
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 18 February 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00041
This article is part of the Research Topic Action Mechanisms of Traditional Medicinal Plants used to Control Type 2 Diabetes or Conditions of Metabolic Syndrome View all 8 articles
 Tarun Belwal1
Tarun Belwal1 Aarti Bisht1
Aarti Bisht1 Hari Prasad Devkota2,3
Hari Prasad Devkota2,3 Hammad Ullah4
Hammad Ullah4 Haroon Khan4
Haroon Khan4 Aseesh Pandey5
Aseesh Pandey5 Indra Dutt Bhatt1*
Indra Dutt Bhatt1* Javier Echeverría6*
Javier Echeverría6*The incidences of diabetic mellitus and other metabolic diseases such as hypertension and hyperlipidemia are increasing worldwide; however, the current treatment is not able to control the rapidly increasing trend in diabetes mortality and morbidity. Studies related to the effectiveness of extracts and pure compounds obtained from plants have shown promising responses in preclinical and clinical studies related to these metabolic diseases. Plants belonging to the genus Berberis (Family: Berberidaceae) are widely distributed with nearly 550 species worldwide. Extracts and compounds obtained from Berberis species, especially Berberine alkaloid, showed effectiveness in the management of diabetes and other metabolic diseases. Various pharmacological experiments have been performed to evaluate the effects of Berberis extracts, berberine, and its natural and chemically synthesized derivatives against various cell and animal disease models with promising results. Various clinical trials conducted so far also showed preventive effects of Berberis extracts and berberine against metabolic diseases. The present review focuses on i) research updates on traditional uses, ii) phytopharmacology and clinical studies on Berberis species, and iii) active metabolites in the prevention and treatment of diabetes and other metabolic diseases with a detailed mechanism of action. Furthermore, the review critically analyzes current research gaps in the therapeutic use of Berberis species and berberine and provides future recommendations.
Diabetes mellitus (DM) is a metabolic disorder that is characterized by an abnormal long-term increase in plasma glucose levels. Diabetes is mainly classified into four types, i.e., type I diabetes (T1DM), type II diabetes (T2DM), gestational diabetes, and specific types of diabetes due to other causes (American Diabetes Association, 2019). Many factors, such as insulin deficiency or resistance as well as altered carbohydrate, protein, and fat metabolisms, are usually the reasons for high blood glucose levels leading to DM. Chronic hyperglycemia related to diabetes is often associated with many other complications, such as cardiovascular, dermatological, neurological, renal, retinal, and nerve diseases. Diabetes is one of the most common chronic disease, and it has shown an increasing rate of occurrence over the past decade (Bullard et al., 2018). According to the World Health Organization (WHO), the total number of people with diabetes worldwide substantially increased from 108 million in 1980 to 422 million in 2014 (World Health Organization, 2016). Along with diabetes, the incidence of other metabolic diseases, such as hyperlipidemia, is also increasing rapidly (Karr, 2017).
Metabolic syndrome (MS) is associated with a group of disease conditions that occur together, and it is composed of central adiposity, hyperglycemia, hypertriglyceridemia, low high-density lipoproteins (HDL)-cholesterol, and hypertension. This disease cluster of diabetes and cardiovascular diseases is also known as “The Deadly Quartet”, “Syndrome X”, and “The Insulin Resistance Syndrome” (Alberti, 2005). Various treatment options are available to mitigate MS, including the diabetic condition and related disorders (Deedwania and Volkova, 2005). As MS is manifested by the cluster of diseases, use of a single drug candidate might not be able to provide necessary therapeutic effects. Plant extracts and isolated compounds can be possible options as adjuvants in such cases. Traditionally, various medicinal plants and their products (extracts and isolated compounds) have been used in the treatment of diabetes and hypertension (Oyedemi et al., 2009; Tabassum and Ahmad, 2011; Rizvi and Mishra, 2013; Ezuruike and Prieto, 2014). Various research showed the protective/curative effect of plant extracts as a whole and/or an individual bioactive compound against diabetes and other metabolic diseases (Tabatabaei-Malazy et al., 2015; Waltenberger et al., 2016).
Plants belonging to the genus Berberis (Family: Berberidaceae) are widely distributed worldwide with nearly 550 species. A decoction prepared from the roots of Berberis plants is one of the common traditional recipes for the treatment of diabetes (Neag et al., 2018). Various studies have reported the traditional uses Berberis plants for the treatment of metabolic diseases (e.g., diabetes and hyperlipidemia) in many countries, including India, Pakistan, China, and Iran (Hamayun et al., 2006; Uniyal et al., 2006; Rahimi Madiseh et al., 2014; Rana et al., 2019). Various bioactive compounds, such as alkaloids, polyphenols, flavonoids, anthocyanins, etc., have been found in Berberis species along with various vitamins and mineral components (Andola et al., 2010; Srivastava et al., 2015; Belwal et al., 2016; Belwal et al., 2017). Berberine (BBR), a quaternary ammonium salt belonging to a group of benzylisoquinoline alkaloids, is the most active compound reported from Berberis species, and it is considered to be highly effective against diabetes and other metabolic diseases (Dong et al., 2012; Lan et al., 2015; Wang H. et al., 2018). BBR is also distributed in various plant species of other genera such as Coptis, Hydrastis, Mahonia, Tinospora, Xanthorhiza, and many others (Neag et al., 2018). In the genus Berberis, the distribution of BBR and other alkaloids is mostly in its root part, followed by the stem bark and the stem itself (Andola et al., 2010). In addition, its presence in trace amounts has been reported from leaves and berries. Various studies have been conducted to evaluate the effectiveness of Berberis extract or bioactive alkaloidal compounds against diabetes and other MS with promising results (Gulfraz et al., 2008; Meliani et al., 2011; Imenshahidi and Hosseinzadeh, 2016; Mirhadi et al., 2018). Moreover, various clinical trials were also conducted on testing their effectiveness against diabetes and other metabolic diseases and showed variable effects (Zhang et al., 2010; Pérez-Rubio et al., 2013).
Considering the Berberis species and their active alkaloidal components, the present review specifically focuses on their effectiveness against diabetes and other metabolic diseases. This review discusses various traditional uses of Berberis against metabolic diseases, along with its cell- and animal-model studies. The pharmacological effects of Berberis extracts and alkaloids against diabetes and other metabolic diseases are also discussed along with the molecular mechanism of action. Furthermore, based on the present studies of Berberis species against diabetes and metabolic diseases, research gaps were highlighted, and future recommendations were made.
The scattered scientific information on Berberis species and isolated compounds used to counteract metabolic diseases was collected and documented. The synonyms of the various species were crosschecked with the plant name database The Plant List (www.theplantlist.org, Retrieved on November 22, 2019). Afterwards, the available articles on respective species were retrieved using popular search engines and various databases, such as SciFinder, ScienceDirect, PubMed, Scopus, Mendeley, JOAP, Microsoft academic, and Google Scholar. The keywords used were Berberis, berberine, diabetes, metabolic diseases, metabolic syndrome, ethnopharmacology, ethnobotany, chemical constituents, alkaloids, in vitro, in vivo, clinical study, and clinical trials. The data were congregated through the Boolean information retrieval method by using a plant name along with an “AND” operator followed by diabetes and metabolic syndrome. No prerequisite limitations on publications, i.e., language, year, and publication type (original contribution, review article, or key editorial note), were taken into consideration.
According to The Plant List database (www.theplantlist.org, retrieved on September 20, 2019), the family Berberidaceae consists of a total of 19 genera. The members of the genus Berberis are reported to be difficult to identify taxonomically due to their extreme morphological variation in relation to the environmental factors and natural hybridization (Ahrendt, 1961; Rao et al., 1998). Various overlapping morphological characters, such as flowers, leaves, stems, and berries—which also depend upon the season—and plant age also make it difficult to identify during field tasks (Rao and Hajra, 1993; Rao et al., 1998; Tiwari and Singh Adhikari, 2011). Berberis species are widely cultivated around the world due to their high medicinal and ornamental value. Most members of the genus Berberis are reported to be tolerant to shade, resistant to drought, and widely distributed in open and wooded habitats and wetlands. These plants are also studied as indicators of habitat degradation in the temperate region due traditionally to their thorny stem and unpalatable shoots (Champion and Seth, 1968). Representative photographs of some Berberis species from the Indian Himalayan Region (IHR) are shown in Figure 1, and their major plant parts used to extract berberine and other bioactive alkaloids are shown in Figure 2.

Figure 1 Some Berberis species of Indian Himalayan Region (IHR). (A) B. aristata DC., (B) B. asiatica Roxb. ex DC., (C) B. jaeschkeana C.K.Schneid., (D) B. lycium Royle, (E) B. pseudumbellata R. Parker, (F) B. thomsoniana Schneider.

Figure 2 Various plant parts of (A) Berberis asiatica collected from Indian Himalayan Region (IHR), includes, (B) roots, (C) stems and (D) stem barks. These parts are the major sources to extract Berberine (yellow color) from Berberis species.
A literature review revealed that the ethnopharmacological uses of Berberis species have been documented from different parts of the world for the treatment of diabetes, hypertension, and obesity, and some of them also revealed the formulation methods. A majority of Berberis species were found to be used in the Himalayan region of India and Pakistan.
B. lycium Royle has been used traditionally for the treatment of diabetes mellitus and other diseases, particularly by the local inhabitants of the Himalayan region (Hamayun et al., 2006). Apart from diabetes, B. lycium is also used to treat bone fractures, diarrhoea, fever, intestinal colic, internal wounds, jaundice, menorrhagia, ophthalmic disorders, piles, rheumatism, sun blindness, and throat pain (Jabeen et al., 2015; Adhikari et al., 2019). Fruits and leaves of B. lycium are also reported to be used for the treatment of diabetes mellitus in south-west of Iran (Rahimi Madiseh et al., 2014) and Pakistan (Zain-Ul-Abidin et al., 2018). The water extract obtained by soaking the root bark in water is used for the treatment of diabetes (Ahmed et al., 2004). The whole plant is used to treat diabetes in Chamba district of Himachal Pradesh, West Himalaya, India (Rana et al., 2019). The Bhotiya tribal community of the Central Himalayan region of India used B. lycium roots with water for the treatment of diabetes (Phondani et al., 2010).
The stem of B. aristata DC. is widely used in Indian traditional medicine for the treatment of diabetes (Upwar et al., 2011), which is also reported in Ayurvedic Pharmacopoeia. The decoction (5–10 mL) of roots or stems of this species prepared with water was taken twice a day for 1–2 weeks to treat diabetes in Uttarakhand region (Kumar et al., 2019). It is also used by Uttarakhand people for the treatment of hypertension (Singh et al., 2019). The root, stem, and fruit also have been used to treat obesity (Chandrasekaran et al., 2018). B. asiatica is also used for the treatment of diabetes by the tribal communities of Chhota Bhangal, Western Himalaya, India. The decoction prepared from the roots is concentrated and dried in shade and then used with the sap of bitter guard for the treatment of diabetes (Uniyal et al., 2006).
In Iranian traditional medicine, B. vulgaris L. is extensively used to treat diabetes and hypertension (Rahimi-Madiseh et al., 2017). Local people use a decoction from the fruits and roots of B. vulgaris to treat hypertension (Baharvand-Ahmadi et al., 2016). The fruits are most frequently used in traditional and modern medicine (Rahimi Madiseh et al., 2014). Dried roots of B. crateagina DC. were recorded to be used as anti-diabetic agents locally in Turkey, and the decoction or infusion prepared from dried roots was taken orally one to two times a day for the treatment of diabetes (Durmuskahya and Öztürk, 2013). The anti-diabetic activity has also been reported for B. brevissima Jafri and B. parkeriana C.K.Schneid. (Alemardan et al., 2013). Bahmani et al. (2016) reported that the inhabitant of Urmia, Iran, use boiled and steamed B. integerrima Bunge extract for the treatment of diabetes.
A large number of studies have been conducted on the isolation and quantification of bioactive compounds from Berberis species. The phytochemical investigations of the genus Berberis have shown the presence of more than 105 compounds with varying structural confirmations. Most of the studies on Berberis species are focused on phytochemical screening; for the presence and estimation of different secondary metabolites, such as alkaloids, flavonoids, steroids, sugars, triterpenoids, tannins, and other preliminary assays such as total ash content, acid soluble ash content, and moisture content (Belwal et al., 2016; Belwal et al., 2017; Andola et al., 2018; Srivastava et al., 2006; Shahid et al., 2009). However, the isolation and characterization of alkaloids from genus Berberis is well documented. Alkaloids are one of the major bioactive chemical constituents of the Berberis species, and they are responsible for various pharmacological activities of either whole extract or isolated individual compounds. Berberine (BBR) is one of the most commonly reported alkaloids from various Berberis species along with palmatine, magnoflorine, and jatrorrhizine, etc. (Figure 3) (Bhardwaj and Kaushik, 2012; Feng et al., 2018). Simple isoquinolone alkaloids are mainly reported from these species; however, studies have also reported their dimmers or dimeric benzylisoquinoline alkaloids (Leet et al., 1983). The detailed list of different alkaloids isolated from various Berberis species are given in Table 1. Among other compounds, BBR and its various natural and synthetic derivatives have also been evaluated and found effective in prevention and treatment of MS (Pérez-Rubio et al., 2013; Li et al., 2015; Zhao et al., 2017).
The effect of different habitat conditions (altitudinal variations and edaphic factors) of Berberis species has been investigated. Chandra and Purohit (1980) investigated eight Berberis species from different altitudinal range for determining the BBR concentration in different parts. Among these, B. asiatica was found to contain higher content of BBR than other species. Lower altitudinal range was found to contain higher BBR content within a species as compared to high altitude habitat. Among plant parts, roots contained a higher concentration of BBR (Chandra and Purohit, 1980). Similarly, variations in the BBR content of five Berberis species (i.e., B. aristata, B. asiatica, B. jaeschkeana, B. lycium, and B. pseudumbellata) depending upon the habitat have also studied. The presence of higher BBR content was recorded from rocky habitats in B. jaeschkeana (Andola et al., 2018). Both altitude and edaphic conditions were found to be responsible for the variation in BBR content in root and stem bark. Lower altitude populations showed significantly higher BBR content and positively correlated with moisture and potassium availability in soil species. Among these, B. asiatica contain significantly higher BBR content as compared to other species (Andola et al., 2010) Seasonal variations in the BBR content revealed higher percentage in summer and lower in rainy season (Andola et al., 2018). Low moisture and high soil potassium level is reported to be well correlated with high BBR content (Andola et al., 2011).
It has been suggested that physical exercise and a proper diet can act as controllers of the cause of T2DM and metabolic diseases. Currently available pharmacological interventions can control many aspects of diabetes and metabolic diseases, like microvascular and macrovascular complications, hypertension, dyslipidemia, and obesity. However, there is also a need for novel therapeutic agents that work alone or in combination with currently available drugs. Within the pharmacological options, phytochemicals have a great potential to act against T2DM, MS, and associated complications (Davì et al., 2010). Extracts of Berberis species and their components, especially alkaloids, have been documented for their potential activity against T2DM and MS in various in vitro studies (Table 2) (Potdar et al., 2012)
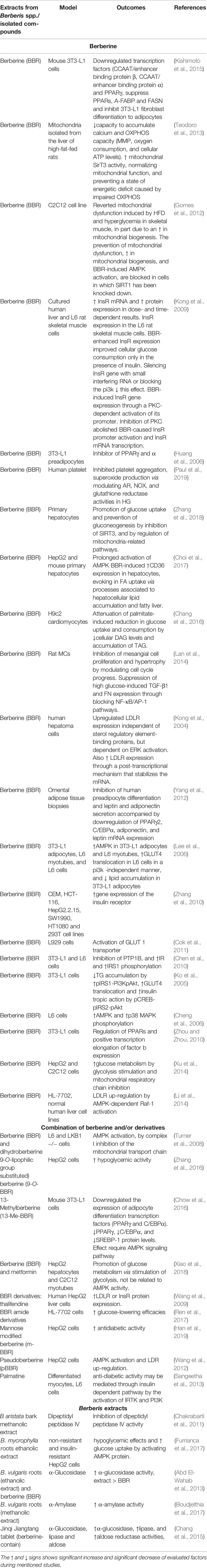
Table 2 In vitro activity of extracts and/or isolated compounds from Berberis species against diabetes and metabolic diseases.
Studies in mouse 3T3-L1 cells suggested that BBR has an pivotal role in regulating adipose tissues (Kishimoto et al., 2015). Experiments in mitochondria isolated from the liver of high-fat-fed rats have shown that BBR exhibited protective effects against MS that was associated with the increased mitochondrial sirtuin-3 (SIRT3) activity, normalizing mitochondrial function, and preventing a state of impaired oxidative phosphorylation (OXPHOS) that caused energetic deficit (Teodoro et al., 2013). In the same way, the preventive effects of BBR on diet-induced insulin resistance (InsR) was suggested to be linked to sirtuin-1 (SIRT1) and mitochondrial biogenesis (Gomes et al., 2012). It has been suggested that BBR is a unique natural medicine against insulin resistance in T2DM and MS (Kong et al., 2009). Different investigations have concluded that BBR as a new hypolipidemic drug works by a different mechanism of action to that of statin drugs (Kong et al., 2004). BBR works on multiple molecular targets as an inhibitor of peroxisome proliferator-activated receptor (PPAR) γ and α and is a potential weight reducing, hypolipidemic, and hypoglycemic agent (Huang et al., 2006). Prolonged activation of AMP-activated protein kinase (AMPK) by BBR improved CD36 expression in hepatocytes and was evoked in fatty acid uptake via processes associated with hepatocellular lipid accumulation (Choi et al., 2017). Also, BBR improved insulin sensitivity (InsS) by inhibiting fat storage and adjusting the adipokine profile in human preadipocytes (Yang et al., 2012). The hypoglycemic effects of BBR have also been attributed to its acute activation of the transport activity of glucose transporter 1 (GLUT1) (Cok et al., 2011).
Numerous studies of BBR in in vitro models have shed light on its positive effect on T2DM. BBR promoted glucose uptake and inhibited gluconeogenesis by inhibiting SIRT3, and regulating the mitochondria-related pathways (Zhang et al., 2018). BBR treatment attenuated a palmitate-induced reduction in glucose uptake and consumption through a reduction in cellular diacylglycerol (DAG) levels and the accumulation of triacylglycerol (TAG) in H9c2 cells (Chang et al., 2016). In addition, BBR displayed beneficial effects in the treatment of diabetes and obesity via stimulation of AMPK activity (Lee et al., 2006). The mechanisms of action of BBR in treatment of T2DM are suggested to be different than that of metformin and rosiglitazone (Zhang et al., 2010). BBR, as an insulin signal activator, had shown insulin-mimicry effects through the inhibition of protein tyrosine phosphatase 1B (PTP1B) activity on both adipocytes and myocytes (Chen et al., 2010) and acted as an effective insulin sensitizing and insulinotropic agent (Ko et al., 2005). Moreover, BBR and metformin promoted glucose metabolism by stimulating glycolysis through the inhibition of mitochondrial respiratory chain complex I and independent of AMPK activation (Xu et al., 2014). Besides, BBR circumvented the insulin signaling pathways and stimulated the glucose uptake through the AMP-AMPK-p38 MAPK pathway (Cheng et al., 2006). BBR modulated metabolism-related PPARs expression and differentiation-related positive transcription elongation factor b (P-TEFb) expression in adipocytes, which are associated with its hypoglycemic and hypolipidemic effects (Zhou and Zhou, 2010). In addition, BBR upregulated LDL receptor expression through Ras-independent (but AMPK-dependent) Raf-1 activation in liver cells (Li et al., 2014). BBR and metformin induced glycolysis and glucose consumption but are not related to the AMPK status (Xiao et al., 2018).
Different natural and synthetic derivatives of berberine are also evaluated for their in vitro activities. A BBR derivative, thalifendine, showed upregulatory activities for both LDLR and InsR, proving to be a potential treatment of both hyperlipidemia and hyperglycemia (Wang et al., 2009). Similarly, BBR amide derivatives improved the glucose-lowering effects (Ren et al., 2017). Mannose-modified BBR derivative exhibited high anti-diabetic activity at both high and low drug concentrations (Han et al., 2019). Palmatine showed anti-diabetic activity mediated through an insulin-dependent pathway by the activation of IRTK and PI3K (Sangeetha et al., 2013). Pseudoberberine (pBBR) has exhibited a potential effect on AMPK activation and LDLR upregulation as compared with BBR (Wang et al., 2012).
In the same way, the effects of extracts of species of the genus Berberis have been studied in several in vitro models and found effective. For instance, B. mycrophylla root extracts showed hypoglycemic effects and stimulated glucose uptake in HepG2 cells with and without resistance by activating AMPK protein (Furrianca et al., 2017). B. aristata bark methanolic extracts also inhibited the dipeptidyl peptidase–IV (DPP-IV) enzyme activity (Chakrabarti et al., 2011). B. vulgaris roots (ethanolic extract) and BBR showed α-glucosidase inhibition, where the inhibition caused by the extract was found to be higher than that of the BBR alone (Abd El-Wahab et al., 2013), and the extract also showed α-amylase inhibition activity (Boudjelthia et al., 2017).
Some of the mechanisms of Berberis species and BBR against diabetes and metabolic diseases are depicted in Figure 4.
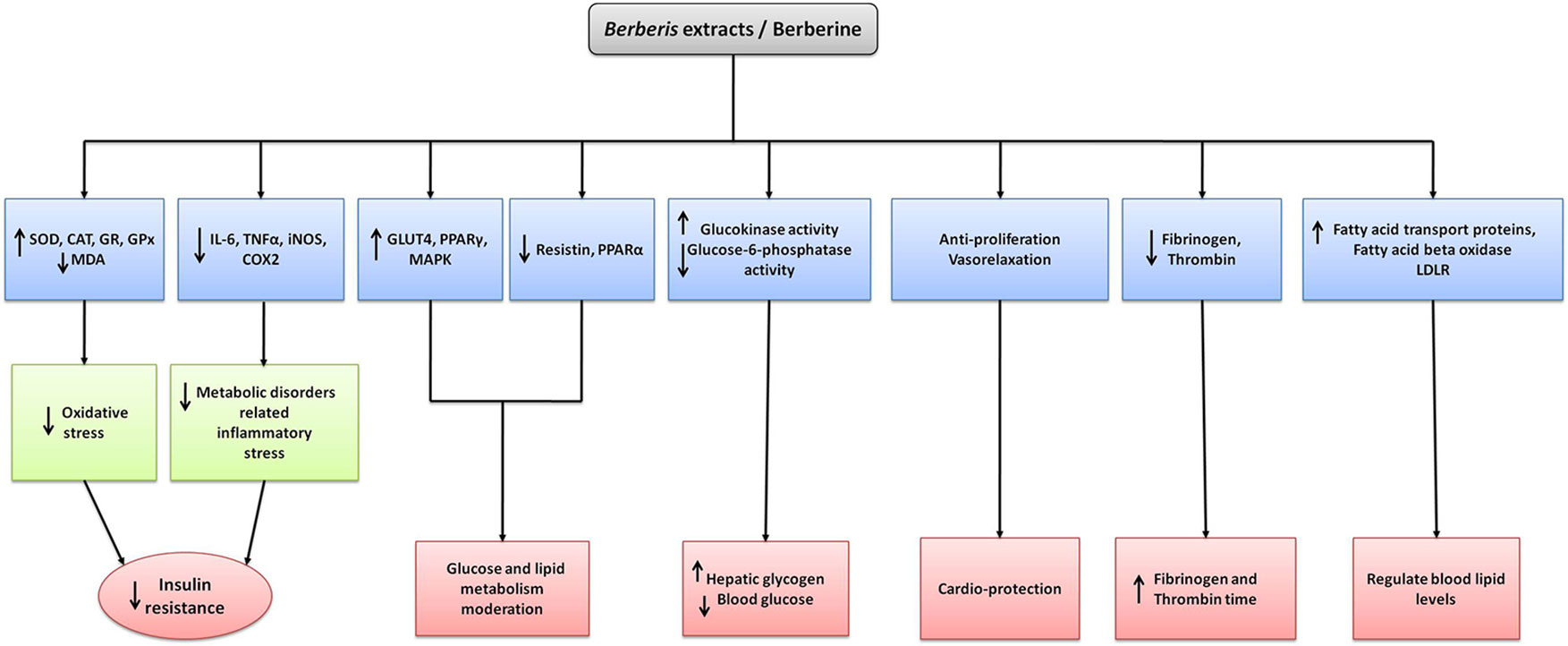
Figure 4 The mechanism of action of extracts and its major isolated alkaloid of Berberis species in the treatment of diabetes and metabolic syndrome. Berberis spp. and berberine upregulate the anti-oxidant enzymes while decreasing reactive oxygen species and inflammatory mediators which in turn decreases oxidative and inflammatory stresses and thus decreasing insulin resistance. Upstream regulating expression of GLUT4, PPARγ, MAPK and downstream regulation of resistin, PPARα results glucose and lipid metabolism moderation. Increase in AMPK and glucokinase activities while decrease in glucose-6-phosphate activity results in decreasing gluconeogenesis, restoring hepatic glycogen and blood glucose. Upregulating AMPK and p38 MAPK activities also cause increasing insulin action and decreasing lipid synthesis. Antiproliferative action and vasorelaxation results in cardioprotection whereas decrease in fibrinogen and thrombin results in increasing fibrinogen and thrombin time respectively. Increasing expression of fatty acid transport proteins, fatty acid beta oxidase and LDLR aids in regulating blood lipid levels.
Extracts of Berberis species and their components, especially alkaloids, have been documented for their potential activity against T2DM and MS in in vivo models (Table 3). In the MS condition, BBR improved vascular inflammation and remodeling that was found to be correlated with the ability to inhibit p38 MAPK activation, ATF-2 phosphorylation, and MMP-2 expression (Li et al., 2015). Long-term treatment with BBR diminished the adipose tissue weight and decreased the renal injury (MS related diseases) in spontaneously hypertensive rats (Kishimoto et al., 2015). In normal diet-fed mice treated with BBR, hepatic CD36 expression and TG levels were increased; however, these effects were prevented when hepatic CD36 was silenced with an adenovirus containing CD36-specific short hairpin RNAs (shRNA) (Choi et al., 2017). BBR also improved the insulin-mediated vasodilatation of mesenteric arteries in diabetic rats through upregulation of insulin receptor-mediated signaling and increasing vascular InsS (Geng et al., 2016). Similarly, BBR increased both InsR and the low-density lipoprotein receptor (LDLR) expression, which resulted in a cellular response against InsR (Kong et al., 2009). In hyperlipidemic hamsters, the cholesterol-lowering effect of BBR was found to be due to its activity on upregulation of hepatic LDLR (Kong et al., 2004). Administration of BBR in hyperlipidemic and InsR rats decreased blood free fatty acid levels and increased the activity of lipoprotein lipase, leading to the amelioration of blood lipid and glucose metabolism (He et al., 2004). BBR administration resulted in the decrease of fasting blood glucose (FBL) level and ameliorated glycogen structural fragility (Li et al., 2019). Furthermore, BBR displayed beneficial effects in the treatment of obesity, and this was in part via improvement of adipose tissue fibrosis (Wang L. et al., 2018). BBR was reported to act in the liver to regulate lipid utilization and to maintain whole-body energy metabolism by mediating autophagy and FGF21 activation (Sun Y. et al., 2018). Additionally, BBR is also reported to reduce the systemic low-grade inflammation of T2DM mice to alleviate disease, and this effect may be achieved through regulating the gut microbes or inhibiting the TLR4 signaling pathway (Cao et al., 2017). Other in vivo investigations also showed the hypoglycemic effects of BBR through the improvement in gut-derived hormones and the attenuation of both intestinal mucosal mechanic and immune barrier damages (Gong et al., 2017). In the same way, the gut microbiota modulation was also suggested to be an effective mechanism of the antidiabetic effect of BBR (Han et al., 2011). The lipid-lowering effect of BBR chloride treatment in hyperlipidemic rats was found to be associated with a global change in the metabolism of lipids, carbohydrates, and amino acids as well as the structure of microbiota (Li et al., 2016).

Table 3 In vivo activity extracts and/or isolated compounds from Berberis species against diabetes and metabolic diseases.
On the other hand, BBR protects against metformin-associated lactic acidosis (MALA) in streptozotocin (STZ)-induced T2DM (Almani et al., 2017). BBR attenuated hyperglycemia and its associated oxidative stress and inflammation through, possibly, the potentiation of the antioxidant defenses and upregulation of PPARγ expression (Mahmoud et al., 2017). BBR decreased 2-hour postprandial plasma glucose (2h-PPG) level in STZ-induced diabetic rats by locally inhibiting intestinal DPP-IV (Wang J. et al., 2016). Moreover, BBR also reduced the blood glucose level in diabetic rats, improving the blood lipid and decreasing the retinal vascular injury, suggesting its association with the reduced expressions of Nrf2/HO-1 (Tao et al., 2017). BBR also upregulated protein expressions of LKB1, AMPK, p-AMPK, and p-TORC2 and also inhibited the translocation of TOCR2 into the cell nucleus (Jiang et al., 2015). Moreover, BBR was also found to be effective in lowering blood glucose and lipid levels, reducing the body weight, and alleviating the oxidative stress in diabetic hamsters (Liu et al., 2015).
The anti-diabetic effect of BBR was suggested to be mainly due to its activity in the regulation of glycometabolism and lipometabolism and the activation of AMPK (Lee et al., 2006; Dong et al., 2016). BBR improved glucose metabolism through an insulin-independent pathway (Xia et al., 2011). BBR also significantly inhibited the progression of diabetes induced by alloxan, and the effect of BBR on diabetes was suggested to be associated with its hypoglycemic effect, modulating lipids metabolic effects and its ability to scavenge free radicals (Tang et al., 2006). BBR improved glucolipid metabolism in diabetic rats both in the blood and liver, possibly through modulating the metabolic related PPARα/δ/γ protein expression in liver (Zhou et al., 2008). BBR targeted MAPK to suppress Th17 and Th1 differentiation in T1DM NOD mice and showed a novel role of ERK in Th17 differentiation through downregulation of STAT3 phosphorylation and RORt expression (Cui et al., 2009). Altered hepatic SREBPs, LXRα, and PPARα transcriptional programs were suggested to be involved in the therapeutic mechanisms of BBR on fat-induced hepatic insulin resistance (FIHIR) in T2DM hamsters (Liu et al., 2010). The inhibitory effect on intestinal disaccharidases and β-glucuronidase of BBR might be one of the mechanisms for BBR as an antihyperglycaemic agent (Liu et al., 2008). BBR caused the glucose metabolism modulation by the GnRH-GLP-1 and MAPK pathway in the gut (Zhang et al., 2014). The treatment of BBR chloride notably protected the blood components (Chandirasegaran et al., 2017) and significantly reversed the abnormal levels of lipids, oxidant status, and insulin signaling molecules in the diabetic rat model (Chandirasegaran et al., 2019). BBR also reduced the release of lipopolysaccharides and ameliorated inflammation by reducing the level of lipolysaccharide binding protein (LBP), thus alleviating intestinal injury and improving InsR (Cui et al., 2018).
The combination of Ortosiphon staminensis, policosanol, red yeast rice extract, BBR, folic acid, and coenzyme Q10 provided an antihypertensive effect, which allowed for an effective control of blood pressure in patients with MS (Rozza et al., 2009). The berberine-metformin hybrid compound BMH473 was found to be beneficial for maintaining glucose and lipid homeostasis in T2DM rats, and it exhibited better anthyperlipidaemic effects compared to metformin and BBR alone (Jia et al., 2019).
Combining timosaponin B2 (TB-2) and BBR in a single formulation enhanced the anti-diabetic efficacy by improving the intestinal absorption (Huang et al., 2019). Glycyrrhizic acid was also reported to improve the oral absorption of BBR by inhibiting P-gp, and it thus increased the anti-diabetic effects of BBR in db/db mice (Qiao et al., 2018). Lipid-lowering effects of BBR were also reported to be increased with resveratrol, which may be associated with upregulation of a low-density-lipoprotein (LDL) receptor (Zhu et al., 2018). Similarly, gelucire44/14 was found to enhance the oral absorption of BBR and thus improve the antidiabetic efficacy of BBR (Sun J. et al., 2018). Berberine organic acids (BOAs) were found to be comparable to berberine hydrochloride (BH) in terms of hypoglycaemic effects, they were but superior with regard to safety from hyperchloraemia in T2DM rats (Li et al., 2017). Coptis chinensis (containing berberine) and BBR exerted similar effects when used for the treatment of T2MD rats, mainly via the stimulation of the pancreatic secretion of insulin (Jiang et al., 2017). Berberine chloride was a stronger antidiabetic agent than BBR or canagliflozin alone with fewer side effects on kidneys in the diabetic mice (Cai-Ming et al., 2016). BBR and ginsenoside Rb1 (Rb1) improve abnormal metabolism of glucose and lipid (Shang et al., 2015).
Extracts of Berberis plants have shown interesting results in in vivo models. The ethanolic extract of B. aristata showed antidiabetic activity due to its significant dose-dependent reduction effect on the blood glucose levels (Semwal et al., 2008; Mittal et al., 2012), which were also reported to be better than glibenclamide (Rameshwar et al., 2009) and comparable to metformin in diabetic rats (Pareek and Suthar, 2010). In addition, the aqueous extract of B. aristata showed significant antidiabetic activity, decreased total cholesterol, increased HDL-C levels, and prevented the body weight loss in diabetic rats (Ahamad et al., 2012).
The aqueous extract of B. lycium roots showed an antihyperlipidemic effect (Ahmad et al., 2008). B. lycium leaf extracts alleviated lipid profile levels and might be used efficiently in hyperglycemic and diabetic patients (Hussain et al., 2017). Also, the root extract of B. lycium reduced the serum glucose levels in normal and diabetic rats (Gulfraz et al., 2007). In chicken Broilers, the powder of B. lycium reduced the serum cholesterol (Chand et al., 2007). The oral administration of extracts of B. lycium showed hypoglycemic activity (Mustafa et al., 2011) and alleviated lipid profile levels (Rahimi Madiseh et al., 2014). Similarly, the methanolic extract of the B. lycium root and its main alkaloid BBR showed hypoglycemic activity (Gulfraz et al., 2008) and showed antiglycation activity (Khan et al., 2014).
On the other hand, in diabetic rats, the beneficial effects of B. vulgaris extracts showed positive effects in attenuating the side effects of T2DM (Karami et al., 2016), ameliorating oxidative stress (Hemmati et al., 2016), decreasing the liver damage by influencing hepatic histopathological and biochemical markers (Rahimi-Madiseh et al., 2017), and showed that the serum cholesterol and serum triglycerides levels were decreased (Meliani et al., 2011).
Other species of Berberis have also been studied. For instance, B. asiatica hydro-ethanolic root extracts have shown to be a potent orally effective antidiabetic extract (Singh and Jain, 2010). Likewise, the B. dictyophylla cortex could significantly reduce the level of fasting blood glucose, ICAM-1, and ANG II expression (Yue et al., 2013). The B. holstii extract showed the reduction of blood glucose levels (Kimani et al., 2017). Furthermore, the aqueous extract of B. integerrima roots improved renal dysfunction in STZ-induced diabetic rats through controlling blood glucose, and it also showed renal protective effects (Ashraf et al., 2013). The anthocyanin fraction of the fruits of B. integerrima also showed hypoglycemic effects (Sabahi et al., 2016). Moreover, the methanolic extract of B. julianae roots was also reported to possess promising beneficial effects for the treatment of T2DM with the possible mechanism via stimulating AMPK activity (Yang et al., 2014).
Other alkaloids isolated from Berberis species have also shown promising activities against T2DM and MS. For example, berbamine increased the activity of metabolic enzymes and preserved the glucose homeostasis in HFD/STZ induced diabetic rats (Chandrasekaran et al., 2018). Jatrorrihizine (JAT) induced an important decrease in FBG in normal and hyperglycemic mice, attributed to improve in aerobic glycolysis (Yan et al., 2005). JAT, BBR, and a combination of BBR and JAT decreased the FBG of diabetic and normal mice at different degrees. JAT also possessed the function of decreasing FBG, which was found less than that of BBR at the same dose level (Fu et al., 2005). Palmatine was also found to decrease FBG and suppressed the increase of blood glucose level in normal rats (Patel and Mishra, 2011).
Several pilot studies as well as pre-clinical studies and clinical trials have evaluated the beneficial effects of Berberis extracts and isolated compounds on diabetes, metabolic syndrome, and other metabolic diseases (Table 4).

Table 4 Studies in diabetic and/or metabolic syndrome patients using treatment with extract and/or isolated compounds of Berberis species.
The administration of BBR in patients with MS was found to be effective in regulating the blood glucose and blood lipid levels, improving the InsR, and reducing the level of inflammatory responses in the body (Cao and Su, 2019). BBR also decreased the waist circumference, systolic blood pressure (SBP), triglycerides, and total insulin secretion along with an increase in InsS (Pérez-Rubio et al., 2013). BBR was suggested as a promising new hypolipidemic drug that acts through signaling pathways distinct from those of statins in the treatment of hyper mild mixed hyperlipidemia patients (Kong et al., 2004). Besides, BBR has been shown to have a good potential as a drug to control lipid metabolism alone or in combination with other drugs for hyperlipidemic hepatitis or liver cirrhosis patients (Zhao et al., 2008). Moreover, BBR improved the InsS by limiting fat storage and adjusting adipokine profile in human preadipocytes and MS patients (Yang et al., 2012), and attenuated some of the metabolic and hormonal derangements in women with polycystic ovary syndrome (PCOS (Wei et al., 2012). The administration of BBR was found to be effective in the regulation of blood glucose and blood lipid in T2DM patients (Ming et al., 2018) and in improving diabetic kidney disease by reducing UACR and serum Cys C (Li et al., 2018). On the other hand, BBR had also shown glucose-lowering activity with a mechanism different from metformin and rosiglitazone (Zhang et al., 2010). In pilot study, BBR demonstrated a potent oral hypoglycemic activity with positive effects on lipid metabolism (Yin et al., 2008). Also, the benefits of BBR in lowering blood glucose, lipids, body weight, and blood pressure have been confirmed in T2DM and MS patients (Zhang et al., 2008). BBR played an important role in the treatment T2DM through downregulating the higher levels of free fatty acids (Gu et al., 2010). In another study, BBR reduced the fasting plasma glucose, post-meal blood glucose, and fructosamine; however, no signification changes were found in lipid profiles, fasting insulin, HOMA-IR, and HOMA-β% in T2DM patients (Rashidi et al., 2018).
In addition, BBR improved the glycemic parameters comparable to metformin in T2DM patients (Dange et al., 2016). BBR significantly ameliorated T2DM via modulation of Bifidobacterium species, TNF-α, and LPS (Chen et al., 2016). BBR improved the blood lipid level in mild hyperlipidemia patients (Wang L, et al., 2016). Likewise, it reduced the plasma LDL-C and TG in mixed hyperlipidaemic subjects (Cicero and Ertek, 2008).
The combination of BBR and simvastatin (SIMVA) in hypercholesterolemic patients significantly improved LDL-receptor upregulation and LDL-cholesterol downregulation compared to monotherapies, and the combined effect also reduce the statins dosage (Kong et al., 2008). The administration of BBR along with red yeast and policosanol on a daily basis was found to be effective in reducing cholesterol levels and was associated with the enhancement of endothelial function and InsS (Affuso et al., 2010). The administration of this supplementation in patients with familial hypercholesterolemia heterozygotes on stable treatment with LDL-C-lowering validated that the supplement reduced the LDL-C superior to that obtained by doubling the dose of statins (Pisciotta et al., 2012).
Also, the dietary supplement Armolipid Plus™ composed of BBR, red yeast rice, policosanol, folic acid, coenzyme Q10, and astaxanthin showed significant reduction of cholesterolemia and positive plasma LDL-C levels in elderly (statin-intolerant) hypercholesterolemic patients (Marazzi et al., 2011). Moreover, it reduced LDL-C levels as well as total cholesterol/HDLc and ApoB/ApoA1 ratios, and it increased the Apo A1; tjos demonstrated the improvements in CVD risk indicators in patients with hypercholesterolemia (Sola et al., 2014) and amelioration of blood lipids and significant reduction of global CVD risk in dyslipidemic patients (Trimarco et al., 2011). In patients with low- to moderate-risk hypercholesterolemia, Armolipid Plus ™ in association with a hypolipidic diet significantly reduced the total cholesterol and LDL-C levels (Gonnelli et al., 2015). In addition, Armolipid Plus ™ improved the lipid profile similar to a low dose of a standard statin and also increased the HDL-C levels and improved the leptin-to-adiponectin ratio in patients with moderate dyslipidemia and MS (Ruscica et al., 2014). Armolipid Plus™ alone or in combination with ezetimibe enhanced the lipid profile in statin-intolerant patients with coronary heart disease (Marazzi et al., 2015). BBR and Armolipid Plus™ could be a useful alternative to correct dyslipidemias and to reduce CVD risk in subjects with moderate mixed dyslipidemias (Cicero et al., 2007).
Other food supplements containing BBR, including Body Lipid™, were suggested as an alternative to pharmacological treatment for patients with mild-to-moderate hypercholesterolemia (D'Addato et al., 2017). A new nutraceutical formulation containing BBR, monacolin K, chitosan, and coenzyme Q10 has proven effective in reducing non-HDL/LDL-C levels, representing an emergent therapeutic strategy in dyslipidemic patients (Spigoni et al., 2017). On the other hand, the combination of BBR and isoflavones was found to be effective in lowering CVD risk factors in menopausal women with moderate dyslipidaemia (Cianci et al., 2012).
Treatment with BBR and rho iso-alpha acids, vitamin D3, and vitamin K1 produced a more favorable bone biomarker profile, indicative of healthy bone metabolism in postmenopausal women with MS (Lamb et al., 2011). In a case–control study, BBR is more effective in decreasing the serum MGO levels and InsR through increasing the glycemic control in newly diagnosed T2DM patients (Memon et al., 2018). The intake of the natural formulation (containing BBR, orthosiphon, red yeast rice equivalent to monacolin, policosanol, folic acid, and coenzyme Q10) has evidenced the effective control of plasma lipids and keeps borderline high blood pressure within normal values compared with diet alone (Manzato and Benvenuti, 2014).
Stem powder of B. aristata was found to be effective in improving glycemic control and lipid profiles with no major adverse effects on T2DM patients (Sharma et al., 2017). The effect of B. vulgaris extract on T2DM and MS patients has been widely studied in humans. The intake of 3 g/d of B. vulgaris fruits aqueous extract for 3 months may have beneficial effects on lipoproteins, apoproteins, glycemic control, and TAC in T2DM patients (Shidfar et al., 2012). B. vulgaris juice reduced oxidative burden in patients with MS (Mohammadi et al., 2014). Other study showed the beneficial effects of processed B. vulgaris on certain atherosclerosis risk factors in T2DM patients (Ebrahimi-Mamaghani et al., 2009). B. vulgaris fruit extract showed beneficial metabolic effects in T2DM patients, improving the glucose catabolism via the glycolysis pathway, stimulating the insulin secretion or improving the insulin function, and later decreasing the glucose uptake (Moazezi and Qujeq, 2014). Another study demonstrated that the B. vulgaris juice evoked regulatory roles on HOMA-IR and improved HOMA-B with the metabolic controlling insulin-related indices in benign breast disease (Asemani et al., 2018). Also, B. vulgaris supplementation in patients with MS significantly diminished anti-HSPs 27 and 60 and hs-CRP levels and improved lipid profiles (Zilaee et al., 2014). It is reported that the Hsp60 protein is able to induce the production of anti-Hsp60 antibodies, which leads to the destruction of β-islet cells. In the same way, Hsp60 acts as a proinflammatory signaling molecule, which plays a role in the non-resolved vascular inflammation, and this is recognized as one of the characteristic of T2DM (Juwono and Martinus, 2016). Others natural formulations containing Berberis have also been tested in humans. A clinical trial demonstrated that daily intake of polyherbal capsule composed by B. aristata and Cyperus rotundus, Cedrus deodara, Emblica officinalis, Terminalia chebula, and T. bellirica decreased the glucose level, enhanced lipid homeostasis, and maintained other serum biochemical levels to the normal in patients with T2DM (Awasthi et al., 2015).
The nutraceutical product Berberol®, containing a B. aristata extract (titrated in 85% BBR) plus a Silybum marianum extract (titrated in 60% silymarin), has been evaluated for its antidiabetic potential in humans. Berberol® was demonstrated to be more effective than BBR alone (administered at the same dose), reducing HbA1c in T2DM patients (Di Pierro et al., 2013). The incorporation of Berberol® into insulin therapy in patients with T1DM has the effect of a diminution of the insulin dose necessary for adequate glycemic control (Derosa et al., 2016). In dyslipidemic patients, Berberol® has proven to be safe and effective in improving lipid profile, InsR, and adipocytokines levels (Derosa et al., 2013). Berberol® also improved the cholesterol-lowering properties of statins and showed the positive effects on liver enzymes and glycemic control in patients with T2DM (Guarino et al., 2015). In addition, Berberol® significantly lowered abdominal adiposity and decreased the circulating uric acid level in overweight/obese patients with T2DM (Guarino et al., 2017). Berberol® was suggested as a good candidate for an adjunctive treatment option in diabetes, especially in patients with suboptimal glycemic control (Di Pierro et al., 2012). Berberol® administered as a single or add-on therapy in statin-intolerant subjects is an effective treatment to improve the lipidic and glycemic profiles in T2DM and hypercholesterolemia patients (Di Pierro et al., 2015). The combination of Berberol® and a reduced dosage of statin is found effective for the treatment of hyperlipidemia in patients intolerant to statins at high dosage (Derosa et al., 2015a) and in dyslipidemic euglycemic patients (Derosa et al., 2015b)
Berberol K®, was found to be a potentially good alternative in primary intervention in low cardiovascular-risk subjects with dyslipidemia, as an add-on therapy in mildly statin-intolerant patients, and as an alternative for dyslipidemic patients with a negative perception of statins (Di Pierro et al., 2017). Berberol K® reduced lipid profile effectively and improved the inflammatory parameters under a safe dose (Derosa et al., 2017). It was also found to be effective in diabetic subjects with dyslipidemia statin intolerant or with diarrhea caused by IBS or metformin (Di Pierro et al., 2018).
Few studies have also reported the effectiveness of BBR in non-alcoholic fatty liver disease (NAFLD). NAFLD is a result of abnormal fat accumulation in the liver due to the reasons other than alcohol, and it is considered to be a hepatic manifestation of MS. NAFLD results in the overproduction of sugars and triglycerides and plays a central role in the development of InsR and various other glucose- and lipid metabolism-related diseases (Yki-Järvinen, 2014). Recently, Yan et al. (2015) conducted a randomized, parallel controlled, open-label clinical trial in 188 NAFLD patients. Patients received lifestyle intervention (LSI) or LSI and 15 mg of pioglitazone qd or LSI and of BBR for 16 weeks. Parameters, including hepatic fat content, serum glucose level, serum lipid profiles, liver enzymes, and serum and urine BBR concentrations, were measured before and after treatment. LSI and BBR showed a reduction in hepatic fat content as compared to LSI and were better than pioglitazone in reducing body weight and resulted in better lipid profiles (Yan et al., 2015). Furthermore, a mechanism-based study revealed that BBR reduced hepatic TG accumulation and decreased the expressions of hepatic stearyl-coenzyme A desaturase 1 (SCD1) and other TG synthesis-related genes (Zhu et al., 2019). Berberine administration was also reported to recruit and activate BAT in both humans and mice (Wu et al., 2019).
Although there are many effective therapeutic drugs for the treatment of metabolic diseases, the current treatment did not control the rapid increasing trend in diabetes mortality and morbidity. Various therapeutic agents from both natural and synthetic sources are being investigated in patients with clinical signs of diabetic and other metabolic diseases. Formulations prepared from the various plant parts of Berberis species were found to be used traditionally in the treatment of diabetes and other metabolic diseases and related complications. A review of the scientific literature revealed that the extracts, isolated alkaloids from Berberis species including BBR and their derivatives, have shown promising effects in the studies related to diabetes and other metabolic diseases. The relatively low cost of BBR or supplements or extracts containing BBR, compared to other synthetic medications, will be of an advantage to the patients living in developing countries with poor socioeconomic circumstances. However, currently available scientific evidence is still not fully sufficient to prove their efficacy clinically. Further randomized double-blind clinical trials with a large number of patients and standardized clinical assessments are required to prove the effectiveness of the Berberis extracts and isolated compounds on metabolic diseases alone or in combinations. Novel pharmacological assessment techniques and analytical techniques will further provide additional opportunities for these agents. Moreover, the development of novel formulations of berberine could be an effective strategy for increasing its effectiveness against diabetes and other metabolic diseases.
TB, IB and JE conceptualized the manuscript. TB, AB, HD, HU, HK, IB and JE wrote the initial manuscript. TB, HD, HU, AP, IB and JE revised the manuscript. All authors agreed on the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
TB and IB are thankful to the Director of GBPNIHESD for providing necessary facilities and to the State Biotech Department, Dehradun, Uttarakhand, for partial financial support under the Grant No. SBP/R&D-02/11/432. JE is grateful for support from CONICYT PAI/ACADEMIA project N° 79160109.
2h-PPG, 2-hour postprandial plasma glucose; A-FABP, Adipocyte fatty acid–binding protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AMPK, AMP-activated protein kinase; BBA, berbamine; BBD, benign breast disease; BBR, berberine; BFFAL, blood free fatty acids level; BF, berberine fumarate; BGL, blood glucose levels; BJ, Berberis juice; b.i.d., twice daily; BMI, body mass index; BP, blood pressure; BW, body weight; BWG, body weight gain; CAT, catalase; C/EBPα, CCAAT enhancer binding protein alpha; CMS, cardio metabolic syndrome; COX2, Cyclooxygenase-2; CPK, serum creatine phosphokinase; DAG, diacylglycerol; DBP, diastolic blood pressure; DVIS, diabetic vascular insulin sensitivity; DPP-IV, dipeptidyl-peptidase IV; DN, Diabetic nephropathy; eNOS, endothelial nitric oxide synthase; EZE, ezetimibe; FA, fructosamine; FASN, fatty acid synthase; FBS, Fasting Blood Sugar; FBGL, fasting blood glucose levels; FOP, fibrodysplasia ossificans progressive; FPG, fasting plasma glucose; FPI, fasting plasma Insulin; FSIL, fasting serum insulin level; GHb, glycosylated hemoglobin; GLP-1, glucagon-like peptide-1; GLUT4, Glucose transporter type 4; GPx, glutathione peroxidase; GR, glutathione reductase; HbA1c, glycated haemoglobin; HDL-C, high density lipoprotein HDL-cholesterol; HFD, High Fat Diet; HOMA-R, Homeostatic Model Assessment; HOMA-IR, and HOMA-β%; IC, insulin concentration; IFG, Impaired fasting glycemia; IL-6, Interleukin-6; iNOS, Inducible nitric oxide synthase; INSR-mRNA, insulin receptor gene messenger RNA; InsR, Insulin resistance; InsS, insulin sensitivity; LDL-C, low density lipoprotein cholesterol; LDLR, Low density lipoprotein receptor; LEL, liver enzyme levels; Lp, lipid profile; MALA, metformin-associated lactic acidosis; MAPK, Mitogen activated protein kinase; MDA, Malondialdehyde; MMP, mitochondrial membrane potential; MS, metabolic syndrome; RCT: randomized, controlled trial; OXPHOS, impaired oxidative phosphorylation; PAB, prooxidant-antioxidant balance; pBBR, pseudoberberine; PG, plasma glucose; PGs, prostaglandins; P-gp, P-glycoprotein; pi3k, phosphoinositol 3 kinase; PKC, protein kinase C; PMBG, post-meal blood glucose; PON1, Paraoxonase-1; PPARα, peroxisome proliferator activated receptor alpha; PPARγ, peroxisome proliferator activated receptor gamma; PPBG, postprandial blood glucose; SBP, systolic blood pressure; SOD, superoxide dismutase; SREBP-1, sterol regulatory element-binding protein 1; STZ, streptozotocin; T1DM: type-1 diabetes mellitus; T2DM, type-2 diabetes mellitus; TAG, triacylglycerol; TC, total cholesterol; TG, triglycerides; TIC, total insulin consumption; TIS, total insulin secretion; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor alpha; ULK1, Unc-51-like autophagy-activating kinase 1.
Abd El-Wahab, A. E., Ghareeb, D. A., Sarhan, E. E. M., Abu-Serie, M. M., El Demellawy, M. A. (2013). In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 13, 218. doi: 10.1186/1472-6882-13-218
Abu Zarga, M. H., Miana, G. A., Shamma, M. (1982). Gandharamine: a new benzylisoquinoline alkaloid from Berberis baluchistanica. Heterocycles 18, 63–65. doi: 10.3987/S(B)-1982-01-0063
Adhikari, M., Thapa, R., Kunwar, R. M., Devkota, H. P., Poudel, P. (2019). Ethnomedicinal uses of plant resources in the machhapuchchhre rural municipality of kaski district, nepal. Medicines 6, 69. doi: 10.3390/medicines6020069
Affuso, F., Ruvolo, A., Micillo, F., Saccà, L., Fazio, S. (2010). Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 20, 656–661. doi: 10.1016/j.numecd.2009.05.017
Ahamad, J., Mir, S. R., Naquivi, K. J. (2012). Hypoglycemic activity of aqueous extract of Berberis aristata stems bark in STZ-induced rats. Int. J. Pharm. Pharm. Sci. 4, 473–474.
Ahamad, J., Naquvi, K. J., Ali, M., Mir, S. R. (2014). New isoquinoline alkaloids from the stem bark of Berberis aristata. Indian J. Chem. - Sect. B. Org. Med. Chem. 53B, 1237–1241.
Ahmad, M., Alamgeer, S. T. (2009). A potential adjunct to insulin: Berberis lycium Royle. Diabetol Croat 38, 13–18.
Ahmad, M., Alamgeer, C. M. Z., Nadeem, M., Sharif, T., Ahmad, B. (2008). Hepatoprotective effect of Berberis lycium (Royle) in hepatotoxic rabbits. Gomal. Uni J. Res. 24, 1–9.
Ahmed, E., Arshad, M., Ahmad, M., Saeed, M., Ishaque, M. (2004). Ethnopharmacological survey of some medicinally important plants of galliyat areas of NWFP, Pakistan. Asian J. Plant Sci. 3, 410–415. doi: 10.3923/ajps.2004.410.415
Ahmed, B., Masoodi, M. H., Khan, S. (2008). Pachycanthine: A new isoquinoline alkaloid and its antihepatotoxic activity from Berberis pachycantha Koehne. Indian J. Chem. - Sect. B. Org. Med. Chem. 47, 945–951. doi: 10.1002/chin.200841201
Ahrendt, L. W. A. (1961). Berberis and Mahonia A taxonomic revision. J. Linn. Soc. London Bot. 57, 1–410. doi: 10.1111/j.1095-8339.1961.tb00889.x
Akhtar, M. S., Sajid, S. M., Akhtar, M. S., Ahmad, M. (2008). Hypoglycaemic effect of Berberis aristata roots, aqueous and methanolic extracts in normal and alloxan-diabetic rabbits. Pharmacologyonline 2, 845–856.
Alamzeb, M., Khan, M. R., Mamoon Ur, R. U. R., Ali, S., Khan, A. A. (2015). A new isoquinoline alkaloid with anti-microbial properties from Berberis jaeschkeana Schneid. var. jaeschkeana. Nat. Prod. Res. 29, 692–697. doi: 10.1080/14786419.2014.981187
Alamzeb, M., Omer, M., Ur-Rashid, M., Raza, M., Ali, S., Khan, B., et al. (2018). NMR, novel pharmacological and in silico docking studies of oxyacanthine and tetrandrine: bisbenzylisoquinoline alkaloids isolated from Berberis glaucocarpa roots. J. Anal. Methods Chem. 2018, 7692913. doi: 10.1155/2018/7692913
Alberti, G. (2005). Introduction to the metabolic syndrome. Eur. Hear. J. Suppl. 7, D3–D5. doi: 10.1093/eurheartj/sui021
Alemardan, A., Asadi, W., Rezaei, M., Tabrizi, L., Mohammadi, S. (2013). Cultivation of iranian seedless barberry (Berberis integerrima ‘bidaneh’): a medicinal shrub. Ind. Crops Prod. 50, 276–287. doi: 10.1016/j.indcrop.2013.07.061
Almani, S. A., Memon, I. A., Shaikh, T. Z., Khoharo, H. K., Ujjan, I. (2017). Berberine protects against metformin-associated lactic acidosis in induced diabetes mellitus. Iran. J. Basic Med. Sci. 20, 511–515. doi:10.22038/IJBMS.2017.8675
American Diabetes Association. (2019). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care 42, S13–S28. doi: 10.2337/dc19-S002
Andola, H. C., Gaira, K. S., Rawal, R. S., Rawat, M. S. M., Bhatt, I. D. (2010). Habitat-dependent variations in berberine content of Berberis asiatica Roxb. ex. DC. in Kumaon, Western Himalaya. Chem. Biodivers. 7, 415–420. doi: 10.1002/cbdv.200900041
Andola, H. C., Rawal, R. S., Bhatt, I. D. (2011). Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res. Int. 44, 2352–2356. doi: 10.1016/j.foodres.2010.07.017
Andola, H. C., Gaira, K. S., Pandey, A., Bhatt, I. D., Rawal, R. S. (2018). Influence of habitat characteristics and altitude on berberine content in Berberis jaeschkeana C.K. Schneid. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 89, 967–972. doi: 10.1007/s40011-018-1014-9
Aribi, I., Chemat, S., Hamdi-Pacha, Y., Luyten, W. (2017). Isolation of berberine tannate using a chromatography activity-guided fractionation from root bark of Berberis hispanica Boiss. & Reut. J. Liq. Chromatogr. Relat. Technol. 40, 894–899. doi: 10.1080/10826076.2017.1381850
Asemani, S., Montazeri, V., Baradaran, B., Tabatabiefar, M. A., Pirouzpanah, S. (2018). The effects of Berberis vulgaris juice on insulin indices in women with benign breast disease: a randomized controlled clinical trial. Iran. J. Pharm. Res. IJPR 17, 110–121.
Ashraf, H., Heidari, R., Nejati, V., Ilkhanipoor, M. (2013). Aqueous extract of Berberis integerrima root improves renal dysfunction in streptozotocin induced diabetic rats. Avicenna J. phytomedicine 3, 82–90.
Awasthi, H., Nath, R., Usman, K., Mani, D., Khattri, S., Nischal, A., et al. (2015). Effects of a standardized Ayurvedic formulation on diabetes control in newly diagnosed type-2 diabetics; a randomized active controlled clinical study. Complement. Ther. Med. 23, 555–561. doi: 10.1016/j.ctim.2015.06.005
Baharvand-Ahmadi, B., Bahmani, M., Eftekhari, Z., Jelodari, M., Mirhoseini, M. (2016). Overview of medicinal plants used for cardiovascular system disorders and diseases in ethnobotany of different areas in Iran. J. HerbMed. Pharmacol. 5, 39–44. doi: 10.15171/jnp.2016.08
Bahmani, M., Zargaran, A., Rafieian-Kopaei, M., Saki, K. (2014). Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Biomed. 7, S348–S354. doi: 10.1016/S1995-7645(14)60257-1
Bai, M., Liu, Y., Zhou, F., Zhang, Y., Zhu, Q., Zhang, L., et al. (2018). Berberine inhibits glucose oxidation and insulin secretion in rat islets. Endocr. J. 65, 469–477. doi: 10.1507/endocrj.EJ17-0543
Bajpai, V., Singh, A., Arya, K. R., Srivastava, M., Kumar, B. (2015). Rapid screening for the adulterants of Berberis aristata using direct analysis in real-time mass spectrometry and principal component analysis for discrimination. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 32, 799–807. doi: 10.1080/19440049.2015.1022885
Belwal, T., Dhyani, P., Bhatt, I. D., Rawal, R. S., Pande, V. (2016). Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 207, 115–124. doi: 10.1016/j.foodchem.2016.03.081
Belwal, T., Giri, L., Bhatt, I. D., Rawal, R. S., Pande, V. (2017). An improved method for extraction of nutraceutically important polyphenolics from Berberis jaeschkeana C.K. Schneid. fruits. Food Chem. 230, 657–666. doi: 10.1016/j.foodchem.2017.03.086
Bhakuni, D. S., Shoeb, A., Popli, S. P. (1968). Medicinal plants. I. Chemical constituents of Berberis asiatica. Indian J. Chem. 6, 123–127.
Bhardwaj, D., Kaushik, N. (2012). Phytochemical and pharmacological studies in genus Berberis. Phytochem. Rev. 11, 523–542. doi: 10.1007/s11101-013-9272-x
Boudjelthia, K., Hammadi, K., Kouidri, M., Djebli, N. (2017). Evaluation of antidiabetic activity of two plants Berberis vulgaris and Zygophyllum geslini. J. Phys. Chem. Biophys. 7, 398–2161. doi: 10.4172/2161-0398.1000236
Brazdovicova, B., Kostalova, D., Slavik, J., Tomko, J. (1975). Alkaloids of Berberis julianae. Chem. Zvesti 29, 265–268.
Bullard, K. M., Cowie, C. C., Lessem, S. E., Saydah, S. H., Menke, A., Geiss, L. S., et al. (2018). Prevalence of diagnosed diabetes in adults by diabetes type — United States 2016. Morb. Mortal. Wkly. Rep. 67, 359–361. doi: 10.15585/mmwr.mm6712a2
Cai-Ming, T., Jiang, X., Ouyang, X.-X., Zhang, Y.-O., Wei-Dong, X. I. E. (2016). Berberine enhances antidiabetic effects and attenuates untoward effects of canagliflozin in streptozotocin-induced diabetic mice. Chin. J. Nat. Med. 14, 518–526. doi: 10.1016/S1875-5364(16)30061-9
Cao, C., Su, M. (2019). Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp. Ther. Med. 17, 3009–3014. doi: 10.3892/etm.2019.7295
Cao, W., Hu, L., Chen, H., Gao, Y., Liang, Y., Wu, Y., et al. (2017). Berberine alleviates chronic inflammation of mouse model of type 2 diabetes by adjusting intestinal microbes and inhibiting TLR4 signaling pathway. Int. J. Clin. Exp. Med. 10, 10267–10276.
Chakrabarti, R., Bhavtaran, S., Narendra, P., Varghese, N., Vanchhawng, L., Mohamed Sham Shihabudeen, H., et al. (2011). Dipeptidyl peptidase-IV inhibitory activity of Berberis aristata. J. Nat. Prod. 4, 158–163.
Champion, S. H., Seth, S. K. (1968). A revised survey of the forest types of India (Delhi: Publisher-Manager of Publications).
Chand, N., Durrani, F. R., Qureshi, M. S., Durrani, Z. (2007). Role of Berberis lycium in reducing serum cholesterol in broilers. Asian-australasian J. Anim. Sci. 20, 563–568. doi: 10.5713/ajas.2007.563
Chandirasegaran, G., Elanchezhiyan, C., Kavisa, G., Hemalatha, S. (2017). Protective role of berberine chloride on blood components in streptozotocin induced diabetic rats. Chem. Pharm. Res. 9, 69–73. doi: 10.7897/2230-8407.079107
Chandirasegaran, G., Elanchezhiyan, C., Ghosh, K. (2019). Modulatory effects of berberine chloride on lipid profile, oxidant status and insulin signaling molecules in Streptozotocin induced diabetic rats. Indian J. Clin. Biochem. 34, 254–262. doi: 10.1007/s12291-018-0754-x
Chandra, P., Purohit, A. N. (1980). Berberine contents and alkaloid profile of Berberis species from different altitudes. Biochem. Syst. Ecol. 8, 379–380. doi: 10.1016/0305-1978(80)90040-X
Chandrasekaran, S., Ramajayam, N., Pachaiappan, P. (2018). Ameliorating effect of berbamine on hepatic key enzymes of carbohydrate metabolism in high-fat diet and streptozotocin induced type 2 diabetic rats. Biomed. Pharmacother. 103, 539–545. doi: 10.1016/j.biopha.2018.04.066
Chang, Y., Ge, A., Donnapee, S., Li, J., Bai, Y., Liu, J., et al. (2015). The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 164, 210–222. doi: 10.1016/j.jep.2015.02.018
Chang, W., Chen, L., Hatch, G. M. (2016). Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1861, 352–362. doi: 10.1016/j.bbalip.2015.12.017
Chen, C., Zhang, Y., Huang, C. (2010). Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 397, 543–547. doi: 10.1016/j.bbrc.2010.05.153
Chen, L., Lu, W., Li, Y. (2016). Berberine ameliorates type 2 diabetes via modulation of Bifidobacterium species, tumor necrosis factor-alpha, and lipopolysaccharide. Int. J. Clin. Exp. Med. 9, 9365–9372.
Cheng, Z., Pang, T., Gu, M., Gao, A.-H., Xie, C.-M., Li, J.-Y., et al. (2006). Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta - Gen. Subj. 1760, 1682–1689. doi: 10.1016/j.bbagen.2006.09.007
Choi, Y.-J., Lee, K.-Y., Jung, S.-H., Kim, H. S., Shim, G., Kim, M.-G., et al. (2017). Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 in mice. Toxicol. Appl. Pharmacol. 316, 74–82. doi: 10.1016/j.taap.2016.12.019
Choudhary, A. S., Sharma, A., Sharma, P., Joshi, Y. C., Sharma, M. C., Dobhal, M. P. (2010). Isolation and characterization of isoquinoline alkaloids from methanolic extract of Berberis chitria Lindl. J. Indian Chem. Soc 87, 635–636.
Chow, Y.-L., Sogame, M., Sato, F. (2016). 13-Methylberberine, a berberine analogue with stronger anti-adipogenic effects on mouse 3T3-L1 cells. Sci. Rep. 6, 38129. doi: 10.1038/srep38129
Cianci, A., Cicero, A. F. G., Colacurci, N., Matarazzo, M. G., De Leo, V. (2012). Activity of isoflavones and berberine on vasomotor symptoms and lipid profile in menopausal women. Gynecol. Endocrinol. 28, 699–702. doi: 10.3109/09513590.2011.652250
Cicero, A. F. G., Ertek, S. (2008). Natural sources of antidyslipidaemic agents: is there an evidence-based approach for their prescription? Med. J. Nutr. Metab. 1, 85–93. doi: 10.3233/s12349-008-0011-6
Cicero, A. F. G., Rovati, L. C., Setnikar, I. (2007). Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. Arzneimittelforschung 57, 26–30. doi: 10.1055/s-0031-1296582
Cok, A., Plaisier, C., Salie, M. J., Oram, D. S., Chenge, J., Louters, L. L. (2011). Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 93, 1187–1192. doi: 10.1016/j.biochi.2011.04.013
Cui, G., Qin, X., Zhang, Y., Gong, Z., Ge, B., Zang, Y. Q. (2009). Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J. Biol. Chem. 284, 28420–28429. doi: 10.1074/jbc.M109.012674
Cui, H.-X., Hu, Y.-N., Li, J.-W., Yuan, K. (2018). Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid. Med. Cell. Longev. 2018, 8930374. doi: 10.1155/2018/8930374
D'Addato, S., Scandiani, L., Mombelli, G., Focanti, F., Pelacchi, F., Salvatori, E., et al. (2017). Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q(10) on lipid levels: a randomized, double-blind, placebo controlled study. Drug Des. Devel. Ther. 11, 1585–1592. doi: 10.2147/DDDT.S128623
Dahlberg, C. J., Ou, J. J., Babish, J. G., Lamb, J. J., Eliason, S., Brabazon, H., et al. (2017). A 13-week low glycemic load diet and lifestyle modification program combining low glycemic load protein shakes and targeted nutraceuticals improved weight loss and cardio-metabolic risk factors. Can. J. Physiol. Pharmacol. 95, 1414–1425. doi: 10.1139/cjpp-2016-0704
Dange, S. V., Shende, S. S., Rane, B. T., Tilak, A. V., Vaidya, M. U., Limaye, M. V. (2016). An observational study of the antidiabetic activity of berberine in newly diagnosed type 2 diabetes mellitus patients. J. Pharm. Biomed. Sci. 6, 230–233.
Davì, G., Santilli, F., Patrono, C. (2010). Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc. Ther. 28, 216–226. doi: 10.1111/j.1755-5922.2010.00179.x
Deedwania, P. C., Volkova, N. (2005). Current treatment options for the metabolic syndrome. Curr. Treat. Options Cardiovasc. Med. 7, 61–74. doi: 10.1007/s11936-005-0007-1
Derosa, G., Bonaventura, A., Bianchi, L., Romano, D., D'Angelo, A., Fogari, E., et al. (2013). Effects of Berberis aristata/Silybum marianum association on metabolic parameters and adipocytokines in overweight dyslipidemic patients. J. Biol. Regul. Homeost. Agents 27, 717–728.
Derosa, G., Romano, D., D'Angelo, A., Maffioli, P. (2015a). Berberis aristata/Silybum marianum fixed combination (Berberol®) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: a randomized, placebo-controlled, clinical trial. Phytomedicine 22, 231–237. doi: 10.1016/j.phymed.2014.11.018
Derosa, G., Romano, D., D'Angelo, A., Maffioli, P. (2015b). Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis 239, 87–92. doi: 10.1016/j.atherosclerosis.2014.12.043
Derosa, G., D'Angelo, A., Maffioli, P. (2016). The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 35, 1091–1095. doi: 10.1016/j.clnu.2015.08.004
Derosa, G., D'Angelo, A., Romano, D., Maffioli, P. (2017). Effects of a combination of Berberis aristata, silybum marianum and monacolin on lipid profile in subjects at low cardiovascular risk; a double-blind, randomized, placebo-controlled trial. Int. J. Mol. Sci. 18, 343. doi: 10.3390/ijms18020343
Di Pierro, F., Villanova, N., Agostini, F., Marzocchi, R., Soverini, V., Marchesini, G. (2012). Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes. Metab. Syndr. Obes. 5, 213–217. doi: 10.2147/DMSO.S33718
Di Pierro, F., Putignano, P., Villanova, N., Montesi, L., Moscatiello, S., Marchesini, G. (2013). Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin. Pharmacol. Adv. Appl. 5, 167–174. doi: 10.2147/CPAA.S54308
Di Pierro, F., Bellone, I., Rapacioli, G., Putignano, P. (2015). Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab. Syndr. Obes. Targets Ther. 8, 89–96. doi: 10.2147/DMSO.S78877
Di Pierro, F., Putignano, P., Ferrara, T., Raiola, C., Rapacioli, G., Villanova, N. (2017). Retrospective analysis of the effects of a highly standardized mixture of Berberis aristata, Silybum marianum, and monacolins K and KA in patients with dyslipidemia. Clin. Pharmacol. Adv. Appl. 9, 1–9. doi: 10.2147/CPAA.S120032
Di Pierro, F., Putignano, P., Villanova, N. (2018). Retrospective analysis of the effects of a highly standardized mixture of Berberis aristata, Silybum marianum, and monacolins K and KA in diabetic patients with dyslipidemia. Acta Biomed. 88, 462–469. doi: 10.23750/abm.v88i4.5851
Dong, H., Wang, N., Zhao, L., Lu, F. (2012). Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evidence-Based Complement. Altern. Med. 2012, 591654. doi: 10.1155/2012/591654
Dong, Y., Chen, Y.-T., Yang, Y.-X., Zhou, X.-J., Dai, S.-J., Tong, J.-F., et al. (2016). Metabolomics study of type 2 diabetes mellitus and the antidiabetic effect of Berberine in Zucker Diabetic fatty rats using Uplc-ESI-Hdms. Phyther. Res. 30, 823–828. doi: 10.1002/ptr.5587
Durmuskahya, C., Öztürk, M. (2013). Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malaysiana. 42, 1431–1438. doi: 10.2174/2210289201304010288
Ebrahimi-Mamaghani, M., Arefhosseini, S. R., Golzarand, M., Aliasgarzadeh, A., Vahed-Jabbary, M. (2009). Long-term effects of processed Berberis vulgaris on some metabolic syndrome components. Iran. J. Endocrinol. Metab. 39, 41–47.
Ezuruike, U. F., Prieto, J. M. (2014). The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J. Ethnopharmacol. 155, 857–924. doi: 10.1016/j.jep.2014.05.055
Fajardo, V., Cárcamo, C., Moreno, B. (1996). Ilicifoline: new berbine dimer alkaloid from Berberis ilicifolia. Heterocycles 43, 949–951. doi: 10.3987/COM-94-6909
Fajardo, V., Araya, M., Cuadra, P., Oyarzun, A., Gallardo, A., Cueto, M., et al. (2009). Pronuciferine N-oxide, a proaporphine N-oxide alkaloid from Berberis coletioides. J. Nat. Prod. 72, 1355–1356. doi: 10.1021/np9000976
Falco, M. R., de Vries, J. X., de Brovetto, A. G., Macció, Z., Rebuffo, S., Bick, I. R. C. (1968). Two new alkaloids from Berberis laurina billb. Tetrahedron Lett. 9, 1953–1959. doi: 10.1016/S0040-4039(01)99064-1
Faskhutdinov, M. F., Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1997). Berberis alkaloids XXXV. the structure of nummularine. Chem. Nat. Compd. 33, 70–72. doi: 10.1007/BF02273928
Fazal Hussain, S., Tariq Siddiqui, M., Shamm, M. (1980). Khyberine and the biogenesis of dimeric aporphine-benzylisqoquinoline alkaloids. Tetrahedron Lett. 21, 4573–4576. doi: 10.1016/0040-4039(80)80076-1
Feng, T., Du, H., Chen, H., Xiao, Q., He, Y., Fan, G. (2018). Comparative analysis of genetic and chemical differences between four Berberis Herbs based on molecular phylogenetic and HPLC methods. Biol. Pharm. Bull. 41, 1870–1873. doi: 10.1248/bpb.b18-00327
Fu, Y., Hu, B. R., Tang, Q., Fu, Q., Zhang, Q., Xiang, J. Z. (2005). Effect of jatrorrhizine, berberine, Huanglian Decoction and compound-mimic prescription on blood glucose in mice. Chin. Tradit. Herb. Drugs 36, 548–551.
Furrianca, M. C., Alvear, M., Zambrano, T., Fajardo, V., Salazar, L. A. (2017). Hypoglycemic effect of Berberis microphylla G Forst root extract. Trop. J. Pharm. Res. 16, 2179–2184. doi: 10.4314/tjpr.v16i9.19
Geng, F., Li, G., Zhang, X., Zhang, P., Dong, M., Zhao, Z., et al. (2016). Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br. J. Pharmacol. 173, 1569–1579. doi: 10.1111/bph.13466
Gomes, A. P., Duarte, F. V., Nunes, P., Hubbard, B. P., Teodoro, J. S., Varela, A. T., et al. (2012). Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1822, 185–195. doi: 10.1016/j.bbadis.2011.10.008
Gong, J., Hu, M., Huang, Z., Fang, K., Wang, D., Chen, Q., et al. (2017). Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front. Pharmacol. 8, 42. doi: 10.3389/fphar.2017.00042
Gonnelli, S., Caffarelli, C., Stolakis, K., Cuda, C., Giordano, N., Nuti, R. (2015). Efficacy and tolerability of a nutraceutical combination (red yeast rice, policosanols, and berberine) in patients with low-moderate risk hypercholesterolemia: a double-blind, placebo-controlled study. Curr. Ther. Res. 77, 1–6. doi: 10.1016/j.curtheres.2014.07.003
Gu, Y., Zhang, Y., Shi, X., Li, X., Hong, J., Chen, J., et al. (2010). Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 81, 766–772. doi: 10.1016/j.talanta.2010.01.015
Guarino, G., Della Corte, T., Sofia, M., Carbone, L., Marino, G., Martedì, E., et al. (2015). Metabolic effects of the association Berberis aristata/Silybum marianum: a preliminary double-blind, placebo-controlled study in obese patients with type 2 diabetes. Nutrafoods 14, 181–188. doi: 10.1007/s13749-015-0052-7
Guarino, G., Strollo, F., Carbone, L., Della Corte, T., Letizia, M., Marino, G., et al. (2017). Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Silybum marianum: a 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents 31, 495–502.
Gulfraz, M., Qadir, G., Nosheen, F., Parveen, Z. (2007). Antihyperglycemic effects of Berberis lyceum Royle in alloxan induced diabetic rats. Diabetol. Croat. 36, 49–54.
Gulfraz, M., Mehmood, S., Ahmad, A., Fatima, N., Praveen, Z., Williamson, E. M. (2008). Comparison of the antidiabetic activity of Berberis lyceum root extract and berberine in alloxan-induced diabetic rats. Phyther. Res. 22, 1208–1212. doi: 10.1002/ptr.2438
Gupta, J. K., Mishra, P., Rani, A., Mazumder, P. M. (2010). Blood glucose lowering potential of stem bark of Berberis aristata Dc in alloxan-induced diabetic rats. Iran. J. Pharmacol. Ther. 9, 20–21.
Hamayun, M., Khan, S. A., Sohn, E. Y., Lee, I.-J. (2006). Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia 11, 101–113. doi: 10.1300/J044v12n04_02
Han, J., Lin, H., Huang, W. (2011). Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 17, RA164–RA167. doi: 10.12659/MSM.881842
Han, L., Sheng, W., Li, X., Sik, A., Lin, H., Liu, K., et al. (2019). Novel carbohydrate modified berberine derivatives: synthesis and in vitro anti-diabetic investigation. MedChemComm 10, 598–605. doi: 10.1039/C9MD00036D
He, M.-K., Lu, F.-E., Wang, K.-F., Leng, S. H., Xu, L. J., Zhou, X. (2004). Effect and mechanisms of berberine on hyperlipidemic and insulin resistant rats. Chin. J. Hosp. Pharm. 24, 389–390.
Hemmati, M., Serki, E., Gholami, M., Hoshyar, R. (2016). Effects of an ethanolic extract of Berberis vulgaris fruits on hyperglycemia and related gene expression in streptozotocin-induced diabetic rats. Clin. Phytosci. 2, 1–7. doi: 10.1186/s40816-016-0017-4
Hošt'álková, A., Novák, Z., Pour, M., Jirošová, A., Opletal, L., Kuneš, J., et al. (2013). Berbanine: a new isoquinoline-isoquinolone alkaloid from Berberis vulgaris (Berberidaceae). Nat. Prod. Commun. 8, 441–442. doi: 10.1177/1934578X1300800407
Hosry, L. E., Boyer, L., Garayev, E. E., Mabrouki, F., Bun, S.-S., Debrauwer, L., et al. (2016). Chemical composition, antioxidant and cytotoxic activities of roots and fruits of Berberis libanotica. Nat. Prod. Commun. 11, 645–648. doi: 10.1177/1934578X1601100523
Hostalkova, A., Marikova, J., Opletal, L., Korabecny, J., Hulcova, D., Kunes, J., et al. (2019). Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of alzheimer's disease. J. Nat. Prod. 82, 239–248. doi: 10.1021/acs.jnatprod.8b00592
Huang, C., Zhang, Y., Gong, Z., Sheng, X., Li, Z., Zhang, W., et al. (2006). Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARγ pathway. Biochem. Biophys. Res. Commun. 348, 571–578. doi: 10.1016/j.bbrc.2006.07.095
Huang, C., Tian, X., Liu, F., Li, Z., Lin, Y., Liu, H., et al. (2019). Enhanced anti-diabetic effect of berberine combined with timosaponin B2 in Goto-Kakizaki rats, associated with increased variety and exposure of effective substances through intestinal absorption. Front. Pharmacol. 10, 19. doi: 10.3389/fphar.2019.00019
Hussain, Q., Mushtaq, W., Ishtiaq, M., Anjum, M., Faisal, M., Mazhar, M. (2017). Comparative In vivo antidiabetic evaluation of leaves and bark of Berberis lyceum Royle in alloxan induced diabetic rabbits. Int. J. Biosci. 11, 91–98. doi: 10.12692/ijb/11.2.91-98
Hussaini, F. A., Shoeb, A. (1985). Isoquinoline derived alkaloids from Berberis chitria. Phytochemistry 24, 633. doi: 10.1016/S0031-9422(00)80794-3
Imenshahidi, M., Hosseinzadeh, H. (2016). Berberis vulgaris and Berberine: an update review. Phyther. Res. 30, 1745–1764. doi: 10.1002/ptr.5693
Istatkova, R., Philipov, S., Tuleva, P., Amgalan, S., Samdan, J., Dangaa, S. (2007). Alkaloids from Mongolian species Berberis sibirica Pall. Comptes Rendus L'Academie Bulg. Des. Sci. 60, 1177–1182.
Jabeen, N., Saleem, A., Anwaar, S., Hussain, Z. (2015). Berberis lycium Royle (Royle 1837): a threatened medicinal plant and its biological activities. EC Agric. 1, 100–108.
Jia, D., Li, Z. W., Zhou, X., Gao, Y., Feng, Y., Ma, M., et al. (2019). A novel berberine-metformin hybrid compound exerts therapeutic effects on obese type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 46, 533–544. doi: 10.1111/1440-1681.13085
Jiang, S.-J., Dong, H., Li, J.-B., Xu, L.-J., Zou, X., Wang, K.-F., et al. (2015). Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. WJG 21, 7777. doi: 10.3748/wjg.v21.i25.7777
Jiang, Y., Cui, H., Wang, J., Liu, H., Dang, M., Zhang, Q., et al. (2017). Protective role of berberine and Coptis chinensis extract on T2MD rats and associated islet Rin−5f cells. Mol. Med. Rep. 16, 6981–6991. doi: 10.3892/mmr.2017.7467
Juwono, J., Martinus, R. D. (2016). Does Hsp60 Provide a link between mitochondrial stress and inflammation in diabetes Mellitus? J. Diabetes Res. 2016, 8017571. doi: 10.1155/2016/8017571
Karami, M., Sepehrimanesh, M., Koohi-Hosseinabadi, O., Fattahi, M., Jahromi, I. R., Mokhtari, M., et al. (2016). Therapeutic effects of hydroalcoholic and aqueous extracts of Berberis vulgaris fruits in streptozotocin induced type 1 diabetes mellitus rats. Rom. J. Diabetes Nutr. Metab. Dis. 23, 239–245. doi: 10.1515/rjdnmd-2016-0028
Karimov, A., Lutfullin, K. L. (1986). Berberis alkaloids. 2'-N-methylisotetrandrine from Berberis oblonga. Khimiya Prir. Soedin. 2, 249–251.
Karimov, A., Shakirov, R. (1993). Berberis alkaloids. XX. investigation of the alkaloids of Berberis iliensis. Chem. Nat. Compd. 29, 69–70. doi: 10.1007/BF00631020
Karimov, A., Telezhenetskaya, M. V., Lutfullin, K. L., Yunusov, S. Y. (1977). Berberis alkaloids. the new alkaloid oblongamine. Chem. Nat. Compd. 13, 68–70. doi: 10.1007/BF00565503
Karimov, A., Butayarov, A. B., Yusupov, M. M., Mirzamatov, R. T., Shakirov, R. S. (1992). Berberis alkaloids XIII. an investigation of the alkaloids of Berberis heteropoda. Chem. Nat. Compd. 28, 523–524. doi: 10.1007/BF00630680
Karimov, A., Abdullaev, N. D., Shakirov, R. (1993a). Berberis alkaloids. XVI. Structure of berpodine. Chem. Nat. Compd. 29, 219–221. doi: 10.1007/BF00630120
Karimov, A., Faskhutdinov, M. F., Abdullaev, N. D., Levkovich, M. G., Mil'grom, E. G., Rashkes, Y. V., et al. (1993b). Berberis alkaloids XXXII. Berberal—A new alkaloid from Berberis heterobotrys. Chem. Nat. Compd. 29, 774–777. doi: 10.1007/BF00629649
Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1993c). Berberis alkaloids. XXIII. structure of turcberine. Chem. Nat. Compd. 29, 63–67. doi: 10.1007/BF00631018
Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1993d). Berberis alkaloids. XXIV. structure of bernumine. Chem. Nat. Compd. 29, 331–334. doi: 10.1007/BF00630532
Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1993e). Berberis alkaloids. XXIX. an investigation of the alkaloids of Berberis sibirica. Chem. Nat. Compd. 29, 361–364. doi: 10.1007/BF00630540
Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1993f). Berberis alkaloids XXXI. the structure of turconidine. Chem. Nat. Compd. 29, 771–773. doi: 10.1007/BF00629648
Karimov, A., Tashkhodzhaev, B., Rashkes, Y. V., Makhmudov, M. K., Mil'grom, E. G. (1993g). Berberis alkaloids. XXI. intebrine—a new N-benzylisoquinoline alkaloid from Berberis integerrima. Chem. Nat. Compd 29, 53–57. doi: 10.1007/BF00631015
Karimov, A., Vinogradova, V. I., Shakirov, R. (1993h). Berberis alkaloids. XXII. interbrinine and intebrimine—New alkaloids from Berberis integerrima. Chem. Nat. Compd. 29, 57–60. doi: 10.1007/BF00631016
Karimov, A., Yusupov, M. M., Shakirov, R. (1993i). Berberis alkaloids. XV. Structure of bargustanine. Chem. Nat. Compd. 29, 35–38. doi: 10.1007/BF00631010
Khamidov, I., Telezhenetskaya, M. V., Karimov, A., Shakirov, R. (1995). Berberis alkaloids. XXXIII. Investigations of the alkaloids of Berberis vulgaris. Chem. Nat. Compd. 31, 417–418. doi: 10.1007/BF01165220
Khamidov, I., Faskhutdinov, M., Telezhenetskaya, M. V., Karimov, A., Levkovich, M. G., Abdullaev, N. D., et al. (1996a). Berberis alkaloids. XXXIV. Turcomanine, a new alkaloid from Berberis turcomanica. Khimiya Prir. Soedin. 1, 74–76. doi: 10.1007/BF01373793
Khamidov, I. I., Aripova, S. F., Telezhenetskaya, M. V., Faskhutdinov, M. F., Karimov, A. K., Dzhepberov, I. (1996b). Berberis alkaloids XXXVI. turcomanidine—a new alkaloid from Berberis turcomanica. Chem. Nat. Compd. 32, 873–875. doi: 10.1007/BF01374018
Khamidov, I. I., Aripova, S. F., Telezhenetskaya, M. V., Karimov, A. K. (1996c). Berberis Alkaloids XXXVIII. turcamine—a new isoquinoline alkaloid from Berberis turcomanica. Chem. Nat. Compd. 32, 880–881. doi: 10.1007/BF01374020
Khamidov, I., Karimo, A. K., Telezhenetskaya, M. V., Tashkhodzhaev, B. (1996d). Berberis alkaloids XXXV. An Investigation of Berberis turcomanica. Chem. Nat. Compd. 32, 89–90. doi: 10.1007/BF01373805
Khamidov, I. I., Aripova, S. F., Karimov, A., Yusupov, M. M. (1997a). Berberis alkaloids. XL. an investigation of the alkaloids of Berberis thunbergii. Chem. Nat. Compd. 33, 599. doi: 10.1007/BF02254817
Khamidov, I. I., Aripova, S. F., Karimov, A., Yusupov, M. M. (1997b). Berberis alkaloids. XL. an investigation of the alkaloids of Berberis thunbergii. Chem. Nat. Compd. 33, 599–599. doi: 10.1007/BF02254817
Khamidov, I. I., Aripova, S. F., Telezhenetskaya, M. V., Karimov, A., Dzhenberov, I. (1997c). Berberis alkaloids XXXIX. new alkaloids from B. densiflora. Chem. Nat. Compd. 33, 323–325. doi: 10.1007/BF02234886
Khamidov, I. I., Aripova, S. F., Karimov, A. K. (2003). Berberis alkaloids. XLI. Alkaloids from leaves of cultivated Berberis oblonga. Chem. Nat. Compd. 39, 407. doi: 10.1023/B:CONC.0000003429.41497.b6
Khan, I., Ahmad, H., Ahmad, B., Azam, S. (2014). Antiglycation and antioxidation properties of Berberis lyceum and Terminalia chebula: possible role in curing diabetes and slowing aging. Pak J. Bot. 46, 1469–1471.
Kimani, N. L., Njangiru, I. K., Njagi, E. N. M., Orinda, G. O. (2017). Antidiabetic activity of administration of aqueous extract of Berberis holstii. J. Diabetes Metab. 8, 11. doi: 10.4172/2155-6156.1000774
Kishimoto, A., Dong, S., Negishi, H., Yasui, N., Sun, J., Ikeda, K. (2015). Effects of berberine on adipose tissues and kidney function in 3T3-L1 cells and spontaneously hypertensive rats. Nat. Prod. Commun. 10, 1543–1546. doi: 10.1177/1934578X1501000914
Ko, B.-S., Choi, S. B., Park, S. K., Jang, J. S., Kim, Y. E., Park, S. (2005). Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol. Pharm. Bull. 28, 1431–1437. doi: 10.1248/bpb.28.1431
Kong, W., Wei, J., Abidi, P., Lin, M., Inaba, S., Li, C., et al. (2004). Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10, 1344–1351. doi: 10.1038/nm1135
Kong, W. J., Wei, J., Zuo, Z. Y., Wang, Y. M., Song, D. Q., You, X. F., et al. (2008). Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 57, 1029–1037. doi: 10.1016/j.metabol.2008.01.037
Kong, W.-J., Zhang, H., Song, D.-Q., Xue, R., Zhao, W., Wei, J., et al. (2009). Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 58, 109–119. doi: 10.1016/j.metabol.2008.08.013
Kumar, A., Aswal, S., Chauhan, A., Semwal, R. B., Kumar, A., Semwal, D. K. (2019). Ethnomedicinal Investigation of Medicinal Plants of Chakrata Region (Uttarakhand) Used in the Traditional Medicine for Diabetes by Jaunsari Tribe. Nat. Prod. Bioprospect. 9, 175–200. doi: 10.1007/s13659-019-0202-5
Lamb, J. J., Holick, M. F., Lerman, R. H., Konda, V. R., Minich, D. M., Desai, A., et al. (2011). Nutritional supplementation of hop rho iso-alpha acids, berberine, vitamin D3, and vitamin K1 produces a favorable bone biomarker profile supporting healthy bone metabolism in postmenopausal women with metabolic syndrome. Nutr. Res. 31, 347–355. doi: 10.1016/j.nutres.2011.03.016
Lan, T., Wu, T., Chen, C., Chen, X., Hao, J., Huang, J., et al. (2014). Berberine attenuates high glucose-induced proliferation and extracellular matrix accumulation in mesangial cells: involvement of suppression of cell cycle progression and NF-κB/AP-1 pathways. Mol. Cell. Endocrinol. 384, 109–116. doi: 10.1016/j.mce.2014.01.022
Lan, J., Zhao, Y., Dong, F., Yan, Z., Zheng, W., Fan, J., et al. (2015). Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 161, 69–81. doi: 10.1016/j.jep.2014.09.049
Lee, Y. S., Kim, W. S., Kim, K. H., Yoon, M. J., Cho, H. J., Shen, Y., et al. (2006). Berberine, a natural plant product, activates AMP-Activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55, 2256 LP–2264. doi: 10.2337/db06-0006
Leet, J. E., Freyer, A. J., Shamma, M., Fajardo, V. (1983). Some dimeric benzylisoquinoline alkaloids with an unusual oxygenation pattern. J. Nat. Prod. 46, 908–912. doi: 10.1021/np50030a013
Li, Z., Jiang, J.-D., Kong, W.-J. (2014). Berberine upregulates hepatic low-density lipoprotein receptor through ras-independent but amp-activated protein kinase-dependent raf-1 activation. Biol. Pharm. Bull. 37, 1766–1775. doi: 10.1248/bpb.b14-00412
Li, X.-X., Li, C.-B., Xiao, J., Gao, H.-Q., Wang, H.-W., Zhang, X.-Y., et al. (2015). Berberine attenuates vascular remodeling and inflammation in a rat model of metabolic syndrome. Biol. Pharm. Bull. Pharm. Bull. 38, 862–868. doi: 10.1248/bpb.b14-00828
Li, M., Shu, X., Xu, H., Zhang, C., Yang, L., Zhang, L., et al. (2016). Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 14, 237–250. doi: 10.1186/s12967-016-0987-5
Li, J., Yuan, K., Shang, S., Guo, Y. (2017). A safer hypoglycemic agent for type 2 diabetes—Berberine organic acid salt. J. Funct. Foods 38, 399–408. doi: 10.1016/j.jff.2017.09.031
Li, Z. Y., Liu, B., Zhuang, X. J., Shen, Y. D., Tian, H. R., Ji, Y., et al. (2018). Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. Zhonghua Yi Xue Za Zhi 98, 3756–3761. doi: 10.3760/cma.j.issn.0376-2491.2018.46.007
Li, C., Gan, H., Tan, X., Hu, Z., Deng, B., Sullivan, M. A., et al. (2019). Effects of active ingredients from traditional Chinese medicines on glycogen molecular structure in diabetic mice. Eur. Polym. J. 112, 67–72. doi: 10.1016/j.eurpolymj.2018.12.039
Liu, C., Beecher, C. W. W., Zhao, S. (1995). A benzylisoquinoline alkaloid from Berberis virgetorum. J. Nat. Prod. 58, 1100–1102. doi: 10.1021/np50121a021
Liu, L., Deng, Y., Yu, S., Lu, S., Xie, L., Liu, X. (2008). Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats. Die Pharm. Int. J. Pharm. Sci. 63, 384–388.doi: 10.1691/ph.2008.7778
Liu, X., Li, G., Zhu, H., Huang, L., Liu, Y., Ma, C., et al. (2010). Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRα and PPARα transcriptional programs. Endocr. J. 57, 881–893. doi: 10.1507/endocrj.K10E-043
Liu, C., Wang, Z., Song, Y., Wu, D., Zheng, X., Li, P., et al. (2015). Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. BioMed. Res. Int. 2015, 313808. doi: 10.1155/2015/313808
Lu, S. S., Yu, Y. L., Zhu, H. J., Liu, X. D., Liu, L., Liu, Y. W., et al. (2009). Berberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J. Endocrinol. 200, 159–165. doi: 10.1677/JOE-08-0419
Mahmoud, A. M., Abdel-Rahman, M. M., Bastawy, N. A., Eissa, H. M. (2017). Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 7, 1–10. doi: 10.7324/JAPS.2017.70401
Manzato, E., Benvenuti, C. (2014). Controlled clinical study on the effect of a patented combination of berberine, red yeast rice and orthosiphon on lipids and borderline high blood pressure versus diet alone in metabolic syndrome. Eur. J. Prev. Cardiol. 21.
Marazzi, G., Cacciotti, L., Pelliccia, F., Iaia, L., Volterrani, M., Caminiti, G., et al. (2011). Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv. Ther. 28, 1105–1113, S116. doi: 10.1007/s12325-011-0082-5
Marazzi, G., Pelliccia, F., Campolongo, G., Quattrino, S., Cacciotti, L., Volterrani, M., et al. (2015). Usefulness of nutraceuticals (Armolipid Plus) versus ezetimibe and combination in statin-intolerant patients with dyslipidemia with coronary heart disease. Am. J. Cardiol. 116, 1798–1801. doi: 10.1016/j.amjcard.2015.09.023
Meliani, N., Dib, M. E. A., Allali, H., Tabti, B. (2011). Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 1, 468–471. doi: 10.1016/S2221-1691(11)60102-0
Memon, M. A., Khan, R. N., Riaz, S., Ain, Q. U., Ahmed, M., Kumar, N. (2018). Methylglyoxal and insulin resistance in berberine-treated type 2 diabetic patients. J. Res. Med. Sci. 23, 110. doi: 10.4103/jrms.JRMS_1078_17
Miana, G. A., Ikram, M. (1970). Alkaloids of Berberis petiolaris Wall. Pakistan J. Sci. Indus Res. 13, 49–51.
Miana, G. A., Foy, J. E., Minard, R. D., Shamma, M. (1979). Baluchistine, a new bisbenzylisoquinoline alkaloid. Experientia 35, 1137–1138. doi: 10.1007/BF01963244
Ming, J., Xu, S., Liu, C., Liu, X., Jia, A., Ji, Q. (2018). Effectiveness and safety of bifidobacteria and berberine in people with hyperglycemia: study protocol for a randomized controlled trial. Trials 19, 72. doi: 10.1186/s13063-018-2438-5
Mirhadi, E., Rezaee, M., Malaekeh-Nikouei, B. (2018). Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 104, 465–473. doi: 10.1016/j.biopha.2018.05.067
Mittal, M., Juyal, V., Singh, A. (2012). Phytochemical, antidiabetic, and cytoprotective properties of Berberis aristata DC. root extracts. Pharm. Crop 3, 64–68. doi: 10.2174/2210290601203010064
Moazezi, Z., Qujeq, D. (2014). Berberis fruit extract and biochemical parameters in patients with type II diabetes. Jundishapur J. Nat. Pharm. Prod. 9, e13490. doi: 10.17795/jjnpp-13490
Mohammadi, A., Sahebkar, A., Kermani, T., Zhilaee, M., Tavallaie, S., Mobarhan, M. G. (2014). Barberry administration and pro-oxidant–antioxidant balance in patients with metabolic syndrome. Iran. Red Crescent Med. J. 16, e16786. doi: 10.5812/ircmj.16786
Mustafa, K., Ganai, B., Akbar, S., Dar, M., Tantry, M., Masood, A. (2011). The extracts of Berberis lycium and diabetes mellitus in alloxan monohydrate induced diabetic rats. J. Pharm. Res. 4, 2570–2573.
Neag, M. A., Mocan, A., Echeverría, J., Pop, R. M., Bocsan, C. I., Crisan, G., et al. (2018). Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 9, 557. doi: 10.3389/fphar.2018.00557
Och, A., Szewczyk, K., Pecio, Ł., Stochmal, A., Załuski, D., Bogucka-Kocka, A. (2017). UPLC-MS/MS Profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxid. Med. Cell. Longev. 2017, 9369872. doi: 10.1155/2017/9369872
Oyedemi, S. O., Bradley, G., Afolayan, A. J. (2009). Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe municipality of South Africa. J. Med. Plants Res. 3, 1040–1044.
Pérez-Rubio, K. G., González-Ortiz, M., Martínez-Abundis, E., Robles-Cervantes, J. A., Espinel-Bermúdez, M. C. (2013). Effect of Berberine Administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 11, 366–369. doi: 10.1089/met.2012.0183
Pareek, A., Suthar, M. (2010). Antidiabetic activity of extract of Berberis aristata root in streptozotocin induced diabetic rats. Pharmacologyonline 2, 179–185.
Patel, M. B., Mishra, S. (2011). Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 18, 1045–1052. doi: 10.1016/j.phymed.2011.05.006
Paul, M., Hemshekhar, M., Kemparaju, K., Girish, K. S. (2019). Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic. Biol. Med. 130, 196–205. doi: 10.1016/j.freeradbiomed.2018.10.453
Petcu, P. (1968). Berberis crataegina DC plants acclimated to Romania. Arch. Pharm. (Weinheim). 301, 680. doi: 10.1002/ardp.19683010906
Phondani, P. C., Maikhuri, R. K., Rawat, L. S., Farooquee, N. A., Kala, C. P., Vishvakarma, S. C. R., et al. (2010). Ethnobotanical uses of plants among the Bhotiya tribal communities of niti valley in central Himalaya, India. Ethnobot. Res. Appl. 8, 233–244. doi: 10.17348/era.8.0.233-244
Pisciotta, L., Bellocchio, A., Bertolini, S. (2012). Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 11, 123. doi: 10.1186/1476-511X-11-123
Potdar, D., Hirwani, R. R., Dhulap, S. (2012). Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 83, 817–830. doi: 10.1016/j.fitote.2012.04.012
Qiao, X., Wang, Q., Wang, S., Kuang, Y., Li, K., Song, W., et al. (2018). A 42-markers pharmacokinetic study reveals interactions of berberine and glycyrrhizic acid in the anti-diabetic Chinese medicine formula gegen-qinlian decoction. Front. Pharmacol. 9, 622. doi: 10.3389/fphar.2018.00622
Quevedo, R., Valderrama, K., Moreno-Murillo, B., Laverde, M., Fajardo, V. (2008). A new bisbenzyltetrahydroisoquinoline alkaloid from Berberis tabiensis (Berberidaceae). Biochem. Syst. Ecol. 36, 812–814. doi: 10.1016/j.bse.2008.07.007
Rahimi Madiseh, M., Heidarian, E., Rafieian-Kopaei, M. (2014). Biochemical components of Berberis lycium fruit and its effects on lipid profile in diabetic rats. J. HerbMed. Pharmacol. 3, 15–19.
Rahimi-Madiseh, M., Karimian, P., Kafeshani, M., Rafieian-Kopaei, M. (2017). The effects of ethanol extract of Berberis vulgaris fruit on histopathological changes and biochemical markers of the liver damage in diabetic rats. Iran. J. Basic Med. Sci. 20, 552–556. doi:10.22038/IJBMS.2017.8681
Rameshwar, N. K., Shenoy, R. R., Theerthahalli, S., Arun, R. C. M. (2009). Effect of Berberis aristata on type I and II diabetes mellitus models in albino rats. Pharmacologyonline 1, 89–96.
Rana, D., Bhatt, A., Lal, B. (2019). Ethnobotanical knowledge among the semi-pastoral Gujjar tribe in the high altitude (Adhwari's) of Churah subdivision, district Chamba, Western Himalaya. J. Ethnobiol. Ethnomed. 15, 10. doi: 10.1186/s13002-019-0286-3
Rao, R. R., Hajra, P. K. (1993). “Berberis“ in Flora of India, ed. Sharma, B. D.. Vol. 1. (New Delhi: Botanical Survey of India), 352–402.
Rao, R. R., Husain, T., Datt, B., Garg, A. (1998). Revision of the family Berberidaceae of the Indian region: 2. Rheedia 8, 109–143.
Rao, A. (2017). Efficacy of berberine hydrochloride on biochemical parameters in Indian type 2 diabetic patients. Endocr. Pract. 23, 18A.
Rashidi, H., Namjoyan, F., Mehraban, Z., Zakerkish, M., Ghaderian, S. B., Latifi, S. M. (2018). The effects of active ingredients of barberry root (Berberine) on Glycemic control and insulin resistance in type 2 diabetic patients. Jundishapur J. Nat. Pharm. Prod. 13, e64180. doi: 10.5812/jjnpp.64180
Ren, G., Wang, Y.-X., Li, Y.-H., Song, D.-Q., Kong, W.-J., Jiang, J.-D. (2017). Structure-activity relationship of berberine derivatives for their glucose-lowering activities. Int. J. Clin. Exp. Med. 10, 5054–5060.
Rizvi, S. I., Mishra, N. (2013). Traditional Indian medicines used for the management of diabetes mellitus. J. Diabetes Res. 2013, 712092. doi: 10.1155/2013/712092
Rozza, F., de Simone, G., Izzo, R., De Luca, N., Trimarco, B. (2009). Nutraceuticals for treatment of high blood pressure values in patients with metabolic syndrome. High Blood Press Cardiovasc. Prev. 16, 177–182. doi: 10.2165/11530420-000000000-00000
Ruscica, M., Gomaraschi, M., Mombelli, G., Macchi, C., Bosisio, R., Pazzucconi, F., et al. (2014). Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 8, 61–68. doi: 10.1016/j.jacl.2013.11.003
Sabahi, Z., Khoshnood-Mansoorkhani, M. J., Namadi, S. R., Moein, M. (2016). Antidiabetic and synergistic effects of anthocyanin fraction from Berberis integerrima fruit on streptozotocin-induced diabetic rats model. Trends Phram. Sci. 2, 43–50.
Sangeetha, M. K., Priya, C. D. M., Vasanthi, H. R. (2013). Anti-diabetic property of Tinospora cordifolia and its active compound is mediated through the expression of Glut-4 in L6 myotubes. Phytomedicine 20, 246–248. doi: 10.1016/j.phymed.2012.11.006
Sehdev, R. K., Handa, K. L., Rao, P. R. (1971). Note on the alkaloids of Berberis lycium Royle. Indian J. Chem. 9, 503.
Semwal, B. C., Shah, K., Chauhan, N. S., Badhe, R., Divakar, K. (2008). Anti-diabetic activity of stem bark of Berberis aristata DC in alloxan induced diabetic rats. Internet J. Pharmacol. 6, 1531–1576. doi: 10.5580/90
Shahid, M., Rahim, T., Shahzad, A., Latif, T. A., Fatma, T., Rashid, M., et al. (2009). Ethnobotanical studies on Berberis aristata DC. root extracts. Afr. J. Bio-technol. 8, 556–563.
Shamma, M., Moniot, J. L., Yao, S. Y., Miana, G. A., Ikram, M. (1973). Pakistanine and Pakistanamine, two new dimeric isoquinoline alkaloids. J. Am. Chem. Soc 95, 5742–5747. doi: 10.1021/ja00798a050
Shamma, M., Foy, J. E., Miana, G. A. (1974). Baluchistanamine. novel type dimeric isoquinoline alkaloid. J. Am. Chem. Soc 96, 7809–7811. doi: 10.1021/ja00832a033
Shang, W., Guo, C., Yu, X., Zhao, J., Z. H. (2015). Effects of combination of ginsenoside Rb1 and berberine on glucose and lipid metabolism in db/db obese diabetic mice. Lishizhen Med. Mater. Med. Res. 3, 518–521.
Sharma, R. K., Sharma, B., Jindal, M., Gupta, A. K., Kunwar, R., Lata, S., et al. (2017). Evaluation of hypolipidemic effect of stem part of Berberis aristata in Type 2 diabetes mellitus patients as add on therapy. Natl. J. Physiol. Pharm. Pharmacol. 7, 1A–11A. doi: 10.5455/njppp.2017.7.0517510062017
Sharma, A., Sharma, R., Kumar, D., Padwad, Y. (2018). Berberis lycium Royle fruit extract mitigates oxi-inflammatory stress by suppressing NF-κB/MAPK signalling cascade in activated macrophages and Treg proliferation in splenic lymphocytes. Inflammopharmacology. 1–20. doi: 10.1007/s10787-018-0548-z
Shidfar, F., Ebrahimi, S. S., Hosseini, S., Heydari, I., Shidfar, S., Hajhassani, G. (2012). The effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran. J. Pharm. Res. IJPR 11, 643.
Singh, P., Jain, S. (2010). Antidiabetic activity of Berberis asiatica (DC) roots. Int. J. Pharm. Sci. Res. 1, 109–112. doi: 10.13040/IJPSR.0975-8232.1(6).109-12
Singh, J., Kakkar, P. (2009). Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 123, 22–26. doi: 10.1016/j.jep.2009.02.038
Singh, A., Bajpai, V., Srivastava, M., Arya, K. R., Kumar, B. (2015). Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry. J. Pharm. Anal. 5, 332–335. doi: 10.1016/j.jpha.2015.05.002
Singh, A., Hart, R., Chandra, S., Nautiyal, M. C., Sayok, A. K. (2019). Traditional Herbal Knowledge among the Inhabitants: A Case Study in Urgam Valley of Chamoli Garhwal, Uttarakhand, India. Evid. Based. Complement. Alternat. Med. 2019, 5656925. doi: 10.1155/2019/5656925
Sola, R., Valls, R.-M., Puzo, J., Calabuig, J.-R., Brea, A., Pedret, A., et al. (2014). Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: a multicenter randomized trial. PloS One 9, e101978. doi: 10.1371/journal.pone.0101978
Spigoni, V., Aldigeri, R., Antonini, M., Micheli, M. M., Fantuzzi, F., Fratter, A., et al. (2017). Effects of a new nutraceutical formulation (berberine, red yeast rice and chitosan) on non-HDL cholesterol levels in individuals with dyslipidemia: results from a randomized, double blind, placebo-controlled study. Int. J. Mol. Sci. 18, 1498. doi: 10.3390/ijms18071498
Srivastava, S. K., Rawat, A. K. S., Srivastava, M., Mehrotra, S. (2006). Pharmacognostic evaluation of the roots of Berberis chitria Lindl. Nat. Prod. Sci. 12, 19–23.
Srivastava, S., Srivastava, M., Misra, A., Pandey, G., Rawat, A. K. S. (2015). A review on biological and chemical diversity in Berberis (Berberidaceae). EXCLI J. 14, 247–267. doi: 10.17179/excli2014-399
Suau, R., Rico, R., López-Romero, J. M., Nájera, F., Cuevas, A. (1998). Isoquinoline alkaloids from Berberis vulgaris subsp. australis. Phytochemistry 49, 2545–2549. doi: 10.1016/S0031-9422(98)00121-6
Sun, J., Bao, H., Peng, Y., Zhang, H., Sun, Y., Qi, J., et al. (2018). Improvement of intestinal transport, absorption and anti-diabetic efficacy of berberine by using Gelucire44/14: in vitro, in situ and in vivo studies. Int. J. Pharm. 544, 46–54. doi: 10.1016/j.ijpharm.2018.04.014
Sun, Y., Xia, M., Yan, H., Han, Y., Zhang, F., Hu, Z., et al. (2018). Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 175, 374–387. doi: 10.1111/bph.14079
Tabassum, N., Ahmad, F. (2011). Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 5, 30–40. doi: 10.4103/0973-7847.79097
Tabatabaei-Malazy, O., Larijani, B., Abdollahi, M. (2015). Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 14, 57. doi: 10.1186/s40200-015-0184-8
Tang, L.-Q., Wei, W., Chen, L.-M., Liu, S. (2006). Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 108, 109–115. doi: 10.1016/j.jep.2006.04.019
Tao, K., Chen, J., Wang, L. (2017). Effects of berberine on the expressions of NRF2 and HO-1 in endothelial cells of diabetic rat. BioMed. Res. 28, 3860–3864. doi: 10.1155/2017/6352858
Teodoro, J. S., Duarte, F. V., Gomes, A. P., Varela, A. T., Peixoto, F. M., Rolo, A. P., et al. (2013). Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion 13, 637–646. doi: 10.1016/j.mito.2013.09.002
Tiwari, K. P., Masood, M. (1977). Alkaloidal constituents of Berberis concina and Berberis acanthifolium. Proc. Natl. Acad. Sci. India Sect. A 47, 93–94.
Tiwari, U. L., Singh Adhikari, B. (2011). Berberis rawatii sp. nov. (Berberidaceae) from India. Nord. J. Bot. 29, 184–188. doi: 10.1111/j.1756-1051.2011.00940.x
Trimarco, B., Benvenuti, C., Rozza, F., Cimmino, C. S., Giudice, R., Crispo, S. (2011). Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med. J. Nutr. Metab. 4, 133–139. doi: 10.1007/s12349-010-0043-6
Turner, N., Li, J.-Y., Gosby, A., To, S. W. C., Cheng, Z., Miyoshi, H., et al. (2008). Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57, 1414–1418. doi: 10.2337/db07-1552
Uniyal, S. K., Singh, K. N., Jamwal, P., Lal, B. (2006). Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2, 14. doi: 10.1186/1746-4269-2-14
Upwar, N., Patel, R., Waseem, N., Mahobia, N. K. (2011). Hypoglycemic effect of methanolic extract of Berberis aristata DC stem on normal and streptozotocin induced diabetic rats. Int. J. Pharm. Pharm. Sci. 3, 222–224.
Valencia, E., Fajardo, V., Freyer, A. J., Shamma, M. (1985). Magallanesine: an isoindolobenzazocine alkaloid. Tetrahedron Lett. 26, 993–996. doi: 10.1016/S0040-4039(00)98494-6
Waltenberger, B., Mocan, A., Smejkal, K., Heiss, E. H., Atanasov, A. G. (2016). Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 807. doi: 10.3390/molecules21060807
Wang, Y.-X., Wang, Y.-P., Zhang, H., Kong, W.-J., Li, Y.-H., Liu, F., et al. (2009). Synthesis and biological evaluation of berberine analogues as novel up-regulators for both low-density-lipoprotein receptor and insulin receptor. Bioorg. Med. Chem. Lett. 19, 6004–6008. doi: 10.1016/j.bmcl.2009.09.059
Wang, Y.-X., Kong, W.-J., Li, Y.-H., Tang, S., Li, Z., Li, Y.-B., et al. (2012). Synthesis and structure–activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg. Med. Chem. 20, 6552–6558. doi: 10.1016/j.bmc.2012.09.029
Wang, P., Liu, X., Hong, Y., Reng, X., Wu, X. (2014). Anti-diabetic effects of Berberin Glycyrrhizinate complex salt on GK rat. Chin. Arch. Tradit. Chin. Med. 12, 2995–2997.
Wang, J., Dai, G., Li, W. (2016). Berberine regulates glycemia via local inhibition of intestinal dipeptidyl peptidase-IV. Zhejiang Da Xue Xue Bao Yi Xue Ban 45, 486–492.
Wang, L., Peng, L., Wei, G., Ge, H. (2016). Therapeutic effects of berberine capsule on patients with mild hyperlipidemia. Zhongguo Zhong Xi Yi Jie He Za Zhi 36, 681–684.
Wang, H., Zhu, C., Ying, Y., Luo, L., Huang, D., Luo, Z. (2018). Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget 9, 10135–10146. doi: 10.18632/oncotarget.20807
Wang, L., Ye, X., Hua, Y., Song, Y. (2018). Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 105, 121–129. doi: 10.1016/j.biopha.2018.05.110
Wei, W., Zhao, H., Wang, A., Sui, M., Liang, K., Deng, H., et al. (2012). A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 166, 99–105. doi: 10.1530/EJE-11-0616
Wu, J., Yu, D., Sun, H., Zhang, Y., Zhang, W., Meng, F., et al. (2015). Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crops Prod. 69, 68–75. doi: 10.1016/j.indcrop.2015.01.053
Wu, L., Xia, M., Duan, Y., Zhang, L., Jiang, H., Hu, X., et al. (2019). Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 10, 1–18. doi: 10.1038/s41419-019-1706-y
Xia, X., Yan, J., Shen, Y., Tang, K., Yin, J., Zhang, Y., et al. (2011). Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PloS One 6, 1–10. doi: 10.1371/journal.pone.0016556
Xiao, Y., Xu, M., Alimujiang, M., Bao, Y., Wei, L., Yin, J. (2018). Bidirectional regulation of adenosine 5′-monophosphate–activated protein kinase activity by berberine and metformin in response to changes in ambient glucose concentration. J. Cell. Biochem. 119, 9910–9920. doi: 10.1002/jcb.27312
Xu, M., Xiao, Y., Yin, J., Hou, W., Yu, X., Shen, L., et al. (2014). Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PloS One 9, e103702. doi: 10.1371/journal.pone.0103702
Yan, F., Benrong, H., Qiang, T., Qin, F., Jizhou, X. (2005). Hypoglycemic activity of jatrorrhizine. J. Huazhong Univ. Sci. Technol. [Med. Sci. 25, 491–493. doi: 10.1007/BF02895996
Yan, H. M., Xia, M. F., Wang, Y., Chang, X. X., Yao, X. Z., Rao, S. X., et al. (2015). Efficacy of berberine in patients with non-alcoholic fatty liver disease. PloS One 10, e0134172. doi: 10.1371/journal.pone.0134172
Yang, J., Yin, J., Gao, H., Xu, L., Wang, Y., Xu, L., et al. (2012). Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evidence-Based Complement. Altern. Med. 2012, 363845. doi: 10.1155/2012/363845
Yang, J., Zhao, P., Wan, D., Zhou, Q., Wang, C., Shu, G., et al. (2014). Antidiabetic effect of methanolic extract from Berberis julianae Schneid. via activation of AMP-activated protein kinase in type 2 diabetic mice. Evidence-Based Complement. Altern. Med. 2014, 106206. doi: 10.1155/2014/106206
Yin, J., Xing, H., Ye, J. (2008). Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 57, 712–717. doi: 10.1016/j.metabol.2008.01.013
Yki-Järvinen, H. (2014). Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910. doi: 10.1016/S2213-8587(14)70032-4
Yue, L., Zhang, Y., Xiang, L., Lai, X., Meng, X. (2013). Study on the effect of Berberis dictyophlla cortex on diabetic retinopathy and the mechanism. Chin. J. Exp. Tradit. Med. Formulae 2013, 43.
Yusupov, M. M., Karimov, A., Levkovich, M. G., Abdullaev, N. D., Shakirov, R. (1993a). Berberis alkaloids. XVII. investigation of the alkaloids of Berberis heteropoda. Chem. Nat. Compd. 29, 43–48. doi: 10.1007/BF00631012
Yusupov, M. M., Karimov, A., Shakirov, R., Gorovoi, P. G., Faskhutdinov, M. F., Levkovich, M. G., et al. (1993b). Berberis alkaloids. XXVI. an investigation of the alkaloids of Berberis amurensis. Chem. Nat. Compd. 29, 338–340. doi: 10.1007/BF00630534
Zain-Ul-Abidin, S., Khan, R., Ahmad, M., Bhatti, M. Z., Zafar, M., Saeed, A., et al. (2018). Ethnobotanical survey of highly effective medicinal plants and phytotherapies to treat diabetes mellitus ii in South-West Pakistan. Indian J. Tradit. Knowl. 17, 682–690.
Zhang, Y., Li, X., Zou, D., Liu, W., Yang, J., Zhu, N., et al. (2008). Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 93, 2559–2565. doi: 10.1210/jc.2007-2404
Zhang, H., Wei, J., Xue, R., Wu, J.-D., Zhao, W., Wang, Z.-Z., et al. (2010). Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 59, 285–292. doi: 10.1016/j.metabol.2009.07.029
Zhang, Q., Xiao, X., Li, M., Li, W., Yu, M., Zhang, H., et al. (2014). Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement. Altern. Med. 14, 188. doi: 10.1186/1472-6882-14-188
Zhang, S., Wang, X., Yin, W., Liu, Z., Zhou, M., Xiao, D., et al. (2016). Synthesis and hypoglycemic activity of 9-O-(lipophilic group substituted) berberine derivatives. Bioorg. Med. Chem. Lett. 26, 4799–4803. doi: 10.1016/j.bmcl.2016.08.027
Zhang, B., Pan, Y., Xu, L., Tang, D., Dorfman, R. G., Zhou, Q., et al. (2018). Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 62, 576–587. doi: 10.1007/s12020-018-1689-y
Zhao, X., Zhang, J.-J., Wang, X., Bu, X.-Y., Lou, Y.-Q., Zhang, G.-L. (2008). Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed. Pharmacother. 62, 567–572. doi: 10.1016/j.biopha.2007.02.009
Zhao, W., Ge, H., Liu, K., Chen, X., Zhang, J., Liu, B. (2017). Nandinine, a derivative of berberine, inhibits inflammation and reduces insulin resistance in adipocytes via regulation of AMP-Kinase activity. Plant. Med. 83, 203–209. doi: 10.1055/s-0042-110576
Zhou, J., Zhou, S. (2010). Berberine regulates peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in diabetic adipocytes. Eur. J. Pharmacol. 649, 390–397. doi: 10.1016/j.ejphar.2010.09.030
Zhou, J. Y., Zhou, S. W., Zhang, ,. K. B., Tang, J. L., Guang, L. X., Ying, Y., et al. (2008). Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 31, 1169–1176. doi: 10.1248/bpb.31.1169
Zhu, X., Yang, J., Zhu, W., Yin, X., Yang, B., Wei, Y., et al. (2018). Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 19, 3903. doi: 10.3390/ijms19123903
Zhu, X., Bian, H., Wang, L., Sun, X., Xu, X., Yan, H., et al. (2019). Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 141, 192–204. doi: 10.1016/j.freeradbiomed.2019.06.019
Zilaee, M., Kermany, T., Tavalaee, S., Salehi, M., Ghayour-Mobarhan, M., Ferns, G. A. A. (2014). Barberry treatment reduces serum anti-heat shock protein 27 and 60 antibody titres and high-sensitivity C-reactive protein in patients with metabolic syndrome: a double-blind, randomized placebo-controlled trial. Phyther. Res. 28, 1211–1215. doi: 10.1002/ptr.5117
Keywords: Berberis, berberine, diabetes, metabolic diseases, pharmacology, clinical studies
Citation: Belwal T, Bisht A, Devkota HP, Ullah H, Khan H, Pandey A, Bhatt ID and Echeverría J (2020) Phytopharmacology and Clinical Updates of Berberis Species Against Diabetes and Other Metabolic Diseases. Front. Pharmacol. 11:41. doi: 10.3389/fphar.2020.00041
Received: 17 September 2019; Accepted: 14 January 2020;
Published: 18 February 2020.
Edited by:
William Cho, Queen Elizabeth Hospital (QEH), Hong KongReviewed by:
Waranya Chatuphonprasert, Mahaasarakham University, ThailandCopyright © 2020 Belwal, Bisht, Devkota, Ullah, Khan, Pandey, Bhatt and Echeverría. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Indra Dutt Bhatt, aWRfYmhhdHRAeWFob28uY29t; Javier Echeverría, amF2aWVyLmVjaGV2ZXJyaWFtQHVzYWNoLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.