
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 31 May 2019
Sec. Pharmacology of Anti-Cancer Drugs
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00557
 Steven R. Chamberlin1*
Steven R. Chamberlin1* Aurora Blucher2
Aurora Blucher2 Guanming Wu1,2,3
Guanming Wu1,2,3 Lynne Shinto4
Lynne Shinto4 Gabrielle Choonoo1,2
Gabrielle Choonoo1,2 Molly Kulesz-Martin2,5
Molly Kulesz-Martin2,5 Shannon McWeeney1,2,3
Shannon McWeeney1,2,3A body of research demonstrates examples of in vitro and in vivo synergy between natural products and anti-neoplastic drugs for some cancers. However, the underlying biological mechanisms are still elusive. To better understand biological entities targeted by natural products and therefore provide rational evidence for future novel combination therapies for cancer treatment, we assess the targetable space of natural products using public domain compound-target information. When considering pathways from the Reactome database targeted by natural products, we found an increase in coverage of 61% (725 pathways), relative to pathways covered by FDA approved cancer drugs collected in the Cancer Targetome, a resource for evidence-based drug-target interactions. Not only is the coverage of pathways targeted by compounds increased when we include natural products, but coverage of targets within those pathways is also increased. Furthermore, we examined the distribution of cancer driver genes across pathways to assess relevance of natural products to critical cancer therapeutic space. We found 24 pathways enriched for cancer drivers that had no available cancer drug interactions at a potentially clinically relevant binding affinity threshold of <100nM that had at least one natural product interaction at that same binding threshold. Assessment of network context highlighted the fact that natural products show target family groupings both distinct from and in common with cancer drugs, strengthening the complementary potential for natural products in the cancer therapeutic space. In conclusion, our study provides a foundation for developing novel cancer treatment with the combination of drugs and natural products.
While treatment for cancer has seen great strides in recent decades, we still face many open challenges in cancer therapy. In particular, adaptive resistance to cancer therapies has necessitated a move into rational combination therapies if we are to achieve a sustained therapeutic benefit for patients. An active area of interest is the inclusion of natural products in combination therapies for cancer treatment. In this work, our core premise is that the target space associated with natural products (NPs) will increase the number of potentially therapeutically accessible targets and lead to novel combination therapies for cancer treatment. To investigate this premise, we will not only quantify these new therapeutic targets and associated molecular pathways, but also assess the functional qualities and complementarity with FDA-approved cancer drugs of this space by using a variety of network methods.
Currently, the National Cancer Institute lists eight categories of cancer treatments: surgery, radiation, chemotherapy, immunotherapy, targeted therapy, stem cell transplant, and precision medicine1. Historically, surgery, radiation, and chemotherapy were the primary forms of treatment. In the late 1990s the FDA started approving targeted therapies for cancer, i.e., therapies directed toward unique molecular characteristics that drive oncogenesis. The first such therapy, imatinib, has shown an 80% decrease in 5-year mortality with chronic myeloid leukemia patients (Druker, 2009). While some of the early targeted therapies have resulted in dramatic clinical responses, drug resistance often develops after an initial positive response. This adaptation to treatment is known as acquired drug resistance, as opposed to intrinsic resistance, which exists prior to any cancer therapy (Holohan et al., 2013).
Acquired drug resistance is seen with both cytotoxic chemotherapies and targeted therapies, although mechanisms differ. Knowledge of the molecular mechanisms of resistance can inform therapeutic strategies. In cancer, these mechanisms can include compensatory and redundant molecular signaling, target mutations acquired during treatment, increased expression of the targeted proteins, inactivation of pro-apoptotic pathways, inhibition of DNA repair mechanisms, epithelial-mesenchymal transition, activation of pro-survival signaling, and upregulation of tumor cell efflux transporters (Holohan et al., 2013; Housman et al., 2014). For drug resistance caused by mutations in drug targets or redundant cell pathways, “rational combinatorial targeted therapy” is a possible solution (Al-Lazikani et al., 2012). This “rational” approach is done within the framework of network pharmacology, which brings together systems biology, network analysis, redundancy, and consideration of all drug target effects, beyond therapeutic intention, for designing therapies (Hopkins, 2008). Knowledge of molecular signaling pathways can be used to design multi-target strategies to block redundant pathways or newly mutated targets. Simultaneous targeting of multiple cancer hallmarks is another approach (Hopkins, 2008). Along with reduction in drug resistance, this approach can also lead to decreased adverse effects and increased efficacy (Hanahan and Weinberg, 2011). For these combinations, multiple therapeutic agents can be used, but these methods can also take advantage of poly-pharmacological characteristics of each single agent (Hu et al., 2014). Drugs can also work together through pharmacokinetic mechanisms, coalistic mechanisms (Jia et al., 2009), and through independent actions when used in combination (Palmer and Sorger, 2017). A interaction is coalisitic when two compounds interact in a biological context to form a new third compound.
Natural products can be broadly defined as any compound derived from a living source (animal, plant, microbial, fungi). This absolute definition would include “natural” cosmetics, “natural” foods, wood, silk, bioplastics and even coal. A more specific definition was based on the following from the National Center for Complementary and Integrative Health (NCCIH) (Milshteyn et al., 2014):
“Natural products include a large and diverse group of substances from a variety of sources. They are produced by marine organisms, bacteria, fungi, and plants. The term encompasses complex extracts from these producers, but also the isolated compounds derived from those extracts. It also includes vitamins, minerals and probiotics.”
We have further refined this definition with the following inclusion and exclusion criteria. Only NPs from plants and fungi were included, and only isolated compounds. In addition, only plants and fungi that have a history of traditional medicinal use were included. These compounds already have some historic use as therapeutic agents, and some are already classified as safe for human consumption by the FDA's Dietary Supplement Health and Education Act of 1994.
Natural product compounds show greater structural diversity, bioactivity and complexity than compounds in synthetic drug libraries, have the ability to inhibit some targets considered “undruggable,” such as protein-protein interactions, and inherently target biologically relevant space since they are mostly secondary metabolites, or signaling molecules (Harvey et al., 2015). There is also limited overlap between the molecular space targeted by natural products and targeted by synthetic drug libraries (Harvey et al., 2015). These characteristics not only indicate the potential for new targets for therapy, but also can help reduce the cost of the development of new treatments, since these molecules already exist in nature, and offer additional options for combination therapies. Natural products, or natural product derivatives, are the source of 33% of cancer drugs developed between 1981 and 2014 (Newman and Cragg, 2016). While the use of natural products for cancer treatment by patients is sometimes considered “complementary medicine,” there is also a body of literature demonstrating in vitro and in vivo natural product synergies with cancer drugs (Cote et al., 2015; Cheng et al., 2016), overcoming drug resistance with the addition of natural products (Pearson et al., 2017), and paradoxical synergy in cancer cells while demonstrating antagonism in healthy tissue (Cote et al., 2015). Therefore, the exploration of the natural product target space could offer the potential to improve existing drug therapy outcomes and reduce side effects. Computational methods, including approaches in graph theory (Sun et al., 2015) and assessment of differential gene expression (Liu and Zhao, 2016), have been successfully developed to predict synergy between compounds in silico. Predicting these effective drug combinations often requires up-to-date, comprehensive knowledge of the target space associated with a set of compounds.
However, natural product-target information in the public domain has several high need areas to address if we are to harness natural products in computational methods for predicting and prioritizing compounds for high throughput screens. There is a need not only to compile a comprehensive set of natural product targets from public sources, but also to characterize these compound-target relationships with respect to their supporting evidence, within a variety of relevant contexts (Figure 1). Such an evidence- characterization was recently completed for all FDA-approved antineoplastic drugs from multiple public resources by Blucher, et al. in the Cancer Targetome (CT) (Blucher et al., 2017). The methods and evidence framework from that project were applied here to develop and characterize a natural product target network (natural product compounds linked to associated targets). By comparing our results with natural products to the results of the Cancer Targetome, we provide a quantitative assessment of the potential of natural products, when added to FDA-approved cancer drug space, to increase novel cancer therapies. This characterization of the natural product network includes assessment of target and pathway coverage, compound promiscuity, established cancer driver genes, and the potential to inhibit molecular compensatory signaling mechanisms. This characterization of NP target space serves to not only identify potential new targets, but also evaluate the importance of these targets in a molecular context for therapeutic targeting in cancer treatment. In particular, we evaluate the dual space of natural products and FDA- approved cancer drugs from the Cancer Targetome for potential synergy in drug combination therapy. To assess this potential, we propose using a variety of network methods to evaluate natural products and their targets in the context of biological networks. First, by mapping natural product targets of interest into large protein-protein interaction networks, we can characterize their importance to the network using measures of “centrality” as well as their proximity to known cancer driver genes. Second, we create a network where entities are biological pathways, which allows us to assess potential pathway cross-talk. This is critical for efforts to prioritize combinations of natural products and FDA-approved drugs to interfere with pathway-based mechanisms of drug resistance. Finally, we create a network that contains both the natural product compounds and FDA-approved cancer drugs as entities, with connections between entities indicating shared protein targets. This network allows us to assess targets with respect to class of compound and can potentially be harnessed in rational combinations, such as combining compounds that target different families of targets for a complementary treatment strategy.
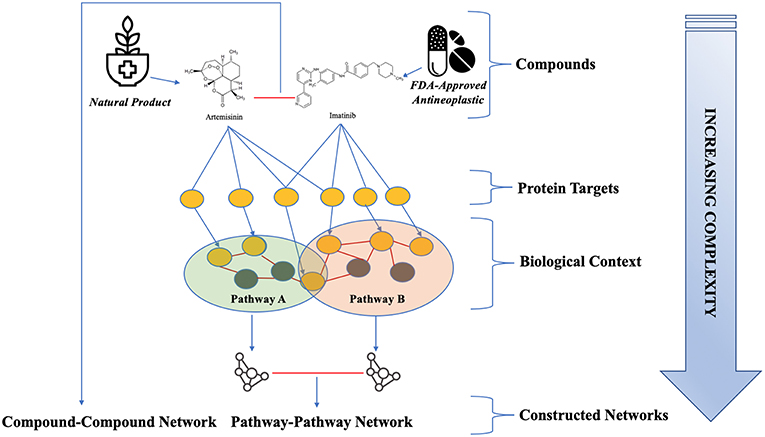
Figure 1. Framework for natural product target network evaluation. The targets associated with both NPs and anti-neoplastic drugs were evaluated in different contexts of increasing complexity. Complementary and distinct coverage of protein targets and pathways by the two compound classes were assessed. Target importance and relationships were evaluated in biological contexts, which include protein-protein interaction networks and molecular pathways. Pathway relationships and shared target space were assessed through the construction of a pathway-pathway network and a compound-compound network. Red lines indicate the existence of an edge between nodes in these networks. Two compounds have an edge if they share at least one target, and two pathways have an edge if they share at least one protein (Mahira and Umehara, 2018; Mykhal, 2004; Hinemash6, 2010).
Seven public databases were used to construct the natural product target network used in this project, as sources of both compounds and targets (Table 1). Two of the seven databases were the source of natural product chemical compounds, TarNet (2016) (Hu et al., 2016), and the Traditional Chinese Medicine Integrated Database for herb molecular mechanism analysis (TCMID, version 2.01) (Xue et al., 2013). These databases were chosen because they contain only compounds from plants used from medicinal traditions.
Four of the target source databases used were not specific to natural product compounds and were also used in the creation of the Cancer Targetome. These included DrugBank, version 5.0.7 (Law et al., 2014); Therapeutic Targets Database, version 4.3.02 (Qin et al., 2014); the International Union of Basic and Clinical Pharmacology (IUPHAR)/British Pharmacological Society (BPS) database, version 2017.4 (Harding et al., 2017); and BindingDB (7/1/2017). Additional human target interactions were included from three natural product databases. These include TarNet, TCMID and the Universal Natural Products Database (Gu et al., 2013). For full details of data analyzed in this article see Data Sheet 1.
The TarNet database, the first of the two sources of NP compounds, contains information about 12,187 compounds derived from 894 medicinal plants. These plants are used in four traditions of botanical medicine: Chinese, Japanese, European and American (Hu et al., 2016). This database also contains 10,783 targets associated with the plant compounds through direct regulation or indirect effect. Both the plant-compound and compound-biotarget relationships were derived by text-mining and manual curation. A random sample of 91 literature references, used by TarNet to support the compound-biotarget relationships, was manually checked for the presence of binding affinity data in the source.
The second source, TCMID, comprises plants, associated compounds, and bio-targets curated from Traditional Chinese Medicine (TCM). The goal of this database is to translate the common factors between modern western medicine and TCM. The TCMID database contains 8,159 plants, 43,413 associated compounds, and 17,521 bio-targets compiled through a combination of other databases and text mining (Hu et al., 2016; Traditional Chinese Medicine Integrated Database, 2018).
The compounds from these two databases were combined and redundancies were removed. This was done through the use of the multiple keys associated with each compound in each database. TarNet compounds were classified by chemical name, Chemical Abstracts Service (CAS) number, simplified molecular-input line-entry system (SMILES), and International Union of Pure and Applied Chemistry's International Chemical Identifier Key (INCHIKEY). TCMID compounds were classified by PubChem Compound ID, SMILES and chemical name. The Chemical Translation Service (CTS) from the University of California at Davis was used to help resolve some of the missing key data in TarNet (Wohlgemuth et al., 2010). The two compound lists were then combined and scrubbed for possible drugs that are not of NP origin using lists from DrugBank (Law et al., 2014) and the FDA. The five keys found in the source databases: chemical name, SMILES, INCHIKEY, Pubchem Compound ID, and CAS number, were then used to retrieve target information from a variety of sources as detailed below.
Molecular target interaction information for the NP compounds was retrieved from seven publicly available data sources. Four widely used data sources were chosen based on the rationale used by Blucher et al. for the Cancer Targetome (Blucher et al., 2017). These sources include DrugBank (Law et al., 2014), Therapeutic Targets Database (Qin et al., 2014), IUPHAR/BPS Guide to Pharmacology (Harding et al., 2017), and BindingDB (Gilson et al., 2016). These four sources contain substantial information about NPs but are not limited to this class of compounds. Three additional sources of target interaction data that are limited only to NPs were also used. These include TarNet (Hu et al., 2016), TCMID (Xue et al., 2013), and the UNPD (Gu et al., 2013). All of the interaction data used for this project were based on experimental data from the literature, no computationally predicted interactions were used.
These data were retrieved by systematically merging each of the five NP keys, from the base NP data, individually to the seven target data sources. The data retrieved by the individual key were then associated back to the unique five key combination of values from which they were derived and data redundancies were resolved to create the final NP target network database.
The Evidence Level framework developed by Blucher et al. (2017) was then applied to these data as follows. Each NP-target interaction could have more than one piece of supporting evidence. Each piece of evidence was assigned one of three levels. Evidence Level I had an entry in only one of the databases for the interaction, without a supporting literature reference or experimental binding value. Evidence Level II had a supporting literature reference in the database but without binding affinity value, and Evidence Level III had both an experimental binding value and a literature reference. For NP-target interactions with multiple evidence entries from different databases, the maximum Evidence Level was assigned. For a single target, the maximum Evidence Level from all associated NP-target interactions was assigned. Analyses for this project considers all levels of evidence when appropriate and also focuses on interactions only with evidence of strong binding affinity, < 100 nM (IC50, EC50, Ki, or KD). Less stringent levels can still be considered biologically relevant, but below 100 nM is considered significant for drug binding (Paolini et al., 2006; Wang et al., 2016).
Analyses compared the two target networks above, i.e., the NP target network and the Cancer Targetome (CT). First, the sets of targets associated with the NP and CT drug networks were mapped to the two associated sets of molecular pathways. The Reactome Pathway Knowledgebase (Fabregat et al., 2017), which leverages biological entity reactions, was the source of the pathway information. A total of 1,944 hierarchically structured pathways was used from this database for the first pathway mapping. For the second pathway mapping, a set of pan-cancer aberrant pathways was derived from the full set of Reactome pathways by performing an over-representation analysis with likely cancer driver genes. The genes cataloged for the Cancer Genome Interpreter (Tamborero et al., 2017) were used for this analysis. These genes have either experimental, clinical or in silico evidence showing that their mutations can drive tumorigenesis. There are 837 genes cataloged, representing 193 different tumor types. A hypergeometric test identified pathways enriched with these driver genes. The Benjamini-Yekutieli method was used to control the false discovery rate for multiple testing with dependencies (Benjamini and Yekutieli, 2001). If a pathway contained at least one molecular target with evidence of an interaction with either a NP or a CT drug, then the entire pathway was considered targeted by the respective compound category. Pathways were then classified as either targeted by NP only, CT drugs only, both NP and CT drugs, or neither. This same mapping classification was also applied at the protein target level, as opposed to the pathway level, to all of the targets associated with both target networks, and to targets only associated with the pan-cancer aberrant pathways. Finally, tumor types were identified that were associated with cancer driver genes targeted only by NPs. Only high-affinity interactions (IC50, EC50, Ki, or KD < 100 nM) were considered for this analysis.
The multi-targeting aspect of these two compound classes (NP and CT drug) is considered the basis for their poly-pharmacological effects (Hu et al., 2014). These effects can be undesirable, such as adverse events, or they could be the mechanism of the therapeutic effect. For this reason, it is important to map and compare the average interactions (targets, pathways, tumor types, and cancer drivers) per compound between the two target networks and to understand how NPs might differ from the CT drugs. For most analyses in this project we considered only < 100 nM target interactions, but for this analysis we considered two binding affinity thresholds: < 1,000 and < 100 nM. Distributions of interactions per compound were then compared between NPs and CT drugs for the four categories mentioned. A two sample Kolmogorov-Smirnov test was used to compare the two distributions, NP and CT drugs.
For molecular interaction analysis, the NP targets, CT drug targets and cancer driver gene products were projected onto biological networks, and the topological features of these targets were evaluated and compared. Target-oriented topology research often uses a large non-specific protein-protein interaction network (PPI) for the biological context, but it has also been suggested that the base network should be either be more specific to the tissue or disease of interest, or have greater biological relevance (Peng and Schork, 2014). For this reason, two specific interaction networks were used for this evaluation. For the first biological network, the protein functional interactions from the Reactome Functional Interaction Network were used. This network integrates uncurated relationships from sources such as PPI databases and others with curated interaction information derived from pathway data in Reactome and other databases (Wu et al., 2010). These functional interactions have a higher likelihood of being functional in a biological context than an interaction from an uncurated PPI database. The second network is a PPI constructed by Wang (2014) from the integration of four manually curated human cancer signaling networks with protein interactions from BioGRID (Awan et al., 2007; Cui et al., 2007; Li et al., 2012; Newman et al., 2013;Chatr-Aryamontri et al., 2015).
Network measures that were considered include degree centrality, betweenness centrality, eigenvector centrality, and average shortest distance to cancer driver genes in the network. Degree centrality is the number of connections a node in a network has to neighboring nodes. In a biological network this could be the number of different interaction partners a single protein might have. Betweenness centrality is the number of shortest paths in a network that pass through a specific node. In a biological network this measure can capture the node's ability to control communication (Peng and Schork, 2014). Eigenvector centrality is a measure of how connected the nodes are that are connected to the node of interest. This is a measure of importance of the node's neighbors in the biological network. The average shortest distance is the path between two nodes that contains the minimum number of nodes, or steps. In a biological network this can be related to the number of reaction steps between two proteins (Yildirim et al., 2007; Li et al., 2012). Proteins that are closer could have a higher likelihood of impacting each other, if used as a therapeutic target.
To compare the topology measures, the nodes in the network were classified in four ways: targeted only by NPs, targeted only by CT drugs, targeted by both, and not targeted by either. These were compared to each other for similarities and differences to evaluate any additional therapeutic potential achieved with the addition of NPs. Only targets with strong binding affinities, < 100 nM, were considered.
Chen et al. (2016), constructed a pathway-pathway interaction network to evaluate drug pair synergy and as a possible model for the inhibition of pathway crosstalk. In this network, a node represents an entire molecular pathway and an edge represents an interaction between two pathways. Three types of interactions were used to construct three networks. The three edges were defined by manually curated interactions from the KEGG database (Kanehisa and Goto, 2000), protein-protein interactions, and shared genes. Two sets of pathways were defined by associating the two drugs via the projection of the associated drug target networks onto the pathways. Chen et al. found that drug synergy was correlated with the average shortest distance between the two sets of pathways in this network, most strongly in the network with edges representing shared genes.
This structure was chosen as a good method to evaluate the NP and CT drug target network's crosstalk inhibition potential, since it is possible to measure global patterns in this framework and assess the two target sets in their entirety. Pathways in this network were then classified as being associated with NPs only, CT drugs only, both or none. A compound was associated with a pathway only if there was at least one target with a strong binding affinity, < 100 nM, in a pathway. Average shortest path distance between NP associated pathways and CT associated pathways was assessed to estimate the possible synergistic potential between NPs and CT drugs, and also to estimate increased cross talk inhibition by considering NPs. Reactome entity level pathways (non-hierarchical) were chosen to construct the network, of which there are 290. In addition, the previously described cancer driver genes were used to do an over-representation analysis on this set of 290 pathways, identifying possible aberrant cancer pathways in this network. Orientation to these cancer pathways in this network was then assessed for the pathways associated with NPs only, CT drugs, and both NPs and CT drugs.
In this network, a node represents a compound, either NP or CT drug, and an edge exists if they share at least one target, both having a binding value of < 100 nM. The edges were also weighted based on the number of targets shared. This network analysis has also been used for FDA approved drugs (Yildirim et al., 2007). Network communities were identified and assessed for enrichment in either NPs or CT drugs to see if they are clustering around the same targets, or separate sets of targets. The targets of each network community were also classified based on protein families, defined in IUPHAR. Multilevel clustering was used as the community detection method. This method is a “greedy” algorithm—that is, it creates an optimal solution at each step to find a good global optimum—that creates communities based on maximizing modularity and is recommended for networks of this size. This method is also appropriate for unconnected networks and can use edge weights to determine community structure (Yang et al., 2016).
The analysis was conducted using R (v3.3.1) (https://www.r-project.org/). Packages used included dplyr (v0.7.4) (https://dplyr.tidyverse.org) and iGraph (v1.1.2) (http://igraph.org). Network visualization was also performed in Cytoscape (v3.4.0) (http://cytoscape.org).
Our final set of natural products, compiled from TarNet and TCMID, contained 50,109 compounds, uniquely identified by the five keys found in the source databases: chemical name, SMILES, INCHIKEY, Pubchem Compound ID and CAS number. Of these compounds, target interaction data was retrieved for 4,991 from seven public databases. To contrast this with approved cancer drug space, there are only 137 anti-neoplastic drugs in the Cancer Targetome, which included all FDA approved drugs for cancer as of 2017.
Most of the NP-target interactions were classified as Evidence Level I (94%), and most CT interactions were classified as Evidence Level III (95%), although the absolute counts of interactions were much larger for NPs given the overall number of NPs (Figure 2A). For stronger affinity interactions (Evidence Level III Exact), the raw numbers of interactions were comparable between natural products and cancer drugs (Figure 2B). The large volume of Level I evidence for NPs is primarily from the TarNet database, which is populated via text mining of the public literature for natural products, combined with manual curation. All of these interactions are supported by literature references (and therefore technically are Evidence Level II), but the linkage to these literature references is not made publicly available in the TarNet database, therefore these interactions are restricted to Evidence Level I due to accessibility issues. Curation of a random sampling of these data showed that approximately 37% of the supporting literature has binding data available and would therefore could potentially be assigned Level III were the linkage to this supporting evidence made accessible.
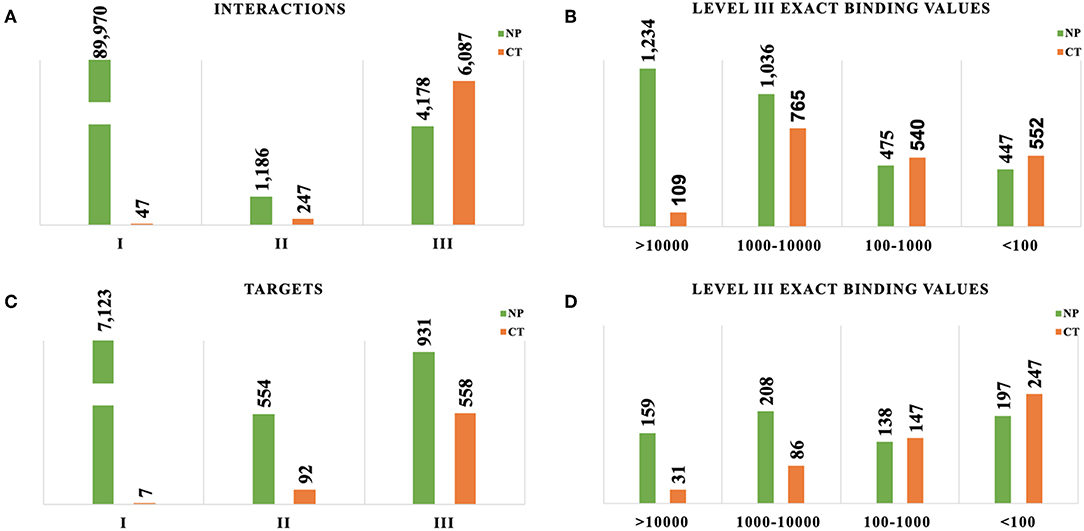
Figure 2. Assessment of evidence levels for target and compound-target interactions. CT, Cancer Targetome; NP, Natural Product Target Network. (A) Comparison of interaction distribution by evidence level between NPs and CT drugs. (B) Comparison of interaction distribution at high affinity only (evidence level III exact values in nM). (C) Comparison of maximum target evidence level distribution between NPs and CT drugs. (D) Comparison of maximum target evidence level distribution at high affinity only (evidence level III exact values in nM).
Targets are reported according to the highest evidence level of all of the associated compound interactions for each target (Figure 2C). This approach allows us to take a target-focused perspective and determine the maximum strength of evidence supporting any compound/drugs interacting with it. For a target set of interest, this framework now us to prioritize those targets with stronger evidence for natural product or drug interactions. As with the target-interaction distribution, most of the NP associated targets had a maximum Evidence Level I (83%) and most of the CT drug associated targets had a maximum Evidence Level III (85%), but the distributions were more comparable within Evidence Level III (Figure 2D).
In addition to descriptively evaluating pathway and target coverage by NP compounds compared to coverage by FDA-approved antineoplastic drugs, it is also important to consider the target sets associated with NPs and CT drugs in the context of molecular interaction networks, such as PPI networks. The topology of these networks can be related to biological function (Winterbach et al., 2013). Some topology measures commonly considered relevant to biological function include degree centrality, betweenness centrality, eigenvector centrality, average shortest paths, and clustering coefficients (Zhu et al., 2007; Pavlopoulos et al., 2011). These measures, and others, have been suggested as a guide to identify potential therapeutic targets for drug development (Hwang et al., 2008; Arrell and Terzic, 2010; Winterbach et al., 2013; Peng and Schork, 2014).
Two biological networks (PPIs) were used to evaluate functional relevance of the targets. One is the Reactome Functional Interaction network, and the other was created by Wang (2014) based on interactions specific to cancer biology. The three target node categories include nodes targeted by NPs only, targeted by CT drugs only, or targeted by both CT drugs and NPs. In Reactome, these three node categories represented 393 of the 12,227 total nodes contained in the network. In the wang (cancer) network, these three node categories represented 364 of the 6,306 total nodes in the network. Comparisons were done for the following topology measures: degree, betweenness, and eigenvector centrality.
In the Reactome network, betweenness for all three target node categories was significantly higher than it was for non-targeted nodes in the network. There was no significant difference between these three categories. Consistent with previous research, betweenness was significantly higher for the cancer driver nodes than for non-driver nodes in this network.
In the wang (cancer) network (Wang, 2014), as with the Reactome network, all three target node categories had higher betweenness than the non-target nodes. The nodes targeted only by NPs were not different from the nodes targeted only by CT drugs, but the betweenness for these nodes was less than that for the nodes targeted by both NPs and CT drugs. Consistent with previous research, the cancer driver nodes were also significantly higher for betweenness than the non-cancer driver nodes.
The results for this statistic were mostly consistent between the two networks. Overall, nodes targeted by NPs are similar for this characteristic to nodes targeted by CT drugs, and different from non-targeted nodes. The average value for the nodes targeted by both NPs and CT drugs was higher for this measure than the average for nodes targeted by either NP only or CT drugs only, although this difference was not significant when compared to CT only drug targets, and there were only 39 nodes in this category.
Betweenness measures the number of shortest paths in a network that pass through a given node. The target with the highest betweenness in the Reactome network, interacting with only NPs at binding affinity < 100 nM, was nuclear factor kappa beta subunit 1 (NFKB1). The interacting NP compound was rocaglamide, extracted from a variety of Aglaia plant species. This gene functions in a dozen large molecular pathways, ranging in size from 72 to 758 genes, most of which are enriched with cancer drivers. This protein is a transcription factor found in almost all cell types and is involved in a wide variety of cell processes, including some that are related to cancer, such as inflammation, tumorigenesis, apoptosis and cell growth. This protein is activated by many intracellular and extracellular stimuli, which is consistent with a high degree of betweenness centrality.
A literature search for the natural product “rocaglamide” returned 68 articles, many of which were in-vitro and mouse xenograph cancer studies with positive results. Cancers tested with positive results included renal cell carcinoma (Nalli et al., 2018), non-small cell lung cancer (Yao et al., 2018), multiple myeloma, T-cell leukemia (Wu et al., 2017). This natural product was also found to protect non-malignant cells (Becker et al., 2014). This body of literature also addressed infectious disease applications for this compound.
In the Reactome network, degree was not significantly different between all three target node categories, but each of these three categories were all significantly higher than non-targeted nodes in the network, as was seen with betweenness. The cancer driver nodes were also higher for this measure than non-driver nodes.
In the wang (cancer) network, the nodes targeted by NPs only were not significantly different from non-targeted nodes, and they were significantly lower for this measure than nodes targeted by CT drugs only or nodes targeted by both NPs and CT drugs. The cancer driver nodes were also significantly higher for this measure in this network.
For degree centrality, the two networks were not in agreement. The nodes targeted only by NPs were similar to CT drug nodes in the Reactome network, but not in the wang (cancer) network. But nodes targeted by both NPs and CT drugs were again higher on average than either of the other two categories, NP only and CT drugs only, although it was only significant for the comparison to the NP-only targeted nodes.
The node with the highest degree targeted only by NPs at a binding affinity < 100 nM in the Reactome network is the same as the top node for betweenness for this category, nuclear factor kappa beta subunit 1 (NFKB1). In this network, there were 515 interactions with other proteins for this protein target. Of these 515 proteins, 90 (17.5%) were cancer drivers.
For this measure, in the Reactome network, nodes targeted only by NPs were not different from non-targeted nodes, were significantly higher than nodes targeted by CT drugs only, and significantly lower than nodes targeted by both CT drugs and NPs. The nodes targeted only by CT drugs were significantly lower than non-targeted nodes, but not different from nodes targeted by both CT drugs and NPs. The nodes targeted by both CT drugs and NPs were significantly higher than non-targeted nodes. Cancer driver nodes were significantly higher for this measure than non-cancer driver nodes in this network.
In the wang (cancer) network, nodes targeted only by NPs were not different from non-targeted nodes but were significantly less than nodes targeted only by CT drugs and nodes that were targeted by both CT drugs and NPs. Nodes targeted by CT drugs only and those targeted by both CT drugs and NPs were significantly higher than non-targeted nodes. CT only nodes were not different from nodes targeted by both CT drugs and NPs. Cancer driver nodes were significantly higher than non-cancer driver nodes for this measure.
The two networks were not in agreement for these node categories for this measure. Generally, nodes targeted by NP only were not the same as other targeted nodes in both networks, but the direction of these differences was not the same. This was partly because the CT drug only nodes in the Reactome network were generally lower than most other categories, including non-targeted nodes.
The node targeted only by NPs that exhibited the highest eigenvector centrality in the Reactome network was Heterogeneous Nuclear Ribonucleoprotein A1 (HNRNPA1). This means that the other nodes directly connected to HNRNPA1 had a high level of connectivity. There were 185 proteins interacting with this protein in this network, with an average degree of 217, which was significantly higher than the network average of 38. While there were only 27 cancer drivers in this list, or 15% of the 185 proteins, one of these proteins, E1A Binding Protein P300 (EP300), has over 1000 interactions listed in Reactome. HNRNPA1 is an abundant core protein found in the cell nucleus and functions in alternative splicing of RNA.
There was a high level of overlap for the top genes ranked by betweenness and degree centrality for the three target categories (Table 2). There was less overlap between these genes and the genes with the highest eigenvector centrality.
In both networks, the average shortest path distances from either NP only, CT drug only, and both CT/NP nodes to cancer driver nodes were significantly shorter than a set of randomly selected nodes. The nodes targeted by both CT drugs and NPs were the closest to the cancer driver nodes, on average. This proximity to cancer drivers indicates fewer reaction steps between the targeted node and the cancer driver node and greater potential to interfere with an oncogenic signal from the driver. These nodes also had higher betweenness and degree. These nodes represent a subset of both CT drug nodes and NP nodes that appear to be more critical in both networks. These 39 nodes also contained a higher percentage of cancer drivers than the other categories.
The use of compensatory and redundant molecular pathways by the cancer cell is a likely mechanism of acquired drug resistance, suggesting that rational drug combination therapy could inhibit these processes. Several methods have been proposed to select and predict drug combinations that could inhibit this phenomenon (Peng and Schork, 2014; Sun et al., 2015; Chen et al., 2016; Jaeger et al., 2017).
For this analysis, we used the pathway network model developed by Chen et al. using shared genes as the criterion for an edge between two pathway nodes. Our network contained 285 pathways (nodes) with 9,152 edges. These 285 were non-hierarchical Reactome entity-level pathways. Of the 285 pathways, 90 were enriched with cancer drivers. The majority of the cancer pathways were targeted by both NPs and CT drugs (73%), and most of the non-cancer pathways were not targeted by either NPs or CT drugs (48%). Only target interactions < 100 nM were considered.
The average shortest path in this network between pathways targeted by CT drugs, those targeted by NPs, and between NP-targeted pathways and CT-drug targeted pathways were all similar, and closer than random controls. Based on previous research, this short distance could correlate with synergy between these classes of compounds, particularly between NPs and CT drugs. Pathways targeted by both CT drugs and NPs were closer on average to cancer pathways than random controls. Pathways targeted by NPs only and those targeted by CT drugs only were not closer to cancer pathways than random controls (Supplemental S1).
There were 559 examples of neighboring pathways (nodes) in this network, where one pathway is targeted by NPs only and the other pathway is a cancer-enriched pathway targeted by either CT drugs or both CT drugs and NPs. An example is DAG and IP3 signaling and Signaling by FGFR1. In the Reactome database, these two pathways share the gene PLCG1 (1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1), which created an edge between them in this network. The pathway Signaling by FGFR1 was enriched with cancer drivers and had a CT drug target with an interaction < 100 nM, BRAF (B-Raf Proto-Oncogene, Serine/Threonine Kinase). This pathway initiates intracellular signaling pathways involved with cell proliferation and migration, and other functions. DAG and IP3 signaling was not enriched with cancer drivers and did not have any low affinity CT drug targets, but did have six high affinity NP targets: PRKCG, E,A,D (Protein Kinase C), PDE1A (Phosphodiesterase 1A) and ADCY1 (Adenylate Cyclase 1). DAG (diacylglycerol) and IP3 (inositol 1,4,5-trisphosphate) are secondary messengers used in intracellular signaling.
There was a total of 253 compounds and 1,238 edges in the compound network, which included 68 CT drugs and 185 NPs. This network contained 26 unconnected subnetworks, with the majority of nodes (163) in one large connected component. Multilevel clustering created 35 communities, most of which contained 5 or fewer compounds. Of interest were the three largest communities, each containing over 30 compounds. One community was dominated by CT drugs, but also contained a substantial number of NPs. The other two communities contained primarily NPs.
The first community contained 32 CT drugs and 11 NPs. The community clustered primarily around kinase and other cancer-related target families (Table 3). Kinases are extremely well-targeted by current FDA-approved cancer drugs and have been an active area of research following the breakthrough kinase inhibitor imatinib. The next largest community comprised 39 NPs and 4 CT drugs. This community contained a variety of cytochrome P450 families and other target families considered therapeutic targets, such as the carbonate dehydratases, which are inhibited for the treatment of glaucoma and other conditions (Table 3). The third largest community contained no CT drugs and 30 NPs. The target protein families here were primarily hormone and neurotransmitter receptors (Table 3).
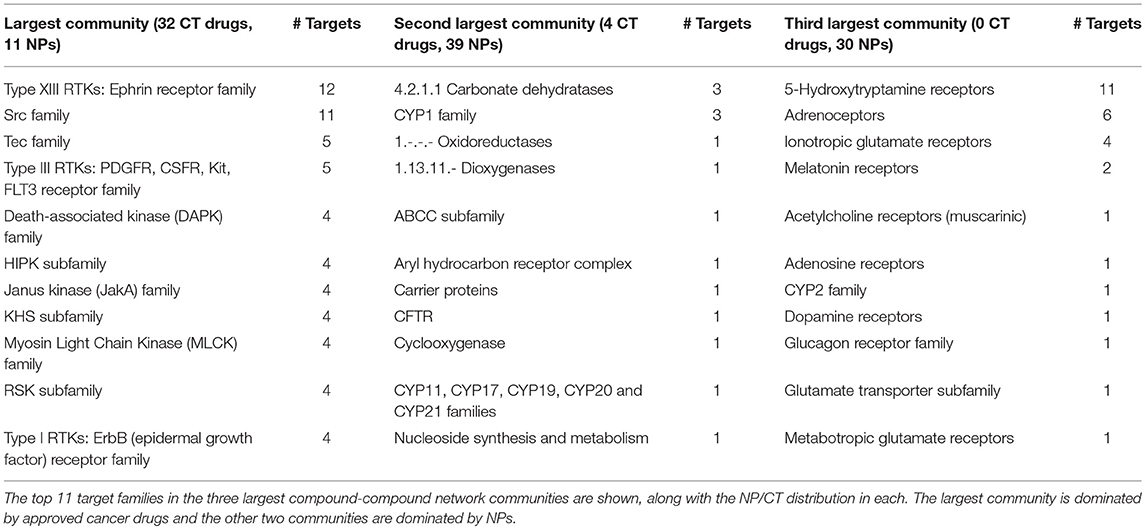
Table 3. Natural products (NP) and approved cancer drugs (CT) interact with disjoint and shared target sets.
Coverage is assessed at two thresholds: (1) at all levels of supporting evidence, and (2) for when restricting to interactions supporting by binding evidence < 100 nM. Of the 1,944 hierarchically structured Reactome pathways, 533 were considered pan-cancer aberrational based on over-representation analysis. For all Reactome pathways, at all levels of evidence, natural products increased coverage by 61%, relative to pathways covered by CT drugs. Reactome pathway coverage at the 100 nM level was increased by 29%, relatively (Table 4). For the aberrational cancer pathways, the NP relative coverage increase for all evidence levels was 12%, and the increase at the 100 nM level was 6% (Table 5). The percentage of aberrational cancer pathways targeted by both NPs and CT drugs was higher than it was for all Reactome pathways, which would be expected since the Cancer Targetome is specific to the disease domain associated with this subset of pathways.
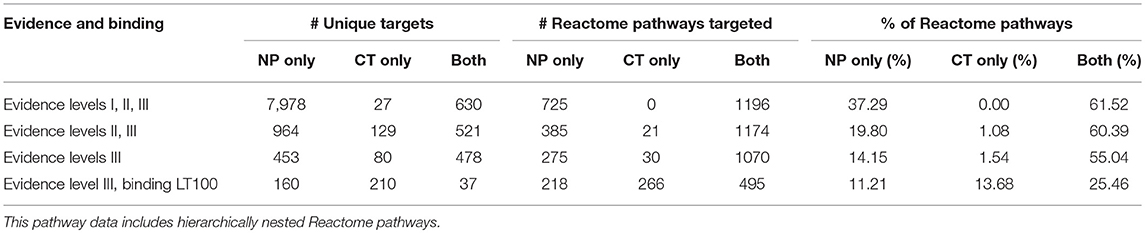
Table 4. Consideration of natural product targets increases both pathway and target coverage in all Reactome pathways.
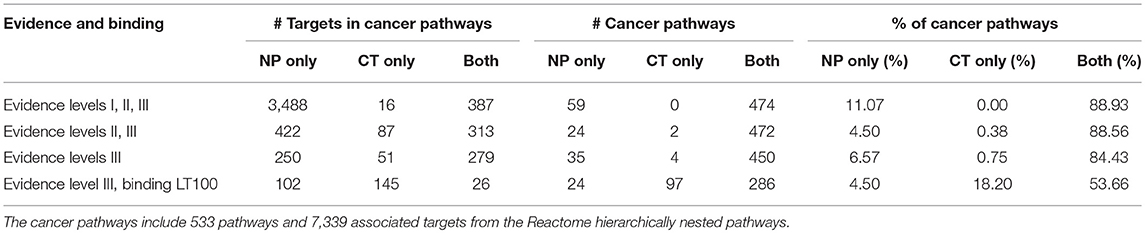
Table 5. Consideration of natural product targets increases both pathway and target coverage in cancer pathways.
When considering the NP contributions within pathways targeted by both NPs and CT drugs, 51% of the individual target interactions in the 1,196 Reactome pathways were with NPs only. For the 495 Reactome pathways targeted by both at the 100 nM level, 43% of the target interactions were with NPs only. For cancer pathways targeted with affinities < 100 nM there was a high degree of overlap by compound classes at the pathway level, but very little overlap at the target level (Figure 3). Not only were the number of pathways targeted increased when considering the NP target space, but coverage of targets within pathways was increased in those pathways already targeted by CT drugs.
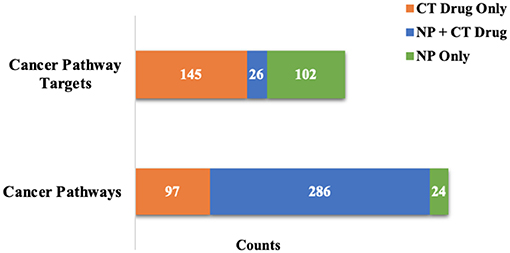
Figure 3. Evaluation of cancer pathway overlap at affinities <100 nM. There is a high level of pathway overlap between those targeted by NPs and CT drugs, but little overlap at the individual target level.
When considering all of the targets contained in both target networks, a large number of interactions were with NPs only (Table 4). We found the same result for the 7,339 targets associated with the aberrational cancer pathways (Table 5). The vast majority of these interactions were Level I evidence from the TarNet database. At the stronger binding affinity (< 100 nM), there is a relative increase in target coverage of 65% for all targets from the two networks, and 60% for targets from the aberrational cancer pathways.
Target interactions were then assessed for the 837 cancer drivers, which are then mapped back to their associated tumor types. There were twelve tumor types for which NPs increased driver coverage (Table 6). For these tumor types, five cancer drivers were targeted only by NPs at < 100 nM, including Adenylate Cyclase 1 (ADCY1), Matrix Metallopeptidase 2 (MMP2), Aryl Hydrocarbon Receptor (AHR), Cyclin Dependent Kinase 2 (CDK2), and Mitogen-Activated Protein Kinase 11 (MAP3K11). Some of the plant derived NPs targeting these drivers include forskolin (from the Indian coleus plant, ADCY1), caffeic acid (found in many foods including coffee, MMP2), kaempferol (found in many common foods including apples, grapes, tomatoes and green tea, AHR), and flavopiridol (a semi-synthetic derivative from the Pithraj tree, CDK2). MAP3K11 is targeted by staurosporinone, which is of bacterial origin so is out of the scope of the NP definition for this paper. While there is a significant body of in-vitro cancer research associated with these natural products, there are 65 clinical trials listed for flavopiridol, many of which are either single agent or combination trials for cancer2.
There were 24 pathways in Reactome that were enriched for cancer drivers, had no CT drug interactions at < 100 nM, and had at least one target interaction with a NP with a binding affinity < 100 nM. In some of these pathways, such as “RUNX1 regulates transcription of genes involved in differentiation of myeloid cells,” the NP interaction was not with a driver gene (Supplemental S2). In this example, the interacting NP is a phorbol ester isolated from croton oil. Phorbol esters have been called “double-edged swords,” showing both tumor promoting and tumor inhibiting activities, depending on the cancer (Goel et al., 2007). This pathway is involved with the differentiation of myeloid progenitors and also with apoptosis of mature myeloid cells (Ntie-Kang et al., 2013). The three driver genes in this pathway are associated with over 20 tumor types, including acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin's lymphoma. In other pathways, such as “TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest,” the NP interaction was directly with a driver gene (Figure 4). The interacting NP for this example is flavopiridol, which is a semisynthetic derivative of a compound extracted from the Pithraj tree. This compound is already in numerous clinical trials for several cancer types (Tse et al., 2016). This pathway primarily inhibits the cell cycle transition from the G1 phase to the S phase through multiple mechanisms (Ntie-Kang et al., 2013). Over 40 tumor types are associated with the five cancer drivers in this pathway, including breast, bladder, esophagus, head and neck, lung, prostate, ovary, and hepatocellular.
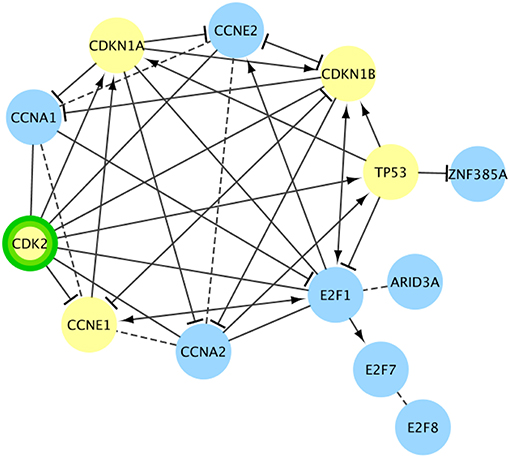
Figure 4. Cancer pathway gene set targeted only by natural products for a cancer driver target. Another Reactome functional interaction network (TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest) targeted only by NPs at <100 nM (green border). No FDA-approved cancer drugs target this pathway with < 100 nM evidence. Cancer drivers are shown in yellow. In this pathway the NP target is also a cancer driver. Dashed lines are predicted interactions.
There were also 286 pathways in Reactome that were enriched for cancer drivers that were targeted by both NPs and CT drugs at binding affinities < 100 nM. One smaller pathway example of this is “MAPK3 (ERK1) activation” (Supplemental S3). The two NP compounds targeting the three proteins in this pathway are flavopiridol, and arctigenin, which is extracted from the burdock plant. This pathway is involved in a wide range of cellular processes, including cytoskeleton remodeling, proliferation, differentiation and regulation of inflammatory responses (Ntie-Kang et al., 2013). Over 20 tumor types are associated with the four cancer drivers found in this pathway, including leukemias, lung cancers, colorectal cancer, melanoma, and head and neck squamous cell cancer.
We found significant differences between the distributions of the number of targets and pathways interacting per compound for NPs vs. CT drugs. Additionally, we found that the significant distribution differences for tumor types per compound and cancer driver interactions per compound were dependent on the binding affinity threshold (Table 7). But it is difficult to draw any further conclusions about these differences, since the motivations for studying the relationships are not necessarily the same between NPs and FDA-approved cancer drugs. Different experiments could be designed to compare promiscuity by running matched assay panels on both NPs and FDA-approved drugs. Previous research has shown an increasing number of target interactions per compound as they progress along the drug development pipeline, with approved drugs having the highest number of interactions (Hu and Bajorath, 2013; Hu et al., 2014). Importantly, we note that the total number of targets tested for each of these compounds (for both NPs and CT drugs), is not captured in the public data resources used for this project. This information is important both to explain therapeutic polypharmacological effects and undesirable adverse events. Finally, there is always the problem of incomplete data for this characteristic since all possible target interactions that occur in a biological context cannot be known.
Natural products show much potential for combination therapy in cancer treatment; but barriers in the public domain of natural product-target interactions make it difficult to harness this information in a high throughput manner. Here, we applied an evidence framework to public domain natural product information and quantitatively assessed the added value of natural product space when used in conjunction with approved cancer drug space. Natural products increase the cancer-relevant targetable space from both a target and a pathway perspective, as well as providing additional coverage of key cancer driver genes. Given current challenges in acquired drug resistance to approved cancer drugs, the inclusion of natural products in combination therapies may allow us to broaden our scope of targetable cancer-relevant pathways for therapeutic advantage.
We have demonstrated that the projection of this natural product target network into various biological contexts can identify candidate natural products for cancer treatment, and provided examples with substantial support in the literature. This previous research is most likely the source of some of the public target data we used. To test the ability of this network to identify novel natural product cancer candidate treatments that have not been previously reported, we are projecting these network targets into molecular pathways associated with head and neck oral squamous cell cancer (HNSCC), identified by using the HNSCC cohort data from The Cancer Genome Atlas. Further, we are testing cell viability of patient derived HNSCC tumor cells exposed to over 160 cancer drugs and natural products. In preliminary analyses we identified two natural products among the top five compounds to which one patient's tumor cells were sensitive, the other three being cancer drugs approved or under development (Vigoda, unpublished). These findings are encouraging for the potential of these public data, efficiently mined through the methods detailed herein, to reveal new natural product candidates for cancer treatment.
The natural product-target interaction space in the public domain still faces challenges that will need addressing in order to more fully harness the therapeutic potential of natural products. For instance, our work here brought attention to several key quality issues in publicly available resources for natural product–target interaction data. The two databases chosen, TarNet and TCMID, are not the only available for NPs, but they were appropriate choices given our criteria for defining natural products. The heavy reliance on text mining for these resources could explain some of the data quality issues that we encountered. For instance, some of these data sources appear to contain drugs that do not have a “true” NP source, or include compounds used in the extraction of these NP compounds. For this reason, we removed any compounds with a synonym match to drugs using FDA and DrugBank compound lists. We also manually curated our high binding affinity natural product compounds and removed about 11% due to quality concerns, such as the inclusion of synthetic compounds. Another issue encountered with these data sources was the inability to download the entire database from the website. There are well-curated proprietary natural product databases, such as NapAlert and The Dictionary of Natural Products, for future application of these methods and findings beyond our current analyses including only public resources.
While we did find an increase in relevant target and pathway coverage by considering NP target interactions in public data sources, it is also important to consider and compare these two target spaces (CT and NP) in a biological context. We used two networks to do this, the Reactome network and the cancer-specific network. Of these two, the Reactome network is more comprehensive. In this network, the new targets identified by considering NPs were similar to drug-targeted nodes and less similar to non-targeted nodes, when considering betweenness and degree. This similarity was not seen for eigenvector centrality. Degree and betweenness are strong measures of criticality in a network and the fact that the new NP targets are similar to CT drug targets could indicate potential for novel combination therapies. Also, of interest is that the nodes targeted by both NPs and CT drugs tended to have higher average values for these measures than either CT drug only or NP only targets.
In general, biological networks are scale-free, meaning that many nodes have low connectivity and are not critical to the function of the network, but some nodes are highly connected and more critical, possibly making good therapeutic targets. Topology measures can guide the identification of these critical nodes. Both degree and betweenness are considered measures of strong importance to the network, but nodes with high values of either can be considered too lethal or toxic to target (Hwang et al., 2008; Arrell and Terzic, 2010; Winterbach et al., 2013; Peng and Schork, 2014). It is possible to identify nodes with network influence that have lower essentiality (Winterbach et al., 2013) by using measures such as eigenvector centrality, bridging centrality, and others (Hwang et al., 2008; Arrell and Terzic, 2010; Peng and Schork, 2014). Eigenvector centrality is a measure of the connectivity of the nodes that are connected to the node of interest. Bridging centrality, developed by Hwang et al. is a measure of how well a node connects separate modular sub-regions in a network. Other strategies for reducing adverse effects consider targets that influence critical nodes without targeting the critical nodes themselves (Peng and Schork, 2014; Laderas et al., 2015). For this reason, assessing the shortest path distances from critical nodes, such as cancer driver genes, is important. This distance could also be considered an estimate of the number of molecular steps from this node (Yildirim et al., 2007). It has also been shown that cancer driver genes have topology measures in biological networks that are distinctly different from other nodes. These differences include higher degree and betweenness, shorter paths between them and weaker clustering coefficients (Sun and Zhao, 2010; Xia et al., 2011; Winterbach et al., 2013).
Biological network topology has also been used to predict therapeutic synergy between compounds, an aspect of interest for designing novel combination therapies (Yin et al., 2014). Several in-silico methods for prediction of synergy in combination therapy use topology measures (Li et al., 2011; Huang et al., 2014; Sun et al., 2015). Generally, higher values of measures, such as degree and betweenness, are preferred in these models.
The clustering patterns seen in the larger communities in the compound-compound network seem to indicate NP target interaction research driven by known therapeutic targets as seen in drug therapeutic classifications. Since the only therapeutic classification for the drugs used in this study is cancer, it follows that there is one large cluster containing a majority of the CT drugs and also some NPs, indicating interest in NPs for cancer research. If this analysis were expanded to include drugs from other therapeutic classifications, the other communities might also contain the associated drugs, along with the NPs. The drugs from these other therapeutic classifications could also be investigated for cancer therapies. In this study, we did not investigate non-cancer drugs that interact with the targets found to interact only with NPs. That we saw compound clustering around target families common to NPs and CT drugs, and also clustering around families unique to NPs, when compared to CT drugs, supports the potential of complementary therapeutic relationships between the two compound classifications.
From the perspective of combination therapy and synergy discovery, the focus of this research was on a pharmacodynamic framework. There is a large body of literature for predictive algorithms for combination therapies that is based on network pharmacology, which influenced this work. Many of these methods use compound-target networks, such as what we have developed for natural products, to derive the predictive features used in these models. However, NPs can also interact with compounds, either synergistically, additively, or antagonistically, through other mechanisms. These can include pharmacokinetic interactions, drug efflux transporter interactions, and other cell microenvironment interactions. Furthermore, some synergy effects of NPs are not mediated by direct interaction with a protein target, but through the up- or down-regulation of the expression of the protein of interest. Since binding assay results do not necessarily provide functional information about the compound-target interaction, the presence of a strong binding assay may not necessarily indicate a positive therapeutic effect for cancer. In fact, some of the interactions could promote tumor growth, as is the case in some cancers whose growth can be stimulated with phorbol esters isolated from croton oil (Goel et al., 2007). Future efforts should consider both the strength of compound-target binding and the functional effect of the interaction. All of these mechanisms could be considered in future algorithms to predict combination therapies.
Overall, the intent of this work was to assess both the natural product therapeutically targetable space and also to assess the potential complementarity of natural products with FDA-approved cancer drugs. As discussed previously, while we were often not able to verify the direct link between supporting literature and the large amount of Level I interactions due to accessibility issues, our random sampling of this space indicates that a sizeable portion of this Level I evidence is actually supported by literature and/or binding assay values. This presents a high need and actionable area for natural product-target curation. Improved curation and accessibility of natural product-target interactions will greatly enable efforts to harness natural products in the cancer therapeutic space. While further work is needed to improve the quality of evidence associated with natural product target interactions, our work here shows that there is a clear benefit to the inclusion of natural products along with FDA-approved cancer drugs in this effort.
SC, AB, and SM conceived of the presented idea. SC and AB developed the framework. SC, AB, and GC conducted the analyses. SC, AB, GW, LS, GC, MK-M, and SM contributed to the study design, development of analysis plan, as well as development and refinement of the manuscript.
This work was supported by National Library of Medicine Informatics Training Grant T15LM007088 (SC and AB), National Cancer Institute1R01CA192405 (GC, MK-M, and SM), National Human Genome Research Institute2U41HG003751 (GW), and National Center for Advancing Translational Sciences5UL1TR000128 (GW and SM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Dr. Rachel Dresbeck for her discussions and input on the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00557/full#supplementary-material
1. ^National Cancer Institute. Types of Treatment. Available online at: https://www.cancer.gov/about-cancer/treatment/types
2. ^ClinicalTrials.gov. Available online at: https://clinicaltrials.gov
Al-Lazikani, B., Banerji, U., and Workman, P. (2012). Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 30, 679–692. doi: 10.1038/nbt.2284
Arrell, D. K., and Terzic, A. (2010). Network systems biology for drug discovery. Clin. Pharmacol. Ther. 88, 120–125. doi: 10.1038/clpt.2010.91
Awan, A., Bari, H., Yan, F., Moksong, S., Yang, S., Chowdhury, S., et al. (2007). Regulatory network motifs and hotspots of cancer genes in a mammalian cellular signalling network. IET Syst. Biol. 1, 292–297. doi: 10.1049/iet-syb:20060068
Becker, M. S., Schmezer, P., Breuer, R., Haas, S. F., Essers, M. A., Krammer, P. H., et al. (2014). The traditional Chinese medical compound Rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression. Cell Death Dis. 5:e1000. doi: 10.1038/cddis.2013.528
Benjamini, Y., and Yekutieli, D. (2001). The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188. doi: 10.1214/aos/1013699998
Blucher, A. S., Choonoo, G., Kulesz-Martin, M., Wu, G., and McWeeney, S. K. (2017). Evidence-based precision oncology with the cancer targetome. Trends Pharmacol. Sci. 38, 1085–1099. doi: 10.1016/j.tips.2017.08.006
Chatr-Aryamontri, A., Breitkreutz, B. J., Oughtred, R., Boucher, L., Heinicke, S., Chen, D., et al. (2015). The BioGRID interaction database: 2015 update. Nucleic Acids Res. 43, D470–D478. doi: 10.1093/nar/gku1204
Chen, D., Zhang, H., Lu, P., Liu, X., and Cao, H. (2016). Synergy evaluation by a pathway-pathway interaction network: a new way to predict drug combination. Mol. Biosyst. 12, 614–623. doi: 10.1039/C5MB00599J
Cheng, Y. T., Yang, C. C., and Shyur, L. F. (2016). Phytomedicine-modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 114, 128–143. doi: 10.1016/j.phrs.2016.10.022
Cote, B., Carlson, L. J., Rao, D. A., and Alani, A. W. G. (2015). Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J. Control. Release 213, 128–133. doi: 10.1016/j.jconrel.2015.06.040
Cui, Q., Ma, Y., Jaramillo, M., Bari, H., Awan, A., Yang, S., et al. (2007). A map of human cancer signaling. Mol. Syst. Biol. 3:152. doi: 10.1038/msb4100200
Druker, B. J. (2009). Perspectives on the development of imatinib and the future of cancer research. Nat. Med. 15, 1149–1152. doi: 10.1038/nm1009-1149
Fabregat, A., Sidiropoulos, K., Garapati, P., Gillespie, M., Hausmann, K., Haw, R., et al. (2017). The reactome pathway knowledgebase. Nucleic Acids Res. 44, D481–D487. doi: 10.1093/nar/gkv1351
Gilson, M. K., Liu, T., Baitaluk, M., Nicola, G., Hwang, L., and Chong, J. (2016). BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 44, D1045–D1053. doi: 10.1093/nar/gkv1072
Goel, G., Makkar, H. P., Francis, G., and Becker, K. (2007). Phorbol esters: structure, biological activity, and toxicity in animals. Int. J. Toxicol. 26, 279–288. doi: 10.1080/10915810701464641
Gu, J., Gui, Y., Chen, L., Yuan, G., Lu, H. Z., and Xu, X. (2013). Use of natural products as chemical library for drug discovery and network pharmacology. PLoS ONE 8:e62839. doi: 10.1371/journal.pone.0062839
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Hallmarks of cancer: the next generation Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Harding, S. D., Sharman, J. L., Faccenda, E., Southan, C., Pawson, A. J., Ireland, S., et al. (2017). The IUPHAR/BPS Guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 46, D1091–D1106. doi: 10.1093/nar/gkx1121
Harvey, A. L., Edrada-Ebel, R., and Quinn, R. J. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14, 111–129. doi: 10.1038/nrd4510
Hinemash6. (2010). Imatinib Chemical Structure.PNG. Available online at: https://commons.wikimedia.org/w/index.php?curid=11965288 (accessed December, 2018).
Holohan, C., Van Schaeybroeck, S., Longley, D. B., and Johnston, P. G. (2013). Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726. doi: 10.1038/nrc3599
Hopkins, A. L. (2008). Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690. doi: 10.1038/nchembio.118
Housman, G., Byler, S., Heerboth, S., Lapinska, K., Longacre, M., Snyder, N., et al. (2014). Drug resistance in cancer: an overview. Cancers (Basel). 6, 1769–1792. doi: 10.3390/cancers6031769
Hu, R., Ren, G., Sun, G., and Sun, X. (2016). TarNet: an evidence-based database for natural medicine research. PLoS ONE 11:e0157222. doi: 10.1371/journal.pone.0157222
Hu, Y., and Bajorath, J. (2013). High-resolution view of compound promiscuity. F1000Research 2:144. doi: 10.12688/f1000research.2-144.v1
Hu, Y., Gupta-Ostermann, D., and Bajorath, J. (2014). Exploring compound promiscuity patterns and multi-target activity spaces. Comput. Struct. Biotechnol. J. 9:e201401003. doi: 10.5936/csbj.201401003
Huang, L., Li, F., Sheng, J., Xia, X., Ma, J., Zhan, M., et al. (2014). DrugComboRanker: drug combination discovery based on target network analysis. Bioinformatics 30, i228–i236. doi: 10.1093/bioinformatics/btu278
Hwang, W. C., Zhang, A., and Ramanathan, M. (2008). Identification of information flow-modulating drug targets: a novel bridging paradigm for drug discovery. Clin. Pharmacol. Ther. 84, 563–572. doi: 10.1038/clpt.2008.129
Jaeger, S., Igea, A., Arroyo, R., Alcalde, V., Canovas, B., Orozco, M., et al. (2017). Quantification of pathway cross-talk reveals novel synergistic drug combinations for breast cancer. Cancer Res. 77, 459–469. doi: 10.1158/0008-5472.CAN-16-0097
Jia, J., Zhu, F., Ma, X., Cao, Z., Cao, Z. W., Li, Y., et al. (2009). Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov. 8, 111–128. doi: 10.1038/nrd2683
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Laderas, T. G., Heiser, L. M., and Sönmez, K. (2015). A network-based model of oncogenic collaboration for prediction of drug sensitivity. Front. Genet. 6:341. doi: 10.3389/fgene.2015.00341
Law, V., Knox, C., Djoumbou, Y., Jewison, T., Guo, A. C., Liu, Y., et al. (2014). DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 42, D1091–D1097. doi: 10.1093/nar/gkt1068
Li, L., Tibiche, C., Fu, C., Kaneko, T., Moran, M. F., Schiller, M. R., et al. (2012). The human phosphotyrosine signaling network: evolution and hotspots of hijacking in cancer. Genome Res. 22, 1222–1230. doi: 10.1101/gr.128819.111
Li, S., Zhang, B., and Zhang, N. (2011). Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 5(Suppl. 1):S10. doi: 10.1186/1752-0509-5-S1-S10
Liu, Y., and Zhao, H. (2016). Predicting synergistic effects between compounds through their structural similarity and effects on transcriptomes. Bioinformatics 32, 3782–3789. doi: 10.1093/bioinformatics/btw509
Mahira, B., and Umehara, T. (2018). The Noun Project. Available online at: https://thenounproject.com (accessed March, 2018).
Milshteyn, A., Schneider, J. S., and Brady, S. F. (2014). Mining the metabiome: identifying novel natural products from microbial communities. Chem. Biol. 21, 1211–1223. doi: 10.1016/j.chembiol.2014.08.006
Mykhal. (2004). Artemisinin 1.png. Available online at: https://commons.wikimedia.org/wiki/File:Artemisinin_1.png (accessed December, 2018).
Nalli, A. D., Brown, L. E., Thomas, C. L., Sayers, T. J., Porco, J. A., and Henrich, C. J. (2018). Sensitization of renal carcinoma cells to TRAIL-induced apoptosis by rocaglamide and analogs. Sci. Rep. 8:17519. doi: 10.1038/s41598-018-35908-0
Newman, D. J., and Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. doi: 10.1021/acs.jnatprod.5b01055
Newman, R. H., Hu, J., Rho, H. S., Xie, Z., Woodard, C., Neiswinger, J., et al. (2013). Construction of human activity-based phosphorylation networks. Mol. Syst. Biol. 9:655. doi: 10.1038/msb.2013.12
Ntie-Kang, F., Zofou, D., Babiaka, S. B., Meudom, R., Scharfe, M., Lifongo, L. L., et al. (2013). AfroDb: a select highly potent and diverse natural product library from African medicinal plants. PLoS ONE 8:e78085. doi: 10.1371/journal.pone.0078085
Palmer, A. C., and Sorger, P. K. (2017). Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell 171, 1678–1691.e13. doi: 10.1016/j.cell.2017.11.009
Paolini, G. V., Shapland, R. H., van Hoorn, W. P., Mason, J. S., and Hopkins, A. L. (2006). Global mapping of pharmacological space. Nat. Biotechnol. 24, 805–815. doi: 10.1038/nbt1228
Pavlopoulos, G. A., Secrier, M., Moschopoulos, C. N., Soldatos, T. G., Kossida, S., Aerts, J., et al. (2011). Using graph theory to analyze biological networks. BioData Min. 4:10. doi: 10.1186/1756-0381-4-10
Pearson, H. E., Iida, M., Orbuch, R. A., McDaniel, N. K., Nickel, K. P., Kimple, R. J., et al. (2017). Overcoming resistance to cetuximab with honokiol, a small-molecule polyphenol. Mol. Cancer Ther. 17, 204–214. doi: 10.1158/1535-7163.MCT-17-0384
Peng, Q., and Schork, N. J. (2014). Utility of network integrity methods in therapeutic target identification. Front. Genet. 5:12. doi: 10.3389/fgene.2014.00012
Qin, C., Zhang, C., Zhu, F., Xu, F., Chen, S. Y., Zhang, P., et al. (2014). Therapeutic target database update 2014: a resource for targeted therapeutics. Nucleic Acids Res. 42, D1118–D1123. doi: 10.1093/nar/gkt1129
Sun, J., and Zhao, Z. (2010). A comparative study of cancer proteins in the human protein-protein interaction network. BMC Genomics 11(Suppl. 3):S5. doi: 10.1186/1471-2164-11-S3-S5
Sun, Y., Sheng, Z., Ma, C., Tang, K., Zhu, R., Wu, Z., et al. (2015). Combining genomic and network characteristics for extended capability in predicting synergistic drugs for cancer. Nat. Commun. 6:8481. doi: 10.1038/ncomms9481
Tamborero, D., Rubio-Perez, C., Deu-Pons, J., Schroeder, M. P., Vivancos, A., Rovira, A., et al. (2017). Cancer genome interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. 10:25. doi: 10.1101/140475
Traditional Chinese Medicine Integrated Database (2018). Available online at: http://www.megabionet.org/tcmid/ (accessed October, 2017).
Tse, K. Y., Lau, S. K., Lui, M. W., Ip, P. P., Cheung, A. N., and Ngan, H. Y. (2016). Patient compliance with cervical smear surveillance in a shared-care setting. Int. J. Gynaecol. Obstet. 135, 177–181. doi: 10.1016/j.ijgo.2016.04.012
Wang, E. (2014). Human Signaling Network. Available online at: http://www.bri.nrc.ca/wang/ (accessed October, 2017).
Wang, Y., Cornett, A., King, F. J., Mao, Y., Nigsch, F., Paris, C. G., et al. (2016). Evidence-based and quantitative prioritization of tool compounds in phenotypic drug discovery. Cell Chem. Biol. 23, 862–874. doi: 10.1016/j.chembiol.2016.05.016
Winterbach, W., Van Mieghem, P., Reinders, M., Wang, H., and de Ridder, D. (2013). Topology of molecular interaction networks. BMC Syst. Biol. 7:90. doi: 10.1186/1752-0509-7-90
Wohlgemuth, G., Haldiya, P. K., Willighagen, E., Kind, T., and Fiehn, O. (2010). The chemical translation service–a web-based tool to improve standardization of metabolomic reports. Bioinformatics 26, 2647–2648. doi: 10.1093/bioinformatics/btq476
Wu, G., Feng, X., and Stein, L. (2010). A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 11:R53. doi: 10.1186/gb-2010-11-5-r53
Wu, Y., Giaisi, M., Köhler, R., Chen, W. M., Krammer, P. H., and Li-Weber, M. (2017). Rocaglamide breaks TRAIL-resistance in human multiple myeloma and acute T-cell leukemia in vivo in a mouse xenogtraft model. Cancer Lett. 389, 70–77. doi: 10.1016/j.canlet.2016.12.010
Xia, J., Sun, J., Jia, P., and Zhao, Z. (2011). Do cancer proteins really interact strongly in the human protein-protein interaction network? Comput. Biol. Chem. 35, 121–125. doi: 10.1016/j.compbiolchem.2011.04.005
Xue, R., Fang, Z., Zhang, M., Yi, Z., Wen, C., and Shi, T. (2013). TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 41, D1089–D1095. doi: 10.1093/nar/gks1100
Yang, Z., Algesheimer, R., and Tessone, C. J. (2016). A comparative analysis of community detection algorithms on artificial networks. Sci. Rep. 6:30750. doi: 10.1038/srep30750
Yao, C., Ni, Z., Gong, C., Zhu, X., Wang, L., Xu, Z., et al. (2018). Rocaglamide enhances NK cell-mediated killing of non-small cell lung cancer cells by inhibiting autophagy. Autophagy 14, 1831–1844. doi: 10.1080/15548627.2018.1489946
Yildirim, M. A., Goh, K. I., Cusick, M. E., Barabási, A. L., and Vidal, M. (2007). Drug-target network. Nat. Biotechnol. 25, 1119–1126. doi: 10.1038/nbt1338
Yin, N., Ma, W., Pei, J., Ouyang, Q., Tang, C., and Lai, L. (2014). Synergistic and antagonistic drug combinations depend on network topology. PLoS ONE 9:e93960. doi: 10.1371/journal.pone.0093960
Keywords: natural product, antineoplastic drug, cancer, synergy, therapeutic targets
Citation: Chamberlin SR, Blucher A, Wu G, Shinto L, Choonoo G, Kulesz-Martin M and McWeeney S (2019) Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 10:557. doi: 10.3389/fphar.2019.00557
Received: 02 February 2019; Accepted: 03 May 2019;
Published: 31 May 2019.
Edited by:
Brion William Murray, Pfizer, United StatesReviewed by:
Marco Falasca, Curtin University, AustraliaCopyright © 2019 Chamberlin, Blucher, Wu, Shinto, Choonoo, Kulesz-Martin and McWeeney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven R. Chamberlin, Y2hhbWJlc3RAb2hzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.