Mequindox Induced Genotoxicity and Carcinogenicity in Mice
- 1National Reference Laboratory of Veterinary Drug Residues (HZAU) and MAO Key Laboratory for Detection of Veterinary Drug Residues, Huazhong Agricultural University, Wuhan, China
- 2Key Laboratory of Preventive Veterinary Medicine in Hubei Province, Huazhong Agricultural University, Wuhan, China
- 3MOA Laboratory for Risk Assessment of Quality and Safety of Livestock and Poultry Products, Huazhong Agricultural University, Wuhan, China
- 4Hubei Collaborative Innovation Center for Animal Nutrition and Feed Safety, Wuhan, China
A Corrigendum on
Mequindox Induced Genotoxicity and Carcinogenicity in Mice
by Liu, Q., Lei, Z., Wu, Q., Huang, D., Xie, S., Wang, X., et al. (2018). Front. Pharmacol. 9:361. doi: 10.3389/fphar.2018.00361
In the original article, there was a mistake in Figures 5B,F as published. The descriptions of Figures 5B,F did not match the figures displayed. The corrected Figure 5 appears below. The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
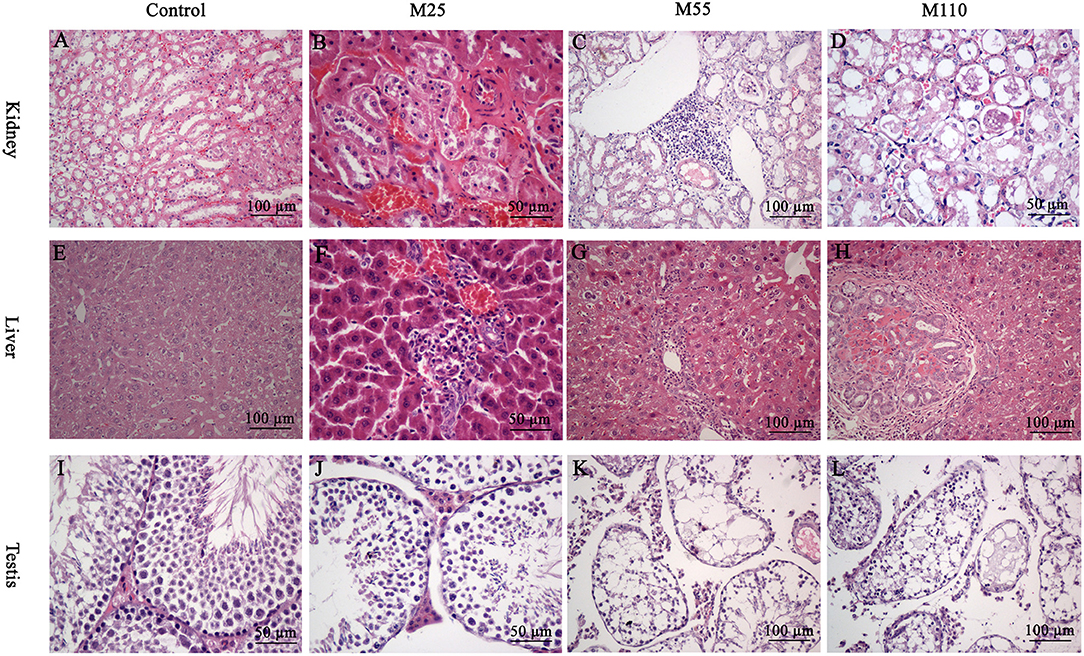
Figure 5. Selected microphotographs of kidney, liver and testis following dietary exposure to MEQ in the carcinogenicity tests (200×, 400×). (A) Kidney from control group (200×); (B) Kidney from the M25 mg/kg group showing kidney interstitial small blood vessels congestion, glomerular congestion (400×); (C) Kidney from the M55 mg/kg group showing aggregation of lymphocyte into a group around the central veins (200×); (D) Kidney from the M110 mg/kg group showing degeneration and necrosis of renal tubular epithelial cells (400×); (E) Liver from control group (200×); (F) Liver from the M25 mg/kg group showing degeneration and necrosis of hepatic cells (400×); (G) Liver from the M55 showing neutrophilic infiltrate within and around bile duct (200×); (H) Liver from the M110 mg/kg group showing proliferation in bile duct epithelium (200×); (I) Testis from the control group (400×); (J) Testis from the M25 mg/kg group showing a broadened testicular interstitium (400×); (K) Testis at the M55 mg/kg group showing an irregular arrangement as well as a decreased number of spermatogenic cells (200×); (L) Testis at the M110 mg/kg group showing necrosis of spermatogonia and spermatocytes in the lumen (200×).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Keywords: mequindox, quinoxaline, carcinogenicity, genotoxicity, KM mice
Citation: Liu Q, Lei Z, Wu Q, Huang D, Xie S, Wang X, Pan Y and Yuan Z (2018) Corrigendum: Mequindox Induced Genotoxicity and Carcinogenicity in Mice. Front. Pharmacol. 9:1387. doi: 10.3389/fphar.2018.01387
Received: 25 October 2018; Accepted: 12 November 2018;
Published: 29 November 2018.
Edited and reviewed by: Eleonore Fröhlich, Medical University of Graz, Austria
Copyright © 2018 Liu, Lei, Wu, Huang, Xie, Wang, Pan and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanhu Pan, cGFueXVhbmh1QGhvdG1haWwuY29t
Zonghui Yuan, eXVhbjU4MDJAbWFpbC5oemF1LmVkdS5jbg==
 Qianying Liu
Qianying Liu Zhixin Lei1
Zhixin Lei1 Zonghui Yuan
Zonghui Yuan