- 1Tropical Medicine Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 2Nephrology and Dialysis Unit, Internal Medicine Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 3Diagnostic & Interventional Radiology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 4Clinical Pathology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 5Medical Microbiology and Immunology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Background and Purpose: Predictors of response to type-1 hepatorenal syndrome (HRS) therapy are urgently needed. This study's purpose is to evaluate the proposed predictors in these patients.
Methods: Forty-two type-1 HRS patients with cirrhosis were treated with albumin and terlipressin. Clinical, biochemical, and demographic parameters taken at the onset of therapy and changes in endothelin-1/nitric oxide (ET-1/NO) ratio during therapy were analyzed to check their predictive value.
Results: Response to treatment (serum creatinine level <1.5 mg/dL at the end of therapy) was shown in 20 patients (48%). Independent predictive variables of response to therapy were early reduction of ET-1/NO ratio ≥0.15 at day 3 of therapy and serum bilirubin baseline <8 mg/dL (area under the receiver operating characteristic curve, 0.751; P < 0.001; specificity, 55%; sensitivity, 85%). Response rates in patients with serum bilirubin level <8 and ≥8 mg/dL were 63% and 20%, respectively (P = 0.008). The corresponding values in patients with an early reduction of ET-1/NO ratio ≥0.15 and <0.15 on day 3 were 85% and 13.6%, respectively (P < 0.001).
Conclusions: Early reduction of ET-1/NO ratio and lower serum bilirubin baseline can predict response to type-1 HRS therapy with albumin and terlipressin. Alternative therapy should be investigated for nonresponder type-1 HRS patients.
Introduction
Hepatorenal syndrome (HRS) is a reversible cause of renal impairment that occurs in patients with cirrhotic ascites, as well as in patients with alcoholic hepatitis or acute liver failure who show no significant abnormalities in the kidneys morphology (Arroyo et al., 1996; Dagher and Moore, 2001; Angeli et al., 2015).
HRS is characterized by marked alterations in cardiovascular function, impaired renal function, and over-activity of renin-angiotensin systems that lead to severe renal vasoconstriction with a significant reduction of the glomerular filtration rate (GFR) as well as vigorous activation of the sympathetic nervous system (Arroyo et al., 1996; Dagher and Moore, 2001).
HRS predicts mortality in cirrhosis. The most important aspect of providing care to patients with HRS is an evaluation of candidacy for orthotopic liver transplantation (Gonwa et al., 1995; Jeyarajah et al., 1997; Nair et al., 2002). Current accessible therapies other than liver transplantation for HRS consist of albumin and the utilization of vasoconstrictors, for example, terlipressin (Angeli and Merkel, 2008).
Recently, only 52% of HRS patients have responded to therapy with terlipressin (Møller et al., 2000; Fabrizi et al., 2006; Gluud et al., 2010; Allegretti et al., 2017). Furthermore, few researches have reported some predictors of response to treatment in HRS patients treated with this regimen.
The endothelium release many vasoactive mediators that change the structure and function of the vascular smooth muscle in response to mechanical and humoral stimuli. Two of the most prominent are ET and NO (Cahill et al., 2001). ET-1 is released in response to infectious complications with an inflammatory response in the form of transcription mediators activation and proinflammatory cytokines (IL-1, IL-6, and TNF-α) production. It also plays an important role in the vascular disruption pathogenesis caused by infection (Charpin et al., 2001; Yeager et al., 2012; Freeman et al., 2014).
The pathophysiology of various disorders is closely related on the ET-1/NO imbalance (Alonso and Radomski, 2003). In cirrhosis and HRS, the ET system dysfunction causes hemodynamic disturbances (Moore et al., 1992; Uchihara et al., 1992).
The identification of patients with low therapeutic response probability is of imperative clinical importance, especially in patients waiting for transplantation. In our study, we evaluated the hypothesized predictors of response to albumin and terlipressin in type-1 HRS and cirrhosis patients treated with this protocol.
Patients and Methods
Study Population
Starting 2015, patients with renal impairment and cirrhosis admitted to the Tropical Medicine Department (Mansoura, Egypt) were assessed using the same diagnostic algorithm (Gine`s et al., 2003), that involves evaluation of the possible causes of hypovolemia, diuretic withdrawal, possible infection, drug nephrotoxicity, and renal parenchymal disorders. To exclude the presence of volume depletion related renal failure, plasma expansion with I.V. albumin was tried. According to the International Ascites Club (Arroyo et al., 1996), patients who met the type-1 HRS criteria, were treated with albumin and terlipressin. The new HRS defining criteria were approved by this study protocol (Angeli et al., 2015). Some patients didn't receive therapy due to having advanced hepatocellular carcinoma, severe cardiovascular disorders and/or being terminal cases (death expected in less than 2 days).
All the differential diagnosis causes of cirrhotic acute kidney injury including: organic (including glomerulonephritis and acute tubular necrosis), prerenal (all accessible hypovolemic causes), and obstructive nephropathy (Garcia-Tsao et al., 2008; Møller et al., 2014) were excluded in this study by meticulous history taking and physical examination, together with complete biochemical analysis and radiological investigations.
The existence of infection was assessed by various methods, for example, culture and sensitivity testing for ascitic fluid, blood, sputum, and urine, evaluation of the ascitic fluid absolute polymorphonuclear (PMN) count, and chest radiography. Patients with infection were precluded from this study and received albumin and terlipressin only when renal failure continued after the infection was successfully treated. According to the type of infection, bacterial infections were initially treated with antibiotics as reported in detail elsewhere (Martin-Llahi et al., 2011; Fernandez et al., 2012). The antibiotic was changed according to the results of the cultures if there was no response to the initial therapy. In patients with negative cultures and not responding to the initial therapy, empiric antibiotics policy consisting of vancomycin and carbapenems combination was used (Barreto et al., 2014).
The current study included 42 consecutive cirrhosis/type-1 HRS patients who were subjected to treatment from March 2015 to February 2019. Twenty nine (69%) of them had bacterial infection induced type-1 HRS including 20 SBP cases, six soft tissue infection cases, and three patients with pneumonia as well as 13 patients with HRS without known risk factors.
Bacterial infections were categorized according to the following criteria: SBP is the presence of an ascitic fluid absolute polymorphonuclear count >250/mm3 and/or a positive ascitic fluid bacterial culture with no apparent source of infection (Rimola et al., 2000); spontaneous bacteremia is positive blood cultures without an evident source of infection; local infections (skin infection, urinary tract infection, pneumonia, gastroenteritis, meningitis, and biliary tract infection) were evaluated utilizing standard criteria; sepsis with negative-culture is leukocytosis with/without band forms, the presence of fever (>38°C), together with negative cultures, after the exclusion of disorders other than infection that could be related to the systemic inflammatory response.
Improvement of SBP was defined as a reduction in polymorphonuclear cell count <250/mm3, absence of clinical signs of infection, negative cultures, and normal leukocyte count. The remaining infections were improved according to the conventional criteria. Septic shock was identified as a mean arterial pressure below 60 mmHg regardless of adequate volume resuscitation and need for vasopressor agents (Dellinger, 2003).
In this study, all specific therapy or treatment was delayed for type-1 HRS until the infection has been cured.
In addition, the control group included 42 cirrhotic patients without renal impairment who were sex- and age-matched subjects (male/female = 27/15).
Treatment Protocol
The response to treatment was considered a primary endpoint, determined by either a decline in serum creatinine level >50% of the pretreatment value, or >1.5 mg/dL during treatment with an end-of-therapy level of ≤1.5 mg/dL.
Terlipressin (Glypressin, Ferring S.A., Saint-Prex, Switzerland) was administrated as follows; 0.5–1 mg/4 h, as intravenous (i.v.) bolus for 3 days with an increase up to 2 mg/4 h for those whose values did not decrease by at least 25% of the pretreatment values. The maximum duration of therapy was 2 weeks. Once serum creatinine is reduced below 1.5 mg/dL, Terlipressin was withheld. If patients developed manifestations matching the ischemic complications, administration of Terlipressin was stopped.
All patients received albumin (Baxter Human Albumin 20%, Baxter AG, Industriestraße 67, A-1221 Vienna, Austria) at a dose of 1 g/kg BW. during the first day, followed by 40 g/d, given for a maximum of 15 days, in order to reach the inferior vena cava collapsibility index (IVCCI) ≥40% and ≤75%. Clinically, IVCCI was evaluated daily in a regular manner throughout the follow-up period. The dose of albumin was decreased to 20 g/d and/or was stopped if IVCCI was <40% (Brennan et al., 2006). All measurements were carried out by qualified ultrasound radiologists. Moreover, if radiological and clinical signs of pulmonary edema developed, the administration of albumin was withheld, and the patients received i.v. doses of furosemide with careful follow-up.
During therapy of HRS, any complications of cirrhosis were treated based on standardized therapeutic measures (Cardenas and Gine`s, 2005).
Sampling
Following an overnight fasting of 12 h, 4 to 8 mL blood were withdrawn through cannulas in superficial forearm veins for all patients [1 mL in EDTA for complete blood count (CBC), 5mL without anticoagulants for serum samples].
Methodology
CBC was assessed using the CELL-DYN Emerald cell counter (Abbott, Wiesbaden, Germany). Serum creatinine, blood urea nitrogen (BUN) and liver function tests were measured on a Dimension Xpand plus chemistry analyzer (Siemens Technology, Princeton, NJ, USA) using commercially available enzyme-based kit and reagents. Quantitative detection of plasma nitric oxide (NO) was measured using ELISA kits provided by MyBiosource (San Diego, CA 92195-3308, USA) Cat No. MBS264607, with detection range 1.56 to 100 nmol/mL (nmol/mL = µmol/L). Though the NO role in various disorders is debatable, the majority of researchers agree that evaluating serum levels of NOx are associated with NO production. (Andrukhov et al., 2013). Quantitative detection of plasma Endothelin-1 (ET-1) was measured using ELISA kits provided by MyBiosource (San Diego, CA 92195-3308, USA) Cat No. MBS025621, with detection range 6.25 to 200 pg/mL (pg/mL = ng/L). Plasma L-Arginine was quantitatively measured using ELISA kits provided by ALPCO (26G Keewaydin Drive, Salem, New Hampshire 3079, United States) Cat No. 30-7733, with detection range 12.5 to 300 µmol/L. The estimated glomerular filtration rate (eGFR) was calculated according to the following formula (Levey et al., 1999): 186 x (Creatinine/88.4)-1.154 x (Age)-0.203 x (0.742 if female) x (1.210 if black). The ET-1/NO ratio was calculated and recorded for all patients.
Ethics
The protocol of this study was completed to acclimate with the Declaration of Helsinki and was affirmed by the Ethics Committee of the Mansoura University. Informed consent was obtained from all enrolled patients or their legal representatives.
Statistical Analysis
The analysis was carried out using SPSS version 20 for Windows (SPSS Inc., Chicago, IL). Results are expressed as the mean ± SD. Comparisons of continuous and categorical variables between patients were carried out using Student t- and X2-tests respectively. A paired Wilcoxon test and Student t-test were used to compare the variables measured at various time points. The multivariate analysis was built using the univariate analysis parameters that had a significant predictive value (P < 0.01). The best cutoff values for variables with independent predictive value were measured using receiver operating characteristic curves. The probability of response was evaluated using the Kaplan–Meier method. The calculation of overall response involved patients who received a liver transplant (n = 3) and the results were viewed redacted at the time of transplantation. P < 0.05 was considered statistically significant.
Results
Patient Characteristics
Only 42 out of the 121 patients with cirrhotic ascites and renal failure were included in this study. The etiology of renal failure was evaluated after admission and HRS was diagnosed following the diagnostic criteria reported by Angeli et al. Of all the excluded patients, 51 were due to previous infection exposure who were discharged from hospital with normal renal function after adequate therapy of infection (32 SBP, 12 soft tissue infections and 7 pneumonia), six due to nephrotoxic agents administration (four aminoglycosides and two contrast agents), seven due to hypovolemic causes (five GIT bleeding and two severe vomiting), and three due to obstructive nephropathy. Also, the 12 patients who showed complete response and marked serum creatinine decline after receiving only albumin as a corrective measure for the pre-renal causes of renal failure were excluded.
Baseline characteristics of patients with type-1 HRS at the time of diagnosis before starting the treatment with albumin and terlipressin are reported in Table 1. Not surprisingly, most patients with type-1 HRS had severe liver failure, as demonstrated by increased international normalized ratio (INR), high Model for End-Stage Liver Disease (MELD) and Child-Pugh scores, low estimated GFR using Modification of Diet in Renal Disease equation, high levels of serum bilirubin, creatinine, L-arginine, NOx, serum ET-1, and ET-1/NO ratio compared to the control group.
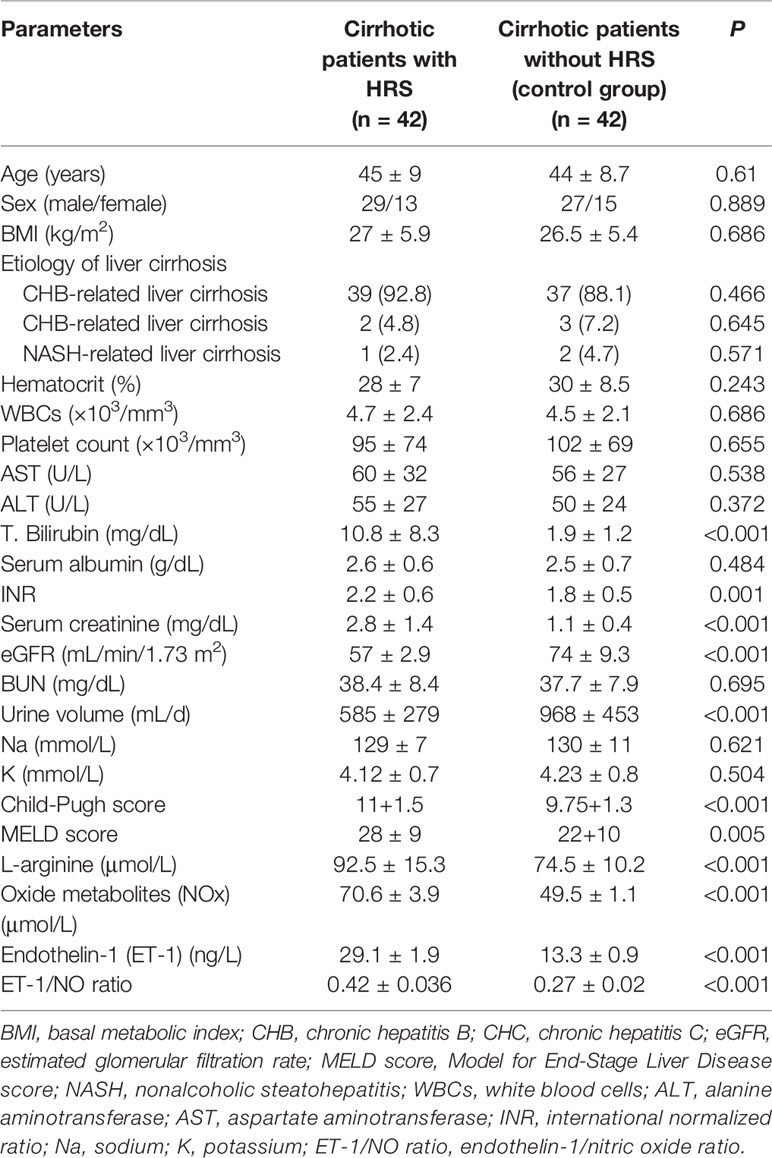
Table 1 Baseline characteristics of patients with type-1 hepatorenal syndrome at the time of diagnosis.
Predictive Factors For Treatment Response
Twenty out of the 42 patients (48%) had a response to therapy. Generally, at the end of therapy, serum creatinine decreased below 1.5 mg/dL, while in two patients serum creatinine decreased >50% of the pretreatment levels but stayed above 1.5 mg/dL (4.8 decreased to 1.8 mg/dL and 3.7 to 1.7 mg/dL). The rest of the patients (52%) failed to reach the response to therapy criteria. Serum creatinine levels during the therapy period in non-responders and responders are summarized in Figure 1. During therapy, the probability of response in all patients is shown in Figure 2. The estimated period for response was approximately 2 weeks. Response to therapy was merely constant in most patients. Recurrence of HRS was 4/20 (20%) in patients who responded to therapy. The average period for recurrence was 2 weeks (range, 3–38 days).
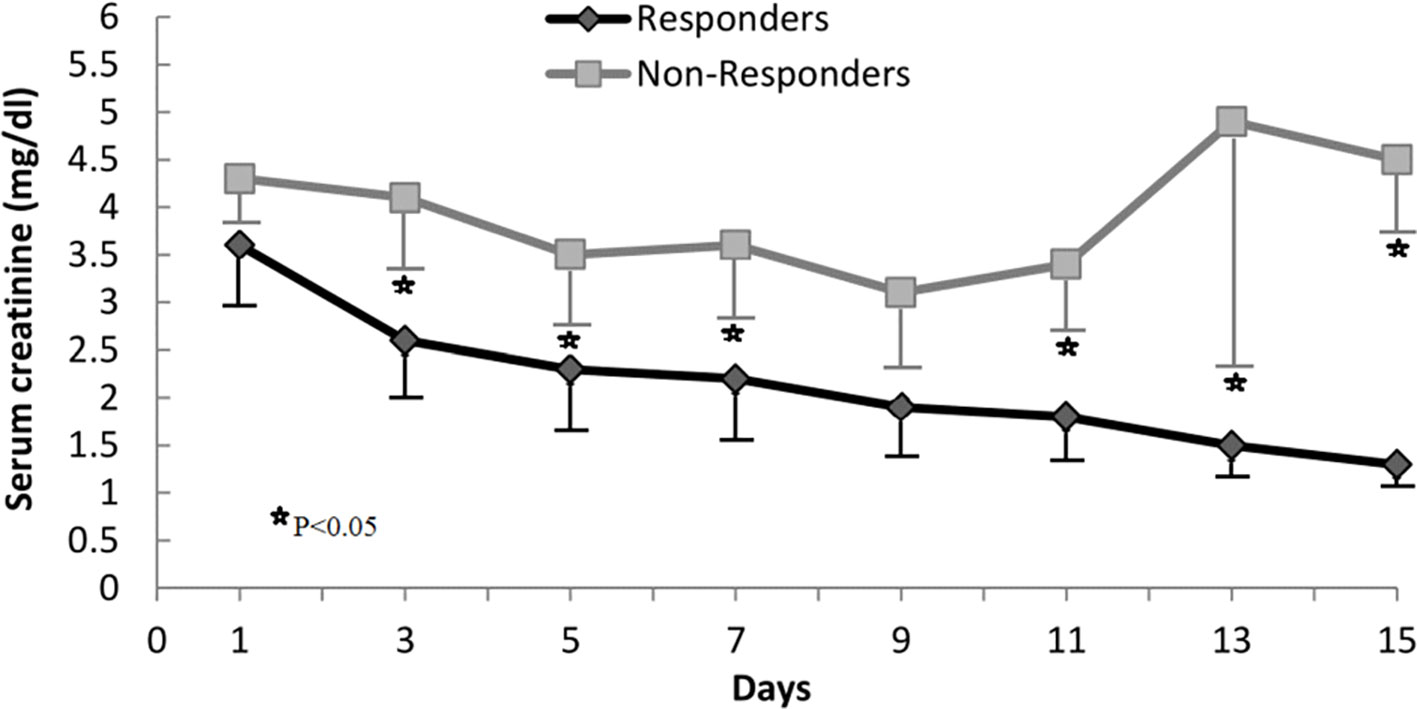
Figure 1 Changes in serum creatinine concentration during treatment in responders and nonresponders.
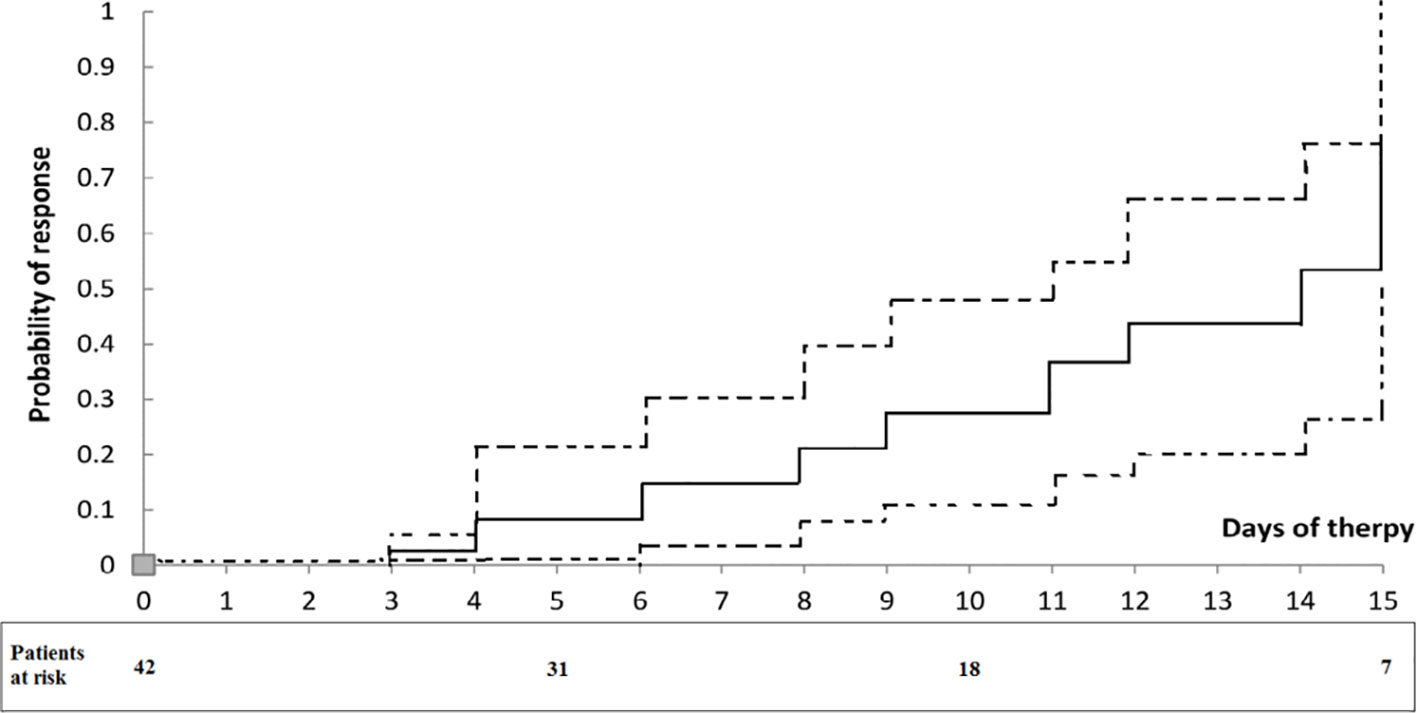
Figure 2 Probability of response to treatment in patients with type 1 HRS treated with albumin and terlipressin. Dotted lines represent 95% confidence intervals. The numbers under the curve are patients at risk at each time.
At baseline, several parameters obtained were assessed for the predictive value of response to therapy (Table 2). Parameters related to response to therapy (P < 0.05) were WBCs count, AST level, serum bilirubin level, urine volume, and MELD score. Furthermore, neither ET-1/NO ratio nor serum creatinine level at baseline was related to response to treatment. The only parameter found to have an independent predictive value in the multivariate analysis was the serum bilirubin level (OR, 14.2; 95% CI, 1.256–160.77; P = 0.032). By receiver operating characteristic curves, serum bilirubin level has a cutoff value (< 8 mg/dL) that well-predicted response to therapy, (AUC = 0.751; P < 0.001; specificity = 55%; sensitivity = 85%; PPV = 63%; and NPV = 80%). Response rates in patients classified based on baseline serum bilirubin level <8 or ≥8 mg/dL were 63% (17/27) and 20% (3/15), respectively (P = 0.008).
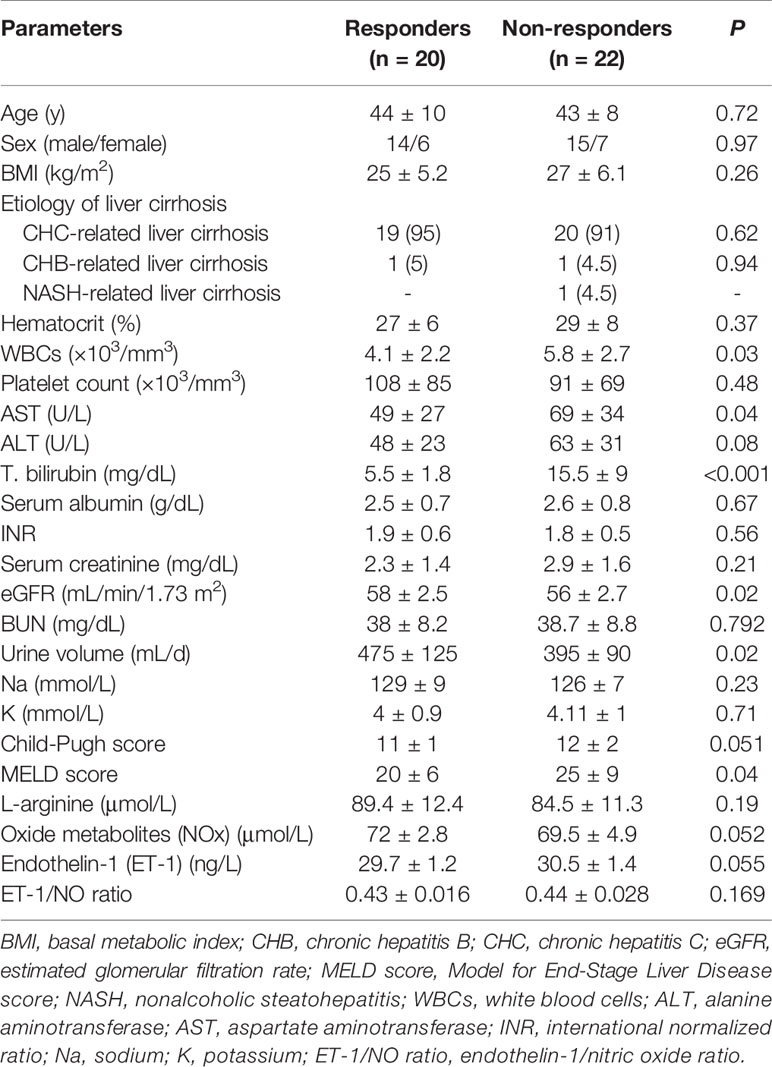
Table 2 Univariate analysis of baseline parameters according to response to treatment with albumin and terlipressin.
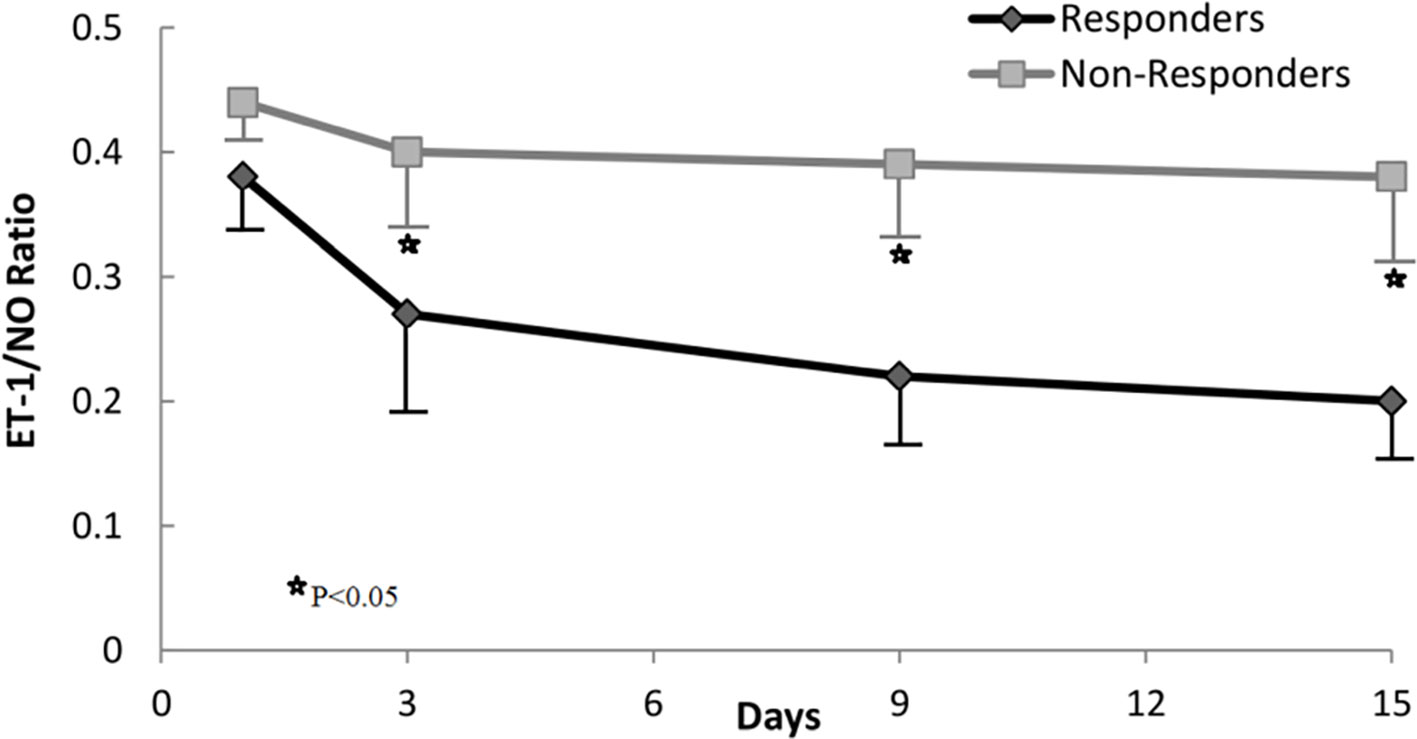
Based on changes in the ET-1/NO ratio evaluated at day 3 of therapy, we analyzed response rates in all patients. The value of the ET-1/NO ratio used was the average value of all measurements of ET-1/NO ratio assessed at day 3; a reduction in ET-1/NO ratio of 0.15 was considered relevant and used as a cutoff value. Values of ET-1/NO ratio throughout therapy in non-responders and responders are summarized in Figure 3. Although the baseline ET-1/NO ratio was not a predictive factor of response, early changes of ET-1/NO ratio within the first days of therapy predicted response. Patients with a reduction in the ET-1/NO ratio equal to or greater than 0.15 at day 3 of therapy had a response rate at the end of treatment of 85% (17/20) compared to 13.6% (3/22) in patients with minimal decreases in the ET-1/NO ratio (P < 0.001). When this decrease in the ET-1/NO ratio of 0.15 or more at day 3 was enclosed in the multivariate analysis with the baseline parameters, the predictive factors of response to treatment were the baseline serum bilirubin level and the decrease in the ET-1/NO ratio ≥0.15 at day 3 (Table 3). Moreover, 2/5 patients (40%) responded if there was a reduction ET-1/NO ratio ≥0.15 in those with baseline serum bilirubin level ≥8 mg/dL, compared to a response in 1/10 (10%) in those with a change in the ET-1/NO ratio <0.15 with baseline serum bilirubin level ≥8 mg/dL (Figure 4).
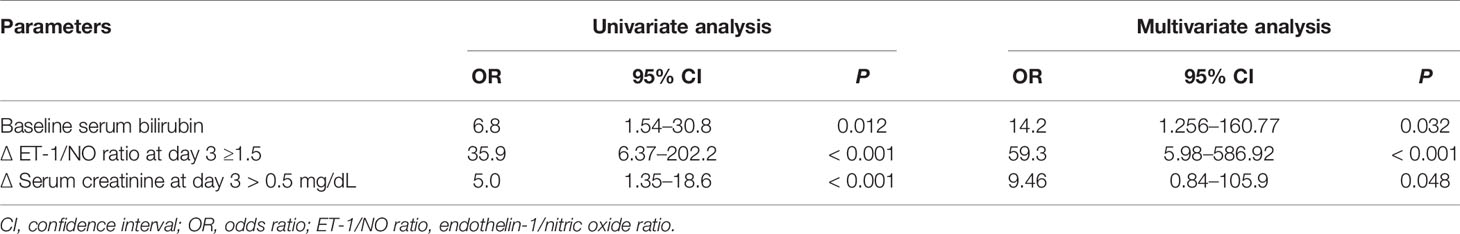
Table 3 Parameters with independent predictive value of response to treatment with albumin and terlipressin in patients with type 1 HRS.
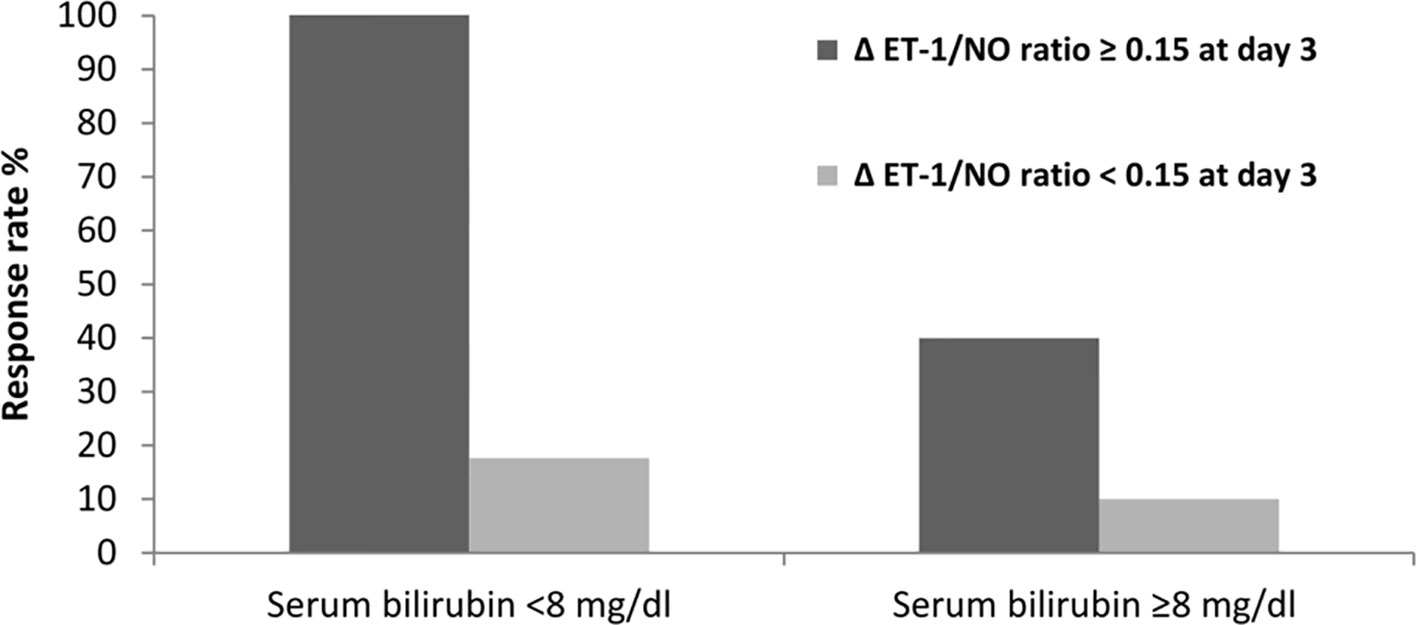
Figure 4 Response rate according to levels of ET-1/NO ratio and its relationship to serum bilirubin at baseline.
In addition, Spearman's correlation analysis showed that there was a significant correlation between the ET-1/NO ratio and serum bilirubin level, serum creatinine level, creatinine clearance, MELD and Child-Pugh scores (rho = 0.63, P < 0.001; rho = 0.72, P < 0.001; rho = −0.66, P < 0.001; rho = 0.72, P < 0.001; rho = 0.69, P < 0.001respectively).
During therapy, the early decrease in serum creatinine was also used as a predictor of response to treatment. Response to therapy was demonstrated in 15 of the 19 patients (79%) in whom serum creatinine decreased by at least 0.5 mg/dL at day 3, compared to only 5 of the 23 patients (22%) in whom serum creatinine did not decrease 0.5 mg/dL or elevated at day 3 compared to the baseline (P < 0.001). When the cutoff value for the change in serum creatinine used was 1 mg/dL instead of 0.5 mg/dL similar figures were demonstrated (89% and 36%, respectively; P = 0.005). The value of the reduction in serum creatinine at day 3 as a predictor of response to treatment was also approved in the multivariate analysis model (odds ratio, 9.46; P = 0.048).
Complications of Cirrhosis
A total of 47 major cirrhotic complications were developed in 37 patients (88%) during therapy: 32 patients with hepatic encephalopathy, five patients with GIT bleeding, and ten patients with bacterial infection. Bacterial infections between responders and non-responders showed no significant difference [4/20 (20%) vs. 6/22 (27%), respectively; P = 0.6]. The median value for WBCs count was 7,100/mm3 and throughout therapy, the occurrence of bacterial infections was partially more common in patients higher count than in those with lower count [7/23 (30.4%) vs. 3/19 (15.8%), respectively; P = 0.275]. At the lower WBCs count baseline, patients acquired pneumonia (n = 1) and urinary tract infections (n = 2) after an average period of 6 days (range, 2–10 days), while at the higher WBCs count baseline, patients acquired pneumonia (n = 2) and sepsis (n = 5) after an average period of 6 days (range, 2–14 days).
Side Effects of Therapy
Eight patients (19%) developed albumin/terlipressin therapy-related side effects. Signs of circulatory overload developed in four patients, that improved after short-lived cessation of albumin but not of terlipressin and furosemide was added in small doses. Intestinal ischemia signs developed in two patients, that disappeared after cessation of therapy. Transient arrhythmia (ventricular extrasystolia) developed in two patients, that did not need persistent cessation of therapy, and improved after therapy withdrawal. We followed up with the patients for at least 3 months after their hospital discharge.
Survival
90 days after the initiation of treatment, 32 (76.2%) patients died, 6 (14.3%) patients were still alive, 3 (7.2%) participants had undergone liver transplantation, and 1 (2.4%) patient was lost to follow-up as well as the survival probability at that time was significantly higher in responders (40%) with median survival of 65 days compared to non-responders (9%) with median survival of 8 days; P < 0.001). Septic shock (n = 16), multiorgan failure (n = 11), liver failure (n = 4), and/or unknown (n = 1) were the causes of death.
Using the original MELD risk equation, Area Under the Receiver Operating Characteristic Curve (AUROC) analysis revealed that a MELD score threshold of 20 corresponded to median survival less than 3-months (Figure 5). Kaplan-Meier survival curves of patients stratified by baseline MELD score below or above 20, which represents threshold for 3-month median survival. Median survival of patients with baseline MELD score <20 significantly greater than those with baseline MELD scores ≥ 20 (62 vs. 15 days, P < 0.001).
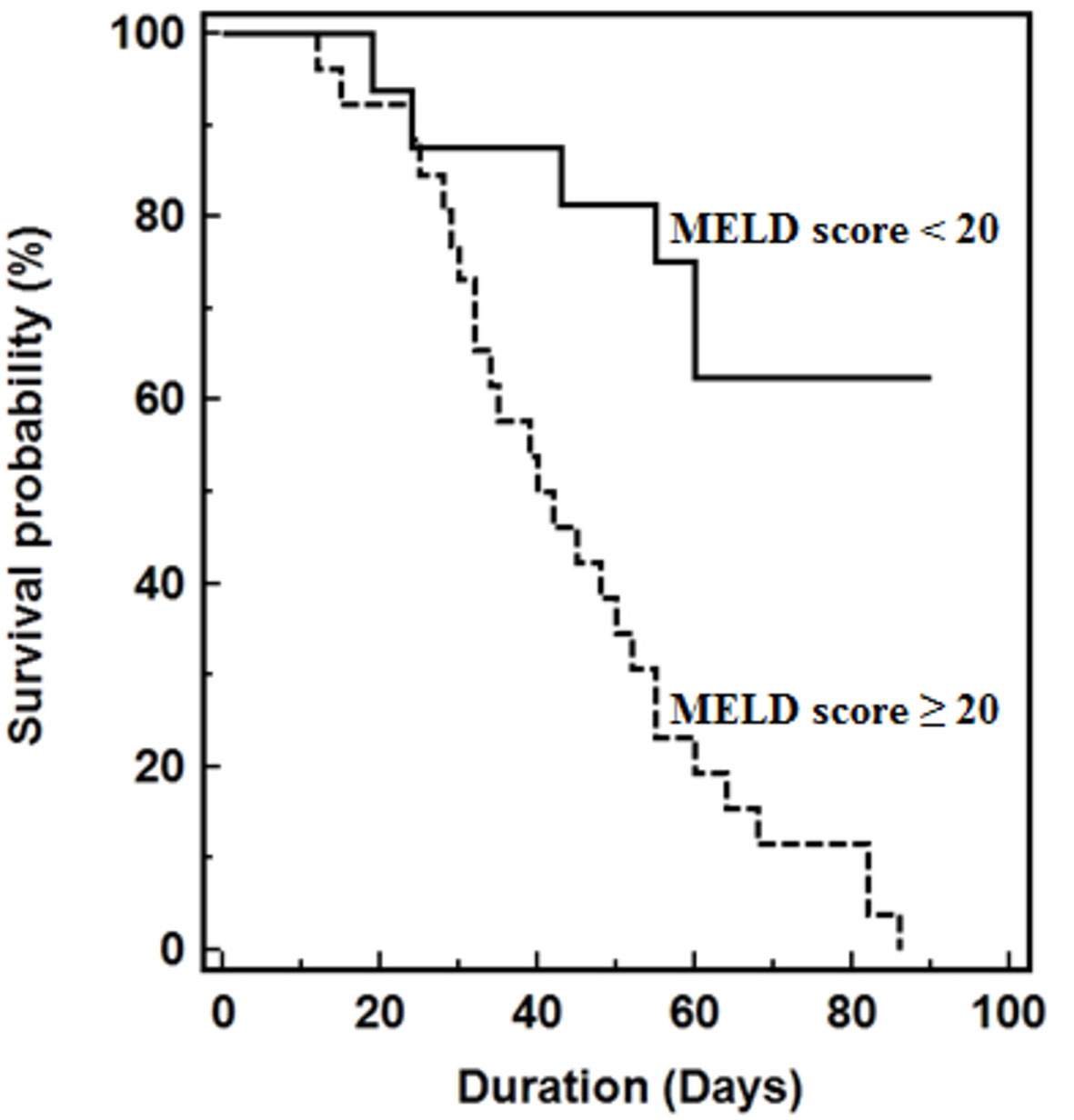
Figure 5 Kaplan-Meier survival curves of patients stratified by baseline MELD score below or above 20, which represents threshold for 3-month median survival. Median survival of patients with baseline MELD score <20 significantly greater than those with baseline MELD scores ≥ 20 (62 vs. 15 days, P < 0.001).
Discussion
This recent study reports that albumin with terlipressin are currently the accepted therapy for type-1 HRS, but over 50% of patients show a suboptimal to no response to treatment (Dagher and Moore, 2001; Angeli et al., 2015). It is therefore of paramount importance that we keep searching and investigating not only for adequate predictors of response but also prognostic factors before beginning the treatment or early afterward. Obviously, patients responding to treatment showed a statistically significant decline in ET-1/NO ratio at the end of therapy; probably because the low effective arterial blood volume of HRS was improved. These outcomes firmly recommend that the gainful impact of terlipressin in the treatment of HRS is identified with its ability to enhance systemic hemodynamics (Fabrizi et al., 2006; Moreau and Lebrec, 2006). We still don't know why in some type-1 HRS patients the systemic hemodynamics were not enhanced when treated with terlipressin. This may be due to the presence of concomitant adrenal insufficiency, and latent infections associated with higher levels of ET-1 (Hocher et al., 1999).
Independent predictors of response to treatment were serum bilirubin levels at baseline and a decline in the ET-1/NO ratio ≥0.15 at day 3 of therapy. Eleven of the eleven patients (100%) with serum bilirubin at baseline <8 mg/dL who demonstrated a decline in the ET-1/NO ratio ≥0.15 at day 3 responded to our therapeutic protocol. While only one of the 10 (10%) patients with serum bilirubin at baseline ≥8 mg/dL and a reduction in the ET-1/NO ratio <0.15 had a response to therapy. In previous studies, predictive variables of response observed in patients with HRS involved MELD and Child-Pugh scores, arterial pressure, and serum creatinine level at the baseline (Moreau et al., 2002; Alessandria et al., 2007; Neri et al., 2008; Sanyal et al., 2008; Sharma et al., 2008). But some drawbacks may be observed as a small number of patients received therapy as well as analysis may have involved patients treated with different vasoconstrictors e.g. norepinephrine instead of terlipressin.
The association between the renal response to terlipressin and the presence of an early reduction of the ET-1/NO ratio shows the significance of the enhancement of systemic hemodynamics in accomplishing type-1 HRS reversal. ET-1 and NO are the most important local vasoconstrictor and vasodilator respectively. They seem to play a role in almost every tissue and organ (Hei et al., 2006). Recent researches reported that the important factors in identifying the harmful versus beneficial effects of NO are the site of NO production, duration of action, and its amount (Hon et al., 2002). It has been proposed that the disequilibrium of ET-1 and NO levels may have an impact on the pathophysiology of local and systemic circulation disorders. This imbalance in the renal microcirculation has been proposed to be in charge of the dynamic disintegration in kidney function in these patients and the development of HRS (Kayali et al., 2009).
ET-1 may be involved in both impaired glomerular perfusion and renal vasoconstriction which causes HRS (Moore, 2004). Renal blood flow is not the only factor contributing to changes in glomerular filtration rate as seen at the microcirculatory level. The enhanced production of vasoactive mediators including ET-1 causes reduction of the surface area available for glomerular filtration and contraction of mesangial cells which contributes to this phenomenon (Ring-Larsen, 1977; Simonson and Dunn, 1990). The intake of ETA and ETB receptors antagonists combination prevented and in sometimes reversed the pathogenesis of renal failure in different experiments (Anand et al., 2002). Physiologically, early reduction of the ET-1/NO ratio is considered a good prognostic marker in HRS patients with end stage liver disease or on the liver transplantation waiting list.
Interestingly, serum bilirubin level at baseline was considered an independent predictive factor of response to treatment. Poor response to therapy and elevated serum bilirubin levels association can't be explained, but looks to be unrelated to the hemodynamic response to terlipressin. The mechanisms explaining the lack of response to terlipressin related to high serum bilirubin levels are interesting and merits examination. The correlation between the ET-1/NO ratio and bilirubin noticed in this study demonstrates that the values of ET-1 are influenced by the excretory liver function. A correlation between ET-1 and bilirubin has been approved also by some (Nozue et al., 1995; Bachmann-Brandt et al., 2000), but not all authors (Isobe et al., 1993). The possible explanation, not determined by our data, maybe owing to indirect (excess pulmonary arteriovenous shunts that increased with the deterioration of liver condition) and direct (reduced clearance of ET-1 by the hepatic tissue) associations. Leakage of bile into the blood and/or disturbance of ET-1 degradation may result in increased serum ET-1 levels since ET-1 is excreted through the biliary system (Cai et al., 1999).
The multivariate analysis model showed that the reduction in serum creatinine level at day 3 can be considered a predictor of response to treatment. It should be noted that up to one-fourth of the patients showed response at the end of therapy though their serum creatinine level was not reduced by day 3. This may be due to increasing the dose of terlipressin in patients whom their serum creatinine level failed to reach an early reduction or showed a delayed renal response with respect to the improvement of hemodynamic status. In these patients, treatment with terlipressin was maintained after 3 days to achieve this delayed response effect. This finding is approved by Nazar et al. (2010).
In the current study, the 90-day survival probability in non-responders was 9% and in responders 40%. These results, in conjunction with the observation that patients with HRS where the albumin and terlipressin enhanced their renal capacity had marvelous posttransplantation results compared to patients without HRS (Restuccia et al., 2004), confirm that albumin plus terlipressin is a successful and helpful choice for HRS patients anticipating liver transplantation. The observations of this study were in accordance with previous reports which stated that type-1 HRS patients responding to therapy with albumin and terlipressin have longer survival expectancy compared to that of non-responders [(Ortega et al., 2002; Martín-Llahí et al., 2008; Neri et al., 2008; Sharma et al., 2008).
Moreover, to our knowledge, no studies published the role of changes in the ET-1/NO ratio as an independent predictor of response to albumin with terlipressin in type-1 HRS. Such results will certainly be much scrutinized, but there are some clear conclusions for the role of ET-1 and NO in the pathophysiology of HRS. Such findings add to our understanding and knowledge of the pathogenesis of HRS in a number of ways and provide a foundation for its management. This study's findings have a number of significant implications for future practice.
Of course, this study has some limitations that should be addressed. First, the sample size is small. Second, the current study is a single center study, and recruiting a large number of patients for such a meticulous study, is mandatory yet difficult due to the unavailability of terlipressin in many centers and type-1 HRS is not a common disorder. Third, the ET-1/NO ratio is not routinely measured in clinical practice. Furthermore, studies from multiple centers are needed to confirm these findings in HRS patients.
In conclusion, the outcomes of the current study, in type-1 HRS patients, indicate that a reduction in the ET-1/NO ratio and lower serum bilirubin baseline are considered good predictors of response to treatment with albumin and terlipressin. Future studies on treatment of type-1 HRS should discuss the possible mechanisms of declined response to pharmacological treatment and should search for new therapeutic options for non-responders.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The study was reviewed and approved by the Ethics Committee of Mansoura University, Egypt. (Proposal code: R.19.06.534). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. AA-R and NM acquired, analyzed and interpreted data; performed statistical analysis; and wrote, edited and reviewed the manuscript. MA, AA, and MT recruited and followed up with patients; acquired, analyzed and interpreted data; performed statistical analysis; critically revised the manuscript. AT recruited and followed up with patients; acquired, analyzed and interpreted data; critically revised the manuscript. AH recruited and followed up with patients; acquired, analyzed and interpreted data; critically revised the manuscript. RE acquired, analyzed and interpreted data; underwent laboratory investigations and revised the manuscript. WE and NE-W acquired, analyzed and interpreted data; performed statistical analysis; underwent laboratory investigations; critically revised the manuscript. All authors approved the final version of the article, including the authorship list.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the staff and patients of the Tropical Medicine Department and the lab specialists for their significant aid.
Abbreviations
HRS, hepatorenal syndrome; ET-1/NO ratio, endothelin-1/nitric oxide ratio; GFR, glomerular filtration rate; PMN, polymorphonuclear; SBP, spontaneous bacterial peritonitis; IVCCI, inferior vena cava collapsibility index; CBC, complete blood count; MELD, Model for End-Stage Liver Disease.
References
Alessandria, C., Ottobrelli, A., Debernardi-Venon, W., Todros, L., Cerenzia, M. T., Martini, S., et al. (2007). Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J. Hepatol. 47, 499–505. doi: 10.1016/j.jhep.2007.04.010
Allegretti, A. S., Israelsen, M., Krag, A., Jovani, M., Goldin, A. H., Schulman, A. R., et al. (2017). Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst. Rev. 6, CD005162. doi: 10.1002/14651858.CD005162.pub4
Alonso, D., Radomski, M. W. (2003). The nitric oxide-endothelin-1 connection. Heart Fail Rev. 8, 107–115. doi: 10.1023/a:1022155206928
Anand, R., Harry, D., Holt, S., Milner, P., Dashwood, M., Goodier, D., et al. (2002). Endothelin is an important determinant of renal function in a rat model of acute liver and renal failure. Gut 50, 111–117. doi: 10.1136/gut.50.1.111
Andrukhov, O., Haririan, H., Bertl, K., Rausch, W. D., Bantleon, H. P., Moritz, A., et al. (2013). Nitric oxide production, systemic inflammation and lipid metabolism in periodontitis patients: possible gender aspect. J. Clin. Periodontol. 40 (10), 916–923. doi: 10.1111/jcpe.12145
Angeli, P., Merkel, C. (2008). Pathogenesis and management of hepatorenal syndrome in patients with cirrhosis. J. Hepatol. 48 (Suppl.), S93–S103. doi: 10.1016/j.jhep.2008.01.010
Angeli, P., Ginès, P., Wong, F., Bernardi, M., Boyer, T. D., Gerbes, A., et al. (2015). Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J. Hepatol. 62, 968–974. doi: 10.1016/j.jhep.2014.12.029
Arroyo, V., Gine`s, P., Gerbes, A. L., Dudley, F. J., Gentilini, P., Laffi, G., et al. (1996). Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Int. Ascites Club. Hepatol. 23, 164–176. doi: 10.1002/hep.510230122
Bachmann-Brandt, S., Bittner, I., Neuhaus, P., Frei, U., Schindler, R. (2000). Plasma levels of endothelin-1 in patients with the hepatorenal syndrome after successful liver transplantation. Transpl. Int. 13, 357–362. doi: 10.1007/s001470050714
Barreto, R., Fagundes, C., Guevara, M., Solà, E., Pereira, G., Rodríguez, E., et al. (2014). Type1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatology 59, 1505–1513. doi: 10.1002/hep.26687
Brennan, J. M., Ronan, A., Goonewardena, S., Blair, J. E., Hammes, M., Shah, D., et al. (2006). Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin. J. Am. Soc Nephrol. 1, 749–753. doi: 10.2215/CJN.00310106
Cahill, P. A., Redmond, E. M., Sitzmann, J. V. (2001). Endothelial dysfunction in cirrhosis and portal hypertension. Pharmacol. Ther. 89, 273–293. doi: 10.1016/s0163-7258(01)00128-0
Cai, L., Wang, G. J., Mukherjee, K., Xu, Z. L., Khalil, M., Cherian, M. G., et al. (1999). Endothelins and their receptors in cirrhotics and neoplastic livers of Canadian and Chinese populations. Anticancer Res. 19, 2243–2247.
Cardenas, A., Gine`s, P. (2005). Management of complications of cirrhosis in patients awaiting liver transplantation. J. Hepatol. 42, S124–S133. doi: 10.1016/j.jhep.2004.12.007
Charpin, J. M., Stern, M., Lebrun, G., Aubin, P., Grenet, D., Israël-Biet, D. (2001). Increased endothelin-1 associated with bacterial infection in lung transplant recipients. Transplantation 71 (12), 1840–1847. doi: 10.1097/00007890-200106270-00022
Dellinger, R. P. (2003). Cardiovascular management of septic shock. Crit. Care 31, 946–955. doi: 10.1097/01.CCM.0000057403.73299.A6
Fabrizi, F., Dixit, V., Martin, P. (2006). Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol. Ther. 24, 935–944. doi: 10.1111/j.1365-2036.2006.03086.x
Fernandez, J., Acevedo, J., Castro, M., Garcia, O., de Lope, C. R., Roca, D., et al. (2012). Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 55, 1551–1561. doi: 10.1002/hep.25532
Freeman, B. D., Machado, F. S., Tanowitz, H. B., Desruisseaux, M. S. (2014). Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 118 (2), 110–119. doi: 10.1016/j.lfs.2014.04.021
Garcia-Tsao, G., Parikh, C. R., Viola, A. (2008). Acute kidney injury in cirrhosis. Hepatology 48, 2064–2077. doi: 10.1002/hep.22605
Gine`s, P., Guevara, M., Arroyo, V., Rodes, J. (2003). Hepatorenal syndrome. Lancet 362, 1819–1827. doi: 10.1016/S0140-6736(03)14903-3
Gluud, L. L., Christensen, K., Christensen, E., Krag, A. (2010). Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology 51 (2), 576–584. doi: 10.1002/hep.23286
Gonwa, T. A., Klintmalm, G. B., Levy, M., Jennings, L. S., Goldstein, R. M., Husberg, B. S. (1995). Impact of pretransplant renal function on survival after liver transplantation. Transplantation 59, 361–365. doi: 10.1097/00007890-199502150-00010
Hei, Z. Q., Huang, H. Q., Luo, C. F., Li, S. R., Luo, G. J. (2006). Changes of nitric oxide and endothelin, thromboxane A2 and prostaglandin in cirrh-otic patients undergoing liver transplantation. World J. Gastroenterol. 12, 4049–4051. doi: 10.3748/wjg.v12.i25.4049
Hocher, B., Brause, M., Mendes, U., Berger, D., Bulher, H., Gross, P. (1999). Impact of endothelin system on water and sodium excretion in patients with liver cirrhosis. Nephrol. Dial Transplant. 14, 1133–1138. doi: 10.1093/ndt/14.5.1133
Hon, W. M., Lee, K. H., Khoo, H. E. (2002). Nitric oxide in liver diseases: friend, foe, or just passerby? Ann. N.Y. Acad. Sci. 962, 275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x
Isobe, H., Satoh, M., Sakai, H., Nawata, H. (1993). Increased plasma endothelin-1 levels in patients with cirrhosis and esophageal varices. J. Clin. Gastroenterol. 17, 227–230. doi: 10.1097/00004836-199310000-00011
Jeyarajah, D. R., Gonwa, T. A., McBride, M., Testa, G., Abbasoglu, O., Husberg, B. S., et al. (1997). Hepatorenal syndrome: combined liver kidney transplants versus isolated liver transplant. Transplantation 64, 1760–1765. doi: 10.1097/00007890-199712270-00024
Kayali, Z., Herring, J., Baron, P., Franco, E., Ojogho, O., Smith, J., et al. (2009). Increased plasma nitric oxide, L-arginine, and arginase-1 in cirrhotic patients with progressive renal dysfunction. J. Gastroenterol. Hepatol. 24, 1030–1037. doi: 10.1111/j.1440-1746.2008.05757.x
Levey, A. S., Bosch, J. P., Lewis, J. B., Greene, T., Rogers, N., Roth, D. (1999). A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study groupm. Ann. Intern. Med. 130, 461–470. doi: 10.7326/0003-4819-130-6-199903160-00002
Møller, S., Hansen, E. F., Becker, U., Brinch, K., Henriksen, J. H., Bendtsen, F. (2000). Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver 20 (1), 51–59. doi: 10.1034/j.1600-0676.2000.020001051.x
Møller, S., Krag, A., Bendtsen, F. (2014). Kidney injury in cirrhosis: pathophysiological and therapeutic aspects of hepatorenal syndromes. Liver Int. 34, 1153–1163. doi: 10.1111/liv.12549
Martin-Llahi, M., Guevara, M., Torre, A., Fagundes, C., Restuccia, T., Gilabert, R., et al. (2011). Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 140, 488–496. doi: 10.1053/j.gastro.2010.07.043
Martín-Llahí, M., Pépin, M. N., Guevara, M., Díaz, F., Torre, A., Monescillo, A., et al. (2008). Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 134, 1352–1359. doi: 10.1053/j.gastro.2008.02.024
Moore, K., Wendon, J., Frazer, M., Karani, J., Williams, R., Badr, K. (1992). Plasma endothelin immunoreactivity in liver disease and hepatorenal syndrome. N. Engl. J. Med. 327, 1774–1778. doi: 10.1056/NEJM199212173272502
Moore, K. (2004). Endothelin and vascular function in liver disease. Gut 53 (2), 159–161. doi: 10.1136/gut.2003.024703
Moreau, R., Lebrec, D. (2006). The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology 43, 385–394. doi: 10.1002/hep.21094
Moreau, R., Durand, F., Poynard, T., Duhamel, C., Cervoni, J. P., Ichaı¨, P., et al. (2002). Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology 122, 923–930. doi: 10.1053/gast.2002.32364
Nair, S., Verma, S., Thuluvath, P. J. (2002). Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology 35, 1179–1185. doi: 10.1080/00365521.2018.1526967
Nazar, A., Pereira, G. H., Guevara, M., Martín-Llahi, M., Pepin, M. N., Marinelli, M., et al. (2010). Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 51, 219–226. doi: 10.1002/hep.23283
Neri, S., Pulvirenti, D., Malaguarnera, M., Cosimo, B. M., Bertino, G., Ignaccolo, L., et al. (2008). Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig. Dis. Sci. 53, 830–835. doi: 10.1007/s10620-007-9919-9
Nozue, T., Kobayashi, A., Uemasu, F., Takagi, Y., Endoh, H., Sako, A. (1995). Plasma endothelin-1 levels of children with cirrhosis. J. Pediatr. Gastroenterol. Nutr. 21, 220–223. doi: 10.1097/00005176-199508000-00015
Ortega, R., Gine`s, P., Uriz, J., Cardenas, A., Calahorra, B., de las Heras, D., et al. (2002). Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology 36, 941–948. doi: 10.1053/jhep.2002.35819
Restuccia, T., Ortega, R., Guevara, M., Gine`s, P., Alessandria, C., Ozdogan, O., et al. (2004). Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J. Hepatol. 40, 140–146. doi: 10.1016/j.jhep.2003.09.019
Rimola, A., García-Tsao, G., Navasa, M., Piddock, L. J., Planas, R., Bernard, B., et al. (2000). Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. Int. Ascites Club. J. Hepatol. 32, 142–153. doi: 10.1016/s0168-8278(00)80201-9
Ring-Larsen, H. (1977). Renal blood flow in cirrhosis: relation to systemic and portal haemodynamics and liver function. Scand. J. Clin. Lab. Invest. 37, 635–642. doi: 10.3109/00365517709100657
Sanyal, A. J., Boyer, T., Garcia-Tsao, G., Regenstein, F., Rossaro, L., Appenrodt, B., et al. (2008). A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 134, 1360–1368. doi: 10.1053/j.gastro.2008.02.014
Sharma, P., Kumar, A., Shrama, B. C., Sarin, S. K. (2008). An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am. J. Gastroenterol. 103, 1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x
Simonson, M. S., Dunn, M. J. (1990). Endothelin-1 stimulates contraction of rat glomerular mesangial cells and potentiates beta-adrenergic-mediated cyclic adenosine monophosphate accumulation. J. Clin. Invest. 85 (3), 790–797. doi: 10.1172/JCI114505
Uchihara, M., Izumi, N., Sato, C., Marumo, F. (1992). Clinical significance of elevated plasma endothelin concentration in patients with cirrhosis. Hepatology 16, 95–99. doi: 10.1002/hep.1840160117
Keywords: hepatorenal syndrome, endothelin-1, nitric oxide, liver cirrhosis, endothelin-1/nitric oxide ratio, terlipressin, albumin
Citation: Abdel-Razik A, Mousa N, Abdelsalam M, Abdelwahab A, Tawfik M, Tawfik AM, Hasan AS, Elhelaly R, El-Wakeel N and Eldars W (2020) Endothelin-1/Nitric Oxide Ratio as a Predictive Factor of Response to Therapy With Terlipressin and Albumin in Patients With Type-1 Hepatorenal Syndrome. Front. Pharmacol. 11:9. doi: 10.3389/fphar.2020.00009
Received: 31 October 2019; Accepted: 06 January 2020;
Published: 31 January 2020.
Edited by:
Md Abdul Hye Khan, Medical College of Wisconsin, United StatesReviewed by:
Chiara Elia, Cardinal Massaia Asti Hospital, ItalyFlemming Bendtsen, University of Copenhagen, Denmark
Copyright © 2020 Abdel-Razik, Mousa, Abdelsalam, Abdelwahab, Tawfik, Tawfik, Hasan, Elhelaly, El-Wakeel and Eldars. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Abdel-Razik, QWhtZWRhYmRlbHJhemlrNzZAZ21haWwuY29t
 Ahmed Abdel-Razik
Ahmed Abdel-Razik Nasser Mousa1
Nasser Mousa1 Mostafa Abdelsalam
Mostafa Abdelsalam