- 1Cancer Research Center, School of Medicine, Xiamen University, Xiamen, China
- 2Department of Traditional Chinese Medicine, School of Medicine, Xiamen University, Xiamen, China
- 3College of Pharmacy, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
Background: Chronic stress has been known to impair the female reproductive function, but the mechanism remains to be further investigated. Chaiyu-Dixian Formula (CYDXF) has been reported to regulate human endocrine disorders clinically. However, whether this formula can affect chronic stress-induced ovarian follicular development is not clear.
Aim of the study: To examine effects of CYDXF on follicular development and explore possible mech anisms in a chronic unpredictable mild stress (CUMS) model.
Materials and Methods: Adult female rats were randomly divided into 5 groups control group, CUMS group (saline treatment), CUMS+Estradiol (E2) (0.1 mg/kg) group, CUMS+CYDXF (2.73 g/kg) group, and CUMS+CYDXF (5.46 g/kg) group. Body weights and behavioral tests were documented. Serum hormone levels were determined by enzyme-linked immunosorbent assay (ELISA). Western blotting was used to detect the protein levels in the PI3K/Akt pathway and brain-derived neurotrophic factor (BDNF). The follicles were analyzed and classified according to their morphological characterization.
Results: CYDXF relieved depression-like behaviors and ameliorated the abnormality in rat estrous cycle within the rat model of CUMS. Moreover, CYDXF could regulate endocrine disorders, increase the proportion of antral follicles as well as decrease the proportion of follicular atresia, which suggested that CYDXF could alleviate abnormal follicular development and improve overall ovarian function. Furthermore, CYDXF also activated the BDNF-mediated PI3K/Akt signaling pathway.
Conclusions: CYDXF (at dose of both 2.73 and 5.46 g/kg) attenuated chronic stress-induced abnormal ovarian follicular development by relieving depression-like behaviors and improving ovarian function through partly the regulation of the BDNF-mediated PI3K/Akt pathway.
Introduction
Chronic stress can affect the body's physiological and mental health, including its metabolic and immune systems. It is one of the risk factors for anxiety, depression, cardiovascular disease, and other diseases (McEwen, 2008; Karisetty et al., 2017). Due to anatomical and physiological structures, women in the reproductive age are more susceptible than men to developing stress-related psychiatric disorders such as depression and anxiety. The prevalence of psychiatric disorders in women is approximately 2 to 3 times higher than in men (Westenbroek et al., 2004; Ter Horst et al., 2009). Some important relationships between sex hormones and depression have been observed (Peltier et al., 2019). Altered reproductive hormone levels have been associated with the pathophysiology of depressive disorders (Smeeth et al., 2019). Moreover, chronic stress has certain impacts on female reproductive functions, which can lead to disorders of ovulation and infertility (Zangeneh and Shooshtary, 2009). For example, repeated restrained stress (RRS) can damage oocyte development (Gao et al., 2016); heat stress can reduce estradiol levels produced by cumulus-oocyte complexes (COCs) (Paes et al., 2016); chronic cold stress may change follicular development, leading to a reduction of antral follicles via an intraovarian sympathetic activation (Dorfman et al., 2003); chronic unpredictable mild stress (CUMS) not only inhibits the development of secondary follicles and antral follicles in mice, but also increases the atresia of follicles (Wu et al., 2012a).
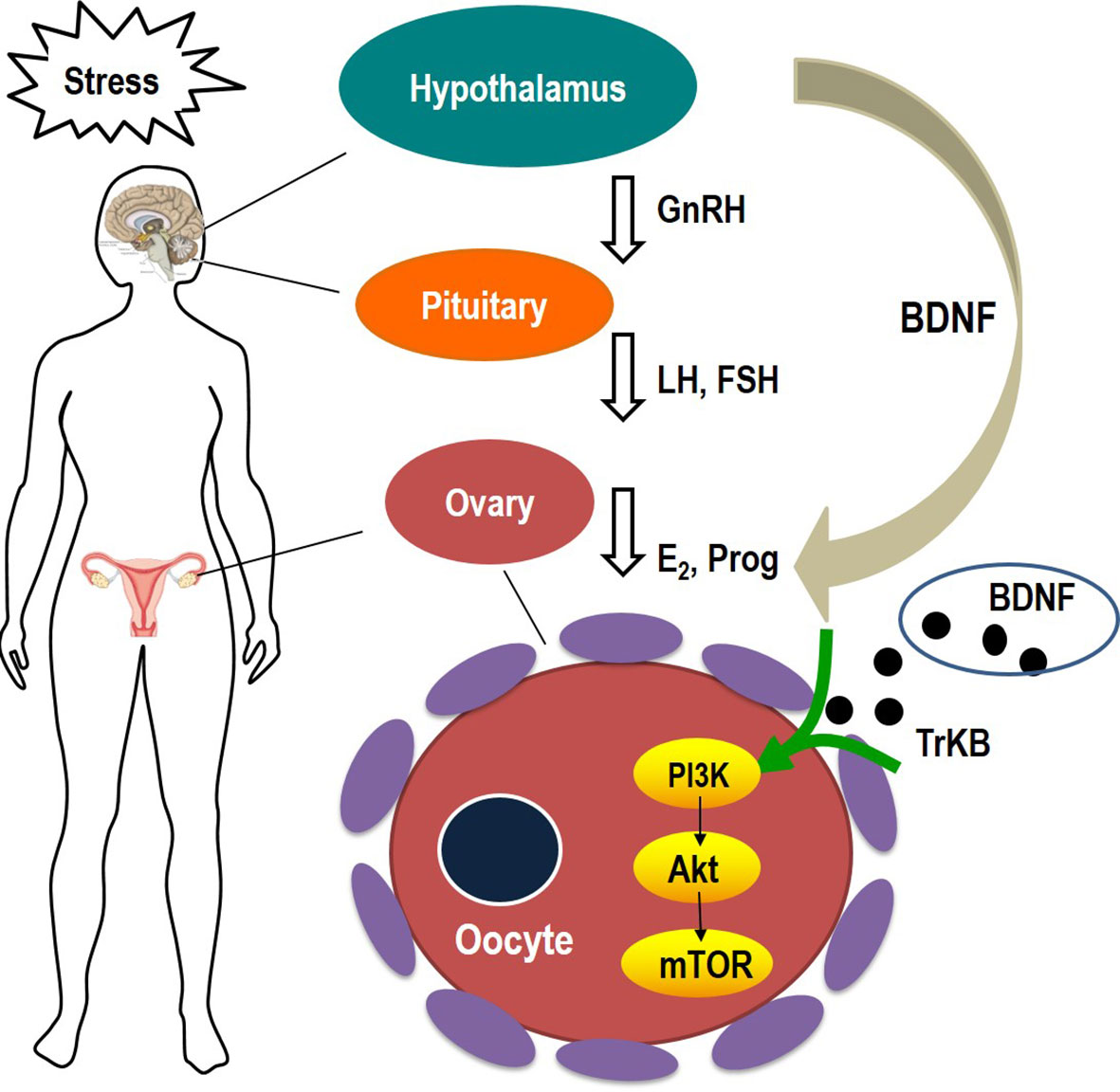
Follicles are the basic units of the ovary. They can produce eggs as well as secrete steroid hormones. In mammalian ovaries, primordial follicles develop during the fetuses (such as primates) or develop early in newborns (such as rodents) (Hirshfield, 1991; Fortune, 1994). The follicles in the primordial follicle pool undergo a series of recruitment, selection, and dominance, eventually resulting in ovulation or atresia. Therefore, maintaining the growth and development of follicles plays an important role in women's reproduction. This process is not only affected by different hormones and growth factors through endocrine, autocrine, and paracrine patterns, but also regulated by a variety of signaling pathways (Wu et al., 2012b; Fernandois et al., 2016; Hayes et al., 2016; Wen et al., 2018). The brain-derived neurotrophic factor (BDNF) is one of these factors. Although BDNF is primarily produced by the brain and has an influence on the growth and survival of neurons, it may be associated with depression (Rasmussen et al., 2009; Larsen et al., 2010). Moreover, BDNF is also expressed in the ovaries (Bodis et al., 2014). After binding to its specific receptor, BDNF activates its downstream phosphoinositide-3 kinase-protein kinase B-mammalian target of rapamycin (PI3K-AKT-mTOR) signaling pathway (Martinowich and Lu, 2008). The PI3K-AKT-mTOR signaling pathway plays a crucial role in cell proliferation, differentiation and apoptosis (Chen et al., 2016), and is involved in the activation of primordial follicles and the meiosis of oocytes (Zheng et al., 2012). Moreover, the BDNF-activated PI3K-AKT-mTOR signaling pathway is reported to be essential in female reproductive diseases such as polycystic and premature ovarian failure, which may be induced by chronic unpredictable mild stress (Divyashree and Yajurvedi, 2016; Sun et al., 2017; Fu et al., 2018).
In recent years, the potential value of traditional Chinese medicine (TCM) has been recognized because TCM exerts profound activity in many diseases and has relatively few side effects (Efferth et al., 2007; Sun et al., 2017). Chaiyu-Dixian Formula (CYDXF) is one of the traditional Chinese empirical formulas, which consists of Radix Bupleuri, Radix Curcumae, Radix Rehmanniae Praeparata, Epimedium Herb, Rhizoma Pinellinae Praeparata, Poria, Radix Codonopsis, Radix Salviae Miltiorrhizae, and Glycyrrhizae. Previous studies showed that CYDXF could regulate many disorders of the endocrine system and had an antidepressant effect (Li and Zhu, 2016; Zhou et al., 2017). Pharmacological studies have shown that major bioactive substances isolated from CYDXF possess activities against gynaecological disorders and can also treat depressive disorders (Papoutsi et al., 2006; Yang et al., 2011; Gong et al., 2016; Yan et al., 2018; Liu et al., 2019). However, the role of CYDXF in chronic stress-induced follicular abnormal development has not been examined.
Our previous study found that the hippocampal BDNF expression was significantly decreased in chronic stress-induced depressive rats (Zhou et al., 2018). However, the effects of ovarian BDNF in chronic stress-induced rats still needs further investigation. At the same time, whether the PI3K-AKT-mTOR signaling pathway is involved in chronic stress-induced follicular development in ovaries needs to be further elucidated. Therefore, this study aims to explore the biological activities and mechanism of CYDXF in a rat model of chronic stress-induced ovarian follicular development.
Materials and Methods
Experimental Animals
Thirty-five adult female Sprague-Dawley rats, weighing 190-200 g and aged 7-8 weeks, were purchased from the SLAC Laboratory Animal Co. Ltd in Shanghai, China [License No. SCXK (Hu) 2016-0003]. Animals were allowed to adapt for seven days before the experimental procedures, and rats with an abnormal estrous cycle were discarded. The remaining rats were housed in polypropylene cages under controlled conditions at 22-24 °C and photoperiod of lights on 07:00 a.m.-19:00 p.m. with free access to food and tap water unless food or water deprivation were needed. They were randomly assigned to 5 groups: control group, CUMS group (0.9% normal saline, 10 mg/kg), CUMS+Estradiol (0.0001 g/kg) group, CUMS+CYDXF (2.73 g/kg) group, and CUMS+CYDXF (5.46 g/kg) group. The control animals were housed separately and had no contact with the stressed groups. Stressed and control animals were kept in separate rooms of the Animal Center of Xiamen University [No. SYXK (Min) 2018-0009]. The body weight of rats was recorded once every week. All protocols in this animal experiment were conducted in strict accordance with the animal care and use guidelines of the Ministry of Science and Technology of the People's Republic of China, and the study was approved by the Laboratory Animal Administration and Ethics Committee of Xiamen University.
Stress Model
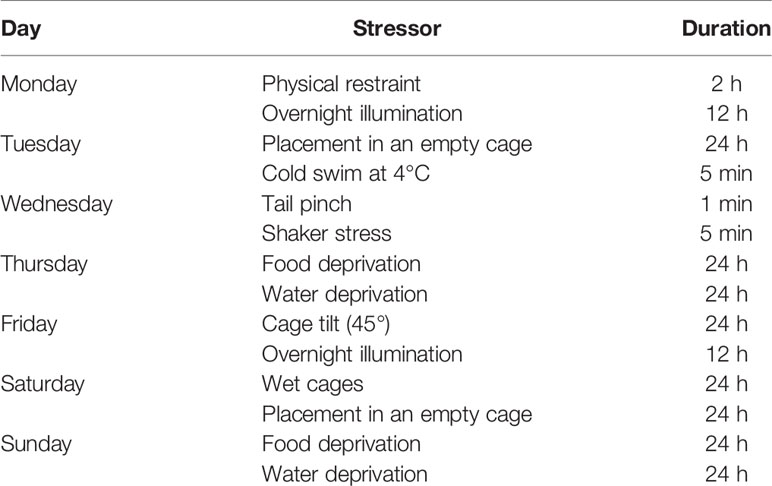
The stressed groups were individually housed and received a 28-day stress procedure according to previous studies, with minor modifications (Willner, 1997). They were administered every day between 9:00 a.m. and 11:00 a.m., excluding the 24 h duration stressors. Stress procedures included: (1) 24-h food deprivation, (2) 24-h water deprivation, (3) 24-h cage tilt (45 degrees from the horizontal), (4) 24-h wet cages, (5) overnight illumination, (6) 2-h physical restraint, (7) 5-min cold swim at 4°C, after which they were toweled dry, (8) 5-min shaker stress, (9) 24-h placement in an empty cage and (10) 1-min tail pinch (1 cm from the end of the tail). Habituation can develop when animals are repeatedly exposed to a predictable stressor—such as the chronic restraint stress (CRS) model. Thus, all stressors were randomly scheduled over a 1-week period and repeated throughout the 4-week experiment (Table 1) to ensure the unpredictability of the stressors.
Experimental Drugs
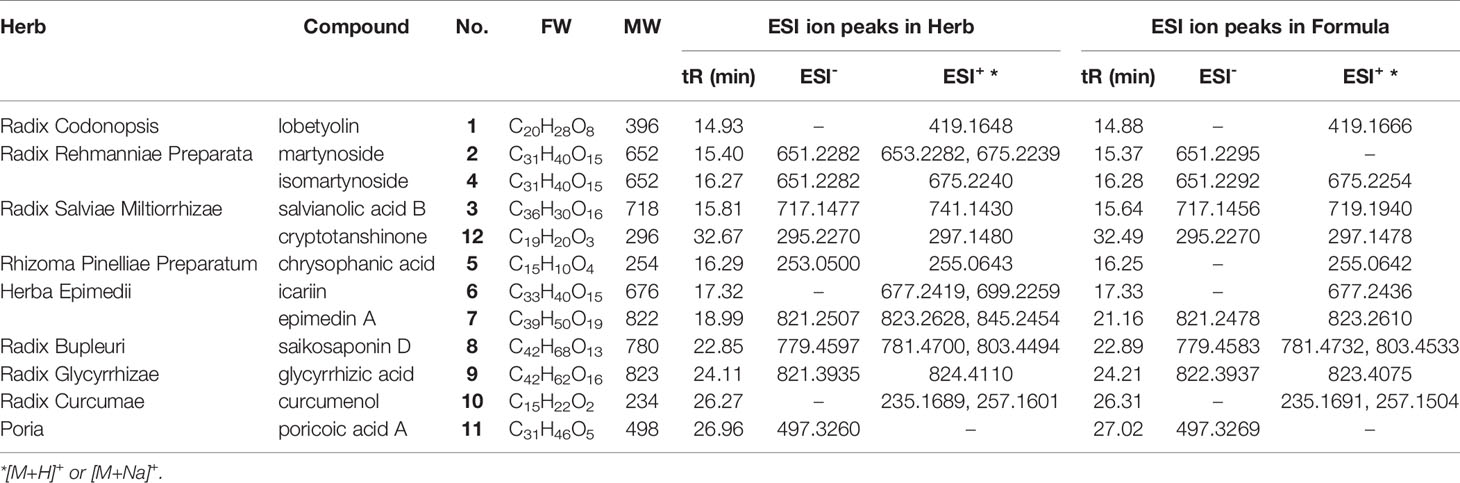
CYDXF consists of Chai Hu (Radix Bupleuri) (8.85%), Yu Jin (Radix Curcumae) (8.85%), Shu Di Huang (Radix Rehmanniae Praeparata) (13.27%), Xian Ling Pi (Epimedium Herb) (13.27%), Fa Ban Xia (Rhizoma Pinellinae Praeparata) (10.62%), Fu Ling (Poria) (13.27%), Dang Shen (Radix Codonopsis) (13.27%), Dan Shen (Radix Salviae Miltiorrhizae) (13.27%) and Gan Cao (Glycyrrhizae) (5.31%) (Table 2). The major bioactive components in CYDXF are saikosaponin D (Li et al., 2017), curcumin (Bamba et al., 2011), iridoids glycosides (Liu et al., 2012), icariin (Xue et al., 2016), pinellic acid (Nagai et al., 2002), the triterpene fraction and the polysaccharide fraction (Rios, 2011), polysaccharides and saponins (Li et al., 2009), tanshinone and cryptotanshinone (Chang et al., 1990) as well as liquiritigenin (Kim et al., 2004). Each herb in CYDXF was purchased from the Xiamen Yanlaifu Pharmaceutical Co., Ltd. (Xiamen, China) and identified by Professor Yingkun Qiu (School of Pharmaceutical Sciences of Xiamen University, China). All voucher specimens were deposited at the Xiamen Botanical Garden (Herbarium Code: XMBG) for future reference. Estradiol valerate (E2) was produced by Bayer Healthcare Co., Ltd. Guangzhou Branch, with a product lot number of J20171038.
Main Reagents and Kits
In the study, the following materials of rabbit polyclonal anti-BDNF antibody were purchased from Proteintech Group, Inc. (Chicago, USA): product lot No. Cat#28205-1-AP, rabbit polyclonal anti-PI3K p110 (beat) antibody, product lot No. Cat#20584-1-AP, rabbit polyclonal anti-AKT antibody, product lot No. Cat#10176-2-AP, and rabbit polyclonal anti-mTOR antibody, product lot No. Cat#20657-1-AP. Rabbit polyclonal anti-p-AKT antibody, product lot No. 4060S and rabbit polyclonal anti-p-mTOR antibody, product lot No. 5536S, were purchased from Cell Signaling Technology, Inc. (Danvers, USA). MLGR-E30267 Rat Estradiol (E2) ELISA Kit, product lot No.ml002871, MLGR-E30274 Rat Follicle-stimulating hormone (FSH) ELISA Kit, product lot No.ml002872, MLGR-E30447 Rat Luteinizing Hormone (LH) ELISA Kit, product lot No.ml002860, and MLGR-E31019 Rat Progesterone (Prog) ELISA Kit, product lot No.ml002894, were produced by Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China).
Medicinal Preparation and Administration
Based on the clinical dosage, CYDXF contains 113g crude herbs, which is equivalent to a dosage of 11.6 g (dried medicinal herb)/kg for rats, according to the formula that converts equivalent drug dosages based on body surface areas from human to rats [Human equivalent dose (HED) = Animal dose (mg/kg) multiplied by (Km factor of a 60 kg adult is 37 and Km factor of a 0.15 kg rat is 6) ] (Reagan-Shaw et al., 2008). To prepare this formula, a total of 113g of mixed CYDXF crude herbs were immersed in 1130 mL of distilled water for 30 min and boiled for 1 h to yield a final volume of 150 mL. The decoction was filtered with 8 layers of surgical gauze. Herb residues were again soaked in 600 mL water, boiled for 1 h, and filtered again. Both filtered decoctions were combined and concentrated with rotary evaporation (Shanghai Yarong Biochemistry Instrument Factory, Shanghai, China) at 580C until a final volume of 90 mL was reached, then lyophilized with freeze dryer (Beijing Songyuan Huaxing Technology Development Co., Ltd., Beijing, China) to get the extract. The weight of the final freeze-dried powder of each dose of CYDXF (113g) was 26.58g. The extraction yield was 23.52% and the concentration of the final filtered decoction was 0.30 g/mL. The lyophilized powder was stored at 4°C until use. The dosages of 2.73 and 5.46 g /kg [equal to 11.6 and 23.2 g (dried medicinal herb)/kg, respectively] of Chaiyu-Dixian Formula (CYDXF) were chosen, which were equivalent to 10 and 20 times of the clinical recommended human daily dose, respectively. The powder was reconstituted with distilled water to 0.27 g/mL, 0.55 g/mL respectively. The model group received normal saline (0.9%, 10 mL/kg). E2 was dissolved in distilled water to a concentration of 0.01 mg/mL. All medicines were administered to rats by gavage in a volume of 10 mL/kg once per day.
Estrous Cycle Determination
The vaginal smears were performed using methylene blue staining at 11:00 a.m. for ten consecutive days, beginning with the 18th day of the CUMS procedure, and were observed under a light microscope (Marcondes et al., 2002). Briefly, there were three types of exfoliated cells that could be observed: the oval nuclear epithelial cells (NE), the irregular-shaped anucleated epithelial keratinocyte cells (EK), and the little round leukocyte cells (L). The four phases of the estrous cycle were determined by the proportion among them. In proestrus, nuclear epithelial cells were mainly observed, occasionally with a few of epithelial keratinocyte cells; in estrus, the vaginal smear primarily consists of cornified cells with no nucleus and a small number of nucleated epithelial cells. When the smear consisted of the same amount of leukocyte, cornified and nucleated epithelial cells, it was considered a metestrus phase; in diestrus, a predominance of leukocytes and a small portion of nuclear epithelial cells were detected.
Behavioral Tests
Animals were placed in the testing room and acclimated for 0.5 h before the test. All tests were conducted between 7:30 a.m. and 11:30 a.m. in a dim and quiet room. After being tested, rats were returned to the holding rooms.
Open Field Test (OFT): The open field test was aimed to evaluate the rodents' spontaneous spatial exploration activity (Jindal et al., 2013). Briefly, the apparatus consisted of a square arena (100 cm × 100 cm × 50 cm) with a black floor and a four-sided black wall. Each rat was placed gently on the center of the area and were allowed to explore freely for 5 min during the test. The activity of rodents, including central and peripheral activity, total movement distance, and times of rearing and grooming behaviors, were recorded by a video camera connected to a computer 1.5 m above the center of the floor. After each test, the apparatus was cleaned with a solution of 75% ethanol and was dried.
Forced Swim Test (FST): The FST was performed to assess the depression-like behavior of rats (Walf et al., 2009). Rats were individually placed in a transparent plexiglass cylindrical container (30 cm in diameter, 80 cm in height) with water at 25°C and filled to 50 cm of depth and videotaped for 5 min. In this container, rats cannot reach the bottom or escape. After the test, total immobility time was analyzed by two independent observers to avoid bias, and average time was recorded. Contrary to activity (swimming or diving behavior), immobility was defined as passive, floating-like behavior lasting longer than 1 second. After each test session, the water was changed and containers were cleaned thoroughly in order to eliminate odor. Rats were dried and returned to their home cages.
Sucrose Preference Test (SPT): The sucrose preference test was used as an index of stress-induced anhedonia in rodents (Patki et al., 2013). Briefly, rats were kept separately in cages and trained to consume from two identical bottles. On the first day of SPT, two bottles filled with 100 mL of 1% sucrose solution were given to rats. On the 2nd day, one bottle was filled with 100 mL of 1% sucrose solution and the other 100 mL of distilled water. In the meantime, the bottles were interchanged after 12 h. On the third day, rats were deprived of food and water. The test was conducted on the fourth day. Two bottles of water and sucrose were given to rats for 24 h, and the placement of them was interchanged after 12 h. The weight of the bottles was measured before and after the test. The sucrose preference was calculated by the following formula: Sucrose preference = sucrose consumption/ [total consumption (sucrose + water)]×100%.
Elevated Plus Maze (EPM): The elevated plus maze was to assess anxiety-like behavior in rats (Aldridge et al., 2005). The apparatus was 50 cm above the floor and consisted of 4 arms (45 cm long, 10 cm wide), of which two were open and two were closed. Four arms extended from a central platform (10 cm× 10 cm) shaping like a plus sign. Each rat was placed gently in the central platform facing the open arms to crawl freely for 5 min. Percentage of time spent in open and closed arms, number of entries into open arms (with all four paws), along with the head dipping and rearing of each rat were recorded. After each trial, the apparatus was wiped down with 75% ethanol.
Biochemical Analysis
Biochemical analysis was conducted after all behavioral tests were finished. Rats were anesthetized and sacrificed on the 30th day. Serum hormone levels and ovarian protein expression were assayed. The ovarian morphological and histological characteristics were observed.
Sample Collection
Peripheral blood was collected and its serum was obtained by centrifugation of blood at a speed of 3000 r/min for 15 min. After that, the supernatant was separated and stored at -80°C until use. For morphological evaluation, the right ovaries were removed and fixed in 4% paraformaldehyde at -20°C for at least 24 h, then dehydrated, embedded in paraffin, serially sectioned (with a thickness of 3 μm), and stained by hematoxylin-eosin. For protein level measurement, the left ovaries were quickly removed, dipped in liquid nitrogen, and stored at -80 °C.
ELISA Analysis
We used the enzyme-linked immunosorbent assay to detect the hormone levels in serum. The contents of follicle-stimulating hormone (FSH), estradiol (E2), luteinizing hormone (LH), and progesterone (Prog) in serum were determined by commercially available ELISA kits, according to the manufacturer's instructions.
Western Blot Analysis
Total protein levels of PI3K, AKT, mTOR, and BDNF were determined by western blotting in the left ovaries. The proteins were extracted by homogenizing the ovaries in radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Nanjing, China) and centrifuged at 12000 rpm for 5 min. The supernatant was obtained and the protein concentration of each sample was assessed using a bicinchoninic acid (BCA) protein assay kit (Beyotime, Nanjing, China). The samples containing equal amounts of total proteins were loaded in each track and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently electro-transferred to polyvinylidene fluoride (PVDF) membranes. After treating with blocking solution (Tris-buffered saline Tween containing 5% skim dry milk), the membranes were incubated overnight at 4 °C with specific primary antibodies. After this process, the membranes were rinsed with TBST and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. After washing with TBST three times, the proteins of the bands were detected by the enhanced chemiluminescence (ECL) detection reagent (Beyotime, Nanjing, China). The optical density of protein bands on the membranes was analyzed by the ONE-DScan program system.
Ovarian Histological Evaluation
The right ovarian tissues were excised, rinsed and fixed in 4% paraformaldehyde. After 12 h fixation, the specimens were embedded in paraffin and serially sectioned (3 μm), mounted on glass slides, and stained with hematoxylin and eosin (HE). The presence of the primordial, primary, secondary, antral, and atretic follicles as well as corpus luteum were analyzed and classified according to their morphological characterization (Osman, 1985; Myers et al., 2004; Yoshida et al., 2009). The follicles were counted in every 10th section of the whole ovaries by two independent researchers.
Briefly, follicle stages were classified under the following definitions. Primordial follicles showed a partial or complete layer of flattened pregranulosa cells surrounding an oocyte. Primary follicles were defined as an oocyte that was encircled by a single layer of columnar or cubic granulosa cells (GCs). Secondary follicles appeared on two or more layers of granulosa cells with no cavity and antral follicles had formed a cavity filled with fluid—the antrum. The antral follicle was counted when nuclei was observed. Follicles were judged as artresia when showing deformation and/or had pyknotic oocyte nuclei, as well as granulosa cells showing shrinkage or fragmentation. Corpus luteum contained luteal cells which had eosinophilic cytoplasm and basophilic nuclei.
UHPLC/ESI-MS Analysis
The major chemical components of CYDXF decoction were examined using ultra-high performance liquid chromatography (UHPLC) equipped with an electrospray ionization mass (ESI-MS) detector. Analysis on HPLC was performed on a Cosmosil 5C18-MS-II column (250 × 4.6 mm i.d., 5 μm, Nakalai Tesque Co. Ltd., Kyoto Japan) with mobile phase consisting of water (A) and acetonitrile (B) in a gradient program: A from 95% to 30% and B from 5% to 70% during 0-35 min, A from 30% to 0% and B from 70% to 100% during 30-31 min. Then A were kept 0% at 31.1-40 min, and lastly, A and B ran back to 95% A and 5% B when 40.1 min and kept there during 40.1-45 min, at a flow rate of 1 mL/min. The column was maintained at room temperature and the injected volume was 5 μL. The mass spectrometer was equipped with a high resolution ESI detector and were used to record the HPLC chromatograms. The MS spectra were recorded on a Thermo Q-Exactive system. The positive and negative electrospray voltages were 3.5 Kv and 2.5 Kv, the capillary temperature was set at 300 °C, and the mass range was 100-1000.
Statistical Analysis
All results were presented as mean ± standard mean of error (SEM) (± sem). Data were analyzed by SPSS 21.0. Normal distribution and homogeneity test of variance between groups were performed. One-way analyses of variance (One-Way ANOVA) was used to compare the means of multiple groups. The least significant difference (LSD) method was selected for post hoc analysis. If there was one inconsistency, the Kruskal-Wallis H test of multiple independent samples in nonparametric statistics was used for statistical processing. GraphPad Prism Version 7 software (GraphPad Software, USA) was used for drawing statistical graphs. A value of P less than 0.05 was considered statistically significant.
Results
Analysis of CYDXF
As shown in Figure 1, CYDXF decoction was separated with a UHPLC system and its chromatographic fingerprinting was established (Figure 1). Twelve compounds (See Table 3) with different retention times were identified as lobetyolin (peak 1), martynoside (peak 2), salvianolic acid B (peak 3), isomartynoside (peak 4), chrysophanic acid (peak 5), icariin (peak 6), epimedin A (peak 7), saikosaponin D (peak 8), glycyrrhizic acid (peak 9), curcumenol (peak 10), poricoic acid A (peak 11), and cryptotanshinone (peak 12) respectively.
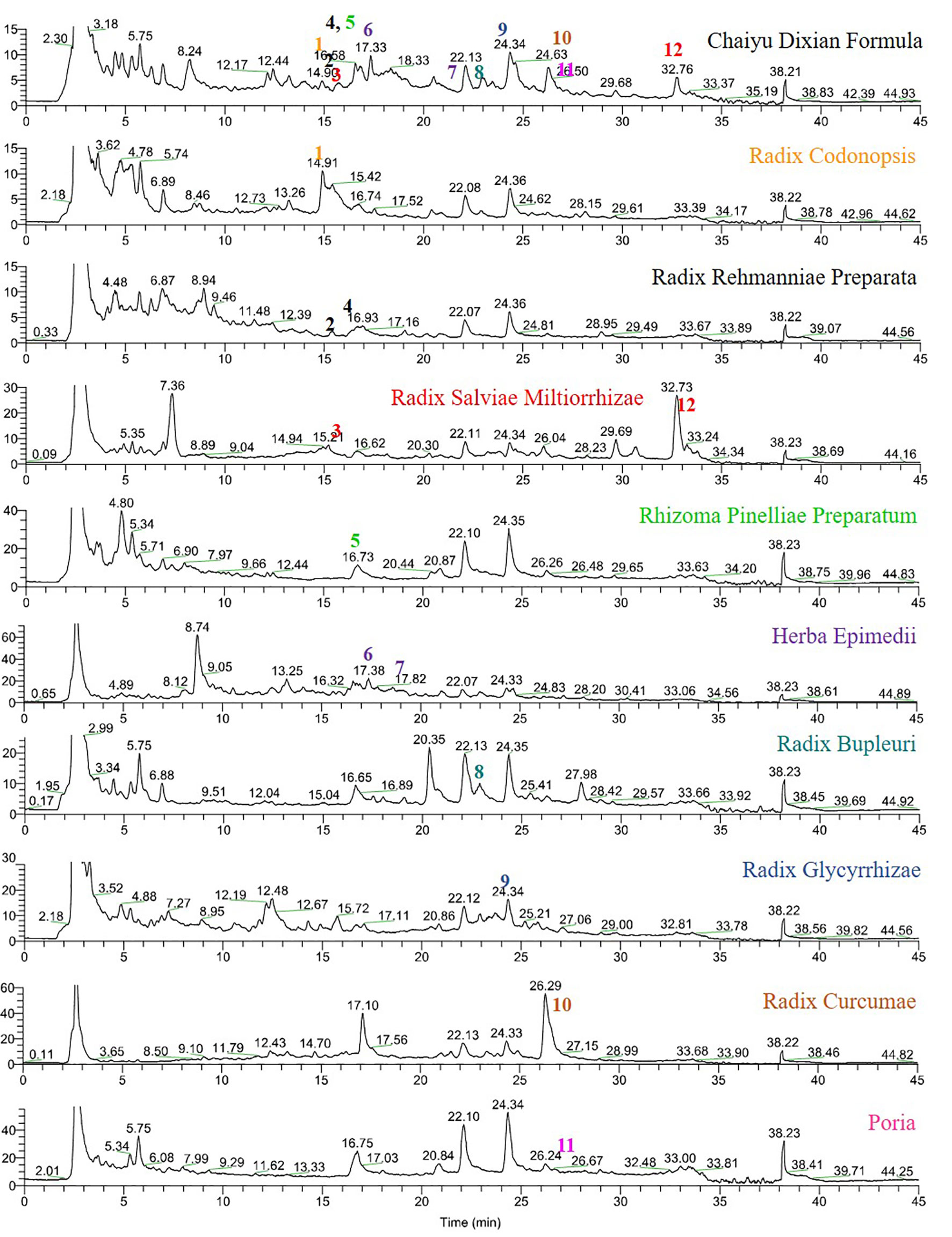
Figure 1 UHPLC-MS fingerprint chromatogram of CYDXF and each herb decoction. Lobetyolin (1), martynoside (2), salvianolic acid B (3), isomartynoside (4), chrysophanic acid (5), icariin (6), epimedin A (7), saikosaponin D (8), glycyrrhizic acid (9), curcumenol (10), poricoic acid A (11) and cryptotanshinone (12).
CYDXF Attenuated Depressive-like Behaviors Induced by CUMS
Body Weight
In order to evaluate the effect of CYDXF on the physical condition of CUMS-induced rats, body weight was recorded once a week. As shown in Figure 2A, CUMS significantly caused loss of body weight compared to control (P<0.001), and both CYDXF (2.73 g/kg) and (5.46 g/kg) treatments significantly alleviated loss of body weight. However, estradiol treatment did not alleviate body weight of CUMS rats. Moreover, on the last day of treatment, both CYDXF (2.73 g/kg) and (5.46 g/kg) did not restore body weight back to that of control but significantly improved body weight compared to no treatment (Figure 2B).
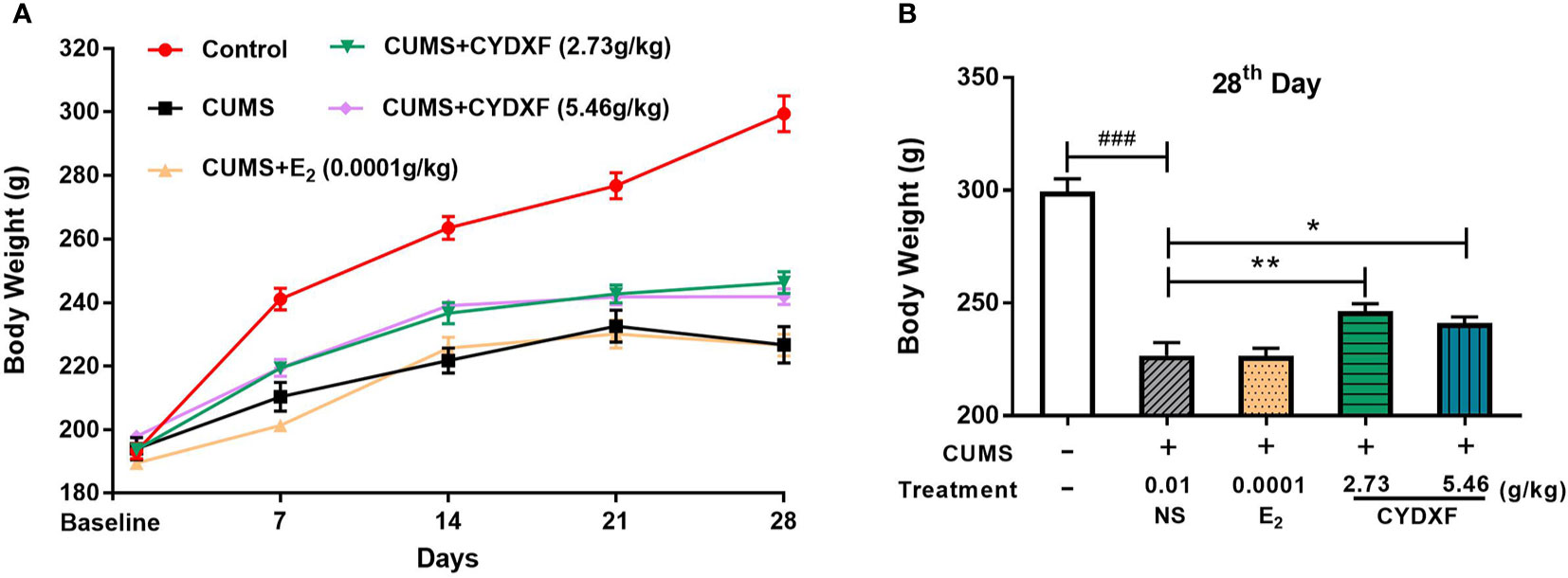
Figure 2 Effect of CYDXF on body weight. (A) Changes in body weight during the modeling period; (B) The body weight on 28th day. Results are presented as mean ± SEM (n=7). ###P<0.001 vs control group; *P<0.05 vs CUMS group; **P<0.01 vs CUMS group.
Behavioral Results
Several behavioral tests were performed on rats to successfully determine the establishment of the chronic unpredictable mild stress model, and used to evaluate the effect of CYDXF on stressed rats. The OFT was used to examine decreased total traveled distance, which is considered a sign of anxiety-like behavior (Frye et al., 2000). Each group had a similar result of total traveled distance in the OFT at baseline on day 0 (Figure 3A, P>0.05), and after modeling for 28 days, the total traveled distance of rats in the CUMS group was significantly decreased compared with the control group (P<0.001), while estradiol (P<0.05) and both CYDXF (P<0.01) treatments could significantly alleviate the reduction of total traveled distance (Figure 3C). Moreover, the SPT was used to evaluate the motivational state in rodents. Decreased sucrose consumption was a sign of anhedonia (Yu et al., 2011). There were no significant differences at baseline on day 0 among each group, (Figure 3B, P>0.05), but differences were obvious after the CUMS procedure for 28 days. The sucrose preference was significantly reduced in the CUMS group as compared to the control group (P<0.05). Rats exposed to CUMS that were treated with estradiol or CYDXF (2.73 g/kg and 5.46 g/kg) had significantly increased sucrose preference which was restored to a level resembled to that of control group (Figure 3D, both P<0.05).
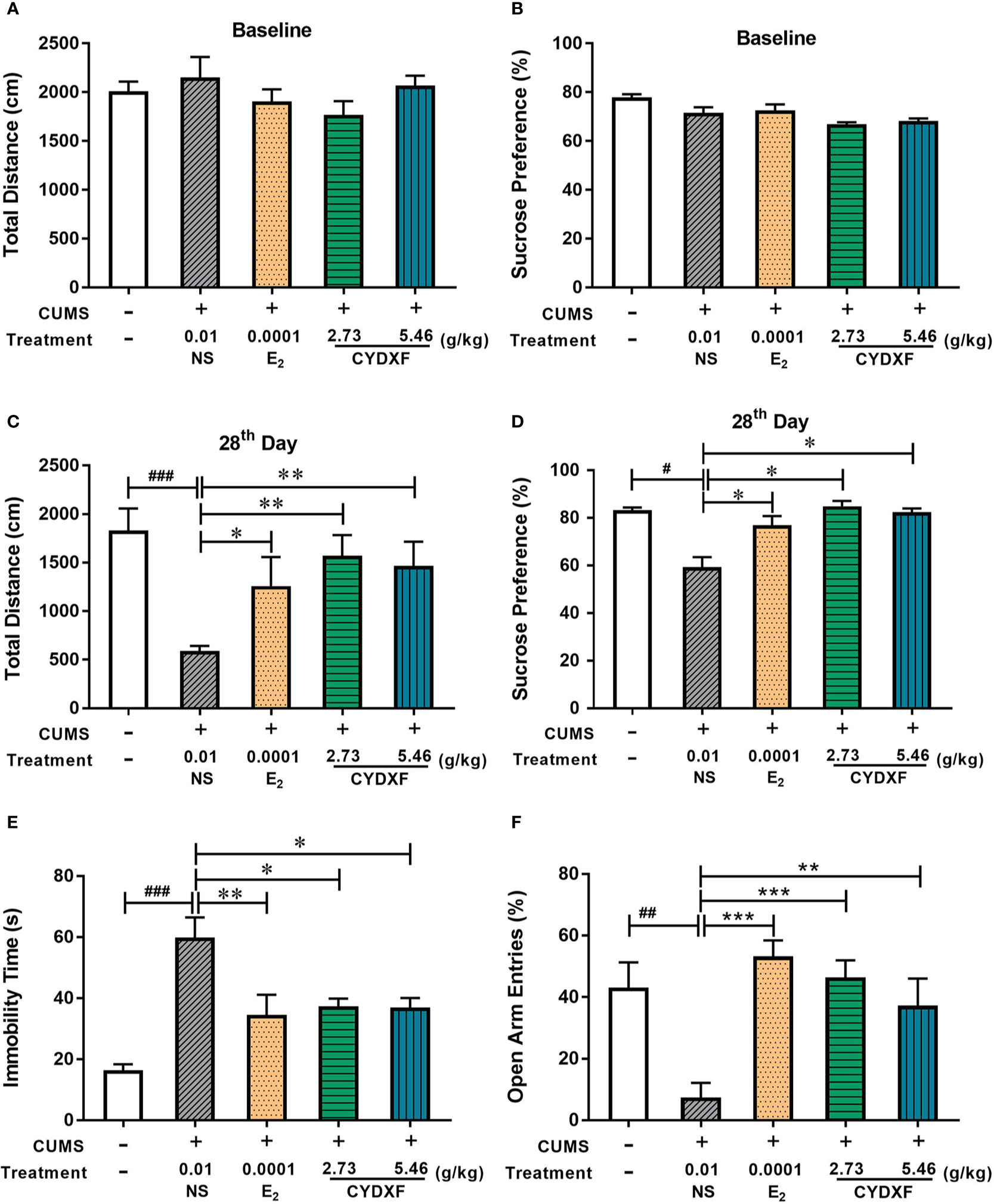
Figure 3 Effect of CYDXF on behavioral tests. (A) OFT results on baseline; (B) SPT results on baseline; (C) The results of total traveled distance on day 28; (D) The results of sucrose preference on day 28; (E) Immobility time spent in FST; (F) Percentage of open arm entries in EPM. Results are presented as mean ± SEM (n=7). #P<0.05 vs control group; ##P<0.01 vs control group; ###P<0.001 vs control group; *P<0.05 vs CUMS group; **P<0.01 vs CUMS group; ***P<0.001 vs CUMS group.
Furthermore, The FST and EPM were performed on the final day of the experiment. As shown in Figure 3E, rats showed less time spent swimming or struggling but more time spent immobile in the CUMS group compared with the control group (P<0.001), indicating that the CUMS caused more depression in rats. However, rats treated with estradiol (P<0.01) or CYDXF (both P<0.05) significantly reduced immobility time. In the EPM (Figure 3F), the percentage of open arm entries in the CUMS rats was significantly lower than the control rats, indicating a high level of anxiety in the CUMS rats. Estradiol- (P<0.001) and CYDXF- (2.73 g/kg and 5.46 g/kg) treated rats showed significant improvement in open arm entries as compared to the CUMS group (P<0.001; P<0.01).
CYDXF Ameliorated the Abnormality of Rat Estrous Cycle Induced by CUMS
The estrous cycle of rats lasts 4-5 days in the control group and is comprised of proestrus, estrus, metestrus, and diestrus, which could be determined according to the cell types through the vaginal smears (Figure 4). Rats in the control group (Figure 5A) showed normal estrous cycles while those in the CUMS group (Figure 5B) experienced irregular estrous cycles. Post-treatments with estradiol (Figure 5C), CYDXF (2.73 g/kg) and (5.46 g/kg) revealed that the irregular estrous cycles induced by CUMS were restored (Figures 5D, E).
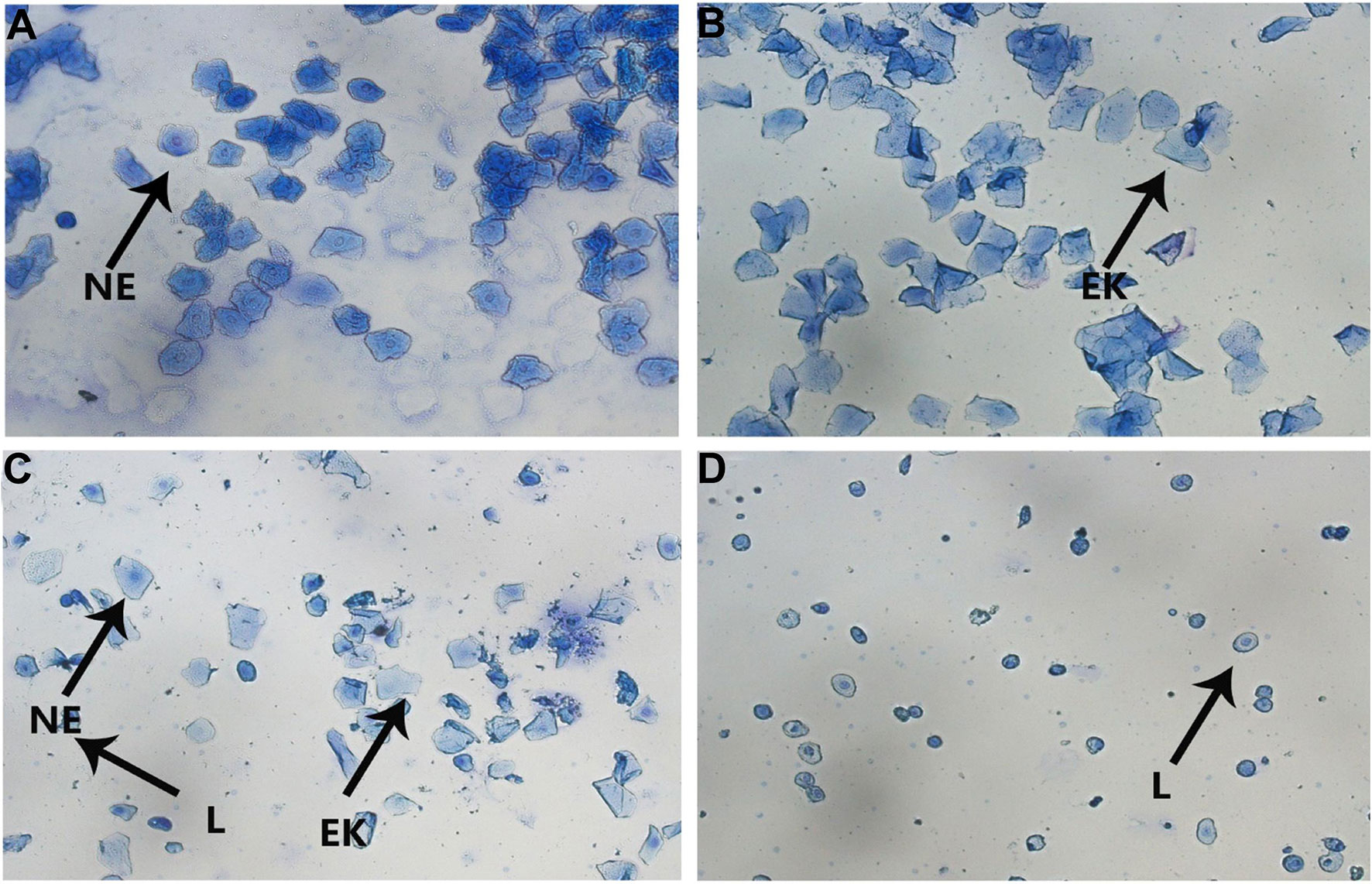
Figure 4 The vaginal smears of rats with different stages of estrous cycle in the control group. (A) Proestrus; (B) Estrus; (C) Metestrus; (D) Diestrus (NE, nuclear epithelial cell; EK, epithelial keratinocyte; L, leukocyte).

Figure 5 Representative images exhibiting the changes of rat estrous cycle for 10 consecutive days. P, proestrus; E, estrus; M, metestrus; D, diestrus. (A) control group; (B) CUMS group; (C) CUMS+Estradiol group; (D) CUMS+CYDXF (2.73 g/kg) group; (E) CUMS+CYDXF (5.46 g/kg) group.
CYDXF Alleviated Rat Endocrine Disorder Induced by CUMS
Daily perceived stress may lead to unknown reproductive disorders (Schliep et al., 2015), which could change the expression of several hormones such as the follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone (Prog), and estradiol (E2) in serum. As shown in Figure 6, rats in the CUMS group showed significantly higher levels of serum FSH and LH (both P<0.001) and significantly lower levels of E2 and Prog (both P<0.001) in comparison to those rats in the control group. Estradiol treatment significantly decreased the serum levels of FSH and LH (P<0.01; P<0.001, Figures 6A, B), and increased the serum levels of E2 and Prog of rats in CUMS treatment (both P<0.001, Figures 6C, D), respectively. Moreover, treatment of CYDXF for rats in CUMS treatment showed significant reversion of these hormones as compared to those of rats in the CUMS group in a dose-dependent manner. Except CYDXF at 2.73 g/kg, which did not significantly alter the level of LH, all other treatments significantly reversed the levels of FSH, LH, E2, and Prog back to the levels of the control group.
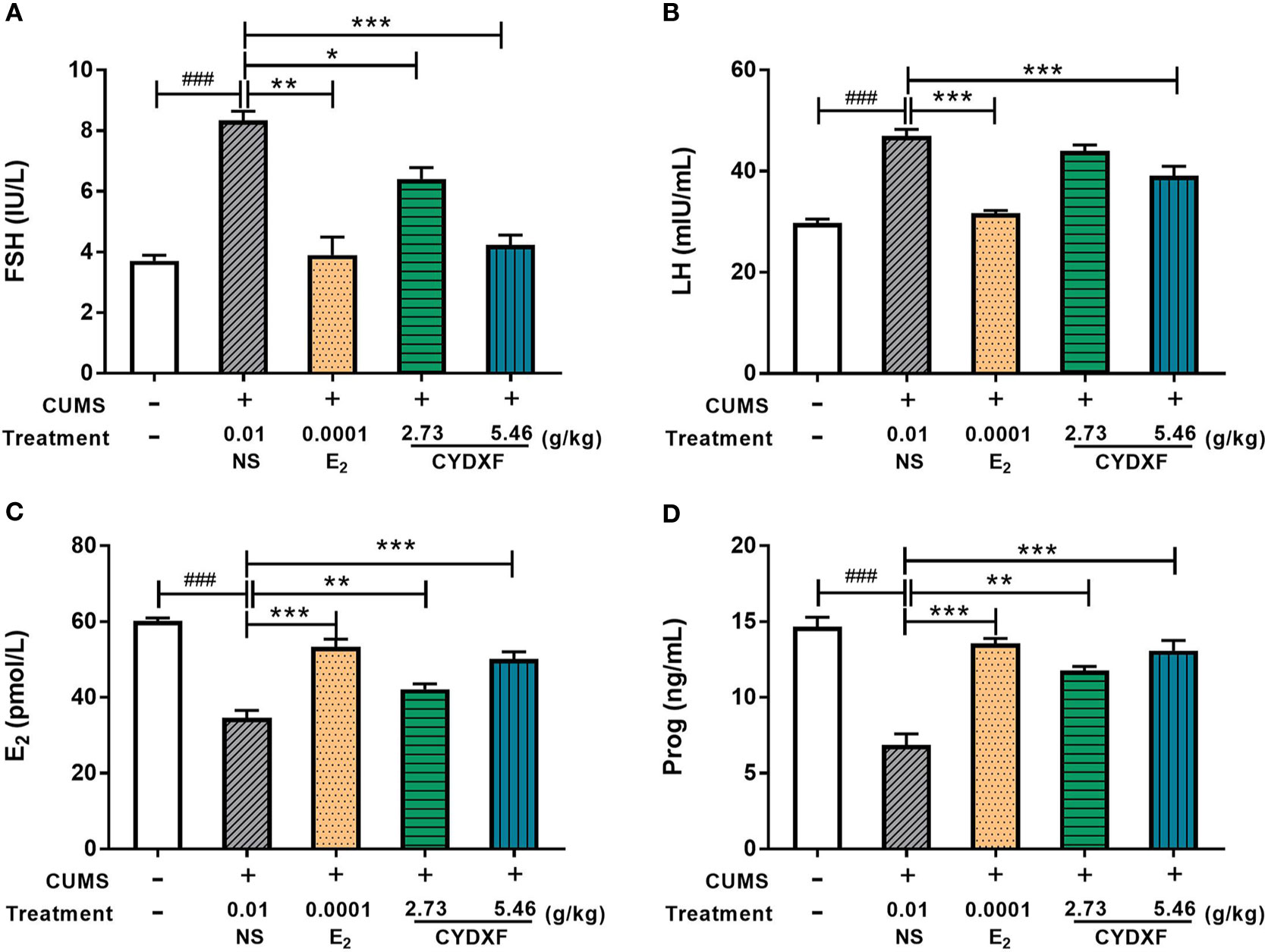
Figure 6 Effect of CYDXF on the concentrations of the hormone levels in serum. (A) FSH concentration; (B) LH concentration; (C) E2 concentration; (D) Prog concentration. Results are presented as mean ± SEM (n=7). ###P<0.001 vs control group; *P<0.05 vs CUMS group; **P<0.01 vs CUMS group; ***P<0.001 vs CUMS group.
CYDXF Alleviated Rat Follicle Development Abnormality
Follicle counts were performed on these rats. As showed in Figures 7 and 8, representative images of different developmental stages of ovarian follicles as well as the percentage of the follicles and corpus luteum are displayed. In the CUMS group, the percentage of antral follicles was sharply decreased (Figure 7D, P<0.01), while the percentage of atresia follicles had significantly increased as compared to the control group (Figure 7E, P<0.001). Treatment with estradiol (P<0.05) and CYDXF (2.73 g/kg and 5.46 g/kg) significantly increased the percentage of antral follicles (P<0.01, P<0.001) (Figure 7D). Moreover, both estradiol and CYDXF (2.73 g/kg and 5.46 g/kg) treatments significantly reduced the percentage of atresia follicles (P<0.001) (Figure 7E). However, there were no significant differences among the percentage of the primordial follicles, the primary follicles, or the secondary follicles, as well as the number of corpus luteum after these treatments (Figures 7A-C, F).
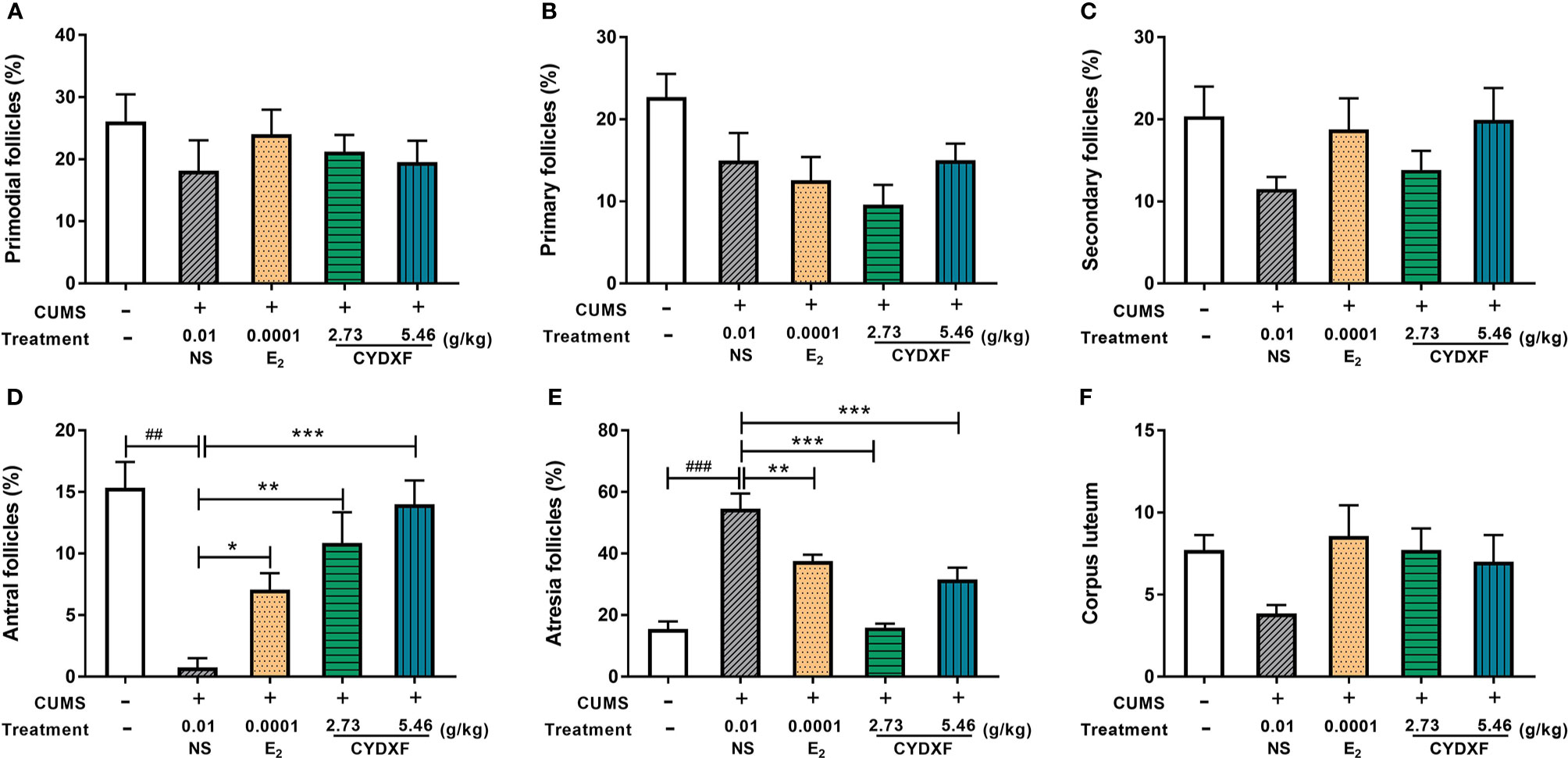
Figure 7 Effect of CYDXF on follicle development. (A) Percentage of primordial follicle number; (B) Percentage of primary follicle number; (C) Percentage of secondary follicle number; (D) Percentage of antral follicle number; (E) Percentage of atresia follicle number; (F) Number of corpus luteum. Results are presented as mean± SEM (n=7). ##P<0.01 vs control group; ###P<0.001 vs control group; *P<0.05 vs CUMS group; **P<0.01 vs CUMS group; ***P<0.001 vs CUMS group.
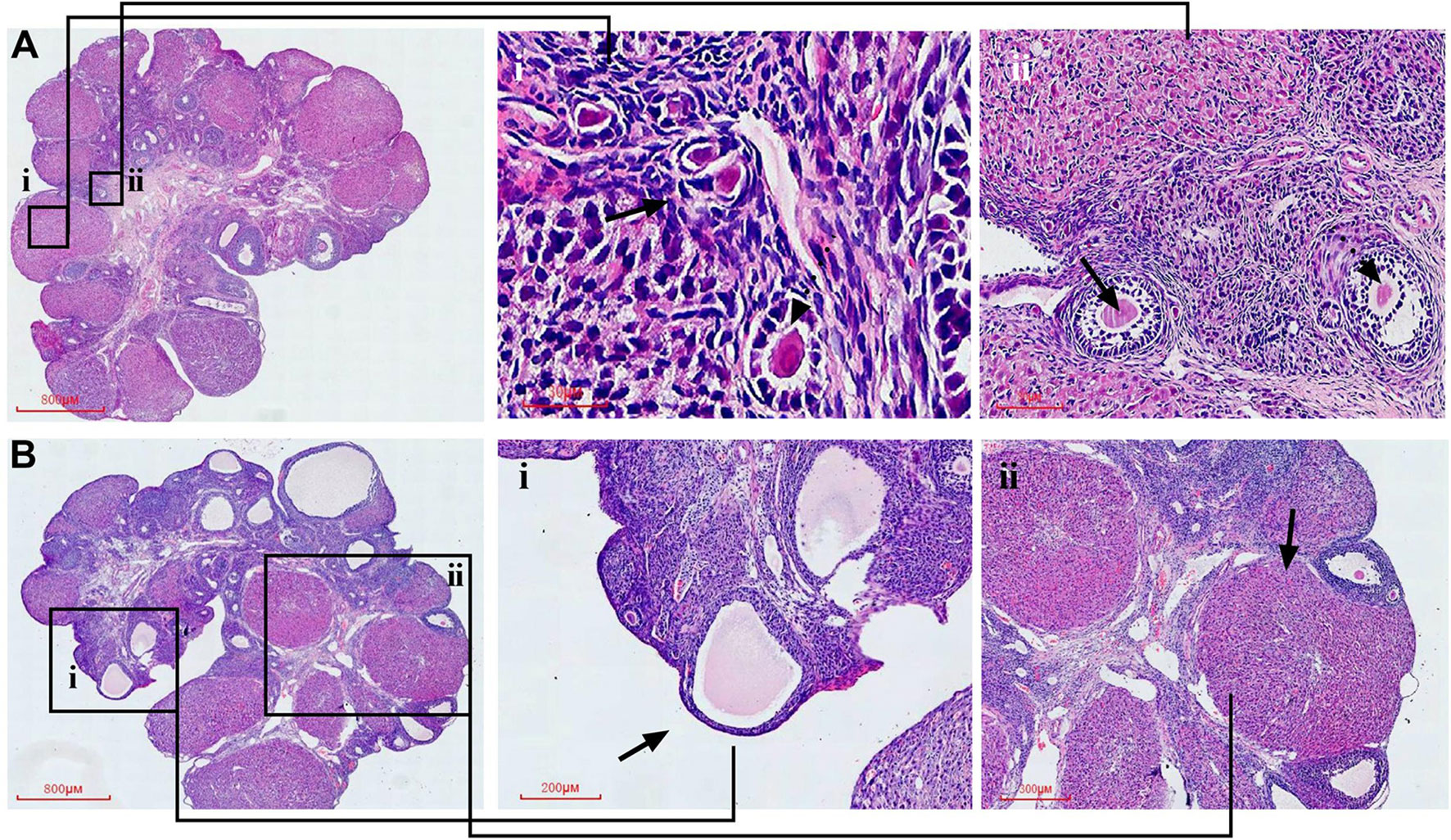
Figure 8 Representative HE staining images of rat ovarian tissues from the control and model group, respectively. Partial enlarged view of the section in (A)-i primordial follicle and primary follicle; (A)-ii secondary follicle and antral follicle; (B)-i atretic follicle; (B)-ii, corpus luteum.
BDNF/PI3K/Akt/mTOR was Involved in CYDXF Treatment of CUMS Rats
In order to examine whether the BDNF-mediated PI3K/AKT signaling pathways were involved in CYDXF-induced ovarian recovery in the CUMS model, the expressions of BDNF/PI3K/Akt/mTOR, as well as the phosphorylation status of Akt and mTOR, were examined. The results indicated that the effects of CYDXF on follicular abnormal development were correlated with the PI3K/AKT signaling pathways activated by BDNF.
As shown in Figure 9, the estradiol and both CYDXF treatments remarkably increased the level of BDNF and PI3K compared with those in CUMS group, respectively (P<0.05). Total Akt and mTOR protein expression were almost the same in each group, whereas the p-Akt and p-mTOR levels were markedly lower in the CUMS group than in the control group, respectively (P<0.05, P<0.01). Compared with those in the CUMS group, estradiol and CYDXF (5.46 g/kg) treatments significantly increased the protein level of p-Akt (P<0.01). Meanwhile, as compared to the model group, the relative level of p-mTOR protein was significantly elevated in the estradiol-treated group and CYDXF-treated group at a dosage of 2.73 g/kg, respectively (P<0.05). Together, the above results revealed that CYDXF could affect the PI3K/AKT signaling pathways activated by BDNF.
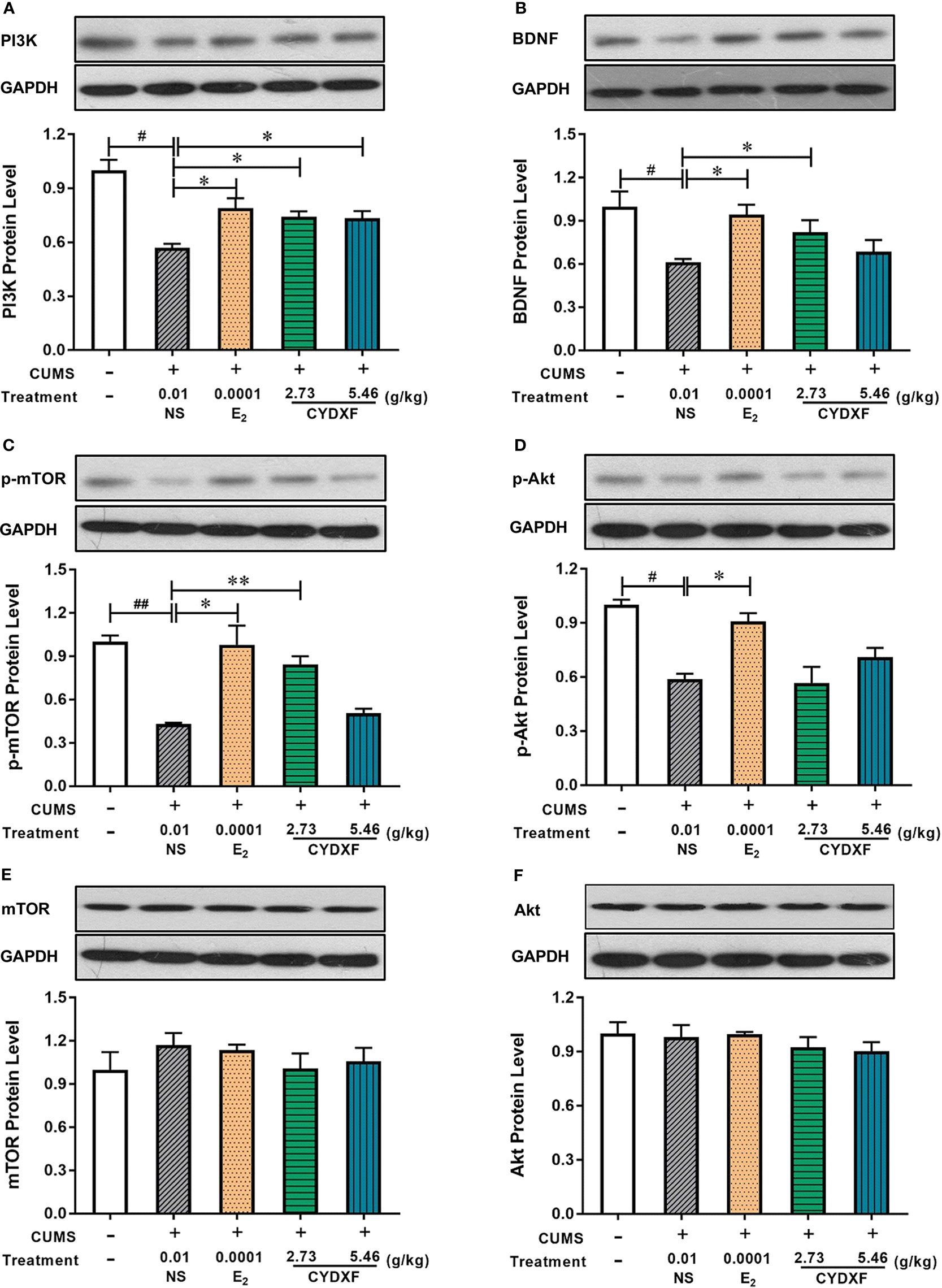
Figure 9 Effect of CYDXF on the protein expression of BDNF mediated PI3K-Akt-mTOR by western blotting. (A) PI3K protein expression level; (B) BDNF protein expression level; (C) p-mTOR (cleaved mTOR) protein expression level; (D) p-Akt (cleaved Akt) protein expression level; (E) mTOR protein expression level; (F) Akt protein expression level. Results are presented as mean ± SEM (n=7). #P<0.05 vs control group; ##P<0.01 vs control group; *P<0.05 vs CUMS group; **P<0.01 vs CUMS group. (The same loading control in Figure 9C and Figure 9E or Figure 9D and Figure 9F was used for uncleaved and cleaved versions of the same protein for ease of comparison).
Discussion
Stress is inevitable in our lives. It is known that stress exerts a detrimental effect on physical and mental status through a healthy person's various systems, including their reproduction system. However, the mechanism of stress that impacts the female reproductive system remains unclear. Many previous studies focused on the effect of stress on the HPA axis. However, in this study, we found that chronic unpredictable mild stress (CUMS) not only induced irregular estrous cycles and endocrine disturbance, but also caused follicle development abnormality, which may be due to the suppression of BDNF-PI3K-Akt-mTOR signaling molecules. CYDXF treatment partly prevented stress-induced downregulation of expression of BNDF/PI3K/Akt/p-mTOR proteins in the ovaries, which was positively correlated with a normalization of hormonal imbalance and most importantly with the stress protective effect of CYDXF on follicular development. The results from this study are in line with those common to stress protecting herbal extracts used to upregulate the PI3K/Akt signalling pathway (Panossian et al., 2018). Follicular maldevelopment could affect female reproduction. Since not all people are suitable for hormone replacement therapy for a long time (Nelson et al., 1997), traditional Chinese medicine showed a great promise in the application for treatment of follicle development abnormality in clinics.
In the current study, we used chronic unpredictable mild stress (CUMS) to induce psychosocial stress in rats. As a putative model of depression, CUMS induced an anxiety-like behavior in the OFT, EPM, as well as a depressive-like behavior in the FST and SPT. Apparently, all the tests above are measuring “behavioral despair” through different methods. In the present study, exposure to stressors caused a decrease in body weight concomitant with a marked change in core behavioral features of depression, including lower motivation, as well as anhedonia (Patki et al., 2013). Taken together, all these indications showed that the rats were experiencing stress and the CUMS model was established successfully.
In TCM theory, follicle development abnormality is based on kidney deficiency and chronic stress can induce liver depression. CYDXF can sooth the liver and nourish the kidney, which is commonly used to treat gynecological diseases and mood disorder. Radix Bupleuri and Radix Curcumae in CYDXF could soothe the liver and relieve depression as well as activate blood circulation and move stagnation. Radix Rehmanniae Praeparata and Epimedium Herb could nourish Yin and Yang together, supplement kidney qi, and promote the viscera function to restore its normal state. Rhizoma Pinellinae Praeparata could dissolve phlegm and dampness as well as strengthen the spleen and stomach. Radix Codonopsis and Poria could fortify the spleen, nourish the heart and calm the mind. The whole formula could invigorate the kidney and soothe the liver, strengthen the spleen and nourish the heart, promote blood circulation and resolve phlegm, which are in line with the basic pathogenesis of chronic stress-induced abnormal ovarian follicular development.
Our findings revealed that CYDXF had multiple functions (Figure 10), including producing antidepressant-like effects and treating gynaecological disorders. Firstly, it could alleviate the incidence of CUMS-induced depression-like behaviors. It could not only increase body weight and sucrose consumption in SPT, but also display higher locomotive activity in OFT, more entrance in the open arms in the EPM, and decrease immobility time in FST. The CYDXF contains a variety of components and twelve major bioactive components were found. Both Saikosaponin D from Bupleurum chinensis DC. and Curcumenol from Curcuma aromatica Salisb. have antidepressant-like activity (Zafir and Banu, 2007; Li et al., 2017). Secondly, CYDXF could regulate the serum hormone levels. In related mechanistic research, stress exerts an impact on the female reproductive system, including the pituitary luteinizing hormone and follicle stimulating hormone, as well as ovarian estrogen and progesterone secretion (Kalantaridou et al., 2004). CYDXF could promote the secretion of estrogen and progesterone and reduce the luteinizing hormone and follicle stimulating hormone, indicating that it may play a role in regulating the hypothalamic-pituitary-ovarian axis to affect female ovulation induction and fertility. We speculate that these actions are probably through the effect of regulating the endocrine system of some components (Zhang et al., 2008). Martynoside, a major component of the Radix Rehmanniae Praeparata, has been shown to have antiestrogenic properties (Papoutsi et al., 2006). Specifically, CYDXF could improve the morphology of ovarian tissue. In the present study, exposure to stressors caused marked changes in the antral follicles as there was a significant increase in the atretic follicles. Conversely, CYDXF could promote follicle maturation while decreasing atretic follicles. As one of the major herbs of CYDXF, Epimedium brevicornu Maxim can partially restore ovarian function and enhance both fertility (Wang et al., 2019) and the identified component icariin (Ming et al., 2013; Wang et al., 2019). In addition, Poria cocos (Schw.) Wolf has antidepressant and immunosuppressive activities (Zhang et al., 2018) and its major component, poricoic acid A, could attenuate oxidative stress and inflammation (Chen et al., 2019). In addition, Radix codonopsis, whose main active component is lobetyolin, could improve the cognitive-function and neuroprotective effects (Weon et al., 2013). Salvia miltiorrhiza Bunge exerts estrogenic effects on reproductive tissues (Xu et al., 2016). Its two major bioactive compounds, Salvianolic acid B and cryptotanshinone, were reported to have an antidepressant-like effect (Zhang et al., 2016) and reverse reproductive and metabolic disturbances through regulating ovarian signaling pathway (Yang et al., 2011), respectively. Glycyrrhizic acid, a bioactive compound of Glycyrrhiza uralensis Fisch., also has a therapeutic effect on neural disease by influencing the PI3K/Akt signaling pathway (Kao et al., 2009). Furthermore, chrysophanic acid has been proven to suppress activation of signaling molecules, such as AKT and mTOR (Lee et al., 2011).
One of the major findings in our study was the involvement of the BDNF-mediated PI3K/Akt/mTOR signaling pathway. We found that the phosphorylation of Akt and mTOR were significantly decreased in CUMS rats. By contrast, CYDXF treatment could obviously increase the phosphorylation of Akt and mTOR. In addition, CUMS caused a reduction of PI3K and BDNF expression in rats, and these changes were attenuated by CYDXF. BDNF was originally discovered in the nervous system as a stress responsive messenger (Larsen et al., 2010; Panossian et al., 2018), but has recently shown to be related to human reproductive diseases including polycystic ovary syndrome, infertility, and impaired ovarian oocyte development (Wu et al., 2012a; Russo et al., 2012; Sadeu et al., 2012). The phosphoinositide-3 kinase (PI3K) signaling pathway plays an essential role in oogenesis, folliculogenesis, as well as ovulation in the ovary (Zheng et al., 2012). Although the basic mechanism of the PI3K/Akt/mTOR signaling pathway has been well studied, much remains to be learned when it comes to reproductive biology. The PI3K pathway, which is characterized by regulating cell survival, growth and proliferation, may also play a critical part in the regulation of oocyte growth and the activation of primordial follicles (Liu et al., 2006). PI3K is also activated by FSH and LH, which are essential hormones for ovulation (Dupont et al., 2012). The protein kinase B (Akt), which cooperates with other kinases to regulate follicular development, determines the pool of primordial follicles which transform from a quiescent to a growing phase in the mammalian ovary (Cecconi et al., 2013). The mammalian target of rapamycin (mTOR) is an important downstream of PI3K/Akt signaling. Recent study has shown that mTOR signaling cascade suppresses granulosa cell autophagy (Choi et al., 2013), and abnormalities in this process may cause reproductive diseases such as premature ovarian failure and polycystic ovary syndrome (Adhikari and Liu, 2010; Yaba and Demir, 2012).
From our study and literature, we speculated that the main reason for the efficacy of CYDXF was probably due to the activation of AKT, while some of the active ingredients were responsible for this effect. Therefore, the restoration of BDNF/PI3K/Akt/mTOR expression by CYDXF may be attributed to, at least in part, the effects of regulating endocrine disorders and/or treating the depressive disorders of these bioactive compounds.
In a word, our study first manifested that CYDXF has multiple functions, including improvement of depression-like behaviors, but is not limited to the regulation of serum hormone levels and promotion of the ovarian function. However, several limitations in our study still exist. First of all, due to the great complexity in Chinese medical formula, it remains unknown which herb or component in CYDXF is mainly responsible for the beneficial effects, how they exert their signaling functions requires further investigation. Secondly, whether CYDXF is effective in other models of depression remains to be further studied. In addition, clinical observations of its randomized trials in humans are needed. The present study which builds the relationship between stress and reproduction is the first step towards this investigation.
Conclusion
Chaiyu-Dixian Formula could attenuate chronic stress-induced abnormal ovarian follicular development through relieving depression-like behaviors and ovarian function. Its mechanism was partly attributed to the regulation of BDNF-mediated PI3K/Akt pathway.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the Laboratory Animal Administration and Ethics Committee of Xiamen University (Permission license No. XMULAC 20190020).
Author Contributions
H-XX carried out the experiment and contributed to the compilation of data and the writing of this paper. YG critically revised the manuscript. H-MZ and S-YX contributed to data analysis and administrative support. S-XL, Z-XH, and C-YZ participated in the experimental process and contributed to the collection of data.
Funding
This work was supported by the Natural Science Foundation of Fujian Province of China (No. 2017J01147), the Traditional Chinese Medicine Foundation of Fujian Ministry of Health of China (No. wzpw201307), the Natural Science Foundation of Fujian Province of China (No. 2018J01136) and the National Natural Science Foundation of China (No. 81202659).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CYDXF, Chaiyu-Dixian Formula; CUMS, Chronic unpredictable mild stress; NS, Normal saline; ELISA, Enzyme-linked immunosorbent assay; BDNF, Brain-derived neurotrophic factor; PI3K, Phosphoinositide-3 kinase; Akt, Protein kinase B; mTOR, Mammalian target of rapamycin; NE, Nuclear epithelial cells; EK, Epithelial keratinocyte; L, Leukocyte; OFT, Open filed test; FST, Forced swim test; SPT, Sucrose preference test; EPM, Elevated plus maze; FSH, Follicle-stimulating hormone; E2, Estradiol; LH, Luteinizing hormone; Prog, Progesterone.
References
Adhikari, D., Liu, K. (2010). mTOR signaling in the control of activation of primordial follicles. Cell Cycle 9, 1673–1674. doi: 10.4161/cc.9.9.11626
Aldridge, J. E., Levin, E. D., Seidler, F. J., Slotkin, T. A. (2005). Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 113, 527–531. doi: 10.1289/ehp.7867
Bamba, Y., Yun, Y. S., Kunugi, A., Inoue, H. (2011). Compounds isolated from Curcuma aromatica Salisb. inhibit human P450 enzymes. J. Nat. Med. 65, 583–587. doi: 10.1007/s11418-011-0507-0
Bodis, J., Papp, S., Vermes, I., Sulyok, E., Tamas, P., Farkas, B., et al. (2014). Platelet-associated regulatory system (PARS) with particular reference to female reproduction. J. Ovarian Res. 7, 55. doi: 10.1186/1757-2215-7-55
Cecconi, S., Mauro, A., Cellini, V., Patacchiola, F. (2013). The role of Akt signalling in the mammalian ovary. Int. J. Dev. Biol. 56, 809–817. doi: 10.1387/ijdb.120146sc
Chang, H. M., Cheng, K. P., Choang, T. F., Chow, H. F., Chui, K. Y., Hon, P. M., et al. (1990). Structure elucidation and total synthesis of new Tanshinones isolated from Salvia-miltiorrhiza Bunge (Danshen). J. Org. Chem. 55, 3537–3543. doi: 10.1021/jo00298a029
Chen, C. H., Sung, C. S., Huang, S. Y., Feng, C. W., Hung, H. C., Yang, S. N., et al. (2016). The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp. Neurol. 278, 27–41. doi: 10.1016/j.expneurol.2016.01.023
Chen, D. Q., Feng, Y. L., Chen, L., Liu, J. R., Wang, M., Vaziri, N. D., et al. (2019). Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/Axl-NF-kappa B/Nrf2 axis. Free Radical Bio. Med. 134, 494–507. doi: 10.1016/j.freeradbiomed.2019.01.046
Choi, J., Jo, M., Lee, E., Choi, D. (2013). AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 147, 73–80. doi: 10.1530/REP-13-0386
Divyashree, S., Yajurvedi, H. N. (2016). Long-term chronic stress exposure induces PCO phenotype in rat. Reproduction 152, 765–774. doi: 10.1530/REP-16-0404
Dorfman, M., Arancibia, S., Fiedler, J. L., Lara, H. E. (2003). Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol. Reprod. 68, 2038–2043. doi: 10.1095/biolreprod.102.008318
Dupont, J., Reverchon, M., Cloix, L., Froment, P., Rame, C. (2012). Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 56, 959–967. doi: 10.1387/ijdb.120134jd
Efferth, T., Li, P. C. H., Konkimalla, V. S. B., Kaina, B. (2007). From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 13, 353–361. doi: 10.1016/j.molmed.2007.07.001
Fernandois, D., Na, E., Cuevas, F., Cruz, G., Lara, H. E., Paredes, A. H. (2016). Kisspeptin is involved in ovarian follicular development during aging in rats. J. Endocrinol. 228, 161–170. doi: 10.1530/JOE-15-0429
Fortune, J. E. (1994). Ovarian follicular growth and development in mammals. Biol. Reprod. 50, 225–232. doi: 10.1095/biolreprod50.2.225
Frye, C. A., Petralia, S. M., Rhodes, M. E. (2000). Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol. Biochem. Behav. 67, 587–596. doi: 10.1016/s0091-3057(00)00392-0
Fu, X. Y., Chen, H. H., Zhang, N., Ding, M. X., Qiu, Y. E., Pan, X. M., et al. (2018). Effects of chronic unpredictable mild stress on ovarian reserve in female rats: feasibility analysis of a rat model of premature ovarian failure. Mol. Med. Rep. 18, 532–540. doi: 10.3892/mmr.2018.8989
Gao, Y., Chen, F., Kong, Q. Q., Ning, S. F., Yuan, H. J., Lian, H. Y., et al. (2016). Stresses on female mice impair oocyte developmental potential: effects of stress severity and duration on oocytes at the growing follicle stage. Reprod. Sci. 23, 1148–1157. doi: 10.1177/1933719116630416
Gong, M. J., Han, B., Wang, S. M., Liang, S. W., Zou, Z. J. (2016). Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats. J. Pharm. Biomed. Anal. 123, 63–73. doi: 10.1016/j.jpba.2016.02.001
Hayes, E., Kushnir, V., Ma, X., Biswas, A., Prizant, H., Gleicher, N., et al. (2016). Intra-cellular mechanism of Anti-Mullerian hormone (AMH) in regulation of follicular development. Mol. Cell. Endocrinol. 433, 56–65. doi: 10.1016/j.mce.2016.05.019
Hirshfield, A. N. (1991). Development of Follicles in the Mammalian Ovary. Int. Rev. Cytol. 124, 43–101. doi: 10.1016/S0074-7696(08)61524-7
Jindal, A., Mahesh, R., Bhatt, S. (2013). Etazolate rescues behavioral deficits in chronic unpredictable mild stress model: modulation of hypothalamic-pituitary-adrenal axis activity and brain-derived neurotrophic factor level. Neurochem. Int. 63, 465–475. doi: 10.1016/j.neuint.2013.08.005
Kalantaridou, S. N., Makrigiannakis, A., Zoumakis, E., Chrousos, G. P. (2004). Stress and the female reproductive system. J. Reprod. Immunol. 62, 61–68. doi: 10.1016/j.jri.2003.09.004
Kao, T. C., Shyu, M. H., Yen, G. C. (2009). Neuroprotective effects of Glycyrrhizic acid and 18 beta-Glycyrrhetinic acid in PC12 cells via modulation of the PI3K/Akt pathway. J. Agr. Food Chem. 57 (2), 754–761. doi: 10.1021/jf802864k
Karisetty, B. C., Maitra, S., Wahul, A. B., Musalamadugu, A., Khandelwal, N., Guntupalli, S., et al. (2017). Differential effect of chronic stress on mouse hippocampal memory and affective behavior: Role of major ovarian hormones. Behav. Brain Res. 318, 36–44. doi: 10.1016/j.bbr.2016.10.034
Kim, S. C., Byun, S. H., Yang, C. H., Kim, C. Y., Kim, J. W., Kim, S. G. (2004). Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage. Toxicology 197, 239–251. doi: 10.1016/j.tox.2004.01.010
Larsen, M. H., Mikkelsen, J. D., Hay-Schmidt, A., Sandi, C. (2010). Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J. Psychiatr. Res. 44, 808–816. doi: 10.1016/j.jpsychires.2010.01.005
Lee, M. S., Cha, E. Y., Sul, J. Y., Song, I. S., Kim, J. Y. (2011). Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway. Phytother. Res. 25 (6), 833–837. doi: 10.1002/ptr.3323
Li, L. L., Zhu, H. M. (2016). Effects of Chaiyu Dixian Formula on behavior and HPG axis of perimenopausal depressive disorder model rats. Tradit. Chin. Med. J. 1564-66, 72. doi: 10.14046/j.cnki.zyytb2002.2016.05.024
Li, C. Y., Xu, H. X., Han, Q. B., Wu, T. S. (2009). Quality assessment of Radix Codonopsis by quantitative nuclear magnetic resonance. J. Chromatogr. A 1216, 2124–2129. doi: 10.1016/j.chroma.2008.10.080
Li, H. Y., Zhao, Y. H., Zeng, M. J., Fang, F., Li, M., Qin, T. T., et al. (2017). Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology 234, 3385–3394. doi: 10.1007/s00213-017-4720-8
Liu, K., Rajareddy, S., Liu, L., Jagarlamudi, K., Boman, K., Selstam, G., et al. (2006). Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev. Biol. 299, 1–11. doi: 10.1016/j.ydbio.2006.07.038
Liu, Y. F., Liang, D., Luo, H., Hao, Z. Y., Wang, Y., Zhang, C. L., et al. (2012). Hepatoprotective Iridoid Glycosides from the Roots of Rehmannia glutinosa. J. Nat. Prod. 75, 1625–1631. doi: 10.1021/np300509z
Liu, L., Yan, J., Ge, F., Xu, X., Lu, J., Shi, H., et al. (2019). Saikosaponin-D improves fear memory deficits in ovariectomized rats via the action of estrogen receptor-alpha in the hippocampus. Mol. Med. Rep. 20 (1), 332–340. doi: 10.3892/mmr.2019.10232
Marcondes, F. K., Bianchi, F. J., Tanno, A. P. (2002). Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 62, 609–614. doi: 10.1590/s1519-69842002000400008
Martinowich, K., Lu, B. (2008). Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83. doi: 10.1038/sj.npp.1301571
McEwen, B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185. doi: 10.1016/j.ejphar.2007.11.071
Ming, L. G., Chen, K. M., Xian, C. J. (2013). Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 228 (3), 513–521. doi: 10.1002/jcp.24158
Myers, M., Britt, K. L., Wreford, N. G. M., Ebling, F. J. P., Kerr, J. B. (2004). Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127, 569–580. doi: 10.1530/rep.1.00095
Nagai, T., Kiyohara, H., Munakata, K., Shirahata, T., Sunazuka, T., Harigaya, Y., et al. (2002). Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int. Immunopharmacol. 2, 1183–1193. doi: 10.1016/s1567-5769(02)00086-3
Nelson, H. D., Humphrey, L. L., Nygren, P., Teutsch, S. M., Allan, J. D. (1997). Postmenopausal hormone replacement therapy - Scientific review. JAMA-J. Am. Med. Assoc. 288, 872–881. doi: 10.1001/jama.288.7.872
Osman, P. (1985). Rate and course of atresia during follicular development in the adult cyclic rat. J. Reprod. Fertil. 73, 261–270. doi: 10.1530/jrf.0.0730261
Paes, V. M., Vieira, L. A., Correia, H. H. V., Sa, N. A. R., Moura, A. A. A., Sales, A. D., et al. (2016). Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus-oocyte complex. Theriogenology 86, 994–1003. doi: 10.1016/j.theriogenology.2016.03.027
Panossian, A., Seo, E. J., Efferth, T. (2018). Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 50, 257–284. doi: 10.1016/j.phymed.2018.09.204
Papoutsi, Z., Kassi, E., Mitakou, S., Aligiannis, N., Tsiapara, A., Chrousos, G. P., et al. (2006). Acteoside and martynoside exhibit estrogenic/antiestrogenic properties. J. Steroid Biochem. Mol. Biol. 98, 63–71. doi: 10.1016/j.jsbmb.2005.07.005
Patki, G., Solanki, N., Atrooz, F., Allam, F., Salim, S. (2013). Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 1539, 73–86. doi: 10.1016/j.brainres.2013.09.033
Peltier, M. R., Flores, J. M., Smith, P. H., Roberts, W., Verplaetse, T. L., Moore, K. E., et al. (2019). Smoking across the menopausal transition in a 10-year longitudinal sample: The role of sex hormones and depressive symptoms. Nicotine Tob. Res. 2019, 1–6. doi: 10.1093/ntr/ntz069.
Rasmussen, P., Brassard, P., Adser, H., Pedersen, M. V., Leick, L., Hart, E., et al. (2009). Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 94, 1062–1069. doi: 10.1113/expphysiol.2009.048512
Reagan-Shaw, S., Nihal, M., Ahmad, N. (2008). Dose translation from animal to human studies revisited. FASEB J. 22, 659–661. doi: 10.1096/fj.07-9574LSF
Rios, J. L. (2011). Chemical Constituents and Pharmacological Properties of Poria cocos. Planta Med. 77, 681–691. doi: 10.1055/s-0030-1270823
Russo, N., Russo, M., Daino, D., Bucci, F., Pluchino, N., Casarosa, E., et al. (2012). Polycystic ovary syndrome: brain-derived neurotrophic factor (BDNF) plasma and follicular fluid levels. Gynecol. Endocrinol. 28, 241–244. doi: 10.3109/09513590.2011.613969
Sadeu, J. C., Doedee, A., Neal, M. S., Hughes, E. G., Foster, W. G. (2012). Neurotrophins (BDNF and NGF) in follicular fluid of women with different infertility diagnoses. Reprod. Biomed. Online 24, 174–179. doi: 10.1016/j.rbmo.2011.11.011
Schliep, K. C., Mumford, S. L., Vladutiu, C. J., Ahrens, K. A., Perkins, N. J., Sjaarda, L. A., et al. (2015). Perceived stress, reproductive hormones, and ovulatory function a prospective cohort study. Epidemiology 26, 177–184. doi: 10.1097/EDE.0000000000000238
Smeeth, D. M., Dima, D., Jones, L., Jones, I., Craddock, N., Owen, M. J., et al. (2019). Polygenic risk for circulating reproductive hormone levels and their influence on hippocampal volume and depression susceptibility. Psychoneuroendocrinology 106, 284–292. doi: 10.1016/j.psyneuen.2019.04.011.
Sun, H. Y., Li, Q., Liu, Y. Y., Wei, X. H., Pan, C. S., Fan, J. Y., et al. (2017). Xiao-Yao-San, a Chinese medicine formula, ameliorates chronic unpredictable mild stress induced polycystic ovary in rat. Front. Physiol. 8, 729. doi: 10.3389/fphys.2017.00729
Ter Horst, G. J., Wichmann, R., Gerrits, M., Westenbroek, C., Lin, Y. (2009). Sex differences in stress responses: focus on ovarian hormones. Physiol. Behav. 97, 239–249. doi: 10.1016/j.physbeh.2009.02.036
Walf, A. A., Paris, J. J., Frye, C. A. (2009). Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology 34, 909–916. doi: 10.1016/j.psyneuen.2009.01.004
Wang, J. L., Liu, B., Zhang, C., Wang, X. M., Zhen, D., Huang, X. M., et al. (2019). Effects of icariin on ovarian function in D-galactose-induced aging mice. Theriogenology 125, 157–167. doi: 10.1016/j.theriogenology.2018.10.028
Wen, X., Xie, J., Zhou, L., Fan, Y., Yu, B., Chen, Q., et al. (2018). The role of combining medroxyprogesterone 17-acetate with human menopausal gonadotropin in mouse ovarian follicular development. Sci. Rep. 8, 4439. doi: 10.1038/s41598-018-22797-6
Weon, J. B., Yun, B. R., Lee, J., Eom, M. R., Ko, H. J., Kim, J. S., et al. (2013). Effect of Codonopsis lanceolata with steamed and fermented process on scopolamine-induced memory impairment in mice. Biomol. Ther. 21 (5), 405–410. doi: 10.4062/biomolther.2013.055
Westenbroek, C., Den Boer, J. A., Veenhuis, M., Ter Horst, G. J. (2004). Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res. Bull. 64, 303–308. doi: 10.1016/j.brainresbull.2004.08.006
Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134, 319–329. doi: 10.1007/s002130050456
Wu, L. M., Hu, M. H., Tong, X. H., Han, H., Shen, N., Jin, R. T., et al. (2012a). Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PloS One 7, e52331. doi: 10.1371/journal.pone.0052331
Wu, L. M., Liu, Y. S., Tong, X. H., Shen, N., Jin, R. T., Han, H., et al. (2012b). Inhibition of follicular development induced by chronic unpredictable stress is associated with growth and differentiation factor 9 and gonadotropin in mice. Biol. Reprod. 86, 121. doi: 10.1095/biolreprod.111.093468
Xu, Y., Chen, T., Li, X., Qu, Y. K., An, J. N., Zheng, H. X., et al. (2016). Salvia miltiorrhiza bunge increases estrogen level without side effects on reproductive tissues in immature/ovariectomized mice. Aging-Us 9, 170–186. doi: 10.18632/aging.101145
Xue, L. M., Jiang, Y. P., Han, T., Zhang, N., Qin, L., Xin, H., et al. (2016). Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu maxim. J. Ethnopharmacol. 192, 370–381. doi: 10.1016/j.jep.2016.07.037
Yaba, A., Demir, N. (2012). The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J. Ovarian Res. 5, 38. doi: 10.1186/1757-2215-5-38
Yan, Z., Dai, Y., Fu, H., Zheng, Y., Bao, D., Yin, Y., et al. (2018). Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 60 (3), 261–271. doi: 10.1530/JME-17-0214
Yang, X. M., Zhang, Y. H., Wu, X. K., Bae, C. S., Hou, L., Kuang, H., et al. (2011). Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of ovarian signaling mechanisms and androgen synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300 (4), R869–R875. doi: 10.1152/ajpregu.00334.2010
Yoshida, M., Sanbuissyo, A., Hisada, S., Takahashi, M., Ohno, Y., Nishikawa, A. (2009). Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J. Toxicol. Sci. 34, SP189. doi: 10.2131/jts.34.s189
Yu, T., Guo, M., Garza, J., Rendon, S., Sun, X. L., Zhang, W., et al. (2011). Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int. J. Neuropsychoph. 14, 303–317. doi: 10.1017/S1461145710000945
Zafir, A., Banu, N. (2007). Antioxidant potential of fluoxetine in comparison to Curcuma longa in restraint-stressed rats. Eur. J. Pharmacol. 572, 23–31. doi: 10.1016/j.ejphar.2007.05.062
Zangeneh, F. Z., Shooshtary, F. S. (2009). Chronic stress and limbic-hypothalamopituitary-adrenal axis (LHPA) response in female reproductive system. J. Fam. Reprod. Health 4, 101–108.
Zhang, R. X., Li, M. X., Jia, Z. P. (2008). Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 117, 199–214. doi: 10.1016/j.jep.2008.02.018
Zhang, J. Q., Wu, X. H., Feng, Y., Xie, X. F., Fan, Y. H., Yan, S., et al. (2016). Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol. Sin. 37 (9), 1141–1153. doi: 10.1038/aps.2016.63
Zhang, W. X., Chen, L., Li, P., Zhao, J. Z., Duan, J. Y. (2018). Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 120, 1696–1704. doi: 10.1016/j.ijbiomac.2018.09.171
Zheng, W., Nagaraju, G., Liu, Z., Liu, K. (2012). Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell. Endocri. 356, 24–30. doi: 10.1016/j.mce.2011.05.027
Zhou, J., Huang, S. Q., Li, L. L., Huo, Z. X., Wang, Y. P., Zhu, H. M. (2017). Effects of Chaiyu Dixian Decoction on ethology and hypothalamus-pituitary-adrenal axis in rats of perimenopausal depressive disorder model rats. Acad. J. Shanghai Univ. Tradit. Chin. Med. 31, 66–70. doi: 10.16306/j.1008-861x.2017.05.016
Zhou, J., Huo, Z. X., Zhu, H. M., Wang, Y. P., Li, L. L., Huang, S. Q., et al. (2018). Effects of Chaiyu Dixian Recipe on ethology and the expressions of BDNF and TrkB in the hippocampus of perimenopausal depressive disorder model rats. Chin. J. Integr. Tradit. West. Med. 38, 1350–1355. doi: 10.7661/j.cjim.201800822.275
Keywords: Chaiyu-Dixian formula, chronic stress, follicle development, PI3K/Akt, brain-derived neurotrophic factor, ovary
Citation: Xu H-X, Lin S-X, Gong Y, Huo Z-X, Zhao C-Y, Zhu H-M and Xi S-Y (2020) Chaiyu-Dixian Formula Exerts Protective Effects on Ovarian Follicular Abnormal Development in Chronic Unpredictable Mild Stress (CUMS) Rat Model. Front. Pharmacol. 11:245. doi: 10.3389/fphar.2020.00245
Received: 20 September 2019; Accepted: 21 February 2020;
Published: 24 March 2020.
Edited by:
Alexander George Panossian, Phytomed AB, SwedenReviewed by:
Germain Sotoing Taiwe, University of Buea, CameroonPing Li, China-Japan Friendship Hospital, China
Xi Huang, Nanjing University of Chinese Medicine, China
Copyright © 2020 Xu, Lin, Gong, Huo, Zhao, Zhu and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Mei Zhu, ejU5MTM3NzhAMTI2LmNvbQ==; Sheng-Yan Xi, eGlzaGVuZ3lhbkB4bXUuZWR1LmNu
 Hui-Xian Xu
Hui-Xian Xu Shu-Xia Lin
Shu-Xia Lin Yuewen Gong
Yuewen Gong Zi-Xuan Huo
Zi-Xuan Huo Cheng-Yun Zhao
Cheng-Yun Zhao Hong-Mei Zhu
Hong-Mei Zhu Sheng-Yan Xi
Sheng-Yan Xi