
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 07 January 2019
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.01467
Background and Objective: It has been suggested to avoid cilostazol, the first-line therapy for peripheral arterial disease, in patients with congestive heart failure (HF). The objective of this study was to evaluate the risk of hospitalization for heart failure (HHF) associated with cilostazol use in the patients of diabetes mellitus.
Methods: This case-crossover study retrieved records on diabetic patients > 20 years of age who were hospitalized for heart failure during the period of 2009–2011 from the Taiwan National Health Insurance Database. The “current” period was defined as 1–30 days prior to HHF whereas the 91–120 days prior to HHF served as the “reference” period. The exposure status just preceding the event is compared with exposure of the same person in one or more referent remote to the event. Adjusted odds ratios (OR) were used to estimate time-varying discordant exposure by the ratio of the number exposed to cilostazol only during the case period to the number exposed to cilostazol only during the control period.
Results: A total of 47,506 diabetic patients were included in the analysis (average age: 72.7 ± 12.4, percentage of males: 48%). A total of 399 patients (0.84%) received cilostazol only in the current period, and 252 (0.53%) received cilostazol only in the reference period. After adjustment for other medications, a significant association was found between cilostazol and HHF (OR: 1.35, 95% CI: 1.14–1.59). After further adjustment for time-varying co-morbidities the ORs remained essentially the same. Sensitivity analyses using different definitions of control period (ranging from 31–60, 61–90, to 121–150 days before index date) yielded adjusted ORs of 1.43 (95% CI: 1.14–1.79), 1.31 (95% CI: 1.09–1.57) and 1.23 (95% CI: 1.06–1.44), respectively suggesting the robustness of our study findings.
Conclusion: Use of cilostazol may be positively related to the risk of HHF. Further studies are warranted to explore the underlying mechanisms and to confirm the association.
Cilostazol is a unique antiplatelet agent that has been commercially available for two decades. It is a potent antiplatelet agent and possesses vasodilatation and antiproliferative effects. It has been broadly applied in the treatment of peripheral artery disease (PAD) and also for patients with PAD undergoing endovascular procedures. In addition, a randomized placebo controlled trial has investigated the role of cilostazol in patients with coronary artery disease (CAD) undergoing primary coronary intervention (PCI) along with the use of aspirin and/or clopidogrel (Rogers et al., 2015).
Cilostazol has multifactorial pharmacological properties and broad spectrum of pharmacological actions. Its main effect includes selective inhibition of cellular phosphodiesterase (PDE) type III, which augments the effect of cyclic adenosine monophosphate (cAMP) metabolism within the platelet (Pan et al., 1994; Schror, 2002). Since cAMP is involved in the pathway which controls platelet aggregation and vasomotor function, inhibition of PDE III could reversibly inhibit platelet aggregation and results in vasodilation (Schror, 2002). Besides, cilostazol has also been shown to reduce triglycerides, raise high-density lipoprotein levels and increase adenosine accumulation in the interstitium, all of which provide protective effects for patients with stroke, CAD and PAD. Nevertheless, inhibition of PDE III may lead to a positive inotropic effect, meta-analysis for randomized trials of PDE III inhibitors versus placebo in heart failure (HF) patients revealed that PDE III inhibitors were responsible for an increase in mortality rate compared with placebo in patients suffering from chronic HF. Thus, the use of PDE III inhibitors is prohibited in HF patients. The above results were mainly obtained from the trials involving intravenous medication for HF (e.g., milrinone, vesnarinone). Whether the use of cilostazol is associated with development of HF or worsening of HF is still unknown.
Diabetes is a major risk factor for development of PAD. The number of prescriptions for cilostazol in diabetic patients have increased gradually and, therefore, understanding the role of cilostazol in diabetic patients has become even more important. Therefore, this national-wide population-based study aimed to examine whether the use of cilostazol was associated with hospitalization for heart failure (HHF) in the diabetic population.
This study was approved by our hospital’s Research Ethics Committee. The case-crossover study design was used to assess the relationship between cilostazol exposure and risk of HF that led to hospitalization. Instead of using propensity-matched controls, each patient served as his/her own control in the case-crossover study, so that stable confounders, unmeasured or poorly measured, would not influence the results. In addition, because diabetic patients might receive complicated anti-hypertensive and anti-diabetic drug therapy and switch from one class of drug to other classes in order to attain optimal blood pressure and glycemic control, it was difficult to find appropriate comparison groups. The major advantage of this design is that stable risk factors such as cigarette smoking or other cardiovascular risk factors will not confound the analysis results, despite the fact that they are not recorded in the claim database.
A universal NHI program was implemented in Taiwan in March 1995. Ninety-six percent of the total Taiwanese population was enrolled in this NHI program. The Taiwan NHI database includes complete outpatient visits, hospitalization data, prescriptions, and disease status for 99% of the 23 million inhabitants of Taiwan. The longitudinal medical history of each beneficiary was established by linking several computerized administrative, claims datasets and National Death Registry through the civil identification number unique to each beneficiary and their date of birth. Data for gender, birth date, medications, and diagnostic codes (based on the International Classification of Diseases, Ninth Revision, Clinical Modification) were retrieved for the analyses performed in this study. Patients were required to have at least 1 year of registration in the National Health Insurance database prior to index date.
As shown in Figure 1, all patients hospitalized for CHF in 2009–2011 were included in the study, based on having an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 428 on inpatient claims. Previous validation studies using a hospital administrative database reported a positive predictive value of 90% with this definition (Wu et al., 2015). For those who had ≥2 hospitalizations for heart failure during the study period, only the first event was included. Date of hospitalization was defined as the index date. Patients who did not have ICD-9-CM code of diabetes (250) and those had not received any anti-diabetic therapy before hospitalization, patients aged < 20 years, and those with missing information regarding gender were excluded. Patients were also excluded: (1) if they were admitted for any reason during 120 days before index date due to clinical instability or (2) due to the inability to ascertain dosage and duration of cilostazol use during hospitalization. We also repeat the same analysis and included patients with mortality and those without to test the effect of cilostazol.
The main exposure of interest in this study was cilostazol use (reimbursed by NHI since 2001 for the indication of relieving intermittent claudication symptoms). Because non-steroidal anti-inflammatory drugs (NSAIDs) have had documented association with CHF, the risk estimates for NSAIDs were calculated as a comparison. Information regarding the type of drug prescribed (according to the anatomic therapeutic chemical ATC classification system), date of prescription, days of supply, and total number of drug pills dispensed from pharmacy prescription database were collected. Other concomitant drug exposure that may also modify the risk of HF are listed in Table 2 and include insulin, oral antidiabetic drugs, antiplatelet agents (aspirin, clopidogrel), warfarin, anti-hypertensives, nitrates, lipid-lowering agents, digitalis, anti-arrhythmics, inhaled and oral bronchodilators (beta-adrenergic agonists, inhaled anticholinergics, aminophylline), oral corticosteroids, and systemic antibiotics (ATC codes were provided in Supplementary Table 1).
McNemar’s tests were used for comparison of drug exposure between case and control period. Information on patient’s age, gender, and comorbidities (including hypertension, ischemic heart disease, myocardial infarction, atrial fibrillation, congestive HF, cerebrovascular disease, ischemic stroke, intracerebral hemorrhage, peripheral vascular disease, chronic renal, liver, and lung disease, depression, and cancer based on ICD-9-CM codes) were also collected (Supplementary Table 1) (Wu et al., 2010). The Charlson’s index (ranging from 0 to 40) was used as a measure of co-morbid conditions, such as chronic liver disease, stroke, or cancer (a total of 22 conditions). Each condition was assigned a score of 1, 2, 3, or 6, depending on the mortality associated with each one. Scores were summed to provide a total score that predicted long-term mortality (Chang et al., 2010).
In the case-crossover design, the exposure status just preceding the event (defined as “case period”) is compared with exposure of the same person in one or more referent “control periods” remote to the event. The odds ratios (ORs) and their 95% confidence interval (CIs) were estimated by the ratio of the number exposed to cilostazol only during the case period to the number exposed to cilostazol only during the control period (i.e., ratio of discordant pairs) by conditional logistic regression. In the main analysis, case period was defined as 1–30 days prior to index date and control period was defined as 91–120 days prior to index date. Persons were considered currently exposed to cilostazol and other concomitant drugs during the start date and end date of a prescription.
In the multivariable analysis, adjusted ORs were calculated simultaneously and controlled for time-varying discordant exposure to medications including metformin, pioglitazone, sitagliptin, aspirin, angiotensin-converting enzyme inhibitors, beta-adrenergic blockers, calcium channel blockers, diuretics, nitrates, digitalis glycoside, inhaled and oral bronchodilators (beta-adrenergic agonists, anticholinergics, aminophylline), oral corticosteroids, and systemic antibiotics between case and control periods except for the medication being analyzed itself. Further adjustment for time-varying confounding co-morbidities (hypertension, ischemic heart disease, cerebrovascular disease, chronic kidney disease) or adjustment for surrogate markers of glycemic control (number of outpatients visits and the number of A1c tests prescribed) were also performed.
In sensitivity analyses, different control periods of 31–60, 61–90, and 121–150 days prior to index date were used, and recent 7 days of drug exposure were excluded to avoid protopathic bias (physicians discontinued cilostazol therapy because of early symptoms/signs of HF) to see whether results would change substantially. In another sensitivity analyses, longer case and control period windows (i.e., 60 or 90 days) were used to capture the risk associated with longer-term exposure of cilostazol (Chang et al., 2015).
Furthermore, stratified analysis was performed to evaluate potential modification effects. The cases were separated according to (1) gender (men vs. women), (2) age (≥65, <65 years), (3) congestive HF, and (4) chronic kidney disease. A formal test of interaction was performed for each subgroup to examine if the difference in size of effect between two subgroups was statistically significant. A two-sided p-value < 0.05 was considered to be statistically significant. All statistical analyses were performed with SAS 9.2 (SAS Institute, Cary, NC, United States).
A total of 79,100 patients with diabetes who were ≥ 20 years of age and who were first hospitalized for CHF between 2009 and 2011 were identified. After further excluding those who had been hospitalized for any reason 120 days prior to index HHF, a total of 47,506 patients (48.2% male, mean age 72.7 years) were included in the final analysis (Figure 1).
Table 1 summarizes the proportion of patients with comorbidities at index hospitalization. Approximately 63.7% of the cohort had hypertension, 47.3% had ischemic heart disease, 12.4% had a history of myocardial infarction, and 20.5% had chronic kidney disease. The exposure of cilostazol, anti-hypertensive, and anti-diabetic agents, as well as concomitant medications during the case period (1–30 days before index hospitalization) and control period (91–120 days before index hospitalization) is shown in Table 2. A higher proportion of patients took cilostazol, anti-diabetic agents (except for metformin), aspirin, angiotensin converting enzyme inhibitors, beta-blockers, calcium channel blockers, diuretics, nitrates, digitalis, inhaled bronchodilators, aminophylline, systemic antibiotics, and NSAIDs in the case period than in the control period.
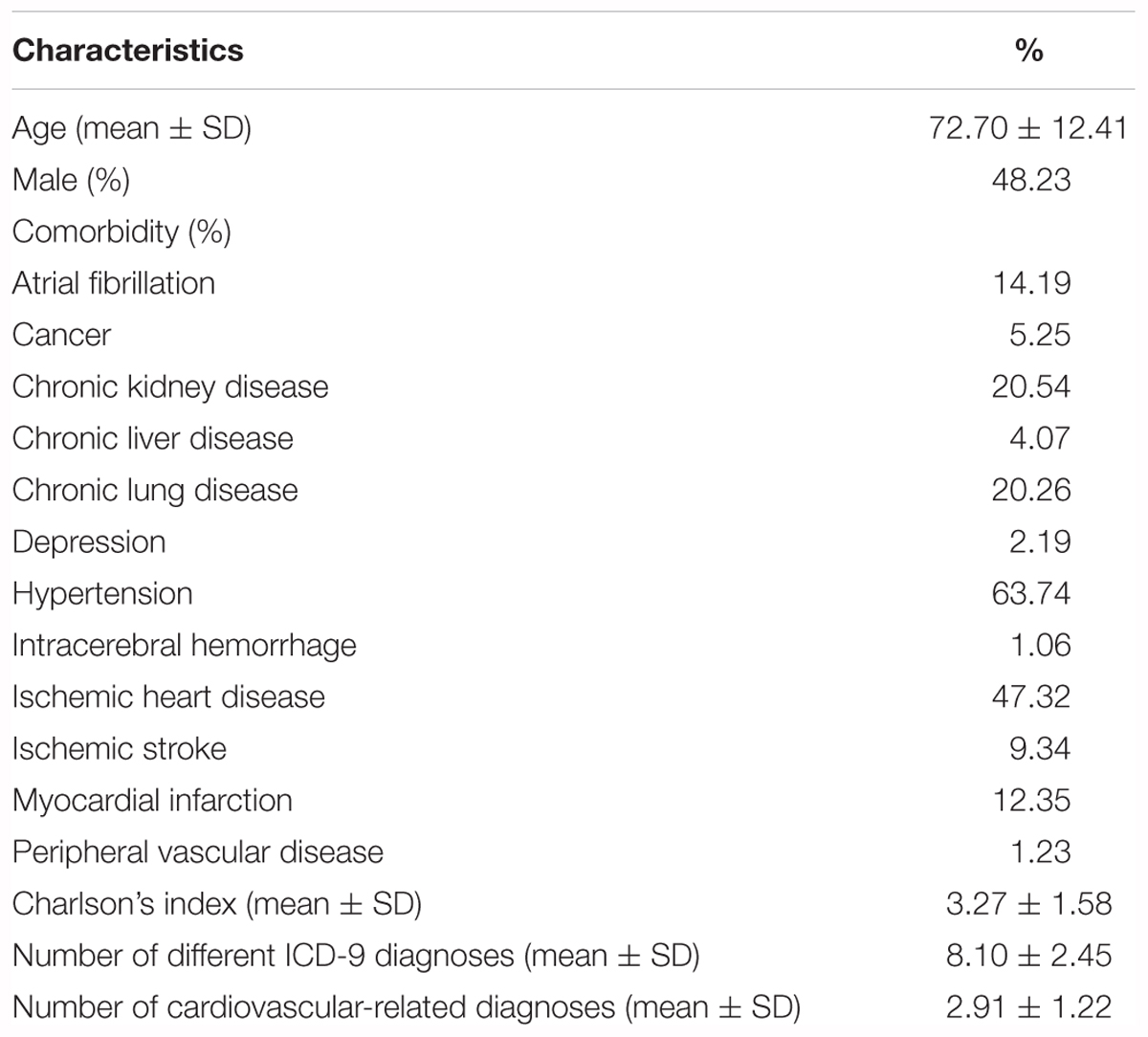
Table 1. Characteristics of the diabetic patients hospitalized for congestive heart failure (N = 47,506).

Table 2. Proportion of patients with concomitant medications use and resource utilization during 1–30 and 91–120 days before hospitalization for congestive heart failure (N = 47,506).
Table 3 presents crude and adjusted ORs and their 95% CIs for cilostazol and NSAIDs by conditional logistic regression. A modestly increased risk of hospitalization for HHF was found for the exposure of cilostazol with a crude OR of 1.57 (95% CI: 1.35–1.84). After controlling for potential time-varying confounders including drugs potentially associated with HF, cilostazol was associated with an increased risk of HHF (OR: 1.35, 95% CI: 1.14–1.59) for hospitalized CHF. A significantly elevated OR was also evident for NSAIDs (adjusted OR 1.69, 95% CI: 1.62–1.76).
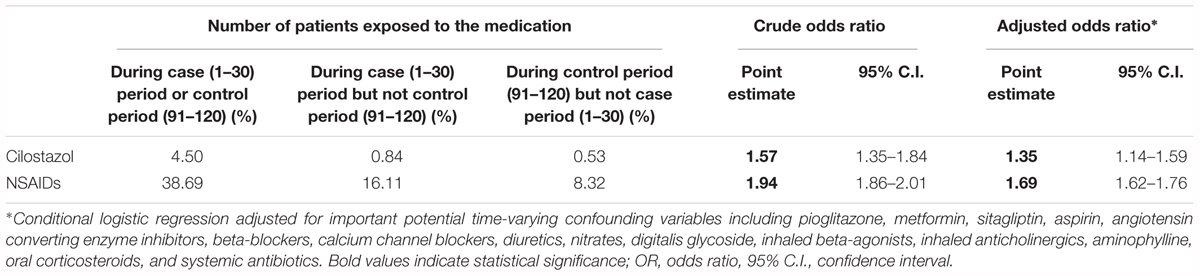
Table 3. Risk of hospitalized heart failure associated with current use of cilostazol and NSAIDs (N = 47,506).
After further adjustment for time-varying co-morbidities including hypertension, ischemic heart disease, cerebrovascular disease, chronic kidney disease, or further adjustment for surrogate measures of glycemic control including number of outpatients visits and the number of A1c test prescribed, the ORs remained essentially the same (Supplementary Table 2).
Sensitivity analyses using different definitions of control period (ranging from 31–60, 61–90, to 121–150 days before index date) yielded adjusted ORs of 1.43 (95% CI: 1.14–1.79), 1.31 (95% CI: 1.09–1.57) and 1.23 (95% CI: 1.06–1.44), respectively (Table 4A). An analysis excluding recent 7 days of drug exposure prior to index date showed similar results suggesting the robustness of our study findings (Table 4A). Additional sensitivity analyses using longer case and control time windows (i.e., 60 and 90 days) showed a similar risk estimate in the analysis using a window length of 60 days; however, the OR were attenuated and became non-significant in the analysis using a window length of 90 days (Table 4B). In contrast, NSAIDs were consistently associated with significantly higher risks, regardless of the definition and window length used for the case and control periods.

Table 4A. Risks of hospitalized heart failure associated with cilostazol and NSAIDs use on different definitions of case period and control period (N = 47,506).
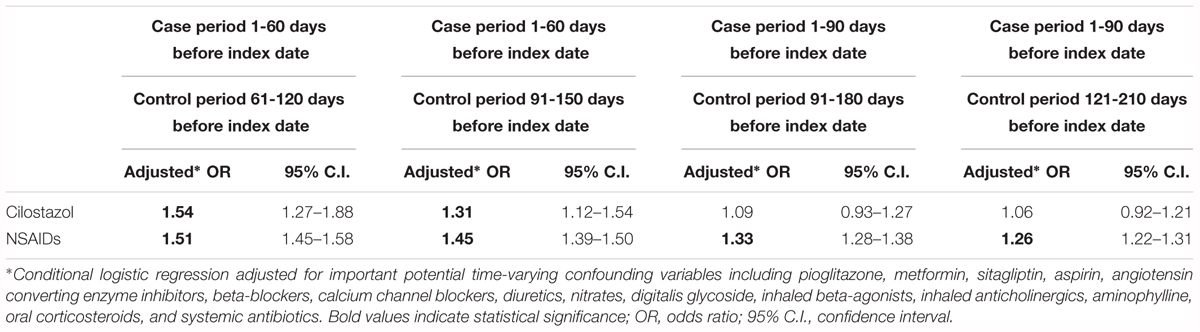
Table 4B. Risks of hospitalized heart failure associated with cilostazol and NSAIDs use on different window lengths of case period and control period (N = 47,506).
The subgroup analysis showed that the increased risk of HHF associated with cilostazol exposure was uniform across pre-defined strata by gender, age, prior history of HF, and chronic kidney disease, suggesting no significant modification effect by these characteristics (Table 5). The same analysis was performed to analysis cilostazol’s effect for overall mortality (Supplementary Table 3). Similar findings were found and recent us of cilostazol was associated with an OR of 3.90 (95% CI: 3.51–4.33) for increased mortality risk (Supplementary Table 4). The results were consistent after adjustment for comorbidities and drug use (Supplementary Table 5).
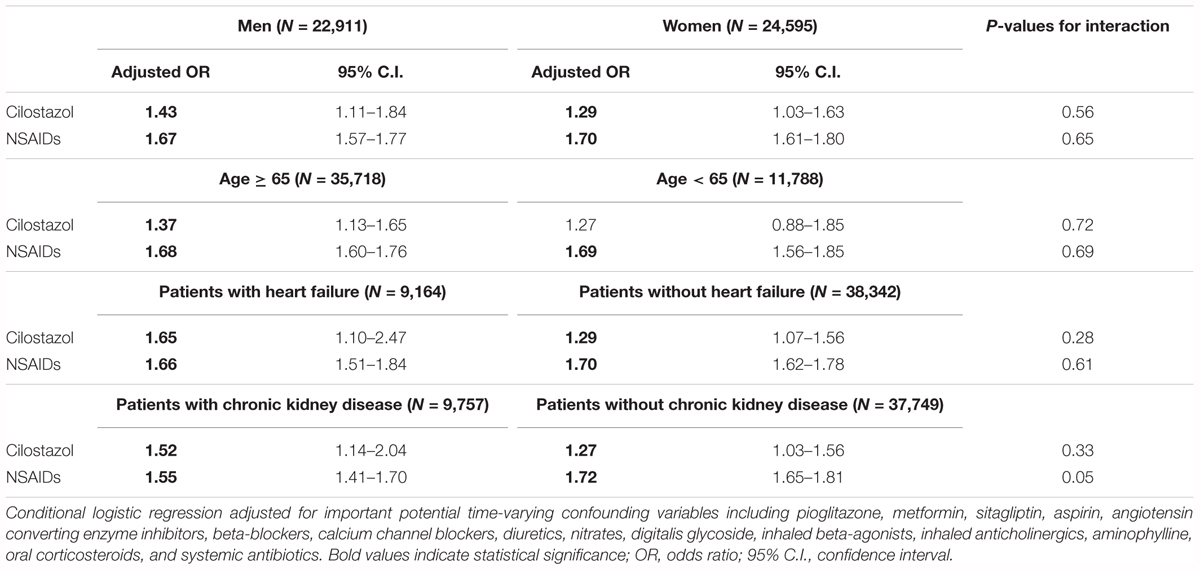
Table 5. Adjusted odds ratio of hospitalized heart failure associated with use of cilostazol and NSAIDs among different subgroups.
In this study, we analyzed a nationwide health insurance claims database and found an increased risk of HHF with use of cilostazol in diabetic patients. The effect of cilostazol was only slightly lower than that associated with the use of non-steroid anti-inflammatory drugs (NSAID). We also observed that the elevated risk of HHF associated with cilostazol exposure was uniform across all pre-defined subgroups after controlling for potential risk factors.
PDE3-selective inhibitors such as milrinone and vesnarinone have been used clinically to treat acute congestive HF (Benotti et al., 1978). Blockage of PDE 3 could lead to increased levels of intracellular cAMP and protein kinase A which could result in smooth muscle cell relaxation and attenuation of myosin phosphorylation (McDaniel et al., 1994). On the other hand, elevated cAMP levels enables L-type Ca2+ channels and a component of a delayed-rectifier potassium channel signal transduction pathway in the sinoatrial node (Kodama-Takahashi et al., 2003). Therefore, drugs that inhibit PDE 3 pathways would cause a positive chronotropic effect and inotropic action which have been demonstrated to increase left ventricular ejection fraction and stroke volume (Baim et al., 1983; Jaski et al., 1985; Rauch et al., 1991). However, clinical studies [such as the Prospective Randomized Milrinone Survival Evaluation (PROMISE) (Packer et al., 1991) and the Vesnarinone trial (VEST) (Feldman et al., 1993) studies] have evaluated the long-term effects of PDE 3 administration in congestive HF patients and reported increased cardiovascular mortality. Several studies have also shown that long-term elevation of cAMP could cause adverse effects on chronic HF patients (Movsesian, 2003; Phrommintikul and Chattipakorn, 2006). Increased levels of cAMP trigger early and delayed after-depolarization and enhance the risk of lethal ventricular arrhythmia. Besides, the inotropic effects of PDE 3 inhibitors could cause higher myocardial oxygen consumption and increase various arrhythmia over time (Naccarelli and Goldstein, 1989; Sunderdiek et al., 2000).
Cilostazol also inhibits PDE 3 selectively. It has potent vasodilating and antithrombotic effects and has been used in the treatment of PAD (Dawson et al., 1998). Therefore, the FDA contraindicated the use of cilostazol in HF of any severity. Nevertheless, no clinical study has directly investigated the effects of cilostazol in HF patients. Although cilostazol is a PDE 3 inhibitor, the effect on myocytes differs from other PDE 3 inhibitors. The weaker inotropic effect of cilostazol compared with milrinone lessens long-term cardiac side effects (Cone et al., 1999). Cilostazol also blocks adenosine uptake, and, subsequently, the interstitial adenosine elevation in the heart could attenuate cAMP response and limit the long-term harmful effects of cAMP activation (Wang et al., 2001). In addition, in smooth muscle and platelets, elevated interstitial and circulating adenosine levels by cilostazol could potentiate its cAMP-raising effect through inhibiting PDE3 and, thereby, augmenting antiplatelet and vasodilatory effects which could be beneficial for prognosis of congestive HF (Liu et al., 2001). For subjects with myocardium ischemic/reperfusion, cilostazol could decreased plasma IL-6, IL-1beta and TNF-alpha levels, and activate the PPARγ/JAK2/STAT3 pathway. Through the above pathway, cilostazol is possible to limit myocardial inflammation and apoptosis after ischemic/reperfusion injury. These results may provide a rationale for new therapy with cilostazol in patients with ischemic cardiomyopathy and may possible restore cardiac function (Li et al., 2017). Taken together, the use of cilostazol in distinct groups should be reinvestigated.
The biological mechanism(s) behind the increased risk of HHF associated with cilostazol is unclear. Atarashi et al. (1998) demonstrated that cilostazol increased heart rate and improved symptoms in patients with symptomatic bradyarrhythmia. There is no cardiac electrophysiological study that has investigated the strength of cilostazol. In contrast, there is evidence for its possible undesirable tachyarrhythmia effects (Barta et al., 2008; Shinohara et al., 2010). Elevated resting heart rate was associated with increased risk for incident HF in asymptomatic high risk patients and higher heart rate was an independent risk factor for HHF in the subclinical group (Opdahl et al., 2014). Few studies specifically evaluated the effect of cilostazol on HF in the patients with diabetes. One recent meta-analysis evaluated 10 randomized case-control trials in the diabetic population, mostly with reported outcomes of major adverse cardiovascular events or platelet aggregation function (Bundhun et al., 2015). However, all the above trials failed to provide information about the risk of HHF associated with cilostazol use.
Our current study compared recent use of cilostazol (1–30 days) with controls (remote use) to document the influence of the drug over a short period. In the sensitivity analysis, we excluded the recent 7 days of drug exposure and found similar risk for the drug. We also used different definitions of control periods or longer case and control time windows (i.e., 60 and 90 days), assuming the statistical significance associated with discordant cilostazol use might diminish. In contrast, the effects of NSAIDs were consistently associated with significantly higher risks, regardless of the definition or window length used for the case and control periods. These findings suggested that exposure to cilostazol caused an accumulated HHF risk with a duration about 2 months but not longer. Instead, the well-known risks for NSAID in diabetic patients are consistent around 3 months. As we have discussed, cilostazol has a much weaker inotropic effect than other PDE3 inhibitors and could lessens long-term cardiac side effects. However, cilostazol might also increase heart rate and hence lead to higher HHF risk during the initial 2-month period. On the other hand, our data was consistent with the previous conclusion that NSAID use is associated with a higher risk of myocardial infarction, stroke, and hospitalizations for both ischemia and HF (Kohli et al., 2014) which further validated the reliability of our cohort study.
A very recent study used five population-based healthcare databases from four European countries and showed that current use of any NSAID (use in preceding 14 days) was found to be associated with a 19% increased risk of HHF (adjusted OR: 1.19, 95% CI: 1.17–1.22), compared with past use of any NSAIDs (use > 183 days in the past). The results were consistent with our findings of an association between NSAID use and HHF. Hence, we verified the higher risk of developing HHF associated with NSAID use compared with that of cilostazol in diabetic patients (Arfe et al., 2016).
Our study had several limitations. First, we did not have information on lifestyle risk factors such as weight, cigarette smoking, and alcohol consumption. However, these lifestyle factors would probably not change substantially during the relatively short study period and thus could be controlled by our case-crossover design. We also relied on the classification from the claims data base for baseline characteristics which may have resulted in a potential bias in terms of disease classification. Usually, doctors will exclude reversible causes for diagnosis of HHF. However, because detailed hematology data were not available, it was not possible to adjust for some potential confounding factors of HHF or mortality. Second, our case-control design evaluated the effect of cilostazol over a certain period. This method justified the effect of cilostazol as compared with controls, but the long-term risk concerning cilostazol is still unknown. In addition, from the results of current study, we suggested that recent use cilostazaol seems to be one of the determinants of HHF. However, because no data on inflammation parameters were available, all proposed mechanisms are still hypothetical. Although we used a cross-over design to control for the most important risk factors for HHF such as age, HTN and comorbid conditions, some unknown factors that may influence the outcomes in this population such as obesity, smoking, alcohol consumption, family history of premature CAD, lifestyle and diet, might still affect the results of current study. Finally, we could not exclude possible time-varying within-subject confounding factors that were associated with disease severity over time or trends in cilostazol use. The multivariable analysis might also over-control some of the intermediate variables responsible for HHF associated with cilostazol.
One of the strength of present study is that we performed the largest National-wide case-crossover study that included 47,506 diabetic patients to find significant association between cilostazol and HF hospitalization. We showed for the first time that use of cilostazol may be positively related to the risk of heart failure hospitalization.
In conclusion, our study found that cilostazol use was associated with a modestly increased risk of HHF. Further studies are warranted to explore underlying mechanisms and to confirm the association.
C-HC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. C-KW and J-WL participated in the study concept and design. L-CW acquired the data. C-KW and J-WL also contributed to the analysis and interpretation of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01467/full#supplementary-material
Arfe, A., Scotti, L., Varas-Lorenzo, C., Nicotra, F., Zambon, A., Kollhorst, B., et al. (2016). Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case-control study. BMJ 354:i4857. doi: 10.1136/bmj.i4857
Atarashi, H., Endoh, Y., Saitoh, H., Kishida, H., and Hayakawa, H. (1998). Chronotropic effects of cilostazol, a new antithrombotic agent, in patients with bradyarrhythmias. J. Cardiovasc. Pharmacol. 31, 534–539. doi: 10.1097/00005344-199804000-00010
Baim, D. S., Mcdowell, A. V., Cherniles, J., Monrad, E. S., Parker, J. A., Edelson, J., et al. (1983). Evaluation of a new bipyridine inotropic agent–milrinone–in patients with severe congestive heart failure. N. Engl. J. Med. 309, 748–756. doi: 10.1056/NEJM198309293091302
Barta, J., Sanganalmath, S. K., Kumamoto, H., Takeda, N., Edes, I., and Dhalla, N. S. (2008). Antiplatelet agents sarpogrelate and cilostazol affect experimentally-induced ventricular arrhythmias and mortality. Cardiovasc. Toxicol. 8, 127–135. doi: 10.1007/s12012-008-9019-x
Benotti, J. R., Grossman, W., Braunwald, E., Davolos, D. D., and Alousi, A. A. (1978). Hemodynamic assessment of amrinone. A new inotropic agent. N. Engl. J. Med. 299, 1373–1377. doi: 10.1056/NEJM197812212992501
Bundhun, P. K., Qin, T., and Chen, M. H. (2015). Comparing the effectiveness and safety between triple antiplatelet therapy and dual antiplatelet therapy in type 2 diabetes mellitus patients after coronary stents implantation: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 15:118. doi: 10.1186/s12872-015-0114-1
Chang, C. H., Lin, J. W., Lin, C. H., Chen, H. C., Hwang, J. J., and Lai, M. S. (2015). Effectiveness and safety of extracranial carotid stent placement: a nationwide self-controlled case-series study. J. Formos. Med. Assoc. 114, 274–281. doi: 10.1016/j.jfma.2014.05.001
Chang, C. H., Shau, W. Y., Kuo, C. W., Chen, S. T., and Lai, M. S. (2010). Increased risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study. Stroke 41, 1884–1890. doi: 10.1161/STROKEAHA.110.585828
Cone, J., Wang, S., Tandon, N., Fong, M., Sun, B., Sakurai, K., et al. (1999). Comparison of the effects of cilostazol and milrinone on intracellular cAMP levels and cellular function in platelets and cardiac cells. J. Cardiovasc. Pharmacol. 34, 497–504. doi: 10.1097/00005344-199910000-00004
Dawson, D. L., Cutler, B. S., Meissner, M. H., and Strandness, D. E. Jr. (1998). Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation 98, 678–686. doi: 10.1161/01.CIR.98.7.678
Feldman, A. M., Bristow, M. R., Parmley, W. W., Carson, P. E., Pepine, C. J., Gilbert, E. M., et al. (1993). Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone study group. N. Engl. J. Med. 329, 149–155. doi: 10.1056/NEJM199307153290301
Jaski, B. E., Fifer, M. A., Wright, R. F., Braunwald, E., and Colucci, W. S. (1985). Positive inotropic and vasodilator actions of milrinone in patients with severe congestive heart failure. Dose-response relationships and comparison to nitroprusside. J. Clin. Invest. 75, 643–649. doi: 10.1172/JCI111742
Kodama-Takahashi, K., Kurata, A., Ohshima, K., Yamamoto, K., Uemura, S., Watanabe, S., et al. (2003). Effect of cilostazol on the ventricular escape rate and neurohumoral factors in patients with third-degree atrioventricular block. Chest 123, 1161–1169. doi: 10.1378/chest.123.4.1161
Kohli, P., Steg, P. G., Cannon, C. P., Smith, S. C. Jr., Eagle, K. A., Ohman, E. M., et al. (2014). NSAID use and association with cardiovascular outcomes in outpatients with stable atherothrombotic disease. Am. J. Med. 127:e51. doi: 10.1016/j.amjmed.2013.08.017
Li, J., Xiang, X., Gong, X., Shi, Y., Yang, J., and Xu, Z. (2017). Cilostazol protects mice against myocardium ischemic/reperfusion injury by activating a PPARγ/JAK2/STAT3 pathway. Biomed. Pharmacother. 94, 995–1001. doi: 10.1016/j.biopha.2017.07.143
Liu, Y., Shakur, Y., Yoshitake, M., and Kambayashi, Ji. J (2001). Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 19, 369–386. doi: 10.1111/j.1527-3466.2001.tb00076.x
McDaniel, N. L., Rembold, C. M., and Murphy, R. A. (1994). Cyclic nucleotide dependent relaxation in vascular smooth muscle. Can. J. Physiol. Pharmacol. 72, 1380–1385. doi: 10.1139/y94-199
Movsesian, M. A. (2003). PDE3 inhibition in dilated cardiomyopathy: reasons to reconsider. J. Card. Fail. 9, 475–480. doi: 10.1016/S1071-9164(03)00135-0
Naccarelli, G. V., and Goldstein, R. A. (1989). Electrophysiology of phosphodiesterase inhibitors. Am. J. Cardiol. 63, 35A–40A. doi: 10.1016/0002-9149(89)90391-3
Opdahl, A., Ambale Venkatesh, B., Fernandes, V. R., Wu, C. O., Nasir, K., Choi, E. Y., et al. (2014). Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (multi-ethnic study of atherosclerosis). J. Am. Coll. Cardiol. 63, 1182–1189. doi: 10.1016/j.jacc.2013.11.027
Packer, M., Carver, J. R., Rodeheffer, R. J., Ivanhoe, R. J., Dibianco, R., Zeldis, S. M., et al. (1991). Effect of oral milrinone on mortality in severe chronic heart failure. The promise study research group. N. Engl. J. Med. 325, 1468–1475. doi: 10.1056/NEJM199111213252103
Pan, X., Arauz, E., Krzanowski, J. J., Fitzpatrick, D. F., and Polson, J. B. (1994). Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families PDE III and PDE IV to attenuate proliferation of rat vascular smooth muscle cells. Biochem. Pharmacol. 48, 827–835. doi: 10.1016/0006-2952(94)90062-0
Phrommintikul, A., and Chattipakorn, N. (2006). Roles of cardiac ryanodine receptor in heart failure and sudden cardiac death. Int. J. Cardiol. 112, 142–152. doi: 10.1016/j.ijcard.2005.11.106
Rauch, B., Zimmermann, R., Kapp, M., Haass, M., Von Molitor, S., Smolarz, A., et al. (1991). Hemodynamic and neuroendocrine response to acute administration of the phosphodiesterase inhibitor BM14.478 in patients with congestive heart failure. Clin. Cardiol. 14, 386–395. doi: 10.1002/clc.4960140506
Rogers, K. C., Oliphant, C. S., and Finks, S. W. (2015). Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs 75, 377–395. doi: 10.1007/s40265-015-0364-3
Schror, K. (2002). The pharmacology of cilostazol. Diabetes Obes. Metab. 4(Suppl. 2), S14–S19. doi: 10.1046/j.1463-1326.2002.0040s2s14.x
Shinohara, Y., Katayama, Y., Uchiyama, S., Yamaguchi, T., Handa, S., Matsuoka, K., et al. (2010). Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 9, 959–968. doi: 10.1016/S1474-4422(10)70198-8
Sunderdiek, U., Korbmacher, B., Gams, E., and Schipke, J. D. (2000). Myocardial efficiency in stunned myocardium. Comparison of Ca(2+)-sensitization and PDE III-inhibition on energy consumption. Eur. J. Cardiothorac. Surg. 18, 83–89. doi: 10.1016/S1010-7940(00)00413-9
Wang, S., Cone, J., Fong, M., Yoshitake, M., Kambayashi, J., and Liu, Y. (2001). Interplay between inhibition of adenosine uptake and phosphodiesterase type 3 on cardiac function by cilostazol, an agent to treat intermittent claudication. J. Cardiovasc. Pharmacol. 38, 775–783. doi: 10.1097/00005344-200111000-00014
Wu, C. K., Lin, J. W., Caffrey, J. L., Chang, M. H., Hwang, J. J., and Lin, Y. S. (2010). Cystatin C and long-term mortality among subjects with normal creatinine-based estimated glomerular filtration rates: NHANES III (third national health and nutrition examination survey). J. Am. Coll. Cardiol. 56, 1930–1936. doi: 10.1016/j.jacc.2010.04.069
Keywords: heart failure hospitalization, cilostazol, diabetes, case-crossover study, outcomes
Citation: Wu C-K, Lin J-W, Wu L-C and Chang C-H (2019) Risk of Heart Failure Hospitalization Associated With Cilostazol in Diabetes: A Nationwide Case–Crossover Study. Front. Pharmacol. 9:1467. doi: 10.3389/fphar.2018.01467
Received: 12 September 2017; Accepted: 30 November 2018;
Published: 07 January 2019.
Edited by:
Changhua Wang, Wuhan University, ChinaReviewed by:
Claudio de Lucia, Temple University, United StatesCopyright © 2019 Wu, Lin, Wu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-Hsuin Chang, Y2hpYWhzdWluMTIzQHlhaG9vLmNvbS50dw==; Y2hva2Fpd3VAeWFob28uY29tLnR3
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.