- 1Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Pediatrics, Pizhou City Hospital of Traditional Chinese Medicine, Pi Zhou, China
- 3Institute of Hypertension, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Background: Breviscapine (Dengzhanhua) injection has been wildly used in clinical treatment for cerebral infarction, cardiovascular disease, diabetic nephropathy, renal impairment of essential hypertension and stroke in China. Breviscapine injection and antihypertensive drugs combination therapy is supposed to be beneficial for hypertension-induced renal damage patients.
Objectives: To evaluate the beneficial and adverse effects of breviscapine injection on hypertension in hypertension-induced renal damage patients, an extensive meta-analysis was performed.
Methods: We searched PubMed, the Cochrane Library, Embase, CNKI, Sino Med, VIP, and Wanfang Data for relevant literature. The timeframe of retrieval was set from the founding date of each database to September 28, 2018.
Results: Fourteen papers were included in this study. The quality of all the studies included was determined to be low. All studies were conducted with Chinese populations. Meta-analysis showed that, compared with single-use antihypertensive drugs, using breviscapine injection in combination with antihypertensive drugs to treat hypertension in hypertension-induced renal damage patients can reduce 24-h urinary total protein (24 h UTP) [WMD = −0.04, 95% CI (−0.05, −0.02), P ≤ 0.001], but does not lower systolic blood pressure (SBP) [WMD = −1.02, 95% CI (−2.88, 0.84), P = 0.281] or diastolic blood pressure (DBP) [WMD = −0.21, 95% CI (−1.71, 1.29), P = 0.786] more effectively. There was also no statistically significant difference in adverse events between experimental groups and control groups.
Conclusion: Breviscapine injection, in combination with antihypertensive drugs, appears to be more effective in improving the 24 h UTP, but overall have no effect on improving the blood pressure in hypertension-induced renal damage patients. Moderate dose of breviscapine injection (10 ml) may have effects on reducing blood pressure in hypertension-induced renal damage patients but high doses of breviscapine injection (20 ml) may increase blood pressure by subgroup analysis. However, the evidence of methodological quality and sample sizes is weak, and thus, further standardized research is required.
Introduction
Hypertension, defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, is a risk factor for stroke, coronary artery disease, heart failure, peripheral vascular disease, and chronic kidney disease (CKD) (Lewington et al., 2002; Forouzanfar et al., 2017). Elevated blood pressure (BP) was the leading global contributor to premature death in 2015, accounting for almost 10 million deaths and over 200 million disability-adjusted life years (Forouzanfar et al., 2017). The main factors leading to the development of hypertension-induced renal damage include: (1) inappropriately elevated sympathetic nervous activity (SNA) (Joles and Koomans, 2004); (2) activation of the renin-angiotensin-aldosterone system (RAAS) (Grisk and Rettig, 2004); (3) increased arterial stiffness (Safar et al., 2001); (4) genetic susceptibility (Freedman et al., 2009); (5) impaired salt and water excretion by the kidney (ALLHAT Officers Coordinators for the ALLHAT Collaborative Research Group, 2002). Hypertensive kidney disease is the second leading cause of end-stage renal disease (ESRD) after diabetes mellitus (USRDS, 2018; Williams et al., 2018). In Europe, according to the European Dialysis and Transplant Association registry, hypertension-induced renal damage is accounted for 12% of new patients starting renal replacement therapy. However, the reported incidence varies among different countries, with France, Italy and United Kingdom, reporting in 25, 17, and 6.1%, with both Japanese and Chinese reporting in 6 and 7%, respectively (Fervenza et al., 2017). The diagnosis of hypertension-induced renal damage is based on the finding of reduced renal function and/or the detection of albuminuria (≥300 mg/d, or ≥300 mg/g albuminuria to-creatinine ratio in the first morning void). CKD is classified according to estimated glomerular filtration rate (eGFR), calculated by the 2009 CKD-Epidemiology Collaboration formula (Levey et al., 2009). Current evidence suggests that in patients with CKD, BP should be lowered to <140/90 mmHg and toward 130/80 mmHg. The combination of renin-angiotensin system blockers with calcium channel blockers (CCB) or diuretics should be used to achieve recommended blood pressure targets in CKD (Whelton et al., 2018; Williams et al., 2018). A recent meta-analysis have shown that BP lowering significantly reduced ESRD in patients with CKD, but only in patients with albuminuria, and had no beneficial effect on cardiovascular events (Lv et al., 2013). In a large retrospective cohort containing 398419 treated hypertensive patients, the nadir SBP and DBP for the lowest risk of ESRD and mortality were 137 and 71 mmHg, respectively, with a clear increase in mortality risk at SBP <120 mmHg (Sim et al., 2014). The evidence with respect to BP targets in patients with CKD is complex.
Breviscapine (Dengzhanhua) injection is extracted from Erigeron breviscapus (Vant.), Erigeron breviscapus also known as herba erigerontis or lamp chrysanthemum, is a traditional Chinese herb that has been in use for more than 600 years, found in Yunnan, Sichuan, Guizhou, and other southwest provinces of China. Breviscapine, as a purified flavonoid extract from this species, was first isolated by Zhang et al. (1988). Breviscapine mainly contains scutellarin (4′,5,6,7-tetrahydroxyflavone-7-O-glucuronide) and apigenin-7-O-glucuronide (Gao et al., 2017). Studies have shown that breviscapine has significant effects on vasodilation; inhibition platelet aggregation, scavenging free radicals, also has a protective effects on myocardial and endothelial structures because of its anti-inflammatory effects, and improve microcirculation; protection against ischemia/reperfusion (I/R); anticoagulation and antithrombosis; reduction of smooth muscle cell migration and proliferation; anticardiac remodeling;antiarrhythmia, and reduction of blood lipids (Jia et al., 2008; Wang et al., 2008, 2010, 2015). Breviscapine has been demonstrated to possess a number of pharmacological functions in addition to its hemodynamic effects; it has been concluded that breviscapine can relax norepinephrine-induced vasoconstriction in a concentration-dependent manner (Zheng et al., 1998); it has been linked to the scavenging of oxygen free radicals, decreasing the expressions ofintercellular adhesion molecule-1 protein in the myocardium and increasing the activities of Na(+)-K(+)-ATPase, Mg(2+)-ATPase, Ca(2+)-ATPase in the myocardial mitochondria (Jia et al., 2008); it has been reported that breviscapine could prevent thrombosis and platelet aggregation and improve the characteristics of haemorheology by restricting the ADP-induced platelet aggregation rate (Song et al., 2011); it could obviously inhibit the proliferation of vascular smooth muscle cell (VSMC) and may prevent atherosclerosis, and the mechanism may be realized partly by regulating NF-κB activity of VSMC (Pang et al., 2004); it has been reported to serve as an anti-oxidative stress agent and a protein kinase C (PKC) inhibitor, can inhibits the glycogen synthase kinase 3β (GSK3β) signaling pathway to promote neurobehavioral function following neurotrauma, and can improve renal function and reduce urinary micro-albuminuria (He et al., 2012; Liu et al., 2016; Jiang et al., 2017; Wang et al., 2018). In the light of these pharmacological activities, an injection preparation of breviscapine (a traditional Chinese patent medicine) has been wildly used in clinical treatment for cerebral infarction, cardiovascular disease, diabetic nephropathy, renal impairment of essential hypertension and stroke in China (Yang and Li, 2007; Liu et al., 2016; Gao et al., 2017; Wang et al., 2018).
However, in the past decades, although numerous clinical trials have been published analyzing the beneficial effects of breviscapine injection as an adjunctive therapy for hypertension-induced renal damage. However, there is no critical appraisal of the evidence on whether breviscapine injection as a complementary therapy could decrease BP for hypertension-induced renal damage patients. Therefore, we did a systematic review and meta-analysis to provide more reliable evidence on the effect of breviscapine injection on BP and other key outcomes.
Materials and Methods
Database and Search Strategies
We designed our systematic review and meta-analysis in accordance with the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. Foreign databases searched included PubMed, Embase, and the Cochrane Library. Chinese databases included the China National Knowledge Infrastructure (CNKI), China Biology Medicine Disc (Sino Med), the VIP information resource integration service platform (VIP), and the Wanfang Data knowledge service platform (Wanfang Data). The retrieval scheme was mainly based on a combination of subject words and free words. The searched words were: “Dengzhanhua,” “Dengzhanhua preparations,” “Dengzhanhua Zhusheye,” “Zhusheyong Dengzhanhua,” “Gaoxueya Shenbing,” “Gaoxueya Shenyan” and “Gaoxueya Shenshunhai,” while the searched English words were: “Breviscapine,” “Breviscapine Injection,” “BVP,” “Hypertension-induced renal damage,” “Hypertensive Nephropathy,” “Hypertension, Renal,” “Hypertensive Kidney Lesion,” and “Hypertensive Renal Damage” (see Supplementary Tables 1, 2 for search strategy). The retrieval language was not limited, and the timeframe of the retrieval was from the founding date of each database to September 28, 2018. There was language limitation. Manual searches of relevant literature supplemented the electronic search.
Inclusion Criteria
Randomized controlled trials (RCTs) that use breviscapine injection in combination with antihypertensive drugs to treat hypertension-induced renal damage, regardless of blinding, were included in this study. Language was also not restricted as to minimize publication bias. There were no serious organic diseases or complications in the selected cases. The diagnosis of hypertension-induced renal damage is based on the finding of reduced renal function and/or the detection of albuminuria. CKD is classified according to eGFR, calculated by the 2009 CKD–Epidemiologyn Collaboration formula (Levey et al., 2009). Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg were based on the Chinese Guidelines for the Prevention and Treatment of Hypertension (2010), and the Eight Joint National Commission (JNC8). We did not limit inclusion based on age, sex, case source, disease course, hypertensive classification, or antihypertensives. Experimental group: Breviscapine Injection combined with antihypertensive drugs[captopril, amlodipine, lisinopril, benazapril, losartan potassium, felodipine, nifedipine, etc. (medication dose, medication time and frequency, and treatment course)]. Control group: Antihypertensive drugs included captopril, amlodipine, lisinopril, benazapril, losartan potassium, felodipine, nifedipine, etc. (medication dose, medication time and frequency, and treatment course). The age, sex, and other baseline conditions of the research objects were well-matched.
Exclusion Criteria
Studies were excluded if they were: (1) clinical trials from which no relevant data could be extracted; (2) studies that were published repeatedly; (3) populated with inconsistent baseline information (age, sex, case source, disease course, hypertensive classification, or antihypertensive drugs); (4) systematic review, important data report, and case reports; no reply from corresponding authors such that further data could not be obtained; and (5) therapeutic measures failing to meet the predetermined inclusion criteria.
Data Extraction
Primary outcomes were SBP and DBP. Secondary outcomes included 24-h urinary total protein (24 h UTP) and adverse effects. Two evaluators independently performed a search according to the search strategy, and preliminary screening was based on independent topics and abstracts of the search results, excluding obviously unqualified documents. A full-text methodology screening was conducted on the literature that might meet the inclusion criteria, and corresponding authors were contacted when there was incomplete information. Then, the studies were cross-checked by two evaluators. Any disagreement on the conclusion of two evaluators was resolved by discussion. If such disagreement could not be resolved through discussion, final judgment and arbitration was made by a third party. Extracted contents included authors' names, year of publication, number of samples, intervention, course of treatment, and observed indicators.
Quality Evaluation
The investigators simultaneously evaluated the bias risk of the included studies based on the “risk of bias” evaluation tool in the Cochrane Handbook for Systematic Reviews (Review Manager, 2014) of interventions and relevant assessment guideline regulations. This risk evaluation tool contains seven items: (1) random sequence generation; (2) allocation concealment; (3) blinding of the participants and personnel; (4) blinding of the outcome data; (5) incomplete outcome data; (6) selective reporting; and (7) other bias., and were evaluated as having a “high risk of bias,” “low risk of bias,” or “unclear risk of bias” according to assessment criteria.
Data Analysis
(1) Stata 14.0 software was used to perform the statistical analysis for the meta-analysis (Higgins and Thompson, 2002). (2) Select effect size: if an index of the included documents is a binary variable, the curative effect analysis statistics can be represented by relative risk (RR) and expressed by its confidence interval (CI); mean difference (MD) and 95% CI were used to represent continuous changes. (3) Homogeneity test: test the variation degree of original research results and clearly include the degree of homogeneity of the experiment. (4) Meta-analysis: according to the result of the heterogeneity test, P ≥ 0.05 and I2 < 50 indicate that the results have good agreement and that the fixed effect model may be used. P < 0.05 and I2 ≥ 50 suggest that the heterogeneity of the results cannot be ignored. If the included studies still have clinical significance, the random effects model may be used. (5) Sensitivity analysis: in those meta-analysis of the comprehensive factors combined with multiple outcomes, possible anomalous studies were ruled out before reevaluation. The results were compared with those of meta-analysis before the exclusion to determine how the excluded studies would influence the combined effect size and the stability of meta-analysis. If there is little difference between the two results, then the sensitivity of the results is relatively low, and the results are stable, indicating high credibility. (6) Subgroup analysis: subgroup analysis was conducted on some indexes with high heterogeneity. For events in which quantitative synthesis was impossible and events with very low incidence, qualitative evaluation may be based on the description. In this study, Stata 14.0 software was used to conduct a sensitivity analysis and subgroup analysis and to create a sensitivity analysis chart. Publication bias occurs when positive data in similar research papers with statistical significance are more likely to be published in journals. This situation is hard to control. The funnel plot method is often used to detect publication bias. Egger's test was performed to detect publication bias in the outcome measures, and a funnel plot was drawn. If a large publication bias was found in a particular research index, the exact reason should be identified.
Results
Search Results
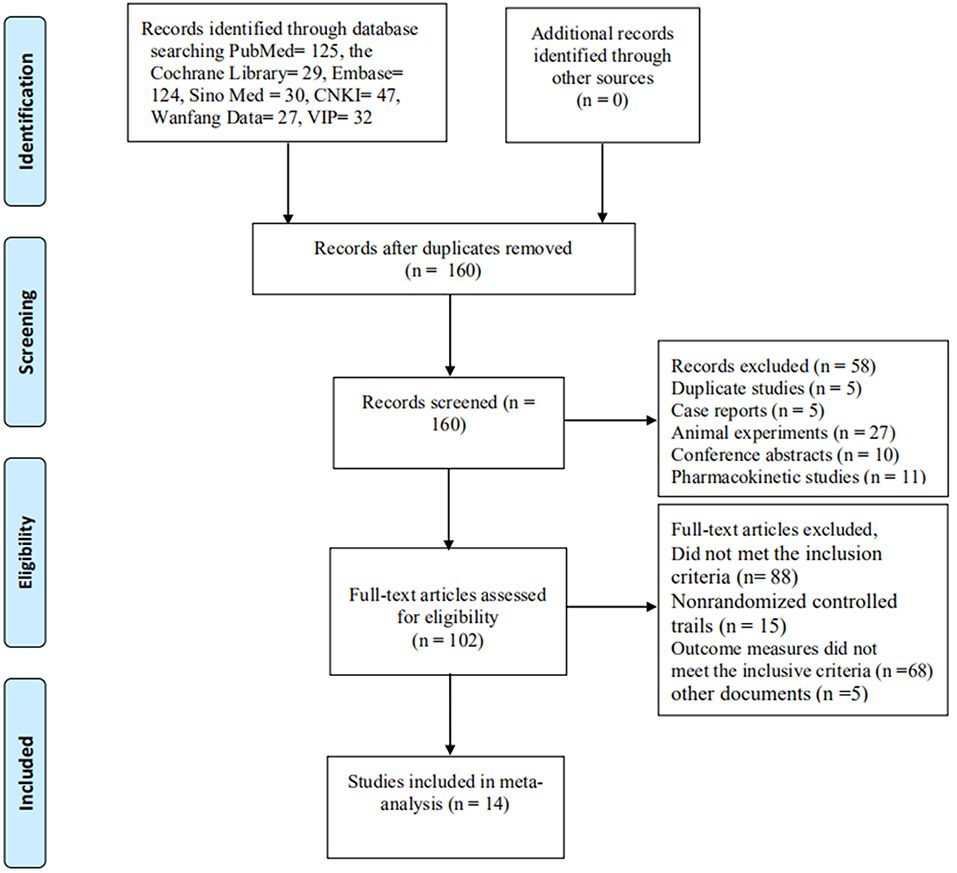
A total of 414 documents [the Cochrane Library (n = 29), PubMed (n = 125), Embase (n = 124), Sino Med (n = 30), CNKI (n = 47), Wanfang Data (n = 27), and VIP (n = 32)] met the data collection and search strategy conditions. NoteExpress, a professional document management software, was employed to check for duplication of the 414 obtained articles that met the relevance requirement. The majority of these trials were excluded because some papers were found in more than one database and some included irrelevant titles and abstracts. Only 160 studies were retrieved. Following a review of the titles and abstracts, several studies were excluded, and only 102 studies remained. Five trials were excluded because of duplicated publications. Twenty-seven trials were excluded for being animal studies, and twenty-six trials were excluded for being non-clinical trials, including case reports, pharmacokinetic studies, and conference abstracts. Eighty-eight out of the remaining 102 articles were excluded based on the inclusion criteria, leaving 14 RCTs to be reviewed in Figure 1.
Study Characteristics
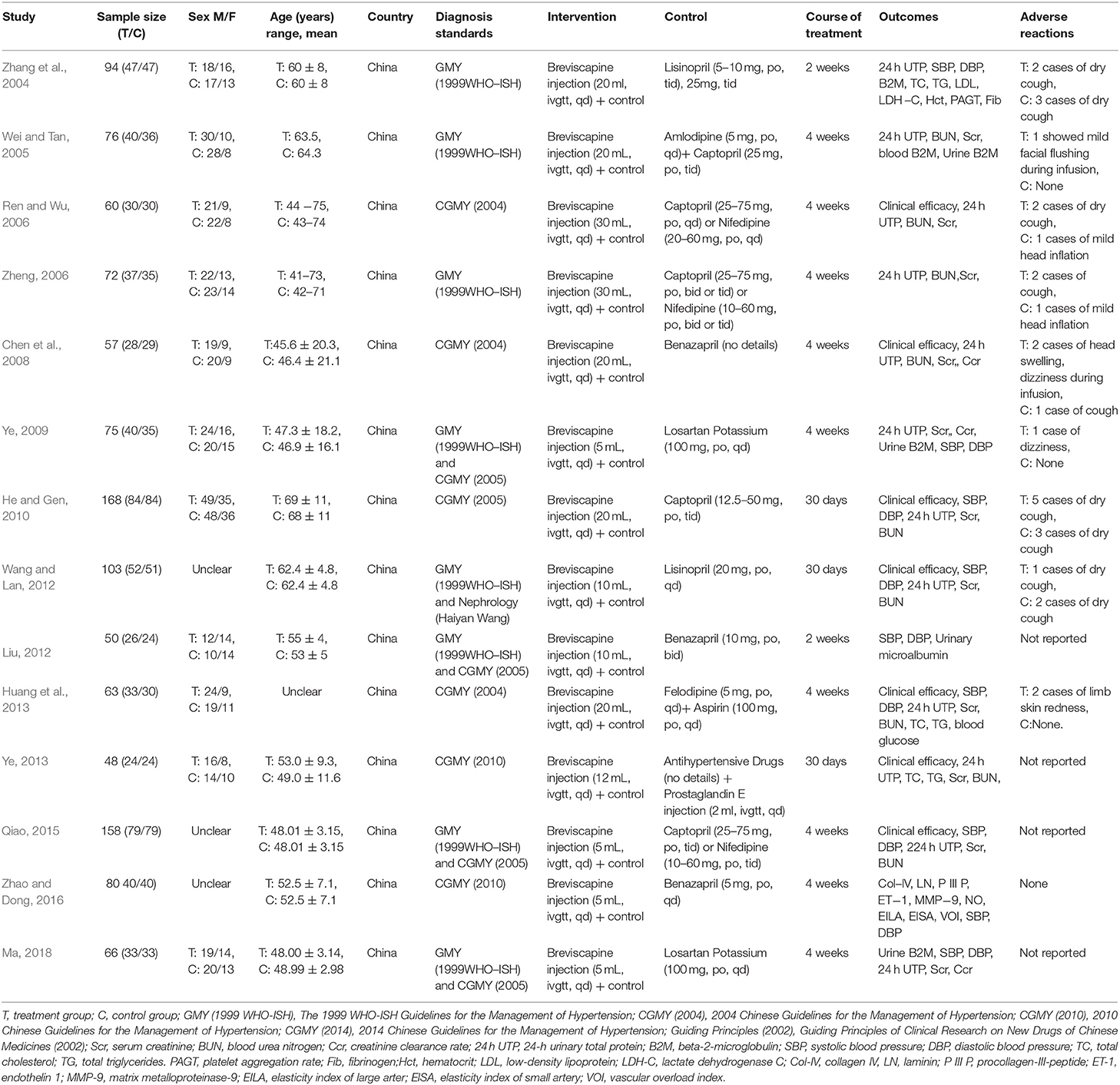
There were 14 randomized controlled trials (Zhang et al., 2004; Wei and Tan, 2005; Ren and Wu, 2006; Zheng, 2006; Chen et al., 2008; Ye, 2009, 2013; He and Gen, 2010; Liu, 2012; Wang and Lan, 2012; Huang et al., 2013; Qiao, 2015; Zhao and Dong, 2016; Ma, 2018) that were included in the present research involving 1,170 patients (593 in the research group and 577 in the control group). These 14 RCTs are summarized in Tables 1, 2.
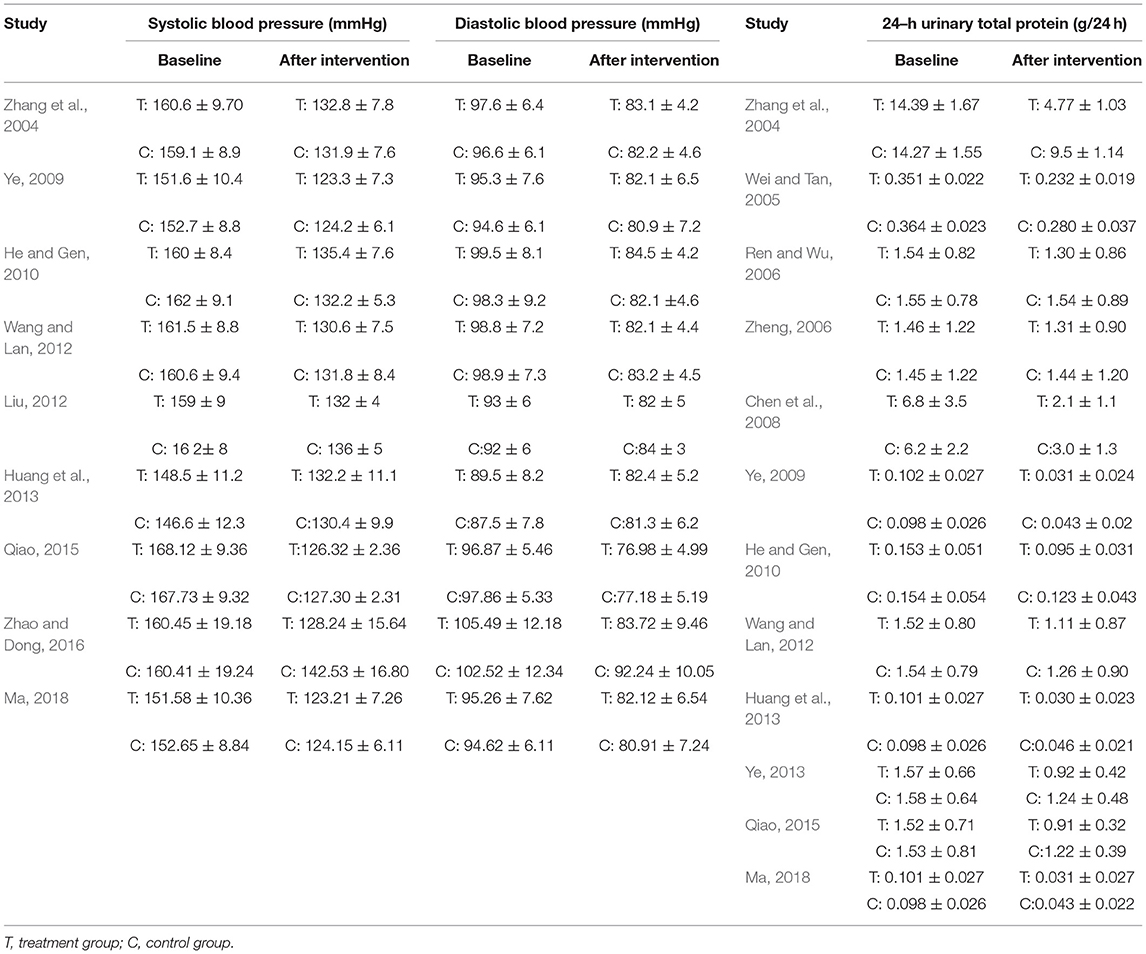
Table 2. Study characteristics: Effect of breviscapine injection on blood pressure and 24–hour urinary total protein in patients with hypertension-induced renal damage.
Risk of Bias in Included Studies
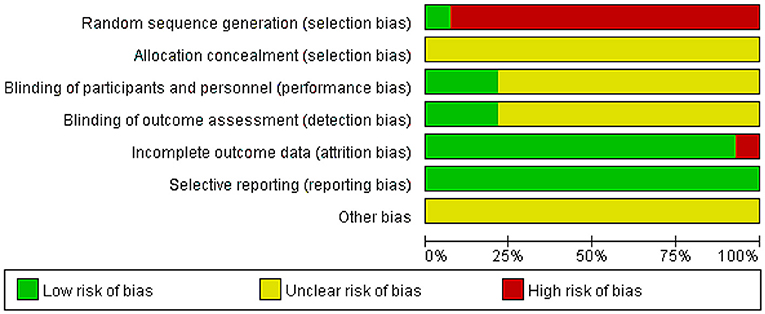
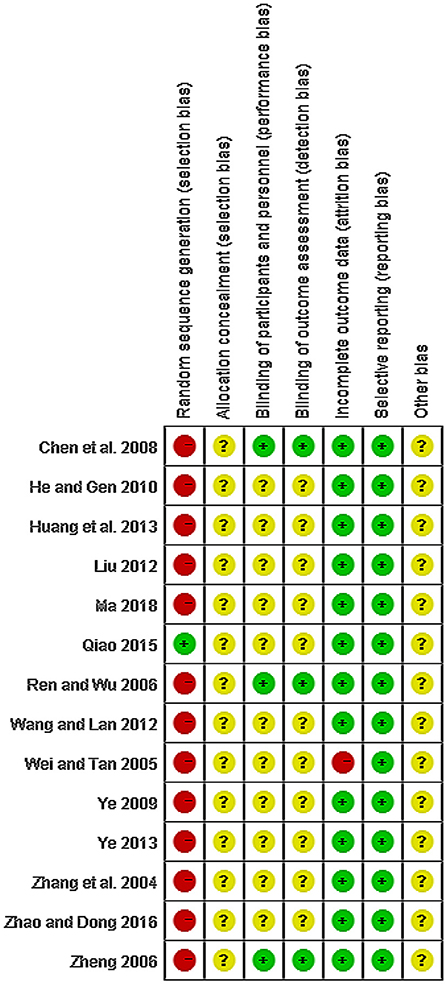
The quality of all studies included was low. All studies were carried out among the Chinese population. Fourteen studies mention the use of random allocation: All studies failed to mention the specific grouping method, and none of the studies discussed allocation concealment, blinding, or evaluator blinding. The quality assessment is shown in Figures 2, 3 (Wu et al., 2018).
Outcome Measures
Systolic Blood Pressure (SBP, mmHg)
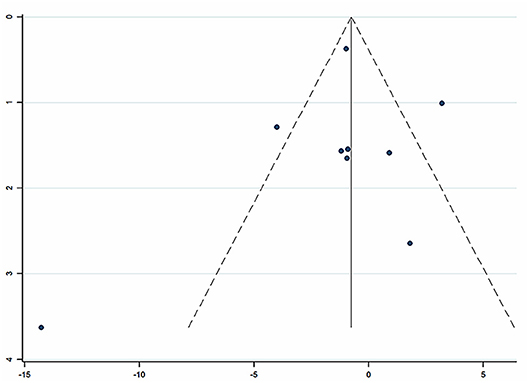
Nine studies (Zhang et al., 2004; Ye, 2009; He and Gen, 2010; Liu, 2012; Wang and Lan, 2012; Huang et al., 2013; Qiao, 2015; Zhao and Dong, 2016; Ma, 2018) involving 857 participants reported on the use of breviscapine injection plus antihypertensive drugs in the treatment of SBP for hypertension-induced renal damage. After the test for heterogeneity (I2 = 79.0%, P ≤ 0.001, Figure 4), we employed a random-effects model. A funnel plot analysis of the 9 trials suggested possible publication bias and inclusion of low quality studies as significant asymmetry is shown in Figure 5. We applied Egger's test to evaluate publication bias. A p (P = 0.757) value more than 0.05 was considered no publication bias (Supplementary Figure 1). The meta-analysis revealed that there was no significant difference between the experimental group and the control group in reducing SBP [WMD = −1.02, 95% CI (−2.88, 0.84), P = 0.281] (Supplementary Figure 2) (Wu et al., 2018).
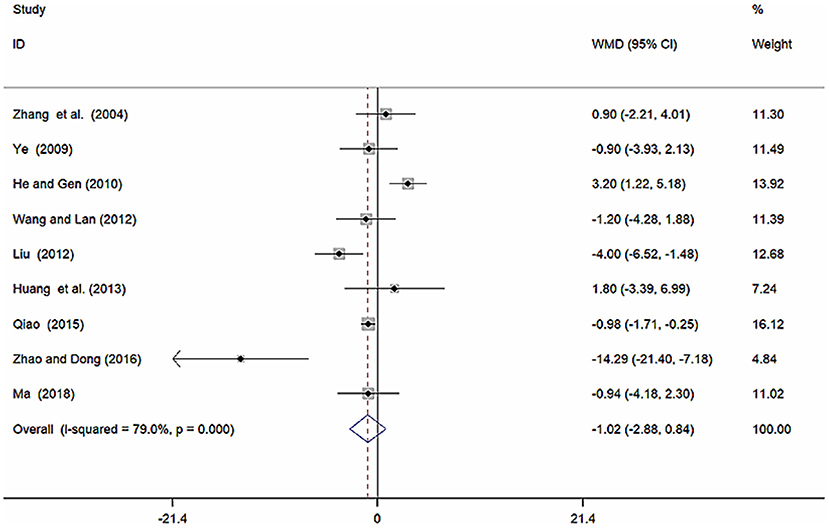
Figure 4. Forest plot of SBP of breviscapine injection plus antihypertensive drugs compared to antihypertensive drugs alone for hypertension-induced renal damage.
Sensitivity Analysis of SBP
We conducted a sensitivity analysis for SBP (Supplementary Figure 3). By seriatim excluding one trial each time and re-performing meta-analysis of the remaining trials, we could observe whether the outcomes have dramatically changed. Sensitivity analysis indicated that the outcomes of Scr were very similar, which had relatively good stability.
Subgroup Analysis of SBP
Because of high heterogeneity of SBP, we conducted subgroup analysis among studies using different different doses of breviscapine injection (20, 10, and 5 ml). Compared with the control groups, the results of subgroup analysis showed significant difference between breviscapine injection (20 ml) and antihypertensive drugs alone[WMD = 2.47, 95% CI (0.88, 4.06), P = 0.002], between breviscapine injection (10 ml) and antihypertensive drugs alone [WMD = −2.74, 95% CI (−5.47, −0.01), P = 0.002], but had no significant difference between breviscapine injection (5 ml) and antihypertensive drugs alone [WMD = −2.55, 95% CI (−5.57, 0.47), P = 0.097] (Figure 6, Supplementary Figure 4).
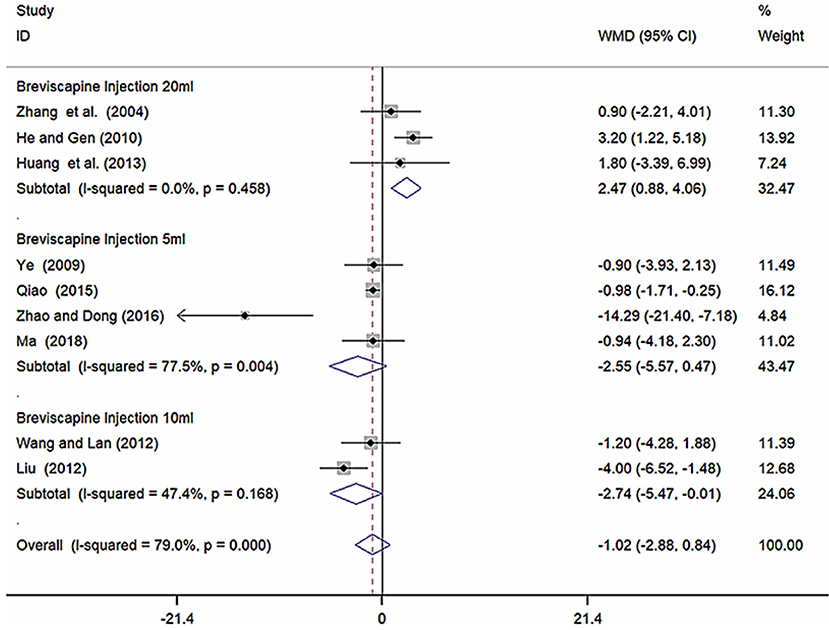
Figure 6. Subgroup analysis of different doses of breviscapine injection plus antihypertensive drugs compared to antihypertensive drugs alone in terms of SBP for hypertension-induced renal damage.
Diastolic Blood Pressure (DBP, mmHg)
Nine studies (Zhang et al., 2004; Ye, 2009; He and Gen, 2010; Liu, 2012; Wang and Lan, 2012; Huang et al., 2013; Qiao, 2015; Zhao and Dong, 2016; Ma, 2018) involving 857 participants reported on the use of breviscapine injection plus antihypertensive drugs in the treatment of DBP for hypertension-induced renal damage. After the test for heterogeneity (I2 = 76.5%, P ≤ 0.001, Figure 7), we employed a random-effects model. We conducted a sensitivity analysis and applied Egger's test (P = 0.160) to evaluate publication bias for DBP (Supplementary Figures 5, 6). Meta-analysis showed a non-significant trend for reduction in DBP between the experimental group and the control group [WMD = −0.21, 95% CI (−1.71, 1.29), P = 0.786] (Supplementary Figure 7).
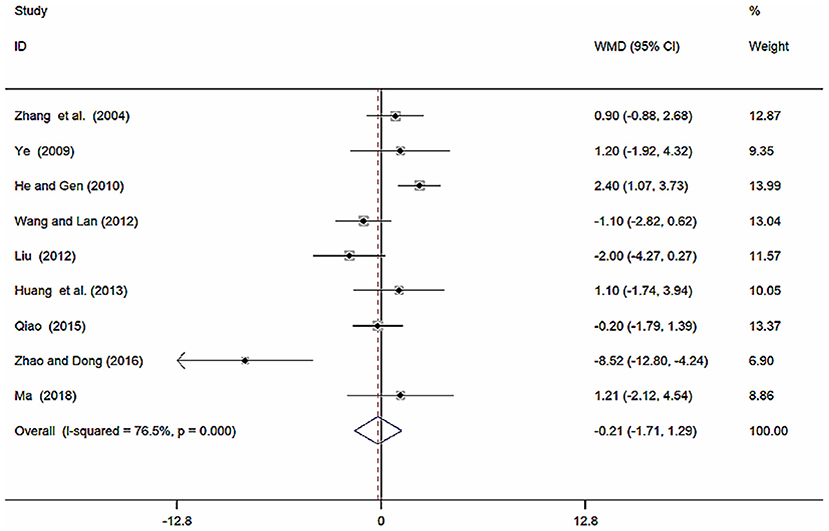
Figure 7. Forest plot of DBP of breviscapine injection plus antihypertensive drugs compared to antihypertensive drugs alone for hypertension-induced renal damage.
Subgroup Analysis of DBP
Because of high heterogeneity of DBP, we conducted subgroup analysis among studies using different different doses of breviscapine injection (20, 10, and 5 ml). Compared with the control groups, the results of subgroup analysis showed significant difference between breviscapine injection (20 ml) and antihypertensive drugs alone [WMD = 1.77, 95% CI (0.77, 2.77), P = 0.001], between breviscapine injection (10 ml) and antihypertensive drugs alone [WMD = −1.43, 95% CI (−2.80, −0.06), P = 0.041], but had non-significant difference between breviscapine injection (5 ml) and antihypertensive drugs alone [WMD = −1.25, 95% CI (−4.59, 2.09), P = 0.462] (Figure 8, Supplementary Figure 8).
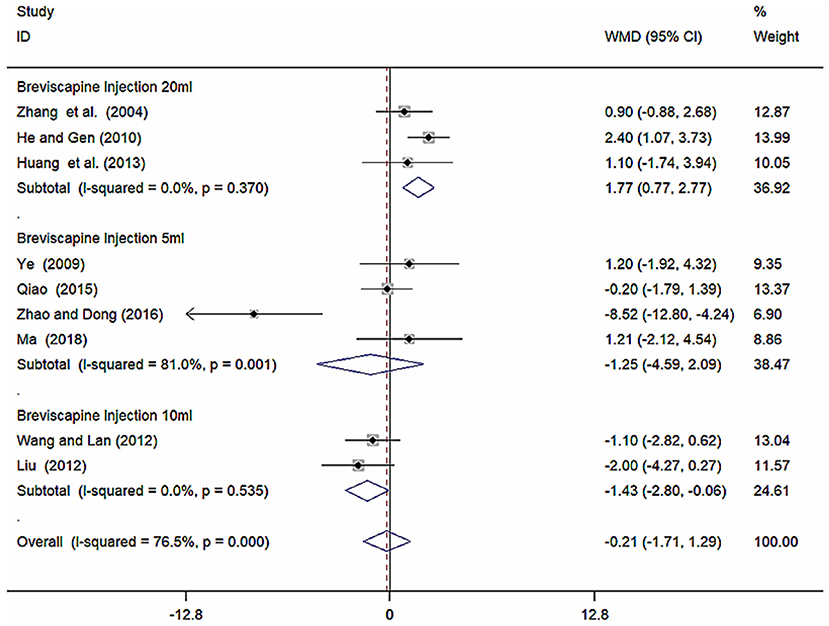
Figure 8. Subgroup analysis of different doses of breviscapine injection plus antihypertensive drugs compared to antihypertensive drugs alone in terms of DBP for hypertension-induced renal damage.
Twenty-Four Hour Urinary Total Protein (24 h UTP, g/d)
Twelve studies (Zhang et al., 2004; Wei and Tan, 2005; Ren and Wu, 2006; Zheng, 2006; Chen et al., 2008; Ye, 2009, 2013; He and Gen, 2010; Wang and Lan, 2012; Huang et al., 2013; Qiao, 2015; Ma, 2018) involving 941 participants reported on the use of breviscapine injection plus antihypertensive drugs in terms of the 24 h UTP for hypertension-induced renal damage. After the test for heterogeneity (I2 = 93.7%, P ≤ 0.001, Figure 9), we employed a random-effects model. We conducted a sensitivity analysis and applied the Egger's test (P = 0.586) to evaluate publication bias for 24 h UTP (Supplementary Figures 9, 10). The meta-analysis revealed that the experimental group performed better than the control group in reducing 24 h UTP [WMD = −0.04, 95% CI (−0.05, −0.02, P ≤ 0.001, Supplementary Figure 11) (Wu et al., 2018).
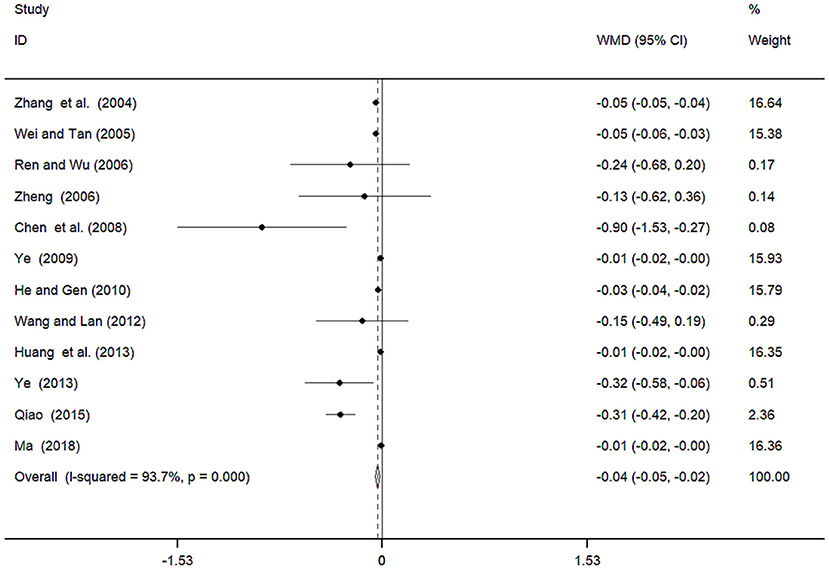
Figure 9. Meta-analyses results of breviscapine injection plus antihypertensive drugs compared to antihypertensive drugs alone in terms of the 24h UTP for hypertension-induced renal damage.
Adverse Effects
Nine of the included trials (Zhang et al., 2004; Wei and Tan, 2005; Ren and Wu, 2006; Zheng, 2006; Chen et al., 2008; Ye, 2009; He and Gen, 2010; Wang and Lan, 2012; Huang et al., 2013) described adverse effects in detail, while the others did not mention adverse events. Only one showed mild facial flushing during intravenous dripping of breviscapine (Wei and Tan, 2005), and after the speed of transfusion was slowed down, the symptom got remitted. Two cases (Huang et al., 2013) showed redness of limb skin in the experimental group. There were two cases (Ren and Wu, 2006; Zheng, 2006) of head inflation during the use of antihypertensive drugs. There were three cases (Chen et al., 2008; Ye, 2009) of head swelling, dizziness during infusionduring the use of breviscapine injection. Twenty-one cases showed dry cough due to the use of ACEI in six trials (Zhang et al., 2004; Ren and Wu, 2006; Zheng, 2006; Chen et al., 2008; He and Gen, 2010; Wang and Lan, 2012). None of the adverse events were serious. These symptoms are self-limited and did not affect the treatment.
Discussion
This meta-analysis included 14 studies with 1,170 total participants comparing breviscapine injection plus antihypertensive drugs vs. antihypertensive drugs alone for hypertension in patients with hypertension-induced renal damage. Hypertension-induced renal damage marks with increased excretion of urinary protein, which is mostly attributed to increased permeability of glomerular filtration membrane, damage of electrostatic barrier, and renal tubular reabsorption. Urinary excretions of albumin and beta-2-microglobulin are sensitive to early functional damage of glomerular filtration and renal tubular reabsorption, respectively. It is defined as a urinary albumin excretion rate ranging from 30 to 300 mg/day, and the definitive measurement is based on a timed urine collection during a 24-h period. Evidence from previous systematic review shows that breviscapine injection in combination with antihypertensive drugs can improve creatinine clearance rate and reduce serum creatinine, blood urea nitrogen, 24-h urinary protein and beta-2-microglobulin in hypertensive nephropathy patients, a reduction in urinary protein may contribute toward the renal protective effect of breviscapine injection in hypertension-induced renal damage patients (Wu et al., 2018). This meta-analysis mainly explores the effects of breviscapine injection on hypertension in hypertension-induced renal damage patients.
Hypertension can affect each renal compartment: vessels, glomeruli and tubulointerstitium. Since the pioneering descriptions of nephrosclerosis by Theodor Fahr and Franz Volhard in 1918, a large number of studies have been published on hypertensive nephropathy (Meyrier, 2015; Seccia et al., 2017). Hypertension-induced renal damage is a common cause of ESRD and is associated with significant morbidity and mortality. To maximize reduction in the cardiovascular and renal mortality associated with hypertension-induced renal damage (Bakris, 2004; Kistorp et al., 2005), physicians and their patients should set specific treatment goals that must be achieved with the least intrusive strategy possible. The most effective strategy to maximally delay CKD progression must include albuminuria reduction and achievement of the BP goal of <130/80 mmHg (Whelton et al., 2018; Williams et al., 2018). This can be done by using multiple antihypertensive agents, including RAAS blockers and diuretics, together with adherence to dietary sodium restriction (Whelton et al., 2018; Williams et al., 2018).
Erigeron breviscapus is a kind of traditional Chinese medicine. It was first recorded in the book of “Yunnan Materia Medica.” According to Chinese medicine theory, erigeron breviscapus is cold-natured, sweet, bitter and pungent in taste, with the function of clearing heat, relieving toxicity, eliminating wind and dampness, activating blood and removing stasis, expediting channel and activating meridian, relieving inflammation, and alleviating pain. The monomer component of Chinese herbal medicine (CHM), also known as the natural pure compound drug, has recently attracted much attention. The natural extract artemisinin and its derivatives are good examples of monomer components of CHM that can treat diseases through various activities, and can be a good starting point to uncover the mechanism of traditional Chinese medicine.
Hypertension-induced renal damage is major target-organ damage due to sustained high BP. Long-term hypertension can cause renal sclerosis and gradually progress to chronic renal failure. Positive control of hypertension is the key to preventing hypertensive renal damage. It is unclear whether breviscapine can reduce blood pressure in animal studies or clinical trials, but breviscapine can improve myocardium in animal studies. Breviscapine could reverse the myocardial, interstitial and vascular remodeling, improve the rigidness of cardiac muscle, thus, has protective effect on heart. The mechanisms of their above-mentioned effects may be related to increase of cardiac myocyte apoptosis triggered by them via upregulation of apoptosis induced gene (Bax gene) expression and downregulation of apoptosis suppressor gene (Bcl-2 gene) expression as well as suppression of PKC activity (Li and Chen, 2002; Zhou et al., 2002). According to Chinese medicine theory, hypertensive renal injury is strongly related to fluid, phlegm and dampness retention syndrome and liver-yang hyperactivity syndrome, which are caused by deficiency syndrome. Chinese medicines, which are used to treat fluid, phlegm, and dampness retention syndrome, deficiency syndrome and liver-yang hyperactivity syndrome, respectively, have certain advantages with regard to treating hypertensive renal injury. Clinical research indicates that CHM might control increased SBP, inhibit the glomerular and tubular hyperplasia caused by high BP and significantly reduce urinary albumin and beta-2-microglobulin by increasing the activity of renal rennin and the level of Ang II (Shih et al., 2005; Yang et al., 2014, 2015; Xiong et al., 2015).
Limitations
Several limiting factors in this study should be considered. First, the quality of the included randomized controlled trials was low. All included trials showed high or undefined risk of bias due to design, reporting, and methodology. There were no definitive, randomized, double-blind, placebo-controlled trials included in this meta-analysis. No trial reported detailed randomization methods, or allocation concealment. Therefore, “so-called” trials may not truly represent true randomized controlled trials, which may undermine the strength of clinical evidence and lead to potential selection bias. No trial was double-blinded, and unfortunately, none of the randomized-controlled trial has a placebo. Second, five trials did not report adverse reactions. Therefore, conclusions about safety cannot be made with confidence. Furthermore, certain active ingredients are chemically unstable, which limits large-scale synthesis. These pressing issues should be resolved in future research. The safety of breviscapine injection needs to be strictly monitored and properly reported in future clinical trials. Third, although most tests were conducted with small samples. We tried our best to avoid language bias and positional prejudice, but cannot rule out potential publication bias.
Overall, our results suggest that moderate dose of breviscapine injection (10 ml) has effects on reducing blood pressure in patients with hypertension-induced renal damage and high doses of breviscapine injection (20 ml) may increase blood pressure. More large-scale, multicenter, rigorously designed randomized controlled trials are needed to provide accurate data to further validate and s explore the mechanism of breviscapine injection for the treatment of hypertensive renal damage.
Conclusions
Evidence from this systematic review shows that breviscapine injection in combination with antihypertensive drugs, can reduce 24-h urinary protein in patients with hypertension-induced renal damage, but overall have no effect on improving the blood pressure in hypertension-induced renal damage patients. Moderate dose of breviscapine injection (10 ml) may have effects on reducing blood pressure in hypertension-induced renal damage patients but high doses of breviscapine injection (20 ml) may increase blood pressure by subgroup analysis. However, the evidence of methodological quality and sample sizes is weak, and thus, further standardized research is required.
Author Contributions
LW and ZF put forward this topic and designed this review. LW, YG, and SZ performed article screening, data collection and extraction, and manuscript writing. LW and YG conducted the data analysis.
Funding
This work is supported by Social Development Key Programs of Science and Technology Commission Foundation of Jiangsu Province (grant BE2015730), and the first level of 333Project of Jiangsu Province (grant BRA2016503).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00118/full#supplementary-material
References
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group (2002). Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288, 2981–2997. doi: 10.1001/jama.288.23.2981
Bakris, G. L. (2004). Clinical importance of microalbuminuria in diabetes and hypertension. Curr. Hypertens. Rep. 6, 352–356. doi: 10.1007/s11906-004-0053-1
Chen, Y. J., Lin, L. H., Huang, X. T., and Xiao, H. (2008). Therapeutic effect of breviscapine combined with benazepril on hypertensive nephropathy. Int. Med. Health Rep. 14, 69–71. doi: 10.3760/cma.j.issn.1007-1245.2008.22.030
Fervenza, F. C., Textor, S. C., Zand, D., and Rosenthal, D. (2017). Nephrosclerosis. Available online at: http://edmedicine.medscape.com (Accessed December 29, 2017).
Forouzanfar, M. H., Liu, P., Roth, G. A., Ng, M., Biryukov, S., Marczak, L., et al. (2017). Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 317, 165–182. doi: 10.1001/jama.2016.19043
Freedman, B. I., Hicks, P. J., Bostrom, M. A., Cunningham, M. E., Liu, Y., Divers, J., et al. (2009). Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 75, 736–745. doi: 10.1038/ki.2008.701
Gao, J. L., Chen, G., He, H. Q., Liu, C., Xiong, X. J., Li, J., et al. (2017). Therapeutic effects of breviscapine in cardiovascular diseases: a review. Front. Pharmacol. 8:289. doi: 10.3389/fphar.2017.00289
Grisk, O., and Rettig, R. (2004). Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc. Res. 61, 238–246. doi: 10.1016/j.cardiores.2003.11.024
He, B. L., and Gen, L. J. (2010). Therapeutic effect of breviscapine combined with captopril on early renal damage in patients with hypertension. Chin. J. Misdiagn. 10, 2329–2330.
He, M., Xue, Z. M., Li, J., and Zhou, B. Q. (2012). Breviscapine inhibits high glucose-induced proliferation and migration of cultured vascular smooth muscle cells of rats via suppressing the ERK1/2 MAPK signaling pathway. Acta Pharmacol. Sin. 33, 606–614. doi: 10.1038/aps.2012.6
Higgins, J. P. T., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Huang, S. X., Mu, X. M., and Zhu, Y. P. (2013). Effect of breviscapine on early renal impairment in patients with essential hypertension. J. Chin. Med. 28, 27–28.
Jia, J. H., Chen, K. P., Chen, S. X., Liu, K. Z., Fan, T. L., and Chen, Y. C. (2008). Breviscapine, a traditional Chinese medicine, alleviates myocardial ischaemia reperfusion injury in diabetic rats. Acta Cardiol. 63, 757–762. doi: 10.2143/AC.63.6.2033394
Jiang, L., Xia, Q. J., Dong, X. J., Hu, Y., Chen, Z. W., Chen, K., et al. (2017). Neuroprotective effect of breviscapine on traumatic brain injury in rats associated with the inhibition of GSK3beta signaling pathway. Brain Res. 1660, 1–9. doi: 10.1016/j.brainres.2017.01.031
Joles, J. A., and Koomans, H. A. (2004). Causes and consequences of increased sympathetic activity in renal disease. Hypertension 43, 699–706. doi: 10.1161/01.HYP.0000121881.77212.b1
Kistorp, C., Raymond, I., Pedersen, F., Gustafsson, F., Faber, J., and Hildebrandt, P. (2005). N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 293, 1609–1616. doi: 10.1001/jama.293.13.1609
Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. R., Feldman, H. I., et al. (2009). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. doi: 10.7326/0003-4819-150-9-200905050-00006
Lewington, S., Clarke, R., Qizilbash, N., Peto, R., and Collins, R. (2002). Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913. doi: 10.1016/S0140-6736(02)11911-8
Li, F. Q., and Chen, Y. Z. (2002). Effects of scutellarein on cardiomyocyte apoptosis and ventricular remodeling in spontaneously hypertensive rats. J. Chongqing Med. Univ. 27, 400–416. doi: 10.3969/j.issn.0253-3626.2002.04.010
Liu, F. (2012). Clinical study of breviscapine combined with benazepril in the treatment of hypertensive renal damage. Chin. J. Pract. Med. 39, 84–85. doi: 10.3760/cma.j.issn.1674-4756.2012.01.040
Liu, X. D., Yao, L., Sun, D., Zhu, X. W., Liu, Q., Xu, T. H., et al. (2016). Effect of breviscapine injection on clinical parameters in diabetic nephropathy: a meta-analysis of randomized controlled trials. Exp. Ther. Med. 12, 1383–1397. doi: 10.3892/etm.2016.3483
Lv, J., Ehteshami, P., Sarnak, M. J., Tighiouart, H., Jun, M., Ninomiya, T., et al. (2013). Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 185, 949–957. doi: 10.1503/cmaj.121468
Ma, H. H. (2018). Clinical effect of breviscapine injection in patients with hypertensive nephropathy. Med. Equipm. 31, 133–134.
Meyrier, A. (2015). Nephrosclerosis: update on a centenarian. Nephrol. Dial. Transplant. 30, 1833–1841. doi: 10.1093/ndt/gfu366
Pang, R. Q., Pan, X H., Long, P. R., Tan, M., and Wu, Y. L. (2004). Effect of breviscapine on the proliferation of rabbit vascular smooth muscle cells. Chin. J. Arterioscler. 12, 395–398. doi: 10.3969/j.issn.1007-3949.2004.04.006
Qiao, S. Q. (2015). Clinical study of breviscapine injection in the treatment of hypertensive nephropathy. TCM Clin. Res. 7, 13–15. doi: 10.3969/j.issn.1674-7860.2015.20.006
Ren, W., and Wu, H. W. (2006). Therapeutic effect of breviscapine combined with western medicine on hypertensive nephropathy. Modern Chinese medicine. 26, 35–36. doi: 10.13424/j.cnki.mtcm.2006.04.020
Review Manager (2014). (RevMan) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Available online at: https://community.cochrane.org.
Safar, M., Chamiot-Clerc, P., Dagher, G., and Renaud, J. F. (2001). Pulse pressure, endothelium function, and arterial stiffness in spontaneously hypertensive rats. Hypertension 38, 1416–1421. doi: 10.1161/hy1201.096538
Seccia, T. M., Caroccia, B., and Calo, L. A. (2017). Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J. Hypertens. 35, 205–212. doi: 10.1097/HJH.0000000000001170
Shih, H. C., Lee, T. H., Chen, S. C., Li, C. Y., and Shibuya, T. (2005). Anti-hypertension effects of traditional Chinese medicine ju-ling-tang on renal hypertensive rats. Am. J. Chin. Med. 33, 913–921. doi: 10.1142/S0192415X05003545
Sim, J. J., Shi, J., Kovesdy, C. P., Kalantar-Zadeh, K., and Jacobsen, S. J. (2014). Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J. Am. Coll. Cardiol. 64, 588–597. doi: 10.1016/j.jacc.2014.04.065
Song, Y., Zhang, H. M., Ma, J. J., and Li, C. L. (2011). Effects of scutellarein on thrombosis and hemorheology in rats. Chin. J. New Drugs 15, 1446–1449. doi: 10.1631/jzus.B1000135
USRDS. (2018). 2017 USRDS Annual Data Report: Executive Summary. Am. J. Kidney Dis. 71, S1–S8. doi: 10.1053/j.ajkd.2018.01.003
Wang, C., Li, Y., Gao, S., Cheng, D., Zhao, S., and Liu, E. (2015). Breviscapine injection improves the therapeutic effect of western medicine on angina pectoris patients. PLoS ONE 10:e0129969. doi: 10.1371/journal.pone.0129969
Wang, M., Xie, C., Cai, R. L., Li, X. H., Luo, X. Z., and Qi, Y. (2008). Studies on antioxidant activities of breviscapine in the cell-free system. Am. J. Chin. Med. 36, 1199–1207. doi: 10.1142/S0192415X08006521
Wang, M., Zhang, W. B., Song, J. L., Luan, Y., and Jing, C. Y. (2018). Effect of breviscapine on recovery of viable myocardium and left ventricular remodeling in chronic total occlusion patients after revascularization: rationale and design for a randomized controlled trial. Med. Sci. Monit. 24, 4602–4609. doi: 10.12659/MSM.906438
Wang, M., Zhang, W. B., Zhu, J. H., Fu, G. S., and Zhou, B. Q. (2010). Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol. 47(Suppl. 1), 209–218. doi: 10.1007/s00592-009-0164-x
Wang, W. L., and Lan, L. D. (2012). “Therapeutic effect of breviscapine combined with lisinopril on hypertensive nephropathy”, in 2012 Zhejiang Medical Association Clinical Pharmacy Academic Annual Conference.
Wei, L., and Tan, J. (2005). Clinical observation on Breviscapine in treating hypertension patients complicated with micro-albuminuria of renal impairment. Chin. J. Integr. Med. 11, 31–33. doi: 10.1007/BF02835745
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. J., Collins, K. J., Dennison, H. C., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324. doi: 10.1161/HYP.0000000000000066
Williams, B., Mancia, G., Spiering, W., Agabiti, R. E., Azizi, M., Burnier, M., et al. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 36, 1953–2041. doi: 10.1097/HJH.0000000000001940
Wu, L. H., Liu, M., and Fang, Z. Y. (2018). Combined therapy of hypertensive nephropathy with breviscapine injection and antihypertensive drugs: a systematic review and a meta-analysis. Evid. Based Complement. Alternat. Med. 2018:2958717. doi: 10.1155/2018/2958717
Xiong, X. J., Wang, P. J., and Li, S. J. (2015). Meta-analysis of the effectiveness of traditional Chinese herbal formula Zhen Wu Decoction for the treatment of hypertension. BMJ Open 5:e007291. doi: 10.1136/bmjopen-2014-007291
Yang, P., Shih, W., Chu, Y., Chen, P., and Wu, C. (2015). Frequency and co-prescription pattern of Chinese herbal products for hypertension in Taiwan: a Cohort study. BMC Complement. Altern. Med. 15:163. doi: 10.1186/s12906-015-0690-8
Yang, Q., He, Y. M., and Wang, W. J. (2014). The protective effect of Liu-Wei-Di-Huang-Fang in salt-sensitive hypertension rats. Clin. Exp. Hypertens. 36, 426–432. doi: 10.3109/10641963.2013.846357
Yang, X. P., and Li, Q. F. (2007). Clinical observation on the therapeutic effect of Brevisca Pine in treatment of acute cerebral infarction. Chin. J. Pract. Nervous Dis. 10, 11–13. doi: 10.3969/j.issn.1673-5110.2007.02.006
Ye, P. (2009). Clinical analysis of breviscapine in the treatment of hypertensive nephropathy. Chin. Contempor. Med. 16, 64–65. doi: 10.3969/j.issn.1674-4721.2009.09.037
Ye, Z. (2013). Clinical study of breviscapine injection in the treatment of hypertensive nephropathy. J. Guangxi Univ. Tradit. Chin. Med. 16, 45–46. doi: 10.3969/j.issn.1008-7486.2013.02.020
Zhang, R. W., Zhang, Y., and Wang, J. (1988). Isolation and identification of flavonoids from shortscape fleabane (Erigeron breviscapus). Zhong Cao Yao 19, 199-201.
Zhang, Y., Wang, J. S., and Zhang, L. M. (2004). Effect of breviscapine combined with lisinopril on urinary microalbumin in patients with hypertension. Hum. J. Trad. Chin. Med. 20, 12–13. doi: 10.16808/j.cnki.issn1003-7705.2004.06.006
Zhao, Q., and Dong, G. (2016). Effect of breviscapine on serum fibrosis index and arterial elasticity index in patients with hypertensive nephropathy. Chin. J. Biochem. Med. 36, 151–153.
Zheng, G. H., Dong, Y. F., Liang, Y. L., Li, W. G., and Xie, C. H. (1998). Effects of breviscapine on isolated rat thoracic aortic ring. Chin. Tradit. Herbal Drugs 10, 680–683.
Zheng, X. L. (2006). Therapeutic effect of breviscapine combined with western medicine on hypertensive nephropathy. Pract. Chin. Med. J. 22, 144–145. doi: 10.3969/j.issn.1004-2814.2006.03.018
Keywords: breviscapine injection, hypertension, systematic review, meta-analysis, effects
Citation: Wu L, Gao Y, Zhang S and Fang Z (2019) The Effects of Breviscapine Injection on Hypertension in Hypertension-Induced Renal Damage Patients: A Systematic Review and a Meta-Analysis. Front. Pharmacol. 10:118. doi: 10.3389/fphar.2019.00118
Received: 31 October 2018; Accepted: 31 January 2019;
Published: 21 February 2019.
Edited by:
Steyner F. Cortes, Federal University of Minas Gerais, BrazilReviewed by:
Ai-Jun Liu, Second Military Medical University, ChinaCarlos R. Tirapelli, University of São Paulo, Brazil
Copyright © 2019 Wu, Gao, Zhang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuyuan Fang, anNzenl5Znp5QDE2My5jb20=
†These authors have contributed equally to this work
 Lihua Wu
Lihua Wu Yanhua Gao2†
Yanhua Gao2†