
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 11 July 2019
Sec. Pharmacology of Anti-Cancer Drugs
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00774
 Guolan Wu1,2†
Guolan Wu1,2† Lihua Wu1,2†
Lihua Wu1,2† Huili Zhou1,2
Huili Zhou1,2 Meihua Lin1,2
Meihua Lin1,2 Ling Peng3
Ling Peng3 Yina Wang4
Yina Wang4 You Zhai1,2
You Zhai1,2 Xingjiang Hu1,2
Xingjiang Hu1,2 Yunliang Zheng1,2
Yunliang Zheng1,2 Duo Lv1,2
Duo Lv1,2 Jian Liu1,2
Jian Liu1,2 Jianzhong Shentu1,2*
Jianzhong Shentu1,2*Purpose: The aim of this study was to compare the pharmacokinetics and safety between two vinorelbine formulations [a new oil-in-water emulsion formulation (ANX) versus a previously marketed solution formulation (Navelbine)] in Chinese patients with advanced non-small cell lung cancer (NSCLC).
Method: This was a single-center, randomized, open-label study. Eligible patients aged 18–70 years who had histologically or cytologically confirmed NSCLC were enrolled. In cycle 1, the patients alternatively received the two formulations (30 mg/m2, given as a 10-min infusion) with a 7-day interval. Samples for pharmacokinetic analysis were taken during cycle 1. For all subsequent 21-day cycles (maximum four cycles), ANX was administered on days 1 and day 8. Bioequivalence analysis was performed on Cmax, AUClast, and AUCinf. The safety profiles and anti-tumor effects were also determined.
Results: From March 2013 to January 2015, 24 patients were enrolled and 20 were eligible for pharmacokinetic evaluation. The 20 subjects in the pharmacokinetic analysis set had a median age of 61 years (range, 37–70 years), and 15 patients were male (75%). Mean vinorelbine Cmax values for ANX and Navelbine were 1,317.40 and 1,446.30 ng/mL, respectively. Corresponding AUClast values were 797.08 and 924.26 ng·h/mL, respectively. AUCinf values were 830.14 and 957.16 ng·h/mL, respectively. Treatment ratios of the geometric means were 90.00% (90% CI, 83.22–99.07%) for Cmax, 86.92% (90% CI, 80.91–93.37%) for AUClast, and 87.44% (90% CI, 82.08–93.16%) for AUCinf. These results met the required 80–125% bioequivalence criteria. The most frequently reported adverse events after vinorelbine administration were neutropenia, leucopenia, neutropenic fever, and constipation.
Conclusion: At therapeutic dosage levels, pharmacokinetic behavior and safety profiles were similar for both formulations. Chinese National Registry Code: ChiCTR-IPR-15005856.
Worldwide, lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) (Bray et al., 2018). Nonsmall cell lung cancer (NSCLC) accounts for ∼85% of all lung cancers (Schvartsman et al., 2016). Nearly 60% of NSCLC cases are metastatic at diagnosis, and the 5-year relative survival rate for metastatic disease is 4.2% (Kaburaki et al., 2017). Many advances have been made in the first-line systemic treatment of NSCLC, and current treatment approaches are markedly different from what they were several years ago. At the onset of the 2000s, first-line management of patients with advanced NSCLC relied on platinum-based doublets chemotherapy. In the last decade, targeted therapies have transformed the management of patients whose tumors harbor an oncogenic mutation (∼15%), but the majority of patients cannot benefit from these treatments (Bylicki et al., 2019). For patients without driver oncogenes, however, improvements in survival were minimal until immunotherapeutic options became available. Since then, these treatments have quickly moved to the first-line setting (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Mok et al., 2019). Yet, it is only a minority of patients who achieve this transcendent, durable benefit from immunotherapy. Overall, the prognosis for lung cancer remains poor. Nevertheless, most lung cancer patients will still need, and continue to receive, chemotherapy over their disease course (Hardin et al., 2017).
Vinorelbine, a semisynthetic vinca alkaloid, is known to be an active agent in NSCLC treatment (Crawford, 1996; Jones and Burris, 1996). Like other vinca, it exhibits cell cycle-specific cytotoxic activity by binding to tubulin and preventing its assembly into microtubules, ultimately inhibiting mitosis and inducing apoptosis (Caffo et al., 2013). Either used alone or in combination, vinorelbine has demonstrated clinical benefits in different settings of lung cancer management (Gridelli, 2001; Reck et al., 2014; Lesueur et al., 2018). Vinorelbine for intravenous (i.v.) administration by label is typically given 25–30 mg/m2 on days 1 and 8 of a 21-day cycle. Its main dose-limiting toxicity is grade 3 leukocytopenia, which develops 8–10 days after each dose. Like other anticancer vinca alkaloids, vinorelbine is a moderate vesicant that is well documented to cause local venous toxicity such as injection site reactions and chemical phlebitis (Winton et al., 2005; Yoh et al., 2007; Li et al., 2013). Although local venous toxicity is not life threatening, it can be discomforting or painful; thus, it may negatively influence patients’ quality of life. The oral formulation provides a valuable option for treating cancer patients because of ease of administration, greater convenience for the patient, and reduced need for hospitalization. However, relatively higher cost and incidence of gastrointestinal toxicities may reduce patient compliance. Consequently, improved i.v. formulations have been sought for vinorelbine. To overcome the local venous toxicity of vinorelbine, drug delivery systems were developed, such as nanoparticles, liposomes, microspheres, and lipid emulsions (Yang et al., 2012; Li et al., 2013). A new bitartrate vinorelbine emulsion has recently been developed in China. The vinorelbine is encapsulated in the oil droplets, thus preventing direct contact of drug molecules with the venous endothelium tissue, and therefore reducing the venous toxicity.
Accurate and comprehensive understanding of safety and PK profiles of new formulation is needed for clinical development. Hence, we conducted a phase I study to compare pharmacokinetic parameters of this new bitartrate emulsion with a previously marketed solution in NSCLC patients. The efficacy and safety profile of this new bitartrate emulsion was also evaluated as an exploratory objective. The study will provide a basis for better clinical use of the drug in Chinese population in the future.
The study protocol and its amendments were reviewed and approved by the ethics committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (approval no.: 2012-EC-60). The trial was designed according to the current revised Declaration of Helsinki and conducted in accordance with Good Clinical Practice guidelines. Written informed consent was obtained from each participating patient before entry into the study.
The study was initiated in March 2013 and closed to accrual in February 2015. All enrolled patients had histologically or cytologically confirmed non-small cell lung cancer. Additional inclusion criteria included the following: age ≥18 and ≤70 years; a performance status ≤2 (ECOG-Zubrod scale); life expectancy greater than 3 months; initial blood parameters of hemoglobin ≥10 g/dl, granulocytes (absolute neutrophil count) ≥1.5 × 109/L, platelets ≥80 × 109/L; adequate hepatic function and renal function [bilirubin < 1.5 mg/dl, transaminases <2.5 times upper limit of normality (ULN) or ≤5.0 × ULN if liver metastases present]; serum total bilirubin ≤1.5 × ULN; calculated creatinine >80 ml/min (Cockcroft–Gault formula); and presence of at least one evaluable or measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.
Additional ineligibility criteria included a history of primary central nervous system tumors or brain metastases, any peripheral neuropathy of grade ≥1 per the Common Terminology Criteria for Adverse Events (CTCAE), impaired cardiac function [left ventricular ejection fraction <45%, complete left bundle branch block, obligate use of a cardiac pacemaker, congenital long QT syndrome, history or presence of significant ventricular or atrial tachyarrhythmias, clinically significant resting bradycardia (<50 beats per minute), QTcF >480 ms on screening electrocardiogram, or other clinically significant heart disease], impairment of gastrointestinal function or gastrointestinal disease, and acute or chronic liver or renal disease. Female subjects with reproductive potential were required to have a negative pregnancy test (urine and/or serum β-human chorionic gonadotropin) and agree to use an effective (>90% reliability) form of birth control during the study. Subjects with an acute infection or fever over 38.0°C within 3 days prior to study entry, cardiovascular disease, lactation, or history of allergic reactions attributed to compounds of similar chemical or biologic composition to vinorelbine were not eligible for the study. Subjects with positive tests for human immunodeficiency virus, hepatitis B virus surface antigen, or anti-hepatitis C virus antibody were also excluded. Patients were required to be able to understand and sign a written informed consent document.
Pretreatment evaluation included a complete medical history and physical examination, a computed tomographic (CT) scan of the chest and abdomen, magnetic resonance imaging (MRI) of the brain, and a bone scintigraphy to assess the extent of disease. Follow-up consisted of physical examination, monitoring of toxic effects, a complete blood count, liver function tests, chest radiography, and CT scan as clinically indicated. Tumor response was assessed using RECIST criteria. Tumor responses were assessed every two cycles of chemotherapy, and patients were evaluated before each new treatment cycle for toxicities.
Vinorelbine treatment by label is typically given on days 1 and 8 of a 21-day cycle. In cycle 1, patients were randomized to receive a single i.v. dose of either vinorelbine bitartrate emulsion injection or vinorelbine solution, then 1 week later crossover to the alternate vinorelbine formulation. All drug administrations were given as 10-min infusions at the dose of 30 mg/m2. No dose reduction was allowed in the first cycle. In following cycles, patients were planned to receive vinorelbine bitartrate emulsion injection on days 1 and 8. Cycles were repeated every 3 weeks if the patient’s blood count had returned to normal and non-hematologic toxicities had resolved. Dosage of subsequent cycles was adjusted according to the observed toxicities that developed during the preceding cycle. The criteria for dose reduction included grade 4 hematological and grade 3 non-hematological toxicity, in which case the vinorelbine dose was reduced by 20%.The treatment continued for a maximum of four cycles or until disease progression.
Vinorelbine pharmacokinetic (PK) parameters were evaluated based on collection of plasma samples during cycle 1. Blood samples (3 ml) were collected from an indwelling venous catheter into heparinized tubes immediately before (within 15 min) the start of infusion and 5, 10, and 30 min, and 1, 2, 3, 6, 12, 24, 48, 72, 96, 120, and 144 h after the start of the infusion for each treatment on days 1 and 8. Vinorelbine concentration was determined using a validated liquid chromatography/mass spectrometry-mass spectrometry (LC/MS-MS) analytical procedure. The assay was validated in the range of 0.36–1,450.38 ng/ml. Pharmacokinetic parameters were calculated by non-compartment method using WinNonlin 6.3 software (Pharsight Corporation; Sunnyvale, CA, USA).
The primary outcome measure was the evaluation of the bioavailability of the test product and the reference product, assessed as extent of exposure (AUClast, area under the curve from time zero to last measurable concentration). Secondary pharmacokinetic outcomes were the pharmacokinetic parameters: maximum plasma concentration (Cmax), area under the curve from first time point extrapolated to infinity (AUCinf), plasma peak concentration time (Tmax), total body clearance (CL), apparent volume of distribution (Vz), and terminal half-life (T1/2).
Injection-site tolerability was assessed in relation to six specific reactions: erythema,itching,swelling, bruising, bleeding, and pain. Pain was assessed using a visual analogue scale (VAS), where 0 mm was equal to no pain and 100 mm was equal to severe pain. Information on adverse events (AEs) was collected throughout the study. Additional safety variables included vital signs, physical examination, 12-lead electrocardiogram, hematology, clinical chemistry, urinalysis, and any general signs or symptoms relating to potential adverse effects of the drug.
All participants for whom primary PK parameters could be calculated for both treatment periods were included in the PK population. The safety population comprised all individuals who received at least one dose of study treatment. AEs were summarized by treatment. Bioequivalence was assessed according to the China Food and Drug Administration (CFDA) Guideline on the Investigation of Bioequivalence. Thus, 90% confidence intervals (CIs) for analysis of variance estimates of the geometric mean ratios of the primary PK parameters for the two products (test/reference) had to lie within the range of 80.00–125.00%. The geometric mean ratios were calculated from least square mean values. The required calculations were performed using Phoenix WinNonlin 6.3. Assuming the above bioequivalence criteria for Cmax and AUClast, a within-participant coefficient of variation (CV%) of 20%, and a “test/reference” mean ratio between 0.95 and 1.05, it was calculated that 24 participants would be needed to show bioequivalence with a power of at least 80% and an alpha level of 0.05.
Thirty individuals were screened for inclusion in the study, and 24 of them were randomized to the study (Figure 1). Six subjects were not randomized, the reasons being consent withdrawal (n = 2) and screening failures (n = 4). Four volunteers withdrew from the study before the second treatment in cycle 1. Therefore, 20 individuals completed the study and were included in the PK population. The baseline characteristics of the patients are summarized in Table 1. Nineteen patients were male and five were female, with a median age of 61 years (range, 37–70 years). The median body weight was 61.0 kg (range, 40.5–77.0 kg), and the median body surface area was 1.68 m2 (range, 1.34–1.82 m2).
The total number of cycles delivered to all patients was 60, with a median number of three treatment cycles delivered per patient (range, 0–4). After the first administration of 30 mg/m2 vinorelbine, four patients (three patients using Navelbine and one patient using ANX) were withdrawn from the study due to either safety reasons or patient decision. As per protocol, a total of 11 patients (45.8%) had a vinorelbine dose reduction. Seven patients had dose reduction for the second cycle due to grade 3 neutropenia (two patients) and grade 4 neutropenia (five patients).One additional dose reduction was applied for cycle 3 in one patient for whom vinorelbine was administered at 25 mg/m2 at cycle 2 due to grade 4 neutropenia. Table 2 displays an overview of dose changes during the study in patients.
For the first cycle, 20 patients provided full and interpretable PK results. The mean plasma concentration–time curves of vinorelbine after an i.v. administration of 30 mg/m2 of the two formulations to Chinese NSCLC patients are shown in Figure 2. The main PK parameters calculated for both formulations are listed in Table 3. Similar Tmax results were observed for the two formulations. The 90% CIs of the test/reference (ANX versus Navelbine) ratios of the natural log-transformed values of AUClast, AUCinf, and Cmax were within the predetermined bioequivalence range of 80.00–125.00% (Figure 3 and Table 3). Individual values of vinorelbine pharmacokinetic parameters (AUClast, AUCinf, and Cmax) obtained in cycle 1 are depicted in Figure 4. Therefore, it was deduced that the test and reference formulations were bioequivalent according to the guidelines.
All enrolled patients were evaluable for safety. No toxic death occurred during the study, and no unexpected adverse events were recorded. Overall toxicity data are summarized in Table 4.
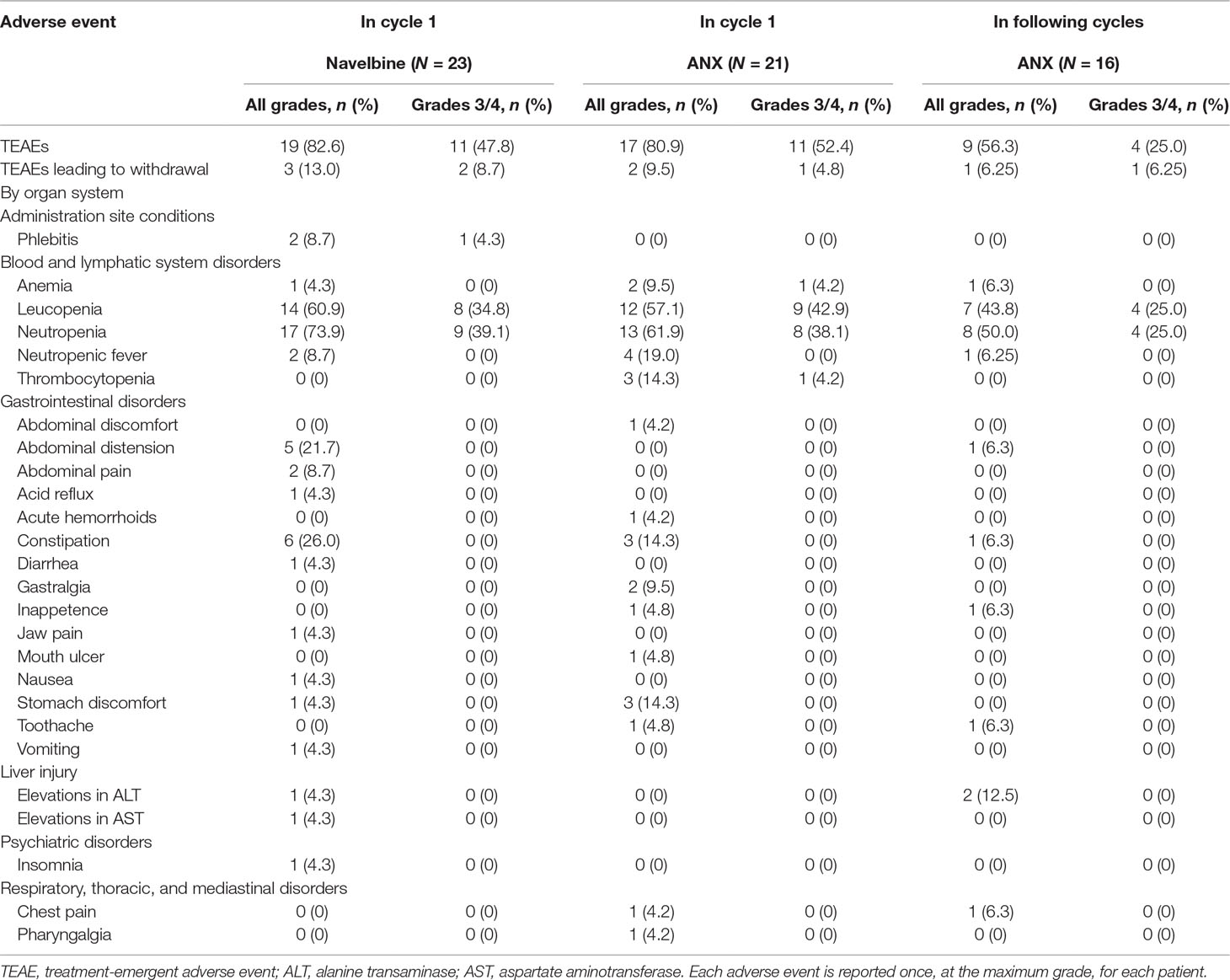
Table 4 Adverse events in all cycles of treatment for all patients receiving at least one treatment.
In total, 19 participants (82.6%) occurred TEAEs following Navelbine administration, and 17 individuals (80.9%) occurred TEAEs after administration of ANX in treatment cycle 1. The most common hematological toxicity was neutropenia, which was grades 3–4 in 40% of patients. Non-hematological toxicities including constipation, abdominal distension, nausea, vomiting, and diarrhea were generally mild to moderate. In this study, infusion site phlebitis was only observed in two patients (8.7%) following Navelbine administration. The schedule of vinorelbine Ton days 1 and 8 every 3 weeks employed in this study offered a similar safety profile as reported by other with i.v. vinorelbine.
While this study was not powered for evaluation of anti-cancer efficacy, among the 24 recruited patients, 16 (66.7%) patients had their best objective response rate evaluated at the end of cycle 2 treatment. Fourteen patients (14/16, 87.5%) had stable disease, 1 patient (6.25%) had partial response, while 1 patient (6.25%) progressed.
Ten subjects completed four cycles of chemotherapy, 8 patients (80%) had stable disease, 1 patient (10%) had partial response, while 1 patient (6.25%) progressed.
This study has shown that the test product (ANX) is bioequivalent to the reference product (Navelbine), in terms of AUClast, AUCinf, and Cmax. The dose of vinorelbine administered in this study is within the range of vinorelbine doses that are used in routine clinical practice. To our knowledge, this is the first published study to characterize the tolerability and pharmacokinetic profile of vinorelbine in Chinese advanced NSCLC patients receiving a dose of 30 mg/m2 by 10 min i.v. When dose adjusted to be comparably equivalence, the pharmacokinetic parameters described here were similar to those observed after i.v. infusion (Marquet et al., 1992; Wargin and Lucas, 1994; Robieux et al., 1996; Marty et al., 2001; Khayat et al., 2004; Lush et al., 2005; Requejo et al., 2008). However, the Cmax and AUC values in our study presented here (as shown in Table 3) were higher than the values previously reported for Chinese (Qian et al., 2011) and Western cancer patients (Schilling et al., 1996) (in 12 Chinese cancer patients receiving a dose of 25 mg/m2 by 20-min i.v. infusion, Cmax 599.5 ± 243.6 ng·h/mL, and AUCinf 476.7 ± 73.2 ng·h/mL; in 13 Western patients with solid tumors following i.v. administration 35 mg/m2, Cmax 676.6 ± 220.2 ng·h/mL, and AUCinf 476.7 ± 73.2 ng·h/mL). This variation might be due to differences in infusion time, the patients’ accompanying diseases, and analytical method for measuring the plasma concentration.
As shown in Figure 2, human pharmacokinetics of vinorelbine can be best described by a three-compartment model. As indicated by the large apparent volume of distribution seen after the i.v. administration of 30 mg/m2 in NSCLC patients, vinorelbine is extensively distributed to tissues. There are considerable inter-patient differences in estimated vinorelbine volume of distribution, some of which may be due to the patients’ characteristics: body surface area, gender, and platelet count before administration (Nguyen et al., 2002). CL and T1/2 did not vary significantly with dosage and route of administration, which were close to the values reported in previous studies (Gebbia and Puozzo, 2005; Caffo et al., 2013).
Neutropenia was the most common AE, and all symptoms related to the drug neurotoxicity are usually reversible and recover after treatment discontinuation. A range of gastrointestinal disturbance AEs was also seen, although these were mild. During the study period, only two patient (2/23, 8.7%) using Navelbine experienced 1–3 grade infusion-site reactions. The incidence of infusion-site reactions in our study was much lower than that reported in previous studies (29%) (Yoh et al., 2007; Schupp et al., 2008). This may due to the fact that quite a number of patients received the test or reference product through a central line in current study. Further studies for the control of local venous toxicity of the new i.v. formulation are warranted. Overall, our results are consistent with previous safety data and show no new safety signal (Marty et al., 2001; Hirsh et al., 2007; Briasoulis et al., 2013; Sutiman et al., 2016; Bilir et al., 2017; Kaburaki et al., 2017).
At present, vinorelbine is available for administration in both i.v. and oral formulations. Previous studies have proved that oral form shows similar inter-individual variability, same metabolism pattern, reproducible intra-patient blood exposure, and same pharmacokinetic–pharmacodynamic relationship (Gebbia and Puozzo, 2005; Hirsh et al., 2007). Several advantages for the i.v. administration compared with the oral one have been suggested. The patients’ compliance can be better controlled when using i.v. administration. Moreover, i.v. vinorelbine may be required for patients with difficulty swallowing or to avoid absorption variability.
This pharmacokinetic study had some limitations, which should be considered. In our study there was an imbalance in the proportion of female and male subjects, with approximately 80% being male. In addition, levels of free drug and the active metabolite 4-O-deacetylvinorelbine were also not measured in our study, which may be higher in patients with liver dysfunction (Gong et al., 2019). Therefore, caution should be exercised when extrapolating these data to patients with abnormal liver function. As vinorelbine continues to remain a viable treatment option in refractory settings and continues development as part of novel combination therapeutic strategies including immunotherapy, ongoing investigations of its safety and pharmacokinetics are warranted.
This study, performed in NSCLC patients, has demonstrated that this new emulsion formulation was pharmacokinetically equivalent to Navelbine. The reported adverse events for the two formulations are similar. It is assumed that ANX would improve tolerability and compliance in patients with cancer, especially those with increased risk of vein toxicity. Further study is warranted to explore its clinical application.
All datasets generated for this study are included in the manuscript and the supplementary files.
The study protocol and its amendments were reviewed and approved by the ethics committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (approval no. 2012-EC-60). The trial was designed according to the current revised Declaration of Helsinki and conducted in accordance with Good Clinical Practice guidelines. Written informed consent was obtained from each participating patient before entry into the study.
All of the authors were involved in the design of the study, acquisition, analysis, or interpretation of data for the work, drafting the manuscript or revising it critically for important intellectual content. The authors were fully involved in all content and editorial decisions, were involved in all stages of manuscript development, and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Qiong Zhao for undertaking the quality control through the entire clinical trial and Meixiang Zhu for coordinating the bioanalytical analysis. The authors would also like to thank the contributions from the study sponsor Hangzhou Minsheng Pharmaceutical Group Co., Ltd.
Bilir, C., Durak, S., Kizilkaya, B., Hacibekiroglu, I., Nayir, E., Engin, H. (2017). Efficacy of metronomic vinorelbine in elderly patients with advanced non-small-cell lung cancer and poor performance status. Curr. Oncol. 24 (3), e199–e204. doi: 10.3747/co.24.3486
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi: 10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi: 10.1056/NEJMoa1504627
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi: 10.3322/caac.21492
Briasoulis, E., Aravantinos, G., Kouvatseas, G., Pappas, P., Biziota, E., Sainis, I., et al. (2013). Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. BMC Cancer 13, 263. doi: 10.1186/1471-2407-13-263
Bylicki, O., Barazzutti, H., Paleiron, N., Margery, J., Assie, J. B., Chouaid, C. (2019). First-line treatment of non-small-cell lung cancer (NSCLC) with immune checkpoint inhibitors. BioDrugs 33 (2), 159–171. doi: 10.1007/s40259-019-00339-4
Caffo, O., Dipasquale, M., Murgia, V., Veccia, A., Galligioni, E. (2013). An evaluation of the pharmacokinetics and clinical use of vinorelbine for NSCLC treatment. Expert Opin. Drug Metab. Toxicol. 9 (8), 1037–1051. doi: 10.1517/17425255.2013.804065
Crawford, J. (1996). Update: vinorelbine (navelbine) in non-small cell lung cancer. Semin. Oncol. 23 ( Suppl 5), 2–7.
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030), 1837–1846. doi: 10.1016/S0140-6736(16)00587-0
Gebbia, V., Puozzo, C. (2005). Oral versus intravenous vinorelbine: clinical safety profile. Expert Opin. Drug Saf. 4 (5), 915–928. doi: 10.1517/14740338.4.5.915
Gong, J., Cho, M., Gupta, R., Synold, T. W., Frankel, P., Ruel, C., et al. (2019). A pilot study of vinorelbine safety and pharmacokinetics in patients with varying degrees of liver dysfunction. Oncologist. doi: 10.1634/theoncologist.2018-0336
Gridelli, C. (2001). The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Oncologist 6 Suppl 1, 4–7. doi: 10.1634/theoncologist.6-suppl_1-4
Hardin, C., Shum, E., Singh, A. P., Perez-Soler, R., Cheng, H. (2017). Emerging treatment using tubulin inhibitors in advanced non-small cell lung cancer. Expert Opin. Pharmacother. 18 (7), 16. doi: 10.1080/14656566.2017.1316374
Hirsh, V., Desjardins, P., Needles, B. M., Rigas, J. R., Jahanzeb, M., Nguyen, L., et al. (2007). Oral versus intravenous administration of vinorelbine as a single agent for the first-line treatment of metastatic nonsmall cell lung carcinoma (NSCLC): a randomized phase II trial. Am. J. Clin. Oncol. 30 (3), 245–251. doi: 10.1097/01.coc.0000256103.21797.e5
Jones, S. F., Burris, H. A., 3rd (1996). Vinorelbine: a new antineoplastic drug for the treatment of non-small-cell lung cancer. Ann. Pharmacother. 30 (5), 501–506. doi: 10.1177/106002809603000513
Kaburaki, K., Isobe, K., Kobayashi, H., Yoshizawa, T., Takai, Y., Homma, S. (2017). A feasibility study of bevacizumab and vinorelbine in patients with previously treated advanced non-squamous non-small-cell lung cancer. Mol. Clin. Oncol. 6 (4), 510–514. doi: 10.3892/mco.2017.1187
Khayat, D., Rixe, O., Brunet, R., Goupil, A., Bugat, R., Harousseau, J. L., et al. (2004). Pharmacokinetic linearity of i.v. Cancer Chemother. Pharmaco.l 54 (3), 193–205. doi: 10.1007/s00280-004-0794-1
Lesueur, P., Martel-Laffay, I., Escande, A., Kissel, M., Locher, C., Gervais, R., et al. (2018). Oral vinorelbine-based concomitant chemoradiotherapy in unresectable stage III non-small cell lung cancer: a systematic review. Expert Rev. Anticancer Ther. 18 (11), 1159–1165. doi: 10.1080/14737140.2018.1518714
Li, Y., Jin, W., Yan, H., Liu, H., Wang, C. (2013). Development of intravenous lipid emulsion of vinorelbine based on drug-phospholipid complex technique. Int. J. Pharm. 454 (1), 472–477. doi: 10.1016/j.ijpharm.2013.06.032
Lush, R. M., McCune, J. S., Tetteh, L., Thompson, J. A., Mahany, J. J., Garland, L., et al. (2005). The absolute bioavailability of oral vinorelbine in patients with solid tumors. Cancer Chemother. Pharmaco.l 56 (6), 578–584. doi: 10.1007/s00280-005-1025-0
Marquet, P., Lachatre, G., Debord, J., Eichler, B., Bonnaud, F., Nicot, G. (1992). Pharmacokinetics of vinorelbine in man. Eur. J. Clin. Pharmacol. 42 (5), 545–547. doi: 10.1007/BF00314866
Marty, M., Fumoleau, P., Adenis, A., Rousseau, Y., Merrouche, Y., Robinet, G., et al. (2001). Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann. Oncol. 12 (11), 1643–1649. doi: 10.1023/A:1013180903805
Mok, T. S. K., Wu, Y. L., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 393 (10183), 1819–1830. doi: 10.1016/S0140-6736(18)32409-7
Nguyen, L., Tranchand, B., Puozzo, C., Variol, P. (2002). Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br. J. Clin. Pharmacol. 53 (5), 459–468. doi: 10.1046/j.1365-2125.2002.01581.x
Qian, J., Wang, Y., Chang, J., Zhang, J., Wang, J., Hu, X. (2011). Rapid and sensitive determination of vinorelbine in human plasma by liquid chromatography-tandem mass spectrometry and its pharmacokinetic application. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879 (9-10), 662–668. doi: 10.1016/j.jchromb.2011.01.039
Reck, M., Popat, S., Reinmuth, N., De Ruysscher, D., Kerr, K. M., Peters, S., et al. (2014). Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25 Suppl 3, iii27–39. doi: 10.1093/annonc/mdu199
Requejo, H., Arboit, L., Arroyo, G., Blajman, C., Fein, L., Quintana, P., et al. (2008). Pharmacokinetic equivalence observed between an emulsion formulation of vinorelbine (anx-530) and vinorelbine solution in a clinical study of patients with advanced cancer. Cancer Res. 68, 270.
Robieux, I., Sorio, R., Borsatti, E., Cannizzaro, R., Vitali, V., Aita, P., et al. (1996). Pharmacokinetics of vinorelbine in patients with liver metastases. Clin. Pharmacol. Ther. 59 (1), 32–40. doi: 10.1016/S0009-9236(96)90021-1
Schilling, T., Fiebig, H. H., Kerpel-Fronius, S., Winterhalter, B., Variol, P., Tresca, P., et al. (1996). Clinical phase I and pharmacokinetic trial of vinorelbine administered as single intravenous bolus every 21 days in cancer patients. Invest. New Drugs 14 (4), 371–378. doi: 10.1007/BF00180813
Schupp, J., Lopez, L. P., Robbins, J. M., Arboit, L., Requejo, H., Arroyo, G., et al. (2008). Tolerability and incidence of infusion site reactions with emulsion formulation of vinorelbine (ANX-530) compared to vinorelbine solution. J. Clin. Oncol. 26 (15), 13549. doi: 10.1200/jco.2008.26.15_suppl.13549
Schvartsman, G., Ferrarotto, R., Massarelli, E. (2016). Checkpoint inhibitors in lung cancer: latest developments and clinical potential. Ther. Adv. Med. Oncol. 8 (6), 460–473. doi: 10.1177/1758834016661164
Sutiman, N., Zhang, Z., Tan, E. H., Ang, M. K., Tan, S. W., Toh, C. K., et al. (2016). Phase I study of oral vinorelbine in combination with erlotinib in advanced non-small cell lung cancer (NSCLC) using two different schedules. PLoS One 11 (5), e0154316. doi: 10.1371/journal.pone.0154316
Wargin, W. A., Lucas, V. S. (1994). The clinical pharmacokinetics of vinorelbine (Navelbine). Semin. Oncol. 21 (5 Suppl 10), 21–27.
Winton, T., Livingston, R., Johnson, D., Rigas, J., Johnston, M., Butts, C., et al. (2005). Vinorelbine plus cisplatin vs. N. Engl. J. Med. 352 (25), 2589–2597. doi: 10.1056/NEJMoa043623
Yang, S. H., Lin, C. C., Lin, Z. Z., Tseng, Y. L., Hong, R. L. (2012). A phase I and pharmacokinetic study of liposomal vinorelbine in patients with advanced solid tumor. Invest. New Drugs 30 (1), 282–289. doi: 10.1007/s10637-010-9522-3
Keywords: pharmacokinetics, bioavailability, safety, vinorelbine, non-small-cell lung cancer
Citation: Wu G, Wu L, Zhou H, Lin M, Peng L, Wang Y, Zhai Y, Hu X, Zheng Y, Lv D, Liu J and Shentu J (2019) A Phase I Comparative Pharmacokinetic and Safety Study of Two Intravenous Formulations of Vinorelbine in Patients With Advanced Non-Small Cell Lung Cancer. Front. Pharmacol. 10:774. doi: 10.3389/fphar.2019.00774
Received: 14 February 2019; Accepted: 14 June 2019;
Published: 11 July 2019.
Edited by:
Jiangjiang Qin, Zhejiang Chinese Medical University, ChinaCopyright © 2019 Wu, Wu, Zhou, Lin, Peng, Wang, Zhai, Hu, Zheng, Lv, Liu and Shentu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzhong Shentu, c3RqekB6anUuZWR1LmNu
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.