- 1Center of Endocrinology, Genetics and Metabolism, National Center for Children’s Health, Beijing Children’s Hospital, Capital Medical University, Beijing, China
- 2Department of Endocrinology, Shenzhen Children’s Hospital, Shenzhen, China
- 3Genetic and Metabolic Central Laboratory, Maternal and Children Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 4Department of Endocrinology, Shanghai Children’s Medical Center, Shanghai Jiao Tong University, Shanghai, China
- 5Department of Endocrinology, Children’s Hospital of Fudan University, Fudan University, Shanghai, China
- 6Department of Endocrinology, Jiangxi Provincial Children’s Hospital, Nanchang, China
- 7Department of Endocrinology, Children’s Hospital of Soochow University, Suzhou, China
- 8Department of Endocrinology, Fuzhou Children’s Hospital, Fuzhou, China
- 9Department of BME, Capital Medical University, Beijing, China
Background: 5α-reductase type 2 deficiency (5αRD) is an autosomal recessive hereditary disease of the group of 46, XY disorders of sex development (DSD).
Objective: To study the growth pattern in Chinese pediatric patients with 5αRD.
Subjects: Data were obtained from 141 patients with 5αRD (age: 0–16 years old) who visited eight pediatric endocrine centers from January 2010 to December 2017.
Methods: In this retrospective cohort study, height, weight, and other relevant data were collected from the multicenter hospital registration database. Baseline luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone (T), and dihydrotestosterone (DHT) after human chorionic gonadotropin (HCG) stimulation test were measured by enzyme enhanced chemiluminescence assay. Bone age (BA) was assessed using the Greulich-Pyle (G-P) atlas. Growth curve was constructed based on λ-median-coefficient of variation method (LMS).
Results: The height standard deviation scores (HtSDS) and weight standard deviation scores (WtSDS) in 5αRD children were in the normal range as compared to normal boys. Significantly higher HtSDS was observed in patients with 5αRD who were <1 year old (t = 3.658, 2.103, P = 0.002, 0.048, respectively), and higher WtSDS in those <6 months old (t = 2.756, P = 0.012). Then HtSDS and WtSDS decreased gradually and fluctuated near the median of the same age until 13 years. WtSDS in 5αRD children from northern China were significantly higher than those from the south (Z = -2.670, P = 0.008). The variation tendency of HtSDS in Chinese 5αRDs was consistent with the trend of stimulating T. HtSDS and stimulating T in the external masculinization score (EMS) <7 group were slightly higher than those in EMS ≥ 7 group without significant difference. Additionally, the ratio of BA over chronological age (BA/CA) was significantly <1 in children with 5αRD.
Conclusion: Children with 5αRD had a special growth pattern that was affected by high levels of T, while DHT played a very small role in it. Their growth accelerated at age <1 year, followed by slowing growth and fluctuating height near normal median boys’ height. The BA was delayed in 5αRD children. Androgen treatment, which may be considered anyway for male 5αRD patients with a micropenis, may also be beneficial for growth.
Highlights
- The first growth curve in children with 5αRD.
- Our study provided evidence for applying androgen products at specific time periods for 5αRD patients with a micropenis.
Introduction
Sex hormones are synthesized in the gonads and adrenal glands (Shen, 2016) and have bioactivity within bone and other target tissues (Vanderschueren et al., 2014). Their biological effects on bone are mediated by different cell types and mechanisms (Almeida et al., 2017) and controlled by gonadotropins via hypothalamic-pituitary feedback. Before puberty, longitudinal bone growth shows no significant sex differences (Nishiyama et al., 2012). During puberty, estrogen shows the biphasic regulation of longitudinal bone growth and epiphyseal closure. Early in puberty, estrogen at low concentrations can stimulate longitudinal growth via indirect effects on GH and insulin-like growth factor I (IGF-I), both of which stimulate growth plate chondrocytes (Veldhuis et al., 2005b, 2011; Almeida et al., 2017). However, in late puberty, higher levels of estrogen can exert inhibitory effects on the growth plate via estrogen receptors in the chondrocytes. Androgens mainly have direct effects on GH and show the influence on circulating IGF-I via peripheral and central aromatization (Veldhuis et al., 2005a, 2009). Whether androgen receptor in chondrocytes contributes to sex differences in longitudinal growth remains unclear. Thus, sex hormones have an essential role for male and female growth.
Disorders of sex development are defined as congenital conditions associated with atypical development of gonadal, chromosomal, or anatomical sex, such as androgen insensitivity syndrome (AIS) (Wang et al., 2017) and 5αRD. With the rapid development of next-generation sequencing and the popularity of precision medicine, DSD as one of rare diseases can be diagnosis earlier and more accurately (Jia and Shi, 2017; Ni and Shi, 2017). Furthermore, different DSDs may have different characteristics and different growth patterns partly owing to different changes in sex hormones. For example, gonadal dysplasia impacts physical development throughout the prenatal period until adulthood (Hughes et al., 2002; Richter-Unruh et al., 2004). In addition, height in children with CAIS who had their gonads removed in the pre-pubertal stage has shown to be lower than those who had it done after the puberty (Han et al., 2008), thus addressing the effect of sex hormones on pre-pubertal height. In our previous study, we found that children with 46, XY DSD are shorter than the normal population (Di et al., 2013). In addition, DSD children with T <100 ng/dL after HCG test are shorter than those with T ≥ 100 ng/dL (Wu et al., 2017). However, published literature on DSD growth is scarce.
5α-reductase type 2 deficiency (OMIM 264600) is an autosomal recessive hereditary disease with an incidence of 11.2–15.5% among patients with 46, XY DSD (Veiga-Junior et al., 2012; Ittiwut et al., 2017) and is caused by loss-of-function mutations of the SRD5A2 gene on chromosome 2. The mutations make the enzyme defective and impair T to DHT conversion (Sultan et al., 2001); therefore, individuals with 5αRD may develop malformation of external genitalia, including pseudovaginalis, ambiguous genitalia, hypospadias, micropenis, and cryptorchid or, in some cases, even a normal phenotype (Choi et al., 2008). Most of the patients with 5αRD are born as females, and more than 50% undergo gender self-reassignment during puberty (Wilson et al., 1993; Kolesinska et al., 2014; Bertelloni et al., 2016; Deeb et al., 2016). While many research studies have focused on gender reassignment (Deeb et al., 2016; Byers et al., 2017; Raveenthiran, 2017), our study investigates the growth and development of 5αRD patients. With the help of growth pattern, doctors can get more information for diagnosis and antidiastole of 5αRD. Perhaps, this will also help in accurate genetic analysis. Thus far, only two studies have reported on a 5αRD growth pattern in children. Ko et al. (2010) examined six Korean children with 5αRD and found a height percentile of P95, P90, and P90th in three cases that did not receive hormone treatment or gonadectomy. Eren et al. (2016) found the HtSDS being -0.31, +0.24, and +0.48 in three untreated Turkish children with 5αRD. In this systemic multicenter study, we aimed to determine the growth of 5αRD children.
Materials and Methods
Patients
All 187 patients with 5αRD (age 0–16 years) and admitted to eight pediatric endocrine centers from January 2010 to December 2017 were included in this retrospective cohort study. Their clinical manifestations included ambiguous genitalia at birth, hypospadias, micropenis, and cryptorchid. The ratio of T over dihydrotestosterone (T/DHT) after HCG stimulation test in all patients fluctuated between 10.67 and 86.56 (M: 32.72). All patients were diagnosed as 5αRD according to their manifestations and ratio of T/DHT >8.5 (Avendano et al., 2018). Then, the diagnosis was confirmed by pathogenic SRD5A2 gene mutations. All patients had 46, XY karyotype and did not require hormonal treatment. The serum T level was >100 ng/dL after HCG stimulation test.
We excluded patients with malformation, abnormal functioning of the liver and kidney, or other with systemic diseases which may affect the physical development. Patients with 17β-hydroxysteroid dehydrogenase type 3, androgen insensitivity syndrome, and other types of 46, XY DSD were excluded by biochemical diagnosis and genetic confirmation. Informed consent for genetic testing was obtained from parents or caregivers of all study subjects.
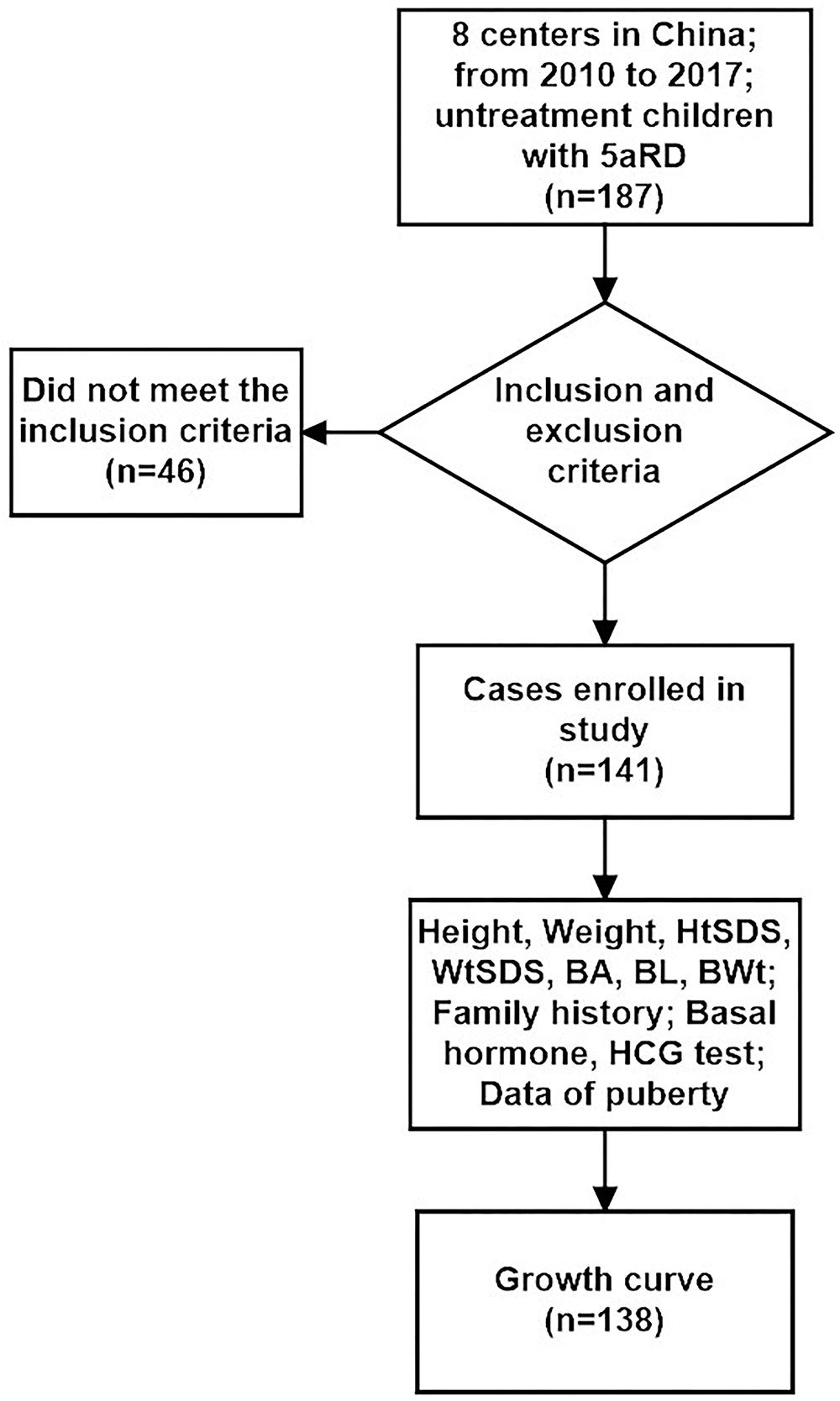
In our study, we enrolled 141 cases and excluded 46 cases according to the inclusion and exclusion criteria. There were no significant differences in initial gender, age, geographical distribution, BWt, BL, BA, LH, FSH, T, and DHT levels after HCG stimulation test between inclusion and exclusion groups (χ2 = 0.983, P = 0.321, t = 1.483, P = 0.140, χ2 = 3.678, P = 0.055, Z = -0.864, P = 0.388, Z = -0.288, P = 0.773, Z = -1.700, P = 0.095, Z = -1.101, P = 0.271, Z = -0.206, P = 0.837, Z = -0.612, P = 0.540, Z = -0.164, P = 0.870, respectively). Also patients with EMS <7 showed no significantly different from those with EMR ≥ 7 between inclusion and exclusion groups (χ2 = 2.841, P = 0.092).
Data Collection
Data on anthropometry, CA, native place, gestational term, BL, BWt, family history, external genitalia and EMS (Ahmed et al., 2000), baseline LH and FSH, BA, T, and DHT levels after HCG stimulation test (Ishii et al., 2015) were recorded. Height was measured as orthostatic height when patients were aged >3 years. Patients aged <3 years were measured based on supine length. For each case, the height and weight were measured three times by experienced nurses, and the average value was considered.
Outcome Measures
The following were main outcomes: HtSDS and WtSDS of different age groups were compared to the control subjects of the same age (Li et al., 2009).
We chose Chinese normal boys as the control group for the following reasons: All patients in the study had the 46, XY karyotype; about 60% of 5αRD children who were assigned the female gender in the period of infancy had marked masculinization and were reassigned as males at puberty (Lee et al., 2006).
HCG Stimulation Test, BA, and Hormone Examination
Bone age, hormonal, and HCG stimulation test data were collected from the Beijing Children’s Hospital. Hormones were tested by enzyme enhanced chemiluminescence assay (Siemens Immulite 2000, Munich, Germany). BA was assessed according to the G-P atlas, by the same endocrinologist based on radiographic films of the left hand.
Growth Curves Plotting
The internationally well accepted method (λ-median-coefficient of variation, LMS) for generating standard curves was adopted to calculate the M, S, and L (after converting the data into a normal distribution, using Box-Cox transformation) (Cole and Green, 1992), which described the growth index in each age band. L, M, and S of smooth curves and the required percentile were calculated using age as an independent variable. Growth curves (P3, P10, P25, P50, P75, P90, P97 percentile curves as well as -2SD, -1SD, 0SD, +1SD, +2SD standard deviation curves) for children in the age groups of 0–36 months and 3–13 years were constructed.
Gene Analysis
All patients underwent genetic testing. Five to ten milliliters of peripheral blood was collected in disposable vacuum tubes for genetic testing. Genomic DNA was isolated using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. After construction according to the standard protocol, whole exon sequencing (100X) of the libraries was performed with SureSelect Human All Exon V6 array on the Illumina HiSeq X Ten Platform with PE150 strategy. To detect the potential variants in the cases, we performed bioinformatic processing and data analysis after receiving the primary sequencing data. Sequence variants were carefully identified with the help of GATK (McKenna et al., 2010) software following the best practice guidelines recommended by GATK (DePristo et al., 2011; Van der Auwera et al., 2013), including local realignment around INDELs, base quality score recalibration, followed by SNVs. INDELs were called simultaneously with the default setting of GATK Unified Genotyper on the realigned and recalibrated reads, followed by SNV and INDEL filtering to eliminate false-positive calls. The pathogenic SRD5A2 gene mutations were confirmed by homozygous or compound heterozygous mutations inherited from parents or de novo. Nucleotide sequences of SRD5A2 gene were compared with the published data. The pathogenicity of unreported SRD5A2 mutations was tested by in silico analysis using two software of “SIFT” (https://sift.bii.a-star.edu.sg/www/code.html) and “Polyphen” (http://genetics.bwh.harvard.edu/pph2/). The interpretation of gene pathogenicity is based on the ACMG (Richards et al., 2015).
Data Analysis
The calculations for the growth curves were performed using LMS-chartmaker Pro software, and curves were drawn using the GraphPad Prism 6 software. SPSS 23.0 software was used for statistical analyses. Data pertaining to quantitative variables were expressed as mean ± SD or quartiles. Intergroup differences between two groups were assessed using the Student’s t-test for normally distributed data and Mann–Whitney U-test for non-normally distributed data. Multiple age group comparisons were assessed using the Kruskal–Wallis H-test and Bonferroni correction. The ratio of BA/CA was assessed using 95% CIs. Intergroup differences between inclusion and exclusion groups were assessed using the Chi-square test for qualitative data. P < 0.05 was considered statistically significant. Geographic difference analysis was based on north and south regions; Qinling Mountains and Huaihe River were selected as geographical boundaries between the south and north.
Results
General Data of Chinese Cases With 5αRD
The initial sex assignment was male in 143/187 (76.5%) and female in 44/187 (23.5%) cases. The clinical presentation was simple micropenis (n = 64, 34.2%); simple hypospadias (n = 43, 23.0%); ambiguous genitalia (n = 37, 19.8%); simple cryptorchid (n = 19, 10.2%); and others (micropenis and/or hypospadias and/or cryptorchid) (n = 24, 12.8%). Only two patients were siblings; the others were unrelated. The details of standard phenotype and gene mutations (Jia and Shi, 2017) were shown in the section of Supplementary Tables 1, 2).
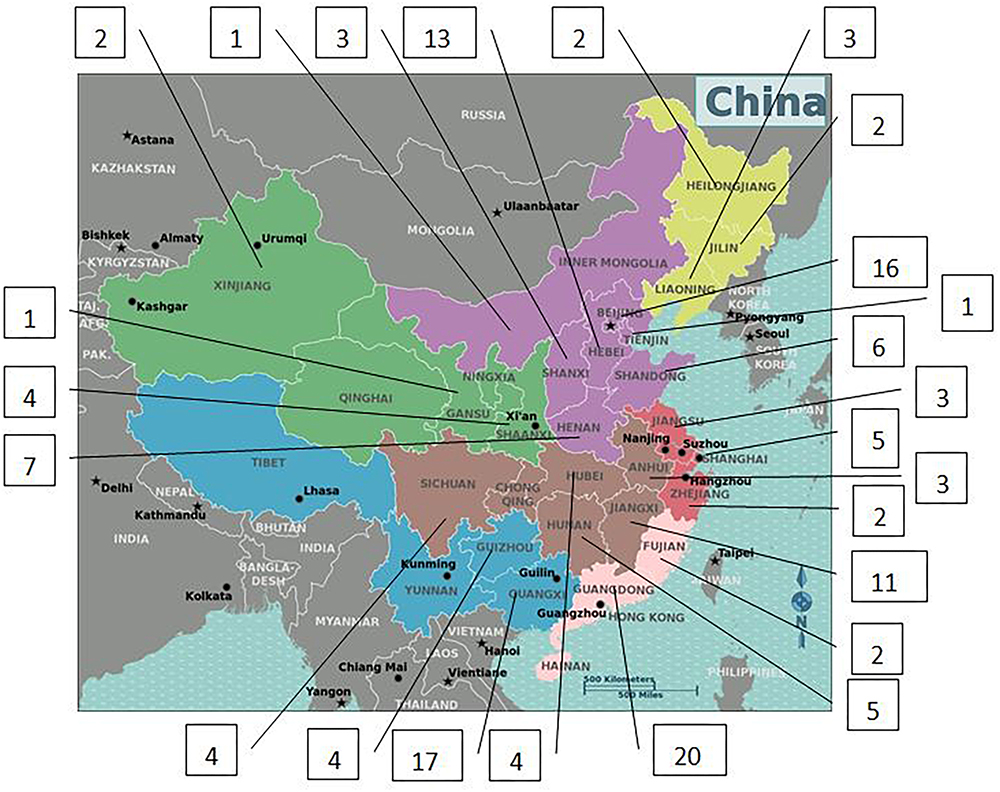
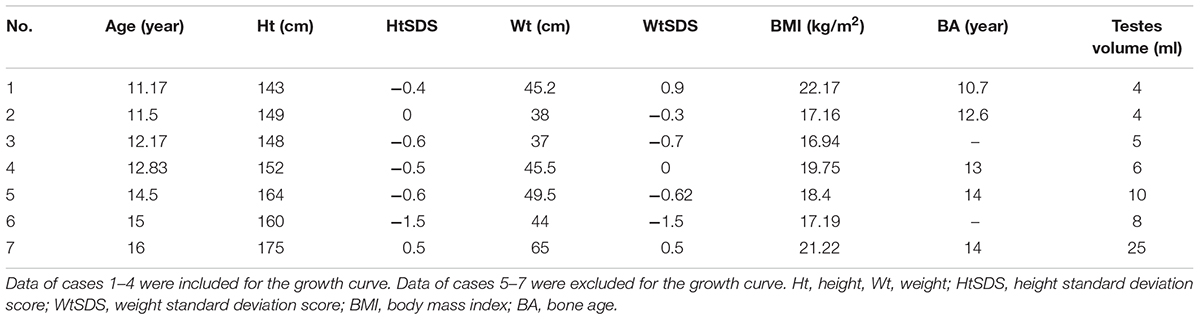
Only 141 of 187 patients with 5αRD who had the data of height and weight and were aged between 0.08 and 16 years (M age: 1.75 years) were enrolled in our study. All 141 children with 5αRD came from 25 provinces and municipalities across China (Figure 1). Seven cases had presentations of puberty, with a testicular volume of ≥4 mL (Table 1). Four cases aged between 10 and 12 years (M age: 11.83 years) were in Tanner stage 2. The other 3 cases aged ≥13 years (M age: 15.16 years) were in Tanner stage 3–5. When plotting growth curve, we enrolled 138 cases aged <13 years because only 3 patients were aged between 13 and 16 years (Table 1, cases 5–7). Patients were divided into the following six groups: 0–5 months, 6–11 months, 1–2 years, 3–5 years, 6–9 years, and 10–12 years. The flow chart of the study is presented in Figure 2.
Physical Parameters of Chinese Children With 5αRD (Table 2)
Height and weight curves are shown in Figures 3–6.
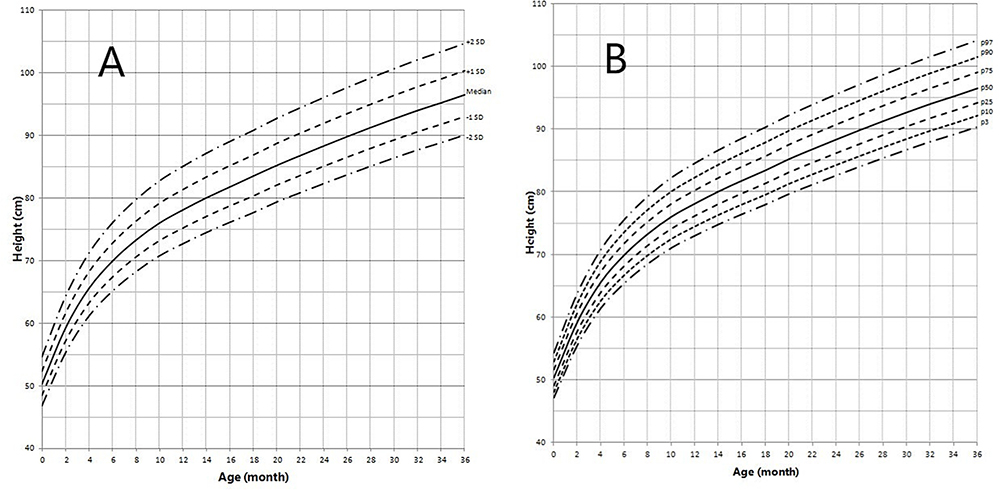
Figure 3. Height in children with 5αRD (0–36 months old). (A) Standard deviation curve; (B) Percentile curve.
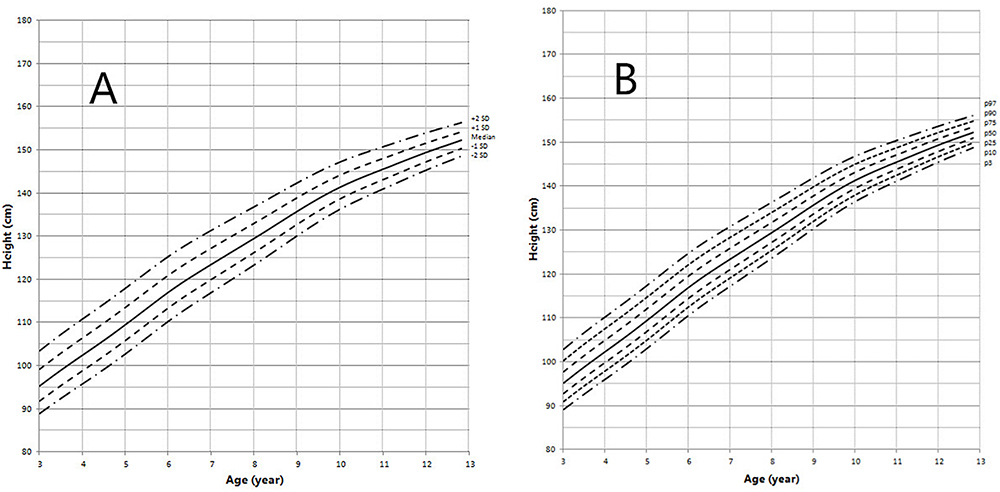
Figure 4. Height in children with 5αRD (3–13 years old). (A) Standard deviation curve; (B) Percentile curve.
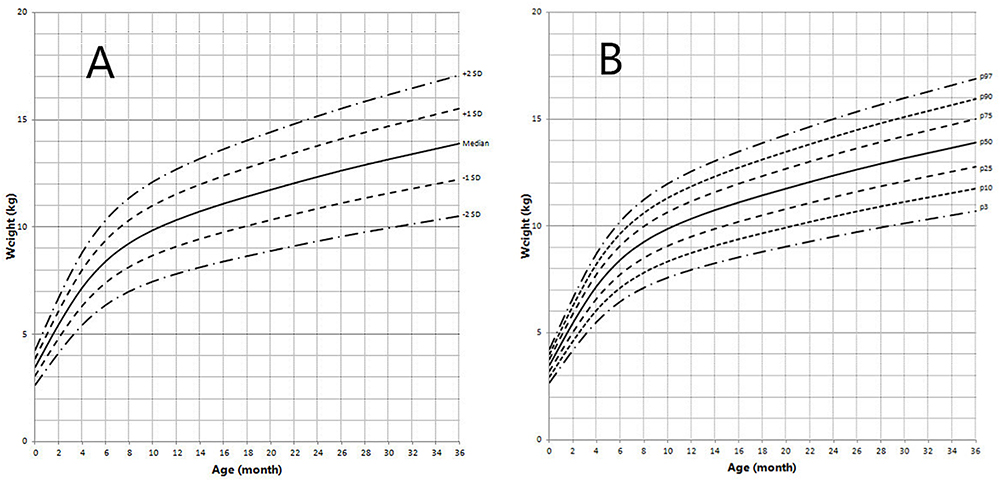
Figure 5. Weight in children with 5αRD (0–36 months old). (A) Standard deviation curve; (B) Percentile curve.
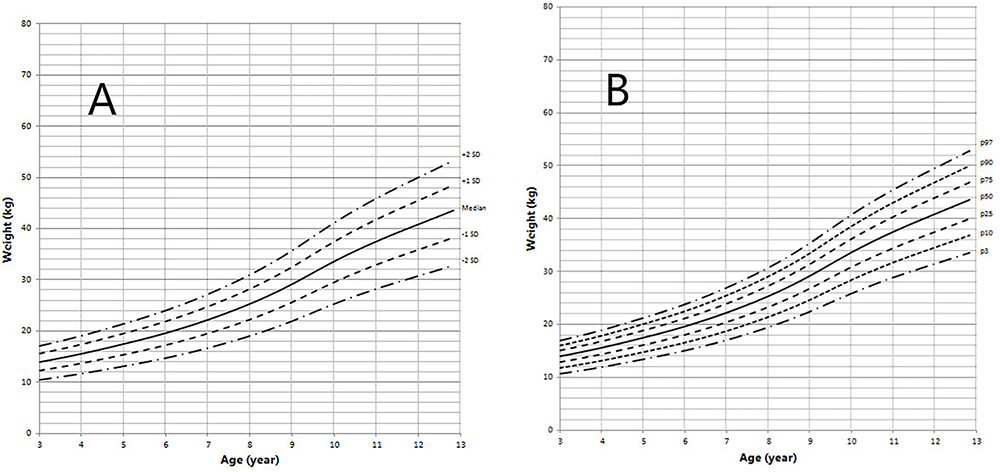
Figure 6. Weight in children with 5αRD (3–13 years old). (A) Standard deviation curve; (B) Percentile curve.
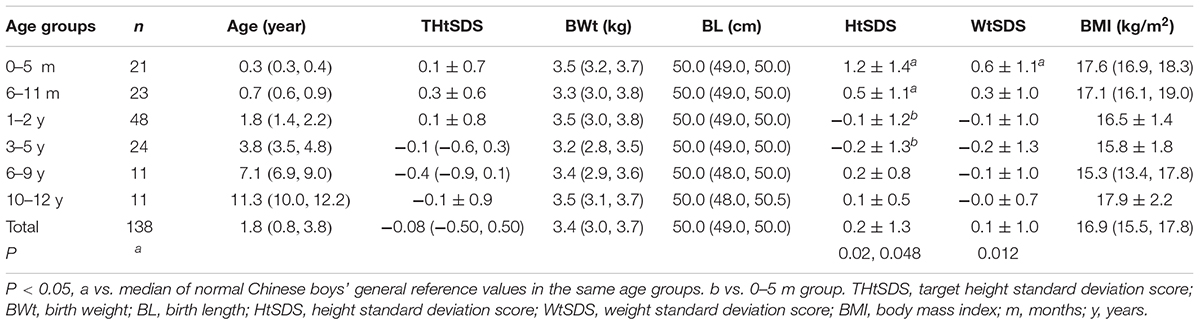
Height standard deviation scores and WtSDS of 138 cases with 5αRD were 0.21 ± 1.25 and 0.10 ± 1.02, respectively and in the normal range. The average BL of 5αRD was 50.00 (49.00, 50.00) cm and the average BWt of 5αRD was 3.40 (3.00, 3.70) kg, within the normal range, which was comparable to the normal reference standard for the Chinese population. Compared to the normal reference values for boys of the same age, HtSDS and WtSDS of 5αRD patients were higher when they were younger than 2 years old (the mean of HtSDS and WtSDS ranged from 0SD to +1SD) with significant difference aged <1 year in HtSDS (t = 3.658, 2.103, P = 0.002, 0.048; respectively) and aged <6 months in WtSDS (t = 2.756, P = 0.012). After that, their height and weight plateaued gradually and fluctuated around the median for normal Chinese boys until the age of 13 years (Figure 7). To sum up, these data showed that the height in 5αRD children increased faster than the normal population before 2 years of age, especially within the first 1 year of life, after which, the growth velocity gradually reduced and fluctuated near the median height of normal Chinese boys of the same age.
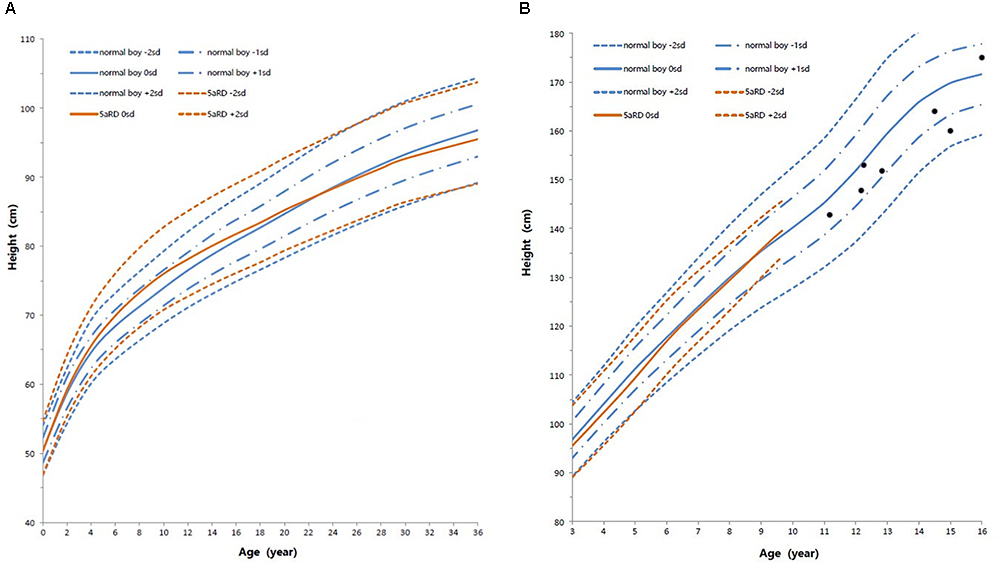
Figure 7. Standard deviation height curves between children with 5aRD and normal Chinese boys (0–16 years old). (A) Aged 0–36 months old; (B) Aged 3–16 years old. Red is 5αRD; Blue is the normal Chinese boy. Black spot is the patients of 5αRD older than 13 years. sd, standard deviation scores.
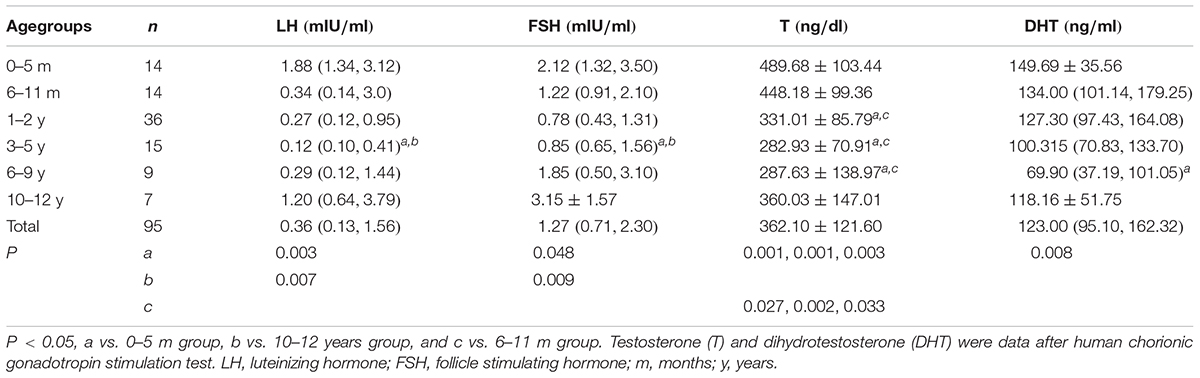
Physical Assessments and Hormone Levels of 5αRD Among Different Age Groups
Physical Assessment for Chinese Children With 5αRD Among Different Age Groups (Table 2)
The highest and lowest HtSDS were found in the 0–5 months and 3–5 years groups. HtSDS in the 0–5 months group had significant differences with the 1–2 years and 3–5 years groups (P = 0.001, 0.004, respectively), while no significant differences were observed in the other groups.
The highest WtSDS was observed in the 0–5 months group; however, no significant differences were observed among the different age groups.
Hormones in Different Age Groups of 5αRD
The baseline levels of LH, FSH and the levels of T and DHT after HCG stimulation test are shown in Table 3. The lowest levels of LH, FSH, and T were found in the 3–5 years group. LH and FSH in the 0–5 months and 10–12 years groups were higher than the other age groups with significant differences to those in the 3–5 years group (P = 0.003, 0.048, 0.007, 0.009, respectively). The top three levels of T were observed in the 0–5 months, 6–11 months, and 10–12 years groups. Further, T in the 0–5 months and 6–11 months groups were significantly higher than in those aged 1–9 years (P = 0.001, 0.001, 0.003, 0.027, 0.002, 0.033, respectively). The lowest DHT level was seen in the 6–9 years group. The highest DHT was seen in the 0–5 months group and showed significant differences with those in the 6–9 years group (P = 0.008). The variation tendency of HtSDS in 5αRDs was consistent with the trend of T.
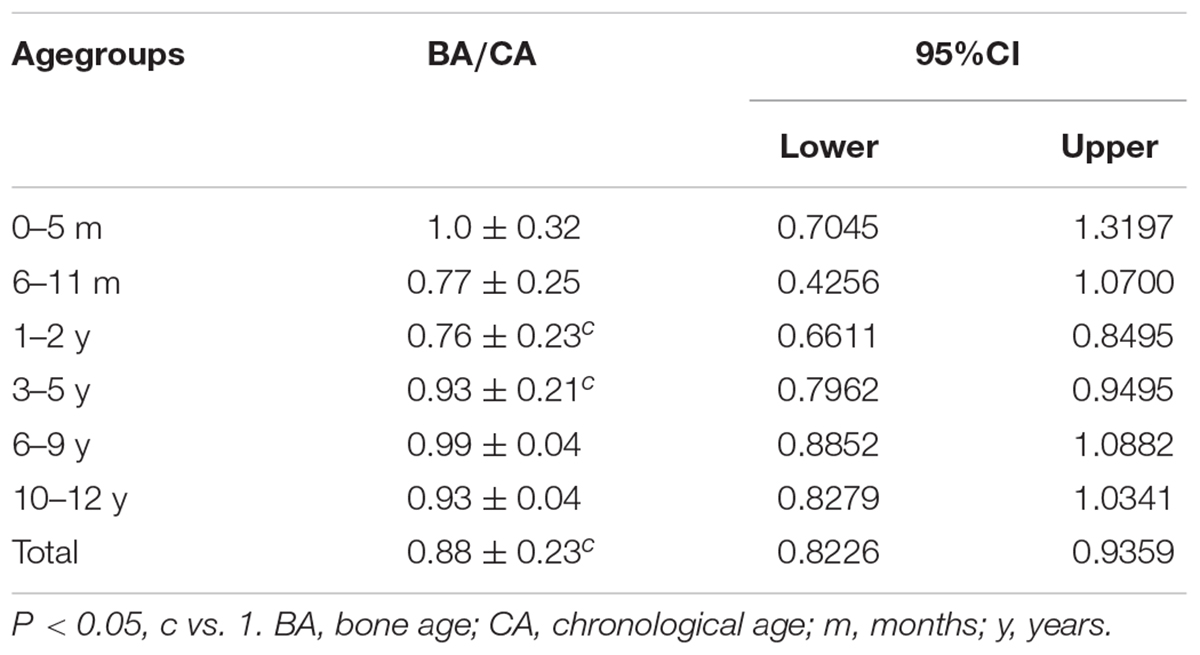
Ratio of BA/CA in Children With 5αRD (Table 4)
The ratio of BA/CA (N = 68) was 0.88 ± 0.23 with 95% CI < 1. Additionally, 95% CI < 1 for BA/CA ratio were found in those aged 1–5 years, which meant that BA in 5αRDs aged between 1 and 5 years was delayed, then BA was almost near to CA in the other age groups.
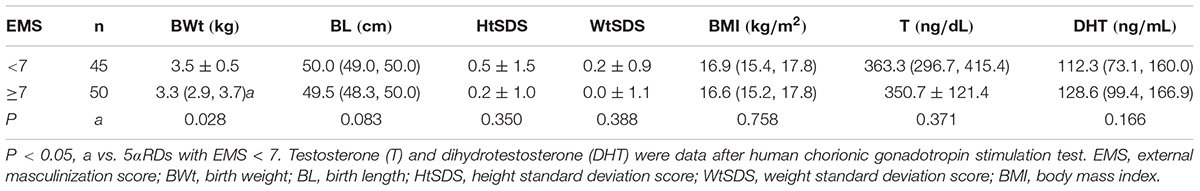
Physical Assessments and Hormone Levels of 5αRD According to EMS (Table 5)
Children with 5αRD were divided into two groups according to EMS (n = 95). One group was severely undervirilized with an EMS < 7, and the other was mildly undervirilized with an EMS ≥ 7. In the EMS < 7 group, HtSDS, WtSDS, BL, and T after HCG stimulation test were higher, and DHT after HCG stimulation test was lower than those in the EMS ≥ 7 group without significant differences. BWt in EMS < 7 group was significantly higher (Z = -2.191, P = 0.028).
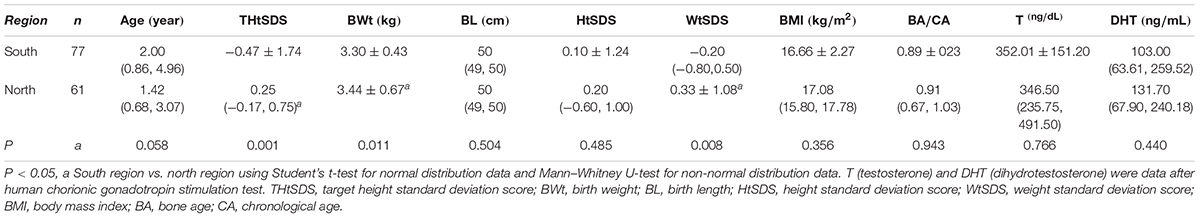
Geographical Difference in Children With 5αRD (Table 6)
Data indicated that THtSDS, BWt, and WtSDS in children with 5αRD from northern China were significantly higher than those from the southern region (Z = -4.556, -2.558, -2.670, P = 0.001, 0.011, 0.008, respectively), and no differences were found in age, BL, HtSDS, BMI, BA/CA, T, and DHT between the two groups.
Discussion
5α-reductase type 2 deficiency is a 5α-reductase isoenzyme 2 formation defect caused by the SRD5A2 gene mutation. Its clinical profile ranges from 46, XY presented as complete female external genitalia to under masculinized male external genitalia (Sinnecker et al., 1996; Mendonca et al., 2016) such as enlarged clitoris, hypospadias, and micropenis (Ng et al., 1990; Gad et al., 1997; Silver and Russell, 1999; Nicoletti et al., 2005; Avendano et al., 2018). With the help of gene analysis, more and more 5αRD can be diagnosed in some cases with the mild phenotype (Wang et al., 2004; Nie et al., 2011). These give clinicians a chance to profile the growth of children with 5αRD. The growth pattern of 5αRD is becoming a concerned focus by clinicians.
Height Features in Chinese Children With 5αRDs
In the present study, the plotted growth chart showed that the average HtSDS in 5αRD patients was within the range for normal reference values of Chinese boys. Children with 5αRD grow faster than normal boys before the age of 2 years, particularly when <1 year old. Between the ages of 2 and 13 years, their growth velocity decreases gradually and height fluctuates around the median height of normal Chinese boys. This trend was concordant with stimulating T fluctuation levels in 5αRD children in the growing age. 5αRD had decreased 5α-reductase type 2 enzymatic activity caused by SRD5A2 gene mutation. In 5αRDs, the lower the residual activity of 5α-reductase type 2 isoenzyme, the greater the severity of the manifestation and higher accumulation of T. Patients with severe undervirilized male external genitalia (EMS < 7) had slightly higher HtSDS, WtSDS, BL, and T after the HCG stimulation test and significantly higher BWt. All these hint at T having an impact on their growth. In 5αRD children, increased activity of the hypothalamic-pituitary-testicular axis during infancy would result in higher T levels because of minipuberty and diminished conversion to DHT. Thereafter, the slowed growth rate is a consequence of lower T during the quiescence of childhood before puberty. Previous studies have shown that growth in DSD children is associated with androgens (Hughes et al., 2002; Richter-Unruh et al., 2004; Han et al., 2008; Di et al., 2013; Wu et al., 2017). For example, Han et al. (2008) assessed the height in patients with CAIS and found that patients who underwent gonadectomy after adolescence or during adulthood were taller than those who underwent the same surgery in the pre-pubertal phase. The same conclusion was drawn when comparing with testicular dysfunction 46, XY DSD (Wu et al., 2017). This suggests that androgen affects growth in the pre-pubertal stage despite being undetectable in childhood. T and DHT are two types of androgen. T increases growth in association with a direct elevation of GH and an indirect elevation of IGF-I, respectively; the latter occurs due to estrogen by peripheral and central aromatization (Veldhuis et al., 2005a,b, 2009; Perry et al., 2008). DHT, on the other hand, could not up-regulate the function of hypothalamo-somatotrope-IGF-I axis directly (Veldhuis et al., 1997) and could not be aromatized to estrogen. There are two 5-alpha-reductase isoenzymes (Wilson, 1972). The type 1 isoenzyme is distributed in the bone, skeletal muscle, osteoblast-like cells, and a few other tissues (Wilson, 1972; Thigpen et al., 1993; Issa et al., 2002; Tria et al., 2004), whereas the type 2 isoenzyme is distributed in the prostate, seminal vesicle epididymis, medulla oblongata, and other tissues (Thigpen et al., 1993; Tria et al., 2004). The capability of T to induce biologic actions in bone depends on localized intraskeletal sex steroid hormone metabolism via type-1 5-alpha-reductase isoenzyme specially expressed in bone tissue (Yarrow et al., 2015). Furthermore, T surges in perinatal periods and consequent imprinting of the GH/IGF-I axis has important positive effects on the growth plate (Sims et al., 2006; Vanderschueren et al., 2014). In conclusion, growth of children with 5αRD was affected by high levels of T, while DHT plays a very minor role in their growth. Individuals with 5αRD have exhibited different residual activity of 5α-reductase. T treatment, which may be considered anyway for 5αRDs patients with micropenis, may also have an extra benefit on their growth.
Bone Age Feature in 5αRDs
Bone age is the best index of growth potential ability. Sex hormones promote bone maturation, which, in different types of DSDs show special characteristics (Bertelloni et al., 2010). Short-term treatment with testosterone undecanoate (Andriol) did not change the BA in our previous study (Chen et al., 2012). The average ratio of BA/CA in this study of 5αRD children was lower than 1, particularly in the ages of 1–5 years. Perhaps, physiological androgen resistance during early childhood could explain the BA delay in presence of high T concentration among 5αRD children. This research addressed concerns related to treatment with androgen products on bone maturation and provided some supporting evidence for androgen therapy in childhood. Suitable T therapy may be beneficial for either micropenis or height growth among children with 5αRD.
Puberty Feature in Chinese 5αRDs
Four of 11 cases aged 10–12 years (M age: 11.83 years) had the signs of puberty with Tanner stage 2. Another 3 cases aged >13 years (M age: 15.16 years) who were excluded from the growth curve were in Tanner stage 3–5. Perhaps these data showed that the onset time of puberty in children with 5αRD was somewhat more delayed than those of normal Chinese boys (M age: 10.55 years) (Pubertal Study Group of the Subspeciality Group of Endocrinologie, Hereditary and Matabolic Diseases, Society of Pediatrics, and Chinese Medical Association, 2010). Growth deceleration before puberty may partly explain the growth retardation in 5αRD during the age of 10–12 years.
Study Limitation
We acknowledge that our study has some limitations. There were relatively few patients older than 10 years; thus, the growth pattern in children with 5αRD during the puberty period was limited. Seven children aged >10 years were in the pubertal stage with Tanner stage 2–5. These data partly reflected the onset time of puberty in children with 5αRD. We need more cases to validate the therapeutic opinions in the follow-up study. Owing to technical constraints in most Chinese labs hereto, serum LH, FSH, T, and DHT were measured by enzyme enhanced chemiluminescence assay and not as per the gold standard of mass spectrometry. Nonetheless, our hormone results had the value to assess the patients’ sex hormones, because there are several publications that have used the same testing methods, and enzyme enhanced chemiluminescence assay is still widely used to measure hormones in many countries currently. The levels of GH, IGF-I, and estrogens were not mentioned in the research due to lack of sufficient data in patients’ medical records.
Conclusion
The growth curve of children with 5αRD revealed the special pattern affected by T, while DHT plays a very minor role in it. Our growth curve can provide the reference for clinical judgment of Chinese children with 5αRD. In addition, patients showed lagging BA. Furthermore, androgen treatment, which may be considered anyway for 5αRD patients with micropenis, may also be beneficial for their growth.
Note
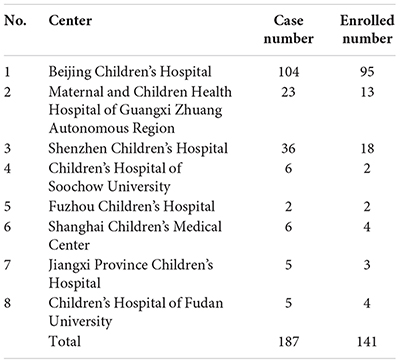
Number of cases with 5αRD in the present study
Ethics Statement
This study was approved by the ethical committee of Beijing Children’s Hospital. This study was approved by the ethical committee of Beijing Children’s Hospital. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
XZ contributed to data collection, data interpretation, and writing of the report. CG contributed to study design and reviewed the paper. YS, SC, XW, FL, YY, LC, RC, DW, and ZS contributed to data collection. HC contributed to statistical analysis.
Funding
This work was funded by the Public Health Project for Residents in Beijing (Z151100003915103) and the National Key Research and Development Program of China (2016YFC0901505).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all patients and their families for participation in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00173/full#supplementary-material
Abbreviations
ACMG, American College of Medical Genetics and Genomics; BA, bone age; BL, birth length; BMI, body mass index; BWt, birth weight; CA, chronological age; CAIS, complete androgen insensitivity syndrome; CIs, confidence intervals; DHT, dihydrotestosterone; DSD, disorders of sex development; EMS, external masculinization score; 5αRD, 5α-reductase type 2 deficiency; FSH, follicle stimulating hormone; GATK, genome analysis toolkit; GH, growth hormone; G-P, Greulich-Pyle; HCG, human chorionic gonadotropin; HtSDS, height standard deviation score; IGF-I, insulin-like growth factor-I; L, coefficient of skewness; LH, luteinizing hormone; LMS, λ-median-coefficient of variation method; M, median; S, coefficient of variation; SD, standard deviation; SNVs, single nucleotide variations; T, testosterone; THtSDS, target height standard deviation score; WtSDS, weight standard deviation score.
References
Ahmed, S. F., Khwaja, O., and Hughes, I. A. (2000). The role of a clinical score in the assessment of ambiguous genitalia. BJU Int. 85, 120–124.
Almeida, M., Laurent, M. R., Dubois, V., Claessens, F., O’Brien, C. A., Bouillon, R., et al. (2017). Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 97, 135–187. doi: 10.1152/physrev.00033.2015
Avendano, A., Paradisi, I., Cammarata-Scalisi, F., and Callea, M. (2018). 5-alpha-Reductase type 2 deficiency: is there a genotype-phenotype correlation? A review. Hormones 17, 197–204. doi: 10.1007/s42000-018-0013-9
Bertelloni, S., Baldinotti, F., Russo, G., Ghirri, P., Dati, E., Michelucci, A., et al. (2016). 5alpha-reductase-2 deficiency: clinical findings, endocrine pitfalls, and genetic features in a large italian cohort. Sex. Dev. 10, 28–36. doi: 10.1159/000445090
Bertelloni, S., Baroncelli, G. I., and Mora, S. (2010). Bone health in disorders of sex differentiation. Sex. Dev. 4, 270–284. doi: 10.1159/000315961
Byers, H. M., Mohnach, L. H., Fechner, P. Y., Chen, M., Thomas, I. H., Ramsdell, L. A., et al. (2017). Unexpected ethical dilemmas in sex assignment in 46,XY DSD due to 5-alpha reductase type 2 deficiency. Am. J. Med. Genet. C Semin. Med. Genet. 175, 260–267. doi: 10.1002/ajmg.c.31560
Chen, J. J., Gong, C. X., Cao, B. Y., Di, W. U., and Yu-chuan, L. I. (2012). Clinical observation of short-term oral testosterone undecanoate treatment for 46, XY DSD Chinese boys with small penis: a self-comparison study. Clin. J. Evid. Based Pediatr. 7, 167–171. doi: 10.3969/j.issn.1673-5501.2012.03.002
Choi, J. H., Kim, G. H., Seo, E. J., Kim, K. S., Kim, S. H., and Yoo, H. W. (2008). Molecular analysis of the AR and SRD5A2 genes in patients with 46,XY disorders of sex development. J. Pediatr. Endocrinol. Metab. 21, 545–553.
Cole, T. J., and Green, P. J. (1992). Smoothing reference centile curves: the LMS method and penalized likelihood. Stat. Med. 11, 1305–1319.
Deeb, A., Al, S. H., Ibukunoluwa, F., and Attia, S. (2016). Phenotype, sex of rearing, gender re-assignment, and response to medical treatment in extended family members with a novel mutation in the SRD5A2 gene. J. Clin. Res. Pediatr. Endocrinol. 8, 236–240. doi: 10.4274/jcrpe.2782
DePristo, M. A., Banks, E., Poplin, R., Garimella, K. V., Maguire, J. R., Hartl, C., et al. (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498. doi: 10.1038/ng.806
Di, W. U., Gong, C. X., and Qin, M. (2013). Analysis of the clinical characteristics and body height in 153 disorders of sex development children without known cause. Chin. J. Evid. Based Pediatr. 8, 46–49.
Eren, E., Edgunlu, T., Asut, E., and Karakas, C. S. (2016). Homozygous Ala65Pro mutation with V89L polymorphism in SRD5A2 deficiency. J. Clin. Res. Pediatr. Endocrinol. 8, 218–223. doi: 10.4274/jcrpe.2495
Gad, Y. Z., Nasr, H., Mazen, I., Salah, N., and El-Ridi, R. (1997). 5 alpha-reductase deficiency in patients with micropenis. Inherit. J. Metab. Dis. 20, 95–101.
Han, T. S., Goswami, D., Trikudanathan, S., Creighton, S. M., and Conway, G. S. (2008). Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. Eur. Endocrinol, J. 159, 179–185. doi: 10.1530/EJE-08-0166
Hughes, I. A., Northstone, K., and Golding, J. (2002). Reduced birth weight in boys with hypospadias: an index of androgen dysfunction? Arch. Dis. Child Fetal Neonatal Ed. 87, F150–F151.
Ishii, T., Matsuo, N., Sato, S., Ogata, T., Tamai, S., Anzo, M., et al. (2015). Human chorionic gonadotropin stimulation test in prepubertal children with micropenis can accurately predict leydig cell function in pubertal or postpubertal adolescents. Horm. Res. Paediatr. 84, 305–310. doi: 10.1159/000439234
Issa, S., Schnabel, D., Feix, M., Wolf, L., Schaefer, H. E., Russell, D. W., et al. (2002). Human osteoblast-like cells express predominantly steroid 5alpha-reductase type 1. J. Clin. Endocrinol. Metab. 87, 5401–5407. doi: 10.1210/jc.2001-011902
Ittiwut, C., Pratuangdejkul, J., Supornsilchai, V., Muensri, S., Hiranras, Y., Sahakitrungruang, T., et al. (2017). Novel mutations of the SRD5A2 and AR genes in Thai patients with 46, XY disorders of sex development. J. Pediatr. Endocrinol. Metab. 30, 19–26. doi: 10.1515/jpem-2016-0048
Jia, J., and Shi, T. (2017). Towards efficiency in rare disease research: what is distinctive and important? Sci. China Life Sci. 60, 686–691. doi: 10.1007/s11427-017-9099-3
Ko, J. M., Cheon, C. K., Kim, G. H., Kim, S. H., Kim, K. S., and Yoo, H. W. (2010). Clinical characterization and analysis of the SRD5A2 gene in six Korean patients with 5alpha-reductase type 2 deficiency. Horm. Res. Paediatr. 73, 41–48. doi: 10.1159/000271915
Kolesinska, Z., Ahmed, S. F., Niedziela, M., Bryce, J., Molinska-Glura, M., Rodie, M., et al. (2014). Changes over time in sex assignment for disorders of sex development. Pediatrics 134, e710–e715. doi: 10.1542/peds.2014-1088
Lee, P. A., Houk, C. P., Ahmed, S. F., and Hughes, I. A. (2006). Consensus statement on management of intersex disorders. International consensus conference on intersex. Pediatrics 118, e488–e500. doi: 10.1542/peds.2006-0738
Li, H., Ji, C. Y., Zong, X. N., and Zhang, Y. Q. (2009). [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi 47, 487–492.
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Mendonca, B. B., Batista, R. L., Domenice, S., Costa, E. M., Arnhold, I. J., Russell, D. W., et al. (2016). Steroid 5alpha-reductase 2 deficiency. J. Steroid Biochem. Mol. Biol. 163, 206–211. doi: 10.1016/j.jsbmb.2016.05.020
Ng, W. K., Taylor, N. F., Hughes, I. A., Taylor, J., Ransley, P. G., and Grant, D. B. (1990). 5 alpha-reductase deficiency without hypospadias. Arch. Dis. Child. 65, 1166–1167.
Ni, X., and Shi, T. (2017). The challenge and promise of rare disease diagnosis in China. Sci. China Life Sci. 60, 681–685. doi: 10.1007/s11427-017-9100-1
Nicoletti, A., Baldazzi, L., Balsamo, A., Barp, L., Pirazzoli, P., Gennari, M., et al. (2005). SRD5A2 gene analysis in an Italian population of under-masculinized 46,XY subjects. Clin. Endocrinol. 63, 375–380. doi: 10.1111/j.1365-2265.2005.02348.x
Nie, M., Zhou, Q., Mao, J., Lu, S., and Wu, X. (2011). Five novel mutations of SRD5A2 found in eight Chinese patients with 46,XY disorders of sex development. Mol. Hum. Reprod. 17, 57–62. doi: 10.1093/molehr/gaq072
Nishiyama, K. K., Macdonald, H. M., Moore, S. A., Fung, T., Boyd, S. K., and McKay, H. A. (2012). Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. Bone J. Miner. Res. 27, 273–282. doi: 10.1002/jbmr.552
Perry, R. J., Farquharson, C., and Ahmed, S. F. (2008). The role of sex steroids in controlling pubertal growth. Clin. Endocrinol. 68, 4–15. doi: 10.1111/j.1365-2265.2007.02960.x
Pubertal Study Group of the Subspeciality Group of Endocrinologie, Hereditary and Matabolic Diseases, Society of Pediatrics, and Chinese Medical Association (2010). Testis volume.pubic hair development and spernmrcheal age in urban Chinese boys. Chinese J. Pediatr. 48, 418–424. doi: 10.3760/cma.j.issn.0578-1310.2010.06.005
Raveenthiran, V. (2017). Controversies of sex re-assignment in genetic males with congenital inadequacy of the penis. Indian Pediatr. J. 84, 700–708. doi: 10.1007/s12098-017-2412-3
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17, 405–424. doi: 10.1038/gim.2015.30
Richter-Unruh, A., Knauer-Fischer, S., Kaspers, S., Albrecht, B., Gillessen-Kaesbach, G., and Hauffa, B. P. (2004). Short stature in children with an apparently normal male phenotype can be caused by 45,X/46,XY mosaicism and is susceptible to growth hormone treatment. Eur. Pediatr. J. 163, 251–256. doi: 10.1007/s00431-004-1406-0
Shen, Z. (2016). What are the biological roots of sexual orientation? Chin. Sci. Bull. 61, 1733–1747. doi: 10.1360/N972015-01212
Silver, R. I., and Russell, D. W. (1999). 5alpha-reductase type 2 mutations are present in some boys with isolated hypospadias. J. Urol. 162(3 Pt 2), 1142–1145.
Sims, N. A., Brennan, K., Spaliviero, J., Handelsman, D. J., and Seibel, M. J. (2006). Perinatal testosterone surge is required for normal adult bone size but not for normal bone remodeling. Am. J. Physiol. Endocrinol. Metab. 290, E456–E462. doi: 10.1152/ajpendo.00311.2005
Sinnecker, G. H., Hiort, O., Dibbelt, L., Albers, N., Dorr, H. G., Hauss, H., et al. (1996). Phenotypic classification of male pseudohermaphroditism due to steroid 5 alpha-reductase 2 deficiency. Am. J. Med. Genet. 63, 223–230.
Sultan, C., Paris, F., Terouanne, B., Balaguer, P., and Georget, V. (2001). Disorders linked to insufficient androgen action in male children. Hum. Reprod. Update 7, 314–322. doi: 10.1093/humupd/7.3.314
Thigpen, A. E., Silver, R. I., Guileyardo, J. M., Casey, M. L., McConnell, J. D., and Russell, D. W. (1993). Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. Clin. J. Invest. 92, 903–910. doi: 10.1172/JCI116665
Tria, A., Hiort, O., and Sinnecker, G. H. (2004). Steroid 5alpha-reductase 1 polymorphisms and testosterone/dihydrotestosterone ratio in male patients with hypospadias. Horm. Res. 61, 180–183. doi: 10.1159/000076279
Van der Auwera, G. A., Carneiro, M. O., Hartl, C., Poplin, R., Del Angel, G., Levy-Moonshine, A., et al. (2013). From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43
Vanderschueren, D., Laurent, M. R., Claessens, F., Gielen, E., Lagerquist, M. K., Vandenput, L., et al. (2014). Sex steroid actions in male bone. Endocr. Rev. 35, 906–960. doi: 10.1210/er.2014-1024
Veiga-Junior, N. N., Medaets, P. A., Petroli, R. J., Calais, F. L., de Mello, M. P., Castro, C. C., et al. (2012). Clinical and laboratorial features that may differentiate 46,XY DSD due to partial androgen insensitivity and 5alpha-reductase type 2 deficiency. Int. Endocrinol. J. 2012:964876. doi: 10.1155/2012/964876
Veldhuis, J. D., Anderson, S. M., Iranmanesh, A., and Bowers, C. Y. (2005a). Testosterone blunts feedback inhibition of growth hormone secretion by experimentally elevated insulin-like growth factor-I concentrations. J. Clin. Endocrinol. Metab. 90, 1613–1617. doi: 10.1210/jc.2004-1303
Veldhuis, J. D., Frystyk, J., Iranmanesh, A., and Orskov, H. (2005b). Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I), IGF binding protein 1 (IGFBP-1), and dimeric IGF-I/IGFBP-1 concentrations. J. Clin. Endocrinol. Metab. 90, 2941–2947. doi: 10.1210/jc.2004-1314
Veldhuis, J. D., Erickson, D., Wigham, J., Weist, S., Miles, J. M., and Bowers, C. Y. (2011). Gender, sex-steroid, and secretagogue-selective recovery from growth hormone-induced feedback in older women and men. J. Clin. Endocrinol. Metab. 96, 2540–2547. doi: 10.1210/jc.2011-0298
Veldhuis, J. D., Metzger, D. L., Martha, J. P. M., Mauras, N., Kerrigan, J. R., Keenan, B., et al. (1997). Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement∗. J. Clin. Endocrinol. Metab. 82, 3414–3420.
Veldhuis, J. D., Mielke, K. L., Cosma, M., Soares-Welch, C., Paulo, R., Miles, J. M., et al. (2009). Aromatase and 5alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex steroid modulation of somatostatin and growth hormone secretagogue actions in healthy older men. J. Clin. Endocrinol. Metab. 94, 973–981. doi: 10.1210/jc.2008-2108
Wang, Y., Gong, C., Wang, X., and Qin, M. (2017). AR mutations in 28 patients with androgen insensitivity syndrome (Prader grade 0-3). Sci. China Life Sci. 60, 700–706. doi: 10.1007/s11427-017-9084-9
Wang, Y., Li, Q., Xu, J., Liu, Q., Wang, W., Lin, Y., et al. (2004). Mutation analysis of five candidate genes in Chinese patients with hypospadias. Eur. Hum, J. Genet. 12, 706–712. doi: 10.1038/sj.ejhg.5201232
Wilson, J. D. (1972). Recent studies on the mechanism of action of testosterone. N. Engl. J. Med. 287, 1284–1291. doi: 10.1056/NEJM197212212872508
Wilson, J. D., Griffin, J. E., and Russell, D. W. (1993). Steroid 5 alpha-reductase 2 deficiency. Endocr. Rev. 14, 577–593. doi: 10.1210/edrv-14-5-577
Wu, D., Chen, H., and Gong, C. (2017). Physical assessment and reference growth curves for children with 46,XY disorders of sex development. Pediatr. Invest. 1, 16–22.
Keywords: dihydrotestosterone, testosterone, 5α-reductase type 2 deficiency, 46, XY DSD, height, children
Citation: Zhao X, Song Y, Chen S, Wang X, Luo F, Yang Y, Chen L, Chen R, Chen H, Su Z, Wu D and Gong C (2019) Growth Pattern in Chinese Children With 5α-Reductase Type 2 Deficiency: A Retrospective Multicenter Study. Front. Pharmacol. 10:173. doi: 10.3389/fphar.2019.00173
Received: 13 July 2018; Accepted: 11 February 2019;
Published: 15 March 2019.
Edited by:
Tieliu Shi, East China Normal University, ChinaReviewed by:
Michaël R. Laurent, University Hospitals Leuven, BelgiumChao Xu, Shandong Qianfoshan Hospital, China
Michel Polak, Necker-Enfants Malades Hospital, France
Copyright © 2019 Zhao, Song, Chen, Wang, Luo, Yang, Chen, Chen, Chen, Su, Wu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiu Gong, Y2h1bnhpdWdvbmdAc2luYS5jb20=
†These authors have contributed equally to this work
 Xiu Zhao
Xiu Zhao Yanning Song
Yanning Song Shaoke Chen
Shaoke Chen Xiumin Wang4
Xiumin Wang4 Feihong Luo
Feihong Luo Linqi Chen
Linqi Chen Hui Chen
Hui Chen Zhe Su
Zhe Su