- 1Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Research and Development Center of TCM, Tianjin International Joint Academy of Biotechnology & Medicine, Tianjin, China
- 3Molecular Cardiology Research Institute, Tufts Medical Center and Tufts University School of Medicine, Boston, MA, United States
Blood activation and stasis removal from circulation is a central principle for treatment of syndromes related to cerebral and cardiovascular diseases in Chinese herbal medicine. However, blood-activating and stasis-removing patent Chinese herbal medicine (BASR-pCHM) widely used with or without prescription in China and elsewhere are highly variable in composition and manufacture standard, making their safety assessment a challenging task. We proposed that an integrated evaluation of multiple toxicity parameters of BASR-pCHM would provide critical reference and guidelines for their safe clinical application. Examination of standardized extracts from 58 compound BASR-pCHM in vivo in VEGFR2-luc mice and in vitro in cardiac, renal, and hepatic cells identified Naoluotong capsule (NLTC) as a potent organ/cell damage inducer. Composition analysis revealed that NLTC was the one that contained nonherbal ingredients among the BASR-pCHM collection. In vivo and in vitro experiments confirmed that NLTC, as well as its chemical supplement tolperisone hydrochloride, caused organ and cell damage by reducing cell viability, mitochondrial mass/activity, while the NLTC herbal components did not. Taken together, our study showed that safety evaluation of patent herbal medicines already on market is still necessary and urgently needed. In addition, chemical/herbal interactions should be considered as an important contributor of potential toxicity when evaluating the safety of herbal medicine.
Introduction
Chinese herbal medicine (CHM) has been used in treating various diseases and maintaining health for Chinese people for more than 2,000 years (Hao et al., 2015). With both unique theories and rich experience, it is increasingly recognized worldwide (Pittler and Ernst, 2003; Fang and Xiong, 2019). Modernization of CHM boosted the manufacture and application of patent CHM (pCHM), which accounts up to one-third total drugs distributed and used on the market in China (Wang L. et al., 2015; Wang et al., 2017). Importantly, patients who used more types of pCHM tended to use much less Western medicine recommended by current guidelines. However, the safety concern of pCHM has constantly attracted attention from both health-care professionals and the public, as some pCHMs have been reported to have toxic side effects (Teschke and Eickhoff, 2015; Wang et al., 2018). Moreover, since CHM formula often consists of dozens of ingredients with innumerous chemical molecules, objective, and quantitative safety evaluation criteria for pCHM are difficult to establish (Teschke and Eickhoff, 2015).
Blood-activating and stasis-removing (BASR) CHMs are used mainly for treating cardiovascular diseases (Zhang, 2018). As an important class of CHM following the principle of synergy and toxin removal, many proprietary BASR pCHMs have been produced by standardized industrial procedures and are used for treating cardiovascular and cerebrovascular patients. The efficacy of the BASR pCHMs has been proven in both animal experiments and in the clinics. However, pCHMs belonging to the BASR category are vast, and the disease distinctions by individual members are vaguely defined. We have previously investigated pharmacological efficacy of BASR-pCHMs on the index of inhibiting platelet aggregation and dilatation of arterial tubes with the adult doses in vivo. Therefore, the collection and preparation process of our drugs are consistent with the previous pharmacodynamic experiments (Lu et al., 2015; Meng et al., 2016; Wang et al., 2017).
Adverse outcome pathways (AOPs) are a toxicological approach developed recently to connect mechanistic information to apical endpoints for regulatory purposes. It links a molecular initiating event to the adverse outcome via key events, in a way specified by key event relationships (Leist et al., 2017). The AOP approach gathers toxicity data using high-throughput cell- and biochemical-based tests to evaluate the combined data to predict potential toxic effects. Since the AOP concept is highly adaptable for CHM, we proposed that a systematic evaluation of multiple toxicity parameters of patent BASR-CHM would provide critical reference for their safe clinical application and briefly explore the material basis of typical adverse drug reactions.
Materials and Methods
Reagents and Drugs
Dulbecco’s modified Eagle’s medium (DMEM) and other cell culture supplies were purchased from Gibco (Grand Island, NY, USA). Hoechst 33342 was obtained from Invitrogen (Eugene, USA). Doxorubicin hydrochloride (Dox, batch number 20120205) was from Meilun Biotech Co. Ltd, (Dalian, China). Hematoxylin and eosin (H&E) staining kit (C0105) was purchased from Beyotime Biotechnology (Shanghai, China). ELISA kits for lactate dehydrogenase (LDH), aspartate aminotransferase (AST), creatine kinase-MB (CK-MB), and serum creatinine (Scr) were purchased from Biosino Bio-Technology and Science Inc. (Beijing, China). Tolperisone hydrochloride (batch number HY.B1139, purity >98% (Patel et al., 2011), was purchased from MedChem Express Corporation (New Jersey, USA). Methyl hesperidin (batch number 111580, purity >98%) was purchased from China Pharmaceutical and Biological Products Inspection Institute (Beijing, China). Vitamin B6 (batch number SV8110, purity >98%) was purchased from Beijing Solarbio Co., Ltd. (Beijing, China). KH2PO4, acetonitrile, methanol, and sodium heptane sulfonate (C7H15NaO3S) were purchased from Sigma (St Louis, Missouri, USA). Fifty-eight BASR-pCHMs, including Naoluotong capsule (NLTC), were purchased from certified local pharmacies and prepared as we described previously (Meng et al., 2016, detailed information in Supplemental Data Sheet 1 and 2).
Animals and Drug Administration
VEGFR2-Luc mice, in which a luciferase reporter (luc) is under the control of the murine VEGFR2 promoter (Zhang et al., 2004), were obtained by breeding transgenic colonies maintained under specific pathogen-free (SPF) conditions at Tianjin International Joint Academy of Biotechnology and Medicine (TJAB). Institute of Cancer Research (ICR) mice were purchased from Beijing Hua Fu Kang (HFK) Bioscience Co. Ltd (License number SCXK-[jun] 2014-001schke3) and maintained under SPF conditions at TJAB. All animal experiments were performed in accord with the international regulations, following the guidelines of Tianjin University of TCM Animal Research Committee (TCM-LAEC2014004) and approved by the animal care and use committee of TJAB (No. TJU20160021).
All BASR-pCHMs were weighed, dissolved in ultrapure water, sonicated for 30 min, and filtered through a 0.22-µm polyvinylidene fluoride filter into stock solutions at a concentration of 10 mg/ml (Meng et al., 2016). Tolperisone hydrochloride, methyl hesperidin, and vitamin B6 were freshly prepared in ddH2O at the time of use.
Animal Administration and Treatment
In order to evaluate whether the effects on the tissues at the clinical dose of commercial pCHMs that are the same as BASR function are consistent with each other, 58 BASR-pCHMs were administered VEGFR2-Luc mice by gavage for 7 days and compared weight/organ ratio and evaluated for histomorphology by H&E staining.
NLTC was tested in ICR mice. As the positive control group (Pugazhendhi A. 2018), 2.5 mg/kg/day dose of doxorubicin was injected for 10 days in a row (Yarana et al., 2018). The low-dose group of NLTC (20 g/7.8 mg/day) and high-dose group of NLTC (20 g/39 mg/day) were administered in mice by gavage for 21 days to evaluate whether the drug is toxic and potentially toxic to tissues compared with blank control. The heart rate was monitored using the Langendorff perfusion heart experiment (Langendorff ADI Australia).
Intraperitoneal injection (2.5 mg/kg) of the drug was given continuously for 10 days (Yarana et al., 2018). Blood sample was collected from the animals, and the heart rate was monitored using the Langendorff perfusion heart experiment (Langendorff ADI Australia). Weighing tissue from the internal organs of the mouse (heart, kidney, and liver) were fixed in 4% paraformaldehyde (PFA) solution for over 24 h and further prepared for paraffin sectioning. Tissue sections (3 µm thick) were cut and stained with H&E.
H&E Staining and Histopathological Scoring
The heart, liver, and kidney tissues were isolated from the mice and weighed and fixed in 4% PFA solution for well over 24 h. Thereafter, specimens were dehydrated through 70−100% ethanol alcohol and cleared in several changes of xylene and embedded in paraffin wax. Transverse serial sections were cut at 3 µm and mounted on glass slides. Sections were stained with H&E. Stained slides were scored according to the degree of organ damage following the reference system by “New Drug Toxicology Experimental Animal Histopathology Map” (Southeast University Press). Evaluation criteria are as shown in Supplemental Tables 3–5. Organ coefficient, a common toxicological indicator, was calculated as: organ coefficient = organ weight/body weight.
Blood Serum Samples Treatment
Serum was obtained from the peripheral blood of ICR mice by centrifugation at 3,000 rpm for 10 min. Level of ALT, AST, LDH, CK-MB, and Scr in the serum were determined by automatic biochemical detector (MK3; Thermo Fisher Scientific, Waltham, MA, USA). Based on the instructions, commercial ELISA kits (R&D Systems, Minneapolis, MN) were used to measure the concentration.
Cardiac, Renal, and Hepatic Cell Culture
H9c2 (cardiac cells), HEK293 (renal cells), and HepG2 (hepatic cells) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM containing 4.5 g/l glucose, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. All the cultured cells were maintained in a humidified incubator with 95% air and 5% CO2 at 37°C. The cell culture medium was replaced every 2–3 days, and the cells were subcultured or subjected to experimental procedures at 80–90% confluence.
Cell Viability Assay
Cells were cultured in 96-well plates for 24 h. The seed plate density of H9c2, HepG2, and HEK293 cells was 6 × 104/ml, 1 × 105/ml, and 1 × 105/ml, respectively, at 100 µl/well. They were incubated for 24 h at 37°C in a 5% CO2 incubator. In 96-well black transvaginal cell culture plates, HepG2 cells and HEK293 cells were each added with 50 µl of 0.025 mg/ml rat tail type I collagen per well overnight, and washed twice with sterile pure water.
The original medium was discarded in the culture plate, and the drug was added at a concentration as a dose standard for examining the effects of nine drugs on the viability of three cell lines, and was placed in a 37°C, 5% CO2 incubator and cultured for 24 h.
The cell parameters were measured by Hoechst 33342, which were obtained from Invitrogen (Eugene, USA). After 24-h incubation, 3 μmol/l Hoechst 33342 was mixed with DMEM/high glucose and then incubated with the cells for 30 min in the dark. The assay plate was imaged and analyzed using the Operetta HCA system (Perkin Elmer, MA, USA) at 25°C with a relative humidity of 45%. Using a 20× objective, fluorescent images of nine fields per well in Hoechst 33342 were measured and calculated by the mean values.
HPLC Analysis
Liquid chromatography was performed on the filtrate using a SunFire-C18 column (4.6 × 250 mm, 5 µm). An isocratic elution program was conducted for chromatographic separation, with the mobile phase A (0.05% KH2PO4) and mobile phase B (acetonitrile), which was employed using a mixture of 0.05% KH2PO4 and acetonitrile (75:25, v/v) at a flowrate of 1.0 ml/min (Li et al., 2010). The column temperature was maintained at 30°C, the detection wavelength was 261 nm, and the injection volume was 10 µl for the detection of tolperisone hydrochloride. Under the same conditions, the detection wavelength was changed to 283 nm, and 10 µl was injected for the detection of methyl hesperidin monomer. Liquid chromatography with a UV detector was fixed at a wavelength of 290 nm (Chi and Yin, 2008). Isocratic elution was employed using a mixture of methanol and 0.06% sodium heptane sulfonate (C7H15NaO3S) aqueous solution (13:87, v/v) at a flowrate of 1.0 ml/min, with injection volume of 20 µl for the vitamin B6 analysis.
Statistical Analysis
All values are expressed as the mean ± SD. Comparisons between multiple-group means were performed using one-way analysis of variance (one-way ANOVA). Multiple comparisons between the groups were performed using least significant difference method. P values < 0.05 were considered to be statistically significant. All graphs are drawn by GraphPad Software (GraphPad Software, Inc. CA, USA).
Results
In Vivo Comparative Toxicology Evaluation of BASR-pCHMs
In order to establish a safety pharmacology standard for BASR-pCHMs, we first collected all 58 pCHMs with BASR function and evaluated them in vivo. Each of the 58 BASR-pCHMs were given daily by intragastric administration to VEGFR2-Luc mice for 7 days, and duplicate mice were used for each drug. Body and organ weights of the VEGFR-2-Luc mice in each group were measured. As shown in Figure 1, the ratios of body weight to heart (Figure 1A), kidney (Figure 1B), and liver (Figure 1C) in most animals treated with BASR-pCHMs were normal. However, the body/heart ratio of animals treated with drugs 5, 10, and 12 were significantly lower. On the other hand, the body/heart ratio of animals treated with drugs 27, 52, and 56 were much higher (Figure 1A). In addition, the body/kidney ratio of animals treated with drugs 10 and 18 were significantly lower and that of 23 was higher. Finally, the body/liver ratio of animals treated with drugs 3 and 10 were lower and those of drugs 4, 23, 27, and 53 were higher. H&E tissue stain of BASR-pCHM-treated animals was examined for possible pathological changes. As shown in Figures 1D–F, according to a pathological score criteria of 0–3 grades (detailed information in Supplemental Tables 3–5), most of the heart (Figure 1D), kidney (Figure 1E), and liver (Figure 1F) tissue stains were normal, except those that were treated with drugs 3 and 4 in kidney. Therefore, a combination of the body/organ ratio and H&E score results identified that treatment by drugs 3, 4, 5, 10, 12, 15, 18, 23, and 27 may cause potential pathological abnormality.
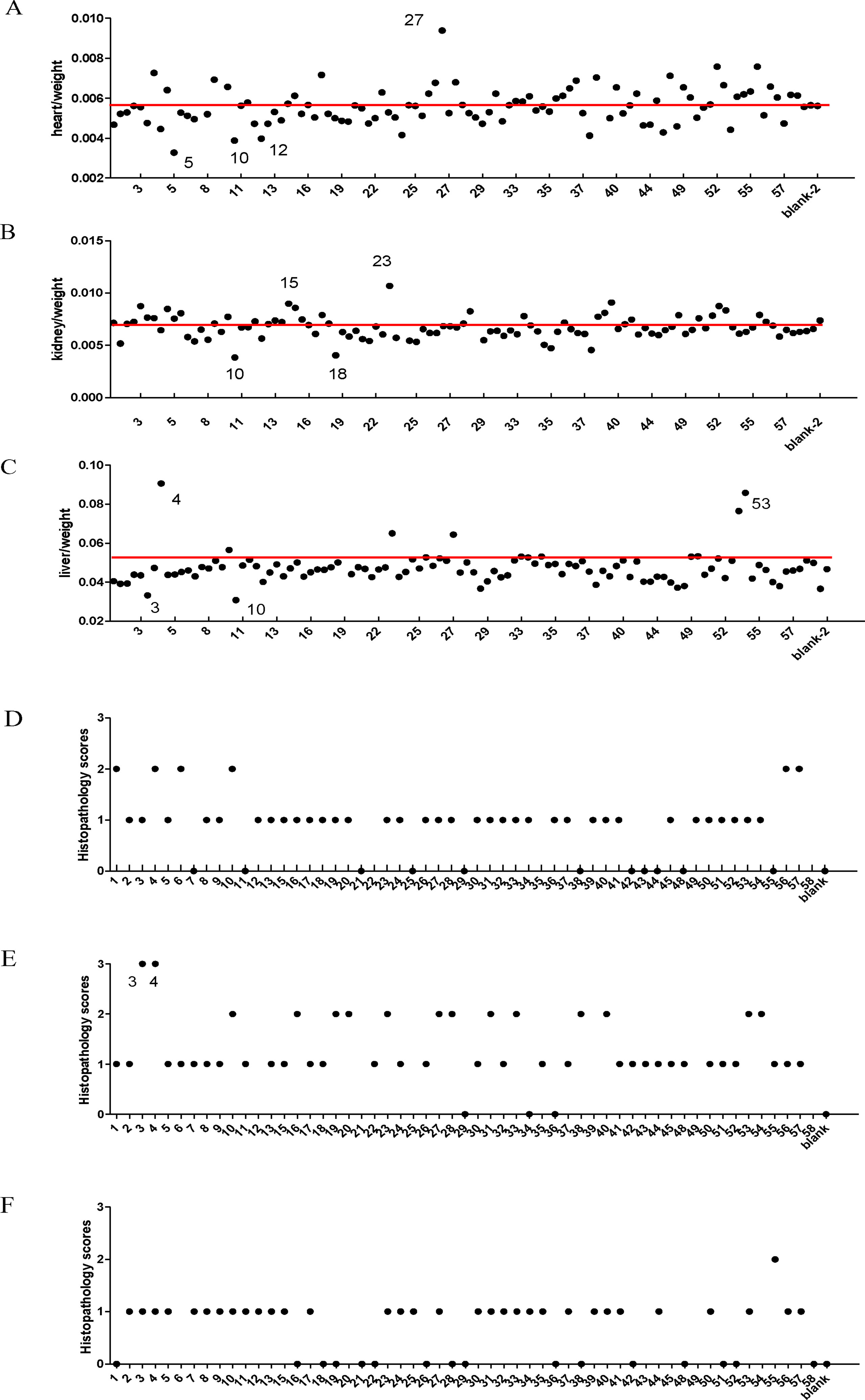
Figure 1 Comparison of organ coefficient and histology scores of BASR-pCHMs-administered mice. (A–C) Organ/body weight ratio. Redline is the average level. (A) Heart/body weight ratio. (B) The kidney/body weight ratio. (C) The liver/body weight ratio. (D) Heart H&E pathology scores. (E) Kidney H&E pathology scores. (F) Liver H&E pathology scores.
In Vitro Toxicity Evaluation in Cardiac, Renal, and Hepatic Cells of the BASR-pCHMs With Organ Damage Potential
Based on the primary evaluation of the in vivo toxicity, drugs 3, 4, 5, 10, 12, 15, 18, 23, and 27 were selected for further investigation in in vitro cell models. H9c2, HepG2, and HEK293 cells were chosen to examine the effect of a given drug at a concentration of 1 mg/ml (a concentration that was five times of the maximum effective dose of a compound Chinese medicine in previous in vitro study in our laboratory) on cell viability. As shown in Figure 2, nuclear numbers of H9c2 (Figure 2A), HEK293 (Figure 2B), and HepG2 (Figure 2C) changed in various fashions after treatment by these nine drugs, but only drug 4 (Naoluotong capsule, NLTC) showed a most dramatic decrease in all of the cardiac, renal, and hepatic cells.
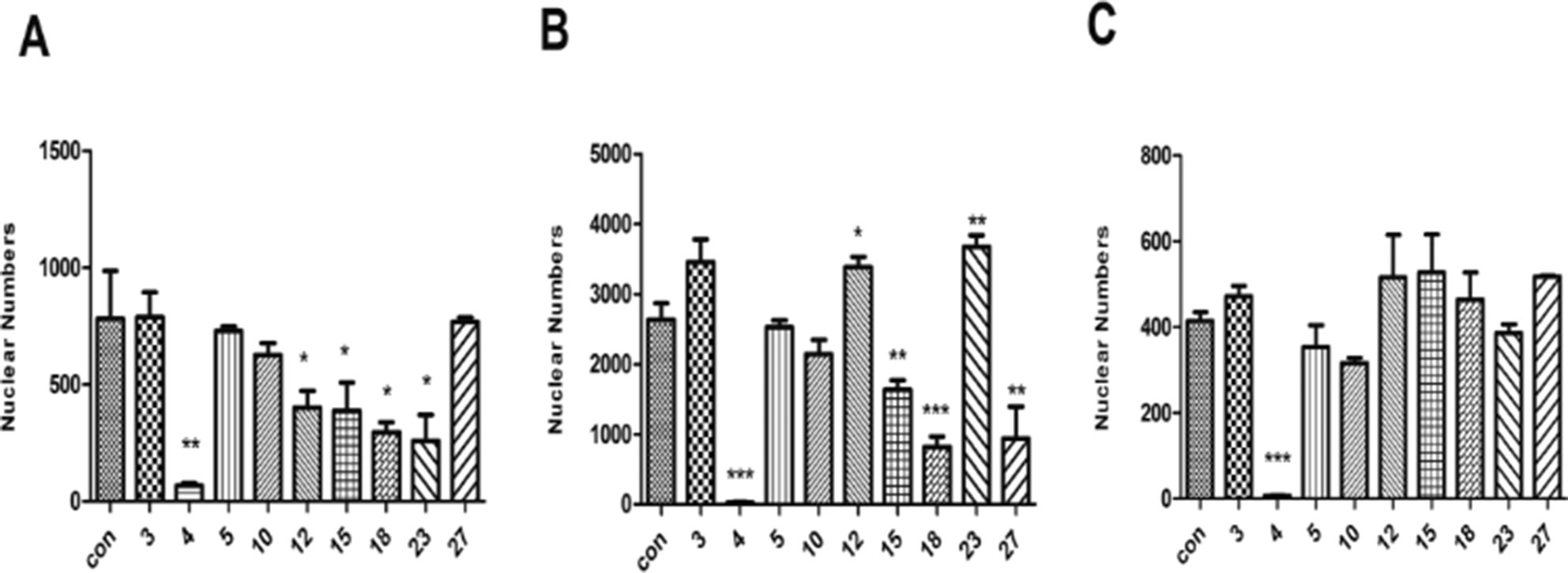
Figure 2 Effects of the nine BASR-pCHMs on the viability of cardiac, renal, and hepatic cells. Hochest-stained cell nuclei were counted after 24 h treatment with the nine BASR-pCHM selected by in vivo toxicity screen. (A) H9c2 cells. (B) HEK293 cells. (C) HepG2 cells. Numbers on the X-axis are the BASR-pCHM code numbers. Data are expressed as mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control.
Composition Analysis of NLTC
To trace the possible toxic components, composition of the 58 BASR-pCHMs were examined according to the Chinese Pharmacopoeia (10th edition) and the manufacture’s documentations. NTLC was found to be only one of the two BASR-pCHMs that contained chemical synthetic drugs in addition to herbal components. NLTC consists of three CHMs (Salvia miltiorrhiza, Ligustrum chuanxiong, and Astragalus membranaceus) and three chemical synthetic drugs [tolperisone hydrochloride (Figure 3E), methyl hesperidin (Figure 3F), and vitamin B6 (Figure 3G, detailed in Supplemental Table 6)]. To determine the contribution of chemical synthetic drugs in NLTC formula, their aqueous extracts were quantified using high-performance liquid chromatography (HPLC) as shown in Figure 3. Mixed standards of methyl hesperidin and tolperisone hydrochloride were detected at 283 nm (top) and 261 nm (bottom), respectively (Figure 3A), and the presence of tolperisone hydrochloride and methyl hesperidin in NLTC was qualified in Figures 3B, C, respectively. As shown in Figure 3D, vitamin B6 standard (top) and its presence in NLTC (bottom) were detected at 290 nm. The quantitative results showed that NLTC contained 8.59% per mg/ml tolperisone hydrochloride, 1.20% per mg/ml methyl-hesperidin, and 0.38% per mg/ml vitamin B6, respectively. In addition, the methanol extracts of the chemical synthetic drugs in NLTC were also quantified, and the results were consistent with those of aqueous extracts (Supplemental Figure 1).
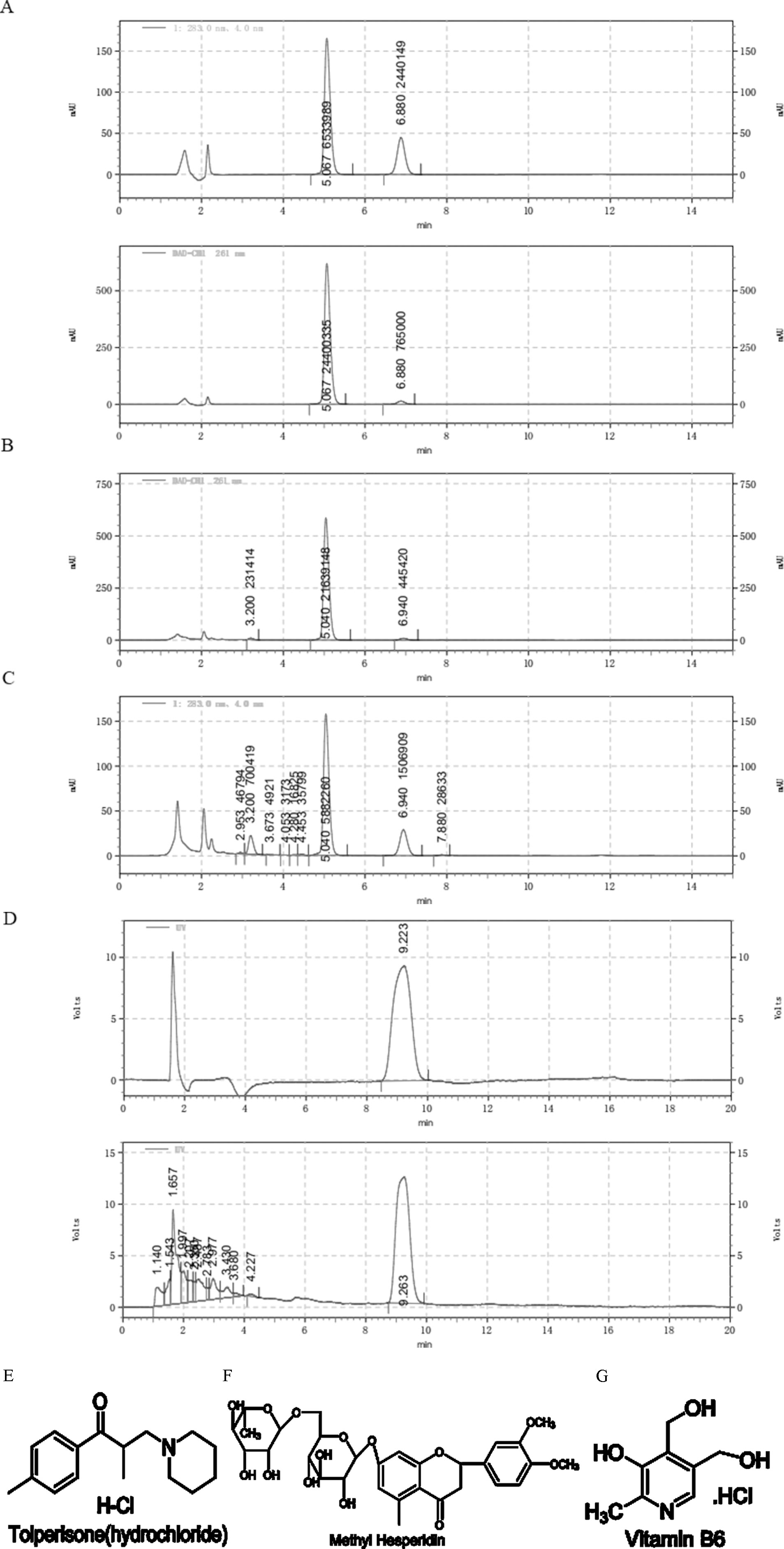
Figure 3 Composition analysis of NLTC. (A) Aqueous solution of mixed standards (methyl hesperidin and tolperisone hydrochloride) was detected at 283 nm (upper panel) and 261 nm (lower panel), respectively. (B) Aqueous solution of NLTC was detected at wavelength of 261 nm. (C) Aqueous solution of NLTC was detected at wavelength of 283 nm. (D) Vitamin B6 standard (upper panel) and aqueous solution of NLTC (lower panel) were detected at wavelength of 290 nm. (E–G) Structures of the chemical synthetic drug components in NLTC. (E) Tolperisone hydrochloride. (F) Methyl hesperidin. (G) Vitamin B6.
Toxicity Effects on Cardiac, Renal, and Hepatic Cells of NLTC Produced by Different Manufacturers
To rule out the possibility that the toxic effect by NLTC might have been caused by the preparation process, 10 batches (all at doses of 1 mg/ml) of NLTC from different manufacturers were analyzed by cellular toxicity assay. As shown in Figure 4, NLTCs from different manufacturers all showed a significant decrease in nuclear numbers of cardiac (Figure 4A), renal (Figure 4B), and hepatic (Figure 4C) cells, supporting that it was the composition, not the production technique, of NLTC that caused cell toxicity.
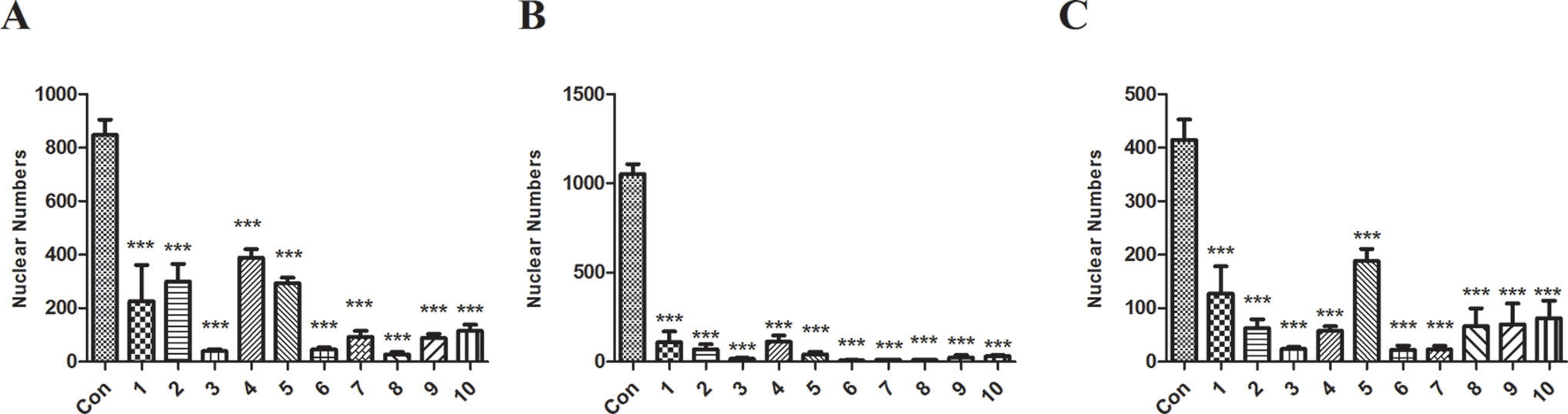
Figure 4 Effects of NLTC produced by different manufacturers on the viability of cardiac, renal, and hepatic cells. Ten different manufacturers of NLTC were represented in the X-axis. Con: vehicle control. (A) H9c2 cells. (B) HEK293 cells. (C) HepG2 cells. All drugs were used at a concentration of 1 mg/ml. Data are expressed as mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control.
In Vivo Verification of NLTC Cellular Toxicity
As shown in Table 1, high doses of NLTC and positive control drug Adriamycin significantly elevated serum levels of cardiac function indicators CK-MB and LDH as well as liver function indicators AST and ALT compared with that of the vehicle control, while low dose of NLTC only elevated CK-MB and LDH levels but not AST and ALT levels. In addition, all the drugs significantly reduced the renal function indicator Scr.
As shown in Figure 5, the morphology of H&E-stained heart (Figure 5A), liver (Figure 5B), and kidney (Figure 5C) indicated typical pathological changes. Quantitation of the H&E stain showed that NLTC significantly increased pathological scores in the heart (Figure 5D), liver (Figure 5E), and kidney (Figure 5F) compared with the control.
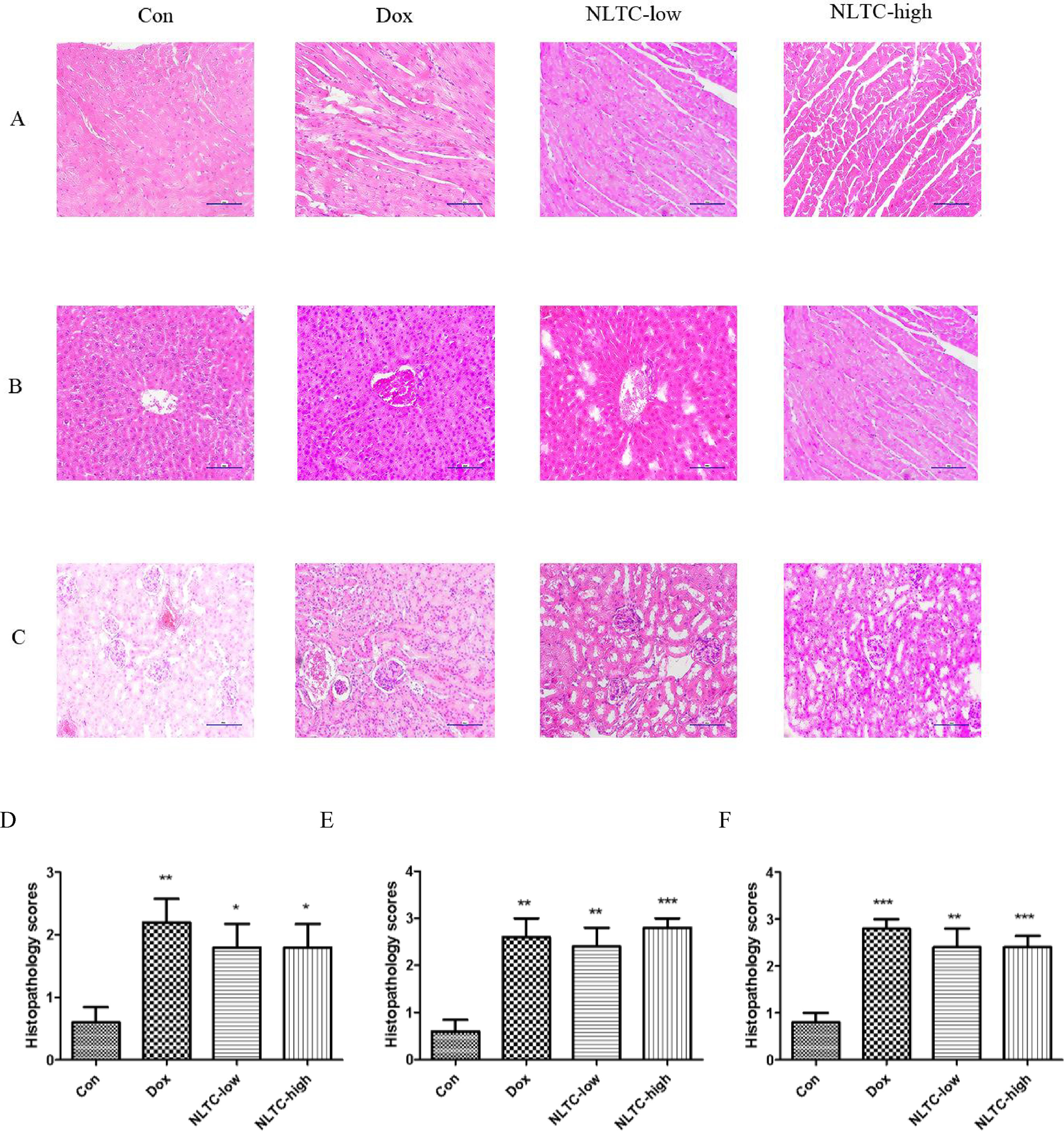
Figure 5 Histopathology of NLTC-treated animals experimental scores. (A–C) Representative images of H&E-stained tissues. (A) cardiac tissue. (B) hepatic tissue. (C) Renal tissue. All images were under 100× microscope. Order of panels from left to right: Con (negative control), Dox (positive control), NLTC-low, and NLTC-high. (D–F) Quantitation of the H&E histopathology scores. (D) cardiac tissue. (E) Hepatic tissue. (F) Renal tissue. Data are expressed as mean ± SD, n = 5. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control.
Toxicity Mechanisms of NLTC and Its Chemical Synthetic Drug Combination
In order to further clarify the contribution of the chemical synthetic drug combination to the cytotoxicity of total NLTC and their toxicity mechanisms, the three chemical synthetic drugs (tolperisone hydrochloride, methyl hesperidin, and vitamin B6, TMV) were formulated according to the same proportion of their presence in NLTC. In a cell-based multiparameter toxicity assay, aqueous extract of NLTC and its chemical synthetic drug combination TMV were examined as shown in Figure 6. Mitochondrial mass (Figures 6A, D, G), calcium ion concentration (Figures 6B, E, H), and mitochondrial membrane potential (Figures 6C, F, I) were determined in HepG2 (Figures 6A–C), HEK293 (Figures 6D–F), and H9c2 (Figures 6G–I) cells. Similar to the positive control drug DOX, both NLTC and TMV affected mitochondrial mass, calcium ion concentration, and mitochondrial membrane potential in the same fashion in most cells, except for the renal HEK293 cells where mitochondrial functional parameters were more severely affected by TMV than NLTC (Figures 6D, F).
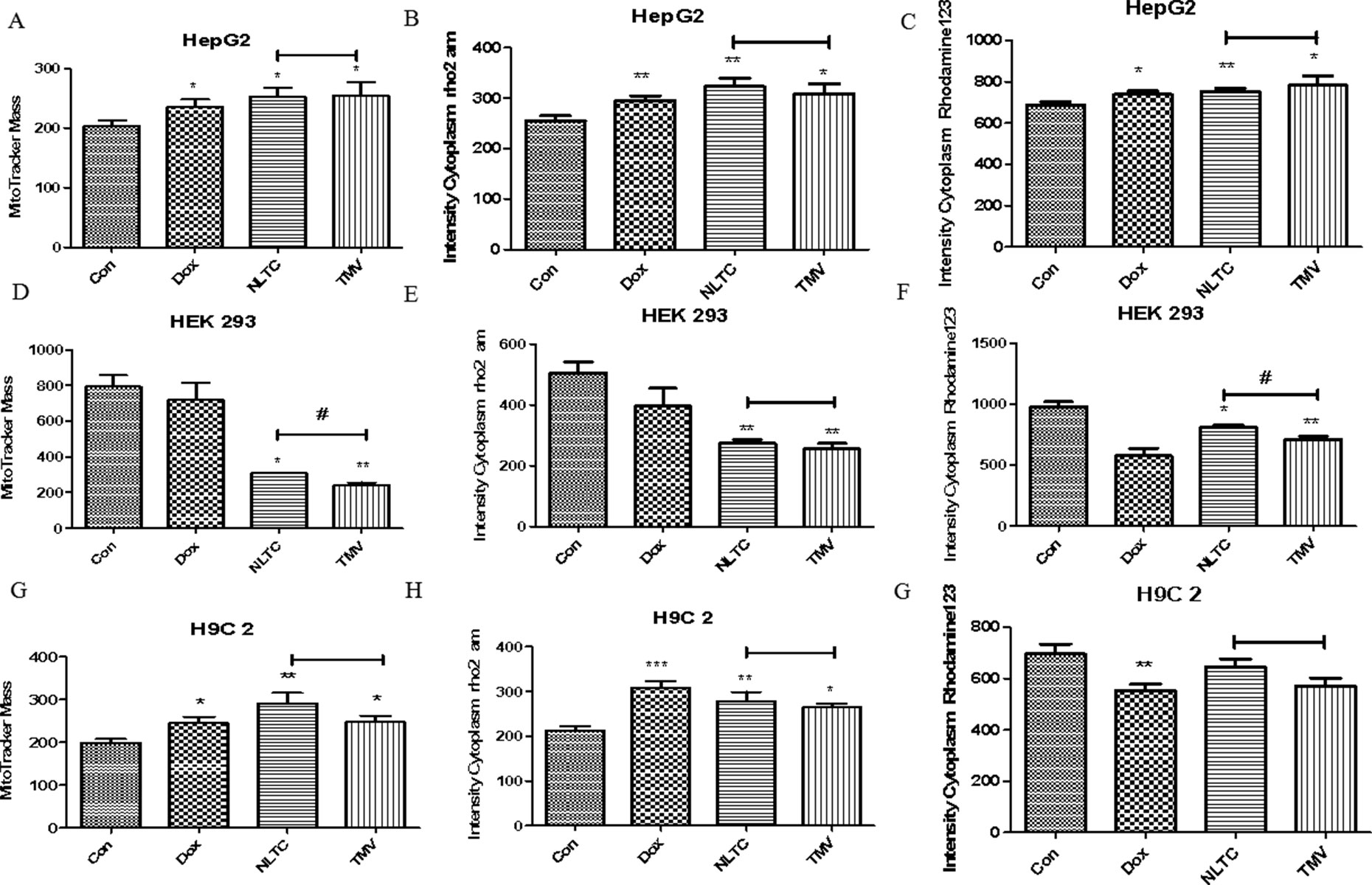
Figure 6 Toxicity mechanisms of NLTC and its chemical synthetic drug combination. Multiparameter cell-based imaging analysis was performed after 24 h drug treatment. Mitochondrial mass, intracellular calcium, and mitochondrial membrane potential were determined in cardiac, renal, and hepatic cells. (A, D, G) Mitochondrial mass. (B, E, H) Intracellular calcium. (C, F, I) Mitochondrial membrane potential. (A–C) HepG2 cells. (D–F) HEK293 cells. (G–I) H9c2 cells. Con: control, Dox: positive control, NLTC (NLTC aqueous extract), TMV: mixtures of tolperisone hydrochloride, methyl hesperidin, and vitamin B6. *P < 0.05, **P < 0.01 compared to the control. #P < 0.05 NLTC compared to TMV.
Attribution of Individual Chemical Synthetic Drugs From NLTC on Cellular Toxicity
Cellular toxicity by tolperisone hydrochloride, methyl-hesperidin, and vitamin B6, the three chemical synthetic drugs in NLTC, was analyzed in cardiac, hepatocyte, and renal cell lines. As shown in Figure 7, compared with that of NLTC (Figure 7A), which had IC50s on H9c2 cell, HepG2, and HEK293 cells of 0.2941, 0.02541, and 0.4985 mg/ml, tolperisone hydrochloride (Figure 7B) significantly caused a lethal effect on H9c2 cell, HepG2, and HEK293 cells with IC50 of 0.004038, 0.0118, and 0.04535 mg/ml, respectively. Methyl-hesperidin (Figure 7C) also significantly caused a lethal effect on H9c2 cell, HepG2, and HEK293 cells with IC50 of 0.001745, 0.002395, and 0.04830 mg/ml, respectively. However, vitamin B6 (Figure 7D) had no effect on these cells. Therefore, the cellular toxicity of NLTC could be attributed mostly to tolperisone hydrochloride, but to a less extent, methyl-hesperidin.
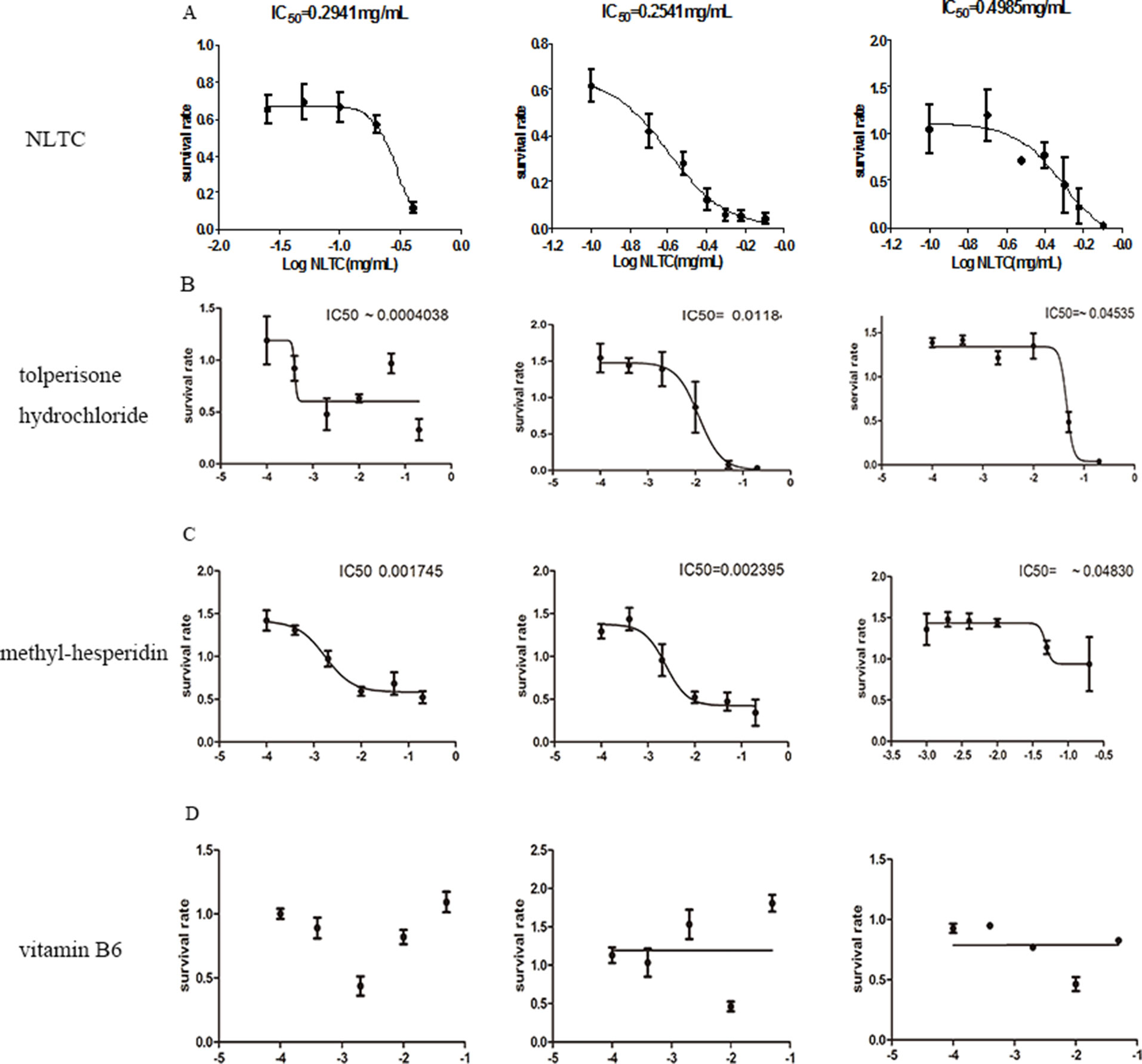
Figure 7 Attribution of individual chemical synthetic drugs from NLTC on cellular toxicity. Survival rate of cells after 24 h treatment by NLTC (A), tolperisone hydrochloride (B), methyl hesperidin (C), and vitamin B6 (D). Left panels: H9c2; middle panels: HepG2; right panels: HEK293. Values of IC50, where available, are indicated in the panel. Data are expressed as mean ± SEM, n = 9.
Discussion
Safety reevaluation of patent Chinese herbal medicine already on market is challenging due to their complex chemical composition and ambiguous clinical indications. To adapt the adverse outcome pathway (AOP) approach for pCHM, 58 BASR-pCHMs were evaluated in vivo and in vitro screening. New discoveries in this study include 1) untargeted screen revealed that the one BASR-pCHM (NLTC) that may cause organ damage is in fact a mixture of CHM and chemical synthetic drug components; 2) NLTC’s multi cellular toxicity is conformed in vivo in a mouse model; and 3) the NLTC chemical synthetic drugs alone, particularly tolperisone hydrochloride, are the cause of cytotoxicity.
Blood activating and stasis removing (BASR) is the main principle in traditional Chinese medicine for treatment of cardiovascular diseases. Previous studies have reported that BASR-CHMs promote angiogenesis by upregulating vascular endothelial growth factor (VEGF) gene in the ischemic model (Xin et al., 2018; Zheng et al., 2018; Shi et al., 2019) and inhibit angiogenesis mainly by downregulating VEGF and decreasing MMP9 expression in tumor tissues (Zeng et al., 2018). There are also reports in the literature of the side effect caused by BASRs in abnormally promoting normal blood vessel growth (Su et al., 2014). We used VEGFR2-luc reporter mice to explore the effect of BASR-pCHMs on angiogenesis in vivo and possible correlation of tissue-specific VEGF gene expression and organ response. Bioluminescence results of luciferase activity showed occasional VEGF elevation induced by certain BASR-pCHM, but it was not related to the toxicity effects reported in this work (data not shown).
According to AOP approach, we integrated animal, cellular, and molecular parameters for the BASR-pCHMs evaluation. The animal level screen of organ toxicity was informative but did not reach statistical significance. Since the organ weight changes may or may not be directly affected by BASR-pCHMs, we considered histopathology score as additional evaluation parameter.
Although NLTC has been used as a cerebral vascular disease treatment medicine in China, its safety has not been systematically examined except occasional reports of adverse reactions such as allergies (Liu et al., 2004; Kang et al., 2017; Bai et al., 2019). Our experimental study suggested that NLTC could potentially cause multiorgan toxicity.
The results of H&E stain in NLTC animal experiments are inconsistent with serum index results in our study. H&E stain was used to evaluate the changes in tissues from mice with different treatments. The results showed that the volume of the myocardium in the doxorubicin (Dox) group was smaller than that in the control group and the CK-MB and LDH levels increased by Dox. This is consistent with the literature report (Deng et al., 2018). In Dox-induced nephrotoxicity animal experiment, renal sections from Dox-treated rats showed intensive desquamation in tubular epithelial cells, single-cell necrosis, tubular atrophy, tubular necrosis, and glomerular necrosis (Altinkaynak et al., 2018). According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, acute kidney injury is defined as an increase in the serum creatinine level of 0.3 mg/dl or greater within 48 h or an increase in serum creatinine levels to 1.5 or greater times the baseline (Joyce et al., 2017). However, the level of renal function parameters of NLTC-treated animals was significantly lower compared to that of control in our results. The results show that the drug damage to the kidney was nonacute injury, but the specific type is affected by many factors and needs further analysis (Takeda et al., 2018). Dox-induced hepatotoxicity significantly increases the levels of ALT and AST in serum (Wang et al., 2014). In our result, serum ALT and AST levels were normal in the low dose of NLTC-treated animals. However, levels of ALT and AST in serum were significantly increased in the high dose of NLTC-treated animals. Therefore, our data suggest that the drug dose is a critical factor for the safe clinical application of NLTC, and potential organ damage depends on the sensitivity of different organs (i.e., kidney vs. liver). However, our experimental data should be interpreted with caution, and a detailed clinical investigation is needed to confirm if NLTC indeed may cause multiorgan toxicity in patients.
The toxicity of toperidone hydrochloride, methyl hesperidin, and vitamin B6 has not been reported before. The side effects of tolperisone hydrochloride reported at present is not clear (Ribi et al., 2003; Sporkert et al., 2012; Martos et al., 2015). In animal studies, methyl hesperidin has not been found to have toxic or side effects before (Kawabe et al., 1993). As for vitamin B6, previous reports have shown that its combination with other drugs may reduce the toxicity or side effects of the later (Wang L.Z. et al., 2015). However, our work suggested that tolperisone hydrochloride may cause cellular toxicity in vitro, although this effect has yet to be confirmed in vivo.
Dox is a well-established anticancer drug, but it broad-spectrum cytotoxicity has also been reported (Nishiyama et al., 2019). Using Dox as a cellular toxicity positive control, we determined each of the chemical synthetic drugs in NLTC for the mechanistic insights, such as mitochondrial mass, mitochondrial membrane potential, and intracellular calcium. The results showed that the administration of the combined chemical synthetic drugs or NLTC aqueous extract exhibited similar effects as Dox in cardiac, renal, and hepatic cells, suggesting that these drugs may share the same toxicity mechanism(s). Our results showed that in cardiomyocytes and hepatocytes, the toxicity levels of the combined chemical synthetic drugs and NLTC aqueous extract were similar, whereas in the renal cells, the toxicity level of the combined chemical synthetic drugs was more significant than that of NLTC. It may suggest that kidney is a more sensitive organ for chemical toxicity than the heart and liver. Alternatively, it is possible that the presence of the herbal components decreased the chemical toxicity in kidney by drug–drug interaction.
One of the limitations of our work is that the chemical synthetic drug components in NLTC were verified only at cellular level, and no in vivo animal experiments were carried out. In addition, we have not ruled out the possibility of drug–drug interaction between the components of chemical synthetic drugs and herbs. In addition, although our cell-based multiparameter assays revealed preliminary mechanisms, further in-depth investigation is required to fully understand if and how NLTC might lead to multiorgan toxicity in clinics.
For the first time, this study evaluated the safety of a series of commercially available pCHMs, from the levels of the whole animal, selected tissues, and targeted cells. Compared with conventional toxicity studies that focus a single CHM formula or a monomer, our approach offers a systematic evaluation which allows an unbiased comparison of multiple drugs.
In conclusion, our study for the first time systematically evaluated a series of patent herbal medicines using an integrated approach and identified the presence of chemical synthetic drug in an herbal medicine as a potential cause of organ toxicity.
Ethics Statement
All animal experiments were performed in accord with the international regulations, following the guidelines of Tianjin University of TCM Animal Research Committee (TCM-LAEC2014004) and approved by the animal care and use committee of TJAB (No. TJU20160021).
Author Contributions
YZ conceived the project and designed the study. XL performed the experiments, contributed to acquisition, analysis, and interpretation of the data. RS supervised the experiment and revised the manuscript. XY, GX, and YF contributed to study design and interpretation of results. XL, RS, and YZ wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
This study was supported by the grants from National Science Foundation of China (81274128, 81873037), Major National Science and Technology Projects (2018YFC1704500), and Innovation Team of Tianjin High Education Commission (TD13-5046).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the members of our laboratory, particularly Drs. Jian Yang and Pengzhi Dong, for the stimulating discussions and sharing reagents and ideas, and Dr. John Orgah for editorial assistance.
Abbreviations
BASR-pCHM, blood-activating and stasis-removing patent Chinese herbal medicine; NLTC, Naoluotong capsule; CHM, Chinese herbal medicine; pCHM, patent Chinese herbal medicine; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; CK-MB, creatine kinase-MB; Scr, serum creatinine; H&E, hematoxylin and eosin.
Supplementary Materials
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00993/full#supplementary-material
References
Altınkaynak, Y., Kural, B., Akcan, B. A., Bodur, A., Özer, S., Yuluğ, E., et al. (2018). Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed. Pharmacother. 108, 1524–1534. doi: 10.1016/j.biopha.2018.09.171
Bai, M., Liu, B., Peng, M., Jia, J., Fang, X., Miao, M. (2019). Effect of sargentodoxa cuneata total phenolic acids on focal cerebral ischemia reperfusion injury rats model. Saudi J. Biol. Sci. 26 (3), 569–576. doi: 10.1016/j.sjbs.2018.11.019
Chi, F. Z., Yin, A. Q. (2008). Determination of Vitamin B6 in naoluotong capsules by HPLC. Drug Stand. China 22 (9), 60–61.
Deng, G., Chen, C., Zhang, J., Zhai, Y., Zhao, J., Ji, A., et al. (2018). Se@SiO2 nanocomposites attenuate doxorubicin-induced cardiotoxicity through combatting oxidative damage. Artif. Cells Nanomed. Biotechnol, 1–10. 46 (sup2), 112–121. doi: 10.1080/21691401.2018.1452250
Fang, N. L., Xiong, Y. (2019). Chinese medicine gains WHO acceptance but it has many critics. CNN’s report. https://edition.cnn.com/2019/05/24/health/traditional-chinese-medicine-who-controversy-intl/index.html.
Hao, P. P., Jiang, F., Chen, Y. G., Yang, J., Zhang, K., Zhang, M. X., et al. (2015). Traditional Chinese medication for cardiovascular disease. Nat. Rev. Cardiol. 12 (6), 318. doi: 10.1038/nrcardio.2014.177
Joyce, E. L., Kane-Gill, S. L., Fuhrman, D. Y., Kellum, J. A. (2017). Drug-associated acute kidney injury: who’s at risk? Pediatr. Nephrol. 32 (1), 59–69. doi: 10.1007/s00467-016-3446-x
Kang, L., Miao, M., Bai, M., Tian, S. (2017). Effect of total flavonoid in rabdosia rubescens on tolerant mice models under cerebral anoxia. Saudi J. Biol. Sci. 24 (8), 1798–1802. doi: 10.1016/j.sjbs.2017.11.015
Kawabe, M., Tamano, S., Shibata, M. A., Hirose, M., Fukushima, S., Ito, N. (1993). Subchronic toxicity study of methyl hesperidin in mice. Toxicol. Lett. 69 (1), 37–44. doi: 10.1016/0378-4274(93)90143-L
Leist, M., Ghallab, A., Graepel, R., Marchan, R., Hassan, R., Bennekou, S. H., et al. (2017). Adverse outcome pathways: opportunities, limitations and open questions. Arch. Toxicol. 91 (11), 3477–3505. doi: 10.1007/s00204-017-2045-3
Li, J. M., Long, Y., Huang, S. R. (2010). The contents of topperidone hydrochloride and methyl hesperidin in naolotong capsules were also determined by HPLC. Chin. J. Pharm. 45 (01), 60–63.
Liu, H., Wu, X., Wu, G. (2004). Clinical study of zhuyutongmai capsule on treating senile cerebral infarction and effect on function of vascular endothelium. Zhong Yao Cai 27 (11), 882–884.
Lu, M., Wang, T. Y., Tian, X. X., Shi, X. H., Fan, G. W., Zhang, Y., et al. (2015). Interaction of anti-thrombotic and anti-inflammatory activities of commonly used traditional Chinese medicine for promoting blood circulation and removing blood stasis revealed by network pharmacology analysis. Acta Pharm. Sin. 50 (9), 1135–1141.
Martos, V., Hofer, K. E., Rauber-Lüthy, C., Schenk-Jaeger, K. M., Kupferschmidt, H., Ceschi, A. (2015). Acute toxicity profile of tolperisone in overdose: observational poison centre-based study. Clin. Toxicol. (Phila) 53 (5), 470–476. doi: 10.3109/15563650.2015.1022896
Meng, Y. Z., Wang, Z. L., Su, J. C., Cheng, L. Y., LV, M., Zhu, Y. (2016). Evaluation of anti-platelet aggregation activity and compatibility patterns of 58 kinds of compound traditional Chinese medicine for promoting blood circulation and removing blood stasis. Chin. J. Exp. Med. 24, 22. doi: 10.13422/j.cnki.syfjx.2016240187
Nishiyama, K., Numaga-Tomita, T., Fujimoto, Y., Tanaka, T., Toyama, C., Nishimura, A., et al. (2019). Ibudilast attenuates doxorubicin-induced cytotoxicity by suppressing formation of TRPC3-Nox2 protein complex. Br. J. Pharmacol. doi: 10.1111/bph.14777
Patel, M. J., Badmanaban, R., Patel, C. N. (2011). Reversed phase-high performance liquid chromatographic method for simultaneous estimation of tolperisone hydrochloride and etodolac in a combined fixed dose oral formulations Pharmaceutical Methods. Pharm. Methods 2 (2), 124–129. doi: 10.4103/2229-4708.84458
Pittler, M. H., Ernst, E. (2003). Systematic review: hepatotoxic events associated with herbal medicinal products. Aliment. Pharmacol. Ther. 18 (5), 451–471. doi: 10.1046/j.1365-2036.2003.01689.x
Pugazhendhi, A., Edison, T. N. J. I., Velmurugan, B. K., Jacob, J. A., Karuppusamy, I. (2018). Toxicity of Doxorubicin(Dox) to different experimental organ systems. LifeSci.
Ribi, C., Vermeulen, C., Hauser, C. (2003). Anaphylactic reactions to tolperisone (Mydocalm). Swiss Med. Wkly. 133 (25–26), 369–371.
Ruffin, T. (2018). Pharmacologic renal therapy. Nurs. Clin. North Am. 53 (4), 491–497. doi: 10.1016/j.cnur.2018.07.009
Shi, W.-L., Zhao, J., Yuan, R., Lu, Y., Xin, Q.-Q., Liu, Y., et al. (2019). Combination of ligusticum chuanxiong and radix paeonia promotes angiogenesis in ischemic myocardium through notch signalling and mobilization of stem cells. Evid. Based Complement. Altern. Med. 2019, 1–12. doi: 10.1155/2019/7912402
Sporkert, F., Brunel, C., Augsburger, M. P., Mangin, P. (2012). Fatal tolperisone poisoning: autopsy and toxicology findings in three suicide cases. Forensic Sci. Int. 215 (1–3), 101–104. doi: 10.1016/j.forsciint.2011.05.025
Su, X. Y., Song, J., Gao, D. (2014). Comparative study of vascular endothelial growth factor and Xuefu Zhuyu decoction promoting angiogenesis. Chin. J. Med Tradit. Chin. Med. 20 (08), 1128–1130.
Takeda, M., Miyagawa, S., Fukushima, S., Saito, A., Ito, E., Harada, A., et al. Development of in vitro drug-induced cardiotoxicity assay by using three-dimensional cardiac tissues derived from human induced pluripotent stem cells. Tissue Eng. Part C Methods 24 (1), 56–67.
Teschke, R., Eickhoff, A. (2015). Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front. Pharmacol. 6. doi: 10.3389/fphar.2015.00072
Wang, B., Ma, Y., Kong, X., Ding, X., Gu, H., Chu, T., et al. (2014). NAD(+) administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem. Biol. Interact. 212, 65–71. doi: 10.1016/j.cbi.2014.01.013
Wang, L. Z., Zhang, H. J., Song, J. (2015). Efficacy of mannatide combined with sodium cantharidate Vitamin B6 in the treatment of malignant pleural effusions. Asian Pac. J. Cancer Prev. 16 (9), 3913–3916. doi: 10.7314/APJCP.2015.16.9.3913
Wang, L., Kong, W., Yang, M., Han, J., Chen, S. (2015). Safety issues and new rapid detection methods in traditional Chinese medicinal materials. Acta Pharm. Sin. B. 5 (1), 38–46. doi: 10.1016/j.apsb.2014.12.005
Wang, X. Y., Li, S. N., Zhu, H. F., Hu, Z. Y., Zhong, Y., Gu, C. S., et al. (2017). RGC32 induces epithelial-mesenchymal transition by activating the Smad/Sip1 signaling pathway in CRC. Sci. Rep. 7, 46078. doi: 10.1038/srep46078
Wang, Y., Fan, L. P., Song, J., Cai, Y.-P., Jinang, T.-T., Wang, Y.-G., et al. (2018). Retrospective analysis and discussion on 74 cases of adverse reactions of traditional Chinese medicine injection. Zhongguo Zhong Yao Za Zhi China journal of Chinese materia medica, 43 (21) 4347–4351.
Xin, M., He, J., Yang, W., Yin, X., Wang, J. (2018). Wenshen Yangxue decoction improves endometrial receptivity recovery and promotes endometrial angiogenesis in a rat model. Pharm. Biol. 56 (1), 573–579. doi: 10.1080/13880209.2018.1510973
Yarana, C., Carroll, D., Chen, J., Chaiswing, L., Zhao, Y., Noel, T., et al. (2018). Extracellular vesicles released by cardiomyocytes in a doxorubicin-induced cardiac injury mouse model contain protein biomarkers of early cardiac injury. Clin. Cancer Res. 24 (7), 1644–1653. doi: 10.1158/1078-0432.CCR-17-2046
Zeng, J., Yan, R., Pan, H, You, F., Cai, T., Liu, W., et al. (2018). Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complement. Altern. Med. 18 (1), 250. doi: 10.1186/s12906-018-2309-3
Zhang, F. (2018). Clozapine causes cardiotoxicity by inducing the apoptosis of zebrafish embryo cardiac cells. Chin. Pharmacol. Bull. 2018 (08), 1088–1093.
Zhang, N., Fang, Z., Contag, P. R., Purchio, A. F., West, D. B. (2004). Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 103 (2), 617–626. doi: 10.1182/blood-2003-06-1820
Keywords: safety pharmacology, Chinese herbal medicine, blood-activating and stasis-removing medicines, herbal–drug interaction, organ damage, toxicity
Citation: Liu X, Shao R, Yang X, Xiao G, He S, Feng Y and Zhu Y (2019) Untargeted Safety Pharmacology Screen of Blood-Activating and Stasis-Removing Patent Chinese Herbal Medicines Identified Nonherbal Ingredients as a Cause of Organ Damage in Experimental Models. Front. Pharmacol. 10:993. doi: 10.3389/fphar.2019.00993
Received: 26 November 2018; Accepted: 05 August 2019;
Published: 12 September 2019.
Edited by:
Aiping Lu, Hong Kong Baptist University, Hong KongReviewed by:
SubbaRao V. Madhunapantula, JSS Academy of Higher Education and Research, IndiaShilin Chen, China Academy of Chinese Medical Sciences, China
Copyright © 2019 Liu, Shao, Yang, Xiao, He, Feng and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhu, eWFuemh1LmhhcnZhcmRAaWNsb3VkLmNvbQ==
†These authors have contributed equally to this work
 Xinyan Liu
Xinyan Liu Rui Shao1,2†
Rui Shao1,2† Yan Zhu
Yan Zhu