- 1Hong Kong Chinese Medicine Clinical Study Centre, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong
- 2Institution of Clinical Research and Evidence Based Medicine, Gansu Provincial Hospital, Lanzhou, China
Background: Cancer incidence and mortality rates keep rising globally. Coriolus versicolor and Ganoderma lucidum related natural products are commonly applied as a complementary therapeutic option for different stages and types of cancers. The aim of this study is to evaluate the efficacy and safety of the products for cancer therapy.
Methods: Randomized controlled trials were identified by systematic search over seven databases from inceptions to May 10, 2019. Two independent reviewers extracted data and assessed the study quality. Meta-analyses were performed to pool hazard ratio (HR), risk ratio (RR), mean differences (MD), and 95% CI using random-effects models. The sources of heterogeneity were explored by subgroup analyses and sensitivity analyses. Publication bias was detected by Funnel plots, Begg’s test, and Egger’s test.
Results: Twenty-three trials involving 4,246 cancer patients were included in this work. C. versicolor and G. lucidum related natural products were significantly associated with lower risks of mortality (HR: 0.82; 95% CI: 0.72, 0.94) and higher total efficacy (RR: 1.30; 95% CI: 1.09, 1.55), but not associated with control rate (RR: 1.05; 95% CI: 0.96, 1.14) compared with control treatment. There was no significant difference between C. versicolor related natural products and control treatment in the effect on relapse-free survival (HR: 1.19; 95% CI: 0.91, 1.55). Compared with control treatment, C. versicolor and G. lucidum related natural products had a favorable effect on elevated levels of CD3 (MD: 9.03%; 95% CI: 2.10, 16.50) and CD4 (MD: 9.2%; 95% CI: 1.01, 17.39), but had no effect on the levels of CD8 (MD: −5.52%; 95% CI: −23.17, 12.13), CD4/CD8 (MD: 0.73; 95% CI:-0.45, 1.91), or NK(MD: 5.87%; 95% CI: −1.06, 12.8).
Conclusion: In this meta-analysis, we found that C. versicolor and G. lucidum related natural products might have potential benefits on the overall survival and quality of life in cancer patients.
Introduction
The burden of cancer continues to increase globally. According to WHO statistics, cancer is the second leading cause of death, accounting for 8.8 million deaths in 2015 (Organization, 2018). Anticancer therapies, for instance, surgery, chemotherapy, radiotherapy, and targeted cancer immunotherapy, are examples on controlling cancer cell growth, prolonging survival time, and improving quality of life to some extent. However, these therapies either alone or in combination have been shown to have various limitations and can result in severe side effects, which include an increased risk of subsequent cancers and lowered quality of life that vary with clinical factors (e.g., cancer type and treatment) and patient characteristics (e.g., age, sex, and comorbidity) (Hodge et al., 2012; Zigler et al., 2013; Miller et al., 2016).
In the past few decades, Coriolus versicolor (taxonomic name, Trametes versicolor; Chinese name; Yun Zhi) and its related mushrooms recorded in traditional Chinese medicine (TCM) literature have found their way to the market in Asian countries as anticancer remedies, and potentially play an important role in the whole course of cancer treatment such as the recovery stage of post-operation, and the stages during and after radiotherapy or chemotherapy (Sanodiya et al., 2009; Zhou et al., 2014). However, they have long been clinically confused based on their similar appearance and nature of medicinals according to the TCM theory (Pharmacopoeia, 2015). In vitro studies suggested that both C. versicolor (Yun Zhi) and Ganoderma lucidum (Ling Zhi) extracts, for instance, polysaccharide krestin (PSK), polysaccharide peptide (PSP) in C. versicolor, and beta-glucans, triterpenes in G. lucidum, possess selective cytotoxic activity against certain tumor cells (Yang et al., 1992; Dong et al., 1997; Jin et al., 2012). They may also activate various types of immune effector cells to enhance their anticancer activity, for instance, B lymphocytes, T lymphocytes, cytotoxic T cells, natural killer cells, and lymphokine activated killer cells (Xu et al., 2011; Yousefi et al., 2017). Furthermore, significant reduction of the tumor size after prolonged administration with the extracts was clearly shown in mice and the extract appeared to be effective for the prophylaxis against cancers (Tsukagoshi et al., 1984; Ren et al., 2012; Chen et al., 2014).
Compared to the supporting evidence from laboratory and animal tests, human trial of Yun Zhi and Ling Zhi extracts is just having its start. In the past 40 years, trials were mainly conducted on patients in Asia with breast cancer, colorectal cancer, gastric cancer, and non-small cell lung cancer, etc. and the trial data were scattered in regional databases (Torisu et al., 1990; Nakazato et al., 1994; Tsang et al., 2003; Kuo et al., 2012). Also, there was no systematic review to integrate the outcome measurements in different trials to form strong evidence. As a result, in order to provide better understanding of their clinical effect for physicians and other health care providers, we summarized trial results using C. versicolor and G. lucidum related natural products as adjuvant cancer treatment in different stages and kinds of cancer lesions from various databases. We hope this review can contribute a comprehensive view of current existing evidence to facilitate development of more effective natural products for public good.
Methods
Search Strategy
The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). We searched PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and Chinese Scientific Journal Database (VIP) from inceptions to May 10, 2019, and identified randomized controlled trials with C. versicolor and G. lucidum related natural products for cancer patients. The search strategy was conducted by medical subject headings with text words. We referred to the published Cochrane protocol about C. versicolor and G. lucidum to initiate our search strategies (Pilkington et al., 2016). We also consulted all the searching names with the Chinese Medicine pharmacists in mainland China and Hong Kong. The completed search terms about C. versicolor and G. lucidum included as follows: C. versicolor, T. versicolor, Polyporus versicolor, Polystictus versicolor, Kawaratake, Yun Zhi, Ling Zhi, polysaccharide-K, PSK, krestin, polysaccharopeptide, polysaccharide-peptide, PSP, VPS, turkey tail, cloud mushroom, and unji mushroom.
In addition, the reference lists of the included studies were also checked, so as to supplement possible relevant literatures.
Study Selection
Two reviewers independently screened and selected the searched articles according to the inclusion criteria (LZ and PY): 1) patients with cancer confirmed by pathology; 2) C. versicolor and G. lucidum related natural products were used as an intervention alone or combined with other drugs, without limitation on drug regimen, dosage and, course of treatment; and 3) must be randomized controlled trials. The following articles were excluded: 1) case series or reviews and conference abstracts; 2) valid original data were unable to obtain even when contacting the author; and 3) similar studies were reported without additional data to analyze and extract.
Data Extraction
Two reviewers (LZ and PY) independently extracted data on participant characteristics from the selected studies in a standardized data extraction form. We extracted the following information from each included article: first author, year of publication, country, the type of tumor, number of participants, participant characteristics, the characteristics of the products (the type, dose, start time, duration of therapy), mean follow-up duration, number of dropout, controlled intervention, and outcome data.
Definition of Outcomes
We included three primary outcomes to compare the effectiveness and safety of C. versicolor and G. lucidum related natural products for cancer in the analysis: 1) overall survival (OS); 2) relapse-free survival (RFS) rate; and 3) clinical efficacy. OS and RFS are defined by the individual study. Clinical efficacy was evaluated by investigators using Macdonald criteria (Macdonald et al., 1990). There were four “response” categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Total efficacy means CR+PR; control rate means CR+PR+SD.
The secondary outcomes recorded were 1) immune-modulating effects including cluster of differentiation 3 (CD3); cluster of differentiation 4 (CD4); cluster of differentiation 8 (CD8); CD4/CD8; and natural killer cell (NK); 2) the post-treatment Karnofsky Performance Status (KPS) Score Change; and 3) adverse events. The post-treatment KPS score change was divided into obvious effectiveness (had an improvement of more than 20 points in the KPS score), effectiveness (an improvement of more than 10 points), stabilization (an improvement of less than 10 points or had no change), and invalid (a decrease in KPS score). Total effectiveness means obvious effectiveness plus effectiveness (Zhou and Lin, 2003).
Risk of Bias Assessment
Two review authors (LZ and PY) assessed potential risks of bias for all included studies using the Cochrane’s tool for assessing risk of bias (Higgins JPT and Sterne, 2011). The tool assesses bias in six different domains: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Each domain receives a score of high, low, or unclear depending on each review author’s judgment. A third review author acted as an adjudicator in the event of disagreement. Where doubt existed as to a potential risk of bias, we contacted the study authors for clarification.
Statistical Analysis
In this meta-analysis, risk ratio (RR) and 95% confidence interval (CI) were considered as the effect size for dichotomous outcomes; weighted mean differences (WMD) with 95% CI were calculated as the effect size for continuous outcomes. For time-to-event data, we will pool hazard ratio (HR). Forest plots were produced to visually assess the effect size and corresponding 95% CI using random-effects models. Heterogeneity between studies was assessed via the forest plot, while I 2 values described the total variation between studies. I 2 values of <25%, 25–50%, and >50% indicated low, moderate, and high heterogeneity, respectively. Subgroup analyses were used to explore and interpret the sources of heterogeneity; to evaluate whether the effects were modified by treatment characteristics, we specified based on experiment type and cancer type. We used sensitivity analyses to explore and interpret the sources of high heterogeneity (Higgins JPT and Sterne, 2011). Funnel plots, Begg’s test (Begg and Mazumdar, 1994), and Egger’s test (Egger et al., 1997) would be adopted to detect publication bias only when there are at least 10 studies reporting the primary outcomes, because when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry (Higgins JPT and Sterne, 2011). Statistical analysis was performed with STATA software, version 13.0 (StataCorp, College Station, TX).
Results
Studies Selection
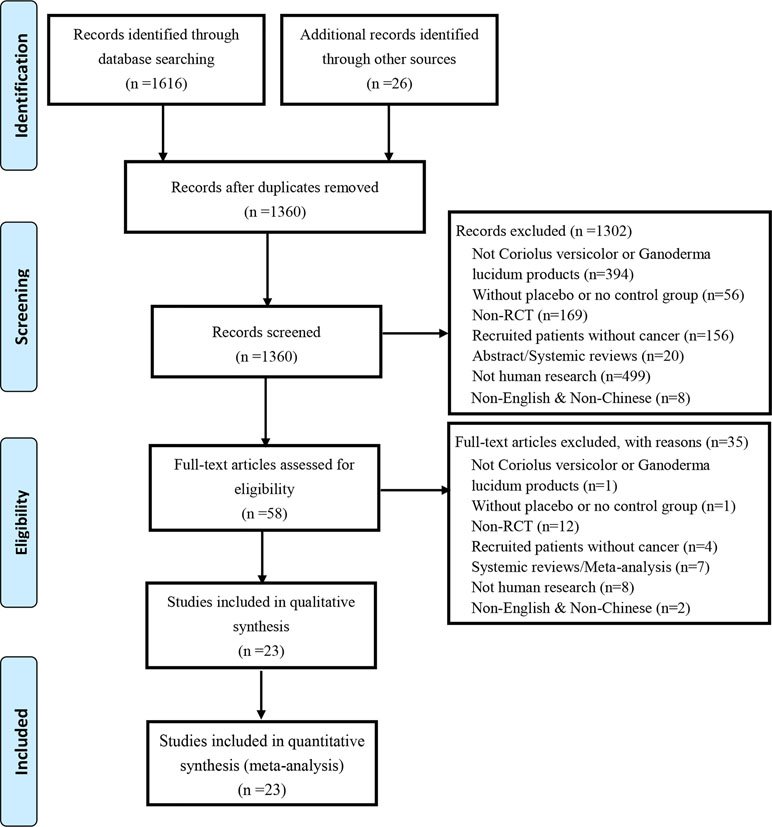
Our literature search yielded 1,616 trials via electronic databases, and 26 trials by hand research. After removing duplicates records, 1,360 trials were screened, and 1,302 trials were excluded by reviewing titles and abstracts. The remaining 58 trials were reviewed by full text. Eventually, 23 trials involving 4,246 cancer patients were included in this work. Study selection flow is detailed by PRISMA flow diagram as shown in Figure 1. All the preparations of C. versicolor and G. lucidum related natural products in each included article were listed in Supplement 1.
Description of Trials Identified
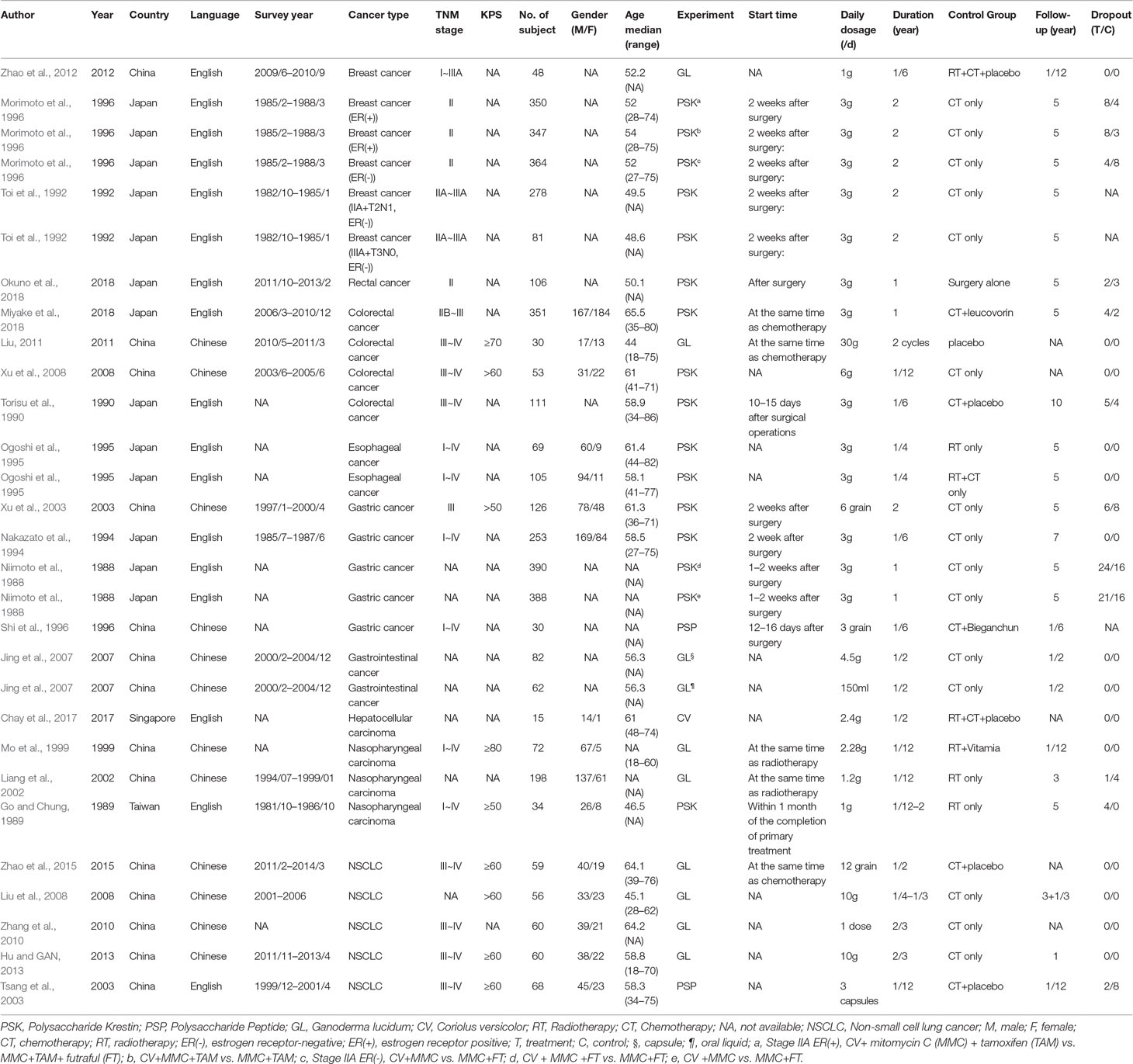
Table 1 presents the characteristics of the 23 included trials (Niimoto et al., 1988; Go and Chung, 1989; Torisu et al., 1990; Toi et al., 1992; Nakazato et al., 1994; Ogoshi et al., 1995; Morimoto et al., 1996; Shi et al., 1996; Mo et al., 1999; Liang et al., 2002; Tsang et al., 2003; Xu et al., 2003; Jing et al., 2007; Liu et al., 2008; Xu et al., 2008; Zhang et al., 2010; Liu, 2011; Zhao et al., 2012; Hu and GAN, 2013; Zhao et al., 2015; Chay et al., 2017; Miyake et al., 2018; Okuno et al., 2018). All the trials used C. versicolor related natural products (n = 14) (Niimoto et al., 1988; Go and Chung, 1989; Torisu et al., 1990; Toi et al., 1992; Nakazato et al., 1994; Ogoshi et al., 1995; Morimoto et al., 1996; Shi et al., 1996; Tsang et al., 2003; Xu et al., 2003; Xu et al., 2008; Chay et al., 2017; Miyake et al., 2018; Okuno et al., 2018) or G. lucidum related natural products (n = 9) (Mo et al., 1999; Liang et al., 2002; Jing et al., 2007; Liu et al., 2008; Zhang et al., 2010; Liu, 2011; Zhao et al., 2012; Hu and GAN, 2013; Zhao et al., 2015). The majority of trials were from China (Shi et al., 1996; Mo et al., 1999; Liang et al., 2002; Tsang et al., 2003; Xu et al., 2003; Jing et al., 2007; Liu et al., 2008; Xu et al., 2008; Zhang et al., 2010; Liu, 2011; Zhao et al., 2012; Hu and GAN, 2013; Zhao et al., 2015), whereas eight papers were from Japan (Niimoto et al., 1988; Torisu et al., 1990; Toi et al., 1992; Nakazato et al., 1994; Ogoshi et al., 1995; Morimoto et al., 1996; Miyake et al., 2018; Okuno et al., 2018), one paper was from in Taiwan (Go and Chung, 1989), and one from Singapore (Chay et al., 2017). Almost half of the 23 trials were published in Chinese (Shi et al., 1996; Mo et al., 1999; Liang et al., 2002; Xu et al., 2003; Jing et al., 2007; Liu et al., 2008; Xu et al., 2008; Zhang et al., 2010; Liu, 2011; Hu and GAN, 2013; Zhao et al., 2015), the others were published in English (Niimoto et al., 1988; Go and Chung, 1989; Torisu et al., 1990; Toi et al., 1992; Nakazato et al., 1994; Ogoshi et al., 1995; Morimoto et al., 1996; Tsang et al., 2003; Zhao et al., 2012; Chay et al., 2017; Miyake et al., 2018; Okuno et al., 2018). In 23 trials, 5 included non-small cell lung cancer (NSCLC) patients (Tsang et al., 2003; Liu et al., 2008; Zhang et al., 2010; Hu and GAN, 2013; Zhao et al., 2015), 3 included breast cancer patients (Toi et al., 1992; Morimoto et al., 1996; Kuo et al., 2012; Zhao et al., 2012), 4 included gastric cancer patients (Niimoto et al., 1988; Nakazato et al., 1994; Shi et al., 1996; Xu et al., 2003), 4 included colorectal cancer patients (Torisu et al., 1990; Xu et al., 2008; Liu, 2011; Miyake et al., 2018), 3 included nasopharyngeal carcinoma patients (Go and Chung, 1989; Mo et al., 1999; Liang et al., 2002), and the other 4 trials included esophageal cancer (Ogoshi et al., 1995), rectal cancer (Okuno et al., 2018), gastrointestinal cancer (Jing et al., 2007), and hepatocellular carcinoma patients (Chay et al., 2017), respectively. The range duration of therapy was 1 to 24 months.
The data in five trials were split into two or three records because there were two kinds of comparison, dosage form or disease staging. The data were split into two records because of the comparison in Ogoshi K’s trial (Ogoshi et al., 1995) [C. versicolor + radiotherapy (RT) vs. RT only and C. versicolor + RT + chemotherapy (CT) vs. RT + CT only] and in Niimoto M’s trial (Niimoto et al., 1988) [C. versicolor+ mitomycin C (MMC)+futraful (FT) vs. MMC+FT and C. versicolor+MMC vs. MMC+FT]; because of the dosage form in Junjie Jing’s trial (Jing et al., 2007) (capsule and oral liquid); because of the disease staging in Toi M’s trial (Toi et al., 1992) (stage IIA+T2N1, ER[-], and stage IIIA+T3N0,ER[-]). The data were split into three records because of the comparison and disease staging in Morimoto T’s trial (Morimoto et al., 1996) (stage IIA ER[-]:C. versicolor+MMC vs. MMC+FT; stage IIA ER[+]:C. versicolor+MMC+ tamoxifen [TAM] vs. MMC+TAM+FT and C. versicolor+MMC+TAM vs. MMC+TAM). Therefore, there were 27 records to be analyzed.
Figures S1 and S2 in the Supplement show the assessment of the risk of bias. All studies were randomized; four studies were double-blinded design; five studies were placebo-controlled trials; six trials described an adequate random sequence generation process; and four trials described the methods used for allocation concealment.
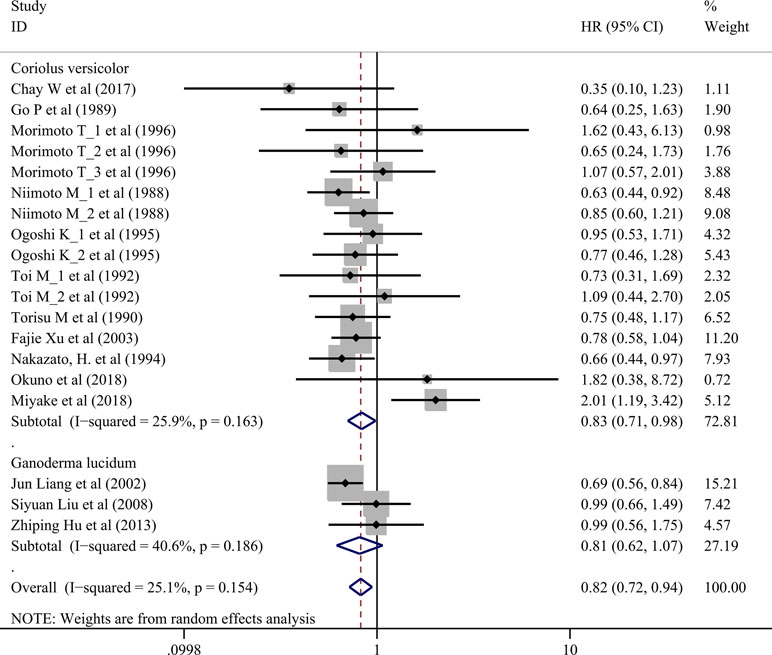
Effects on Overall Survival
Seventeen trials involving 3,682 cancer patients compared the effect on survival. Compared with control treatment, using of C. versicolor and G. lucidum related natural products was associated with a lower risk of mortality (HR: 0.82 95% CI: 0.72, 0.94; P = 0.005). Subgroup analysis for experiment type showed this effect was consistent for trial using C. versicolor related natural products (HR: 0.83; 95% CI: 0.71, 0. 98; P = 0.030, 16 studies), but no difference was found in trial using G. lucidum related natural products (HR: 0.81; 95% CI: 0.62, 1.07; P = 0.139; three studies) (Figure 2).
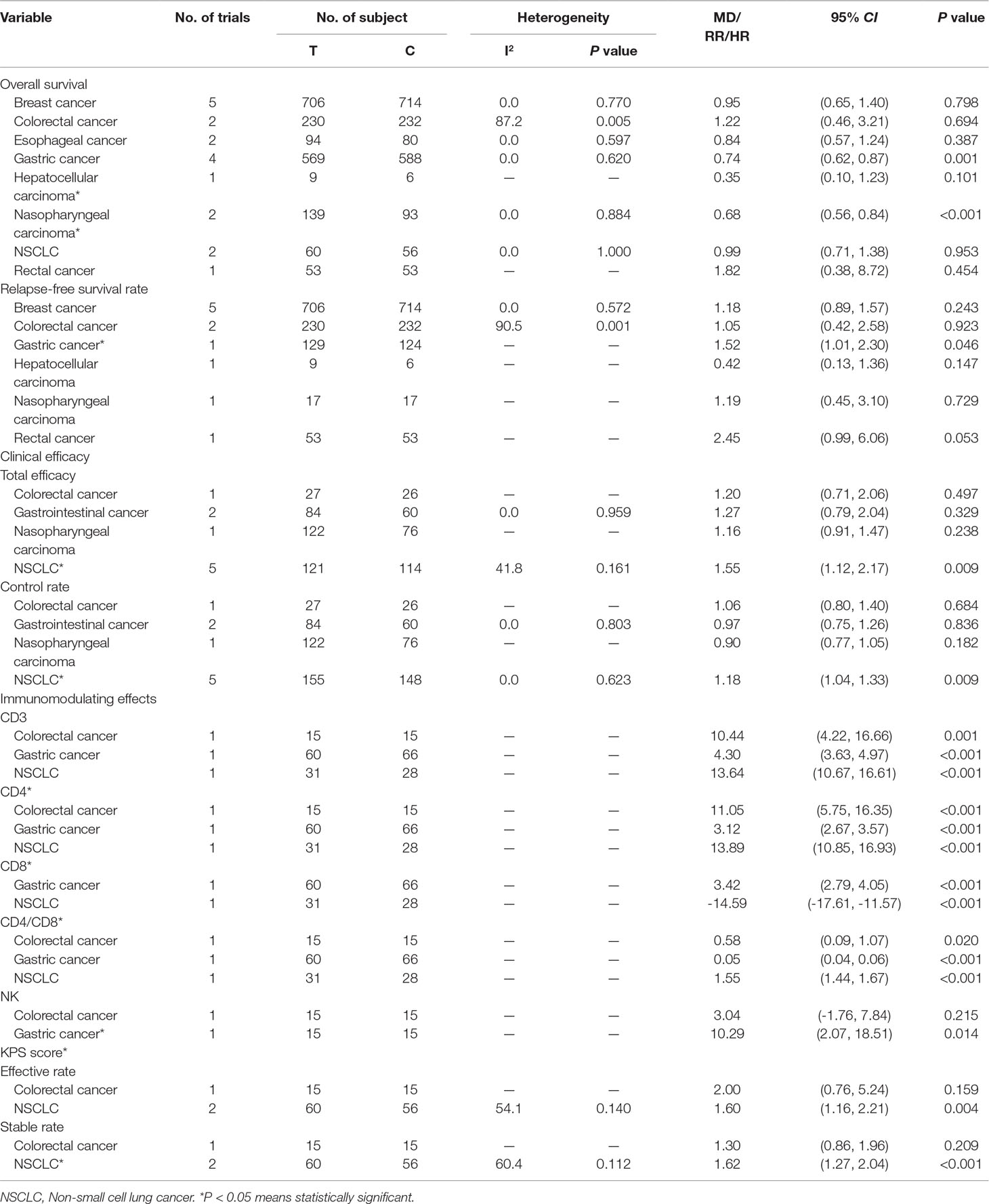
Subgroup analysis for cancer type showed, compared with control treatment, trials using C. versicolor and G. lucidum related natural products were associated with lower risk of mortality in gastric cancer (HR: 0.74; 95% CI: 0.62,0.87; P = 0.001; four trials) and nasopharyngeal carcinoma (HR: 0.68; 95% CI: 0.56,0.84; P < 0.001; two trials). However, no differences were found in breast cancer (HR: 0.95; 95% CI: 0.65,1.40; P = 0.798; five trials), colorectal cancer (HR: 1.22; 95% CI: 0.46, 3.21; P = 0.694; two trials), esophageal cancer (HR: 0.84; 95% CI: 0.57,1.24; P = 0.387; two trials), hepatocellular carcinoma (HR: 0.35; 95% CI: 0.10,1.23; P = 0.101; one trial), NSCLC (HR: 0.99; 95% CI: 0.71,1.38; P = 0.953; two trials), and rectal cancer (HR: 1.82; 95% CI: 0.38,8.72; P = 0.454; one trial) (Table 3).
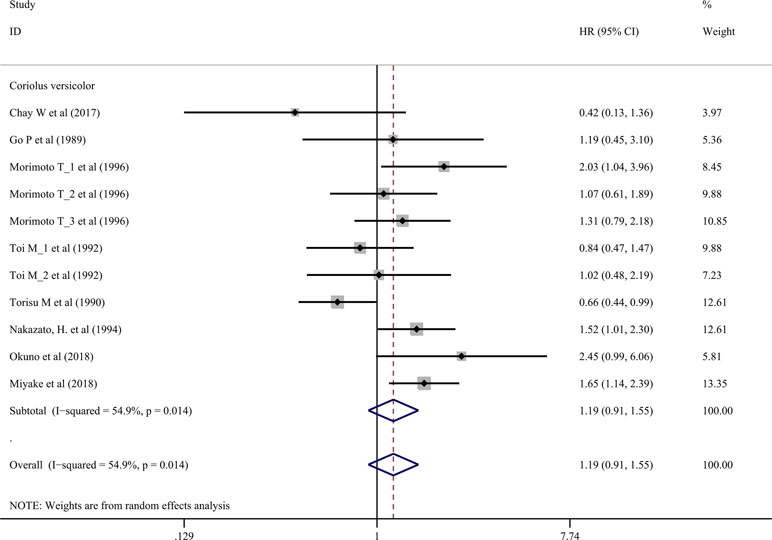
Effects on Relapse-Free Survival Rate
Nine trials involving 1,155 cancer patients compared the effect on RFS rate. All nine trials used C. versicolor related natural products. As shown in Figure 3, there was no significant association of C. versicolor related natural products with RFS (HR: 1.19; 95% CI: 0.91, 1.55; P = 0.2) compared with control treatment (Figure 3). Subgroup analysis for cancer type showed, compared with control treatment, trials using C. versicolor and G. lucidum related natural products were associated with higher risk of relapse in gastric cancer (HR: 1.52; 95% CI: 1.01,2.30; P = 0.046; one trial). There were no significant associations of C. versicolor related natural products with risk of relapse in breast cancer (HR: 1.18; 95% CI: 0.89,1.57; P = 0.243; five trials), colorectal cancer (HR: 1.05; 95% CI: 0.42,2.58; P = 0.923; two trials), hepatocellular carcinoma (HR: 0.42; 95% CI: 0.13,1.36; P = 0.147; one trial), nasopharyngeal carcinoma (HR: 1.19; 95% CI: 0.45,3.10; P = 0.729; one trial), and rectal cancer (HR: 2.45; 95% CI: 0.99,6.06; P = 0.053; one trial) (Table 3).
Clinical Efficacy
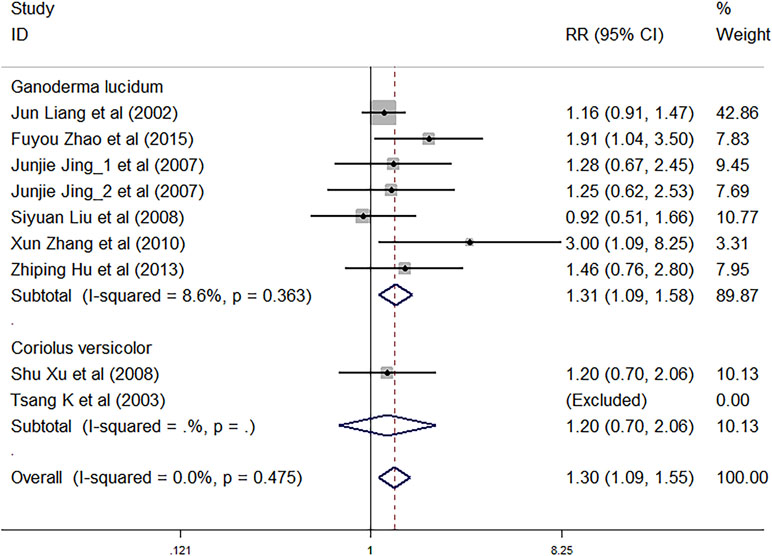
Nine trials involving 1,883 cancer patients assessed the total efficacy. Compared with control treatment, using C. versicolor and G. lucidum related natural products was associated with a higher total efficacy (RR: 1.30; 95% CI: 1.09, 1.55; P = 0.003). Subgroup analysis for experiment type showed trials using G. lucidum related natural products with a higher total efficacy (RR: 1.31; 95% CI: 1.09, 1.58; P = 0.004; seven studies) compared with control treatment. However, there was no significant association of C. versicolor related natural products with total efficacy (RR: 1.20; 95% CI: 0.70, 2.06; P = 0.497) compared with control treatment. Only two trials used C. versicolor related natural products, and one of them was excluded because the total efficacy of the trial was 0% (Figure 4).
Subgroup analysis for cancer type showed, compared with control treatment, trials using C. versicolor and G. lucidum related natural products were associated with a higher total efficacy in NSCLC (RR: 1.55; 95% CI: 1.12, 2.17; P = 0.009; five trials), but no differences were found in colorectal cancer (RR: 1.20; 95% CI: 0.71, 2.06; P = 0.497; one trial), gastrointestinal cancer (RR: 1.27; 95% CI: 0.79,2.04; P = 0.329; two trials), and nasopharyngeal carcinoma (RR: 1.16; 95% CI: 0.91, 1.47; P = 0.238; one trial) (Table 3).
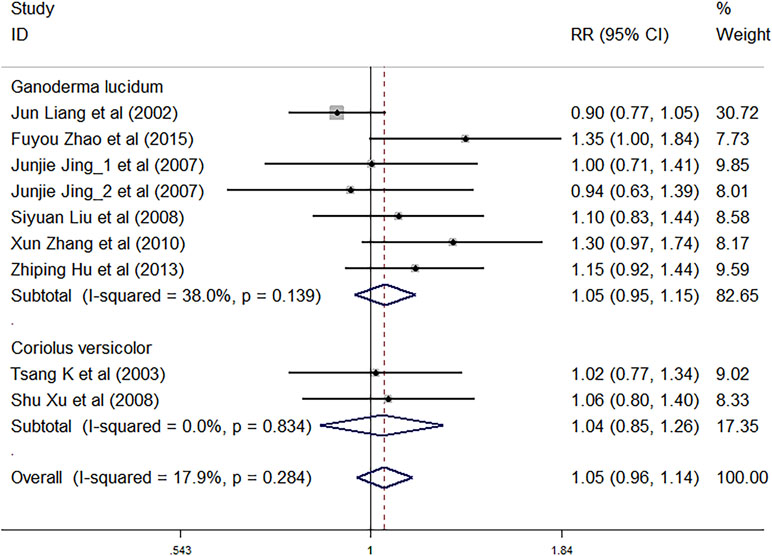
Nine trials involving 1,883 cancer patients were compared with the control rate. There was no significant association of C. versicolor and G. lucidum related natural products and control rate (RR: 1.05; 95% CI: 0.96, 1.14; P = 0.321) compared with control treatment. Subgroup analysis for experiment type showed no significant interactions with experiment type for the primary outcome of control rate. Control rate did not differ significantly between trials using G. lucidum related natural products (RR: 1.05; 95% CI: 0.95, 1.15; P = 0.355; seven studies) or trials using C. versicolor related natural products (RR: 1.04; 95% CI: 0.85, 1.26; P = 0.725; two studies) and control treatment (Figure 5).
Subgroup analysis for cancer type showed, compared with control treatment, trials using C. versicolor and G. lucidum related natural products were associated with a higher control rate in NSCLC (RR: 1.18; 95% CI: 1.04, 1.33; P = 0.009; five trials), but no differences were found in colorectal cancer (RR: 1.06; 95% CI: 0.80, 1.40; P = 0.684; one trial), gastrointestinal cancer (RR: 0.97; 95% CI: 0.75,1.26; P = 0.836; two trials), and nasopharyngeal carcinoma (RR: 0.90; 95% CI: 0.77, 1.05; P = 0.182; one trial) (Table 3).
Immunomodulating Effects
Table 2 summarizes results of meta-analysis and subgroup analyses of the immunomodulating effects for C. versicolor and G. lucidum related natural products. Compared with control treatment, using C. versicolor and G. lucidum related natural products had a favorable effect on elevated levels of CD3 (MD: 9.03%; 95% CI: 2.10, 16.50; P = 0.011) and CD4 (MD: 9.2%; 95% CI: 1.01, 17.39; P = 0.028). However, no difference between C. versicolor and G. lucidum related natural products and control treatment was seen in the effect on the levels of CD8 (MD: -5.52%; 95% CI: -23.17, 12.13; P = 0.540) and CD4/CD8 (MD: 0.73; 95% CI: -0.45, 1.91; P = 0.227). In terms of NK, only two trials reported the NK[9, 16]; one trial used G. lucidum related natural products in a patient with colorectal cancer (MD: 3.04%; 95% CI: -1.76, 7.84; P = 0.215), while the other used C. versicolor related natural products in a patient with gastric cancer (MD: 10.29%; 95% CI: 2.07, 18.15; P = 0.014); there was no difference between C. versicolor and G. lucidum related natural products and control treatment in the effect on NK (MD: 5.87%; 95% CI: -1.06, 12.8; P = 0.097) (Tables 2 and 3).
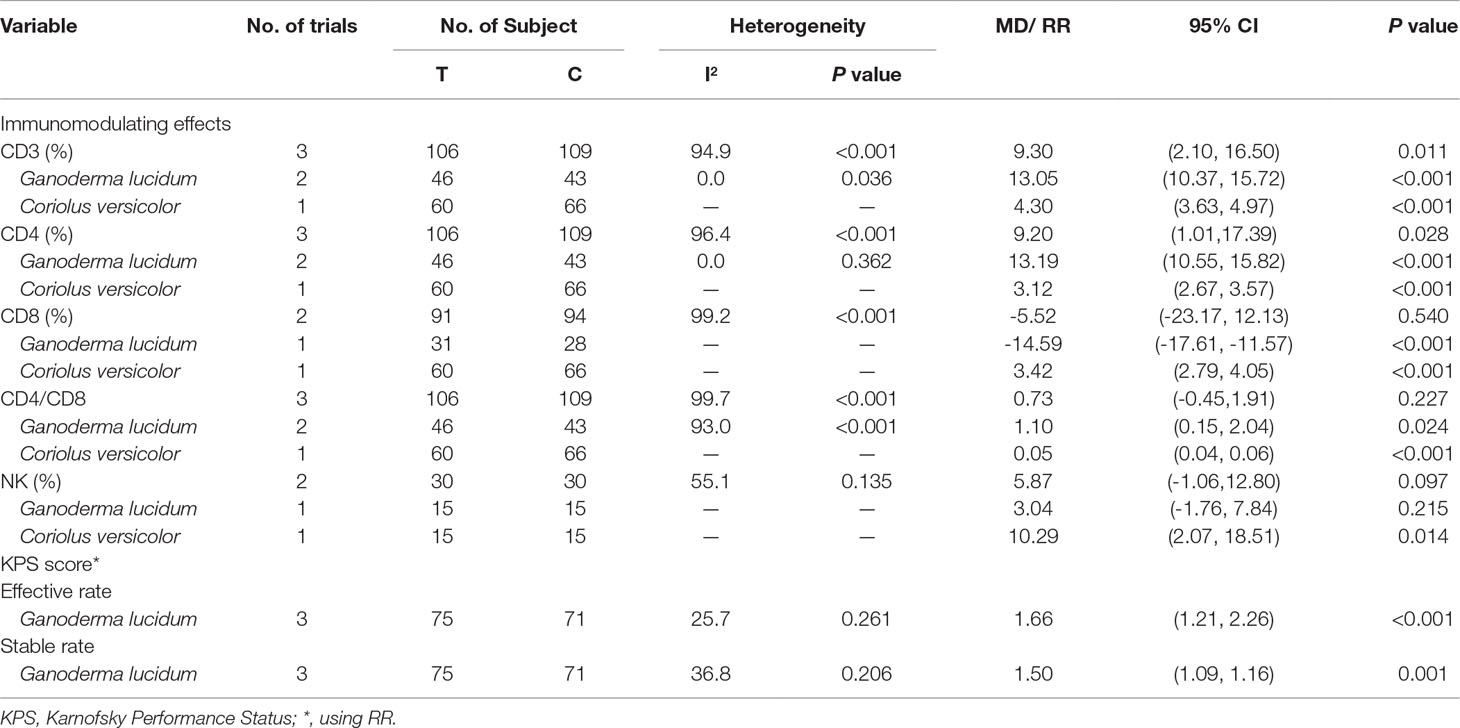
Table 2 Meta-analysis and subgroup analysis of immunomodulating effects and KPS for Coriolus versicolor and Ganoderma lucidum related natural products.
Subgroup analysis for experiment type of CD3 showed the favorable effect on elevating the levels of CD3 both for trials using G. lucidum related natural products (MD: 13.05%; 95% CI: 10.37, 15.72; P < 0.001; two trials) and C. versicolor related natural products (MD: 4.30%; 95% CI: 3.63, 4.97; P < 0.001; one trials) compared with control group. The subgroup analysis of CD4 was consistent with CD3; trials using G. lucidum related natural products (MD: 13.19%; 95% CI: 10.55, 15.82; P < 0.001; two trials) or C. versicolor related natural products (MD: 3.12%; 95% CI: 2.67, 3.57; P < 0.001; one trials) had favorable effect on elevating the levels of CD4 (Table 2).
Subgroup analysis for experiment type of CD8 showed the favorable effect on felling the level of CD8 both for one trial using G. lucidum related natural products (MD: -14.59%; 95% CI: -17.61, -11.57; P < 0.001) and one trial using C. versicolor related natural products (MD: 3.42%; 95% CI: 2.79, 4.05; P < 0.001) compared with the control group. Subgroup analysis of CD4/CD8 showed the favorable effect on elevating the levels of CD4/CD8 both for trials using G. lucidum related natural products (MD: 1.1 95% CI: 0.15, 2.04; P = 0.024; two trials) and C. versicolor related natural products (MD: 0.05; 95% CI: 0.04, 0.06; P < 0.001; one trials) compared with the control group (Table 2).
Table 3 summarizes results of subgroup analyses for cancer type and immunomodulating effects. There was one trial that reported that C. versicolor and G. lucidum related natural products had a favorable effect on elevating the levels of CD3, CD4, and CD4/CD8 in colorectal cancer, gastric cancer, and NSCLC, respectively. One trial in gastric cancer reported that the products had a favorable effect on elevating the levels of CD8, but one trial in NSCLC reported that the products had an effect on felling the levels of CD8 (Table 3).
Post-Treatment KPS Score Change
Three trials using G. lucidum related natural products were compared with the post-treatment KPS score change. Compared with the control treatment, using G. lucidum related natural products was associated with a higher total efficacy (RR: 1.66; 95% CI: 1.21, 2.26; P < 0.001) and higher stable rate (RR: 1.50; 95% CI: 1.09, 1.16; P = 0.001) (Table 2). Subgroup analyses for cancer type showed, compared with control treatment, that using G. lucidum related natural products was associated with a higher total efficacy (RR: 1.60; 95% CI: 1.16, 2.21; P = 0.004; two trials) and higher stable rate (RR: 1.62; 95% CI: 1.27, 2.04; P < 0.001; two trials) in NSCLC. However, one trial reported the association between G. lucidum related natural products and post-treatment KPS score change in colorectal cancer (Table 3).
Adverse Events
In all 23 trials, one trial in gastric cancer mentioned the severe adverse events (Shi et al., 1996); seven trials (two in NSCLC; one in breast cancer; one in nasopharyngeal carcinoma; one in gastric cancer; two in colorectal cancer) reported that adverse effects fell by using C. versicolor and G. lucidum related natural products (Morimoto et al., 1996; Liang et al., 2002; Xu et al., 2003; Xu et al., 2008; Zhang et al., 2010; Zhao et al., 2015; Miyake et al., 2018). The most common adverse effects happened in including nausea or/and vomiting, leucopenia, diarrhea, thrombocytopenia, liver dysfunction, general fatigue, and anorexia.
Publication Bias and Sensitivity Analyses
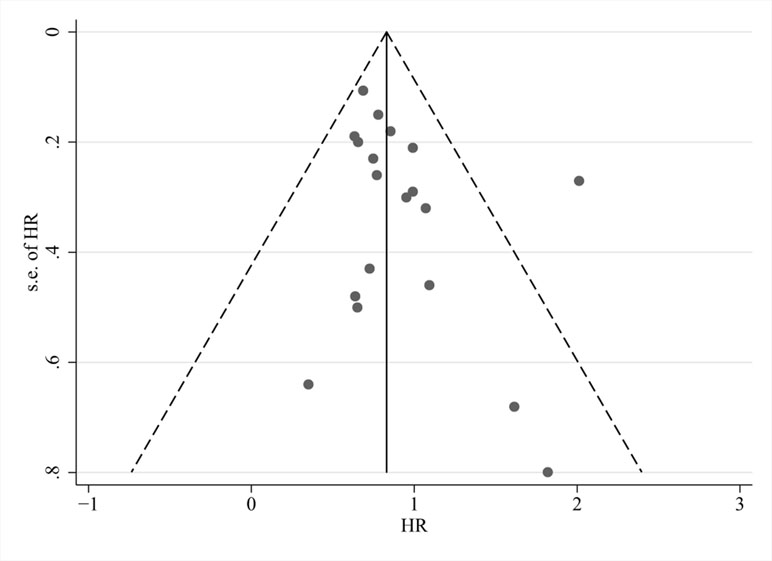
Visual inspection of funnel plots (Figure 6), Begg’s test (P = 0.294), and Egger’s test (P = 0.162) revealed no evidence of publication bias for the examined primary outcomes. We did sensitivity analyses by excluding four trials using the decoction (Liu et al., 2008; Zhang et al., 2010; Liu, 2011; Hu and GAN, 2013); effects on OS (HR: 0.81; 95% CI: 0.69, 0.93; P = 0.004), total efficacy (RR: 1.26; 95% CI: 1.04, 1.53; P = 0.018), and control rate (RR: 1.00; 95% CI: 0.90, 1.11; P = 0.957) showed that the results did not change.
Discussion
To the best of our knowledge, this study is the first meta-analysis focusing on C. versicolor and G. lucidum related natural products as adjuvant treatment on cancer patients. The pooled analysis demonstrated that C. versicolor and G. lucidum related natural products were significantly associated with lower risks of mortality in patients with cancers, and the pooled HR of patient in both groups is 0.82. Moreover, the effect on RFS is only available for C. versicolor related trials and results no significant difference across the patients. The side effect profiles show that these products were well tolerated. As a result, from aspects of clinical efficacy and safety, this study suggested that both C. versicolor and G. lucidum related therapy can be considered as an additional treatment option over different stages and types of cancers, although this recommendation cannot be specifically conclusive because the review only included limited kinds and stages of cancers.
With more evidence proving the anticancer effect of C. versicolor and G. lucidum extracts, lack of guidelines to support the clinical use for patients with cancers gradually becomes an issue. Especially in Asia, several companies have been dedicated to prepare anti-cancer formulae from C. versicolor and G. lucidum extracts using new technology (Patel and Goyal, 2012). As the companies put huge amount of resources in marketing, as supplements, there is a booming demand of C. versicolor and G. lucidum related natural products for preventing and treating of cancers, and thus more companies are expected to participate in the potential market. However, based on the amount of clinical trials found in this review study, it is necessary to accelerate human trials of C. versicolor and G. lucidum extracts to verify their efficacy and safety in various types and stages of cancers. For cancer patients and their families, clinical evidence and guidelines recommending C. versicolor and G. lucidum related natural products as an additional treatment with conventional cancer therapies are critical to improve the survival chance. In this study, meta-analysis on the immunomodulating effects also showed that both C. versicolor and G. lucidum extracts can significantly elevate the levels of CD3 and CD4 T cell. The CD3 and CD4 T cell count alongside other immunological parameters are critical in monitoring immune function, and the CD4 T cell subset is used as a standard for assessing the progression of disease. Lower levels of CD3 and CD4 T cell are related to immunosuppression of chemotherapy or radiotherapy (Tsegaye et al., 1999). The results of increasing the levels of CD3 and CD4 indicated that these products can help to reduce the immunosuppression of chemotherapy or radiotherapy.
The anticancer values of C. versicolor and G. lucidum have progressed for decades since the analysis of their extracts. Regarding the fact that clinicians usually confuse C. versicolor and G. lucidum since their dried herbs have similar appearance and nature of medicinals, this review has demonstrated distinguishable benefit of C. versicolor and G. lucidum extracts in different types and stages of cancer therapies as supplements. However, one of the best ways to prevent confusion is to promote proprietary Chinese medicine, for instance, capsule, pill, or dissolvable granule, and develop regular drugs for regions outside of East Asia. Learnt from this review, the future direction of C. versicolor and G. lucidum extract trials can be aimed to compare efficacy based on different standard dosages, or their independent therapeutic effect on patients with end stage cancers.
Several limitations are encountered during this study. First, the number of clinical trials is limited and mainly conducted in Asia. There is a lack of a large number of patients with the same type and stage of cancer, and the generalizability is limited. Second, the C. versicolor and G. lucidum extracts in trials are prepared differently and with various dosages. Therefore, we could not effectively examine the dosage effect of treatment across the outcomes. Third, although the adverse events (AE)/serious adverse events (SAE) profile is an important factor for choosing treatment options, it was not possible to perform an analysis to deal with such a concern because AE/SAE are not fully reported in all included trials. Fourth, we lack the dose-response analysis of these products because most of the original data did not mention the dose response; we could not conduct the dose-response analysis. In addition, as some patients are undergoing post-surgery and/or radiotherapy or chemotherapy, causation between the events and C. versicolor and G. lucidum extracts is hard to be evaluated. Fourth, in the included trials, C. versicolor and G. lucidum extracts can be independently applied or combined with other drugs as interventions; therefore, some of the therapeutic effect can be due to the interacted result between C. versicolor and G. lucidum extracts and other components.
Conclusion
In this meta-analysis, we found that C. versicolor and G. lucidum related natural products could increase the OS in cancer patients. Besides, it seems likely that the products provide clinical and life quality benefits for cancer patients with low side effects. Large sample size and high-quality randomized controlled trial (RCT) in different continents with various types and stages of cancer are needed to further evaluate the effect of the products on patients in the future.
Author Contributions
ZB designed and supervised this study. LZ, PY, and WL wrote the manuscript. LZ and PY searched the data and extracted the data. LY and PY provided the searching strategy and data analysis. PY and WL assessed the risk of bias. All authors read and approved the final manuscript.
Funding
The present work was supported by a grant from the Innovation and Technology Fund from Hong Kong Innovation and Technology Commission (ITS/134/15FX).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with the authors at the time of review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00703/full#supplementary-material
Figure S1 | Number/proportions of trials that met each criterion for risk of bias.
Figure S2 | Results of the risk of bias for 23 included trials.
Table S1 | The preparation of Coriolus versicolor and Ganoderma lucidum related natural products in each included articles.
References
Begg, C. B., Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi: 10.2307/2533446
Chay, W., Tham, C., Toh, H., Lim, H., Tan, C., Lim, C., et al. (2017). Coriolus versicolor (Yunzhi) use as therapy in advanced hepatocellular carcinoma patients with poor liver function or who are unfit for standard therapy. J. Altern. Complement. Med. (New York, N.Y). 23, 648–652. doi: 10.1089/acm.2016.0136
Chen, S. N., Chang, C. S., Hung, M. H., Chen, S., Wang, W., Tai, C. J., et al. (2014). The effect of mushroom beta-glucans from solid culture of ganoderma lucidum on inhibition of the primary tumor metastasis. Evid. Based Complement. Alternat. Med. 2014, 252171. doi: 10.1155/2014/252171
Dong, Y., Yang, M. M., Kwan, C. Y. (1997). In vitro inhibition of proliferation of HL-60 cells by tetrandrine and coriolus versicolor peptide derived from Chinese medicinal herbs. Life Sci. 60, Pl135–140. doi: 10.1016/S0024-3205(96)00695-9
Egger, M., Smith, G. D., Schneider, M., Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634. doi: 10.1136/bmj.315.7109.629
Go, P., Chung, C. (1989). Adjuvant PSK immunotherapy in patients with carcinoma of the nasopharynx. J. Int. Med. Res. 17, 141–149. doi: 10.1177/030006058901700205
Higgins, JPT, A. D., Sterne, J., (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Online]. London: the cochrane collaboration. Available: www.cochrane-handbook.org. [Accessed].
Hodge, J. W., Ardiani, A., Farsaci, B., Kwilas, A. R., Gameiro, S. R. (2012). The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin. Oncol. 39, 323–339. doi: 10.1053/j.seminoncol.2012.02.006
Hu, Z., Gan, N. (2013). A randomized parallel controlled study of TCM of Bailonglingshatang combinated chemotherapy in the treatment of advanced non-small cell lung cancer. J. Pract. Tradit. Chin. Intern. Med. 27, 54–57. doi: 10.3969/j.issn.1671-7813.2013.09(x).25
Jin, X., Ruiz Beguerie, J., Sze, D. M., Chan, G. C. (2012). Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev., Cd007731. doi: 10.1002/14651858.CD007731.pub2
Jing, J., Zhu, X., Li, B., Ying, J. (2007). The clinical observation of bailong capsule and oral liquid treating gastroenterology cancers. Shanxi Med. J. 36, 631–633. doi: 10.3969/j.issn.0253-9926.2007.07.027
Kuo, W. H., Yao, C. A., Lin, C. H., Chang, K. J. (2012). Safety and efficacy of Tien-Hsien liquid practical in patients with refractory metastatic breast cancer: a randomized, double-blind, placebo-controlled, parallel-group, Phase IIa Trial. Evid. Based Complement. Alternat. Med. 2012, 1–8. doi: 10.1155/2012/803239
Liang, J., Li, Y.-R., Wang, S.-W. (2002). Effect of radiotherapy combined with a medicine Lingzhi-912 on treatment of esophageal cancer. J. Fourth Military Med. Univ. 23, 278–280. doi: 10.3321/j.issn:1000-2790.2002.03.025
Liu, S. (2011). The cinical observation of Yiqiyangying combined with FOLFO 6 treating middle and advanced colorectal cancer. Beijing University of Chinese Medicine: M.M.
Liu, S., Xu, J., Rao, Q. (2008). The clinical observation of Feiliufang treating non-small-cell lung cancer with 28 cases. J. New Chin. Med. 40, 17–18+18. doi: 10.3969/j.issn.0256-7415.2008.04.012
Macdonald, D. R., Cascino, T. L., Schold, S. C., Jr., Cairncross, J. G. (1990). Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 8, 1277–1280. doi: 10.1200/JCO.1990.8.7.1277
Miller, K. D., Siegel, R. L., Lin, C. C., Mariotto, A. B., Kramer, J. L., Rowland, J. H., et al. (2016). Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66, 271–289. doi: 10.3322/caac.21349
Miyake, Y., Nishimura, J., Kato, T., Ikeda, M., Tsujie, M., Hata, T., et al. (2018). Phase III trial comparing UFT + PSK to UFT + LV in stage IIB, III colorectal cancer (MCSGO-CCTG). Surg. Today 48, 66. doi: 10.1007/s00595-017-1555-1
Mo, H., Hong, M., Zhang, J., Chen, D., Ma, J., Su, Y., et al. (1999). Effect of Wuse-Lingzhi-Jiaonang on reducing side-effects of radiotherapy and improving immune function in patients with nasophrygeal cancer. Chin. J. Clin.Oncol. 26, 216–218.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and the, P. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Morimoto, T., Ogawa, M., Orita, K., Sugimachi, K., Toge, T., Dohi, K., et al. (1996). Postoperative adjuvant randomised trial comparing chemoendocrine therapy, chemotherapy and immunotherapy for patients with stage II breast cancer: 5-year results from the nishinihon cooperative study group of adjuvant chemoendocrine therapy for breast cancer (ACETBC) of Japan. Eur. J. Cancer 32, 235–242. doi: 10.1016/0959-8049(95)00579-X
Nakazato, H., Koike, A., Saji, S., Ogawa, N., Sakamoto, J. (1994). Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Lancet 343, 1122–1126. doi: 10.1016/S0140-6736(94)90233-X
Niimoto, M., Hattori, T., Tamada, R., Sugimachi, K., Inokuchi, K., Ogawa, N. (1988). Postoperative adjuvant immunochemotherapy with mitomycin C, futraful and PSK for gastric cancer. Jpn J. Surg. 18, 681–686. doi: 10.1007/BF02471530
Ogoshi, K., Satou, H., Isono, K., Mitomi, T., Endoh, M., Sugita, M. (1995). Immunotherapy for esophageal cancer. Am. J. Clin. Oncol 18, 216–222. doi: 10.1097/00000421-199506000-00007
Okuno, K., Aoyama, T., Oba, K., Yokoyama, N., Matsuhashi, N., Kunieda, K., et al. (2018). Randomized phase III trial comparing surgery alone to UFT + PSK for stage II rectal cancer (JFMC38 trial). Cancer Chemother. Pharmacol. 81, 65. doi: 10.1007/s00280-017-3466-7
Organization W. H. (2018). Cancer [Online]. Available: http://www.who.int/news-room/fact-sheets/detail/cancer [Accessed].
Patel, S., Goyal, A. (2012). Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech 2, 1–15. doi: 10.1007/s13205-011-0036-2
Pharmacopoeia (2015). The Pharmacopoeia of the People’s Republic of China. Beijing: China Medicine Science and Technology Press.
Pilkington, K., Leach, J., Teng, L., Storey, D., Liu, J. P. (2016). Coriolus versicolor mushroom for colorectal cancer treatment. Cochrane Database Syst. Rev. CDO12053. doi: 10.1002/14651858.CD012053
Ren, L., Perera, C., Hemar, Y. (2012). Antitumor activity of mushroom polysaccharides: a review. Food Funct. 3, 1118–1130. doi: 10.1039/c2fo10279j
Sanodiya, B. S., Thakur, G. S., Baghel, R. K., Prasad, G. B., Bisen, P. S. (2009). Ganoderma lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 10, 717–742. doi: 10.2174/138920109789978757
Shi, J., Chen, T., Zhao, R., Sun, Y., Lian, Z. (1996). The effect of immunological function for the patients with gastric cancer after operation during chemotherapy using PSP. Chin. J. Clin. Oncol. Rehabil. 3, 3–4. doi: 10.13455/j.cnki.cjcor.1996.02.003
Toi, M., Hattori, T., Akagi, M., Inokuchi, K., Orita, K., Sugimachi, K., et al. (1992). Randomized adjuvant trial to evaluate the addition of tamoxifen and PSK to chemotherapy in patients with primary breast cancer: 5-Year results from the Nishi-Nippon Group of the Adjuvant Chemoendocrine Therapy for Breast Cancer Organization. Cancer 70, 2475–2483. doi: 10.1002/1097-0142(19921115)70:10<2475::AID-CNCR2820701014>3.0.CO;2-P
Torisu, M., Hayashi, Y., Ishimitsu, T., Fujimura, T., Iwasaki, K., Katano, M., et al. (1990). Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunol. Immunother. 31, 261–268. doi: 10.1007/BF01740932
Tsang, K. W., Lam, C. L., Yan, C., Mak, J. C., Ooi, G. C., Ho, J. C., et al. (2003). Coriolus versicolor polysaccharide peptide slows progression of advanced non-small cell lung cancer. Respir. Med. 97, 618–624. doi: 10.1053/rmed.2003.1490
Tsegaye, A., Messele, T., Tilahun, T., Hailu, E., Sahlu, T., Doorly, R., et al. (1999). Immunohematological reference ranges for adult Ethiopians. Clin. Diagn. Lab. Immunol. 6, 410–414.
Tsukagoshi, S., Hashimoto, Y., Fujii, G., Kobayashi, H., Nomoto, K., Orita, K. (1984). Krestin (PSK). Cancer Treat. Rev. 11, 131–155. doi: 10.1016/0305-7372(84)90005-7
Xu, F., Han, S., Xu, Y. (2003). Effect of postoperative adjuvant immunochtherapy for stage III gastric cancer. Chin. J. Clin. Oncol. Rehabil. 10, 53–56. doi: 10.1023/A:1026336321738
Xu, S., Song, Y., Li, H., Cai, H. (2008). The clinical observation of coriolus versicolor polysaccharide combined with XELOX treating advanced colorectal cancer. Acta Universitatis Medicinalis Nanjing (Natural Science) 28, 1616–1618.
Xu, Z., Chen, X., Zhong, Z., Chen, L., Wang, Y. (2011). Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 39, 15–27. doi: 10.1142/S0192415X11008610
Yang, M. M., Chen, Z., Kwok, J. S. (1992). The anti-tumor effect of a small polypeptide from Coriolus versicolor (SPCV). Am. J. Chin. Med. 20, 221–232. doi: 10.1142/S0192415X92000230
Yousefi, H., Yuan, J., Keshavarz-Fathi, M., Murphy, J. F., Rezaei, N. (2017). Immunotherapy of cancers comes of age. Expert. Rev. Clin. Immunol. 13, 1001–1015. doi: 10.1080/1744666X.2017.1366315
Zhang, X., Ye, L., Peng, H., Zhang, Y. (2010). Clinical investigation of treating advanced non small cell lung cancer (NSCLC) by tonifying deficiency and resolving toxin prescription combined with chemotherapy. J. Liaoning Univ. TCM 12, 23–24. doi: 10.13194/j.jlunivtcm.2010.09.25.zhangx.095
Zhao, F., Wu, Q., Li, Y., Wang, R. (2015). The efffect of immunological function and cinical observation of compound ganoderma lucidum spore capsule combined with chemotherapy treating non-small-cell lung cancer. Chin. J. Gerontol., 10, 2721–2722. doi: 10.3969/j.issn.1005-9202.2015.10.059
Zhao, H., Zhang, Q., Zhao, L., Huang, X., Wang, J., Kang, X. (2012). Spore powder of ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid. Based Complement. Alternat. Med. 2012, 1–8. doi: 10.1155/2012/809614
Zhou, D.-H., Lin, L.-Z. (2003). Clinical Chinese medical oncology. Beijing: People’s Medical Publishing House.
Zhou, J., Zhou, T., Jiang, M., Wang, X., Liu, Q., Zhan, Z., et al. (2014). Research progress on synergistic anti-tumor mechanisms of compounds in traditional Chinese medicine. J. Tradit. Chin. Med. 34, 100–105. doi: 10.1016/S0254-6272(14)60062-5
Keywords: Coriolus versicolor, Ganoderma lucidum, natural products, cancer therapies, systematic review, meta-analysis
Citation: Zhong L, Yan P, Lam WC, Yao L and Bian Z (2019) Coriolus Versicolor and Ganoderma Lucidum Related Natural Products as an Adjunct Therapy for Cancers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 10:703. doi: 10.3389/fphar.2019.00703
Received: 25 August 2018; Accepted: 31 May 2019;
Published: 03 July 2019.
Edited by:
Hongjie Zhang, Hong Kong Baptist University, Hong KongReviewed by:
Ulrike Lindequist, University of Greifswald, GermanyGuo-qing Zheng, The Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, China
Copyright © 2019 Zhong, Yan, Lam, Yao and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoxiang Bian, Ymlhbnp4aWFuZ0BnbWFpbC5jb20=
†These authors have contributed equally to this work.
 Linda Zhong
Linda Zhong Peijing Yan2†
Peijing Yan2† Wai Ching Lam
Wai Ching Lam Zhaoxiang Bian
Zhaoxiang Bian