
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 02 August 2019
Sec. Pharmacology of Ion Channels and Channelopathies
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00771
 Ibrahim El-Battrawy1,2*
Ibrahim El-Battrawy1,2* Johanna Besler1
Johanna Besler1 Xin Li1,2
Xin Li1,2 Huan Lan1,2
Huan Lan1,2 Zhihan Zhao1,2
Zhihan Zhao1,2 Volker Liebe1
Volker Liebe1 Rainer Schimpf1
Rainer Schimpf1 Siegfried Lang1,2
Siegfried Lang1,2 Christian Wolpert1
Christian Wolpert1 Xiaobo Zhou1,2
Xiaobo Zhou1,2 Ibrahim Akin1,2†
Ibrahim Akin1,2† Martin Borggrefe1,2†
Martin Borggrefe1,2†Short QT syndrome (SQTS) is associated with sudden cardiac arrest. There are limited data on the impact of antiarrhythmic drugs on the outcome of SQTS.
Materials and Methods: We studied data that describe the clinical outcome of 62 SQTS patients treated with antiarrhythmic drugs, who were recruited from a pool of patients diagnosed in our institution and also from known databases after a systematic search of the published literature.
Results: Sixty-two SQTS patients treated with antiarrhythmic drugs were followed up over a median timeframe of 5.6 years (interquartile range 1.6–7.7 years). Six patients, in particular, received multiple drugs as a combination. Of the 55 patients treated with hydroquinidine (HQ), long-term prophylaxis was documented in 41 patients. Fourteen patients stopped treatment due to the following reasons: gastrointestinal intolerance (n = 4), poor compliance (n = 8), and no QTc prolongation (n = 2). Of the 41 patients treated with HQ, the QTc interval increased from 313.5 ± 17.2 to 380.1 ± 21.2 ms. Thirteen of the 41 patients suffered from at least one or more ventricular tachyarrhythmias (VAs) before HQ initiation. VAs are reduced in incidence after HQ treatment (13/41: 31% versus 3/41: 7.3%, p < 0.001).
Conclusion: HQ increases the corrected QT interval and prevents VAs in the majority of the patients in this cohort. HQ is safe for use in SQTS patients, particularly due to its low rate of side effects. Other antiarrhythmic drugs might be useful, but the data justifying their use are sparse.
Short QT syndrome (SQTS) is a rare channelopathy, and patients affected with this disorder are predisposed to a higher risk of developing ventricular tachyarrhythmias (VAs; Giustetto et al., 2011; Mazzanti et al., 2014). Although significant progress has been made in the last decade to better understand this channelopathy, there are still significant challenges that physicians face in its management. Since its first description in 2000 (Gussak et al., 2000), causative mutations have been identified in eight different genes: SQTS1 to SQTS3 are associated with gain-of-function mutations in potassium channels, whereas SQTS4 to SQTS6 are caused by loss-of-function of calcium channels, demonstrating some overlap with Brugada syndrome. A loss-of-function mutation in SCN5A was detected in a patient with SQTS and Brugada syndrome (Hong et al., 2012). Although there are no conclusive data regarding the association of this variant with SQTS, it was described as SQTS7. Recently, a mutation in the cardiac Cl/HCO3 exchanger AE3 was found to also cause SQTS and described as SQTS8 (Thorsen et al., 2017). Recently, clinical guidelines have recommended hydroquinidine (HQ) to treat SQTS patients (Priori et al., 2015b). Although different antiarrhythmic drugs have been tested in a small series of SQTS patients (Schimpf et al., 2007;Zhao et al., 2019), there are few data describing the long-term outcome of treated patients.
A recently published report by our research group showed a significant reduction of arrhythmia-like events in human cardiomyocytes from induced pluripotent stem cells established from patients with SQTS1 in the presence of N588K mutation after incubation of cells with 10 µM quinidine (El-Battrawy et al., 2018).
The aim of the present study was to consolidate our experience treating SQTS patients with reports from other groups using a systematic method of review.
We defined SQTS according to the Gollob criteria published in 2011 and the ESC criteria published in 2015 (Gollob et al., 2011; Priori et al., 2015b). Syncope was described as the transient loss of consciousness in the absence of other causes. An arrhythmic event was defined as a documented ventricular fibrillation (VF)/ventricular tachycardia (VT).
Of the 10 families admitted to our hospital with suspected SQTS, 7 families fulfilled the prescribed SQTS criteria. The family screening cascade further permitted the diagnosis of SQTS in 9 affected relatives. The QT interval was measured using the tangent method in the precordial lead presenting the highest T-wave amplitude in V2 or V3. The diagnosis of SQTS was independently reviewed by two experienced blinded cardiologists. DNA sequencing using next-generation sequencing of affected genes (KCNH2, KCNQ1, KCNJ2, CACNA1C, CACNB2, and CACNA2D1) for SQTS1 to SQTS6 was performed for the purpose of genetic screening and then analyzed for results.
The study was approved by the local ethics committee of the University Hospital Mannheim, University of Heidelberg, Germany. A written informed consent was not required by the local ethics committee due to the observational and retrospective characteristics of the study.
A literature search (PubMed, Web of Science, Cochrane Library, and Cinahl) with limits defining the publication dates up to 2018, use of the English language, and studies with human subjects was performed. Clinical studies earmarked included SQTS patients with well-described therapeutic lines of management (Figure 1). All these studies were based on data recorded from electrocardiograms (ECGs) and principally excluded patients with structural heart disease. Additionally, case reports and studies not reporting the outcome of antiarrhythmic drug use were excluded.
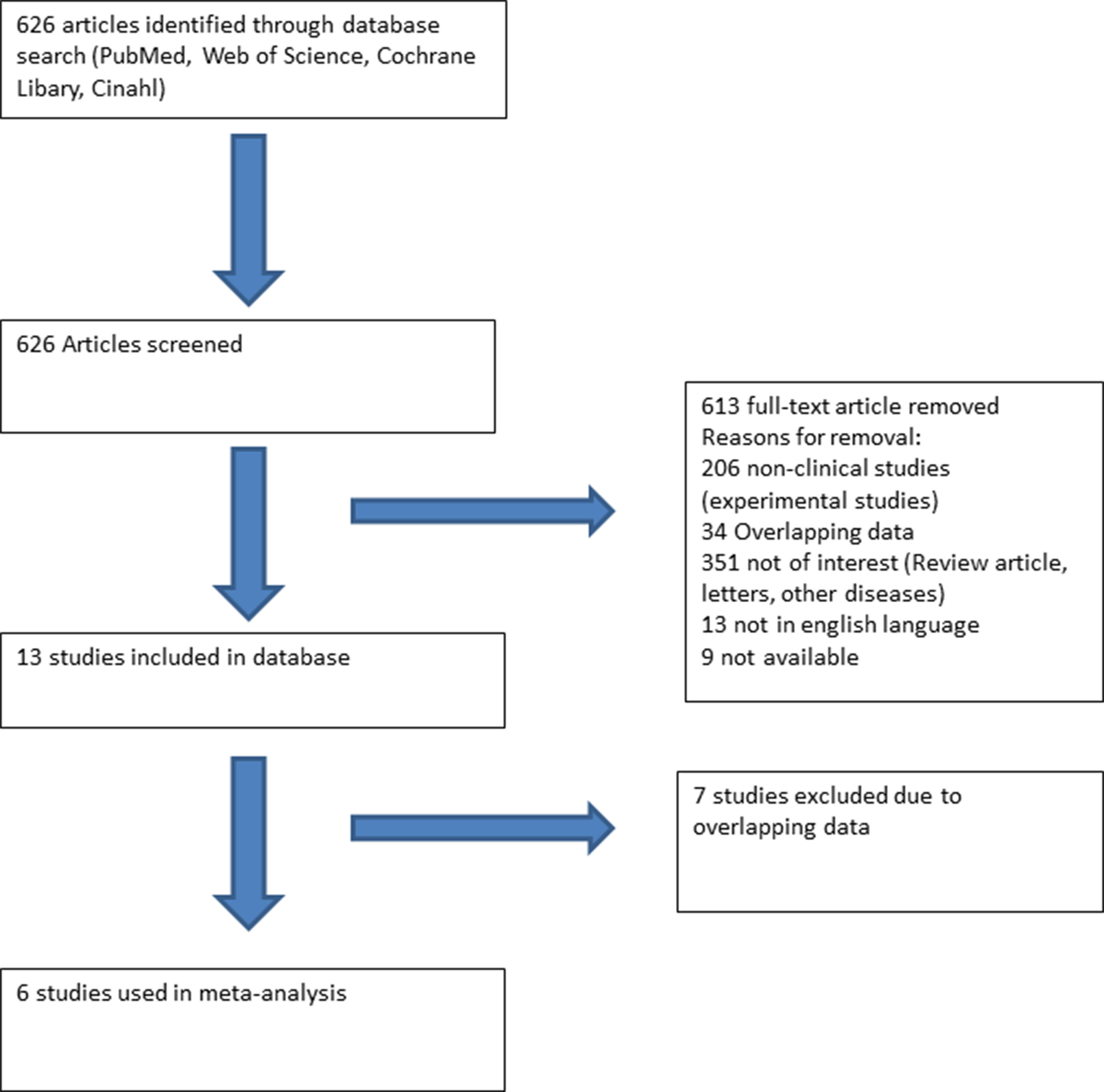
Figure 1 Flow chart presenting the systematic literature review using PubMed, Web of Science, Cochrane Library, and Cinahl. Six studies reporting the use and outcome of drugs in SQTS patients were included.
Data are presented as mean ± SD for continuous variables with a normal distribution, median [interquartile range (IQR)] for continuous variables with a nonnormal distribution, and frequency (%) for categorical variables. Kolmogorov-Smirnov test was used to assess normal distribution. Student’s t-test and Mann-Whitney U-test were used to compare continuous variables with normal and nonnormal distributions, respectively. Chi-square test or Fisher’s exact test was used to compare categorical variables.
The baseline characteristics of SQTS patients are illustrated in Table 1. Seven of the 10 SQTS families (n = 17 patients) fulfilled the criteria for SQTS and were retrospectively followed. Majority of the patients presented symptoms, including syncope (n = 5), palpitation (n = 8), and atrial fibrillation and atrial flutter (n = 9). Aborted sudden cardiac arrest (SCA) was documented in 2 (12%) patients with a confirmed short QT interval at admission and in 1 patient after receiving implantable cardioverter defibrillator (ICD) implantation. Eight (47%) patients were discharged with antiarrhythmic drugs, including HQ (n = 6, 35%) and bisoprolol (n = 2, 12%). Five (29%) patients underwent an ICD implantation, 3 of them were based on a high risk of SCA due to the abbreviated QTc interval of <300 ms and the presence of recurrent familial SCA.
We conducted a systematic literature review to identify SQTS patients and their outcomes after antiarrhythmic drug use. A total of six studies/cases were deemed eligible for follow-up. The patient pool from our center included 8 patients, which were consequently added to this analysis, thus leading to a total of 62 SQTS patients available for this study (Giustetto et al., 2011; Bun et al., 2012; Villafane et al., 2013; Suzuki et al., 2014; Giustetto et al., 2015; Mazzanti et al., 2017;El-Battrawy et al., 2018). The baseline characteristics of the included studies are illustrated in Table 1.
Different drugs were tested: HQ (n = 55), bisoprolol (n = 2), flecainide (n = 1), sotalol (n = 5), sotalol plus propafenone (n = 1), propafenone plus digoxin (n = 1), dofetilide plus digoxin (n = 1), disopyramide (n = 3), amiodarone (n = 1), and amiodarone plus metoprolol (n = 1). HQ prolonged the QTc interval in 52 of 55 (94%) patients (Table 3). Other treatment regimens, including sotalol, sotalol plus propafenone, flecainide, amiodarone, and bisoprolol failed to increase the QTc interval in treated patients. However, digoxin plus dofetilide, amiodarone plus metoprolol, and disopyramide with the higher dosage (400 mg/day) presented a QTc prolongation in ECG (Table 2 and Table S1).
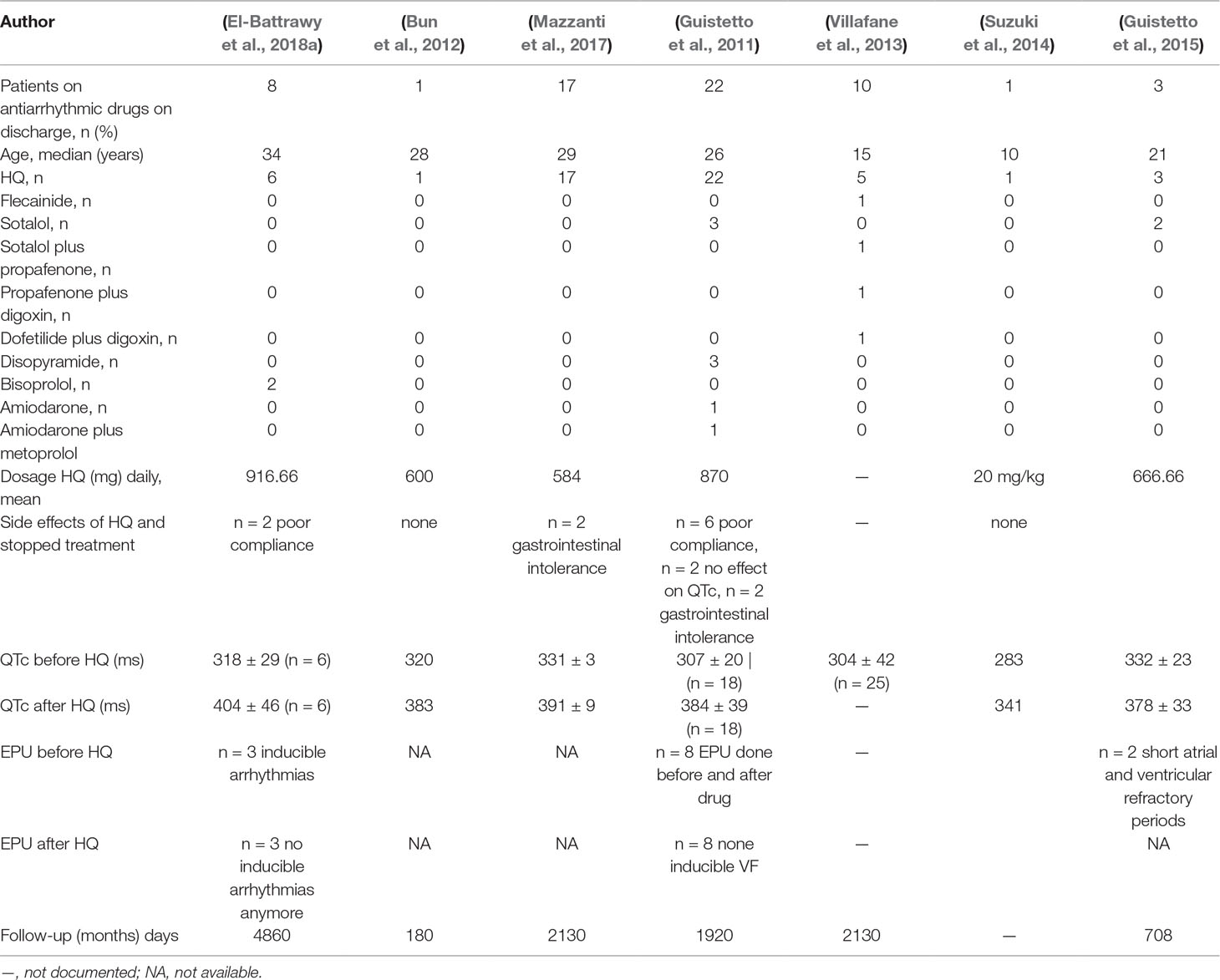
Table 2 Drug treatment strategy in SQTS patients (of Table 1) and side effects.
Of the 55 SQTS patients treated by HQ, only 41 patients reported long-term use after a median follow-up time of 5.6 years (IQR 0.5–13.5 years). The QTc interval was increased in all reported studies, except in two cases (Figure 2A). Thirteen patients suffered from at least one or more VA event before HQ initiation. VAs have been dropped after HQ treatment (31% versus 7.3%, p < 0.001; Figure 2B). There was no significant change in the rates of atrial fibrillation (Figure 2B). Fourteen patients stopped treatment due to the following reasons: gastrointestinal intolerance (n = 4), poor compliance (n = 8), and no QTc prolongation (n = 2).
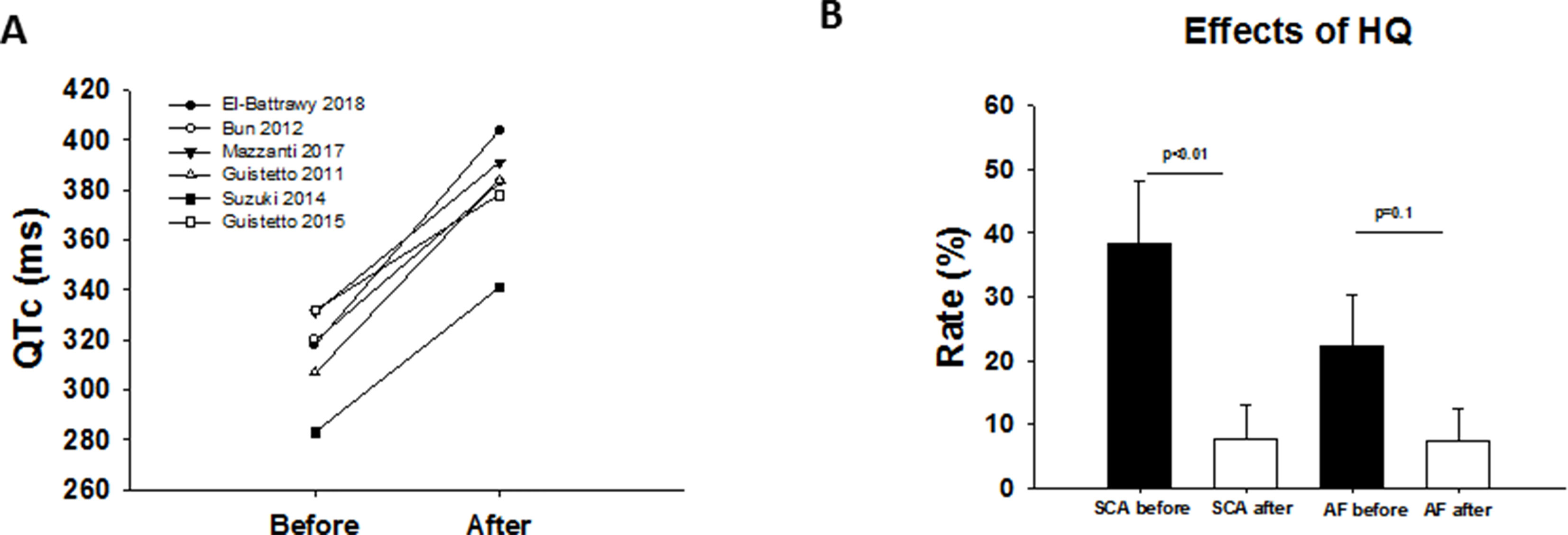
Figure 2 (A) Overview showing the QTc interval before and after drug treatment (HQ) in different studies. (B) Effect of HQ on VAs and atrial fibrillation rate.
In two clinical studies, an electrophysiological study was conducted before and after HQ treatment (n = 11; Figure 3A). In these cases, an VF/VT was found to be inducible before treatment. However, these could not be reproduced once treatment was initiated.
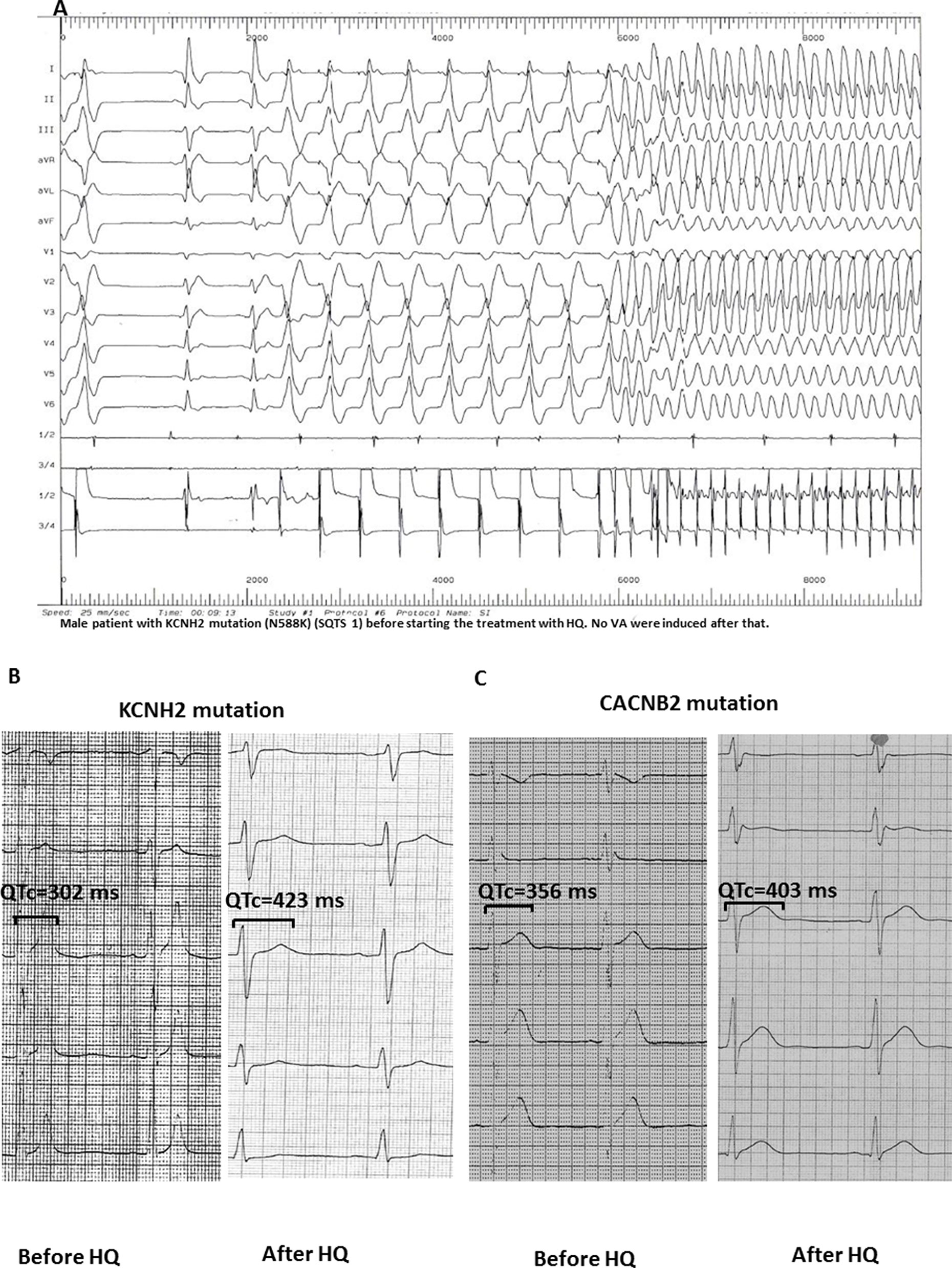
Figure 3 (A) Induction of ventricular flutter (CL 150 ms) in a patient with KCNH2 mutation (N588K; SQTS1) before starting HQ treatment. (B and C) After the application of HQ, the QTc interval (corrected by Bazett formula) was significantly increased in SQTS1 and SQTS5 patients without induction of arrhythmias in the electrophysiology study.
Table 3 includes all studies with a detailed description of baseline characteristics such as gender, QTc, gene mutation, and symptoms including atrial fibrillation and VAs of patients treated with HQ. Two studies (Giustetto et al., 2011; Villafane et al., 2013) were excluded due to the absence of details of every patient. Using cases with available information about the SQTS-related gene, the outcome data, a detailed analysis of the mutation-specific effects of HQ on QTc and arrhythmias was performed. In the mutation group, seven patients had a mutation in KCNH2 [either N588K (n = 4) or T618I (n = 3)] and four other patients had mutations in CACNA1C [G490R (n = 1)], CACNB2B [C1422T (n = 1)], KCNQ1 [R259H (n = 1)], and KCNJ2 [D172N (n = 1)]. In all patients, except two patients with KCNH2 mutation in T618I, life-threatening arrhythmias were suppressed. Although the QTc interval of the whole population was significantly increased, a detailed grouping of patients regarding gene mutations showed some differences. For example, although the QTc was prolonged in the presence of KCNH2 mutations (Figure 4A), this prolongation was only significant in the presence of N588K mutation (Figure 4B) but not in the presence of T618I mutation (Figure 4C). In patients with SQTS2 to SQTS5 (mutations in CACNA1C, CACNB2B, KCNQ1, and KCNJ2), QTc was also significantly prolonged by HQ (Figure 4D). Taking all these data together, it seems like that patients with T618I mutation of KCNH2 does not respond to HQ treatment.
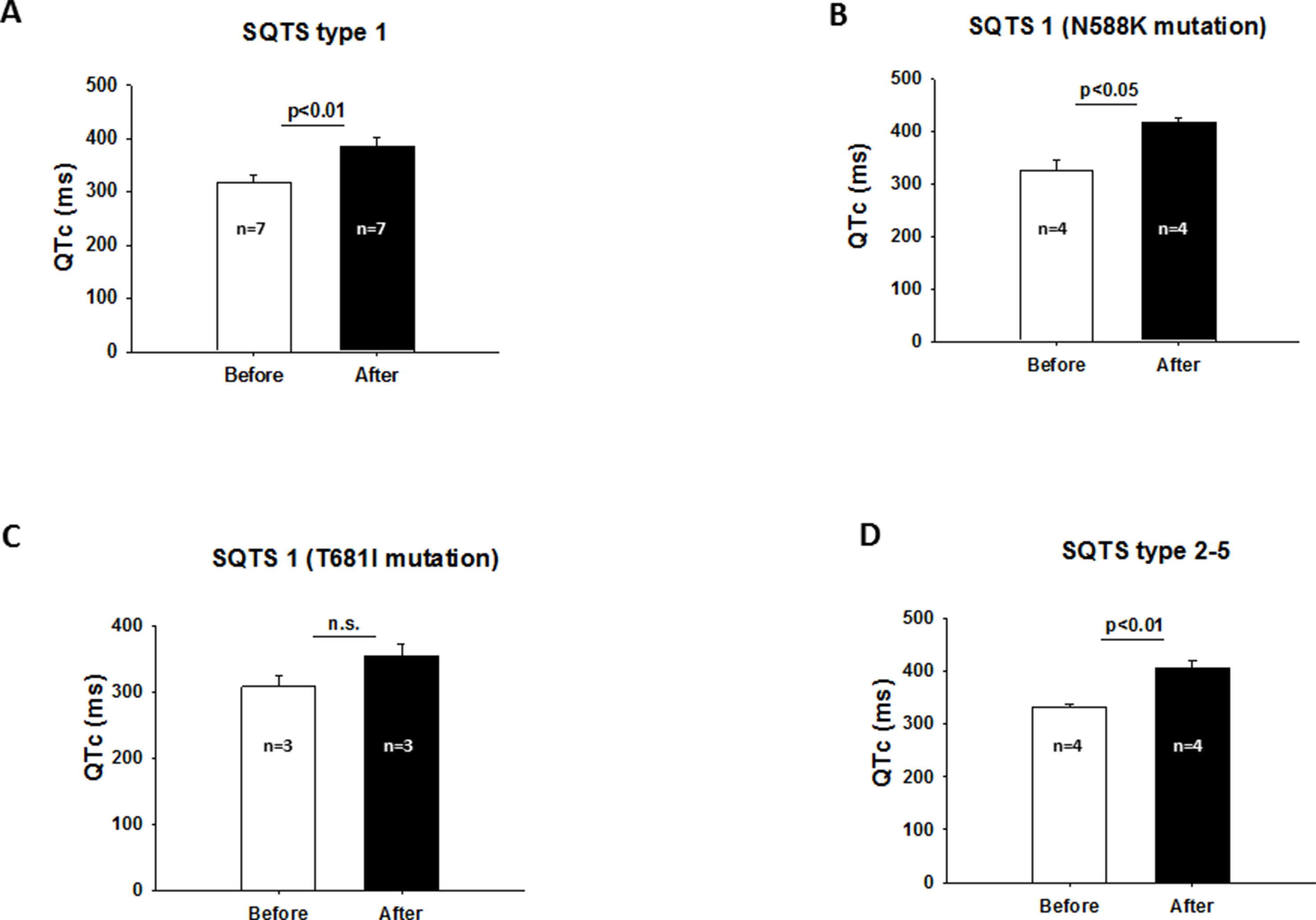
Figure 4 (A) Increased QTc interval after HQ treatment in the presence of SQTS1. (B) QTc interval was significantly prolonged in the presence of N588K mutation of KCNH2 after HQ treatment. (C) QTc interval was not significantly prolonged after HQ use in the presence of KCNH2 mutation in T618I. (D) Effect of HQ on QTc interval in the presence of SQTS2 to SQTS5.
All studies with a detailed description of the baseline characteristics of SQTS treatment with other drugs are summarized in Table S1. Digoxin plus dofetilide and amiodarone plus metoprolol showed efficacy for preventing arrhythmias in SQTS patients.
We have described the short- and long-term impact of antiarrhythmic drugs in SQTS patients from our hospital and those from a systematic literature review and can ascertain the following: i) HQ is safe for use among SQTS patients; ii) HQ significantly reduces VAs and consequently ICD shocks; iii) the benefit of HQ might be higher in SQTS patients of unknown origin; and iv) sotalol, sotalol plus propafenone, flecainide, amiodarone, disopyramide, and bisoprolol failed to show beneficial effects on SQTS.
ICD implantation has been recommended for SQTS patients (Priori et al., 2015a). However, ICD implantation is associated with adverse events and survivors of VA events are at high risk of recurrence several years later with risk of repeated ICD shocks. Therefore, a medical approach needs to be considered for SQTS patients.
In our center, the medical line of management was pursued among eight affected patients. HQ was initiated in six patients, with poor compliance in two of these patients. Two patients, who were started on beta-blockers, did not show changes of the QTc interval.
A systematic review of the literature showed that HQ use significantly reduced the recurrence of VAs. Additionally, in electrophysiological studies, VT/VF could not be induced after HQ use (11 of 11 patients). About 7% of the cases in the patient cohort initiated on HQ reported side effects such as gastrointestinal intolerance, leading to the termination of treatment. Importantly, HQ did not induce arrhythmias. Sotalol was used in five SQTS patients with no effect on the QTc interval.
HQ is a multichannel blocker. Due to the block of the IKr, it is able to prolong the action potential duration and consequently the QTc interval. However, sotalol and amiodarone, which are also IKr blockers and prototypical QT-prolonging drugs, were ineffective in patients with SQTS1. Of note, whereas amiodarone is a multichannel blocker, sotalol is a beta-blocker. It has been suggested that drug effects in SQTS might be based on the type of mutation. Some studies have demonstrated that the site mutation of N588K in HERG channel attenuates the inactivation of the channel and thus increases the whole-cell channel current at physiological voltages by shifting rightward the voltage dependence of inactivation (Cordeiro et al., 2005; Mcpate et al., 2005). Strikingly, this inactivation defect reduces strongly the sensitivity of the channel to some IKr blockers such as sotalol and E-4031, which have the highest effect in the inactivated state of HERG and are therefore less effective in inhibiting the channel currents (Vitali Serdoz et al., 2019). HQ has a similar effect in the open and inactivated states of HERG channels and is therefore effective in inhibiting IKr and treating SQTS1 patients.
Another study demonstrated that double mutations in HERG channel further enhance the impairment of the channel gating and channel response to drugs (Mcpate et al., 2008). In the presence of N588K and S631A mutations, the channel inactivation and the effect of HQ were more strongly attenuated compared to N588K or S631A mutation alone. Taken together, mutations in an ion channel can influence both channel gating and drug effects. In our present systematic review, two of three patients carrying a KCNH2 mutation in T618I recurrently suffered from VAs despite HQ treatment and the QTc interval was not significantly increased. Therefore, it might be speculated that T618I mutation impacts the sensitivity of the channel to HQ and therefore its antiarrhythmic effect in SQTS patients, although the effects of HQ on IKr mediated by HERG channels with T618I needs to be examined.
This study is consistent with recently published data by our research group studying human cardiomyocytes from induced pluripotent stem cells derived from SQTS donors (El-Battrawy et al., 2018). Our in vitro study showed that quinidine led to a prolongation of the action potential duration, consistent with a prolongation of the QTc interval, leading to a significant reduction in arrhythmia-like events among SQTS patients, whereas other antiarrhythmic drugs such as sotalol and metoprolol did not show any effect.
As the prevalence of SQTS is low (Bjerregaard, 2018), there are little data documenting the experience of drug treatments in this scenario. The documented adverse events in 7% of patients treated with HQ may provide an impetus to test other drugs. Our systematic literature analysis showed serial cases of use of treatment regimens such as digoxin plus dofetilide, amiodarone plus metoprolol, and disopyramide, which caused QTc prolongation (Gaita et al., 2004; Wolpert et al., 2005; Mizobuchi et al., 2008; Giustetto et al., 2011). However, these data need to be confirmed in the laboratory followed by testing in a clinical scenario involving a higher number of patients.
HQ, an off-label drug, increases the QTc interval and reduces VA events in SQTS patients. Due to the adverse effects and ineffectiveness of HQ in some patients, searching for other antiarrhythmic drugs is required.
First, indications for HQ use have not been homogeneous throughout the study. HQ use was dependent on the experience of the clinical team and no control group has been identified. Second, these studies were not randomized, and no comparative analyses were drawn to other drugs. Third, it is not excluded that SQTS-related genes were not screened; therefore, the SQTS-related gene is underestimated. Finally, although this study represents the largest data accumulation among the recently published reports, it may still not be sufficient to fully understand the true outcome of SQTS patients initiated on HQ treatment. A further significant limitation is that QT interval measurement using the tangent method in the precordial lead presenting the highest T-wave amplitude in V2 or V3 might be biased by interobserver variability and may not be consistent with those data from the our institution.
IE-B, XL, ZZ, JB, MB, and IA contributed to the conception and design of the work. RS, SL, and CW contributed to the acquisition, analysis, and interpretation of data for the work. IE-L, MB and IA drafted the manuscript. XZ, VL, RS, and CW critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00771/full#supplementary-material
Bjerregaard, P. (2018). The diagnosis and management of short QT syndrome. Heart Rhythm. 15 (8), 1261–1267. doi: 10.1016/j.hrthm.2018.02.034
Bun, S. S., Maury, P., Giustetto, C., Deharo, J. C. (2012). Electrical storm in short-QT syndrome successfully treated with isoproterenol. J. Cardiovasc. Electrophysiol. 23, 1028–1030. doi: 10.1111/j.1540-8167.2012.02295.x
Cordeiro, J. M., Brugada, R., Wu, Y. S., Hong, K., Dumaine, R. (2005). Modulation of I(Kr) inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc. Res. 67, 498–509. doi: 10.1016/j.cardiores.2005.02.018
El-Battrawy, I., Besler, J., Liebe, V., Schimpf, R., Tulumen, E., Rudic, B., et al. (2018). Long-term follow-up of patients with short QT syndrome: clinical profile and outcome. J. Am. Heart Assoc. 7 (7). (23), e010073.
El-Battrawy, I., Lan, H., Cyganek, L., Zhao, Z., Li, X., Buljubasic, F., et al. (2018). Modeling short QT syndrome using human-induced pluripotent stem cell-derived cardiomyocytes. J. Am. Heart Assoc. 7(7). doi: 10.1161/JAHA.117.007394
Gaita, F., Giustetto, C., Bianchi, F., Schimpf, R., Haissaguerre, M., Calo, L., et al. (2004). Short QT syndrome: pharmacological treatment. J. Am. Coll. Cardiol. 43, 1494–1499. doi: 10.1016/j.jacc.2004.02.034
Giustetto, C., Schimpf, R., Mazzanti, A., Scrocco, C., Maury, P., Anttonen, O., et al. (2011). Long-term follow-up of patients with short QT syndrome. J. Am. Coll. Cardiol. 58, 587–595. doi: 10.1016/j.jacc.2011.03.038
Giustetto, C., Scrocco, C., Giachino, D., Rapezzi, C., Mognetti, B., Gaita, F. (2015). The lack of effect of sotalol in short QT syndrome patients carrying the T618I mutation in the KCNH2 gene. HeartRhythm Case Rep. 1, 373–378. doi: 10.1016/j.hrcr.2015.07.001
Gollob, M. H., Redpath, C. J., Roberts, J. D. (2011). The short QT syndrome: proposed diagnostic criteria. J. Am. Coll. Cardiol. 57, 802–812. doi: 10.1016/j.jacc.2010.09.048
Gussak, I., Brugada, P., Brugada, J., Wright, R. S., Kopecky, S. L., Chaitman, B. R., et al. (2000). Idiopathic short QT interval: a new clinical syndrome? Cardiology 94, 99–102. doi: 10.1159/000047299
Hong, K., Hu, J., Yu, J., Brugada, R. (2012). Concomitant Brugada-like and short QT electrocardiogram linked to SCN5A mutation. Eur. J. Hum. Genet. 20, 1189–1192. doi: 10.1038/ejhg.2012.63
Mazzanti, A., Kanthan, A., Monteforte, N., Memmi, M., Bloise, R., Novelli, V., et al. (2014). Novel insight into the natural history of short QT syndrome. J. Am. Coll. Cardiol. 63, 1300–1308. doi: 10.1016/j.jacc.2013.09.078
Mazzanti, A., Maragna, R., Vacanti, G., Kostopoulou, A., Marino, M., Monteforte, N., et al. (2017). Hydroquinidine prevents life-threatening arrhythmic events in patients with short QT syndrome. J. Am. Coll. Cardiol. 70, 3010–3015. doi: 10.1016/j.jacc.2017.10.025
Mcpate, M. J., Duncan, R. S., Hancox, J. C., Witchel, H. J. (2008). Pharmacology of the short QT syndrome N588K-hERG K+ channel mutation: differential impact on selected class I and class III antiarrhythmic drugs. Br. J. Pharmacol. 155, 957–966. doi: 10.1038/bjp.2008.325
Mcpate, M. J., Duncan, R. S., Milnes, J. T., Witchel, H. J., Hancox, J. C. (2005). The N588K-HERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 degrees C. Biochem. Biophys. Res. Commun. 334, 441–449. doi: 10.1016/j.bbrc.2005.06.112
Mizobuchi, M., Enjoji, Y., Yamamoto, R., Ono, T., Funatsu, A., Kambayashi, D., et al. (2008). Nifekalant and disopyramide in a patient with short QT syndrome: evaluation of pharmacological effects and electrophysiological properties. Pacing Clin. Electrophysiol. 31, 1229–1232. doi: 10.1111/j.1540-8159.2008.01169.x
Priori, S. G., Blomstrom-Lundqvist, C., Mazzanti, A., Blom, N., Borggrefe, M., Camm, J., et al. (2015a). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 36, 2793–2867. doi: 10.1093/eurheartj/ehv316
Priori, S. G., Blomstrom-Lundqvist, C., Mazzanti, A., Blom, N., Borggrefe, M., Camm, J., et al. (2015b). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 17, 1601–1687. doi: 10.1714/2174.23496
Schimpf, R., Veltmann, C., Giustetto, C., Gaita, F., Borggrefe, M., Wolpert, C. (2007). In vivo effects of mutant HERG K+ channel inhibition by disopyramide in patients with a short QT-1 syndrome: a pilot study. J. Cardiovasc. Electrophysiol. 18, 1157–1160. doi: 10.1111/j.1540-8167.2007.00925.x
Suzuki, H., Hoshina, S., Ozawa, J., Sato, A., Minamino, T., Aizawa, Y., et al. (2014). Short QT syndrome in a boy diagnosed on screening for heart disease. Pediatr. Int. 56, 774–776. doi: 10.1111/ped.12308
Thorsen, K., Dam, V. S., Kjaer-Sorensen, K., Pedersen, L. N., Skeberdis, V. A., Jurevicius, J., et al. (2017). Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat. Commun. 8, 1696. doi: 10.1038/s41467-017-01630-0
Villafane, J., Atallah, J., Gollob, M. H., Maury, P., Wolpert, C., Gebauer, R., et al. (2013). Long-term follow-up of a pediatric cohort with short QT syndrome. J. Am. Coll. Cardiol. 61, 1183–1191. doi: 10.1016/j.jacc.2012.12.025
Vitali Serdoz, L., Rittger, H., Furlanello, F., Bastian, D. (2019). Quinidine — a legacy within the modern era of antiarrhythmic therapy. Pharmacol. Res. 144, 257–263. doi: 10.1016/j.phrs.2019.04.028
Wolpert, C., Schimpf, R., Giustetto, C., Antzelevitch, C., Cordeiro, J., Dumaine, R., et al. (2005). Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J. Cardiovasc. Electrophysiol. 16, 54–58. doi: 10.1046/j.1540-8167.2005.04470.x
Keywords: short QT syndrome, sudden cardiac arrest, channelopathy, congenital disease, arrhythmia
Citation: El-Battrawy I, Besler J, Li X, Lan H, Zhao Z, Liebe V, Schimpf R, Lang S, Wolpert C, Zhou X, Akin I and Borggrefe M (2019) Impact of Antiarrhythmic Drugs on the Outcome of Short QT Syndrome. Front. Pharmacol. 10:771. doi: 10.3389/fphar.2019.00771
Received: 28 January 2019; Accepted: 14 June 2019;
Published: 02 August 2019.
Edited by:
Jean-Yves Le Guennec, Université de Montpellier, FranceReviewed by:
Daniel M. Johnson, University of Birmingham, United KingdomCopyright © 2019 El-Battrawy, Besler, Li, Lan, Zhao, Liebe, Schimpf, Lang, Wolpert, Zhou, Akin and Borggrefe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim El-Battrawy, SWJyYWhpbS5lbC1iYXR0cmF3eUB1bW0uZGU=
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.