
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Pharmacol., 09 January 2019
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.01532
This article is part of the Research TopicAdvanced Technologies for the Quality Control and Standardization of Plant Based MedicinesView all 20 articles
This study presented a rapid, simple and environmentally friendly method of employing AQ C18-based vortex-homogenized matrix solid-phase dispersion with ionic liquid (AQ C18-IL-VHMSPD) for the extraction of compounds with different polarities from Platycladi Cacumen (PC) samples by ultra high-performance liquid chromatography with PDA detection. AQ C18 (aqua C18) and ionic liquid ([Bmim]BF4) were used as the adsorbent and green elution reagent in vortex-homogenized MSPD procedure. The AQ C18-IL-VHMSPD conditions were optimized by studying several experimental parameters including the type of ionic liquid, the type of adsorbent, ratio of sample to adsorbent, the concentration and volume of ionic liquid, grinding time and vortex time. The recoveries of the target compounds were in the range of 96.9–104% with relative standard deviation values no more than 2.8%. The limits of detection and limits of quantitation were in the range of 0.2–1.2 and 1.0–5.4 ng mL-1, respectively. Compared with the traditional ultrasonic-assisted extraction, the developed AQ C18-IL-VHMSPD method required less sample, reagent and time. It was concluded that the AQ C18-IL-VHMSPD method was a powerful method for the extraction and quantification of the high polarity and low polarity compounds in traditional Chinese medicines samples.
Platycladi Cacumen (PC), namely Cebaiye, derived from the dry twigs and leaves of Platycladus orientalis (L.) Franco, is one of the most commonly used traditional Chinese medicines (TCMs). It has been applied in many TCM formulations for thousands of years. It was recorded that the PC could cool the blood to stanch bleeding, dispel pathogenic wind, remove dampness and resolve phlegm (Chinese Pharmacopoeia Commission, 2015). Recently, it was confirmed that PC has antimicrobial, anti-tumor, anti-inflammatory, anti-oxidant activities and neuroprotective effect (Hassanzadeh et al., 2001; Emami et al., 2011; Zaugg et al., 2011; Fan et al., 2012; Zhang et al., 2013). Flavonoids are regarded as the material basis for the efficacy. The main flavonoid glycosides consists of myricitrin, isoquercitrin, and quercitrin. In addition, there is a large amount of biflavonoids in PC. Among these biflavonoids, hinokiflavone, and amentoflavone are the representative ones (Lu et al., 2006). These five flavonoids with different polarities have been reported to exert a therapeutic effect on diseases in previous literatures (Ma and Wu, 2013). Thus, the complete extraction and precise analysis of the five compounds (Figure 1) with different polarities are particularly crucial for quality control and pharmacological investigations of herbal PC.
The conventional methods for the extraction of PC included the ultrasonic-assisted extraction (UAE) with HPLC-UV or UPLC-DAD (Zhuang et al., 2017, 2018), the microwave-based extraction with ultraviolet–visible detection, and heat reflux extraction with UPLC-DAD (Chen et al., 2007; Shan et al., 2018). However, these methods normally require a great deal of organic reagent, which arouse the environment pollution. Besides, the longer extraction time was another shortage. Matrix solid-phase dispersion (MSPD) is a comprehensive sample preparation method in which sample homogenization, disruption, extraction, fractionation and purification were simultaneously performed in one step and a shorter extraction time and less organic solvent were required (Barker et al., 1989; García-López et al., 2008). At present, many MSPD methods have been successfully applied for various samples (Liu et al., 2008; Vela-Soria et al., 2014). Recently, a vortex-homogenized MSPD (VHMSPD) technique not only retained the superiority of the traditional MSPD method but also overcame the shortage of the loss of analytes in complex procedures (Du et al., 2018a,b). However, no information about VHMSPD was available for the analysis of Platycladi Cacumen in the literature.
The adsorbent used in the MSPD procedure acts a pivotal part in improving the extraction efficiency of target analytes (Cao et al., 2016). Most applications of MSPD have utilized the silica gel matrix material and absorptive matrix materials. PSA, NH2, CN, COOH, C8, C18 (end capped), C18-N (no end capped) and AQ C18 were silica gel matrix material. Florisil was absorptive matrix materials. Compared with C18, more silanol groups linking to the surface of C18-N, which could provide the additional polar interactions. A certain proportion of polar functional groups were added to the surface of non-polar materials of AQ C18, which not only has the higher adsorption capacity on low polarity target compounds, but also greatly increases the adsorption capacity of high polarity target compounds. Thus, AQ C18 could be used to extract high polarity and low polarity target compounds from traditional Chinese medicines. To our knowledge, no reference on the use of AQ C18 as an adsorbent for the extraction of TCMs by VHMSPD has been reported.
Ionic liquids (ILs) are characterized by diverse combinations of organic or inorganic anions and organic cations. Recently, they have been widely used as green elution reagent for microextraction due to the advantages of high thermal stability, negligible vapor pressure and good solubility for inorganic and organic compounds (Han et al., 2012). It was reported that ILs could interact with analytes by various mechanisms such as π-π, electrostatic force, hydrogen bonding, ion-dipole, inclusion complex etc. (Qiu et al., 2012). Thus, the green ILs were applied for extracting analytes using VHMSPD method in the TCMs.
In this study, an AQ C18-based vortex-homogenized matrix solid phase dispersion with ionic liquids (AQ C18-IL-VHMSPD) technique with UHPLC-PDA was first established for simultaneous determination of compounds with different polarities from Platycladi Cacumen including three high polarity flavonoid glycosides (myricitrin, isoquercitrin and quercitrin) and two low polarity bioflavonoids (hinokiflavone and amentoflavone). The combination of AQ C18 and ionic liquid was applied to extract natural compounds in the vortex-homogenized matrix solid phase dispersion procedure. Some parameters such as type of adsorbent, sample/adsorbent ratio, and type and concentration of eluent were optimized to obtain a good extraction efficiency in detail. Furthermore, the five compounds were extracted by using the conventional ultrasonic-assisted extraction to evaluate the feasibility of the developed AQ C18-IL-VHMSPD method.
Reference standards of myricitrin, isoquercitrin, quercitrin, amentoflavone and hinokiflavone were purchased from Chengdu Desite Bio-Technology Co., Ltd., (Chengdu, China). PSA (40–60 μm, 60 A), NH2 (40–60 μm, 60 A), CN (50 μm, 60 A), COOH (50 μm, 60 A), Florisil (60–100 μm, 80 A), C8 (50 μm, 60 A), C18 (50 μm, 60 A), C18-N (50 μm, 60 A) and AQ C18 (50 μm, 60 A) were supplied from Agela Technologies. 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4), 1-hexyl-3-methylimidazolium tetrafluoroborate ([Hmim]BF4), 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]BF4), 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim] PF6) and 1-octyl-3-methylimidazolium hexafluorophosphate ([Omim]PF6) were purchased from Shanghai Chengjie Chemical Co., Ltd., HPLC-grade acetonitrile and methanol were purchased from Dikma Technologies Inc., United States. HPLC-grade formic acid was purchased from Tedia Company, Inc. (Tedia, Fairfield, OH, United States). Deionized water was purified from a Milli-Q academic ultra-pure water system (Millipore, Milford, MA, United States). Other chemical reagents were of analytical grade. All the solutions were filtered through a 0.22 μm filter membrane before UPLC analysis.
A total of 8 batches of Platycladi Cacumen and its processed products were collected from different regions of China and authenticated by Dr. Yan-xu Chang (Tianjin University of Traditional Chinese Medicine). All samples were pulverized using a pulverizer (Zhongcheng Pharmaceutical Machinery) after being dried at 60°C for 24 h, then passed through a 100-mesh sieve.
The standard stock solutions of myricitrin, isoquercitrin, quercitrin, hinokiflavone and amentoflavone were separately prepared in methanol. Myricitrin and quercitrin were 2 mg mL-1. Isoquercitrin, hinokiflavone and amentoflavone were 1 mg mL-1. The appropriate amount solution of all standards was diluted with methanol to obtain eight different appropriate concentrations for calibration curves. The concentrations of myricitrin, isoquercitrin, quercitrin, hinokiflavone and amentoflavone were in the range of 0.4–100, 0.08–20, 0.8–200, 0.2–50, and 0.2–50 μg mL-1, respectively. The related standard solutions were stored at 4°C.
The chromatographic analysis was performed on a Waters ACQUITY UPLC System (Waters Co., Milford, MA, United States) that consisted of a photodiode array (PDA). The workstation controlled by Empower 2 software was employed to collect and analyze data. The separation was performed on an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm, Waters) at the flow rate of 0.3 mL min-1. The mobile phase consisted of water with 0.1% formic acid (eluent A) and acetonitrile (eluent B) using a gradient elution: 0–2 min, 5–37% B; 2–9 min, 37–67% B; 9–10 min, 67–85% B; 10–13 min, 85–95% B; 13–15 min, 95–5% B, then post run 6 min. The column temperature was maintained at 30°C and the injection volume was 1 μL. The detection wavelength was set at 340 nm. Under the above chromatographic conditions, the chromatographic peaks of analytes included samples and standard solutions were separated excellently (Figure 2).
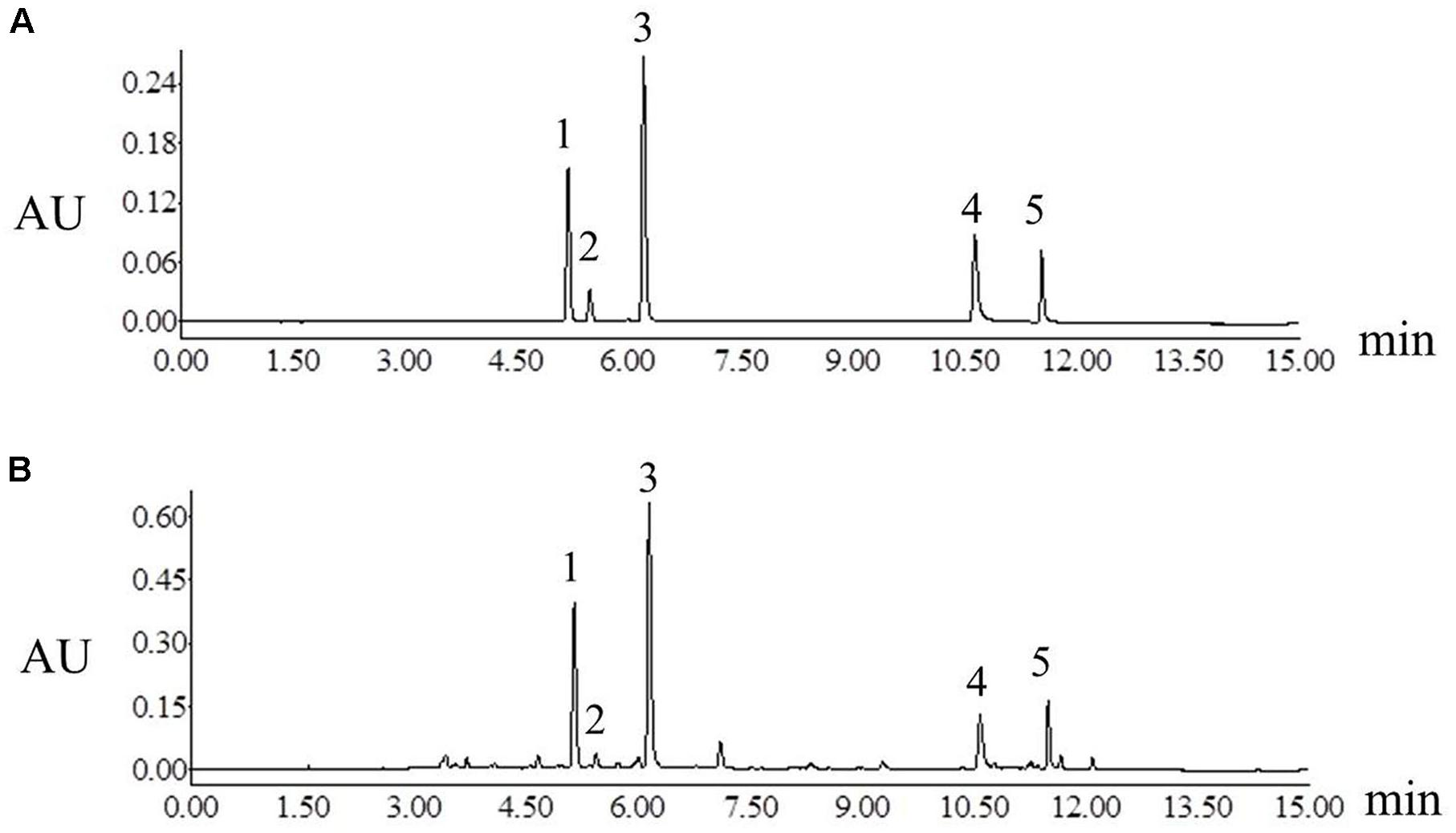
Figure 2. Ultra high-performance liquid chromatograms of mixture of standard compounds (A) Platycladi Cacumen samples (B). Peak: 1, myricitrin; 2, isoquercitrin; 3, quercitrin; 4, amentoflavone; 5, hinokiflavone.
An aliquot of 25 mg of the previously crushed sample and 50 mg adsorbents (AQ C18) were put into an agate mortar gradually. The mixture was grinded with a pestle for 3 min. Once completely dispersed, the mixture was transferred into a 4 mL polypropylene tube. 1.5 mL elution reagent ([Bmim]BF4) was added and then thoroughly shaken by vortex for 45 s. Subsequently, the tubes were placed into a centrifuge at 14000 rpm for 10 min. The supernatant liquor was collected and 1 uL was injected into the UHPLC for analysis. The schematic diagram of AQ C18-IL-VHMSPD method was exhibited in Figure 3.
According to the Chinese Pharmacopoeia 2015, the dried PC samples (0.500 g) were precisely weighed and introduced into a 100 mL Erlenmeyer flask, then mixed with 20 mL methanol. Finally the mixture was extracted ultrasonically (40 kHz, 96% power) for 30 min and the weight loss of the solution was complemented with methanol. All the extract solution was filtrated through a 0.22 μm filter membrane and 1 μL filter liquor was injected into the UPLC-PDA for further analysis.
To obtain a good extraction efficiency of the target compounds, several experimental parameters including the type of adsorbent, ratio of sample to adsorbent, type and concentration of the eluting solvent, and grinding time were investigated. Each test was repeated in triplicate.
Several adsorbents were investigated including PSA, NH2, CN, COOH, Florisil, C8, C18, C18-N and AQ C18. An aliquot of 25 mg PC samples and 50 mg adsorbents were transferred into an agate mortar, then grinded for 3 min. The eluent was 1.5 mL 90 mM ILs and 3 min was chosen as vortex time. Different types of ILs such as [Bmim]BF4, [Hmim]BF4, [Omim]BF4, [Bmim]PF6 and [Omim]PF6 were optimized. Then, four levels of concentration of [Bmim]BF4 (50, 70, 90, and 110 mM) and volumes of IL (0.5–2 mL) were considered to be optimized. The ratio of sample to adsorbent (1:0, 1:1, 1:2, and 1:3) was considered to investigate while the other conditions remained unchanged. In addition, the vortex time (15, 30, 45, and 60 s) and grinding time (0, 1, 2, 3, and 4 min) were also tested.
In the development of the MSPD procedure, it is crucial to employ a suitable dispersing adsorbent. The adsorbent is not only used as a disruption and dispersion agent that destroys the structure of samples for the extraction of the target compounds, but also as a purificant that removes the interfering substance of matrix. In the present study, nine types of adsorbent were evaluated. As Figure 4A shows, the analytes has the strongly retention when PSA, NH2, CN, COOH, Florisil and C8 were used as adsorbent. The reason was that the analytes bonded with the adsorbent too tightly to be eluted effectively. C18-N and C18 produced the equivalent extraction efficiency, but not dramatically better than AQ C18. One probable explanation was that the abundant Si-O-Si and Si-OH groups formed hydrogen bonds between adsorbent and analytes. Additionally, the interaction force may be supplied by the silica, including hydrogen bonding and electrostatic interaction. Therefore, AQ C18 was chosen as the optimal adsorbent for the subsequent extraction procedure.
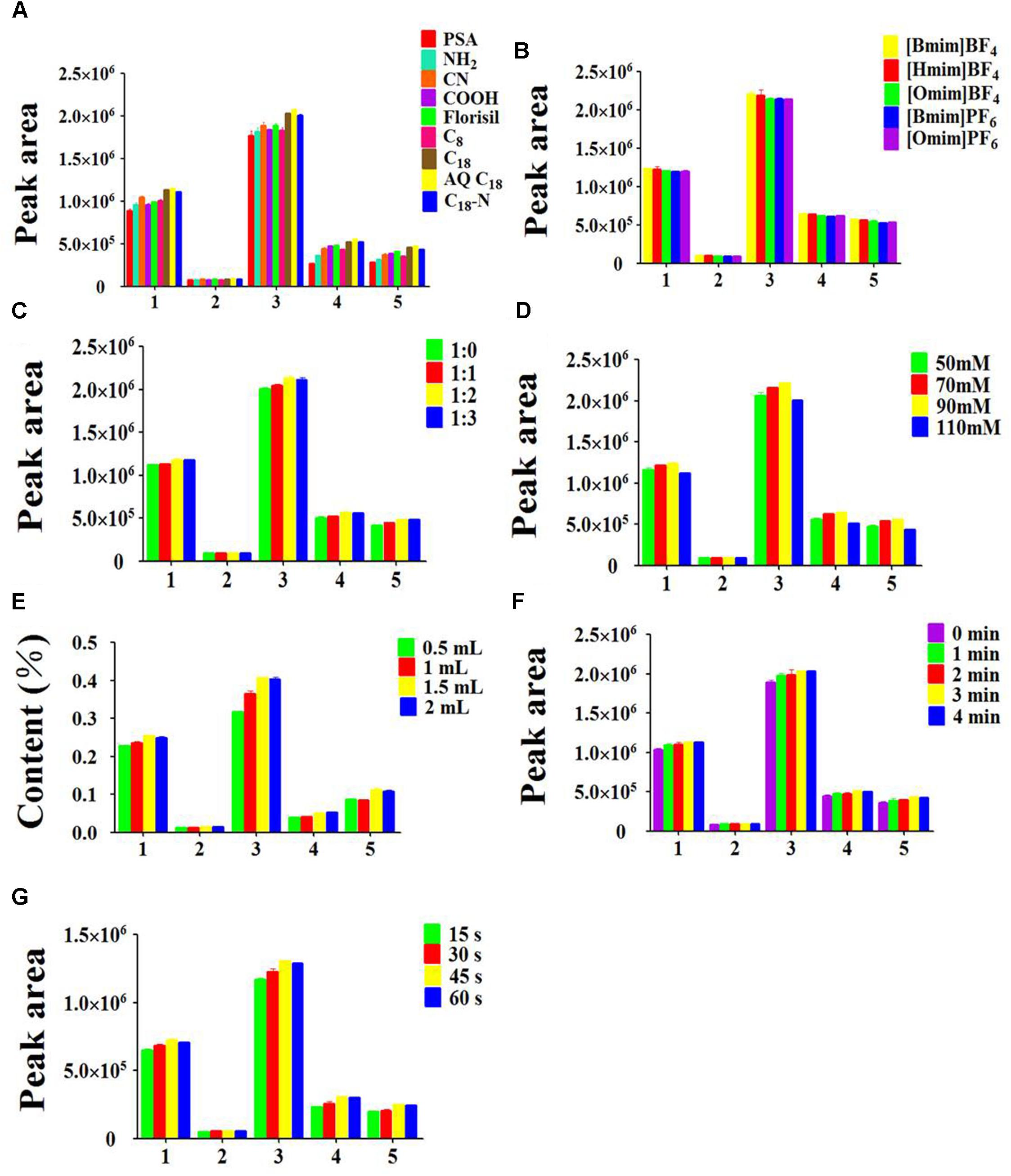
Figure 4. Effects of parameters on extraction efficiency of five peak: (1) myricitrin, (2) isoquercitrin, (3) quercitrin, (4) hinokiflavone, (5) amentoflavone, (A) type of the adsorbent, (B) type of the ionic liquid, (C) ratio of sample to adsorbent, (D) ionic liquid concentration, (E) ionic liquid volume, (F) grinding time, (G) vortex time. The errors bars represent RSD (n = 3).
An appropriate type of elution solvent is a significant parameter to obtain good extraction efficiency for target analytes. The elution solvent should have a similar polarity as target analytes, which is easy to disrupt the interaction between the target analytes and the adsorbent (García-Mayor et al., 2012). [Bmim]BF4, [Hmim]BF4, [Omim]BF4, [Bmim]PF6 and [Omim]PF6 are five common ionic liquids. To select the most appropriate one, these five ILs were evaluated based on the extraction efficiency of the target analytes in the MSPD procedure. As shown in Figure 4B, the elution efficiency of ILs was affected by the alkyl chain length of cation and the types of anion. It was quite obvious that the peak areas of the five target compounds were significantly increased from [Omim]BF4 to [Bmim]BF4. The mechanism of this phenomenon is that the hydrogen bonding interaction was weakening between the target analytes and imidazolium rings when alkyl chain length was increased with the same anion BF4-. Although [Bmim]BF4 and [Bmim]PF6 had the same cation, the highest peak areas of all analytes were observed with the BF4- anion. The possible reason is that the combination of anion BF4- and analytes generated the stronger electrostatic interaction. Overall, [Bmim]BF4 was chosen as the optimum elution solvent for the next experiment.
The mass ratio of adsorbent to sample is a significant parameter affecting the extraction yield of the target analytes from the samples (Rodrigues et al., 2010; Xu et al., 2016). An appropriate ratio of sample to adsorbent not only guarantees that the sample is completely homogenized and dispersed in the adsorbent, but also decreases the loss of sample in the blending procedure. It can be seen in Figure 4C that the peak areas of the five target compounds were distinctly increased when the sample/adsorbent ratio increased from 1:0 to 1:2. The possible reason was that the larger the amount of adsorbent, the stronger the molecular interaction produced, such as hydrogen bonding and electrostatic force between analytes and adsorbents. However, further increment of the sample/adsorbent ratio led to a slightly decrease of the extraction efficiency. The reason may be that the excessive adsorbent generated such a strong interaction between the five compounds and AQ C18 that eluent could not completely eluted. Thus, sample/sorbent ratio of 1:2 was selected.
The concentration and volume of [Bmim]BF4 are also crucial parameters affecting the extraction yield of the target analytes in the elution process. The results (Figure 4D) showed that the peak areas of target compounds were gradually increased from 50 to 90 mM. A possible reason was that the π-π, electrostatic and hydrogen-bond interactions between the [Bmim]BF4 and target analytes were stronger than the interaction of the analytes and adsorbent, while the extraction efficiency was slightly decreased following the increment of the concentration of [Bmim]BF4. This phenomenon could be ascribed to the intensive viscosity, which gave rise to poor capacity to transfer the target analytes from adsorbent into eluent. Thus, 90 mM [Bmim]BF4 seemed to be the optimum concentration for further experiments.
To attain the highest extraction efficiency with the minimum volumes of ILs, 0.5 mL to 2 mL volume of 90 mM [Bmim]BF4 were investigated. Figure 4E demonstrates that the contents of the five compounds were increased from 0.5 to 1.5 mL. The reason could be that the stronger interaction was generated between analytes and eluant along with the increase of the volume of eluant. Nevertheless, the peak areas of the five compounds remained unchanged when the volume kept increasing. T-test was introduced to evaluate the differences between two groups by Microsoft excel (version 2010). The results showed that there was no significant difference between 1.5 and 2 mL of 90 mM [Bmim]BF4. Consequently, 1.5 mL of [Bmim]BF4 was used in the following MSPD extraction procedure.
The grinding time is a vital parameter of great concern in the VH-MSPD method. In order to evaluate the effect of different grinding times, five time intervals at 0, 1, 2, 3, and 4 min were tested. As the results show in Figure 4F, the peak areas of the 5 target analytes were increased with increasing grinding time from 1 to 3 min. It was likely that the longer grinding time caused a stronger interaction force between AQ C18 and analytes, facilitating the transfer of the target analytes from samples into the adsorbent. However, the extraction efficiency employing 3 min-grinding was approximately equal to that of 4 min-grinding. Thus, the grinding time for 3 min was chosen for further experiments.
Previous studies have proven that vortex time is an important factor influencing the extraction efficiency. As shown in Figure 4G, the peak areas of the five target compounds were significantly increased as the vortex time increased from 15 to 30 s. However, increasing the vortex time to 60 s resulted in a slightly decline of the peak areas. Thus, 45 s was chosen as the optimal vortex time. Eventually, the optimized AQ C18-IL-VHMSPD conditions were determined to be 25 mg PC sample, 50 mg AQ C18, grinding time of 3 min, 1.5 mL 90 mM [Bmim]BF4 as the elution solvent and 45 s vortex time.
The calibration curves (n = 8) of 5 analytes were obtained by performing the peak areas as Y-axis, versus the concentration in μg mL-1 as X-axis, which ranged from 0.08 to 200 μg mL-1. The correlation coefficients (R2) of each analyte were higher than 0.9997 (Table 1).
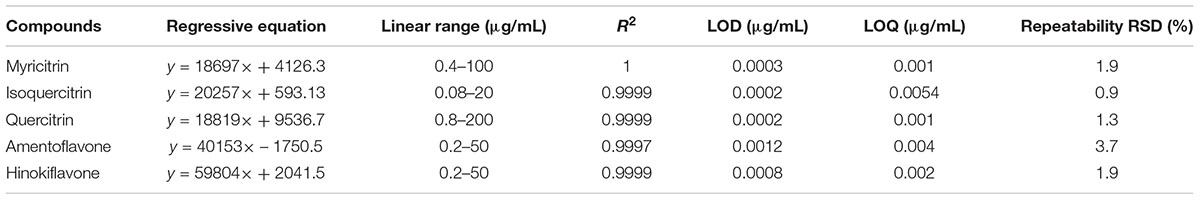
Table 1. Linearity, limits of detection (LOD), limit of quantification (LOQ), and repeatability of the proposed method (n = 6).
Limits of detection (LOD) and limit of quantification (LOQ) were employed to assess the sensitivity of the developed method. They were estimated as the concentrations of the analytes when the signal-to-noise (S/N) ratio reached 3 and 10 individually. The LODs of the five compounds ranged from 0.0002 to 0.0012 μg mL-1, while the LOQs ranged from 0.001 to 0.0054 μg mL-1 (Table 1).
The repeatability was evaluated by six parallel AQ C18-IL-VHMSPD extracts of a PC sample. As summarized in Table 1, the values of relative standard deviations (RSDs) were all less than 3.7%. It was confirmed from the results that the developed method had good reproducibility during experiment.
Instrumental precision was expressed as intra-day and inter-day precision by determining the relative standard deviations (RSDs) at three levels of concentrations in six replicates of each compounds. Intra-day precision and inter-day precision were tested in a single day and within continuous 3 days, respectively. The validation results are presented in Table 2, the accuracies were within the range of 95.2–104.2% (RSD ≤ 3.3%) and 96.2–114.6% (RSD ≤ 3.1%) for intra-day precision and inter-day precision, respectively.
The stability was investigated by analyzing the accuracies of three levels of concentrations of five compounds at room temperature condition over 24 h. The accuracies of five compounds were in a range of 95.8–114.8%, while with the RSDs were both no more than 2.6%, showing that the analytes were stable from 0 to 24 h at room temperature.
To verify the accuracy of the proposed method, recovery tests were performed by analyzing the spiked sample in triplicate. Unspiked samples and spiked samples were simultaneously extracted using the optimum AQ C18-IL-VHMSPD procedures. The results are listed in Table 3. The mean recoveries of 5 compounds were all in the range of 96.9–103.6% and the RSDs were all less than 2.8%, which demonstrated that the proposed AQ C18-IL-VHMSPD method was reliable and effective.
All of the above analysis results demonstrated that the proposed method had applicable value. Thus, the developed AQ C18-IL-VHMSPD method was employed to analyze the target compounds with different polarities using the last-optimized conditions in six batches of crude Platycladi Cacumen and two batches of carbonized Platycladi Cacumen obtained from various producing areas. The contents of myricitrin, isoquercitrin, quercitrin, amentoflavone and hinokiflavone in crude PC were in the range of 1.64–2.10, 0.11–0.16, 3.41–4.13, 0.40–0.48, and 0.20–0.26 mg g-1, individually (Table 4). Furthermore, the contents of myricitrin, isoquercitrin, quercitrin, amentoflavone and hinokiflavone in carbonized PC were in the range of 0.06–0.17, 0.00–0.01, 0.03–0.25, 0.02–0.06, and 0.02–0.04 mg g-1, respectively. The results clearly revealed that the contents of the five target compounds were remarkably decreased after processing.
In order to compare the proposed AQ C18-IL-VHMSPD method with conventional ultrasonic-assisted extraction from Pharmacopoeia of China 2015, the contents of the five target compounds from the same batches of PC were determined by these two methods. It was found that there was no significantly difference between the contents of the five components by two methods. The results indicated that the developed AQ C18-IL-VHMSPD method had the almost same effectiveness as the method of Pharmacopoeia of China 2015 for extracting PC.
To evaluate the performance of the proposed AQ C18-IL-VHMSPD method, several methods including ultrasonic assisted extraction and reflux extraction were introduced to compare the sample amount, extraction solvent, solvent volume, extraction time and detection time. As summarized in Table 5, it is obviously observed that the developed AQ C18-IL-VHMSPD method has the lower extraction time and detection time in contrast with other methods. Except for the method 2 in Table 5 (deep eutectic solvents based ultrasonic assisted extraction) and the AQ C18-IL-VHMSPD method, other methods all employed a large volume of organic solvent. Moreover, the proposed method required less extraction time and detection time than deep eutectic solvents based ultrasonic assisted extraction method. Overall, these results indicated that the AQ C18-IL-VHMSPD method was a rapid, simple and environmentally friendly method for the extraction of the high polarity and low polarity compounds in PC samples.
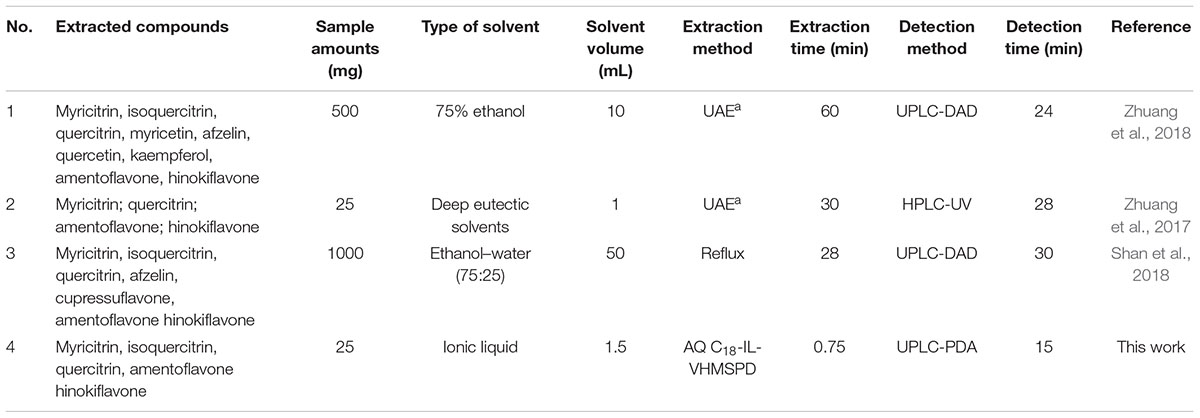
Table 5. Comparison of the AQ C18-IL-VHMSPD method with other methods in the determination of compounds in Platycladi Cacumen sample.
An environmentally friendly sample pretreatment method, AQ C18-based vortex-homogenized matrix solid-phase dispersion with ionic liquid was successfully developed to extract and quantify the target analytes with different polarity from Platycladi Cacumen by UHPLC-PDA. AQ C18 was employed as the adsorbent to improve the adsorption capacity of compounds of different polarity. The use of green ionic liquids reduced the environment pollution. Compared with other extraction methods (UAE and reflux extraction), the present method is rapid, time-saving and efficient. This proposed method could be used for determination of compounds with different polarity from other traditional Chinese medicines.
Y-xC, GT, XG, and JL designed the experiments. MD and SZ performed the experiments. MD wrote the manuscript.
This research was supported National Natural Science Foundation of China (81374050 and 81703702) and Special Program of Talents Development for Excellent Youth Scholars in Tianjin of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Barker, S. A., Long, A. R., and Short, C. R. (1989). Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 475, 353–361. doi: 10.1016/S0021-9673(01)89689-8
Cao, J., Peng, L. Q., and Xu, J. J. (2016). Microcrystalline cellulose based matrix solid phase dispersion microextration for isomeric triterpenoid acids in loquat leaves by ultrahigh-performance liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 1472, 16–26. doi: 10.1016/j.chroma.2016.10.034
Chen, L. G., Ding, L., Yu, A. M., Yang, R. L., Wang, X. P., Li, J. T., et al. (2007). Continuous determination of total flavonoids in Platycladus orientalis (L.) Franco by dynamic microwave-assisted extraction coupled with on-line derivatization and ultraviolet-visible detection. Anal. Chim. Acta 596, 164–170. doi: 10.1016/j.aca.2007.05.063
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the People’s Republic of China, Vol. 1. Beijing: China Medical Science and Technology Press.
Du, K. Z., Li, J., Bai, Y., An, M. R., Gao, X. M., and Chang, Y. X. (2018a). A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus corni by ultra-high performance liquid chromatography. Food Chem. 244, 190–196. doi: 10.1016/j.foodchem.2017.10.057
Du, K. Z., Li, J., Tian, F., and Chang, Y. X. (2018b). Non-ionic detergent triton X-114 based vortex-synchronized matrix solid-phase dispersion method for the simultaneous determination of six compounds with various polarities from Forsythiae Fructus by ultra high-performance liquid chromatography. J. Pharm. Biomed. Anal. 150, 59–66. doi: 10.1016/j.jpba.2017.12.003
Emami, S. A., Asgary, S., Ardekani, M. R. S., Naderi, G. A., Kasher, T., Aslani, S., et al. (2011). Antioxidant activity in some in vitro oxidative systems of the essential oils from the fruit and the leaves of Platycladus orientalis. J. Essent. Oil Res. 23, 83–90. doi: 10.1016/j.jep.2011.05.019
Fan, S. Y., Zeng, H. W., Pei, Y. H., Li, L., Ye, J., Pan, Y. X., et al. (2012). The anti-inflammatory activities of an extract and compounds isolated from Platycladus orientalis (Linnaeus) Franco in vitro and ex vivo. J. Ethnopharmacol. 141, 647–652. doi: 10.1016/j.jep.2011.05.019
García-López, M., Canosa, P., and Rodríguez, I. (2008). Trends and recent applications of matrix solid-phase dispersion. Anal. Bioanal. Chem. 391, 963–974. doi: 10.1007/s00216-008-1898-y
García-Mayor, M. A., Gallego-Picó, A., Garcinuño, R. M., Fernández-Hernando, P., and Durand-Alegría, J. S. (2012). Matrix solid-phase dispersion method for the determination of macrolide antibiotics in sheep’s milk. Food Chem. 134, 553–558. doi: 10.1016/j.foodchem.2012.02.120
Han, D. D., Tang, B. K., Lee, Y. R., and Row, K. H. (2012). Application of ionic liquid in liquid phase microextraction technology. J. Sep. Sci. 35, 2949–2961. doi: 10.1002/jssc.201200486
Hassanzadeh, M. K., Rahimizadeh, M., Fazly Bazzaz, B. S., Emami, S. A., and Assili, J. (2001). Chemical and antimicrobial studies of Platycladus orientalis essential oils. Pharm. Biol. 39, 388–390. doi: 10.1076/phbi.39.5.388.5894
Liu, H. C., Li, Q. W., Li, S. P., Zou, Y. H., and Gu, A. Y. (2008). The rapid determination of artemisinin by post-column derivatization high-performance liquid chromatography using matrix solid-phase dispersion method. J. Chromatogra. Sci. 46, 122–126. doi: 10.1093/chromsci/46.2.122
Lu, Y. H., Liu, Z. Y., Wang, Z. T., and Wei, D. Z. (2006). Quality evaluation of Platycladus orientalis (L.) Franco through simultaneous determination of four bioactive flavonoids by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 41, 1186–1190. doi: 10.1016/j.jpba.2006.02.054
Ma, R., and Wu, S. B. (2013). Research progress about pharmacological effect mechanism of flavonoids in traditional Chinese medicine. Chin. Pharmacovigil. J. 10, 286–290. doi: 10.3969/j.issn.1672-8629.2013.05.008
Qiu, H. D., Mallik, A. K., Takafuji, M., Liu, X., Jiang, S. X., and Ihara, H. (2012). A new imidazolium-embedded C18 stationary phase with enhanced performance in reversed phase liquid chromatography. Anal. Chim. Acta 738, 95–101. doi: 10.1016/j.aca.2012.06.018
Rodrigues, S. A., Caldas, S. S., and Primel, E. G. (2010). A simple; efficient and environmentally friendly method for the extraction of pesticides from onion by matrix solid-phase dispersion with liquid chromatography-tandem mass spectrometric detection. Anal. Chim. Acta 678, 82–89. doi: 10.1016/j.aca.2010.08.026
Shan, M. Q., Li, S. F. Y., Yu, S., Qian, Y., Guo, S. C., Zhang, L., et al. (2018). Chemical fingerprint and quantitative analysis for the quality evaluation of Platycladi cacumen by ultra-performance liquid chromatography coupled with hierarchical cluster analysis. J. Chromatogr. Sci. 56, 41–48. doi: 10.1093/chromsci/bmx079
Vela-Soria, F., Rodríguez, I., Ballesteros, O., Zafra-Gómez, A., Ballesteros, L., Cela, R., et al. (2014). Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J. Chromatogr. A 1371, 39–47. doi: 10.1016/j.chroma.2014.10.063
Xu, J. J., Cao, J., Peng, L. Q., Cao, W., Zhu, Q. Y., and Zhang, Q. Y. (2016). Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 1436, 64–72. doi: 10.1016/j.chroma.2016.01.046
Zaugg, J., Khom, S., Eigenmann, D., Baburin, I., Hamburger, M., and Hering, S. (2011). Identification and characterization of GABA(A) receptor modulatory diterpenes from Biota orientalis that decrease locomotor activity in mice. J. Nat. Prod. 74, 1764–1772. doi: 10.1021/np200317p
Zhang, J. F., Sun, G. L., Zhang, B., Sun, Z. G., and Li, Y. (2013). Study progress of oriental arborvitae pharmacological effects. Lishizhen Med. Mater. Med. Res. 24, 2231–2233. doi: 10.3969/j.issn.1008-0805.2013.09.087
Zhuang, B., Bi, Z. M., Wang, Z. Y., Duan, L., Lai, C. J. S., and Liu, E. H. (2018). Chemical profiling and quantitation of bioactive compounds in Platycladi cacumen by UPLC-Q-TOF-MS/MS and UPLC-DAD. J. Pharm. Biomed. Anal. 154, 207–215. doi: 10.1016/j.jpba.2018.03.005
Keywords: AQ C18, ionic liquid, Platycladi Cacumen, UHPLC, vortex-homogenized matrix solid-phase dispersion
Citation: Ding M, Li J, Zou S, Tang G, Gao X and Chang Y-x (2019) Simultaneous Extraction and Determination of Compounds With Different Polarities From Platycladi Cacumen by AQ C18-Based Vortex-Homogenized Matrix Solid-Phase Dispersion With Ionic Liquid. Front. Pharmacol. 9:1532. doi: 10.3389/fphar.2018.01532
Received: 23 July 2018; Accepted: 13 December 2018;
Published: 09 January 2019.
Edited by:
Caroline Howard, Medicines and Healthcare Products Regulatory Agency, United KingdomReviewed by:
Johanna Mahwahwatse Bapela, University of Pretoria, South AfricaCopyright © 2019 Ding, Li, Zou, Tang, Gao and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-xu Chang, VGNtY3l4QDEyNi5jb20=; dGNtY3l4QHRqdXRjbS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.