- 1Research Division of Clinical Pharmacology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of GCP Office, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 3School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China
Type 2 diabetes mellitus (T2DM) is prevalent, with a dramatic increase in recent years. Moreover, its microvascular and macrovascular complications cause significant societal issues. The demand for new and effective antidiabetic therapies grows with each passing day and motivates organizations and individuals to pay more attention to such products. In this article, we focused on oral antihyperglycemic drugs patented in China and introduced them according to their antihyperglycemic mechanisms. By searching the website of State Intellectual Property Office of the People’s Republic of China (http://www.sipo.gov.cn), 2,500 antihyperglycemic patents for T2DM were identified and analyzed. These consisted of 4 patents for derivatives of herbal extracts (0.2%), 162 patents for herbal extracts (6.5%), 61 compositions for traditional Chinese medicine (TCM) (2.4%), 2,263 patents for synthetic compounds (90.5%), and 10 (0.4%) patents of the combination of synthetic compounds and TCM. As the most common drugs for diabetes mellitus, synthetic compounds can also be classified into several categories according to their working mechanisms, such as insulin secretion promotor agents, insulin sensitizer agents, α-glucosidase inhibitors, and so forth. This article discussed the chemical structure, potential antihyperglycemic mechanism of these antihyperglycemic drugs in patents in China.
Expert opinion: Insulin sensitivity and β-cell function could be improved by weight loss to prevent prediabetes into T2DM. However, 40–50% patients with impaired glucose tolerance (IGT) still progress to T2DM, even after successful long-term weight loss.
Antihyperglycemic remedies provide a treatment option to improve insulin sensitivity and maintain β-cell function. Combination therapy is the best treatment for diabetes. Combination therapy can reduce the dosage of each single drug option, and avoid the side effects. Drugs with different mechanisms are complementary, and are better adapted to patients with changing conditions. Classical combination therapies include combinations such as sulfonylureas plus biguanides or glucosidase inhibitors, biguanide plus glucosidase inhibitors or insulin sensitizers, insulin treatment plus biguanides or glucosidase inhibitors. The general principle of combination therapy is that two drugs with different mechanisms are selected jointly, and the combination of three types of hypoglycemic drugs is not recommended. After reading a large amount of literature, we have rarely found a case of three oral hypoglycemic agents, which may mean that the combination of three oral hypoglycemic agents is unnecessary and has unpredictable risks. There is no objection to the idea of multi-drug therapy. But multiple drugs can only be used when it shows a significant benefit to the patients. Combined use of multiple antidiabetic drugs poses a risk to patients due to drug interactions and overtreatment.
Introduction
Diabetes mellitus is classified as type 1 diabetes (T1DM), type 2 diabetes mellitus (T2DM), gestational diabetes, and other types of diabetes mellitus in terms of their different etiology, genetics, and clinical manifestation (American Diabetes Association, 2014). In 2000, there were 150 million patients with diabetes mellitus, and the number increased to 200 million in 2010 (Mulnier et al., 2006). Among these, T2DM attracts more attention due to its high morbidity and mortality. It accounts for 90% of diabetes mellitus patients (Rao Kondapally Seshasai et al., 2011; Rahelić, 2016; Zheng et al., 2018; Deng et al., 2016). In China, the overall prevalence of T2DM was 7.9% (Han et al., 2017). T2DM is diagnosed by clinical manifestations of diabetes mellitus and elevated plasma glucose in laboratory tests.
T2DM is also called noninsulin-dependent diabetes mellitus, which is induced by pancreatic β-cell dysfunction and insulin resistance. Initially, there is a compensatory increase in insulin secretion, followed by a decrease in insulin secretion because of the damage of β cells. The initial insulin response to secretagogue is also reduced, which is the first phase of the reaction of chronic hyperglycemia state (Dinneen et al., 1992). For the pathogenesis of T2DM, insulin resistance is the original factor and the damage of pancreatic β cells is the key factor (Gulli et al., 1992; Martin et al., 1992; Defronzo, 2009). Pancreatic β cells belong to islet cells, accounting for 70% of the total number of islet cells, and are mainly located in the central part of the pancreas. They can secrete insulin to regulate blood glucose. A prospective study found that islet function was about 50% of normal at the time of diagnosis, and a reduction in β-cell mass of about 60% was shown at necropsy (Neutzsky-Wulff et al., 2012). Pancreatic β cells play their role by activation of glucagon like peptide-1 receptor (GLP-1R) and glucose-dependent insulinotropic polypeptide receptor (GIPR) on their surface. In T2DM patients, after a meal, food reaches the gastrointestinal tract, stimulates the pancreas and promotes glucagon-like peptide-1 (GLP-1) inactivation under the catalysis action of dipeptide peptidase-IV (DPP-IV). Glucose-dependent insulinotropic polypeptide (GIP) is an incretin synthesized and secreted by the K cells in the duodenum and jejunum. It is mainly stimulated by diet, especially fat. Excessive secretion of GIP leads to the deposition of lipids in peripheral tissues (liver, muscle, etc.) and islet β cells, which causes impaired insulin resistance and secretory function, while inhibition of GIP secretion can significantly improve T2DM associated with obesity. GLP-1 is an incretin synthesized and secreted by distal ileal L cells. Glucose and fat in food are the most important nutrients to stimulate GLP-1 release. GLP-1 can promote glycogen synthesis and insulin expression, delay gastric empty and glucagon secretion, while also promote proliferation of pancreatic β cells (Urbano et al., 2016). Pancreatic β cells also express G protein-coupling receptor 119 (GPR119), which can protect and promote pancreatic β cells to stimulat the secretion of insulin, together with GIPR and GLP-1R. GLP-1R binds to stimulatory G protein (Gs), activates adenylate cyclase (AC), and further catalyzes the production of cyclic adenosine monophosphate (cAMP) by adenosine triphosphate (ATP). cAMP is the first signal transduction molecule that GLP-1 stimulates β cells to produce insulin. Increasing cAMP can increase the insulin stimulation of β cells (Dov et al., 2008). GLP-1 also reduces brain satiety and inhibits gastric empty. They play a common role in regulating blood glucose, as shown in Figure 1.
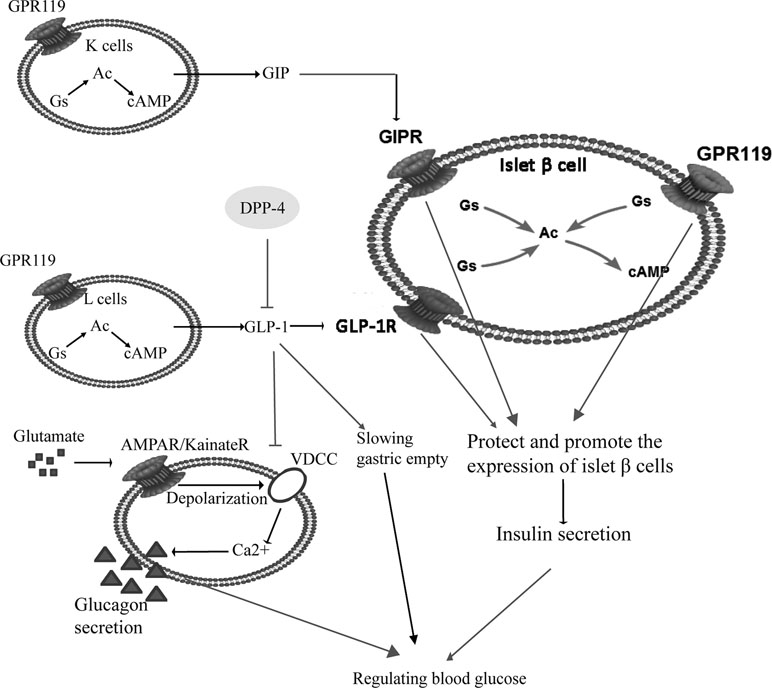
Figure 1 The pathogenesis of type 2 diabetes mellitus (T2DM) and related anti-hyperglycemia targets. Pancreatic β cells secrete insulin, and regulate blood sugar by activation of GLP-1R and GIPR on their surface. In patients with T2DM, after a meal food reaches the gastrointestinal tract and promotes GLP-1 inactivation under the catalysis of DPP-IV. K cells and L cells work together to regulate insulin secretion. GIP and GLP-1 bind with GIPR and GLP-1R respectively, together with GPR119 to protect pancreatic β cells and stimulate the secretion of insulin. Increased GLP-1 inhibit αcells secreting glucagon to lower blood glucose. Meanwhile, GLP-1 reduces brain satiety and slows gastric emptying. Abbreviations: GPR119, G protein-coupling receptor 119; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon like peptide-1; GIPR, glucose-dependent insulinotropic polypeptide receptor; GLP-1R, glucagon like peptide-1 receptor; Ac, adenylate cyclase; Gs, stimulatory G protein; cAMP, cyclic adenosine monophosphate.
In some patients with mild diabetes, blood glucose can be successfully controlled by exercise and diet without drugs (American Diabetes Association, 1998; Balducci et al., 2014). However, many patients require additional insulin or oral hypoglycemic agents to achieve euglycemia. The nodes of pathogenesis of T2DM become targets of the invention of antihyperglycemic drugs, such as insulin secretagogues, insulin sensitizer agents, α-glucosidase inhibitors, GLP-1 analogs, and DPP-4 inhibitors. However, many side effects have been reported, such as hypoglycemia and gastrointestinal reactions. Therefore, it is necessary to summarize the categories of existing patents and find new drugs to better guide the clinical treatment of T2DM.
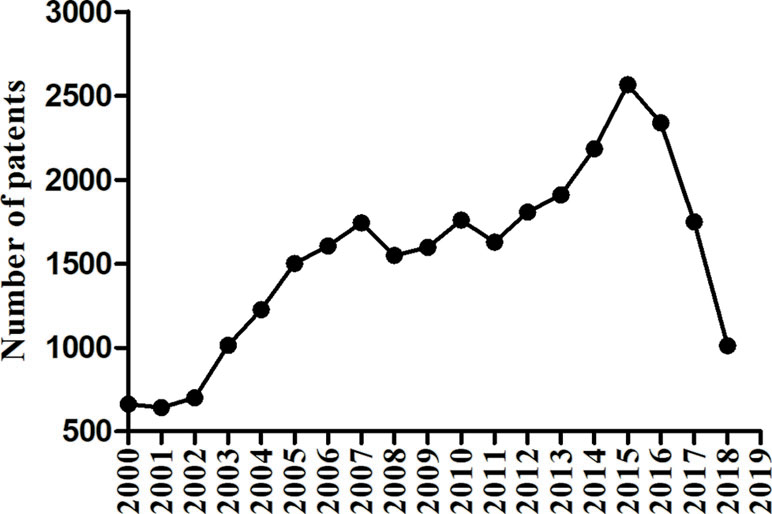
The number of patents on drugs for diabetes mellitus has been increasing in recent years, even with some decline in the latter years, as shown in Figure 2. And many pharmaceutical companies and organizations are devoting themselves to finding or inventing new products. The aim at this review is to document the current drug patents in China of antihyperglycemic, and guide the invention of new drugs and their clinical use.
Materials and Methods
Data Retrieval Method
All data were obtained from the website of State Intellectual Property Office of the People’s Republic of China: http://www.sipo.gov.cn. In our searches, we used the keywords “diabetes mellitus,” “diabetes,” “hyperglycemia” “type 2 diabetes mellitus,” and “T2DM.” These keywords can be used either independently or as a phrase by adding “or.” After careful reading, screening, and deleting invalid patents, we have obtained 2,500 patents on antihyperglycemic therapies for T2DM.
Patent Review
A total of 2,500 patents for anti-T2DM remedies were found. The 2,500 patents consisted of 4 patents for derivatives of herbal extracts (0.2%), 162 patents for herbal extracts (6.5%), 61 compositions for traditional Chinese medicine (TCM) (2.4%), 2,263 patents for synthetic compounds (90.5%), and 10 (0.4%) patents of a combination of synthetic compounds and TCM. The distribution of these patents is shown in Figure 3. Compared to TCM, antidiabetic synthetic compounds appeared more often in prescriptions because of their clear pharmacological action and adverse reactions. Currently, patents on antihyperglycemic drugs for T2DM include insulin secretagogues, insulin sensitizer agents, α-glucosidase inhibitors, GLP-1 analogs, DPP-4 inhibitors, cannabinoid receptor type 1 antagonists, and endothelin receptor antagonists (Srinivasan et al., 2008). There are also some new drug targets, such as GPR119 agonists, and G protein-coupled receptor 40 (GPR40) agonists. In the following part of this article, we will describe these categories in details.
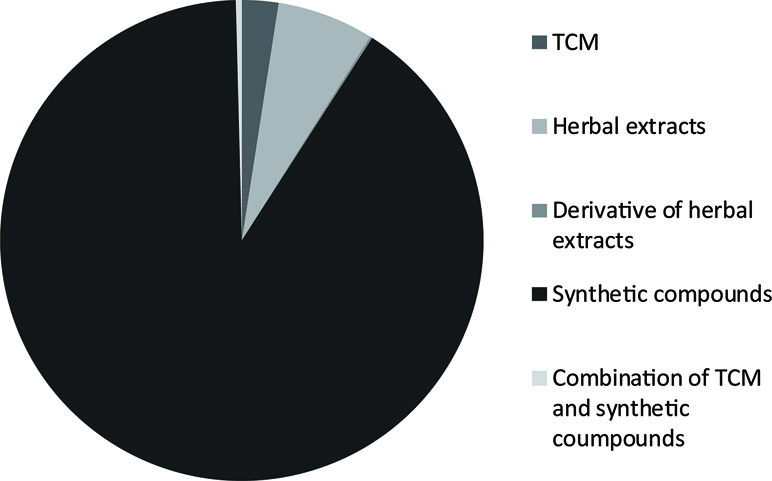
Figure 3 Distribution and composition of traditional Chinese medicine (TCM), herbal extracts, derivatives of herbal extracts, synthetic compounds and combinations of synthetic compounds and TCM patents for anti-T2DM therapies issued in China.
Insulin Secretagogues
Thiazolidinediones
Thiazolidinediones (TZDs) are identified as insulin sensitizers that directly protect islet β cells and promote insulin secretion (Lebovitz and Banerji, 2001; Leiter, 2005; Campbell and Mariz, 2007). Furthermore, TZDs increase the sensitivity of liver, adipose, and muscle tissue to insulin. They also promote hepatic glucose production and inhibit peripheral glucose utilization (Hara et al., 2006; Riera-Guardia and Rothenbacher, 2008).
As a representative TZD, pioglitazone is a “wonder drug” with several functions. In the Actos Now for prevention of diabetes (ACT NOW) study subjects, the results in the pioglitazone group showed that pioglitazone inhibited the progression from IGT to T2DM by 72% (p < 0.001) (Podar et al., 2001; Wentworth et al., 2014). A recent study suggested the combination therapy of pioglitazone and DPP-4 inhibitor provided better glycemic control than pioglitazone monotherapy, and reflected on HbA1c and FPG levels (Wang et al., 2018). As another classical TZD, rosiglitazone has shown its pharmacological effects in preclinical and clinical experiments. Rosiglitazone can improve the insulin resistance, control blood glucose and prevent rebound (Finegood et al., 1999). However, rosiglitazone also causes some adverse effects, such as edema, weight increase and serious osteoporosis (Malinowski and Bolesta, 2000). Therefore, it is necessary to design safe and effective TZDs. A protected patent describes a series of TZD N-substituted derivatives that were synthesized to improve the activity of the TZD ring, reduce side effects and extend its applications (Hu Xiangnan et al., 2012). The invention utilizes the active hydrogen of the nitrogen atom on the rosiglitazone TZD ring without affecting the pharmacological TZD ring, and is chemically modified to link a series of small molecule substituents. The N-substituting derivatives were designed to improve their properties, reduce toxic side effects, and increase their range of application (Hu Xiangnan et al., 2012).
Sulfonylureas
Sulfonylureas (SUs) are the earliest and the most frequently used hypoglycemic agents in clinic. They are especially suited for patients who cannot achieve ideal blood glucose even with exercise and diet. SUs act on the SU receptor 1 (Sur1), which is expressed on the surface of pancreatic β cells. When Sur1 is combined with a SU, Ca2+ channels are opened and Ca2+ flows into intracellular regions to stimulate insulin secretion. This category has the same structure (R1-SO4NHCONH-R2). Because of its serious side effects, drugs of the first generation like toluene SU were withdrawn (Harrower, 2000). The latter glibenclamide and glimepiride are still on the markets. Glimepiride is a long-acting hypoglycemic agent to stimulate insulin secretion and improve the sensitivity of insulin in the peripheral tissues, with a higher hypoglycemic effect than that of glipizide. Glimepiride can be used alone to control the blood glucoses, especially in patients who cannot control the blood glucose only with diet and exercise. It can also cooperate with other hyperglycemic agents or insulin, with the advantages of high efficiency, long-acting safety, low dose, low toxicity, and fewer adverse effects. However, it is limited by the poor stability low dissolution and bioavailability when glimepiride acts as oral and solid preparation. Consequently, it is necessary to provide a glimepiride solution delivery system with high bioavailability, fast onset, and better stability to obtain better hypoglycemic effect. A protected patent for sublingual administration may solve this problem. Compare with the solution administration system, the patented formulation is administered by spraying to achieve quick effect, high bioavailability and avoid adverse effects on gastrointestinal and liver. It works for postprandial blood glucose control, especially in T2DM patients whose blood glucose cannot be controlled by diet and exercise alone. The manufacturing process is simple, feasible, and suitable for industrial production (Gao Yongliang et al., 2008).
Biguanides
Biguanides mainly act on islet tissue to inhibit intestinal epithelial cells from absorbing glucose. It increases the sensitivity of peripheral tissues to insulin, and increase noninsulin-dependent liver glycogen regeneration. Biguanides stimulate the anaerobic glycolysis of glucose, inhibit hepatic gluconeogenesis, and decrease plasma glucagon levels (Miller et al., 2013).
In Chinese, Li et al. (2002) found that metformin can reduce fasting blood glucose and blood glucose 1h after an oral glucose tolerance test (OGTT), and delay the progression to T2DM. Metformin hydrochloride plays a significant role among the guanidine oral hypoglycemic agents in the drug treatment of T2DM. The hypoglycemic effect of metformin is obvious, probably because that metformin hydrochloride increases the uptake and utilization of glucose in peripheral tissue, enhances tissue insulin sensitivity, inhibits gluconeogenesis and decomposition, reduces the high glycogen output, and delays the absorption of glucose in the intestine. It can reduce glycosylated hemoglobin and the free fatty acid concentration without stimulating insulin secretion from pancreatic β cells and causing premature failure of pancreatic β cells (Yang et al., 2017). When the blood glucose level is regular, there will be no hypoglycemic effect and the weight gain. However, there are also other hypotheses suggesting that metformin hydrochloride may improve the sensitivity of insulin (Giannarelli et al., 2003).
By a pathway depend on peroxisome proliferator-activated receptor-α (Maida et al., 2011), biguanides regulate the transcription of insulin-responsive genes to improve the insulin tolerance and have a hypoglycemic effect. There is one patent combines metformin hydrochloride and pioglitazone hydrochloride in a sustained-release pellets preparation. The pellet capsule formulation is a polydispersion system, with the advantages of simple production processes, large drug loading, great liquidity (Ali et al., 2009). When pioglitazone hydrochloride is combined with metformin hydrochloride, it enhances the sensitivity of tissue to insulin, and improves anaerobic glycolysis in peripheral tissues. The two hypoglycemic agents are complementary regarding onset and duration of action, mutual overlap effects, offsetting side effects. The combination of metformin and pioglitazone is an ideal treatment for early phase of T2DM to prevent insulin resistance (Perez et al., 2009).
α-Glucosidase Inhibitors
α-Glucosidases are catalyticase to catalyze the hydrolysis of α-glucosidase groups from the non-reducing ends of α-glucoside substrate. They are widely distributed among organisms and participate in food digestion, glycoprotein biosynthesis. α-Glucosidase inhibitors are oral hypoglycemic agents that delay the absorption of intestinal carbohydrates and achieve effective treatment of T2DM. α-glucosidase inhibitors can competitively inhibit the various α-glucosidases to inhibit glucose absorption in the small intestine. Studies have shown that α-glucosidase inhibitors can improve postprandial hyperglycemia and alleviate hyperinsulinemia while increasing glucose tolerance (van de Laar, 2008).
Tan et al. extracted one kind of α-glucosidase inhibitor from akebia plants, the new 23, 29-drop oleanolic acid compounds. Akebia plants are a rich source of these compounds, and can be used to extract the medicine when the fruit is harvested. Its activity in inhibiting α-glucosidase is five times greater than that of acarbose. Acarbose is currently a first-line drug for T2DM. Therefore, the new 23, 29-drop oleanolic acid compounds are highly likely to become marketed drugs (Tan Jianwen et al., 2013).
GLP-1 Agonist
GLP-1 is secreted from gastrointestinal L cells (Theodorakis et al., 2006) and appears in the plasma within several minutes after ingestion of food (Herrmann et al., 1995; Orskov et al., 1996). GLP-1 regulates plasma glucose in the postprandial period by stimulating insulin secretion, inhibiting glucagon secretion and slowing gastric emptying (Komatsu et al., 1989; Flint et al., 2001; Holst and Gromada, 2004; Drucker, 2006; Hare et al., 2010). The effect of GLP-1 is initially mediated by its effect delaying gastric emptying, which leads to delayed entry of nutrients into the circulation (Chen and Yang, 2014). The effect of incretin drops off in T2DM patients, which contributes to postprandial hyperglycemia (Holst et al., 2011). GLP-1 agonists help stabilize glycemic control.
Exenatide is a member of the GLP-1 family. It can bind to and activate GLP-1R. GLP-1R is involved in the synthesis of glucose-dependent insulin by intracellular signaling mechanisms and stimulates β cells secreting insulin. Exenatide can promote insulin release from β cells and improve blood glucose control in patients with T2DM by controlling fasting and postprandial blood glucose levels (Grossman, 2014). However, the half-life of GLP-1 in the serum is only 3 to 5 min, that limits clinical applications. A patent invented by Li et al. in Tianjin Sanli Patent Co., Ltd showed a GLP-1 analogue, of which some amino acids were replaced by cysteine. It has a half-life of 72 to 96 h, much longer than that of GLP-1 itself. This finding created the foundation for the widespread clinical use of GLP-1 analogues (Li Ying and Yuan, 2014).
DPP-4 Inhibitors
GIP and GLP-1 both stimulate insulin secretion, and can be hydrolyzed by DPP-4. To date, seven DPP-4 inhibitors have been sequentially listed worldwide: sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin, gemigliptin and teneligliptin. In 2009, the European Medicines Evaluation Administration (EMEA) approved the use of saxagliptin in combination with metformin, TZDs, or SUs to treat T2DM (Dhillon, 2015).
Saxagliptin is a powerful and selective DPP-4 inhibitor which can specifically prolongs the inhibitory effect of DPP-4, prolongs endogenous GLP-1 and GIP duration, and reduces blood glucose (Augeri et al., 2005; Zhao et al., 2005). The most frequent adverse reactions reported during the phase III trial of pravastatin were headache, upper respiratory tract infection, and urinary tract infection. In combination therapies, the incidence of adverse reactions to saxagliptin and placebo was similar (Defronzo et al., 2009; Hollander et al., 2009). The National Institute for Health and Clinical Excellence (NICE) recommended that DPP-4 inhibitors be considered the first-line treatment for patients with T2DM who are unable to tolerate metformin or SUs when the patient has significant risk of hypoglycemia (Adler et al., 2009).
A new compound invented by Zhang Xiaoqing et al. in 2012 (Zhang Xiaoqing et al., 2012) inhibits the activity of DPP-4. It is an amidation reaction of existing DPP-4 inhibitors and their derivatives to prepare novel polymer DPP-4 inhibitors. Compared with other DPP-4 inhibitors, it has higher inhibitory activity and provide a new choice for the treatment of T2DM.
Cannabinoid-1 Receptor Inhibitors
The cannabinoid-1 (CB1) receptors distribute among the brain and other tissues. A study showed that activation of the CB1 receptor increased feeding behavior. Therefore, CB1 receptor antagonists have been developed as potential agents to treat obesity and other metabolic diseases, such as T2DM. Rimonabant, a CB1 receptor antagonist, has effects on controlling obesity and the metabolic syndrome (Carai et al., 2005; Van Gaal et al., 2005). A study in North America showed that 20 mg rimonabant everyday could lose weight, decrease waist circumference and triglycerides, increased high-density lipoprotein (HDL) cholesterol, and hindered the progress of metabolic syndrome (Pi-Sunyer, 2006). Currently, there is no patent for a CB1 receptor inhibitor in the protected period, which suggests its limited clinical value and uncertain future.
G Protein-Coupled Receptor 40 Agonists
GPR40 is reported to be expressed in the brain, with unclear functions and mechanisms at present. GPR40 is activated by endogenous ligands such as linoleic acid, decanoic acid, and docosahexaenoic acid (DHA) (Katayama et al., 1994; Nakamoto et al., 2011). Until now, many synthetic patents of GPR40 agonists have been reported and the optimal compound was TAK-875 from Takeda Pharmaceutical Company (Negoro et al., 2010). However, due to its signs of hepatotoxicity in patients, phase III clinical trials involving TAK-875 was finally discontinued (Mohammad, 2016). In the Langerhans islets isolated from GPR40 knockout mice, the glucose-responsive insulin secretion-promoting effect of fatty acids was reduced compared to normal mice (Zhang Yingjun et al., 2014). A new GPR40 agonist was developed by Zhang et al. The invention provides a composition which has good stability and rapid dissolution. It contains the active ingredient 2-((S)-6-((2’,6’-dimethyl-4’-((2’,6’-dimethyl-4’-(((R)-tetrahydrofuran-3-yl)oxy)-[1,1’-biphenyl]-3-yl)methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid or medicinal salts, and polyvinylpyrrolidone. When the amount of polyvinylpyrrolidone in the composition is from 0.5 to 20% by weight, it has good compressibility and fluidity. It has the advantages of good solubility and stability (Zhang Daimei and Tingting, 2017).
GPR119 Agonists
GPR119 is a G protein-coupled receptor expressed on β cells, L cells and K cells predominantly in the pancreas and gastrointestinal tract. GPR119 has revealed two classes of possible endogenous ligands, phospholipids and fatty acid amides, of which, fatty acid amides have attracted attention due to its known effects of reducing food intake and losing weight. GPR119 agonists can increase intracellular cAMP levels. GPR119 can also stimulate insulin-releasing by enteroendocrine cells. The effects of GPR119 agonists in animal models of diabetes and obesity were reviewed by Overton et al. (2008), and the potential value of these compounds in T2DM therapies are discussed (Overton et al., 2008).
Other Chinese Medicines
Treatment of diabetes mellitus has a long history by TCM. The first patent for a TCM to treat diabetes mellitus was issued in 1999. The preparation consisted radix bupleuri, mulberry leaf, mulberry, radix astragali, radix puerariae, fructus lycii, and other ingredients (Gang, 1998). Subsequently, Liu Xin and Kun (2009) produced a powder containing ginseng, rhizoma anemarrhenae, gypsum, bitter gourd, cocoon, astragalus, etc. Tan Xiaozhong et al. (2015) prepared a powder containing ginseng, poria, atractylodes, radix rehmanniae, radix astragali, yam, etc. These herbs are in accordance with the TCM principle of gout “nourishing yin, replenishing qi, tonifying kidney, promoting blood circulation and removing blood stasis.”
Among 61 TCM patents, 20 active ingredients are the most frequently mentioned (Table 1, Figure 4). The unclear mechanisms limit their widespread application, and a discussion of those issues is beyond the scope of this review.
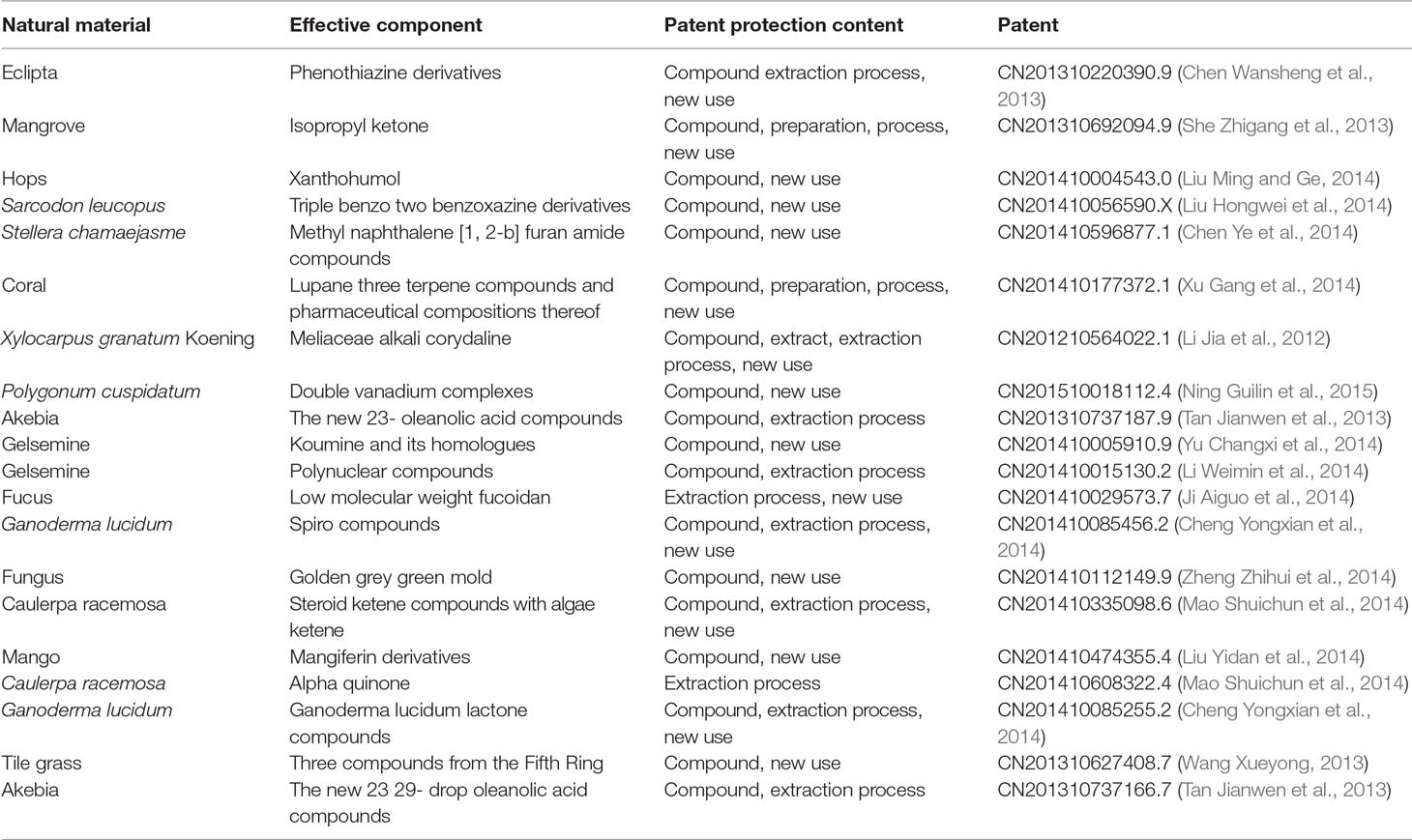
Table 1 Twenty patents on phytochemical ingredients of herbal extracts in anti-diabetic properties, and their natural sources, and their protected content.

Figure 4 Structure of active phytochemical ingredients of herbal extracts in anti-diabetic properties in Chinese patents.
Conclusions
Our search found 2,500 published patents, including 4 patents of derivatives of herbal extracts (0.2%), 162 patents for herbal extracts (6.5%), 61 compositions for traditional Chinese medicine (TCM) (2.4%), 2,263 patents of synthetic compounds (90.5%), and 10 (0.4%) patents of a combination of synthetic compounds and TCM. There are fewer patents for derivatives of herbal extracts and herbal extracts themselves than the others, especially synthetic compounds which target islet β cells, α-glucosidase, and GLP-1. We expect more compounds and more effective preparations could be developed to improve therapeutic benefits for T2DM patients.
Expert Opinion
In view of the global spread of T2DM and the actual low rate of blood glucose compliance, clinicians should take more actions to address this problem. These include lower hypoglycemic targets [glycosylated hemoglobin (HbA1c) <6.5%], earlier drug therapy, more frequent detection, and more drug options such as GLP-1 analogs and DPP-4 inhibitors.
Clinicians should determine the utility of different drugs by analyzing drug safety, hypoglycemic strength, hypoglycemic risk, expected patient compliance, and total treatment costs (not just drug costs). The treatment choices depend primarily on the level of HbA1c at the patient’s baseline and previous treatments. For the treatment of patients with HbA1c ≤7.5%, monotherapy can reduce HbA1c to ≤6.5%. The antihyperglycemic drugs include metformin, TZDs, DPP-4 inhibitors, and α-glucosidase inhibitors.
Insulin sensitivity and β-cell function can be improved by weight loss to prevent prediabetes into T2DM. However, 40–50% patients with IGT still progress to T2DM, even after successful long-term weight loss. Chemotherapy provides an alternative strategy to improve insulin sensitivity and preserve β-cell function. Combination therapy is the best treatment for T2DM. Combination therapy are complementary to reduce the dosage of each drug and avoid side effects, which can be to better adapt to patients with changing conditions. Classical combination therapies include SUs plus biguanides or glucosidase inhibitors, biguanides plus glucosidase inhibitors or insulin sensitizers, insulin treatment plus biguanides or glucosidase inhibitors and others. The general principle of combination therapy is that two kinds of drugs with different mechanism of hypoglycemic action are selected jointly, and the combination of three types of hypoglycemic drugs is not recommended. The final aim of clinical pharmacy is to achieve an individual medication regimen for each patient. We expect the increasing number of patent submissions for antihyperglycemic therapies soon. Our study could help better catch the opportunities in research on antihyperglycemic drugs in China and promote the development of appropriate antihyperglycemic therapies.
Author Contributions
JFW and HY designed the article. JFW revised the article. WZ collected the data, analyzed the data, and wrote this review. The other authors collected data and revised the article.
Funding
This project was sponsored by the grants from the National Natural Science Foundation of China (81571568 and 81871265); CAMS Innovation Fund for Medical Sciences (CIFMS:2016-I2M-1003); and Innovation wTeam of Jiangsu Provincial Commission of Health and Family Planning (CXTDA2017049).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adler, A. I., Shaw, E. J., Stokes, T., Ruiz, F. (2009). Newer agents for blood glucose control in type 2 diabetes: summary of NICE guidance. BMJ (Clin. Res. Ed.) 338, b1668. doi: 10.1136/bmj.b1668
Ali, A. M., De, M. M., York, P., Rowe, R. C. (2009). Influence of pellet aggregate populations on the variability of pellet filling into hard shell capsules: a comparison of experiment and computer simulation. Eur. J. Pharm. Sci. 38, 197–205. doi: 10.1016/j.ejps.2009.07.001
American Diabetes Association. (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care 37 (Suppl 1), S81–90. doi: 10.2337/dc14-S081
American Diabetes Association. (1998). Nutrition recommendations and principles for people with diabetes mellitus. J. Florida Med. Assoc. 85, 25–29. doi: 10.2337/diacare.21.1.S32
Augeri, D. J., Robl, J. A., Betebenner, D. A., Magnin, D. R., Khanna, A., Robertson, J. G., et al. (2005). Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 48, 5025–5037. doi: 10.1021/jm050261p
Balducci, S., Sacchetti, M., Haxhi, J., Orlando, G., D’Errico, V., Fallucca, S., et al. (2014). Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 30 (Suppl 1), 13–23. doi: 10.1002/dmrr.2514
Campbell, I.W., Mariz, S. (2007). Beta-cell preservation with thiazolidinediones. Diabetes Res. Clin. Prac. 76, 163–176. doi: 10.1016/j.diabres.2006.08.015
Carai, M. A., Colombo, G., Gessa, G. L. (2005). Rimonabant: the first therapeutically relevant cannabinoid antagonist. Life Sciences 77, 2339–2350. doi: 10.1016/j.lfs.2005.04.017
Chen Wansheng, X. F., Wu, Z., Li, C., Han, J., Liang, F. (2013). Preparation and application of thiophene derivatives CN201310220390.9.
Chen Ye, W. Y., Li, W., Chen, Y., Zhang, L., Ren, C., Yin, L. (2014). Preparation and application of methylnaphtho[1,2-b]furanamides and their salts CN201410596877.1.
Chen, X., Yang, W. (2014). Epidemic trend of diabetes in China: for the Xiaoren Pan Distinguished Research Award in AASD. J. Diabetes Investig. 5, 478–481. doi: 10.1111/jdi.12254
Cheng Yongxian, W. X., Ping, F., Qing, Lv. (2014). Preparation and application of spiro-ganoderma lucidum and its pharmaceutical composition CN201410085456.2.
Cheng Yongxian, Y. Y., Xinlong, W., Linhong, Z., Jinhua, M., Ping, F. (2014). Preparation and application of erythritol lactones and their pharmaceutical compositions CN201410085255.2.
Defronzo, R. A., Hissa, M. N., Garber, A. J., Luiz, G. J., Yuyan, D. R., Ravichandran S., et al. (2009). The Efficacy and Safety of Saxagliptin When Added to Metformin Therapy in Patients With Inadequately Controlled Type 2 Diabetes With Metformin Alone. Diabetes Care 32, 1649–1655. doi: 10.2337/dc08-1984
Deng, C., Wang, X., Liao, Y. (2016). Current recommendations on managing tuberculosis patients with diabetes & its epidemiology. Microbial Pathogenesis 92, 43–45. doi: 10.1016/j.micpath.2015.12.005
Dinneen, S., Gerich, J., Rizza, R. (1992). Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 327, 707–713. doi: 10.1056/NEJM199209033271007
Dov, A., Abramovitch, E., Warwar, N., Nesher, R. (2008). Diminished phosphodiesterase-8B potentiates biphasic insulin response to glucose. Endocrinology 149, 741–748. doi: 10.1210/en.2007-0968
Drucker, D. J. (2006). The biology of incretin hormones. Cell. Metab. 3, 153–165. doi: 10.1016/j.cmet.2006.01.004
Finegood, D. T., Mcarthur, M. D., Kojwang, D., Thomas, M. J., Buckingham, R. E., Leonard, T. B. (1999). The PPAR[Gamma] Agonist Rosiglitazone (RSG) Decreases Net Loss of Pancreatic [Beta]-Cell Mass in Zucker Diabetic Fatty Rats. Diabetes, SA237.
Flint, A., Raben, A., Ersbøll, A. K., Holst, J. J., Astrup, A. (2001). The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int. J. Obesity 25, 781–792. doi: 10.1038/sj.ijo.0801627
Gang, W. (1998). Preparation and method of traditional Chinese medicine for treating diabetes CN98100516.0.
Gao Yongliang, T. J., Liu, J., Wang, J., Li, J. (2008). Preparation of glimepiride aqueous solution delivery system CN200810119045.5.
Giannarelli, R., Aragona, M., Coppelli, A., Del Prato, S. (2003). Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 29, 6s28–35. doi: 10.1016/S1262-3636(03)72785-2
Grossman, S. S. (2014). Pathophysiological and pharmacological rationale for the use of exenatide once weekly in patients with type 2 diabetes. Adv. therapy 31, 247–263. doi: 10.1007/s12325-014-0101-4
Gulli, G., Ferrannini, E., Stern, M., Haffner, S., DeFronzo, R. A. (1992). The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 41, 1575–1586. doi: 10.2337/diab.41.12.1575
Han, C., Zhang, M., Luo, X., Wang, C., Yin, L., Pang, C., et al. (2017). Secular trends in the prevalence of type 2 diabetes in adults in China from 1995 to 2014: a meta-analysis. J. Diabetes 9, 450–461. doi: 10.1111/1753-0407.12440
Hara, K., Horikoshi, M., Yamauchi, T., Yago, H., Miyazaki, O., Ebinuma, H., et al. (2006). Measurement of the High–Molecular Weight Form of Adiponectin in Plasma Is Useful for the Prediction of Insulin Resistance and Metabolic Syndrome. Diabetes Care 29, 1357. doi: 10.2337/dc05-1801
Hare, K. J., Vilsbøll, T., Asmar, M., Deacon, C. F., Knop, F. K., Holst, J. J. (2010). The Glucagonostatic and Insulinotropic Effects of Glucagon-Like Peptide 1 Contribute Equally to Its Glucose-Lowering Action. Diabetes 59, 1765. doi: 10.2337/db09-1414
Harrower, D. A. D. B. (2000). Comparative Tolerability of Sulphonylureas in Diabetes Mellitus. Drug Safety 22, 313–320. doi: 10.2165/00002018-200022040-00004
Herrmann, C., Göke, R., Richter, G., Fehmann, H. C., Arnold, R., Göke, B. (1995). Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56, 117–126. doi: 10.1159/000201231
Hollander, P., Li, J., Allen, E., Chen, R. (2009). Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J. Clin. Endocrinol. Metab. 94, 4810. doi: 10.1210/jc.2009-0550
Holst, J. J., Gromada, J. (2004). Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 287, E199–206. doi: 10.1152/ajpendo.00545.2003
Holst, J. J., Knop, F. K., Vilsbøll, T., Krarup, T., Madsbad, S. (2011). Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 34, S251–S257. doi: 10.2337/dc11-s227
Hu Xiangnan, X. X., Liu, Z., Feng, Y., Chen, Y., Ming, Y., Tao, Z., et al. (2012). Preparation and application of thiazolidinedione derivative CN201210202455.2.
Ji Aiguo, S. S., Liang, H., Wang, X. (2014). Preparation of low molecular fucoidan sulfate and its application to diabetic nephropathy CN201410029573.7.
Katayama, Y., Kawamata, T., Maeda, T., Tsubokawa, T. (1994). Free fatty acid liberation and cellular swelling during cerebral ischemia: the role of excitatory amino acids. Acta. Neurochir. Suppl. (Wien) 60, 242–245. doi: 10.1007/978-3-7091-9334-1_64
Komatsu, R., Matsuyama, T., Namba, M., Watanabe, N., Itoh, H., Kono, N., et al. (1989). Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes 38, 902–905. doi: 10.2337/diab.38.7.902
Lebovitz, H. E., Banerji, M. A. (2001). Insulin resistance and its treatment by thiazolidinediones. Recent Prog. Horm. Res. 56, 265–294. doi: 10.1210/rp.56.1.265
Leiter, L. A. (2005). β-cell preservation: a potential role for thiazolidinediones to improve clinical care in Type 2 diabetes. Diabetic Med. J. Br. Diabetic Assoc. 22, 963. doi: 10.1111/j.1464-5491.2005.01605.x
Van Gaal, L. F., Rissanen, A. M., Scheen, A. J., Ziegler, O., Rössner, S., RIO-Europe Study Group. (2005). Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. ACC Curr. J. Rev. 14, 1389–1397.
Li Jia, G. Y., Zhou, Z., Liu, H., Gao, L. (2012). Preparation and application of saponin A CN201210564022.1.
Li Weimin, Y. X., Rong, X., Zhou, D. (2014). Preparation and application of polynuclear compounds synthesized from berberine and magnolol CN201410015130.2.
Li, Y., Lu, J., Pan, C., Tian, H., Li, C., Yang, G., et al. (2002). Prevention of type 2 diabetes in the population with impaired glucose tolerance by metformin and diet fibre. Chin. J. Diabetes 10, 340–343.
Li Ying, G. J., Yuan, Y. (2014). Preparation and application of long-acting enterosteroid polypeptide analogues for the treatment of type 2 diabetes CN201410243272.4.
Liu Hongwei, M. K., Han, J., Bao, L. (2014). Preparation and application of two terphenyldioxazine derivatives CN201410056590.X.
Liu Ming, M. J., Ge, L. (2014). Preparation and application of a xanthohumol for preparing medicine or health care product for inhibiting α-glucosidase activity CN201410004543.0.
Liu Xin, Z. Z., Zhao, K. (2009). Traditional Chinese medicine for treating diabetes CN200910117610.9.
Liu Yidan, S. Z., Xujuan, Y., Liming, S., Ping, W., Xiaoying, L., Zhang, J., et al. (2014). Application of mangiferin derivatives in the preparation of drugs for diabetes and its complications CN201410474355.4.
Maida, A., Lamont, B. J., Cao, X., Drucker, D. J. (2011). Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia 54, 339–349. doi: 10.1007/s00125-010-1937-z
Malinowski, J. M., Bolesta, S. (2000). Rosiglitazone in the treatment of type 2 diabetes mellitus: a critical review. Clin. Ther. 22, 1151–1168. doi: 10.1016/S0149-2918(00)83060-X
Mao Shuichun, L. J., Guo, Y., Liu, D. (2014). Method for extracting α-tocopherol in the genus Pteridium CN201410608322.4.
Mao Shuichun, L. J., Guo, Y., Liu, D., Yang, P. (2014). Preparation and application of steroidal ketone ketone CN201410335098.6.
Martin, B. C., Warram, J. H., Krolewski, A. S., Bergman, R. N., Soeldner, J. S., Kahn, C. R. (1992). Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet (London, England) 340, 925–929. doi: 10.1016/0140-6736(92)92814-V
Miller, R. A., Chu, Q., Xie, J., Foretz, M., Viollet, B., Birnbaum, M. J. (2013). Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260. doi: 10.1038/nature11808
Mohammad, S. (2016). GPR40 Agonists for the Treatment of Type 2 Diabetes Mellitus: Benefits and Challenges. Curr. Drug Targets 17, 1292–1300. doi: 10.2174/1389450117666151209122702
Mulnier, H. E., Seaman, H. E., Raleigh, V. S., Soedamah-Muthu, S. S., Colhoun, H. M., Lawrenson, R. A. (2006). Mortality in people with type 2 diabetes in the UK. Diabetic Med. J. Br. Diabetic Assoc. 23, 516–21. doi: 10.1111/j.1464-5491.2006.01838.x
Nakamoto, K., Nishinaka, T., Matsumoto, K., Kasuya, F., Mankura, M., Koyama, Y., et al. (2011). Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 1432, 74–83. doi: 10.1016/j.brainres.2011.11.012
Negoro, N., Sasaki, S., Mikami, S., Ito, M., Suzuki, M., Tsujihata, Y., et al. (2010). Discovery of TAK-875: a potent, selective, and orally bioavailable GPR40 Agonist. ACS Med. Chem. Lett. 1, 290–294. doi: 10.1021/ml1000855
Neutzsky-Wulff, A. V., Andreassen, K. V., Hjuler, S. T., Feigh, M., Bay-Jensen, A. C., Zheng, Q., et al. (2012). Future detection and monitoring of diabetes may entail analysis of both beta-cell function and volume: how markers of beta-cell loss may assist. J. Transl. Med. 10, 214. doi: 10.1186/1479-5876-10-214
Ning Guilin, D. L., Ye, J., Gong, W., Lin, Y., Na, D. (2015). Preparation method of diaphorin vanadium complex with hypoglycemic activity CN201510018112.4.
Orskov, C., Wettergren, A., Holst, J. J. (1996). Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 31, 665–670. doi: 10.3109/00365529609009147
Overton, H. A., Fyfe, M. C. T., Reynet, C. (2008). GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 153 (Suppl 1), S76–81. doi: 10.1038/sj.bjp.0707529
Perez, A., Zhao, Z., Jacks, R., Spanheimer, R. (2009). Efficacy and safety of pioglitazone/metformin fixed-dose combination therapy compared with pioglitazone and metformin monotherapy in treating patients with T2DM. Curr. Med. Res. Opin. 25, 2915–2923. doi: 10.1185/03007990903350011
Pi-Sunyer, F. X. (2006). Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295 (7), 761–75.
Podar, T., Solntsev, A., Karvonen, M., Padaiga, Z., Brigis, G., Urbonaite, B., et al. (2001). Increasing incidence of childhood-onset type 1 diabetes in 3 Baltic countries and Finland. Diabetologia 44 (Suppl 3), B17–20.
Rahelić, D. (2016). [7th edition of IDF Diabetes Atlas--call for immediate action]. Lijec Vjesn 138 (1–2), 57–58.
Rao Kondapally Seshasai, S., Kaptoge, S., Thompson, A., Di Angelantonio, E., Gao, P., Sarwar, N., et al. (2011). Diabetes mellitus, fasting glucose, and risk of cause-specific death. New Engl. J. Med. 364, 829–841. doi: 10.1056/NEJMoa1008862
Riera-Guardia, N., Rothenbacher, D. (2008). The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obesity Metab. 10, 367–375. doi: 10.1111/j.1463-1326.2007.00755.x
She Zhigang, L. Y., Ma, L., Li, H., Xia, G., Lu, Y. (2013). Preparation and application of an isobenzofuranone compound CN201310692094.9.
Srinivasan, B. T., Jarvis, J., Khunti, K., Davies, M. J. (2008). Recent advances in the management of type 2 diabetes mellitus: a review. Postgraduate Med. J. 84, 524. doi: 10.1136/pgmj.2008.067918
Tan Jianwen, W. J., Xu, Q., Zhou, Z., Ren, H. (2013). Preparation of a new 23,29-norsole oleic acid compound and the application for preparing glycosidase inhibitor drugs CN201310737166.7.
Tan Jianwen, W. J., Zhou, Z., Ren, H., Xu, Q. (2013). Preparation of a new 23-lower oleic acid compound and its role in glycosidase inhibitor drugs CN201310737187.9.
Tan Xiaozhong, S. Y., Qu, Q., Wang, L., Fu, S., Li, Z., Zhang, S. (2015). Preparation for one traditional Chinese medicine CN201510188449.X.
Theodorakis, M. J., Carlson, O., Michopoulos, S., Doyle, M. E., Juhaszova, M., Petraki, K., et al. (2006). Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 290, E550–9. doi: 10.1152/ajpendo.00326.2004
Urbano, F., Filippello, A., Di, P. A., Barbagallo, D., Di, M. S., Pappalardo, A., et al. (2016). Altered expression of uncoupling protein 2 in GLP-1-producing cells after chronic high glucose exposure: implications for the pathogenesis of diabetes mellitus. Ajp Cell Physiol. 310, C558. doi: 10.1152/ajpcell.00148.2015
van de Laar, F. A. (2008). Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vascular Health Risk Manage 4, 1189–1195. doi: 10.2147/VHRM.S3119
Wang Xueyong, Z. B. (2013). Application of valerian pentacyclic triterpenoid saponin for preparing hypoglycemic drugs CN201310627408.7.
Wang, B., Sun, Y., Sang, Y., Liu, X., Liang, J. (2018). Comparison of dipeptidyl peptidase-4 inhibitors and pioglitazone combination therapy versus pioglitazone monotherapy in type 2 diabetes: a system review and meta-analysis. Medicine 97, e12633. doi: 10.1097/MD.0000000000012633
Wentworth, J. M., Hensman, T., Playfair, J., Laurie, C., Ritchie, M. E., Brown, W. A., et al. (2014). Laparoscopic adjustable gastric banding and progression from impaired fasting glucose to diabetes. Diabetologia 57, 463–468. doi: 10.1007/s00125-013-3129-0
Xu Gang, S. X., Ma, J., Tan, J. (2014). Preparation and application of lupin-type triterpenoids and their pharmaceutical compositions CN201410177372.1.
Yang, X., Xu, Z., Zhang, C., Cai, Z., Zhang, J. (2017). Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim. Biophys. Acta. Mol. Basis Dis. 1863 (8), 1984–1990.
Yu Changxi, L. M., Ling, Q., Xu, Y., Huang, H., Yang, J., Su, Y. (2014). Application of scrotonin and its homologues in the preparation of drugs for treating diabetic complications CN201410005910.9.
Zhang Daimei, D. H., Tingting, Z. (2017). Method for preparing a GPR40 agonist pharmaceutical composition CN201711429787.3.
Zhang Xiaoqing, S. Z., Bao, J., Jiang, Y. (2012). Method for preparing a polymer as DPP-4 inhibitor CN201210418156.2.
Zhang Yingjun, Y. X., Wang, X., Ma, F., Wu, S., Zheng, C., Xu, J. (2014). Preparation and application of novel urea-substituted biphenyl compounds and their derivatives CN201410429055.4.
Zhao, G., Al, E., Zhao, G., Taunk, P. C., Magnin, D. R., Simpkins, L. M., et al. (2005). Diprolyl Nitriles as Potent Dipeptidyl Peptidase IV Inhibitors. Cheminform 36, 3992–3995. doi: 10.1002/chin.200552163
Zheng Zhihui, L. X., Xu, Y., Zheng, H., Ke, A., Ren, X., Li, Y., et al. (2014). New application of golden gray chlorophyll-like compounds CN201410112149.9.
Keywords: type 2 diabetes mellitus, antihyperglycemic, patent, review, therapy
Citation: Zhu W, Huang W, Xu Z, Cao M, Hu Q, Pan C, Guo M, Wei J-F and Yuan H (2019) Analysis of Patents Issued in China for Antihyperglycemic Therapies for Type 2 Diabetes Mellitus. Front. Pharmacol. 10:586. doi: 10.3389/fphar.2019.00586
Received: 01 November 2018; Accepted: 07 May 2019;
Published: 21 May 2019.
Edited by:
Lei Xi, Virginia Commonwealth University, United StatesReviewed by:
Sudheer Kumar Ravuri, Steadman Philippon Research Institute, United StatesNazareno Paolocci, Johns Hopkins University, United States
Copyright © 2019 Zhu, Huang, Xu, Cao, Hu, Pan, Guo, Wei and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Fu Wei, d2VpamlmdUBob3RtYWlsLmNvbQ==; Hongyu Yuan, aHl5dWFuMjAwMkBzb2h1LmNvbQ==
 Wei Zhu
Wei Zhu Wen Huang
Wen Huang Zhiqiang Xu1
Zhiqiang Xu1 Qiaoli Hu
Qiaoli Hu Ji-Fu Wei
Ji-Fu Wei