- 1Guangdong Provincial Key Laboratory of Molecular Target & Clinical Pharmacology, School of Pharmaceutical Sciences and the Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 2Institute of Respiratory and Occupational Diseases, Collaborative Innovation Center for Cancer, Medical College, Shanxi Datong University, Datong, China
- 3School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China
- 4Infinitus (China) Company Ltd., Jiangmen, China
As a quinonemethide triterpenoid extracted from species of the Celastraceae and Hippocrateaceae, pristimerin has been shown potent anti-cancer effects. Specifically, it was found that pristimerin can affect many tumor-related processes, such as apoptosis, autophagy, migration and invasion, vasculogenesis, and drug resistance. Various molecular targets or signaling pathways are also involved, such as cyclins, reactive oxygen species (ROS), microRNA, nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and PI3K/AKT/mammalian target of rapamycin (mTOR) pathways. In this review, we will focus on the research about pristimerin-induced anti-cancer activities to achieve a deeper understanding of the targets and mechanisms, which offer evidences suggesting that pristimerin can be a potent anti-cancer drug.
Introduction
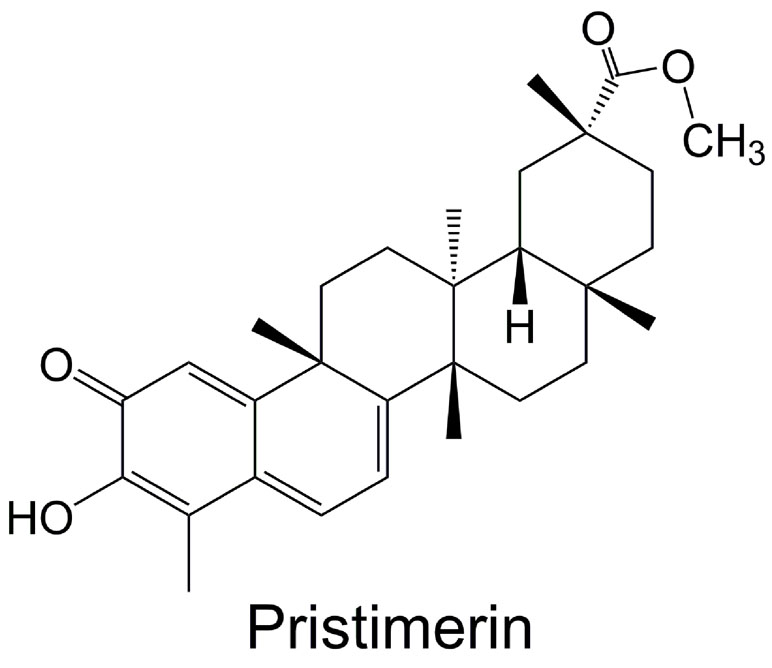
In recent years, natural compound has received more and more attention for use in treating human diseases and conditions, due to their long history of use and various pharmacological therapeutic effects (Tao et al., 2015; Zhang et al., 2015; Peng et al., 2016; Zhang et al., 2016; Lin et al., 2017), especially their relative safety (fewer and less severe side effects) than chemical drugs. Naturally occurring triterpenoid can be used as anti-cancer, anti-inflammatory, anti-malarial, and insecticidal agent (Deeb et al., 2012; Larsen et al., 2012; Kim et al., 2013; Deeb et al., 2014a). It has been proven that some natural or synthetic triterpenoids have promising clinical potential, exhibiting both therapeutic and chemopreventive activities for cancer (Salminen et al., 2008; Alessia et al., 2009; Ke et al., 2016). Pristimerin (20α-3-hydroxy-2-oxo-24-nor-friedela-1-10,3,5,7-tetraen-carboxylic acid-29-methylester, molecular formula: C30H40O4) (Figure 1), a methyl ester of celastrol, is a quinonemethide triterpenoid which has been extracted from a variety of species of the Celastraceae and Hippocrateaceae families, such as Hippocratea excels (Mena-Rejon et al., 2007), Maytenus heterophylla (Murayama et al., 2007), and Celastrus aculeatus Merr. (Tang et al., 2014). Pristimerin was first isolated in 1951 from Pristimerae indica and P. grahami and was first identified in 1954 to confirm its molecular structure (Kulkarni and Shah, 1954). Pristimerin has displayed different pharmacological effects, such as anti-cancer, anti-oxidant, anti-inflammatory, anti-bacterial, anti-malarial, and insecticidal activities (Figueiredo and Sequin, 1998; Avilla et al., 2000; Haroldo Jeller et al., 2004; Lopez et al., 2011; Kim et al., 2013; Wu et al., 2019). As such, it is being developed as a potential anti-cancer drug (Yousef et al., 2017). Here, we present and discuss current research findings with regard to pristimerin emphasis on the anti-cancer effect.
Pristimerin: Broad-Spectrum Anti-Cancer Effect
Cancer is a complicated disease, which starts with a normal change through the activation of proto-oncogenes or the suppression of tumor suppressor genes (Elmore, 2007). These alterations result in diversed and interactive changes at the level of cellular processes which are involved in the regulation of proliferation, differentiation, apoptosis, migration, and tissue homeostasis. Finally, biological properties for cancer cells are acquired, including infinite proliferation potential, independent exogenous growth factors, and resistance to death signals (Brattain et al., 1994; Dent and Aranda-Anzaldo, 2019; Petho et al., 2019; Shen et al., 2019).
Pristimerin exerts its effects influencing a series of biological properties of cancer cells. Recent studies on a wide range of cancer cell lines of different origins, such as oral cancer (Wu et al., 2019), colorectal cancer (Yousef et al., 2018), glioma (Yan et al., 2013), leukemia (Lu et al., 2010), breast cancer (Xie et al., 2016), lung cancer (Zhang et al., 2019), and prostate cancer (Liu et al., 2013), and also in cancer stem cells (Cevatemre et al., 2018). These results have proved that pristimerin possesses strong anti-proliferative activities with involvement of mitochondrial apoptosis, autophagy, and inhibition of nuclear factor kappa B (NF-κB), Akt (protein kinase B, PKB) and mitogen-activated protein kinase (MAPK) (Guo et al., 2013; Liu et al., 2013; Yan et al., 2013; Gao et al., 2014; Deeb et al., 2015).
In view of the potent anti-cancer effect in a broad spectrum (cancer cell lines and molecular targets), it possesses a great potential for pristimerin to develop as a multiple-target anti-cancer drug.
Pristimerin: Anti-Cancer Activities
Growth Inhibition
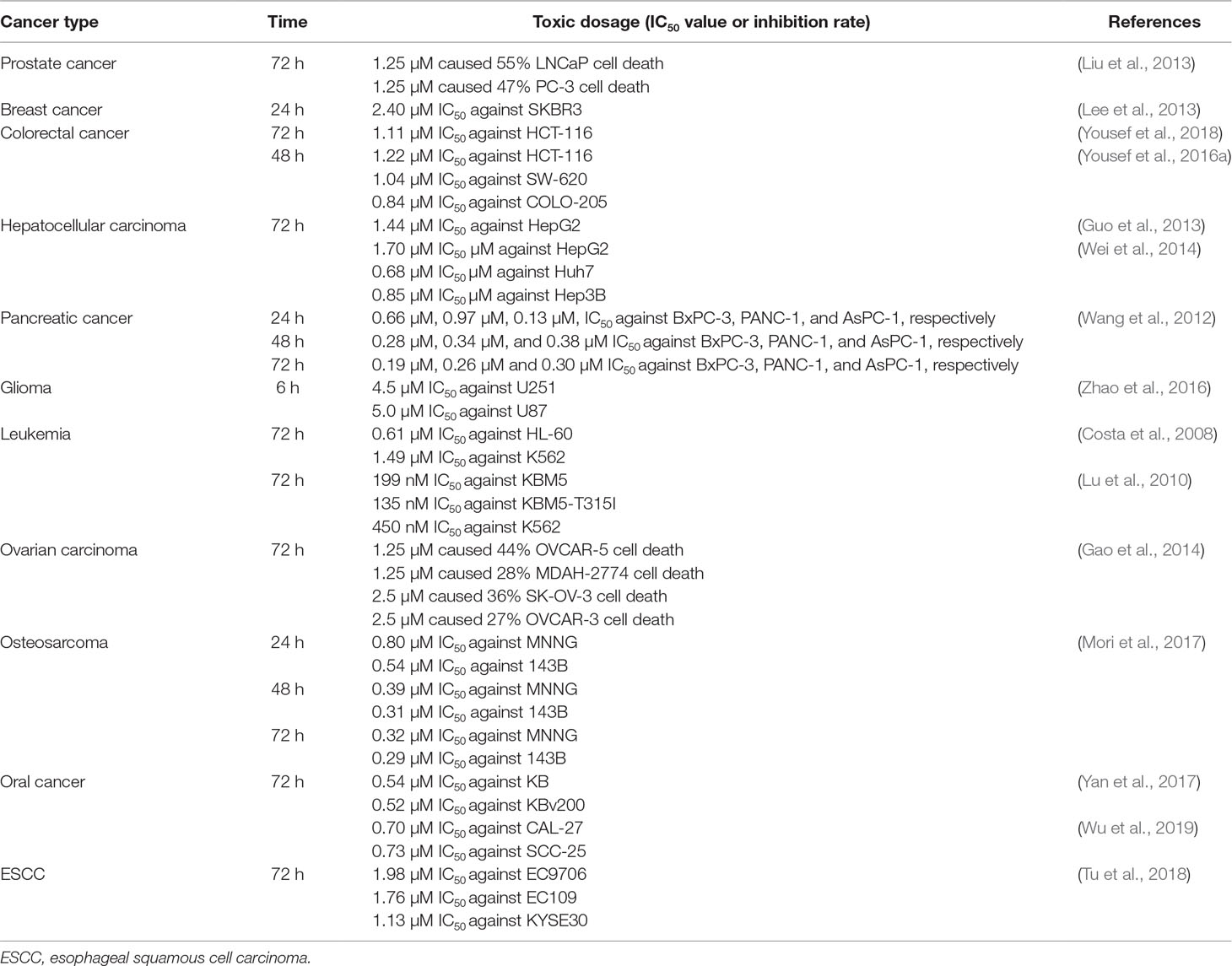
Pristimerin induces a potent effect of growth inhibition within wide range types of human tumors; the cytotoxicity of pristimerin in different cancer cell lines is summarized in Table 1.
Apoptosis Induction
Apoptosis is a kind of programmed cell death, whose activation is regulated by a series of genes, in the purpose of eliminating redundant, damaged, even infected cells to maintain homeostasis (Ke et al., 2016). Anti-cancer agents killing tumor cells by the induction of apoptosis is generally studied (Wu et al., 2017; Xiao et al., 2018; Qi et al., 2019). Two main subtypes of apoptosis have been divided into the intrinsic mitochondrial pathway and the extrinsic death receptor pathway (Elmore, 2007).
In the mitochondrial pathway, Bcl-2 family members converge on mitochondria (Kale et al., 2018), regulating release of various mitochondrial components to form the apoptosome (Dorstyn et al., 2018), such as cytochrome c associated with Apaf-1 and procaspase-9 (Estaquier et al., 2012). In the death receptor pathway, stimulation of death receptors, including Fas and tumor necrosis factor (TNF) receptor-1, results in the assembly of death-inducing signaling complex, containing the adapter protein (Gupta, 2001), Fas-associated death domain, and initiator caspases, such as caspase-8 (Pecina-Slaus, 2009).
Pristimerin-induced apoptotic effects were mainly due to mitochondrial dysfunction, activation of both extrinsic and intrinsic caspases, and cleavage of poly ADP-ribose polymerase (PARP). It has been reported that pristimerin can induce caspase-dependent apoptosis in human glioma cancer cells (Yan et al., 2013), pancreatic cancer cells (Deeb et al., 2014b), and hepatoma cancer cells (Gao et al., 2014). Pristimerin-induced inhibition of Bcl-2 (as well as Bcl-2 mRNA) is sufficient to promote mitochondrial permeability transition and release of cytochrome c mediated by Bax and Bak without the inhibition of Bcl-xL in pancreatic cancer cells (Deeb et al., 2014b). On the other hand, caspase inhibitor failed to antagonize the effects of pristimerin, indicating that the lethal effect of pristimerin may not be caspase-dependent in human glioma U251 and U87 cells (Zhao et al., 2016).
The apoptotic effect of pristimerin is related to Bcl-2, and it mediates down-regulation of Bcl-2 through reactive oxygen species (ROS)-dependent ubiquitin-proteasomal degradation pathway in human prostate cancer LNCaP and PC-3 cells (Liu et al., 2013). ROS-induced apoptosis by pritimerin was also reported in hepatocellular carcinoma HepG2 cells, involving EGFR and Akt proteins (Guo et al., 2013). In colorectal carcinoma cells, the associated induction of JNK activation and MMP loss was observed (Yousef et al., 2016b), similar with the results in cervical cancer cells (Byun et al., 2009).
In human colon cancer cells, pristimerin caused cell cycle arrest and apoptosis through cyclin-CDK, mitochondrial dysfunction, and caspase-dependent mechanisms. Besides, the inhibition of DNA synthesis in HL-60 was also associated with pristimerin-induced apoptosis (Costa et al., 2008).
Pristimerin-induced apoptosis could be mediated by microRNA (miRNA). miRNAs exert a post-transcriptional gene silencing effect through binding to target mRNA and endonucleolytic cleavage of the mRNA by protein argonaute-2 (AGO2) (Kobayashi and Tomari, 2016). It was reported that pristimerin induced apoptosis through inhibiting AGO2 and PTPN1 expression via miR-542-5p in glioma cancer cells U373 (Li et al., 2019). Synergization with cisplatin, pristimerin led to apoptosis via inhibiting the miR-23a, regulating PTEN/Akt signaling-related PTEN and the phosphorylation of Akt and GSK3β in lung carcinoma NCI-H446 and A549 cells (Zhang et al., 2019).
Autophagy Induction
As another programmed necrosis, autophagy is a homeostatic cellular self-digestive process. Autophagy triggered by various cellular stress plays vital role in cell death, providing novel target for developing anti-cancer drug (Mizushima et al., 2008; Ravanan et al., 2017). LC3-II promotes the expansion and maturation of autophagy, which is considered as signal of autophagy activation. Pristimerin-induced autophagy was reported in human breast cancer MDA-MB-231 (Cevatemre et al., 2018; Lee et al., 2018) and MCF-7 cells (Cevatemre et al., 2018). As evidenced by the increase of p62 and LC3-II with an unfolded protein response (UPR), pristimerin induced an incompleted autophagy through Wnt signaling. Although endoplasmic reticulum (ER) stress is also a trigger of autophagy (Smith and Wilkinson, 2017), it was not concluded whether the observed ER stress by pristimerin induced autophagy (Cevatemre et al., 2018). Additionally, a combination treatment of pristimerin and paclitaxel strengthened the extracellular signal-related kinase (ERK)-dependent autophagic cell death, with increase of p62 degradation and beclin1 expression (Lee et al., 2018).
On the contrary, pristimerin suppressed autophagy, downregulating LC3BII and beclin1 to sensitize the apoptosis caused by cisplatin in lung carcinoma A549 and NCI-H446 cells (Zhang et al., 2019).
Inhibition of Metastasis, Migration, Invasion, Angiogensis, and Cancer Stem Cell
The cancer metastases include a series of process, such as the completion of a complex succession of cell-biological event, cancer cell invasion, migration, and forming metastatic colonization in clinic (Valastyan and Weinberg, 2011). Pristimerin was reported to inhibit migration and invasion via targeting G protein signaling 4 (RGS4) in breast cancer MDA-MB-231 cells (Mu et al., 2012a) and HER2 in human breast carcinoma SKBR3 cells (Lee et al., 2013). Furthermore, mammalian target of rapamycin (mTOR) may be associated with its upstream Akt in pristimerin-induced inhibition of migration and invasion in colorectal cancer HCT-116 cells (Yousef et al., 2016b). Pristimerin suppressed the invasion of human prostate cancer PC-3 cells through inhibition of epithelial-to-mesenchymal transition (EMT), which was confirmed by the EMT-related markers (Chaffer et al., 2016), including N-cadherin, fibronectin, vimentin and ZEB1 (Zuo et al., 2015). MMP2 and MMP9, which are important proteins regulating invasion and metastasis, were decreased by pristimerin in esophageal cancer EC9706 and EC109 cells in a dose-dependent manner, resulting in inhibition of migration and invasion (Tu et al., 2018).
To supply nutrients and clear metabolic wastes, novel capillary blood vessels grow from pre-existing vasculature, which is called angiogenesis. However, aberrant angiogenesis plays a key role in cancer development (Valastyan and Weinberg, 2011). Thus, anti-angiogenic therapy is promising and under development (Li et al., 2018). Pristimerin was reported to in vivo inhibit the neovascularization of chicken chorioallantoic membrane (CAM) and vessel ex vivo sprout in rat aortic ring assay, through a vascular endothelial growth factor (VEGF)-dependent mechanism (Mu et al., 2012b). Also, the decreased-VEGF by pristimerin was reported through the inhibition of HIF-1α via the SPHK-1 signaling pathway in hypoxic prostate cancer PC-3 cells (Lee et al., 2016). In addition, pristimerin-induced cancer stem cell toxicity was observed in breast cancer stem cells (Cevatemre et al., 2018) and esophageal squamous cell carcinoma (ESCC) (Tu et al., 2018).
Reversal of Drug Resistance
Multi-drug resistance (MDR) is defined as the resistance of cancer cells not limited to a specific chemotherapeutic drug through different structures and mechanisms of action (Wu et al., 2014). ABCB1 (P-glycoprotein, Pgp) is recognized as putative drug transporter, which is encoded by the ABCB1 gene, one of (ATP)-binding cassette (ABC) transporter family (Dewanjee et al., 2017). Pristimerin may overcome ABCB1-mediated chemotherapeutic drug resistance through disturbing the stability of ABCB1 independent of its mRNA expression in human oral epidermoid carcinoma cells KBv200 (Yan et al., 2017). In addition, with inhibition of NF-κB and Bcr-Abl, pristimerin is effective in vitro and in vivo against imatinib-resistant chronic myelogenous leukemia cells (Lu et al., 2010). Additionally, Akt signaling was related to the reversal of MDR in multidrug-resistant MCF-7/ADR breast cancer cells (Xie et al., 2016).
Synergization With Chemotherapeutic Drugs
Drug combination for cancer treatment has been well established to strengthen the anti-tumor action in varied aspects (Ho and Cheung, 2014; Andre et al., 2018), including therapeutic drug combination with natural product (Efferth, 2017; Sanchez et al., 2019). Pristimerin was reported to synergize with paclitaxel in human breast cancer cells (Lee et al., 2018), with 5-fluorouracil (5-FU) in esophageal ESCC (Tu et al., 2018). In cervical cancer cells, combination with taxol could induce cell death through ROS-mediated mitochondrial dysfunction (Eum et al., 2011). In NCI-H446 and A549 lung carcinoma cells, combination with cisplatin could induce cell apoptosis through inhibiting the miRNA-23a and Akt/GSK3β signaling pathway (Zhang et al., 2019). In pancreatic cancer cells, pristimerin could potentiate the cytotoxic effect of gemcitabine with the possible mechanism being the inhibition of gemcitabine-induced NF-κB activation (Wang et al., 2012).
In Vivo Anti-Tumor Activities
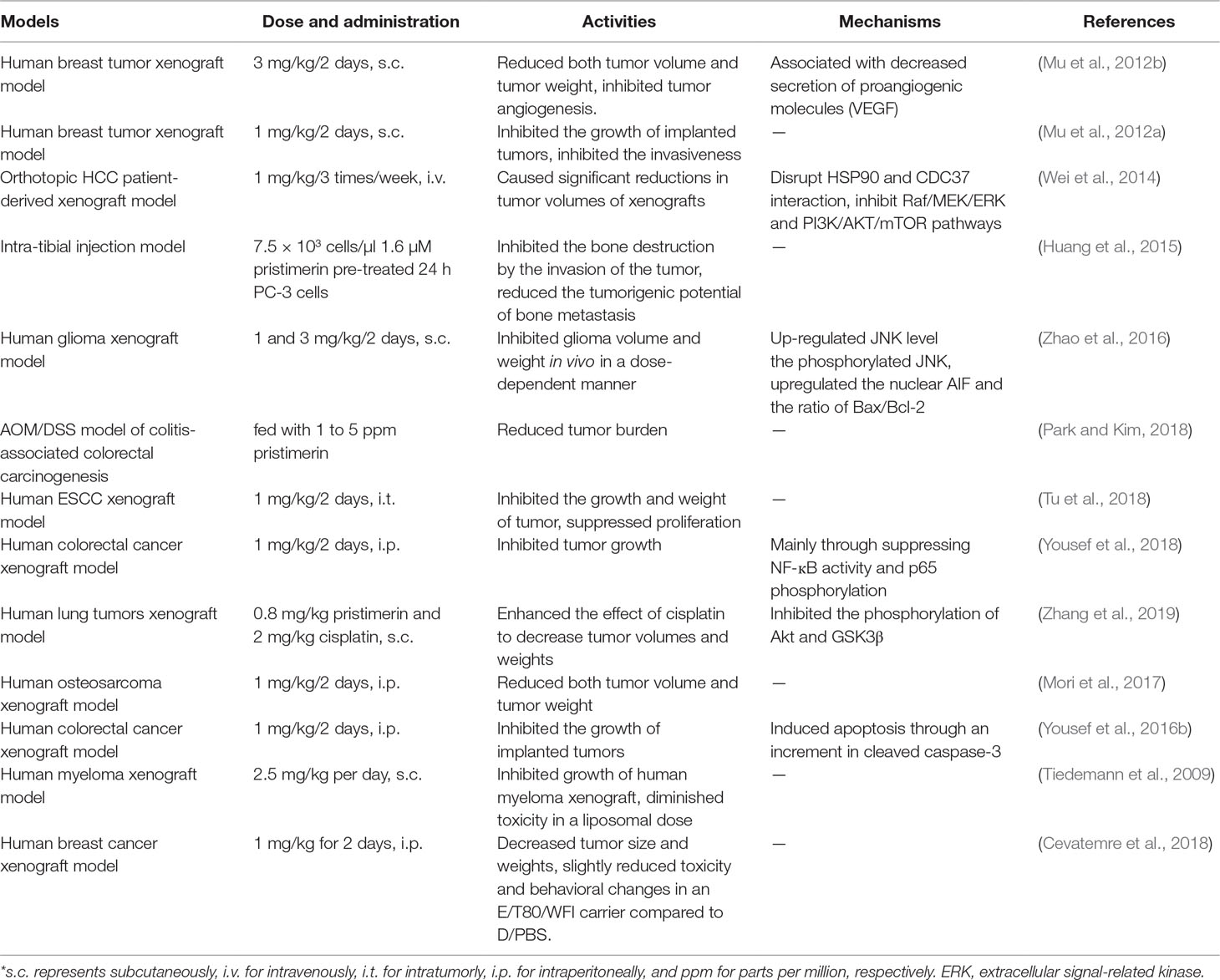
Pristimerin was widely reported its in vivo anti-tumor activities, which is summarized in Table 2.
Pristimerin in Tumors: Targets and Pathways
Proteasome
As another important mechanism of maintaining homeostasis, proteasome-mediated degradation is associated with essential cellular processes, regulating the vast majority of cellular proteins (Livneh et al., 2016). Consistent with triterpenoids being reported to target proteasome (Chintharlapalli et al., 2007; Tiedemann et al., 2009), pristimerin also showed a potent activity to inhibit proteasome activity in prostate cancer cells (Yang et al., 2010; Liu et al., 2013; Liu et al., 2014), breast cancer cells (Mu et al., 2012a), cervical carcinoma cells (Eum et al., 2011), and myeloma cells (Tiedemann et al., 2009).
The β subunits of proteasome contain active protease sites with different peptidase activities, including caspase-like or peptidyl-glutamyl peptide-hydrolyzing-like (β1), trypsin-like post basic (β2), and chymotrypsin-like (β5) activities (Mayor et al., 2016). Pristimerin was associated with the N-terminal threonine of the β5 subunit through its conjugated ketone carbon C6, exerting a chymotrypsin-like activity (Yang et al., 2010), which is also associated with RGS4 (Mu et al., 2012a).
Pristimerin can inhibit Bcl-2, finally induced mitochondrial cell death via an ROS-dependent ubiquitin-proteasomal degradation pathway (Liu et al., 2013). Pristimerin combination with taxol caused mitochondrial apoptosis due to ROS generation and direct proteasome inhibition (Eum et al., 2011). In addition, pristimerin-induced inhibition of proteosome and IKK phosphorylation of IκB together led to UPR and suppression of NF-κB activity and cyclin D2 expression in myeloma cells H929 and U266 (Tiedemann et al., 2009).
Telomerase
Telomere is a ribonucleoprotein complex located in the end of chromosomes, maintaining telomere length homeostasis to keep chromosomal stability (Wang and Feigon, 2017). Due to the differences in telomere homeostasis between cancer and normal cells, targeting telomerase may be a promising approach to find effective and safe anti-cancer treatments (Armstrong and Tomita, 2017).
Pristimerin can inhibit telomerase activity in human prostate cancer LNCaP and PC-3 cells (Liu et al., 2015). The mechanism is related to inhibition of human telomerase reverse transcriptase (hTERT) and its mRNA expression, which codes the catalytic subunit of the telomerase. At the same time, knocking-down of hTERT strengthened the effects of pristimerin. Furthermore, hTERT regulatory proteins c-Myc, Sp1, p-STAT3, and p-Akt were inhibited in a dose-dependent manner (Liu et al., 2015).
MAPK Pathway
The generic MAPK signaling pathway is co-regulated by four different cascades including extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases (JNK1/2/3), p38-MAPK, and ERK5 (Sun et al., 2015). MAPK/ERK pathway regulates the cell proliferation (Sun et al., 2017), differentiation (Wang et al., 2017), migration (Tao et al., 2018) and apoptosis (Wang and Zhu, 2018).
Pristimerin-induced autophagy was reported via ERK1/2 in human breast cancer cells when combination with paclitaxel (Lee et al., 2018). ERK1/2 may be involved in pristimerin-induced intrinsic apoptosis in human oral epidermoid carcinoma cells (Yan et al., 2017) and in human glioma cells (Yan et al., 2013). Both JNK and PARP-1 via ROS pathway are essentially required for the pristimerin-induced intrinsic apoptosis in human cervical cancer cells (Byun et al., 2009). In addition, ERK1/2 suppression occurred in VEGF-induced capillary-like structure formation of human umbilical vascular endothelial cells (HUVECs) (Mu et al., 2012b). These activities were accompanied with Akt inhibition (Mu et al., 2012b; Yan et al., 2017; Lee et al., 2018).
PI3K/AKT/mTOR Pathway
The phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway cascade containing PI3K, AKT, and mTOR is the most frequently altered pathway in human for cancer development, such as cell cycle, cell survival, metabolism, motility, angiogenesis, chemoresistance, and genomic instability (Mabuchi et al., 2015).
Pristimerin showed a potent apoptosis-inducing anti-proliferative activity in human osteosarcoma cells (Mori et al., 2017) by PI3K/AKT/mTOR pathway. The pristimerin-induced ROS-dependent mitochondrial cell apoptosis was also associated with the inhibition of EGFR and Akt in human glioma cells (Yan et al., 2013). It was confirmed that PI3K/AKT/mTOR pathway-activated activities were accompanied by the downstream Foxo-3α, cyclin D1 and Bcl-XL (Akt), p-S6K1, and p-4E-BP1 (mTOR) as well as p21, p27, and PKCε in human ovarian cancer cells (Deeb et al., 2014b; Gao et al., 2014; Park and Kim, 2018). Furthermore, downstream Bad and Bcl-xL pointed to drug resistance in MCF-7/ADR human breast cancer cells (Xie et al., 2016). In addition, pristimerin suppressed angiogenesis through VEGF-induced Akt, ERK1/2, mTOR, and ribosomal protein S6 kinase (Mu et al., 2012b).
NF-κB Pathway
NF-κB family transcription factors are crucial regulators of cell survival and inflammatory processes (Napetschnig and Wu, 2013). The inactive NF-κBs are isolated from nucleus by inhibitor of NF-κB (IκB) proteins. When activated IKK (IκB kinase) makes a proteasomal degradation of IκB, the subsequent process will occur, including the release of NF-κB, translocation of NF-κB nuclear and activation of gene transcription. NF-κB can be activated by both intracellular and extracellular stimuli, including cytokines (TNF-α, IL-1β), bacterial, and viral products (LPS) (Xia et al., 2014).
NF-κB-regulated anti-apoptotic Bcl-2, Bcl-xL, c-IAPl, and surviving in human ovarian carcinoma cells (Gao et al., 2014), Cox-2 and VEGF in human pancreatic cancer cells (Deeb et al., 2014b). NF-κB pathway may link anti-tumor activity of pristimerin and its anti-inflammatory properties (Park and Kim, 2018). Pristimerin suppressed the translocation of NF-κB nuclear; however, there was no change of the total NF-κB protein in pancreatic cancer (Wang et al., 2012). In contrast, pristimerin inhibited both genetic expression and activation of NF-кB protein with suppression of p65 mRNA in human colorectal cancer cells (Yousef et al., 2018). TNFα-induced NF-κB activation was observed by the downstream MMP9, cyclin D1, and c-Myc in ESCC cells (Tu et al., 2018). When combined with pristimerin, the inactivation of Bcr-Abl by imatinib did not interfere with the TNFα-induced NF-κB activation, which implicated that NF-κB inactivation and Bcr-Abl inhibition may be parallel mechanisms of pristimerin-induced activity in human chronic myelogenous leukemia cells (Lu et al., 2010). G1 phase arrest was also associated with NF-κB pathway in human pancreatic cancer cells (Wang et al., 2012), as well as proteosome in human myeloma cells (Tiedemann et al., 2009). Moreover, pristimerin inhibited expression of miR-542-5p targeting PTPN1, which encodes protein tyrosine phosphatase 1B (PTP1B) related to NF-κB pathway (Li et al., 2019).
Wnt/β-Catenin Pathway
Wnt proteins are key mediators in a series of important cellular process. The abnormal activation of Wnt/β-catenin pathway can cause a wide range of diseases including cancers (Krishnamurthy and Kurzrock, 2018; Pedone and Marucci, 2019). Pristimerin was reported to suppress Wnt/β-catenin pathway through targeting and inhibiting the expression of LRP6 and its phosphorylation, which may contribute to autophagy in human breast cancer MCF-7 cells (Cevatemre et al., 2018).
Conclusions and Perspective
Plants, particularly medicinal herbs, have become increasingly popular due to their potent therapeutic effects. Pristimerin, a quininemethide triterpenoid compound isolated from species of the Celastraceae and Hippocrateaceae families, has displayed biological and pharmacological activities, particularly inhibiting cancer. This review summarizes the reported results on anti-cancer activities and related mechanisms of pristimerin.
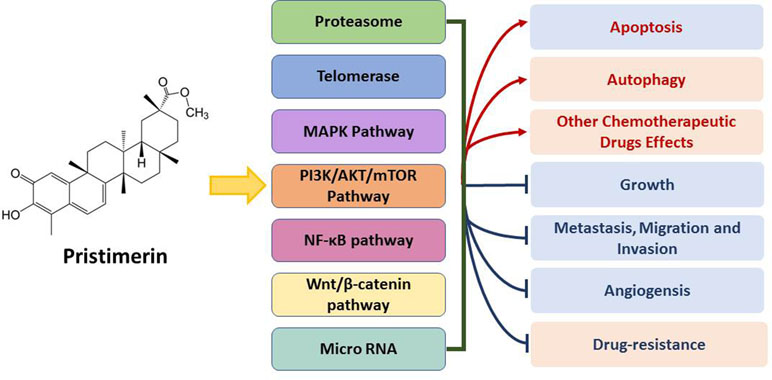
Pristimerin has shown anti-cancer potency in vivo (Table 2) and in vitro (Table 3) via specific mechanisms (Figure 2). Like many other chemotherapeutic drugs, pristimerin exerts cytotoxicity largely related to apoptosis, while the mechanism of autophagy is merely reported. The cross-talk of apoptosis and autophagy mediated by pristimerin is still remained to be explored. So far, the mechanism study of pristimerin has little reported on lung cancer, epigenetic regulation, and combination with immunotherapy. Furthermore, pristimerin has been reported to have poor selective toxicity in some cancer cells or compared with its derivatives (Costa et al., 2008; Wei et al., 2014). Comprehensive evaluation of pristimerin toxicity is yet to be carried out (as well as clinical trials). In summary, pristimerin possesses potent anti-cancer effect and further study will bring about novel drug development based on pristimerin.
Data Availability
All datasets analyzed for this study are included in the manuscript and the supplementary files.
Author Contributions
JZ and HC conceived this review; JL and YY wrote the article. HS, YL, and CS revised the article.
Funding
The work was supported by National Natural Science Foundation of China (81473320 and 81773888), Fund of Guangdong Science and Technology Department (2016A020226024), Fund of Guangzhou Science and Technology Program (201707010048), Fund of Guangdong Education Department (2015KTSCX112), and Fund of Construction of High Level Universities in Guangdong (Nanshan Scholars Program and Academic Backbone Program).
Conflict of Interest Statement
HS was employed by Infinitus (China) Company Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alessia, P., Gaetano, P., Ugo, T. (2009). Triterpenoids as new promising anticancer drugs. Anticancer Drugs 20, 880–892. doi: 10.1097/CAD.0b013e328330fd90
Andre, P., Denis, C., Soulas, C., Bourbon-Caillet, C., Lopez, J., Arnoux, T., et al. (2018). Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743. doi: 10.1016/j.cell.2018.10.014
Armstrong, C. A., Tomita, K. (2017). Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells. Open Biol. 7, 160338. doi: 10.1098/rsob.160338
Avilla, J., Teixidò, A., Velázquez, C., Alvarenga, N., Ferro, E., Canela, R. (2000). Insecticidal activity of Maytenus Species (Celastraceae) Nortriterpene Quinone Methides against codling moth, Cydia pomonella (L). (Lepidoptera: Tortricidae). J. Agric. Food Chem. 48, 88–92. doi: 10.1021/jf990008w
Brattain, M. G., Howell, G., Sun, L. Z., Willson, J. K. (1994). Growth factor balance and tumor progression. Curr. Opin. Oncol. 6, 77–81. doi: 10.1097/00001622-199401000-00011
Byun, J.-Y., Kim, M.-J., Eum, D.-Y., Yoon, C.-H., Seo, W.-D., Park, K. H., et al. (2009). Reactive oxygen species-dependent activation of Bax and Poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol. Pharmacol. 76, 734–744. doi: 10.1124/mol.109.056259
Cevatemre, B., Erkısa, M., Aztopal, N., Karakas, D., Alper, P., Tsimplouli, C., et al. (2018). A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol. Res. 129, 500–514. doi: 10.1016/j.phrs.2017.11.027
Chaffer, C. L., San Juan, B. P., Lim, E., Weinberg, R. A. (2016). EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 35, 645–654. doi: 10.1007/s10555-016-9648-7
Chintharlapalli, S., Papineni, S., Ramaiah, S. K., Safe, S. (2007). Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 67, 2816–2823. doi: 10.1158/0008-5472.CAN-06-3735
Costa, P. M. D., Ferreira, P. M. P., Bolzani, V. D. S., Furlan, M., Corsino, J., De Moraes, M. O., et al. (2008). Antiproliferative activity of pristimerin isolated from Maytenus ilicifolia (Celastraceae) in human HL-60 cells. Toxicol. In Vitro 22, 854–863. doi: 10.1016/j.tiv.2008.01.003
Deeb, D., Brigolin, C., Gao, X., Liu, Y., Pindolia, K. R., Gautam, S. C. (2014a). Induction of Apoptosis in Pancreatic Cancer Cells by CDDO-Me Involves repression of telomerase through epigenetic pathways. J. Carcinog. Mutagen. 5, 177. doi: 10.4172/2157-2518.1000177
Deeb, D., Gao, X., Liu, Y., Kim, S. H., Pindolia, K. R., Arbab, A. S., et al. (2012). Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity. Biochem. Biophys. Res. Commun. 422, 561–567. doi: 10.1016/j.bbrc.2012.05.024
Deeb, D., Gao, X., Liu, Y., Pindolia, K., Gautam, S. C. (2015). Inhibition of hTERT/telomerase contributes to the antitumor activity of pristimerin in pancreatic ductal adenocarcinoma cells. Oncol. Rep. 34, 518–524. doi: 10.3892/or.2015.3989
Deeb, D., Gao, X., Liu, Y. B., Pindolia, K., Gautam, S. C. (2014b). Pristimerin, a quinonemethide triterpenoid, induces apoptosis in pancreatic cancer cells through the inhibition of pro-survival Akt/NF-kappaB/mTOR signaling proteins and anti-apoptotic Bcl-2. Int. J. Oncol. 44, 1707–1715. doi: 10.3892/ijo.2014.2325
Dent, M.a.R., Aranda-Anzaldo, A. (2019). Lessons we can learn from neurons to make cancer cells quiescent. J. Neurosci. Res. doi: 10.1002/jnr.24428
Dewanjee, S., Dua, T. K., Bhattacharjee, N., Das, A., Gangopadhyay, M., Khanra, R., et al. (2017). Natural products as alternative choices for P-glycoprotein (P-gp) Inhibition. Molecules 22, 871. doi: 10.3390/molecules22060871
Dorstyn, L., Akey, C. W., Kumar, S. (2018). New insights into apoptosome structure and function. Cell Death Differ. 25, 1194–1208. doi: 10.1038/s41418-017-0025-z
Efferth, T. (2017). Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 139, 56–70. doi: 10.1016/j.bcp.2017.03.019
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516. doi: 10.1080/01926230701320337
Estaquier, J., Vallette, F., Vayssiere, J. L., Mignotte, B. (2012). The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 942, 157–183. doi: 10.1007/978-94-007-2869-1_7
Eum, D.-Y., Byun, J.-Y., Yoon, C.-H., Seo, W.-D., Park, K.-H., Lee, J.-H., et al. (2011). Triterpenoid pristimerin synergizes with taxol to induce cervical cancer cell death through reactive oxygen species-mediated mitochondrial dysfunction. Cell Death Dis. 22, 763–773. doi: 10.1097/CAD.0b013e328347181a
Figueiredo, J. B., Sequin, U. (1998). Novel quinone methides from Salacia kraussii with in vitro antimalarial activity. J. Nat. Prod. 61, 718–723. doi: 10.1021/np9704157
Gao, X., Liu, Y., Deeb, D., Arbab, A. S., Gautam, S. C. (2014). Anticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-kappaB/mTOR signaling. J. Exp. Ther. Oncol. 10, 275–283.
Guo, Y., Zhang, W., Yan, Y. Y., Ma, C. G., Wang, X., Wang, C., et al. (2013). Triterpenoid pristimerin induced HepG2 cells apoptosis through ROS-mediated mitochondrial dysfunction. J. BUON. 18, 477–485.
Gupta, S. (2001). Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sci. 69, 2957–2964. doi: 10.1016/S0024-3205(01)01404-7
Haroldo Jeller, A., Helena Siqueira Silva, D., Morais Lião, L., Da Silva Bolzani, V., Furlan, M. (2004). Antioxidant phenolic and quinonemethide triterpenes from Cheiloclinium cognatum. Phytochemistry 65, 1977–1982. doi: 10.1016/j.phytochem.2004.03.039
Ho, J. W., Cheung, M. W. (2014). Combination of phytochemicals as adjuvants for cancer therapy. Recent Pat. Anticancer Drug Discov. 9, 297–302. doi: 10.2174/1574892809666140619154838
Huang, S., He, P., Peng, X., Li, J., Xu, D., Tang, Y. (2015). Pristimerin inhibits prostate cancer bone metastasis by targeting PC-3 stem cell characteristics and VEGF-induced vasculogenesis of BM-EPCs. Cell. Physiol. Biochem. 37, 253–268. doi: 10.1159/000430350
Kale, J., Osterlund, E. J., Andrews, D. W. (2018). BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 25, 65–80. doi: 10.1038/cdd.2017.186
Ke, B., Tian, M., Li, J., Liu, B., He, G. (2016). Targeting programmed cell death using small-molecule compounds to improve potential cancer therapy. Med. Res. Rev. 36, 983–1035. doi: 10.1002/med.21398
Kim, H. J., Park, G. M., Kim, J.-K. (2013). Anti-inflammatory effect of pristimerin on lipopolysaccharide-induced inflammatory responses in murine macrophages. Arch. Pharm. Res. 36, 495–500. doi: 10.1007/s12272-013-0054-1
Kobayashi, H., Tomari, Y. (2016). RISC assembly: coordination between small RNAs and argonaute proteins. Biochim. Biophys. Acta 1859, 71–81. doi: 10.1016/j.bbagrm.2015.08.007
Krishnamurthy, N., Kurzrock, R. (2018). Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat. Rev. 62, 50–60. doi: 10.1016/j.ctrv.2017.11.002
Kulkarni, A. B., Shah, R. C. (1954). Structure of pristimerin. Nature 173, 1237–1238. doi: 10.1038/1731237b0
Larsen, H., Muz, B., Khong, T. L., Feldmann, M., Paleolog, E. M. (2012). Differential effects of Th1 versus Th2 cytokines in combination with hypoxia on HIFs and angiogenesis in RA. Arthritis Res. Ther. 14, R180. doi: 10.1186/ar3934
Lee, J. S., Yoon, I. S., Lee, M. S., Cha, E. Y., Thuong, P. T., Diep, T. T., et al. (2013). Anticancer activity of pristimerin in epidermal growth factor receptor 2-positive SKBR3 human breast cancer cells. Biol. Pharm. Bull. 36, 316–325. doi: 10.1248/bpb.b12-00685
Lee, S.-O., Kim, J.-S., Lee, M.-S., Lee, H.-J. (2016). Anti-cancer effect of pristimerin by inhibition of HIF-1α involves the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer 16, 701. doi: 10.1186/s12885-016-2730-2
Lee, Y., Lee, M., Cha, E., Sul, J., Park, J., Lee, J. (2018). Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation. Mol. Med. Rep. 18, 4281–4288. doi: 10.3892/mmr.2018.9488
Li, T., Kang, G., Wang, T., Huang, H. (2018). Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 16, 687–702. doi: 10.3892/ol.2018.8733
Li, Z., Hu, C., Zhen, Y., Pang, B., Yi, H., Chen, X. (2019). Pristimerin Inhibits Glioma Progression by Targeting AGO2 and PTPN1 Expression via miR-542-5p. Biosci. Rep. 39. doi: 10.1042/BSR20182389
Lin, M., Tang, S., Zhang, C., Chen, H., Huang, W., Liu, Y., et al. (2017). Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway. Acta Pharm. Sin. B 7, 59–64. doi: 10.1016/j.apsb.2016.06.008
Liu, Y. B., Gao, X., Deeb, D., Arbab, A. S., Gautam, S. C. (2013). Pristimerin Induces Apoptosis in Prostate Cancer Cells by Down-regulating Bcl-2 through ROS-dependent ubiquitin-proteasomal degradation pathway. J. Carcinog. Mutagen. Suppl 6, 5. doi: 10.4172/2157-2518.S6-005
Liu, Y. B., Gao, X., Deeb, D., Brigolin, C., Zhang, Y., Shaw, J., et al. (2014). Ubiquitin-proteasomal degradation of antiapoptotic survivin facilitates induction of apoptosis in prostate cancer cells by pristimerin. Int. J. Oncol. 45, 1735–1741. doi: 10.3892/ijo.2014.2561
Liu, Y. B., Gao, X., Deeb, D., Pindolia, K., Gautam, S. C. (2015). Role of telomerase in anticancer activity of pristimerin in prostate cancer cells. J. Exp. Ther. Oncol. 11, 41–49.
Livneh, I., Cohen-Kaplan, V., Cohen-Rosenzweig, C., Avni, N., Ciechanover, A. (2016). The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res. 26, 869–885. doi: 10.1038/cr.2016.86
Lopez, M. R., De Leon, L., Moujir, L. (2011). Antibacterial properties of phenolic triterpenoids against Staphylococcus epidermidis. Planta. Med. 77, 726–729. doi: 10.1055/s-0030-1250500
Lu, Z., Jin, Y., Chen, C., Li, J., Cao, Q., Pan, et al. (2010). Pristimerin induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation by blocking NF-κB signaling and depleting Bcr-Abl. Mol. Cancer 9, 112. doi: 10.1186/1476-4598-9-112
Mabuchi, S., Kuroda, H., Takahashi, R., Sasano, T. (2015). The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 137, 173–179. doi: 10.1016/j.ygyno.2015.02.003
Mayor, T., Sharon, M., Glickman, M. H. (2016). Tuning the proteasome to brighten the end of the journey. Am. J. Physiol., Cell Physiol. 311, C793–C804. doi: 10.1152/ajpcell.00198.2016
Mena-Rejon, G. J., Perez-Espadas, A. R., Moo-Puc, R. E., Cedillo-Rivera, R., Bazzocchi, I. L., Jimenez-Diaz, I. A., et al. (2007). Antigiardial activity of triterpenoids from root bark of Hippocratea excelsa. J. Nat. Prod. 70, 863–865. doi: 10.1021/np060559y
Mizushima, N., Levine, B., Cuervo, A. M., Klionsky, D. J. (2008). Autophagy fights disease through cellular self-digestion. Nature 451, 1069. doi: 10.1016/j.cell.2004.11.046
Mori, Y., Shirai, T., Terauchi, R., Tsuchida, S., Mizoshiri, N., Hayashi, D., et al. (2017). Antitumor effects of pristimerin on human osteosarcoma cells in vitro and in vivo. Onco. Targets Ther. 10, 5703–5710. doi: 10.2147/OTT.S150071
Mu, X.-M., Shi, W., Sun, L.-X., Li, H., Wang, Y.-R., Jiang, Z.-Z., et al. (2012a). Pristimerin Inhibits Breast Cancer Cell Migration by Up-regulating Regulator of G Protein Signaling 4 Expression. Asian Pac. J. Cancer Prev. 13, 1097–1104. doi: 10.7314/APJCP.2012.13.4.1097
Mu, X., Shi, W., Sun, L., Li, H., Jiang, Z., Zhang, L. (2012b). Pristimerin, a triterpenoid, inhibits tumor angiogenesis by targeting VEGFR2 activation. Molecules 17, 6854–6868. doi: 10.3390/molecules17066854
Murayama, T., Eizuru, Y., Yamada, R., Sadanari, H., Matsubara, K., Rukung, G., et al. (2007). Anticytomegalovirus activity of pristimerin, a triterpenoid quinone methide isolated from Maytenus heterophylla (Eckl. & Zeyh). Antivir. Chem. Chemother. 18, 133–139. doi: 10.1177/095632020701800303
Napetschnig, J., Wu, H. (2013). Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 42, 443–468. doi: 10.1146/annurev-biophys-083012-130338
Park, J. H., Kim, J. K. (2018). Pristimerin, a naturally occurring triterpenoid, attenuates tumorigenesis in experimental colitis-associated colon cancer. Phytomedicine 42, 164–171. doi: 10.1016/j.phymed.2018.03.033
Pecina-Slaus, N. (2009). Genetic and molecular insights into apoptosis. Acta Med. Croatica 63 Suppl 2, 13–19.
Pedone, E., Marucci, L. (2019). Role of beta-catenin activation levels and fluctuations in controlling cell fate. Genes (Basel) 10, 176. doi: 10.3390/genes10020176
Peng, S., Hang, N., Liu, W., Guo, W., Jiang, C., Yang, X., et al. (2016). Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-kappaB pathways. Acta Pharm. Sin. B 6, 205–211. doi: 10.1016/j.apsb.2016.02.002
Petho, Z., Najder, K., Bulk, E., Schwab, A. (2019). Mechanosensitive ion channels push cancer progression. Cell Calcium 80, 79–90. doi: 10.1016/j.ceca.2019.03.007
Qi, S., Guo, L., Yan, S., Lee, R. J., Yu, S., Chen, S. (2019). Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm. Sin. B 9, 279–293. doi: 10.1016/j.apsb.2018.12.004
Ravanan, P., Srikumar, I. F., Talwar, P. (2017). Autophagy: the spotlight for cellular stress responses. Life Sci. 188, 53–67. doi: 10.1016/j.lfs.2017.08.029
Salminen, A., Lehtonen, M., Suuronen, T., Kaarniranta, K., Huuskonen, J. (2008). Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 65, 2979–2999. doi: 10.1007/s00018-008-8103-5
Sanchez, B. G., Bort, A., Mateos-Gomez, P. A., Rodriguez-Henche, N., Diaz-Laviada, I. (2019). Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Cancer Cell Int. 19, 54. doi: 10.1186/s12935-019-0769-2
Shen, S., Dean, D. C., Yu, Z., Duan, Z. (2019). Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: therapeutic potential of targeting CDKs signaling pathway. Hepatol. Res. doi: 10.1111/hepr.13353
Smith, M., Wilkinson, S. (2017). ER homeostasis and autophagy. Essays Biochem. 61, 625–635. doi: 10.1042/EBC20170092
Sun, Q., Liang, Y., Zhang, T., Wang, K., Yang, X. (2017). ER-alpha36 mediates estrogen-stimulated MAPK/ERK activation and regulates migration, invasion, proliferation in cervical cancer cells. Biochem. Biophys. Res. Commun. 487, 625–632. doi: 10.1016/j.bbrc.2017.04.105
Sun, Y., Liu, W. Z., Liu, T., Feng, X., Yang, N., Zhou, H. F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 35, 600–604. doi: 10.3109/10799893.2015.1030412
Tang, W.-H., Bai, S.-T., Tong, L., Duan, W.-J., Su, J.-W., Chen, J.-X., et al. (2014). Chemical constituents from Celastrus aculeatus Merr. Biochem. Syst. Ecol. 54, 78–82. doi: 10.1016/j.bse.2014.01.001
Tao, L., Zhang, C. Y., Guo, L., Li, X., Han, N. N., Zhou, Q. (2018). MicroRNA-497 accelerates apoptosis while inhibiting proliferation, migration, and invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1. J. Cell. Physiol. 233, 6578–6588. doi: 10.1002/jcp.26272
Tao, Y. W., Lin, Y. C., She, Z. G., Lin, M. T., Chen, P. X., Zhang, J. Y. (2015). Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med. Chem. 15 (2), 258–266. doi: 10.2174/1871520614666140825112255
Tiedemann, R. E., Schmidt, J., Keats, J. J., Shi, C. X., Zhu, Y. X., Palmer, S. E., et al. (2009). Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood 113, 4027–4037. doi: 10.1182/blood-2008-09-179796
Tu, Y., Tan, F., Zhou, J., Pan, J. (2018). Pristimerin targeting NF-κB pathway inhibits proliferation, migration, and invasion in esophageal squamous cell carcinoma cells. Cell Biochem. Funct. 36, 228–240. doi: 10.1002/cbf.3335
Valastyan, S., Weinberg, R. A. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292. doi: 10.1016/j.cell.2011.09.024
Wang, H., Shan, X. B., Qiao, Y. J. (2017). PDK2 promotes chondrogenic differentiation of mesenchymal stem cells by upregulation of Sox6 and activation of JNK/MAPK/ERK pathway. Braz. J. Med. Biol. Res. 50, e5988. doi: 10.1590/1414-431x20165988
Wang, K., Zhu, Y. (2018). Dexmedetomidine protects against oxygen-glucose deprivation/reoxygenation injury-induced apoptosis via the p38 MAPK/ERK signalling pathway. J. Int. Med. Res. 46, 675–686. doi: 10.1177/0300060517734460
Wang, Y., Feigon, J. (2017). Structural biology of telomerase and its interaction at telomeres. Curr. Opin. Struct. Biol. 47, 77–87. doi: 10.1016/j.sbi.2017.06.010
Wang, Y., Zhou, Y., Zhou, H., Jia, G., Liu, J., Han, B., et al. (2012). Pristimerin causes G1 arrest, induces apoptosis, and enhances the chemosensitivity to gemcitabine in pancreatic cancer cells. PLoS One 7, e43826. doi: 10.1371/journal.pone.0043826
Wei, W., Wu, S., Wang, X., Sun, C. K., Yang, X., Yan, X., et al. (2014). Novel celastrol derivatives inhibit the growth of hepatocellular carcinoma patient-derived xenografts. Oncotarget 5, 5819–5831. doi: 10.18632/oncotarget.2171
Wu, H., Li, L., Ai, Z., Yin, J., Chen, L. (2019). Pristimerin induces apoptosis of oral squamous cell carcinoma cells via G1 phase arrest and MAPK/Erk1/2 and Akt signaling inhibition. Oncol. Lett. 17, 3017–3025. doi: 10.3892/ol.2019.9903
Wu, Q., Yang, Z., Nie, Y., Shi, Y., Fan, D. (2014). Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 347, 159–166. doi: 10.1016/j.canlet.2014.03.013
Wu, T., Geng, J., Guo, W., Gao, J., Zhu, X. (2017). Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin. B 7, 65–72. doi: 10.1016/j.apsb.2016.04.003
Xia, Y., Shen, S., Verma, I. M. (2014). NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2, 823–830. doi: 10.1158/2326-6066.CIR-14-0112
Xiao, J., Xing, F., Liu, Y., Lv, Y., Wang, X., Ling, M. T., et al. (2018). Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta Pharm. Sin. B 8, 575–586. doi: 10.1016/j.apsb.2017.10.003
Xie, G. E., Yu, X., Liang, H., Chen, J., Tang, X., Wu, S., et al. (2016). Pristimerin overcomes adriamycin resistance in breast cancer cells through suppressing Akt signaling. Oncol. Lett. 11, 3111–3116. doi: 10.3892/ol.2016.4335
Yan, Y.-Y., Wang, F., Zhao, X.-Q., Wang, X.-K., Chen, Y.-F., Liu, H., et al. (2017). Degradation of P-glycoprotein by pristimerin contributes to overcoming ABCB1-mediated chemotherapeutic drug resistance in vitro. Oncol. Rep. 37, 31–40. doi: 10.3892/or.2016.5230
Yan, Y. Y., Bai, J. P., Xie, Y., Yu, J. Z., Ma, C. G. (2013). The triterpenoid pristimerin induces U87 glioma cell apoptosis through reactive oxygen species-mediated mitochondrial dysfunction. Oncol. Lett. 5, 242–248. doi: 10.3892/ol.2012.982
Yang, H., Landis-Piwowar, K. R., Lu, D., Yuan, P., Li, L., Reddy, G. P., et al. (2010). Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J. Cell. Biochem. 103, 234–244. doi: 10.1002/jcb.21399
Yousef, B. A., Guerram, M., Hassan, H. M., Hamdi, A. M., Zhang, L.-Y., Jiang, Z.-Z. (2016a). Pristimerin demonstrates anticancer potential in colorectal cancer cells by inducing G1 phase arrest and apoptosis and suppressing various pro-survival signaling proteins. Oncol. Rep. 35, 1091–1100. doi: 10.3892/or.2015.4457
Yousef, B. A., Hassan, H. M., Guerram, M., Hamdi, A. M., Wang, B., Zhang, L.-Y., et al. (2016b). Pristimerin inhibits proliferation, migration and invasion, and induces apoptosis in HCT-116 colorectal cancer cells. Biomed. Pharmacother. 79, 112–119. doi: 10.1016/j.biopha.2016.02.003
Yousef, B. A., Hassan, H. M., Zhang, L.-Y., Jiang, Z.-Z. (2018). Pristimerin exhibits in vitro and in vivo anticancer activities through inhibition of nuclear factor-кB signaling pathway in colorectal cancer cells. Phytomedicine 40, 140–147. doi: 10.1016/j.phymed.2018.01.008
Yousef, B. A., Hassan, H. M., Zhang, L. Y., Jiang, Z. Z. (2017). Anticancer potential and molecular targets of pristimerin: a mini-review. Curr. Cancer Drug Targets 17, 100–108. doi: 10.2174/1568009616666160112105824
Zhang, J. Y., Lin, M. T., Tung, H. Y., Tang, S. L., Yi, T., Zhang, Y. Z., et al. (2016). Bruceine D induces apoptosis in human chronic myeloid leukemia K562 cells via mitochondrial pathway. Am. J. Cancer Res. 6, 819.
Zhang, J. Y., Lin, M. T., Zhou, M. J., Yi, T., Tang, Y. N., Tang, S. L., et al. (2015). Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules 20, 11524–11534. doi: 10.3390/molecules200611524
Zhang, Y., Wang, J., Hui, B., Sun, W., Li, B., Shi, F., et al. (2019). Pristimerin enhances the effect of cisplatin by inhibiting the miR23a/Akt/GSK3beta signaling pathway and suppressing autophagy in lung cancer cells. Int. J. Molec. Med. 43, 1382–1394. doi: 10.3892/ijmm.2019.4057
Zhao, H., Wang, C., Lu, B., Zhou, Z., Jin, Y., Wang, Z., et al. (2016). Pristimerin triggers AIF-dependent programmed necrosis in glioma cells via activation of JNK. Cancer Lett. 374, 136–148. doi: 10.1016/j.canlet.2016.01.055
Keywords: pristimerin, anti-cancer, mechanism, molecular target, pharmaceutical effect, apoptosis, autophagy
Citation: Li J-j, Yan Y-y, Sun H-m, Liu Y, Su C-y, Chen H-b and Zhang J-y (2019) Anti-Cancer Effects of Pristimerin and the Mechanisms: A Critical Review. Front. Pharmacol. 10:746. doi: 10.3389/fphar.2019.00746
Received: 26 January 2019; Accepted: 11 June 2019;
Published: 12 July 2019.
Edited by:
Syed Nasir Abbas Bukhari, Al Jouf University, Saudi ArabiaCopyright © 2019 Li, Yan, Sun, Liu, Su, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-ye Zhang, amlhbnllekBnemhtdS5lZHUuY24=; Hubiao Chen, aGJjaGVuQGhrYnUuZWR1Lmhr
†These authors have contributed equally to this work.
 Jia-jun Li1†
Jia-jun Li1† Yan-yan Yan
Yan-yan Yan Jian-ye Zhang
Jian-ye Zhang