- 1Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmacy, Xi’an Jiaotong University, Xi’an, China
- 2Center for Drug Safety and Policy Research, Xi’an Jiaotong University, Xi’an, China
Objectives: To assess the effects on medicine price, a new public medicine procurement policy (NPMPP) undertaken in western China in 2015.
Methods: An interrupted time series analysis was used to evaluate the impact of NPMPP on the prices of emergency medicines, gynaecological medicines, and paediatric medicines in Shaanxi Province, western China. Based on the procurement records in all the public health institutions in Shaanxi Province, we built three regression models. The monthly average price growth rate of the three categories of medicines was analysed covering the period 2015 to 2017.
Findings: Before the intervention, there was an increasing trend in the monthly average growth rate of the three categories of medicines, but significant only in emergency medicines and paediatric medicines. After the introduction of NPMPP, the increasing trend was accelerated for both the emergency medicines (coefficient = 0.114, P < 0.001) and gynaecological medicines (coefficient = 0.078, P < 0.05), whereas the increasing trend for paediatric medicines was slowed down after the intervention (coefficient = −0.024, P < 0.05).
Conclusion: Using interrupted time series analysis, we identified a statistically significant increase in the price growth rate of emergency medicines and gynaecological medicines, but a statistically significant decrease in the price growth rate of paediatrics, following the introduction of NPMPP. The impact of NPMPP on emergency medicines was greater than that on gynaecological medicines. To inhibit the growth trend of drug price, effective policies need to be introduced.
Introduction
Drug shortages were a serious problem in China (Fan et al., 2018). By literature review, 148 drugs were identified in shortage from 2006 to 2015, and most of which were cheap and essential medicines (Wu et al., 2016). During the period of 2016 to 2017, the number of drugs in short supply monitored by the central government reached 1,000 (Wang et al., 2018). In a 29-province study in China, nearly two-thirds of the physicians reported that drug shortages occurred at least once a month (Yang et al., 2018). Among the three geographical regions (i.e., eastern, central, and western China), the eastern and western regions experienced more serious drug shortages than did the central region (Fan et al., 2018). One study in Shaanxi Province, western China, reported that there were almost 100 drugs in short supply in only 20 hospitals (12 secondary hospitals and 8 tertiary hospitals) in 2016 (Yang et al., 2016). The ongoing drug shortages crisis had exacted a significant effect on patient care and brought great challenges for the government (Du et al., 2018).
To address this drug shortage problem, the Chinese government employed several pharmaceutical policies. A new public medicine procurement policy (NPMPP) was introduced in January 2015, as the old procurement scheme was criticised as the primary cause of drug shortage (Cui and Lyu, 2018; Xiong et al., 2019). Previously, each province had a medicine tender bidding centre to select medicine manufacturers and to determine medicine prices for government-run healthcare institutions using a “2-envelope” system (Hu, 2017). The first envelope was quality, and the second envelope was the price (Hu, 2017). A composite score was calculated to determine the winner (usually the lowest price wins). To win the tender, the manufacturers usually offered a very low price that would not change for a long time (typically 5 years for most provinces) (Hu, 2017). In the NPMPP, as long as the manufactures met the technical and quality requirements, these manufacturers could be the procurement sources for all the public hospitals (NHFPC, 2015). Besides, manufacturers could negotiate on medicine price with healthcare institutions more frequently (NHFPC, 2015). Therefore, the situation of drug shortage caused by fixed low price may be greatly mitigated under the new medicine procurement scheme (Fang et al., 2015).
The central government issued the NPMPP at first, and then provincial governments started to implement it successively (SNHFPC, 2015). Up to now, the NPMPP has been implemented in 23 provinces. Unlike other types of medicines, there was no ceiling price when the NPMPP was implemented in paediatric, gynaecological, and emergency medicines (used in the emergency department and the prehospital setting).
After the implementation of NPMPP, the shortages of some low-priced medicines have been eased to a certain degree. However, at the same time, the prices of some medicines were reported to rise substantially since the NPMPP (Baidu Research, 2019; Financial Network, 2019; Quanzhou Network, 2019). One study carried out in a hospital in Guangxi Province found that, after the implementation of the NPMPP, the average price increased by 53% in 2016 and 93% in 2017 for 43 medicines (Zheng, 2018). Compared with other kinds of medicines, paediatric, gynaecological, and emergency medicines usually have lower market demands or fewer manufacturers, so monopoly was more common for these kinds of medicines (Cheng and Li, 2016; He et al., 2018). And when no ceiling price was set, medicine prices may increase considerably. Hence, we assumed that NPMPP would increase prices for paediatric, gynaecological, and emergency medicines.
The medicine price directly affected healthcare cost (Wang and Long, 2010). Currently, the continuous increasing of healthcare expenditure was one of the biggest healthcare issues in China (Yan et al., 2019). The per capita expenditure on healthcare for urban residents rose from 1,136 RMB ($169.14, $1 = 6.7162RMB on March 23, 2019) in 2013 to 1,777 RMB ($264.58) in 2017, with a growth rate of 56% during these 5 years in China (The State Council, 2018). Among the total healthcare expenditure, the pharmaceutical cost was one essential constituent. Even in 2017, the zero markup drug policy was implemented in all the hospitals (previously, hospitals were allowed to earn a 15% markup on the sale of pharmaceutical products); for inpatient, the pharmaceutical expenditure still took account for more than 30% of the total cost; for outpatient, the proportion was 42.7% (NHFPC, 2018). The problem of huge pharmaceutical cost was serious, and rapidly rising medicine price may make it worse.
To the best of our knowledge, the effect of this NPMPP on medicine price has not been characterised. In the context of increasing pharmaceutical expenditure in China, identifying and understanding the unintended consequences of this NPMPP on medicine price were highly relevant. Thus, the aim of this study was to assess the impacts of the NPMPP on medicine price.
Methods
Study Design
The NPMPP was introduced by China’s NHFPC in January 2015 (SNHFPC, 2015). After the announcement of this policy, they provided a medicine list recommending for using this procurement scheme in September of the same year. This medicine list contained only paediatric, gynaecological, and emergency medicines. After checking all the provincial policies, we found that up to now 23 provinces have implemented this NPMPP. However, the implementation time and medicine lists varied among different provinces. To ensure the veracity of assessment of this NPMPP, we focused on one single province, Shaanxi Province, by monitoring contextual factors that might influence our interests.
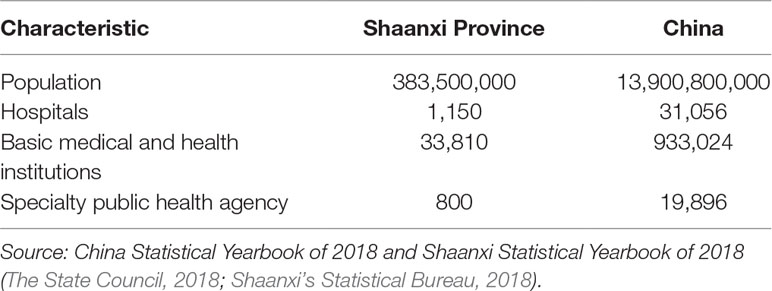
Shaanxi Province was chosen for study because of the following reasons. First, Shaanxi Province was one of the earliest provinces implementing the NPMPP, which guaranteed enough data for evaluation. Following the NHFPC’s version of NPMPP, the Shaanxi Health and Family Planning Commission issued a corresponding document on the NPMPP in August 2015 and announced the first batch of medicines that would be applied to this policy since April 2016 (SNHFPC, 2015). Second, Shaanxi was broadly representative of the typical health and health system status of the 12 western provinces of China. It has a population of 38.35 million and 11 areas in its jurisdiction, ranked 14th for gross domestic product per capita in Mainland China (31 provinces in total in the Mainland) in 2017 (The State Council, 2018). In Shaanxi Province, there were 1,150 hospitals, 33,810 basic medical and health institutions, and 800 specialty public health agencies (Table 1). Third and most important, the Shaanxi Provincial government was able and willing to collaborate in this study.
Similar to NHFPC, the first batch of medicines in Shaanxi still contained only paediatric, gynaecological, and emergency medicines. Later, in 2017 some common low-priced medicines and basic infusions were added in the new procurement processing, and ceiling prices were set for these medicines. In this article, we tried to assess the impact of NPMPP on the prices of paediatric, gynaecological, and emergency medicines.
Medicine Selection
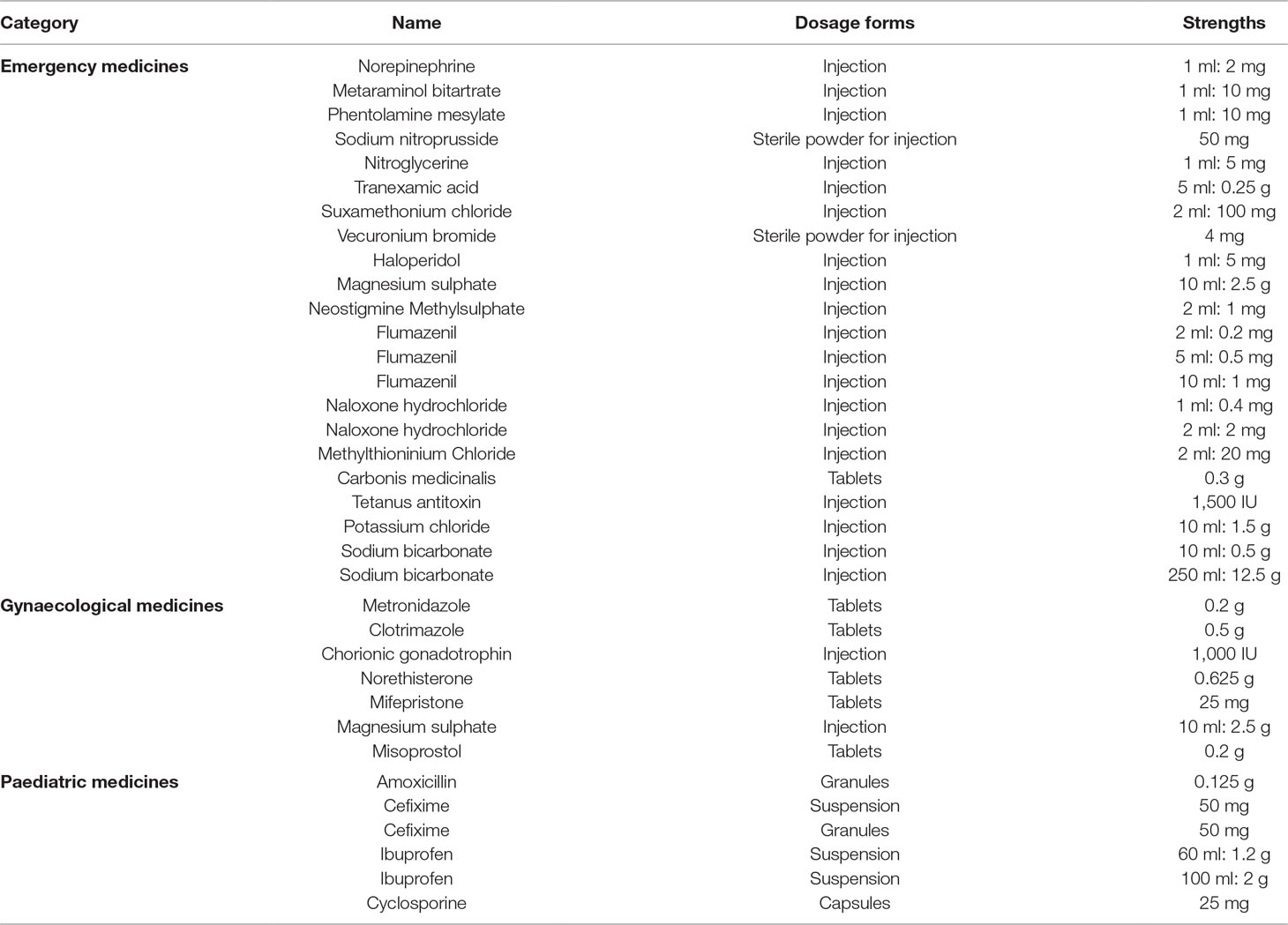
One medicine was selected for study only when it was both in the medicine list recommended by the China NHFPC and the first batch of medicines announced by the Shaanxi Health and Family Planning Commission. Finally, 95 medicines (same medicine name, different dosage forms or strengths were considered as different medicines) were selected, including 55 emergency medicines, 14 gynaecological medicines, and 26 paediatric medicines.
Data Collection and Management
Data were gathered from the Shaanxi Public Health Administration Department, which was responsible for centralised transactions in pharmaceutical and medical products procurement in public hospitals in Shaanxi. We extracted monthly purchasing information (procurement price and quantity) of all the public health institutions in Shaanxi Province for the 95 medicines, from January 2015 to December 2017. It would be taken as missing data for 1 medicine if no hospital purchased this medicine in a certain month.
Among the 95 medicines, we retained 35 medicines (including 22 emergency medicines, 7 gynaecological medicines, and 6 paediatric medicines), and 59 medicines were excluded because of missing data. The exclusion criterions were as follows: 1) if no data were available for more than three consecutive months for 1 medicine, then this medicine would not be included in the analysis; 2) if no data were available for more than 6 months for 1 medicine in total, then this medicine would be excluded.
For the final 35 medicines (Table 2), when 1 medicine had no purchasing record in a certain month, we used the purchase price of the previous month to represent the price and set quantity as zero for the current month. For better comparison, all the price data were adjusted according to the consumer price index.
Outcome Measurements
To analyse the changes in drug price, we adopted purchasing price growth rate to describe the speed of price change. The price growth rate of drug j in month i was calculated according to the following equation:
where p0j means the average purchasing price of drug j in the first month (January 2015) for all the public healthcare institutions in Shaanxi; pij means the average purchasing price of drug j in the ith month. The monthly average price growth rate for each category was defined as MPRe (for emergency medicines), MPRg (for gynaecological medicines), and MPRp (for paediatric medicines). And the MPRe in month i was calculated as follows:
Similarly, MPRgi and MPRpi were calculated.
Statistical Analysis
Interrupted time series analysis was considered to be the strongest, quasi-experimental design to evaluate the longitudinal effects of time-delimited interventions (Cook and Campbell, 1979; Rashidian et al., 2013). Segmented regression analysis of interrupted time series data can statistically assess how much an intervention changed an outcome of interest, transiently or in the long term (Wagner et al., 2002).
We carried an interrupted time series analysis of the price growth rate on each category of medicines. For each category of medicines, two segments with one interruption point were constructed. As the NPMPP was implemented in April 2016, the first segment was February 2015 (January 2015 was considered as base month; there were no MPRe/MPRg/MPRp values in this month) to March 2016 (14 months before intervention). To avoid incorrect specification of intervention effects, we excluded April 2016 from the analysis as it was a month lag in the effect of the intervention. Hence, the second segment was from May 2016 to December 2017 (20 months after the intervention). We assumed that there could be an immediate increase in the price increasing rate in May 2016 for the three categories of medicines, and the monthly trend in the price increasing rate after the intervention could differ from that before the intervention. We also assumed that the trend in the increasing price rate after intervention would differ among different kinds of medicines.
We tested for serial correlation by assuming a first-order autoregressive correlation structure. Breusch–Pagan statistic was used to check for heteroscedasticity in the residuals, and when heteroscedasticity existed, robust regression was adopted to correct the heteroscedasticity. We used Stata SE 12.0 (Stata Corporation, College Station, TX, USA), for estimation.
Results
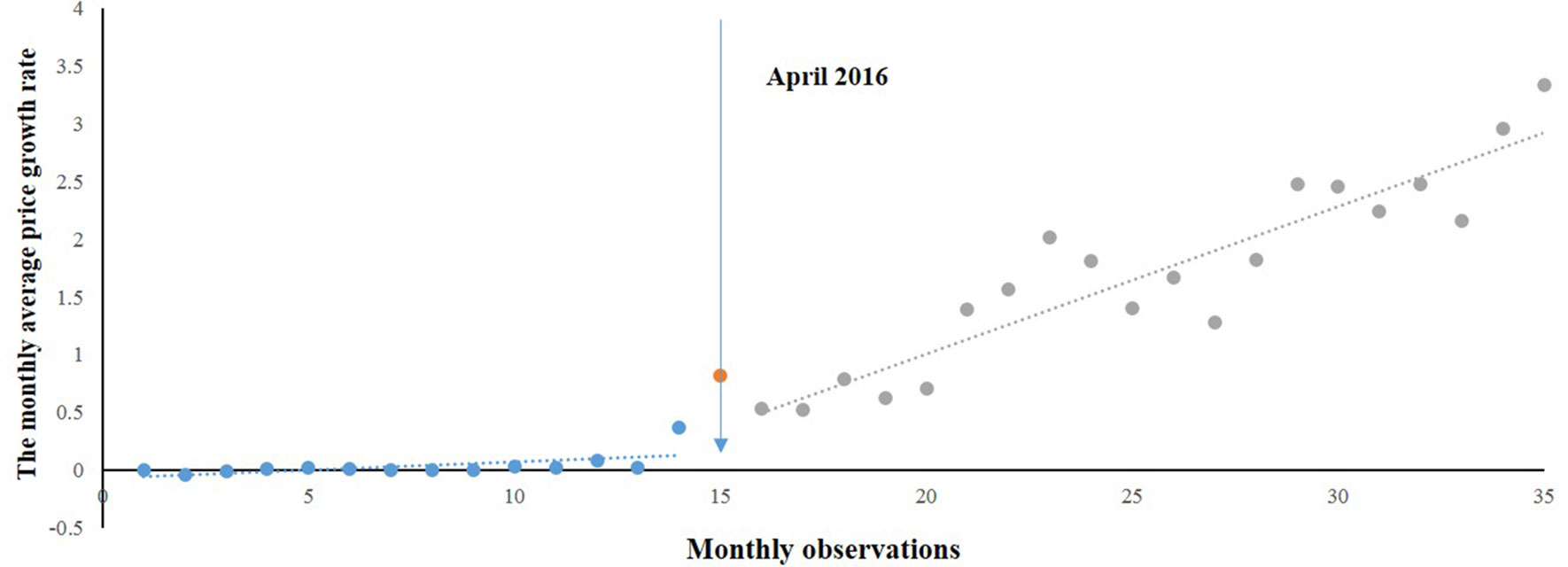
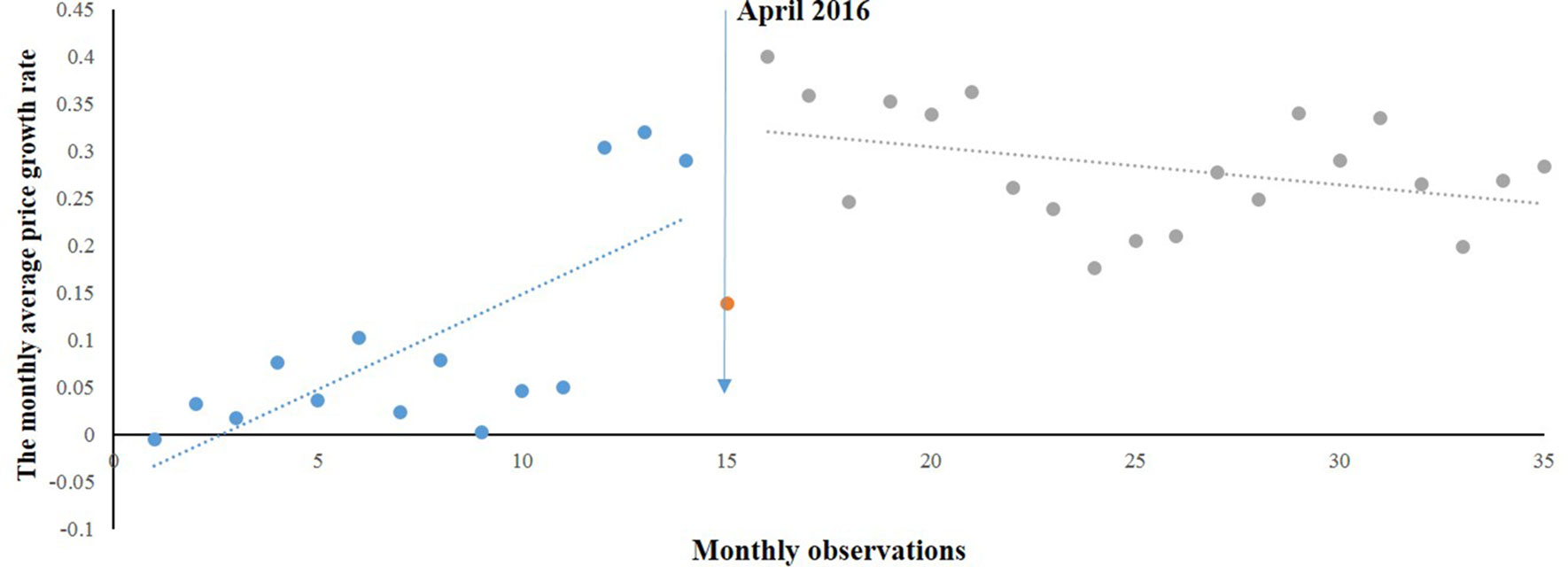
As Figure 1 shows, the overall prices of paediatric, gynaecological, and emergency medicines were increasing during the period of 2015 to 2017. Among the three categories of medicines, the prices of gynaecological and emergency medicines increased dramatically after the intervention, whereas the price of paediatric medicines was relatively stable.
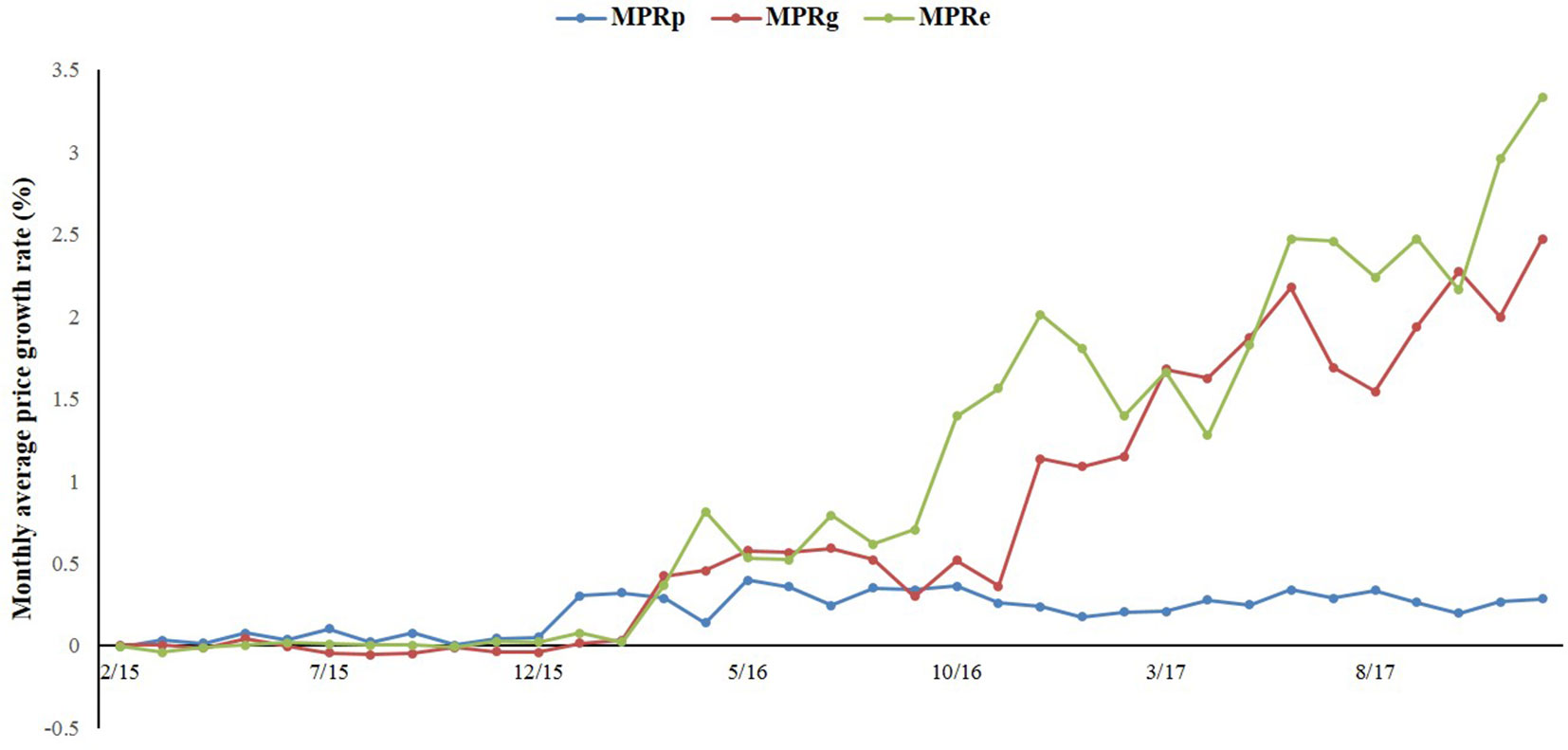
Figure 1 The trend in the monthly average price growth rate of paediatric, gynaecological and emergency medicines in Shaanxi Province from 2015 to 2017.
Monthly Average Price Growth Rate of Emergency Medicines
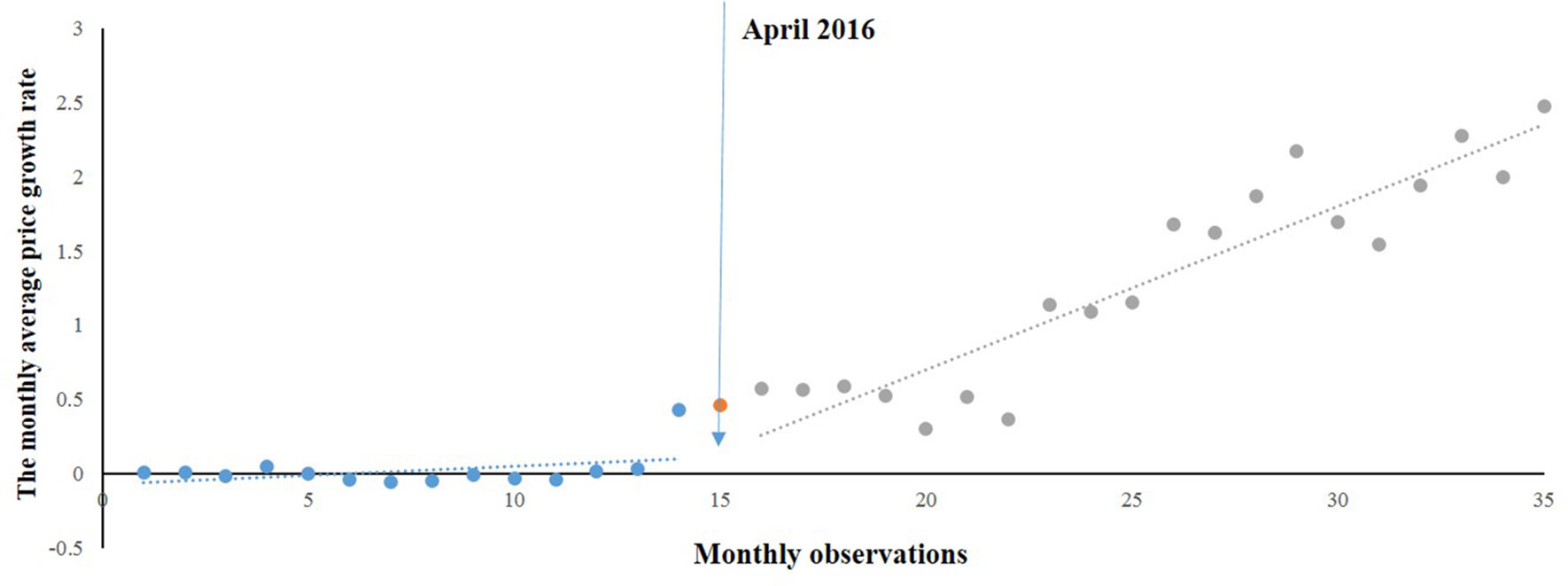
Figure 2 shows the time series of MPRe from 2015 to 2017. Before the implementation of NPMPP, there was a slight increasing trend in the MPRe. After the intervention, a little change in level was noted, and change can also be observed in the trend. We developed a regression model for MPRe:
where T was a preintervention slope, P was the change in slope, and D was the change in the intercept.
As Table 1 shows, we found an increasing rate (0.014) in the MPRe from month to month before the intervention (P = 0.088). After the intervention, significant increases in the regression slope (coefficient = 0.114, P < 0.001) and in the level were noted (coefficient = 0.243, P = 0.096).
Monthly Average Price Growth Rate of Gynaecological Medicines
According to the time series of monthly average price growth rate of gynaecological medicines (Figure 3), changes in the level and trend were similar to those of emergency medicines. A regression model was developed:
where MPRg^ was the MPRg after correcting for autocorrelation (MPRgt − q * MPRgt−1), and q was the autocorrelation parameter and equaled 0.375.
Likewise, a positive but no significant rise in Table 3 was observed in the MPRg from month to month before the intervention. After the intervention, a significant increase (0.078) in the regression slope (P < 0.05) was noted. The level also showed an increase (0.026) but not statistically significant (P = 0.9).
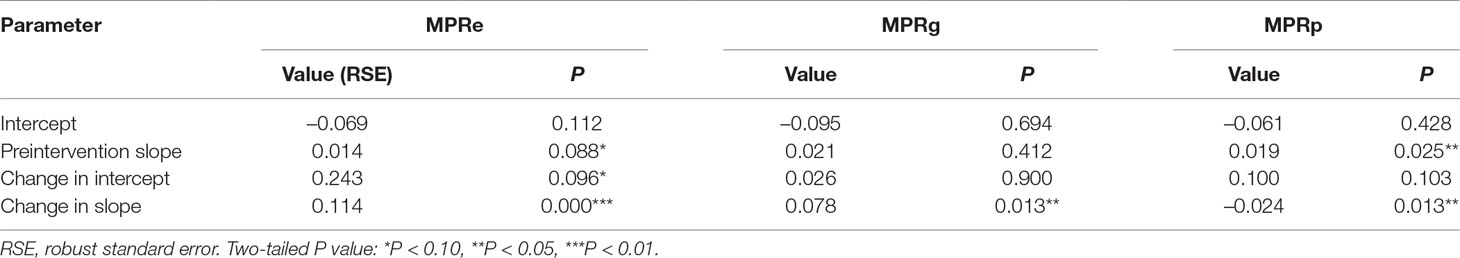
Table 3 Estimated coefficients of segmented regression models for the MPRe, MPRg, and MPRp before and after the NPMPP, Shaanxi Province, February 2015 to December 2017.
Monthly Average Price Growth Rate of Paediatric Medicines
As shown in Figure 4, the trend of MPRp was stable during the whole period. A regression model was also generated:
where MPRp^ was the MPRp after correcting for autocorrelation (MPRpt − q* MPRpt−1), and q was the autocorrelation parameter and equaled 0.428.
The trend of MPRp was different from MPRe and MPRg. Before the intervention, a significant increase (0.019) was found in the average price growth rate of paediatric medicines (P = 0.025). However, a significant decrease (−0.024) was noted in the regression slope (P < 0.05) after the intervention. An increase (0.100) but not statistically significant (P = 0.103) in the level was found.
Discussion
In this study, we identified the effects of the NPMPP, which aimed to address this drug shortage problem, on the price of emergency, gynaecological, and paediatric medicines. Most of the previous studies related to NPMPP were about the implementation mode of this policy in different provinces (Chen, 2017; Fang et al., 2015). To the best of our knowledge, this was the first quantitative study to investigate the effects of the NPMPP on medicine price in China. An interrupted time series analysis showed the NPMPP in Shaanxi Province resulted in an acceleration of the price growth rate of emergency medicines and gynaecological medicines, but a slight deceleration of the price growth rate of paediatric medicines. The changes in price growth rate varied; however, the medicines prices of the three categories were still higher compared with the prices before the intervention.
Before the introduction of NPMPP, the monthly average price growth rates of the three categories of medicines revealed a trend of slow increase. The intervention accelerated the monthly average price growth rates of emergency medicines and gynaecological medicines significantly. The relative increased rate of MPRe (0.114/0.014) was bigger than that of MPRg (0.078/0.021). This suggested that the impact of on MPRe was greater than that on MPRg. However, the NPMPP decreased the monthly average price growth rate of paediatric medicines. We noted two possible explanations for this result. First, the median of the number of manufacturers for emergency medicines and gynaecological medicines was 4, which was fewer than that for paediatric medicines (eight suppliers). Therefore, the monopoly was more common for emergency and gynaecological medicines. While for paediatric medicines, the NPMPP enlarged the number of suppliers, intensified the competition between manufacturers, and finally resulted in a decrease in the price growth rate. Second, the prices of active ingredients for many emergency medicines increased greatly during last 2 years (Baidu Research, 2019; Financial Network, 2019; Quanzhou Network 2019). For example, the price of inosine (an active ingredient for leukopenia, various heart diseases, chronic hepatitis, cirrhosis, etc.) rose from 92 to 95 RMB/kg in early 2015 to 600 RMB/kg in July 2018 (Zhan, 2018), and the price of raw materials of nitroglycerin injection rose sharply, almost tripled from 2015 to 2018 (Sina Finance, 2019).
Although we observed a decreasing trend in the MPRp, the prices of all the 35 medicines were still increasing after the NPMPP. This result was different compared with a previous study. Based on the annual tender prices of medicines in 31 provinces, Shang et al. (2019) found that there was an instant increase for the overall price of these medicines (including emergency, gynaecological, and paediatric medicines; common low-priced medicines and basic infusions, recommended using NPMPP by China’s NHFPC) in 2015, but a stable decrease from 2016 to 2017. This difference could be attributed to the use of different methods, as well as different medicine samples and different study areas. Especially in China, there was a great disparity of medicine prices among different provinces, while Shaanxi was ranked fourth for high drug price (Pan and Wang, 2019). Therefore, using the average price data of 31 provinces would generate different results, comparing with our research, which included price information only of Shaanxi Province.
The study design had two advantages. First, no other related policies were implemented during 2015 to 2017, which guaranteed that we can perform a natural experiment and use robust research method. Second, we controlled for possible confounding by using the price information for all the public healthcare institutions in Shaanxi Province and taking the monthly average price growth rate for each category as our outcome indicator.
Limitations of this study included that the study was conducted in only one province, whereas ideally it should have been conducted in a nationally representative sample. However, the NPMPP was carried out on a province level, which means that the implementation time and drug catalogue were different in different provinces. Hence, it was difficult to extend our research to the whole country. The second limitation was the lack of a control group. Although it was not necessary to build a control group to establish a causal relationship between an intervention and an outcome, the control group may help better understand the effects of the intervention. On the other hand, the choice of medicines in the control group would be subjective, which may lead to bias.
Our study had important policy implications. The NPMPP needs to be modified to restrain the upward trending in prices of emergency and gynaecological medicines. Generally, no ceiling price needs to be cautiously considered, especially for medicines with a small number of manufacturers and for countries with no strong antitrust regulation.
Conclusion
We investigated the effects of an NPMPP on medicine price in Shaanxi Province, western China. By interrupted time series analysis, we identified a statistically significant increase in the price growth rate of emergency medicines and gynaecological medicines, but a statistically significant decrease in the price growth rate of paediatrics, following the introduction of NPMPP. The impact of NPMPP on emergency medicines was greater than that on gynaecological medicines. Other effective policies were needed to inhibit the price growth rates of emergency and gynaecological medicines.
Data Availability
The data analyzed in this study was obtained from Shaanxi Health and Family Planning Commission. Requests to access these datasets should be directed to Caijun Yang,
Author Contributions
Conceived and designed the experiments: CY, SH. Performed the experiments: SH, CC. Analysed the data: SH, CC, CY, SY, FX. Wrote the paper: SH. Critical revision of the manuscript: SH, CC, CY, SY, FX, LS. Approval of the final version of the manuscript: SH, CC, CY, SY, FX, LS, YF.
Funding
This work is supported by the National Natural Science Foundation of China (7150319) and “the Fundamental Research Funds for the Central Universities.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Shaanxi Public Health Administration Department for their support and cooperation.
References
Baidu Research Engines. (2019). How does nitroglycerin tablet go up from a few to more than 30 RMB; c2019 [cited 2019 Apr 6]. Chengdu evening news. Available from: https://baijiahao.baidu.com/s?id=1625786952527807381&wfr=spider&for=pc.
Chen, Z. (2017). Study on new medicine procurement policy in public hospitals of Shandong province. Science and Technology & Innovation 16, 51+54–55.
Cheng, M., Li, Q. (2016). Analysis of the development environment and market of pediatric drugs in China. Prog. Pharm. Sci. 9, 653–664.
Cook, T. D., Campbell, D. T. (1979). Quasi-Experimentation: Design & Analysis Issues for Field Settings. Boston, MA: Houghton Mifflin Company.
Cui, Z., Lyu, L. (2018). Implementation effects, risk prediction and policy suggestion of “Two-Invoice System”in the field. China Pharmacy 8, 1009–1014. doi: 10.6039/j.issn.1001-0408.2018.08.01
Du, W., Xu, W., Cai, G, Ma, L. (2018). The analysis of low-cost medicine’s accessibility in China: based on the empirical study from 24 provinces and cities. Chin. J. Health Policy 3, 72–77. doi: 10.3969/j.issn.1674-2982.2018.03.013
Fan, J., Wang, Z., Zhang, J., Han, S., Shi, L., Guan, X., et al. (2018). Analysis of drug shortages in China’s hospitals. Chin. J. New Drugs 17, 1964–1967.
Fang, L., Zuo, G., Jia, L. (2015). The comparative study on low-cost drugs online procurement policy in China. Chin. Health Serv. Manage. 8, 610–612.
Financial Network. (2019). Who is driving up drug prices? Some drugs have risen as much as 600 percent in three years; c2019 [cited 2019 Apr 6]. Industrial economics channel. Available from: http://industry.caijing.com.cn/20190304/4567156.shtml.
He, J., Li, X., Liu, R., Wang, Q., Lu, S., Wang, L., et al. (2018). Survey and analysis of drug shortages in 61 medical institutions from Yunnan Province. China Pharm. 14, 1882–1885. doi: 10.6039/j.issn.1001-0408.2018.14.03
Hu, S. (2017). The Theory and practice of “Two-Invoice System” in the drug procurement Vol. 4, China Academic Journal Electronic Publishing House, 8–10.
NHFPC. (2015). Notice on the procurement and supply of emergency medicine. Division of Drug Policy and Essential Drug Policy. Beijing: China’s National Health and Family Planning Commission.
NHFPC. (2018). Statistical bulletin on the development of health undertakings in China in 2017. Department of planning, development and information technology. Beijing: China’s National Health and Family Planning Commission.
Pan, J., Wang, Y. (2019). Comparative analysis of drug price levels in 23 provinces and cities in China under the background of regional differentiation. Health Econ. Res. 2, 49–52. doi: 10.14055/j.cnki.33-1056/f.2019.02.014
Quanzhou Network. (2019). “Life-saving medicine” nitroglycerin tablets were out of stock throughout the city, and the price rose from 4 RMB a bottle to nearly 20 RMB; c2019 [cited 2019 Apr 6]. Hot topic. Available from: http://www.qzcns.com/qznews/2019/0227/568850.html.
Rashidian, A., Joudaki, H., Khodayari-Moez, E., Omranikhoo, H., Geraili, B., Arab, M. (2013). The impact of rural health system reform on hospitalization rates in the Islamic Republic of Iran: an interrupted time series. Bull. World Health Organ 12, 942–949. doi: 10.2471/BLT.12.111708
Shaanxi’s Statistical Bureau. (2018). Shaanxi: National Bureau of Statistics of Shaanxi Province. Xi’an: The Statistical Bureau.
Shang, L., Chang, F., Lu, Y. (2019). Analysis of China’s drug price situation after drug price reform-an empirical study based on Provincial Panel Data in China. Health Econ. Res. 4, 59–63. doi: 10.14055/j.cnki.33-1056/f.2019.04.018
Sina Finance. (2019). Nitroglycerin tablets are in short supply with 10 times higher price; c2019 [cited 2019 Apr 6]. Industrial economics channel. Available from: http://finance.sina.com.cn/roll/2019-03-02/doc-ihsxncvf9055596.shtml.
SNHFPC. (2015). Shaanxi Provincial Health and Family Planning Commission’s notice on the new medicine procurement policy. Division of Drug Policy and Essential Drug Policy. Xi’an: National Health and Family Planning Commission.
Wagner, A. K., Soumerai, S. B., Zhang, F., Ross-Degnan, D. (2002). Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 4, 299–309. doi: 10.1046/j.1365-2710.2002.00430.x
Wang, S., Zhu, J., Gao, Z., Wu, W., Yao, L. (2018). Discussion on key drugs in shortage and their supply safeguard measures. Chin. Pharm. Aff. 8, 1034–1042. doi: 10.16153/j.1002-7777.2018.08.005
Wang, Y., Long, Y. (2010). Characteristics, problems, and recommendations of the mid-and-low income group’s medical service demand: based on the survey of 1642 families. Chin. J. Health Policy 3, 9–15. doi: 10.3969/j.issn.1674-2982.2010.03.004
Wu, L., Fang, Y., Yang, C., Shen, Q., Chang, J., Zhu, W., et al. (2016). A review of research on drug shortages in China. Chin. Pharm. Aff. 5, 458–465. doi: 10.16153/j.1002-7777.2016.05.009
Xiong, K., Chen, H., Zhang, Z, Xu, J. (2019). Feasibility demonstration and comparative study of “Two-Vote System” of pharmaceutical procurement of public medical institutions. Chin. Hosp. Manage. 2, 21–23.
Yan, J., Lin, H., Zhao, D., Hu, Y., Shao, R. (2019). China’s new policy for healthcare cost control based on global budget: a survey of 110 clinicians in hospitals. BMC Health Serv. Res. 1, 84. doi: 10.1186/s12913-019-3921-8
Yang, C., Cai, W., Li, Z., Page, A. T., Fang, Y. (2018). The current status and effects of emergency drug shortages in China: perceptions of emergency department physicians. Plos One 10, e0205238. doi: 10.1371/journal.pone.0205238
Yang, C., Wu, L., Cai, W., Zhu, W., Shen, Q., Li, Z., et al. (2016). Current situation, determinants, and solutions to drug shortages in Shaanxi Province, China: a qualitative study. Plos One 10, e0165183. doi: 10.1371/journal.pone.0165183
Zhan, L. (2018). Who is behind the maneuver with the price of API soaring 99%? Qianjiang Evening News.
Keywords: medicine price, interrupted time series analysis, medicine procurement policy, policy effect, China
Citation: Hu S, Chen C, Yuan S, Xue F, Shi L, Fang Y and Yang C (2019) The Effects of a New Public Medicine Procurement Policy on Medicine Price in Shaanxi Province, Western China: An Interrupted Time Series Analysis. Front. Pharmacol. 10:950. doi: 10.3389/fphar.2019.00950
Received: 22 April 2019; Accepted: 25 July 2019;
Published: 29 August 2019.
Edited by:
Dan Kibuule, University of Namibia, NamibiaReviewed by:
Florentina Ligia Furtunescu, Carol Davila University of Medicine and Pharmacy, RomaniaYaser Mohammed Al-Worafi, Ajman University of Science and Technology, United Arab Emirates
Copyright © 2019 Hu, Chen, Yuan, Xue, Shi, Fang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caijun Yang, eWFuZ2NqQHhqdHUuZWR1LmNu
 Shuchen Hu
Shuchen Hu Chen Chen
Chen Chen Shengfang Yuan
Shengfang Yuan Fei Xue
Fei Xue Li Shi
Li Shi Yu Fang
Yu Fang Caijun Yang
Caijun Yang