receptors in immune cells, thus acquiring anti-inflammatory profiles in response to dopamine. Accordingly, recent data obtained from clinical trials has suggested that the usage of anti-inflammatory and/or dopaminergic drugs as adjuvant therapy for schizophrenia might give a significant improvement in the symptomatology involved in this disorder. Recent evidence indicates that significant inflammatory events occurring during embryonic or early postnatal age lead to alterations in microglial cells which later in life result in imbalance of dopaminergic transmission and the consequent development of neuropsychiatric disorders. Further research is necessary in this area to decipher the molecular and cellular underlying mechanisms and, consequently, to be able to design therapeutic strategies to target specific detrimental processes without affecting the general function of the immune system. Studies about the long-term safety of combined therapies (i.e. anti-psychotics with anti-inflammatory drugs) are also still necessaries, as chronic administration of anti-inflammatory drugs could yield hepatic failure and/or an immunosuppressive state that may be involve increased susceptibility to infections and cancer. Finally, another aspect that should be further explored in the upcoming future is the validation of the cross-talk between immune system and CNS in the development of cognitive and behavioral changes, as most research in this field has been done in animal models.
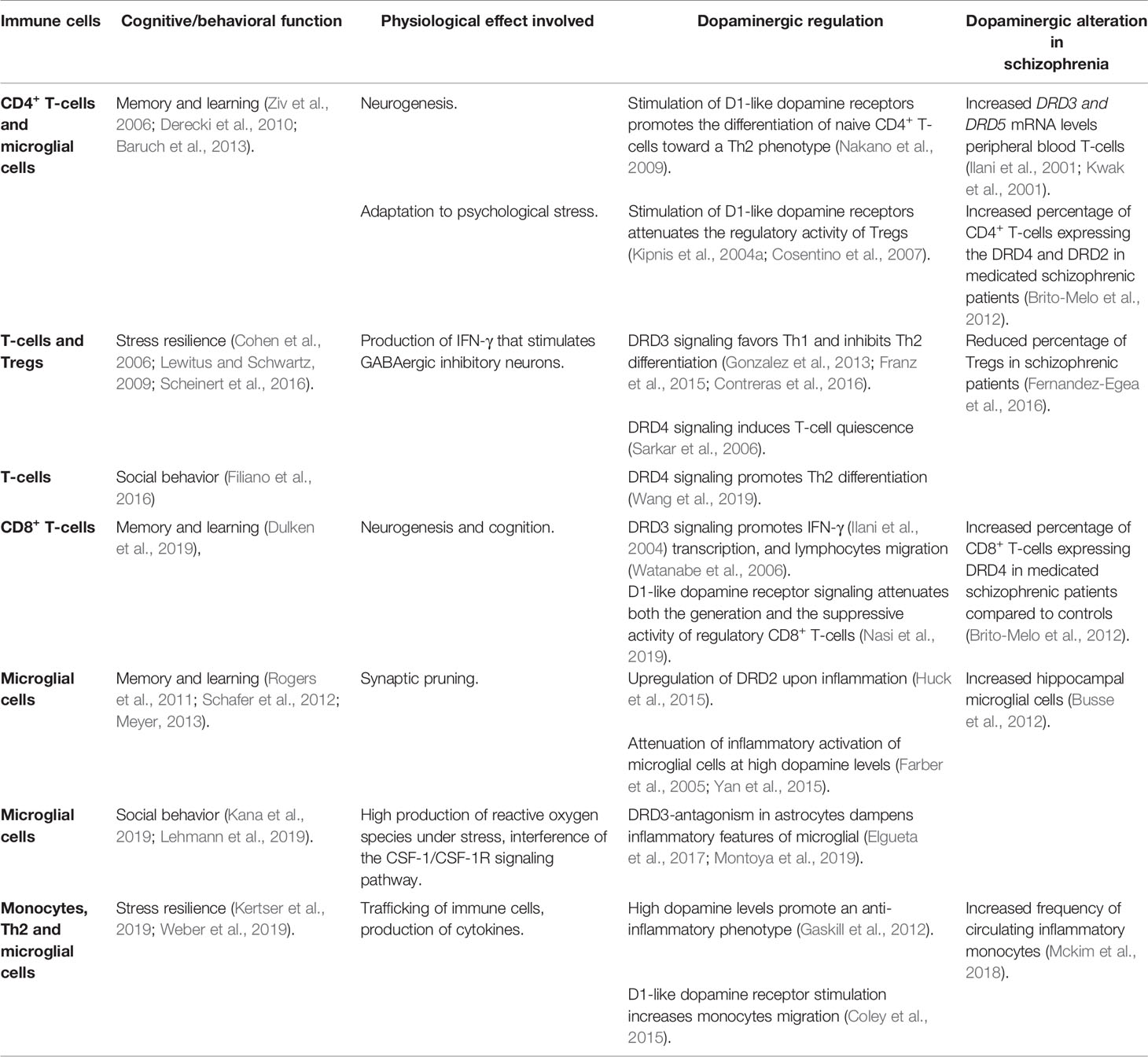
Table 1 Dopaminergic regulation of the immune system associated with cognitive/behavioral functions.
Author Contributions
Both authors contributed in analyzing the bibliography and writing the paper.
Funding
This work was supported by Programa de Apoyo a Centros con Financiamiento Basal AFB-170004 (to Fundación Ciencia & Vida) from “Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT)” and by grants FONDECYT-1170093 (to RP) from “Fondo Nacional de Desarrollo Científico y Tecnológico de Chile” and the FAA 02/2019 from Universidad Católica de la Santísima Concepción (to PV).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Akil, M., Edgar, C. L., Pierri, J. N., Casali, S., Lewis, D. A. (2000). Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol. Psychiatry 47, 361–370. doi: 10.1016/S0006-3223(99)00282-6
Arce-Sillas, A., Sevilla-Reyes, E., Alvarez-Luquin, D. D., Guevara-Salinas, A., Boll, M. C., Perez-Correa, C. A., et al. (2019). Expression of Dopamine Receptors in Immune Regulatory Cells. Neuroimmunomodulation. 26 (3), 159–166. doi: 10.1159/000501187
Avram, M., Brandl, F., Bauml, J., Sorg, C. (2018). Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology 43, 2239–2248. doi: 10.1038/s41386-018-0059-z
Avram, M., Brandl, F., Cabello, J., Leucht, C., Scherr, M., Mustafa, M., et al. (2019). Reduced striatal dopamine synthesis capacity in patients with schizophrenia during remission of positive symptoms. Brain 142, 1813–1826. doi: 10.1093/brain/awz093
Baruch, K., Ron-Harel, N., Gal, H., Deczkowska, A., Shifrut, E., Ndifon, W., et al. (2013). CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl. Acad. Sci. U. S. A 110, 2264–2269. doi: 10.1073/pnas.1211270110
Basilico, B., Pagani, F., Grimaldi, A., Cortese, B., Di Angelantonio, S., Weinhard, L., et al. (2019). Microglia shape presynaptic properties at developing glutamatergic synapses. Glia 67, 53–67. doi: 10.1002/glia.23508
Bauman, M. D., Lesh, T. A., Rowland, D. J., Schumann, C. M., Smucny, J., Kukis, D. L., et al. (2019). Preliminary evidence of increased striatal dopamine in a nonhuman primate model of maternal immune activation. Transl. Psychiatry 9, 135. doi: 10.1038/s41398-019-0449-y
Benros, M. E., Nielsen, P. R., Nordentoft, M., Eaton, W. W., Dalton, S. O., Mortensen, P. B. (2011). Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am. J. Psychiatry 168, 1303–1310. doi: 10.1176/appi.ajp.2011.11030516
Bergquist, J., Ohlsson, B., Tarkowski, A. (2000). Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Ann. N. Y. Acad. Sci. 917, 281–289. doi: 10.1111/j.1749-6632.2000.tb05394.x
Boksa, P. (2010). Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav. Immun. 24, 881–897. doi: 10.1016/j.bbi.2010.03.005
Brachman, R. A., Lehmann, M. L., Maric, D., Herkenham, M. (2015). Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 35, 1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015
Brady, R. O., Jr., Gonsalvez, I., Lee, I., Ongur, D., Seidman, L. J., Schmahmann, J. D., et al. (2019). Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am. J. Psychiatry 176, 512–520. doi: 10.1176/appi.ajp.2018.18040429
Brisch, R., Saniotis, A., Wolf, R., Bielau, H., Bernstein, H. G., Steiner, J., et al. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front. Psychiatry 5, 47. doi: 10.3389/fpsyt.2014.00047
Brito-Melo, G. E., Nicolato, R., De Oliveira, A. C., Menezes, G. B., Lelis, F. J., Avelar, R. S., et al. (2012). Increase in dopaminergic, but not serotoninergic, receptors in T-cells as a marker for schizophrenia severity. J. Psychiatr. Res. 46, 738–742. doi: 10.1016/j.jpsychires.2012.03.004
Brown, A. S. (2011). The environment and susceptibility to schizophrenia. Prog. Neurobiol. 93, 23–58. doi: 10.1016/j.pneurobio.2010.09.003
Brynskikh, A., Warren, T., Zhu, J., Kipnis, J. (2008). Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 22, 861–869. doi: 10.1016/j.bbi.2007.12.008
Busse, S., Busse, M., Schiltz, K., Bielau, H., Gos, T., Brisch, R., et al. (2012). Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav. Immun. 26, 1273–1279. doi: 10.1016/j.bbi.2012.08.005
Castellani, G., Contarini, G., Mereu, M., Albanesi, E., Devroye, C., D’amore, C., et al. (2019). Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav. Immun. 81, 138–150 doi: 10.1016/j.bbi.2019.06.006
Chaves, C., Marque, C. R., Maia-De-Oliveira, J. P., Wichert-Ana, L., Ferrari, T. B., Santos, A. C., et al. (2015). Effects of minocycline add-on treatment on brain morphometry and cerebral perfusion in recent-onset schizophrenia. Schizophr. Res. 161, 439–445. doi: 10.1016/j.schres.2014.11.031
Chong, H. Y., Teoh, S. L., Wu, D. B., Kotirum, S., Chiou, C. F., Chaiyakunapruk, N. (2016). Global economic burden of schizophrenia: a systematic review. Neuropsychiatr. Dis. Treat 12, 357–373. doi: 10.2147/NDT.S96649
Chua, J. S., Cowley, C. J., Manavis, J., Rofe, A. M., Coyle, P. (2012). Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice. Brain Behav. Immun. 26, 326–336. doi: 10.1016/j.bbi.2011.10.002
Cohen, H., Ziv, Y., Cardon, M., Kaplan, Z., Matar, M. A., Gidron, Y., et al. (2006). Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J. Neurobiol. 66, 552–563. doi: 10.1002/neu.20249
Coley, J. S., Calderon, T. M., Gaskill, P. J., Eugenin, E. A., Berman, J. W. (2015). Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PloS One 10, e0117450. doi: 10.1371/journal.pone.0117450
Contreras, F., Prado, C., Gonzalez, H., Franz, D., Osorio-Barrios, F., Osorio, F., et al. (2016). Dopamine Receptor D3 Signaling on CD4+ T Cells Favors Th1- and Th17-Mediated Immunity. J. Immunol. 196, 4143–4149. doi: 10.4049/jimmunol.1502420
Corona, A. W., Huang, Y., O’connor, J. C., Dantzer, R., Kelley, K. W., Popovich, P. G., et al. (2010). Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflamm. 7, 93. doi: 10.1186/1742-2094-7-93
Cosentino, M., Fietta, A. M., Ferrari, M., Rasini, E., Bombelli, R., Carcano, E., et al. (2007). Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109, 632–642. doi: 10.1182/blood-2006-01-028423
Coyle, P., Tran, N., Fung, J. N., Summers, B. L., Rofe, A. M. (2009). Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 197, 210–218. doi: 10.1016/j.bbr.2008.08.022
Da Silva Alves, F., Figee, M., Van Amelsvoort, T., Veltman, D., De Haan, L. (2008). The revised dopamine hypothesis of schizophrenia: evidence from pharmacological MRI studies with atypical antipsychotic medication. Psychopharmacol. Bull. 41, 121–132.
Delpech, J. C., Wei, L., Hao, J., Yu, X., Madore, C., Butovsky, O., et al. (2016). Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 57, 79–93. doi: 10.1016/j.bbi.2016.06.006
Demjaha, A., Murray, R. M., Mcguire, P. K., Kapur, S., Howes, O. D. (2012). Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 169, 1203–1210. doi: 10.1176/appi.ajp.2012.12010144
Derecki, N. C., Cardani, A. N., Yang, C. H., Quinnies, K. M., Crihfield, A., Lynch, K. R., et al. (2010). Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080. doi: 10.1084/jem.20091419
Derecki, N. C., Quinnies, K. M., Kipnis, J. (2011). Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 25, 379–385. doi: 10.1016/j.bbi.2010.11.009
Diaz-Aparicio, I., Paris, I., Sierra-Torre, V., Plaza-Zabala, A., Rodriguez-Iglesias, N., Marquez-Ropero, M., et al. (2020). Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 40 (7), 1453–1482. doi: 10.1523/JNEUROSCI.0993-19.2019
Dolan, R. J., Fletcher, P., Frith, C. D., Friston, K. J., Frackowiak, R. S., Grasby, P. M. (1995). Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 378, 180–182. doi: 10.1038/378180a0
Dominguez-Meijide, A., Rodriguez-Perez, A. I., Diaz-Ruiz, C., Guerra, M. J., Labandeira-Garcia, J. L. (2017). Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 62, 277–290. doi: 10.1016/j.bbi.2017.02.013
Domyo, T., Kurumaji, A., Toru, M. (2001). An increase in [3H]SCH23390 binding in the cerebral cortex of postmortem brains of chronic schizophrenics. J. Neural Transm. (Vienna) 108, 1475–1484. doi: 10.1007/s007020100021
Dulken, B. W., Buckley, M. T., Navarro Negredo, P., Saligrama, N., Cayrol, R., Leeman, D. S., et al. (2019). Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210. doi: 10.1038/s41586-019-1362-5
Elgueta, D., Aymerich, M. S., Contreras, F., Montoya, A., Celorrio, M., Rojo-Bustamante, E., et al. (2017). Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology 113, 110–123. doi: 10.1016/j.neuropharm.2016.09.028
Elgueta, D., Contreras, F., Prado, C., Montoya, A., Ugalde, V., Chovar, O., et al. (2019). Dopamine Receptor D3 Expression Is Altered in CD4(+) T-Cells From Parkinson’s Disease Patients and Its Pharmacologic Inhibition Attenuates the Motor Impairment in a Mouse Model. Front. Immunol. 10, 981. doi: 10.3389/fimmu.2019.00981
El-Khodor, B., Boksa, P. (2001). Caesarean section birth produces long term changes in dopamine D1 receptors and in stress-induced regulation of D3 and D4 receptors in the rat brain. Neuropsychopharmacology 25, 423–439. doi: 10.1016/S0893-133X(01)00228-7
Fan, Y., Chen, Z., Pathak, J. L., Carneiro, A. M. D., Chung, C. Y. (2018). Differential Regulation of Adhesion and Phagocytosis of Resting and Activated Microglia by Dopamine. Front. Cell Neurosci. 12, 309. doi: 10.3389/fncel.2018.00309
Farber, K., Pannasch, U., Kettenmann, H. (2005). Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell Neurosci. 29, 128–138. doi: 10.1016/j.mcn.2005.01.003
Felger, J. C., Cole, S. W., Pace, T. W., Hu, F., Woolwine, B. J., Doho, G. H., et al. (2012). Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychol. Med. 42, 1591–1603. doi: 10.1017/S0033291711002868
Fernandez-Egea, E., Vertes, P. E., Flint, S. M., Turner, L., Mustafa, S., Hatton, A., et al. (2016). Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PloS One 11, e0155631. doi: 10.1371/journal.pone.0155631
Filiano, A. J., Xu, Y., Tustison, N. J., Marsh, R. L., Baker, W., Smirnov, I., et al. (2016). Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535, 425–429. doi: 10.1038/nature18626
Franz, D., Contreras, F., Gonzalez, H., Prado, C., Elgueta, D., Figueroa, C., et al. (2015). Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production. J. Neuroimmunol. 284, 18–29. doi: 10.1016/j.jneuroim.2015.05.003
Frydecka, D., Krzystek-Korpacka, M., Lubeiro, A., Stramecki, F., Stanczykiewicz, B., Beszlej, J. A., et al. (2018). Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immun. 71, 28–36. doi: 10.1016/j.bbi.2018.05.002
Garay, P. A., Hsiao, E. Y., Patterson, P. H., Mcallister, A. K. (2013). Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 31, 54–68. doi: 10.1016/j.bbi.2012.07.008
Gaskill, P. J., Carvallo, L., Eugenin, E. A., Berman, J. W. (2012). Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J. Neuroinflamm. 9, 203. doi: 10.1186/1742-2094-9-203
Gonzalez, H., Pacheco, R. (2014). T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflamm. 11, 201. doi: 10.1186/s12974-014-0201-8
Gonzalez, H., Contreras, F., Prado, C., Elgueta, D., Franz, D., Bernales, S., et al. (2013). Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J. Immunol. 190, 5048–5056. doi: 10.4049/jimmunol.1203121
Gonzalez, H., Contreras, F., Pacheco, R. (2015). Regulation of the Neurodegenerative Process Associated to Parkinson’s Disease by CD4+ T-cells. J. Neuroimmune. Pharmacol. 10, 561–575. doi: 10.1007/s11481-015-9618-9
Greene, C., Kealy, J., Humphries, M. M., Gong, Y., Hou, J., Hudson, N., et al. (2018). Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry 23, 2156–2166. doi: 10.1038/mp.2017.156
Gurevich, E. V., Bordelon, Y., Shapiro, R. M., Arnold, S. E., Gur, R. E., Joyce, J. N. (1997). Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch. Gen. Psychiatry 54, 225–232. doi: 10.1001/archpsyc.1997.01830150047009
Gururajan, A., Van De Wouw, M., Boehme, M., Becker, T., O’connor, R., Bastiaanssen, T. F. S., et al. (2019). Resilience to chronic stress is associated with specific neurobiological, neuroendocrine and immune responses. Brain Behav. Immun. 80, 583–594. doi: 10.1016/j.bbi.2019.05.004
Hadar, R., Dong, L., Del-Valle-Anton, L., Guneykaya, D., Voget, M., Edemann-Callesen, H., et al. (2017). Deep brain stimulation during early adolescence prevents microglial alterations in a model of maternal immune activation. Brain Behav. Immun. 63, 71–80. doi: 10.1016/j.bbi.2016.12.003
Hellwig, S., Brioschi, S., Dieni, S., Frings, L., Masuch, A., Blank, T., et al. (2016). Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 55, 126–137. doi: 10.1016/j.bbi.2015.11.008
Howes, O. D., Egerton, A., Allan, V., Mcguire, P., Stokes, P., Kapur, S. (2009a). Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr. Pharm. Des. 15, 2550–2559. doi: 10.2174/138161209788957528
Howes, O. D., Montgomery, A. J., Asselin, M. C., Murray, R. M., Valli, I., Tabraham, P., et al. (2009b). Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry 66, 13–20. doi: 10.1001/archgenpsychiatry.2008.514
Huck, J. H., Freyer, D., Bottcher, C., Mladinov, M., Muselmann-Genschow, C., Thielke, M., et al. (2015). De novo expression of dopamine D2 receptors on microglia after stroke. J. Cereb. Blood Flow Metab. 35, 1804–1811. doi: 10.1038/jcbfm.2015.128
Ilani, T., Ben-Shachar, D., Strous, R. D., Mazor, M., Sheinkman, A., Kotler, M., et al. (2001). A peripheral marker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc. Natl. Acad. Sci. U. S. A 98, 625–628. doi: 10.1073/pnas.98.2.625
Ilani, T., Strous, R. D., Fuchs, S. (2004). Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells. FASEB J. 18, 1600–1602. doi: 10.1096/fj.04-1652fje
Inta, D., Meyer-Lindenberg, A., Gass, P. (2011). Alterations in postnatal neurogenesis and dopamine dysregulation in schizophrenia: a hypothesis. Schizophr. Bull. 37, 674–680. doi: 10.1093/schbul/sbq134
Juckel, G., Manitz, M. P., Brune, M., Friebe, A., Heneka, M. T., Wolf, R. J. (2011). Microglial activation in a neuroinflammational animal model of schizophrenia–a pilot study. Schizophr. Res. 131, 96–100. doi: 10.1016/j.schres.2011.06.018
Kana, V., Desland, F. A., Casanova-Acebes, M., Ayata, P., Badimon, A., Nabel, E., et al. (2019). Disruption of the CSF-1-CSF-1R axis alters cerebellar microglia and is associated with motor and social interaction defects. bioRxiv, 639526. doi: 10.1101/639526
Kapur, S., Zipursky, R., Jones, C., Remington, G., Houle, S. (2000). Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am. J. Psychiatry 157, 514–520. doi: 10.1176/appi.ajp.157.4.514
Kazumori, H., Ishihara, S., Rumi, M. A., Ortega-Cava, C. F., Kadowaki, Y., Kinoshita, Y. (2004). Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G508–G514. doi: 10.1152/ajpgi.00269.2003
Kegeles, L. S., Abi-Dargham, A., Frankle, W. G., Gil, R., Cooper, T. B., Slifstein, M., et al. (2010). Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry 67, 231–239. doi: 10.1001/archgenpsychiatry.2010.10
Kelly, D. L., Li, X., Kilday, C., Feldman, S., Clark, S., Liu, F., et al. (2018). Increased circulating regulatory T cells in medicated people with schizophrenia. Psychiatry Res. 269, 517–523. doi: 10.1016/j.psychres.2018.09.006
Kennedy, D. P., Adolphs, R. (2012). The social brain in psychiatric and neurological disorders. Trends Cognit. Sci. 16, 559–572. doi: 10.1016/j.tics.2012.09.006
Kertser, A., Baruch, K., Deczkowska, A., Weiner, A., Croese, T., Kenigsbuch, M., et al. (2019). Corticosteroid signaling at the brain-immune interface impedes coping with severe psychological stress. Sci. Adv. 5, eaav4111. doi: 10.1126/sciadv.aav4111
Kestler, L. P., Walker, E., Vega, E. M. (2001). Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behav. Pharmacol. 12, 355–371. doi: 10.1097/00008877-200109000-00007
Khandaker, G. M., Zimbron, J., Dalman, C., Lewis, G., Jones, P. B. (2012). Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr. Res. 139, 161–168. doi: 10.1016/j.schres.2012.05.023
Khandaker, G. M., Stochl, J., Zammit, S., Lewis, G., Jones, P. B. (2014). Childhood Epstein-Barr Virus infection and subsequent risk of psychotic experiences in adolescence: a population-based prospective serological study. Schizophr. Res. 158, 19–24. doi: 10.1016/j.schres.2014.05.019
Khandaker, G. M., Cousins, L., Deakin, J., Lennox, B. R., Yolken, R., Jones, P. B. (2015). Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2, 258–270. doi: 10.1016/S2215-0366(14)00122-9
Kim, S., Hwang, Y., Lee, D., Webster, M. J. (2016). Transcriptome sequencing of the choroid plexus in schizophrenia. Transl. Psychiatry 6, e964. doi: 10.1038/tp.2016.229
Kinon, B. J., Lieberman, J. A. (1996). Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacol. (Berl) 124, 2–34. doi: 10.1007/BF02245602
Kipnis, J., Avidan, H., Caspi, R. R., Schwartz, M. (2004a). Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc. Natl. Acad. Sci. U. S. A 101 Suppl 2, 14663–14669. doi: 10.1073/pnas.0404842101
Kipnis, J., Cohen, H., Cardon, M., Ziv, Y., Schwartz, M. (2004b). T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci. U. S. A 101, 8180–8185. doi: 10.1073/pnas.0402268101
Kitagami, T., Yamada, K., Miura, H., Hashimoto, R., Nabeshima, T., Ohta, T. (2003). Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 978, 104–114. doi: 10.1016/S0006-8993(03)02776-8
Kopec, A. M., Smith, C. J., Ayre, N. R., Sweat, S. C., Bilbo, S. D. (2018). Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 9, 3769. doi: 10.1038/s41467-018-06118-z
Kosaka, J., Takahashi, H., Ito, H., Takano, A., Fujimura, Y., Matsumoto, R., et al. (2010). Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 86, 814–818. doi: 10.1016/j.lfs.2010.03.018
Kumarasinghe, N., Beveridge, N. J., Gardiner, E., Scott, R. J., Yasawardene, S., Perera, A., et al. (2013). Gene expression profiling in treatment-naive schizophrenia patients identifies abnormalities in biological pathways involving AKT1 that are corrected by antipsychotic medication. Int. J. Neuropsychopharmacol. 16, 1483–1503. doi: 10.1017/S1461145713000035
Kwak, Y. T., Koo, M. S., Choi, C. H., Sunwoo, I. (2001). Change of dopamine receptor mRNA expression in lymphocyte of schizophrenic patients. BMC Med. Genet. 2, 3. doi: 10.1186/1471-2350-2-3
Laruelle, M., Abi-Dargham, A., Van Dyck, C. H., Gil, R., D’souza, C. D., Erdos, J., et al. (1996). Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U. S. A 93, 9235–9240. doi: 10.1073/pnas.93.17.9235
Lehmann, M. L., Weigel, T. K., Poffenberger, C., Herkenham, M. (2019). The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J. Neurosci. 39 (28), 5594–5605. doi: 10.1523/JNEUROSCI.0184-19.2019
Levite, M. (2016). Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. (Oxf) 216, 42–89. doi: 10.1111/apha.12476
Levkovitz, Y., Mendlovich, S., Riwkes, S., Braw, Y., Levkovitch-Verbin, H., Gal, G., et al. (2010). A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 71, 138–149. doi: 10.4088/JCP.08m04666yel
Lewitus, G. M., Schwartz, M. (2009). Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol. Psychiatry 14, 532–536. doi: 10.1038/mp.2008.103
Lewitus, G. M., Cohen, H., Schwartz, M. (2008). Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 22, 1108–1114. doi: 10.1016/j.bbi.2008.05.002
Li, W., Knowlton, D., Woodward, W. R., Habecker, B. A. (2003). Regulation of noradrenergic function by inflammatory cytokines and depolarization. J. Neurochem. 86, 774–783. doi: 10.1046/j.1471-4159.2003.01890.x
Li, P., Snyder, G. L., Vanover, K. E. (2016). Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 16, 3385–3403. doi: 10.2174/1568026616666160608084834
Lieberman, J. A., Davis, R. E., Correll, C. U., Goff, D. C., Kane, J. M., Tamminga, C. A., et al. (2016). ITI-007 for the Treatment of Schizophrenia: A 4-Week Randomized, Double-Blind, Controlled Trial. Biol. Psychiatry 79, 952–961. doi: 10.1016/j.biopsych.2015.08.026
Liu, X., Nemeth, D. P., Mckim, D. B., Zhu, L., Disabato, D. J., Berdysz, O., et al. (2019). Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity 50, 764–766. doi: 10.1016/j.immuni.2019.02.012
Lodge, D. J., Grace, A. A. (2007). Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 27, 11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007
Macdowell, K. S., Caso, J. R., Martin-Hernandez, D., Madrigal, J. L., Leza, J. C., Garcia-Bueno, B. (2014). Paliperidone prevents brain toll-like receptor 4 pathway activation and neuroinflammation in rat models of acute and chronic restraint stress. Int. J. Neuropsychopharmacol. 18, pyu070 1–11. doi: 10.1093/ijnp/pyu070
Maggi, L., Trettel, F., Scianni, M., Bertollini, C., Eusebi, F., Fredholm, B. B., et al. (2009). LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R). J. Neuroimmunol. 215, 36–42. doi: 10.1016/j.jneuroim.2009.07.016
Mattei, D., Djodari-Irani, A., Hadar, R., Pelz, A., De Cossio, L. F., Goetz, T., et al. (2014). Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav. Immun. 38, 175–184. doi: 10.1016/j.bbi.2014.01.019
Mattei, D., Ivanov, A., Ferrai, C., Jordan, P., Guneykaya, D., Buonfiglioli, A., et al. (2017). Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 7, e1120. doi: 10.1038/tp.2017.80
Mccutcheon, R., Beck, K., Jauhar, S., Howes, O. D. (2018). Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophr. Bull. 44, 1301–1311. doi: 10.1093/schbul/sbx180
Mccutcheon, R. A., Abi-Dargham, A., Howes, O. D. (2019). Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 42, 205–220. doi: 10.1016/j.tins.2018.12.004
Mckenna, F., Mclaughlin, P. J., Lewis, B. J., Sibbring, G. C., Cummerson, J. A., Bowen-Jones, D., et al. (2002). Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J. Neuroimmunol. 132, 34–40. doi: 10.1016/S0165-5728(02)00280-1
Mckim, D. B., Weber, M. D., Niraula, A., Sawicki, C. M., Liu, X., Jarrett, B. L., et al. (2018). Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431. doi: 10.1038/mp.2017.64
Mcneill, E., Crabtree, M. J., Sahgal, N., Patel, J., Chuaiphichai, S., Iqbal, A. J., et al. (2015). Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 79, 206–216. doi: 10.1016/j.freeradbiomed.2014.10.575
Meyer, U., Nyffeler, M., Schwendener, S., Knuesel, I., Yee, B. K., Feldon, J. (2008). Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology 33, 441–456. doi: 10.1038/sj.npp.1301413
Meyer, U. (2013). Developmental neuroinflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 20–34. doi: 10.1016/j.pnpbp.2011.11.003
Miller, B. J., Buckley, P., Seabolt, W., Mellor, A., Kirkpatrick, B. (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry 70, 663–671. doi: 10.1016/j.biopsych.2011.04.013
Montoya, A., Elgueta, D., Campos, J., Chovar, O., Falcon, P., Matus, S., et al. (2019). Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. J. Neuroinflamm. 16, 258. doi: 10.1186/s12974-019-1652-8
Moreno-Kustner, B., Martin, C., Pastor, L. (2018). Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PloS One 13, e0195687. doi: 10.1371/journal.pone.0195687
Mueller, H. T., Haroutunian, V., Davis, K. L., Meador-Woodruff, J. H. (2004). Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res. Mol. Brain Res. 121, 60–69. doi: 10.1016/j.molbrainres.2003.11.004
Muller, N., Krause, D., Dehning, S., Musil, R., Schennach-Wolff, R., Obermeier, M., et al. (2010). Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr. Res. 121, 118–124. doi: 10.1016/j.schres.2010.04.015
Muller, N., Myint, A. M., Krause, D., Weidinger, E., Schwarz, M. J. (2013). Anti-inflammatory treatment in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 146–153. doi: 10.1016/j.pnpbp.2012.11.008
Muller, N. (2010). COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr. Opin. Invest. Drugs 11, 31–42. doi: 10.1016/j.schres.2010.04.015
Nakajima, S., Gerretsen, P., Takeuchi, H., Caravaggio, F., Chow, T., Le Foll, B., et al. (2013). The potential role of dopamine D(3) receptor neurotransmission in cognition. Eur. Neuropsychopharmacol. 23, 799–813. doi: 10.1016/j.euroneuro.2013.05.006
Nakano, K., Higashi, T., Takagi, R., Hashimoto, K., Tanaka, Y., Matsushita, S. (2009). Dopamine released by dendritic cells polarizes Th2 differentiation. Int. Immunol. 21, 645–654. doi: 10.1093/intimm/dxp033
Nasi, G., Ahmed, T., Rasini, E., Fenoglio, D., Marino, F., Filaci, G., et al. (2019). Dopamine inhibits human CD8+ Treg function through D1-like dopaminergic receptors. J. Neuroimmunol. 332, 233–241. doi: 10.1016/j.jneuroim.2019.02.007
Niraula, A., Wang, Y., Godbout, J. P., Sheridan, J. F. (2018). Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 38, 2328–2340. doi: 10.1523/JNEUROSCI.2568-17.2018
Norden, D. M., Muccigrosso, M. M., Godbout, J. P. (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96, 29–41. doi: 10.1016/j.neuropharm.2014.10.028
Norden, D. M., Faw, T. D., Mckim, D. B., Deibert, R. J., Fisher, L. C., Sheridan, J. F., et al. (2019). Bone Marrow-Derived Monocytes Drive the Inflammatory Microenvironment in Local and Remote Regions after Thoracic Spinal Cord Injury. J. Neurotrauma 36, 937–949. doi: 10.1089/neu.2018.5806
Olingy, C. E., San Emeterio, C. L., Ogle, M. E., Krieger, J. R., Bruce, A. C., Pfau, D. D., et al. (2017). Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 7, 447. doi: 10.1038/s41598-017-00477-1
Orlovska, S., Vestergaard, C. H., Bech, B. H., Nordentoft, M., Vestergaard, M., Benros, M. E. (2017). Association of Streptococcal Throat Infection With Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry 74, 740–746. doi: 10.1001/jamapsychiatry.2017.0995
Osorio-Barrios, F., Prado, C., Contreras, F., Pacheco, R. (2018). Dopamine Receptor D5 Signaling Plays a Dual Role in Experimental Autoimmune Encephalomyelitis Potentiating Th17-Mediated Immunity and Favoring Suppressive Activity of Regulatory T-Cells. Front. Cell Neurosci. 12, 192. doi: 10.3389/fncel.2018.00192
Pacheco, R., Contreras, F., Zouali, M. (2014). The dopaminergic system in autoimmune diseases. Front. Immunol. 5, 117. doi: 10.3389/fimmu.2014.00117
Pacheco, R. (2017). Targeting dopamine receptor D3 signalling in inflammation. Oncotarget 8, 7224–7225. doi: 10.18632/oncotarget.14601
Papa, I., Saliba, D., Ponzoni, M., Bustamante, S., Canete, P. F., Gonzalez-Figueroa, P., et al. (2017). TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547, 318–323. doi: 10.1038/nature23013
Pape, K., Tamouza, R., Leboyer, M., Zipp, F. (2019). Immunoneuropsychiatry - novel perspectives on brain disorders. Nat. Rev. Neurol. 15, 317–328. doi: 10.1038/s41582-019-0174-4
Park, J. H., Park, H. J., Lee, S. E., Kim, Y. S., Jang, G. Y., Han, H. D., et al. (2019). Repositioning of the antipsychotic drug TFP for sepsis treatment. J. Mol. Med. (Berl) 97, 647–658. doi: 10.1007/s00109-019-01762-4
Parkhurst, C. N., Yang, G., Ninan, I., Savas, J. N., Yates, J. R., Lafaille, J. J., et al. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609. doi: 10.1016/j.cell.2013.11.030
Patel, N. H., Vyas, N. S., Puri, B. K., Nijran, K. S., Al-Nahhas, A. (2010). Positron emission tomography in schizophrenia: a new perspective. J. Nucl. Med. 51, 511–520. doi: 10.2967/jnumed.109.066076
Paz, R. D., Tardito, S., Atzori, M., Tseng, K. Y. (2008). Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur. Neuropsychopharmacol. 18, 773–786. doi: 10.1016/j.euroneuro.2008.06.005
Pycock, C. J., Carter, C. J., Kerwin, R. W. (1980). Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J. Neurochem. 34, 91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x
Radjavi, A., Smirnov, I., Derecki, N., Kipnis, J. (2014a). Dynamics of the meningeal CD4(+) T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 19, 531–533. doi: 10.1038/mp.2013.79
Radjavi, A., Smirnov, I., Kipnis, J. (2014b). Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav. Immun. 35, 58–63. doi: 10.1016/j.bbi.2013.08.013
Ragland, J. D., Yoon, J., Minzenberg, M. J., Carter, C. S. (2007). Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int. Rev. Psychiatry 19, 417–427. doi: 10.1080/09540260701486365
Reshef, R., Kreisel, T., Beroukhim Kay, D., Yirmiya, R. (2014). Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain Behav. Immun. 41, 239–250. doi: 10.1016/j.bbi.2014.04.009
Rimmerman, N., Schottlender, N., Reshef, R., Dan-Goor, N., Yirmiya, R. (2017). The hippocampal transcriptomic signature of stress resilience in mice with microglial fractalkine receptor (CX3CR1) deficiency. Brain Behav. Immun. 61, 184–196. doi: 10.1016/j.bbi.2016.11.023
Roberts, A. C., De Salvia, M. A., Wilkinson, L. S., Collins, P., Muir, J. L., Everitt, B. J., et al. (1994). 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J. Neurosci. 14, 2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994
Rodriguez-Iglesias, N., Sierra, A., Valero, J. (2019). Rewiring of Memory Circuits: Connecting Adult Newborn Neurons With the Help of Microglia. Front. Cell Dev. Biol. 7, 24. doi: 10.3389/fcell.2019.00024
Rogers, J. T., Morganti, J. M., Bachstetter, A. D., Hudson, C. E., Peters, M. M., Grimmig, B. A., et al. (2011). CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 31, 16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011
Sakai, N., Kaufman, S., Milstien, S. (1995). Parallel induction of nitric oxide and tetrahydrobiopterin synthesis by cytokines in rat glial cells. J. Neurochem. 65, 895–902. doi: 10.1046/j.1471-4159.1995.65020895.x
Sarkar, C., Das, S., Chakroborty, D., Chowdhury, U. R., Basu, B., Dasgupta, P. S., et al. (2006). Cutting Edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J. Immunol. 177, 7525–7529. doi: 10.4049/jimmunol.177.11.7525
Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. doi: 10.1016/j.neuron.2012.03.026
Scheinert, R. B., Haeri, M. H., Lehmann, M. L., Herkenham, M. (2016). Therapeutic effects of stress-programmed lymphocytes transferred to chronically stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 70, 1–7. doi: 10.1016/j.pnpbp.2016.04.010
Schmidt, K., Kolesnik, B., Gorren, A. C., Werner, E. R., Mayer, B. (2014). Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling. Biochem. Pharmacol. 90, 246–253. doi: 10.1016/j.bcp.2014.05.010
Scianni, M., Antonilli, L., Chece, G., Cristalli, G., Di Castro, M. A., Limatola, C., et al. (2013). Fractalkine (CX3CL1) enhances hippocampal N-methyl-D-aspartate receptor (NMDAR) function via D-serine and adenosine receptor type A2 (A2AR) activity. J. Neuroinflamm. 10, 108. doi: 10.1186/1742-2094-10-108
Sellgren, C. M., Gracias, J., Watmuff, B., Biag, J. D., Thanos, J. M., Whittredge, P. B., et al. (2019). Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 22, 374–385. doi: 10.1038/s41593-018-0334-7
Sellner, S., Paricio-Montesinos, R., Spiess, A., Masuch, A., Erny, D., Harsan, L. A., et al. (2016). Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol. Commun. 4, 102. doi: 10.1186/s40478-016-0374-8
Shao, W., Zhang, S. Z., Tang, M., Zhang, X. H., Zhou, Z., Yin, Y. Q., et al. (2013). Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 494, 90–94. doi: 10.1038/nature11748
Shi, W., Meininger, C. J., Haynes, T. E., Hatakeyama, K., Wu, G. (2004). Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem. Biophys. 41, 415–434. doi: 10.1385/CBB:41:3:415
Shi, Q., Colodner, K. J., Matousek, S. B., Merry, K., Hong, S., Kenison, J. E., et al. (2015). Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J. Neurosci. 35, 13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015
Shi, Q., Chowdhury, S., Ma, R., Le, K. X., Hong, S., Caldarone, B. J., et al. (2017). Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 9, eaaf6295 1–31. doi: 10.1126/scitranslmed.aaf6295
Sierra, A., Encinas, J. M., Deudero, J. J., Chancey, J. H., Enikolopov, G., Overstreet-Wadiche, L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. doi: 10.1016/j.stem.2010.08.014
Simpson, E. H., Kellendonk, C., Kandel, E. (2010). A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65, 585–596. doi: 10.1016/j.neuron.2010.02.014
Slifstein, M., Kolachana, B., Simpson, E. H., Tabares, P., Cheng, B., Duvall, M., et al. (2008). COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol. Psychiatry 13, 821–827. doi: 10.1038/mp.2008.19
Slifstein, M., Van De Giessen, E., Van Snellenberg, J., Thompson, J. L., Narendran, R., Gil, R., et al. (2015). Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72, 316–324. doi: 10.1001/jamapsychiatry.2014.2414
Snyder, G. L., Vanover, K. E., Zhu, H., Miller, D. B., O’callaghan, J. P., Tomesch, J., et al. (2015). Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacol. (Berl) 232, 605–621. doi: 10.1007/s00213-014-3704-1
Snyder-Keller, A., Stark, P. F. (2008). Prenatal inflammatory effects on nigrostriatal development in organotypic cultures. Brain Res. 1233, 160–167. doi: 10.1016/j.brainres.2008.07.106
Sommer, I. E., Van Westrhenen, R., Begemann, M. J., De Witte, L. D., Leucht, S., Kahn, R. S. (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr. Bull. 40, 181–191. doi: 10.1093/schbul/sbt139
Swerdlow, N. R., Van Bergeijk, D. P., Bergsma, F., Weber, E., Talledo, J. (2009). The effects of memantine on prepulse inhibition. Neuropsychopharmacology 34, 1854–1864. doi: 10.1038/npp.2009.7
Takahashi, H., Kato, M., Takano, H., Arakawa, R., Okumura, M., Otsuka, T., et al. (2008). Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J. Neurosci. 28, 12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008
Tanaka, K., Okada, Y., Kanno, T., Otomo, A., Yanagisawa, Y., Shouguchi-Miyata, J., et al. (2008). A dopamine receptor antagonist L-745,870 suppresses microglia activation in spinal cord and mitigates the progression in ALS model mice. Exp. Neurol. 211, 378–386. doi: 10.1016/j.expneurol.2008.02.004
Torres, L., Danver, J., Ji, K., Miyauchi, J. T., Chen, D., Anderson, M. E., et al. (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain Behav. Immun. 55, 6–16. doi: 10.1016/j.bbi.2015.09.001
Torres-Rosas, R., Yehia, G., Pena, G., Mishra, P., Del Rocio Thompson-Bonilla, M., Moreno-Eutimio, M. A., et al. (2014). Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 20, 291–295. doi: 10.1038/nm.3479
Tracey, K. J. (2009). Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428. doi: 10.1038/nri2566
Vanryzin, J. W., Marquardt, A. E., Argue, K. J., Vecchiarelli, H. A., Ashton, S. E., Arambula, S. E., et al. (2019). Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron 102, 435–449 e436. doi: 10.1016/j.neuron.2019.02.006
Vidal, P. M., Pacheco, R. (2019). Targeting the Dopaminergic System in Autoimmunity. J. Neuroimmune. Pharmacol. doi: 10.1007/s11481-019-09834-5
Vidal, P. M., Ulndreaj, A., Tetreault, L., Hong, J., Fehlings, M. G. (2019). The changes in systemic monocytes in humans undergoing surgical decompression for degenerative cervical myelopathy may influence clinical neurological recovery. J. Neuroimmunol. 336, 577024. doi: 10.1016/j.jneuroim.2019.577024
Villeda, S. A., Luo, J., Mosher, K. I., Zou, B., Britschgi, M., Bieri, G., et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. doi: 10.1038/nature10357
Wagner, M. J., Kim, T. H., Savall, J., Schnitzer, M. J., Luo, L. (2017). Cerebellar granule cells encode the expectation of reward. Nature 544, 96–100. doi: 10.1038/nature21726
Wang, S. M., Han, C., Lee, S. J., Jun, T. Y., Patkar, A. A., Masand, P. S., et al. (2017). Investigational dopamine antagonists for the treatment of schizophrenia. Expert Opin. Invest. Drugs 26, 687–698. doi: 10.1080/13543784.2017.1323870
Wang, W., Cohen, J. A., Wallrapp, A., Trieu, K. G., Barrios, J., Shao, F., et al. (2019). Age-Related Dopaminergic Innervation Augments T Helper 2-Type Allergic Inflammation in the Postnatal Lung. Immunity 51, 1102–1118 e1107. doi: 10.1016/j.immuni.2019.10.002
Watanabe, Y., Nakayama, T., Nagakubo, D., Hieshima, K., Jin, Z., Katou, F., et al. (2006). Dopamine Selectively Induces Migration and Homing of Naive CD8+ T Cells via Dopamine Receptor D3. J. Immunol. 176, 848–856. doi: 10.4049/jimmunol.176.2.848
Wattananit, S., Tornero, D., Graubardt, N., Memanishvili, T., Monni, E., Tatarishvili, J., et al. (2016). Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 36, 4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016
Weber, M. D., Mckim, D. B., Niraula, A., Witcher, K. G., Yin, W., Sobol, C. G., et al. (2019). The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat. Biol. Psychiatry 85, 667–678. doi: 10.1016/j.biopsych.2018.10.009
Weinstein, J. J., Chohan, M. O., Slifstein, M., Kegeles, L. S., Moore, H., Abi-Dargham, A. (2017). Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 81, 31–42. doi: 10.1016/j.biopsych.2016.03.2104
Winter, C., Djodari-Irani, A., Sohr, R., Morgenstern, R., Feldon, J., Juckel, G., et al. (2009). Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 12, 513–524. doi: 10.1017/S1461145708009206
Wohleb, E. S., Mckim, D. B., Shea, D. T., Powell, N. D., Tarr, A. J., Sheridan, J. F., et al. (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981. doi: 10.1016/j.biopsych.2013.11.029
Wolf, S. A., Steiner, B., Akpinarli, A., Kammertoens, T., Nassenstein, C., Braun, A., et al. (2009). CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 182, 3979–3984. doi: 10.4049/jimmunol.0801218
Xiang, Y. Q., Zheng, W., Wang, S. B., Yang, X. H., Cai, D. B., Ng, C. H., et al. (2017). Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. Eur. Neuropsychopharmacol. 27, 8–18. doi: 10.1016/j.euroneuro.2016.11.012
Xie, X., Luo, X., Liu, N., Li, X., Lou, F., Zheng, Y., et al. (2017). Monocytes, microglia, and CD200-CD200R1 signaling are essential in the transmission of inflammation from the periphery to the central nervous system. J. Neurochem. 141, 222–235. doi: 10.1111/jnc.13972
Yamamoto, S., Ohta, N., Matsumoto, A., Horiguchi, Y., Koide, M., Fujino, Y. (2016). Haloperidol Suppresses NF-kappaB to Inhibit Lipopolysaccharide-Induced Pro-Inflammatory Response in RAW 264 Cells. Med. Sci. Monit. 22, 367–372. doi: 10.12659/MSM.895739
Yan, Y., Jiang, W., Liu, L., Wang, X., Ding, C., Tian, Z., et al. (2015). Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. doi: 10.1016/j.cell.2014.11.047
Ziv, Y., Ron, N., Butovsky, O., Landa, G., Sudai, E., Greenberg, N., et al. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275. doi: 10.1038/nn1629
Keywords: schizophrenia, dopamine receptors, T cells, microglia, peripheral monocytes, neuroimmunology, behavior
Citation: Vidal PM and Pacheco R (2020) The Cross-Talk Between the Dopaminergic and the Immune System Involved in Schizophrenia. Front. Pharmacol. 11:394. doi: 10.3389/fphar.2020.00394
Received: 29 October 2019; Accepted: 16 March 2020;
Published: 31 March 2020.
Edited by:
Jean-Claude Martel, Pierre Fabre, FranceReviewed by:
Dietmar Fuchs, Innsbruck Medical University, AustriaBelinda Lennox, University of Oxford, United Kingdom
Marie-Eve Tremblay, Laval University, Canada
Copyright © 2020 Vidal and Pacheco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo Pacheco, cnBhY2hlY29AY2llbmNpYXZpZGEub3Jn
 Pia M. Vidal
Pia M. Vidal Rodrigo Pacheco
Rodrigo Pacheco