- 1Industrial Bioresource Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, South Korea
- 2Lee's Biotech Co., KRIBB, Daejeon, South Korea
- 3College of Medicine, Myunggok Medical Research Institute, Konyang University, Daejeon, South Korea
- 4R & D, Kolmar BNH Co., Ltd., Sejong, South Korea
Helicobacter pylori is one of the most widespread infections involved in the pathogenesis of chronic gastritis, peptic ulcer, and gastric cancer. Hence, there is an urgent need to develop medications against H. pylori. This study aimed to evaluate synergistic effect of Rubus crataegifolius (RF) and Ulmus macrocarpa Hance (UL) against H. pylori. Antibacterial susceptibility of each extract either separately or in combination was studied against two H. pylori standard strains and 11 clinical isolates using agar dilution method. The effect of the extracts on H. pylori inoculated Balb/c mice model was also studied using single dosing (100 mg/kg each) approach. The MIC50 of RF and UL were more than 100 and 200 µg/ml, respectively, against the tested strains. However, simultaneous treatment with RF and UL at 75 and 50 µg/ml, respectively, showed decreased viable cell number, MIC70, and at 75 µg/ml each showed synergic effect with MIC90. On H. pylori inoculated Balb/c mice model, RF and UL separately (100 mg/kg each) showed moderate anti-H. pylori effect, while simultaneous treatment of RF and UL with same dose showed significant synergistic anti-gastric effects in stomach. The results showed a significant synergistic effect of plants extract against H. pylori infection and eventually gastric mucosal damage. Our finding could be considered a valuable support in the treatment of H. pylori induced gastritis and may contribute to the development of new and safe combined herbal product as anti-H. pylori regimens.
Introduction
Helicobacter pylori is a pathogenic bacterium that can persist in the stomach of an infected person for their entire life. Approximately 50% of the earth's population is infected with Helicobacter pylori, which has been implicated in the etiology of chronic gastritis and peptic ulcer, both in adults and children (Liou et al., 2016a). The WHO has declared H. pylori as a carcinogen, which causes gastric cancer, the third most common cause of cancer related mortality worldwide (GBD 2013 Mortality and Causes of Death Collaborators, 2015). Several drug treatments have been developed to be efficacious in H. pylori infections. Triple therapy consisting of clarithromycin given for 7 to 14 days in one of the most commonly used regimens in the first line therapy (Malfertheiner et al., 2012). However, the eradication rate of standard first line therapy has fallen below 80% in many Asian countries, including China, India, and Korea (Liou et al., 2015; Liou et al., 2016b). It is noteworthy that metronidazole resistant rate was higher than 60% in Asian counties including China and India (Liou et al., 2015). In the face of the declining eradication rate of standard triple therapy, several alternative strategies have been proposed to increase the first line treatment (Rimbara et al., 2011; Gisbert and Calvet, 2012; Venerito et al., 2013). Despite advances in antimicrobial therapy, there is still no ideal treatment and indications due to increasing resistance, side effects, and falling eradication rates. Hence, considerable interest has focused on alternative/adjuvant approaches for the eradication of H. pylori. Some of them including herbal treatment, novel antibiotics, or classical ones from natural product in different combinations, using probiotics, etc. (Brown and Jiang, 2013; Takeuchi et al., 2014; Goderska et al., 2018).
In this work, we report in vitro anti-H. pylori activity of two popular Korean medicinal plants Rubus crataegifolius Bunge (RF, Family: Rosaceae) and Ulmus macrocarpa Hance (UL, Family:Ulmaceae) either separately or in combination and in vivo anti-gastritic effect of the extracts against H. pylori infected animal model.
Materials and Methods
Reagents
Dimethyl sulfoxide (DMSO), ethanol, formalin, HCl, amoxicillin, clarithromycin, omeprazole, cimetidine, ellagic acid and catechin glycoside were purchased from Sigma (Sigma Aldrich Inc., MO, USA). Fetal bovine serum (FBS) and trypsin-EDTA were obtained from GIBCO (Invitrogen INC., NY. USA). Brucella agar medium was purchased from Becton and Dickinson Company (BD). All other reagents were pharmaceutical or analytical grade.
Plant Materials and Preparation of Extracts
The unripened fruit of RF and the stem bark of UL were purchased from Kyung Dong Medicinal Herb market at Seoul, Korea. The species of plants were verified by International Biological Material Research Center at KRIBB (Korea Research Institute of Bioscience and Biotechnology). Access number for RF Bunge (KRIBB 0001387) and Ulmus macrocarpa Hance (KRIBB 0002361) are kept in the herbarium of KRIBB. The experimental extracts of RF and UL were prepared using extraction, concentration and spray drying in Sam Woo-Dayeon company (Kum San, Chung Nam province, Korea). In brief, the dried fruits of RF and stem bark of UL (1 kg each) were pulverized separately using electric blender and extracted two times at 60°C for 6 hours in extraction COD water bath (JSEB-62T). Fifty percent ethanol and 100% H2O were used as extraction solvent (each 5 L) for RF and UL, respectively. The extracted solution was then filtered (50 mesh), concentrated with an evaporator under vacuum, spray dried, and stored at −20° C until further use. The extract yields of RF and UL were 28% and 15%, respectively. For high performance liquid chromatography (HPLC) analysis and in vitro assay 20% DMSO was used whereas for in vivo assay 5% tween 80 in H2O was used to solubilize the extracts.
HPLC Analysis for Standardization of RF and UL
The quantitative analysis of the main components used as standard in R. crataegifolius and U. macrocarpa were performed using a HPLC analysis system (Agilent Technologies 1260 infinity) equipped with auto sampler (G1329B) and UV (G1316A) detector. Chromatographic separation was achieved at 35°C on Agilent reversed-phase C-18 (4.6 × 150 mm, 5 µm) column. Mobile phase A (0.1% aqueous TFA) and B (acetonitrile) with flow rate 0.8 ml/min and injection volume of 10 μl were used in analysis. The wavelength used for detection was 254 nm for ellagic acid in R. crataegifolius and 280 nm for catechin-7-O-β-D-apiofuranoside in U. macrocarpa. The quantity of ellagic acid and catechin-7-O-β-D-apiofuranoside in samples were measured from standard calibration curve (concentration vs area curve) of ellagic acid and catechin-7-O-β-D-apiofuranoside using method described by He et al., 2013.
Helicobacter pylori Strains and Growth Condition
Two reference strains (ATCC-43504, SS1) obtained from American Type Tissue Culture collection (ATCC, Ritzville, ND, USA) and 11 clinically isolated strains from the Department of Microbiology, Gyeongsang National University, Korea were used for antibacterial assay. H. pylori (1 × 108 CFU, equivalent to 1 McFarland turbidity standard unit) was seeded in brucella media containing 10% defibrinated FBS, and incubated for 24 hours at 37°C (85% N2, 10% CO2, 5% O2). After 3 days of incubation, number of colonies was counted. Amoxicillin was used as positive control.
Minimal Inhibitory Concentration (MIC) Test
The minimal inhibitory concentrations were determined using the agar dilution procedure (Gisbert and Calvet, 2012) according to the guidelines described by the National Committee for Clinical Laboratory Standards (KFDA, Korea). Twenty mg of plants extract were dissolved in 1 ml of 20% DMSO and stored at −20°C. Final concentrations consisting 0, 10, 50, 75, 100, 150, and 200 µg/ml plants extract were prepared using brucella media containing 10% FBS in the Petri plates. For MIC test, each H. pylori strain was inoculated onto 10% FBS containing agar plates and incubated at 37°C (85% N2, 10% CO2, 5% O2) for 24 hours. An inoculum of each isolate of H. pylori strain was prepared by suspending cultured bacteria in brucella broth media to get a final inoculum concentration of 1 × 106 CFU/spot. The plates were incubated at 37°C (85% N2, 10% CO2, 5% O2) for 3 days and the results were expressed as MIC30 (concentration at which inhibition of 30% of colonization occurred) or MIC90 values (concentration at which inhibition of 90% or more colonization).
Animals
Male Balb/c mice, weighing 30–40 g, were purchased from Orient Bio Animal Laboratories, Kyunggi-do, Korea, and were acclimatized to standard laboratory conditions (24 ± 2°C, 45 ± 5% humidity and 12 hour light/dark cycle) for 7 days. All the procedures were performed in compliance with the guiding principles in the care of Animals and the Animal Welfare Committee of Korea Research Institute of Bioscience and Biotechnology (KRIBB, Approval No: KRIBB-AEC-14098).
Effect of Plants Extract on H. pylori Induced Gastritis In Balb/c Mice
Four weeks old mice were fasted for 24 hours and then H. pylori culture was orally inoculated by gavage (0.5 ml, 2 × 108 CFU/animal, n = 10 for each group). After inoculation, each animal was kept without food and drink for 4 hours and then given a basal diet. After 4 weeks, experimental group were received either single dose of RF and UL (100 mg/kg BW/day) or combined dose of RF and UL (100 mg/kg BW/day, 1:1 ratio) until the end of the experimental periods. The animals were monitored daily for their general health and their body weight were measured once in a week. Eight weeks after the inoculation of H. pylori, all animals were sacrificed under the ether anaesthesia, stomach were resected, opened along the greater curvature, and washed twice with saline. Then, gastric lesions (edema and hemorrhage) using macroscope were observed followed by measurement of the wet weight of the whole stomach including the forestomach and glandular stomach. Half of the glandular mucosa was scraped for detection of colonizing H. pylori, and the residual part was formalin-fixed and embedded in paraffin for histological observation. Pathological diagnosis of gastritis was made according to the criteria described previously (Takeuchi et al., 2014). A microscopic score, varying from 0 to 7, was used as a measure of the level of gastritis. To detect H. pylori colonization, scraped mucosa samples were homogenized, inoculated onto segregating agar plates for H. pylori and incubated at 37°C under microaerobic conditions. After 5 days, the colonies were counted to determine the level of H. pylori colonization for each stomach.
Statistical Analysis
All experiments were performed three times and data were expressed as means ± SD. Multiple comparisons for two groups were done by independent sample T-test. P-values below 0.05 were considered as statistically significant. Analysis was performed using SPSS 18.0 (SPSS, Chicago, IL, United States).
Results
HPLC Chromatograms for Standardization of RF and UL
As described in Materials and Methods, the RF and UL used in the experiment were standardized by determining the major components using HPLC chromatography. Rubus crataegifolius and Ulmus macrocarpa samples were analyzed at 254 nm for ellagic acid and 280 nm for catechin-7-O-β-D-apiofuranoside, respectively. The Chromatogram is shown in Figure 1. The contents of ellagic acid in RF and catechin-7-O-ß-D-apiofuranoside in UL calculated for standardization were 14.2 and 30.5 mg/g dry extract, respectively (He et al., 2013).
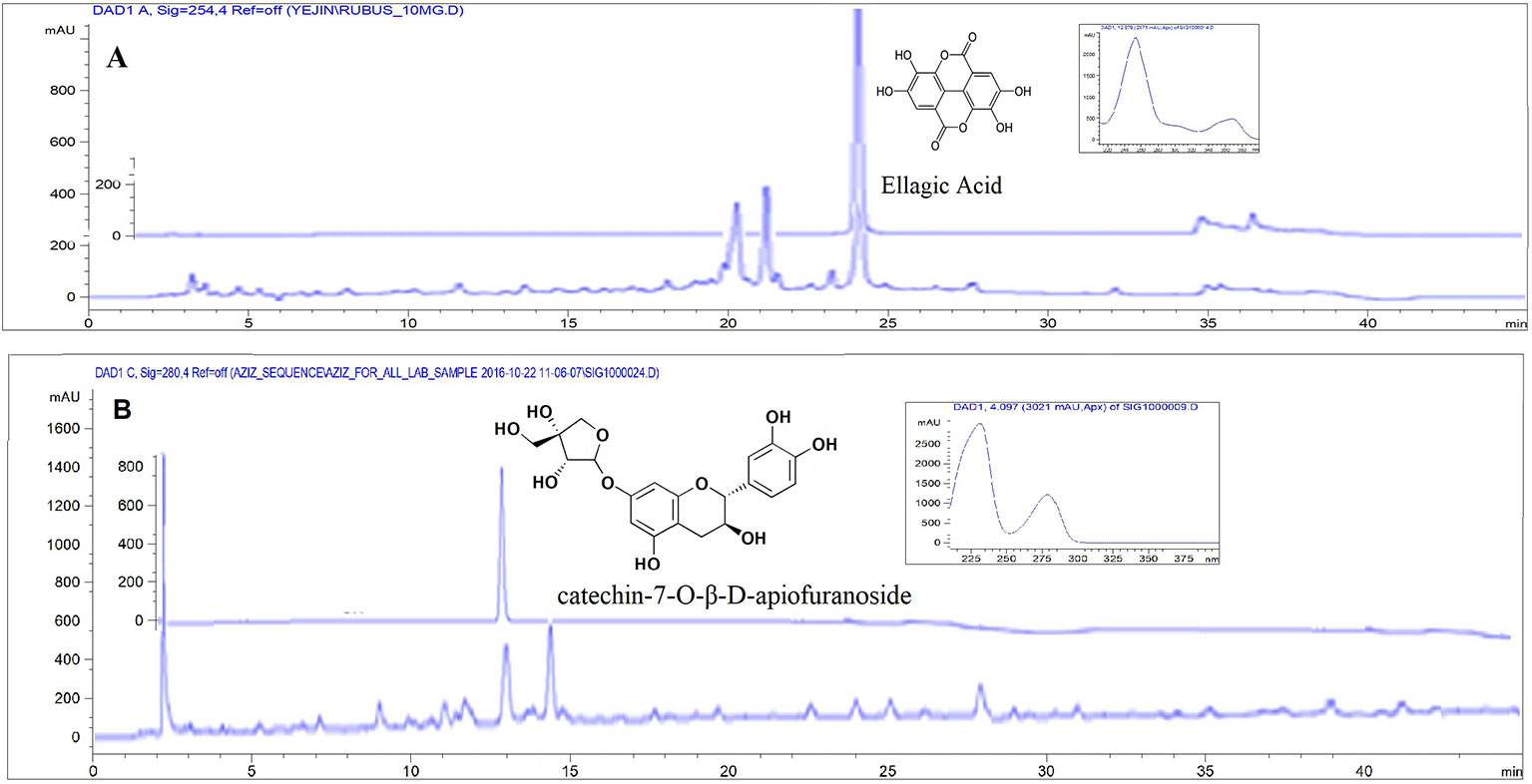
Figure 1 HPLC-DAD chromatogram for standardization of sample. (A) Rubus crataegifolius extracts and standard ellagic acid (overlaid) at 254 nm, (B) Ulmus macrocarpa extracts and standard catechin-7-O-β-D-apiofuranoside (overlaid) at 280 nm.
Susceptibility Study of H. pylori Strains
A total of 11 clinically isolated H. pylori strains originated from gastric ulcer of gastritis patients collected from Kyung Sang University, Korea and two reference strains obtained from ATCC were characterized for their susceptibility to amoxicillin, clarithromycin and omeprazole. The antimicrobial susceptibility results of the strains are presented in Table 1. All 13 strains showed to be sensitive to the tested drugs.
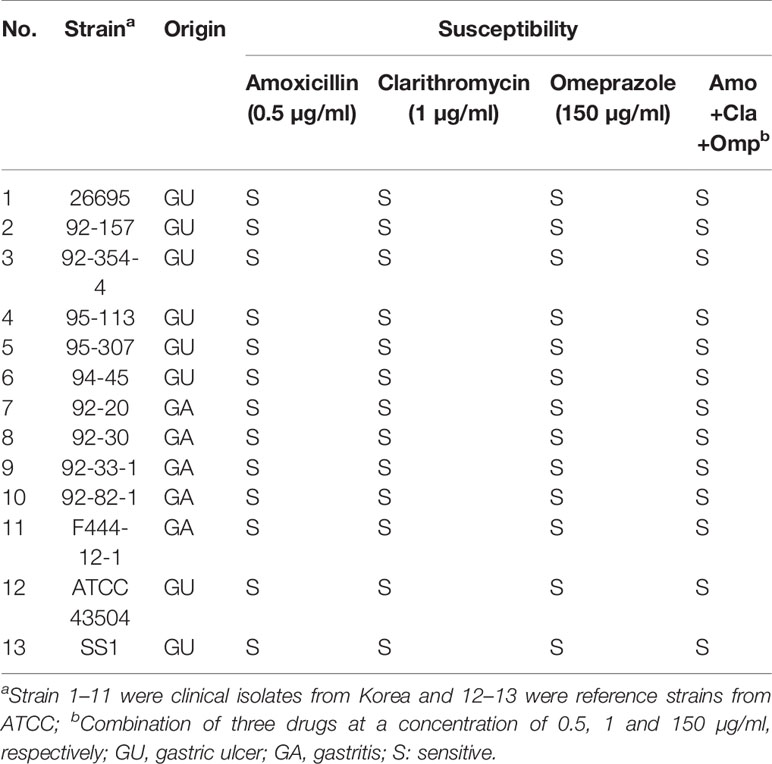
Table 1 Susceptibility test of H. pylori clinical and reference strains to amoxicillin, clarithromycin and omeprazole.
Synergistic Anti-H. pylori Activity of RF and UL Extracts
From the in vitro experiment using agar dilution method it was found that RF extract completely inhibited the colonization of H. pylori at 150 µg/ml (Figure 2) whereas UL showed MIC50 at 200 µg/ml concentrations as shown in Table 2 and Figure 2. UL extract did not show any inhibition at 150 µg/ml. As shown in Table 2, RF or UL did not show any anti-H. pylori effect, at 50 or 75 µg/ml, while RF in combination with UL at 75 and 50 µg/ml, respectively, showed strong synergistic effect, MIC70. Combination of RF and UL at 75 µg/ml each showed complete inhibition of H. pylori colonization (Figure 2). All 11 clinically isolated H. pylori strains and two reference strains were tested for the synergistic effect of RF and UL. The antibiotic complex OAC (amoxicillin, clarithromycin, omeprazole) showed similar inhibitory effect on H. pylori colony formation (Table 2).
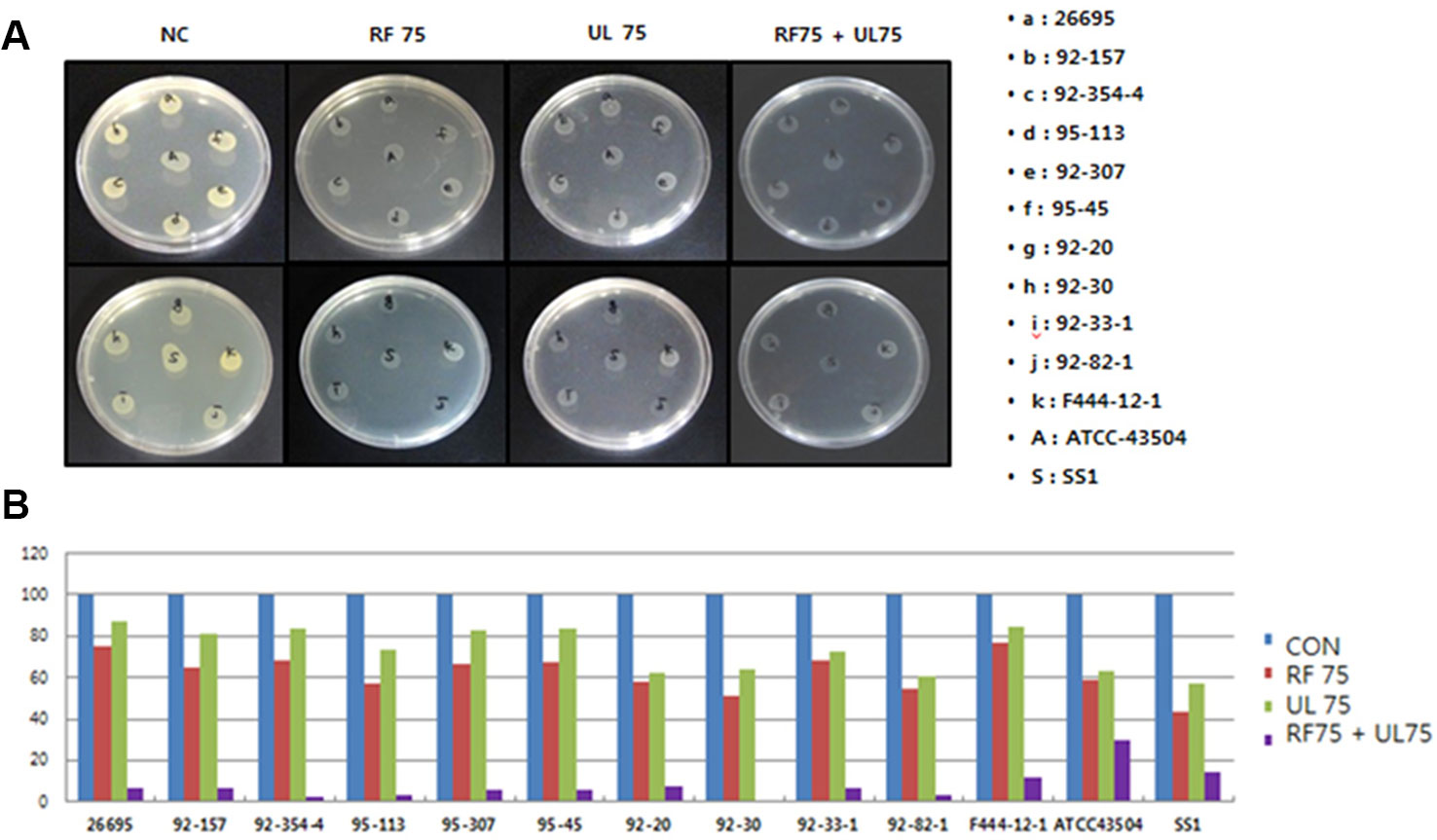
Figure 2 Synergistic effects of plant extracts on the colonization of H. pylori. (A) Effects of plant extracts (Rubus crataegifolius, Ulmus macrocarpa) on the colonization of 13 (represented as alphabet) H. pylori strains determined by agar dilution method, (B) Percentage of inhibition of colonization of each extract represented as graph. RF combined with UL at 75 µg/ml each showed complete inhibition of H. pylori growth (MIC90) on complex treated agar plates. CON indicated normal control and 75 indicated 75 µg/ml.
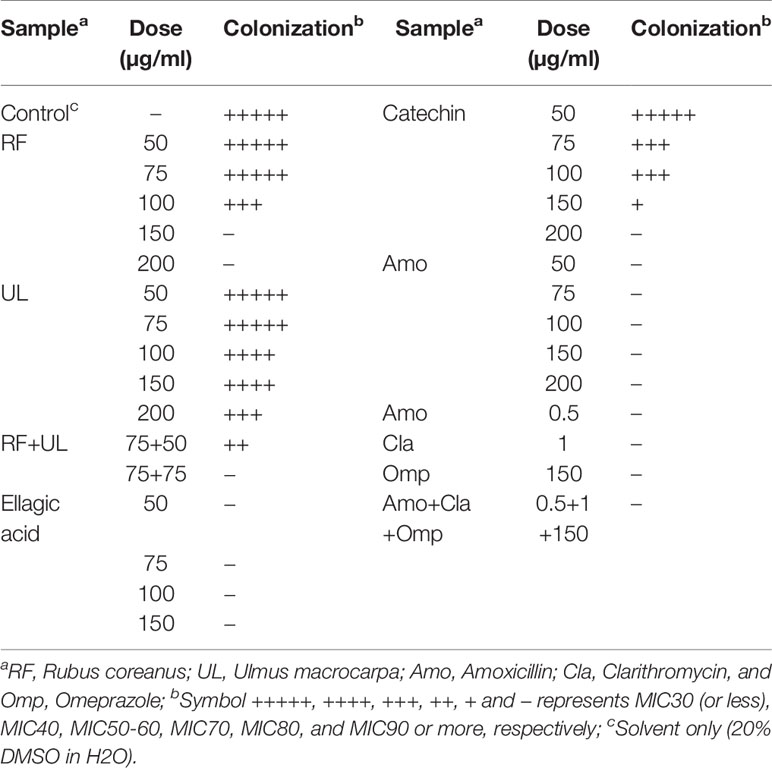
Table 2 Comparison of the effects of RF, UL, RF combined UL, and standard drugs on the colonization of H. pylori.
Suppression of H. pylori-Induced Gastritis by RF, UL, and RF Combined UL Extracts in Balb/c Mice Models
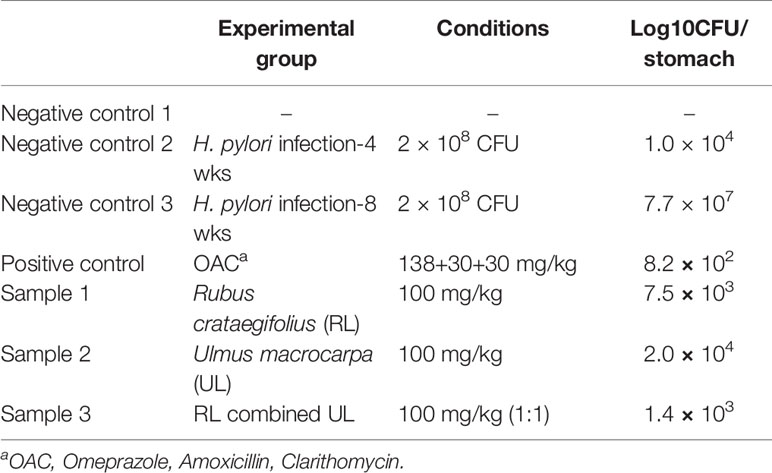
The results on the effect of plant extracts on H. pylori infected Balb/c mice are shown in Table 3. Regarding the average number of the viable bacteria, the control groups (control 2 and 3) which were infected by H. pylori showed 1 × 104 CFU/stomach and 1 × 107 CFU/stomach while positive control group (OAC treated) showed 8.2 × 102 CFU/stomach. The treatment of RF and UL showed 7.5 × 103 and 2.0 × 104 CFU/stomach, respectively, while RF combined UL showed a significant decrement of viable H. pylori (1.4 × 103 CFU/stomach).
Histological analysis (H & E staining) is represented in Figure 3. Microscopic erosions with infiltration featuring many polymorphonuclear leukocyte and lymphocytes were observed in H. pylori infected control mouse (Figures 3A, B). Gastric changes were severe in the pyloric region, but moderate in fundic region. The average microscopic score for gastritis of the H. pylori inoculated control animal was 3.0 (Figure 3B). Though H. pylori induced gastritis showed increased immune cells in mice stomach (Figures 3A, B), each plants extract (Figures 3D–F) and triple therapy group (Figure 3C) showed suppressed immune cells against inflammation in mice stomach. The average stomach weight of control Balb/c mice inoculated with H. pylori was approximately 1.5-fold of that for animals without inoculation (data not shown).
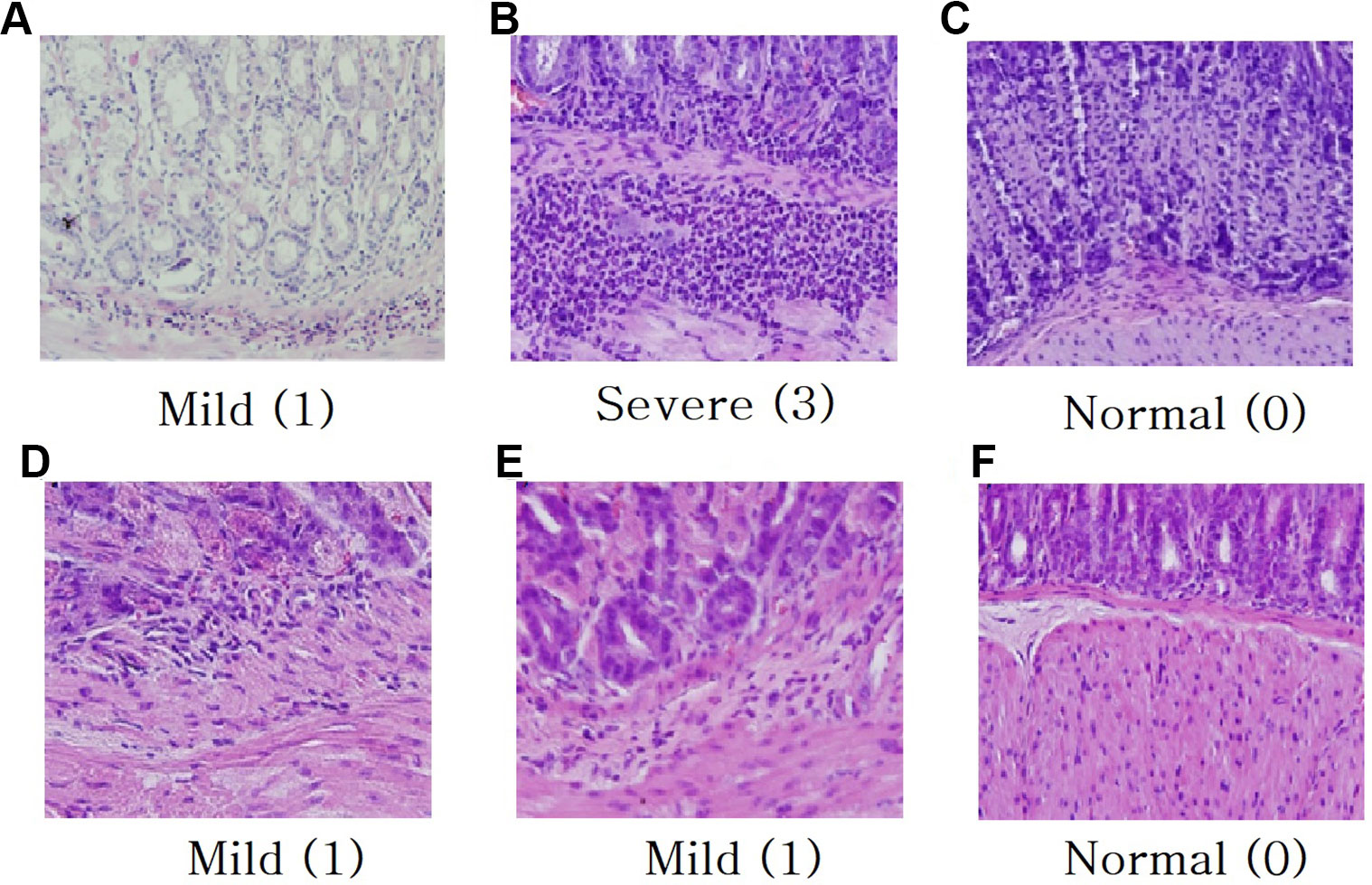
Figure 3 Histological analysis for H & E staining (magnification × 40). (A) H. pylori infected control mouse 4 weeks, (B) H. pylori infected control mouse 8 weeks, (C) Treatment with triple therapy (OAC), (D) Treatment with R. crataegifolius, (E) Treatment with U. macrocarpa, (F) Treatment with R. crataegifolius combined U. macrocarpa. Each plant extract suppresses immune cells against inflammation in mice stomach. Histological scoring of inflammation: normal (0), mild (1), moderate (2), and severe (3).
Discussion
Rubus crataegifolius Bunge, commonly known as “red raspberry” is used as a traditional oriental medicine in Korea. Unripened R. crataegifolius is extensively used in combination with other herbal preparations in food, beverages and in Korean folk medicine for its management of impotence, inflammatory, hepatotoxicity, enuresis, and allergic disease (Cha et al., 2001; Oh et al., 2007; Yang et al., 2008). The plant is also shown to possess antibacterial (Jeon et al., 2012) as well as antiulcer (Kim et al., 2011) activity. The main constituents of RF are polyphenols including sanguine, coreanoside-F1, niga-ichigoside, gallic acid, and ellagic acid (Oh et al., 2007; Kim et al., 2011; Jeon et al., 2012; Lim et al., 2012). Among these, antioxidant phenolic compound ellagic acid possesses strong chemoprotective and anti-Helicobacter properties (Chung, 1998). In our present study, we also found strong anti H. pylori effect of the plant probably due to the presence of ellagic acid at a concentration of 14.2 mg/g of dry extract. Further studies need to be done for determination whether ellagic acid is the main component which shows anti-H. pylori effect in RF extract.
Elm tree (UL), Ulmus macrocarpa Hance is a wide spread deciduous tree in Korea. The stem and root bark of this plant have long been used in oriental medicine to treat inflammation, edema, and mastitis (Yun et al., 2008; Rimbara et al., 2011; Kim et al., 2012). The plant was [reported to possess anti-gastritis, chemopreventive, and anti-Helicobacter activity (Yun et al., 2008; Kim et al., 2012; Hosny et al., 2014). The reported main constituents of the plant are polyphenols (Hosny et al., 2014) and it was reported that catechin showed strong anti-Helicobacter activity (Mabe et al., 1999). In our study, we also found moderate anti H. pylori effect of the plant probably due to the presence of catechin at a concentration of 30.5 mg/g of dry extract.
The significant synergistic anti-H. pylori and anti-gastritis effect of R. crataegifolius and U. macrocarpa is reported for the first time. The new findings are in line with the previously reported data for both plants that might encourage researchers for novel therapy schemes including phytotherapy as alternative approaches to cure H. pylori or as novel alimentary regimens as a more plant-based diet with an intake of compounds having chemoprotective and chemopreventive effects. Further clinical follow-up studies will be necessary to investigate the effect of active plant extracts when combined with antimicrobial agents commonly used for H. pylori eradications.
Conclusion
In conclusion, the synergistic effect of two plants, RF and Ulmus macrocarpa against H. pylori infection and gastric mucosal damage is demonstrated. The results presented in this report suggest that protective synergistic effect of two well-known plants could be considered as a valuable support in the treatment of H. pylori induced gastritis and may contribute to the development of new and safe agents of inclusion in anti-H. pylori regimens.
Data Availability Statement
All datasets generated for this study are included in the article.
Ethics Statement
The animal study was reviewed and approved by the Care of Animals and the Animal Welfare Committee, Korea Research Institute of Bioscience and Biotechnology (KRIBB).
Author Contributions
JP: Experimental design for Helicobacter and MIC test using RF and UL. JC: Purchasing and processing of RF and UL. JK and HK: MIC test and gastritis experiment. YJ: Histological analysis, H & E staining. MR: HPLC analysis and in vitro Helicobacter activity test. YL: Conception and design, participated in general coordination of the study, writing and revising the manuscript.
Funding
The research was supported by Korea Ministry of SMEs (Small and Medium Enterprise) and Startups with grant number #2410060 and Lee's Biotech Co., KRIBB, Korea. Lee's Biotech provided funding/resources for analysis including extraction and HPLC analysis.
Conflict of Interest
Authors JC and MR are employed by Lees Biotech Co. Authors HK and YJ are employed by Kolmar BNH Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Brown, J. C., Jiang, X. (2013). Activities of muscadine grape skin and polyphenolic constituents against Helicobacter pylori. J. Appl. Microbiol. 114 (4), 982–991. doi: 10.1111/jam.12129
Cha, H.-S., Park, M.-S., Park, K.-M. (2001). Physiological activities of Rubus coreanus Miquel. Korean J. Food Sci. Technol. 33 (4), 409–415.
Chung, J. G. (1998). Inhibitory actions of ellagic acid on growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Microbios 93 (375), 115–127.
GBD 2013 Mortality and Causes of Death Collaborators (2015). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386 (10010), 2287–2323. doi: 10.1016/S0140-6736(15)00128-2
Gisbert, J. P., Calvet, X. (2012). Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin. Exp. Gastroenterol. 5, 23–34. doi: 10.2147/CEG.S25419
Goderska, K., Agudo Pena, S., Alarcon, T. (2018). Helicobacter pylori treatment: antibiotics or probiotics. Appl. Microbiol. Biotechnol. 102 (1), 1–7. doi: 10.1007/s00253-017-8535-7
He, J. M., Sun, N., Wu, W. D., Fan, L. J., Guo, M. L. (2013). Determination of ellagic acid, flavonoids and goshonoside-F5 in Rubi Fructus by HPLC. Zhongguo Zhong Yao Za Zhi 38 (24), 4351–4356. doi: 10.4268/cjcmm20132431
Hosny, M., Zheng, M. S., Zhang, H., Chang, H. W., Woo, M. H., Son, J. K., et al. (2014). (–)-Catechin glycosides from Ulmus davidiana. Arch. Pharm. Res. 37 (6), 698–705. doi: 10.1007/s12272-013-0264-6
Jeon, Y.-H., Sun, X., Kim, M.-R. (2012). Antimicrobial activity of the ethanol extract from Rubus coreanum against microorganisms related with foodborne illness. Korean J. Food Cookery Sci. 28 (1), 9–15. doi: 10.9724/kfcs.2012.28.1.009
Kim, S. J., Lee, H. J., Kim, B. S., Lee, D., Lee, S. J., Yoo, S. H., et al. (2011). Antiulcer activity of anthocyanins from Rubus coreanus via association with regulation of the activity of matrix metalloproteinase-2. J. Agric. Food Chem. 59 (21), 11786–11793. doi: 10.1021/jf104192a
Kim, T.-W., Youm, S.-Y., Shin, S.-K., Kim, D. J., Hong, J. T., Kim, Y., et al. (2012). Chemopreventive effects of elm tree bark extract on Helicobacter pylori-associated mouse gastric carcinogenesis. Basic Appl. Pathol. 5, 2, 31–38. doi: 10.1111/j.1755-9294.2012.01125.x
Lim, J. W., Hwang, H. J., Shin, C. S. (2012). Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J. Agric. Food Chem. 60 (20), 5121–5127. doi: 10.1021/jf205350k
Liou, J. M., Chang, C. Y., Chen, M. J., Chen, C. C., Fang, Y. J., Lee, J. Y., et al. (2015). The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors-a nationwide study. PloS One 10 (5), e0124199. doi: 10.1371/journal.pone.0124199
Liou, J. M., Wu, M. S., Lin, J. T. (2016a). Treatment of Helicobacter pylori infection: where are we now? J. Gastroenterol. Hepatol. 31 (12), 1918–1926. doi: 10.1111/jgh.13418
Liou, J. M., Chen, C. C., Chang, C. Y., Chen, M. J., Chen, C. C., Fang, Y. J., et al. (2016b). Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut 65 (11), 1784–1792. doi: 10.1136/gutjnl-2015-310142
Mabe, K., Yamada, M., Oguni, I., Takahashi, T. (1999). In vitro and in vivo activities of tea catechins against Helicobacter pylori. Antimicrob. Agents Chemother. 43 (7), 1788–1791. doi: 10.1128/AAC.43.7.1788
Malfertheiner, P., Megraud, F., O'Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of Helicobacter pylori infection–the Maastricht IV/Florence consensus report. Gut 61 (5), 646–664. doi: 10.1136/gutjnl-2012-302084
Oh, M. S., Yang, W. M., Chang, M. S., Park, W., Kim, D. R., Lee, H. K., et al. (2007). Effects of Rubus coreanus on sperm parameters and cAMP-responsive element modulator (CREM) expression in rat testes. J. Ethnopharmacol. 114 (3), 463–467. doi: 10.1016/j.jep.2007.08.025
Rimbara, E., Fischbach, L. A., Graham, D. Y. (2011). Optimal therapy for Helicobacter pylori infections. Nat. Rev. Gastroenterol. Hepatol. 8 (2), 79–88. doi: 10.1038/nrgastro.2010.210
Takeuchi, H., Trang, V. T., Morimoto, N., Nishida, Y., Matsumura, Y., Sugiura, T. (2014). Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 20 (27), 8971–8978. doi: 10.3748/wjg.v20.i27.8971
Venerito, M., Krieger, T., Ecker, T., Leandro, G., Malfertheiner, P. (2013). Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 88 (1), 33–45. doi: 10.1159/000350719
Yang, H. M., Oh, S.-m., Lim, S. S., Shin, H.-K., Oh, Y.-S., Kim, J.-K. (2008). Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother. Res. 22, 1, 102–107. doi: 10.1002/ptr.2274
Keywords: Rubus crataegifolius, Ulmus macrocarpa, MIC, Helicobacter pylori, gastritis
Citation: Park JU, Cho JS, Kim JS, Kim HK, Jo YH, Rahman MAA and Lee YI (2020) Synergistic Effect of Rubus crataegifolius and Ulmus macrocarpa Against Helicobacter pylori Clinical Isolates and Gastritis. Front. Pharmacol. 11:4. doi: 10.3389/fphar.2020.00004
Received: 24 June 2019; Accepted: 03 January 2020;
Published: 20 February 2020.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Simone Carradori, University “G. d'Annunzio” of Chieti-Pescara, ItalyPinarosa Avato, University of Bari Aldo Moro, Italy
Copyright © 2020 Park, Cho, Kim, Kim, Jo, Rahman and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young Ik Lee, eWlsZWVAa3JpYmIucmUua3I=
 Jung Uoon Park1
Jung Uoon Park1 Md Aziz Abdur Rahman
Md Aziz Abdur Rahman Young Ik Lee
Young Ik Lee