
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 29 January 2020
Sec. Ethnopharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01629
This article is part of the Research Topic Systems Pharmacology and Traditional Chinese Medicine View all 19 articles
 Songhong Yang1†
Songhong Yang1† Jinlian Zhang1†
Jinlian Zhang1† Yiqi Yan2†
Yiqi Yan2† Ming Yang1
Ming Yang1 Chao Li1
Chao Li1 Junmao Li1
Junmao Li1 Lingyun Zhong1*
Lingyun Zhong1* Qianfeng Gong1*
Qianfeng Gong1* Huan Yu1*
Huan Yu1*Chronic gastritis (CG) is an inflammatory disease. Atractylodes macrocephala Koidz (AMK) is employed in traditional Chinese medicine (TCM) to treat various disorders. AMK can be efficacious against CG, but the active ingredients, drug targets, and its exact molecular mechanism are not known. We employed network pharmacology to analyze the active ingredients, drug targets, and key pathways of AMK in CG treatment. Seventy-seven AMK candidate ingredients were selected from four databases, and 27 active ingredients were selected for CG treatment. Twenty-five overlapping gene symbols related to CG and drugs were obtained from GeneCards and OMIM databases. A protein–protein interaction (PPI) network and TCM comprehensive network (Drug–Ingredients–Gene symbols–Disease network) were constructed, and 528 Gene Ontology (GO) terms and 26 pathways were obtained by analyses of enrichment of GO pathways and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. We suggest that the interleukin-17 signaling pathway, C-type lectin receptor signaling pathway, tumor necrosis factor signaling pathway, and AGE-RAGE signaling pathway in diabetic complications might serve as the key points and principal pathways for CG treatment. We also evaluated the reliability of some important active ingredients and targets by in vitro experiments. We showed that AMK probably influences the inflammatory response, amino acid synthesis, and energy metabolism when treating CG. This study provides novel insights for researchers to explore the mechanism of action of TCM systematically.
Chronic gastritis (CG) is an inflammatory disease in which the epithelium of the gastric mucosa is invaded by various pathogenic factors, which results in persistent and chronic inflammatory changes. CG can be divided into three categories: chronic non-atrophic, chronic atrophic, and “special.” If not treated in a timely manner, CG can transform into gastric cancer (Ohata et al., 2004; Yue et al., 2018; Osaki et al., 2019; Han et al., 2019). Most patients with CG do not have obvious symptoms, and the main symptom is dyspepsia, which is nonspecific. Some CG patients can present with abdominal pain, bloating, and other symptoms of indigestion.
Traditional Chinese medicine (TCM) has been used to treat various diseases for thousands of years. In TCM theory, CG is divided into six types according to the pattern of: accumulation of damp heat in the spleen–stomach; dampness obstructing the spleen–stomach; spleen–stomach Qi deficiency; spleen–stomach deficiency cold; liver–Qi stagnation; stagnant heat in the liver–stomach (Zhu et al., 2014).
Atractylodes macrocephala Koidz. (AMK) can invigorate the spleen and Qi, reduce dampness and moisture, act as an antiperspirant, and stop fever developing. In TCM, it is often used to treat spleen deficiency, malnutrition, abdominal distension, diarrhea, dizziness, palpitations, edema, spontaneous sweating, and fetal restlessness (The State Commission of Chinese Pharmacopoeia, 2010; Tang et al., 2012; Zhao, 2013).
In recent years, network pharmacology has been used widely in TCM research. By using “web”-based approaches, network pharmacology can systematically determine the effects and mechanisms of drugs used to treat complex diseases at molecular, cellular, tissue, and biologic levels.
Danlu capsules, the Maxing Ganshi Decoction, Wei Pi Xiao Decoction and Xiaoyao powder have been adopted widely in TCM (Liu et al., 2016; Huang et al., 2017; Song et al., 2018; Yang et al., 2019). AMK can be efficacious for CG treatment, but the active ingredients, drug targets, and the exact molecular mechanism are not known.
We used network pharmacology to analyze the active ingredients, drug targets, and key pathways of AMK in CG treatment (Figure 1). Results suggested that AMK may have a therapeutic effect against CG because it can regulate key factors in the inflammatory pathway.
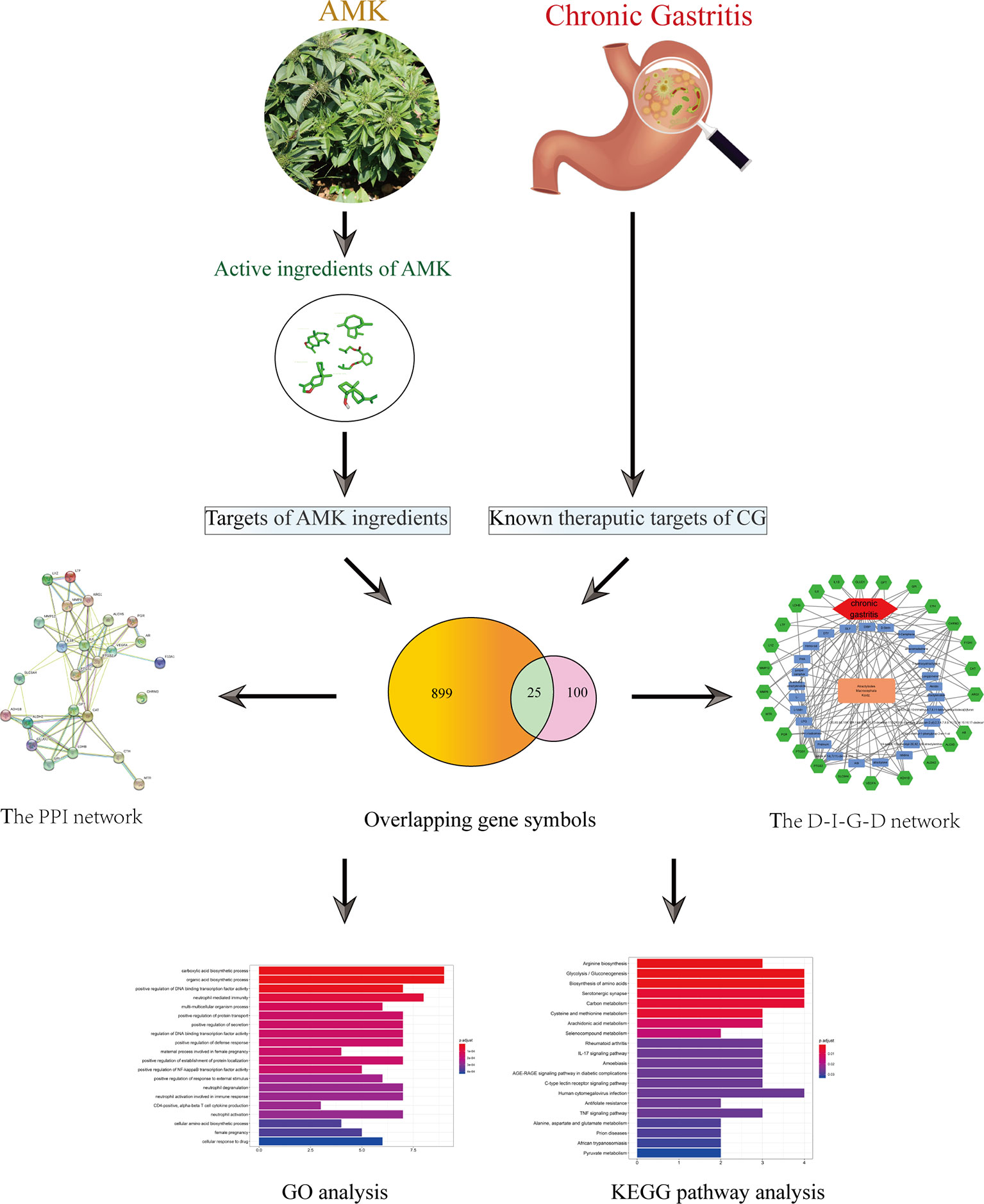
Figure 1 Flowchart of a network pharmacology-based strategy to investigate the pharmacologic mechanisms of Atractylodes macrocephala Koidz. for treatment of chronic gastritis.
All ingredients contained in AMK were searched for in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP; http://lsp.nwu.edu.cn/tcmsp.php), Chinese Academy of Sciences Chemistry Database (www.organchem.csdb.cn), TCM Database@taiwan (http://tcm.cmu.edu.tw), and Traditional Chinese Medicine Integrated Database (https://omictools.com/tcmid-too). TCMSP is a special platform based on systems pharmacology for Chinese herbal medicines that includes the relationships between drugs, targets, and diseases. TCMSP has information on 500 drugs and >12,000 ingredients (Ru et al., 2014).
“Oral bioavailability” (OB) refers to the percentage of unmodified drugs that enters the circulatory system after oral administration (Xu et al., 2012; Liu et al., 2013). OB is also an important indicator for objective evaluation of the internal quality of drugs (Alam et al., 2014). The higher the OB of an ingredient, the higher is the likelihood that it can be used clinically. “Drug likeness” (DL) is a vague concept that refers to the similarity between ingredients and known drugs (Walters and Murcko, 2002; Tao et al., 2013). Ingredients with DL properties are not drugs, but can become drugs. This class of ingredient comprises drug-like molecules or drug analogs. The Tanimoto coefficient was used to evaluate the DL index of the molecules in AMK using the following formula:
where α is the molecular property of the AMK ingredient on the basis of Dragon software (www.talete.mi.it/products/dragon_description.htm) and β denotes the average molecular property for all drugs in the DrugBank database (www.drugbank.ca/) (Mauri et al., 2006).
Most ingredients in Chinese formulations have poor pharmacologic properties, so they cannot bind to specific protein targets on cells efficaciously. Therefore, several researchers have recommended that molecules with OB ≥ 30% or DL ≥ 0.18 are considered to have better pharmacologic effects, and are selected as candidate ingredients for further analyses (Liu et al., 2016; Huang et al., 2017; Yang et al., 2019). We used this principle to screen for active ingredients to be used in further analyses.
Target forecasts and target-prediction methods can be divided into four categories based on: the predictions of a ligand (chemical similarity and pharmacophore model); the predictions of a receptor (molecular docking); machine learning (the molecules in a database must have a clear correspondence with the target, and the name of the target must be standardized); a combined forecast. Considering the limitations of the experimental conditions of molecular docking and machine learning and our previous experience, we chose a prediction method based on ligands for subsequent research (Yu et al., 2012; Wu et al., 2016; Wang et al., 2017).
All the protein targets of the ingredients in AMK were retrieved from TCMSP (http://lsp.nwu.edu.cn/tcmsp.php). We removed redundant information and retained only those targets that could interact directly with each of these ingredients in AMK. Then, the target was transformed using the UniProt knowledge database (www.uniprot.org) with the selected species as Homo sapiens. After deletion of redundant items, data were merged to obtain gene symbols.
We collected gene targets for CG from two sources. The first source was the GeneCards v4.9.0 (www.genecards.org/). GeneCards is a searchable, comprehensive database that offers all comments and predicts human genetic information comprehensively in a user-friendly manner (Rebhan, 1997; Safran et al., 2010). We used the keyword “chronic gastritis” to search this database. The second source was the Online Mendelian Inheritance in Man (OMIM) database (www.omim.org/, updated on 28 February 2019) (Hamosh et al., 2005).
First, we intersected the obtained drug targets with the genes associated with a disease and obtained a Venn diagram of the intersected gene symbols. Then, we built a network of complex information based on interactions between the drug (AMK), ingredients, gene symbols, and disease (CG). Next, we used Cytoscape v3.7.1 (www.cytoscape.org/) (Shannon, 2003; Su et al., 2014) to undertake visual analyses of the D–I–G–D network (which is a graphical display of network analyses and editing software).
PPI data were obtained from the String v11.0) https://string-db.org/, updated on 19 January 2019) (Hsia et al., 2015). Then, the target was transformed using the UniProt knowledge database. After deletion of redundant items, data were merged to obtain gene symbols. Finally, we searched these gene symbols using the “multiple proteins” option while simultaneously setting the organism to Homo sapiens. Then, the PPI network of AMK active ingredients–target symbols and chronic gastritis-related target symbols was constructed.
GO is an international standard classification system for gene function (Ashburner et al., 2000). We used Bioconductor (R) v3.8 (http://bioconductor.org/, released on 31 October 2018) for analyses.
The KEGG database analyzes the metabolic pathways of gene products in cells and the functions of these gene products (Kanehisa and Susumo, 2000). We used Bioconductor (R) v3.8 (http://bioconductor.org/, released on 31 October 2018) for analyses.
We wished to ascertain the interaction between active ingredients and their protein targets and explore their binding modes. Hence, we selected three active ingredients and three targets, a total of four ingredients–targets interactions for verification of molecular docking. We used GOLD v5.1 (a genetic algorithm-based docking program to dock protein–ligand complexes). We obtained the X-ray crystal structures of interleukin (IL)-6, IL-1β, and muscarinic acetylcholine receptor M3 (CHRM3) from the RCSB Protein Data Bank (PDB) (www.rcsb.org); the PDB entry code for these proteins is 1ALU, 2NVH, and 4DAJ, respectively. During molecular docking, we adopted the GOLD Score fitness function. The GOLD fitness function comprised three main terms: a hydrogen energy; a pairwise van der Waals steric energy between the protein and the ligand; and an internal energy term for the ligand.
Atractylenolide-I (ATL-I; purity ≥98%) was purchased from Must Biotechnology (Chengdu, China). A stock solution of 100 mM ATL-I in dimethyl sulfoxide was prepared and stored at 4°C. Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and IL-1β were purchased from Elabscience Biotechnology (Wuhan, China).
RAW264.7 cells were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). RAW264.7 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Billings, MT, USA) with 10% fetal bovine serum (Gibco). Cells were cultured at 37°C in an atmosphere of 5% CO2 for all experiments.
RAW264.7 cells in the logarithmic phase were seeded at 8 × 103 cells/well in 96-well culture plates. After incubation for 24 h, Raw264.7 cells were exposed to ATL-I (0, 20, 40, 60, 80, or 100 μmol/L). After treatment for 24 h, 20 μL of Cell Counting Kit (CCK-8) assay solution (Biosharp, Hefei, China) was added to each well, and cells were incubated for 4 h at 37°C in an atmosphere of 5% CO2. The absorbance at 450 nm was measured by a microplate reader. Cell survival was calculated as: absorbance/absorbance of control ×100%.
RAW264.7 cells (5 × 103 cells/well) were incubated with lipopolysaccharide (LPS; 1 μg/mL) for 24 h and then treated with ATL-I (20, 40, or 60 μM) for 24 h. Supernatants were harvested and the level of IL-6 and IL-1β determined by ELISA kits (Biosharp).
Total RNA was extracted with TRIzol® Reagent (Thermo Scientific, Waltham, MA, USA), and reverse-transcribed with oligo-DT using HiScript™ Reverse Transcriptase (Vazyme, Beijing, China) according to manufacturer instructions. The primers used were synthesized by Tsingke (Beijing, China). The sequences were (forward and reverse, respectively) 5′-TCAGGCAGGCAGTATCACTC-3′ and 5′-AGCTCATATGGGTCCGACAG-3′ for IL-1β; 5′-CACAGAGGATACCACTCCCAACAGA-3′ and 5′-ACAATCAGAATTGCCATTGCACAAC-3′ for IL-6; 5′-ATGGGTGTGAACCACGAGA-3′ and 5′-CAGGGATGATGTTCTGGGCA-3′ for the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
qRT-PCR was done using SYBR™ Green Master Mix (Vazyme) in the QuantStudio 6 Flex system (Applied Biosystems, Foster City, CA, USA). The PCR cycling profile was: one cycle at 50°C for 2 min and 95°C for 10 min, 40 cycles at 95°C and 60°C for 30s. Fluorescence signals were detected using the QuantStudio 6 Flex system. Gene-expression data were normalized to that of the endogenous control GAPDH. The 2−ΔΔCT method was the basis for relative expression of genes.
Data are the mean ± SD. The significance of results was determined based on one-way analysis of variance using Prism 8.0.1 (Graphpad, San Diego, CA, USA). p < 0.05 was considered significant. All experiments were repeated at least three times.
Seventy-seven AMK candidate ingredients were selected from three databases (Supplementary Table S1). To identify the active ingredients of AMK, two classical absorption, distribution, metabolism, and excretion (ADME) parameters, OB and DL, were used for screening. Some ingredients were not in accordance with the standard of screening ingredients and were also likely to produce therapeutic effects in the human body. To study this issue more comprehensively, although they did not meet the screening criteria, these candidate ingredients were retained as active ingredients in our study. For example, ATL-I had a low DL, but it was retained as an active ingredient because it is the major constituent of AMK (Zhang et al., 1998; Li et al., 2007). We found that proliferation of human gastric cancer (mgc-803) cells was inhibited by ATL-I in a time-dependent and dose-dependent manner (p < 0.05). ATL-I inhibited expression of Notch-1, Hey-1, Jagged-1, and Hes-1 in the Notch signaling pathway. ATL-I inhibited expression of the mRNA of Notch-1, Hey-1, Jagged-1, and Hes-1 (p < 0.05). ATL-I has been shown to inhibit proliferation of mgc-803 cells in human gastric cancer by inhibiting the Notch signaling pathway (Ma et al., 2014). ATL-I could inhibit the increase in vascular permeability caused by acetic acid and can resist proliferation of granuloma tissue. AMK also has a therapeutic effect on acute or chronic inflammation (Chen, 1987; Li et al., 2007). It is important to note that although the pharmacokinetic values of several ingredients were relatively low, they had biologic activity, so they were also regarded as candidate ingredients. In addition, we expanded the criteria for screening beyond the ADME principle. We postulated that, as long as the candidate ingredients in AMK intersect with the targets of CG, they can be considered to be active ingredients.
In summary, 27 ingredients were selected as active ingredients in AMK (Table 1).
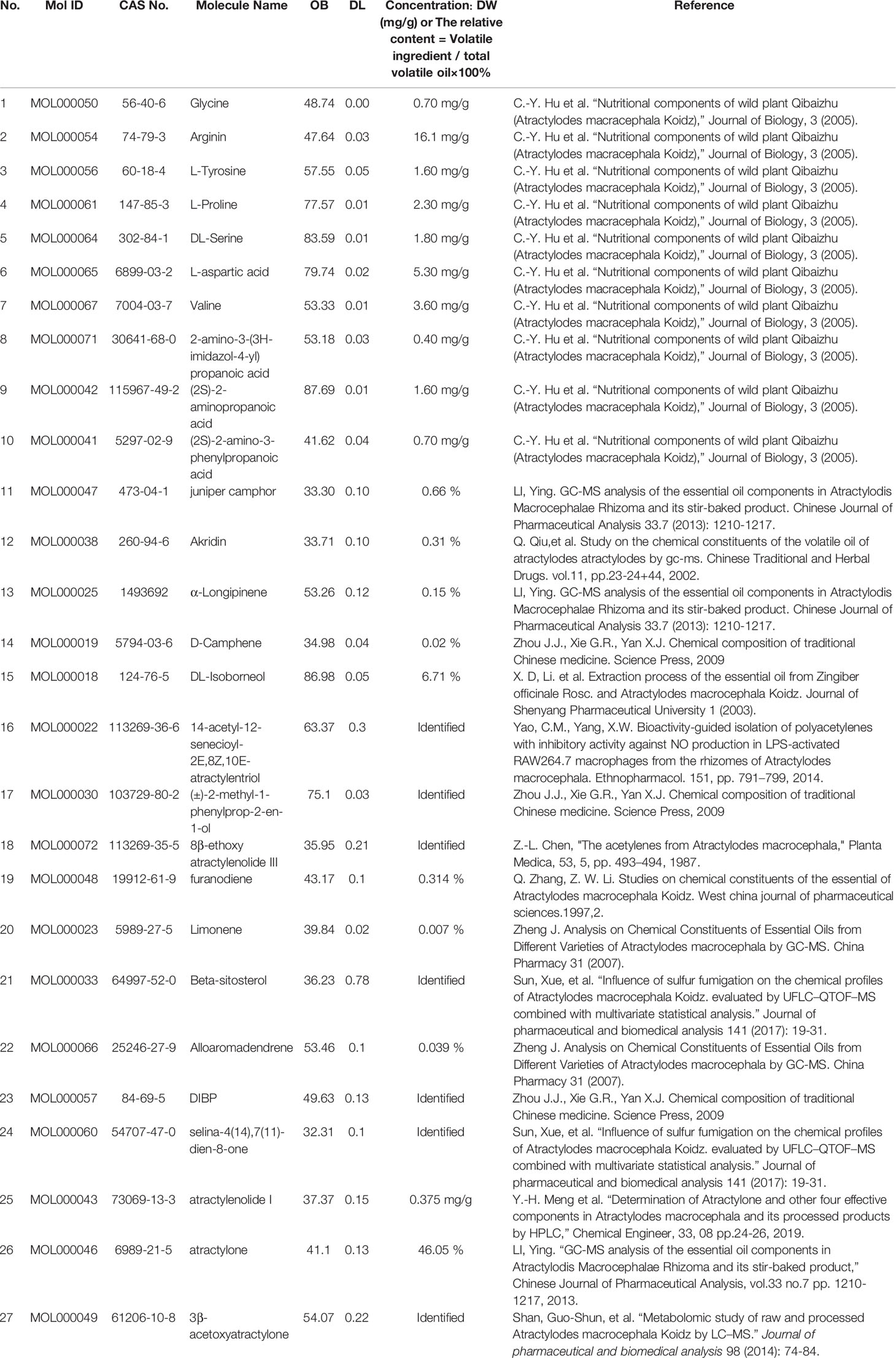
Table 1 A total of twenty-seven ingredients were selected as the details of the active ingredients of AMK in this study.
After collection from the TCMSP database, conversion into the UniProt database, and deletion of redundant items, 27 ingredients in AMK and 100 known target symbols related to them were obtained (Supplementary Table S2).
A total of 901 known therapeutic target symbols for CG were collected from the GeneCards database. In addition, 23 known therapeutic targets for CG were obtained from the OMIM database. After elimination of redundancies, 899 known therapeutic targets for CG were collected (Supplementary Table S3).
Figure 2A shows that 899 gene symbols for disease and 100 gene symbols for drugs had 25 overlaps. That is, 25 gene symbols may be the key for CG treatment by AMK. The 25 overlapping gene symbols are detailed in Supplementary Table S4.
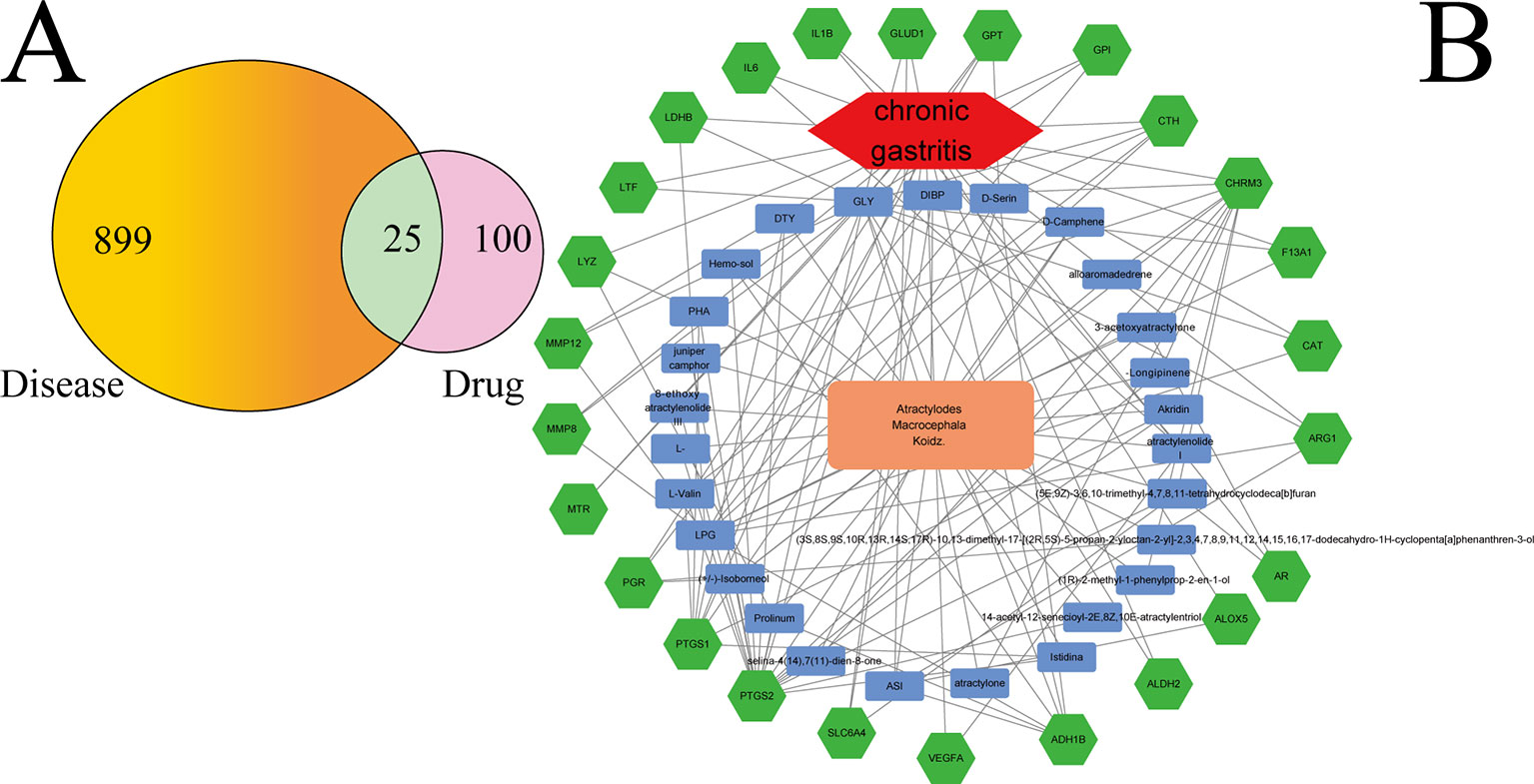
Figure 2 (A) Twenty-five overlapping gene symbols between the disease and drug. (B) D-I-G-D network. The orange node represents AMK and the red node represents CG. The 27 blue nodes represent the active ingredients in AMK; The 25 green nodes represent the overlapping gene symbols between the disease and drug. The edges denote that nodes can interact with each other.
To clarify how AMK may act against CG, we used Ctyoscape to build a D–I–G–D network (Figure 2B); the orange node represents AMK and the red node represents CG. Also, the 27 blue nodes represent the active ingredients in AMK; the 25 green nodes represent the overlapping gene symbols between the disease and drug. The edges denote that nodes can interact with each other.
We conducted further network analyses by evaluating centralization and heterogeneity, which were 0.432 and 1.077, respectively. Hence, some nodes were more concentrated in the network than others. That is, the ingredient−target symbol space tended to veer toward certain ingredients and target symbols. Hence, the network included some ingredients with multiple target symbols, such as glycine (GLY; degree = 17), (L)-Alanine (LPG; degree = 12), (5E,9Z)-3,6,10-trimethyl-4,7,8,11-tetrahydrocyclodeca[b]furan (5), 3β-acetoxyatractylone (4), ATL-I (4), and atractylone (2). Moreover, it meant that AMK could act on multiple targets through the same active ingredient. For example, ATL-I could have an effect on vascular endothelial growth factor A (VEGFA), IL-6, and IL-1β if used for CG treatment. Wang and colleagues showed that ATL-I can achieve anti-inflammatory effects by inhibiting expression of IL-6 and IL-1β (Wang et al., 2009). Detailed information regarding active ingredients and gene symbols are described in Supplementary Table S5.
We constructed a PPI network consisting of 25 nodes and 77 edges (Figure 3A). This was based on the premise that proteins have more interactions among themselves than would be expected for a random set of proteins of similar size, drawn from the genome. Such an enrichment indicates that the proteins are at least partially biologically connected, as a group.
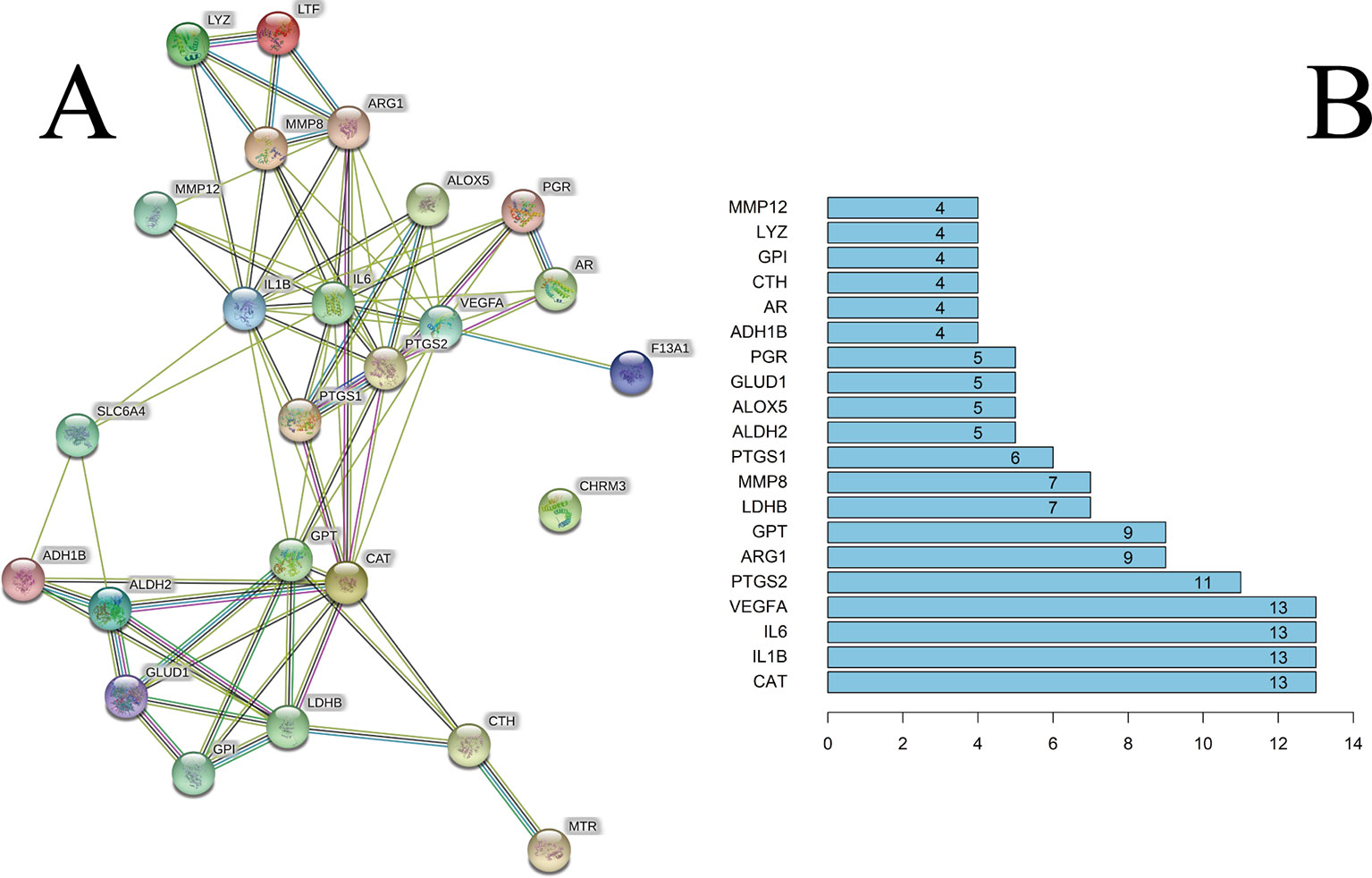
Figure 3 (A) The PPI network. (B) The bar plot of the PPI network. The x-axis represents the number of neighboring proteins of the target protein. The y-axis represents the target protein.
The light-blue edges denote known interactions from curated databases. The pink edges denote that the known interactions were determined by experimental methods. The green edges denote that the predicted interactions arose from the neighborhood gene. The red edges denote that the predicted interactions arose from gene fusions. The dark-blue edges denote that the predicted interactions arose from gene co-occurrence. The yellow edges denote that the predicted interactions arose from text-mining. The black edges denote that the predicted interactions arose from co-expression. The lavender edges denote that the predicted interactions arose from protein homology. The details of the PPI network are described in Supplementary Table S6.
We took the first 20 proteins in the PPI network. As seen from Figure 3B, catalase (CAT), IL-6, IL-1β, and VEGFA could be related to the other 13 proteins. These results suggested that these four proteins would be the focus of our research of PPIs. Prostaglandin G/H synthase 2 (PTGS2) could be related to the other 11 proteins. CHRM3 was not associated with other proteins in this PPI network.
To ascertain if the 25 gene symbols were related to CG, we conducted analyses of enrichment of GO pathways to clarify the relevant biologic processes (p < 0.01) (Figure 4A). The y-axis represents GO terms. The x-axis indicates the number of genes enriched in that term. The redder the color, the smaller the value of p.adjust (FDR); it also denotes greater credibility and greater importance. In contrast, the bluer the color, the greater is the value of p.adjust. For a brief demonstration, we intercepted the first 20 terms from small to large according to the p-value. The details of GO analyses are described in Supplementary Table S7. The results indicated that numerous biologic processes were involved in CG treatment, including carboxylic acid biosynthetic process (GO:0046394), organic acid biosynthetic process (GO:0016053), positive regulation of DNA binding transcription factor activity (GO:0051091), positive regulation of the defense response (GO:0031349), and neutrophil activation involved in the immune response (GO:0002283).
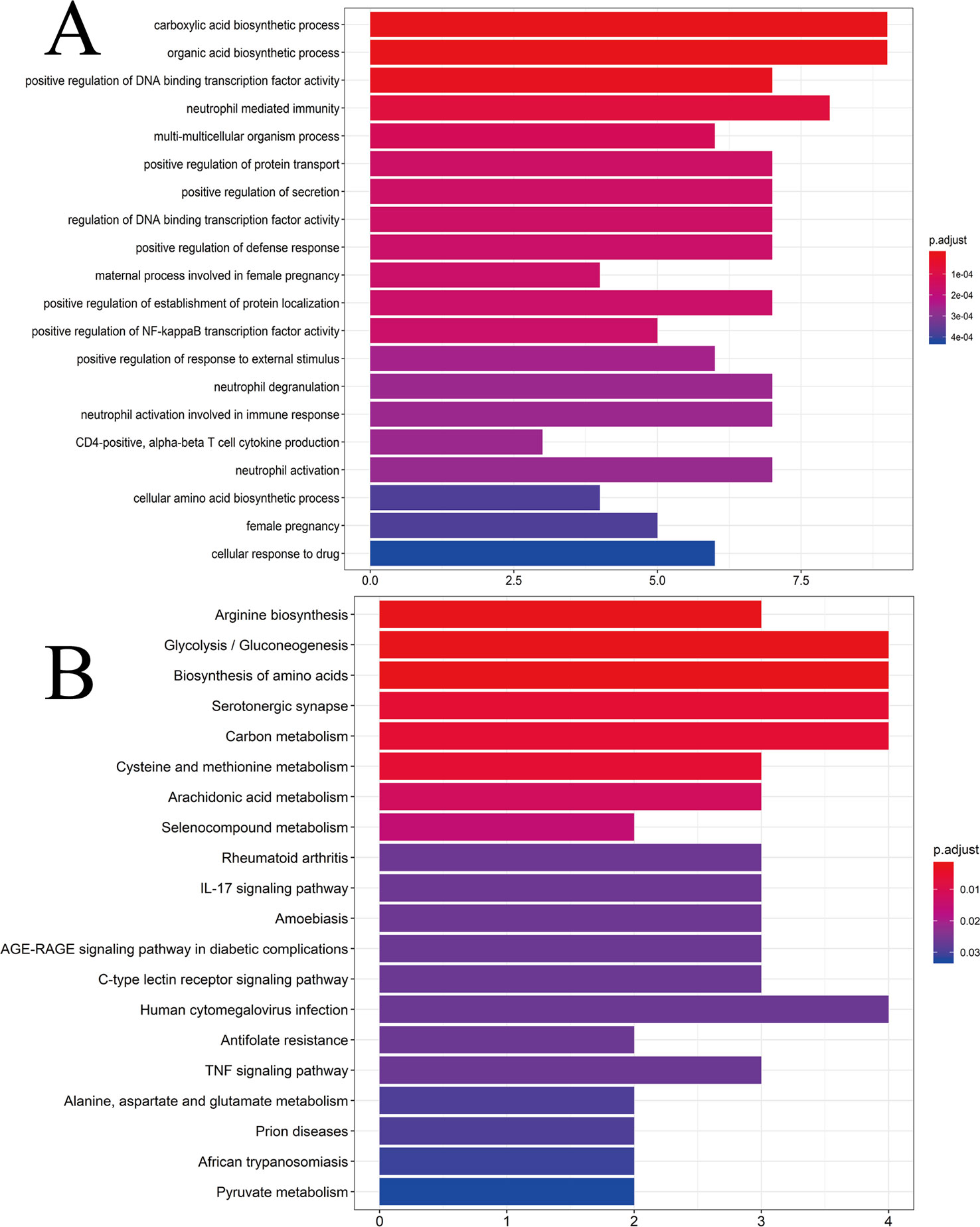
Figure 4 (A) GO analyses of the 25 gene symbols associated with chronic gastritis. The x-axis represents significant enrichment in the counts of these terms. The y-axis represents the categories of “biological process” in the GO of the target genes (p < 0.01). (B) KEGG pathway enrichment analyses. The x-axis represents the counts of the target symbols in each pathway; the y-axis represents the main pathways (p < 0.01).
Analyses of enrichment of the KEGG pathway were carried out using Bioconductor (R) (p < 0.01) (Figure 4B). The y-axis represents the KEGG pathway. The x-axis indicates the number of genes enriched in that pathway. The redder the color, the smaller the value of p.adjust; it also denotes greater credibility and greater importance. In contrast, the bluer the color, the greater the value of p.adjust. The 25 overlapping gene symbols were mapped to 26 pathways after enrichment of the KEGG pathway. For a brief demonstration, we intercepted the first 20 pathways from small to large according to the p-value. The details of enrichment of the KEGG pathway are described in Supplementary Table S8.
Enrichment of the KEGG pathway could be divided approximately into modules of amino acid synthesis, energy metabolism, and inflammation. As shown in Figure 4B, the 25 overlapping gene symbols interacted closely with the pathways involved in the IL-17 signaling pathway (hsa04657), C-type lectin receptor signaling pathway (hsa04625), tumor necrosis factor (TNF) signaling pathway (hsa04668), and AGE-RAGE signaling pathway in diabetic complications (hsa04933). These pathways may be the key pathways responsible for CG treatment. Such analyses offer a new way to study CG treatment.
In general, the number and strength of a ligand bound to a receptor is determined largely by the inhibitory efficiency (Wang et al., 2017). Therefore, we explored the interactions and binding modes between the inflammatory factors IL-6, IL-1β, and gastrointestinal regulatory target CHRM3 with their active ingredients by molecular docking.
ATL-I has special pharmacologic effects and high content in AMK. Hence, we first conducted molecular docking of ATL-I with IL-6 (Figure 5A). We can see clearly from Figure 5A that the O atoms in ATL-I and arginine (ARG)-179 (3.0 Å) and ARG-179 (3.2 Å) interact with each other between each N atom. In Figure 5B, the molecular-docking result of ATL-I with IL-1β shows that the O atoms on the lactone ring in ATL-I can interact with the N atoms on lysine (LYS)-92 (3.1Å) of IL-1β. The results of molecular docking were also consistent with our cell-experiment results, which demonstrated that ATL-I had a significant anti-inflammatory effect.
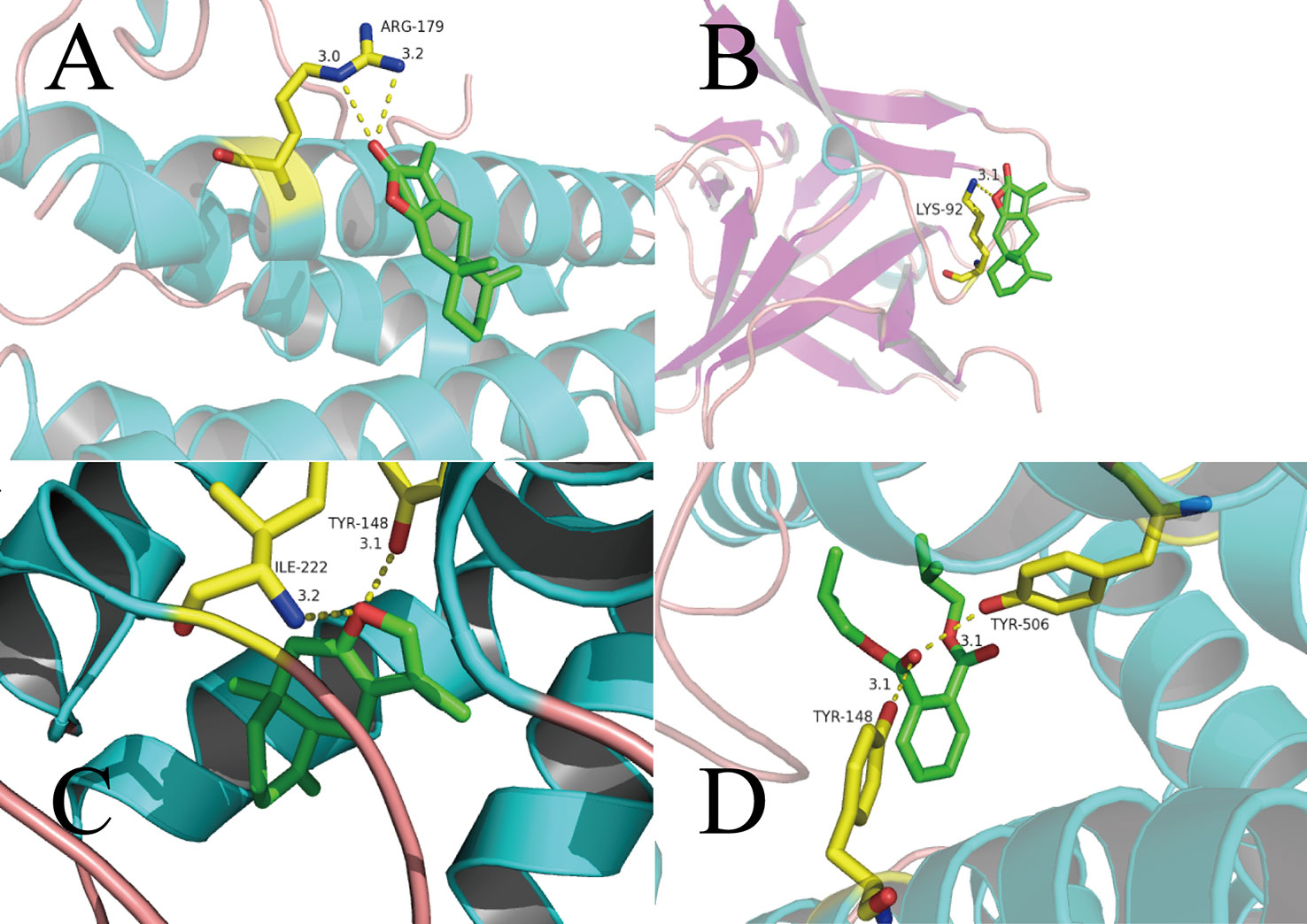
Figure 5 Binding studies of selected ingredients–targets interactions. (A) ATL-I with IL-6; (B) ATL-I with IL-1β; (C) atractylone with CHRM3; (D) DIBP with CHRM3. Molecules are represented by a ball-and-stick model, the hydrogen bonds are represented by a dotted line, and the distance is in angstroms. Atoms C, O, and N are green, red, and blue, respectively.
As another active ingredient with high content in AMK, atractylone was docked with CHRM3 (Figure 5C). The O atoms in atractylone interact with N and O atoms in isoleucine (ILE)-222 (3.2Å) and tyrosine (TYR)-148 (3.1Å), respectively, in CHRM3. This interaction also ensures a tight fit between atractylone and CHRM3. In Figure 5D, the carbonyl group in bis(2-methylpropyl) benzene-1,2-dicarboxylate (DIBP) is associated with the O atoms in TYR-148 (3.1Å) and TYR-506 (3.1Å) in CHRM3.
Based on these data, we can consider that the interaction between these active ingredients and targets is the basis of their biologic activity. It also means that AMK has multiple ingredients and multiple targets.
First, we determined the effects of different doses of ATL-I on the viability of RAW264.7 cells using the CCK-8 assay (Figure 6A). ATL-I at <60 μmol/L had high cell viability (>90%). Therefore, three concentrations were selected (20, 40, 60 μmol/L) for subsequent experiments.
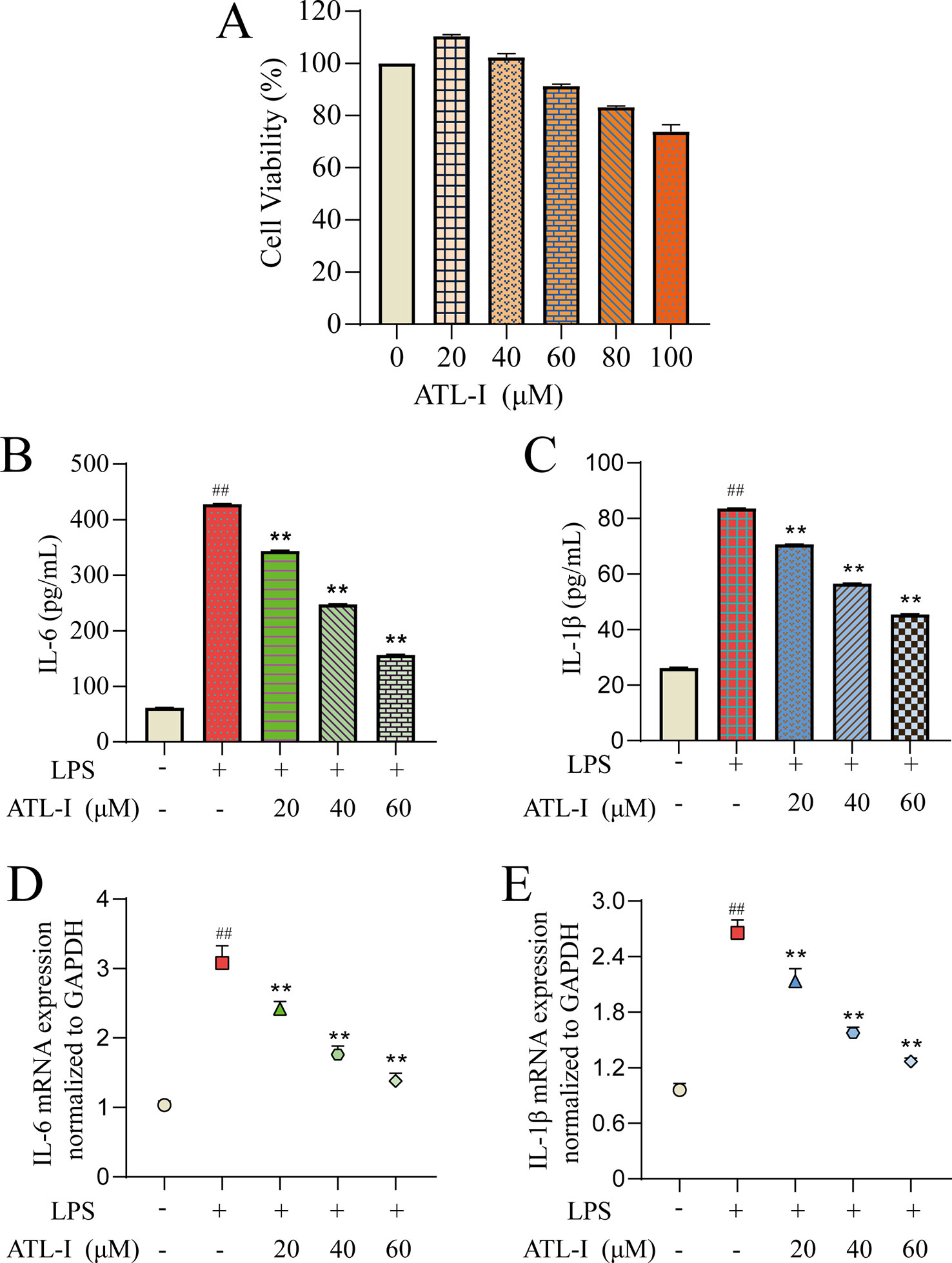
Figure 6 Action of ATL-I on RAW264.7 cells. RAW264.7 cells were incubated with LPS (1 μg/mL) for 24 h and then treated with ATL-I (20, 40, or 60 μM) for 24 h. The effects of ATL-I (A) on the viability of RAW264.7 cells using the CCK-8 assay. Production of IL-6 (B) and IL-1β (C) was determined by ELISAs. Protein expression of IL-6 (D) and IL-1β (E) was determined by qRT-PCR. ##p < 0.01 versus blank control group. **p < 0.01 LPS-treated group.
To further evaluate the results obtained in systematic pharmacologic analyses, ATL-I was selected from AMK to examine potential anti-inflammatory effects using LPS (1 μg/mL)-stimulated RAW264.7 cells. We undertook ELISAs and qRT-PCR for IL-6 and IL-1β to confirm the predicted anti-inflammatory effects of the ingredients. IL-6 production showed a significant decreasing trend with increasing ATL-I concentration (Figure 6B). Simultaneously, a significant decrease in IL-6 mRNA expression was observed (Figure 6C). Production and mRNA expression of IL-1β also decreased significantly with increasing ATL-I concentration (Figures 6D, E).
To summarize, these data suggested that ATL-I from AMK may have a therapeutic effect against CG because it can regulate expression of IL-6 and IL-1β to inhibit inflammation. In vitro studies also provided additional information for screening ingredients with potential anti-inflammatory effects, and demonstrated the rationality of molecular-docking results and the reliability of a screening strategy based on systems pharmacology.
Western medicine often uses antibacterial therapy and gastric-mucosa protection for CG. However, this strategy can lead to bacterial resistance and, increasingly, drugs cannot control CG efficaciously. Some scholars have suggested that TCM can achieve similar (or even better) curative effects than those of western medicine (Qian et al., 2007; Niu and Meng, 2013; Jia et al., 2017). A detailed study showed that traditional Chinese formulations, which include AMK, have biologic effects in patients with gastric diseases of spleen-deficiency and Qi-stagnation syndrome (Zhang et al., 2013). However, the specific pharmacologic mechanism is incompletely understood. Therefore, we explored the mechanism of action of AMK in CG treatment. We applied network pharmacology combined with screening of active ingredients, drug target symbols, and network/pathway analyses.
We showed that 27 active ingredients in AMK influence 25 overlapping gene symbols that have important roles in CG treatment. Thirty GO terms and 26 pathways were obtained by analyses of enrichment of GO pathways and KEGG pathways. We postulate that the IL-17 signaling pathway (hsa04657), C-type lectin receptor signaling pathway (hsa04625), TNF signaling pathway (hsa04668), and AGE-RAGE signaling pathway in diabetic complications (hsa04933) might serve as the key points and principal signaling pathways in CG treatment.
ATL-I and atractylone have been identified as bioactive ingredients by researchers (Chen, 1987; Hwang et al., 1996; Wang et al., 2006). In addition, in TCM theory, AMK is considered to replenish QI. Therefore, when we screened the active ingredients of AMK, although the DL of some amino acids was <0.18 (e.g., GLY, LPG), they were not removed. Our hypothesis was confirmed in analyses of enrichment of GO pathways and KEGG pathways. For example, ingredients such as GLY and LPG can affect alanine aminotransferase 1 (GPT), but GPT is enriched in carbon metabolism, arginine biosynthesis, glycolysis/gluconeogenesis, biosynthesis of amino acids, alanine, aspartate, and glutamate metabolism. Hence, AMK can act through synthetic or metabolic pathways involved in CG treatment.
In addition, the same target can be linked to multiple active ingredients, for example, PTGS2 can be associated with D-camphene, α-longipinene, akridin, and 14-acetyl-12-senecioyl -2E,8Z, 10E-atractylentriol. These data suggest that AMK can act on the same target through multiple active ingredients. ATL-I can be associated with IL-6, IL-1β, and VEGFA, which also suggests that AMK can act on multiple targets through the same active ingredient.
Analyses of a PPI network showed that CAT, IL-6, IL-1β, and VEGFA were the most correlated proteins, followed by PTGS2. In combination with analyses of enrichment of GO pathways and KEGG pathways, we believe that the mechanism of action of AMK in CG treatment is closely related to inflammation regulation (Graham et al., 2000; Bartchewsky et al., 2009; Chen et al., 2018). For example, the target symbol PTGS2 (also named cyclo-oxygenase (COX)-2) can be associated with 18 active ingredients in AMK which can affect the IL-17 signaling pathway, TNF signaling pathway, and C-type lectin receptor signaling pathway and, thus, the regulation of inflammation. If not treated in a timely manner, CG can transform into gastric cancer. Studies have shown that COX-2 plays an important part in the pathogenesis of gastric cancer (Sheu et al., 2003; Bartchewsky et al., 2009). In addition, Zhang and colleagues showed that a reduction in the COX-2 level is related to regression of precancerous lesions (Zhang et al., 2015).
To some extent, the data mentioned above also suggest that AMK has multiple ingredients, multiple targets, and multiple approaches. Such data provide the basis for multi-ingredient synergies in future research.
To summary, network pharmacology has been used widely in TCM research (Yang et al., 2017; Wang et al., 2017).
In China, AMK can be used to treat constipation, irritable bowel syndrome (IBS) and diarrhea (Pan et al., 2009; Xiao et al., 2015; Li and Li, 2015). In TCM theory, patients with spleen deficiency and dampness can suffer diarrhea after taking AMK. The interactions between the herbs in AMK merit investigation. Studies have shown that solute carrier family 6 member 4 (SLC6A4) can have an effect on IBS (Kim et al., 2004; Yuan et al., 2014). The target is associated with diarrhea and constipation. Therefore, we speculate that AMK may have side effects by affecting SLC6A4. But more proof is needed to verify this hypothesis.
We explored, systematically, how AMK may operate in terms of CG treatment. Our data may offer insights into how TCM can be employed in CG treatment.
The data used to support the findings of our study are included within the article, or within the Supplementary Material.
SY and HY conceived and designed the study. YY conceived and designed the experimental validation in vitro. All authors participated in drafting of the manuscript and revising it before final submission. These authors: SY, JZ and YY have contributed equally to this work and share first authorship.
This work was supported by the National Key Research and Development Project of China (2018YFC1707206).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to Jiangxi University of Traditional Chinese Medicine for the help in conducting this study.
ADME, absorption, distribution, metabolism, and excretion; AMK, Atractylodes macrocephala Koidz.; ATL-I, atractylenolide-I; CG, chronic gastritis; CHRM3, muscarinic acetylcholine receptor M3; DL, drug-likeness; GO, Gene Ontology; IL, interleukin; KEGG, Kyoto Encyclopedia of Genes and Genomes; LPS, lipopolysaccharide; OB, oral bioavailability; OMIM, Online Mendelian Inheritance in Man; PPI, protein–protein interaction; PTGS2, prostaglandin G/H synthase 2; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins; TCM, traditional Chinese medicine; TCMSP, Traditional Chinese Medicine Systems Pharmacology Database.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01629/full#supplementary-material
Alam, M., Al-Jenoobi, F., Al-Mohizea, A., Ali, R. (2014). Understanding and managing oral bioavailability: physiological concepts and patents. Recent Pat. Anti-Cancer Drug Discovery 10 (1), 87–96. doi: 10.2174/1574892809666140917103834
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Chery, J. M., et al. (2000). Gene ontology: tool for the unification of biology. Nat. Genet. 25 (1), 25–29. doi: 10.1038/75556
Bartchewsky, W., Jr., Martini, M. R., Masiero, M., Squassoni, A. C., Alvarez, M. C., Ladeira, M. S., et al. (2009). Effect of helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand. J. Gastroenterology 44 (2), 153–161. doi: 10.1080/00365520802530853
Chen, Y., Wang, X., Yu, Y., Xiao, Y., Huang, J., Yao, Z., et al. (2018). Serum exosomes of chronic gastritis patients infected with Helicobacter pylori mediate IL-1α expression via IL-6 trans-signalling in gastric epithelial cells. Clin. Exp. Immunol. 194 (3), 339–349. doi: doi:10.1111/cei.13200
Graham, D. Y., Gutierrez, G. O., Gomez, M., El-Zimaity, H. M., Yamaoka, Y. (2000). Effect of antibiotic therapy on acid secretion H. pylori density, inflammation and mucosal interleukin-I (IL-1B levels in corpus gastritis. Gastroenterology 118 (4), A740. doi: 10.1016/s0016-5085(00)85096-9
Hamosh, A., Scott, A. F., Amberger, J. S., Bocchini, C. A., Mckusick, V. A. (2005). Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 30 (1), 52–55. doi: 10.1093/nar/30.1.52
Han, T.-S., Voon, D. C.-C., Oshima, H., Nakayama, M., Echizen, K., Sakai, E., et al. (2019). Interleukin 1 up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology 156 (4), 1140–1155, e1144. doi: 10.1053/j.gastro.2018.11.059
Hsia, C.-W., Ho, M.-Y., Shui, H.-A., Tsai, C.-B., Tseng, M.-J. (2015). Analysis of dermal papilla cell interactome using STRING database to profile the ex vivo hair growth inhibition effect of a vinca alkaloid drug, colchicine. Int. J. Mol. Sci. 16 (2), 3579–3598. doi: 10.3390/ijms16023579
Huang, J., Tang, H., Cao, S., et al. (2017). Molecular targets and associated potential pathways of danlu capsules in hyperplasia of mammary glands based on systems pharmacology. Evidence-Based Complement. Altern. Med. 2017 1–10. doi: 10.1155/2017/1930598
Hwang, J.-M., Tseng, T.-H., Hsieh, Y.-S., Chou, F.-P., Wang, C. J., Chu, C.-Y. (1996). Inhibitory effect of atractylon on tert-butyl hydroperoxide induced DNA damage and hepatic toxicity in rat hepatocytes. Arch. Toxicol. 70 (10), 640–644. doi: 10.1007/s002040050323
Jia, Y., Liu, X., Jia, Q., Zhang, W., Sun, C., Yuan, D., et al. (2017). The anti-hyperplasia of mammary gland effect of protein extract HSS from Tegillarca granosa. Biomed. Pharmacother. 85, 1–6. doi: 10.1016/j.biopha.2016.11.109
Kanehisa, M., Susumo, G. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 27 (1), 27–30. doi: 10.1093/nar/28.1.27
Kim, H. J., Camilleri, M., Carlson, P. J., Cremonini, F., Ferber, I., Stephens, D. (2004). Association of distinct α2 adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut 53 (6), 829–837. doi: 10.1136/gut.2003.030882
Li, C. Y., Li, S. C. (2015). “Treatment of irritable bowel syndrome in China: a review,”. World J. Gastroenterology: WJG 21 (8), 2315. doi: 10.3748/wjg.v21.i8.2315
Li, C.-Q., He, L.-C., Dong, H.-Y., Jin, J.-Q. (2007). Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala koidz. J. Ethnopharmacology 114 (2), 212–217. doi: 10.1016/j.jep.2007.08.002
Liu, H., Wang, J., Zhou, W., Wang, Y., Yang, L. (2013). Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J. Ethnopharmacology 146 (3), 773–793. doi: 10.1016/j.jep.2013.02.004
Liu, H., Zeng, L., Yang, K., Zhang, G. (2016). A network pharmacology approach to explore the pharmacological mechanism of Xiaoyao powder on anovulatory infertility. Evidence-Based Complement. Altern. Med. 2016, 1–13. doi: 10.1155/2016/2960372
Ma, L., Mao, R., Shen, K., Zheng, Y., Li, Y., Liu, J., Ni, L. (2014). Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 450 (1), 353–359. doi: 10.1016/j.bbrc.2014.05.110
Mauri, A., Consonni, V., Pavan, M., Todeschini, R. (2006). DRAGON software: an easy approach to molecular descriptor calculations. Match Commun. In Math. In Comput. Chem. 56 (2), 237–248.
Niu, Y., Meng, Q. -X. (2013). Chemical and preclinical studies on Hedyotis diffusawith anticancer potential. J. Asian Natural Products Res. 15 (5), 550–565. doi: 10.1080/10286020.2013.781589
Ohata, H., Kitauchi, S., Yoshimura, N., Mugitani, K., Iwane, M., Nakamura, H., et al. (2004). Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 109 (1), 138–143. doi: 10.1002/ijc.11680
Osaki, L. H., Bockerstett, K. A., Wong, C. F., Ford, E. L., Madison, B. B., DiPaolo, R. J., et al. (2019). Interferon-γ directly induces gastric epithelial cell death and is required for progression to metaplasia. J. Pathol. 247 (4), 513–523. doi: 10.1002/path.5214
Pan, F., Zhang, T., Zhang, Y., Xu, J., Chen, F. (2009). Effect of Tongxie Yaofang granule in treating diarrhea-predominate irritable bowel syndrome. Chin. J. Integr. Med. 15 (3), 216–219. doi: 10.1007/s11655-009-0216-7
Qian, L.-Q., Pei, X.-H., Xu, Z.-Y., Wang, C. (2007). Clinical observation on treatment of hyperplasia of mammary gland by lirukang granule. Chin. J. Integr. Med. 13 (2), 120–124. doi: 10.1007/s11655-007-0120-y
Rebhan, M. (1997). GeneCards: integrating information about genes, proteins and diseases. Trends In Genet. 13 (4), 163. doi: 10.1016/s0168-9525(97)01103-7
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6 (1), 13. doi: 10.1186/1758-2946-6-13
Safran, M., Dalah, I., Alexander, J., Rosen, N., Iny Stein, T., Shmoish, M., et al. (2010). GeneCards version 3: the human gene integrator. Database (Oxford) 2010, baq020. doi: 10.1093/database/baq020
Shannon, P. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 (11), 2498–2504. doi: 10.1101/gr.1239303
Sheu, B. S., Yang, H. B., Sheu, S. M., Huang, A. H., Wu, J. J. (2003). Higher gastric cycloxygenase-2 expression and precancerous change in Helicobacter pylori-infected relatives of gastric cancer patients. Clin. Cancer Res. 9 (1), 5245–5251.
Song, W., Ni, S., Fu, Y., Wang, Y. (2018). Uncovering the mechanism of maxing ganshi decoction on asthma from a systematic perspective: a network pharmacology study. Sci. Rep. 8 (1). doi: 10.1038/s41598-018-35791-9
Su, G., Morris, J. H., Demchak, B., Bader, G. D. (2014). Biological network exploration with cytoscape 3. Curr. Protoc. In Bioinf. 47 (1), 8.13.11–18.13.24. doi: 10.1002/0471250953.bi0813s47
Tang, X.-D., Liu, B., Zhou, L.-Y., Zhan, S., Li, Z., Li, B., et al. (2012). “Clinical practice guideline of Chinese medicine for chronic gastritis,”. Chin. J. Integr. Med. 18 (1), pp.57–pp.71. doi: 10.1007/s11655-012-0960-y
Tao, W., Xu, X., Wang, X., Li, B., Wang, Y., Li, Y., et al. (2013). Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal radix curcumae formula for application to cardiovascular disease. J. Ethnopharmacology 145 (1), 1–10. doi: 10.1016/j.jep.2012.09.051
The State Commission of Chinese Pharmacopoeia (2010). Pharmacopoeia of People’s Republic of China, Part I (Beijing, China: Chemical Industry Press).
Walters, W. P., Murcko, M. A. (2002). Prediction of ‘drug-likeness’. Adv. Drug Deliv. Rev. 54 (3), 255–271. doi: 10.1016/S0169-409X(02)00003-0
Wang, C.-C., Lin, S.-Y., Cheng, H.-C., Hou, W.-C. (2006). Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem. Toxicol. 44 (8), 1308–1315. doi: 10.1016/j.fct.2006.02.008
Wang, C., Duan, H., He, L. (2009). Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur. J. Pharmacol. 612 (1-3), 143–152. doi: 10.1016/j.ejphar.2009.04.001
Wang, S., Wang, H., Lu, Y. (2017). Tianfoshen oral liquid: a CFDA approved clinical traditional Chinese medicine, normalizes major cellular pathways disordered during colorectal carcinogenesis. Oncotarget 8 (9), 14549–14569. doi: 10.18632/oncotarget.14675
Wang, J., Li, Y., Yang, Y., Chen, X., Du, J., Zeng, Q., Liang, Z., et al. (2017). A new strategy for deleting animal drugs from traditional chinese medicines based on modified yimusake formula. Sci. Rep. 7 (1), pp.1504–1526. doi: 10.1038/s41598-017-01613-7
Wang, J. H., Li, Y., Yang, Y. F., Du, J., Zhao, M., Lin, F., et al. (2017). Systems pharmacology dissection of multiscale mechanisms of action for herbal medicines in treating rheumatoid arthritis. Mol. Pharmaceutics 14 (9), 3201–3217. doi: 10.1021/acs.molpharmaceut.7b00505
Wu, C. W., Lu, L., Liang, S. W., Chen, C., Wang, S. M. (2016). Application of drug-target prediction technology in network pharmacology of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 41 (3), 377–382. doi: 10.4268/cjcmm20160303
Xiao, H. T., Zhong, L., Tsang, S. W., Lin, Z. -S., Bian, Z. -X. (2015). Traditional Chinese medicine formulas for irritable bowel syndrome: from ancient wisdoms to scientific understandings. Am. J. Chin. Med. 43 (1), 1–23. doi: 10.1142/s0192415x15500019
Xu, X., Zhang, W., Huang, C., Li, Y., Yu, H., Wang, Y., et al. (2012). A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 13 (6), 6964–6982. doi: 10.3390/ijms13066964
Yang, Y., Li, Y., Wang, J., Sun, K., Tao, W., Wang, Z., et al. (2017). Systematic investigation of Ginkgo biloba leaves for treating cardio-cerebrovascular diseases in an animal model. ACS Chem. Biol. 12 (5), pp.1363–1372. doi: 10.1021/acschembio.6b00762
Yang, L., Liu, W., Hu, Z., Yang, M., Li, J., Fan, X., et al. (2019). A systems pharmacology approach for identifying the multiple mechanisms of action of the Wei Pi Xiao decoction for the treatment of gastric precancerous lesions. Evidence-Based Complement. Altern. Med. 2019, 1–15. doi: 10.1155/2019/1562707
Yu, H., Chen, J., Xu, X., Li, Y., Zhao, H., Fang, Y., et al. (2012). A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PloS One 7 (5), e37608. doi: 10.1371/journal.pone.0037608
Yuan, J., Kang, C. Y., Wang, M., Wang, Q., Li, P., Liu, H., et al. (2014). Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PloS One 9 (1), e84414. doi: 10.1371/journal.pone.0084414
Yue, H., Shan, L., Bin, L. (2018). The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric. Cancer 21 (4), 579–587. doi: 10.1007/s10120-018-0812-3
Zhang, Q. F., Luo, S. D., Wang, H. Y. (1998). Two new sesquiterpenes from Atractylodes macrocehpala. Chin. Chem. Lett. 9 (12), 1097–1100.
Zhang, S., Zhao, L., Wang, H., Wang, C., Huang, S., Shen, H., et al. (2013). Efficacy of modified LiuJunZi decoction on functional dyspepsia of spleen-deficiency and qi-stagnation syndrome: a randomized controlled trial. BMC Complement. Altern. Med. 13 (1), 54. doi: 10.1186/1472-6882-13-54
Zhang, Y., Pan, K. F., Zhang, L., Ma, J., Zhou, T., Li, J., et al. (2015). Helicobacter pylori, cyclooxygenase-2 and evolution of gastric lesions: results from an intervention trial in China. Carcinogenesis 36 (12), 1572–1579. doi: 10.1093/carcin/bgv147
Zhao, Z.-H. (2013). Review of pharmacological study and clinical application of Xiangsha Liujunzi Decoction. J. Liaoning Univ. Tradit. Chin. Med. 15 (5), 245–247. doi: 10.13194/j.jlunivtcm.2013.05.247.zhangzhh.089
Keywords: network pharmacology, Atractylodes macrocephala Koidz., chronic gastritis, bioactive ingredients, mechanism of action
Citation: Yang S, Zhang J, Yan Y, Yang M, Li C, Li J, Zhong L, Gong Q and Yu H (2020) Network Pharmacology-Based Strategy to Investigate the Pharmacologic Mechanisms of Atractylodes macrocephala Koidz. for the Treatment of Chronic Gastritis. Front. Pharmacol. 10:1629. doi: 10.3389/fphar.2019.01629
Received: 18 June 2019; Accepted: 13 December 2019;
Published: 29 January 2020.
Edited by:
Yonghua Wang, Northwest A&F University, ChinaReviewed by:
Yinfeng Yang, Anhui University of Chinese Medicine, ChinaCopyright © 2020 Yang, Zhang, Yan, Yang, Li, Li, Zhong, Gong and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingyun Zhong, bHkxNjM4MTYzQDE2My5jb20=; Qianfeng Gong, Z29uZ3FmMjAwMkAxNjMuY29t; Huan Yu, eXVodWFuaGViZWlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.