Corrigendum: The Fruits of Siraitia grosvenorii: A Review of a Chinese Food-Medicine
- 1Department of Pharmacy, Baotou Medical College, Baotou, China
- 2Guangxi Key Laboratory of Medicinal Resources Protection and Genetic Improvement, Guangxi Botanical Garden of Medicinal Plants, Nanning, China
- 3Agricultural College, Inner Mongolia University for Nationalities, Tongliao, China
- 4Pharmaceutical Laboratory, Inner Mongolia Autonomous Region Academy of Chinese Medicine, Hohhot, China
- 5Department of Technology, Chifeng Institute for Drug Control, Chifeng, China
- 6Department of Pharmacy, Inner Mongolia Medical University, Hohhot, China
- 7Inner Mongolia Key Laboratory of Characteristic Geoherbs Resources Protection and Utilization, Baotou Medical College, Baotou, China
Siraitia grosvenorii (Swingle) C. Jeffrey, a member of the family Cucurbitaceae, is a unique economic and medicinal plant grown in China. For more than 300 years, S. grosvenorii has been used as a natural sweetener and as a traditional medicine for the treatment of pharyngitis, pharyngeal pain, as well as an anti-tussive remedy in China. It is one of the first approved medicine food homology species in China. It has been widely studied as a natural product with high development potential. Therefore, the present paper provides a review of the botanical characterization, traditional uses and ethnopharmacology, food and nutritional values, chemical constituents, pharmacological effects, toxicology, and development direction for the future of S. grosvenorii. Phytochemical studies have revealed that the chemical composition of this plant mainly includes iridoid and phenylpropanoid glycosides. Several compounds such as triterpenoids, flavonoids, and amino acids have been isolated from the plant. S. grosvenorii and its active constituents possess broad pharmacological properties, such as antioxidant, hypoglycemic, immunologic, anti-tussive and sputum-reducing, hepatoprotective, and antimicrobial activities, etc. By documenting the comprehensive information of S. grosvenorii, we hope to establishes the groundwork for further research on the mechanism of action of S. grosvenorii and its development as a new health food in the future.
Introduction
Siraitia grosvenorii (Swingle) C. Jeffrey is a species of the genus Siraitia Merr. (Cucurbitaceae) and is native to the southern parts of China, mainly the Guangxi Province (Lu and Zhang, 1984). There is a total of seven species belonging to this genus; four of them are native to China, of which S. grosvenorii and Siraitia siamensis (Craib) C. Jeffrey ex Zhong et D. Fang have medicinal value (Chen Y et al., 2006).The formal Chinese name of S. grosvenorii is luo han guo (Chinese: 罗汉果), and it is locally known as lahanguo, jiakugua, changshouguo, or guangguomubie (Lu and Zhang, 1984).
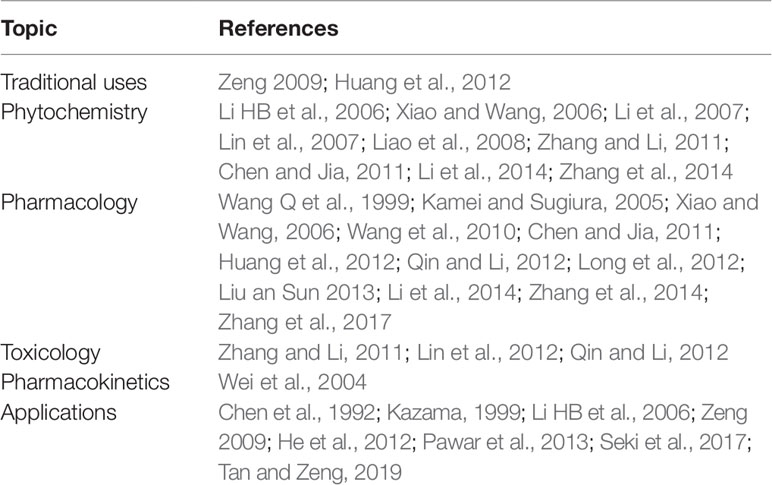
S. grosvenorii is used not only as a food ingredient but also as an herbal medicine, particularly in China, where it has been used as a natural antitussive and expectorant for 300 years (Lu and Zhang, 1984). Furthermore, it is one of the first approved medicine food homology (MFH) species in China (The concept of “medicine and food homology” was mentioned in the Huang Di Nei Jing Su Wen: “eating on an empty stomach as food and administering to the patient as medication, ” which reflects the theory of MFH, i.e., there are food classes that can also be used as drugs.) (Jiang et al., 2015). Phytochemical studies have revealed that the chemical composition of this plant mainly includes iridoid and phenylpropanoid glycosides. Several compounds such as triterpenoids, flavonoids, and amino acids have been isolated from the plant (Qing et al., 2017; Chu et al., 2019). Currently, this species is used as a pulmonary demulcent and an emollient for curing sore throat, dire thirst, and constipation (Li and Zhang, 2000). Modern medicinal research shows that the extract of its ripe fruit can be commercially used as a supplement and as a sweetener in sugar-free health foods and drinks because the fruits contain sweet glycosides that are naturally low in calories (FX, 1996; Soejarto et al., 2019). In addition, the crude extracts and purified compounds from S. grosvenorii have been found to possess various biological activities including antioxidant, hypoglycemic, immunologic, anti-tussive and sputum-reducing, hepatoprotective, and antimicrobial activities (Liu et al., 2018; Chen et al., 2018; Abdel-Hamid et al., 2019; Nie et al., 2019; Zhang et al., 2019).Consequently, S. grosvenorii is a natural product with high development potential, which has increasingly been the focus of scientific research and commercial attention (Wang et al., 2010; Zhang and Li, 2011). The previous reviews focusing on the traditional uses, phytochemistry, pharmacology, toxicology, pharmacokinetics, and applications different aspects of S. grosvenorii are listed in Table 1.
In this review, all of during the last 36 years (from 1983 to 2019) available information on S. grosvenorii was collected from the literary resources, such as PubMed, SciFinder Scholar, CNKI, TPL (www.theplantlist.org), Google Scholar, Baidu Scholar, and Web of Science. We aimed to provide comprehensive information on the botanical characterization, traditional uses and ethnopharmacology, food and nutritional values, chemical constituents, pharmacological effects, and toxicology of S. grosvenorii, and to assess valuable data for further applications to the S. grosvenorii, as well as suggestions regarding development direction.
Botanical Characterization
S. grosvenorii is a perennial vine with yellow-brown pubescence and black glandular scales. The roots are enlarged, fusiform, or subglobose (China national medicinal materials corporation, 1994); the stems and branches are slightly robust. The petioles are 3–10 cm; and the leaf blades are ovate-cordate, 12–23 × 5–17 cm, membranous, apex acuminate or long acuminate, sinus semicircular or broadly ovate-cordate. The male flowers are inflorescence racemose, 6−10-flowered; peduncle 7–13 cm; pedicels slender, 5–15 mm; calyx tube broadly campanulate, 4–5 × ca. 8 mm, usually with 3 membranous scales; segments triangular, ca. 4.5 × 3 mm, 3-veined, apex long acuminate; corolla yellow; segments oblong, 10–15 × 7–8 mm, 5-veined, apex acute; filaments puberulent, ca. 4 mm; anthers ca. 3 mm. The female flowers are solitary or 2–5 on a 6–8 mm peduncle; calyx and corolla as in male flowers but slightly larger; staminodes 2–2.5 mm; ovary oblong, 10–12 × 5-6 mm, densely yellow-brown velvety, base obtuse-rounded; style ca. 2.5 mm; stigmas 3, enlarged, ca. 1.5 mm. The fruits are globose or oblong, 6–11 × 4–8 cm, densely yellow-brown velvety and black glandular-scaly, ultimately glabrous. The seeds are numerous, pale yellow, broadly ovate, compressed, 15–18 × 10–12 mm, base obtuse-rounded, with 2-layered wings, wings sinuate (Figure 1) (Flora of China Editorial Board of the Chinese Academy of Sciences, 2004).
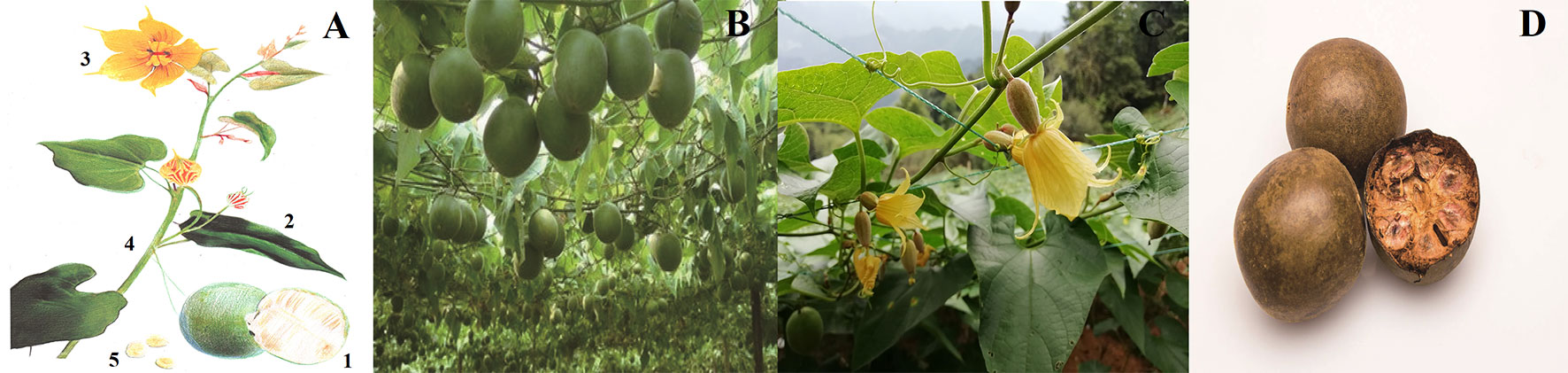
Figure 1 Images of S. grosvenorii. (A) Line drawing of S. grosvenorii: 1. fruit; 2. leaf; 3. flower; 4. stem; 5. seed. (B) and (C) Plant of S. grosvenorii. (D) The medicinal material of S. grosvenorii.
Traditional Uses and Ethnopharmacology
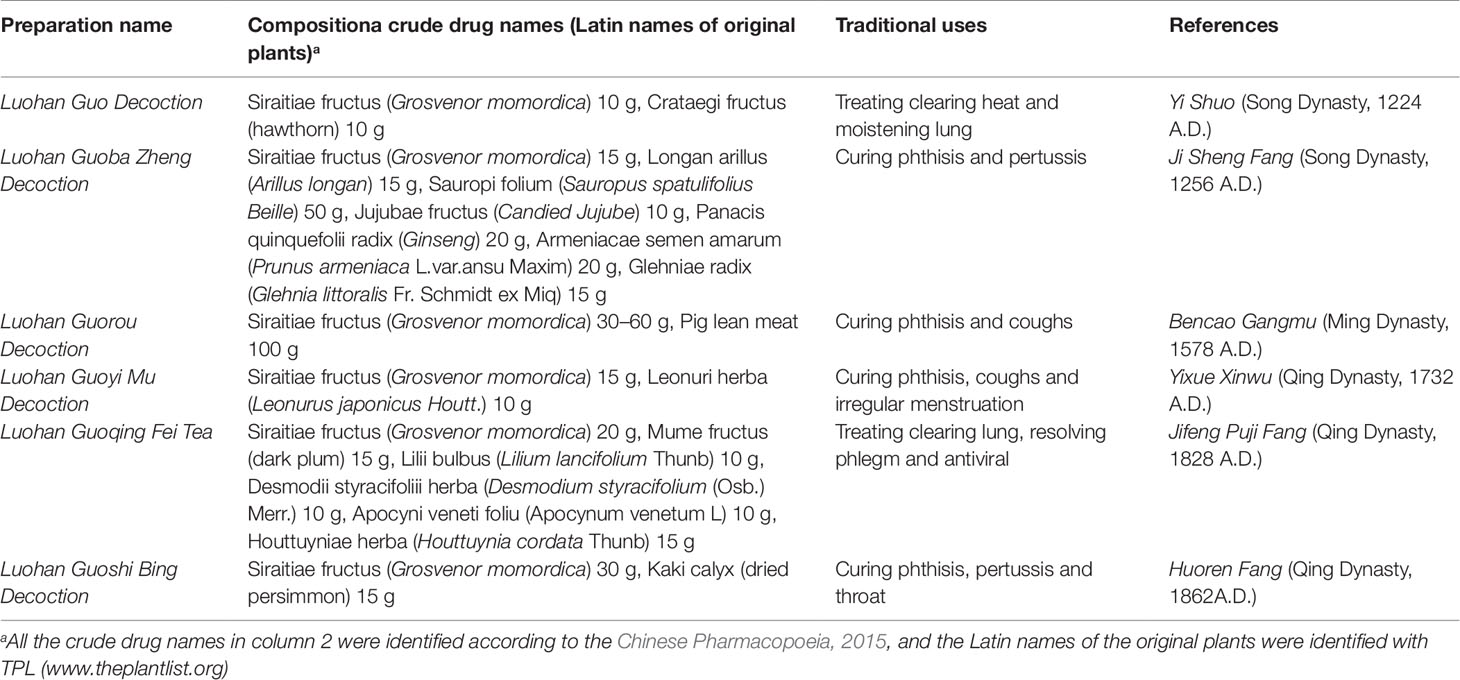
As recorded in the Committee for the Pharmacopoeia of PR China (2015), S. grosvenorii has remarkable efficacy in treating coughs, sore throat, and excessive phlegm (Committee for the Pharmacopoeia of PR China, 2015). Bencao Gangmu, which is a famous monograph written on traditional Chinese medicine during the Ming Dynasty, is the first book to list the applications of this plant in China. The record of the use of S. grosvenorii as an expectorant for relieving sore throat, clearing heat, and moistening the lungs dates to 2,000 years ago. Furthermore, S. grosvenorii is used as a functional food because it contains several nutritious compounds, including mogrosides, trace elements, linoleic acid, vitamin C, and other unsaturated fatty acids (Li et al., 2014). Finally, S. grosvenorii is also used as a food source in East Asian countries, such as China, Japan, and South Korea. The traditional uses of S. grosvenorii in China are shown in Table 2.
Food and Nutritional Value
Commercially, S. grosvenorii is available as dried fruits, which are consumed after processing. In the 1990s, the China Food and Drug Administration (CFDA) approved the use of S. grosvenorii as a sweetener in foods (Cheng et al., 2015). In 1996, it was approved as a substitute for sweeteners in health foods for obesity and diabetes patients (Ren et al., 2009). As a new low-calorie, non-sugar sweetener, S. grosvenorii can be consumed as a health-promoting juice or as an additive or used to prepare sugar-free foods (Yang et al., 2016).
Several S. grosvenorii products are patented; one among such products is a health-promoting sugar-free S. grosvenorii beverage that preserves the medicinal effects of S. grosvenorii. This beverage is prepared by completely dissolving a powder formulation of S. grosvenorii in water and is suitable for treating diabetes (Zhang, 2011). Despite being a low-calorie product, it has high sweetness, making it an ideal new source of sugar for diabetes and obesity patients (Wu, 2011). Medicinal products developed from this plant include Luohanguo Paoteng Tablet and Luohanguo yanhou Tablet, which are used to alleviate pharynx discomfort (Jin et al., 1997; Jiang et al., 2011; Chen et al., 2015). Luohanguo Lvcha Granules are used to enhance immunity, and Luohanguo Sydney Cream and Jinyinhua Luohanguo Lozenges are used to cure sore throat (Zhang et al., 2017).
Stemoninine-mogroside V at mass ratios of 2:1 and 1:1 exerts effects in relieving cough and reducing sputum (Wu et al., 2017). The addition of concentrated S. grosvenorii juice to tobacco markedly reduces the harmful pulmonary effects of smoking, reduces pharynx discomfort, and promotes smoking cessation (Li D et al., 2006).
In addition, foods developed using S. grosvenorii include Luohanguo-fermented wine, Luohanguo cake, Luohanguo preserved fruit, and Luohanguo fruit compound beverage (Li D et al., 2006). Moreover, there are many patented products of S. grosvenorii that find applications in the cosmetic industry due to their skin whitening and moisturizing effects (Zhang and Zhang, 2011; Yang, 2016).
Chemical Constituents
Based on the existing literature, more than 100 compounds have been isolated from S. grosvenorii, including at least 46 triterpenoids, 7 flavonoids, 19 amino acids, and 2 polysaccharides.
Triterpenoids
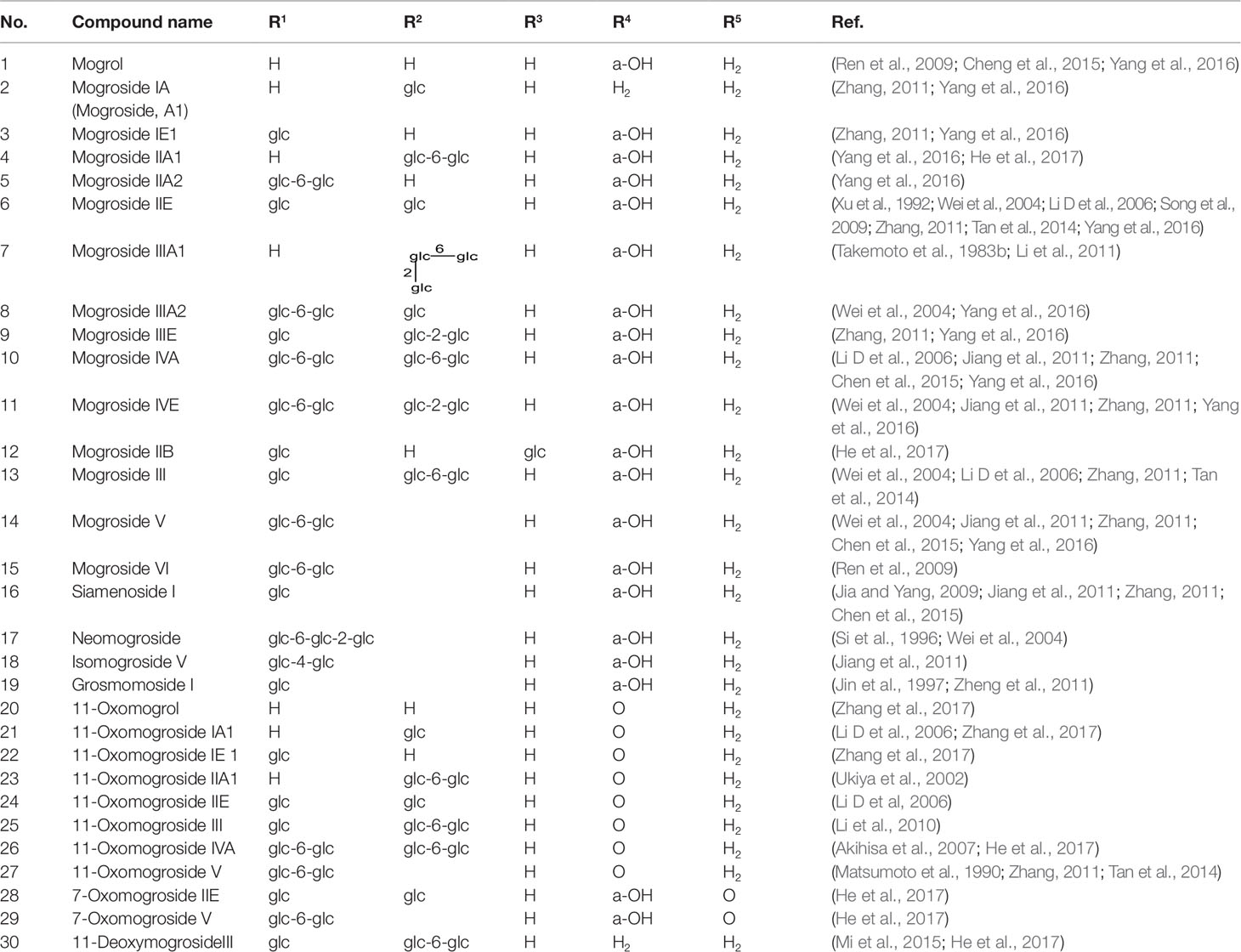
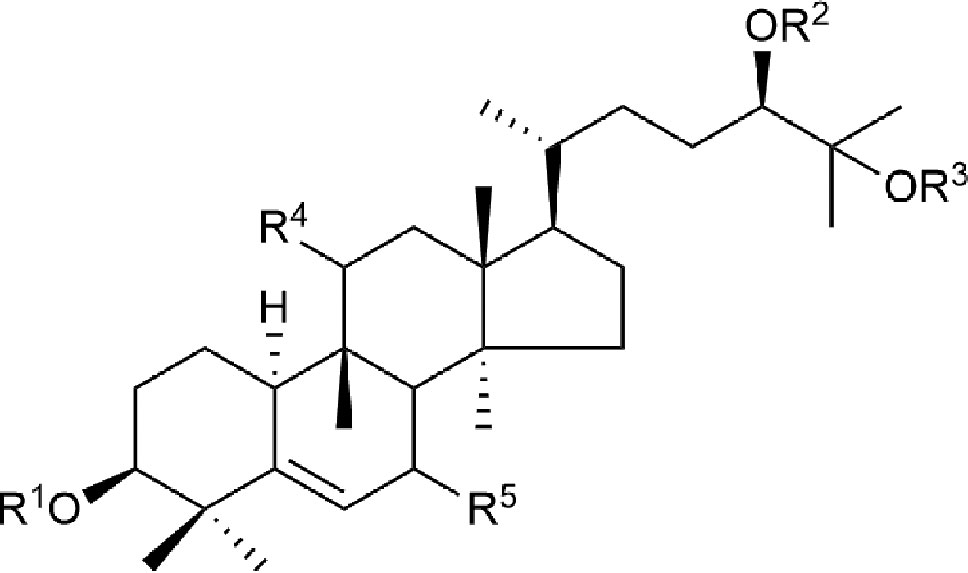
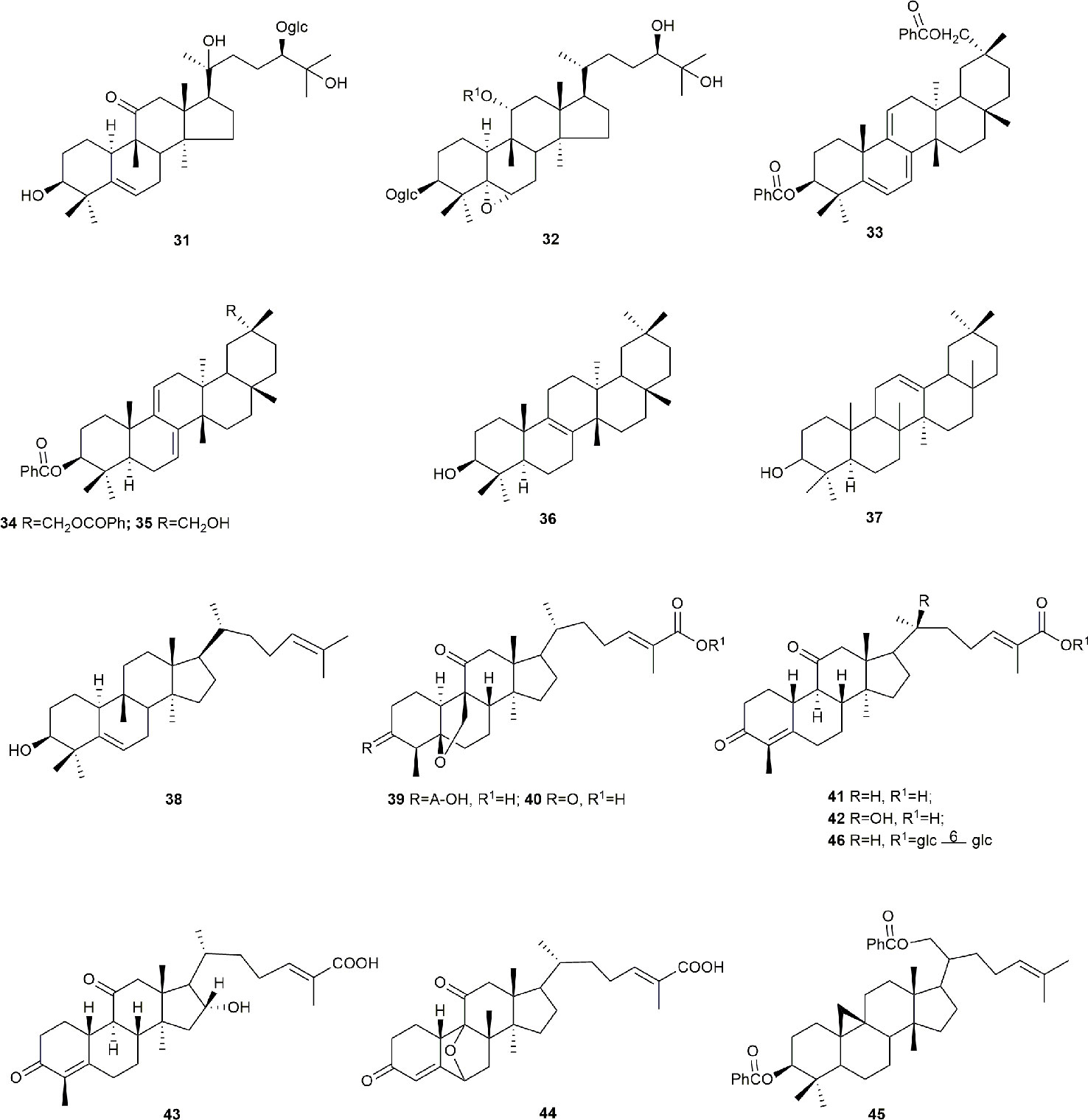
Cucurbitane glycosides are the active ingredients of S. grosvenorii fruits. Mogrosides IV, V, and VI were successfully isolated from S. grosvenorii fruits in 1983 (Takemoto et al., 1983a; Takemoto et al., 1983b). Simultaneously, more than 30 similar compounds have been obtained from the fruits, and these compounds have the mogrolaglycone structure, [10-cucurbit-5-ene-3, 11, 24(R), 25-tetraol], with different numbers of glucose units attached (Table 3, Figures 2 and 3).
Flavonoids
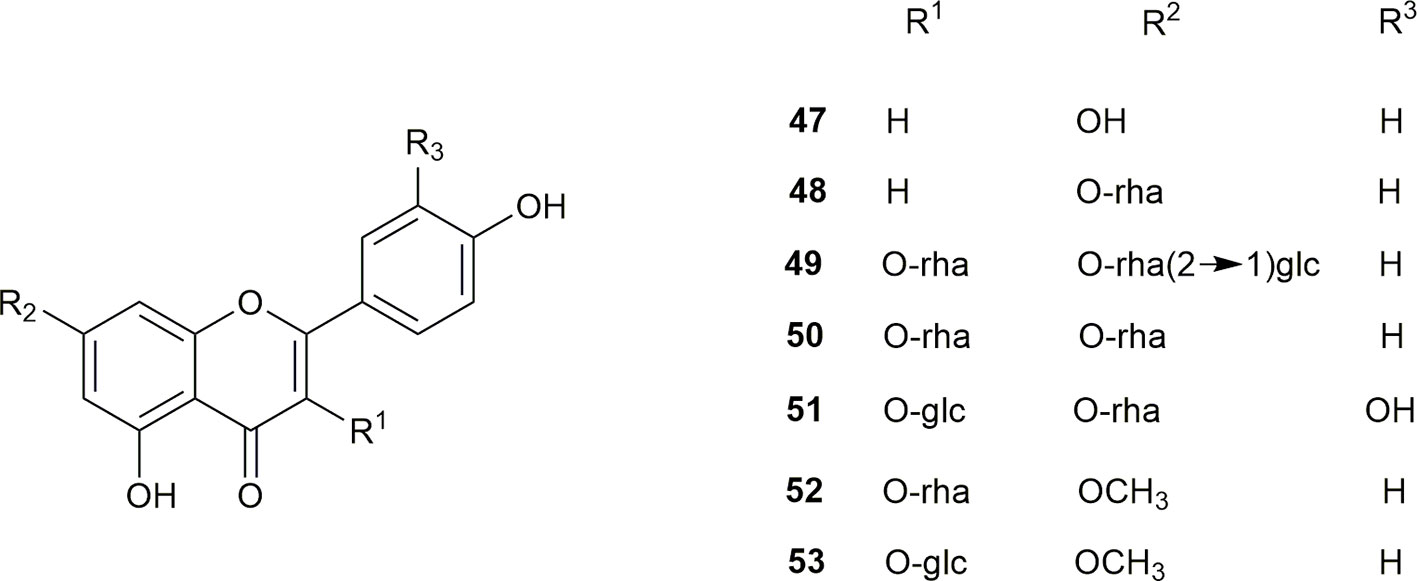
Seven flavonoids have been isolated from the flowers of S. grosvenorii, namely, kaempferol (47), kaempferol 7-O-L-rhamnopyranoside (48), kaempferol 3-O-L-rhamnopyranoside-7-O-[β-D-glucose-based-(1-2)-α-L-rhamnoside] (49), 3-O-L-rhamnopyranoside (52), and 3-O-D-glucopyranoside (53). Kaempferol 3,7-di-O-L-rhamnopyranoside (kaempferitrin) (50) and quercetin-3-O-D-glucopyranoside-7-O-L-rhamnopyranoside (51) were isolated from the leaves of S. grosvenorii (Li et al., 2003; Li et al., 2007; Liao et al., 2008). The flavonoid structures are shown in Figure 4.
Others
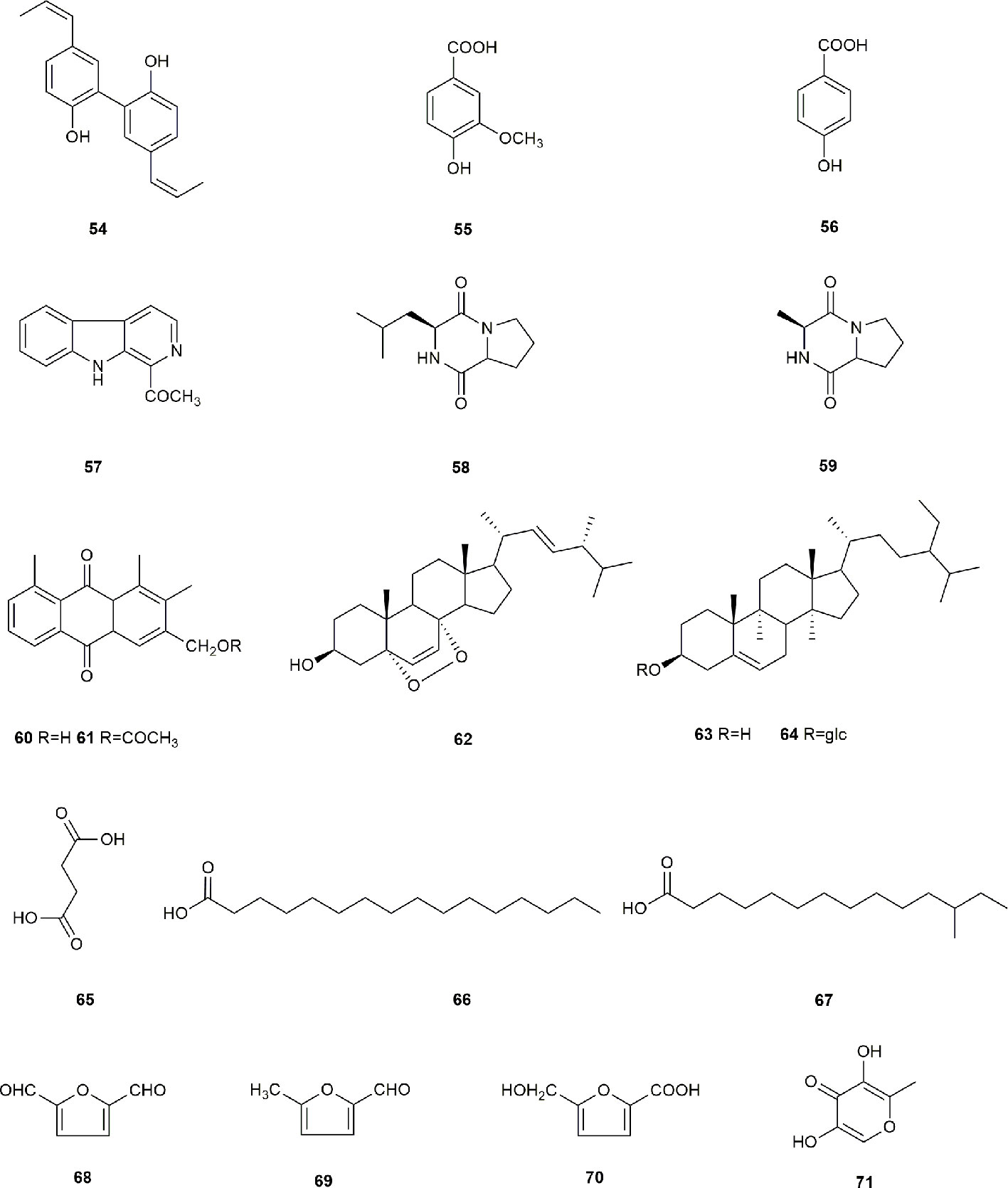
Other types of compounds have also been identified from S. grosvenorii, such as magnolol (54), vanillic acid (55), p-hydroxybenzylic acid (56), 1-acetyl-β-carboline (57), cyclo-(leu-pro) (58), cyclo-(ala-pro) (59), aloe emodin (60), aloe-emodin acetate (61), 5, 8-epidioxy-24(R)-methylcholesta-6, 22-dien-3β-ol (62), β-sitosterol (63), daucosterol (64), succinic acid (65), n-hexadecanoic acid (66), 12-methyltetradecanoic acid (67), 5-hydroxymethylfurfural (68), 5, 5'-oxydimethylenebis(2-furfural) (69), 5-(hydroxymethyl)-furoic acid (70), and 5-hydroxymaltol (71) (Chen et al., 2004; Chen QB et al., 2006; Yao et al., 2008). The structures of these compounds are shown in Figure 5.
Pharmacological Effects
Antioxidant Effects
Hossen have reported that the S. grosvenorii extract prevents the generation of superoxide anion and inhibits histamine release from the mast cells and histamine-induced nose scratching behavior in ICR mice (Hossen and Sun, 2006). These functions are related to the antioxidant activity of S. grosvenorii. The scavenging capacity of mogrosides on reactive oxygen species (ROS) was studied by the chemiluminescence method. The results showed that crude extracts of s. grosvenorii have high scavenging capacity and that the antioxidant effect was almost the same as that of ascorbic acid (VC) (Zhu et al., 2015). Mogroside extract effectively eliminates free radicals, reduces the incidence of hemolysis of Fe2+, and alleviates hydrogen peroxide induced-oxidative damage to hepatic tissues. Hydroxyl radicals and superoxide anion radicals are involved in eliminating free radicals; with increasing concentration of mogroside extract, the scavenging effect gradually increases, thereby exhibiting a dose-effect relationship (Lan et al., 2018).
In a study assessing the in vitro antioxidant activity, it was suggested that the sweet cucurbitane glycosides mogroside V (14) and 11-oxo-mogroside V (27) from the fruits of S. grosvenorii have remarkable ROS scavenging ability, with different antioxidant effects in different systems. Furthermore, as compared to mogroside V (EC50 = 16.52 mg/ml), 11-oxo-mogroside V (EC50 = 4.79 mg/ml) exhibited stronger scavenging activity against superoxide anions and hydrogen peroxide, and stronger inhibitory effect on DNA damage, but weaker scavenging effect on hydroxyl radical. Therefore, these two compounds could be effective as free radical-scavenging agents (Chen et al., 2007).
Exhaustive exercise causes increased oxygen consumption, hypoxia in local tissues, and accumulation of toxic metabolites, which affects the oxidative function of the mitochondria. A study was conducted to establish an animal model of exhaustive exercise and to explore the effects of flavonoids from the leaves of S. grosvenorii on the myocardial mitochondria of rats subjected to vigorous exercise. The flavonoids effectively eliminate the endogenous capacity of production of ROS caused by vigorous exercise in the rats. Thus, it was revealed that flavonoids from the leaves of S. grosvenorii can promote blood circulation and exert a strong antioxidant activity (Xie, 2018).
Four types of extracts of S. grosvenorii have been successfully prepared and their antioxidant effects have been determined. The results showed that the four extracts had antioxidant and radical scavenging activities and that the strength of their antioxidant effects follows the following order: ethyl acetate extract > water extract > methanol extract > ethanol extract. Thus, this evidence indicates that S. grosvenorii might be valuable as a health medicine and food (Li et al., 2008).
Hypoglycemic Effects
It has been revealed that mogroside is the main active ingredient responsible for the hypoglycemic effect of S. grosvenorii. The extract of S. grosvenorii alleviates alloxan-induced damage and repairs β cells to alleviate symptoms in diabetic mice (Qi et al., 2003). Studies have shown that the S. grosvenorii powder and extracts have no effects on blood glucose level and glucose tolerance in normal mice; however, a significant hypoglycemic effect was observed in alloxan-induced diabetic mice. In contrast, serum levels of triglycerides and cholesterol abnormally increased and the serum high-density lipoprotein cholesterol and blood lipid level increased; blood lipid level tended to be normal to prevent lipid metabolism disorders caused by diabetes. In addition, the S. grosvenorii extract can induce repair of islet β cells to relieve the symptoms of diabetes in mice (Qi et al., 2003). In addition, active substances of S. grosvenorii responsible for the hypoglycemic effect include flavonoids and polysaccharides. Currently, the main hypoglycemic mechanism of S. grosvenorii involves the repair of pancreatic injury, promotion of insulin secretion, scavenging of free radicals and anti-lipid peroxidation, and inhibition of intestinal α-glucosidase activity (Wan et al., 2016).
In a study by Suzuki, rats with type II diabetes were fed 40% mogroside extract for 13 weeks. Consequently, it was observed that insulin response in the rats was improved; blood sugar levels, urine amount, and protein levels were reduced; and diabetic complications were prevented. The S. grosvenorii extract and mogroside V have been further studied in rat β cell line RIN-5F. The results showed that S. grosvenorii extract and mogroside V can significantly promote insulin secretion and regulate blood sugar levels (Suzuki et al., 2007). In addition, the S. grosvenorii extract not only reduces blood sugar level but also prevents oxidative stress-related complications in alloxan-induced diabetic mice. The extract reduces the development of diabetes and vascular endothelial injury as well as the incidence of diabetic nephropathy (Zhang et al., 2006).
The mogroside extract also significantly decreases the activity of heme oxygenase-1 (HO-1), manganese superoxide dismutase (MnSOD), and glutathione peroxidase; inhibits the mRNA expression of HO-1 and MnSOD; increases serum HDL-C level; and regulates the activity of antioxidant enzymes in the liver, thereby decreasing the symptoms of diabetic nephropathy in mice with diabetes. The mogroside extract effectively improves clinical symptoms, increases insulin secretion, and decreases the pathological damage of islets in insulin-dependent diabetic mice (Wang Q et al., 1999).
Immunologic Effects
S. grosvenorii polysaccharides (SGP) significantly increased the weight of mouse thymus, spleen, and other immune organs; percent of phagocytic cells; level of serum hemolysin; transformation rate of lymphocytes; and function of the immune system. It has been reported that oral administration of SPG at 1,200 and 100 Mg/Kg increases serum hemolysin (IgM) level, lymphocyte transformation rate, and thymus and spleen indexes. The results indicated that Sgp obviously enhances humoral and nonspecific immunity. intraperitoneal injection of Sgp to mice not only significantly increases thymus and spleen indexes but also improves NO0 and H2O2 levels, SOD activity, and hydroxyl radical scavenging capacity. Furthermore, SGP affects immune functions by adjusting the level of free radicals (Wang et al., 1994).
To study the effects of SGP on the immune function of immunosuppressive mice, experimental mice were randomly divided into three groups, viz. normal group, model group, and treated groups (25, 50, and 100 mg/kg). A mouse model of immunosuppression was established via the intragastric administration of cyclophosphamide (20 mg/kg) for 14 days. The effect of SGP on the immune function of the immunosuppressed mice was investigated by examining the levels of immunoglobulin (Ig)G, IgM, interleukin (IL)-2, IL-4, IL-6, and tumor necrosis factor (TNF)-α. The results indicated that SGP significantly improves the immune function of immunocompromised mice (Zhang et al., 2018).
Anti-Tussive and Sputum-Reducing Effects
Chen Y et al. (2006) studied the antitussive activities of mogrosides by testing the coughing frequency induced by ammonium hydroxide in mice and the expectorant activities by testing the amount of phlegm secreted in mice. The oral dose of mogrosides was higher than 15 g/kg that retrained mice's cough resulted in ammonia water, increased the density of phenol red secreted from windpipe of mice. According to the sequential method for the principle of median effective dose, the antitussive effect of S. grosvenorii was observed and the median time to observe antitussive effect induced by ammonia water in mice was calculated. Tang et al. (2015) investigated the effects of different factors on the antitussive effect of S. grosvenorii. The results showed that S. grosvenorii in different habitats had different antitussive effects, and the fruit growth period and commercial specifications influenced the antitussive effect of S. grosvenorii.
Research has shown the sputum-reducing effect of the S. grosvenorii extracts by increasing the excretion of phenol red from the rat trachea, as well as the excretion of phlegm from the rat trachea (Liu and Sun, 2013). It was reported in a rat model study that mogrosides at doses of 400 and 800 mg/kg could significantly increase the excreted amount of phlegm dose-dependently. And the sputum-reducing effect of 800 mg/kg dosage group was equal to that of ammonium chloride positive group (Chen Y et al., 2006). Furthermore, in order to investigate the relationship between expectorant efficacy and active components of S. grosvenorii, the phenol red secretion of trachea in mice and the HPLC fingerprint of S. grosvenorii extract were determined (Wang et al., 2017). Grey relational analysis was applied to confirm the contribution degree of each common peak from HPLC fingerprints for expectoration efficacy, while partial least-squares regression was utilized to confirm either positive or negative relationship, and to identify the contribution degree of S. grosvenorii extract. The results indicated that expectorant efficacy of S. grosvenorii is contributed by a combined action of multi-components rather than one component. And among them, oxomogroside V and mogroside V (14) have sputum-reducing effect and high contribution degree (Wang et al., 2017).
Hepatoprotective Effects
Mogroside V (14) is the major saponin in S. grosvenorii. Gang evaluated the effect of mogrosides (the main constituents of mogroside V, 14) on carbon tetrachloride-induced liver injury in Kunming mice, and Bacillus Calmette–Guerin (BCG)- and lipopolysaccharide (LPS)-induced liver injury. Mogrosides exert no effect on enzyme activities in normal liver but reduce the serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and malondialdehyde (MDA). The activity of SOD was significantly reduced in the liver tissue, proving that mogrosides exert a protective effect in mice with acute liver injury (Xiao and Wang, 2008). In another study, it was revealed that the S. grosvenorii extract significantly improved the swimming time of mice. It effectively increases the activity of SOD and glutathione peroxidase (GSH-Px), removes blood lactic acid (BLA), enhances the body's antioxidant capacity, promotes hemoglobin synthesis, and reduces the amount of BLA generated (Wang T et al., 1999). Thus, S. grosvenorii extract was shown to exert a significant protective effect against liver injury.
Antimicrobial Effects
The leaf, fruit, and stem extracts of S. grosvenorii possess strong inhibitory effect against Pseudomonas aeruginosa, Escherichia coli, and Streptococcus mutans. To examine the antibacterial effect of S. grosvenorii fruits, crude extracts of dried S. grosvenorii fruits were prepared, and 50 components were separated by high-performance liquid chromatography. The results revealed that compounds 18−19 and 34−35 have strong inhibitory activity, whereas the other compounds did not exhibit any or exhibited only weak activity. In addition, mogroside V had no antibacterial activity (Zhou and Huang, 2008). Qi et al. (2006) studied the antimicrobial effect of S. grosvenorii ethanol extract on P. aeruginosa, Staphylococcus aureus, and Candida albicans. The inhibitory rate of the extract against these different strains was measured using the dichotomy method. It was found that the ethanol extracts of leaves and stems of S. grosvenorii have antimicrobial activity, with inhibition values of 70.2% against P. aeruginosa and 50% or less against S. aureus and C. albicans.
Other Effects
Besides the pharmacological effects mentioned above, S. grosvenorii also possesses other activities, such as anticancer and anti-fatigue effects. In a previous study, dimethyl benzanthracene was used as an initiator and 12-O-tetradecanoyl-phorbol-13-acetate as an accelerator in a two-stage mouse skin carcinogenicity assay. The results showed that mogroside V (14) has a good inhibitory effect (Suzuki et al., 2007). In addition, the tubers of S. grosvenorii have been shown to possess significant anticancer activity in vitro (Takasaki et al., 2003). It is found that S. grosvenorii extract inhibited the expression of Cyp1a1, which plays a role in inhibiting liver cancer (Matsumoto et al., 2009). Furthermore, Zhang et al. studied the anti-fatigue effect of S. grosvenorii extract in 144 male ICR mice, and the results showed that liver and muscle concentrations of glycogen in the low-, medium-, and high-dose S. grosvenorii extract group were significantly higher than those in the control group (p < 0.05). It has been proven that S. grosvenorii fruit extract has obvious anti-fatigue effect in mice with exercise fatigue (Zhang et al., 2017).
Toxicology
Currently, the toxicity of S. grosvenorii is considered low and its use as MFH species is safe (Zhang and Li, 2011). Qin et al. investigated the safety of PureLo, a non-caloric powdered concentrate of S. grosvenorii, which derives its sweetening properties from triterpene glycosides called mogrosides. Male and female dogs were administered 3,000 mg/kg bw/day PureLo for either 28 or 90 days. Body weight, blood chemistry, food consumption, urinalysis, organ weight, and other indexes were evaluated to analyze the toxicity of PureLo. The results showed no changes in body weight, organ weight, and food consumption. There were no significant effects on blood chemistry analysis and urinalysis results. The results indicated that PureLo does not induce any organ or systemic toxicity (Qin et al., 2006). In another study, 20 Kunming mice were administered S. grosvenorii extract (7,200 mg/kg bw) for 12 h and then observed for one week. No changes in organ morphology and no short-term toxicity were observed. Furthermore, Salmonella typhimurium TA97, TA98, TA100, and TA102 were used in Ames assay with mogroside (50, 5, 0.5, 0.05, and 0.002 mg/ml) in the presence or absence of S9 metabolic activator. The result showed that mogroside exerts no genotoxic effect (Hussain et al., 1990). Marone et al. conducted an experiment on Sprague-Dawley rats to evaluate the safety of PureLo. Twenty rats (10/sex/group) were fed PureLo (0, 10,000, 30,000, or 100,000 ppm) for 28 days (OECD, Redbook 2000). The results showed no significant adverse effects. There were no differences in body weight and feed efficiency, but there was a decrease in bilirubin level and an increase in total protein content. Overall, the no observed adverse effect level (NOAEL) of PureLo in the diet was 100,000 ppm, which is equivalent to 7.07 and 7.48 g/kg bw/day for male and female rats, respectively (Marone et al., 2008).
Conclusions and Future Perspectives
Numerous studies have been conducted to investigate the pharmacological activities of S. grosvenorii, including its antioxidant, hepatoprotective, hypoglycemic, immunologic, and anti-inflammatory activities, etc. In this review, the research progress of the traditional uses and pharmacological activities of S. grosvenorii during the last 36 years is summarized. We believe that these pharmacological still deserve further research. Information on the mechanism of action of this plant will help its development as a new health food and a novel therapeutic agent in the future.
Only a few studies have been conducted on its pharmacokinetics and clinical applications. Among the several classes of biologically active compounds identified in S. grosvenorii, mogrosides are assumed to be the main active components responsible for most of the pharmacological actions of S. grosvenorii. However, the biological effects of the other chemical components as well as the interactions of mogrosides with the other compounds cannot be ruled out. Further studies are needed to elucidate the complex pharmacological actions and the complete phytochemical profile of the plant (Li et al., 2014). Moreover, clinical studies should also be conducted to thoroughly evaluate the therapeutic effects, adverse effects, and toxicity of S. grosvenorii.
S. grosvenorii is an important traditional Chinese medicinal herb and commodity. With increasing demand from international markets, the need for high-quality S. grosvenorii has increased to satisfy the higher standards expected (Meng and Liao, 2018). However, there are no uniform standard systems and quality grade standards for the cultivation and management of S. grosvenorii (Wang et al., 2010). Li determined the content of Cu and Cd in the fruits of four S. grosvenorii-producing areas by flame atomic absorption spectrometry, and the results showed that the Cd content seriously exceeded the standard (Li F et al., 2006). Because heavy metal pollution is a threat to human health, we should focus on establishing a GAP standard in the management of Chinese medicinal herbs. This can assist in the sustainable production and management of S. grosvenorii and further improve its efficacy and safety.
In a word, phytochemical and pharmacological studies of S. grosvenorii have received great interest, and an increasing number of extracts and active compounds have been isolated that have demonstrated antioxidant, hypoglycemic, immunologic, anti-tussive and sputum-reducing, hepatoprotective, and antimicrobial activities. However, validating the correlations of the chemical composition and pharmacological effects should be carried out further, and pharmacokinetics and clinical applications of S. grosvenorii also should be studied systematically. Meanwhile, the market demand and quality control also should be highly regarded.
Author Contributions
ML and JW conceived the review. XG, NC, KR, and LZ wrote the manuscript. JJ, and YL collected the literatures. KW and ML edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 81874336); Natural Science Foundation of Inner Mongolia Autonomous Region (No.2018ZD13); China Agriculture Research System (No.CARS-21); the Fourth National Traditional Chinese Medicine Resources Survey Project; Science and Technology Innovation Guidance Project, Inner Mongolia; the TCM (Traditional Chinese Medicine) Standardization Project From State Administration of TCM (No.ZYY-2017-069); Inner Mongolia autonomous region science and technology innovation leading project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdel-Hamid, M., Romeih, E., Huang, Z., Enomoto, T., Huang, L., Li, L. (2019). Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 303, 125400. doi: 10.1016/j.foodchem.2019.125400
Akihisa, T., Hayakawa, Y., Banno, N., Shimizu, N., Suzuki, T., Kimura, Y. (2007). Cucurbitane glycosides from the fruitss of Siraitia grosvenorii and their inhibitory effects on Epstein-Barr virus activation. J. Nat. Prod. 70, 783–788. doi: 10.1021/np068074x
Chen, Y., Jia, E. L. (2011). Advances in studies on chemical constituents and pharmacological effects of luohanguo. Pharm. J. Chin. People's Liberation Army 27, 171–174. doi: 10.3969/j.issn.1008-9926.2011.02.024
Chen, D., Si, J. Y., Chang, Q., Li, C. J., Shen, L. G. (1992). Studies and uses of natural nonsugar sweeteners from Luohanguo (fruit of Siraitia grosvenorii). Nat. Prod. Res. Dev. 4, 72. doi: 10.16333/j.1001-6880.1992.01.013
Chen, Q. B., Cheng, Z. Q., Xu, Z. J., Yi, X. H. (2004). Study on the extraction and properties of oil from seeds of Siraitia grosvenorii (Swingle) C. Jeffery. Sci. Technol. Food Oil 12, 25–27. doi: 10.3969/j.issn.1007-7561.2004.02.011
Chen, Q. B., Cheng, Z. Q., Yang, J. X., Yi, X. H., Dong, C. M. (2006). Extraction and structure identification of Siraitia grosvenorii squalene. Guihaia. 26, 687–689. doi: 10.3969/j.issn.1000-3142.2006.06.024
Chen, Y., Fan, X. B., Wang, Y. X., Hang, X. M. (2006). Functional study of natural food sweetener mogrosides. China Food Addit. 1, 41–43. doi: 10.3969/j.issn.1006-2513.2006.01.009
Chen, W. J., Wang, J., Qi, X. Y., Xie, B. J. (2007). The antioxidant activities of natural sweeteners, mogrosides, from fruits of Siraitia grosvenorii. Int. J. Food Sci. Nutr. 58, 548–556. doi: 10.1080/09637480701336360
Chen, B., Chen, W., Zhang, Y. M., Wu, W. (2015). Studies on the technics of preparing effervescent tablets of Siraitia grosvenorii. Acta Med. Sin. 28, 101–103.
Chen, G., Liu, C., Meng, G., Zhang, C., Chen, F., Tang, S., et al. (2018). Neuroprotective effect of mogrol against Aβ1-42-induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 71, 869–877. doi: 10.1111/jphp.13056
Cheng, Y. J., Tang, H. H., Wu, X. X., Li, J. K., Liu, J. S. (2015). Processing of Compound Health Drink with Boat-fruitsed Sterculia Flos Lonicerae and Fructus Momordicae. Farm. Prod. Process. 3, 18–21. doi: 10.3969/jissn.1671-9646(X).2015.02.006
China national medicinal materials corporation (1994). Chinese traditional medicine resources. Beijing: Science Press.
Chinese Pharmacopoeia Commission (2015). Chinese Pharmacopoeia (Edition 2015). Beijing: China Medical Science and Technology Press.
Chu, D., Yaseen, A., Wang, L., Chen, B., Wang, M., Hu, W., et al. (2019). Two New Cucurbitane Glycosides from the Fruits of Siraitia grosvenorii Swingle. Chem. Pharm. Bull. 67, 721–724. doi: 10.1248/cpb.c19-00210
Committee for the Pharmacopoeia of PR China (2015). Pharmacopoeia of PR Chin. (Part I). China: China Medical Science and Technology Press.
Flora of China Editorial Board of the Chinese Academy of Sciences (2004). Flora of China. Beijing: Science Press.
FX, C. (1996). Newly Organized Chinese Patent Drug Manual. Beijing: China Medicine Science and Technology Press.
He, W. P., Zhu, X. Y., He, C. W. (2012). Research progress in application of Siraitia grosvenorii swingle fruit and problems in product development. Food Ind. Technol. 33, 400–402.
He, W. P., Liu, L. J., He, C. W., Li, A. Y., Li, X. M. (2017). Study on low glycemic index (CI) food and pharmacological effects of S. grosvenorii. J. Light Ind. 27, 1–2. doi: 10.3969/j.issn.1003-2673.2011.07.001
Hossen, M. A., Sun, L. Q. (2006). Effects of Siraitia grosvenorii on nasal and scratching behaviors in ICR mice. Int. J. Tradit. Chin. Med. 5, 294–294. doi: 10.1248/bpb.28.238
Huang, S. S., Liu, S. H., Ban, Q., Song, L. Y., Hu, C., Bai, J. F. (2012). Research and Application Progress on the Special Local Natural Fruit of Siraitia grosvenorii in Guangxi. Enterp. Technol. Dev. 21, 19–22. doi: 10.3969/j.issn.1674-0688.2012.21.009
Hussain, R. A., Lin, Y. M., Poveda, L. J., Bordas, E., Chung, B. S., Pezzuto, J. M., et al. (1990). Plant-derived sweetening agents: saccharide and polyol constituents of some sweet-tasting plants. J. Ethnopharmacol. 28, 103–115. doi: 10.1016/0378-8741(90)90067-4
Jia, Z. H., Yang, X. G. (2009). A minor, sweet cucurbitane glycoside from Siraitia grosvenorii. Nat. Prod. Commun. 4, 769–772. doi: 10.1002/mnfr.200800199
Jiang, S. C., He, L. M., Wu, Y., Luo, C. R. (2011). An additive for smoked Siraitia grosvenorii, their preparation and Application: CN Patent 101633866.
Jiang, C. X., Chen, J. G., Luo, T. (2015). Nutritional Value Analysis of Medicinal and Edible Plant. J. Anhui Agric. Sci. 43, 282–284. doi: 10.3969/j.issn.0517-6611.2015.11.105
Jin, C. H., Jiang, X. L., Hong, T., Wang, Y. J., Xu, T. H., Zhang, D. M. (1997). Pharmacological studies of Luohanguo Yanhou Tablet. J. Chin. Med. Mate. 20, 574–577. doi: 10.13863/j.issn1001-4454.1997.11.017
Kamei, C., Sugiura, M. (2005). New pharmacological functions of Luo Han Guo. Foods Food Ingredients J. Jpn. 210, 244–254.
Kazama, M. (1999). Intense sweetener from fresh fruits of Lo Han Kuo, a Chinese folk medicine. Its new extract and utilization as foods. Gekkan Fudo Kemikaru. 15, 63–71.
Lan, Q., Jin, C. Z., Mo, Y. W. (2018). Antioxidant and Antimicrobial Activities of Crude Extractions Components from Tuberous Root of Siraitia grosvenorii (swingle). North Horticult. 10, 144–149. doi: 10.11937/bfyy.20173116
Li, D. P., Zhang, H. R. (2000). Studies and uses of Chinese medicine Luohanguo-A special local product of Guangxi. Guihaia. 20, 270–276. doi: 10.3969/j.issn.1000-3142.2000.03.012
Li, S., Wang, H. S., Zhang, G. Y. (2003). The analysis of seed oil from Siraitia grosvenorii. Guangxi Med. J. 25, 850–852. doi: 10.3969/j.issn.0253-4304.2003.05.094
Li, D., Ikeda, T., Matsuoka, N., Nohara, T., Zhang, H., Sakamoto, T., et al. (2006). Cucurbitane glycosides from unripe fruitss of Lo Han Kuo (Siraitia grosvenorii). Chem. Pharm. Bull. 55, 1425–1428. doi: 10.1002/chin.200714189
Li, F., Wang, C. N., Zhou, Y., Xiong, W. W. (2006). The Analyse of Content of Copper and Cadmium in Siraitia grosvenorii. Stud. Trace Elem. Heal. 23, 30–31. doi: 10.3969/j.issn.1005-5320.2006.06.013
Li, H. B., Wang, Y., Li, J. F., Li, X. M. (2006). Chemical constituent of luohanguo and applied studies. Food Res. Dev. 27, 85–87. doi: 10.3969/j.issn.1005-6521.2006.02.028
Li, J., Huang, X. S., Zhang, Y. J., He, X. C., Su, X. J. (2007). Chemical constituents of Siraitia grosvenorii (Swingle) C. Jeffrey. China J. Chin. Mater Med. 32, 548–549.
Li, H. Y., Wang, X. L., Pan, Y. M., Li, W. L. (2008). Antioxidant and activated oxygen free radical scavenging activities of different extract from Siraitia grosvenortt. Guihaia. 28, 698–702. doi: 10.3969/j.issn.1000-3142.2008.05.029
Li, D. P., Ikeda, T., Nohara, T., Liu, J. L., Wen, Y. X., Sakamoto, T., et al. (2010). Cucurbitane Glycosides from Unripe Fruitss of Siraitia grosvenorii. Chem. Pharm. Bull. 38, 1425–1428. doi: 10.1248/cpb.55.1082
Li, C., Lin, L. M., Luo, M., Ma, C. F., Wang, Z. M. (2011). A new natural saponin from fruitss of Siraitia grosvenorii. China J. Chin. Mater Med. 6, 721. doi: 10.1007/s10008-010-1224-4
Li, C., Lin, L. M., Sui, F., Wang, Z. M., Huo, H. R., Dai, L., et al. (2014). Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 12 (2), 89–102. doi: 10.1016/s1875-5364(14)60015-7
Liao, R. Q., Li, J., Huang, X. S., Huang, Y., He, X. C., Su, X. J. (2008). Chemical constituents of Siraitia grosvenorii (swingle) C. Jeffrey. Acta BotBoreal-Occident Sin. 28, 1250–1254. doi: 10.7666/d.d042549
Lin, S., Gao, X. L., Yue, P. X. (2007). The progress in research on the effective components extraction of Siraitia grosvenorii. Chin. Food Addit. 4, 77–80. doi: 10.3969/j.issn.1006-2513.2007.04.017
Lin, K., Mo, C. M., Ma, X. J., Huang, D. P., Miu, J. H., Huang, W. Q., et al. (2012). Study on the Quality Safety Standard of Food Additive—lo-han-kuo Extract. Mod. Sci. Instrum. 5 (20-24), 27.
Liu, J., Sun, Y. (2013). Studies on pharmacological effects of S. grosvenorii and its extracts. Heilongjiang Sci. Technol. Inform. 5, 99. doi: 10.3969/j.issn.1673-1328.2013.05.093
Liu, H., Wang, C., Qi, X., Zou, J., Sun, Z. (2018). Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 55, 1880–1888. doi: 10.1007/s13197-018-3105-2
Long, Y., Dong, W., Wang, Q., Tang, H. Q., Tian, H., Tan, H. H. (2012). Analysis on the research status of new efficacy of Siraitia grosvenorii. Shandong Med. J. 52, 95–97.
Marone, P. A., Borzelleca, J. F., Merkel, D., Heimbach, J. T., Kennepohl, E. (2008). Twentyeight-day dietary toxicity study of Luo Han fruits concentrate in Hsd: SD rats. Food Chem. Toxicol. 46, 910–919. doi: 10.1016/j.fct.2007.10.013
Matsumoto, K., Kasai, R., Ohtani, K., Tanaka, O. (1990). Minor cucurbitane-glycosides from fruitss of Siraitia grosvenorii (Cucurbitaceae). Chem. Pharm. Bull. 38, 2030–2032. doi: 10.1248/cpb.38.2030
Matsumoto, S., Jin, M., Dewa, Y., Nishimura, J., Moto, M., Murata, Y., et al. (2009). Suppressive effect of Siraitia grosvenorii extract on dicyclanil-promoted hepatocellular proliferative lesions in male mice. J. Toxicol. Sci. 34, 109–118. doi: 10.2131/jts.34.109
Meng, M. S., Liao, Y. J. (2018). Brief analysis on cultivation techniques of luohanguo with high quality and high yield. South Chin. Agric. 12, 39–43. doi: 10.19415/j.cnki.1673-890x.2018.06.022
Mi, H., Long, Y., Xiao, X. Q., Tang, H. Q., Lan, F. S., Wang, Q., et al. (2015). Overview of research and development of Siraitia grosvenorii products. J. Youjiang Med. Univ. Nation. 5, 745–747. doi: 10.3969/j.issn.1001-5817.2015.05.033
Nie, J., Yan, K., Sui, L., Zhang, H., Zhang, H., Yang, X., et al. (2019). Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 141, 35–40. doi: 10.1016/j.theriogenology.2019.09.010
Pawar, R. S., Krynitsky, A. J., Rader, J. I. (2013). Sweeteners from plants—with emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 405 (13), 4397–4407. doi: 10.1007/s00216-012-6693-0
Qi, X. Y., Chen, W. J., Song, Y. F., Xie, B. J. (2003). Hypoglycemic effect of Siraitia grosvenorii extract on diabetic mice. Chin. J. Public Heal. 19, 1226–1227. doi: 10.3321/j.issn:1002-6630.2003.12.032
Qi, X. Y., Chen, W. J., Zhang, L. Q., Shan, X. F., Song, Y. F. (2006). Study on the inhibitory effects of natural sweetner mogrosides on radical and lipid peroxidation. Sci. Agric. Sin. 39, 382–388. doi: 10.3321/j.issn:0578-1752.2006.02.024
Qin, Y. Y., Li, X. H. (2012). Advances in pharmacology and toxicology of Siraitia grosvenorii. Modern Med. Health 28, 2794–2796.
Qin, X., Xiao, J. S., Ronggan, L., Yu, X. W., Zhu, N. T., Shou, J. G., et al. (2006). Subchronic 90-day oral (gavage) toxicity study of a luo han guo mogroside extract in dogs. Food Chem. Toxicol. 44, 2106–2109. doi: 10.1016/j.fct.2006.07.023
Qing, Z. X., Zhao, H., Tang, Q., Mo, C., Huang, P., Cheng, P., et al. (2017). Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. BioMed. Anal. 138, 240–248. doi: 10.1016/j.jpba.2017.01.059
Ren, X. E., Yang, F., Yan, Z., Huang, Y. C. (2009). Development of compound low-sugar drink of momordica grosvenorrii swingle and Rubus suavissimus S.Lee. Food Mach. 25, 123–126. doi: 10.13652/j.issn.1003-5788.2009.02.024
Seki, H., Tamura, K., Muranaka, T. (2017). Plant-derived isoprenoid sweeteners: recent progress in biosynthetic gene discovery and perspectives on microbial production. Biosci. Biotech. Bioch. 82 (6), 927–934. doi: 10.1080/09168451.2017.1387514
Si, J., Chen, D., Chang, Q., Shen, L. (1996). Isolation and determination of cucurbitane-glycosides from fresh fruitss of Siraitia grosvenorii. Acta Bot. Sin. 38, 489–494.
Soejarto, D. D., Addo, E. M., Kinghorn, A. D. (2019). Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. J. Ethnopharmacol. 243, 112056. doi: 10.1016/j.jep.2019.112056
Song, Z. D., Wen, Z. Y., Li, X. Y., Liu, Y. (2009). Study on Extraction Technology of Fufang luohanguo zhikepian. Tradit. Chin. Med. Patent Prescription 31, 1773–1774.
Suzuki, Y. A., Tomoda, M., Murata, Y., Inui, H., Sugiura, M., Nakano, Y. (2007). Antidiabetic effect of long-term supplementation with Siraitia grosvenorii on the spontaneously diabetic Goto-Kakizaki rat. Br. J. Nutr. 97, 770–775. doi: 10.1017/S0007114507381300
Takasaki, M., Konoshima, T., Murata, Y., Sugiura, M., Nishino, H., Tokuda, H., et al. (2003). Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Siraitia grosvenorii. Cancer Lett. 198, 37. doi: 10.1016/s0304-3835(03)00285-4
Takemoto, T., Arihara, S., Nakajima, T., Okuhira, M. (1983a). Studies on the constituents of fructus Momordicae. I. On the sweet princple. Yakugaku Zasshi. 103, 1151–1154. doi: 10.1079/BJN19830137
Takemoto, T., Arihara, S., Nakajima, T., Okuhira, M. (1983b). Studies on the constituents of fructus Momordicae. II. Structure of sapogenin. Yakugaku Zasshi. 103, 1155–1166. doi: 10.1248/yakushi1947.103.11_1155
Tan, J. C., Zeng, S. E. (2019). Study and Development of Medicinal Function of Siraitia grosvenorii. Chin. Continues Med. Educ. 11, 147–149. doi: 10.3969/j.issn.1674-9308.2019.13.063
Tan, Q. F., Lan, X. Q., Wen, Q. W., Liu, T., Lu, D., Wei, H. Y., et al. (2014). Determination of Mogroside V in Fufang Luohanguo Zhike Granule by HPLC-ELSD. Chin. J. Mod. Appl. Pharm. 31, 850–852. doi: 10.13748/j.cnki.issn1007-7693.2014.07.020
Tang, H. Q., Long, Y., Dong, W., Xiao, X. Q., Mi, H., Wang, Q., et al. (2015). Effect of various factors such as habitat, growth period, and commercial specification on antitussive effect of Siraitia. Chin. Traditional Herbal Drugs 46, 3051–3054. doi: 10.7501/j.issn.0253-2670.2015.20.015
Ukiya, M., Akihisa, T., Tokuda, H., Toriumi, M., Mukainaka, T., Banno, N., et al. (2002). Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruits of Siraitia grosvenorii on epstein-barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 50, 6710. doi: 10.1021/jf0206320
Wan, Y. J., Wu, J. L., Wu, Q. P. (2016). A Review of the Hypoglycemic Activity of Siraitia grosvenorii. Food Res. Dev. 37, 188–191. doi: 10.3969/j.issn.1005-6521.2016.11.045
Wang, M., Song, Z. J., Ke, M. Z., N, F. L., Wang, Q. (1994). Effects of different doses of Siraitia grosvenorii Swingle on the immune function of rats. Acta Acad. Med. Guangxi. 11, 408–410 doi: 10.16190/j.cnki.45-1211/r.1994.04.02
Wang, Q., Li, A. Y., Li, X. P. (1999). Pharmacological effects of S. grosvenorii fruits. China J. Chin. Mater Med. 1999 (24(7)), 425–428. doi: 10.3321/j.issn:1001-5302.1999.07.019
Wang, T., Huang, Z. J., Jiang, Y. M., Zhou, S., Su, L., Jiang, S. Y., et al. (1999). Studies on the pharmacological profile of mogrosides. Chin. Tradit. Herb. Drugs 30, 914–916. doi: 10.3321/j.issn:0253-2670.1999.12.016
Wang, Q., Qin, H., Wang, W., Qiu, S. P. (2010). The pharmacological research progress of Siraitia grosvenorii. [dissertation/master's thesis]. J. Guangxi Tradit. Chin. Med. Univ. 13, 75–76. doi: 10.3969/j.issn.1008-7486.2010.03.040
Wang, Q., Xiao, X. Q., Dong, W., Tang, H. Q., Lu, L. F., Li, Y. H., et al. (2017). Spectrum-effect relationship on expectorant efficacy of Siraitia grosvenorii. Guihaia. 37, 606–609. doi: 10.11931/guihaia.gxzw201603010
Wei, Z. Q., Zhou, Z., Liu, Y. K., Chen, B. S., You, B. X. (2004). Pharmacodynamics of Qingfeiluohanguotangjiang. Chin. J. Exp. Tradi Me Formulae. 10, 51–53. doi: 10.3969/j.issn.1005-9903.2004.02.020
Wu, Y., Jiang, R. W., Zhao, B. (2017). Study on the Relieving Cough and Eliminating Phlegm Effects of Stemoninine Combined with Mogroside V on Mice. J. China Pharm. 28, 1755–1757. doi: 10.6039/j.issn.1001-0408.2017.13.08
Wu, Q. M. (2011). Preparation of Yuanzhen Sugar. Modern Food Sci. Technol. 27, 92–95. doi: 10.3969/j.issn.1673-9078.2011.01.023
Xiao, G., Wang, Q. (2006). Research Advance of Siraitia grosvenorii Swingle. Shanghai J. Tradit. Chin. Med. 40, 71–73. doi: 10.3969/j.issn.1007-1334.2006.11.033
Xiao, G., Wang, Q. (2008). Protective Effect of Mogrosides on Experimental Liver Injury in Mice. China Pharm. 19, 163–165.
Xie, Y. F. (2018). Effects of Flavonoids from Siraitia grosvenorii on myocardial mitochondria in exhaustive exercise rats. Sci. Technol. Inf. 16, 227–228. doi: 10.16661/j.cnki.1672-3791.2018.12.227
Xu, W. K., Li, M. S., Li, Z. Y. (1992). Isolation and identification of a bitter constituent from Luohanguo's unripe fruitss. Guihaia 12, 136–138.
Yang, Y. L., Guo, S. J., Zhang, Y. Z., Yang, D. J., Zhang, Z. X. (2016). Study on the processing technology of Siraitia grosvenorii and Zingiber officinale beverage. China Brewing. 35, 156–160. doi: 10.11882/j.issn.0254-5071.2016.02.036
Yao, J. W., Tang, H., Zhou, L., Shen, W. H., Li, Y. X., Tian, X. Z. (2008). Effect of Siraitia grosvenorii extract on the movement endurance and liver tissue injury of mice. Chin. J. Sports Med. 27, 221–223. doi: 10.3969/j.issn.1000-6710.2008.02.022
Zeng, X. L. (2009). Research progress on Siraitia grosvenorii, a special plant of Guangxi. Guangxi Med. 31, 1182–1186.
Zhang, H., Li, X. H. (2011). Research Progress on Chemical Compositions of Fructus Momordicae. J. Anhui Agric. Sci. 39, 4555–4556. doi: 10.3969/j.issn.0517-6611.2011.08.058 4559.
Zhang, Z. T., Zhang, T. T. (2011). Medicine for improving male sperm quantity and vitality, preparation and preparation method: CN Patent 07737056 A.
Zhang, L. Q., Qi, X. Y., Chen, W. J., Song, Y. F. (2006). Effect of Mogroside extracts on blood glucose, blood lipid and antioxidation of hyperglycemic mice induced by Alloxan. Chin. Pharmacol. Bull. 2, 237–240. doi: 10.3321/j.issn:1001-1978.2006.02.025
Zhang, W., Wang, B., Zhou, L., Gong, J., Han, K., Li, X. J. (2014). Research progress in chemical composition and pharmacology of Siraitia grosvenorii swingle. Sci. Technol. Food Ind. 35, 393–397. doi: 10.13386/j.issn1002-0306.2014.12.077
Zhang, Q. L., Huang, J., Wu, Z. H., Pi, F. J. (2017). Research overview of pharmacology and application development of Siraitia grosvenorii. J. Pharm. Res. 36, 164–165. doi: 10.13506/j.cnki.jpr.2017.03.011
Zhang, H. Q., Huang, Q. Y., Zhen, G. J., Zeng, Z. F., Xu, D. N., Nong, K. L. (2018). Effects of Siraitia grosvenorii polysaccharides on the immune function in immunosuppressed mice induced by cyclophosphamide. Guihaia. doi: 10.11931/guihaia.gxzw201808009
Zhang, Y. L., Zhou, G. S., Peng, Y., Wang, M. Y., Li, X. B. (2019). Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J. Ethnopharmacol. 247, 112273. doi: 10.1016/j.jep.2019.112273
Zhang, Y. S. (2011). Siraitia grosvenorii fruits sweet crystal and its preparation method: CN Patent 101204221.
Zheng, Y., Huang, W., Jae-Gil, Y., Jeffrey, L. E., Huang, C. B. (2011). Antibacterial compounds from Siraitia grosvenorii leaves. Nat. Prod. Res. 25, 890–897. doi: 10.1080/14786419.2010.490212
Zhou, Y., Huang, C. F. (2008). Identification of the Antibacterial Activity from Leaf, Vine and Root of Lo Han Kuo (Siraitia grosvenorii). Lishizhen Med. Mater Med. Res. 19, 1797–1799. doi: 10.3969/j.issn.1008-0805.2008.07.134
Keywords: Siraitia grosvenorii, ethnopharmacology, food and nutritional value, chemical constituent, antioxidant
Citation: Gong X, Chen N, Ren K, Jia J, Wei K, Zhang L, Lv Y, Wang J and Li M (2019) The Fruits of Siraitia grosvenorii: A Review of a Chinese Food-Medicine. Front. Pharmacol. 10:1400. doi: 10.3389/fphar.2019.01400
Received: 12 May 2019; Accepted: 01 November 2019;
Published: 22 November 2019.
Edited by:
Alexander N. Shikov, Saint-Petersburg State Chemical Pharmaceutical Academy, RussiaReviewed by:
Vincenzo De Feo, University of Salerno, ItalyFawzi Mohamad Mahomoodally, University of Mauritius, Mauritius
Copyright © 2019 Gong, Chen, Ren, Jia, Wei, Zhang, Lv, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Wang, bnlfd2poNTEzQDE2My5jb20=; Minhui Li, cHJvZl9saW1pbmh1aUB5ZWFoLm5ldA==
†These authors have contributed equally to this work
 Xue Gong1†
Xue Gong1† Minhui Li
Minhui Li