- 1Department of Biological Science and Technology, National Chiao Tung University, Hsinchu, Taiwan
- 2Division of Cardiovascular Surgery, Department of Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 3Department of Pharmacy Practice, Tri-Service General Hospital, Taipei, Taiwan
- 4School of Pharmacy, National Defense Medical Center, Taipei, Taiwan
- 5Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 6Biobank Management Center, Tri-Service General Hospital, Taipei, Taiwan
- 7School of Public Health, National Defense Medical Center, Taipei, Taiwan
- 8Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan
To date, population-based studies on the healthcare service utilization among stable heart, kidney, and liver transplant recipients with different calcineurin inhibitors are still scarce. Therefore, we used the Taiwan National Health Insurance Research Database to conduct a nationwide cross-sectional study to estimate the healthcare utilization of stable transplant recipients with tacrolimus or cyclosporine (n = 3,482). The sampled patients in this study comprised 377 heart, 1,693 kidney, and 1,412 liver transplant recipients between 1 January 2011 and 31 December 2011. Each subject was followed for a 1-year period to evaluate his/her healthcare service utilization. Outcome variables of the healthcare service utilization were stated as below: numbers of outpatient visits, outpatient costs, numbers of inpatient days, inpatients costs, and total costs of all healthcare services. As for all healthcare service utilization, stable transplant recipients on tacrolimus had significantly more outpatient visits (40.7 vs. 38.6), outpatient costs (US$10,383 vs. US$8,155), and total costs (US$12,516 vs. US$10,372) of all healthcare services than those on cyclosporine during the 1-year follow-up period. Additionally, further analysis showed that heart transplant recipients receiving tacrolimus incurred 1.7-fold higher inpatient costs compared to patients receiving cyclosporine. We concluded that transplant recipients using tacrolimus had significantly higher utilization of all healthcare services than those receiving cyclosporine as immunosuppressive therapy.
Introduction
Solid organ transplantation is expensive and exerts a huge financial burden on transplant recipients. The average reported cost of a solid organ transplant ranges from $334,300 for a single kidney transplant to more than $2.3 million for combined heart and lung transplants (Bentley and Hanson, 2014). The use of immunosuppressive medications after transplantation is essential for the success of organ transplantation because immunosuppressive regimens can reduce acute rejection rates and graft loss resulting from acute rejection (Meier-Kriesche et al., 2004). In the early 1980s, cyclosporine, a calcineurin inhibitor, was introduced in the market and provided excellent renal graft survival rates (Starzl et al., 1980; Gjertson et al., 1995). This agent impairs the transcription of interleukin-2 and several other cytokines in T lymphocytes (Jasiak and Park, 2016). However, cyclosporine may lead to adverse reactions such as nephrotoxicity, chronic hemolytic uremic syndrome, hyperlipidemia, hypertension, gingival hyperplasia, diabetes mellitus, and tremors. Since its Food and Drug Administration (FDA) approval in the early 1990s, tacrolimus has been widely used as an alternative immunosuppressive medication after all types of solid organ transplants; it is a more potent calcineurin inhibitor. Compared with cyclosporine, tacrolimus provides lower acute rejection rates and potentially higher graft survival rates, as demonstrated by several randomized controlled trials and meta-analyses; however, tacrolimus is associated with a higher risk of posttransplant diabetes mellitus (Gjertson et al., 1995; Knoll and Bell, 1999; Webster et al., 2005a). Tacrolimus may plausibly result in additional costs of managing adverse events and increase the non-transplant-related health service utilization of transplant recipients.
Until now, few studies have estimated the healthcare service utilization of stable heart, kidney, and liver transplant recipients receiving different calcineurin inhibitors. Studies have shown that compared with cyclosporine, tacrolimus-based immunosuppression is associated with economic advantages among kidney or liver transplant recipients due to its lower acute rejection rates (Lake et al., 1995; Neylan et al., 1998; Rabkin et al., 2001; Lazzaro et al., 2002; Miners et al., 2007). However, cost-effectiveness analyses in Brazil and Germany have revealed that tacrolimus treatment entails higher total health service expenditures than cyclosporine treatment after renal transplantation (Jurgensen et al., 2010; Guerra Junior et al., 2015). Previous reports have indicated inconsistent economic impacts of calcineurin inhibitors on the healthcare system. In addition, to date, no study has explored healthcare service utilization in heart transplant recipients receiving calcineurin inhibitors. Thus, this population-based study investigated the differences in healthcare service utilization among stable heart, kidney, and liver transplant recipients receiving cyclosporine or tacrolimus-based treatment during a 1-year follow-up period in Taiwan.
Materials and Methods
Database
This study used data of the de-identified medical claims from the Taiwan National Health Insurance Research Database (NHIRD). This database includes medical records and registry files for approximately 99% of the Taiwanese population (n = 23 million) since 1995. The NHIRD has been released to investigators in Taiwan for research purposes, and researchers are permitted to track the longitudinal records of the enrollees. The need for ethics approval was waived by the Tri-Service General Hospital Institutional Review Board because it only used de-identified secondary data. The data sets for this manuscript are not publicly available, because the data are handled and stored by the Health and Welfare Data Science Center (HWDC). Requests to access the data sets should be directed to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Study Sample
In this nationwide cross-sectional study, 3,902 transplant recipients (including heart, kidney, and liver transplant recipients) receiving cyclosporine or tacrolimus between 1 January 2011 and 31 December 2011 were first identified. The use of the study drug was determined on the basis of the Anatomical Therapeutic Chemical codes (L04AD01 for cyclosporine and L04AD02 for tacrolimus). Generally, given that acute rejection mostly occurs within weeks to 1 year after transplantation, the index date was defined as the last date on which patients received cyclosporine or tacrolimus during the study period to minimize the acute rejection factor. To ensure equal follow-up periods among all selected stable transplant recipients, we excluded 175 patients who died within the study period after the index date. We further excluded 245 patients aged <18 years to ensure that adult transplant recipients were recruited. Accordingly, 3,482 transplant recipients receiving cyclosporine or tacrolimus were recruited into this study. In addition, 2,741 tacrolimus users were identified as the study group, and 741 cyclosporine users were defined as the comparison group. We further divided the study patients into three groups: heart transplant recipients, kidney transplant recipients, and liver transplant recipients.
Variables of Interest
In order to carry out the healthcare service utilization assessments and evaluate patients’ healthcare service visits and costs, all transplant recipients in this study were tracked for 1 year following the index date. Healthcare service in this study included those of physician diagnoses, medications, surgery, and laboratory tests covered by National Health Insurance (NHI) program. Variables of outpatient service utilization during the 1-year follow-up period in this study were defined as follows: 1) mean numbers of outpatient visits, 2) mean total costs of outpatient services, 3) mean costs of outpatient study drugs (cyclosporine and tacrolimus), and 4) mean costs of other outpatient services (excluding costs of study drugs to avoid the effects due to different drug prices between cyclosporine and tacrolimus). Variables of inpatient service utilization were identified as follows: 1) mean numbers of inpatient days, 2) mean total costs of inpatient services, 3) mean costs of inpatient study drugs, and 4) mean costs of other inpatient services (excluding costs of study drugs to avoid the effects due to different drug prices between cyclosporine and tacrolimus). Additionally, variables of all NHI healthcare services’ costs were defined as follows: 1) mean total costs, 2) mean costs of study drugs, and 3) mean costs of other healthcare services (excluding costs of study drugs to avoid the effects due to different drug prices between cyclosporine and tacrolimus).
Statistical Analysis
Pearson chi-squared tests were conducted to compare the differences in sex, urbanization level (five levels, with 1 being the most urbanized and 5 being the least urbanized), monthly income, transplanted organs, and comorbidities between cyclosporine and tacrolimus users (Liu et al., 2006). Independent t tests were performed to compare the differences in the utilization and costs between cyclosporine and tacrolimus users. A two-sided p value of <0.05 was used to evaluate statistical significance. All statistical analyses were performed using the SAS system (version 9.4, SAS System for Windows).
Results
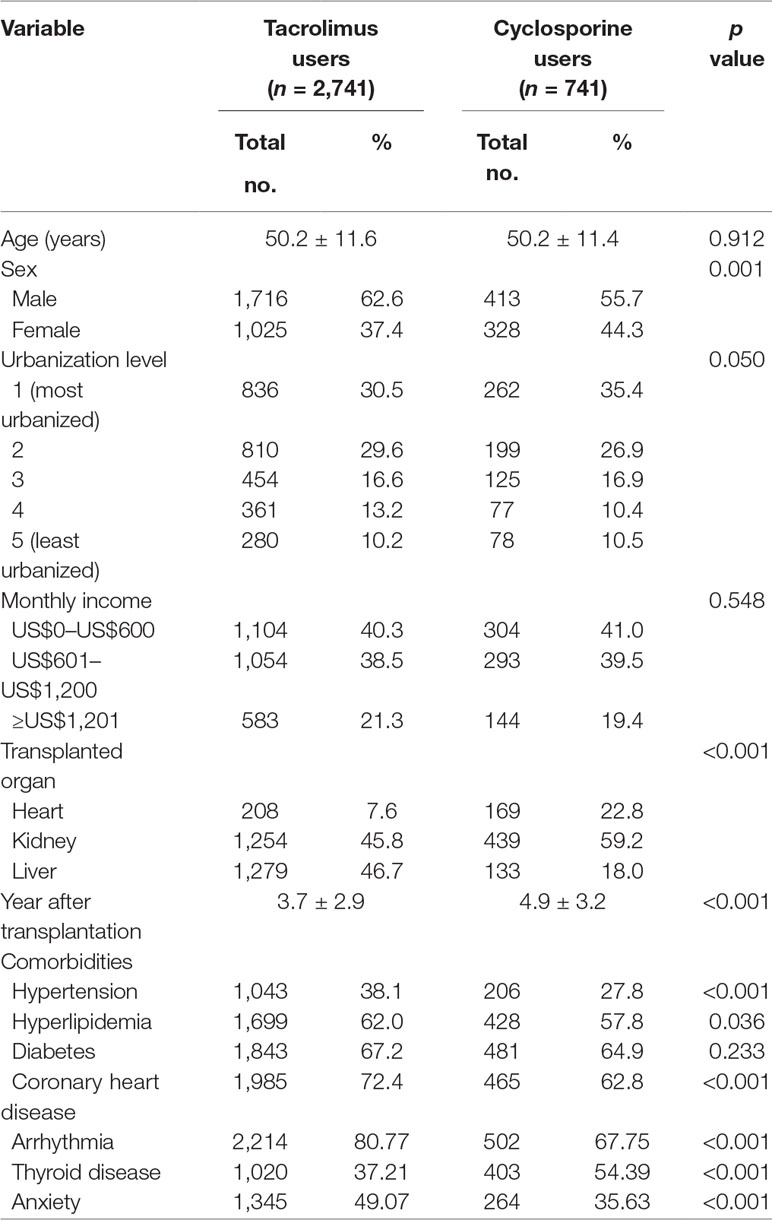
This study included a total of 3,482 stable transplant recipients who received tacrolimus or cyclosporine. Among the sampled transplant recipients in this study, 2,741 were defined as tacrolimus users, and 741 were identified as cyclosporine users. The demographic characteristics and comorbidities of the sampled patients are presented in Table 1. The mean age of tacrolimus and cyclosporine users was 50.2 ± 11.6 and 50.2 ± 11.4 years, respectively (p = 0.912). Although the mean number of years after transplantation was statistically fewer in tacrolimus users (3.7 ± 2.9 years) than in cyclosporine users (4.9 ± 3.2 years), the time after transplantation was sufficiently long to consider these recipients as being relatively stable. Other findings revealed that the distributions of sex, hypertension, hyperlipidemia, coronary heart disease, arrhythmia, thyroid disease, anxiety, and transplanted organ were significantly different between tacrolimus and cyclosporine users. However, no significant difference was observed in age, urbanization level, monthly income, and diabetes between the two groups.
Table 2 presents the utilization and costs of outpatient services, inpatient services, and all healthcare services within the 1-year study period following the index date for transplant recipients receiving cyclosporine or tacrolimus. Regarding outpatient service utilization, tacrolimus users had a significantly higher number of outpatient visits (40.7 vs. 38.6) and total outpatient costs (US$10,383 vs. US$8,155) than cyclosporine users. However, no significant difference was observed in inpatient days and total inpatient costs between tacrolimus and cyclosporine users. Additionally, regarding all healthcare services, tacrolimus users had higher total costs (US$12,516 vs. US$10,372) than cyclosporine users.
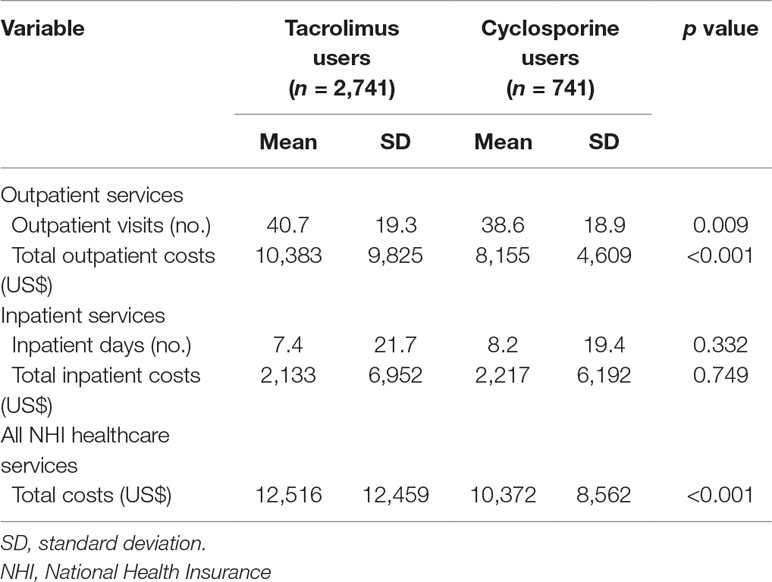
Table 2 Utilization and costs of outpatient services, inpatient services, and all healthcare services within 1 year for tacrolimus and cyclosporine users (N = 3,482).
Table 3 presents the total health service utilization, outpatient service utilization, and inpatient service utilization within 1 year for tacrolimus and cyclosporine users among heart, kidney, and liver transplant recipients. Among heart transplant recipients, tacrolimus users had a significantly higher number of outpatient visits (37.3 vs. 32.2), total outpatient costs (US$11,991 vs. US$9,407), outpatient study drug costs (US$4,197 vs. US$2,394), total inpatient costs (US$4,729 vs. US$2,737), inpatient study drug costs (US$202 vs. US$119), and other inpatient costs (US$4,527 vs. US$2,618) than cyclosporine users. Regarding all healthcare service utilization among heart transplant recipients, tacrolimus users had significantly higher total costs (US$16,720 vs. US$12,144), study drug costs (US$4,399 vs. US$2,513), and other costs (US$12,321 vs. US$963) than cyclosporine users.
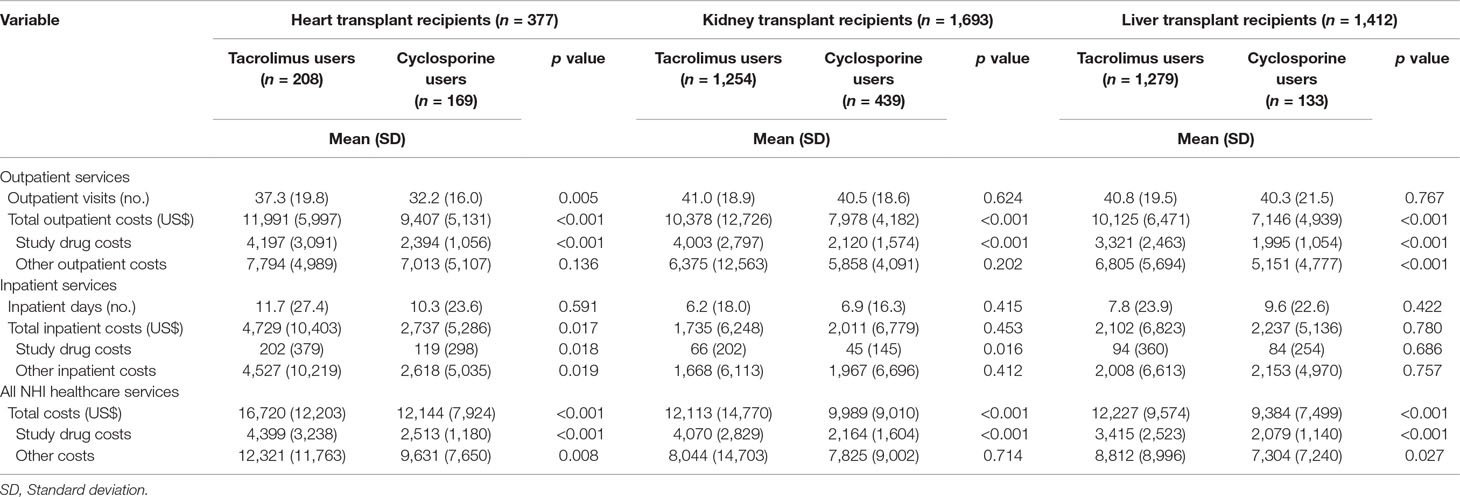
Table 3 Utilization and costs of healthcare services within 1 year for cyclosporine and tacrolimus users, stratified by different transplantations.
Among kidney transplant recipients (Table 3), tacrolimus users had higher total costs and study drug costs for outpatient services and all healthcare services than cyclosporine users. In addition, tacrolimus users had higher inpatient study drug costs than cyclosporine users. Table 3 also shows the utilization of liver transplant recipients. The findings revealed that tacrolimus users had higher total costs (US$10,125 vs. US$7,146), study drug costs (US$3,321 vs. US$1,995), and other costs (US$6,805 vs. US$5,151) for outpatient services than cyclosporine users. Furthermore, regarding all healthcare services, tacrolimus users had higher total costs (US$12,227 vs. US$9,384), study drug costs (US$3,415 vs. US$2,079), and other costs (US$8,812 vs. US$7,304) than cyclosporine users.
Discussion
This population-based study found that stable transplant recipients receiving tacrolimus had higher healthcare service utilization, including outpatient visits, outpatient costs, and total costs of all NHI healthcare services, than transplant recipients receiving cyclosporine (Table 2). The higher outpatient visits among tacrolimus users than among cyclosporine users may be caused by the high prevalence of some chronic diseases, including hypertension, hyperlipidemia, and coronary heart disease, in tacrolimus users (Table 1). A possible explanation is that tacrolimus might be preferred for patients with hypertension, sodium retention, and hypercholesterolemia. Additionally, cyclosporine might be the more favorable choice for patients with diabetes mellitus, and it has milder neurological side effects (Danovitch, 1997; Pirsch et al., 1997; Webster et al., 2005a; Haddad et al., 2006). Therefore, stable transplant recipients receiving tacrolimus may experience more comorbidities, including hypertension, hyperlipidemia, and coronary heart disease, than cyclosporine users, thereby resulting in more outpatient visits. Moreover, no difference was observed in the distribution of diabetes mellitus between tacrolimus and cyclosporine users (Table 1), although previous studies have found that patients receiving tacrolimus had a higher incidence of de novo insulin-requiring diabetes mellitus (Webster et al., 2005a; Haddad et al., 2006). This finding might be explained reasonably by the fact that cyclosporine is more commonly prescribed for transplant recipients with diabetes mellitus. Furthermore, unsurprisingly, outpatient study drug costs in tacrolimus users were higher than those in cyclosporine users in this study due to the higher actual wholesale price of tacrolimus (James and Mannon, 2015). Our findings are in agreement with those of previous economic studies indicating that regimens containing cyclosporine were more cost-effective than tacrolimus-based regimens in renal transplant recipients (Guerra Junior et al., 2010; Jurgensen et al., 2010; Guerra Junior et al., 2015).
In the United States, previous studies comparing cyclosporine with tacrolimus in renal transplant recipients concluded that tacrolimus use led to higher cost savings because of the lower rates of hospitalization as a result of fewer acute rejection episodes (James and Mannon, 2015; Kamel et al., 2016). However, our study found no difference in days and total costs of inpatient services between stable transplant recipients receiving tacrolimus and those receiving cyclosporine in Taiwan (Table 2). It is reasonably speculated that a similar rate of graft survival was observed among stable transplant recipients regardless of the use of different study drugs after the acute rejection period (Opelz, 1999; Orme et al., 2003; Webster et al., 2005b). In Brazil, Gomes et al. showed poorer graft survival rates (survival rates of 64.8% and 71.9%, respectively) and increased risk of death or return to dialysis (hazard ratio = 1.194; 95% CI 1.082–1.318) in patients treated with tacrolimus compared with those treated with cyclosporine after a 10-year follow-up (Gomes et al., 2016). Therefore, different ethnicities may contribute to consistent outcomes from these studies.
To the best of our knowledge, this is the first study to provide a real-world assessment of healthcare service utilization, including complete utilization information and medical costs, among stable heart transplant recipients receiving calcineurin inhibitors through analysis of the nationwide population-based data set NHIRD. To date, studies on healthcare service utilization among heart transplant recipients receiving different calcineurin inhibitors are limited. Studies have indicated that heart transplant recipients treated with cyclosporine presented higher rates of cytomegalovirus infection (Rodriguez-Serrano et al., 2014), thereby increasing the need for treatment by approximately eightfold (Bond et al., 2018), than those treated with tacrolimus. Compared with tacrolimus as baseline immunosuppressive therapy, cyclosporine may also produce higher risks of obesity (Lopez-Vilella et al., 2015) and a more pronounced deterioration of renal function (Helmschrott et al., 2015) after heart transplantation. The abovementioned side effects of cyclosporine may be a key factor for the increase in healthcare service utilization. However, among stable heart transplant recipients, we found that tacrolimus users had approximately five more outpatient visits than cyclosporine users. Regarding inpatient services, although no difference was observed in the number of inpatient days between heart transplant recipients receiving tacrolimus and those receiving cyclosporine, tacrolimus users had approximately 1.7-fold higher total inpatient costs, especially nonstudy drug–related costs, than cyclosporine users (Table 3).
Several limitations of this study should be considered. First, this study did not evaluate self-paid healthcare services (such as over-the-counter medicines) and indirect costs in transplant recipients. Secondly, many undocumented factors may potentially affect the utilization and costs of healthcare services in this study. Third, the study findings should be generalized to other ethnicities with discretion because most of the patients included in this study were Chinese Han.
In conclusion, this population-based study revealed that stable transplant recipients receiving tacrolimus had higher outpatient healthcare service utilization than those receiving cyclosporine. Additionally, heart transplant recipients receiving tacrolimus had approximately 1.7-fold higher total costs of inpatient healthcare services than those receiving cyclosporine, but no difference was noted in inpatient days within the 1-year study period following the index date between transplant recipients receiving cyclosporine and those receiving tacrolimus. Based on the study results, we suggest that physicians should consider the economic impact of tacrolimus on lower-income stable transplant recipients, especially heart transplant recipients. Additional studies should be conducted to further investigate the potential factors leading to elevated healthcare service utilization by stable transplant recipients receiving tacrolimus.
Data Availability
The datasets for this manuscript are not publicly available because the data are handled and stored by the Health and Welfare Data Science Center (HWDC). Requests to access the datasets should be directed to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Ethics Statement
This study was approved by the Institutional Review Board of Tri-Service General Hospital.
Author Contributions
C-SL, J-HS, and L-TK conceived of this study and participated in study design. Y-CL wrote and revised the manuscript. C-ST and I-HL provided guidance and edited the paper. Y-TT and T-YH participated in the performance of the research. K-FL participated in data analysis. L-TK performed statistical analysis and supervised the entire project. All authors critically reviewed content and approved the final version for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bentley, T. S., Hanson, S. G. (2014). 2014 US organ and tissue transplant cost estimates and discussion. Milliman Research Report, Seattle WA.
Bond, M. M. K., Bond, M. M. K., Sehn, A., Dias, V. H., Said, T. L., Dos Santos, C. C., et al. (2018). Cyclosporine versus tacrolimus: which calcineurin inhibitor has influence on cytomegalovirus infection in cardiac transplantation? Transplant. Proc. 50, 809–814. doi: 10.1016/j.transproceed.2018.02.046
Danovitch, G. M. (1997). Cyclosporin or tacrolimus: which agent to choose? Nephrol. Dial. Transplant. 12, 1566–1568. doi: 10.1093/ndt/12.8.1566
Gjertson, D. W., Cecka, J. M., Terasaki, P. I. (1995). The relative effects of FK506 and cyclosporine on short- and long-term kidney graft survival. Transplantation 60, 1384–1388. doi: 10.1097/00007890-199560120-00002
Gomes, R. M., Guerra Junior, A. A., Lemos, L. L., Costa Jde, O., Almeida, A. M., Alvares, J., et al. (2016). Ten-year kidney transplant survival of cyclosporine- or tacrolimus-treated patients in Brazil. Expert Rev. Clin. Pharmacol. 9, 991–999. doi: 10.1080/17512433.2016.1190270
Guerra Junior, A. A., Acurcio Fde, A., Andrade, E. I., Cherchiglia, M. L., Cesar, C. C., Queiroz, O. V., et al. (2010). [Cyclosporine versus tacrolimus in kidney transplants in Brazil: a cost comparison]. Cad. Saude Publica 26, 163–174. doi: 10.1590/S0102-311X2010000100017
Guerra Junior, A. A., Silva, G. D., Andrade, E. I., Cherchiglia, M. L., Costa Jde, O., Almeida, A. M., et al. (2015). Cyclosporine versus tacrolimus: cost-effectiveness analysis for renal transplantation in Brazil. Rev. Saude Publica 49, 13. doi: 10.1590/S0034-8910.2015049005430
Haddad, E. M., Mcalister, V. C., Renouf, E., Malthaner, R., Kjaer, M. S., Gluud, L. L. (2006). Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst. Rev. 18 (4) CD005161. doi: 10.1002/14651858.CD005161.pub2
Helmschrott, M., Rivinius, R., Ruhparwar, A., Schmack, B., Erbel, C., Gleissner, C. A., et al. (2015). Advantageous effects of immunosuppression with tacrolimus in comparison with cyclosporine A regarding renal function in patients after heart transplantation. Drug Des. Devel. Ther. 9, 1217–1224. doi: 10.2147/DDDT.S79343
James, A., Mannon, R. B. (2015). The cost of transplant immunosuppressant therapy: is this sustainable? Curr. Transplant. Rep. 2, 113–121. doi: 10.1007/s40472-015-0052-y
Jasiak, N. M., Park, J. M. (2016). Immunosuppression in solid-organ transplantation: essentials and practical tips. Crit. Care Nurs. Q. 39, 227–240. doi: 10.1097/CNQ.0000000000000117
Jurgensen, J. S., Arns, W., Hass, B. (2010). Cost-effectiveness of immunosuppressive regimens in renal transplant recipients in Germany: a model approach. Eur. J. Health Econ. 11, 15–25. doi: 10.1007/s10198-009-0148-3
Kamel, M., Kadian, M., Srinivas, T., Taber, D., Posadas Salas, M. A. (2016). Tacrolimus confers lower acute rejection rates and better renal allograft survival compared to cyclosporine. World J. Transplant. 6, 697–702. doi: 10.5500/wjt.v6.i4.697
Knoll, G. A., Bell, R. C. (1999). Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ 318, 1104–1107. doi: 10.1136/bmj.318.7191.1104
Lake, J. R., Gorman, K. J., Esquivel, C. O., Wiesner, R. H., Klintmalm, G. B., Miller, C. M., et al. (1995). The impact of immunosuppressive regimens on the cost of liver transplantation—results from the U.S. FK506 multicenter trial. Transplantation 60, 1089–1095. doi: 10.1097/00007890-199511270-00005
Lazzaro, C., Mckechnie, T., Mckenna, M. (2002). Tacrolimus versus cyclosporin in renal transplantation in Italy: cost-minimisation and cost-effectiveness analyses. J. Nephrol. 15, 580–588.
Liu, C. Y., Hung, Y. T., Chuang, Y. L., Chen, Y. J., Weng, W. S., Liu, J. S., et al. (2006). Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 4, 1–22. doi: 10.29805/JHM.200606.0001
Lopez-Vilella, R., Sanchez-Lazaro, I. J., Martinez-Dolz, L., Almenar-Bonet, L., Marques-Sule, E., Melero-Ferrer, J., et al. (2015). Incidence of development of obesity after heart transplantation according to the calcineurin inhibitor. Transplant. Proc. 47, 127–129. doi: 10.1016/j.transproceed.2014.11.025
Meier-Kriesche, H. U., Schold, J. D., Srinivas, T. R., Kaplan, B. (2004). Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am. J. Transplant. 4, 378–383. doi: 10.1111/j.1600-6143.2004.00332.x
Miners, A. H., Yao, G., Raftery, J., Taylor, R. S. (2007). Economic evaluations of calcineurin inhibitors in renal transplantation: a literature review. Pharmacoeconomics 25, 935–947. doi: 10.2165/00019053-200725110-00004
Neylan, J. F., Sullivan, E. M., Steinwald, B., Goss, T. F. (1998). Assessment of the frequency and costs of posttransplantation hospitalizations in patients receiving tacrolimus versus cyclosporine. Am. J. Kidney Dis. 32, 770–777. doi: 10.1016/S0272-6386(98)70132-5
Opelz, G. (1999). Effect of immunosuppressive therapy on graft half-life projections. The collaborative transplant study. Transplant. Proc. 31, 31S–33S. doi: 10.1016/S0041-1345(99)00791-5
Orme, M. E., Jurewicz, W. A., Kumar, N., Mckechnie, T. L. (2003). The cost effectiveness of tacrolimus versus microemulsified cyclosporin: a 10-year model of renal transplantation outcomes. Pharmacoeconomics 21, 1263–1276. doi: 10.2165/00019053-200321170-00003
Pirsch, J. D., Miller, J., Deierhoi, M. H., Vincenti, F., Filo, R. S. (1997). A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 kidney transplant study group. Transplantation 63, 977–983. doi: 10.1097/00007890-199704150-00013
Rabkin, J. M., Corless, C. L., Rosen, H. R., Olyaei, A. J. (2001). Pharmacoeconomic study of tacrolimus-based versus cyclosporine-based immunosuppressive therapy following liver transplantation. Transplant. Proc. 33, 1532–1534. doi: 10.1016/S0041-1345(00)02585-9
Rodriguez-Serrano, M., Sanchez-Lazaro, I., Almenar-Bonet, L., Martinez-Dolz, L., Portoles-Sanz, M., Rivera-Otero, M., et al. (2014). Does the calcineurin inhibitor have influence on cytomegalovirus infection in heart transplantation? Clin. Transplant. 28, 88–95. doi: 10.1111/ctr.12282
Starzl, T. E., Richard Weil Iii, S. I., Klintmalm, G., Schröter, G. P., Koep, L. J., Iwaki, Y., et al. (1980). The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg. Gynecol. Obstet. 151, 17.
Webster, A., Woodroffe, R. C., Taylor, R. S., Chapman, J. R., Craig, J. C. (2005a). Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev., 19 (4) CD003961. doi: 10.1002/14651858.CD003961.pub2
Keywords: healthcare service utilization, tacrolimus, cyclosporine, heart transplant, kidney transplant, liver transplant
Citation: Lin Y-C, Tsai C-S, Li I-H, Tsai Y-T, Huang T-Y, Lee K-F, Lin C-S, Shih J-H and Kao L-T (2019) Transplant Recipients Using Tacrolimus Had Higher Utilization of Healthcare Services Than Those Receiving Cyclosporine in Taiwan. Front. Pharmacol. 10:1074. doi: 10.3389/fphar.2019.01074
Received: 22 March 2019; Accepted: 23 August 2019;
Published: 19 September 2019.
Edited by:
Brian Godman, Karolinska Institute (KI), SwedenReviewed by:
Yaser Mohammed Al-Worafi, Ajman University of Science and Technology, United Arab EmiratesAmer Hayat Khan, University of Science, Malaysia
Copyright © 2019 Lin, Tsai, Li, Tsai, Huang, Lee, Lin, Shih and Kao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ting Kao, a2FvbGl0aW5nQG1haWwubmRtY3RzZ2guZWR1LnR3
†These authors have contributed equally to this work
 Yi-Chang Lin
Yi-Chang Lin Chien-Sung Tsai2
Chien-Sung Tsai2 Jui-Hu Shih
Jui-Hu Shih Li-Ting Kao
Li-Ting Kao